Abstract
Objectives:
We developed a straightforward stretching device for the wrist and hand. To assess the device’s effectiveness in managing spasticity among chronic stroke patients.
Methods:
The device, primarily constructed from plastic, comprises a forearm support module, a wrist module, and a finger module. Twenty stroke patients used the device four times daily, 7 days a week, for 1 month. Spasticity severity was measured using the Modified Ashworth Scale (MAS) for the wrist, thumb, and index fingers. A questionnaire evaluated the device’s feasibility and areas for improvement.
Results:
Before treatment, the mean MAS scores for the wrist, thumb, and index finger flexors were 1.50 ± 0.36, 1.52 ± 0.34, and 1.50 ± 0.30, respectively, compared with 1.25 ± 0.26, 1.27 ± 0.30, and 1.32 ± 0.33 post-intervention. Patients and occupational therapists expressed satisfaction with the device, citing its ease of use, effectiveness in stretching the wrist and fingers, and overall ease of manipulation. Half of the patients reported that all fingers were easily extended. The rigid plastic finger module was subsequently replaced with an inflatable, flexible rubber ball, providing a more comfortable contour for the stretched fingers, which increased user satisfaction.
Conclusion:
The stretching device effectively reduced spasticity in the wrist and hand, and the upgraded device enhanced patient satisfaction.
1 Introduction
Spasticity is a form of hypertonus characterized by increased muscle tension in response to stimuli, which intensifies with the velocity of joint movement (1, 2). It is a common sequela of central nervous system disorders, including stroke, traumatic brain injury, spinal cord injury, multiple sclerosis, and cerebral palsy. After a stroke, approximately 65% of patients experience spasticity (3). This condition can lead to muscle tightness and joint stiffness in the affected extremity, resulting in functional disability (4, 5). Therefore, effective management of spasticity is crucial for stroke patients.
Several methods are currently employed to manage spasticity in stroke patients, including oral medications, botulinum toxin or alcohol injections, bracing, serial casting, and stretching exercises (6–10). Among these therapeutic options, stretching exercises, which involve moving joints through their full range of motion via an external force, are one of the most fundamental approaches (11, 12). Stretching exercises alleviate spasticity in stroke patients through several proposed mechanisms (13–15). Prolonged passive stretching reduces stretch reflex excitability primarily via modulation of Ia afferent input to α-motor neurons and decreased γ-motor drive, leading to reduced sensitivity of muscle spindle (13, 15). Additionally, Golgi tendon organs contribute through autogenic inhibition, further suppressing hyperexcitable motor neuron pools. This, in turn, reduces muscle spindle hyperactivity and promotes viscoelastic changes in muscle-tendon units, thereby increasing joint range of motion (ROM) and reducing resistance to movement. Moreover, repetitive stretching can induce neuroplastic adaptations in supraspinal structures, improving motor control and reducing involuntary muscle contractions.
However, stretching exercises are typically performed manually, requiring a therapist to administer repetitive exercises regularly (11, 16). This manual approach is time-consuming, and outcomes can vary depending on the therapist’s experience. To address these limitations, various stretching devices have been developed, demonstrating positive effects in reducing spasticity (17–20). However, most devices are designed for therapist-assisted use, require considerable time and labor, and are unsuitable for independent operation by patients. If patients could independently wear and use these stretching devices without the assistance of therapists, or with minimal help, it would save therapists’ time and enhance the effectiveness of spasticity management.
We developed a wrist and hand stretching device designed for patients to use conveniently. The device consists of three primary modules: a forearm support module, a wrist module, and a finger module. The forearm support module stabilizes the forearm in a neutral position, reducing strain on the wrist and hand during stretching. The wrist module features a rotational axis that enables controlled wrist extension and can be locked at the desired angle for sustained stretching. The finger module is ergonomically designed to gradually separate the thumb from the other fingers, enabling targeted stretching of the finger flexors. All components are primarily fabricated from lightweight plastic using 3D printing technology, ensuring portability and ease of use without electronic or motorized systems.
We evaluated its effectiveness and assessed feasibility through patient feedback, leading to subsequent device upgrades based on this feedback.
