- Department of Rehabilitation, Shengjing Hospital of China Medical University, Shenyang, China
The pathogenesis of Parkinson’s disease (PD) is gradually evolving from a central neurodegeneration-centered concept to a multi-pathway pathological model at the gut-brain system level. Studies have shown that PD patients commonly exhibit dysbiosis, reduced short-chain fatty acids (SCFAs; microbial fermentation products of dietary fiber that play key roles in host metabolism and immune regulation), abnormal tryptophan metabolism, and impaired gut barrier function. These alterations may contribute to dopaminergic neuronal damage through mechanisms including neuroinflammation, oxidative stress, and α-synuclein (α-syn) aggregation. The vagus nerve plays a critical role in bidirectional gut-brain signaling, and its dysfunction may represent a key route for pathological protein transmission from the periphery to the brain. In response, remote rehabilitation and gut-targeted interventions—including probiotics, prebiotics, dietary modulation, fecal microbiota transplantation (FMT), and transcutaneous vagus nerve stimulation (tVNS)—have shown potential in improving neurological function and inflammation in both animal and clinical studies. Multimodal data analyses have revealed significant associations between SCFA levels in fecal metabolomics and brain imaging features. Despite ongoing challenges in mechanistic extrapolation, biomarker sensitivity, and translational implementation, the integration of metagenomics, metabolomics, neuroimaging, and digital therapeutics—collectively referred to as multi-omics and digital profiling techniques—represents an emerging research direction with the potential to inform future clinical paradigms for precision remote management of PD.
1 Introduction
Parkinson’s disease (PD) is a neurological disorder characterized by the degenerative loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) and abnormal aggregation of α-synuclein (α-syn) (1–3). Pathological deposition of α-syn disrupts mitochondrial function and induces endoplasmic reticulum stress responses, thereby driving neuronal apoptosis (4, 5). This process is closely linked to the core motor symptoms of PD, essentially resulting from the imbalance of the basal ganglia-thalamocortical loop caused by the loss of nigrostriatal dopaminergic projections (6). Notably, non-motor symptoms often precede motor dysfunction, suggesting that PD pathology may originate in the peripheral nervous system (7, 8). Postmortem studies have shown that α-syn pathology initially localizes in the enteric nervous system (ENS) and subsequently propagates to the midbrain via the vagus nerve (9). This phenomenon is supported by epidemiological findings indicating that vagotomy reduces the risk of developing PD by 40% (10).
In recent years, regulatory mechanisms of the gut-brain axis—the bidirectional communication network between the gastrointestinal system and the central nervous system—have offered a novel perspective for PD research (11–13). Clinical cohort analyses reveal that up to 80% of PD patients experience gastrointestinal dysfunction 10–20 years before the onset of motor symptoms (14), and their fecal microbiota exhibit a marked decrease in Prevotellaceae and overgrowth of Enterobacteriaceae (15). This dysbiosis may result in dual pathological effects: on one hand, decreased synthesis of SCFAs compromises their regulatory capacity on microglial M2 polarization, exacerbating neuroinflammation mediated by the TLR4/NF-κB pathway and increasing proinflammatory cytokines such as IL-1β and TNF-α (16); on the other hand, enhanced intestinal permeability facilitates the translocation of lipopolysaccharide (LPS) into circulation, which activates α-syn fibrillization and impairs the blood–brain barrier, accelerating the central propagation of pathological proteins (17). Animal experiments further confirm that intragastrointestinal injection of α-syn fibrils can be transported retrogradely to the substantia nigra via the vagus nerve, inducing selective loss of dopaminergic neurons (18). These lines of evidence collectively suggest that disruption of the gut-brain axis is not only an early event in PD pathogenesis but may also serve as a critical target for therapeutic intervention.
2 Molecular and neural mechanisms of gut-brain axis modulation
2.1 Signal transmission of gut microbiota metabolites
Metabolites of the gut microbiota are involved in the central pathological processes of PD through multiple pathways, particularly playing important roles in regulating neuroinflammation, synaptic function, and oxidative stress (19). Short-chain fatty acids (SCFAs), the major products of microbial fermentation of dietary fiber, play a key role in modulating central nervous inflammation (20, 21). Butyrate promotes microglial polarization toward an anti-inflammatory phenotype (M2 type) by inhibiting histone deacetylases (HDACs), thereby downregulating the activity of the Toll-like receptor 4 (TLR4)/nuclear factor-κB (NF-κB) signaling pathway (22). Clinical studies have shown that butyrate concentration in the feces of PD patients is significantly negatively correlated with IL-6 levels in cerebrospinal fluid (23). Propionate activates free fatty acid receptor 2 (FFAR2) on enterochromaffin cells, promoting the synthesis of serotonin (5-HT), thereby regulating the synaptic plasticity of nigrostriatal dopaminergic neurons (24). Notably, the effect of propionate on dopamine metabolism is dose-dependent: low concentrations of propionate (<1 mM) enhance the activity of tyrosine hydroxylase (TH), while high concentrations (>5 mM) induce neuronal apoptosis through the accumulation of mitochondrial reactive oxygen species (mtROS) (25).
The bidirectional regulation of tryptophan metabolism further highlights the complexity of microbiota-host interaction (26). Indole derivatives derived from gut microbiota can activate aryl hydrocarbon receptors (AhR), upregulate the expression of aquaporin 4 (AQP4) in astrocytes, and promote the clearance of β-amyloid protein (Aβ) (27). In contrast, the host’s metabolic shift toward the kynurenine pathway of tryptophan leads to the accumulation of quinolinic acid, which triggers oxidative stress through activation of NADPH oxidase (NOX2) and enhances the resistance of α-syn to proteasomal degradation (28). Mass spectrometry analysis has confirmed that the plasma quinolinic acid/tryptophan ratio in PD patients is positively correlated with iron deposition in the substantia nigra (evaluated by quantitative susceptibility mapping, QSM) (29). SCFAs and tryptophan metabolites jointly participate in the immune modulation and metabolic regulation of the central nervous system in PD, and the mechanistic basis will be further elaborated in the next section, “Vagal Connectivity Anatomy.”
2.2 Functional anatomy of gut-Nigral network connectivity
The vagus nerve, as the core communication pathway of the bidirectional gut-brain axis, projects from the dorsal motor nucleus (DMV) to the myenteric plexus of the gut, while its afferent fibers transmit intestinal mechanical/chemical signals to the nucleus tractus solitarius (NTS) via the nodose ganglion (30). Viral tracing experiments have confirmed that injection of pseudorabies virus (PRV) into the gut can retrogradely label the multisynaptic pathway from the NTS to the substantia nigra pars compacta (SNc) (31). Optogenetic studies have shown that specific activation of gut vagal C fibers can enhance theta rhythmic oscillations (4–8 Hz) of dopaminergic neurons in the SNc, thereby improving motor coordination (32). This mechanism may explain the clinical efficacy of transcutaneous vagus nerve stimulation (tVNS) in improving motor symptoms in PD patients (33).
