Abstract
Background:
Postoperative delirium (POD) is a common central nervous system complication in older adult surgical patients. At present, the mechanism for POD is still unclear. Lipocalin-2 (LCN2) may have an impact on cognitive function, but the relationship between LCN2 and POD has remained unclear. Therefore, we sought to investigate the relationship between the levels of LCN2 in plasma and cerebrospinal fluid (CSF) and the occurrence of POD in older adults undergoing hip fracture surgery.
Methods:
We conducted a prospective observational cohort study involving 186 older adults (≥65 years old) who underwent hip fracture surgery under spinal anesthesia. CSF and blood samples were collected. The levels of LCN2, interleukin-6 (IL-6), and interleukin-1 (IL-1) were measured using an enzyme-linked immunosorbent assay (ELISA). We used the 3-min diagnostic interview to evaluate delirium defined by the Confusion Assessment Method (3D-CAM), to screen for POD, and the Memorial Delirium Assessment Scale (MDAS) to evaluate the severity of delirium. Multivariable logistic regression was applied to identify independent predictive factors for POD. The relationship between CSF LCN2 levels and POD risk was assessed through receiver operating characteristic (ROC) curve analysis. Correlation analysis was used to investigate the association between CSF LCN2 and MDAS scores as well as IL-6.
Results:
Among the 186 patients ultimately included, 29 (15.6%) developed POD. Their preoperative CSF LCN2 level was significantly higher than that of those without POD (p = 0.001). The multivariable logistic regression analysis revealed that an elevated preoperative CSF LCN2 level [odds ratio (OR) 2.546, 95% confidence interval (CI) 1.345–4.822; p = 0.004] was an independent predictor of POD. Moreover, among POD group patients, preoperative CSF LCN2 levels were positively correlated with the MDAS scores (r = 0.688, p < 0.001) and CSF IL-6 levels (r = 0.379, p = 0.043). ROC analysis of preoperative CSF LCN2 showed an area under the curve of 0.713 (95% CI 0.615–0.810) with a specificity of 75.0%, and sensitivity of 58.6%.
Conclusion:
Elevated preoperative CSF LCN2 levels are associated with an increased risk and severity of POD in older adults undergoing hip fracture surgery.
Clinical trial registration:
https://www.chictr.org.cn/, ChiCTR2200061407.
1 Introduction
Hip fracture is a major disease threatening the health and safety of adults aged 65 years or older. The incidence increases with the aging trend of the population (1). Fracture reduction and internal fixation surgery are the main treatment methods for hip fractures. Postoperative delirium (POD) is a common complication in such patients (2). POD is an acute fluctuating mental state change that occurs in patients after surgery under anesthesia, often accompanied by decreased consciousness, attention disorders, psychomotor disorders, and sleep–wake cycle disorders. According to the patient’s mental activity level, it can be categorized into indifferent, restless, and mixed types (3). POD often occurs within 7 days after surgery or before discharge, and the symptoms can last from hours to weeks (4). The incidence is approximately 4–61% (5), which can increase mortality, hospital costs, and hospital stay (6), constituting a major public health problem.
At present, the mechanism for delirium has remained unclear and is the result of a combination of multiple factors (7, 8), including the neuroinflammatory hypothesis. Central nervous system (CNS) inflammation is closely related to the occurrence of POD (9).
Elevated levels of IL-6 in preoperative CSF are associated with the occurrence of postoperative delirium (10, 11). Another key molecule implicated in neuroinflammation is LCN2, also known as neutrophil gelatinase-associated lipocalin (NGAL), belonging to the lipocalin family (12). Although NGAL is expressed in various peripheral tissues (13–19), its role within the CNS has garnered substantial attention. Under physiological conditions, LCN2 expression in the brain is low (20). However, in response to neuroinflammation, its expression is markedly upregulated (21, 22), primarily in activated astrocytes (23), neuron (24, 25), microglia (24), and endothelial cells (26). Mosialou et al. reported that bone-derived LCN2 can cross the blood–brain barrier (BBB) and bind to melanocortin-4 receptors on hypothalamic neurons (27). However, current research on the mechanism of its crossing the BBB is limited. Two primary mechanisms have been proposed, though neither is fully elucidated. First, it may be facilitated by inflammatory conditions that increase BBB permeability (28, 29). Second, the possibility of receptor-mediated transport exists (29). Within the CNS, LCN2 exerts pleiotropic effects (30). It is implicated in iron homeostasis (31), synaptic plasticity (32), and neuronal apoptosis (33). Crucially, LCN2 acts as a key mediator in the neuroinflammatory cascade, often acting with downstream pyroptosis to promote proinflammatory responses (34). Its involvement in the pathophysiology of various neurodegenerative disorders, including Alzheimer disease and Parkinson disease, is well-documented (35–37). Notably, alterations in LCN2 concentrations in CSF have been linked to cognitive impairment (38, 39), suggesting its potential as both a biomarker and a therapeutic target for neurological conditions.