2 Methods
2.1 Subjects
We prospectively recruited 20 consecutive stroke patients (M:F = 9:11, age = 68.7 ± 4.4, cerebral infarct:cerebral hemorrhage = 7:13, right hemiplegia:left hemiplegia = 11:9, time between onset and start of clinical trial = 13.4 ± 1.1; Fugl-Meyer Upper Extremity = 34.8 ± 7.5; Barthel Index = 72.4 ± 10.2) based on the following inclusion criteria: (1) ≥ 12 months after stroke onset; (2) hemiparesis or hemiplegia due to stroke; (3) sufficient cognitive ability to understand the clinical trial process and respond to our questionnaire (Mini-Mental State Examination score of <25); (4) spasticity in the wrist flexor, thumb flexor, and index finger flexor with a Modified Ashworth Scale (MAS) score between 1 and 2; (5) no history of musculoskeletal disease (e.g., arthritis, musculotendinous injury, or bone fracture) or peripheral nerve injury in the affected upper extremity; (6) no invasive procedure for spasticity management (injection of botulinum toxin, alcohol, or phenol) within 6 months prior to the initiation of this study. The Ethics Committee of the Ministry of Health and Welfare approved this protocol (P01-202410-01-012), and all patients provided written informed consent before participating in the study.
2.2 Stretching device and intervention
The device comprises a forearm support module, a wrist module, and a finger module. Most components were fabricated from plastic using 3D printing techniques (Figure 1). The device lacks electronic controls or a motorized system, ensuring ease of use and safety for users. The primary function of the device is to stretch and extend the spastic wrist and fingers, allowing individuals to operate it independently without assistance. The forearm support module stabilizes the patient’s forearm during stretching (Figure 1), providing a base upon which the wrist module can rotate to achieve dorsal wrist extension. A rotational axis between the hand and forearm modules allows for easy wrist stretching, and the wrist can be securely fixed at any point using a locking system. The finger module, designed ergonomically for spastic hands and fingers, comfortably and securely holds the patient’s fingers. It stretches the fingers by widening the gap between the thumb and the other fingers, controlled by rotating a wheel. The module can also be fixed at any desired position. The stretching angle for the wrist module was typically set between 45° and 70° of extension. The mechanical design of the device allows a maximum wrist flexion of 90° and a maximum extension of 90°, providing a built-in ROM limit to prevent overstretching. Participants used the device independently, four times daily, 7 days a week, for 1 month. Each stretching session lasted 15 min. The end-point ROM for both the wrist and fingers was determined individually, limited either by patients’ pain tolerance or by firm end-range resistance. Patients’ subjective feedback was used to determine the optimal endpoint, ensuring sufficient stretching intensity to engage target muscles without causing discomfort. Adherence was monitored using daily log sheets completed by patients (or their caregivers, if assistance was required), which recorded session completion. The research team reviewed the logs weekly and conducted brief phone follow-ups to confirm compliance.
Figure 1

Illustration of device usage. (A) Initial setup: Place the patient’s wrist and hand into the device and secure the forearm with Velcro tape. (B) Stretch the wrist by rotating the wrist module and locking it in place. (C) Stretch the fingers by rotating the wheel. (D) Final target position: Fully extended wrist and fingers.
2.3 Assessment of spasticity
The degree of spasticity was evaluated using the MAS for the wrist flexor, thumb flexor, and index finger flexor (20). All MAS assessments were performed by a single physician with 20 years of experience in stroke rehabilitation to minimize inter-rater variability. Because this was a single-arm pre-post study without random allocation, blinding of the assessor was not applicable. We acknowledge that the assessor was aware of the intervention, which might introduce a potential source of bias. MAS assessments were conducted immediately before the first therapeutic session with the device (pre-treatment) and again 1 day after the final session (post-treatment). To avoid potential confounding from transient changes immediately following stretching, post-treatment MAS evaluations were performed 1 day after the final intervention rather than immediately after a session. The MAS scores were defined as follows: 1—slight increase in muscle tone, indicated by a catch and release or minimal resistance at the end of the ROM during flexion or extension; 1 + —slight increase in muscle tone, indicated by a catch followed by minimal resistance throughout less than half of the ROM; 2—more marked increase in muscle tone through most of the ROM, although the affected part(s) could still be moved easily. For statistical analysis, scores of 1, 1+, and 2 were assigned values of 1, 1.5, and 2, respectively.
2.4 User feasibility assessment
A questionnaire was administered to the participants to identify the device’s potential shortcomings and assess its convenience. The questionnaire included the following items:
Donning and doffing
Is it easy to don and doff?
How long does it take to don and doff?
Does the device provide optimal positioning for the wrist and fingers?
How many helpers are required to don and doff?