2.3 Immuno-neural interaction of systemic inflammation
Destruction of intestinal barrier integrity leads to the translocation of pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS) into the bloodstream, aggravating central neurodegeneration via dual mechanisms: on the one hand, peripheral monocytes migrate to brain parenchyma via C-C chemokine receptor 2 (CCR2), differentiate into proinflammatory macrophages, and release IL-1β to activate microglial NLRP3 inflammasomes (34); on the other hand, LPS enhances the nucleation efficiency of α-syn fibrils via TLR4-dependent mechanisms, and the strength of this effect is positively correlated with the abundance of Gram-negative bacteria in the gut microbiota (35). Animal experiments have shown that knockout of TLR4 can significantly reduce dopaminergic neuron loss in the substantia nigra induced by α-syn preformed fibrils (PFFs) (36). Clinical neuroimaging evidence further supports this mechanism: serum LPS-binding protein (LBP) levels in PD patients are significantly correlated with the extent of microglial activation in the substantia nigra (quantified by ^11C-PK11195 PET) (37) (Figure 1).
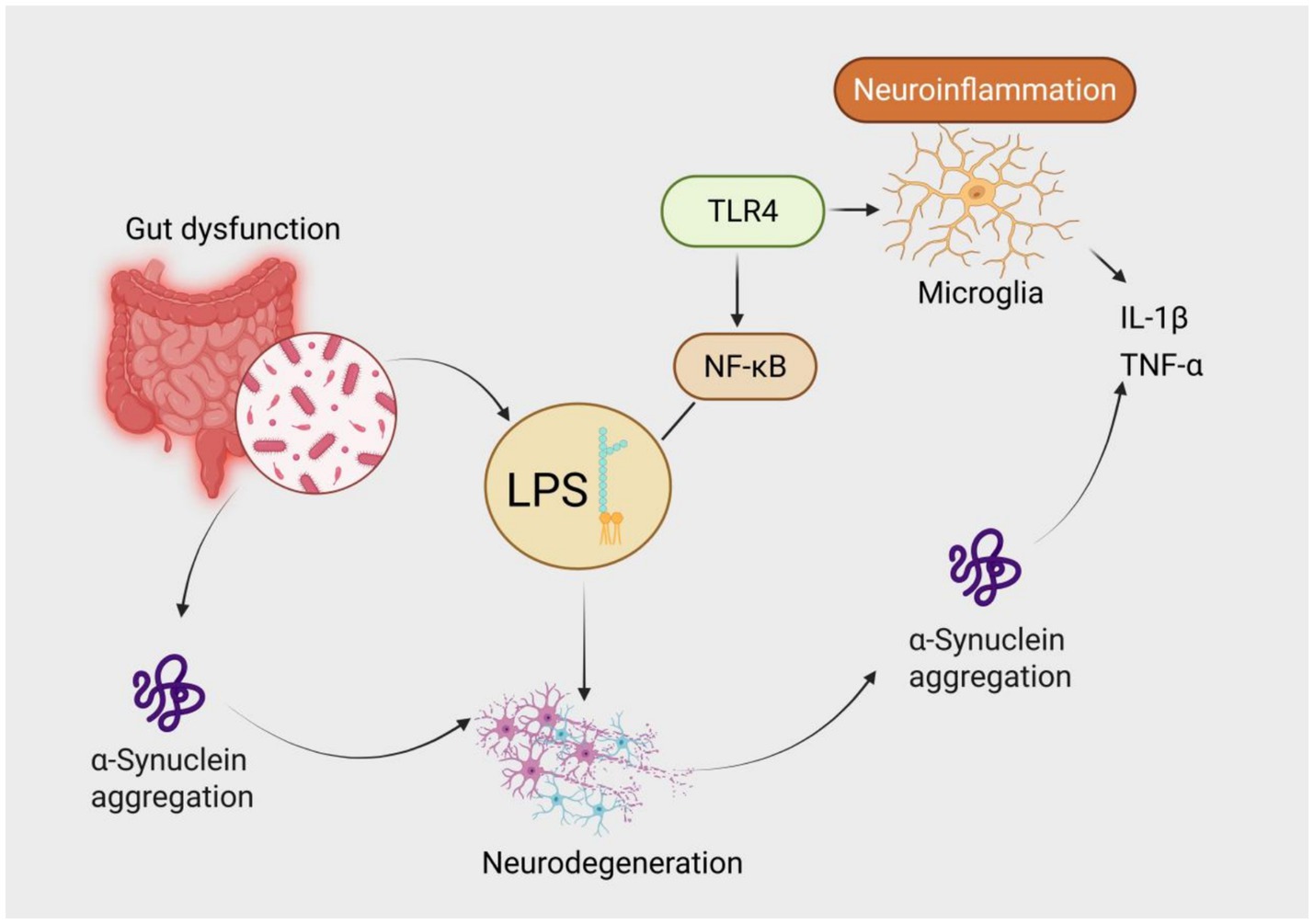
Figure 1. Mechanism of lipopolysaccharide (LPS)-mediated neuroinflammation in the gut-brain axis contributing to Parkinson’s disease pathogenesis. Gut dysfunction leads to dysbiosis and overgrowth of Gram-negative bacteria, resulting in elevated LPS translocation into systemic circulation. LPS activates Toll-like receptor 4 (TLR4) and triggers the NF-κB signaling cascade, leading to microglial activation and release of proinflammatory cytokines such as IL-1β and TNF-α. Both LPS and inflammation promote α-synuclein aggregation, ultimately driving neurodegeneration—a core pathological hallmark of Parkinson’s disease.
2.4 Preclinical evidence linking the gut microbiome to PD
Multiple animal studies have demonstrated a causal relationship between gut microbiota alterations and Parkinsonian pathology. Sampson et al. showed that fecal microbiota transplants (FMT) from PD patients into germ-free mice exacerbated motor deficits and microglial activation compared to transplants from healthy controls, directly implicating gut dysbiosis in disease progression (19). In MPTP-induced PD mouse models, antibiotic treatment or SCFA supplementation modulated both motor behavior and neuroinflammation, indicating that gut-derived metabolites influence the central disease process (38). DSS-induced colitis, by disrupting the intestinal barrier and promoting systemic inflammation, has been shown to aggravate nigrostriatal neurodegeneration in PD-prone mice (39). Additionally, irradiation-based microbiota depletion followed by recolonization further confirmed that gut microbial composition can alter the severity of α-synuclein pathology and dopaminergic neuronal loss (40). These findings provide robust mechanistic support for a gut-origin component in PD pathogenesis.
3 Intervention strategies of remote rehabilitation on the gut-brain Axis
As the gut–brain axis becomes increasingly recognized as a therapeutic target in PD, a variety of strategies have emerged aiming to restore microbial homeostasis, reduce inflammation, and enhance barrier integrity. Table 1 provides a concise overview of the main pathological features associated with gut dysbiosis in PD and the interventions currently under investigation.
