Abstract
Background:
Postoperative delirium is a frequent and severe complication among elderly surgical patients. Electroencephalogram (EEG)-guided anesthesia, which optimizes sedation depth, holds promise for preventing postoperative delirium; however, current research findings remain inconsistent. This meta-analysis incorporates the most recent trials to evaluate the effectiveness of EEG-guided anesthesia in reducing postoperative delirium incidence in older adults.
Methods:
We conducted a comprehensive literature search of PubMed, the Cochrane Central Register of Controlled Trials, and Embase from their inception to February 1, 2025, to identify eligible studies. This systematic review and meta-analysis aimed to evaluate the effectiveness of electroencephalogram-guided anesthesia in preventing postoperative delirium among elderly surgical patients. Pooled effect estimates for all outcomes were calculated using a random-effects model. The quality and certainty of the evidence were assessed using the GRADE methodology. The primary outcome was the occurrence of postoperative delirium.
Results:
Of the 3,151 studies screened, 12 were deemed eligible for inclusion, encompassing a total of 7,441 patients, of whom 3,707 received EEG-guided anesthesia. Compared with standard care, EEG-guided anesthesia demonstrated a beneficial effect in reducing postoperative delirium among elderly patients (RR = 0.76, 95% CI: 0.61–0.96), as well as the incidence of postoperative infections (RR = 0.74, 95% CI: 0.58–0.95). Subgroup analyses revealed no significant interaction based on type of surgery (p = 0.18).
Conclusion:
EEG-guided anesthesia is associated with a reduced incidence of postoperative delirium in elderly surgical patients.
Introduction
Postoperative delirium (POD) is a common and severe neurological complication in elderly surgical patients, with incidence rates ranging from 5 to 50% (1, 2). With the accelerating global aging population, it is projected that by 2050, the number of elderly individuals worldwide will reach 1.5 billion, which will significantly increase the public health burden associated with POD (3). POD has been shown to significantly prolong hospital stays and increase healthcare costs and is closely associated with postoperative cognitive decline and higher mortality rates (4). Therefore, identifying effective strategies for preventing POD has become a critical priority in perioperative care for older adults (5).
EEG-guided anesthesia involves real-time monitoring of brain electrical activity to precisely adjust anesthesia depth, preventing both excessive and insufficient anesthesia (6). Maintaining the bispectral index (BIS) within the range of 40–60 helps avoid burst suppression patterns, which are associated with postoperative cognitive dysfunction (6, 7). The real-time feedback from EEG monitoring enables anesthesiologists to fine-tune anesthetic dosages, reducing exposure to anesthesia and, consequently, lowering the risk of delirium (7). Furthermore, an optimal anesthetic depth helps stabilize intraoperative hemodynamics, preventing low perfusion and a decrease in cardiac output (8), which in turn reduces immune suppression (9, 10), minimizes intraoperative stress (10), and alleviates inflammatory responses, thereby decreasing the risk of postoperative infections (11). In particular, in elderly patients with weakened immune systems, EEG-guided anesthesia may enhance hemodynamic stability and immune function, providing additional protection against postoperative infections (8–10).
A previous meta-analysis (12) combining five randomized controlled trials with a total of 3,433 participants found no significant association between EEG-guided anesthesia and a reduced incidence of POD. Trial sequential analysis indicated that the required sample size was 4,180 participants, suggesting insufficient sample size and limiting the robustness of the conclusions. Recent studies (13–15) have reported inconsistent findings, further highlighting the ongoing debate in this field. To address this, we have incorporated four of the most recent randomized controlled trials in our analysis to provide a more comprehensive comparison and further validate the potential of EEG-guided anesthesia in reducing POD and postoperative infections.
We conducted a systematic review and meta-analysis, including only randomized controlled trials evaluating the effects of EEG-guided anesthesia on postoperative outcomes in elderly patients. This study aims to provide more precise evidence to inform clinical guidelines and enhance perioperative neuroprotection and comprehensive management strategies for the elderly.
Methods
Protocol and guidance
This study was conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16), and the research protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, https://www.crd.york.ac.uk/PROSPERO) under registration number CRD420251053668.
Information sources and search strategy
This study conducted a systematic search of Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, MEDLINE, Web of Science, Embase, and ClinicalTrials.gov. The search spanned from the inception of each database to February 1, 2025, encompassing preprints and articles published online ahead of print. To supplement the search, reference lists of included studies and relevant systematic reviews were examined, conference abstracts and grey literature (including unpublished reports) were screened, and experts in the field were consulted. No restrictions on language, country, or region were applied to minimize language and geographical bias. Literature screening was independently performed by two reviewers (XD and LC) in a blinded manner, with discrepancies resolved through discussion and adjudication by a third reviewer (XL). Studies were eligible for inclusion if their titles, abstracts, original titles, substance names, subject headings, keyword fields, or unique identifiers contained all of the following Medical Subject Headings (MeSH) and keywords: “Electroencephalography,” “postoperative delirium,” “elderly patients,” “older patients,” “aged patients,” “geriatric patients,” and “randomized controlled trial.”
