Abstract
Background:
Neuroinflammation plays an important role in the development and progression of epilepsy. It can be both a result and a potential cause of those seizures. MiR-155 plays a crucial role in inflammatory responses, and its expression is upregulated under various neuroinflammatory conditions. This study aims to compare the expression levels of miR-155 in the brain tissue or serum of epilepsy patients and healthy controls, assessing the correlation between miR-155 levels and epilepsy.
Objective:
The meta-analysis evaluates to assess and compare the levels of miR-155 in the brain tissue and serum of epilepsy patients and healthy controls.
Methods:
The databases PubMed, Embase, Cochrane Library, CNKI, VIP, and WanFang DATA were searched from inception until Dec 30, 2024 by two researchers. The relative expression level of miR-155 in the tissues or serum was the primary outcome of the search. After extracting data independently, the two researchers used the Cochrane Collaboration’s tool for assessing risk of bias. All data were analyzed using Review Manager (V.5.4.1) statistical software.
Results:
This meta-analysis (nine studies, 394 patients) reveals elevated miR-155 in epilepsy patients vs. controls (SMD = 1.62, p = 0.001), especially in brain tissue. Subgroups confirm consistency across ages/regions. Subgroup analysis revealed no significant differences by country (p = 0.31), age category (p = 0.63) and sample source (p = 0.15), indicating robust consistency of the primary findings. MiR-155 may serve as a neuroinflammatory biomarker in epilepsy.
Conclusion:
Compared to healthy controls, the relative expression level of miR-155 in the brain tissue or serum of epilepsy patients increased.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/, identifier: CRD42024558255.
1 Introduction
Epilepsy is a chronic, non-communicable brain disorder that affects individuals of all ages and is characterized by recurrent seizures resulting from abnormal electrical signals produced by damaged brain cells (1). Approximately 70 million people worldwide have epilepsy, with 80% living in low and middle-income countries. It is estimated that 5 million people are diagnosed with epilepsy each year, with a prevalence rate of 6–7%. In China, there are about 9 million epilepsy patients, with around 5–6 million experiencing active epilepsy (2). Poverty, lack of medical resources, and health facilities are major factors affecting the effective treatment of epilepsy patients in above-mentioned countries (3). As a chronic neurological disorder, epilepsy remains a significant public health issue affecting millions of people globally. Patients with epilepsy require long-term medical care and management, but due to the uneven distribution of healthcare resources, many of them do not have access to timely and effective treatment, leading to exacerbation of their condition and further aggravating the pressure on the public health system.
Currently, the diagnosis of epilepsy primarily relies on clinical assessment, including medical history, seizure description, and electroencephalogram (EEG) (4). Imaging techniques, such as magnetic resonance imaging (MRI) and computed tomography (CT), also play a crucial role in identifying potential brain structural abnormalities associated with epilepsy (5, 6). However, despite the availability of these tools, there are still considerable challenges in accurately diagnosing epilepsy, especially when traditional test results are inconclusive. This is where biomarkers emerge as promising adjuncts in the diagnosis and treatment of epilepsy. Several biomarkers such as neurotransmitters and inflammatory markers have been identified for use in diagnosis or monitoring of epilepsy.
MicroRNAs (miRNAs) are single-stranded RNAs universally present in eukaryotic organisms and serve as major regulators of gene expression (7). Individual miRNA can affect multiple proteins across different molecular pathways and networks. Among them, the study of miR-155 as a diagnostic marker for epilepsy gradually develops, with some evidence suggesting its potential in the diagnosis of epilepsy. First, miR-155 plays an important role in various inflammatory responses, and the inflammatory mechanism is believed to play a key role in the pathology of epilepsy (8, 9). Second, epilepsy may not have obvious clinical manifestations in its early stages, especially during the interictal period, where routine tests may fail to capture abnormalities. However, abnormal expression of miR-155 may appear early or even before seizures occur, suggesting its potential to aid in early diagnosis. Nonetheless, there is still no direct evidence linking miR-155 to epilepsy. This meta-analysis aims to observe the expression levels of miR-155 in the brain tissue or serum of epilepsy patients compared to healthy controls, assessing the correlation between miR-155 levels and epilepsy.
2 Methods
This study adheres to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement 2020 and has been registered on the PROSPERO website (registration number: CRD42024558255).
2.1 Search strategy and selection criteria
We researchers independently conducted a literature search in the PubMed, Embase, Cochrane Library, CNKI, WanFang DATA, and VIP databases for articles published prior to December 30, 2024. The search terms used were: (“epilepsy” OR “epilepsies” OR “seizure disorder” OR “seizure disorders”) AND (“miRNAc-155” OR “miR-155”). Inclusion criteria consisted of case–control that reported on the levels of miR-155 in the serum and tissue of epilepsy patients and control groups. Exclusion criteria included studies with overlapping populations, animal studies, case reports, systematic reviews, commentaries, letters, and reviews.
2.2 Data extraction
Two researchers (YW and LMD) independently extracted data from the included studies and cross-validated their results. They initially screened the literature by reviewing titles and abstracts for relevance, followed by a full-text review. Information extracted included the title, authors, publication year, country, article type, study population, research methodology, conclusions, sample size, age and gender of both epilepsy patients and controls, type of epilepsy, source of samples, and relative expression levels of miR-155. In cases of disagreement between the two reviewers, a third reviewer was consulted for adjudication. The literature was managed using EndNote (version X9; Thomson Reuters LLC, Philadelphia, PA, USA), and a manual review was conducted to finalize the selection of studies included in the review and meta-analysis. The risk of bias in randomized controlled trials (RCTs) was assessed using the Cochrane Risk of Bias tool (RoB 2), while the quality of non-randomized studies was evaluated using the Newcastle-Ottawa Scale (NOS).
2.3 Meta-analysis
We conducted the meta-analysis using Review Manager version 5.4. Given that the measurement methods across studies varied slightly, we calculated the effect size using the standardized mean difference (SMD). The SMD represents the difference in miR-155 levels between epilepsy patients and their corresponding controls. For studies with small sample sizes, we utilized Hedges’ g as the effect size measure to mitigate bias associated with small sample sizes. The I2 statistic was employed to describe the heterogeneity among the included studies; p < 0.00001 and an I2 value of 69% indicated significant heterogeneity among the studies. In light of this heterogeneity, we performed a random-effects analysis. Potential sources of heterogeneity included patient characteristics (sex, age, socioeconomic status) and study variations (geographical location, sample sizes). To explore the sources of heterogeneity, we conducted subgroup analyses.
3 Results
3.1 Study selection
By conducting a search in PubMed, Embase, Cochrane Library, CNKI, WanFang DATA, and VIP databases, we identified a total of 75 references. After excluding 28 duplicate articles, a detailed screening was performed on the remaining 47 publications. Among these, nine review articles, 1 meta-analysis and 9 articles were excluded due to missing data. Additionally, nine articles were removed because their results were not relevant to this analysis, and three articles had unavailable full texts with no response received after contacting the authors. In addition, we also screened seven animal experimental studies related to this analysis (10–16). Ultimately, a total of 9 studies were included in the analysis (17–25). The detailed process of study selection is illustrated in Figure 1.