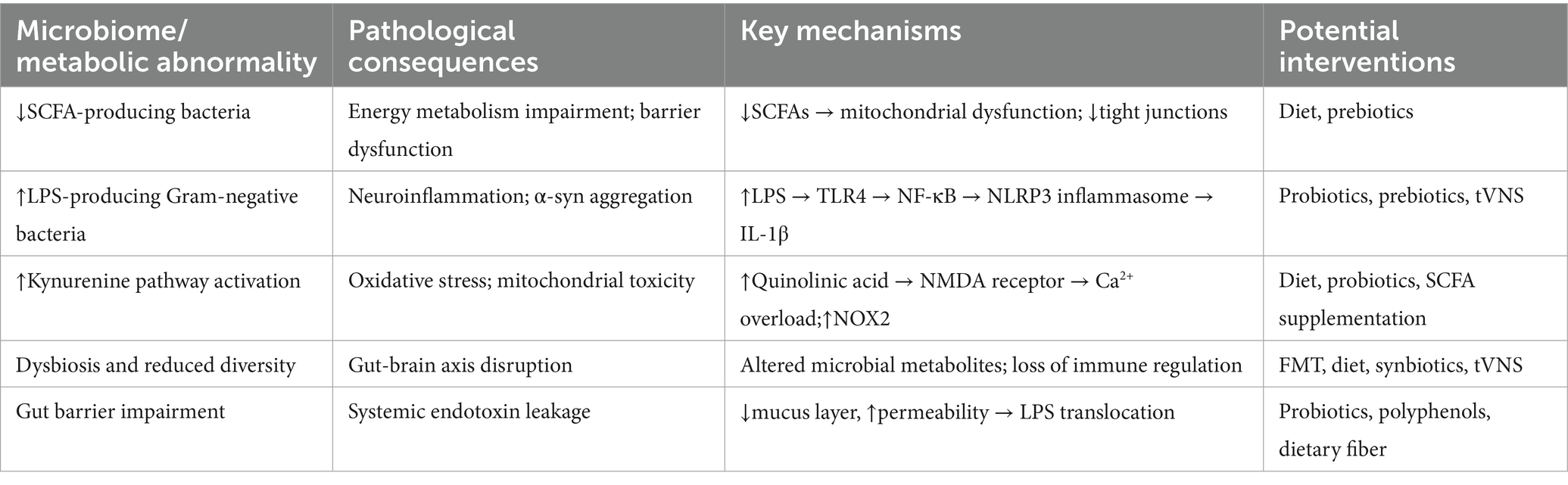
Table 1. Key gut microbiota-associated pathological changes in Parkinson’s disease and corresponding intervention strategies.
3.1 Neuromodulation interventions
To address vagus nerve signaling impairment and central circuit dysfunction, current studies have proposed non-pharmacological remote intervention strategies centered on neuromodulation. Digital therapeutics—software-driven tools that deliver therapeutic effects—provide a precise intervention path for gut-brain axis regulation (41). Biofeedback systems based on wearable devices dynamically assess autonomic nervous tone by continuously monitoring heart rate variability (HRV), allowing for real-time adjustment of the intensity and frequency of transcutaneous vagus nerve stimulation (tVNS) (42). Virtual reality (VR) technology reshapes neural plasticity through multi-sensory integration training. Functional near-infrared spectroscopy (fNIRS) shows that VR tasks induce gamma-band oscillations (30–80 Hz), which are preliminarily associated with improved synchronization of the substantia nigra–thalamus–cortex circuit and may facilitate motor initiation in PD (43). However, these findings remain primarily observational and require further validation in large-scale clinical trials. In animal models of neurodegenerative disease, multi-sensory stimulation interventions such as environmental enrichment, which refers to housing animals in a complex environment with toys, running wheels, and varied stimuli to promote sensory and motor engagement have demonstrated potential regulatory effects—e.g., increased striatal dopamine transporter (DAT) density in α-synuclein transgenic mice after 12 weeks of training. Whether such effects translate to human PD patients remains to be established (44). Based on these preclinical findings, VR technology is hypothesized to exert DAT-modulating effects in PD by mimicking visual–motor integration seen in enriched environments. However, its neuroprotective potential in humans remains speculative and warrants systematic clinical investigation.
3.2 Gut-targeted dietary interventions
Dietary interventions aim to modulate gut microbiota composition and metabolic capacity, potentially improving SCFA deficiency and LPS translocation. Personalized dietary programs are dynamically adjusted via mobile applications: low-FODMAP diets reduce the proliferation of gas-producing bacteria in the gut, thereby lowering serum lipopolysaccharide-binding protein (LBP) levels (45); meanwhile, the Mediterranean diet increases the abundance of Akkermansia muciniphila, promotes butyrate synthesis, and improves fecal microbiota alpha diversity (46).
3.3 Probiotic/prebiotic interventions
Combined probiotic and prebiotic interventions have shown potential for regulating gut-brain axis function through multiple mechanisms. Studies have shown that Lactobacillus reuteri DSM 17938 can improve gut microecology and exert anti-inflammatory effects. Intervention with Lactobacillus reuteri DSM 17938 reduces the abundance of potentially pro-inflammatory bacteria, such as hydrogen sulfide-producing species, thereby indirectly improving host mitochondrial function and metabolic status (47). Although the specific mechanisms remain under investigation, improvements in mitochondrial energy metabolism indicators (e.g., ATP levels) have been observed in animal experiments. A small-scale randomized controlled trial suggested that combined use of probiotics and prebiotics may increase brain-derived neurotrophic factor (BDNF) levels in cerebrospinal fluid (48). These findings are preliminary and require replication in larger cohorts. A meta-analysis reported associative improvements in gastrointestinal symptoms following combined probiotic–prebiotic supplementation in PD patients, with reductions in symptom scores and inflammatory markers such as serum IL-6 (49). However, due to heterogeneity in included studies and modest effect sizes, causal conclusions remain tentative. These findings suggest that combined probiotic-prebiotic interventions may simultaneously target gut microbiota metabolism, intestinal barrier protection, and systemic inflammation alleviation, holding broad prospects for application.
4 Multimodal biomarkers and efficacy evaluation
4.1 Integrated analysis of Fecal metabolomics and neuroimaging
Fecal metabolomics analyses have revealed systematic disturbances in gut-derived metabolites in PD patients, particularly the reduction of short-chain fatty acids (SCFAs) such as butyrate, which is closely related to disease pathogenesis (50–52). Yang, X et al. (53) found that the concentrations of butyrate, propionate, and acetate were significantly reduced in the feces of PD patients, which may impair gut barrier function and influence neuroinflammatory states. Resting-state functional magnetic resonance imaging (rs-fMRI) studies have shown that the functional connectivity of the default mode network (DMN) is closely associated with cognitive performance in PD patients (54). Decreased DMN connectivity in PD patients correlates with cognitive impairment (55). Although direct studies linking fecal metabolomic data to neuroimaging indicators (e.g., nigral iron deposition) are currently lacking, the above findings suggest that changes in gut metabolic profiles may influence brain network function and participate in PD pathogenesis.
