Abstract
Background:
Post-stroke dysphagia (PSD) is a common complication with a high incidence rate, significantly impairing patients’ quality of life and health. Although conventional swallowing training is widely used, its efficacy depends on interindividual heterogeneity. Surface electromyographic biofeedback (sEMG-BF) is an emerging rehabilitation technology that shows promising potential in improving swallowing function. However, there is a lack of systematic and comprehensive evaluation and high-quality evidence to support its clinical application.
Objective:
This study aims to systematically evaluate and conduct a network meta-analysis comparing the efficacy of sEMG-BF, NMES, and conventional therapy in improving electrophysiological outcomes, swallowing function, and quality of life for patients with PSD.
Methods:
A systematic review and network meta-analysis were conducted by searching databases, including PubMed, EMBASE, Cochrane Library, Web of Science, and Scopus for prospective randomized controlled trials on the application of sEMG-BF in patients with PSD. We included randomized controlled trials that compared sEMG-BF, NMES, or conventional therapy in patients with PSD. The study focused on the effects of sEMG-BF on electrophysiological outcomes in these patients.
Results:
Six studies were included in the analysis. sEMG-BF was found to significantly increase mean amplitude (MD = 6.45, 95% CI: 3.53, 9.38) and reduce swallowing duration (MD = −0.22, 95% CI: −0.26, −0.18). The network meta-analysis revealed the following SUCRA ranking: sEMG-BF, neuromuscular electrical stimulation (NMES), and conventional therapy. sEMG-BF significantly improved the Standardized Swallowing Assessment (SSA) score (MD = −6.43, 95% CI: −9.74, −3.11). For Swallowing Quality of Life (SWAL-QOL), the pooled estimate was MD = 29.36 (95% CI: −14.96, 73.69), which did not reach statistical significance. The network meta-analysis demonstrated that sEMG-BF outperformed NMES and conventional therapy in improving swallowing function, consistent with direct comparison results.
Conclusion:
This study suggests that both sEMG-BF and NMES may provide benefits for PSD. Although sEMG-BF demonstrated superior effects in the majority of outcomes, the evidence is limited by small sample sizes and heterogeneity. Further high-quality trials are needed to confirm its efficacy. By enhancing the amplitude of electromyographic signals in swallowing-related muscles and improving muscle contraction capacity, sEMG-BF improves swallowing function; however, the pooled SWAL-QOL estimate was not statistically significant.
1 Introduction
Globally, stroke is the second leading cause of mortality and long-term impairment, representing a major public health challenge. It is frequently complicated by post-stroke dysphagia (PSD), with an estimated incidence of 34.4–80% (1–3). PSD not only significantly increases the risk of aspiration pneumonia but also elevates mortality (4). Dysphagia often leads to malnutrition and dehydration due to impaired swallowing, further exacerbating the deterioration of patients’ health (5). In addition, severe dysphagia restricts patients’ ability to participate in social activities, resulting in social isolation, diminished quality of life, and increased risk of depression (6, 7). Although conventional swallowing exercises, such as Shaker exercise and Mendelsohn maneuver, have been widely adopted, their efficacy is often limited by patients’ cognitive impairment or loss of proprioception, leading to significant interindividual heterogeneity in treatment outcomes (8, 9). Given these limitations, there is growing interest in exploring alternative therapeutic approaches.
In recent years, with advancements in neuroscience and rehabilitation medicine, various swallowing rehabilitation therapies have emerged. Surface electromyography biofeedback (sEMG-BF) therapy, a novel rehabilitation technique, has gained increasing attention and demonstrated promising potential in the treatment of PSD (10). sEMG-BF enhances the amplitude of electromyographic signals in swallowing-related muscles, improves muscle contraction capacity, and promotes upper esophageal sphincter opening and epiglottis elevation, thereby improving pharyngeal transit efficiency and bolus clearance while simultaneously enhancing airway protection mechanisms during deglutition (11–13). Furthermore, sEMG-BF can augment patients’ self-awareness and control over swallowing movements, promoting active participation in therapy and further optimizing treatment outcomes (14).
As a safe, effective, and feasible rehabilitation modality, sEMG-BF holds broad prospects for the treatment of PSD. However, systematic and comprehensive evaluation are still lacking, and high-quality evidence regarding its specific efficacy and advantages in this context remains limited. This study systematically evaluates the efficacy of sEMG-BF and NMES for PSD through network meta-analysis, comparing their therapeutic benefits against conventional therapy to establish evidence-based clinical recommendations.
2 Methods
2.1 Study design
Systematic review and network meta-analysis were conducted and reported in accordance with the PRISMA 2020 guidelines and the PRISMA extension for network meta-analyses (PRISMA-NMA). A dual methodological approach that combines systematic evidence synthesis with advanced network meta-analytical techniques was used to rigorously assess the therapeutic effectiveness of sEMG-BF for managing swallowing disorders following cerebrovascular accidents, aiming to provide high-quality evidence for clinical decision-making. This review was prospectively registered with the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY; Registration number: INPLASY202550028).
2.2 Literature search
A comprehensive literature search was conducted across five major databases, including Scopus, EMBASE, Web of Science, Cochrane CENTRAL, and PubMed/MEDLINE. The search encompassed all available records from each database’s inception through April 1, 2025, with language restrictions limited to English and Chinese. Search terms included “surface electromyography biofeedback,” “post-stroke dysphagia,” and related variations. Tailored search strategies were developed for each database to ensure comprehensiveness and accuracy.
For instance, the PubMed search strategy is as follows:
(“stroke” OR “cerebrovascular accident” OR “CVA” OR “brain attack”) AND (“dysphagia” OR “swallowing disorder” OR “swallowing difficulty” OR “feeding difficulty”) AND (“surface electromyography” OR “sEMG” OR “electromyography” OR “bioelectrical activity”).
2.3 Inclusion and exclusion criteria
The Population, Intervention, Comparison, Outcomes, Study Design (PICOS) framework was applied to determine eligible studies, as outlined in Table 1.
Table 1
| Dimension | Detail |
|---|---|
| Population | Adults aged ≥18 years with post-stroke dysphagia |
| Intervention | Surface electromyography-guided swallowing facilitation (sEMG-SF) |
| Comparison | Conventional swallowing rehabilitation training or neuromuscular electrical stimulation (NMES) |
| Outcomes | Primary outcomes: Electromyographic signals of submental muscle groups (mean amplitude and swallowing duration); Secondary outcomes: Quality of life and swallowing function scores |
| Study design | Randomized controlled trials (RCTs) |
PICOS criteria for literature inclusion.
2.3.1 Exclusion criteria
Studies were excluded based on the following criteria: (1) non-randomized studies, including case reports and conference abstracts; (2) studies involving patients with comorbid neurodegenerative disorders (e.g., Parkinson’s disease) or head/neck tumors; (3) interventions incorporating invasive biofeedback techniques such as intraluminal manometry; (4) duplicate publications, studies with incomplete data, or those for which full texts were inaccessible; and (5) studies with insufficient sample sizes that did not allow for meaningful statistical analysis.
2.3.2 Literature screening and data extraction
The study selection process was performed independently by two investigators using a dual-phase screening approach. In the primary phase, all retrieved citations underwent title and abstract review to identify potentially relevant publications, followed by comprehensive full-text assessment of selected articles in the secondary phase. Inter-rater disagreements were addressed through consensus discussions or arbitration by a senior researcher when required. A customized data collection template was used to capture key study elements, including publication metadata (author names, publication year, country of origin, and research design), population characteristics (sample size, demographic parameters, and stroke classification), intervention specifications (treatment frequency, duration, biofeedback parameters, and adjunct therapies), comparator details (sham procedures and standard care protocols), efficacy metrics (primary and secondary outcomes with corresponding assessment instruments), and temporal evaluation points (post-treatment follow-up intervals). This systematic approach ensured consistent and thorough data acquisition while minimizing selection bias. All extracted data were cross-verified by both researchers to ensure accuracy and completeness prior to analysis.
2.3.3 Quality assessment
The methodological quality of the included randomized controlled trials was rigorously assessed using the Cochrane Collaboration’s revised Risk of Bias tool (ROB 2.0), which systematically evaluates potential biases across five critical domains. The tool examined the adequacy of random sequence generation and allocation concealment in the randomization process, potential biases introduced by deviations from the protocol, including non-adherence or unintended unblinding, handling of missing data with particular attention to dropout rates and adherence to intention-to-treat analysis, objectivity in outcome measurement through assessor blinding and instrument validity, and consistency between pre-specified and reported outcomes to detect selective reporting. Each trial received domain-specific judgments of “low risk,” “some concerns,” or “high risk” of bias, with these evaluations subsequently synthesized and presented through both summary tables and visual plots for comprehensive interpretation. This systematic approach enabled transparent evaluation of study quality while identifying potential limitations in the evidence base.