Eligibility criteria
The studies included in this paper were selected according to the PICOS criteria, as outlined below:
Population: this meta-analysis included studies on adults with an average age of over 60 years who underwent different surgeries under general anesthesia.
Intervention: included studies evaluated EEG-guided anesthesia management, using real-time brain activity monitoring (e.g., bispectral index or spectral edge frequency) to guide anesthesia protocols.
Comparison intervention: control groups received standard anesthesia care without EEG monitoring, with anesthesia depth adjusted by clinical signs (heart rate, blood pressure) or physician experience.
Outcome: the primary outcome was postoperative delirium. Secondary outcomes included length of hospital stay, postoperative infection and all-cause mortality at the longest follow-up.
Study design: only randomized controlled trials were included.
Study selection
Two authors, XD and LC, independently undertook the screening of titles and abstracts obtained through systematic search. After the initial screening, the same two authors conducted full-text reviews of the selected articles. During this process, any discrepancies were resolved through discussion between the two to maximize the reduction of bias. In cases where consensus could not be reached, a reviewer would make the final decision.
Data extraction
Data were independently extracted by two reviewers (XD and LC) using a predesigned data extraction form. Extracted variables included author, year, country, study design, sample size, mean or median age, sex distribution, type of surgery (cardiac vs. non-cardiac), EEG monitoring modality (e.g., BIS, raw EEG), method and timing of delirium assessment (e.g., CAM, CAM-ICU, DSM-IV), postoperative infection definition, and duration of follow-up for mortality. Long-term outcomes were included when reported. All extracted data were cross-checked by a third reviewer (XL).
Risk of bias assessment
Two independent reviewers, XD and LC, assessed the risk of bias in the included studies based on the guidelines outlined in the Cochrane Risk of Bias Assessment Tool (17). This tool evaluates bias across seven domains—participant involvement, attrition, measurement of prognostic factors, outcome assessment, confounding variables, and statistical analysis and reporting—providing a comprehensive appraisal of methodological quality. Studies were categorized as having a high risk of bias if they were rated as “high” in one or more domains. A low risk of bias was assigned when all six domains were rated as “low.” Studies not meeting the criteria for either low or high risk were classified as having a moderate risk of bias. The systematic approach ensures a thorough evaluation of potential sources of bias within the included trials. Disagreements between the two reviewers were resolved through detailed discussion. If consensus could not be reached, a third reviewer (XL) made the final decision.
Confidence of evidence
Two independent authors, XD and LC, conducted a systematic evaluation using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (18). This methodology comprehensively assesses trials across dimensions such as study design, risk of bias, inconsistency, imprecision, and indirectness. Subsequently, evidence was classified into four grades—high, moderate, low, or very low—based on the aforementioned criteria. In the event of discrepancies between reviewers, discussions were prioritized for resolution; if consensus remained unattained, a reviewer would make the final ruling.
Data analysis
Statistical analyses were conducted following a predefined meta-analysis protocol using Review Manager software (version 5.4.1, Cochrane Collaboration). Given the clinical and methodological variability among included studies, a random-effects model was applied to each study’s data to account for between-study heterogeneity, providing more conservative and generalizable pooled effect estimates.
For dichotomous outcomes, relative risks with corresponding 95% confidence intervals were calculated, while mean differences were used for continuous outcomes. Two-sided p-values less than 0.05 were considered statistically significant (19). To assess methodological rigor and the consistency of results, heterogeneity was quantified using the I2 statistic, with thresholds defined as follows: I2 < 25% indicating low heterogeneity, 25–50% moderate, 50–75% substantial, and >75% considerable heterogeneity (20, 21). Publication bias was evaluated through visual inspection of funnel plots to identify potential small-study effects influencing the overall findings.
Sensitivity analysis
To assess the robustness of our findings, we performed a sensitivity analysis using the following approaches: (1) using a fixed-effect model, (2) excluding trial assessed as unclear or high-risk, and (3) each study was systematically excluded in turn.
Subgroup analysis
To better understand the potential sources of heterogeneity in the effectiveness of EEG-guided anesthesia for preventing postoperative delirium in elderly patients, subgroup analyses of the primary outcome were conducted based on the following criteria: type of surgery (Cardiac surgery vs. Non-cardiac surgery).
Results
Our initial literature search identified 3,151 records. After removing duplicates, 2,762 unique articles remained for screening. Following a rigorous assessment of titles, abstracts, and full texts, 12 randomized controlled trials were deemed eligible for inclusion in this systematic review (Figure 1). Table 1 summarizes the characteristics of each trial included in this analysis. These trials were published between 2013 and 2024, with sample sizes ranging from 81 to 1,545 patients. The included studies exhibited a certain degree of heterogeneity in terms of participant characteristics and study protocols. Although the average age of participants was over 60 years, variations were observed in sex distribution and types of surgery. In addition, intraoperative electroencephalogram (EEG) monitoring methods varied across studies. Eight trials (14, 22–27) employed the bispectral index (BIS) for EEG monitoring, while three studies (13, 28, 29) utilized the SEDLine Brain Function Monitor to assess brain activity. The tools used to evaluate postoperative delirium also differed: eight studies (13, 15, 22, 24–26, 28, 29) used the Confusion Assessment Method (CAM), one study (27) applied both the 3D Confusion Assessment Method (3D-CAM) and the Intensive Care Unit version of CAM (CAM-ICU), and another study (23) adopted the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. The timing of delirium assessments ranged from once daily until postoperative day 3 or 7. Furthermore, several studies reported long-term outcomes: three (13, 14, 30) evaluated 30-day mortality, two (22, 23) assessed 90-day mortality, and another three (15, 25, 27) reported 1-year mortality. The remaining studies did not report relevant data.
