Abstract
Purpose:
The aim of the study was to assess the effects of rehabilitation using the Biometrics device on the re-education of hand function in late stroke patients.
Methods:
The data were collected from 1 August 2022 to 28 February 2023. The study was conducted among 120 people after stroke, who were randomly assigned to the test (n = 60) or control groups (n = 60). Both groups were provided with a 3-week rehabilitation program for 2 h a day. While the control group received traditional physiotherapy, the test group additionally underwent biofeedback training. Examinations were performed on the first day and the final day of the 3-week intervention program. The primary measurement included assessment of hand grip strength (key, three jaw chuck, tip-to-tip) using an electronic dynamometer and a Biometrics E-link pinchmeter. Secondary outcomes included hand motor function assessment, using the Fugl–Meyer Assessment-Hand Function scale, hand motor dexterity with the Box and Blocks test, hand grip functions according to the Frenchay scale, and functional fitness with the Barthel index.
Results:
In the test group, significant rehabilitation effects were observed for the assessment of grip strength, finger compression strength, manual hand dexterity, grip function and activities of daily living (p < 0.001; p = 0.001), while in the control group results were improved for grip strength and finger compression strength (key and three-jaw chuck) of the right hand (p = 0.012; p = 0.017; p = 0.001) and manual dexterity (p < 0.001), motor abilities and activities of daily living (p < 0.001).
Conclusion:
The study showed positive effects of hand function rehabilitation in chronic stroke patients in both groups. However, in the test group, which additionally underwent training that stimulated the central nervous system using the biofeedback method with the Biometrics device, better hand and finger grip function as well as hand motor and manual function were noted.
Clinical trial registration:
https://clinicaltrials.gov/, Identifier NCT05486052.
1 Introduction
Stroke is one of the leading causes of disability worldwide. People who survive a stroke show up to 80% of motor disorders in the upper limb and hand (1, 2).
Impairment of hand function makes it difficult to perform activities of daily living. The upper limb is one of the most utilized parts of the body, therefore regaining its function should be treated as a priority in brain injury. However, its improvement is considered one of the most difficult in the rehabilitation process, because complications after a stroke include impaired sensation, limitation of motor functions, dexterity, coordination, abnormal muscle strength and tension, which disrupt the ability of such people to function in society (3–5). New methods and various techniques of working with stroke patients are mainly aimed at improving this ability.
Brain plasticity is the ability to permanently transform the brain at the level of structures and functions based on information supplied to it, which allows us to learn, remember, and undergo developmental and compensatory changes. Research confirms that neurogenesis is constantly occurring in the brain, thanks to which a stroke can be followed by a brain healing effect, so-called neural network reprogramming, potentially leading to faster recovery (6–8). The biofeedback phenomenon is related to processes that take place throughout the body, and so it can be used to stimulate a patient’s nervous system during treatment after stroke. The use of a biofeedback mechanism allows feedback to be provided to the patient, teaching them how to perform movements correctly, which is an important element of therapy when motor deficits occur (8, 9). Research shows that the use of biofeedback in robotic devices facilitates the phenomenon of brain neuroplasticity through multiple repetitions of a given movement, affecting the sensorimotor cortex (10).
Among the various techniques for using surrogate feedback, visual and auditory afferentation are most often used. This type of rehabilitation facilitates intensive training, which enables adaptation to new conditions. Research shows that working with such equipment not only improves motivation to exercise but also supports regeneration. Supplementing rehabilitation with modern devices supports the process of helping patients after stroke to regain functional fitness. In the case of functional disorders of the hand or the entire upper limb following a stroke, biofeedback methods are applied using devices such as: Biometrics (11), Armeo (12, 13), Luna (14, 15), Pablo (9) Gloreha glove (16), HandTutor (17), and Amadeo (18). Many researchers use robotic biofeedback devices to treat stroke patients, but most focus on the entire upper limb (19, 20).
The Biometrics device is a diagnostic and measurement tool that uses the biofeedback method as the basis of hand and finger grip strength exercises that make it possible to perform training to restore hand dexterity and the function of the entire upper limb (12, 21). Biofeedback allows the patient to visualize movements they are performing, which has a positive effect on engagement and increases the range of movement and muscle strength. The Biometrics device has been used many times by researchers for rehabilitation of patients with various disorders (22, 23).
A literature review showed that the Biometrics LTD device has been used in the treatment of patients with rheumatoid arthritis (24), cerebral palsy (25), and studies have been conducted using the device to improve the performance of prosthetic hands (26) as well as with spinal cord injury (27). However, no studies were found on the rehabilitation of chronic stroke patients. Although chronic stroke patients typically exhibit limited potential for recovery due to plateaued neuroplastic processes, biofeedback-based therapy may still activate residual neuroplasticity through targeted, repetitive, and feedback-driven training. Therefore, the aim of our study was to assess the effects of rehabilitation using the Biometrics device on the re-education of hand function in chronic stroke patients.
2 Materials and methods
2.1 Study design
The research was conducted as a two-group randomized controlled trial.
The research received a positive opinion from the Local Bioethics Commission of the University (No. 2022/085). The study was registered in the clinical trials register at the site ClinicalTrials.gov (registration number NCT05486052). Registration date (18.07.2022). The data were collected from 1 August 2022 to 28 February 2023.
2.2 Setting
The study was conducted among chronic stroke patients in the Spa and Rehabilitation Hospital.
2.3 Sample size calculation
The required sample was taken a priori based on the minimal clinically important difference (MCID) for the FMA-UE scale, 4.25 points (28). Using the G*Power program (version 3.1.9.4; F. Faul, University of Cologne, Germany), with a statistical power of 90% (1-β) and a significance level of α = 0.05, a minimum required sample size was calculated as 38 participants in each group (76 in total). However, due to the specific characteristics of those examined, more conservative assumptions regarding oversampling (approximately 25–30%) were deliberately made. 120 patients were enrolled in the study. After applying a 0% elimination estimate, the final sample size, distributed across units, ultimately yielded very high statistical power.
2.4 Study population
Chronic stroke patients were randomly assigned to two groups (test and control). Randomization was performed by the double-blind method, in which both participants and outcome assessors were blinded to group allocation. Due to the nature of the intervention, the therapists administering the treatment were not blinded. Randomization was performed using a computer-generated random number sequence created in Microsoft Excel (Microsoft Corp., Redmond, WA, USA). To ensure concealment of allocation, sealed, opaque envelopes were prepared by an investigator not involved in participant recruitment or outcome. The inclusion criteria were: first ischemic stroke, medical examination confirming participation in exercises, basic gripping ability for the upper limbs and hand, modified Ashworth scale not higher than 3, Brunnström scale 4–5, Rankin scale 3, time since stroke greater than 6 months, written informed consent to participate in the study. The exclusion criteria were: non-ischemic stroke, lack of informed consent from the patient to participate in the study, mechanical, thermal injuries and comorbidities that may impair hand-grip function, unstable health state.