Wrist extension
Is the wrist module easy to operate?
Can the wrist be stretched to the desired angle?
Can the extended posture and angle be easily maintained?
Finger extension
Is the finger module easy to operate?
Can the fingers be stretched to the desired angle?
Can the extended posture and angle be easily maintained?
Are all the fingers equally extended and stretched?
General points
Is the device too heavy to carry?
Does the baseplate provide firm support?
Are there any inconveniences during use?
Would you be willing to use the device daily to manage hand spasticity?
2.5 Statistical analysis
Data were analyzed using SPSS Statistics for Windows, version 27.0 (IBM Corp., Armonk, NY, USA). To verify normal data distributions, Kolmogorov–Smirnov tests were performed prior to each analysis. As the data were not normally distributed, we compared pre-treatment and post-treatment MAS scores using the Wilcoxon signed-rank test. Statistical significance was set at p < 0.05.
3 Results
The mean MAS scores at pre-treatment were 1.50 ± 0.36 for the wrist flexor, 1.52 ± 0.34 for the thumb flexor, and 1.50 ± 0.30 for the index finger flexor. After treatment, the mean MAS scores were significantly reduced to 1.25 ± 0.26, 1.27 ± 0.30, and 1.32 ± 0.33, respectively. Statistical analysis confirmed that the reductions in MAS scores for the wrist flexor, thumb flexor, and index finger flexor were significant when compared to pre-treatment values (wrist flexor, p = 0.002, Z = −3.162; thumb flexor, p = 0.002, Z = −3.162; index finger flexor, p = 0.020, Z = −2.333).
Overall, patient feedback was positive (Table 1). However, when assessing finger extension, only 10 patients (50%) reported that all fingers were easily extended, suggesting that the finger module required improvement to ensure equal extension of all fingers. Based on this feedback, we focused on enhancing the finger module by incorporating a ballooning ball mechanism (Figure 2). This design features a flexible and resilient rubber ball, a metal upright pole, and an air nozzle connected to a manually operated cuff derived from a sphygmomanometer (Figure 3).
Table 1
| Donning and doffing | |||
| Easy to don and doff | Easy (18) | Moderate (2) | Difficult (0) |
| Duration (minutes) | <3 (15) | 3 ~ 5 (4) | >5 (1) |
| Optimal positioning | Yes (18) | Moderate (2) | No (0) |
| How many helpers? | 0 (18) | 1 (2) | 2 (0) |
| Wrist extension | |||
| Easy to operate? | Easy (20) | Moderate (0) | Difficult (0) |
| Stretch to wanted angle? | Easy (19) | Moderate (1) | Difficult (0) |
| Sustain the extended posture? | Easy (20) | Moderate (0) | Difficult (0) |
| Finger extension | |||
| Easy to operate? | Easy (20) | Moderate (0) | Difficult (0) |
| Stretch to wanted angle? | Easy (20) | Moderate (0) | Difficult (0) |
| Sustain the extended posture? | Easy (20) | Moderate (0) | Difficult (0) |
| All the fingers extended equally? | Easy (10) | Moderate (5) | Difficult (5) |
| General point | |||
| Too heavy? | No (20) | A bit (0) | Heavy (0) |
| Firm support? | Yes (20) | A bit (0) | No (0) |
| Any trouble? | No (18) | A bit (2) | Yes (0) |
| Willing to use? | Yes (20) | A bit (0) | No (0) |
User feasibility test results.
The numbers in parentheses indicate the tallies of patients who responded.
