Abstract
Objective:
This meta-analysis aimed to investigate the effect of body weight support treadmill training (BWSTT) on lower limb motor function and daily living activities in a person with a stroke while also exploring the optimal training strategy.
Methods:
Six databases (PubMed, Web of Science, The Cochrane Library, CNKI, Wanfang, and SinoMed) were searched up to August 2025. Randomized controlled trials involving persons with stroke, BWSTT, and outcomes measured by the Fugl-Meyer assessment of lower extremity and Barthel Index scores were included. The risk of bias was assessed using the RoB-2 tool of the Cochrane Collaboration, and the certainty of evidence was assessed using the GRADE tool.
Results:
25 studies with 1,749 people with stroke were incorporated into the meta-analysis. The meta-analysis demonstrated that BWSTT significantly outperformed the control group in improving both the Fugl-Meyer lower extremity score (MD = 4.80, 95% CI: 2.90–6.71, p < 0.001) and Barthel Index score (MD = 10.53, 95% CI: 7.61–13.46, p < 0.001). The certainty of evidence was rated as “very low.” The most effective interventions were observed in persons with a disease duration of 3–6 months (Fugl-Meyer: MD = 4.72, 95% CI: 1.54–7.89, p = 0.004; Barthel: MD = 17.58, 95% CI: 11.75–23.40, p < 0.001), intervention time of 4–8 weeks (Fugl-Meyer: MD = 5.78, 95% CI: 3.80–7.76, p < 0.001; Barthel: MD = 12.85, 95% CI: 3.84–21.87, p = 0.005), body weight support over 30% (Fugl-Meyer: MD = 4.51, 95% CI: 1.75–7.28, p = 0.001; Barthel: MD = 10.79, 95% CI: 6.91–14.67, p < 0.001), and gait speeds of 0.2 m/s or higher (Fugl-Meyer: MD = 4.01, 95% CI: 1.62–6.40, p = 0.001; Barthel: MD = 10.61, 95% CI: 1.13–20.10, p = 0.03).
Conclusion:
BWSTT improved the lower limb function and daily activities of persons with stroke, with optimal outcomes at disease duration of 3–6 months or undergoing interventions for 4–8 weeks, and more than 30% of the maximum body weight support level or using a gait speed exceeding 0.2 m/s. It is unclear whether persons with disease durations of 3–6 months could achieve the same outcomes as those undergoing 4–8 weeks of intervention. The very low quality of evidence suggests that the conclusions require further validation through high-quality randomized controlled trials.
Systematic review registration:
http://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42023486562.
1 Introduction
Stroke ranks as the second primary cause of mortality worldwide (1). The principal aim of early rehabilitation for a person with a stroke is to recover lower limb motor function to enhance their self-care capabilities (2, 3). Research indicates that more than 80% of people with stroke suffer from lower limb motor impairment, with a considerable number restricted to minimal movement within their residences and unable to participate in social activities (4). This restriction significantly diminishes a person’s quality of life and imposes a considerable psychological burden. As such, identifying effective intervention strategies to assist persons with stroke in recovering their lower limb motor function and daily living activities is a critical challenge in the field of rehabilitation (5). Unfortunately, despite numerous studies on rehabilitation, the best strategy to help people recover lower limb motor function and activity of daily living is still unclear.
Body weight support treadmill training (BWSTT) is a crucial method in early stroke rehabilitation, aiding in weight reduction through overhead harness or pneumatic techniques to facilitate exercise, thereby promoting quicker recovery (3). In recent years, BWSTT has been widely adopted for stroke rehabilitation and has demonstrated considerable efficacy (6–8). Prior research has established that BWSTT significantly enhances lower limb motor function, self-care capabilities, and overall quality of life, facilitating people’s reintegration into familial and societal contexts (9–11). A recent study by Jiang examined the impact of BWSTT on balance and walking ability in persons with stroke, revealing that disease duration and training parameters (including intervention time and load) significantly influenced rehabilitation outcomes (3). Nonetheless, previous studies have mainly focused on the efficacy of BWSTT in improving walking and balance abilities of stroke patients. There is a lack of comprehensive systematic reviews or meta-analyses focusing on lower limb motor function and daily activities.
Therefore, this study aimed to systematically and quantitatively assess the effects of BWSTT on lower limb motor function and activities of daily living in persons with stroke and to explore the optimal intervention strategies to provide a reference for optimizing the effectiveness of clinical interventions.
2 Methods
2.1 Protocol and registration
This meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (12), and the study protocol is registered in the International Prospective Register of Systematic Reviews database (identifier: CRD42023486562).
2.2 Information sources and search strategy
The PubMed, Web of Science, The Cochrane Library, CNKI, Wanfang, and Chinese SinoMed Databases were searched through inception to August 1, 2025. Search terms used included “stroke,” “cerebral vascular accident,” “antigravity treadmill,” “body weight support,” and their respective synonyms. Using the Cochrane Library database as a case study, the precise search approach is outlined as follows:
-
#1 MeSH descriptor: [Stroke] explode all trees.
-
#2 Cerebral stroke.
-
#3 Cerebral vascular accident.
-
#4 CVA.
-
#5 #1 OR #2 OR #3 OR #4.
-
#6 Antigravity treadmill.
-
#7 Body weight support.
-
#8 #6 OR #7.
-
#9 #5 AND #8 in Trials.
2.3 Eligibility criteria
Inclusion criteria: (1) Type of study: Randomized Controlled Trial (RCT); (2) Study participants: person with the clinical diagnosis of stroke; (3) Interventions: the experimental group received BWSTT combined with usual rehabilitation, while the control group received only usual rehabilitation; (4) Outcome indexes: Fugl-Meyer assessment of lower extremity score and Barthel Index score. The Fugl-Meyer assessment of the lower extremity and the Barthel Index are two of the most commonly used methods for evaluating lower limb motor function and activity of daily living. The results of previous studies have revealed that they have good reliability for detecting changes over time for persons after stroke rehabilitation and are suitable as outcomes for stroke research and practice (13, 14).
Exclusion criteria: (1) Studies that did not involve BWSTT; (2) Studies with missing or inconsistent outcome measures; (3) Conference abstracts and dissertation papers; and (4) Duplicated publications by the same research team using the same study at different points.
2.4 Study selection and data extraction
EndNote X9 software was utilized to eliminate duplicate literature during the study selection process. Two coauthors (YS and JL) independently evaluated the titles and abstracts of the retrieved records utilizing a double-blind methodology, adhering to the established inclusion and exclusion criteria. Full texts of studies that potentially met the inclusion criteria were downloaded for further screening. In cases of disagreement between the two coauthors, a third coauthor (XZ) participated in a joint discussion to determine whether to include the study.
Two coauthors (YS and JL) separately extracted data from the selected studies utilizing a pre-designed form throughout the data extraction process. The extracted data comprised: (1) fundamental study information: first author, year of publication; (2) participant details: sample size, age, disease duration; (3) intervention parameters: intervention time, frequency, body weight support level, and gait speed; (4) baseline and endpoint outcome data (test results before the start of the intervention and test results after the last intervention).