2.5 Interventions
The rehabilitation program lasted 3 weeks (from Monday to Friday) and took 2 h a day for both groups. All patients participated in individual and group exercises, massages, physical treatments and treatments using natural resources. In addition, patients in the test group participated in 30 min per day of exercises using a biofeedback method, which were performed on the Biometrics dynamometer device and aimed at improving hand motor function (29). These activities were conducted within the existing 2-h sessions, rather than as extra therapy time. The training took place on the basis of tasks stimulating the central nervous system with the help of visual and acoustic biofeedback. These tasks consisted of catching the correct colors of balls for a basket (a), shooting balls into a goal (b), laying colored blocks (c) (Figure 1).
Figure 1
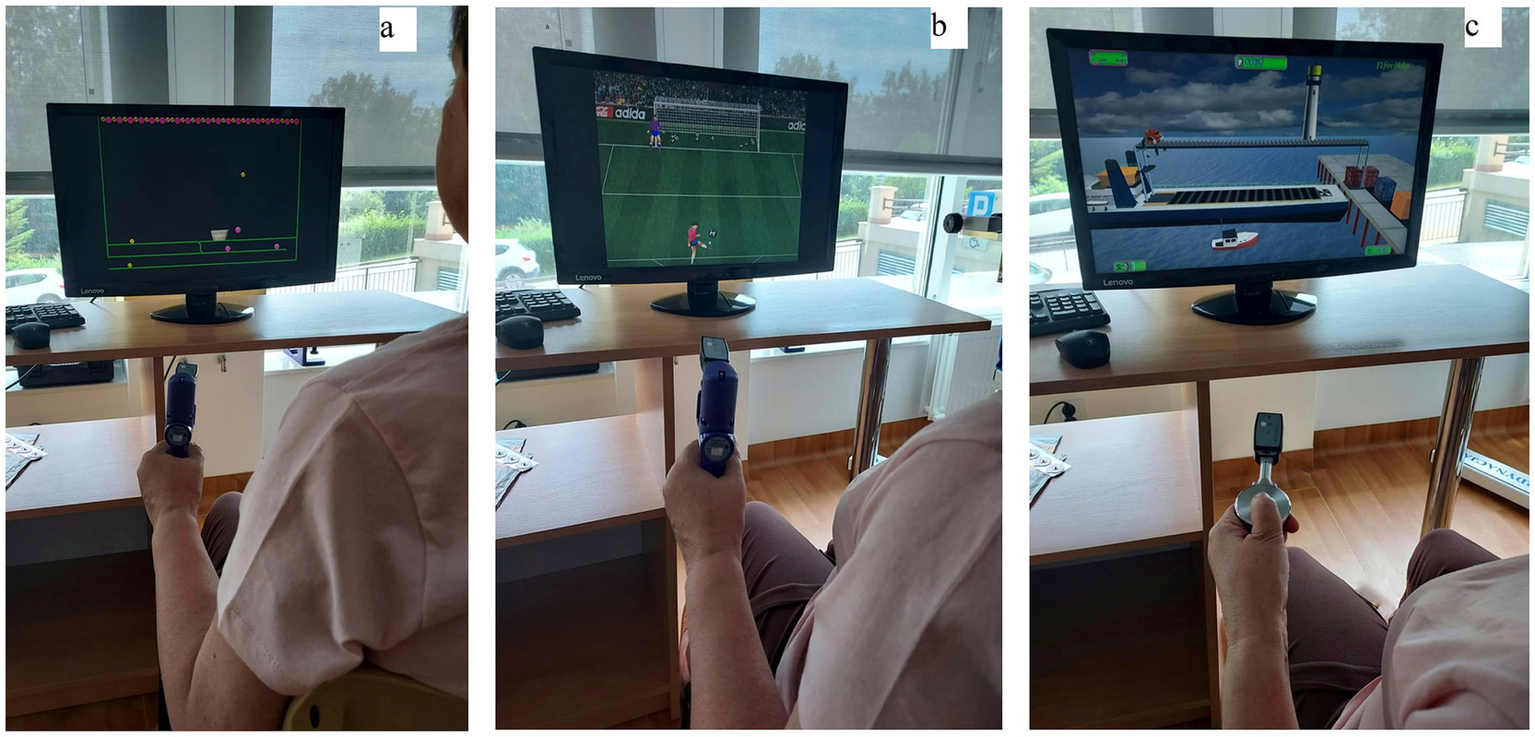
Biofeedback exercises: (a) catching the correct colors of balls for a basket, (b) shooting balls into a goal, (c) laying colored blocks.
2.6 Outcome measures
The first examination was performed on the first day of rehabilitation, and the second on the last day of the 3-week intervention program (at discharge).
The primary measurement of hand grip and finger strength (key, three jaw chuck, tip-to-tip) was based on an objective method, using an electronic dynamometer and a Biometrics E-link pinchmeter. The former registers forces from less than 0.1 kg/lb. to 90 kg (200 lb) while the latter records finger pinch strength up to 22 kg (50 lb) (29, 30).
All measurements were taken according to the American Society of Hand Therapists (ASHT) guidelines and the Biometrics E-link device reliability assessment methodology for assessing hand grip and finger pinch strength in healthy individuals (29, 31).
Secondary results included an assessment of hand motor function, performed using the Fugl–Meyer Assessment-Hand Function (FMA-Hand) scale (32) to assess precise movements and grips: motor control of finger flexion and extension, thumb adduction, finger resistance, cylindrical and spherical grip. The patient received 2 points for making a full movement, 1 point for a partial movement, 0 points for no movement. The Fugl–Meyer Motor Assessment Scale for Upper Extremity (FMA-UE) consists of 7 items (FMA-UE headings 24 to 30) giving a maximum possible 14 points (5, 33–35). Hand motor skills were assessed using the Box and Blocks (BBT) test. The patient was asked to move as many blocks (2.5 cm) as possible in a wooden box (53.7 cm x 25.4 cm x 8.5 cm) divided by a partition into two parts within 60 s. The higher the number of blocks moved, the better the patient’s manual hand dexterity (36–38). Hand grip was assessed according to the Frenchay scale, which consists of 7 tasks, for which the patient receives 1 point when performing them correctly, and 0 points for failing to perform them, giving a maximum possible 7 points. The higher the patient’s score, the better their hand dexterity (39, 40). The patients’ functional status was assessed using the Barthel Index (BI), which consists of 10 items assessing activities of daily living. The results of all items are added together to determine the patient’s condition: 86–100 pts. – patient’s condition “light”; 21–85 points – patient’s condition “moderately severe”; 0–20 points – patient’s condition “very severe” (41–43).