Figure 2
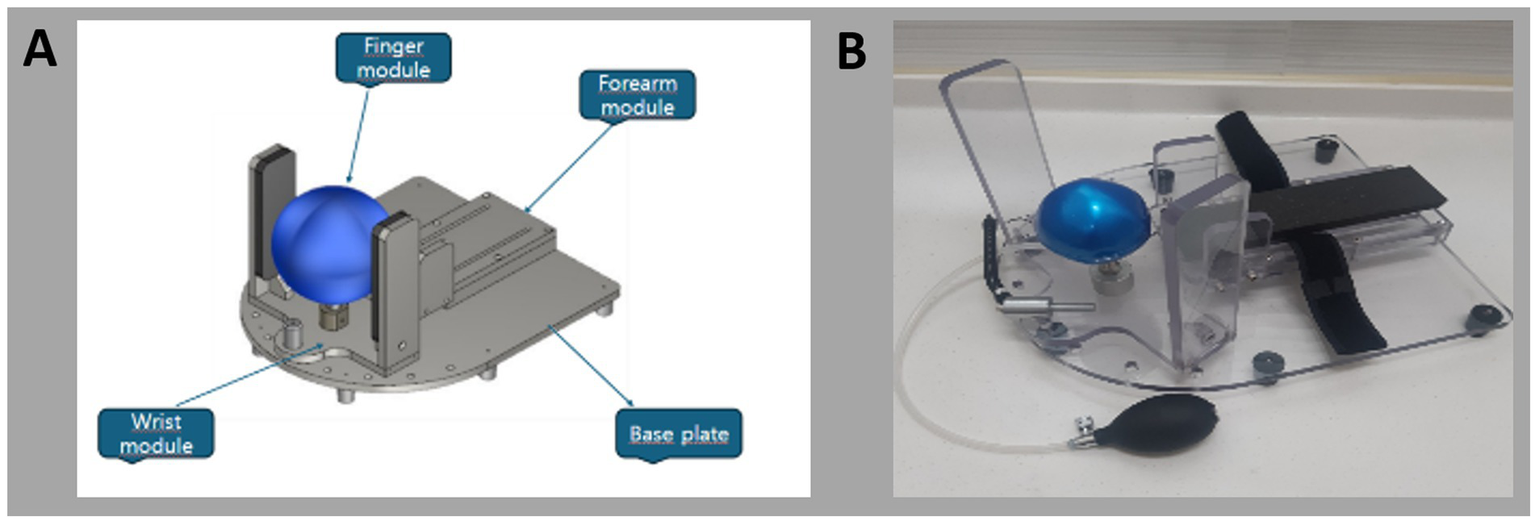
(A) 2D schematics and (B) photograph of the newly developed device.
Figure 3
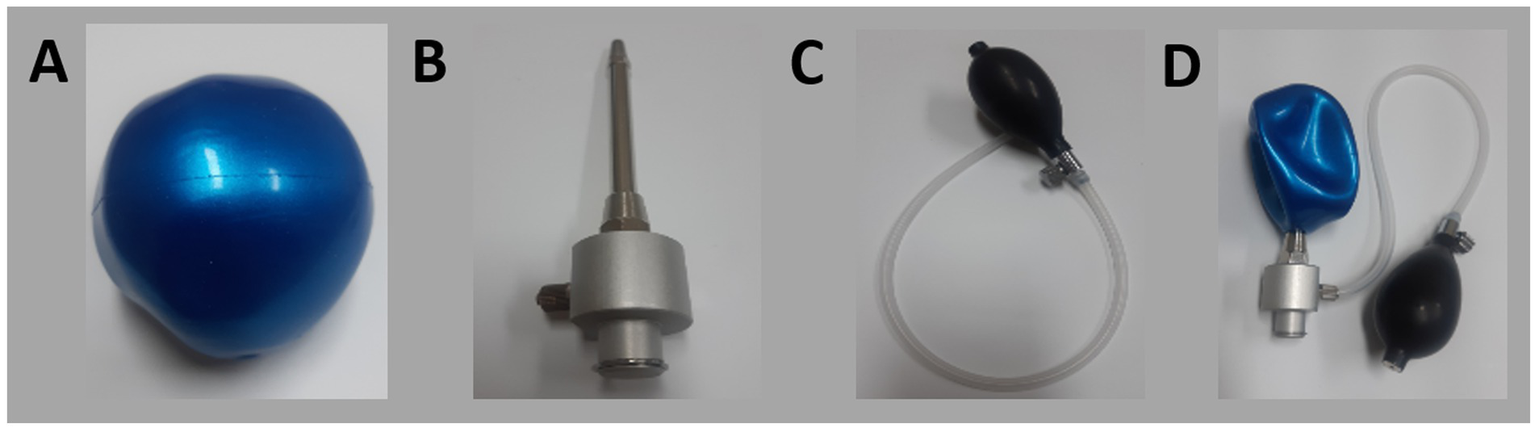
Components of the newly developed device: (A) inflatable rubber ball, (B) upright bar, (C) air pump, (D) fully assembled device.
In its resting state, the ball remains deflated, allowing the metal upright pole to support the spastic hand as it fits into the finger module. When the cuff is used to inflate the ball, the expanding ball stretches the spastic hand and fingers (Figure 4). We applied the upgraded device to all 20 patients and reassessed the user feasibility, specifically focusing on finger extension (Table 2). Following the upgrade, all patients reported that all fingers were equally and easily extended. Additionally, all patients indicated that they could easily operate the device, achieve the desired angle of extension, and maintain the extended posture.
