- 1Department of Neurology, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Neurology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Department of Neurology, ChongGang General Hospital, Chongqing, China
- 4Department of Neurology, Qujing First People's Hospital, Qujing, China
- 5Department of Neurology, Sichuan Mianyang 404 Hospital, Mianyang, China
- 6Department of Neurosurgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 7Department of Neurology, Xinqiao Hospital and The Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China
Background: There is a lack of data to predict futile recanalization (FR) after endovascular treatment (EVT) in acute anterior circulation large vessel occlusion (ACLVO) with large core infarction.
Methods: This analysis included patients from a national multicenter stroke registry (November 2021 to February 2023). Patients who achieved successful recanalization (expanded Thrombolysis in Cerebral Infarction [eTICI] score ≥2b) after EVT were categorized into two groups: meaningful recanalization (MR; 90-day modified Rankin scale [mRS] 0–3) and FR (mRS 4–6). Multivariate logistic regression was performed to identify independent predictors of FR.
Results: Among 313 patients with successful recanalization, 171 (54.6%) experienced FR, and 142 (45.4%) achieved MR. Multivariate analysis showed that a higher baseline NIH Stroke Scale score (p < 0.001), older age (p < 0.001), elevated blood glucose (p = 0.003), poor collateral circulation (p = 0.004), and incomplete recanalization (eTICI 2b vs. 3; p < 0.001) were predictors of FR.
Conclusion: In patients with ACLVO and large core infarction, age, hyperglycemia, baseline NIHSS, poor collaterals, and incomplete recanalization were independent predictors of FR. These findings may be used to guide treatment decisions and optimize management processes.
1 Introduction
Acute anterior circulation large vessel occlusion (ACLVO) with large core infarct accounts for approximately 20% of large vessel occlusion (LVO) strokes. It is a devastating cerebrovascular disease associated with high mortality and disability rates (1). Endovascular thrombectomy (EVT) with successful recanalization (expanded Thrombolysis in Cerebral Infarction [eTICI] ≥ 2b) is the proven effective treatment for ACLVO with large core infarction. This conclusion is supported by two landmark randomized controlled trials: SELECT2 (2023) (2) and ANGEL-ASPECT (2023) (3). Notably, both trials demonstrated that 53% (ANGEL-ASPECT) and 61.8% (SELECT2) of patients still experienced poor functional outcomes (90-day modified Rankin scale [mRS] 4–6) despite successful recanalization—a phenomenon termed futile recanalization (FR), which similarly affects other high-risk patient subgroups (4–6).
Patient characteristics including age, blood glucose, blood pressure, prehospital time, NIH Stroke Scale (NIHSS) scores, and other clinical variables have been established as independent predictors of FR across diverse populations. Predictive models integrating these factors can demonstrate accuracy and play a pivotal role in bridging fundamental research, imaging evaluation, and clinical decision-making (7–9). Despite the study of FR predictors being a global research priority, few studies have specifically focused on ACLVO with large core infarction selected solely by non-contrast CT (NCCT)-based Alberta Stroke Program Early CT Score (ASPECTS) scores of 3–5.
In this study, we analyzed data from the Prospective Multicenter Registry on Early Management of Acute Ischemic Stroke (MAGIC) to identify FR predictors in this specific population with successful recanalization after EVT.
2 Methods
2.1 Patient selection
Patients included in this study received treatment between November 1, 2021, and February 8, 2023. Our study was a subanalysis utilizing data from a nationwide, prospective registry in China, which enrolled patients presenting with acute large vessel occlusion and received standard treatment within 24 h of their last known well state in China (URL: http://www.chictr.org.cn. Uniform identifier: ChiCTR2100051664). Ethical approval for this study was granted by the institutional review board at the Second Affiliated Hospital of Army Medical University, and additional authorization was obtained from all participating centers’ ethics committees. From the originally published cohort (10), we conducted a subanalysis focusing exclusively on patients who met two additional criteria: (1) large ischemic core on NCCT (defined as an ASPECTS of 3–5); (2) eTICI ≥2b after EVT plus standard medical treatment (SMT).
2.2 Clinical evaluation and outcome
Baseline characteristics included: (1) demographic data; (2) stroke risk factors; (3) laboratory results; (4) stroke severity assessed by NIHSS (11); (5) collateral status evaluated using the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology collateral grading system (ASITN/SIR) (12); (6) stroke etiology classified according to the Trial of ORG10172 in Acute Stroke Treatment (TOAST) classification (13); (7) workflow durations; (8) EVT methodology; (9) recanalization grade (eTICI); (10) location of occlusion; (11) baseline core infarct volume determined by the NCCT-based ASPECTS; (12) Procedure-related complications.