Figure 1

The diagram illustrating the systematic literature search.
3.2 Details of studies
The detailed information of the included studies is presented in Table 1. All included clinical trial studies were case–control studies, and no cohort studies were included as this research focuses on retrospective analysis. While the majority of studies originated from China (17–23) (n = 5), our analysis also included one Netherlands (24) study one South Korea study (25). The samples used for miR-155 measurement primarily consisted of serum and brain tissue. Among the nine included studies, two did not specify the age of the samples, three involved children epilepsy patients, and the remaining four targeted adults. The quality of the included studies ranged from 5 to 7, with a mean quality score of 5.89 (Table 2). All studies met the predefined inclusion criteria.
Table 1
| Country | Sample sizes (TLE: CO) | Age category | Epilepsy type | Type of miRNAs | Sample source | Assay type | Mean age | miR-155 relative expression (Mean ± sd) | p-value |
|---|---|---|---|---|---|---|---|---|---|
| China | TLE: CO = 12:11 | NA | TLE | miR-155 | brain tissue | RT-qPCR | TLE (age, 9–69) | TLE:1.62 ± 1.18 CO:0.53 ± 0.66 |
<0.05 |
| China | TLE: CO = 68:42 | NA | TLE | miR-155 | brain tissue | RT-qPCR | TLE (age, 9–69) | TLE:2.08 ± 1.81 CO:1.14 ± 0.52 |
<0.01 |
| China | TLE: CO = 7:8 | Adult | TLE | miR-155 | brain tissue | RT-qPCR | TLE (age, 19–43) CO (age, 16–48) |
TLE:5.6 ± 1.2 CO:3 ± 0.55 |
<0.01 |
| Netherlands | TLE: CO = 16:6 | Adult | TLE | miR-155 | brain tissue | RT-qPCR | TLE (age, 24–49) CO (age, 25–86) |
TLE:3 ± 1.28 CO:1 ± 0.42 |
<0.001 |
| China | TLE: CO = 8:8 | Minor | MTLE | miR-155 | brain tissue | RT-qPCR | TLE (age, 8–13) CO (age, 8–12) |
TLE:0.8 ± 0.17 CO:0.4 ± 0.1 |
<0.05 |
| China | TLE: CO = 41:39 | Adult | TLE | miR-155 | brain tissue | RT-qPCR | TLE (age, 33–39) CO (age, 33–39) |
TLE:1.1 ± 0.83 CO:0.22 ± 0.13 |
<0.05 |
| China | TLE: CO = 23:8 | Adult | TLE | miR-155 | brain tissue | RT-qPCR | TLE (age, 15–80) CO (age, 15–80) |
TLE:2.95 ± 1.05 CO:1.1 ± 0.08 |
<0.001 |
| China | TLE: CO = 43:43 | Minor | TLE | miR-155 | Serum | RT-qPCR | TLE (age, 6–11) CO (age, 6–11) |
TLE:1.82 ± 0.39 CO:1.18 ± 0.39 |
<0.05 |
| Korea | TLE: CO = 8:3 | Minor | TLE | miR-155 | brain tissue | RT-qPCR | TLE (age, 7) CO (age, 6) |
TLE:9.8 ± 2.69 CO:1.3 ± 0.64 |
<0.05 |
Characteristics of the included studies.
NA, Not applicable.
Table 2
| Year | Studies | Selection | Comparability | Exposure | Total |
|---|---|---|---|---|---|
| 2013 | Ashhab, Muhammad | 4 | 1 | 1 | 6 |
| 2014 | Lee, Ji Yeoun | 4 | 1 | 1 | 6 |
| 2017 | L-G. Huang | 4 | 0 | 1 | 5 |
| 2018 | Li, Tao-Ran | 4 | 1 | 1 | 6 |
| 2018 | Zhang, Zhijie | 4 | 1 | 2 | 7 |
| 2018 | Korotkov, Anatoly | 4 | 1 | 1 | 6 |
| 2019 | Fu, Huajun | 4 | 0 | 1 | 5 |
| 2021 | Zhu, Zhujun | 4 | 1 | 1 | 6 |
| 2022 | Liu, Ya | 4 | 1 | 1 | 6 |
The quality of the selected studies was assessed using the NOS.
3.3 Mean result between miR-155 and epilepsy
We systematically generated forest plots encompassing data from 9 clinical trial studies, followed by comprehensive subgroup analyses. The meta-analysis revealed a statistically significant positive correlation between miR-155 relative expression levels and epilepsy (SMD = 1.62; 95% CI, 1.14–2.10, p = 0.001) (Figure 2). This finding is consistent with multiple lines of evidence demonstrating miR-155 involvement in cellular proliferation and apoptosis pathways, particularly in predicting the risk of post-traumatic epilepsy, thereby suggesting its crucial role in epileptogenesis (26). Notably, investigations focusing on children epilepsy populations have further substantiated miR-155’s regulatory function in epileptogenic processes (27). Moreover, emerging evidence from molecular biomarker correlation studies has identified miR-155 as a promising candidate biomarker for both acute seizure events and status epilepticus (28).
Figure 2
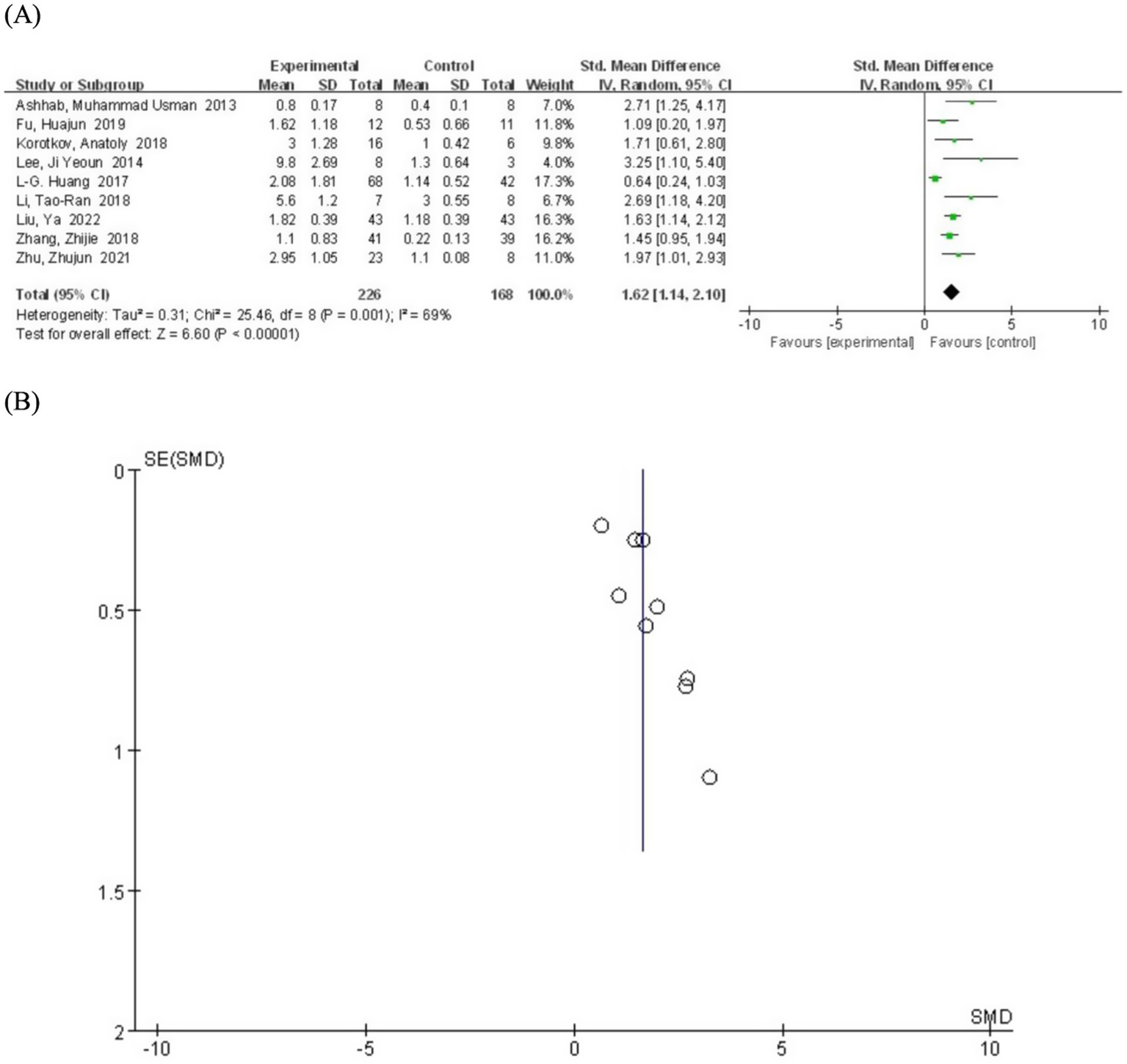
(A) Forest plot depicting the relationship between miR-155 level and epilepsy. (B) A funnel plot to assess publication bias.
