Abstract
Background:
Migraine occurs two to three times more frequently in women than in men, exhibiting different clinical characteristics in both sexes. The present study aims to investigate further and extend the findings of sex-specific migraine phenotypes in a large cohort of subjects with migraine enrolled in the “Italian Headache Registry” (RICe).
Methods:
This is a post-hoc analysis of prospectively collected data including subjects with episodic (EM) and chronic (CM) migraine, with or without medication-overuse headache (MOH), registered in the RICe database by 24 Italian headache centers. Migraine demographic and clinical characteristics were recorded, including quality and intensity of pain, pain localization at onset, concomitant symptoms, and monthly headache days (MHD).
Results:
We included 2,841 migraine subjects (80.0% women; mean age: 45.7 ± 14.3 years; mean MHDs 12.3 ± 9). Among them, 2,087 subjects had EM (73.5%), 754 (26.5%) had CM, and 273 (36.2%) had MOH. When considering individuals with EM and CM as a whole group, women reported higher pain intensity compared to men (NRS scale women [mean 7.6 ± 1.7] vs. men [7.0 ± 2.1], p = 0.006). This difference was also confirmed when comparing intensity categories (severe, moderate/severe, and moderate/mild) (p = 0.020). Moderate/mild attacks occurred more frequently in men than in women (14.9 vs. 7.7%, p = 0.0014). Furthermore, women reported more frequent migraine-associated symptoms such as photophobia/phonophobia (women: 72.7% vs. men: 62.3%, p = 0.006) and nausea/vomiting (women: 44.3% vs. men: 36.0%, p = 0.006). No sex differences were reported in terms of MHDs (p = 0.571) or baseline diagnoses (EM vs. CM, p = 0.269). Focusing on EM individuals, significant sex differences emerged in the summarized intensity categories (p = 0.012), as well as in the percentage of concomitant symptoms, which women more frequently reported.
Conclusion:
Women with EM or CM have higher pain intensity and more frequent concomitant migraine symptoms when compared to men. No sex-related differences were found in the frequency of MOH.
1 Introduction
Migraine is a common neurological disorder and one of the most common causes of disability worldwide (1). It is characterized by recurrent headache attacks accompanied by a plethora of concomitant symptoms (1). Noteworthy, the prevalence of migraine is three times higher in women than in men, and there is growing evidence that sex differences affect age at presentation and clinical features (2). Women with migraine are characterized by a longer duration of attacks, higher reported disability and a greater number of concomitant symptoms and trigger factors compared to men (3, 4). This is in line with advanced neuroimaging findings revealing significant microstructural and functional differences in the brains of men and women with migraine (5), supporting the notion of a ‘sex phenotype’, consistent with the observations of a sex-related neurotransmitter profile (6). On the other hand, to date, there is divergent evidence on whether female biological sex could be considered a risk factor for the conversion from episodic migraine (EM) to chronic migraine (CM) (7).
Beyond environmental and genetic factors, sex hormones, per se, are thought to play a key role in epidemiologic and clinical manifestations of migraine (8). This is supported by migraine changes during the female reproductive period. For instance, during the woman’s fertile period, fluctuations in sex hormones may lower the so-called “migraine threshold” and increase susceptibility to more severe migraine attacks (9).
While biological sex also seems to play a role in stress and emotional responses (10), a recent online survey of an Italian migraine population revealed that migraine phenotypes are independent of gender (11). Indeed, it is still a matter of debate whether sex could affect the progression of migraine severity over time, as well as whether it is possible to distinguish different sex-specific phenotypes. The exploration of these putative differences in large cohorts of migraine individuals could compensate for the bias due to the lower prevalence of migraine in men (12). Herein, we describe sex-related differences in migraine clinical features in a large cohort of migraine subjects enrolled in the Italian Headache Registry (RICe).
For this study, we use the term “sex” to refer to the biological category of male or female.
2 Methods
2.1 Study design and ethics
The RICe (Registro Italiano per le Cefalee) was established in 2019 to study the epidemiology of primary headache disorders in Italy. This study involves all migraine subjects visiting Italian headache centers (at all levels of headache healthcare) included in the registry.
To investigate sex-related differences in migraine clinical characteristics, we performed a post hoc analysis of prospectively collected data from the RICe, from 24 headache centers that expressed interest in joining the project. This is a multicenter, descriptive, cross-sectional study that included demographic and clinical data from all consecutive outpatients who underwent a first or a follow-up visit for migraine between January 2019 and October 2022. All individuals provided their informed consent to participate in the RICe study.
Subjects were then assessed by expert headache specialists using a semi-structured questionnaire containing detailed information on demographic and migraine characteristics. The study adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
2.2 Patient features and variables collected
The study recruited adult individuals (≥18 years old) receiving a diagnosis of migraine without aura [1.1 of International Classification of Headache Disorders 3rd Edition- ICHD-3], migraine with aura [1.2 of ICHD-3], chronic migraine [1.3 of ICHD-3] or Medication overuse headache (MOH) [8.2 of ICHD-3] according to the ICHD-3 criteria (13).
Individuals aged <18 years and subjects with incomplete or missing demographic data or with multiple ICHD-3 diagnoses (other than CM and MOH) were excluded. The variables collected included age, sex, age at migraine onset, presence of aura, migraine frequency (number of monthly headache days - MHDs), pain intensity (Numeric Rating Scale [NRS], ranging from 1 to 10), pain quality (pulsating, pressing/tightening), localization at the onset of migraine attacks (unilateral/bilateral), accompanying symptoms including nausea and/or vomiting (nausea/vomiting) and photophobia and/or phonophobia (photophobia/phonophobia), aggravation by, or avoidance of, routine physical activity, presence of allodynia (assessed using 12 items of the allodynia questionnaire) (14) or dizziness during the attacks. The intensity of pain was also reported as summarized intensity categories (i.e., severe, moderate/severe, or moderate/mild pain). All variables were documented electronically and extracted using the RICe registry.