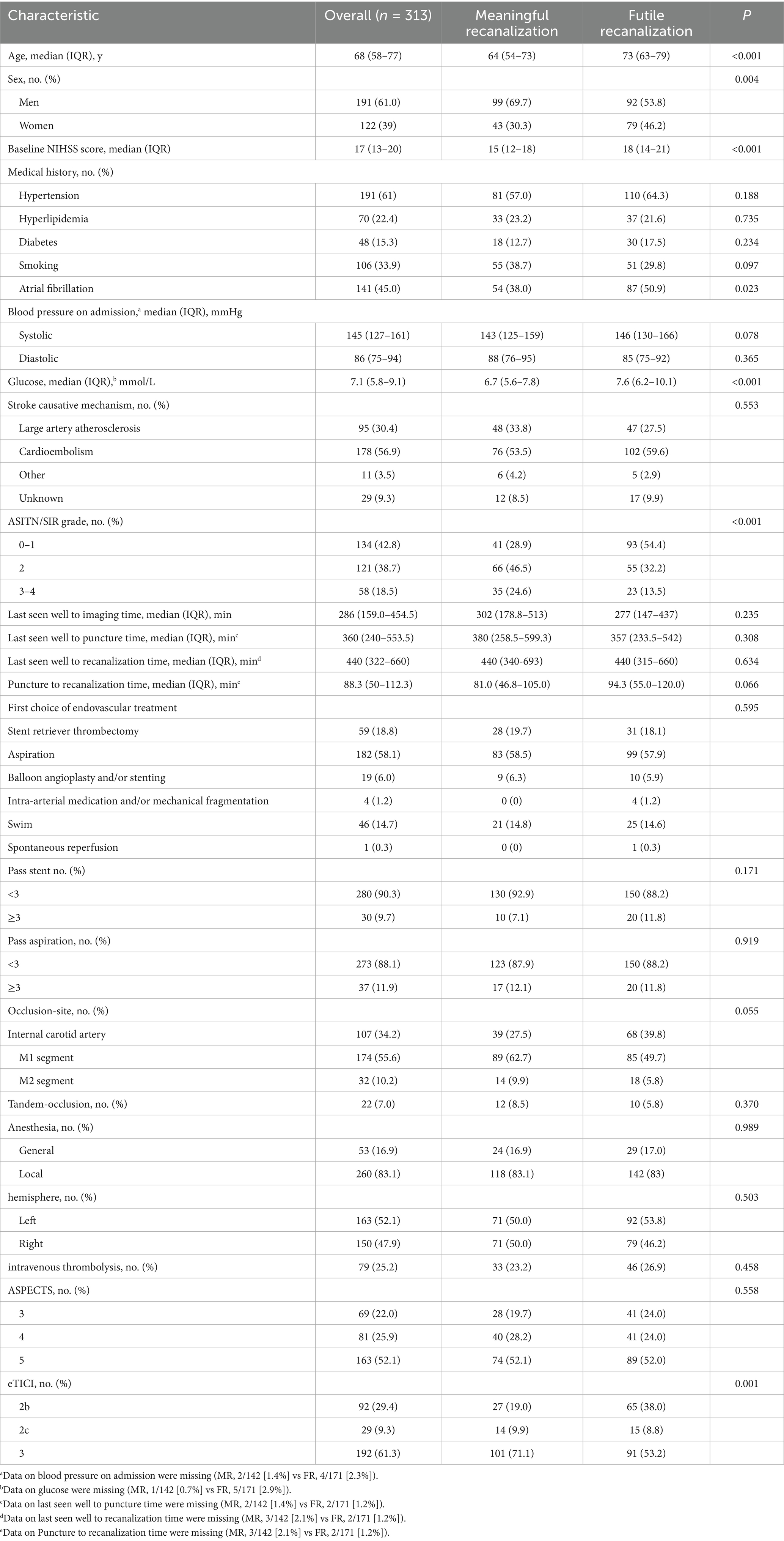
An independent imaging core lab was blinded to treatment allocation and clinical results. Two trained neuroradiologists analyzed all imaging data; they independently evaluated the baseline NCCT-based ASPECTS and assessed occlusion sites using supplemental angiographic imaging. In cases of discrepancies, a third senior neuroradiologist adjudicated any discrepancies. Successful recanalization was defined as eTICI ≥2b (blood flow to greater than 50%). In our study, 90-day functional outcomes were assessed using the mRS through follow-up visits or telephone interviews conducted by trained and experienced local physicians. According to the 90-day mRS, patients were categorized into two groups: FR group (90-day mRS 4–6) and meaningful recanalization (MR) group (90-day mRS 0–3). We present details of the data elements in Table 1.
2.3 Statistical analysis
We performed statistical analyses using SPSS 29.0, employing Mann–Whitney U tests for continuous variables and χ2/Fisher’s exact tests for categorical variables. Significant univariate predictors (p < 0.05) and variables with established clinical relevance as key determinants of infarct progression and recanalization outcomes in ACLVO infarction were included in our multivariable logistic regression model (3, 14). The adjusted covariates comprised age, sex, history of atrial fibrillation, baseline NIHSS score, blood glucose (random admission glucose), ASITN/SIR score, and eTICI grade. The results of multivariable logistic regression were expressed as adjusted odds ratios (aOR) and 95% confidence intervals (CI). Missing data were minimal (0.6–2.9% per variable, see Table 1 footnotes) and handled via complete-case analysis, which is consistent with recommendations for negligible missingness (15). To evaluate the predictive performance of continuous variables in our multivariable logistic regression model, we generated individual ROC curves for each univariate predictor (age, glucose, NIHSS score) and multivariate combinations (age + baseline NIHSS, glucose + baseline NIHSS, glucose + age, age + glucose + NIHSS) using GraphPad Prism 10. The area under the curve (AUC) was used to measure both independent contributions and synergistic predictive effects. To explore potential synergistic effects, we generated distribution surface plots using SigmaPlot 15 to visualize predicted outcome probability variations. For subgroup analyses, we similarly incorporated variables meeting both statistical significance (p < 0.05) and clinical relevance into logistic regression models to explore the occurrence of FR.