2.5 Risk of Bias assessment
The risk of bias for the selected studies was evaluated utilizing the RoB-2 tool of the Cochrane Collaboration (15). The assessment comprised six primary components: (1) Randomization process, (2) Deviations from intended interventions, (3) Missing outcome data, (4) Measurement of the outcome, (5) Selection of the reported result, and (6) Overall. The process of evaluating the quality of the literature was carried out independently by two coauthors (YS and JL). In case of disagreement, a third coauthor (XZ) was added to discuss the matter until a consensus was reached.
2.6 Data synthesis
Meta-analyses were performed for the pre-post change outcomes in the intervention and control groups using Review Manager version 5.4. All outcome parameters of the studies included in this meta-analysis were continuous variables, and the same outcome was measured in the same way, so mean differences (MD) with 95% confidence intervals (CI) were used as pooled effect sizes (16). The Chi2 test and I2 statistic were utilized to evaluate heterogeneity, and a random-effects model was employed when significant heterogeneity was detected (I2 > 50%; p<0.05). Otherwise, a fixed-effects model was employed (17). Sensitivity analyses were performed by removing studies one by one on heterogeneous outcomes to evaluate the robustness of the findings (3). Subgroup analyses were conducted according to person characteristics and training parameters to determine optimal training strategies, while meta-regression analyses were utilized to investigate sources of heterogeneity (18). Publication bias was tested by drawing funnel plots using Stata 14.0 software and further quantified using Egger’s test. When significant publication bias existed, the effect of publication bias on the results of Meta-analysis was further analyzed by the Duvaland Tweedie trim and fill method (19). A p-value less than 0.05 was deemed statistically significant.
2.7 Grading of evidence
Two authors (YS and XZ) evaluated the grading of evidence for the Fugl-Meyer assessment of lower extremity score and Barthel Index score outcomes according to the GRADEpro Guideline Development Tool (20). The evaluations were categorized into risk of bias, inconsistency, indirectness, imprecision, and publication bias. Each domain was categorized as “not serious, ““serious,” or “very serious” according to the evaluation criteria, and the overall certainty of the evidence was categorized into four grades: very low, low, moderate, or high.
3 Results
3.1 Study selection
The initial database search produced 800 records. Following the removal of duplicates via EndNote, 576 records were retained. After evaluating titles and abstracts, 462 studies deemed irrelevant were excluded. One hundred fourteen records were subjected to full-text screening, excluding 89 studies that failed to meet the inclusion criteria. A total of 25 studies were incorporated into the meta-analysis (9–11, 21–42). Figure 1 illustrates the PRISMA flowchart for the study selection process.
Figure 1

PRISMA flowchart for the study selection process.
3.2 Methodological quality assessment
During the RoB-2 tool assessment, each study was categorized as “low risk,” “some concerns” or “high risk.” If all components were assessed as “low risk,” the overall risk of study bias was defined as low risk; if one or more components were assessed as “some concerns” risk, the overall risk of study bias was defined as “some concerns”; if any one component was categorized as “high risk,” the overall risk of study bias was defined as “high risk” (15). A risk of bias assessment of the 25 included studies showed that five studies (21, 25–28) were considered low risk of bias because no bias in the assessment was detected. Twenty studies (9–11, 22–24, 29–42) were rated at high risk of bias, mainly due to deviation from the intended intervention. In addition, one study (9) observed uncertainty in the measurement of the outcome and the missing data on the outcome. Detailed assessment result is presented in Supplementary Figure S1.
3.3 Descriptive characteristics of included studies
The 25 studies included in this meta-analysis involved 1,749 people, most of whom were middle-aged or older adults. The shortest disease duration among the people was 15 days, while the longest was 50 months. In terms of support mechanisms, most studies used overhead harnesses. Regarding intervention time, the shortest duration was 3 weeks, and the longest was 4 months. Regarding body weight support, the minimum level was 8%, while the maximum ranged from 65 to 100%. For outcomes, 20 studies assessed the Fugl-Meyer assessment of lower extremity score, and 16 evaluated the Barthel Index score. In terms of usual rehabilitation training, the Normal limb Position, Acupuncture, PNF, Stand-up, Balance, Gait, Muscle Strength training, and so on were included. Detailed characteristics of the included studies are presented in Supplementary Table S1.
3.4 Effect of BWSTT on Fugl-Meyer assessment of lower extremity score
Fugl-Meyer assessment of lower extremity scores was analyzed for 20 of the 25 included studies (10, 11, 22, 24–29, 31–39, 41, 42). A random effects model was employed for the meta-analysis due to the significant heterogeneity of the combined results (I2 = 94%). The combined effect size significantly enhanced the Fugl-Meyer assessment of lower extremity scores for the BWSTT group relative to the control group (MD = 3.60, 95% CI: 1.23–5.98, p = 0.003). Figure 2 presents the results.
Figure 2
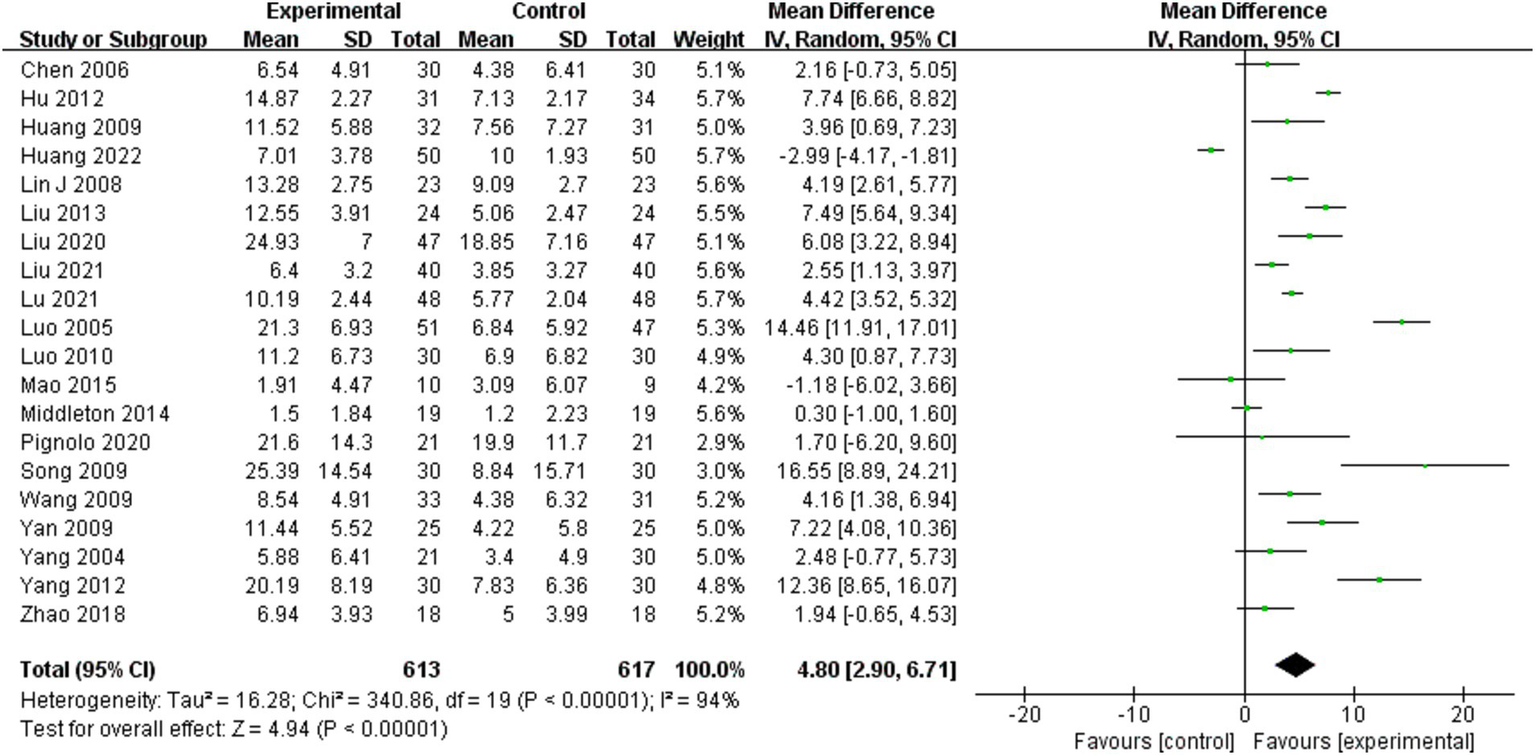
Effect of body weight support training on Fugl-Meyer lower extremity scores.
