Abstract
Objective:
The objective of this study is to assess the impact of acupuncture regarding Blood–Brain barrier (BBB) permeability and provide a data foundation for clinical practice.
Methods:
A database search was carried out in PubMed, Embase, Cochrane Library, and Web of Science to collect controlled animal experiments that investigated the impact of acupuncture on BBB permeability. BBB permeability was primarily assessed by indicators such as Evans Blue (EB) extravasation. The SYRCLE risk-of-bias tool was utilized to assess the quality of the comprised studies, and statistical software was employed for data evaluation. For continuous outcomes, a random-effects model was used to calculate pooled standardized mean differences (SMD) with 95% confidence intervals (CI), and heterogeneity was quantified using the I2 statistic.
Results:
Thirty-two papers were incorporated. Outcomes from the meta-analysis showed that acupuncture significantly reduced the EB leakage in brain tissue (SMD = −0.65, 95% CI [−0.94, −0.37], p < 0.001), indicating its effectiveness in improving BBB permeability. Additionally, acupuncture upregulated the levels of occludin, claudin-5, and ZO-1, inhibited the activity of matrix metalloproteinase-9 (MMP-9), reduced the amounts of glial activation markers (Iba-1, GFAP) and inflammatory mediators (IL-1β, IL-6, TNF-α), and regulated the levels of aquaporin-4 (AQP4).
Conclusion:
acupuncture may improve BBB integrity by means of multiple mechanisms, including the enhancement of tight junction protein production in endothelial cells, inhibiting MMP-9 mediated extracellular matrix (ECM) degradation, modulating glial cell activation and inflammatory responses, and downregulating AQP4-dependent edema.
Systematic review registration:
https://inplasy.com/inplasy-2025-2-0102/, identifier: INPLASY202520102.
1 Introduction
The Blood–Brain barrier (BBB), a highly selective barrier that exists between the central nervous system (CNS) and the peripheral circulation. It is mainly composed of brain microvascular endothelial cells, tight junctions, the basement membrane, pericytes, and astrocytes. The main role of the BBB is to preserve homeostasis of the CNS, protect brain tissue from toxic substances, and ensure the supply of essential molecules required for neuronal activity (1). The BBB primarily relies on tight junctions (TJs) between endothelial cells. These junction complexes consist of transmembrane proteins (claudin-5, occludin, tricellulin, marvelD3) and cytoplasmic membrane-associated proteins (ZO-1, ZO-2, ZO-3) (2). Claudin-5, as a BBB-specific transmembrane protein, maintains intercellular barrier integrity by forming tightly sealed transmembrane strands (3). Matrix metalloproteinases (MMPs), particularly MMP-9, regulate BBB permeability by degrading extracellular matrix and tight junction proteins. Abnormal elevation of their activity constitutes a key mechanism in BBB damage (4). Overexpression of glial cell activation markers Iba-1 (microglia) and GFAP (astrocytes) drives neuroinflammatory responses. These markers disrupt endothelial tight junctions through the release of pro-inflammatory factors (IL-1β, IL-6, TNF-α), ultimately increasing BBB permeability (5, 6). Aquaporin 4 (AQP4) is primarily distributed in astrocyte foot processes, and its abnormal expression is closely associated with cerebral edema formation and BBB dysfunction (7). Notably, breakdown of the BBB is frequently seen in a range of neurological disorders, such as stroke, Alzheimer's disease, and Parkinson's disease, and is strongly linked to the onset and progression of these diseases (8).
Acupuncture, a classic Chinese therapeutic technique, has demonstrated distinct advantages in clinical interventions for neurological diseases due to its simplicity, safety, and low risk of side effects (9, 10). As a classic treatment technology of traditional Chinese medicine, acupuncture shows unique advantages in the clinical intervention of nervous system diseases, and its mechanism involves the coordinated regulation of multiple molecular targets. Recent studies have demonstrated that acupuncture can influence BBB permeability through multiple mechanisms. Specifically, acupuncture reduces LPS, TNF-α, and IL-1β levels while positively regulating intestinal flora and addressing Blood–Brain barrier dysfunction (11). Additionally, acupuncture inhibits Blood–Brain barrier damage by modulating autophagy-apoptosis balance (12). Furthermore, acupuncture suppresses the ERK1/2-Cx43 signaling cascade, thereby alleviating astrocyte-mediated neurotoxicity. Promoting Blood–Brain Barrier Recovery (13). Recent research indicates that acupuncture may influence the permeability of BBB through multiple pathways, for example, bloodletting at the twelve well points of the hand improves BBB permeability by downregulating occludin and claudin-5 (14), while Acupuncture modulates the levels of metalloproteinase isoforms (MMP-2), transmembrane water channel proteins (AQP4, AQP9), and inflammatory cell infiltration in a rat model of middle cerebral artery occlusion (MCAO) (15).
Although a number of researches have explored the impact of acupuncture concerning BBB permeability, the findings derived from these experiments exhibit certain discrepancies, which present challenges for the clinical translation of acupuncture's impact on BBB permeability. First, inconsistencies in research findings: Significant variations exist among studies regarding acupuncture parameters (acupoint selection, stimulation frequency, treatment duration), animal models (ischemic stroke, traumatic brain injury, neurodegenerative diseases), and biomarkers, leading to inconclusive conclusions. Second, lack of systematic mechanism analysis: Current research predominantly focuses on single-target or pathway investigations, lacking comprehensive understanding of acupuncture's multi-target synergistic mechanisms. Third, insufficient clinical translation evidence: A substantial gap persists between preclinical studies and clinical practice, with unclear optimal parameters and indications for acupuncture's protective effects on Blood–Brain barrier (BBB) permeability. To date, no systematic meta-analysis has been conducted to improve BBB permeability through acupuncture, leaving the evidence base inadequate for evidence-based medical practice (16). Thus, this study strives to integrate existing preclinical experiments and evaluate acupuncture's effects on BBB permeability through meta-analysis, while also delving into the potential mechanisms, with the goal of offering a theoretical basis and scientific support for acupuncture's application in the therapy of neurological diseases.
2 Methods
This study follows the PRISMA guidelines (17) for Animal Research Systematic Reviews and Meta-Analyses, and was prospectively registered on the INPLASY platform on February 22, 2025 (Registration ID: INPLASY202520102) and can be accessed via the link https://doi.org/10.37766/inplasy2025.2.0102.
2.1 Database search strategy
The following electronic reference databases were searched: PubMed, Embase, Cochrane Library, and Web of Science, with the database updated until December 2024. The main search terms are as follows: [(Acupuncture) OR (Acupuncture Therapy) OR (Acupuncture, Ear) OR (Acupuncture Points)] AND (Blood–Brain Barrier), as shown in Table 1 (For other database retrieval strategies, please refer to Supplementary Tables 1–3).
Table 1
| #1 | (((Acupuncture[MeSH Terms]) OR (Acupuncture Therapy[MeSH Terms])) OR (Acupuncture, Ear[MeSH Terms])) OR (Acupuncture Points[MeSH Terms]) |
|---|---|
| #2 | ((((((((((((((((((((((((((((((Acupuncture) OR (Acupuncture Therapy)) OR (Pharmacopuncture)) OR (Acupuncture Treatment)) OR (Acupuncture Treatments)) OR (Treatment, Acupuncture)) OR (Therapy, Acupuncture)) OR (Pharmacoacupuncture Treatment)) OR (Treatment, Pharmacoacupuncture)) OR (Pharmacoacupuncture Therapy)) OR (Therapy, Pharmacoacupuncture)) OR (Acupotomy)) OR (Acupotomies)) OR (Acupuncture, Ear)) OR (Acupunctures, Ear)) OR (Ear Acupunctures)) OR (Acupuncture, Auricular)) OR (Acupunctures, Auricular)) OR (Auricular Acupunctures)) OR (Auricular Acupuncture)) OR (Ear Acupuncture)) OR (Acupuncture Points)) OR (Acupuncture Point)) OR (Point, Acupuncture)) OR (Points, Acupuncture)) OR (Acupoints)) OR (Acupoint)) OR (Acupuncture Analgesia)) OR (Analgesia, Acupuncture)) OR (Acupuncture Anesthesia)) OR (Anesthesia, Acupuncture) |
| #3 | (#1) OR (#2) |
| #4 | Blood–Brain Barrier[MeSH Terms] |
| #5 | ((((((((((((((Blood–Brain Barrier) OR (Barrier, Blood–Brain)) OR (Barriers, Blood–Brain)) OR (Blood Brain Barrier)) OR (Blood–Brain Barriers)) OR (Hemato-Encephalic Barrier)) OR (Barrier, Hemato-Encephalic)) OR (Barriers, Hemato-Encephalic)) OR (Hemato Encephalic Barrier)) OR (Hemato-Encephalic Barriers)) OR (Brain-Blood Barrier)) OR (Barrier, Brain-Blood)) OR (Barriers, Brain-Blood)) OR (Brain Blood Barrier)) OR (Brain-Blood Barriers) |
| #6 | (#4) OR (#5) |
| #7 | (#3) AND (#6) |
Search strategy on PubMed.