2.7 Data analysis
Statistical analysis of the collected material was performed in the Statistica 13.3 package. The database and the graphical elaboration of the results were prepared in Microsoft Excel and Microsoft Word.
Descriptive statistics were calculated: number, mean value, median, minimum and maximum values, upper and lower quartile and standard deviation. For the assessment of statistical differences between the test and control groups in the first and second examinations, and for the assessment of the effects of rehabilitation, the student-t test for independent samples was used, or due to non-compliance with the assumptions of parametric tests (lack of compliance of the variable distribution with the normal distribution verified by the Shapiro–Wilk test or a dependent variable of an ordinal character), the non-parametric Mann Whitney U test was used. The Wilcoxon non-parametric test was used to assess the effects of rehabilitation between the first and second examinations in the test and control groups because of the lack of compliance of the distribution of differences with the normal distribution.
The level of statistical significance was p < 0.05.
3 Results
Among 200 patients admitted to the Spa and Rehabilitation Hospital, preliminary qualification was performed based on the criteria of inclusion and exclusion for the study. 64 people did not meet the criteria, and 16 people did not agree to participate in the study. The study included 120 stroke patients (n = 60 test group, n = 60 control group) who completed the 3-week rehabilitation program (Figure 2).
Figure 2
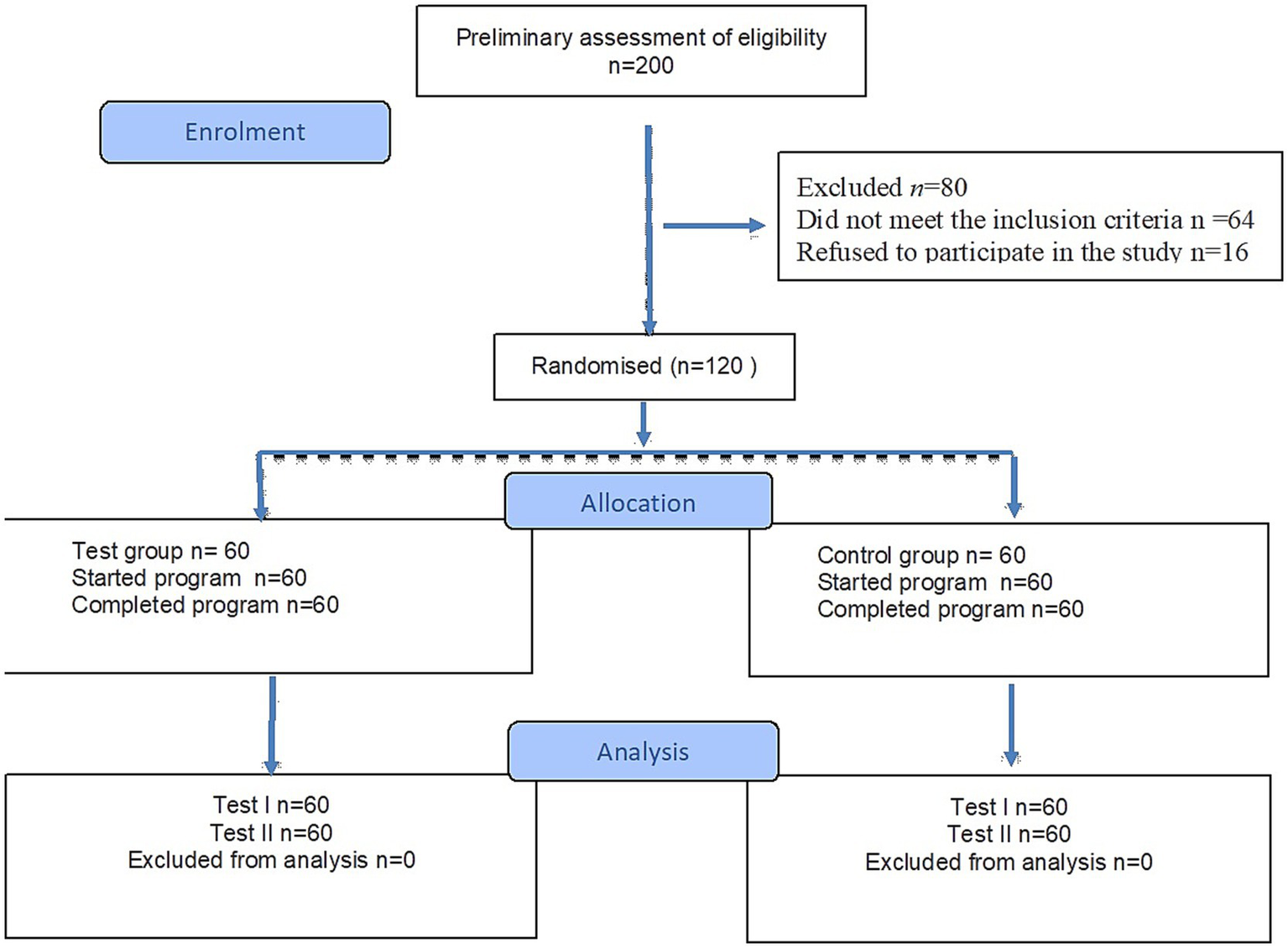
Flow diagram.
In both groups, a slight and insignificant majority consisted of men (66.7 and 65.0%) and people with left-sided paresis (58.3% each). The mean age in the test group was x̄ = 62.7, and in the control group x̄ = 63.6. The mean time since stroke in the test group was x̄ = 43.4 months and in the control group x̄ = 43.5 (Table 1).