Four moderating variables were identified to examine the impact of person characteristics and training parameters on the study outcomes: person disease duration, intervention time, maximum body weight support level, and maximum gait speed. The subgroup analysis based on the above moderating variables is shown in Table 1. The results of the study found that the intervention effect was better in persons with a disease duration within the period of 3–6 months (MD = 4.72, 95% CI: 1.54 to 7.89, p = 0.004) than in those with a duration of 1–3 months (MD = 4.03, 95% CI: 2.38 to 5.68, p < 0.001), while the intervention effect was not statistically significant in person with a disease duration of 6 months or more (MD = -1.36, 95% CI: - 4.58 to 1.87, p = 0.41). Intervention time of 4–8 weeks (MD = 5.78, 95% CI: 3.80 to 7.76, p < 0.001) was better than 1–4 weeks (MD = 4.25, 95% CI: 1.48 to 7.02, p = 0.003), while intervention time of 8 weeks or more (MD = 3.86, 95% CI: −3.18 to 10.91, p = 0.28) was not statistically significant. Interventions with a maximum body weight support of 30% or more (MD = 4.51, 95% CI: 1.75 to 7.28, p = 0.001) were significantly more effective in improving the Fugl-Meyer assessment of lower extremity score, while interventions with 0–30% (MD = 3.82, 95% CI: −1.49 ~ 9.13, p = 0.16) had no statistically significant effect. A maximum gait speed of 0–0.2 m/s or more (MD = 4.01, 95% CI: 1.62 ~ 6.40, p = 0.001) significantly improved the effect of the Fugl-Meyer assessment of lower extremity score, while 0–0.2 m/s (MD = 5.68, 95% CI: −0.46 to 11.82, p = 0.07) had no statistically significant.
Table 1
| Subgroup | Sample size | Number of studies | Effect size and 95% CI | I2 (%) | P-value |
|---|---|---|---|---|---|
| Disease duration | |||||
| 1–3 months | 468 | 9 | 4.03 (2.38, 5.68) | 71 | <0.001 |
| 3–6 months | 246 | 4 | 4.72 (1.54, 7.89) | 87 | 0.004 |
| More than 6 months | 138 | 2 | -1.36(−4.58, 1.87) | 94 | 0.41 |
| Intervention time | |||||
| 1–4 weeks | 453 | 7 | 4.25 (1.48, 7.02) | 92 | 0.003 |
| 4–8 weeks | 471 | 8 | 5.78 (3.80, 7.76) | 76 | <0.001 |
| More than 8 weeks | 86 | 2 | 3.86(−3.18, 10.91) | 97 | 0.28 |
| Maximum body weight support level | |||||
| 0–30% | 343 | 5 | 3.82(−1.49, 9.13) | 95 | 0.16 |
| More than 30% | 483 | 9 | 4.51 (1.75, 7.28) | 95 | 0.001 |
| Maximum gait speed | |||||
| 0–0.2 m/s | 326 | 5 | 5.68(−0.46, 11.82) | 98 | 0.07 |
| More than 0.2 m/s | 344 | 7 | 4.01 (1.62, 6.40) | 79 | 0.001 |
Subgroup analysis of moderating variables affecting Fugl-Meyer assessment of lower extremity score.
As the combined results heterogeneity I2 > 50%, the reasons for the heterogeneity were explored by meta-regression analysis (3). The results indicated (Table 2) that maximum body weight support level (p = 0.835), intervention time (p = 0.249), and maximum gait speed (p = 0.266) did not significantly affect heterogeneity. The disease duration (p = 0.042) demonstrated a statistically significant result, suggesting that it might have been the main cause of heterogeneity.
Table 2
| Moderating variables | β-regression coefficient | Standard error | t-value | P>│t│ | 95%CI |
|---|---|---|---|---|---|
| Disease duration | 1.24 | 0.68 | 1.82 | 0.042 | (0.248, 2.736) |
| Intervention time | 0.85 | 0.71 | 1.2 | 0.249 | (−0.670, 2.379) |
| Maximum body weight support level | −0.21 | 0.98 | −0.21 | 0.835 | (−2.324, 1.907) |
| Maximum gait speed | 1.30 | 1.11 | 1.18 | 0.266 | (−1.165, 3.774) |
Meta-regression analysis of different moderating variables on Fugl-Meyer assessment of lower extremity score.
3.5 Effect of BWSTT on Barthel Index score
Barthel Index scores were analyzed for 16 of the 25 included studies (9, 10, 21–25, 30, 32–35, 37, 40–42). A random effects model was employed for the meta-analysis due to the significant heterogeneity of the combined results (I2 = 98%). The effect size indicated a significant enhancement in Barthel Index scores for the BWSTT group relative to the control group (MD = 10.53, 95% CI: 7.61–13.46, P<0.001). Figure 3 presents the results.