3 Results
3.1 Patient characteristics
The MAGIC registry collected data from 38 comprehensive stroke centers throughout China, a total of 745 eligible patients (November 2021 and February 2023). We excluded 255 patients who received only SMT, 135 patients with NCCT-based ASPECTS scores of 0–2, and 42 patients who did not achieve successful recanalization (eTICI ≥ 2b). Ultimately, 313 patients were included in the final analysis. Among these, 142 patients (45.4%) were categorized into the meaningful recanalization (MR) group, while 171 patients (54.6%) were classified into the FR group. The study flowchart is presented in Figure 1.
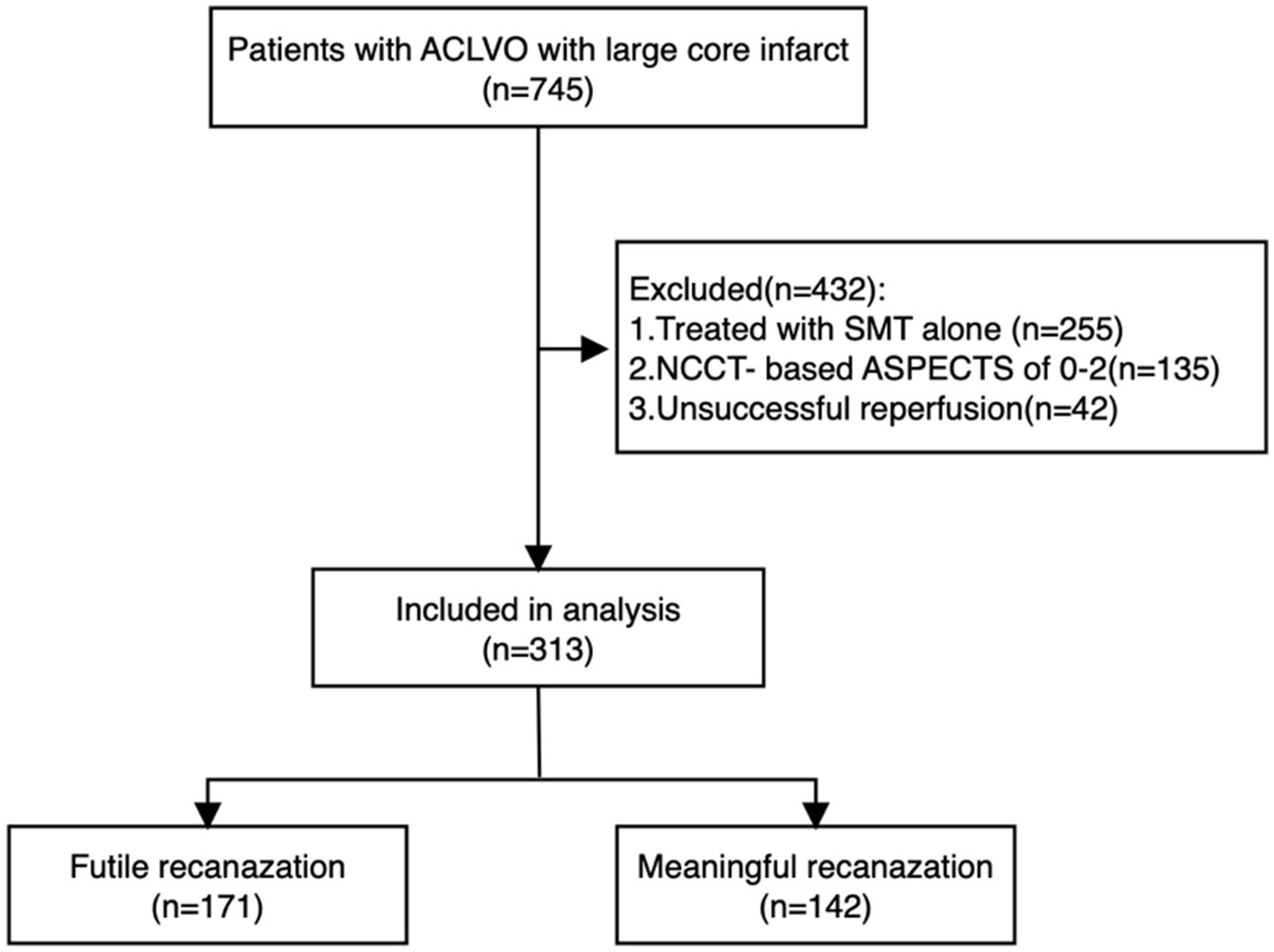
Figure 1. Flow diagram of our study. Flow diagram of our study. ACLVO, anterior circulation large vessel occlusion; SMT, standard medical treatment. NCCT-based ASPECTS, non-contrast computed tomography-Alberta Stroke Program Early CT Score.
Compared with the MR group, patients in the FR group were significantly older, had higher blood glucose levels, and higher baseline NIHSS scores. Additionally, the FR group showed a greater proportion of male patients, a higher percentage of atrial fibrillation, and a worse distribution of ASITN/SIR grades and eTICI grades (Table 1).