3.4 Subgroup analysis by country
We conducted subgroup analyses based on the countries of the study participants to explore potential regional differences in intervention effects. The main results by country are as follows: the miR-155 levels in epilepsy patients were significantly higher than those in the control population in China (SMD = 1.53; 95% CI, 1.02–2.05; p = 0.001) (Figure 3), Netherlands (SMD = 1.71; 95% CI, 0.61–2.80; p = 0.002), and South Korea (SMD = 3.25; 95% CI, 1.10–5.40, p = 0.003). Although the data for the Netherlands and South Korea were derived from single studies (precluding calculation of I2 heterogeneity values), the pooled effect size from subgroup analysis (SMD = 1.62; 95% CI, 1.14–2.10; p = 0.001) demonstrates consistent elevation of miR-155 levels in epilepsy patients. Nevertheless, Future studies with larger sample sizes from these countries are needed to confirm this observation.
Figure 3
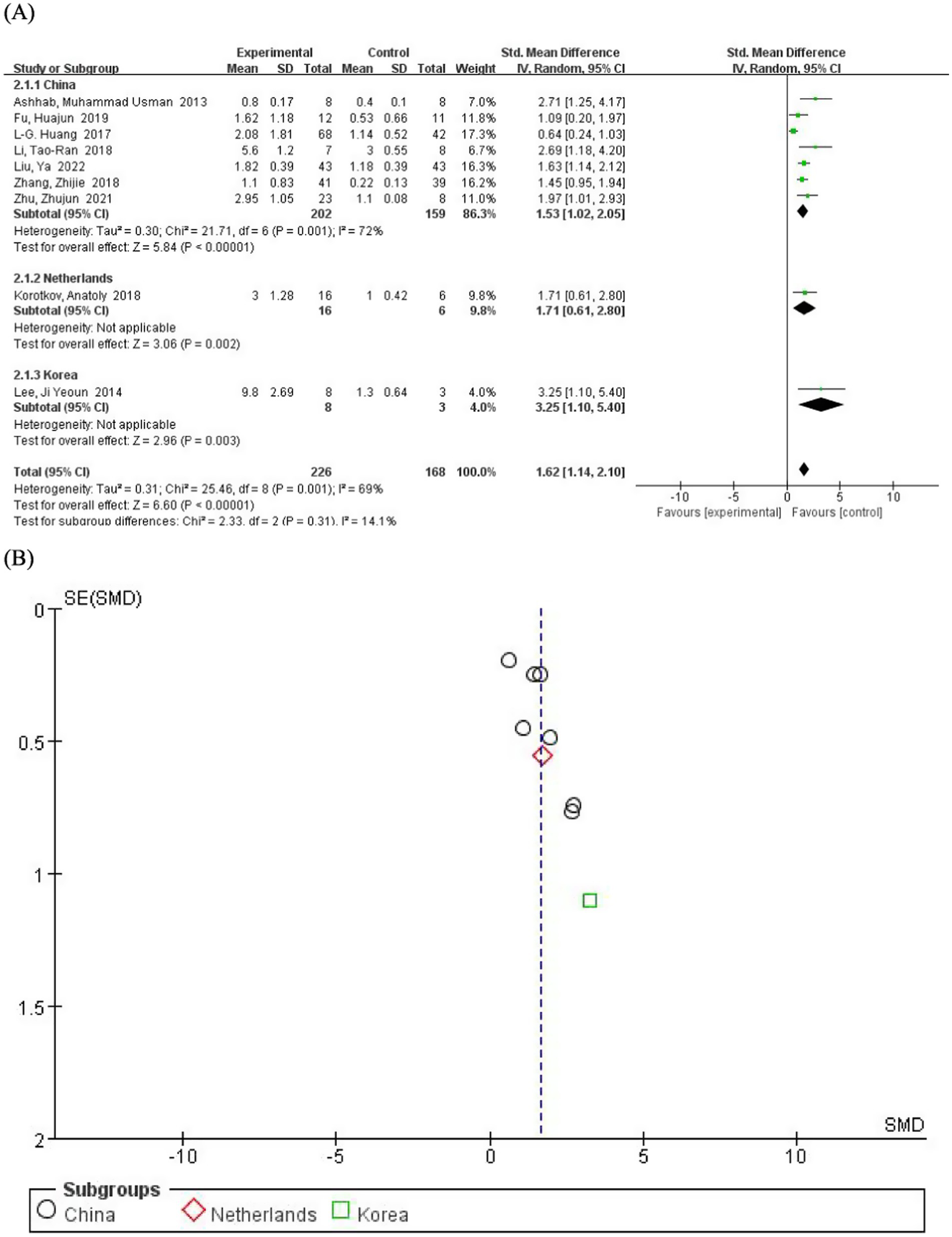
(A) Subgroup analysis by country demonstrating the association between serum miR-155 level and epilepsy. (B) A funnel plot to assess publication bias.
3.5 Subgroup analysis by age category
We performed subgroup analyses based on age to evaluate the potential influence of age on miR-155 levels in epilepsy patients. The participants were divided into adults (≥18 years) and childrens (<18 years). The pooled analysis included four studies involving adult patients. The results demonstrated that miR-155 levels were significantly elevated in adult epilepsy patients compared to the control group (SMD = 1.65; 95% CI, 1.26–2.05; p < 0.00001) (Figure 4). The heterogeneity was negligible (I2 = 0%), indicating low variability. Three other included studies involving children patients. Similarly, miR-155 levels were significantly higher in children with epilepsy compared to the control group (SMD = 1.80; 95% CI, 1.35–2.26; p < 0.00001). Moderate heterogeneity was observed (I2 = 46%), suggesting low to moderate variability.