2.2 Inclusion criteria
(1) Controlled experiment; (2) Rodent models (mice and rats) were considered (18), with no restrictions on sex, age, or weight; (3) The experimental group received acupuncture intervention and/or acupuncture pre-intervention, while the control group received carrier solutions, isotonic saline, sham acupuncture, clinically proven effective drugs, or no intervention;(4) Studies must include outcome measures related to Blood–Brain barrier permeability, such as indicators of BBB integrity, tight junction proteins, matrix metalloproteinases, glial activation markers, inflammatory mediators, or water channel proteins.
2.3 Exclusion criteria
(1) Uncontrolled studies; (2)Studies that did not use rodent models (mice and rats); (3) Reviews, meta-analyses, conference abstracts, editorials, and letters; (4) Research not involving acupuncture intervention or acupuncture pre-intervention; (5) Studies for which data could not be obtained; (6) Studies that did not include any outcome measures related to Blood–Brain barrier permeability were excluded.
2.4 Study screening
EndNote was used for literature screening and exclusion. Initially, 2 investigators independently conducted a preliminary screening of the titles to exclude duplicate studies, non-controlled trials, reviews, conference papers, protocols, editorials, and communications. Next, the two researchers performed an abstract review, assessing the abstracts to determine eligible studies for inclusion and reject those that do not satisfy the inclusion criteria. Finally, the 2 investigators conducted a full-text review, reading the studies left in their entirety and further determining their inclusion. During this phase, 2 investigators autonomously assessed the publications, the leftover studies were compared. If consensus was reached, the study was included. In case of disagreement, a third researcher was consulted to discuss and resolve the discrepancy.
2.5 Data extraction
Two investigators conducted data extraction independently. A predefined data collection template was employed to document the data included in the study, which consisted of the researcher's name, year of release, nation of experimentation, experimental animal strain, gender, weight, age, disease model type, acupuncture points, acupuncture parameters, duration of each intervention, total number of interventions, and primary outcomes.
2.6 Quality assessment
The Laboratory Animal Experimentation (SYRCLE) tool was used by two investigators to independently assess the risk of bias in the included studies. This instrument includes ten domains: (1) Randomized sequence generation, (2) Similarity in baseline characteristics, (3) Concealing group assignment, (4) Randomization of animal housing, (5) Blinding of researchers, (6) Result evaluation with randomization, (7) Blinded result assessment, (8) Missing data reporting, (9) Result reporting bias, and (10) Other biases. Risk of bias in the assessment was classified as “Low,” “High,” or “Unclear”.
2.7 Data analysis
Data synthesis for the meta-analysis, including generating forest plots, was conducted using Review Manager (RevMan, version 5.4). Additional statistical assessments, such as sensitivity analysis and funnel plot creation, were performed using STATA (version 15.1). The standardized mean difference (SMD) and 95% confidence ranges (95% CI) were used to describe continuous variables, and when measurement units differed across studies, the standardized mean difference (SMD) was employed for standardization. Heterogeneity between Studies are evaluated with I2 and Cochran's Q test. Sensitivity analysis is performed using the single-study exclusion approach to test the robustness of the analysis. Publication bias analysis was conducted if the study count reached 10 or more. Funnel plot examination, egger's regression analysis, begg's correlation test and sensitivity analysis were used to assess potential publication bias in the systematic review.
3 Results
3.1 Study eligibility screening process
Five hundred and eighty five articles were collected from electronic databases. Once duplicates were eliminated, we screened the titles and abstracts of the 348 remaining articles, which led to the exclusion of 309 articles. We then read the full texts of the remaining 39 articles, and 7 articles were excluded (reasons for exclusion: data could not be extracted or the number of studies with the same outcome measures was less than five). Finally, 32 articles were included incorporated into this study (11, 12, 14, 15, 19–46) (Figure 1).
Figure 1

PRISMA flow diagram.
3.2 Study characteristics and outcome determination
3.2.1 Study characteristics
This study included 32 controlled trials, encompassing 424 rodent models rats/mice (212 animals in the intervention group and 212 in the comparison group). The acupuncture interventions contained electroacupuncture (15, 19–29, 32, 33, 35–37, 39, 41–43, 45, 46) (accounting for 72% of the studies), manual acupuncture (11, 12, 34, 38, 40), bloodletting acupuncture (14, 30, 31), and moxibustion (44) (together comprising 28%). In the electroacupuncture studies, 9 studies (15, 20, 23, 24, 26, 37, 39, 41, 45) used a continuous wave mode, and 14 studies (19, 21, 22, 25, 27–29, 32, 33, 35, 36, 42, 43, 46) used a sparse-dense wave mode. Three studies (22, 29, 32) applied an additional 6-s stimulation/6-s interval cycle on the sparse-dense wave. Manual acupuncture primarily used low-frequency stimulation (60–210 rotations per minute) (12, 34, 38, 40), mostly in an intermittent stimulation mode. Bloodletting at the twelve well-points was controlled at a dose of 15–20 μL per acupoint (14, 30). Only one study included moxibustion (44), which used mild moxibustion for continuous stimulation. The study characteristic table (Table 2; Supplementary Table 4) indicates that the selection of acupoints, stimulation parameters, intervention duration, and treatment frequency are also factors influencing the final effect size. Figure 2 further illustrates the frequency distribution of acupoint usage.