2.3 Statistical analysis and missing data
Given the descriptive nature of the study, the sample size was not determined based on statistical considerations. Data from all consecutive outpatients enrolled during the study period were included and analyzed (see section 2.3).
We reported mean ± standard deviation [SD] for continuous variables and number (percentage) for categorical data. Categorical variables were dichotomized where appropriate (presence/absence), and comparisons between sex (male/female) and presence/absence of clinical characteristics were analyzed.
The distribution of each numerical variable was tested using the Shapiro–Wilk test. Comparisons between the two groups (men and women) were performed using the chi-square χ2 test for categorical variables and the Mann–Whitney U test for continuous variables. p-values were adjusted for multiple comparisons using the Benjamini–Hochberg false discovery rate (FDR) procedure. No imputation was performed for missing data, and the number of subjects analyzed for a single variable is indicated in the figure and table legends where applicable. SPSS software version 26.0 (IBM Corp. SPSS Statistics, Armonk, NY, United States), and R (R Foundation for Statistical Computing), run in RStudio (Posit, PBC) were used for all data analyses.
3 Results
3.1 Cohort characteristics
Starting from a dataset of 3,244 individuals, after inclusion/exclusion criteria evaluation, we included in the final analysis 2,841 subjects (80.0% women) with a mean age of 46.1 ± 13.9 years (Figure 1). In the whole cohort, 754 individuals (26.5%) had a CM diagnosis, of whom 273 (36.2%) had associated MOH. The mean MHDs were 12.3 ± 9.1 for the entire cohort. In the EM group (1,653 women, 79.2%), the mean MHD was 9.3 ± 7.6, and in the CM group (620 women, 82.2%), the mean MHD was 20.4 ± 8.0.
Figure 1

Flowchart of subjects.
The mean age at migraine onset was 19.0 ± 12.0 years (19.2 ± 11.0 years for the EM group and 18.3 ± 14.5 for the CM group). All demographic and clinical characteristics are listed in Table 1.
Table 1
| Overall population (n = 2,841) | |
|---|---|
| Demographics | |
| Age [years], mean ± SD | 46.1 ± 13.9 |
| Sex female, n (%) | 2,272 (80.0) |
| Migraine features | |
| Age at onset [years], mean ± SD | 19.0 ± 12.0 |
| Chronic migraine (CM), n (%) | 754 (26.5) |
| Medication overuse headache (MOH), n (%)a | 273 (36.2)a |
| Migraine with aura, n (%) | 390 (13.7) |
| Monthly headache days (MHD), mean ± SD | 12.3 ± 9.1 |
| NRS, mean ± SD | 7.5 ± 1.8 |
Patients demographic and clinical features.
Percentages are expressed on the column total if not otherwise specified.
Calculated on CM patients. NRS, Numeric Rating Scale; SD, standard deviation.
3.2 Sex differences in migraine clinical features in the whole cohort
Males and females did not differ significantly, considering both age (p = 0.571) and age at migraine onset (p = 0.974). No sex-related differences were found between the frequency of EM and CM (p = 0.269), CM with or without MOH (p = 0.566), as well as MHDs (p = 0.571).
Including the whole cohort (subjects with EM and subjects with CM, n = 2,841), women reported a higher headache pain intensity compared to men [NRS scale: women (7.6 ± 1.7) vs. men (7.0 ± 2.1; p = 0.006)]. The distribution of summarized reported pain intensity categories (severe, moderate/severe or moderate/mild) also differed significantly between men and women (p = 0.020). In particular, the moderate/mild intensity category was strongly represented in men compared to women (14.9% vs. 7.6%, p = 0.0014), while no sex-related differences were found for the moderate/severe (p = 0.347) and severe (p = 0.571) intensities.
Regarding pain location at the onset, quality (pulsating or pressing/tightening quality) of pain, and aggravation by, or avoidance of, physical activity, there were no significant sex-related differences. Conversely, women reported higher prevalence of hypersensitivity symptoms, namely photophobia and/or phonophobia (females 72.7% vs. males 62.3%, p = 0.006) and neurovegetative symptoms (females 44.3% vs. males 36.0%, p = 0.006). No significant differences were found in interictal allodynia (p = 0.741) and dizziness (p = 0.570) between men and women.
Detailed clinical migraine features are presented in Table 2 and Figure 2.
Table 2
| Females (n = 2,272) | Males (n = 569) | p-value* | |
|---|---|---|---|
| Demographics | |||
| Age [years], mean ± SD | 45.2 ± 14.1 | 45.6 ± 14.1 | 0.571 |
| Migraine features | |||
| Age at onset [years], mean ± SD | 16.7 ± 8.8 | 17.1 ± 10.4 | 0.974 |
| Chronic migraine (CM), n (%) | 620 (27.3) | 134 (23.5) | 0.269 |
| Medication overuse headache (MOH), n (%)* | 225 (9.9) | 48 (8.4) | 0.566 |
| Aura, n (%) | 301 (13.2) | 89 (15.6) | 0.345 |
| Monthly headache days (MHD), mean ± SD | 10.0 ± 8.4 | 11.4 ± 9.3 | 0.571 |
| NRS, mean ± SD | 7.6 ± 1.7 | 7.0 ± 2.1 | 0.006 |
| Features of the attack, n (%) | |||
| Pain localization | |||
| Unilaterala | 742 (33.6) | 178 (32.8) | 0.776 |
| Bilateralb | 193 (25.3) | 52 (30.6) | 0.566 |
| Pain quality | |||
| Pulsatingc | 981 (62.9) | 227 (57.9) | 0.748 |
| Pressing/tighteningd | 171 (23.4) | 45 (26.9) | 0.566 |
| Aggravated by physical activitye | 611 (27.6) | 138 (25.4) | 0.566 |
| Pain intensity | |||
| Mild/moderatef | 56 (7.6) | 25 (14.9) | 0.014 |
| Moderate/severeg | 762 (40.9) | 177 (37.3) | 0.347 |
| Severeh | 283 (38.3) | 57 (34.8) | 0.571 |
| Accompanying symptoms | |||
| Photophobia/phonophobiai | 1,618 (72.7) | 342 (62.3) | 0.006 |
| Nausea/vomitingi | 989 (44.3) | 196 (36.0) | 0.006 |
| Ictal allodynial | 121 (16.6) | 25 (15.1) | 0.741 |
| Ictal dizzinessm | 121 (22.1) | 22 (18.3) | 0.570 |
Sex differences in migraine clinical features including the whole cohort (n = 2,841).