Figure 1

Search strategy and final included and excluded studies.
Table 1
| First authors | Year | Number of participants | Sex distribution | Age, mean ± SD (years) | Countries | Type of pEEG monitor | Delirium assessment method | Mortality follow-up period | Type of surgery | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EEG Female, n (%) | Usual care Female, n (%) | EEG | Usual care | ||||||||
| Chan et al. | 2013 | 902 | 37.8 | 39.6 | 68.1 ± 8.2 | 67.6 ± 8.3 | China | BIS | CAM | 3 months | Elective major surgery expected to last 2 h |
| Radtke et al. | 2013 | 1,155 | 44.7 | 47.6 | 69.7 ± 6.3 | 70.1 ± 6.5 | Germany | Bilateral BIS | DSM IV criteria applied by psychiatrists | 3 months | Elective surgery expected to last 60 min or more |
| Zhou et al. | 2018 | 81 | 29.3 | 32.5 | 68.29 ± 2.81 | 68.90 ± 2.99 | China | BIS | CAM | NR | Colorectal surgery with expected duration >2 h |
| Wildes et al. | 2019 | 1,213 | 45.9 | 45.5 | 69.7 ± 2.4 | 69.8 ± 2.8 | USA | BIS | CAM or CAM-ICU and supplemental note review | 30 days | Major elective surgery excluding neurosurgery |
| Kunst et al. | 2020 | 82 | 21.4 | 15.0 | 71.6 ± 5.0 | 72.0 ± 4.3 | UK | BIS | CAM | 1 year | Cardiac surgery |
| Tang et al. | 2020 | 204 | 52.0 | 52.0 | 72.1 ± 5.6 | 71.8 ± 5.3 | USA | SEDLine brain function monitor | CAM | NR | Major elective noncardiac surgery, excluding neurosurgery |
| Brown et al. | 2021 | 217 | 61.8 | 67.0 | 72.5 ± 2.0 | 72.3 ± 1.8 | USA | BIS | CAM | NR | Noncardiac surgery |
| Evered et al. | 2021 | 515 | 36.0 | 35.1 | 70.8 ± 6.9 | 71.1 ± 6.8 | Australia, China, USA | BIS | 3D-CAM or CAM-ICU | 1 year | Major noncardiac surgery with expected duration >2 h |
| Xu et al. | 2021 | 255 | 14.2 | 11.7 | 62.31 ± 8.02 | 63.16 ± 7.17 | China | SEDLine brain function monitor | CAM | NR | Carotid Endarterectomy |
| Wang et al. | 2022 | 1,545 | 43.5 | 40.7 | 61.5 ± 3.0 | 61.0 ± 3.0 | China | SEDLine brain function monitor | CAM | 30 days | Laparoscopic Surgery |
| He et al. | 2023 | 141 | 36.1 | 32.8 | 68.7 ± 5.9 | 69.3 ± 6.7 | China | BIS | Narcotrend | 30 days | elective major abdominal surgery |
| Deschamps et al. | 2024 | 1,131 | 21.9 | 27.6 | 70.0 ± 2.5 | 71.3 ± 2.3 | Canada | NR | CAM | 1 year | cardiac surgery |
Characteristics of the included RCTs.
pEEG, processed electroencephalography; CAM, Confusion Assessment Method; CAM-ICU, Confusion Assessment Method for the Intensive Care Unit; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; 3D-CAM, 3-Minute Diagnostic Interview for CAM-Defined Delirium; NR, not reported; UK, United Kingdom; US, United States.
A total of 3,707 patients received EEG-guided anesthesia, with a lower incidence of postoperative delirium observed compared to 3,734 patients in the standard care group. The overall incidence of postoperative delirium was 14.94% (554/3,707) in the EEG-guided group and 17.73% (662/3,734) in the standard care group. The pooled relative risk for postoperative delirium was 0.76 (95% CI: 0.61–0.96) in the EEG-guided group compared to the standard care group (Figure 2). In addition, the incidence of postoperative infections was lower in the EEG-guided group, with an incidence of 13.54% (91/672) versus 18.21% (122/670) in the standard care group (RR = 0.74; 95% CI: 0.58–0.95) (Figure 3). However, the intervention demonstrated no significant effect on all-cause mortality at the longest follow-up among elderly patients. The mortality incidence was 2.23% (60/2,689) in the EEG-guided group and 2.62% (71/2,707) in the standard care group (RR = 0.80; 95% CI: 0.43–1.51) (Figure 4). Subgroup analyses revealed no statistically significant interaction based on type of surgery (p for interaction = 0.18) (Figure 5). We performed a sensitivity analysis using a fixed-effects model, excluding studies with unclear or high risk of bias. Additionally, each study was sequentially removed to assess its impact on the overall results. The findings remained robust throughout all analysis(Supplementary Table 2). The funnel plot for the primary outcome appeared symmetrical, suggesting no significant publication bias (Supplementary Figure 3).