Figure 4
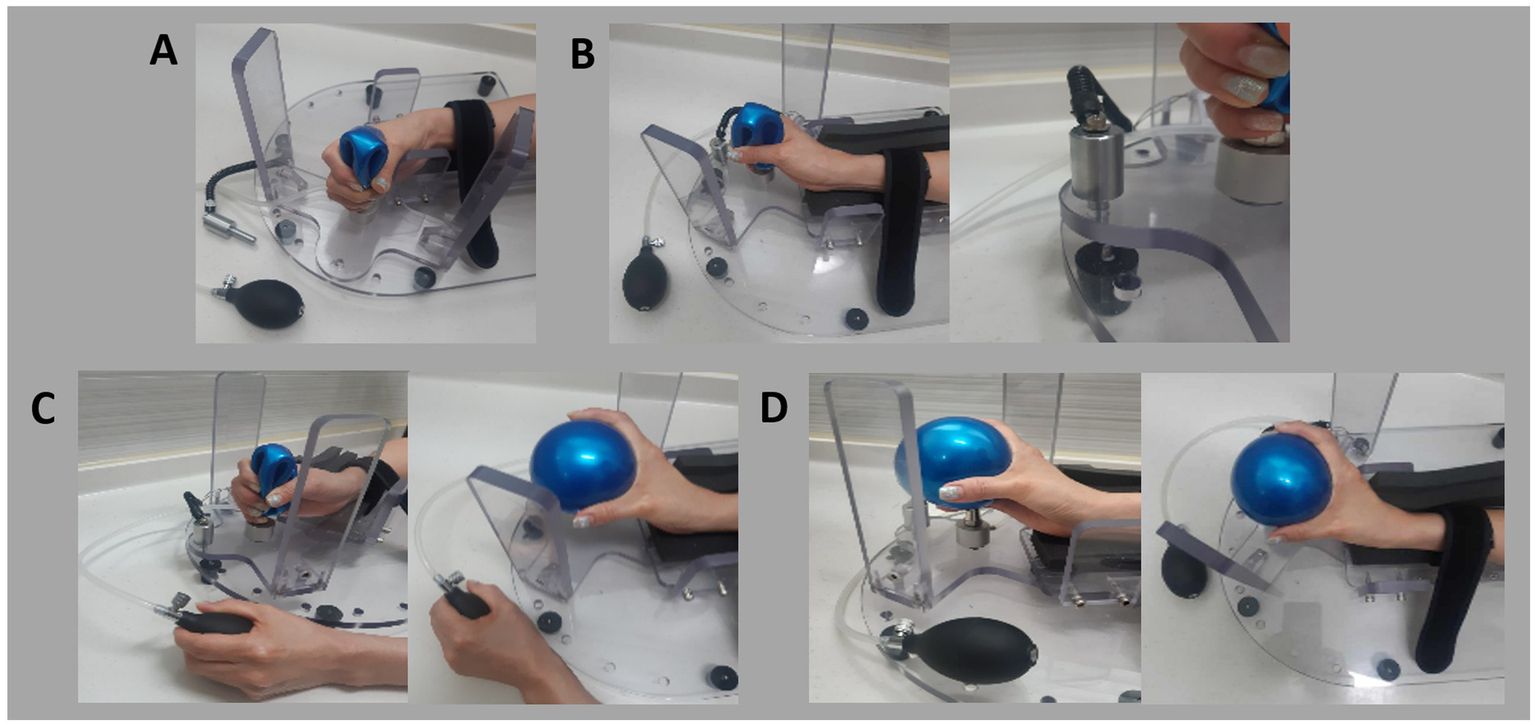
Steps for using the newly developed device: (A) Initial setup: Position the patient’s hand in the device and secure the forearm with Velcro tape. (B) Stretch the wrist by rotating the wrist module and locking it in place. (C) Stretch the fingers by inflating the ball using the cuff. (D) Final target position with extended fingers and wrist.
Table 2
| Finger extension | |||
| Easy to operate? | Easy (20) | Moderate (0) | Difficult (0) |
| Stretch to wanted angle? | Easy (20) | Moderate (0) | Difficult (0) |
| Sustain the extended posture? | Easy (20) | Moderate (0) | Difficult (0) |
| All the fingers extended equally? | Easy (20) | Moderate (0) | Difficult (0) |
User feasibility test results with the newly developed device.