*p-values adjusted for multiple comparisons using the Benjamini–Hochberg false discovery rate (FDR) procedure. Percentages are expressed on the column total. NRS, Numeric Rating Scale; SD, standard deviation. Calculated on: a2750 patients, b905 patients, c1951 patients, d899 patients, e2752 patients, f900 patients, g2320 patients, h902 patients; 2,775 patients, l894 patients, m667 patients. *Calculated on CM patients. Values in bold are statistically significant.
Figure 2
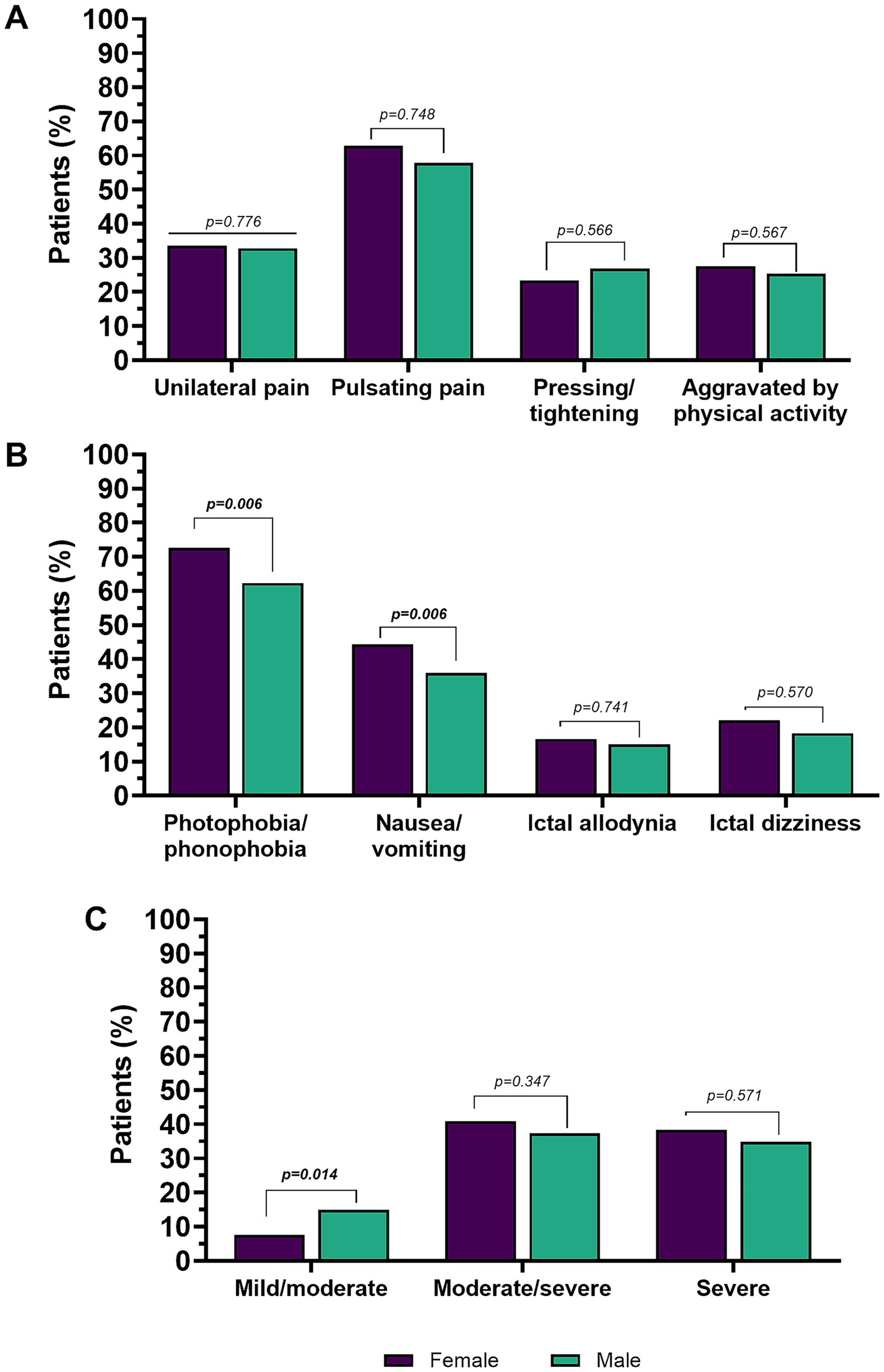
Pain features (A), accompanying symptoms (B), and pain intensity in the overall population (C). Percentages are calculated on the total number of males or females, respectively. Values in bold are statistically significant.