3.2 Predictors of futile recanalization
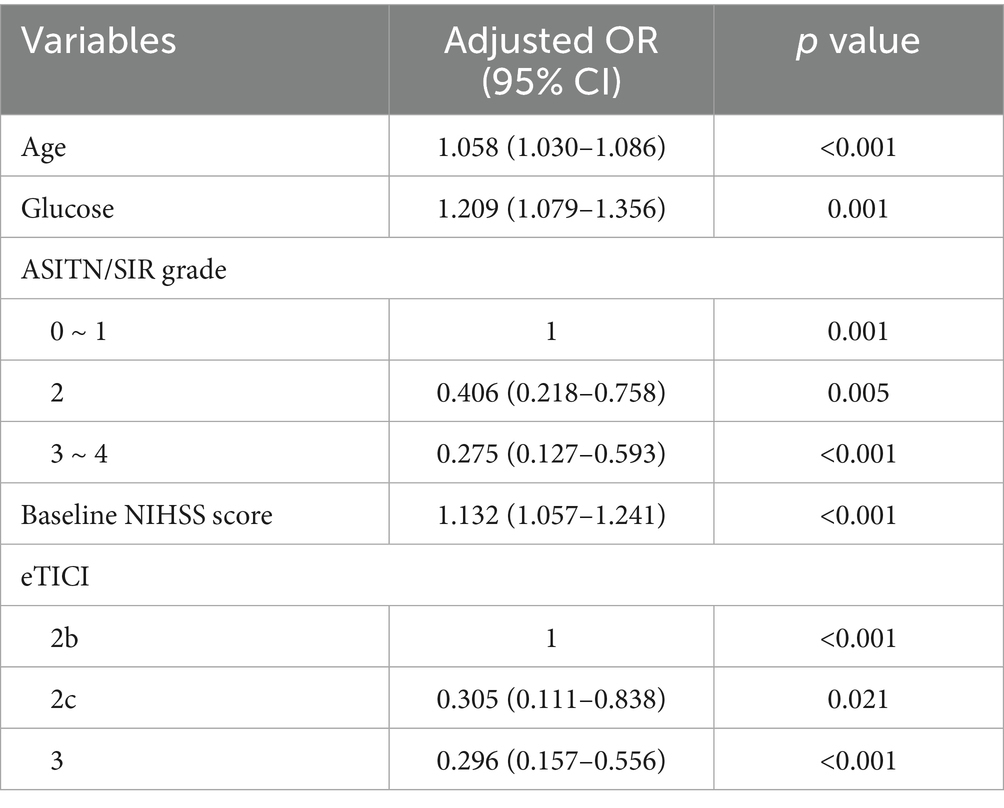
A multivariate logistic regression model was developed to identify independent predictors of FR in patients who achieved successful recanalization after EVT. The analysis revealed that higher baseline NIHSS scores, older age, elevated blood glucose, poor collateral circulation, and incomplete recanalization were significantly associated with FR (Table 2).
ROC analysis confirmed the predictive utility of these three independent predictors, with combined models showing improved discrimination (AUC = 0.7546, 95% CI, 0.7015–0.8078, p < 0.001) (Figure 2). To enhance clinical applicability, we derived optimal glucose cut-off values (7.5 mmol/L, which could effectively distinguish between MR and FR) using the maximum Youden’s index for subsequent subgroup analyses.
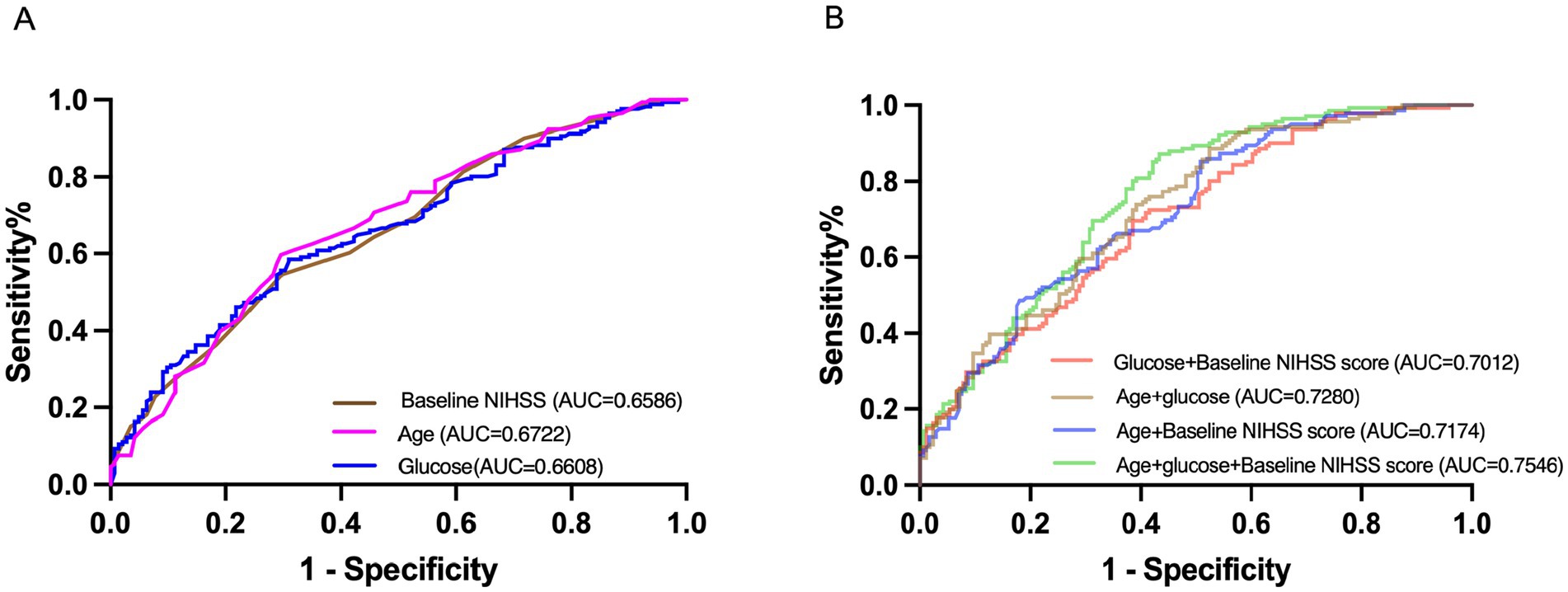
Figure 2. Receiver-operating characteristic curves. (A) Showed the single variables: baseline NIHSS, age, and glucose for futile recanalization. (B) Showed the multivariate variables: age + baseline NIHSS, age + glucose, glucose + baseline NIHSS, and age + glucose + baseline NIHSS for futile recanalization. AUC, area under the curve; NIHSS, National Institutes of Health Stroke Scale.