LCN2 may be involved in the occurrence of postoperative cognitive dysfunction (40, 41), and a correlation between LCN2 in plasma and POD is suggested (42). However, clinical studies are limited, and to our knowledge, the correlation between LCN2 and POD in CSF has not been examined. Therefore, we sought to determine the relationship between LCN2 levels in plasma and CSF and the occurrence of POD.
2 Materials and methods
2.1 Research design and ethical approval
This study was a single-center, prospective observational cohort study conducted at Jishuitan Hospital in Beijing. The protocols were rigorously reviewed and approved by the Ethics Committee of Beijing Jishuitan Hospital (Institutional Review Committee: JLKS202204-08; International Clinical Website Registration Number: ChiCTR2200061407). Research protocols strictly adhered to the principles outlined in the Declaration of Helsinki and its latest amendment (2013). All participants or their legally authorized representatives documented their written informed consent by signing a form before enrollment and undergoing any research-related procedures. This study followed the STROBE guidelines for reporting observational epidemiological studies (43).
2.2 Patients and setting
We recruited patients aged 65 years and over who planned hip fracture surgery under spinal anesthesia between March 2023 and December 2023. All patients were admitted to the orthopedic ward for older adult patients. Inclusion criteria: diagnosed hip fracture site is only unilateral; age ≥65 years; American Society of Anesthesiologists (ASA) Physical Status Classification System levels I, II, and III; no history of allergy to anesthetic drugs; agreed to be a patient participant. Exclusion criteria: those who have not undergone surgery within 48 h after admission; severe comorbidities in other systems; contraindications for nerve block (needle site infection, local anesthesia drug allergy, coagulation dysfunction, international standardized ratio>1.4, platelet count <80 × 109); pathological fracture; metabolic bone disease; old fractures; history of stroke within 6 months before surgery; dementia, mental illness, and preoperative delirium; alcohol dependence or drug addiction; transferred to an intensive care unit (ICU) after surgery, with aphasia and hearing impairment; patients with Parkinson disease. We initially screened 515 patients, and ultimately included 186 in the present study. A flow diagram of the patient recruitment process is shown in Figure 1.
Figure 1

Flow diagram. We initially screened 515 patients for the study, and 186 patients were ultimately included in the data analysis. MMSE, Mini-Mental State Examination; ICU, intensive care unit; POD, postoperative delirium.
2.3 Data collection
We visited the patients 1 day before the surgery and collected baseline data, including age, sex, body mass index (BMI), ASA grading, Mini-Mental State Examination (MMSE) score, Age-adjusted Charlson Comorbidity Index (ACCI) score, Pittsburgh Sleep Quality Index (PSQI) score, and smoking status. Based on the patient’s medical records, other information was also collected, including comorbidities, medication use, medical history, fracture classification, anesthesia and surgical type, and time from injury to surgery. All medical history collection, physical assessment, and cognitive assessment related to dementia were conducted by physicians specializing in geriatrics.
2.4 Anesthesia and analgesia
As a key component of our standardized protocol designed to minimize confounding variables, all enrolled patients underwent spinal anesthesia as their sole method of anesthesia. This choice was based on institutional preference and clinical evidence suggesting potential benefits for older patients with hip fracture, including effective pain control and a possibly reduced incidence of POD compared with that with general anesthesia (44–46). After the patients entered the operating room, electrocardiogram monitoring, pulse oximetry, and invasive blood pressure monitoring via radial artery catheterization were initiated. Before positioning, the high iliac fascial space tissue (“funnel sign”) was identified on the affected side, and each patient was given 30 mL of 0.33% ropivacaine. Subsequently, the patient was placed in a lateral position with the affected side facing upwards, and a dose of 8–10 mg of 0.3% ropivacaine was administered at the L3–L4 intervertebral space. The anesthesia level reached T10, and no intravenous sedatives or anticholinergic drugs were used during the operation. Standard aseptic techniques were employed. Postoperative patient-controlled analgesia (PCA) was administered intravenously, with a specific medication regimen of 100 μg of sufentanil, 200 mg of flurbiprofen axetil, 10 mg of tropisetron hydrochloride, and 100 mL of physiological saline (background infusion rate of 2 mL/h, PCA compression dose of 0.5 mL, locking time of 15 min). For patients in generally poor condition, the dosage of medications was adjusted as appropriate. The analgesic pump was removed after the dosage was fully delivered. For patients who required additional analgesics, intramuscular injection of duromeprazole (50 mg) or oral administration of acetaminophen could be given according to the patient’s condition to alleviate pain.