3.3 Sex differences in migraine clinical features in episodic and chronic migraine
3.3.1 Episodic migraine
Considering only EM individuals (n = 2,087), no sex-related differences were found for age (p = 0.536), and age of migraine onset (p = 0.684). Furthermore, no differences were found in the MHDs (p = 0.684). Women with EM had a higher pain intensity (NRS) than men (7.5 ± 1.8 and 6.8 ± 2.0, respectively, p = 0.005). This was confirmed by the distribution of pain intensity levels (severe, moderate/severe, and moderate/mild), men more frequently reporting mild to moderate headache intensity (women 7.6% vs. men 16.7%, p = 0.012). Women more frequently reported severe headache intensity (39.5% vs. 28.7% in men), with a difference that approached but did not reach statistical significance (p = 0.08).
There were no significant sex-specific differences in the localization of the headache at onset, the pulsating quality, and the pressing or pulling quality. Exacerbation by, or avoidance of, physical activity and interictal allodynia were equally distributed among both sexes.
In terms of accompanying symptoms, women were more likely to report photophobia/phonophobia and nausea/vomiting compared to men (both p = 0.005). The clinical features of migraine in men and women with EM are shown in Table 3 and Figure 3.
Table 3
| Females (n = 1,652) | Males (n = 435) | p-value* | |
|---|---|---|---|
| Demographics | |||
| Age [years], mean ± SD | 45.2 ± 13.7 | 44.4 ± 13.7 | 0.536 |
| Migraine features | |||
| Age at onset, mean ± SD | 19.3 ± 11.0 | 18.9 ± 10.7 | 0.684 |
| Monthly headache days (MHD), mean ± SD | 9.3 ± 7.6 | 9.1 ± 7.6 | 0. 684 |
| NRS, mean ± SD | 7.5 ± 1.8 | 6.8 ± 2.0 | 0.005 |
| Features of the attack, n (%) | |||
| Pain localization | |||
| Unilaterala | 549 (34.5) | 127 (30.9) | 0.477 |
| Bilateralb | 130 (24.2) | 35 (26.5) | 0.684 |
| Pain quality | |||
| Pulsatingc | 700 (62.1) | 176 (58.9) | 0.536 |
| Pressing/tighteningd | 119 (22.2) | 32 (24.2) | 0.684 |
| Aggravated by physical activitye | 430 (27.9) | 102 (24.8) | 0.554 |
| Pain intensity | |||
| Mild/moderated | 41 (7.6) | 22 (16.7) | 0.012 |
| Moderate/severef | 555 (41.9) | 139 (38.7) | 0.536 |
| Severeg | 214 (39.5) | 37 (28.7) | 0.08 |
| Accompanying symptoms | |||
| Photophobia/phonophobiah | 1,158 (72.0) | 257 (61.6) | 0.005 |
| Nausea/vomitingi | 710 (44.0) | 146 (35.4) | 0.005 |
| Ictal allodynial | 71 (13.4) | 16 (12.3) | 0.885 |
| Ictal dizzinessm | 92 (23.1) | 17 (18.1) | 0.536 |
Sex differences in migraine clinical features in patients with episodic migraine (n = 2,087).
Percentages are expressed on the column total. NRS, Numeric Rating Scale; SD, standard deviation. Calculated on: a2004 patients, b670 patients, c1427 patients, d669 patients, e2005 patients, f1685 patients, g671 patients, h2026 patients, i2025 patients, l661 patients, m492 patients. *p-values adjusted for multiple comparisons using the Benjamini–Hochberg false discovery rate (FDR) procedure. Values in bold are statistically significant.
Figure 3

Pain features (A), accompanying symptoms (B), and pain intensity in subjects with episodic migraine (C). Percentages are calculated on the total number of males or females, respectively. Values in bold are statistically significant.
3.3.2 Chronic migraine
In the CM group (n = 754), there were no sex-related differences in terms of age (p = 0.538) and age of migraine onset (p = 0.754). There were also no differences in MHDs.
Pain intensity (NRS) did not differ significantly between men and women with CM (p = 1.0). No significant sex-related difference was found in the summarized reported pain intensity categories.
There were no significant sex-related differences in the location of the headache at onset and the pain quality. Other characteristics were also equally represented in both sexes, such as aggravation by, or avoidance of, physical activity and interictal allodynia.
There were no differences in accompanying symptoms. The clinical features of migraine in men and women subjects with CM are shown in Table 4 and Figure 4.
Table 4
| Females (n = 620) | Males (n = 134) | p-value* | |
|---|---|---|---|
| Demographics | |||
| Age [years], mean ± SD | 48.9 ± 13.8 | 50.2 ± 13.9 | 0.538 |
| Migraine features | |||
| Age at onset, mean ± SD | 18.1 ± 14.7 | 19.3 ± 13.4 | 0.754 |
| Medication overuse headache (MOH), n (%) | 225 (36.3) | 48 (35.8) | 1.0 |
| Monthly headache days (MHD), mean ± SD | 20.3 ± 8.2 | 21.2 ± 6.9 | 0.724 |
| NRS, mean ± SD | 8.0 ± 1.4 | 7.7 ± 2.0 | 1.0 |
| Features of the attack, n (%) | |||
| Pain localization | |||
| Unilaterala | 193 (31.4) | 51 (38.9) | 0.416 |
| Bilateralb | 63 (32.0) | 17 (44.7) | 0. 416 |
| Pain quality | |||
| Pulsatingc | 281 (65.2) | 51 (54.8) | 0. 416 |
| Pressing/tighteningd | 52 (26.7) | 13 (37.1) | 0.476 |
| Aggravated by physical activitye | 181 (29.4) | 36 (27.5) | 1.0 |
| Pain intensity | |||
| Mild/moderatef | 15 (7.7) | 3 (8.3) | 1.0 |
| Moderate/severeg | 107 (20.6) | 28 (24.3) | 0.476 |
| Severef | 69 (35.2) | 20 (57.1) | 0.195 |
| Accompanying symptoms | |||
| Photophobia/phonophobiah | 460 (74.4) | 85 (64.4) | 0.195 |
| Nausea/vomitingi | 279 (45.1) | 50 (37.9) | 0.416 |
| Ictal allodynial | 50 (25.4) | 9 (25.0) | 1.0 |
| Ictal dizzinessm | 29 (19.5) | 5 (19.2) | 1.0 |
Sex differences in migraine clinical features in patients with chronic migraine (n = 754).