All variables in Figure 3 are continuous. The figure demonstrates the relationships between various predictors and the probability of FR. Figure 3A reveals a linear association between increasing blood glucose levels and higher FR probability when NIHSS scores are held constant. Notably, patients with higher NIHSS scores exhibit a steeper glucose-FR probability slope. Figure 3B demonstrates that concurrent elevations in both blood glucose and age are associated with increased probability of FR.
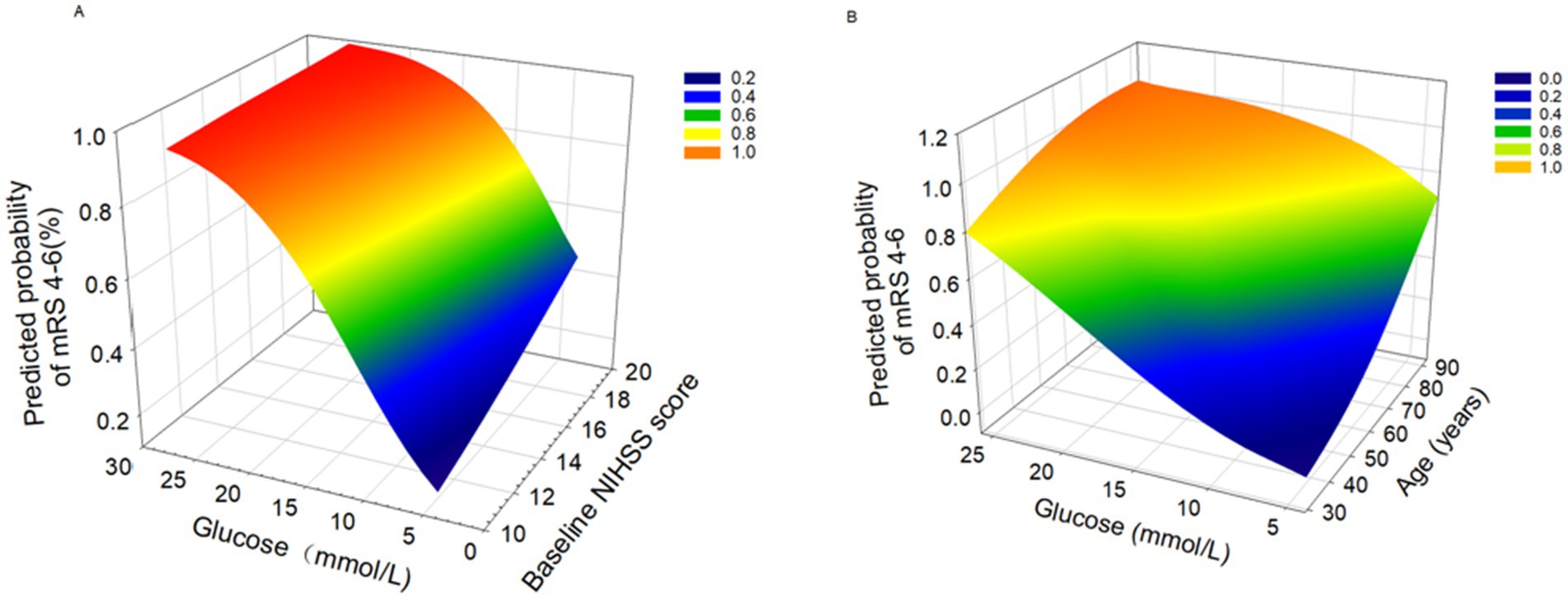
Figure 3. 3D surface plots of predictors for futile rencanalization (defined as 90-day modified Rankin Scale [mRS] 4-6). (A) Association between glucose levels and baseline NIHSS score with predicted probability of mRS 4-6. (B) Association between glucose levels and age with predicted probability of mRS 4-6.
3.3 Subgroup analysis
Establishing population-specific treatment thresholds for blood glucose is critical. Hyperglycemia in hospitalized patients was defined as any glucose level ≥140 mg/dL (7.8 mmol/L) (16). We adopted the optimal predictive cutoff value (7.5 mmol/L, closely aligned to 7.8 mmol/L) identified in this analysis as the dichotomization threshold to explore the relationship and mechanisms between glucose levels and FR in our study.