Figure 2
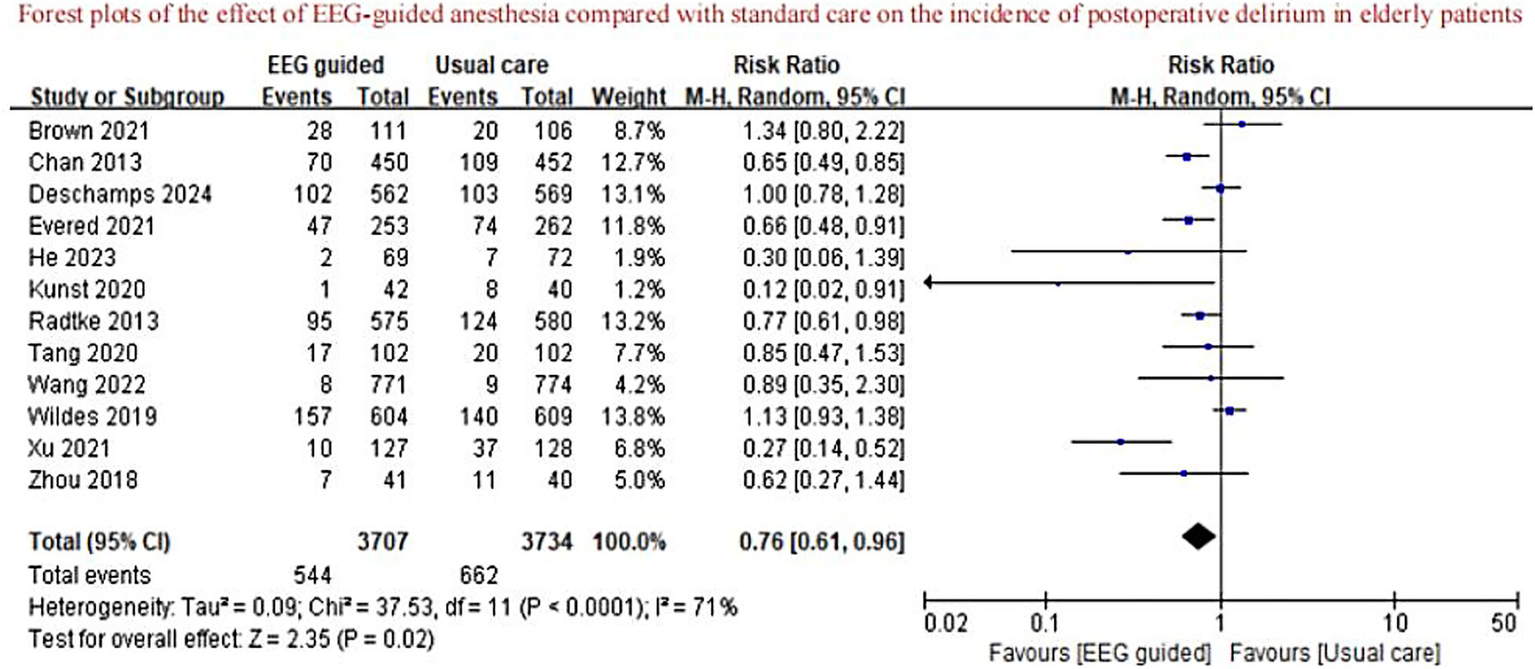
Forest plots of the effect of EEG-guided anesthesia compared with standard care on the incidence of postoperative delirium in elderly patients.
Figure 3

Forest plots of the effect of EEG-guided anesthesia compared with standard care on the incidence of postoperative infection in elderly patients.
Figure 4

Forest plots of the effect of EEG-guided anesthesia compared with standard care on mortality at the longest postoperative follow-up in elderly patients.