2.3.4 Statistical analysis
The synthesis comprised two components: traditional pairwise meta-analysis and network meta-analysis (NMA).
2.3.5 Traditional meta-analysis
For continuous outcomes (e.g., motor function scores), mean differences (MDs) with 95% confidence intervals (CIs) were calculated using sample sizes, means, and standard deviations. When studies reported medians and interquartile ranges, these values were converted to means and SDs using the method described by Wan et al. (15), with explicit notation of this transformation in the results. Heterogeneity was quantified via the I2 statistic and Cochran’s Q test; a random-effects model was employed if I2 exceeded 50%. Subgroup analyses explored potential sources of heterogeneity (e.g., stroke phase and intervention dosage).
2.3.6 Network meta-analysis
A frequentist framework was adopted using STATA 17.0’s network package (StataCorp, College Station, TX, USA). The geometry of the intervention network was visualized with nodes (treatments) sized by sample volume and edges (direct comparisons) weighted by study count. Consistency between direct and indirect evidence was tested via node-splitting (p > 0.10 indicating agreement). Treatment rankings were derived from surface under the cumulative ranking curve (SUCRA) values, where higher percentages denoted superior efficacy.
3 Results
3.1 Literature screening process
A systematic search was conducted across six databases (PubMed, Scopus, Web of Science, EMBASE, CNKI, and WanFang), yielding 1,253 records. After removing 715 duplicate records, 538 records underwent preliminary screening. Following the exclusion of 457 non-clinical studies, 81 full-text articles were selected for further evaluation. Subsequently, 48 non-randomized controlled trials were excluded, leaving 33 reports for full-text review. After detailed assessment, 27 studies were excluded due to insufficient data or inability to extract relevant outcomes, resulting in the final inclusion of six studies for meta-analysis (Figure 1).
Figure 1

Literature screening process.
3.2 Characteristics of included studies
The six included studies comprised three two-arm trials and three three-arm trials. For multi-arm studies, only data pertinent to this meta-analysis were extracted. Interventions were categorized into three groups: conventional swallowing rehabilitation training (CT) (CT, e.g., effortful swallow, Mendelsohn maneuver, and skill-based training), swallowing training combined with neuromuscular electrical stimulation (NMES), and swallowing training combined with surface electromyographic biofeedback (sEMG-BF). For sEMG-BF, the specific exercise types varied across studies (see Table 2), including effortful swallow (16, 17), the Mendelsohn maneuver (18), and skill-based or task-specific swallowing exercises (17, 19–21). Methodological quality was assessed using the ROB tool. Due to the nature of the interventions, blinding of participants was not feasible, resulting in “some concerns” in this domain. All included studies were rated as moderate quality (Figure 2).
Table 2
| Author (Ref) | Year | Enrollments | Intervention | Control | Sample Size | Type of swallowing exercise (with sEMG-BF) |
|---|---|---|---|---|---|---|
| Cheng (16) | 2023 | Dysphagia after acute hemorrhagic stroke | Swallowing training + sEMG-BF | Swallowing training + NMES | 49 vs. 42 | Effortful swallow |
| Gu (18) | 2021 | Post-stroke dysphagia | Swallowing training + sEMG-BF | ① Swallowing rehabilitation training ② Swallowing training + NMES | 40 vs. 40 vs. 40 | Mendelsohn maneuver |
| Hou (19) | 2024 | Early post-stroke dysphagia | Swallowing training + sEMG-BF | Swallowing training + NMES | 44 vs. 44 | Skill-based swallowing training |
| Zhang (20) | 2022 | Post-stroke dysphagia | Swallowing training + sEMG-BF | Conventional swallowing training | 32 vs. 32 | Task-specific swallowing training |
| Min (17) | 2014 | Post-stroke dysphagia | Swallowing training + sEMG-BF | ① Swallowing rehabilitation training ② Swallowing training + NMES | 23 vs. 22 vs. 25 | Effortful swallow |
| Wang (21) | 2019 | Post-stroke dysphagia | Swallowing training + sEMG-BF | ① Swallowing rehabilitation training ② Swallowing training + NMES | 20 vs. 20 | Skill-based swallowing training |
Basic information of included literature.
Figure 2
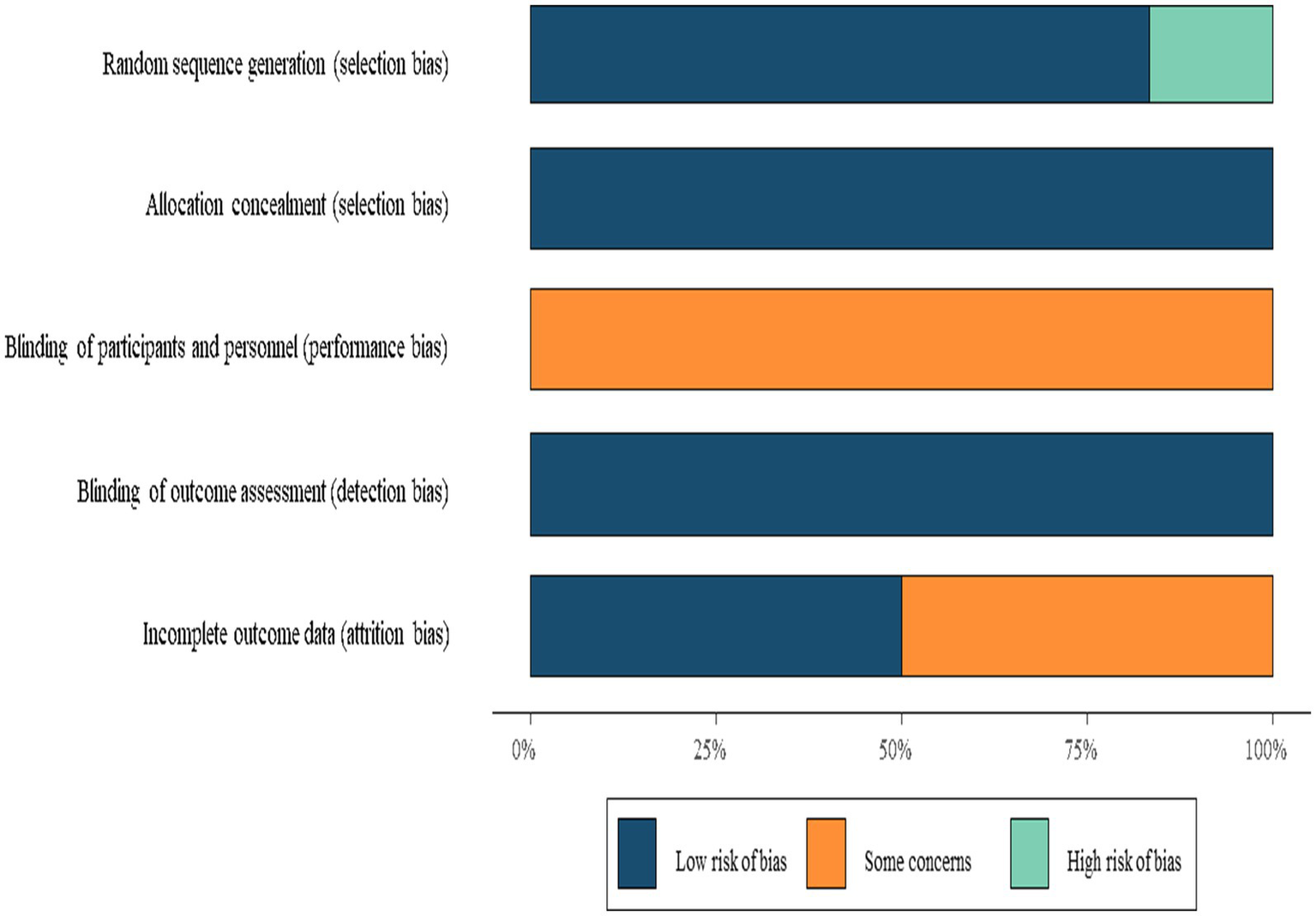
Risk of bias summary.
3.3 sEMG outcomes
Post-intervention sEMG outcomes included mean amplitude (reported in six studies) and swallowing duration (reported in four studies). The network geometry (Figure 3) indicated that sEMG-BF was most frequently compared with NMES, followed by CT.