After excluding 6 cases with missing data, the final cohort comprised 307 patients. We used the cutoff value (7.5 mmol/L) to stratify patients into two groups: low glucose (LG) group (glucose <7.5 mmol/L, n = 170) and high glucose (HG) group (glucose ≥7.5 mmol/L, n = 137). Univariate analysis results are presented in Supplementary Table 1.
In the multivariate logistic regression analysis, age was identified as an independent predictor in both groups. Additionally, in the LG group, higher baseline NIHSS scores were associated with FR. Interestingly, in the HG group, baseline NIHSS scores were not a predictor of FR. We also found that FR was significantly associated with ASITN/SIR grade in the HG group, whereas in the LG group, FR showed a correlation with the eTICI grade (Supplementary Table 2). We assessed collinearity between age, high glucose levels (glucose ≥ 7.5 mmoL/L), and collateral status (ASTIN/SIR grade). All VIF values were < 2 (age: 1.042, glucose: 1.000, collateral status: 1.042).
There were 94 (68.6%) patients with FR in the HG group, and 72 (42.4%) patients with FR in the LG group. We also found that among 260 (84.7%) non-diabetic patients and 47 (15.3%) diabetic patients in the cohort, the LG group, 7 (4.1%) patients had diabetes, with 5 (5.1%) cases in the MR group and 2 (2.8%) cases in the FR group. In contrast, the HG group contained 40 (29.2%) diabetic patients, comprising 13 (30.2%) in the MR group and 27 (28.7%) in the FR group. Notably, diabetes prevalence was 7-fold higher in the HG stratum (29.2% vs. 4.1%), yet diabetic status itself did not modify FR risk within either group (HG: 28.7% FR in diabetics vs. 30.2% MR; LG: 2.8% vs. 5.1%) (Supplementary Table 3). These findings suggest that acute hyperglycemia, rather than chronic diabetic status, drives FR.
4 Discussion
This study selected patients with ACLVO and large core infarction based solely on NCCT-based ASPECTS scores of 3–5. Our results demonstrated an FR incidence of 54.6%, closely mirroring the 53% reported by Huo et al. (3). However, their study enrolled patients based on Computed Tomography Perfusion (CTP). These findings suggest that functional outcomes are comparable between the two imaging modalities, and the findings align with prior research (17, 18). This does not, however, negate the potential benefits of advanced imaging techniques in improving outcomes. While advanced neuroimaging may improve patient selection for EVT (19, 20), strict reliance on these modalities risks excluding potentially eligible candidates, delaying treatment, and increasing the likelihood of unfavorable outcomes. Moreover, such imaging techniques remain inaccessible at many hospitals, particularly in resource-limited settings. In contrast, NCCT is widely available and routinely used in acute stroke workflows, offering a practical alternative for timely decision-making (21, 22).
Our study’s predictors: age, NIHSS score, eTICI grade, and ASITN/SIR grade are fundamental and strong in most models (23–25). Their predictive value is well-established: age reflects recovery capacity; NIHSS score quantifies baseline stroke severity. These factors are clinically easy to assess. Additionally, achieving higher eTICI grades directly improves outcomes. This is a key focus in neurointerventional practice. The mechanisms behind these predictors are clear and measurable. They provide reliable clinical guidance.
The relationship between acute hyperglycemia and FR remains controversial (26–28). Two large-scale RCTs have demonstrated that intensive perioperative glycemic control did not improve clinical outcomes (29, 30), which predominantly suggested no significant association between hyperglycemia and FR. However, our study supports a robust correlation between acute hyperglycemia and futile recanalization (FR), and these findings align with both the RESCUE-Japan LIMIT trial subgroup analysis and the conclusions of Tang et al.—despite some differences in specific thresholds and FR rates. Collectively, they point to a consistent trend regarding the relationship between glycemia and outcomes in such patients (16, 31). We argue that the negative results from glycemic control trials should not be interpreted as disproving a hyperglycemia-FR relationship, since treatment failure may reflect intervention timing rather than mechanism irrelevance. The rapid-onset pathological effects of acute hyperglycemia—including oxidative stress, inflammatory activation, and microthrombosis-likely cause irreversible damage before treatment initiation (32). Although current mechanistic understanding remains incomplete, these findings strongly support the need for further investigation into the underlying pathways.
Furthermore, the SELECT2 trial highlights that post-stroke care quality, rehabilitation strategies, and socioeconomic factors may significantly influence long-term patient outcomes. However, our current study is limited by the lack of longer-term longitudinal follow-up data (1-year or 3-year functional outcome). Future studies incorporating extended follow-up periods and comprehensive socioeconomic assessments are warranted to validate these observations.
4.1 Limitations
As with all multicenter prospective clinical studies, center-specific effects are inevitable. Due to the limited sample size in our study, we were unable to further validate the robustness of our findings using mixed-effects models, and the subanalysis’s results should also be interpreted with caution. Additionally, the lack of documentation regarding whether random admission blood glucose levels were measured under fasting conditions introduces a potential limitation to our subgroup analyses based on dichotomized cut-off values.