Percentages are expressed on the column total. NRS, Numeric Rating Scale; SD, standard deviation. Calculated on: a746 patients, b235 patients, c524 patients, d230 patients, e747 patients, f231 patients, g635 patients, h749 patients, i750 patients, l233 patients, m175 patients. *p-values adjusted for multiple comparisons using the Benjamini–Hochberg false discovery rate (FDR) procedure. Values in bold are statistically significant.
Figure 4
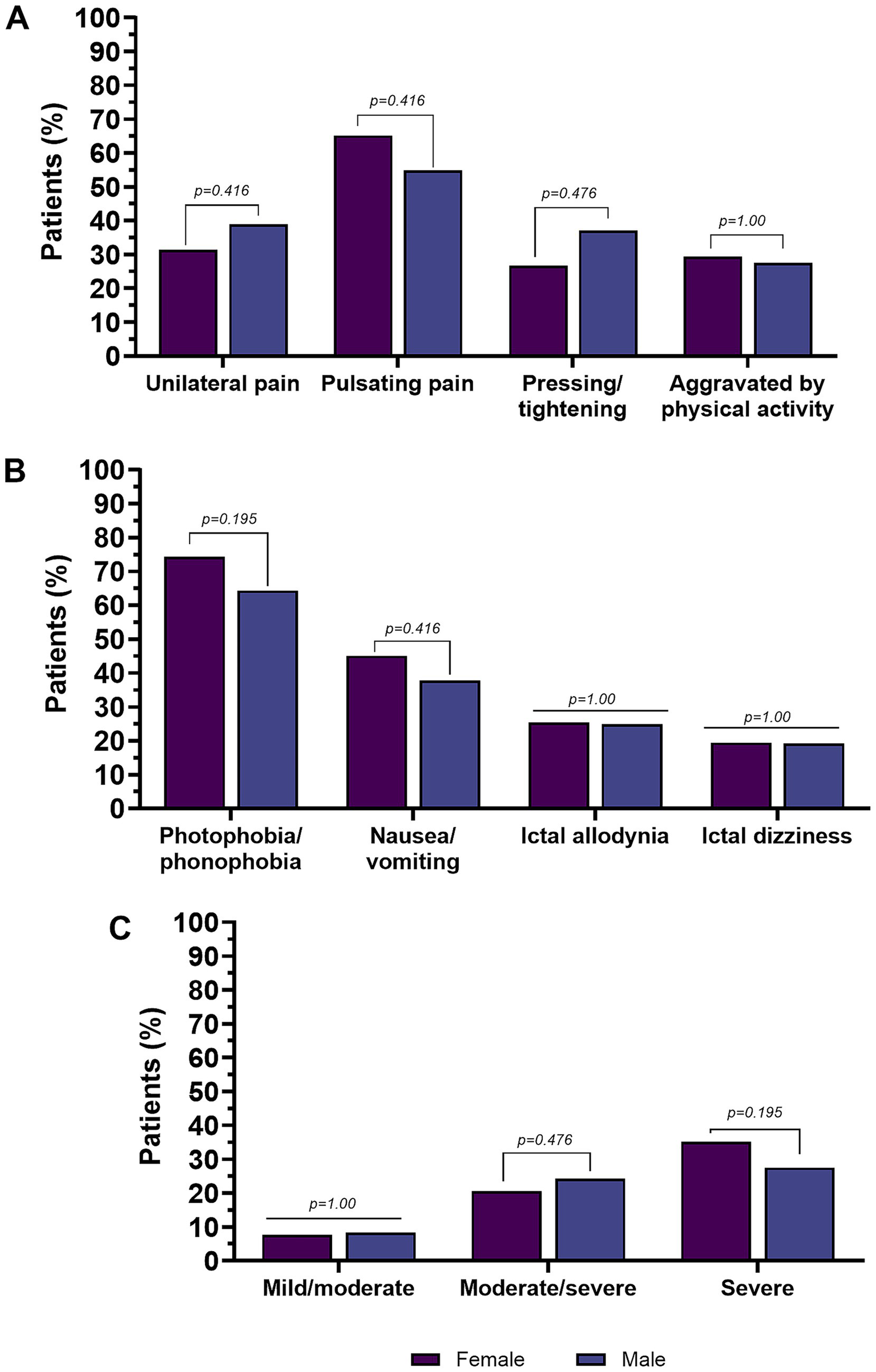
Pain features (A), accompanying symptoms (B), and pain intensity in subjects with chronic migraine (C). Percentages are calculated on the total number of males or females, respectively. Values in bold are statistically significant.
4 Discussion
In the present study, we analyzed sex-related demographic and clinical differences within a large cohort of 2,841 individuals enrolled in the Italian Headache Registry (RICe), of whom 80.0% were women. This data is in line with the well-known epidemiology of migraine (15).
Overall, our results showed that women with EM tend to experience more severe attacks and more frequently report accompanying symptoms compared to men, whereas in the CM group, there were no significant differences between sexes. Finally, we found no significant sex-related differences in migraine frequency or prevalence of CM with MOH.
A growing body of evidence has recently highlighted sex-related differences in clinical features of migraine, such as a significantly longer duration and higher frequency of the attacks, and more severe pain intensity in women, along with more severe headache-related disability (8, 16, 17). In a recent work, women scored significantly higher on the MIDAS scale, with this impact particularly evident in questions related to household chores and social outings (16).