Table 1
| Characteristics | Test group (n = 60) | Control group (n = 60) | p-value |
|---|---|---|---|
| Age (years) | 62.7 (7.80) | 63.6 (6.04) | 0.551 |
| 63.5 (55.5–69.5) | 64.5 (59.0–68.0) | ||
| Body weight (kg) | 76.4 (11.09) | 77.3 (12.01) | 0.633 |
| 77.8 (69.1–81.2) | 78.8 (70.2–83.2) | ||
| Body height (cm) | 170.3 (7.21) | 169.1 (6.98) | 0.364 |
| 170.0 (165.0–176.0) | 169.0 (164.0–173.0) | ||
| BMI (kg/m2) | 26.3 (3.28) | 27.0 (3.43) | 0.300* |
| 26.8 (24.8–28.0) | 26.6 (24.9–28.7) | ||
| Time since stroke (months) | 43.4 (45.63) | 43.5 (43.27) | 0.904 |
| 24.0 (10.5–60.5) | 23.5 (10.0–77.0) |
Differences in age, body weight [kg], body height [cm], BMI and time since stroke in the test group and control group.
Data are presented in two rows: the first row shows Mean (Standard Deviation), and the second row shows Median (Lower Quartile – Upper Quartile).
p-values were calculated using the independent t-test* or Mann–Whitney U test to compare the test and control groups at baseline.
The analysis of the results from the first (baseline) study did not show statistically significant differences between the test and control groups in any of the evaluated demographic and clinical variables (p > 0.05 for all comparisons). The groups were comparable in terms of age, body weight, BMI, and time since stroke. Similarly, no significant between-group differences were found in baseline measurements of grip and finger pinch strength, manual dexterity, or functional assessments. This confirms that the randomization process successfully created two homogeneous groups before the intervention began.
Using a dynamometer and a pinchmeter, respectively, hand grip strength and finger compression strength (key) in the test and control groups were examined in the first and second examinations. In both groups, the effects of rehabilitation on the right hand were shown to be effective (p < 0.001; p = 0.012). Left hand scores were significantly improved only in patients participating in rehabilitation supplemented with biofeedback therapy (p < 0.001), which also translated into better rehabilitation outcomes (p < 0.001) in this group (Table 2).
Table 2
| Variable | Group | Baseline | Post-intervention | p-valuea | p-valueb |
|---|---|---|---|---|---|
| Hand grip strength Right hand (kg) | Test (n = 60) |
23.6 (12.76) | 28.9 (15.45) | <0.001 | 0.246 |
| 21.3 (17.6–28.0) | 26.7 (20.0–34.5) | ||||
| Control (n = 60) |
20.3 (12.55) | 23.6 (17.39) | 0.012 | ||
| 18.4 (12.5–24.0) | 19.4 (10.0–32.3) | ||||
| Hand grip strength - Left hand (kg) | Test (n = 60) |
20.2 (17.55) | 25.3 (19.41) | <0.001 | 0.001 |
| 15.1 (8.8–26.7) | 22.7 (13.8–28.7) | ||||
| Control (n = 60) |
20.8 (10.59) | 21.8 (12.21) | 0.213 | ||
| 20.0 (13.0–28.8) | 18.6 (12.0–29.6) | ||||
| Key pinch - Right hand (kg) | Test (n = 60) |
6.4 (4.43) | 8.3 (6.08) | <0.001 | 0.159 |
| 5.9 (4.1–7.8) | 7.2 (5.5–9.0) | ||||
| Control (n = 60) |
5.7 (3.91) | 6.4 (4.06) | 0.017 | ||
| 5.0 (3.3–6.9) | 5.0 (3.9–8.3) | ||||
| Key pinch - Left hand (kg) | Test (n = 60) |
5.3 (4.42) | 6.8 (5.12) | <0.001 | 0.008 |
| 3.9 (2.4–6.7) | 5.9 (3.3–8.3) | ||||
| Control (n = 60) |
5.9 (3.44) | 6.2 (3.75) | 0.433 | ||
| 5.4 (3.3–7.0) | 5.1 (3.9–8.1) |
Comparison of hand grip and key pinch strength outcomes.
Data are presented in two rows for each group: the first row shows Mean (Standard Deviation), and the second row shows Median (Lower Quartile – Upper Quartile).
p-valuea for within-group changes was calculated using the Wilcoxon signed-rank test (Baseline vs. Post-intervention).
p-valueb for the comparison of changes between groups was calculated using the Mann–Whitney U test.
There were no inter-group differences (p > 0.05) for the finger compression strength (three-jaw chuck) assessment. After the therapy, the results for the right hand were significantly improved in both groups (p < 0.001; p = 0.001), while for the left hand only in people undergoing rehabilitation supplemented with biofeedback (p = 0.001). A similar situation is presented in assessment of the results of the finger compression strength (tip-tip) in the right and left hands. Only in the test group were significant effects of rehabilitation reported (p < 0.001). After the second measurement, people in the test group were characterized by higher finger compression strength in the right hand than people in the control group. In the left hand, the effects of therapy were more beneficial in the test group than in the control group (p = 0.017) (Table 3).
Table 3
| Variable | Group | Baseline | Post-intervention | p-valuea | p-valueb |
|---|---|---|---|---|---|
| Three-jaw chuck - Right hand (kg) | Test | 6.4 (4.58) | 7.8 (5.28) | <0.001 | 0.808 |
| 5.3 (4.0–7.8) | 7.0 (4.3–9.3) | ||||
| Control | 5.3 (3.76) | 6.5 (4.91) | 0.001 | ||
| 4.6 (2.4–6.7) | 4.7 (3.5–7.6) | ||||
| Three-jaw chuck - Left hand (kg) | Test | 5.4 (4.41) | 6.5 (4.50) | 0.001 | 0.237 |
| 4.0 (2.2–7.6) | 5.0 (3.8–7.8) | ||||
| Control | 5.2 (3.35) | 5.8 (3.64) | 0.129 | ||
| 4.5 (3.1–6.9) | 4.7 (3.5–8.3) | ||||
| Tip to tip pinch - Right hand (kg) | Test | 5.8 (4.31) | 6.5 (4.09) | <0.001 | 0.199 |
| 4.8 (3.7–6.6) | 5.8 (4.4–7.2) | ||||
| Control | 4.5 (2.57) | 5.0 (2.98) | 0.054 | ||
| 4.2 (2.8–5.3) | 4.2 (2.8–6.0) | ||||
| Tip to tip pinch - Left hand (kg) | Test | 4.2 (3.00) | 5.4 (3.62) | <0.001 | 0.017 |
| 3.4 (2.0–5.2) | 4.4 (3.0–6.9) | ||||
| Control | 4.3 (2.83) | 4.6 (2.51) | 0.138 | ||
| 3.7 (2.2–6.1) | 3.9 (2.8–6.1) |
Comparison of finger pinch strength outcomes.