5 Conclusion
In conclusion, a significant proportion (54.6%) of ACLVO with large core infarct and NCCT-based ASPECTS scores of 3–5 experienced FR. The key predictors included higher NIHSS scores, older age, elevated blood glucose, poor collateral circulation, and incomplete recanalization. Further investigation into modifiable factors—particularly hyperglycemia’s role in reperfusion injury—may reveal therapeutic targets to reduce FR rates.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Second Affiliated Hospital of the Army Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of our previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QL: Investigation, Writing – original draft, Writing – review & editing, Conceptualization. CD: Writing – review & editing, Investigation, Writing – original draft. BC: Investigation, Writing – review & editing, Writing – original draft. ZT: Investigation, Writing – review & editing. YC: Formal analysis, Writing – review & editing. LL: Investigation, Writing – review & editing. NY: Writing – review & editing, Investigation. JS: Writing – review & editing, Investigation. JY: Investigation, Writing – review & editing. CG: Investigation, Writing – review & editing. JH: Investigation, Writing – review & editing. WZ: Writing – review & editing, Supervision, Resources. ZY: Supervision, Funding acquisition, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The project was supported by the Chongqing Science and Health Project (2025ZDXM003). Chongqing Natural Science Foundation (CSTB2023NSCQ-MSX0711.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1630438/full#supplementary-material
References
1. Sarraj, A, Hassan, AE, Savitz, S, Sitton, C, Grotta, J, Chen, P, et al. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient’s selection for endovascular treatment in acute ischemic stroke (SELECT) study. JAMA Neurol. (2019) 76:1147–56. doi: 10.1001/jamaneurol.2019.2109
2. Yoshimura, S, Sakai, N, Yamagami, H, and Uchida, K. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. (2024) 390:388. doi: 10.1056/NEJMx230009
3. Huo, X, Ma, G, Tong, X, Zhang, X, Pan, Y, Nguyen, TN, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. (2023) 388:1272–83. doi: 10.1056/NEJMoa2213379
4. Sang, H, Li, F, Yuan, J, Liu, S, Luo, W, Wen, C, et al. Values of baseline posterior circulation acute stroke prognosis early computed tomography score for treatment decision of acute basilar artery occlusion. Stroke. (2021) 52:811–20. doi: 10.1161/STROKEAHA.120.031371
5. Yang, J, Jin, Z, Song, J, Guo, C, Xie, D, Yue, C, et al. Futile recanalization after endovascular treatment in patients with acute basilar artery occlusion. Neurosurgery. (2023) 92:1006–12. doi: 10.1227/neu.0000000000002313
6. Meinel, TR, Lerch, C, Fischer, U, Beyeler, M, Mujanovic, A, Kurmann, C, et al. Multivariable prediction model for futile recanalization therapies in patients with acute ischemic stroke. Neurology. (2022) 99:e1009–18. doi: 10.1212/wnl.0000000000200815
7. Liu, Y, Yu, Y, Ouyang, J, Jiang, B, Yang, G, Ostmeier, S, et al. Functional outcome prediction in acute ischemic stroke using a fused imaging and clinical deep learning model. Stroke. (2023) 54:2316–27. doi: 10.1161/strokeaha.123.044072
8. Denorme, F, Portier, I, Rustad, JL, Cody, MJ, De Araujo, CV, Hoki, C, et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. (2022) 132:e154225. doi: 10.1172/jci154225
9. MacDougall, NJJ, and Muir, KW. Hyperglycaemia and infarct size in animal models of middle cerebral artery occlusion: systematic review and Meta-analysis. J Cereb Blood Flow Metab. (2011) 31:807–18. doi: 10.1038/jcbfm.2010.210
10. Guo, C, Li, L, Huang, J, Yang, J, Song, J, Huang, J, et al. Endovascular treatment versus standard medical treatment in patients with established large infarct: a cohort study. Int J Surg. (2024) 110:4775–84. doi: 10.1097/JS9.0000000000001539
11. Brott, T, Adams, HP, Olinger, CP, Marler, JR, Barsan, WG, Biller, J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.str.20.7.864
12. Higashida, RT, and Furlan, AJ. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. (2003) 34:e109–37. doi: 10.1161/01.STR.0000082721.62796.09
13. Hp, A, Bh, B, Lj, K, J, B, Bb, L, Dl, G, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.str.24.1.35
14. Yoshimura, S, Sakai, N, Yamagami, H, Uchida, K, Beppu, M, Toyoda, K, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. (2022) 386:1303–13. doi: 10.1056/nejmoa2118191
15. Graham, JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. (2009) 60:549–76. doi: 10.1146/annurev.psych.58.110405.085530
16. Tanaka, K, Yoshimoto, T, Koge, J, Yamagami, H, Imamura, H, Sakai, N, et al. Detrimental effect of acute hyperglycemia on the outcomes of large ischemic region stroke. J Am Heart Assoc. (2024) 13:e034556. doi: 10.1161/jaha.124.034556