Figure 4
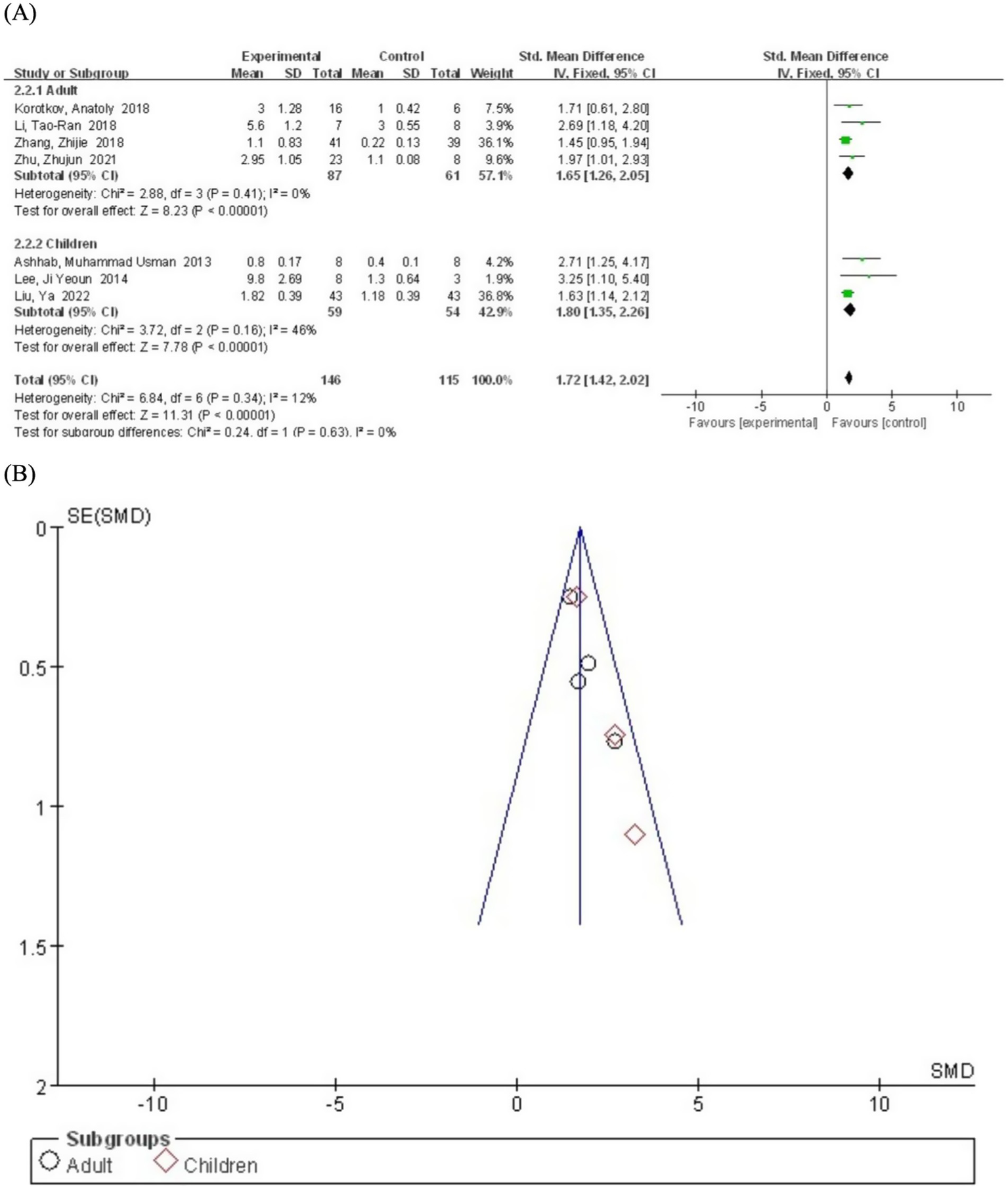
(A) Subgroup analysis by age category demonstrating the association between serum miR-155 level and epilepsy. (B) A funnel plot to assess publication bias.
3.6 Subgroup analysis by sample source
We conducted subgroup analyses based on sample sources (serum and brain tissue) to explore potential sources of heterogeneity and differences in serum miR-155 levels between epilepsy patients and the control population. The serum subgroup included only one study. The results showed that miR-155 levels were significantly higher in epilepsy patients compared to the control group (SMD = 1.63; 95% CI, 1.14–2.12; p < 0.00001) (Figure 5). Heterogeneity assessment was not performed for the serum subgroup as it included only one study. The brain tissue subgroup comprised eight studies. The pooled analysis revealed that miR-155 levels were significantly elevated in epilepsy patients compared to the control group (SMD = 1.22; 95% CI, 0.96–1.48). However, significant heterogeneity was observed (I2 = 70%, df = 7), possibly due to differences in tissue collection methods, epilepsy subtypes, or experimental protocols across studies. Notably, 8 of the 9 included studies utilized brain tissue samples. While these specimens provide direct pathological evidence, their invasive nature substantially limits clinical applicability. In contrast, cerebrospinal fluid (CSF) - by virtue of its direct contact with neural pathology - may represent a superior biological matrix compared to peripheral blood. Although insufficient data precluded CSF analysis in the current study, its anatomical proximity to epileptogenic foci warrants prioritized investigation in future research.