2.5 Delirium assessment
We used the 3-min diagnostic interview to evaluate delirium defined by the Confusion 3D-CAM (47), to screen for POD, and the MDAS (48) to evaluate the severity of delirium. This evaluation can be completed within an average of 3 min and has excellent performance compared with other methods of evaluation. The evaluation mainly includes the following four characteristics: acute onset and fluctuating condition; inability to concentrate; disordered thinking; and change in consciousness level. Specialists in geriatrics trained by professional psychiatrists administered the 3D-CAM to patients twice daily (in the morning and afternoon) during the first two postoperative days.
2.6 Blood sample and CSF collection
We collected 4 mL arterial blood samples from all patients before inducing anesthesia and immediately after surgery. Blood samples were drawn into EDTAK2-containing tubes (BD Biosciences, San Jose, CA, United States) kept at 4°C and centrifuged (3,000 × g for 10 min) within 4 h to separate plasma and blood cells. All plasma samples were stored at −80°C and sent to the laboratory of Peking University Third Hospital for further processing.
Before administering local anesthesia for a subarachnoid block, we slowly extracted 2 mL of CSF using a syringe and placed it in a sterile polypropylene tube. The CSF was immediately centrifuged (3,000 × g for 10 min) at 4°C to remove cells (49). Then we separated and reserved the supernatant, which was stored at −80°C until assayed.
2.7 Biochemical analysis
We used an enzyme immunoassay (Proteintech, Chicago, IL, United States) to measure the concentration of LCN2, with a detection limit of 0.0781 ng/mL. The levels of indicators such as albumin, creatinine, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured using a blood biochemistry analyzer (Hitachi, Tokyo, Japan); white blood cell (WBC), red blood cell (RBC), platelet, and hemoglobin levels were measured using a blood analyzer (Sysmex, Kobe, Japan). IL-1 β and IL-6 levels were also measured using enzyme immunoassays (Boster, Wuhan, China).
2.8 Participant sample size
In the present study, a binary logistic regression model was used to analyze the correlation between LCN2 content in preoperative CSF and POD. Five events per variable (EPV) is the widely used minimum standard for sample size analysis (50). It was estimated that five variables could be included in the final model. For a given number of EPVs, 5 EPV events were required to analyze the sample. At least 25 events (patients with POD) were necessary for analysis. Factoring in a dropout rate of 10%, 28 POD patients were needed. Moreover, a previous study showed that approximately 16% of patients undergoing hip replacement surgery developed POD (46), hence, the minimum sample size was 175.
2.9 Statistical analysis
All statistical analyses were performed using IBM SPSS Software for Windows (version 25.0; IBM Corp., Armonk, NY) and GraphPad Prism (version 8.0; GraphPad Software, San Diego, CA). A Shapiro–Wilk test was used to assess the normality of continuous data distributions. Normally distributed continuous data are presented as mean ± standard deviation (SD) and were compared using an independent samples Student t-test. Non-normally distributed data are reported as median and interquartile range (IQR) and were compared using a Mann–Whitney U-test. Categorical data are expressed as frequency (n) and percentage (%) and were compared using chi-square or Fisher exact tests, as appropriate. To identify independent predictors for POD, variables with clinical relevance or a p < 0.10 in univariable analysis were entered into a multivariable binary logistic regression model using a forward conditional method. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. Given a non-normal distribution of the data, a Spearman rank correlation coefficient was used to assess the association between preoperative CSF LCN2 levels and other variables (CSF IL-6, MDAS score). The predictive performance of preoperative CSF LCN2 for POD was evaluated by constructing a receiver operating characteristic (ROC) curve. The area under the curve (AUC) and its 95% CI were calculated. The Youden index (J = sensitivity + specificity − 1) was used to determine the optimal cutoff value. For all analyses, a two-sided p < 0.05 was considered significant.