The numbers in parentheses indicate the tallies of patients who responded.
4 Discussion
In this study, we examined the effects of a simple stretching device for the wrist and hand, developed specifically to manage spasticity in chronic stroke patients. After using the device for 1 month, patients exhibited significant reductions in spasticity, as measured by the MAS, in the wrist flexor, thumb flexor, and index finger flexor muscles. Additionally, patient feedback revealed a key limitation of the initial device: not all fingers were easily extended. In response to this feedback, we modified the device by incorporating a ballooning ball mechanism, which effectively allowed for the equal extension of all fingers. All participants reported satisfaction with the modified device.
Previous efforts to develop stretching devices for managing hand spasticity have been made (17–20). However, these devices were limited in their ability to control spasticity in the fingers without addressing wrist spasticity. Additionally, earlier devices often required assistance for donning and operation, limiting their practicality for independent use. In contrast, our device addresses spasticity in both the fingers and wrist and is designed for independent use by the patient. This independence allows patients to use the device as frequently as needed, facilitating more consistent management of spasticity. Feedback from our patient survey further highlighted the device’s ease of use, including its straightforward donning and doffing process, user-friendly module manipulation, and portability.
Furthermore, existing devices such as static splints, continuous passive motion (CPM) devices, and dynamic orthoses show several quantitative shortcomings. Static splints lack angle-control precision, with most providing only fixed extension positions without fine adjustment (14). CPM devices allow repetitive motion but often lack individualized torque or velocity control, resulting in inconsistent therapeutic effects (11). Dynamic orthoses, while more adaptable, still provide limited real-time biofeedback and their angle-control accuracy is typically within 5–10°, insufficient for tailoring to spastic muscles with narrow tolerance ranges. A recent systematic review concluded that many stretching devices fail to achieve reproducible, patient-spastic adjustments and recommended integration of feedback-controlled systems for improved outcomes (10, 11). Compared with these approaches, our device enables individualized adjustment of both wrist and finger joints with mechanical locking at specific extension angles, offering greater reproducibility for independent patient use.
Another critical advantage of our device is its ability to maintain the forearm in a neutral position. Previous devices often positioned the forearm in a prone position, complicating the fitting process and causing discomfort in the spastic upper limb (17–20). When the wrist and fingers are flexed due to spasticity, a prone forearm position hinders proper accommodation of the flexed joints, potentially leading to additional strain on the wrist during stretching. Our device avoids these issues by supporting the forearm in a neutral position, making it easier and more comfortable to fit the spastic limb into the device. Moreover, the device applies stretching forces that are evenly aligned with the wrist and finger joints, preventing undue stress on the joints and ensuring a more effective treatment process.
Our study was conducted without a control group. However, we specifically recruited patients who were at least 12 months post-stroke. Given this timeframe, it is unlikely that the observed reductions in spasticity were due to natural recovery. Previous longitudinal studies have shown that most spontaneous neurological recovery, along with associated reductions in spasticity, occur within the first 3–6 months post-stroke, with minimal further change beyond 12 months (21, 22). Thus, it is unlikely that natural recovery contributed significantly to the improvements observed in our patients. Therefore, although we did not compare the therapeutic outcomes of our stretching device with a control group, our results suggest that the device is effective in managing spasticity in the wrists and hands of chronic stroke patients.
The user survey revealed that patients had some issues with the original finger module, particularly with uneven stretching of the fingers. The rigid plastic module was not well-suited to accommodate spastic fingers, especially at the interphalangeal joints. In response to this feedback, we redesigned the finger module, incorporating an inflatable ball with an upright bar and an air nozzle to serve as a guide pole. This design made it easier for spastic hands to fit into the module. We integrated a puffing device from a sphygmomanometer into the air infusion system, allowing the ballooning ball to provide a flexible and comfortable contour for the fingers, both at rest and during stretching. With the initial device, some severely spastic fingers could escape during stretching, exacerbating the spastic posture. The redesigned module, with its ballooning ball concept, applied fluid pressure evenly across the fingers, ensuring uniform stretching.