Figure 5
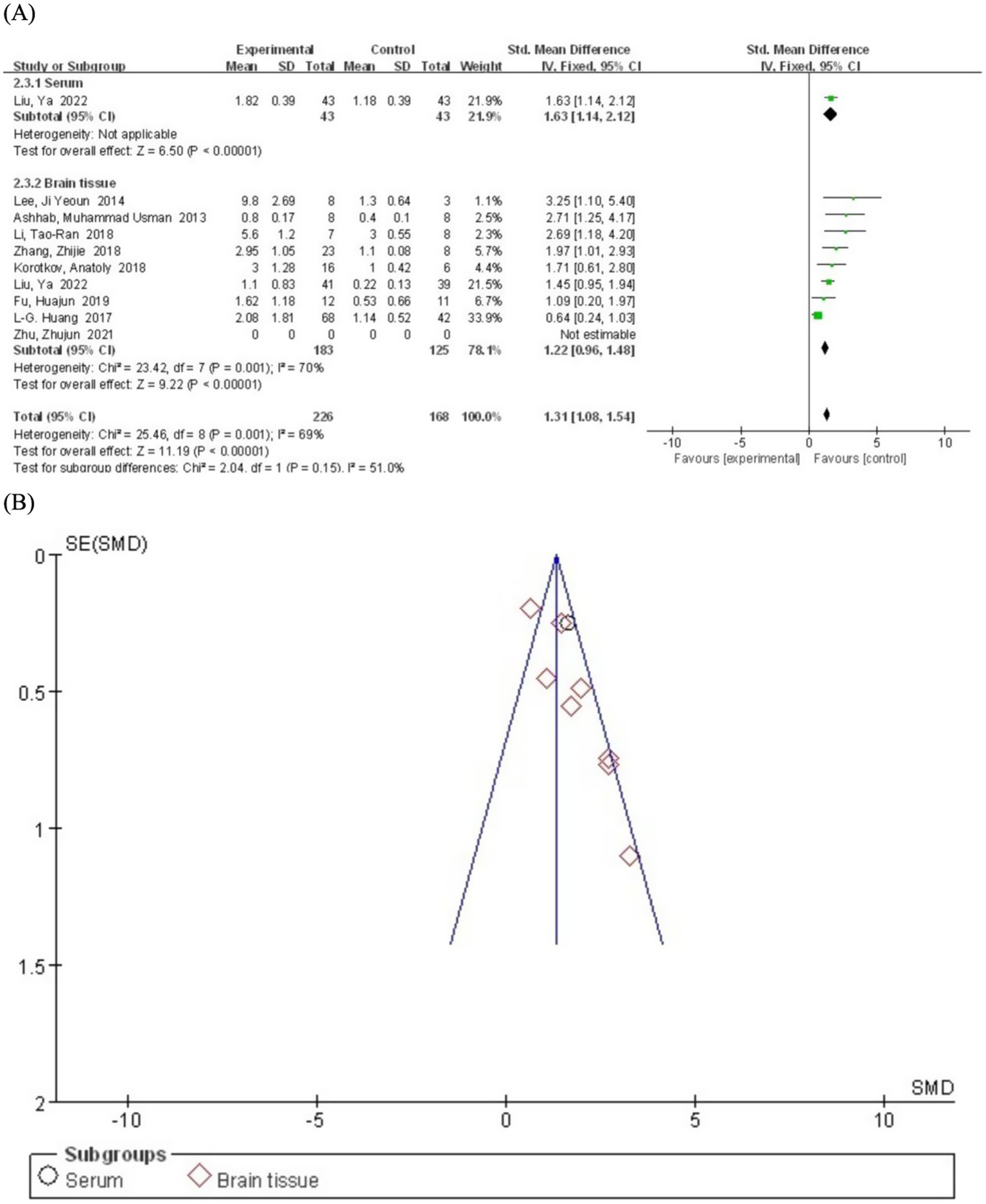
(A) Subgroup analysis by sample source category demonstrating the association between serum miR-155 level and epilepsy. (B) A funnel plot to assess publication bias.
4 Discussion
The accurate diagnosis and classification of epilepsy, combined with precise therapeutic interventions, are the cornerstones of optimal clinical management. Identifying biomarkers for early diagnosis is therefore crucial. Growing evidence implicates miRNAs in epileptogenesis through mechanisms including neuronal circuit reorganization, apoptosis, glial proliferation, neuroinflammation, and oxidative stress (29, 30). Unlike other epilepsy-associated miRNAs that primarily function in single pathways [e.g., miR-146a in neuroinflammation (31) or miR-132 in synaptic plasticity (32)], miR-155 exhibits multimodal regulation of epileptogenesis through simultaneous modulation of neuroinflammation, blood–brain barrier integrity, and neuronal excitability control. Consistent with prior findings, our clinical data confirm significantly elevated miR-155 level in epilepsy patients versus healthy controls. Ashhab et al. (17) reported coordinated upregulation of miR-155 and TNF-α across developmental stages in a rat model of status epilepticus and suggested their dual role in neuroinflammation and barrier dysfunction. Both TNF-α and miR-155 showed significant upregulation during all three stages of mesial temporal lobe epilepsy (MTLE). This co-expression pattern correlated with inflammatory responses and blood–brain barrier dysfunction—processes also implicated in autoimmune disorders, where miR-155 regulates immune activation. Lind et al. (33) reported that overexpression of miR-155 in dendritic cells can reduce SH2-containing inositol phosphatase (SHIP), thereby inducing an autoimmune response. Given the close association between autoimmunity and neurological disorders, particularly degenerative diseases, there appears to be a significant relationship between miR-155 and various neurological conditions.
Moreover, some studies have explored the signaling pathways of miR-155 in order to provide a more comprehensive understanding of its role in the pathogenesis of epilepsy. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway is an essential conduit for “survival signals” and is also referred to as the “antiapoptotic” pathway (34). Duan et al. (34) demonstrated that overexpression of miR-155 inhibits the PI3K/Akt signaling pathway in HT22 cells under glutamate stimulation. This finding suggests that miR-155 may participate in the pathogenesis of epilepsy through the PI3K/Akt/mTOR signaling pathway. However, this experimental approach differs from the methodologies described previously. Zhang et al. (15) demonstrated that the delivery of miR-155 inhibitors directly to the brain improved post-ischemic non-convulsive seizures (NCS) by modulating neuroinflammation and GABA signaling pathways in the parietal cortex, hippocampus, and amygdala. Clinical and animal studies by Huang et al. (19) indicated that silencing miR-155 may significantly suppress the pathological features associated with epilepsy by promoting the expression of Sesn3. Furthermore, animal experiments conducted by Zhou et al. (16) revealed that miR-155 plays a critical role in biological processes by negatively regulating multiple target mRNAs. These findings may offer new therapeutic targets for the treatment of epilepsy (Figure 6).