4.2 Integrated application of clinical assessment tools
With the continuous development of multimodal intervention strategies, a corresponding system of integrated evaluation indicators is gradually being established to enable closed-loop analysis among mechanisms, symptoms, and interventions. The Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) is the standard tool for assessing both motor and non-motor symptoms in PD patients (56–58). Biomarkers of intestinal permeability in PD, such as elevated serum zonulin levels, are positively correlated with MDS-UPDRS scores, suggesting that gut barrier dysfunction may be associated with the severity of motor symptoms (50). In terms of motor function assessment, wearable inertial sensors have been used to capture gait features in PD patients (59–62). One study employed machine learning algorithms such as XGBoost to analyze gait data and successfully distinguished PD patients from healthy controls with high accuracy (63). Although this study did not directly associate gait analysis with gut microbiota diversity, it indicates that the integration of multimodal data may contribute to individualized evaluation of PD. However, current assessment methods still face many challenges in longitudinal tracking, causal inference, and cross-center standardization, requiring further refinement in future research.
5 Challenges and future directions
5.1 Limitations of mechanistic studies
Although a large number of studies have elucidated the pathogenesis of PD from classical pathways such as α-synuclein aggregation, most of these findings are derived from animal models, which differ substantially from the actual human disease course (3, 64). In animal models, bilateral loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) can be observed 6 months after striatal injection of α-synuclein preformed fibrils (PFFs), with α-synuclein inclusions forming within 2 months; the extent of neuronal loss is dose-dependent on the injected PFFs (65). However, in clinical practice, PD is typically diagnosed when neuronal loss has already reached 60 to 80%, suggesting that interventions in the prodromal phase may be more effective (66). This discrepancy underscores a major translational gap: animal models often rely on rapid-onset, artificially induced PD phenotypes that do not fully recapitulate the gradual, multifactorial progression seen in humans. Moreover, many preclinical interventions showing promise in rodents have failed to replicate efficacy in clinical trials, reflecting fundamental differences in pathophysiology and treatment response.
Inter-individual genetic differences also influence the efficacy of interventions. For example, PINK1 and Parkin genes play essential roles in maintaining mitochondrial quality control, and their mutations are closely associated with PD onset (67). These findings underscore the importance of integrating multi-omics data to develop personalized therapeutic strategies.
5.2 Translational bottlenecks in technology
As remote monitoring and data collection gradually enter clinical practice, sample processing and behavioral adherence assessment have become key technical bottlenecks in the implementation of intervention strategies. In remote biosample collection, storage conditions significantly affect metabolite stability (68). Studies have shown that the concentration of butyrate in fecal samples decreases by 37% after 24 h at room temperature but only by 8% when stored at 4 °C (69). Differences in sampling tools may also cause deviations in microbial abundance detection, affecting the accuracy of research results (70). Beyond sampling inconsistencies, another critical bottleneck lies in the validation and regulatory approval of digital biomarkers derived from wearable data. Many machine learning-based models lack external validation and suffer from limited generalizability across populations. Moreover, regulatory frameworks for integrating microbiome-based or digital readouts into clinical decision-making are still in their infancy, delaying real-world implementation.
In adherence assessment for digital therapeutics, traditional questionnaire-based methods are often subjective and error-prone (71, 72). The introduction of blockchain technology (e.g., Hyperledger Fabric framework) enables real-time encrypted recording of data, enhancing data reliability and transparency (73). Baucas et al. (74) proposed a fog computing–IoT platform integrating federated learning and blockchain for wearable device applications in predictive medicine. This platform aims to ensure the security of patient data and the integrity of predictive services through a distributed structure and privacy protection mechanisms. The results showed that the proposed implementation effectively protects patient privacy and maintains the integrity of predictive services (74).
Wearable sensors combined with machine learning algorithms have shown promising potential in the diagnosis and severity assessment of PD though their integration with microbiome-based monitoring remains an emerging field under active development (75–77). One study used wearable sensors to collect gait data, and applied XGBoost algorithms to successfully distinguish PD patients from healthy controls with high accuracy (78).
5.3 Exploration of cutting-edge cross-disciplinary fields
The relationship between the gut microbiota and PD is receiving increasing attention (79–81). Krueger et al. (82) found that the abundance of butyrate-producing bacteria was reduced in the gut of PD patients, leading to lower butyrate levels, which may affect gut barrier function and neuroinflammatory status. The signaling mechanisms between the gut and brain are also being progressively revealed (83, 84). Vitetta et al. reported that neuroepithelial cells in the gut can rapidly transmit signals to the brain via the vagus nerve, influencing neuronal activity (85). Optogenetic technology provides new tools for studying the gut–brain axis (86, 87). By specifically activating light-sensitive channels in intestinal enterochromaffin (EC) cells, serotonin (5-HT) release can be modulated, thereby affecting central nervous system function (88). These emerging technologies offer unprecedented methodological support for elucidating peripheral–central signaling coupling mechanisms and developing non-invasive intervention approaches, which warrant systematic integration and validation in future studies.