3 Results
3.1 Participant characteristics
We screened 515 patients with hip fractures for the present study. As shown in the patient flowchart (Figure 1), 315 patients were excluded, mainly owing to their age being under 65 years (n = 69), pre-existing delirium (n = 45), and diagnosis of dementia (n = 31). Therefore, 200 patients met the criteria and agreed to participate. After further exclusion owing to accidental discharge or withdrawal of consent, a cohort of 186 patients was ultimately included and completed the study protocol. Among these 186 patients, 29 (15.6%) developed POD after surgery, while the remaining 157 patients did not, forming the non-POD group.
The patients in the POD group were significantly older than those in the non-POD group (median age: 80.0 years vs. 76.0 years, p = 0.018). Before surgery, those in the POD group showed significantly poorer cognitive function, with lower MMSE scores (median: 25.0 vs. 26.0, p = 0.005) and higher ACCI scores (mean: 5.14 vs. 4.41, p = 0.005). In addition, the prevalence of diabetes in the POD group was significantly higher (48.28% vs. 28.66%, p = 0.037), and the proportion of insulin or other hypoglycemic drugs was also higher (44.83% vs. 24.84%, p = 0.028). We found no significant differences between the two groups in terms of sex, BMI, ASA classification, Activities of Daily Living (ADL) score, PSQI score, or preoperative pain score (Table 1).
Table 1
| Variable | Non-POD group (n = 157) | POD group (n = 29) | p |
|---|---|---|---|
| Age, years | 76.0 (70.0,85.0) | 80.0 (76.5,86.0) | 0.018* |
| Sex, female, n (%) | 119 (75.8) | 23 (79.3) | 0.682 |
| BMI, kg/m2 | 23.2 ± 3.9 | 23.1 ± 3.5 | 0.827 |
| ASA physical status class | |||
| II, n (%) | 69 (59.5) | 18 (62.1) | 0.533 |
| III, n (%) | 47 (40.5) | 11 (37.9) | |
| MMSE score | 26.0 (25.0,26.0) | 25.000 (24.0,26.0) | 0.005** |
| ADL score | 100.0 (90.0,100.0) | 100.0 (80.0,100.0) | 0.237 |
| PSQI score | 14.0 (10.0,20.0) | 16.0 (13.5,20.0) | 0.070 |
| ACCI score | 4.4 ± 1.3 | 5.1 ± 1.3 | 0.005** |
| Resting NRS | 2.4 ± 1.1 | 2.5 ± 1.2 | 0.701 |
| Motion NRS | 5.0 (4.0,6.5) | 5.0 (5.0,6.0) | 0.460 |
| Smoke, n (%) | 26 (16.6) | 2 (6.9) | 0.181 |
| Hypertension, n (%) | 98 (62.4) | 19 (65.5) | 0.751 |
| Diabete, n (%) | 45 (28.7) | 14 (48.3) | 0.037* |
| Ischemic heart disease, n (%) | 30 (19.1) | 5 (17.2) | 0.813 |
| Chronic obstructive pulmonary disease, n (%) | 15 (9.6) | 2 (6.9) | 0.648 |
| Stroke, n (%) | 30 (19.1) | 5 (17.2) | 0.813 |
| Using β-blockers, n (%) | 18 (11.5) | 4 (13.8) | 0.721 |
| Using calcium channel blocker, n (%) | 69 (44.0) | 12 (41.4) | 0.798 |
| Using ACEI/ARB, n (%) | 38 (24.2) | 5 (17.2) | 0.414 |
| Using statins, n (%) | 44 (28.0) | 9 (31.0) | 0.742 |
| Using insulin/hypoglycemic drugs, n (%) | 39 (24.8) | 13 (44.8) | 0.028* |
| Preoperative laboratory examination | |||
| WBC, ×109/L | 9.7 (7.6, 11.6) | 9.5 (8.4, 10.9) | 0.963 |
| RBC, ×1012/L | 3.9 ± 0.6 | 4.0 ± 0.6 | 0.481 |
| PLT, ×109/L | 194.0 (146.0, 236.0) | 190.0 (158.5, 243.0) | 0.774 |
| Glycosylated hemoglobin, % | 6.0 (5.6, 6.5) | 6.1 (5.8, 6.7) | 0.387 |
| tP1NP, μg/L | 41.0 (31.6, 56.0) | 47.3 (31.4, 71.7) | 0.160 |