17. Nogueira, RG, Haussen, DC, Liebeskind, D, Jovin, TG, Gupta, R, Jadhav, A, et al. Stroke imaging selection modality and endovascular therapy outcomes in the early and extended time windows. Stroke. (2021) 52:491–7. doi: 10.1161/strokeaha.120.031685
18. Miao, J, Sang, H, Li, F, Saver, JL, Lei, B, Li, J, et al. Effect of imaging selection paradigms on endovascular thrombectomy outcomes in patients with acute ischemic stroke. Stroke. (2023) 54:1569–77. doi: 10.1161/strokeaha.122.042203
19. Kobeissi, H, Ghozy, S, Adusumilli, G, Bilgin, C, Tolba, H, Amoukhteh, M, et al. CT perfusion vs noncontrast CT for late window stroke thrombectomy: a systematic review and Meta-analysis. Neurology. (2023) 100:e2304–11. doi: 10.1212/wnl.0000000000207262
20. Dhillon, PS, Butt, W, Podlasek, A, McConachie, N, Lenthall, R, Nair, S, et al. Perfusion imaging for endovascular thrombectomy in acute ischemic stroke is associated with improved functional outcomes in the early and late time windows. Stroke. (2022) 53:2770–8. doi: 10.1161/strokeaha.121.038010
21. García-Tornel, Á, Campos, D, Rubiera, M, Boned, S, Olivé-Gadea, M, Requena, M, et al. Ischemic core overestimation on computed tomography perfusion. Stroke. (2021) 52:1751–60. doi: 10.1161/STROKEAHA.120.031800
22. Wintermark, M, Luby, M, Bornstein, NM, Demchuk, A, Fiehler, J, Kudo, K, et al. International survey of acute stroke imaging used to make revascularization treatment decisions. Int J Stroke. (2015) 10:759–62. doi: 10.1111/ijs.12491
23. Heitkamp, C, Heitkamp, A, Winkelmeier, L, Thaler, C, Flottmann, F, Schell, M, et al. Predictors of futile recanalization in ischemic stroke patients with low baseline NIHSS. Int J Stroke. (2024) 19:1102–12. doi: 10.1177/17474930241264737
24. Kniep, H, Meyer, L, Broocks, G, Bechstein, M, Heitkamp, C, Winkelmeier, L, et al. Thrombectomy for M2 occlusions: predictors of successful and futile recanalization. Stroke. (2023) 54:2002–12. doi: 10.1161/strokeaha.123.043285
25. Heitkamp, A, Hierholzer, SM, Heitkamp, C, Winkelmeier, L, Meyer, L, Bechstein, M, et al. Key to better outcomes in stroke intervention: early versus complete reperfusion in first pass recanalization. J Neurol. (2025) 272:504. doi: 10.1007/s00415-025-13235-5
26. Merlino, G, Romoli, M, Ornello, R, Foschi, M, Del Regno, C, Toraldo, F, et al. Stress hyperglycemia is associated with futile recanalization in patients with anterior large vessel occlusion undergoing mechanical thrombectomy. Eur Stroke J. (2024) 9:613–22. doi: 10.1177/23969873241247400
27. Torbey, MT, Pauls, Q, Gentile, N, Falciglia, M, Meurer, W, Pettigrew, CL, et al. Intensive versus standard treatment of hyperglycemia in acute ischemic stroke patient: a randomized clinical trial subgroups analysis. Stroke. (2022) 53:1510–5. doi: 10.1161/strokeaha.120.033048
28. Zhang, J, Dong, D, Zeng, Y, Yang, B, Li, F, Chen, X, et al. The association between stress hyperglycemia and unfavorable outcomes in patients with anterior circulation stroke after mechanical thrombectomy. Front Aging Neurosci. (2023) 14:14. doi: 10.3389/fnagi.2022.1071377
29. Gray, CS, Hildreth, AJ, Sandercock, PA, O’Connell, JE, Johnston, DE, Cartlidge, NE, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK glucose insulin in stroke trial (GIST-UK). Lancet Neurol. (2007) 6:397–406. doi: 10.1016/s1474-4422(07)70080-7
30. Johnston, KC, Bruno, A, Pauls, Q, Hall, CE, Barrett, KM, Barsan, W, et al. Intensive vs standard treatment of hyperglycemia and functional outcome in patients with acute ischemic stroke: the SHINE randomized clinical trial. JAMA. (2019) 322:326–35. doi: 10.1001/jama.2019.9346
31. Tang, T, Zhang, D, Wang, F, Fan, T, Thomas, AM, Lan, X, et al. Impact of neutrophil-to-lymphocyte ratio on the effect of hyperglycemia at admission on clinical outcomes after endovascular thrombectomy. Neuroprotection. (2024) 2:196–202. doi: 10.1002/nep3.55
Keywords: anterior circulation large vessel occlusion, large core ischemic stroke, endovascular treatment, futile recanalization, modified Rankin scale
Citation: Li Q, Ding C, Chen B, Tian Z, Chen Y, Li L, Yu N, Song J, Yang J, Guo C, Huang J, Zi W and Yang Z (2025) Predictors of futile recanalization after endovascular therapy in anterior circulation stroke with large core infarction. Front. Neurol. 16:1630438. doi: 10.3389/fneur.2025.1630438
Edited by:
Xiaoyan Lan, Affiliated Central Hospital of Dalian University of Technology, ChinaReviewed by:
Qazi Zeeshan, University of Pittsburgh Medical Center, United StatesChunrong Tao, Anhui Provincial Hospital, China
Tao Tang, Capital Medical University, China
Copyright © 2025 Li, Ding, Chen, Tian, Chen, Li, Yu, Song, Yang, Guo, Huang, Zi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhao Yang, eWFuZ3poYW81MTQwQHNvaHUuY29t; Wenjie Zi, eml3ZW5qaWVAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Qinhong Li1†
Qinhong Li1† Yujie Chen
Yujie Chen Jiaxing Song
Jiaxing Song Jiacheng Huang
Jiacheng Huang Wenjie Zi
Wenjie Zi Zhao Yang
Zhao Yang