Figure 3
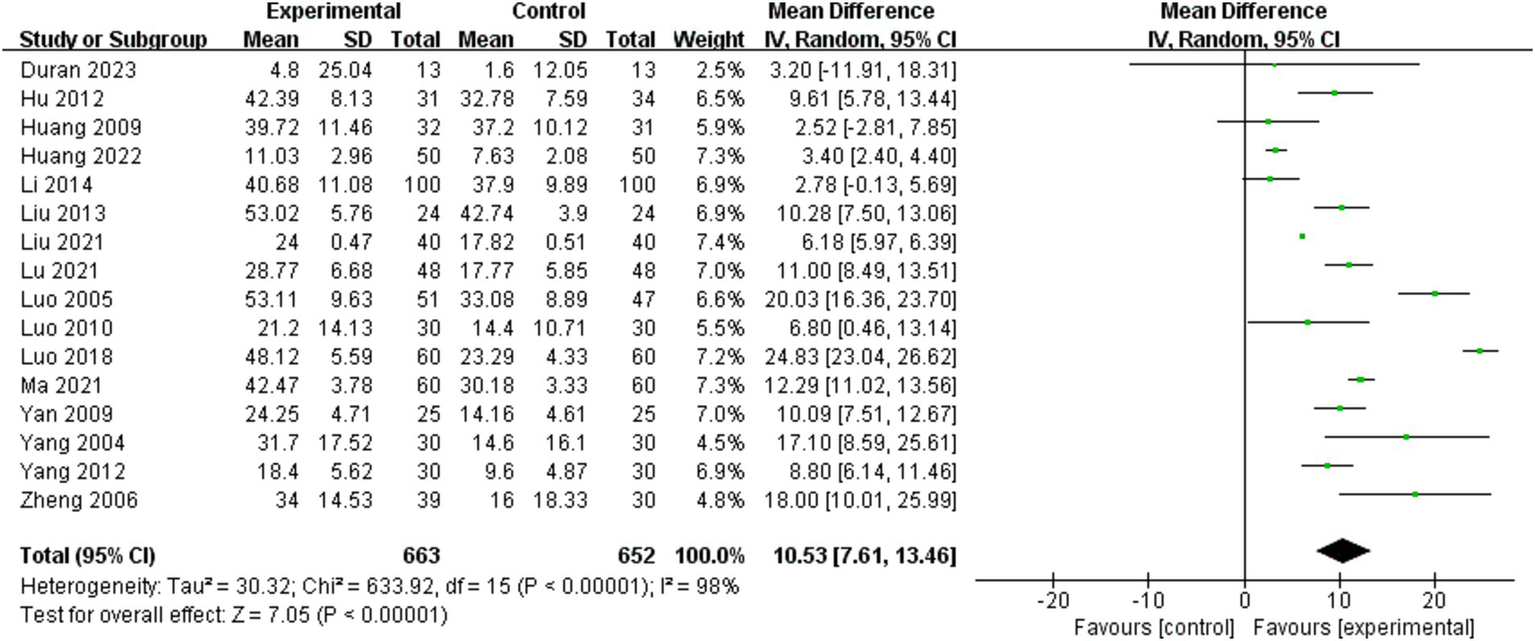
Effect of body weight support training on Barthel Index scores.
Subgroup analyses were performed to investigate the influence of pertinent moderating variables on study outcomes. Table 3 presents the subgroup analysis according to the specified moderating variables. The results of the study found that the intervention effect was better in persons with a disease duration within the period of 3–6 months (MD = 17.58, 95% CI: 11.75 to 23.40, p < 0.001) than in those with a duration of 1–3 months (MD = 9.65, 95% CI: 4.63 to 14.66, p < 0.001) and more than 6 months or more (MD = 3.40, 95% CI: 2.40 to 4.40, p < 0.001). Intervention time of 4–8 weeks (MD = 12.85, 95% CI: 3.83 to 21.87, p = 0.005) was better than 1–4 weeks (MD = 9.93, 95% CI: 5.86 to 13.99, p < 0.001) and more than 8 weeks or more (MD = 8.71, 95% CI: 4.42 to 12.99, p < 0.001). Interventions with a maximum body weight support of 30% or more (MD = 10.79, 95% CI: 6.91 to 14.67, p < 0.001) were significantly more effective in improving the Barthel Index score, while interventions with 0–30% (MD = 9.36, 95% CI: −1.22 to 19.94, p = 0.08) had no statistically significant effect. A maximum gait speed of 0–0.2 m/s or more (MD = 10.61, 95% CI: 1.13 to 20.10, p = 0.03) was better than 0–0.2 m/s (MD = 9.08, 95% CI: 2.72 to 15.45, p = 0.005).
Table 3
| Subgroup | Sample size | Number of studies | Effect size and 95% CI | I2 (%) | P-value |
|---|---|---|---|---|---|
| Disease duration | |||||
| 1–3 months | 741 | 8 | 9.65 (4.63, 14.66) | 99 | <0.001 |
| 3–6 months | 129 | 2 | 17.58 (11.75, 23.40) | 0 | <0.001 |
| More than 6 months | 126 | 2 | 3.40 (2.40, 4.40) | 0 | <0.001 |
| Intervention time | |||||
| 1–4 weeks | 620 | 6 | 9.93 (5.86, 13.99) | 97 | <0.001 |
| 4–8 weeks | 358 | 5 | 12.85 (3.83, 21.87) | 97 | 0.005 |
| More than 8 weeks | 337 | 5 | 8.71 (4.42, 12.99) | 90 | <0.001 |
| Maximum body weight support level | |||||
| 0–30% | 403 | 5 | 9.36(−1.22, 19.94) | 99 | 0.08 |
| More than 30% | 648 | 8 | 10.79 (6.91, 14.67) | 92 | <0.001 |
| Maximum gait speed | |||||
| 0–0.2 m/s | 210 | 3 | 9.08 (2.72, 15.45) | 94 | 0.005 |
| More than 0.2 m/s | 572 | 6 | 10.61 (1.13, 20.10) | 98 | 0.03 |
Subgroup analysis of moderating variables affecting Barthel Index score.
The meta-regression analysis results indicated (Table 4) that disease duration (p = 0.065), maximum body weight support level (p = 0.761), and maximum gait speed (p = 0.439) did not significantly influence heterogeneity. The intervention time (p = 0.044) demonstrated a statistically significant result, suggesting that it might be the main cause of heterogeneity.
Table 4
| Moderating variables | β-regression coefficient | Standard error | t-value | P>│t│ | 95%CI |
|---|---|---|---|---|---|
| Disease duration | 4.45 | 2.15 | 2.07 | 0.065 | (−0.34, 9.23) |
| Intervention time | 4.06 | 1.84 | 2.21 | 0.044 | (0.12, 7.99) |
| Maximum body weight support level | 1.00 | 3.23 | 0.31 | 0.761 | (−6.11, 8.12) |
| Maximum gait speed | 1.55 | 1.89 | 0.82 | 0.439 | (−2.92, 6.03) |
Meta-regression analysis of different moderating variables on Barthel Index score.
3.6 Sensitivity analyses
Due to the significant heterogeneity among studies, a study-by-study culling approach was employed to evaluate each study’s influence on the overall effect size derived from the collective research (43). The results indicated that the impact on overall heterogeneity after the exclusion of one study was minimal, suggesting the robustness and reliability of the findings for these outcomes (Supplementary Figure S2).