Figure 3
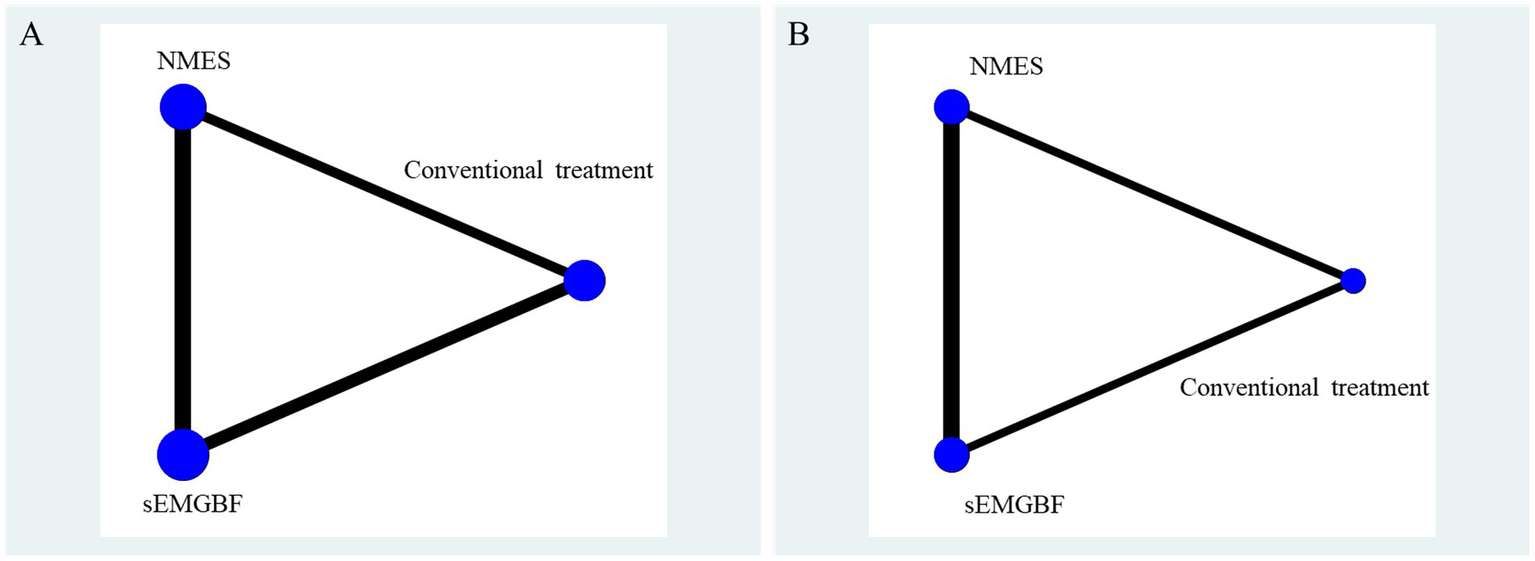
Network relationship diagram of surface electromyography results.
A represents average amplitude (μV); B represents swallowing duration(s).
For mean amplitude, four direct comparisons between sEMG-BF and CT showed significant heterogeneity (I2 > 50%), with a pooled MD of 6.45 (95% CI: 3.53–9.38) using a random-effects model. Five direct comparisons between sEMG-BF and NMES also exhibited heterogeneity, yielding an MD of 6.50 (95% CI: 1.75–11.24). Direct meta-analysis confirmed the superiority of sEMG-BF over both CT and NMES in improving mean amplitude (Figure 4).
Figure 4
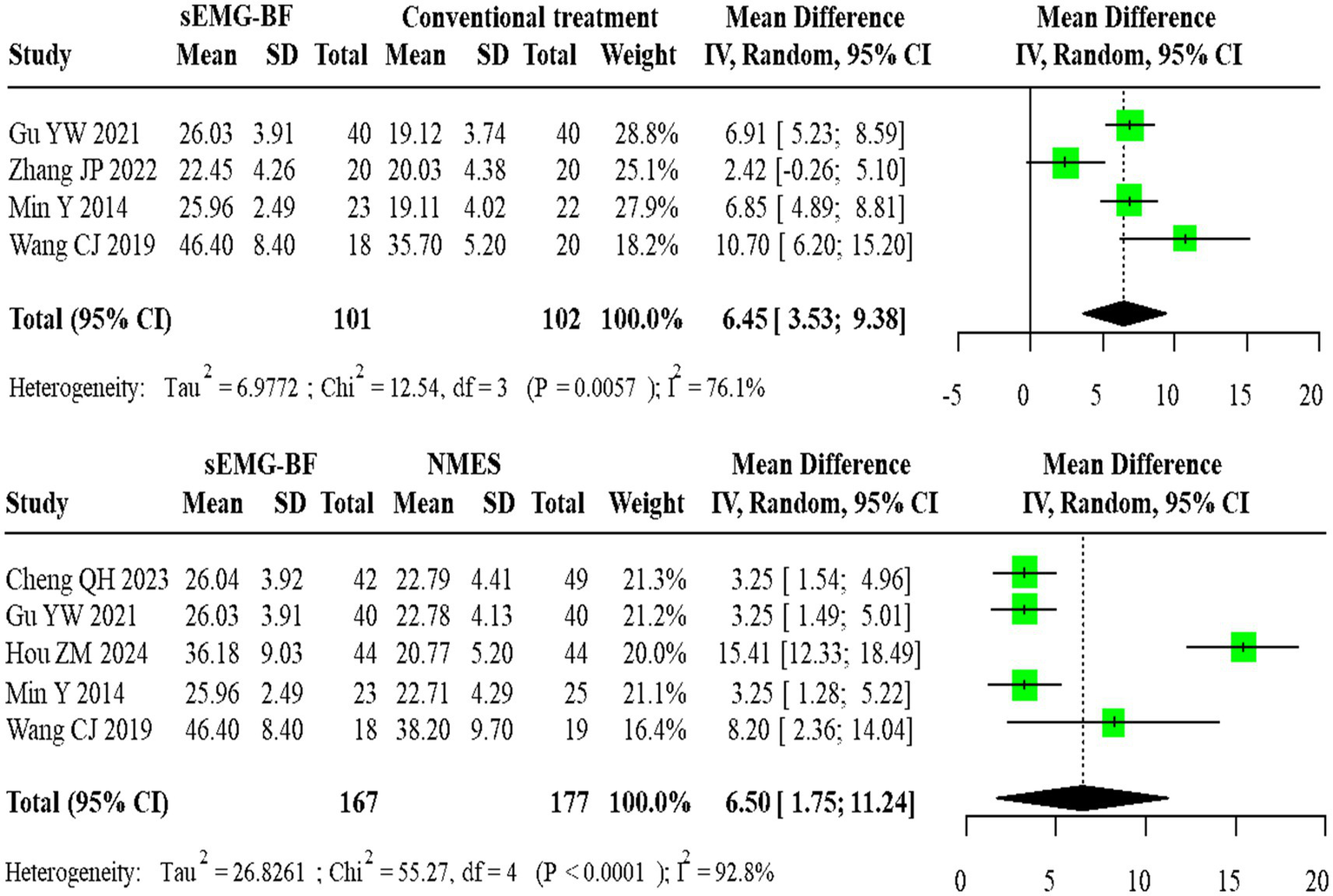
Forest plot of direct comparison meta-analysis for average amplitude.
For swallowing duration, two homogeneous studies comparing sEMG-BF with CT (fixed-effect model) showed an MD of −0.22 (95% CI: −0.26 to −0.18). Four heterogeneous studies comparing sEMG-BF with NMES (random-effects model) demonstrated an MD of −0.15 (95% CI: −0.22 to −0.09). Direct meta-analysis revealed that sEMG-BF significantly reduced swallowing duration compared to both CT and NMES (Figure 5).