Data regarding the higher pain intensity experienced by females with migraine (regardless of experiencing EM and CM) compared to males are in line with the present findings (16, 18).
Several hypotheses for the sex-related differences in pain intensity have been proposed, including lower pain tolerance and threshold for noxious stimuli (18). Furthermore, using advanced neuroimaging techniques, higher functional response in brain regions involved in emotional processing was observed during nociceptive stimuli in women with migraine compared to men, likely representing the underlying neuronal correlates of the greater unpleasantness reported by women in response to painful experience (19). It cannot be excluded that the data of a mean longer headache duration in women reported in the literature may explain the impact of the perception of headache intensity (3). On the other hand, the influence of gender (different but still closely related to biological sexes), role expectations, and social constructs should not be underestimated in the context of pain conditions, where women tend to report pain and seek medical treatment more than men (20, 21).
The finding of a higher percentage, although not statistically significant, of men reporting severe pain in the CM group is of interest and need evaluation in future studies, accurately collecting pain severity. Indeed, a direct comparison with previous studies on sex-related differences is challenging, as in earlier works, there were no distinctions between pain severity reported by individuals with EM and CM, except for a single study that reported a lower pain intensity in EM men compared to women. In contrast, in line with our results, no sex-related differences in pain intensity and other migraine features were observed among subjects with CM, probably suggesting that the mechanism associated with migraine chronicization may equally affect women and men (22).
Interestingly, previous findings showed a peculiar biological profile in men with CM compared to age-matched healthy controls, reporting evidence of lower total testosterone levels that are known to have antinociceptive effects (23). Moreover, studies on basal mechanisms of nociception highlight reduced adaptation and habituation to continuous pain in men than women. The same phenomenon may also occur in cases of repetitive attacks experienced by individuals with CM. Altogether, these findings suggest that men with CM may have a profile of more severe headaches and distress, although further research on male populations is mandatory, as they are often underrepresented (24).
Similarly to our study, previous literature did not observe sex-related differences in patients with EM and CM (22). Lastly, the prevalence of CM with MOH in our study was similar among males and females, according to previous studies (25).
When considering clinical features, previous literature supports a higher female prevalence of accompanying symptoms, allodynia, and migraine triggers (17, 20, 25). Interestingly, the aforementioned findings align with the present results in patients with EM.
Neurovegetative symptoms during migraine attacks, such as nausea and vomiting, are likely due to fluctuations in dopamine levels and altered dopamine sensitivity in migraine (6). Gruber and colleagues demonstrated that dopamine levels were elevated during the headache-free period in women, while no increase was observed in men with migraine (6). On the other hand, neurosensory symptoms, such as photophobia and phonophobia, result from cortical hyperresponsiveness of the visual and auditory cortices (26). Specifically, sex-related differences may exist when considering photophobia, with women potentially exhibiting heightened light sensitivity during the follicular phase of the menstrual cycle (27). This may tie in with the observation that women with migraine usually report exposure to bright light more frequently as a trigger for migraine attacks (17). However, it remains unclear whether these differences in light sensitivity can mirror ictal photophobia (27). Photophobia, as well as phonophobia, has also been linked to increased levels of calcitonin gene-related peptide (CGRP) in the trigeminovascular system. CGRP levels seem, in turn, modulated by estrogen fluctuations in women, potentially explaining the higher prevalence of neurosensory symptoms found in females (28). Hence, this pathophysiological interpretation could be relevant not only for interpreting sex-related differences in accompanying symptoms but also for considering differences in treatment response, particularly concerning the novel acute and preventive therapies specifically targeting the CGRP pathway among women and men (29).
Interestingly, no sex-related differences were found in ictal cutaneous allodynia. A recent study in a monocentric cohort found a marked prevalence of ictal cutaneous allodynia in the female group (25). However, the same authors found that, although allodynia was the more discriminating factor among females and males, a strong overlap existed between the sexes (25). This study found that in a larger patient population, features of sensitization, particularly cutaneous allodynia, are commonly observed in men with migraine. The present study only considered the presence/absence of allodynia; future research should aim to quantify the intensity of cutaneous allodynia and to distinguish between ictal and interictal allodynia.
The present study has several strengths, including the large sample size, the inclusion of consecutive outpatients regardless of clinical characteristics or migraine diagnosis, and the use of a semi-structured face-to-face interview conducted by trained specialists. Nevertheless, limitations must also be acknowledged: first of all, the descriptive, cross-sectional design of the study limits the ability to draw causal conclusions. Additionally, some critical data, such as the duration of attacks and information on symptomatic or preventive treatments, as well as data on migraine disability, were not available for the current analysis.
As expected, due to the study design, some data were missing, as stated in the manuscript. Given these drawbacks, the registry has already been implemented to include these variables for future larger and more comprehensive studies. Lastly, the study cohort was recruited exclusively from tertiary headache centers, which may limit the generalizability of our findings to the broader migraine population. Furthermore, the pronounced gender imbalance (approximately 80% women) may have reduced statistical power for analyses in men, increasing the risk of type II error in that subgroup.