3.7 Publication Bias
The funnel plot and Egger’s test were used for the publication bias test. The results showed that the Fugl-Meyer assessment of the lower extremity score funnel plot was more evenly distributed on both sides (Supplementary Figure S3). There was no statistical difference between the test results of Egger’s test (p = 0.164, Supplementary Table S2). Still, the Barthel index score funnel plot was unevenly distributed on both sides (Supplementary Figure S3), and there was a statistical difference between Egger’s test results (p = 0.002, Supplementary Table S2). Therefore, the Duvaland Tweedie trim and fill method was used to further evaluate the effect of publication bias on the Barthel Index score results. The study results showed that after four iterations and a total of 18 documents after trimming and filling, the amount and significance of the combined effect did not change significantly before and after trimming and filling, indicating that the results of this study were stable. The corrected funnel plot of the supplementary material is shown in Supplementary Figure S4.
3.8 Grading of evidence
Using the GRADE tool, the quality of evidence for the included studies was found to be of very low quality (Supplementary Table S3).
4 Discussion
Lower limb motor dysfunction and impaired daily living ability are key factors hindering persons with stroke’s self-care and reintegration into family and society (2, 3). This meta-analysis indicates that BWSTT significantly enhances lower limb motor function and daily living ability compared to without BWSTT, consistent with prior research findings (21, 23, 24, 26, 29, 33, 41). The theoretical foundation of BWSTT is rooted in the central pattern generator theory, motor control dynamic systems theory, and the theory of forced use (44). Its mechanism primarily involves high-intensity, task-specific repetitive gait training under reduced weight-bearing conditions. This approach regulates gait speed and body load, facilitating improved lower limb coordination through repetitive pattern generation, enhanced cardiovascular fitness via prolonged aerobic exercise, motor relearning through error correction and sensory feedback, and neural pathway reorganization via central pattern generator activation (44, 45).
Therapists can modify the training load and weight support based on the people’s specific pathology during training, effectively incorporating weight-bearing, stepping, and balance components (3). For individuals unable to train autonomously, BWSTT may improve proprioceptive feedback in the lumbar spinal cord, refine motor neural pathways, and reinforce typical movement patterns (40). Previous research (3) has explored how BWSTT enhances motor function and daily life ability, focusing on motor control, neural pathway transmission, and psychological factors (40). However, from the point of view of the development of rehabilitation training programs, there is a lack of research examining how individual differences in training and variations in training intensity, frequency, and duration affect treatment outcomes. Based on this, the present study explored the optimal training strategy for BWSTT regarding disease duration and training parameters (the study results were subdivided into two aspects: time and load parameters), and the overall findings are shown in Figure 4.
Figure 4
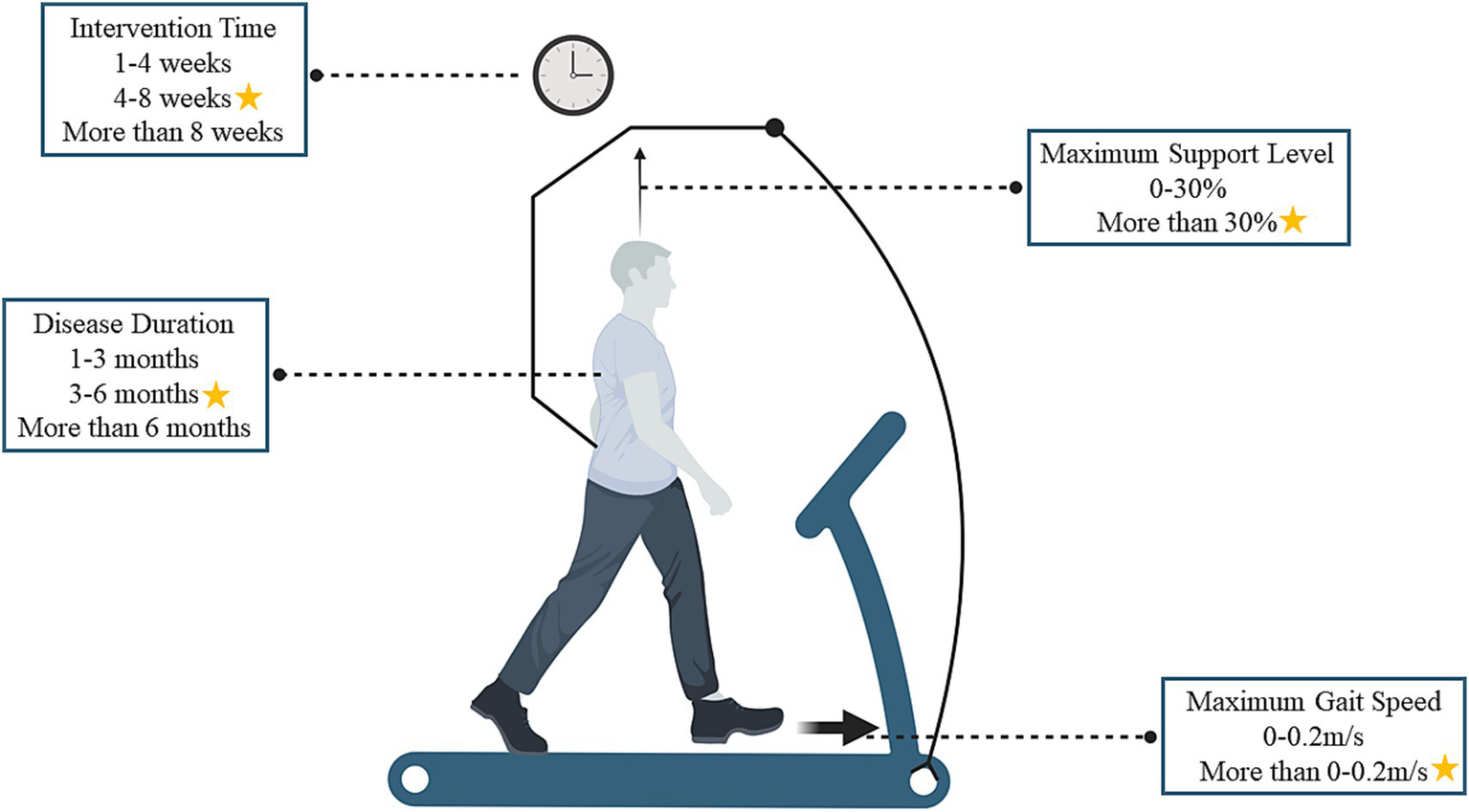
Optimal training strategy for body weight support training (the asterisk indicates the optimal training strategy, using an overhead harness as an example, which also includes pneumatic technology).