The reduction in spasticity observed in this study is clinically meaningful, as it can improve joint mobility and improve function in daily activities, and the absence of adverse events confirms its safety. These effects are likely attributable to biomechanical changes, such as increased muscle-tendon length and improved connective tissue flexibility, as well as neurophysiological mechanisms, including reduced muscle spindle sensitivity and enhanced autogenic inhibition via Golgi tendon organs (23–25).
In conclusion, our stretching device effectively alleviated wrist and hand spasticity in chronic hemiparetic stroke patients, and its feasibility was confirmed through user feedback. Moreover, by incorporating the insights from the user survey, we upgraded the device, leading to enhanced patient satisfaction. However, our study has limitations. It was conducted without a control group, and we did not evaluate the therapeutic outcomes of the modified device. Additionally, we did not monitor serial changes in MAS scores during the 1-month treatment period, nor did we investigate the long-term effects of the treatment. Therefore, further studies are warranted to address these limitations.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ministry of Health and Welfare. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PK: Investigation, Software, Writing – review & editing, Methodology, Writing – original draft, Conceptualization, Formal analysis, Visualization, Data curation, Resources, Project administration, Validation. JK: Project administration, Data curation, Methodology, Visualization, Validation, Conceptualization, Software, Writing – original draft, Resources, Writing – review & editing, Formal analysis, Investigation. PC: Writing – review & editing, Writing – original draft, Software, Investigation, Resources, Data curation, Formal analysis, Project administration, Conceptualization, Visualization, Validation, Methodology. MC: Writing – original draft, Formal analysis, Project administration, Methodology, Visualization, Resources, Data curation, Investigation, Validation, Conceptualization, Writing – review & editing, Supervision, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Research Foundation of Korea grant funded by the Korean government (MSIT) (No. RS-2023-00219725).
Conflict of interest
PK was employed by J&P Robotics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ivanhoe CB Reistetter TA . Spasticity: the misunderstood part of the upper motor neuron syndrome. Am J Phys Med Rehabil. (2004) 83:S3–9. doi: 10.1097/01.phm.0000141125.28611.3e
2.
Raciti L Raciti G Ammendolia A de Sire A Onesta MP Calabrò RS . Improving spasticity by using botulin toxin: an overview focusing on combined approaches. Brain Sci. (2024) 14:631. doi: 10.3390/brainsci14070631
3.
Gallichio JE . Pharmacologic management of spasticity following stroke. Phys Ther. (2004) 84:973–81. doi: 10.1093/ptj/84.10.973
4.
Ryu JS Lee JW Lee SI Chun MH . Factors predictive of spasticity and their effects on motor recovery and functional outcomes in stroke patients. Top Stroke Rehabil. (2010) 17:380–8. doi: 10.1310/tsr1705-380
5.
Shin YI Kim SY Lee HI Kim DY Lee J Sohn MK et al . Association between spasticity and functional impairments during the first year after stroke in Korea: the KOSCO study. Am J Phys Med Rehabil. (2018) 97:557–64. doi: 10.1097/PHM.0000000000000916
6.
Chang MC Boudier-Revéret M . Management of elbow flexor spasticity with ultrasound-guided alcohol neurolysis of the musculocutaneous nerve. Acta Neurol Belg. (2020) 120:983–4. doi: 10.1007/s13760-020-01300-x
7.
Chang MC Choi GS Boudier-Revéret M . Ultrasound-guided ethyl alcohol injection to the deep branch of the ulnar nerve to relieve hand spasticity in stroke patients: a case series. Transl Neurosci. (2021) 12:346–50. doi: 10.1515/tnsci-2020-0188
8.
Chang MC Choo YJ Kwak SG Nam K Kim SY Lee HJ et al . Effectiveness of extracorporeal shockwave therapy on controlling spasticity in cerebral palsy patients: a meta-analysis of timing of outcome measurement. Children. (2023) 10:332. doi: 10.3390/children10020332
9.
Hampton C . The use of long-arm serial casting to manage multiple sclerosis spasticity: a case report. Int J MS Care. (2024) 26:144–8. doi: 10.7224/1537-2073.2023-024
10.
Nawv T de Groot V Meskers CGM . The effectiveness of early interventions for post-stroke spasticity: a systematic review. Disabil Rehabil. (2025) 47:900–11. doi: 10.1080/09638288.2024.2363963
11.
Selles RW Li X Lin F Chung SG Roth EJ Zhang LQ . Feedback-controlled and programmed stretching of the ankle plantarflexors and dorsiflexors in stroke: effects of a 4-week intervention program. Arch Phys Med Rehabil. (2005) 86:2330–6. doi: 10.1016/j.apmr.2005.07.305
12.