Figure 5
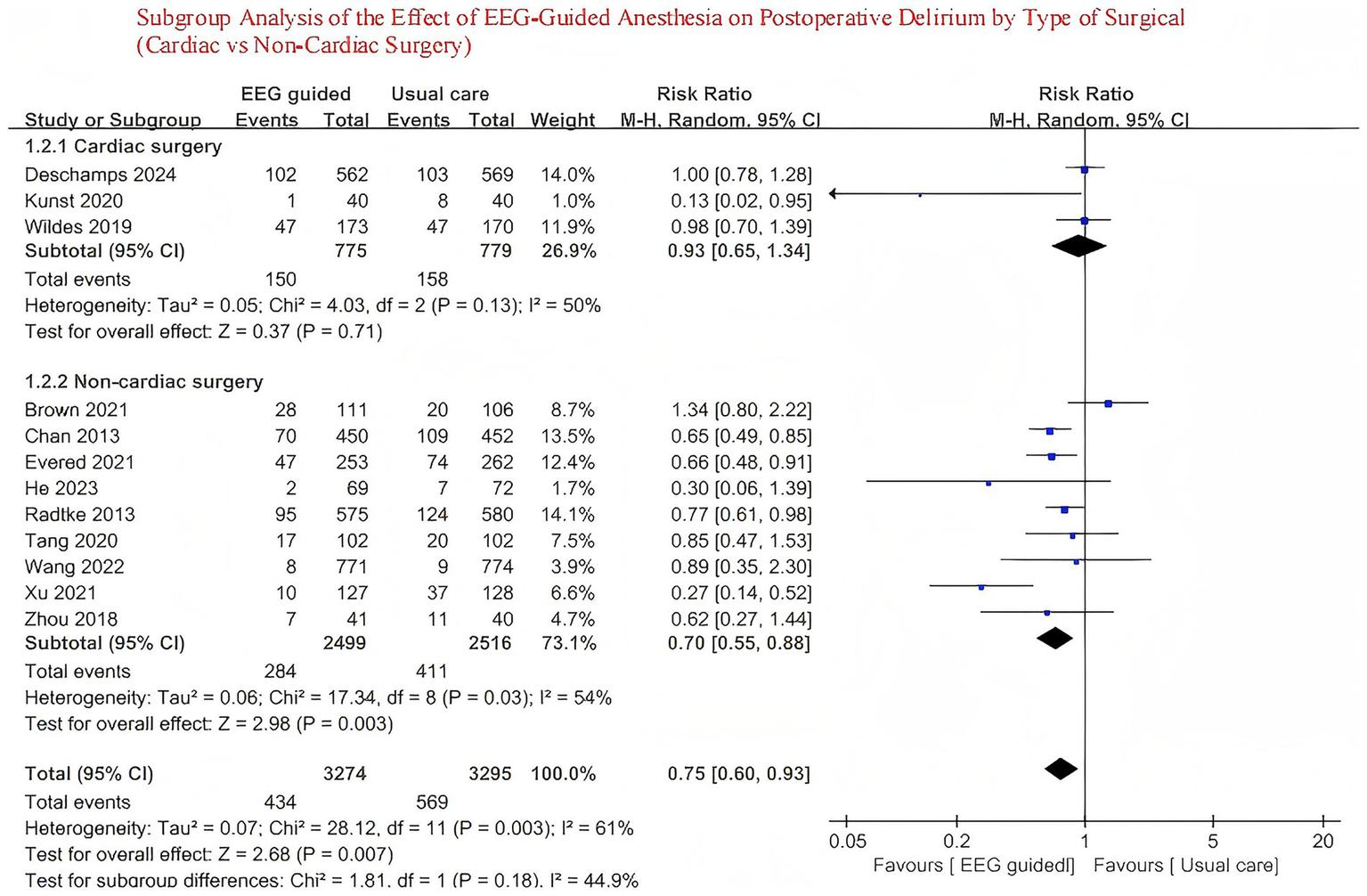
Subgroup analysis of the effect of EEG-guided anesthesia on postoperative delirium by type of surgical (cardiac vs. non-cardiac surgery).
Table 1 in the Data Supplement summarizes the risk-of-bias assessment. Among the included trials, six were rated as having a low risk of bias. Four studies were judged to have some unclear, primarily due to potential biases related to intervention implementation and outcome reporting. The remaining two trials were assessed as high risk, mainly attributable to deficiencies in outcome measurement and selective reporting. Based on the GRADE evaluation, the overall certainty of the evidence for the primary outcome was deemed low.
Discussion
This meta-analysis included 12 randomized controlled trials with a total of 7,441 elderly surgical patients, among whom 3,707 received EEG-guided anesthesia. Our findings demonstrate that EEG-guided anesthesia significantly reduces the incidence of postoperative delirium (RR = 0.76; 95% CI: 0.61–0.96) and postoperative infection (RR = 0.74; 95% CI: 0.58–0.95), while showing no statistically significant effect on long-term all-cause mortality (RR = 0.80; 95% CI: 0.43–1.51). These findings strengthen the evidence supporting the role of EEG guidance in perioperative neuroprotection and are consistent with our predefined hypothesis (Table 2).
Table 2
| Outcome | No. of patients (trials) | Relative effect, risk ratio (95% CI) | Absolute effect estimates (per 1,000) | Quality of the evidence | ||
|---|---|---|---|---|---|---|
| EEG-guided | Usual care | Difference | ||||
| The primary outcome | ||||||
| Postoperative delirium | 7,441 (12) | 0.85 (0.73, 0.98) | 133 | 173 | −43 [−12, −69] | Moderate* |
| The secondary outcome | ||||||
| Postoperative infection | 1,342 (4) | 0.74 (0.58, 0.95) | 135 | 182 | −47 [−9, −76] | High |
| Mortality at the longest postoperative follow-up | 5,396 (6) | 0.80 (0.43, 1.51) | 22 | 26 | −5 [−15, 13] | High |
Summary of findings and strength of evidence.
Inconsistency.
CI, confidence interval; RR, risk ratio.