4.1 Identifying optimal BWSTT time parameters
Subgroup analyses utilizing moderator variables indicated that an intervention duration of 4–8 weeks yielded optimal outcomes for enhancing lower limb motor function and daily living capabilities in stroke persons with a 3–6 months disease duration. This finding aligns with Jiang et al.’s study on walking function and balance in persons with stroke, further supporting the reliability of the present study (3). Although this study found that persons with a disease duration of 1–3 months who received BWSTT had significantly improved Fugl-Meyer scores (MD = 4.03) and Barthel Index scores (MD = 9.65) compared with the control group, the degree of improvement was lower than that of persons with a disease duration of 3–6 months. Previous studies have suggested that early and appropriate rehabilitation training promotes the regeneration of brain cells around the lesion (46). It is postulated that it induces compensation and reorganization of motor function in the contralateral cerebral hemisphere, accelerating the recovery of lower limb function. Current research generally advocates for early intervention in routine rehabilitation for persons with stroke. However, a study by The AVERT Trial Collaboration group showed that a high-dose, very early mobilization protocol reduces the favorable outcome of persons (47). Meanwhile, a study by Dong et al. on weight support training also suggested that the best outcomes are achieved when the person’s disease duration is less than 1 month. The Lovett unassisted muscle test results were at least grade 2 (as determined by the ability to perform the full range of motion of the joints in a gravity-eliminating position) (48). This may be because, during the acute/subacute early stage (<3 months), the person’s neurological injury status may be more unstable, and inflammatory responses, edema, etc., may affect training tolerance and efficacy (46). Additionally, persons in the early stages may have poorer physical fitness, endurance, and cardiopulmonary function, limiting the maximization of BWSTT training intensity and duration. Therefore, while BWSTT remains an effective rehabilitation modality for persons with a 1–3 month disease course, clinical practice may require more individualized protocols, such as starting with lower intensity and shorter durations, gradually increasing the load, and closely monitoring the person’s tolerance and response. Concurrently, other early rehabilitation strategies should be integrated.
In contrast, the present study found that rehabilitation was most effective for persons with a disease duration of 3–6 months, which differs from Dong et al.’s findings (48). This discrepancy may be due to differences in person inclusion criteria. Dong et al.’s study focused on persons with a disease duration of less than 2 months, not accounting for longer-term persons. In this study, persons in the chronic phase with a disease duration exceeding six months did not show statistically significant improvements in Fugl-Meyer assessment of lower extremity scores. While there was an improvement in the Barthel Index, the magnitude of improvement was minimal (MD = 3.40). Previous studies have suggested that the golden period for neuroplasticity typically occurs within the first 6 months after onset, and the potential for neural remodeling in the chronic phase is relatively reduced, which may lead to a weakened response to training (46, 47). Additionally, persons in the chronic phase may have already developed fixed abnormal movement patterns or compensatory strategies, and altering these patterns may require longer durations and higher intensities of specific training. The standard BWSTT protocol may not break these patterns, affecting training outcomes effectively. Furthermore, the number of studies included in this meta-analysis targeting persons with a disease duration of over 6 months was limited (Fugl-Meyer: n = 2; Barthel: n = 2), and the sample sizes were relatively small, restricting the statistical power of the results. Therefore, more high-quality studies are needed to confirm the precise efficacy of BWSTT in the chronic phase. This suggests that applying BWSTT in the chronic phase may require combining other intervention methods or adopting higher-intensity, longer-duration, and more personalized BWSTT protocols to address the specific functional impairments and adaptive changes that chronic-phase persons face. Future research should focus on exploring optimized training strategies for BWSTT in chronic stroke patients. Additionally, the results of this study also found that the timing of interventions substantially impacts rehabilitation outcomes. Like this study, Jiang et al. identified 4–8 weeks as the optimal intervention time (3). Unfortunately, the literature lacks additional studies exploring the timing of BWSTT interventions. Future research should validate these findings by including people at different disease stages and using randomized controlled trials to standardize intervention timing.
4.2 Identifying optimal BWSTT load parameters
In terms of training load parameters, this study focused on the effects of the maximum body weight support level and maximum gait speed on rehabilitation outcomes. Prior research has yielded inconsistent findings concerning the ideal level of body weight support (3). Jang et al. demonstrated that a body weight support level of 30% or greater was most effective, aligning with the current study’s findings (3). However, Hesse et al.’s study on lower limb EMG suggested that the maximum body weight support level should not exceed 30% (49). This discrepancy may be due to variations in person characteristics. Hesse et al.’s study involved persons with a disease duration of around 40 days and a limited sample size, in contrast to the current study’s wider person range and larger sample size. Further research is needed to clarify these differences.
Regarding the maximum gait speed, Klaske et al. concluded that lower speeds should be avoided during gait training, as they may reduce muscle activation and lead to abnormal gait patterns (50). The present study supports this, showing that a maximum gait speed of 0.2 m/s or more was optimal for improving lower limb motor function and daily living ability in persons with stroke. This indirectly confirms Klaske et al.’s finding that slower speeds are less effective and provide a minimum threshold for gait training speed. However, other studies present different findings. For instance, Wu et al.’s research on different gait speeds indicated that a maximum speed of 0.3 m/s resulted in the most substantial enhancement in motor feedback for persons with stroke (51). In contrast, increased speeds (0.45 m/s) did not improve motor recovery.
Training loads should be tailored to people’s mobility level (3, 52). Persons with lower mobility may benefit from greater body weight support or slower gait speeds, whereas more mobile people may experience diminishing returns from such adjustments. The subgroup analysis of this study did not consider variations in person mobility, and due to the limitations of the included studies, it could not further classify body weight support levels or gait speeds. Future research should investigate the impact of different body weight support ratios and gait training speeds on the rehabilitation of persons with stroke, considering individual mobility variations and appropriately adjusting training loads.
4.3 Study limitations
The study presents specific limitations. First, the quality of evidence for the included studies was found to be very low overall, which may compromise the reliability. Second, certain subgroup analyses relied on a restricted number of studies, necessitating further validation of the objectivity of these findings. Future research must emphasize randomized controlled trials with larger sample sizes to improve the rigor of the testing process. Third, this study only focused on BWSTT, which limits the generalizability of the results. Fourth, this study focused on the lower limb motor function and the activities of daily living of persons with stroke. The outcomes mainly used the Fugl-Meyer assessment of lower extremity and the Barthel Index scores. Future studies can use other outcomes to validate the conclusions of this study, according to the purpose of the rehabilitation treatment. Furthermore, conducting more thorough analyses to consider variations in people’s characteristics and interventions based on different device models and support mechanisms of body weight support training (such as an overhead harness or pneumatic) is essential, enhancing the understanding of optimal rehabilitation strategies for persons with stroke. Finally, the high heterogeneity observed in our meta-analysis (I2 = 94% for Fugl-Meyer scores; I2 = 98% for Barthel Index scores) warrants careful interpretation. We attribute this heterogeneity to methodological (protocol variations, bias risk, and control group heterogeneity) and clinical factors (person disease duration and baseline function). We addressed this via random-effects models, sensitivity analyses, and subgroup analyses (Tables 1–4). However, residual heterogeneity persists. Future trials should standardize protocols using core outcome sets and stratify participants by disease duration to reduce clinical heterogeneity.