Table 2
| Study | Animal model | Acupoints | Acupuncture protocol | Outcome |
|---|---|---|---|---|
| Deng et al. (19) | SD, VD | GV20, GV14, BL23 | EA 6/w, 4w | EB; IL-1β |
| Dong et al. (20) | SD, MCAO | GV20 | EA 1/d, 5d | EB; MMP-9 |
| Fan et al. (21) | SD, BI | GV20, GV26 | EA 1/d, 1d | EB; MMP-9 mRNA; IL-1β, TNF-α & IL-6mRNA |
| Gong et al. (22) | SD, Aging rats | GV26, ST2 | EA 1/d, 1d | EB; Occludin |
| He et al. (23) | SD, MCAO | GV20, ST36 | EA 6/w, 8w | ZO-1; Iba-1; IL-1β, TNF-α, IL-6 |
| Jung et al. (24) | C57 BL/6J, MCAO | GV20, GV14 | EA 1/d, 3d | EB; occludin, claudin-5, ZO-1; GFAP |
| Lang et al. (25) | SD, SAH | GV20, GV14 | EA 1/d, 3d | EB; occludin, claudin-5; MMP-9; Iba-1;IL-1β, TNF-α, IL-6 |
| Li et al. (26) | SD, ICH | GV20, GB7 | EA 1/d, 8d | EB |
| Lin et al. (27) | SD, MCAO | GV20, GV24 | EA 1/d, 7d | MMP-9 |
| Lin et al. (28) | SD, MCAO | GV20, GV26 | EA 1/d, 15d | MMP-9 |
| Lin et al. (29) | SD, MCAO | GV20, GV26 | EA 1/d, 1d | EB; Occludin, ZO-1 |
| Liu et al. (30) | SD, TBI | Twelve Jing-Well Points | BL 2/d, 2d | EB; MMP9; AQP4 |
| Lu et al. (31) | Wistar, MCAO | Twelve Jing-Well Points | BL 2/d, 3d | EB |
| Ma et al. (32) | SD, PT | GV20, GV26 | EA 1/d, 1d | EB; Occludin |
| Peng et al. (33) | SD, MCAO | GV20, GV26 | EA 1/d, 1d | AQP4 |
| Wang et al. (34) | SAMP8,AD | CV17, CV12, CV6, ST36, SP10 | MA 6/w, 4w | EB; Occludin, claudin-5, ZO-1 |
| Wang et al. (35) | SAMP8,AD | GV29, LI20 | EA 5/w, 4w | claudin-5, ZO-1; Iba-1; IL-1β, TNF-α, IL-6 |
| Wu et al. (36) | SD, MCAO | GV20, GV26 | EA 1/d, 1d | EB |
| Xin et al. (37) | SD, SAE | GV20, ST36 | EA 1/d, 4d | Occludin, ZO-1; Iba-1, GFAP; IL-6, IL-1β, TNF-α |
| Xu et al. (15) | SD, CIRI | GV20, ST36 | EA 1/d, 2d | AQP4 |
| Yao et al. (38) | SD, MCAO | Anterior Tem-poral Line | MA 1/d, 6d | EB; Occludin & ZO-1 mRNA; IL-1β |
| Yu et al. (39) | KM, Normal | GV20, GV15 | EA 1/d, 1d | EB |
| Yu et al. (14) | Wistar, pMCAO | Twelve Jing-Well Points | BL 1/d, 4d | EB; Occludin & claudin-5 mRNA |
| Zhang et al. (40) | SD, ICH | GV20, GB7 | MA 1/d, 7d | EB; Occludin |
| Zhang et al. (41) | SD, MCAO | GV20, GV26 | EA 1/d, 1d | EB |
| Zhang et al. (42) | SD, Nomal | GV20, GV26 | EA 1/d, 1d | EB; Occludin, claudin-5, ZO-1; Iba1, GFAP; AQP4 |
| Zhang et al. (43) | SD, MCAO | GV26, PC6 | EA 1/d, 1d | EB; claudin-5, ZO-1; MMP-9 |
| Zhang et al. (11) | APP/PS1, AD | GV20, GV29, ST36 | MA 1/d, 38d | EB; Occludin, ZO-1 |
| Zhang et al. (12) | SD, MCAO | GV20, PC6 | MA 1/d, 1d | EB; Occludin, ZO-1 |
| Zhou et al. (44) | SD, AD | GV20, BL23, GV29 | Mox 1/d, 21d | EB; MMP-9 |
| Zhu et al. (45) | APP/PS1, POCD | GV20, GV14, ST36, LI11 | EA 1/d, 9d | Occludin;Iba-1, GFAP; IL-1β, TNF-α, IL-6 |
| Zou et al. (46) | SD, MCAO | GV20 | EA 1/d, 5d | Occludin, claudin-5 |
Summary of study characteristics.
VD, Vascular Dementia; MCAO, Middle Cerebral Artery Occlusion Model; BI, Brain X-ray Radiation; SAH, Subarachnoid Hemorrhage; ICH, Spontaneous Intracerebral Hemorrhage; TBI, Traumatic Brain Injury; PT, C6 Glioma Model; AD, Alzheimer's Disease; SAE, Sepsis-Associated Encephalopathy; CIRI, Cerebral Ischemia/Reperfusion Injury; pMCAO, Permanent Middle Cerebral Artery Occlusion; POCD, Postoperative Cognitive Dysfunction; EA, Electroacupuncture; MA, Manual acupuncture; Mox, moxibustion; KM, Kun ming mice; BL, Bloodletting; NR, No mention was made of this in the study.
Figure 2

Statistical analysis of acupuncture point selection.
3.2.2 Selection of outcome target molecules
We identified the outcome target molecules through a frequency analysis, defining inclusion as the molecule being reported in a minimum of four studies (Figure 3).
Figure 3

Frequency-based selection of outcome target molecules (threshold: ≥4 studies).
3.3 Quality evaluation
Thirty two articles were included in the quality assessment, as shown in Figure 4. Seventy eight percent of the articles (12, 14, 15, 19, 20, 22, 24–28, 30, 31, 33–35, 37, 38, 40–46) had a randomization statement, but only 19% explicitly described the randomization method (24, 28, 30, 35, 38, 44). Thirteen percent of the included studies (22, 25, 30, 45) had a high risk of selective reporting. No studies explicitly described allocation concealment, randomization of animal placement, random outcome assessment, blinded outcome evaluation, or blinding of the experimenters.
Figure 4
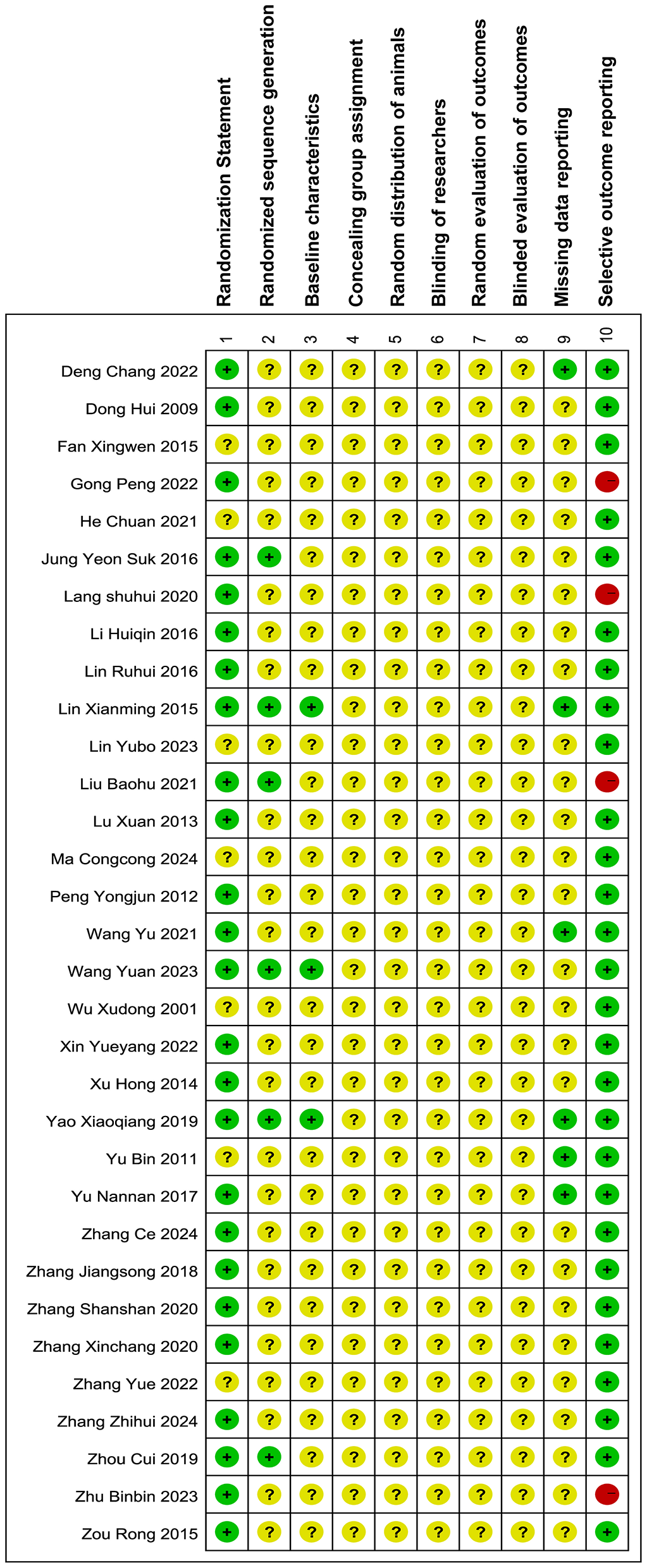
Bias risk of the included researches. The assessment of the 32 papers was carried out with RevMan 5.4.1 along with SYRCLE's quality assessment tool. An additional point regarding the description of randomization methods (Item 1) was included, while the “Additional sources of bias” section was excluded from this review. Notations utilized:  : low risk;
: low risk;  :unclear risk;
:unclear risk;  : high risk.
: high risk.