Figure 5
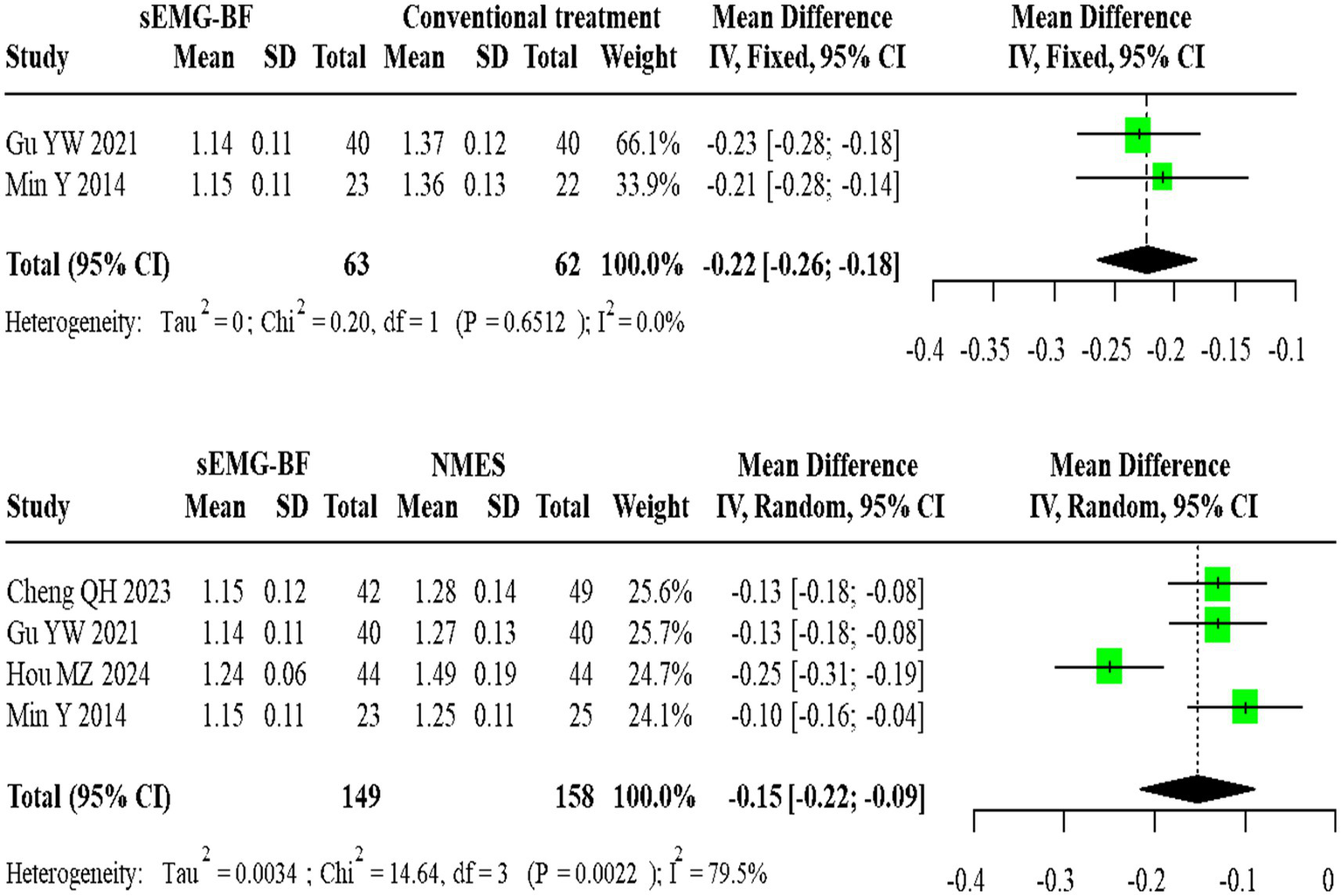
Forest plot of direct comparison meta-analysis for swallowing duration.
Network meta-analysis (NMA) results ranked the interventions as follows: sEMG-BF > NMES > CT for both mean amplitude (Figure 6) and swallowing duration (Figure 7). The analysis confirmed that sEMG-BF outperformed NMES and CT in improving amplitude and shortening swallowing duration, consistent with direct analysis findings. No significant differences were observed between NMES and CT for either outcome (Table 3).
Figure 6
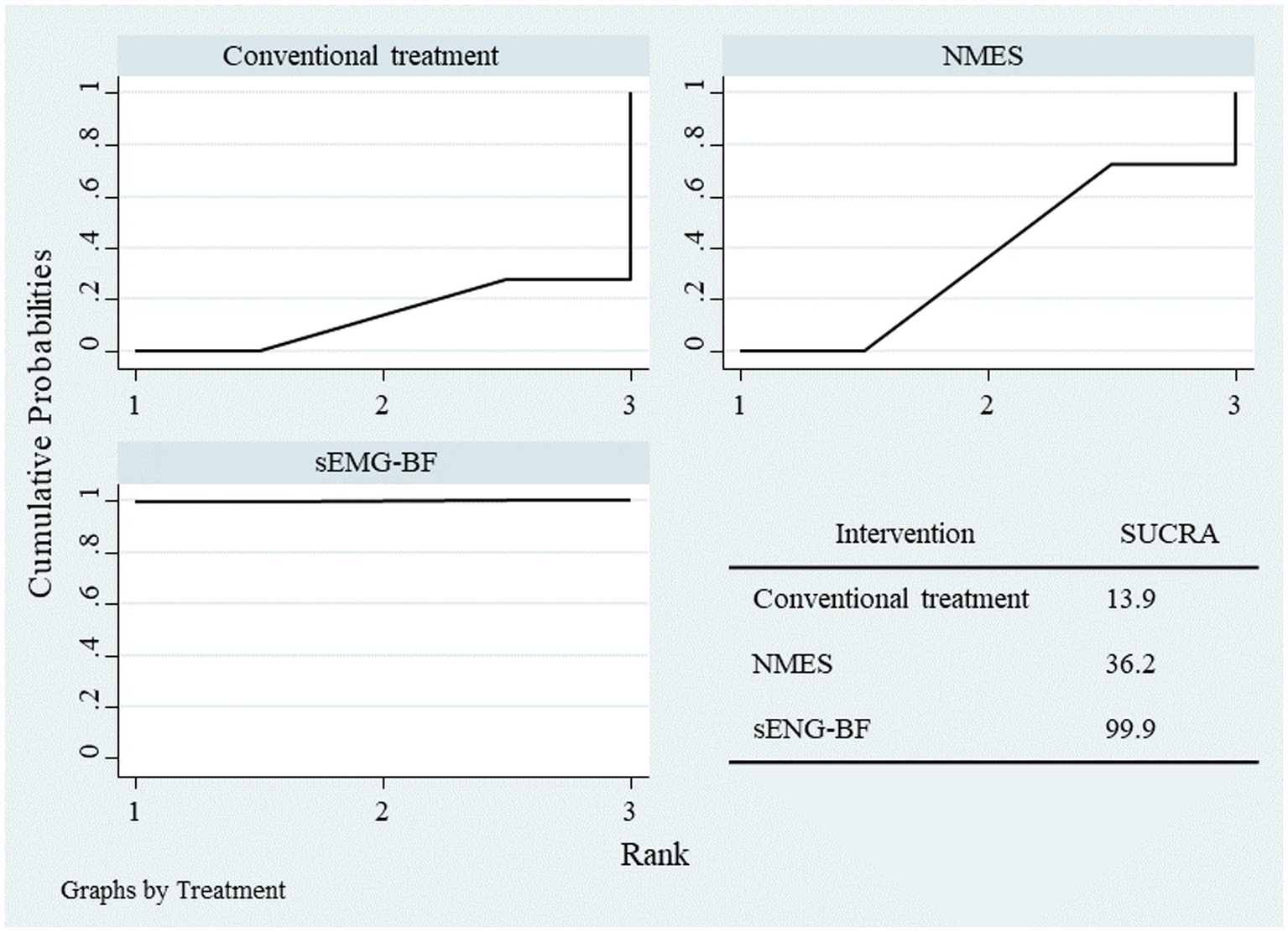
SUCRA plot for average amplitude.
Figure 7
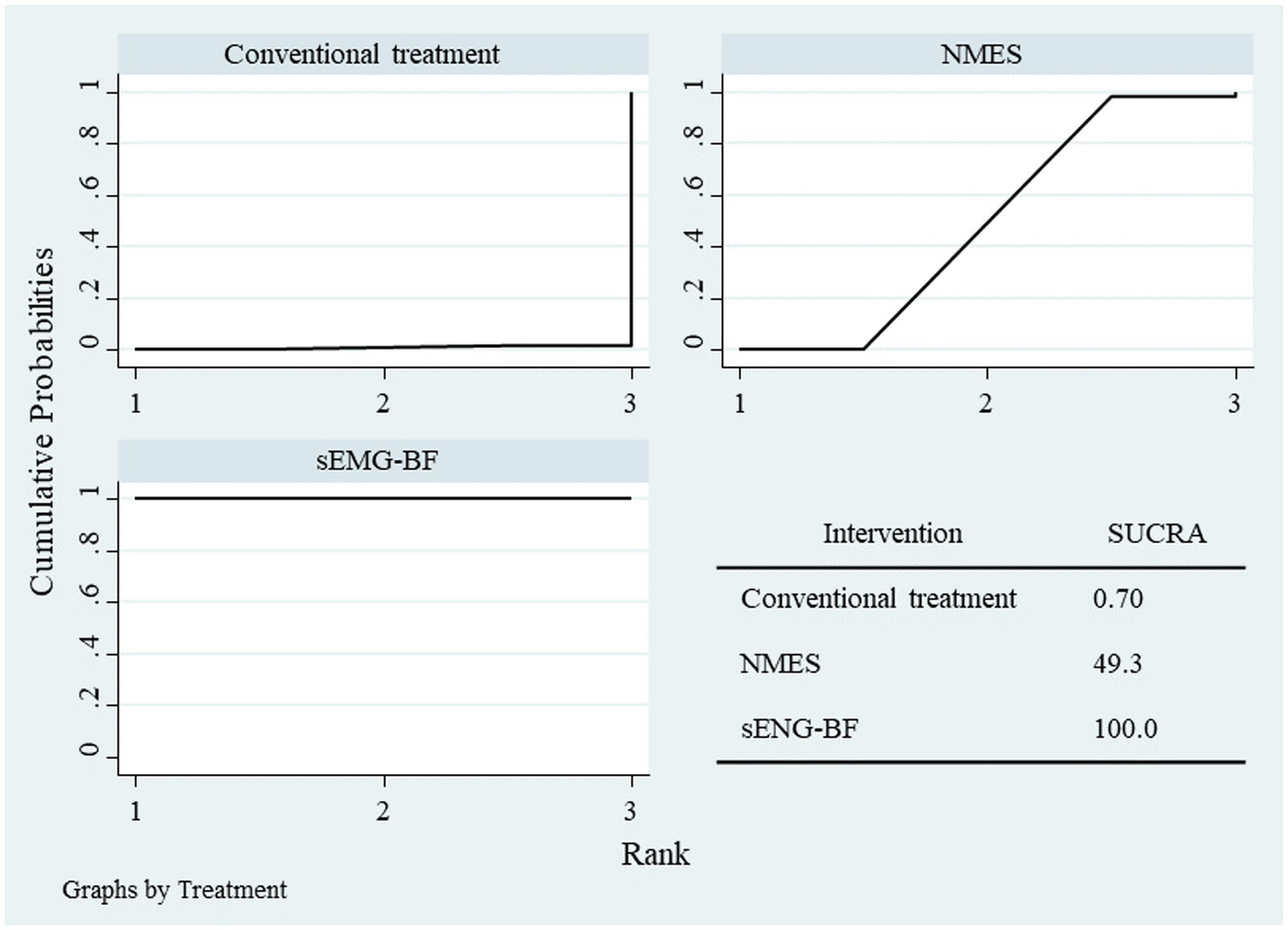
SUCRA plot for swallowing duration.