This meta-analysis, encompassing 7,441 participants from 12 trials (13–15, 22–29), demonstrates that EEG-guided anesthesia is effective in reducing the incidence of postoperative delirium in elderly patients. Our findings differ from previous meta-analysis (12), which did not observe a significant reduction in the incidence of postoperative delirium among elderly patients receiving EEG-guided anesthesia. However, that study acknowledged the limitation of an insufficient sample size. The trial sequential analysis conducted in the previous review estimated that a minimum sample size of 4,180 participants was required to achieve adequate statistical power. However, with only 3,433 participants included, the study may have been underpowered, raising the possibility of a type II error. In addition, several randomized controlled trials (13–15) published in recent years have reported inconsistent findings, underscoring the ongoing uncertainty in this field. So, our meta-analysis incorporates a larger and more up-to-date collection of randomized controlled trials (13–15, 22–29), including a total of 7,441 participants, substantially improving the statistical power and reliability of the conclusions. The results indicate that EEG-guided anesthesia is associated with a reduced risk of Postoperative Delirium in older patients. This finding not only enriches the existing evidence base but also suggests that the potential benefits of Electroencephalogram monitoring in preventing Postoperative Delirium are becoming increasingly apparent as more data accumulates. We also performed subgroup analyses based on surgical sites, which revealed no significant difference between the subgroups.
Our results regarding postoperative infection are in agreement with previous study (8), which suggests that optimal anesthetic depth can stabilize intraoperative hemodynamics and reduce systemic inflammatory responses, thereby lowering the risk of infection. EEG monitoring enables individualized anesthetic titration, helping to prevent hypotension (31), hypoperfusion (32), and overexposure to anesthetic agents (33)—all of which have been associated with postoperative immune suppression. In elderly patients, who are more vulnerable to infection due to immunosenescence (34), EEG-guided anesthesia may offer additional protection by preserving physiological stability and reducing surgical stress (30).
In contrast, our findings did not demonstrate a statistically significant reduction in long-term all-cause mortality. This result differs from several prior studies (35, 36) that have reported potential survival benefits associated with optimized anesthesia depth. One possible explanation is the limited number of trials (n = 4) and patients contributing to this specific outcome, resulting in wide confidence intervals and reduced statistical power. Therefore, this may represent a type II error or false-negative finding. Further large-scale trials are warranted to validate this association.
The mechanisms by which EEG-guided anesthesia may reduce the risk of postoperative delirium remain incompletely understood. One proposed explanation is that maintaining the bispectral index within the range of 40–60 (6,7) during surgery helps to avoid excessively deep anesthesia and reduces the occurrence of burst suppression patterns, which have been strongly associated with postoperative cognitive impairment. EEG monitoring provides real-time feedback on cerebral activity, enabling anesthesiologists to more precisely titrate anesthetic dosages and thereby potentially lower the risk of delirium (37). Studies (38, 39) have shown that both inhalational and intravenous anesthetics may induce neuronal and glial cell damage or apoptosis when used in high doses or for prolonged periods—particularly in brain regions such as the hippocampus, which are closely related to cognitive function. EEG-guided anesthesia may help minimize unnecessary exposure to anesthetics, thereby preserving neural integrity and further reducing the likelihood of Postoperative Delirium (37).
This meta-analysis indicates that EEG-guided anesthesia significantly reduces the incidence of postoperative delirium in elderly patients. Given the substantial impact of POD on recovery and cognitive outcomes, EEG monitoring may serve as a practical strategy to enhance anesthesia safety in this population. However, the underlying mechanisms remain unclear (40). Future research should explore the relationship between anesthetic depth, burst suppression, and cognitive outcomes, and conduct large-scale, multicenter randomized controlled trials to further assess its efficacy and clinical feasibility.
This study has several limitations. First, there was considerable heterogeneity in the diagnostic criteria, assessment tools (e.g., CAM-ICU, 3D-CAM), and follow-up durations used across the included studies. Second, variations in anesthetic agents, dosages, and delivery methods (including inhalational VS. intravenous anesthesia) across trials may have influenced the overall results.
Conclusion
Our study suggests that EEG-guided anesthesia may contribute to a lower incidence of postoperative delirium in elderly surgical patients, highlighting its potential as an effective strategy in perioperative neuroprotection.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
XD: Formal analysis, Writing – original draft, Methodology, Software, Visualization, Data curation, Investigation, Validation, Supervision, Project administration, Writing – review & editing, Resources, Conceptualization. XiL: Writing – review & editing, Conceptualization, Formal analysis. AD: Formal analysis, Writing – original draft. LC: Writing – original draft, Data curation. YM: Data curation, Writing – original draft, Writing – review & editing. XuL: Investigation, Writing – original draft, Resources, Visualization, Software, Validation, Conceptualization, Formal analysis, Data curation, Supervision, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1638282/full#supplementary-material
Abbreviations
EEG, electroencephalogram; RR, relative risk; MD, mean difference; CI, confidence interval; RCTs, randomized controlled trials; POD, postoperative delirium.
References
1.
Demeure MJ Fain MJ . The elderly surgical patient and postoperative delirium. J Am Coll Surg. (2006) 203:752–7. doi: 10.1016/j.jamcollsurg.2006.07.032
2.