5 Conclusion
Overall, the available results confirm that women with migraine experience more intense attacks and a higher frequency of accompanying symptoms than men. Despite pathophysiological, psychosocial, or cultural factors that clearly differentiate females and males, migraine phenotype seems to be similar in several aspects independently of biological sex. This would describe a common and shared disability profile with important implications for the clinical presentation and management of migraine in both females and males.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study is part of the Registro Italiano Cefalee (RICe) study approved by the local Ethics committee (Comitato Etico Area Vasta Toscana Centro; CEAVC Studio RICe, 14591_oss and subsequent amendments 2022–609; 2024). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MR: Investigation, Writing – original draft, Methodology, Formal analysis, Writing – review & editing, Project administration. LI: Investigation, Methodology, Writing – review & editing, Formal analysis, Data curation, Project administration, Writing – original draft. MS: Investigation, Visualization, Writing – review & editing, Data curation, Validation. GP: Data curation, Writing – review & editing. StS: Writing – review & editing, Data curation. SB: Writing – review & editing, Data curation. RO: Data curation, Conceptualization, Writing – review & editing. SiS: Validation, Writing – review & editing, Conceptualization, Data curation, Supervision, Visualization. FS: Data curation, Conceptualization, Writing – review & editing. IR: Validation, Visualization, Supervision, Data curation, Writing – review & editing, Conceptualization. AM: Data curation, Conceptualization, Writing – review & editing. GaS: Validation, Data curation, Writing – review & editing. CA: Data curation, Writing – review & editing, Validation. PS: Data curation, Visualization, Conceptualization, Validation, Writing – review & editing, Supervision. IC: Writing – review & editing, Data curation. GV: Validation, Writing – review & editing, Data curation, Conceptualization. RI: Supervision, Data curation, Writing – review & editing, Conceptualization. GrS: Supervision, Data curation, Conceptualization, Writing – review & editing. CT: Investigation, Validation, Conceptualization, Writing – review & editing, Supervision, Funding acquisition, Software, Formal analysis, Project administration, Methodology, Visualization, Data curation, Resources. SG: Supervision, Data curation, Conceptualization, Writing – review & editing, Validation, Visualization. FLC: Data curation, Writing – review & editing, Conceptualization. AG: Writing – review & editing, Data curation. LB: Writing – review & editing, Data curation. FDC: Writing – review & editing, Data curation, Supervision. AB: Writing – review & editing, Data curation. GV: Writing – review & editing, Supervision, Data curation. MC: Data curation, Writing – review & editing. MG: Data curation, Writing – review & editing. PC: Writing – review & editing, Visualization, Validation, Supervision. MP: Writing – review & editing, Conceptualization, Visualization, Data curation, Supervision, Validation. AR: Writing – review & editing, Project administration, Conceptualization, Methodology, Supervision, Writing – original draft, Validation, Investigation, Visualization, Data curation. MT: Formal analysis, Project administration, Writing – original draft, Data curation, Methodology, Supervision, Visualization, Investigation, Writing – review & editing, Validation, Conceptualization.
Group members of the RiCe study group
Giovanni Rinaldi12; Marco Alabiso12; Giulia Settembrini12; Annalisa Di Dio6,7; Giulia Vigani16; Adriana Fallacara18; Silvia Grimaldi18; Cinzia Tamborino18; Gianni Di Fonzo18.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The “Società Italiana per lo Studio delle Cefalee” (SISC) is acknowledged for the “Registro Italiano delle Cefalee (RiCe).”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ashina M . Migraine. N Engl J Med. (2020) 383:1866–76. doi: 10.1056/NEJMra1915327
2.
Rossi MF Tumminello A Marconi M Gualano MR Santoro PE Malorni W et al . Sex and gender differences in migraines: a narrative review. Neurol Sci. (2022) 43:5729–34. doi: 10.1007/s10072-022-06178-6
3.
Vetvik KG MacGregor EA . Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. (2017) 16:76–87. doi: 10.1016/S1474-4422(16)30293-9
4.
Verhagen IE van der Arend BWH van Casteren DS le Cessie S MaassenVanDenBrink A Terwindt GM . Sex differences in migraine attack characteristics: a longitudinal E-diary study. Headache. (2023) 63:333–41. doi: 10.1111/head.14488
5.
Maleki N Linnman C Brawn J Burstein R Becerra L Borsook D . Her versus his migraine: multiple sex differences in brain function and structure. Brain. (2012) 135:2546–59. doi: 10.1093/brain/aws175
6.
Gruber HJ Bernecker C Pailer S Lechner A Horejsi R Möller R et al . Increased dopamine is associated with the cGMP and homocysteine pathway in female migraineurs. Headache. (2010) 50:109–16. doi: 10.1111/j.1526-4610.2009.01533.x
7.
Bigal ME Serrano D Buse D Scher A Stewart WF Lipton RB . Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. (2008) 48:1157–68. doi: 10.1111/j.1526-4610.2008.01217.x
8.
Al-Hassany L Haas J Piccininni M Kurth T Maassen Van Den Brink A Rohmann JL . Giving researchers a headache - sex and gender differences in migraine. Front Neurol. (2020) 11:549038. doi: 10.3389/fneur.2020.549038
9.
Granella F Sances G Allais G Nappi RE Tirelli A Benedetto C et al . Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia. (2004) 24:707–16. doi: 10.1111/j.1468-2982.2004.00741.x
10.
Slatculescu AM Chen Y . Synergism between female gender and high levels of daily stress associated with migraine headaches in Ontario, Canada. Neuroepidemiology. (2018) 51:183–9. doi: 10.1159/000492503
11.
Delussi M Piraino G Guerzoni S Castro FL Sances G Guaschino E et al . Gender-related stress factors and emotional perception in migraine: a structured online questionnaire in migraine patients and controls. Neurol Sci. (2024) 45:1645–54. doi: 10.1007/s10072-023-07152-6
12.
Ornello R Ahmed F Negro A Miscio AM Santoro A Alpuente A et al . Is there a gender difference in the response to onabotulinumtoxinA in chronic migraine? Insights from a real-life European multicenter study on 2879 patients. Pain Ther. (2021) 10:1605–18. doi: 10.1007/s40122-021-00328-y
13.
Headache Classification Committee of the International Headache Society (IHS) . The international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102413485658
14.