Table 3
| Parameters | Interventions | Mean difference | 95%CI |
|---|---|---|---|
| Average amplitude | NMES vs. CT | 1.36 | −4.72, 7.67 |
| sEMGBF vs. CT | 7.37 | 1.63, 13.25 | |
| sEMGBF vs. NMES | 5.96 | 0.91, 11.26 | |
| Swallowing duration | NMES vs. CT | −0.09 | −0.21, 0.04 |
| sEMGBF vs. CT | −0.24 | −0.36, −0.12 | |
| sEMGBF vs. NMES | −0.15 | −0.25, −0.06 |
Results of network meta-analysis for amplitude and swallowing duration.
3.4 Swallowing function and quality of life
Outcomes included the Standardized Swallowing Assessment (SSA) (five studies) and Swallowing Quality of Life (SWAL-QOL) (three studies) scores. The network graph is presented in Figure 8.
Figure 8
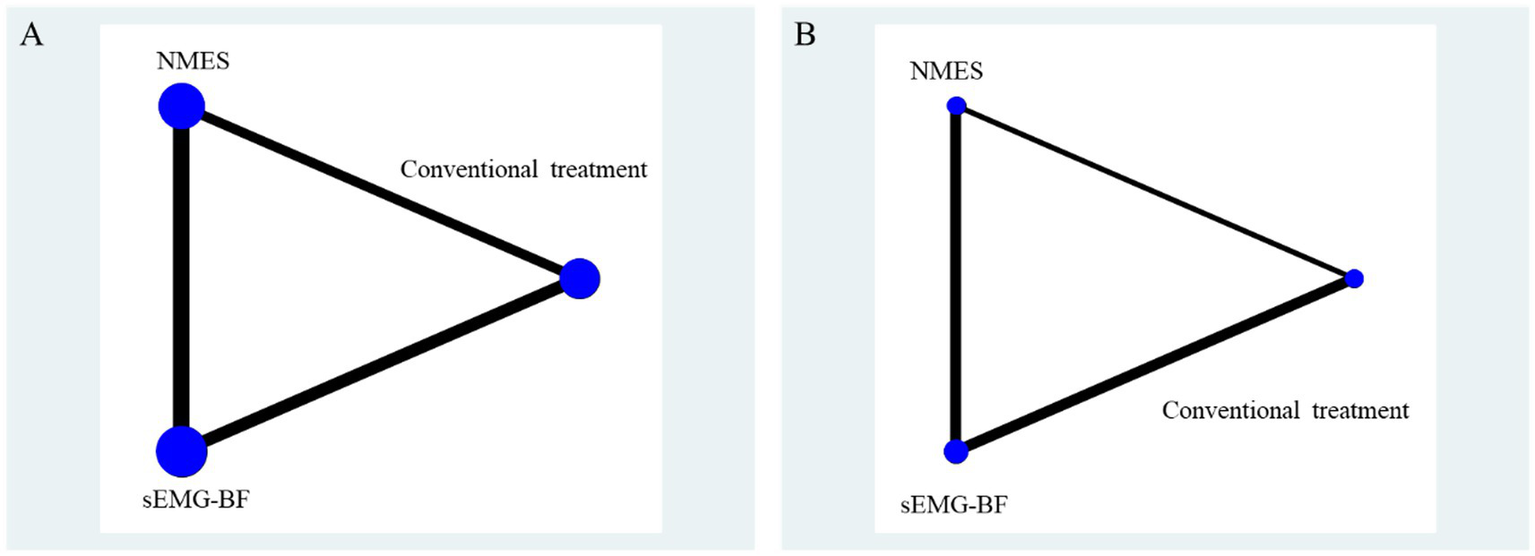
Network relationship diagram of swallowing function and quality of life results. (A) represents SSA score; (B) represents swallowing QOL scores.
For SSA scores, four heterogeneous studies comparing sEMG-BF with CT (random-effects model) showed an MD of −6.43 (95% CI: −9.74 to −3.11). Four homogeneous studies comparing sEMG-BF with NMES (fixed-effect model) yielded an MD of −4.72 (95% CI: −5.69 to −3.75). Direct meta-analysis indicated that sEMG-BF significantly improved swallowing function compared to both CT and NMES (Figure 9).
Figure 9
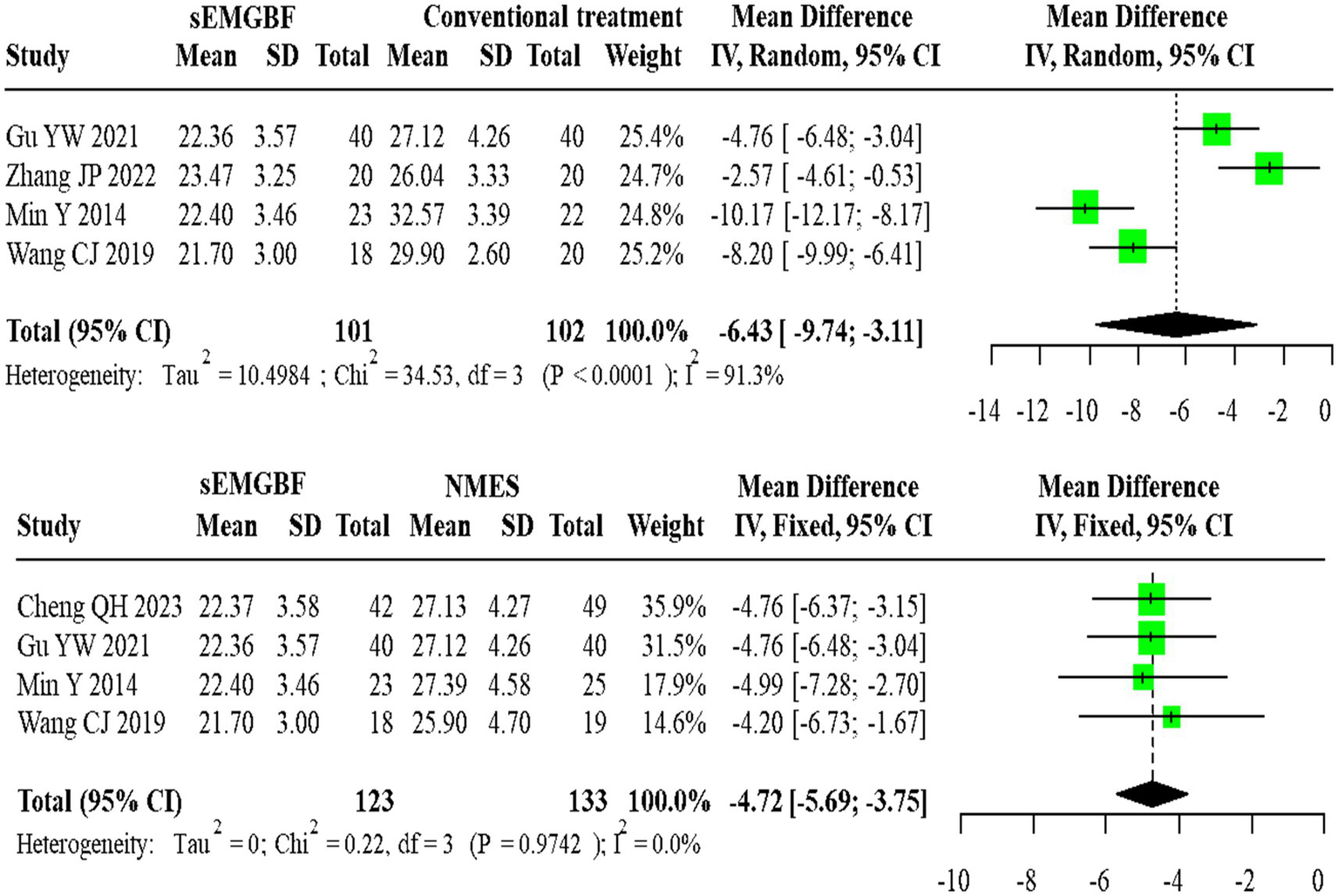
Forest plot of direct comparison meta-analysis for SSA scores.