Figure 6
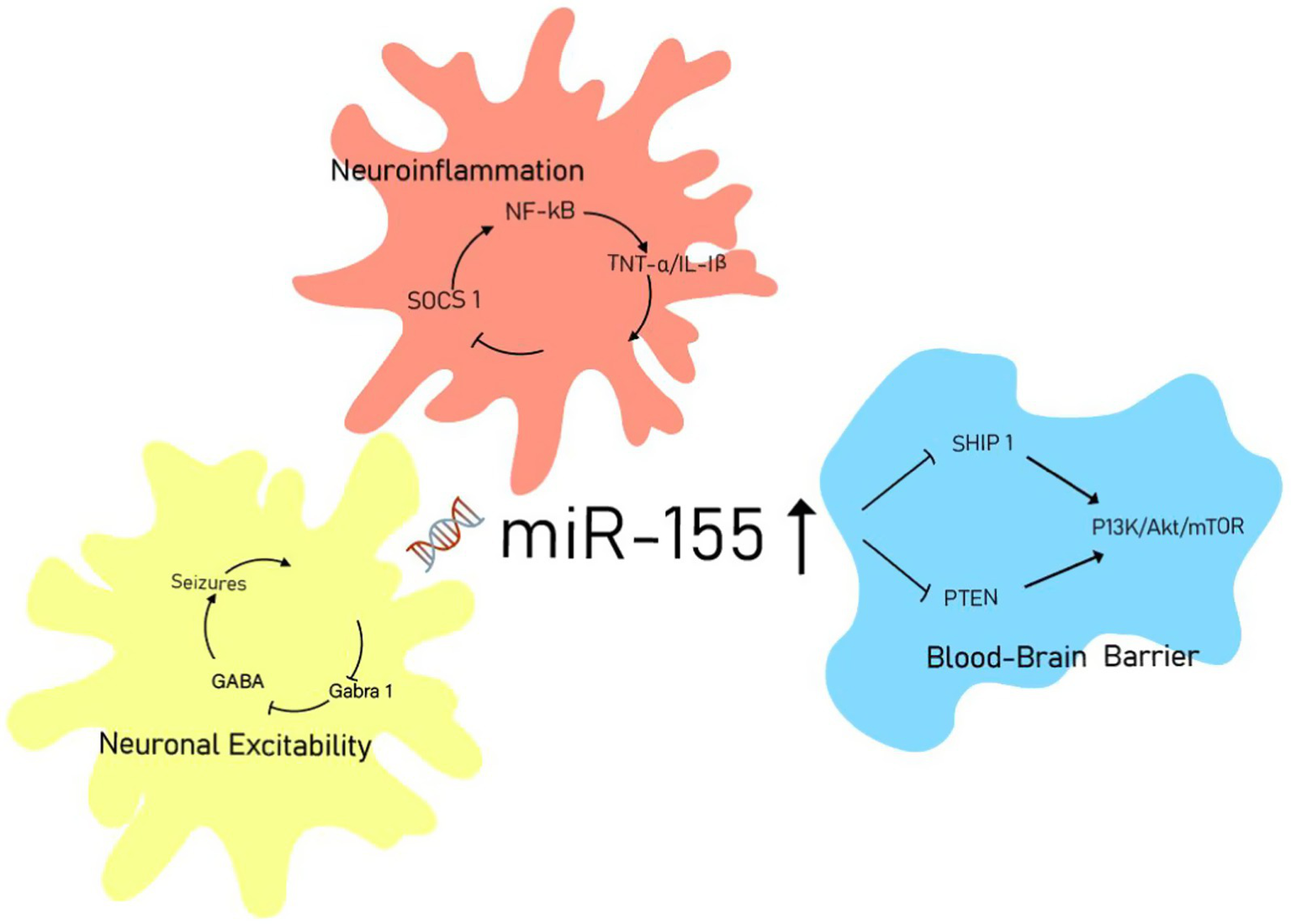
miR-155 signaling network in epileptogenesis.
Notably, miR-155 may promote the development and progression of epilepsy by regulating RNA transcription. Research by Gong et al. (35) has demonstrated that miR-155 can modulate gene expression by regulating circular RNAs (circRNAs) during transcription or post-transcriptionally. CircRNA acts as a sponge that helps miR-155 enhance the expression of FOXO3a (forkhead box O3), thereby exerting its effects in temporal lobe epilepsy (TLE). Various miRNAs have shown dysregulation in brain tissue and serum samples from epilepsy patients, as well as in different animal models of this neurological disorder (36). Additionally, the role of genetic variations within miRNA-encoding genes in the risk of epilepsy or drug resistance to antiepileptic medications represents a promising area for further research exploration.
To the best of our knowledge, this study is the first systematic review and meta-analysis of the association between miR-155 and epilepsy. Current research indicates a positive correlation between elevated level of miR-155 and the occurrence of seizures, which is consistent with previous findings (32). This article reviews the existing literature on the association between miR-155 level in brain tissue and serum with epilepsy, and evaluates the potential of miR-155 as a biomarker for epilepsy. Among the 9 included studies, one lacked quantifiable data, resulting in a final analysis of 226 patients and 168 matched controls. The results indicate that economic status and social development appear to significantly influence the occurrence and progression of epilepsy. Improving economic development and social conditions can notably reduce the incidence of epilepsy and enhance the quality of life for patients (37, 38). However, the age subgroup analysis did not reveal clear evidence suggesting a direct relationship between the expression levels of miR-155 in epilepsy patients and age. Although age did not directly correlate with miR-155 expression, current evidence suggests that seizure-induced neuronal damage may be age-dependent, potentially indirectly influencing miR-155 levels.
Beyond demographic factors, clinical variables also significantly influence miR-155 expression, including seizure duration/frequency and antiepileptic drug regimens. Huang et al. (39) demonstrated that sodium valproate pretreatment reduced miR-155 upregulation during epileptic seizures, whereas immunomodulator use was associated with elevated miR-155 levels in post-traumatic epilepsy patients (40). However, the lack of standardized reporting of these variables in the included studies precluded subgroup analysis of these potentially important confounders.
Although the results of this meta-analysis suggest that miR-155 holds promise as a diagnostic biomarker for epilepsy, there are several limitations to consider. First, significant heterogeneity (I2 = 69%) was observed among included studies, potentially stemming from variations in patient demographics, epilepsy subtypes, and methodological differences in sample collection and detection protocols. Although subgroup analyses showed no significant effects (p = 0.31) of country, age, or sample source, these methodological variations may still introduce potential bias to the conclusions. Second, the geographical distribution of studies was imbalanced, with Chinese studies comprising 7 of the 9 included reports. While no significant inter-country differences were detected, this regional predominance may limit the generalizability of the findings. Third, the sample type distribution was highly skewed, with only one study using serum samples versus eight utilizing brain tissue, substantially constraining the evaluation of miR-155 as a non-invasive biomarker. Notably, CSF - which offers more direct pathological relevance due to its contact with neural tissues - could not be analyzed due to insufficient data, representing an important target for future investigation. Furthermore, inconsistent reporting of key clinical variables such as epilepsy classification, seizure frequency, and medication regimens, along with the lack of longitudinal data tracking miR-155 changes throughout disease progression or treatment response, significantly hinders a comprehensive assessment of its clinical significance. Collectively, these limitations define the prerequisite multicenter efforts needed to bridge miR-155 biomarker potential with clinical translation.
Building on these findings, the translational pathway for miR-155—from biomarker discovery to clinical implementation—requires addressing three interdependent challenges that emerged from our analysis: Although miR-155 shows significant potential as a biomarker, its clinical application faces multiple interrelated challenges in terms of technology, validation, and treatment: the instability of miR-155 in serum without RNase inhibitors (41), and the invasive nature of cerebrospinal fluid collection. These constitute significant technical obstacles. However, emerging exosome miRNA analysis methods may provide solutions (42). These technical limitations exacerbate the significant heterogeneity in miR-155 levels reported in our meta-analysis, and guiding principles for establishing epilepsy biomarker studies may provide a direction for solutions (43). Critically, preclinical studies using antagomirs have confirmed their therapeutic potential. Preclinical studies have demonstrated that intranasal delivery of miR-155-5p antagomir can reduce seizure severity and mitigate hippocampal damage (16). However, it must be emphasized that these findings are derived exclusively from animal models. Significant translational challenges remain, including blood–brain barrier permeability limitations and interspecies variations. Current discussions regarding targeted therapies such as antagomirs and intranasal delivery systems (44) should therefore be considered exploratory hypotheses requiring validation in human clinical trials.