5.4 Conclusion
The pathogenesis of Parkinson’s disease is gradually expanding from the traditional concept of central nervous system degeneration to the interplay between the gut and brain axis (as illustrated in Figure 2).
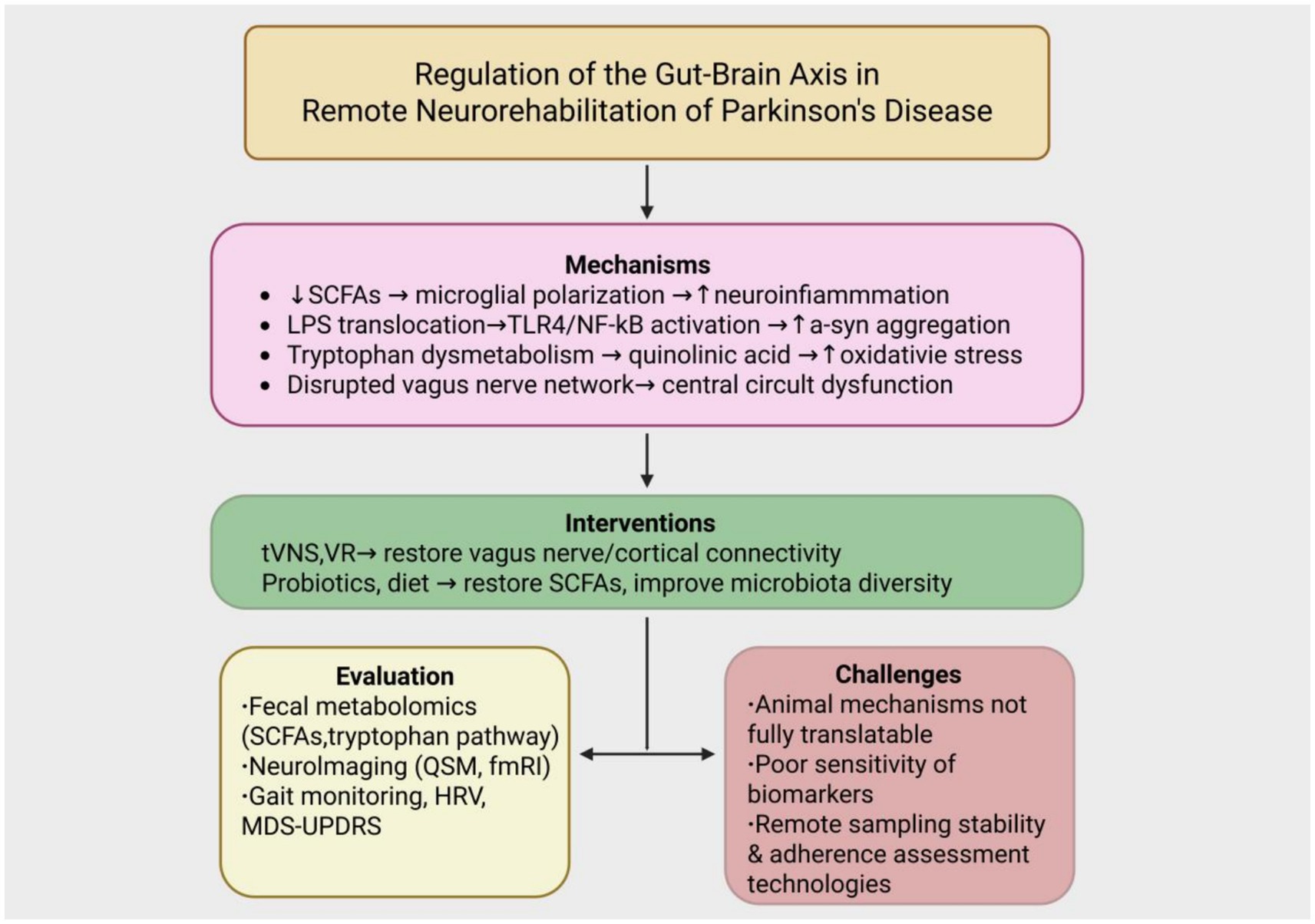
Figure 2. Integrated framework of gut–brain axis regulation in remote neurorehabilitation of Parkinson’s disease. The diagram illustrates the pathological mechanisms of PD involving reduced short-chain fatty acids (SCFAs), LPS translocation, tryptophan dysmetabolism, and vagus nerve dysfunction. These are targeted by non-pharmacological interventions such as transcutaneous vagus nerve stimulation (tVNS), virtual reality (VR), and gut microbiota modulation (probiotics and diet). Multimodal tools for evaluation include fecal metabolomics, neuroimaging, and gait/HRV monitoring. Key translational challenges include limited clinical applicability of animal models, low biomarker sensitivity, and inadequate remote adherence tracking systems.
Current studies suggest that factors such as gut microbiota imbalance, abnormal metabolite profiles, and impaired gut barrier function may play roles as early as the prodromal phase of the disease. These factors contribute to neuronal injury via multiple mechanisms, including neuroinflammation, mitochondrial dysfunction, and α-synuclein aggregation. The vagus nerve, as a key pathway of the gut–brain axis, not only mediates signal transmission under physiological conditions but may also act as a conduit for pathological protein propagation from the periphery to the brain under pathological conditions.
In terms of intervention strategies, remote rehabilitation techniques such as transcutaneous vagus nerve stimulation, virtual reality training, and wearable device monitoring offer new tools for personalized management of Parkinson’s disease. Meanwhile, gut-targeted approaches including probiotics, prebiotics, and dietary interventions have shown potential in preclinical and early clinical studies to modulate gut microbiota, attenuate systemic inflammation, and support gut barrier function; however, their efficacy and mechanisms in PD patients remain to be fully validated. Integrating fecal metabolomics, brain imaging, motor parameters, and immune markers into a multimodal framework may support early detection and treatment monitoring. The envisioned integration of microbiome biomarker analytics with wearable technologies represents a promising but as yet unrealized frontier in precision remote management of PD. While the convergence of omics technologies, wearable devices, and digital therapeutics holds transformative promise, substantial translational hurdles remain. These include the poor replicability of animal-derived findings in heterogeneous human populations, the lack of standardized pipelines for digital biomarker validation, and the limited regulatory infrastructure to support clinical adoption.
Despite current limitations such as unclear mechanisms, lack of sensitive biomarkers, and inadequate remote data collection and adherence assessment tools, ongoing advances in biotechnology, artificial intelligence, and interdisciplinary integration are expected to further advance gut–brain axis research and intervention strategies for Parkinson’s disease. These developments will provide a more solid foundation for early diagnosis and comprehensive treatment.
Author contributions
YJ: Writing – original draft, Visualization, Conceptualization. HW: Writing – original draft, Conceptualization. JS: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yan, S, Jiang, C, Janzen, A, Barber, TR, Seger, A, Sommerauer, M, et al. Neuronally derived extracellular vesicle α-Synuclein as a serum biomarker for individuals at risk of developing Parkinson disease. JAMA Neurol. (2024) 81:59–68. doi: 10.1001/jamaneurol.2023.4398
2. Kam, TI, Mao, X, Park, H, Chou, SC, Karuppagounder, SS, Umanah, GE, et al. Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson's disease. Science. (2018) 11-02) 362:eaat8407. doi: 10.1126/science.aat8407
3. Diaz-Ortiz, ME, Seo, Y, Posavi, M, Carceles Cordon, M, Clark, E, Jain, N, et al. GPNMB confers risk for Parkinson's disease through interaction with α-synuclein. Science. (2022) 377:eabk0637. doi: 10.1126/science.abk0637
4. Hou, X, Chen, TH, Koga, S, Bredenberg, JM, Faroqi, AH, Delenclos, M, et al. Alpha-synuclein-associated changes in PINK1-PRKN-mediated mitophagy are disease context dependent. Brain Pathol. (2023) 33:e13175. doi: 10.1111/bpa.13175
5. Lehtonen, Š, Jaronen, M, Vehviläinen, P, Lakso, M, Rudgalvyte, M, Keksa-Goldsteine, V, et al. Inhibition of excessive oxidative protein folding is protective in MPP(+) toxicity-induced Parkinson's disease models. Antioxid Redox Signal. (2016) 25:485–97. doi: 10.1089/ars.2015.6402
6. Kim, T, Kim, M, Jung, WH, Kwak, YB, Moon, SY, Lho, K, et al. Unbalanced fronto-pallidal neurocircuit underlying set shifting in obsessive-compulsive disorder. Brain. (2022) 145:979–90. doi: 10.1093/brain/awab483
7. Armstrong, MJ, and Okun, MS. Diagnosis and treatment of Parkinson disease: a review. JAMA. (2020) 323:548–60. doi: 10.1001/jama.2019.22360
8. Schapira, AHV, Chaudhuri, KR, and Jenner, P. Non-motor features of Parkinson disease. Nat Rev Neurosci. (2017) 18:435–50. doi: 10.1038/nrn.2017.62
9. Challis, C, Hori, A, Sampson, TR, Yoo, BB, Challis, RC, Hamilton, AM, et al. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci. (2020) 23:327–36. doi: 10.1038/s41593-020-0589-7
10. Liu, B, Fang, F, Pedersen, NL, Tillander, A, Ludvigsson, JF, Ekbom, A, et al. Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology. (2017) 88:1996–2002. doi: 10.1212/WNL.0000000000003961