For SWAL-QOL scores, two heterogeneous studies comparing sEMG-BF with CT (random-effects model) demonstrated an MD of 29.36 (95% CI: −14.96 to 73.69). Two homogeneous studies comparing sEMG-BF with NMES (fixed-effect model) showed an MD of 20.82 (95% CI: 13.96–27.69). Direct meta-analysis did not show a statistically significant difference in SWAL-QOL between sEMG-BF and CT or between sEMG-BF and NMES (Figure 10); for the sEMG-BF versus CT comparison, the pooled estimate (MD = 29.36, 95% CI − 14.96 to 73.69) crossed zero.
Figure 10
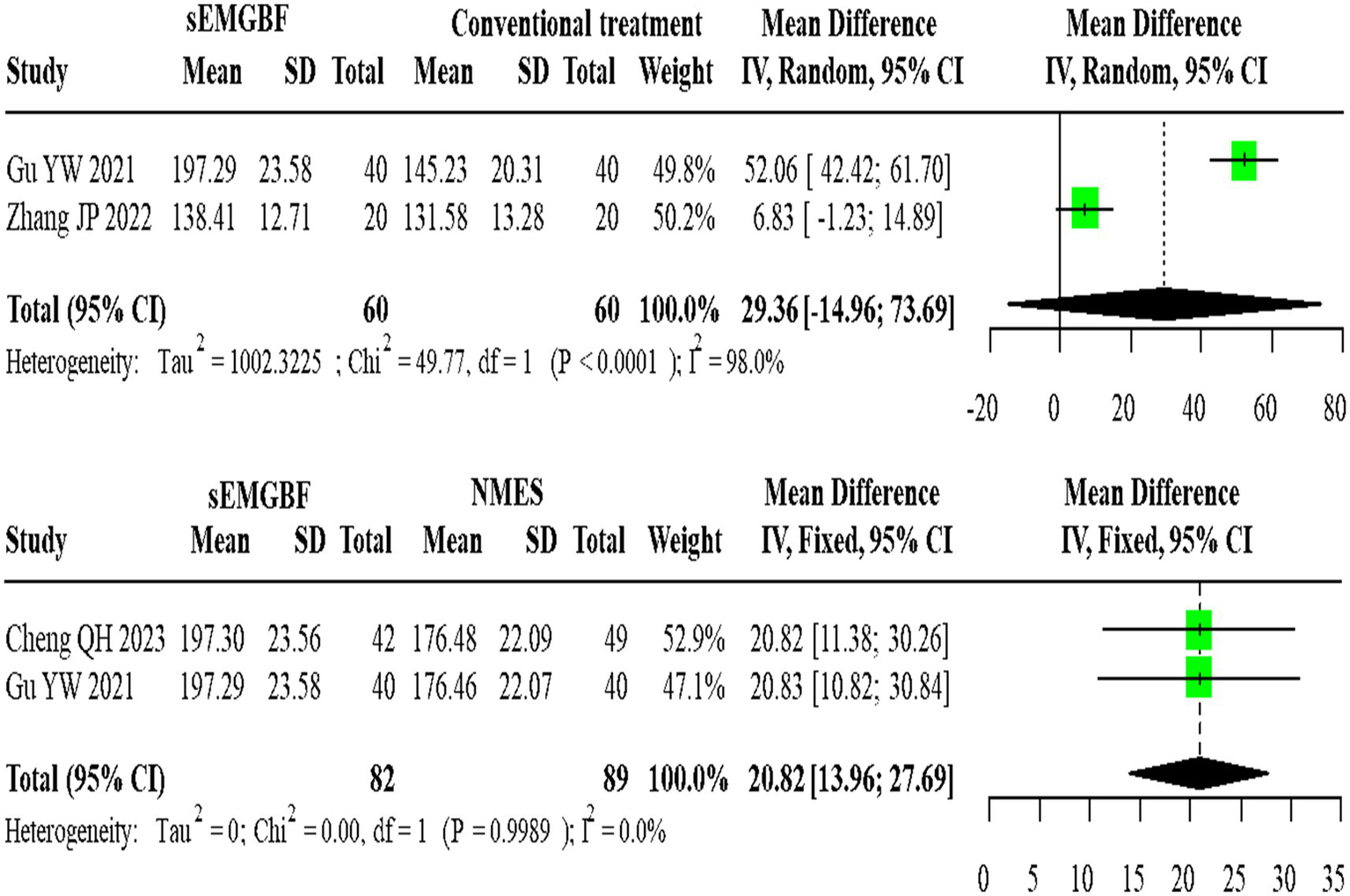
Forest plot of direct comparison meta-analysis for swallowing QOL scores.
NMA results ranked the interventions as sEMG-BF > NMES > CT for both SSA (Figure 11) and SWAL-QOL (Figure 12). The analysis confirmed that sEMG-BF was superior to NMES and CT in improving swallowing function, while NMES outperformed CT. No significant differences were observed among interventions for SWAL-QOL improvement (Table 4).
Figure 11

SUCRA plot for SSA scores.
Figure 12
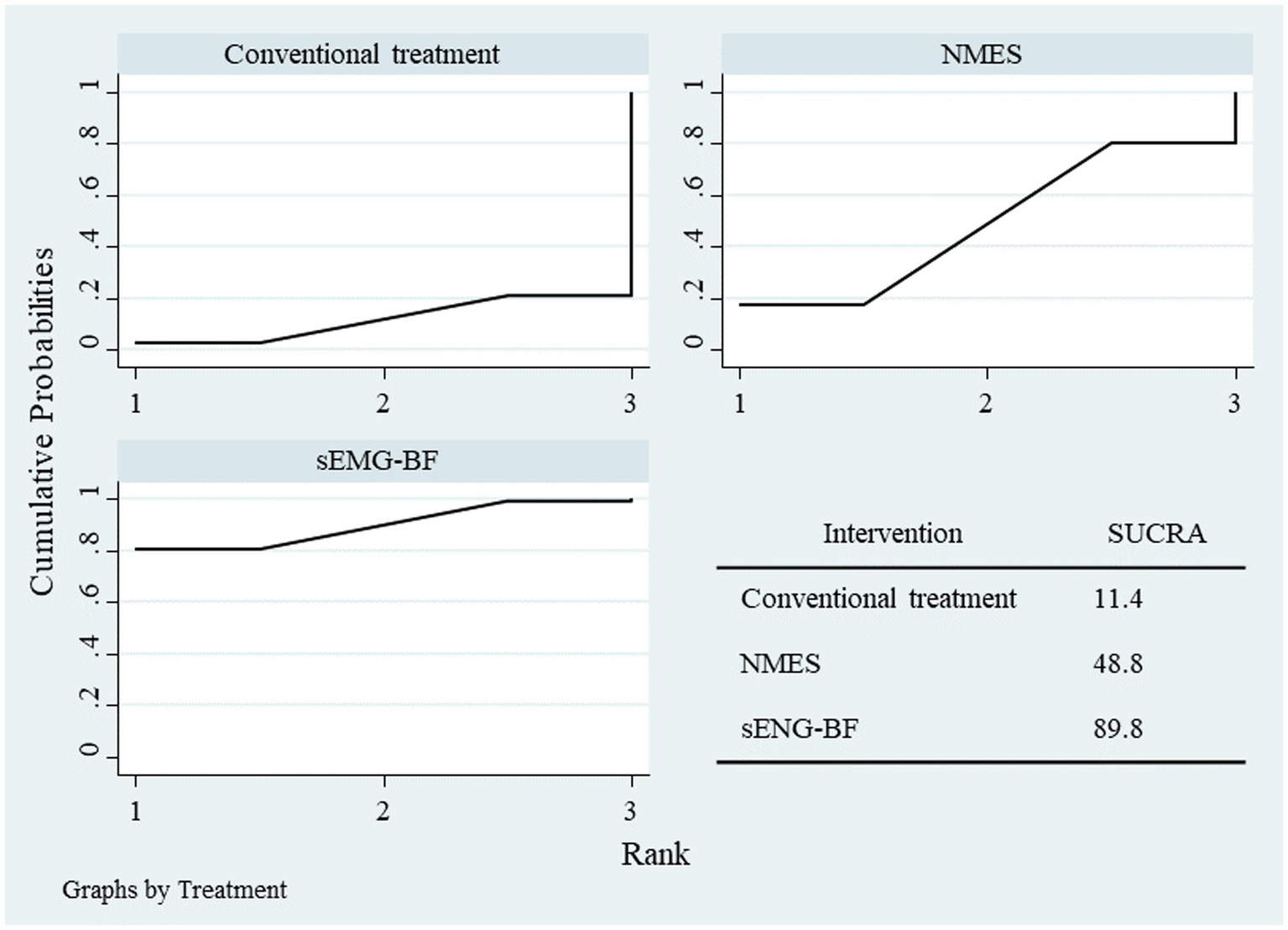
SUCRA plot for swallowing QOL scores.