5 Conclusion
In summary, miR-155 demonstrates potential as a diagnostic biomarker for epilepsy, facilitating early detection and elucidating disease pathophysiology. Furthermore, miR-155 could help classify epilepsy subtypes mediated by neuroinflammation. Notably, it represents a rare theranostic target whose elevation provides an actionable pathway for antagomir-based therapy. Current evidence is largely derived from animal studies, whose translational limitations—including interspecies biological differences and simplified disease models—must be acknowledged. Thus, although preclinical data establish mechanistic foundations, multi-center clinical trials with standardized protocols are imperative to validate the diagnostic utility of miR-155. Translating miR-155 research into clinical practice requires rigorous validation of its reliability as both a diagnostic biomarker and therapeutic target in human epilepsy, pending advances in CNS delivery technologies.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Writing – review & editing, Writing – original draft. CC: Writing – original draft, Investigation. CY: Writing – review & editing. JC: Writing – original draft. BL: Writing – original draft. JL: Writing – review & editing. QW: Writing – review & editing. LD: Writing – review & editing, Funding acquisition. HL: Methodology, Writing – original draft, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Guangxi Science and Technology Base and Talent Special Project (GUI KE AD22035168), and the National Natural Science Foundation of China (82360875).
Acknowledgments
We express our gratitude to the Frontiers in Neuroscience editors and reviewers for their insightful comments on our manuscripts.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Shi J Xie J Li Z He X Wei P Sander JW et al . The role of Neuroinflammation and network anomalies in drug-resistant epilepsy. Neurosci Bull. (2025) 41:881–905. doi: 10.1007/s12264-025-01348-w
2.
Coll M Oliva A Grassi S Brugada R Campuzano O . Update on the genetic basis of sudden unexpected death in epilepsy. Int J Mol Sci. (2019) 20:1979. doi: 10.3390/ijms20081979
3.
Newton CR Garcia HH . Epilepsy in poor regions of the world. Lancet. (2012) 380:1193–201. doi: 10.1016/S0140-6736(12)61381-6
4.
Fisher RS Acevedo C Arzimanoglou A Bogacz A Cross JH Elger CE et al . ILAE official report: a practical clinical definition of epilepsy. Epilepsia. (2014) 55:475–82. doi: 10.1111/epi.12550
5.
Kini LG Gee JC Litt B . Computational analysis in epilepsy neuroimaging: a survey of features and methods. Neuroimage Clin. (2016) 11:515–29. doi: 10.1016/j.nicl.2016.02.013
6.
Sidhu MK Duncan JS Sander JW . Neuroimaging in Epilepsy. Curr Opin Neurol. (2018) 31:371–8. doi: 10.1097/WCO.0000000000000568
7.
Thompson JW Hu R Huffaker TB Ramstead AG Ekiz HA Bauer KM et al . Microrna-155 plays selective cell-intrinsic roles in brain-infiltrating immune cell populations during neuroinflammation. J Immunol. (2023) 210:926–34. doi: 10.4049/jimmunol.2200478
8.
Alsharafi WA Xiao B Abuhamed MM Luo Z . Mirnas: biological and clinical determinants in epilepsy. Front Mol Neurosci. (2015) 8:59. doi: 10.3389/fnmol.2015.00059
9.
Kretschmann A Danis B Andonovic L Abnaof K van Rikxoort M Siegel F et al . Different MicroRNA profiles in chronic epilepsy versus acute seizure mouse models. J Mol Neurosci. (2015) 55:466–79. doi: 10.1007/s12031-014-0368-6
10.
Aloi MS Prater KE Sánchez R Beck A Pathan JL Davidson S et al . Microglia specific deletion of Mir-155 in Alzheimer's disease mouse models reduces amyloid-β pathology but causes Hyperexcitability and seizures. J Neuroinflammation. (2023) 20:60. doi: 10.1186/s12974-023-02745-6
11.
Cai Z Li S Li S Song F Zhang Z Qi G et al . Antagonist targeting Microrna-155 protects against Lithium-pilocarpine-induced status epilepticus in C57bl/6 mice by activating brain-derived neurotrophic factor. Front Pharmacol. (2016) 7:129. doi: 10.3389/fphar.2016.00129
12.
Gao W Bi Y Ding L Zhu W Ye M . Ssa ameliorates the Glu Uptaking capacity of astrocytes in epilepsy via Ap-1/mir-155/GLAST. Biochem Biophys Res Commun. (2017) 493:1329–35. doi: 10.1016/j.bbrc.2017.09.139
13.
Hunsberger JG Fessler EB Wang Z Elkahloun AG Chuang DM . Post-insult Valproic acid-regulated Micrornas: potential targets for cerebral ischemia. Am J Transl Res. (2012) 4:316–32.
14.
Romá-Mateo C Lorente-Pozo S Márquez-Thibaut L Moreno-Estellés M Garcés C González D et al . Age-related MicroRNA overexpression in Lafora disease male mice provides links between Neuroinflammation and oxidative stress. Int J Mol Sci. (2023) 24:1089. doi: 10.3390/ijms24021089
15.
Zhang W Wang L Pang X Zhang J Guan Y . Role of Microrna-155 in modifying Neuroinflammation and γ-aminobutyric acid transporters in specific central regions after post-ischaemic seizures. J Cell Mol Med. (2019) 23:5017–24. doi: 10.1111/jcmm.14358
16.
Zhou X Chen J Tao H Cai Y Huang L Zhou H et al . Intranasal delivery of Mir-155-5p Antagomir alleviates acute seizures likely by inhibiting hippocampal inflammation. Neuropsychiatr Dis Treat. (2020) 16:1295–307. doi: 10.2147/NDT.S247677
17.
Ashhab MU Omran A Kong H Gan N He F Peng J et al . Expressions of tumor necrosis factor alpha and Microrna-155 in immature rat model of status epilepticus and children with mesial temporal lobe epilepsy. J Mol Neurosci. (2013) 51:950–8. doi: 10.1007/s12031-013-0013-9
18.
Fu H Cheng Y Luo H Rong Z Li Y Lu P et al . Silencing Microrna-155 attenuates Kainic acid-induced seizure by inhibiting microglia activation. Neuroimmunomodulation. (2019) 26:67–76. doi: 10.1159/000496344
19.
Huang LG Zou J Lu QC . Silencing Rno-mir-155-5p in rat temporal lobe epilepsy model reduces pathophysiological features and cell apoptosis by activating Sestrin-3. Brain Res. (2018) 1689:109–22. doi: 10.1016/j.brainres.2017.11.019
20.
Li TR Jia YJ Wang Q Shao XQ Zhang P Lv RJ . Correlation between tumor necrosis factor alpha MRNA and Microrna-155 expression in rat models and patients with temporal lobe epilepsy. Brain Res. (2018) 1700:56–65. doi: 10.1016/j.brainres.2018.07.013
21.
Liu Y Yu G Ding YY Zhang YX . Expression of Mir-155 in serum exosomes in children with epilepsy and its diagnostic value. Dis Markers. (2022) 2022:7979500. doi: 10.1155/2022/7979500
22.
Zhang Z Wang Z Zhang B Liu Y . Downregulation of Microrna-155 by preoperative Administration of Valproic Acid Prevents Postoperative Seizures by upregulating Scn 1A. Mol Med Report. (2018) 17:1375–81. doi: 10.3892/mmr.2017.8004
23.
Zhu Z Wang S Cao Q Li G . Circubqln1 promotes proliferation but inhibits apoptosis and oxidative stress of hippocampal neurons in epilepsy via the Mir-155-mediated sox 7 upregulation. J Mol Neurosci. (2021) 71:1933–43. doi: 10.1007/s12031-021-01838-2
24.