11. Matheoud, D, Cannon, T, Voisin, A, Penttinen, AM, Ramet, L, Fahmy, AM, et al. Intestinal infection triggers Parkinson's disease-like symptoms in Pink1−/− mice. Nature. (2019) 571:565–9. doi: 10.1038/s41586-019-1405-y
12. Tan, AH, Lim, SY, and Lang, AE. The microbiome-gut-brain axis in Parkinson disease - from basic research to the clinic. Nat Rev Neurol. (2022) 18:476–95. doi: 10.1038/s41582-022-00681-2
13. Lee, HS, Lobbestael, E, Vermeire, S, Sabino, J, and Cleynen, I. Inflammatory bowel disease and Parkinson's disease: common pathophysiological links. Gut. (2021) 70:408–17. doi: 10.1136/gutjnl-2020-322429
14. Savica, R, Carlin, JM, Grossardt, BR, Bower, JH, Ahlskog, JE, Maraganore, DM, et al. Medical records documentation of constipation preceding Parkinson disease: a case-control study. Neurology. (2009) 73:1752–8. doi: 10.1212/WNL.0b013e3181c34af5
15. Nishiwaki, H, Ito, M, Ishida, T, Hamaguchi, T, Maeda, T, Kashihara, K, et al. Meta-analysis of gut dysbiosis in Parkinson's disease. Mov Disord. (2020) 35:1626–35. doi: 10.1002/mds.28119
16. Wei, H, Yu, C, Zhang, C, Ren, Y, Guo, L, Wang, T, et al. Butyrate ameliorates chronic alcoholic central nervous damage by suppressing microglia-mediated neuroinflammation and modulating the microbiome-gut-brain axis. Biomed Pharmacother. (2023) 160:114308. doi: 10.1016/j.biopha.2023.114308
17. Matsumoto, J, Stewart, T, Sheng, L, Li, N, Bullock, K, Song, N, et al. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson's disease? Acta Neuropathol Commun. (2017) 5:71. doi: 10.1186/s40478-017-0470-4
18. Holmqvist, S, Chutna, O, Bousset, L, Aldrin-Kirk, P, Li, W, Björklund, T, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. (2014) 128:805–20. doi: 10.1007/s00401-014-1343-6
19. Sampson, TR, Debelius, JW, Thron, T, Janssen, S, Shastri, GG, Ilhan, ZE, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell. (2016) 167:1469–80. doi: 10.1016/j.cell.2016.11.018
20. Tao, W, Zhang, Y, Wang, B, Nie, S, Fang, L, Xiao, J, et al. Advances in molecular mechanisms and therapeutic strategies for central nervous system diseases based on gut microbiota imbalance. J Adv Res. (2025) 69:261–78. doi: 10.1016/j.jare.2024.03.023
21. Hosseinkhani, F, Heinken, A, Thiele, I, Lindenburg, PW, Harms, AC, and Hankemeier, T. The contribution of gut bacterial metabolites in the human immune signaling pathway of non-communicable diseases. Gut Microbes. (2021) 13:1–22. doi: 10.1080/19490976.2021.1882927
22. Huang, C, Wang, P, Xu, X, Zhang, Y, Gong, Y, Hu, W, et al. The ketone body metabolite β-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia. (2018) 66:256–78. doi: 10.1002/glia.23241
23. Zhou, ZL, Jia, XB, Sun, MF, Zhu, YL, Qiao, CM, Zhang, BP, et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson's disease mice via gut microbiota and metabolites. Neurotherapeutics. (2019) 16:741–60. doi: 10.1007/s13311-019-00719-2
24. Reigstad, CS, Salmonson, CE, Rainey, JF, Szurszewski, JH, Linden, DR, Sonnenburg, JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. (2015) 29:1395–403. doi: 10.1096/fj.14-259598
25. Hoyles, L, Snelling, T, Umlai, UK, Nicholson, JK, Carding, SR, Glen, RC, et al. Microbiome-host systems interactions: protective effects of propionate upon the blood-brain barrier. Microbiome. (2018) 6:6. doi: 10.1186/s40168-018-0439-y
26. Xu, M, Zhou, EY, and Shi, H. Tryptophan and its metabolite serotonin impact metabolic and mental disorders via the brain-gut-microbiome axis: a focus on sex differences. Cells. (2025) 14:384. doi: 10.3390/cells14050384
27. Li, L, Yang, C, Jia, M, Wang, Y, Zhao, Y, Li, Q, et al. Synbiotic therapy with clostridium sporogenes and xylan promotes gut-derived indole-3-propionic acid and improves cognitive impairments in an Alzheimer's disease mouse model. Food Funct. (2024) 15:7865–82. doi: 10.1039/d4fo00886c
28. Schwarcz, R, Bruno, JP, Muchowski, PJ, and Wu, HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. (2012) 13:465–77. doi: 10.1038/nrn3257
29. Ward, RJ, Zucca, FA, Duyn, JH, Crichton, RR, and Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. (2014) 13:1045–60. doi: 10.1016/S1474-4422(14)70117-6
30. Hong, GS, Zillekens, A, Schneiker, B, Pantelis, D, de Jonge, WJ, Schaefer, N, et al. Non-invasive transcutaneous auricular vagus nerve stimulation prevents postoperative ileus and endotoxemia in mice. Neurogastroenterol Motil. (2019) 31:e13501. doi: 10.1111/nmo.13501
31. Muhammad, B, Li, H, Gu, Y, Xue, S, Gao, Y, Xu, Z, et al. IL-1β/IL-1R1 signaling is involved in the propagation of α-synuclein pathology of the gastrointestinal tract to the brain. J Neurochem. (2023) 166:830–46. doi: 10.1111/jnc.15886
32. Han, W, Tellez, LA, Perkins, MH, Perez, IO, Qu, T, Ferreira, J, et al. Neural circuit for gut-induced reward. Cell. (2018) 175:665–78. doi: 10.1016/j.cell.2018.10.018
33. Shan, J, Li, Z, Ji, M, Zhang, M, Zhang, C, Zhu, Y, et al. Transcutaneous vagus nerve stimulation for Parkinson's disease: a systematic review and meta-analysis. Front Aging Neurosci. (2024) 16:16. doi: 10.3389/fnagi.2024.1498176
34. Sullivan, JL. Misconceptions in the debate on the iron hypothesis. J Nutr Biochem. (2001) 12:33–7. doi: 10.1016/s0955-2863(00)00142-x
35. Zhao, Z, Li, F, Ning, J, Peng, R, Shang, J, Liu, H, et al. Novel compound FLZ alleviates rotenone-induced PD mouse model by suppressing TLR4/MyD88/NF-κB pathway through microbiota-gut-brain axis. Acta Pharm Sin B. (2021) 11:2859–79. doi: 10.1016/j.apsb.2021.03.020
36. Stefanova, N, Fellner, L, Reindl, M, Masliah, E, Poewe, W, and Wenning, GK. Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am J Pathol. (2011) 179:954–63. doi: 10.1016/j.ajpath.2011.04.013
37. Forsyth, CB, Shannon, KM, Kordower, JH, Voigt, RM, Shaikh, M, Jaglin, JA, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson's disease. PLoS One. (2011) 6:6. doi: 10.1371/journal.pone.0028032
38. Pavón, L, Besedosky, H, Bottasso, O, Hernández, R, Velasco, M, and Loria, R. Clinical and experimental immunomodulation. Clin Dev Immunol. (2013) 2013:801251. doi: 10.1155/2013/801251