3.4 Meta-analysis results
3.4.1 BBB permeability and tight junction proteins
3.4.1.1 BBB permeability
A total of 23 studies (11, 12, 14, 19–22, 24–26, 29–32, 34, 36, 38–44) were comprised in the analysis. and the meta-analysis (Figure 5) demonstrated that, in comparison with the control group, acupuncture significantly reduced Evans Blue (EB) extravasation in brain tissue (SMD = −0.65, 95% CI: −0.94 to −0.37, P < 0.01), indicating that acupuncture can inhibit EB leakage into the brain tissue across the BBB and reduce BBB permeability. Funnel plot analysis (Supplementary Figure 1A) was used to visually assess potential publication bias, showing a roughly symmetric distribution of scatter points, suggesting no significant asymmetry. Further quantitative verification was conducted using the Egger linear regression test, and the findings indicated that the test statistic P = 0.053 > 0.05, displaying no statistically significant publication bias. To assess the reliability of the findings, sensitivity analysis was conducted by sequentially removing individual studies. The results (Supplementary Figure 1B) showed minimal fluctuation in the combined effect size (SMD = −2.34 to −0.51). This indicates that the conclusions of the current meta-analysis have low dependence on individual studies, demonstrating high robustness, with conclusions not significantly affected by outliers or bias from individual studies.
Figure 5

Forest plot of EB extravasation.
3.4.1.2 Tight junction proteins
-
(1) Occludin.
A total of 15 studies (11, 12, 14, 22, 24, 25, 29, 32, 34, 37, 38, 40, 42, 45, 46) were comprised in the examination, and the meta-analysis (Figure 6) demonstrated that, relative to the comparison group, the expression of occludin protein and mRNA in brain tissue was significantly upregulated by acupuncture (SMD = 1.73, 95% CI: 0.14–3.32, P < 0.01).The findings point out that acupuncture could enhance occludin expression in the cerebral tissue, potentially strengthening BBB integrity. Funnel plot analysis (Supplementary Figure 2A) showed a roughly symmetric distribution of scatter points, and Egger linear regression test results (P = 0.059 > 0.05) confirmed no major publication bias. Sensitivity evaluation revealed that the combined effect size range remained stable (SMD = 0.30–3.70, Supplementary Figure 3A), suggesting robust results, with conclusions not significantly dependent on individual studies.
Figure 6

Forest plot of tight junction protein expression. (A) Occludin. (B) Claudin-5. (C) ZO-1.
-
(2) Claudin-5.
Eight studies (14, 24, 25, 34, 35, 42, 43, 46) were included, and the meta-analysis (Figure 6) demonstrated that, in comparison to the reference group, acupuncture substantially promoted the production of claudin-5 protein and mRNA in brain tissue (SMD = 4.44, 95% CI: 2.26–6.62, P < 0.01). This result suggests acupuncture may enhance claudin-5 levels in brain tissue. Sensitivity analysis, conducted by sequentially removing individual studies (Supplementary Figure 3B), showed that the range of the combined effect size remained stable (SMD = 2.54–7.07), indicating robust results with conclusions not significantly dependent on individual studies.
-
(3) ZO-1.
The assessment included eleven studies (11, 12, 23, 24, 29, 34, 35, 37, 38, 42, 43), and the meta-analysis (Figure 6) implied that, as opposed to the comparison group, acupuncture significantly increased the level of ZO-1 protein and mRNA in brain tissue (SMD = 2.62, 95% CI: 0.75–4.5, P < 0.01). This indicates that acupuncture could elevate ZO-1 expression. The Egger linear regression analysis revealed a notable publication bias (P = 0.01 < 0.05). To correct for potential bias, the data were adjusted using the trim-and-fill method. After supplementing two imputed missing studies, the updated funnel plot (Supplementary Figure 2B) showed that the effect size points of each study tended to be symmetrically distributed on both sides, indicating partial correction of publication bias. Sensitivity analysis (Supplementary Figure 3C) indicated that the combined effect size range remained stable (SMD = 1.01–5.04), demonstrating robust results, with conclusions not significantly dependent on individual studies.
3.4.2 Matrix metalloproteinase
The analysis comprised eight studies (20, 21, 25, 27, 28, 30, 43, 44), and the meta-analysis (Figure 7) revealed that, in contrast to the comparison group, acupuncture markedly decreased the MMP-9 protein and mRNA levels in brain tissue (SMD = −3.29, 95% CI: −5.18 to −1.40, P < 0.001). This points to the possibility that acupuncture could reduce the expression of MMP-9. Sensitivity analysis applying the leave-one-out approach (Supplementary Figure 4) revealed that the combined effect size range remained stable (SMD = −5.70 to −1.63), indicating the robustness of the results and that the conclusions are not significantly dependent on individual studies.
Figure 7

Forest plot of MMP-9 expression.
3.4.3 Glial activation markers and inflammatory mediators
3.4.3.1 Glial activation markers
-
(1) Iba-1.
Six studies (23, 25, 35, 37, 42, 45) incorporated into the evaluation. The evaluation (Figure 8) revealed that, relative to the comparison group, acupuncture significantly inhibited the production of Iba-1 in brain tissue (SMD = −3.06, 95% CI: −4.90 to −1.22, P < 0.001). This indicates that acupuncture has the potential to effectively suppress the activation of microglial cells. Sensitivity analysis with the leave-one-out procedure (Supplementary Figure 5A) revealed that the combined effect size range remained stable (SMD = −5.48 to −1.45), indicating the robustness of the results and that the conclusions are not significantly dependent on individual studies.
Figure 8

Forest plot of the expression of glial activation markers. (A) Iba-1. (B) GFAP.
-
(2) GFAP.
Four studies (24, 37, 42, 45) were incorporated into the examination, and the meta-analysis (Figure 8) demonstrated that, relative to the reference group, acupuncture significantly inhibited the production of GFAP in brain tissue (SMD = −3.22, 95% CI: −6.38 to −0.07, P < 0.001). This indicates that acupuncture can effectively suppress the activation of astrocytes. Sensitivity analysis utilizing the leave-one-out strategy (Supplementary Figure 5B) revealed that the combined effect size range remained stable (SMD = −7.27 to −0.21), indicating the robustness of the results and that the conclusions are not significantly dependent on individual studies.
3.4.3.2 Inflammatory mediators
-
(1) IL-1β.
The assessment comprised eight studies (19, 21, 23, 25, 35, 37, 38, 45), and the meta-analysis (Figure 9) revealed that, as opposed to the comparison group, acupuncture significantly inhibited the IL-1β protein and mRNA amounts in brain tissue (SMD = −3.35, 95% CI: −4.84 to −1.86, P < 0.001). This points to the possibility that acupuncture could suppress the production of IL-1β in brain tissue. Sensitivity analysis based on the leave-one-out method technique (Supplementary Figure 6A) demonstrated that the combined effect size range remained stable (SMD = −5.52 to −2.24), indicating the robustness of the results and that the conclusions are not significantly dependent on individual studies.
Figure 9

Forest plot of the expression of inflammatory mediators. (A) IL-1β. (B) TNF-α. (C) IL-6.
-
(2) TNF-α.
The assessment comprised six studies (21, 23, 25, 35, 37, 45), and the meta-analysis (Figure 9) confirmed that, as opposed to the comparison group, acupuncture significantly inhibited the amounts of TNF-α protein and mRNA in brain tissue (SMD = −4.41, 95% CI: −7.07 to −1.74, P < 0.001). This Indicates that acupuncture could suppress the release of TNF-α in brain tissue. Sensitivity analysis employing the leave-one-out technique (Supplementary Figure 6B) showed that the combined effect size range remained stable (SMD = −7.77 to −2.04), indicating the robustness of the results and that the conclusions are not significantly dependent on individual studies.
-
(3) IL-6.
Six studies (21, 23, 25, 35, 37, 45) incorporated into the final evaluation, and the meta-analysis (Figure 9) showed that, as opposed to the reference group, the acupuncture intervention led to a significant reduction in the protein and mRNA levels of IL-6 in brain tissue. (SMD = −3.29, 95% CI: −5.19 to −1.40, P < 0.001). This implies that acupuncture may inhibit the production of IL-6 within brain tissue. Sensitivity analysis applying the leave-one-out approach (Supplementary Figure 6C) showed that the combined effect size range remained stable (SMD = −5.85 to −1.71), indicating that the results are robust and that the conclusions are not significantly dependent on individual studies.