Korotkov A Broekaart D van Scheppingen J Anink JJ Baayen JC Idema S et al . Increased expression of matrix metalloproteinase 3 can be attenuated by inhibition of Microrna-155 in cultured human astrocytes. J Neuroinflammation. (2018) 15:211. doi: 10.1186/s12974-018-1245-y
25.
Lee JY Park AK Lee ES Park WY Park SH Choi JW et al . MiRNA expression analysis in cortical dysplasia: regulation of MTOR and Lis1 pathway. Epilepsy Res. (2014) 108:433–41. doi: 10.1016/j.eplepsyres.2014.01.005
26.
Wang J Guo J Fan H . Mir-155 regulates the proliferation and apoptosis of pancreatic Cancer cells through targeting Socs3. Eur Rev Med Pharmacol Sci. (2019) 23:5168–75. doi: 10.26355/eurrev_201906_18181
27.
Fang Q Cai Y Chi J Yang Y Chen Q Chen L et al . Silencing Mir-155-5p alleviates hippocampal damage in Kainic acid-induced epileptic rats via the Dusp14/MAPK pathway. Brain Res Bull. (2024) 217:111057. doi: 10.1016/j.brainresbull.2024.111057
28.
Divi SN Markova DZ Fang T Guzek R Kurd MF Rihn JA et al . Circulating Mir-155-5p as a novel biomarker of lumbar degenerative disc disease. Spine (Phila Pa 1976). (2020) 45:E499–507. doi: 10.1097/BRS.0000000000003322
29.
Korotkov A Broekaart D Banchaewa L Pustjens P van Scheppingen J Anink JJ et al . Microrna-132 is overexpressed in glia in temporal lobe epilepsy and reduces the expression of pro-epileptogenic factors in human cultured astrocytes. Glia. (2020) 68:60–75. doi: 10.1002/glia.23700
30.
Ludhiadch A Bhardwaj N Gotra P Kumar R Munshi A . Common Micrornas in epilepsy and migraine: their possibility as candidates for biomarkers and therapeutic targets during comorbid onset of both conditions. CNS Neurol Disord Drug Targets. (2023) 22:698–710. doi: 10.2174/1871527321666220426103253
31.
Ma H Dong Y Sun K Wang S Zhang Z . Protective effect of Mir-146 on renal injury following cardiopulmonary bypass in rats through mediating Nf-κB signaling pathway. Bioengineered. (2022) 13:593–602. doi: 10.1080/21655979.2021.2012405
32.
Qian Y Song J Ouyang Y Han Q Chen W Zhao X et al . Advances in roles of Mir-132 in the nervous system. Front Pharmacol. (2017) 8:770. doi: 10.3389/fphar.2017.00770
33.
Lind EF Millar DG Dissanayake D Savage JC Grimshaw NK Kerr WG et al . Mir-155 upregulation in dendritic cells is sufficient to break tolerance in vivo by negatively regulating Ship1. J Immunol. (2015) 195:4632–40. doi: 10.4049/jimmunol.1302941
34.
Duan W Chen Y Wang XR . Microrna-155 contributes to the occurrence of epilepsy through the Pi3k/akt/mTOR signaling pathway. Int J Mol Med. (2018) 42:1577–84. doi: 10.3892/ijmm.2018.3711
35.
Gong GH An FM Wang Y Bian M Wang D Wei CX . Comprehensive circular RNA profiling reveals the regulatory role of the Circrna-0067835/mir-155 pathway in temporal lobe epilepsy. Cell Physiol Biochem. (2018) 51:1399–409. doi: 10.1159/000495589
36.
Ghafouri-Fard S Hussen BM Abak A Taheri M Jalili Khoshnoud R . Aberrant expression of Mirnas in epilepsy. Mol Biol Rep. (2022) 49:5057–74. doi: 10.1007/s11033-022-07188-5
37.
Ngugi AK Bottomley C Kleinschmidt I Sander JW Newton CR . Estimation of the burden of active and life-time epilepsy: a Meta-analytic approach. Epilepsia. (2010) 51:883–90. doi: 10.1111/j.1528-1167.2009.02481.x
38.
Shah R Arora S Dunkley C Raja N Bottle A Hargreaves DS . Is geographical variation in emergency epilepsy admissions related to variation in new epilepsy diagnoses among children and young people across England? An observational study using linked datasets. BMJ Open. (2025) 15:e082637. doi: 10.1136/bmjopen-2023-082637
39.
Trécul A Morceau F Gaigneaux A Schnekenburger M Dicato M Diederich M . Valproic acid regulates Erythro-megakaryocytic differentiation through the modulation of transcription factors and MicroRNA regulatory Micro-networks. Biochem Pharmacol. (2014) 92:299–311. doi: 10.1016/j.bcp.2014.07.035
40.
Cui B Liu W Wang X Chen Y du Q Zhao X et al . Brucella Omp25 upregulates Mir-155, Mir-21-5p, and Mir-23b to inhibit Interleukin-12 production via modulation of programmed Death-1 signaling in human monocyte/macrophages. Front Immunol. (2017) 8:708. doi: 10.3389/fimmu.2017.00708
41.
Manshouri T . Ruxolitinib, a Janus kinase (jak)-1 and −2 inhibitor modulates Microrna (mir) levels in patients with myelofibrosis (MF): MiR levels might predict MF treatment outcome. Blood. (2011) 118:1744. doi: 10.1182/blood.v118.21.1744.1744
42.
Zhou Z Zhang H Deng T Ning T Liu R Liu D et al . Exosomes carrying Microrna-155 target Forkhead box O3 of endothelial cells and promote angiogenesis in gastric Cancer. Mol Ther Oncolytics. (2019) 15:223–33. doi: 10.1016/j.omto.2019.10.006
43.
Gliske SV Stacey WC . The BEST conceivable way to talk about epilepsy biomarkers. Epilepsy Curr. (2023) 23:175–8. doi: 10.1177/15357597231159714
44.
Aly AE Caron NS Black HF Schmidt ME Anderson C Ko S et al . Delivery of mutant huntingtin-lowering antisense oligonucleotides to the brain by Intranasally administered apolipoprotein A-I Nanodisks. J Control Release. (2023) 360:913–27. doi: 10.1016/j.jconrel.2023.07.027
Summary
Keywords
epilepsy, miR-155, neuroinflammation, biomarker, meta-analysis
Citation
Wang Y, Chen C, Ya C, Chen J, Lu B, Liu J, Wu Q, Diao L and Liu H (2025) The levels of miR-155 in epilepsy patients: a meta-analysis. Front. Neurol. 16:1630581. doi: 10.3389/fneur.2025.1630581
Received
14 June 2025
Accepted
28 July 2025
Published
13 August 2025
Volume
16 - 2025
Edited by
Daniyah A. Almarghalani, Taif University, Saudi Arabia
Reviewed by
Kursat Bora Carman, Eskişehir Osmangazi University, Türkiye
Sharath M., King Edward Memorial Hospital and Seth Gordhandas Sunderdas Medical College, India
Updates
Copyright
© 2025 Wang, Chen, Ya, Chen, Lu, Liu, Wu, Diao and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Limei Diao, diaolimei0810@163.com; Huihua Liu, huihualiu2003@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.