Data are presented in two rows for each group: the first row shows Mean (Standard Deviation), and the second row shows Median (Lower Quartile – Upper Quartile).
p-valuea for within-group changes was calculated using the Wilcoxon signed-rank test (Baseline vs. Post-intervention).
p-valueb for the comparison of changes between groups was calculated using the Mann–Whitney U test.
In terms of manual hand dexterity, it was noted that both conventional and biofeedback rehabilitation brought the assumed benefits (p < 0.001). The effects of the therapy on the non-dominant hand were significantly better in the test group (p = 0.028). Hand grip function and hand motor abilities were significantly improved in both groups after measurement II (p < 0.001). Significantly better results of therapy were reported in people using rehabilitation supplemented with biofeedback. The final tool used was the Barthel scale, which assessed activities of daily living. There were no inter-group differences in either the first or second examinations, or in the effect assessment, but it was noted that both groups obtained more favorable results after rehabilitation than before the therapy (p < 0.001) (Table 4).
Table 4
| Variable | Group | Baseline | Post-intervention | p-valuea | p-valueb |
|---|---|---|---|---|---|
| Box & Blocks - Dominant hand (blocks) | Test | 30.9 (13.01) | 35.1 (13.76) | <0.001 | 0.267 |
| 32.0 (24.0–40.0) | 37.5 (25.5–44.5) | ||||
| Control | 28.6 (11.06) | 32.4 (13.20) | <0.001 | ||
| 29.0 (21.0–34.0) | 31.5 (23.5–39.5) | ||||
| Box & Blocks - Non-dominant hand (blocks) | Test | 26.5 (11.95) | 31.3 (12.27) | <0.001 | 0.028 |
| 25.5 (17.0–33.0) | 30.0 (23.0–37.0) | ||||
| Control | 27.6 (10.71) | 30.1 (10.48) | <0.001 | ||
| 26.0 (20.0–34.0) | 29.5 (23.0–36.0) | ||||
| Frenchay scale (points) | Test | 4.1 (0.91) | 5.5 (0.69) | <0.001 | <0.001 |
| 4.5 (3.3–5.0) | 5.5 (5.0–6.0) | ||||
| Control | 4.4 (0.88) | 5.2 (0.89) | <0.001 | ||
| 4.5 (4.0–5.0) | 5.5 (4.5–6.0) | ||||
| Fugl-Meyer scale (points) | Test | 8.8 (1.28) | 10.3 (1.39) | <0.001 | <0.001 |
| 9.0 (8.0–9.0) | 11.0 (9.0–11.0) | ||||
| Control | 9.1 (1.62) | 9.6 (1.63) | <0.001 | ||
| 9.0 (9.0–10.0) | 10.0 (9.0–11.0) | ||||
| Barthel Index (points) | Test | 80.6 (8.34) | 85.5 (7.46) | <0.001 | 0.053 |
| 85.0 (75.0–85.0) | 87.5 (80.0–90.0) | ||||
| Control | 79.1 (8.26) | 82.4 (8.71) | <0.001 | ||
| 80.0 (75.0–85.0) | 85.0 (75.0–90.0) |
Comparison of functional and dexterity outcomes.
Data are presented in two rows for each group: the first row shows Mean (Standard Deviation), and the second row shows Median (Lower Quartile – Upper Quartile).
p-valuea for within-group changes was calculated using the Wilcoxon signed-rank test (Baseline vs. Post-intervention).
p-valueb for the comparison of changes between groups was calculated using the Mann–Whitney U test.
4 Discussion
The main aim of the study was to check the effects of rehabilitation of chronic stroke patients in terms of changes in hand motor function and self-reliance, and then to determine the differences in these effects depending on the method used, i.e., biofeedback method and conventional method. The study showed that patients improved manual dexterity in both the test and control groups, but better effects of therapy were noted for the group of patients using rehabilitation combined with biofeedback.
A similar study was conducted by Dziemian et al., using biofeedback exercises during hand rehabilitation. As in our own study, a statistically significant improvement in hand function was shown on the Fugl–Meyer scale and manual hand dexterity in the Box and Blocks test after biofeedback therapy in the test group. The authors draw conclusions on the benefits of implementing biofeedback therapy to improve impaired upper limb function. However, it is worth adding that the study included only 10 patients after stroke, including 8 after ischemic stroke and 2 after haemorrhagic stroke, so the group was heterogeneous and small (44). In our own study, we examined a relatively large and homogeneous group of 120 patients in the chronic phase after a single ischemic stroke, all at a similar motor recovery level. This sample size strengthens the reliability of our findings regarding the positive effects of biofeedback in post-stroke rehabilitation.
In our own study, in measurements using a dynamometer and pinchmeter, patients who used biofeedback equipment also performed better. The analysis of the finger compression strength measurements was carried out in three positions – key, tip-tip, three-jaw chuck. An important element of the study was to record improvements in finger compression strength (in the key and three-jaw chuck positions) for patients who received biofeedback-enhanced therapy. Similar effects were noted for the tip-tip compression strength. Bayidir et al. also assessed grip and finger pinch strength (tip-tip) in a randomized-controlled study of patients following stroke, and their results confirmed better effects for a group of patients who received biofeedback-enriched therapy (17). Hsu et al. studied the effects of robot-assisted training, in combination with conventional rehabilitation, on hand function chronic stroke patients. Their observations showed an improvement in hand function. However, the test group was small, at just 12 people, so the authors recommend further research to confirm the validity of their reports (45).