3.4.4 Water channel protein
Four studies (15, 30, 33, 42) were contained in the assessment, and the meta-analysis (Figure 10) showed that, in contrast to the comparison group, the acupuncture group notably diminished the AQP4 protein and mRNA levels in brain tissue (SMD = −4.36, 95% CI: −7.78 to −0.93, P < 0.001). This suggests that acupuncture may inhibit the level of AQP4 in brain tissue. Sensitivity analysis with the leave-one-out procedure (Supplementary Figure 7) showed that the combined effect size range was unstable (SMD = −9.23 to −1.58).
Figure 10
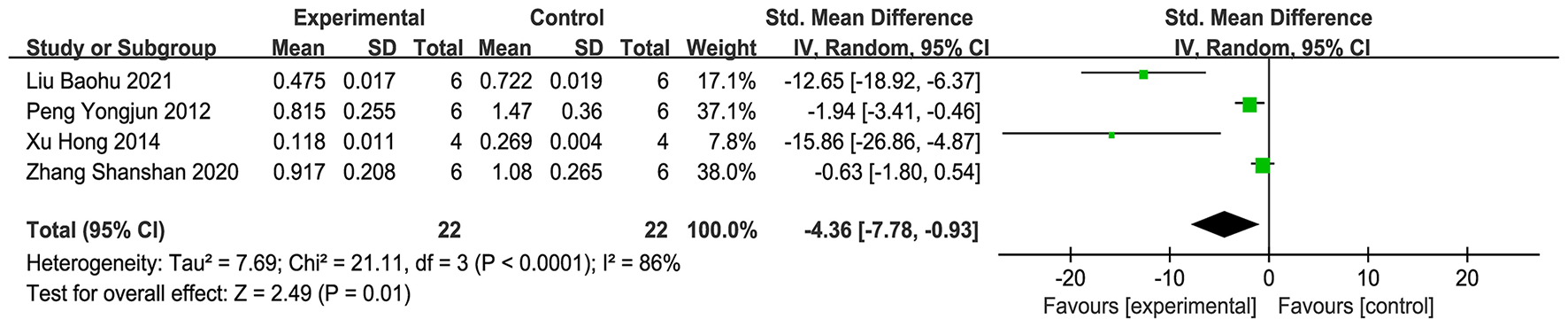
Forest plot of AQP4 expression.
4 Discussion
This study, through the preclinical Meta-analysis, integrated preclinical data on acupuncture's influence regarding BBB permeability. The findings indicate that acupuncture treatment notably decreased EB leakage in brain tissue, upregulated the production of tight junction proteins (occludin, claudin-5, ZO-1), and simultaneously inhibited the expression of matrix metalloproteinase-9 (MMP-9), glial cell activation markers (Iba-1, GFAP), inflammatory mediators (IL-1β, IL-6, TNF-α), and aquaporin-4 (AQP4). These findings indicate that acupuncture might improve BBB integrity with a multi-target synergistic effect.
4.1 Acupuncture's regulation of BBB structure and tight junction proteins
Our research results show that acupuncture intervention can decrease EB extravasation in brain tissue and upregulate the synthesis of tight junction proteins (occludin, claudin-5, ZO-1). EB is a commonly used marker to evaluate BBB permeability. The reduction in EB levels in the acupuncture intervention group directly indicates that acupuncture can reduce the dye's entry into the brain tissue, reflecting that acupuncture may decrease BBB permeability, thereby preventing large molecules from entering the brain from the bloodstream. The protective role of the BBB depends on the integrity of tight junctions (TJs) between endothelial cells, with occludin, claudin-5, and ZO-1 being the core components of TJs (8). Therefore, occludin and claudin-5 may enhance the mechanical strength of endothelial cell junctions upon upregulation, while ZO-1, as a key anchoring molecule that connects tight junction (TJ) proteins to the actin cytoskeleton, may further stabilize the spatial conformation of the TJs complex (47). The lack of ZO-1 results in the disruption of the BBB in many neurodegenerative and acute CNS diseases (8). The regulation of TJs by acupuncture suggests that it may influence endothelial cell function through pathways such as RhoA/ROCK II/MLC 2 (40), Wnt/β-catenin (48, 49) and HIF-1α (50, 51).
4.2 MMP-9 inhibition and extracellular matrix protection
Our research results show that acupuncture intervention can inhibit the amount of matrix MMP-9 expression. MMP-9 is primarily secreted by glial types like astrocytes, microglia and endothelial cells within the central nervous system (52). It can disrupt the BBB structure and exacerbate BBB leakage by degrading the extracellular matrix (ECM) and TJ proteins (52, 53). The reduction in MMP-9 expression by acupuncture may be achieved through two main mechanisms as follows. Firstly inhibition of NF-κB or MAPK signaling pathways, which reduces the transcriptional activation of MMP-9 (30, 43, 54–56). Secondly upregulation of tissue inhibitors of metalloproteinases (TIMPs), restoring the MMP-9/TIMP-1 balance (57, 58). For example, Acupuncture bloodletting at the twelve Jing-well acupoints on the hands, reduces BBB permeability, and downregulates MMP9 expression in rats suffering from severe traumatic brain injury by inhibiting the MAPK signaling pathway (30). The suppression of MMP-9 not only protects the structure of TJs but may also reduce inflammatory cell infiltration, forming a positive feedback loop (25, 59).
4.3 Synergistic inhibition of glial cell activation and inflammatory response
Our research findings demonstrate that acupuncture intervention can inhibit the amount of astrocyte activation markers (GFAP), microglia activation markers (Iba-1), pro-inflammatory factors (IL-1β, IL-6, TNF-α), and aquaporin-4 (AQP4). The excessive activation of microglia (Iba-1+) and astrocytes (GFAP+) is a key driver of neuroinflammation and Blood–Brain barrier (BBB) disruption (60, 61). Acupuncture significantly reduces Iba-1 and GFAP protein levels, demonstrating a suppressive effect on neuroinflammatory glial responses. The suppression of glial activation may reduce BBB damage through two main mechanisms as follows. First, decreasing the secretion of pro-inflammatory substances (TNF-α, IL-6, IL-1β), blocking their destructive effects on endothelial tight junctions (25, 35, 45, 62). Second, downregulating the production of harmful agents like reactive oxygen species (ROS) and nitric oxide (NO), alleviating oxidative stress (21, 24, 35, 63). Meanwhile, inhibiting the generation of inflammatory molecules like TNF-α and IL-1β can alleviate glial cell activation (37), forming a negative feedback loop, thereby reducing NF-κB pathway activation and ultimately improving Blood–Brain barrier permeability (23, 64). Moreover, the reduction in AQP4 expression may be associated with the alleviation of astrocytic end-foot swelling, which reduces the mechanical pressure of vasogenic edema on the BBB, further maintaining barrier stability (65).
4.4 Potential association between AQP4 downregulation and cerebral edema
Our research results show that acupuncture intervention can reduce the protein and mRNA levels of AQP4. AQP4, as the principal water transport protein in astrocytic end-feet, is closely associated with increased BBB permeability and brain edema when its expression is abnormal (66, 67). Acupuncture reduces AQP4 protein and mRNA levels, potentially through two main mechanisms as follows. Firstly, inhibiting the transcriptional regulation of AQP4 by inflammatory signals (30) (e.g., MAPK). Secondly, reducing the disruption of AQP4 polarity distribution caused by glial cell activation (68). The downregulation of AQP4 may limit the transmembrane movement of water molecules, thereby alleviating vasogenic or cytotoxic edema, and working synergistically with tight junction repair to maintain BBB homeostasis (24).
The above findings indicate that acupuncture's protective role in the BBB may involve a multi-target regulatory mechanism. It achieves this by coordinating the interactions between endothelial cells, glial cells (69), and immune signaling molecules, thereby promoting the functional coordination and homeostatic reconstruction of various cellular components within the neurovascular unit (NVU). For example, inhibiting MMP-9 reduces the degradation of tight junctions (TJs), while decreased levels in IL-6 and TNF-α may inhibit glial cell activation via the NF-KB pathway. Additionally, the downregulation of AQP4 further blocks the vicious cycle of edema and inflammation (70).