Wu CL Huang MH Lee CL Liu CW Lin LJ Chen CH . Effect on spasticity after performance of dynamic-repeated-passive ankle joint motion exercise in chronic stroke patients. Kaohsiung J Med Sci. (2006) 22:610–7. doi: 10.1016/S1607-551X(09)70361-4
13.
Avela J Kyröläinen H Komi PV . Altered reflex sensitivity after repeated and prolonged passive muscle stretching. J Appl Physiol (1985). (1999) 86:1283–91. doi: 10.1152/jappl.1999.86.4.1283
14.
Bovend'Eerdt TJ Newman M Barker K Dawes H Minelli C Wade DT . The effects of stretching in spasticity: a systematic review. Arch Phys Med Rehabil. (2008) 89:1395–406. doi: 10.1016/j.apmr.2008.02.015
15.
Budini F Rafolt D Christova M Gallasch E Tilp M . The recovery of muscle spindle sensitivity following stretching is promoted by isometric but not by dynamic muscle contractions. Front Physiol. (2020) 11:905. doi: 10.3389/fphys.2020.00905
16.
Zhang LQ Chung SG Bai Z Xu D van Rey EM Rogers MW et al . Intelligent stretching of ankle joints with contracture/spasticity. IEEE Trans Neural Syst Rehabil Eng. (2002) 10:149–57. doi: 10.1109/TNSRE.2002.802857
17.
Chang PH Lee SH Gu GM Lee SH Jin SH Yeo SS et al . The cortical activation pattern by a rehabilitation robotic hand: a functional NIRS study. Front Hum Neurosci. (2014) 8:49. doi: 10.3389/fnhum.2014.00049
18.
Jo HM Song JC Jang SH . Improvements in spasticity and motor function using a static stretching device for people with chronic hemiparesis following stroke. NeuroRehabilitation. (2013) 32:369–75. doi: 10.3233/NRE-130857
19.
Jung YJ Hong JH Kwon HG Song JC Kim C Park S et al . The effect of a stretching device on hand spasticity in chronic hemiparetic stroke patients. NeuroRehabilitation. (2011) 29:53–9. doi: 10.3233/NRE-2011-0677
20.
Kim EH Chang MC Seo JP Jang SH Song JC Jo HM . The effect of a hand-stretching device during the management of spasticity in chronic hemiparetic stroke patients. Ann Rehabil Med. (2013) 37:235–40. doi: 10.5535/arm.2013.37.2.235
21.
Kwakkel G Kollen B Lindeman E . Understanding the pattern of functional recovery after stroke: facts and theories. Restor Neurol Neurosci. (2004) 22:281–99. PMID: 15502272. doi: 10.3233/RNN-2004-00282
22.
Sommerfeld DK Eek EU Svensson AK Holmqvist LW von Arbin MH . Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. (2004) 35:134–9. doi: 10.1161/01.STR.0000105386.05173.5E
23.
Bressel E McNair PJ . The effect of prolonged static and cyclic stretching on ankle joint stiffness, torque relaxation, and gait in people with stroke. Phys Ther. (2002) 82:880–7.
24.
Harvey LA Herbert RD . Muscle stretching for treatment and prevention of contracture in people with spinal cord injury. Spinal Cord. (2002) 40:1–9. doi: 10.1038/sj.sc.3101241
25.
Sharman MJ Cresswell AG Riek S . Proprioceptive neuromuscular facilitation stretching: mechanisms and clinical implications. Sports Med. (2006) 36:929–39. doi: 10.2165/00007256-200636110-00002
Summary
Keywords
spasticity, stretching therapy, stroke, device, survey
Citation
Kim PS, Kim J, Chen P and Chang MC (2025) Development of a wrist and hand stretching device for managing spasticity in stroke patients: a pilot study. Front. Neurol. 16:1646697. doi: 10.3389/fneur.2025.1646697
Received
13 June 2025
Accepted
08 September 2025
Published
19 September 2025
Volume
16 - 2025
Edited by
Hewei Wang, Fudan University, China
Reviewed by
Guidi Zou, Huizhou Third Peoole's Hospital, China
Yanzheng Zhang, Shanghai Yuhua Rehabilitation Hospital, China
Updates
Copyright
© 2025 Kim, Kim, Chen and Chang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Cheol Chang, wheel633@ynu.ac.kr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.