The above results of both our own and other authors’ research indicate that the use of biofeedback methods together with traditional rehabilitation gives good therapeutic effects in terms of manual function and hand grip strength in chronic stroke patients. It is worth adding, however, that there are conflicting reports in the literature regarding rehabilitation using biofeedback methods. It should be noted that although significant differences were obtained, we did not obtain clinically important differences (MCID). The MCID for the FMA = grasping ability 4.25, releasing ability 5.25 (28), and our patients improved by 1.28–1.63 points. The late post-stroke period in which our subjects were present may have influenced this result. However, we noted the need for continued exercise and ongoing hand rehabilitation in our patients. When analyzing the literature on this topic, numerous studies indicate positive effects from using robotic equipment with biofeedback for improving upper limbs, including the hand, after a stroke (19, 46–49). However, while some studies unambiguously confirm the benefits of robotic therapy, others find no significant differences in fitness improvement between classical rehabilitation and biofeedback methods (50). Therefore, there was a need and rationale to conduct this study in a homogeneous group of stroke patients in order to assess the effects of hand rehabilitation using biofeedback methods. The obtained results allowed us to confirm the hypothesis of higher effectiveness of biofeedback methods compared to the conventional method in hand rehabilitation of chronic stroke patients. The practical application of these studies will enable the development of rehabilitation programs for chronic stroke patients, in whom the adaptive use of a fixed movement pattern may have occurred. The observed advantage of biofeedback-based therapy can be explained by its underlying neurophysiological mechanisms. Firstly, sensory feedback – visual, auditory or proprioceptive information – provided during exercise, improves sensorimotor integration and enables error correction, which promotes the phenomenon of neuroplasticity – the strengthening of synaptic connections through the interaction of sensory and motor cortices. A review of biofeedback studies for neuromotor rehabilitation by Huang et al. showed that biofeedback can increase plasticity by engaging additional sensory stimuli (51). Second, biofeedback requires intense attentional engagement, which modulates cortical excitability and facilitates learning-dependent changes in the motor cortex. A review by Proulx et al. on somatosensory, visual, and auditory feedback and their interactions applied to upper limb neurorehabilitation technology showed that the response of the somatosensory cortex is crucial for improving motor skills after stroke (52). Third, task repetition and specificity are key indicators of neuroplasticity for motor learning. Research indicates that repetition of intentional movements leads to long-term potentiation, reorganization of cortical maps and unblocking of synaptic latencies, even in chronic stages after stroke (53–55).
Importantly, our study found significant functional improvements in people in chronic stages after stroke, a population often thought to have reached a plateau in functional recovery. However, abundant scientific evidence from biofeedback shows that plasticity still exists when a rich sensory environment and task-specific training are provided (56–60). The mechanism of action includes cortical remapping, thanks to activity dependent on the type of training and repetition, additionally sensory stimulation and exercise increase connectivity in the somatomotor network, (53). Biofeedback therapy, by combining sensory stimuli, cognitive engagement, and intense motor repetition, likely reactivates dormant neural pathways and promotes reorganization in cortical areas. This provides a physiological rationale for the observed improvement and supports the inclusion of biofeedback even in rehabilitation protocols for the late stage after stroke.
4.1 Limitations
One of the limitations is the age of the patients, as it was conducted in people over 50 years of age. Scientific reports show that stroke occurs more often in people over that age, but since the incidence of stroke is becoming more frequent among younger people, they should also be included in further studies. Another limitation is the time since stroke. The studies were conducted among chronic stroke patients, a group of patients that is not often analyzed in terms of improving their fixed hand patterns. Further studies should also include people in the early period and also analyze their hand function during this time. Although both groups received the same total duration of therapy (2 h per day over 3 weeks), the integration of biofeedback within the intervention sessions may have introduced differences in cognitive engagement or patient motivation. This potential influence, while not related to therapy time per se, could be considered a confounding factor and should be taken into account when interpreting the superiority of biofeedback-based rehabilitation. Further studies are warranted to isolate the specific contribution of biofeedback mechanisms.
5 Conclusion
The study showed positive effects of hand function rehabilitation in chronic stroke patients in both groups. However, in the test group, which additionally underwent training that stimulated the central nervous system using the biofeedback method with the Biometrics device, better hand and finger grip function as well as hand motor and manual function were noted. Therefore, it can be concluded that exercises using the Biometrics device have clinical application in the re-education of hand function.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Bioethics Committee of the University of Rzeszów. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JL: Conceptualization, Writing – original draft, Formal analysis, Investigation, Writing – review & editing, Data curation, Methodology. BP: Methodology, Data curation, Investigation, Supervision, Writing – review & editing. GG: Data curation, Software, Formal analysis, Supervision, Writing – review & editing, Project administration. AG: Project administration, Formal analysis, Writing – review & editing, Supervision, Writing – original draft, Investigation, Methodology, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Chen Z Xia N He C Gu M Xu J Han X et al . Action observation treatment-based exoskeleton (AOT-EXO) for upper extremity after stroke: study protocol for a randomized controlled trial. Trials. (2021) 22:222. doi: 10.1186/s13063-021-05176-x
2.
Chen ZJ He C Xu J Zheng CJ Wu J Xia N et al . Exoskeleton-assisted anthropomorphic movement training for the upper limb after stroke: the EAMT randomized trial. Stroke. (2023) 54:1464–73. doi: 10.1161/STROKEAHA.122.041480
3.
Wang H Wu X Li Y Yu S . Efficacy of robot-assisted training on rehabilitation of upper limb function in patients with stroke: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2024) 9:1498–513. doi: 10.1016/j.arrct.2024.100387
4.
Tseng KC Wang L Hsieh C Wong AM . Portable robots for upper-limb rehabilitation after stroke: a systematic review and meta-analysis. Ann Med. (2024) 56:2337735. doi: 10.1080/07853890.2024.2337735
5.
Hernandez ED Forero SM Galeano CP Forero SM Nordin Å Sunnerhagen KS et al . Intra- and inter-rater reliability of Fugl-Meyer assessment of upper extremity in stroke. J Rehabil Med. (2019) 51:652–9. doi: 10.2340/16501977-2590
6.
Hermann DM Chopp M . Promoting brain remodelling and plasticity for stroke recovery: therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. (2012) 11:369–80. doi: 10.1016/S1474-4422(12)70039-X
7.
Koroleva ES Kazakov SD Tolmachev IV Loonen AJM Ivanova SA Alifirova VM . Clinical evaluation of different treatment strategies for motor recovery in poststroke rehabilitation during the first 90 days. J Clin Med. (2021) 10:3718. doi: 10.3390/jcm10163718
8.
Mencel J . Biofeedback - skrypt dla studentów fizjoterapii. Poland: Wydawnictwo AWF Wrocław (2024).
9.
Wieczorek M Makuch M . Evaluation of the effectiveness of upper limb exercise performed in the virtual reality using biofeedback in patients after ischemic stroke - preliminary reports. Aktual Probl Biomech. (2019) 17:123–9.
10.
Dimyan M Cohen LG . The effects of post stroke rehabilitation-constraint induced movement therapy in relation to neuroplasticity recovery processes. Nat Rev Neurol. (2011) 7:76–85. doi: 10.1038/nrneurol.2010.200
11.
Ortmann S Kesselring J Kool J . Reliability and validity of measuring grip strength with a robotic-assisted device in patients after stroke. Int Ther J Rehabil. (2018) 4:1–10. doi: 10.12968/ijtr.2019.0018
12.
El-Kafy EMA Alshehri MA El-Fiky AA Guermazi MA . The effect of virtual reality-based therapy on improving upper limb functions in individuals with stroke: a randomized control trial. Front Aging Neurosci. (2021) 13:731343. doi: 10.3389/fnagi.2021.731343
13.