5 Conclusion
BWSTT demonstrated greater efficacy in improving lower limb motor function and activities of daily living in persons with stroke, with optimal outcomes at disease duration of 3–6 months or undergoing interventions for 4–8 weeks, and more than 30% of the maximum body weight support level or using a gait speed exceeding 0.2 m/s. It is unclear whether persons with disease durations of 3–6 months could achieve the same outcomes as those undergoing 4–8 weeks of intervention. However, due to the limited number and quality of included studies, these conclusions require further validation through high-quality randomized controlled trials.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YS: Methodology, Writing – original draft, Formal analysis, Visualization, Investigation, Software, Conceptualization, Data curation. JL: Formal analysis, Data curation, Writing – original draft, Methodology. XZ: Conceptualization, Methodology, Project administration, Supervision, Data curation, Writing – review & editing, Writing – original draft, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Sports Administration Decision-Making Consultation 2024 Key Project (Project No. 2024-B-04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1649246/full#supplementary-material
References
1.
Bonkhoff AK Grefkes C . Precision medicine in stroke: towards personalized outcome predictions using artificial intelligence. Brain. (2022) 145:457–75. doi: 10.1093/brain/awab439
2.
Wang J . Application prospects of specific rehabilitation task training in upper limb function rehabilitation of stroke sequelae patients. Altern Ther Health Med. (2024) 30:10286.
3.
Jiang Z Zhang X Fu Q Tao Y . Effects of body weight support training on balance and walking function in stroke patients: a systematic review and meta-analysis. Front Neurol. (2024) 15:1413577. doi: 10.3389/fneur.2024.1413577
4.
Kim E Kim K . Effects of purposeful action observation on kinematic patterns of upper extremity in individuals with hemiplegia. J Phys Ther Sci. (2015) 27:1809–11. doi: 10.1589/jpts.27.1809
5.
Mehrholz J Pohl M Kugler J Elsner B . The improvement of walking ability following stroke. Dtsch Arztebl Int. (2018) 115:639–45. doi: 10.3238/arztebl.2018.0639
6.
Kim K Lee S Lee K . Effects of progressive body weight support treadmill forward and backward walking training on stroke patients’ affected side lower extremity’s walking ability. J Phys Ther Sci. (2014) 26:1923–7. doi: 10.1589/jpts.26.1923
7.
Lura DJ Venglar MC van Duijn AJ Csavina KR . Body weight supported treadmill vs. overground gait training for acute stroke gait rehabilitation. Int J Rehabil Res. (2019) 42:270–4. doi: 10.1097/MRR.0000000000000357
8.
Zhang X Rong X Luo H . Optimizing lower limb rehabilitation: the intersection of machine learning and rehabilitative robotics. Front Rehabil Sci. (2024) 5:1246773. doi: 10.3389/fresc.2024.1246773
9.
Luo HB Chen TX Chen YX . Effects of body weight support training on lower limb rehabilitation in 60 patients with hemiplegia after cerebral infarction. Biped Health. (2018) 27:42–3.
10.
Luo XP Yuan GH Chen H . Effects of body weight support training on motor function and ADLs in hemiplegic patients with early cerebral infarction. Chin J Rehabil. (2010) 25:42–3.
11.
Wang XF Li Y . The efficacy of body weight support training in the treatment of lower limb dysfunction after hemiplegia in 64 cases. J Qiqihar Med Univ. (2009) 30:1313–4. doi: 10.3969/j.issn.1002-1256.2009.11.019
12.
Moher D Liberati A Tetzlaff J Altman DG . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
13.
Quinn TJ Langhorne P Stott DJ . Barthel index for stroke trials: development, properties, and application. Stroke. (2011) 42:1146–51. doi: 10.1161/STROKEAHA.110.598540
14.
Hsieh YW Wu CY Lin KC Chang YF Chen CL Liu JS . Responsiveness and validity of three outcome measures of motor function after stroke rehabilitation. Stroke. (2009) 40:1386–91. doi: 10.1161/STROKEAHA.108.530584
15.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
16.
Zhang X Jing F Liu Y Tang J Hua X Zhu J et al . Effects of non-invasive brain stimulation on walking and balance ability in Parkinson’s patients: a systematic review and meta-analysis. Front Aging Neurosci. (2022) 14:1065126. doi: 10.3389/fnagi.2022.1065126
17.
Zheng B Luo Y Li Y Gu G Jiang J Chen C et al . Prevalence and risk factors of stroke in high-altitude areas: a systematic review and meta-analysis. BMJ Open. (2023) 13:e071433. doi: 10.1136/bmjopen-2022-071433
18.
Borenstein M Higgins JP . Meta-analysis and subgroups. Prev Sci. (2013) 14:134–43. doi: 10.1007/s11121-013-0377-7
19.
Reinwein J . Does the modality effect exist? And if so, which modality effect?J Psycholinguist Res. (2012) 41:1–32. doi: 10.1007/s10936-011-9180-4
20.
Guyatt GH Oxman AD Vist G Kunz R Brozek J Alonso-Coello P et al . GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol. (2011) 64:407–15. doi: 10.1016/j.jclinepi.2010.07.017
21.
Duran UD Duran M Tekin E Demir Y Aydemir K Aras B et al . Comparison of the effectiveness of antigravity treadmill exercises and underwater walking exercises on cardiorespiratory fitness, functional capacity and balance in stroke patients. Acta Neurol Belg. (2023) 123:423–32. doi: 10.1007/s13760-022-02012-0
22.
Huang J Tan XL Guan HY Lan JN . A study of weight-supported plate walking training for improving lower limb function in stroke patients. Chin Manip Rehabil Med. (2022) 13:33–7. doi: 10.19787/j.issn.1008-1879.2022.06.011
23.
Ma L Liu Y . Suspension weight support training in the rehabilitation of hemiplegic patients after stroke. J Med Forum. (2021) 42:120–2.
24.
Lu MS Gao HL . Observation on the application effect of weight-loss walking training in patients with early hemiplegia after stroke. Clin Med Eng. (2021) 28:1371–2. doi: 10.3969/j.issn.1674-4659.2021.10.1371
25.
Liu Z Liang TJ . Effect of Mirror therapy combined with body weight supported treadmill training on walking function in Poststroke hemiplegia patients. Chin Gen Pract. (2021) 24:2680–5. doi: 10.12114/j.issn.1007-9572.2021.00.482
26.
Liu S . Application of weight-loss walking training in stroke patients and improvement of walking function. Med Inf. (2020) 33:128–9. doi: 10.3969/j.issn.1006-1959.2020.24.036
27.
Pignolo L Basta G Carozzo S Bilotta M Todaro MR Serra S et al . A body-weight-supported visual feedback system for gait recovering in stroke patients: a randomized controlled study. Gait Posture. (2020) 82:287–93. doi: 10.1016/j.gaitpost.2020.09.020
28.