Table 4
| Parameters | Interventions | Mean difference | 95%CI |
|---|---|---|---|
| SSA | NMES vs. CT | −3.87 | −7.84, −0.15 |
| sEMGBF vs. CT | −7.90 | −11.3, −4.14 | |
| sEMGBF vs. NMES | −4.02 | −7.55, −0.48 | |
| Swallowing QOL | NMES vs. CT | 15.5 | −40.8, 68.5 |
| sEMGBF vs. CT | 30.6 | −14.0, 78.3 | |
| sEMGBF vs. NMES | 15.3 | −29.6, 61.4 |
Results of network meta-analysis for swallowing function and quality of life.
4 Discussion
As an emerging rehabilitation technology, sEMG-BF has demonstrated considerable potential in rehabilitation, particularly in the recovery of neurological and motor functions (3). However, systematic evaluation and high-quality evidence are necessary to fully determine its efficacy and optimize its clinical application. This study aimed to conduct a systematic review and network meta-analysis to thoroughly investigate the therapeutic effects of sEMG-BF in PSD, clarify its efficacy advantages, and provide high-quality evidence to support clinical practice. The results suggest that sEMG-BF was superior to conventional therapy and NMES in improving swallowing function and sEMG parameters; however, the overall quality-of-life effects were not statistically significant, and the estimates were limited due to small sample sizes and heterogeneity. Specifically, sEMG-BF significantly increased the mean amplitude and reduced swallowing duration in sEMG metrics. Regarding swallowing function, sEMG-BF markedly improved the SSA score. In terms of quality of life, some studies suggested improvement with sEMG-BF; however, the pooled estimate did not achieve statistical significance, indicating uncertainty about this outcome. These findings indicate that sEMG-BF offers substantial clinical advantages in the treatment of PSD.
Previous studies have shown that traditional swallowing exercises, such as the Shaker exercise and Mendelsohn maneuver, although widely adopted, exhibit significant interindividual variability in efficacy due to limitations such as cognitive impairment or impaired proprioception in patients (8, 22–24). NMES can enhance muscle contraction through electrical stimulation but may cause discomfort or skin irritation and has minimal effects on the central nervous system (25). In the included studies, NMES was used as an independent intervention without biofeedback, in contrast to the sEMG-BF intervention. We recommend retaining these studies as comparators because NMES is a widely used clinical intervention and provides necessary context for evaluating the relative effectiveness of sEMG-BF. To avoid confusion, we have clarified in the discussion that NMES was applied without biofeedback. The findings of this study are consistent with prior research, further confirming the superiority of sEMG-BF in improving swallowing function and quality of life. sEMG-BF has been shown to significantly enhance swallowing function. A preliminary randomized controlled trial reported that patients receiving sEMG-BF rehabilitation exhibited improved pharyngeal transit efficiency and bolus clearance while simultaneously enhancing airway protection mechanisms during deglutition (11). Another study demonstrated that sEMG-BF combined with game-based training yielded greater improvements in swallowing indices compared to conventional rehabilitation alone (26). sEMG-BF facilitates increased muscle activity during swallowing exercises. One study found that both healthy participants and PSD patients generated significantly greater muscle activity when using sEMG biofeedback compared to non-biofeedback conditions, which is critical for effective swallowing rehabilitation (27). Moreover, sEMG-BF is generally well-accepted by patients, with high compliance. Studies indicate that the intervention is comfortable, manageable, and perceived as beneficial, making it a highly acceptable therapeutic approach (28).
sEMG-BF demonstrates potential benefits in improving various aspects of swallowing function, particularly in dysphagia patients, which can be attributed to multiple mechanisms. sEMG-BF is associated with enhanced upper esophageal sphincter (UES) function. One study found that sEMG-BF significantly prolonged UES relaxation duration and increased pharyngeal pressure, both of which are essential for effective swallowing (29). sEMG-BF combined with conventional swallowing training has been shown to significantly improve maximum hyoid displacement, a parameter closely associated with epiglottic elevation during swallowing (30). sEMG-BF effectively improves pharyngeal clearance, reduces residue, and enhances swallowing safety, which is particularly important in PSD patients (11). In addition, sEMG-BF provides real-time visual feedback, enabling patients to more accurately perceive and adjust swallowing muscle activity, thereby enhancing therapeutic efficacy. This approach overcomes the limitations of traditional swallowing exercises by offering a more intuitive and active training modality (31, 32). As a non-invasive technique, sEMG-BF has potential complementary utility with videofluoroscopic swallowing studies (VFSS) and may serve as an adjunctive tool for screening, diagnosing, and monitoring dysphagia. In patients with chronic low back pain, sEMG-BF can reduce muscle tension and improve physical function, thereby alleviating pain and enhancing sleep and mental health (33). Among patients suffering from spinal cord injuries, sEMG-BF can augment muscle activation and engagement (34).
Despite the comprehensive inclusion and analysis of relevant studies through systematic review and network meta-analysis, this study has several limitations. First, publication bias or the omission of unpublished negative results may affect the comprehensiveness of the findings. Second, the included studies exhibited considerable heterogeneity in patient characteristics, intervention details, and follow-up durations, which may influence the stability of the results. For instance, some studies had small sample sizes or short follow-up periods, limiting the interpretation of long-term effects. Furthermore, data on certain outcome measures, such as SWAL-QOL scores, were limited, potentially reducing the statistical power of the analysis.
Future research should focus on large-scale, long-term follow-up studies to evaluate the sustained efficacy and safety of sEMG-BF. In addition, the effectiveness of sEMG-BF may be influenced by the design and usability of electrodes and feedback systems, necessitating tailored approaches to maximize its benefits (35). Further exploration of combined therapies integrating sEMG-BF with other treatment modalities is warranted to optimize rehabilitation strategies for PSD. Feasibility studies on sEMG-BF in low- and middle-income countries are also recommended to assess its potential applicability in resource-limited settings.
5 Conclusion
This study indicates that sEMG-BF may be beneficial in the management of PSD. However, due to small sample sizes, heterogeneity, and limited data for some outcomes (e.g., SWAL-QOL), the evidence remains preliminary. Larger, high-quality studies are needed to confirm these findings. By enhancing the amplitude of sEMG signals and strengthening muscle contraction, sEMG-BF improves swallowing function; quality-of-life findings were inconclusive at the pooled level. Despite certain limitations, sEMG-BF holds promising prospects for the rehabilitation of PSD. Future high-quality studies are needed to further validate its long-term efficacy and safety and to explore its synergistic effects with other therapies to refine rehabilitation strategies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
NL: Writing – original draft, Methodology, Formal analysis, Investigation. QJ: Methodology, Formal analysis, Investigation, Writing – original draft. YZ: Writing – review & editing, Formal analysis, Data curation. LZ: Software, Writing – original draft, Methodology. XP: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Abubakar SA Jamoh BY . Dysphagia following acute stroke and its effect on short-term outcome. Niger Postgrad Med J. (2017) 24:182–6. doi: 10.4103/npmj.npmj_96_17
2.
Al-Mamari RS Lazarus ER Al-Harrasi M Al-Noumani H Al ZO . Prevalence, severity, and predictors of dysphagia among patients with acute stroke in Oman. J Educ Health Promot. (2024) 13:351. doi: 10.4103/jehp.jehp_1704_23
3.
Roje-Bedeković M Dimitrović A Breitenfeld T Supanc V Vargek Solter V . Reliable predicting factors for post-stroke dysphagia – our experience. Neurol Psychiatr Brain Res. (2020) 38:97–101. doi: 10.1016/j.npbr.2020.10.006
4.
Labeit B Michou E Hamdy S Trapl-Grundschober M Suntrup-Krueger S Muhle P et al . The assessment of dysphagia after stroke: state of the art and future directions. Lancet Neurol. (2023) 22:858–70. doi: 10.1016/S1474-4422(23)00153-9
5.