Widmer M Held JP Wittmann F Lambercy O Lutz K Luft AR . Does motivation matter in upper-limb rehabilitation after stroke? ArmeoSenso-Reward: study protocol for a randomized controlled trial. Trials. (2017) 18:580. doi: 10.1186/s13063-017-2328-2
14.
Olczak A Truszczyńska-Baszak A . Influence of the passive stabilization of the trunk and upper limb on selected parameters of the hand motor coordination, grip strength and muscle tension, in post-stroke patients. J Clin Med. (2021) 11:2402. doi: 10.3390/jcm10112402
15.
Leszczak J Pniak B Drużbicki M Poświata A Mikulski M Roksela A et al . Assessment of inter-rater and intra-rater reliability of the Luna EMG robot as a tool for assessing upper limb proprioception in patients with stroke-a prospective observational study. PeerJ. (2024) 12:e17903. doi: 10.7717/peerj.17903
16.
Lee HC Kuo FL Lin YN Liou TH Lin JC Huang SW . Effects of robot-assisted rehabilitation on hand function of people with stroke: a randomized, crossover-controlled, assessor-blinded study. Am J Occup Ther. (2021) 1:7501205020. doi: 10.5014/ajot.2021.038232
17.
Bayındır O Akyüz G Sekban N . The effect of adding robot-assisted hand rehabilitation to conventional rehabilitation program following stroke: a randomized-controlled study. Turk J Phys Med Rehabil. (2022) 68:254–61. doi: 10.5606/tftrd.2022.8705
18.
Torrisi M Maggio MG De Cola MC Zichittella C Carmela C Porcari B et al . Beyond motor recovery after stroke: the role of hand robotic rehabilitation plus virtual reality in improving cognitive function. J Clin Neurosci. (2021) 92:11–6. doi: 10.1016/j.jocn.2021.07.053
19.
Kim CY Lee JS Lee JH Kim YG Shin AR Shim YH et al . Effect of spatial target reaching training based on visual biofeedback on the upper extremity function of hemiplegic stroke patients. J Phys Ther Sci. (2015) 27:1091–6. doi: 10.1589/jpts.27.1091
20.
Rong W Yu TK Ling HX Sz KH . Effects of electromyography-driven robot-aided hand training with neuromuscular electrical stimulation on hand control performance after chronic stroke. Disabil Rehabil Assist Technol. (2015) 10:149–59. doi: 10.3109/17483107.2013.873491
21.
Allen D Barnett F . Reliability and validity of an electronic dynamometer for measuring grip strength. Int J Ther Rehabil. (2011) 18:258–64. doi: 10.12968/ijtr.2011.18.5.258
22.
McDonald S Aguilar L Levine D . Comparison of hand range of motion during the use of normal and adaptive (built–up) silverware. Am J Occup Ther. (2016) 4:7011500081. doi: 10.5014/ajot.2016.70S1-PO7101
23.
Aimola E Valle MS Casabona A . Effects of predictability of load magnitude on the response of the flexor digitorum superficialis to a sudden fingers extension. PLoS One. (2014) 10:09067. doi: 10.1371/journal.pone.0109067
24.
Yeager L. (2019). Effectiveness of adaptive utensils on hand and finger range of motion in individuals with rheumatoid arthritis, University of Tennessee at ChattanoogaChattanooga, TN. 2–19.
25.
Sanpablo A. I. P. Disselhorst-Klug C. Penaloza A. M. Elisa R. A. Juan M. I. Z. Muscular activation during low resistance elbow's motion of children with and without cerebral palsy. 2019 16th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE) (2019) Mexico: IEEE, 1–6.
26.
Jarque-Bou N Vergara M Sancho-Bru JL . Hand kinematics characterization while performing activities of daily living through kinematics reduction. IEEE Trans Neural Syst Rehabil Eng. (2020) 7:1556–65. doi: 10.1109/TNSRE.2020.2998642
27.
Casabona A Valle MS Dominante C Laudani L Onesta MP Cioni M . Effects of functional electrical stimulation cycling of different duration on viscoelastic and electromyographic properties of the knee in patients with spinal cord injury. Brain Sci. (2020) 23:7. doi: 10.3390/brainsci11010007
28.
Page SJ Fulk GD Boyne P . Clinically important differences for the upper-extremity Fugl-Meyer scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther. (2012) 92:791–8. doi: 10.2522/ptj.20110009
29.
Leszczak J Pniak B Drużbicki M Guzik A . The reliability of a biometrics device as a tool for assessing hand grip and pinch strength, in a polish cohort-a prospective observational study. PLoS One. (2024) 19:e0303648. doi: 10.1371/journal.pone.0303648
30.
Biometrics ELINK (2025) Medical evaluation. Available at: https://www.biometricsltd.com/medical-grippinch.htm (Accessed November 21, 2024).
31.
Bohannon RW Schaubert KL . Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. (2005) 18:426–8. doi: 10.1197/j.jht.2005.07.003
32.
Chen P Liu TW Tse MMY Lai CKY Tsoh J Ng SSM . The predictive role of hand section of Fugl-Meyer assessment and motor activity log in action research arm test in people with stroke. Front Neurol. (2022) 13:926130. doi: 10.3389/fneur.2022.926130
33.
Duncan PW Propst M Nelson SG . Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. (1983) 63:1606–10. doi: 10.1093/ptj/63.10.1606
34.
Lundquist CB Maribo T . The Fugl-Meyer assessment of the upper extremity: reliability, responsiveness and validity of the Danish version. Disabil Rehabil. (2017) 39:934–9. doi: 10.3109/09638288.2016.1163422
35.
Heesoo K Jingang H Jooyeon K Dong-sik P Ji-Hea W Youngyoul Y . Reliability, concurrent validity, and responsiveness of the Fugl-Meyer assessment (FMA) for hemiplegic patients. J Phys Ther Sci. (2012) 9:893–9. doi: 10.1589/jpts.24.893
36.
Kontson K Marcus I Myklebust B Civillico E . Targeted box and blocks test: normative data and comparison to standard tests. PLoS One. (2017) 12:e0177965. doi: 10.1371/journal.pone.0177965
37.
Ceravolo MG . Action observation as a tool for upper limb recover. Fiz Rehabil Med. (2016) 28:144–50.
38.
Platz T Pinkowski C van Wijck F Kim IH di Bella P Johnson G . Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer test, action research arm test and box and block test: a multicentre study. Clin Rehabil. (2005) 19:404–11. doi: 10.1191/0269215505cr832oa
39.