5 Limitations and future perspectives
The limitations of this study are as follows: (1) The impact of acupuncture on the BBB in both physiological and pathological conditions, as well as its effects during the acute and recovery phases of disease, were not explored; (2) The study included four types of acupuncture—electroacupuncture, manual acupuncture, bloodletting, and moxi-bustion—but did not investigate whether these different acupuncture methods vary in their effects on the BBB or their effect sizes; (3) The influence of acupuncture parameters, such as acupoint, frequency, and intervention duration, on effect size was not further analyzed; (4) The small number of included studies and limited sample size might result in an overestimation of effect size; (5) The execution of randomization, allocation concealment, and blinding in most studies was unclear, which may also result in an overestimation of effect size; (6) Some outcome measures were assessed using different methods, which may introduce heterogeneity in measurement techniques, such as using different antibodies to detect Occludin; (7) The pathological process in animal models (e.g., MCAO stroke model) may differ from the natural progression of human disease, making it difficult to fully extrapolate the preclinical evidence on acupuncture's effect on BBB permeability to clinical settings. Additional clinical evidence is required to assess if acupuncture is a viable option in clinical practice.
In future research, we anticipate further exploration in the following areas: (1) Investigating the impact of acupuncture upon the BBB in various physiological and pathological states, particularly during the acute and recovery phases of disease. Comparative studies of disease models at these different stages will help elucidate the healing effects and pathways of acupuncture throughout various stages of disease progression; (2) Examining the differences in the effects of various acupuncture interventions—such as electroacupuncture, manual acupuncture, bloodletting, and moxibustion—on BBB permeability, as well as their respective indications. This will provide clearer guidance for clinical treatment; (3) Deepening the study of acupuncture parameter optimization, with a focus on key parameters such as acupoint combinations (single point or multiple points), stimulation frequency, and intervention timing (prevention, treatment, or rehabilitation), to understand their effects on BBB regulation and provide data for clinical translation.
6 Conclusion
In summary, acupuncture may improve BBB integrity by means of multiple mechanisms, including the enhancement of tight junction protein production in endothelial cells, inhibiting MMP-9 mediated extracellular matrix (ECM) degradation, modulating glial cell activation and inflammatory responses, and downregulating AQP4-dependent edema. These results establish a data foundation for acupuncture in the management of neurological illnesses like stroke and Alzheimer's disease, emphasizing the importance of an integrated treatment strategy targeting the neurovascular unit (NVU).
Statements
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The data in this study were extracted from 32 included studies, and the specific sources are listed in the references section of the main text.
Author contributions
KZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. YL: Data curation, Formal analysis, Investigation, Writing – original draft. YW: Data curation, Formal analysis, Investigation, Writing – original draft. TY: Data curation, Formal analysis, Software, Validation, Writing – review & editing. KM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 82104979), the Youth Fund Project of the National Natural Science Foundation (8200150802),the Guangdong Provincial Natural Science Foundation Project (2021A1515110847), and the Key Enterprise-School Joint Project of the Guangzhou Municipal Science and Technology Bureau (2024A03J0796). The funders had no role in the design of the study, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
All individuals who contributed to this work are included as authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1648117/full#supplementary-material
References
1.
Nasrollahi A Yao Y . Laminins and the Blood–Brain barrier. Matrix Biol. (2025) 137:33–41. doi: 10.1016/j.matbio.2025.02.005
2.
Zheng X Ren B Gao Y . Tight junction proteins related to Blood–Brain barrier and their regulatory signaling pathways in ischemic stroke. Biomed Pharmacother. (2023) 165:115272. doi: 10.1016/j.biopha.2023.115272
3.
Berselli A Alberini G Cerioni L Benfenati F Maragliano L A . multi-pore model of the blood–brain barrier tight junction strands recapitulates the permeability features of wild-type and mutant claudin-5. Protein Sci. (2025) 34:e70271. doi: 10.1002/pro.70271
4.
Hashimoto Y Campbell M . Tight junction modulation at the Blood–Brain barrier: Current and future perspectives. Biochimica et Biophysica Acta (BBA) - Biomembranes. (2020) 1862:183298. doi: 10.1016/j.bbamem.2020.183298
5.
Wei X Zhou Y Song J Zhao J Huang T Zhang M et al . Hyperglycemia aggravates blood–brain barrier disruption following diffuse axonal injury by increasing the levels of inflammatory mediators through the PPARγ/Caveolin-1/TLR4 pathway. Inflammation. (2023) 46:129–45. doi: 10.1007/s10753-022-01716-y
6.
Barabási B Barna L Santa-Maria AR Harazin A Molnár R Kincses A et al . Role of interleukin-6 and interleukin-10 in morphological and functional changes of the blood–brain barrier in hypertriglyceridemia. Fluids Barriers CNS. (2023) 20:15. doi: 10.1186/s12987-023-00418-3
7.
Adiele RC Tham M Lucchinetti CF Popescu BFG A . systematic study of the distribution and expression of aquaporin water channels in normal adult human brain. J Neurol. (2025) 272:574. doi: 10.1007/s00415-025-13304-9
8.
Sweeney MD Zhao Z Montagne A Nelson AR Zlokovic BV . Blood–brain barrier: from physiology to disease and back. Physiol Rev. (2019) 99:21–78. doi: 10.1152/physrev.00050.2017
9.
Guo X Ma T . Effects of acupuncture on neurological disease in clinical- and animal-based research. Front Integr Neurosci. (2019) 13:47. doi: 10.3389/fnint.2019.00047
10.
Jang JH Park S An J Choi JD Seol IC Park G et al . Gait disturbance improvement and cerebral cortex rearrangement by acupuncture in Parkinson's disease: a pilot assessor-blinded, randomized, controlled, parallel-group trial. Neurorehabil Neural Repair. (2020) 34:1111–23. doi: 10.1177/1545968320969942
11.
Zhang Y Ding N Hao X Zhao J Zhao Y Li Y et al . Manual acupuncture benignly regulates Blood–Brain barrier disruption and reduces lipopolysaccharide loading and systemic inflammation, possibly by adjusting the gut microbiota. Front Aging Neurosci. (2022) 14:1018371. doi: 10.3389/fnagi.2022.1018371
12.
Zhang Z Lu T Li S Zhao R Li H Zhang X et al . Acupuncture extended the thrombolysis window by suppressing blood–brain barrier disruption and regulating autophagy-apoptosis balance after ischemic stroke. Brain sciences. (2024) 14:399. doi: 10.3390/brainsci14040399
13.
Zhang Z-H Gu Y Huang Z Liu X-Y Xu W-T Zhang X-C et al . Acupuncture regulates astrocyte neurotoxic polarization to protect blood–brain barrier integrity in delayed thrombolysis through mediating ERK1/2/Cx43 axis. IBRO Neurosci Reports. (2025) 18:604–18. doi: 10.1016/j.ibneur.2025.04.005
14.
Yu NN Wang ZG Chen YC Yang JT Lu X Guo Y et al . The ameliorative effect of bloodletting puncture at hand twelve Jing-well points on cerebral edema induced by permanent middle cerebral ischemia via protecting the tight junctions of the Blood–Brain barrier. BMC Complement Altern Med. (2017) 17:470. doi: 10.1186/s12906-017-1979-6
15.
Xu H Zhang YM Sun H Chen SH Wang FM . Effects of acupuncture at GV20 and ST36 on the expression of matrix metalloproteinase 2, aquaporin 4, and aquaporin 9 in rats subjected to cerebral ischemia/reperfusion injury. PLoS ONE. (2014) 9:e97488. doi: 10.1371/journal.pone.0097488
16.
Xu N Gong P Xu S Chen Y Dai M Jia Z et al . Electroacupuncture stimulation enhances the permeability of the Blood–Brain barrier: a systematic review and meta-analysis of preclinical evidence and possible mechanisms. PLoS ONE. (2024) 19:e0298533. doi: 10.1371/journal.pone.0298533
17.