Dasgupta M Dumbrell AC . Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc. (2006) 54:1578–89. doi: 10.1111/j.1532-5415.2006.00893.x
3.
United Nations, Department of Economic and Social Affairs, Population Division (2019). World population ageing 2019: highlights (ST/ESA/SER.A/430). Available online at: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (Accessed April 30, 2025)
4.
Vacas S Cole DJ Cannesson M . Cognitive decline associated with anesthesia and surgery in older patients. JAMA. (2021) 326:863–4. doi: 10.1001/jama.2021.4773
5.
Jin Z Hu J Ma D . Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. (2020) 125:492–504. doi: 10.1016/j.bja.2020.06.063
6.
Pawar N Barreto Chang OL . Burst suppression during general anesthesia and postoperative outcomes: mini review. Front Syst Neurosci. (2022) 15:767489. doi: 10.3389/fnsys.2021.767489
7.
Pérez-Otal B Aragón-Benedí C Pascual-Bellosta A Ortega-Lucea S Martínez-Ubieto J Ramírez-Rodríguez JM et al . Neuromonitoring depth of anesthesia and its association with postoperative delirium: a systematic review and meta-analysis. Sci Rep. (2022) 12:16466. doi: 10.1038/s41598-022-16466-y
8.
Wesselink EM Kappen TH Torn HM Slooter AJ van Klei WA . Intraoperative hypotension and the risk of postoperative adverse outcomes: a systematic review. Br J Anaesth. (2018) 121:706–21. doi: 10.1016/j.bja.2018.04.036
9.
Nguyen XD Horn A Fischer D Beck G Spannenberger CC Gaudilliere B et al . Suppressive effects of deep balanced anesthesia on cellular immunity and protein expression: a randomized-controlled pilot study. BMC Anesthesiol. (2025) 25:129. doi: 10.1186/s12871-025-02980-9
10.
Guo R Yang W Zhong M Rao P Luo X Liao B et al . The relationship between anesthesia, surgery and postoperative immune function in cancer patients: a review. Front Immunol. (2024) 15:1441020. doi: 10.3389/fimmu.2024.1441020
11.
Zhang X Li Y Wang Z Chen H Liu J Huang Y . Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: a narrative review. Br J Anaesth. (2019) 123:777–89. doi: 10.1016/j.bja.2019.09.019
12.
Chew WZ Teoh WY Sivanesan N Loh PS Shariffuddin II Ti LK et al . Bispectral index (BIS) monitoring and postoperative delirium in elderly patients undergoing surgery: a systematic review and meta-analysis with trial sequential analysis. J Cardiothorac Vasc Anesth. (2022) 36:4449–59. doi: 10.1053/j.jvca.2022.07.004
13.
Wang E Wang L Ye C Luo N Zhang Y Zhong Y et al . Effect of electroencephalography spectral edge frequency (SEF) and patient state index (PSI)-guided propofol-remifentanil anesthesia on delirium after laparoscopic surgery: the eMODIPOD randomized controlled trial. J Neurosurg Anesthesiol. (2022) 34:113–21. doi: 10.1097/ANA.0000000000000823:contentReference[oaicite:7]{index=7}
14.
He Z Zhang H Xing Y Liu J Gao Y Gu E et al . Effect of raw electroencephalogram-guided anesthesia administration on postoperative outcomes in elderly patients undergoing abdominal major surgery: a randomized controlled trial. BMC Anesthesiol. (2023) 23:337. doi: 10.1186/s12871-023-02297-5
15.
Deschamps A Ben Abdallah A Jacobsohn E Saha T Djaiani G et al . Electroencephalography-guided anesthesia and delirium in older adults after cardiac surgery: the ENGAGES-Canada randomized clinical trial. JAMA. (2023) 330:441–9. doi: 10.1001/jama.2023.10499
16.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
17.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
18.
Guyatt GH Oxman AD Vist GE Kunz R Falck-Ytter Y Alonso-Coello P et al . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
19.
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
20.
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
21.
Harbord RM Egger M Sterne JA . A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. (2006) 25:3443–57. doi: 10.1002/sim.2380
22.
Chan MTV Cheng BCP Lee TMC Gin T , the CODA Trial Group . BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. (2013) 25:33–42. doi: 10.1097/ANA.0b013e3182712fba
23.
Radtke FM Franck M Lendner J Krüger S Wernecke KD Spies CD . Monitoring depth of anesthesia in a randomised trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. (2013) 110:i98–i105. doi: 10.1093/bja/aet055
24.
Zhou Y Li Y Wang K . Bispectral index monitoring during anesthesia promotes early postoperative recovery of cognitive function and reduces acute delirium in elderly patients with colon carcinoma: a prospective controlled study using the attention network test. Med Sci Monit. (2018) 24:CLR7785–7793. doi: 10.12659/MSM.910124
25.
Kunst G Gauge N Salaunkey K Spazzapan M Amoako D Ferreira N et al . Intraoperative optimization of both depth of anesthesia and cerebral oxygenation in elderly patients undergoing coronary artery bypass graft surgery—a randomized controlled pilot trial. J Cardiothorac Vasc Anesth. (2020) 34:1172–81. doi: 10.1053/j.jvca.2019.10.054
26.