Jakubowski M Silberstein S Ashkenazi A Burstein R . Can allodynic migraine patients be identified interictally using a questionnaire?Neurology. (2005) 65:1419–22. doi: 10.1212/01.wnl.0000183358.53939.38
15.
Chen Z-f Kong X-m Yang C-h Li X-y Guo H Wang Z-w . Global, regional, and national burden and trends of migraine among youths and young adults aged 15–39 years from 1990 to 2021: findings from the global burden of disease study 2021. J Headache Pain. (2024) 25:131. doi: 10.1186/s10194-024-01832-0
16.
Waliszewska-Prosół M Marschollek K Budrewicz S Więckiewicz M Nowaczewska M Straburzyński M . Migraine in men – differences in phenotype and treatment patterns: results from the migraine in Poland cross-sectional national survey. J Headache Pain. (2025) 26:173. doi: 10.1186/s10194-025-02117-w
17.
van Casteren DS Verhagen IE Onderwater GL MaassenVanDenBrink A Terwindt GM . Sex differences in prevalence of migraine trigger factors: a cross-sectional study. Cephalalgia. (2021) 41:643–8. doi: 10.1177/0333102420974362
18.
Russo A Coppola G Pierelli F Parisi V Silvestro M Tessitore A et al . Pain perception and migraine. Front Neurol. (2018) 9:576. doi: 10.3389/fneur.2018.00576
19.
Hashmi JA Davis KD . Deconstructing sex differences in pain sensitivity. Pain. (2014) 155:10–3. doi: 10.1016/j.pain.2013.07.039
20.
Buse DC Loder EW Gorman JA Stewart WF Reed ML Fanning KM et al . Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American migraine prevalence and prevention (AMPP) study. Headache. (2013) 53:1278–99. doi: 10.1111/head.12150
21.
Robinson ME Riley JL 3rd Myers CD Papas RK Wise EA Waxenberg LB et al . Gender role expectations of pain: relationship to sex differences in pain. J Pain. (2001) 2:251–7. doi: 10.1054/jpai.2001.24551
22.
Muñoz Gómez E Aguilar Rodríguez M Serra Añó P Sempere Rubio N Mollà Casanova S Inglés M . Sex-related differences in migraine clinical features by frequency of occurrence: a cross-sectional study. Scand J Pain. (2023) 23:553–62. doi: 10.1515/sjpain-2022-0152
23.
Shields LBE Seifert T Shelton BJ Plato BM . Testosterone levels in men with chronic migraine. Neurol Int. (2019) 11:8079. doi: 10.4081/ni.2019.8079
24.
Fitzek MP Boucherie DM de Vries T Handtmann C Fathi H Raffaelli B et al . Migraine in men. J Headache Pain. (2025) 26:3. doi: 10.1186/s10194-024-01936-7
25.
Paparella G Clemente L Scannicchio S Delussi M De Tommaso M . Sex differences in the expression of central sensitization symptoms in migraine: an observational study. J Womens Health (Larchmt). (2024) 33:1656–64. doi: 10.1089/jwh.2024.0297
26.
Boulloche N Denuelle M Payoux P Fabre N Trotter Y Géraud G . Photophobia in migraine: an interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. (2010) 81:978–84. doi: 10.1136/jnnp.2009.190223
27.
Vidafar P Spitschan M . Light on shedding: a review of sex and menstrual cycle differences in the physiological effects of light in humans. J Biol Rhythm. (2023) 38:15–33. doi: 10.1177/07487304221126785
28.
Alpuente A Gallardo VJ Asskour L Caronna E Torres-Ferrus M Pozo-Rosich P . Salivary CGRP can monitor the different migraine phases: CGRP (in)dependent attacks. Cephalalgia. (2022) 42:186–96. doi: 10.1177/03331024211040467
29.
Porreca F Dodick DW . Considering patient sex in prescribing CGRP receptor antagonists for short-term treatment of migraine. JAMA Neurol. (2023) 80:885–6. doi: 10.1001/jamaneurol.2023.2335
Summary
Keywords
migraine, sex, associated symptoms, headache intensity, gender
Citation
Romozzi M, Iannone LF, Silvestro M, Paparella G, Scannicchio S, Battistini S, Ornello R, Sacco S, De Santis F, Rainero I, Marcinnò A, Sebastianelli G, Abagnale C, Sarchielli P, Corbelli I, Vaghi G, De Icco R, Sances G, Tassorelli C, Guerzoni S, Castro FL, Granato A, Bartole L, De Cesaris F, Burgalassi A, Volta GD, Cortinovis M, Gentile M, Calabresi P, Prudenzano MP, Russo A, de Tommaso M and the RiCe study group (2025) Sex differences in the clinical features of 2,841 patients with migraine: a post-hoc, multicenter, cross-sectional study. Front. Neurol. 16:1649718. doi: 10.3389/fneur.2025.1649718
Received
18 June 2025
Accepted
25 August 2025
Published
04 September 2025
Volume
16 - 2025
Edited by
Parisa Gazerani, Oslo Metropolitan University, Norway
Reviewed by
Lanfranco Pellesi, University of Southern Denmark, Denmark
Marta Waliszewska-Prosół, Wroclaw Medical University, Poland
Updates
Copyright
© 2025 Romozzi, Iannone, Silvestro, Paparella, Scannicchio, Battistini, Ornello, Sacco, De Santis, Rainero, Marcinnò, Sebastianelli, Abagnale, Sarchielli, Corbelli, Vaghi, De Icco, Sances, Tassorelli, Guerzoni, Castro, Granato, Bartole, De Cesaris, Burgalassi, Volta, Cortinovis, Gentile, Calabresi, Prudenzano, Russo, de Tommaso and the RiCe study group.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Russo, dottor.russo@gmail.com
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.