Mehdi Zaidi SM Alvi MH Fatmi SAA Abbasi L Hayat A Irfan Q et al . Effectiveness of intermittent theta burst stimulation (iTBS) for managing post-stroke dysphagia: systematic review and meta-analysis. Top Stroke Rehabil. (2024) 8:1–10. doi: 10.1080/10749357.2024.2437325
6.
Brady MC Clark AM Dickson S Paton G Barbour RS . The impact of stroke-related dysarthria on social participation and implications for rehabilitation. Disabil Rehabil. (2011) 33:178–86. doi: 10.3109/09638288.2010.517897
7.
White J Dickson A Magin P Tapley A Attia J Sturm J et al . Exploring the experience of psychological morbidity and service access in community dwelling stroke survivors: a follow-up study. Disabil Rehabil. (2014) 36:1600–7. doi: 10.3109/09638288.2013.859748
8.
Hoffman MR Mielens JD Ciucci MR Jones CA Jiang JJ McCulloch TM . High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia. (2012) 27:418–26. doi: 10.1007/s00455-011-9385-6
9.
Bodén K Hallgren A Witt Hedström H . Effects of three different swallow maneuvers analyzed by videomanometry. Acta Radiol. (2006) 47:628–33. doi: 10.1080/02841850600774043
10.
O'Kane L Groher ME Silva K Osborn L . Normal muscular activity during swallowing as measured by surface electromyography. Ann Otol Rhinol Laryngol. (2010) 119:398–401. doi: 10.1177/000348941011900606
11.
Nordio S Arcara G Berta G Dellai A Brisotto C Koch I et al . Biofeedback as an adjunctive treatment for post-stroke dysphagia: a pilot-randomized controlled trial. Dysphagia. (2021) 37:1207–16. doi: 10.1007/s00455-021-10385-2
12.
Bahia MM Lowell SY . Surface Electromyographic activity of the masseter muscle during regular and effortful saliva swallows: a preliminary study. Dysphagia. (2024) 39:231–40. doi: 10.1007/s00455-023-10605-x
13.
Bogaardt HCA Grolman W Fokkens WJ . The use of biofeedback in the treatment of chronic dysphagia in stroke patients. Folia Phoniatr Logop. (2009) 61:200–5. doi: 10.1159/000227997
14.
Bahia MM Carpenter J Cherney LR . Barriers and facilitators in using surface electromyography in swallowing management: an implementation science study. Am J Speech Lang Pathol. (2025) 34:44–69. doi: 10.1044/2024_AJSLP-24-00215
15.
Wan X Wang W Liu J Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
16.
Qing Hua C Beilei C Xue X Haixia C . The effect of electromyographic biofeedback and electrical stimulation therapy on clinical curative effect and swallowing function of patients with dysphagia after acute hemorrhagic stroke. Practical J Clin Med. (2023) 20:59–63. (in Chinese).
17.
Min Y Yan H Huang Z Gao Y Huang Z . The effects of electromyographic biofeedback in the treatment of dysphagia after stroke. Chin J Physical Med Rehabilitation. (2014) 36:583–6. (in Chinese).
18.
Yiwen G Jin S . Effects of surface electromyography biofeedback and neuromuscular electrical stimulation on the efficacy of dysphagia and quality of life in stroke patients. Chin J Rehabilitation. (2021) 36:599–603. (in Chinese).
19.
Muzhao H Yijie Z Hongling L . Efficacy observation of surface electromyography biofeedback therapy for dysphagia after early stroke. J Brain and Nervous Dis. (2024) 32:138–43. (in Chinese).
20.
Jianping Z Haipeng D Daiyan J Jie Z . Clinical study on high - frequency repetitive transcranial magnetic stimulation combined with electromyographic biofeedback in the treatment of dysphagia after stroke. Chin J Brain Diseases and Rehabilitation. (2022) 12:285–90. (in Chinese).
21.
Chuanjie W Jie Z . Observation of the therapeutic effect of electromyographic biofeedback therapy on swallowing disorders after early stroke. Chin J Physical Med Rehabilitation. (2019) 41:266–8. (in Chinese).
22.
Galek KE Bice EM Allen K . Influence of three feedback conditions on performing a swallow motor pattern in healthy adults. Folia Phoniatr Logop. (2023) 75:13–22. doi: 10.1159/000525634
23.
Lee CL Banda KJ Chu YH Liu D Lee CK Sung CM et al . Efficacy of swallowing rehabilitative therapies for adults with dysphagia: a network meta-analysis of randomized controlled trials. GeroScience. (2025) 47:2047–65. doi: 10.1007/s11357-024-01389-5
24.
Sayaca C Serel-Arslan S Sayaca N Demir N Somay G Kaya D et al . Is the proprioceptive neuromuscular facilitation technique superior to shaker exercises in swallowing rehabilitation?Eur Arch Otorrinolaringol. (2020) 277:497–504. doi: 10.1007/s00405-019-05772-3
25.
Byeon H . Combined effects of NMES and mendelsohn maneuver on the swallowing function and swallowing-quality of life of patients with stroke-induced sub-acute swallowing disorders. Biomedicine. (2020) 8:12. doi: 10.3390/biomedicines8010012
26.
Hou M Zhao Y Zhao L Yuan X Liu Z Li H . Efficacy of game training combined with surface electromyography biofeedback on post-stroke dysphagia. Geriatr Nurs. (2024) 55:255–62. doi: 10.1016/j.gerinurse.2023.11.019
27.
Archer SK Smith CH Newham DJ . Surface Electromyographic biofeedback and the effortful swallow exercise for stroke-related dysphagia and in healthy ageing. Dysphagia. (2021) 36:281–92. doi: 10.1007/s00455-020-10129-8
28.
Benfield JK Hedstrom A Everton LF Bath PM England TJ . Randomized controlled feasibility trial of swallow strength and skill training with surface electromyographic biofeedback in acute stroke patients with dysphagia. J Oral Rehabil. (2023) 50:440–51. doi: 10.1111/joor.13437
29.
Lan Y Wang X Xu G . The effect of surface electromyogram biofeedback and neuro muscular electrical stimulation on pharyngeal and upper esophageal sphincter function in brain stem injury patients with dysphagia. Chin J Rehabilitation Med. (2014) 29:405–9. (in Chinese).
30.
Huimin Z Yongchao Y Jiang R Li L Yao W Weibo S et al . Effect of surface electromyographic biofeedback on the pharyngeal phase activities in patients with dysphagia after stroke. Chin J Cerebrovascular Diseas. (2015) 12:572–6. (in Chinese).
31.
Huckabee ML Mills M Flynn R Doeltgen S . The evolution of swallowing rehabilitation and emergence of biofeedback modalities. Curr Otorhinolaryngol Rep. (2023) 11:144–53. doi: 10.1007/s40136-023-00451-8
32.
Lekavičiūtė R Paldauskaitė S Stučinskaitė S Trakinienė G . The effect of clear aligner treatment on masticatory muscles (masseter, temporalis) activity in adults: a systematic review and meta-analysis. Eur J Orthod. (2024) 46:cjae030. doi: 10.1093/ejo/cjae030
33.
Sadora J Vilsmark E Bashara A Burton D Paschali M Pester B et al . Electromyography-biofeedback for chronic low back pain: a qualitative cohort study. Complement Ther Med. (2023):73. doi: 10.1016/j.ctim.2023.102922
34.
Conceição MIG Da Silva GL . The biofeedback use in motor learning of people with spinal cord injury. Avances en Psicologia Latinoamericana. (2009) 27:177–91. doi: 10.1067/mmt.2000.108138f
35.
Peres SC Verona D Nisar T Ritchey P . Towards a systematic approach to real-time sonification design for surface electromyography. Displays. (2017) 47:25–31. doi: 10.1016/j.displa.2016.05.006
Summary
Keywords
surface electromyographic biofeedback, neuromuscular electrical stimulation, stroke, dysphagia, network meta-analysis, systematic review
Citation
Li N, Jin Q, Zhang Y, Zhang L and Peng X (2025) Surface electromyographic biofeedback versus neuromuscular electrical stimulation for post-stroke dysphagia: a systematic review and network meta-analysis. Front. Neurol. 16:1649961. doi: 10.3389/fneur.2025.1649961
Received
19 June 2025
Accepted
24 September 2025
Published
16 October 2025
Volume
16 - 2025
Edited by
Elisa Kallioniemi, New Jersey Institute of Technology, United States
Reviewed by
Irene Battel, Trinity College Dublin, Ireland
Pragna Landge, Drs. Kiran & Pallavi Patel Global University, India
Updates
Copyright
© 2025 Li, Jin, Zhang, Zhang and Peng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiapei Peng, Pengxiapei@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.