Hooijmans CR Rovers MM de Vries RB Leenaars M Ritskes-Hoitinga M Langendam MW . SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
18.
El Amine B Fournier J Minoves M Baillieul S Roche F Perek N et al . Cerebral oxidative stress, inflammation and apoptosis induced by intermittent hypoxia: a systematic review and meta-analysis of rodent data. Eur Respir Rev. (2024) 33:240162. doi: 10.1183/16000617.0162-2024
19.
Deng C Zou YJ Zhang H Chen DF Qiu RR Xu YY et al . Effects of electroacupuncture on the Blood–Brain-barrier and proinflammatory cytokine IL-1β and IL-18 in the hippocampus of rats with vascular dementia. Zhen ci yan jiu = Acupuncture Res. (2022) 47:885–90.
20.
Dong H Fan YH Zhang W Wang Q Yang QZ Xiong LZ . Repeated electroacupuncture preconditioning attenuates matrix metalloproteinase-9 expression and activity after focal cerebral ischemia in rats. Neurol Res. (2009) 31:853–8. doi: 10.1179/174313209X393960
21.
Fan XW Chen F Chen Y Chen GH Liu HH Guan SK et al . Electroacupuncture prevents cognitive impairments by regulating the early changes after brain irradiation in rats. PLoS ONE. (2015) 10:e0122087. doi: 10.1371/journal.pone.0122087
22.
Gong P Zhang S Ren L Zhang J Zhao Y Mao X et al . Electroacupuncture of the trigeminal nerve causes N-methyl-D-aspartate receptors to mediate Blood–Brain barrier opening and induces neuronal excitatory changes. Front Cell Neurosci. (2022) 16:1020644. doi: 10.3389/fncel.2022.1020644
23.
He C Huang ZS Yu CC Wang XS Jiang T Wu M et al . Preventive electroacupuncture ameliorates D-galactose-induced Alzheimer's disease-like inflammation and memory deficits, probably via modulating the microbiota-gut-brain axis. Iran J Basic Med Sci. (2021) 24:341–8. doi: 10.22038/ijbms.2021.49147.11256
24.
Jung YS Lee SW Park JH Seo HB Choi BT Shin HK . Electroacupuncture preconditioning reduces ROS generation with NOX4 down-regulation and ameliorates Blood–Brain barrier disruption after ischemic stroke. J Biomed Sci. (2016) 23:32. doi: 10.1186/s12929-016-0249-0
25.
Lang SH Yan XG Wang CH Wu Y Liu X Ma CX et al . The poly-ADP ribose polymerase-1/apoptosis-inducing factor pathway may help mediate the protective effect of electroacupuncture on early brain injury after subarachnoid hemorrhage. Neuroreport. (2020) 31:605–12. doi: 10.1097/WNR.0000000000001445
26.
Li HQ Li Y Chen ZX Zhang XG Zheng XW Yang WT et al . Electroacupuncture exerts neuroprotection through caveolin-1 mediated molecular pathway in intracerebral hemorrhage of rats. Neural Plast. (2016) 2016:7308261. doi: 10.1155/2016/7308261
27.
Lin RH Yu KQ Li XJ Tao J Lin YK Zhao CK et al . Electroacupuncture ameliorates post-stroke learning and memory through minimizing ultrastructural brain damage and inhibiting the expression of MMP-2 and MMP-9 in cerebral ischemia-reperfusion injured rats. Mol Med Rep. (2016) 14:225–33. doi: 10.3892/mmr.2016.5227
28.
Lin XM Chen LP Yao X . The impact of different duration of EA-pretreatment on expression of MMP-9 and VEGF in blood–brain barrier in rats with cerebral ischemia-reperfusion injury. Zhen ci yan jiu = Acupuncture Res. (2015) 40:40–4. doi: 10.13702/j.1000-0607.2015.01.008
29.
Lin YB Gan L Ren L Ma CC Dai MY Qian KC et al . Acupuncture with specific mode electro-stimulation effectively and transiently opens the BBB through Shh signaling pathway. Neuroreport. (2023) 34:873–86. doi: 10.1097/WNR.0000000000001970
30.
Liu BH Zhou D Guo Y Zhang S Guo YM Guo TT et al . Bloodletting puncture at hand twelve Jing-well points relieves brain edema after severe traumatic brain injury in rats via inhibiting MAPK signaling pathway. Chin J Integr Med. (2021) 27:291–9. doi: 10.1007/s11655-021-3326-5
31.
Lu X Chen ZL Guo Y Gao L Jiang LY Li ZZ et al . Blood-letting punctures at twelve jing-well points of the hand can treat cerebral ischemia in a similar manner to mannitol. Neural Regen Res. (2013) 8:532–9. doi: 10.3969/j.issn.1673-5374.2013.06.009
32.
Ma C Ye Q Qian K Dai M Gan L Yang J et al . Anti-glioma effect of paclitaxel mediated by specific mode electroacupuncture stimulation and the related role of the Hedgehog pathway. Brain Res Bull. (2024) 213:110985. doi: 10.1016/j.brainresbull.2024.110985
33.
Peng Y Wang H Sun J Chen L Xu M Chu J . Electroacupuncture reduces injury to the Blood–Brain barrier following cerebral ischemia/reperfusion injury. Neural Regen Res. (2012) 7:2901–6. doi: 10.3969/j.issn.1673-5374.2012.36.004
34.
Wang Y Kan B Zhao L Shi H Jia Y . Improvement effect of Sanjiao acupuncture on permeability of Blood–Brain barrier in SAM mice and its mechanism. J Jilin Univ Med Ed. (2021) 47:1086–91. doi: 10.1007/s11655-023-3592-5
35.
Wang Y Wang Q Luo D Zhao P Zhong SS Dai B et al . Electroacupuncture improves blood–brain barrier and hippocampal neuroinflammation in SAMP8 mice by inhibiting HMGB1/TLR4 and RAGE/NADPH signaling pathways. Chin J Integr Med. (2023) 29:448–58. doi: 10.1007/s11655-023-3592-5
36.
Wu XD Du LN Wu GC Cao XD . Effects of electroacupuncture on blood–brain barrier after cerebral ischemia-reperfusion in rat. Acupunct Electrother Res. (2001) 26:1–9. doi: 10.3727/036012901816356063
37.
Xin Y Wang J Chu T Zhou Y Liu C Xu A . Electroacupuncture alleviates neuroinflammation by inhibiting the HMGB1 signaling pathway in rats with sepsis-associated encephalopathy. Brain Sci. (2022) 12:1732. doi: 10.3390/brainsci12121732
38.
Yao XQ Li XL Du XZ Wang JH Yuan B Zhang TZ et al . Effect of scalp acupuncture stimulation on expression of pentraxin 3 in striatum in acute ische-mic cerebrovascular disease rats. Zhen ci yan jiu = Acupuncture Res. (2019) 44:793–8. doi: 10.13702/j.1000-0607.180899
39.
Yu B Lü GH Sun Y Lin X Fang TH . Effect of electroacupuncture combined with intragastric administration of borneol on the permeability of Blood–Brain barrier in the mouse. Zhen ci yan jiu = Acupuncture Res. (2011) 36:335–40.
40.
Zhang C Zheng J Yu XP Kuang BL Dai XH Zheng L et al . “Baihui” (DU20)-penetrating “Qubin” (GB7) acupuncture on Blood–Brain barrier integrity in rat intracerebral hemorrhage models via the RhoA/ROCK II/MLC 2 signaling pathway. Animal Models Exp Med. (2024). doi: 10.1002/ame2.12374
41.
Zhang JS Lin XM Zhou H Chen YY Xiao SK Jiao JY et al . Electroacupuncture: a new approach to open the Blood–Brain barrier in rats recovering from middle cerebral artery occlusion. Acupuncture Med. (2018) 36:377–85. doi: 10.1136/acupmed-2017-011496
42.
Zhang SS Gong P Zhang JS Mao XQ Zhao YB Wang H et al . Specific frequency electroacupuncture stimulation transiently enhances the permeability of the blood–brain barrier and induces tight junction changes. Front Neurosci. (2020) 14:582324. doi: 10.3389/fnins.2020.582324
43.