39. Kendall, GE, Underwood, CF, and Parr-Brownlie, LC. A novel rat model for inflammatory gut-brain interactions in Parkinson's disease. Eur J Neurosci. (2025) 61:e16667. doi: 10.1111/ejn.16667
40. Esteves, AR, Munoz-Pinto, MF, Nunes-Costa, D, Candeias, E, Silva, DF, Magalhães, JD, et al. Footprints of a microbial toxin from the gut microbiome to mesencephalic mitochondria. Gut. (2023) 72:73–89. doi: 10.1136/gutjnl-2021-326023
41. Iacucci, M, Santacroce, G, Majumder, S, Morael, J, Zammarchi, I, Maeda, Y, et al. Opening the doors of precision medicine: novel tools to assess intestinal barrier in inflammatory bowel disease and colitis-associated neoplasia. Gut. (2024) 73:1749–62. doi: 10.1136/gutjnl-2023-331579
42. Stavrakis, S, Elkholey, K, Morris, L, Li, Y, and Po, S. P3512 Sex differences in rats with heart failure with preserved ejection fraction and the effect of autonomic modulation. Eur Heart J. (2019) 40. doi: 10.1093/eurheartj/ehz745.0376
43. Laver, KE, Adey-Wakeling, Z, Crotty, M, Lannin, NA, George, S, and Sherrington, C. Telerehabilitation services for stroke. Cochrane Database Syst Rev. (2020) 1:CD010255. doi: 10.1002/14651858.CD010255.pub3
44. Faustini, G, Longhena, F, Bruno, A, Bono, F, Grigoletto, J, La Via, L, et al. Alpha-synuclein/synapsin III pathological interplay boosts the motor response to methylphenidate. Neurobiol Dis. (2020) 138:104789. doi: 10.1016/j.nbd.2020.104789
45. Gibson, PR, and Shepherd, SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. (2012) 107:657–66; quiz 667. doi: 10.1038/ajg.2012.49
46. Dilmore, AH, Martino, C, Neth, BJ, West, KA, Zemlin, J, Rahman, G, et al. Effects of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer's disease. Alzheimers Dement. (2023) 19:4805–16. doi: 10.1002/alz.13007
47. Riezzo, G, Chimienti, G, Orlando, A, D'Attoma, B, Clemente, C, and Russo, F. Effects of long-term administration of Lactobacillus reuteri DSM-17938 on circulating levels of 5-HT and BDNF in adults with functional constipation. Benef Microbes. (2019) 10:137–47. doi: 10.3920/BM2018.0050
48. Liu, X, Du, ZR, Wang, X, Sun, XR, Zhao, Q, Zhao, F, et al. Polymannuronic acid prebiotic plus Lacticaseibacillus rhamnosus GG probiotic as a novel synbiotic promoted their separate neuroprotection against Parkinson's disease. Food Res Int. (2022) 155:111067. doi: 10.1016/j.foodres.2022.111067
49. Hong, CT, Chen, JH, and Huang, TW. Probiotics treatment for Parkinson disease: a systematic review and meta-analysis of clinical trials. Aging. (2022) 14:7014–25. doi: 10.18632/aging.204266
50. Aho, VTE, Houser, MC, Pereira, PAB, Chang, J, Rudi, K, Paulin, L, et al. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson's disease. Mol Neurodegener. (2021) 02-08) 16:6. doi: 10.1186/s13024-021-00427-6
51. Shin, C, Lim, Y, Lim, H, and Ahn, TB. Plasma short-chain fatty acids in patients with Parkinson's disease. Mov Disord. (2020) 35:1021–7. doi: 10.1002/mds.28016
52. Cannon, T, Sinha, A, Trudeau, LE, Maurice, CF, and Gruenheid, S. Characterization of the intestinal microbiota during Citrobacter rodentium infection in a mouse model of infection-triggered Parkinson's disease. Gut Microbes. (2020) 12:1–11. doi: 10.1080/19490976.2020.1830694
53. Yang, X, Ai, P, He, X, Mo, C, Zhang, Y, Xu, S, et al. Parkinson's disease is associated with impaired gut-blood barrier for short-chain fatty acids. Mov Disord. (2022) 37:1634–43. doi: 10.1002/mds.29063
54. Delli Pizzi, S, Franciotti, R, Chiacchiaretta, P, Ferretti, A, Edden, RA, Sestieri, C, et al. Altered medial prefrontal connectivity in Parkinson's disease patients with somatic symptoms. MOVEMENT DISORD. (2022) 37:2226–35. doi: 10.1002/mds.29187
55. Spetsieris, PG, Ko, JH, Tang, CC, Nazem, A, Sako, W, Peng, S, et al. Metabolic resting-state brain networks in health and disease. Proc Natl Acad Sci USA. (2015) 112:2563–8. doi: 10.1073/pnas.1411011112
56. Lang, AE, Siderowf, AD, Macklin, EA, Poewe, W, Brooks, DJ, Fernandez, HH, et al. Trial of cinpanemab in early Parkinson's disease. N Engl J Med. (2022) 08-04) 387:408–20. doi: 10.1056/NEJMoa2203395
57. Devos, D, Labreuche, J, Rascol, O, Corvol, JC, Duhamel, A, Guyon Delannoy, P, et al. Trial of deferiprone in Parkinson's disease. N Engl J Med. (2022) 387:2045–55. doi: 10.1056/NEJMoa2209254
58. Krishna, V, Fishman, PS, Eisenberg, HM, Kaplitt, M, Baltuch, G, Chang, JW, et al. Trial of globus pallidus focused ultrasound ablation in Parkinson's disease. N Engl J Med. (2023) 388:683–93. doi: 10.1056/NEJMoa2202721
59. Zadka, A, Rabin, N, Gazit, E, Mirelman, A, Nieuwboer, A, Rochester, L, et al. A wearable sensor and machine learning estimate step length in older adults and patients with neurological disorders. NPJ Digit Med. (2024) 7:142. doi: 10.1038/s41746-024-01136-2
60. Ullrich, M, Roth, N, Kuderle, A, Richer, R, Gladow, T, Gasner, H, et al. Fall risk prediction in Parkinson's disease using real-world inertial sensor gait data. IEEE J Biomed Health Inform. (2023) 27:319–28. doi: 10.1109/JBHI.2022.3215921
61. Nouriani, A, Jonason, A, Jean, J, McGovern, R, and Rajamani, R. System-identification-based activity recognition algorithms with inertial sensors. IEEE J Biomed Health Inform. (2023) 27:3119–28. doi: 10.1109/JBHI.2023.3265856
62. Sotirakis, C, Su, Z, Brzezicki, MA, Conway, N, Tarassenko, L, FitzGerald, JJ, et al. Identification of motor progression in Parkinson's disease using wearable sensors and machine learning. NPJ Parkinsons Dis. (2023) 9:142. doi: 10.1038/s41531-023-00581-2
63. Gao, C, Sun, H, Wang, T, Tang, M, Bohnen, NI, Müller, MLTM, et al. Model-based and model-free machine learning techniques for diagnostic prediction and classification of clinical outcomes in Parkinson's disease. Sci Rep. (2018) 8:8. doi: 10.1038/s41598-018-24783-4
64. Yang, Y, Shi, Y, Schweighauser, M, Zhang, X, Kotecha, A, Murzin, AG, et al. Structures of α-synuclein filaments from human brains with Lewy pathology. Nature. (2022) 610:791–5. doi: 10.1038/s41586-022-05319-3
65. Tapias, V, Hu, X, Luk, KC, Sanders, LH, Lee, VM, and Greenamyre, JT. Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell Mol Life Sci. (2017) 74:2851–74. doi: 10.1007/s00018-017-2541-x