Krzemińska S Bekus A Borodzicz A Arendarczyk M . Analysis and evaluation of subjective quality of life in a group of patients after ischemic stroke. J Neurosci Nurs. (2016) 5:4. doi: 10.15225/pnn.2016.5.2.4
40.
Baude M Mardale V Loche CM Hutin E Gracies JM Bayle N . Intra- and inter-rater reliability of the modified Frenchay scale to measure active upper limb function in hemiparetic patients. Ann Psych Rehabil. (2016) 59:59–60. doi: 10.1016/j.rehab.2016.07.138
41.
Shah S Cooper B Maas F . The Barthel index and ADL evaluation in stroke rehabilitation in Australia, Japan, the UK and the USA. Aust Occup Ther J. (1992) 39:5–13. doi: 10.1111/j.1440-1630.1992.tb01729.x
42.
Liu F Tsang RC Zhou J Zhou M Zha F Long J et al . Relationship of Barthel index and its short form with the modified Rankin scale in acute stroke patients. J Stroke Cerebrovasc Dis. (2020) 29:105033. doi: 10.1016/j.jstrokecerebrovasdis.2020.105033
43.
Musa KI Keegan TJ . The change of Barthel index scores from the time of discharge until 3-month post-discharge among acute stroke patients in Malaysia: a random intercept model. PLoS One. (2018) 13:e0208594. doi: 10.1371/journal.pone.0208594
44.
Dziemian K Kiper A Baba A . The effect of robot therapy assisted by surface EMG on hand recovery in post-stroke patients. A pilot study. Med Rehabil. (2017) 4:4–10. doi: 10.5604/01.3001.0011.7401
45.
Hsu C Wu C Huang C Shie H Tsai Y . Feasibility and potential effects of robot-assisted passive range of motion training in combination with conventional rehabilitation on hand function in patients with chronic stroke. J Rehabil Med. (2022) 54:jrm00323. doi: 10.2340/jrm.v54.1407
46.
Straudi S Fregni F Martinuzzi C Pavarelli C Salvioli S Basaglia N . tDCS and robotics on upper limb stroke rehabilitation: effect modification by stroke duration and type of stroke. Biomed Res Int. (2016) 2016:1–8. doi: 10.1155/2016/5068127
47.
Kakuda W . Future directions of stroke rehabilitation. Rinsho Shinkeigaku. (2020) 3:181–6. doi: 10.5692/clinicalneurol.cn-001399
48.
Grant VM Gibson A Shields N . Somatosensory stimulation to improve hand and upper limb function after stroke-a systematic review with meta-analyses. Top Stroke Rehabil. (2018) 25:150–60. doi: 10.1080/10749357.2017.1389054
49.
Franceschini M Goffredo M Pournajaf S Paravati S Agosti M de Pisi F et al . Predictors of activities of daily living outcomes after upper limb robot-assisted therapy in subacute stroke patients. PLoS One. (2018) 13:e0193235. doi: 10.1371/journal.pone.0193235
50.
Kwakkel G Kollen BJ Krebs HI . Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. (2008) 22:111–21. doi: 10.1177/1545968307305457
51.
Huang H Wolf SL He J . Recent developments in biofeedback for neuromotor rehabilitation. J Neuroeng Rehabil. (2006) 3:11. doi: 10.1186/1743-0003-3-11
52.
Proulx CE Louis Jean MT et al . Somesthetic, visual, and auditory feedback and their interactions applied to upper limb neurorehabilitation technology: a narrative review to facilitate contextualization of knowledge. Front Rehabil Sci. (2022) 3:789479. doi: 10.3389/fresc.2022.78947
53.
Schranz C Vatinno A Ramakrishnan V Seo NJ . Neuroplasticity after upper-extremity rehabilitation therapy with sensory stimulation in chronic stroke survivors. Brain Commun. (2022) 4:fcac191. doi: 10.1093/braincomms/fcac191
54.
Conforto AB dos Anjos SM Bernardo WM Silva AA Conti J Machado AG . Repetitive peripheral sensory stimulation and upper limb performance in stroke: a systematic review and meta-analysis. Neurorehabil Neural Repair. (2018) 32:863–71. doi: 10.1177/1545968318798943
55.
Marconi B Filippi GM Koch G et al . Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair. (2011) 25:48–60. doi: 10.1177/154596831037675
56.
Spencer J Wolf SL Kesar TM . Biofeedback for post-stroke gait retraining: a review of current evidence and future research directions in the context of emerging technologies. Front Neurol. (2021) 12:637199. doi: 10.3389/fneur.2021.637199
57.
Kober SE Schweiger D Reichert JL Neuper C Wood G . Upper alpha based Neurofeedback training in chronic stroke: brain plasticity processes and cognitive effects. Appl Psychophysiol Biofeedback. (2017) 42:69–83. doi: 10.1007/s10484-017-9353-5
58.
Wang T Mantini D Gillebert CR . The potential of real-time fMRI neurofeedback for stroke rehabilitation: a systematic review. Cortex. (2018) 107:148–65. doi: 10.1016/j.cortex.2017.09.006
59.
Renton T Tibbles A Topolovec-Vranic J . Neurofeedback as a form of cognitive rehabilitation therapy following stroke: a systematic review. PLoS One. (2017) 12:e0177290. doi: 10.1371/journal.pone.0177290
60.
Drużbicki M Kwolek A Depa A Przysada G . The use of a treadmill with biofeedback function in assessment of relearning walking skills in post-stroke hemiplegic patients--a preliminary report. Neurol Neurochir Pol. (2010) 44:567–73. doi: 10.1016/s0028-3843(14)60154-7
Summary
Keywords
rehabilitation, upper limb, stroke, biometrics, hand function
Citation
Leszczak J, Pniak B, Gazda G and Guzik A (2025) Assessment of the effects of rehabilitation of hand function using a biometrics device in people after stroke – a randomized controlled trial. Front. Neurol. 16:1643336. doi: 10.3389/fneur.2025.1643336
Received
08 June 2025
Accepted
22 August 2025
Published
16 September 2025
Volume
16 - 2025
Edited by
Giorgio Scivoletto, Santa Lucia Foundation (IRCCS), Italy
Reviewed by
Seyoung Shin, Sungkyunkwan University, Republic of Korea
Aparna Bachkaniwala, Veer Narmad South Gujarat University, India
Updates
Copyright
© 2025 Leszczak, Pniak, Gazda and Guzik.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justyna Leszczak, jleszczak@ur.edu.pl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.