Zhang XC Gu YH Xu WT Song YY Zhang A Zhang ZH et al . Early electroacupuncture extends the rtPA time window to 6 h in a male rat model of embolic stroke via the ERK1/2-MMP9 pathway. Neural Plasticity. (2020) 2020:8851089. doi: 10.1155/2020/8851089
44.
Zhou C Li MY Liu ZX Li KR Ye H Lei LF et al . Effects of moxibustion on the structure and function of blood brain barrier in Alzheimer's disease model rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. (2019) 35:443–6. doi: 10.12047/j.cjap.5819.2019.096
45.
Zhu BB Zhou YL Zhou WJ Chen CQ Wang JH Xu SJ et al . Electroacupuncture modulates gut microbiota in mice: a potential target in postoperative cognitive dysfunction. Anat Rec Adv Integr Anat Evol Biol. (2023) 306:3131–43. doi: 10.1002/ar.25065
46.
Zou R Wu ZQ Cui SY . Electroacupuncture pretreatment attenuates Blood–Brain barrier disruption following cerebral ischemia/reperfusion. Mol Med Rep. (2015) 12:2027–34. doi: 10.3892/mmr.2015.3672
47.
Abdullahi W Tripathi D Ronaldson PT . Blood–Brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am J Physiol Cell Physiol. (2018) 315:C343–C56. doi: 10.1152/ajpcell.00095.2018
48.
Porkolab G Meszaros M Szecsko A Vigh JP Walter FR Figueiredo R et al . Synergistic induction of Blood–Brain barrier properties. Proc Natl Acad Sci U S A. (2024) 121:e2316006121. doi: 10.1073/pnas.2316006121
49.
Jean LeBlanc N Menet R Picard K Parent G Tremblay ME ElAli A . Canonical Wnt pathway maintains blood–brain barrier integrity upon ischemic stroke and its activation ameliorates tissue plasminogen activator therapy. Mol Neurobiol. (2019) 56:6521–38. doi: 10.1007/s12035-019-1539-9
50.
Che P Zhang J Yu M Tang P Wang Y Lin A et al . Dl-3-n-butylphthalide promotes synaptic plasticity by activating the Akt/ERK signaling pathway and reduces the Blood–Brain barrier leakage by inhibiting the HIF-1alpha/MMP signaling pathway in vascular dementia model mice. CNS Neurosci Ther. (2023) 29:1392–404. doi: 10.1111/cns.14112
51.
Devraj G Guerit S Seele J Spitzer D Macas J Khel MI et al . HIF-1alpha is involved in Blood–Brain barrier dysfunction and paracellular migration of bacteria in pneumococcal meningitis. Acta Neuropathol. (2020) 140:183–208. doi: 10.1007/s00401-020-02174-2
52.
Cheng L Zhang Y Lv M Huang W Zhang K Guan Z et al . Impaired learning and memory in male mice induced by sodium arsenite was associated with MMP-2/MMP-9-mediated Blood–Brain barrier disruption and neuronal apoptosis. Ecotoxicol Environ Saf. (2024) 285:117016. doi: 10.1016/j.ecoenv.2024.117016
53.
Pittayapruek P Meephansan J Prapapan O Komine M Ohtsuki M . Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. (2016) 17:868. doi: 10.3390/ijms17060868
54.
Feng L Li Y Lin M Xie D Luo Y Zhang Y et al . Trilobatin attenuates cerebral ischaemia/reperfusion-induced blood–brain barrier dysfunction by targeting matrix metalloproteinase 9: the legend of a food additive. Br J Pharmacol. (2024) 181:1005–27. doi: 10.1111/bph.16239
55.
Guo P Liu L Yang X Li M Zhao Q Wu H . Irisin improves BBB dysfunction in SAP rats by inhibiting MMP-9 via the ERK/NF-kappaB signaling pathway. Cell Signal. (2022) 93:110300. doi: 10.1016/j.cellsig.2022.110300
56.
Zhu H Dai R Zhou Y Fu H Meng Q . TLR2 Ligand Pam3CSK4 regulates MMP-2/9 expression by MAPK/NF-kappaB signaling pathways in primary brain microvascular endothelial cells. Neurochem Res. (2018) 43:1897–904. doi: 10.1007/s11064-018-2607-7
57.
Sellner J Leib SL . In bacterial meningitis cortical brain damage is associated with changes in parenchymal MMP-9/TIMP-1 ratio and increased collagen type IV degradation. Neurobiol Dis. (2006) 21:647–56. doi: 10.1016/j.nbd.2005.09.007
58.
Wu J Zhao D Wu S Wang D . Ang-(1-7) exerts protective role in blood–brain barrier damage by the balance of TIMP-1/MMP-9. Eur J Pharmacol. (2015) 748:30–6. doi: 10.1016/j.ejphar.2014.12.007
59.
Jin X Wang T Liao Y Guo J Wang G Zhao F et al . Neuroinflammatory reactions in the brain of 1,2-DCE-intoxicated mice during brain edema. Cells. (2019) 8:987. doi: 10.3390/cells8090987
60.
Gao C Jiang J Tan Y Chen S . Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct Target Ther. (2023) 8:359. doi: 10.1038/s41392-023-01588-0
61.
Huang X Hussain B Chang J . Peripheral inflammation and Blood–Brain barrier disruption: effects and mechanisms. CNS Neurosci Ther. (2021) 27:36–47. doi: 10.1111/cns.13569
62.
Kwon HS Koh SH . Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. (2020) 9:42. doi: 10.1186/s40035-020-00221-2
63.
Yang QQ Zhou JW . Neuroinflammation in the central nervous system: symphony of glial cells. Glia. (2019) 67:1017–35. doi: 10.1002/glia.23571
64.
Liu LR Liu JC Bao JS Bai QQ Wang GQ . Interaction of microglia and astrocytes in the neurovascular unit. Front Immunol. (2020) 11:1024. doi: 10.3389/fimmu.2020.01024
65.
Alvarez JI Katayama T Prat A . Glial influence on the blood brain barrier. Glia. (2013) 61:1939–58. doi: 10.1002/glia.22575
66.
Nagelhus EA Ottersen OP . Physiological roles of aquaporin-4 in brain. Physiol Rev. (2013) 93:1543–62. doi: 10.1152/physrev.00011.2013
67.
Salman MM Kitchen P Halsey A Wang MX Tornroth-Horsefield S Conner AC et al . Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain. (2022) 145:64–75. doi: 10.1093/brain/awab311
68.
Wang LM Zhao TT Zhou HP Zhou ZY Huang S Ling YL et al . Effect of electroacupuncture on recognition memory and levels of Abeta, inflammatory factor proteins and aquaporin 4 in hippocampus of APP/PS1 double transgenic mice. Zhen ci yan jiu = Acupuncture Res. (2020) 45:431–7. doi: 10.13702/j.1000-0607.190923
69.
Cheslow L Alvarez JI . Glial-endothelial crosstalk regulates Blood–Brain barrier function. Curr Opin Pharmacol. (2016) 26:39–46. doi: 10.1016/j.coph.2015.09.010
70.
Liu S Mao J Wang T Fu X . Downregulation of aquaporin-4 protects brain against hypoxia ischemia via anti-inflammatory mechanism. Mol Neurobiol. (2017) 54:6426–35. doi: 10.1007/s12035-016-0185-8
Summary
Keywords
acupuncture, blood–brain barrier, tight junction, neuroinflammation, meta-analysis
Citation
Zhang K, Liang Y, Wang Y, Yin T and Ming K (2025) Acupuncture improves blood–brain barrier integrity through multi-targeted mechanisms: a preclinical meta-analysis. Front. Neurol. 16:1648117. doi: 10.3389/fneur.2025.1648117
Received
18 June 2025
Accepted
13 October 2025
Published
07 November 2025
Volume
16 - 2025
Edited by
Muhammad Thohawi Elziyad Purnama, Airlangga University, Indonesia
Reviewed by
Kusdiantoro Mohamad, IPB University, Indonesia
Yeni Dhamayanti, Airlangga University, Indonesia
Updates
Copyright
© 2025 Zhang, Liang, Wang, Yin and Ming.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kangwen Ming, mkw1976_medicine@cqu-edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.