66. Pagan, FL. Improving outcomes through early diagnosis of Parkinson's disease. Am J Manag Care. (2012) 18:S176–82.
67. Narendra, D, Walker, JE, and Youle, R. Mitochondrial quality control mediated by PINK1 and parkin: links to parkinsonism. Cold Spring Harb Perspect Biol. (2012) 4:a011338. doi: 10.1101/cshperspect.a011338
68. Hansen, BK, Jacobsen, MW, Middelboe, AL, Preston, CM, Marin, R, Bekkevold, D, et al. Remote, autonomous real-time monitoring of environmental DNA from commercial fish. Sci Rep. (2020) 10:13272. doi: 10.1038/s41598-020-70206-8
69. Cunningham, JL, Bramstång, L, Singh, A, Jayarathna, S, Rasmusson, AJ, Moazzami, A, et al. Impact of time and temperature on gut microbiota and SCFA composition in stool samples. PLoS One. (2020) 15:e0236944. doi: 10.1371/journal.pone.0236944
70. Kool, J, Tymchenko, L, Shetty, SA, and Fuentes, S. Reducing bias in microbiome research: comparing methods from sample collection to sequencing. Front Microbiol. (2023) 14:14. doi: 10.3389/fmicb.2023.1094800
71. Forbes, A, Keleher, MR, Venditto, M, and DiBiasi, F. Assessing patient adherence to and engagement with digital interventions for depression in clinical trials: systematic literature review. J Med Internet Res. (2023) 25:e43727. doi: 10.2196/43727
72. Lam, WY, and Fresco, P. Medication adherence measures: an overview. Biomed Res Int. (2015) 2015:217047. doi: 10.1155/2015/217047
73. Bergman, S, Asplund, M, and Nadjm-Tehrani, S. Permissioned blockchains and distributed databases: a performance study. Concurrency Computat Pract Exper. (2020) 32:e5227. doi: 10.1002/cpe.5227
74. Baucas, MJ, Spachos, P, and Plataniotis, KN. Federated learning and Blockchain-enabled fog-IoT platform for wearables in predictive healthcare. IEEE Transactions on Computational Social Systems. (2023) 10:1732–41. doi: 10.1109/TCSS.2023.3235950
75. Teymourian, H, Tehrani, F, Longardner, K, Mahato, K, Podhajny, T, Moon, JM, et al. Closing the loop for patients with Parkinson disease: where are we? Nat Rev Neurol. (2022) 18:497–507. doi: 10.1038/s41582-022-00674-1
76. Salomon, A, Gazit, E, Ginis, P, Urazalinov, B, Takoi, H, Yamaguchi, T, et al. A machine learning contest enhances automated freezing of gait detection and reveals time-of-day effects. Nat Commun. (2024) 06-06) 15:4853. doi: 10.1038/s41467-024-49027-0
77. Mirelman, A, Ben Or Frank, M, Melamed, M, Granovsky, L, Nieuwboer, A, Rochester, L, et al. Detecting sensitive mobility features for Parkinson's disease stages via machine learning. Mov Disord. (2021) 36:2144–55. doi: 10.1002/mds.28631
78. Li, A, Lian, J, and Vardhanabhuti, V. Multi-modal machine learning approach for early detection of neurodegenerative diseases leveraging brain MRI and wearable sensor data. PLoS Digit Health. (2025) 4:e0000795. doi: 10.1371/journal.pdig.0000795
79. Lubomski, M, Tan, AH, Lim, SY, Holmes, AJ, Davis, RL, and Sue, CM. Parkinson's disease and the gastrointestinal microbiome. J Neurol. (2020) 267:2507–23. doi: 10.1007/s00415-019-09320-1
80. Mancha Chahuara, M, Lopez Tufino, LDM, and Mugruza-Vassallo, CA. Gastrointestinal microbiome and cholelithiasis: Prospect in the nervous system. World J Gastroenterol. (2023) 29:5091–3. doi: 10.3748/wjg.v29.i34.5091
81. Lubomski, M, Tan, A, Lim, S, Holmes, A, Davis, R, and Sue, C. 064 Parkinson’s disease and the gastrointestinal microbiome: clinicopathological correlations and controversies. J Neurol Neurosur Psychiatry. (2019) 5:90. doi: 10.1136/jnnp-2019-anzan.56
82. Krueger, ME, Boles, JS, Simon, ZD, Alvarez, SD, McFarland, NR, Okun, MS, et al. Comparative analysis of Parkinson's and inflammatory bowel disease gut microbiomes reveals shared butyrate-producing bacteria depletion. NPJ Parkinsons Dis. (2025) 11:50. doi: 10.1038/s41531-025-00894-4
83. Pama, C. Socialization by bacteria. Science. (2019) 364:39.1–39.39. doi: 10.1126/science.364.6435.39-a
84. Pang, S, Ren, Z, Ding, H, and Chan, P. Short-chain fatty acids mediate enteric and central nervous system homeostasis in Parkinson's disease: innovative therapies and their translation. Neural Regen Res. (2025) 21:938–56. doi: 10.4103/NRR.NRR-D-24-01265
85. Vitetta, L, Vitetta, G, and Hall, S. The brain-intestinal mucosa-appendix-microbiome-brain loop. Diseases. (2018) 6:23. doi: 10.3390/diseases6020023
86. Anastasiades, PG, Collins, DP, and Carter, AG. Mediodorsal and ventromedial thalamus engage distinct L1 circuits in the prefrontal cortex. Neuron. (2021) 109:314–30. doi: 10.1016/j.neuron.2020.10.031
87. Backman, V, and Roy, HK. Optical spectroscopic markers of cancer. Dis Markers. (2008) 25:279. doi: 10.1155/2008/561471
Keywords: Parkinson’s disease, gut-brain axis, short-chain fatty acids (SCFAs), α-synuclein, neuroinflammation, remote rehabilitation, vagus nerve stimulation
Citation: Jin Y, Wang H and Song J (2025) Gut-brain axis modulation in remote rehabilitation of Parkinson’s disease: reconstructing the fecal metabolome and nigral network connectivity. Front. Neurol. 16:1644490. doi: 10.3389/fneur.2025.1644490
Edited by:
Simona Bonavita, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Carsten Theiss, Ruhr University Bochum, GermanyJacob Raber, Oregon Health and Science University, United States
Copyright © 2025 Jin, Wang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinan Song, MTU5MDQyMTg4NzdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yuting Jin†
Yuting Jin† Jinan Song
Jinan Song