Zhao QY Lin Q Yang T Xia P Li XP . The effeet of body weight supported treadmill training and muscle tone on motor function of lower extremities of stroke survivors. Chin J Phys Med Rehabil. (2018) 40:821–5. doi: 10.3760/cma.j.issn.0254-1424.2018.11.004
29.
Mao Y Lo WL Lin Q Li L Xiao X Raghavan P et al . The effect of body weight support treadmill training on gait recovery, proximal lower limb motor pattern, and balance in patients with subacute stroke. Biomed Res Int. (2015) 2015:175719. doi: 10.1155/2015/175719
30.
Li JT Zhao X Huo HY Cao L Yuan ZL . The effect of weight-supported walking training on the recovery of lower limb mobility in stroke patients. Shandong Med J. (2014) 54:40–1. doi: 10.3969/j.issn.1002-266X.2014.29.016
31.
Middleton A Merlo-Rains A Peters DM Greene JV Blanck EL Moran R et al . Body weight-supported treadmill training is no better than Overground training for individuals with chronic stroke: a randomized controlled trial. Top Stroke Rehabil (2014) 21: 462–476. doi: 10.1310/tsr2106-462
32.
Liu XD Dong JG Sun L . Efficiency of the body weight supported treadmill training (BWSTT) on lower limbs motor function in stroke hemiplegic patients. China J Mod Med. (2013) 23:85–8.
33.
Yang MX Lu JC Liang SJ . The effect of body weight support treadmill training on hemiplegia gait patients after stroke. Gansu Med J. (2012) 31:410–3. doi: 10.15975/j.cnki.gsyy.2012.06.032
34.
Hu RL Yu JM Li GD Chen SL Lai LP Liu T . The effect on the rehabilitation of hemiplegia with the body weight support treadmill training for ischemic stroke patients. China Prac Med. (2012) 7:27–9. doi: 10.3969/j.issn.1673-7555.2012.18.014
35.
Yan Y Lai YC . Effects of weight-supported walking training on lower limb motor function in stroke patients with hemiplegia. J Mod Med Health. (2009) 25:63–4.
36.
Song XJ Feng F Yin WY Li F . The effect of weight-supported plate walking training on walking ability in stroke patients. J Xiangnan Univ. (2009) 11:53–5. doi: 10.3760/cma.j.issn.0254-1424.2009.08.012
37.
Huang Y Chen RL Wan XL Pan CH . Effects of partial body weight support treadmill training on motor function of lower limbs in patients with hemiparalysis after cerebral infarction. J Pract Med. (2009) 25:2270–2. doi: 10.3969/j.issn.1006-5725.2009.14.023
38.
Lin JQ Sun XM Gong YF Ye H . Effect of body-weight supported treadmill training on hemiplegic patients after stroke. Chin J Rehabil Theory Pract. (2008) 14:826–7. doi: 10.3969/j.issn.1006-9771.2008.09.009
39.
Chen LN Zong Y Yang JL Chen TX Fan ZY Xu LF . The effect of weight-supported walking training on the recovery of walking ability in hemiplegic patients with early stage stroke. Chin J Phys Med Rehabil. (2006) 28:343–5. doi: 10.3760/j:issn:0254-1424.2006.05.019
40.
Zheng SC Zhu SW Li YZ Song CZ Wu J Ma PX . The effect of early weight-supported plate walking training on hemiplegic patients with stroke. Chin J Rehabil Theory Pract. (2006) 11:463–4. doi: 10.3969/j.issn.1006-9771.2005.06.025
41.
Luo AH Pan CH Ye T Wan XL . Effects of weight support treadmill training on hemiplegic patients after cerebral infarction. Acad J Guangzhou Med Univ. (2005) 33:18–20. doi: 10.3969/j.issn.1008-1836.2005.04.006
42.
Yang YQ Zhang T . The effects of body weight support treadmill training on hemiplegic gait after stroke. Chin J Rehabil Med. (2004) 19:731–3. doi: 10.3969/j.issn.1001-1242.2004.10.003
43.
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
44.
Trueblood PR . Partial body weight treadmill training in persons with chronic stroke. Neuro Rehabil. (2001) 32:51–6. doi: 10.1044/0161-1461(2001/005)
45.
Harris-Love ML Forrester LW Macko RF Silver KH Smith GV . Hemiparetic gait parameters in overground versus treadmill walking. Neurorehabil Neural Repair. (2001) 15:105–12. doi: 10.1177/154596830101500204
46.
Miu HS . Theory of functional recovery after central nerve injury. Chin J Rehabil. (1998) 13:97–9. doi: 10.3870/j.issn.1001-2001.1998.03.001
47.
AVERT Trial Collaboration Group . Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. (2015) 386:46–55. doi: 10.1016/S0140-6736(15)60690-0
48.
Dong JP Zhao CY Deng XQ Wang LH Xiao Q . Partial body weight support on walking ability for stroke following hemiplegia at early stage. Chin J Rehabil Theory Pract. (2010) 16:958–60. doi: 10.3969/j.issn.1006-9771.2010.10.020
49.
Hesse S Helm B Krajnik J Gregoric M Mauritz KH . Treadmill training with partial body weight support: influence of body weight release on the gait of Hemiparetic patients. Neurorehabil Neural Repair. (1997) 11:15–20. doi: 10.1177/154596839701100103
50.
Van KK Boonstra A Reinders-Messelink H den Otter R . The combined effects of body weight support and gait speed on gait related muscle activity: a comparison between walking in the Lokomat exoskeleton and regular treadmill walking. PLoS One. (2014) 9:e107323. doi: 10.1371/journal.pone.0107323
51.
Wu H Gu XD Fu JM Yao YH Li Y Fu XW et al . Effects of different forms of weight-supported plate training on walking speed and lower limb joint motion in stroke patients. Zhejiang Med J. (2016) 38:1196–9.
52.
Crompton S Khemlani M Batty J Ada L Dean C Katrak P . Practical issues in retraining walking in severely disabled patients using treadmill and harness support systems. Aust J Physiother. (2001) 47:211–3. doi: 10.1016/s0004-9514(14)60268-3
Summary
Keywords
body weight support treadmill training, stroke, lower limb motor function, activity of daily living, meta-analysis
Citation
Shi Y, Liu J and Zhang X (2025) Optimal training strategy for body weight support treadmill training to enhance lower limb motor function and activity of daily living in persons with stroke: a systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 16:1649246. doi: 10.3389/fneur.2025.1649246
Received
18 June 2025
Accepted
15 August 2025
Published
02 September 2025
Volume
16 - 2025
Edited by
Fan Gao, University of Kentucky, United States
Reviewed by
Meng Zhang, Shanghai University of Sport, China
Katsuyuki Morishita, Josai International University, Japan
Updates
Copyright
© 2025 Shi, Liu and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxin Zhang, zhangxx9606@snnu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.