Brown CH Edwards C Lin C Jones EL Yanek LR Esmaili M et al . Spinal anesthesia with targeted sedation based on bispectral index values compared with general anesthesia with masked bispectral index values to reduce delirium: the SHARP randomized controlled trial. Anesthesiology. (2021) 135:992–1003. doi: 10.1097/ALN.0000000000004015
27.
Evered LA Chan MTV Han R Chu MHM Cheng BP Scott DA et al . Anaesthetic depth and delirium after major surgery: a randomised clinical trial. Br J Anaesth. (2021) 127:704–12. doi: 10.1016/j.bja.2021.07.021
28.
Tang CJ Jin Z Sands LP Pleasants D Tabatabai S Hong Y et al . Adapt-2: a randomized clinical trial to reduce intraoperative EEG suppression in older surgical patients undergoing major noncardiac surgery. Anesth Analg. (2020) 131:1228–36. doi: 10.1213/ANE.0000000000004713
29.
Xu N Li L-X Wang T-L Jiao L-Q Hua Y Yao D-X et al . Processed multiparameter electroencephalogram-guided general anesthesia management can reduce postoperative delirium following carotid endarterectomy: a randomized clinical trial. Front Neurol. (2021) 12:666814. doi: 10.3389/fneur.2021.666814
30.
Wildes TS Mickle AM Ben Abdallah A Maybrier HR Oberhaus J Budelier TP et al . Effect of electroencephalography-guided anesthetic administration on postoperative delirium: the ENGAGES randomized clinical trial. JAMA. (2019) 321:473–83. doi: 10.1001/jama.2018.22005
31.
Thomsen KK Sessler DI Krause L Hoppe P Opitz B Kessler T et al . Processed electroencephalography-guided general anesthesia and norepinephrine requirements: a randomized trial in patients having vascular surgery. J Clin Anesth. (2024) 95:111459. doi: 10.1016/j.jclinane.2024.111459
32.
Georgii M-T Kreuzer M Fleischmann A Schuessler J Schneider G Pilge S . Targeted interventions to increase blood pressure and decrease anaesthetic concentrations reduce intraoperative burst suppression: a randomised, interventional clinical trial. Front Syst Neurosci. (2022) 16:786816. doi: 10.3389/fnsys.2022.786816
33.
Romagnoli S Franchi F Ricci Z . Processed EEG monitoring for anesthesia and intensive care practice. Minerva Anestesiol. (2019) 85:1219–30. doi: 10.23736/S0375-9393.19.13478-5
34.
Oh S-J Lee JK Shin OS . Aging and the immune system: the impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. (2019) 19:e37. doi: 10.4110/in.2019.19.e37
35.
Xu Y Shan Z Zhao Y Xiu H Xu K . Association between depth of anesthesia and postoperative outcome: a systematic review and meta-analysis. Int J Clin Exp Med. (2018) 11:3023–32. https://www.researchgate.net/publication/333390084_Association_between_depth_of_anesthesia_and_postoperative_outcome_a_systematic_review_and_meta-analysis
36.
Liu Y-H Qiu D-J Jia L Tan J-T Kang J-M Xie T et al . Depth of anesthesia measured by bispectral index and postoperative mortality: a meta-analysis of observational studies. J Clin Anesth. (2019) 56:119–25. doi: 10.1016/j.jclinane.2019.01.046
37.
Sun Y Ye F Wang J Ai P Wei C Wu A et al . Electroencephalography-guided anesthetic delivery for preventing postoperative delirium in adults: an updated Meta-analysis. Anesth Analg. (2020) 131:712–9. doi: 10.1213/ANE.0000000000004746
38.
Zhou Z Ma D . Anaesthetics-induced neurotoxicity in developing brain: an update on preclinical evidence. Brain Sci. (2014) 4:136–49. doi: 10.3390/brainsci4010136
39.
Cheng Y He L Prasad V Wang S Levy RJ . Anesthesia-induced neuronal apoptosis in the developing retina: a window of opportunity. Anesth Analg. (2015) 121:1325–35. doi: 10.1213/ANE.0000000000000714
40.
Evered LA . EEG-guided anesthetic titration and perioperative cognitive disorders. Alzheimers Dement. (2025) 20:4904. doi: 10.1002/alz.084904
Summary
Keywords
postoperative delirium, older adults, meta-analysis, electroencephalogram-guided anesthesia, EEG
Citation
Da X, Li X, Dong A, Chen L, Ma Y and Li X (2025) Effect of electroencephalogram-guided anesthesia on postoperative delirium in older adults after surgery: a systematic review and meta-analysis. Front. Neurol. 16:1638282. doi: 10.3389/fneur.2025.1638282
Received
30 May 2025
Accepted
04 August 2025
Published
28 August 2025
Volume
16 - 2025
Edited by
Somchai Amornyotin, Mahidol University, Thailand
Reviewed by
Vlasios Karageorgos, University of Crete, Greece
Maria Bruzzone, University of Florida, United States
Updates
Copyright
© 2025 Da, Li, Dong, Chen, Ma and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Li, 18717042824@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.