| β-CTX, pg./mL | 0.3 (0.2, 0.5) | 0.4 (0.2, 0.5) | 0.902 |
| 25-OHVD, ng/mL | 17.2 (12.2, 23.8) | 14.8 (11.4, 21.8) | 0.121 |
| Albumin, g/L | 41.3 (38.6, 43.7) | 41.2 (39.3, 43.0) | 0.707 |
| Creatinine, mmol/L | 53.0 (44.0, 66.0) | 56.0 (46.5, 74.0) | 0.238 |
| ALT, U/L | 15.0 (12.0, 19.0) | 14.0 (11.0, 20.5) | 0.964 |
| AST, U/L | 18.0 (15.5, 23.0) | 17.0 (15.5, 23.0) | 0.768 |
| PaO2, mmHg | 79.0 (70.0, 88.0) | 75.0 (68.0, 86.5) | 0.391 |
| Lactic acid, mmol/L | 1.0 (0.8, 1.6) | 1.2 (0.9, 2.0) | 0.305 |
| Intraoperative data | |||
| Anesthesia time, minutes | 75.0 (72.5, 90.0) | 75.0 (72.5, 92.5) | 0.895 |
| Surgery time, minutes | 60.0 (60.0, 70.0) | 60.0 (60.0, 65.0) | 0.532 |
| Blood loss, mL | 200.0 (100.0, 200.0) | 200.0 (100.0, 200.0) | 0.791 |
| Postoperative laboratory examination | |||
| WBC, ×109/L | 10.3 (7.8, 12.3) | 11.6 (9.7, 13.7) | 0.012* |
| RBC, ×1012/L | 3.6 (3.3, 3.9) | 3.5 (3.3, 4.1) | 0.928 |
| PLT, ×109/L | 172.0 (133.0, 231.0) | 199.0 (156.0, 223.5) | 0.380 |
| Postoperative data | |||
| Subjective sleep rating | 6.0 (4.0, 7.0) | 7.0 (3.0, 8.0) | 0.697 |
| Resting NRS | 2.0 (1.0, 3.0) | 3.0 (1.0, 3.0) | 0.073 |
| Motion NRS | 4.0 (4.0, 5.0) | 4.0 (3.0, 6.0) | 0.746 |
| Remedial analgesia, n (%) | 100 (63.7) | 23 (79.3) | 0.103 |
Patients’ baseline characteristics and intraoperative and postoperative data.
The categorical variables are expressed as n (%). Normal data are given as mean ± SD, whereas non-normaldata are expressed as median (25th percentile, 75th percentile). BMI, body mass index; ASA, American Society of Anesthesiologists Classification; MMSE, Mini-Mental State Examination; ADL, Activities of Daily Living; PSQI, Pittsburgh Sleep Quality Index; ACCI, Age-adjusted Charlson Comorbidity Index; NRS, numeric rating scale; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; tP1NP, total procollagen type I N-terminal propeptide; β-CTX, β-C-terminal telopeptide of type I collagen; 25-OHVD, 25-hydroxy vitamin D; WBC, white blood cells; RBC, red blood cells; PLT, platelets; ALT, alanine aminotransferase; AST, aspartate transaminase; PaO2, oxygen partial pressure. *p < 0.05, **p < 0.01.
3.2 CSF concentrations of LCN2, IL-1, and IL-6
The median preoperative CSF LCN2 concentration in the POD group was 1.8 ng/mL (IQR 1.3, 2.6), which was significantly higher than the observed 1.3 ng/mL (IQR 1.0, 1.8) in the non-POD group (p = 0.001) (Table 2; Figure 2). The preoperative IL-6 concentration in the CSF of patients in the POD group were significantly higher than those in the non-POD group (median: 16.135 vs. 19.248 pg./mL, p = 0.031), whereas there was no difference in the preoperative IL-1 concentration between the two group patients (p = 0.688) (Table 2; Figure 2).
Table 2
| Variable | Non-POD group | POD group | p |
|---|---|---|---|
| Preoperative plasma LCN2 (ng/mL) | 73.9 (58.2, 98.4) | 89.2 (70.5, 106.7) | 0.067 |
| Postoperative plasma LCN2 (ng/mL) | 110.5 (78.1, 178.9) | 102.9 (80.4, 157.6) | 0.649 |
| D-value of plasma LCN2 (ng/mL) | 22.2 (3.3, 72.1) | 6.1 (−2.2, 37.0) | 0.084 |
| Preoperative CSF LCN2 (ng/mL) | 1.3 (1.0, 1.8) | 1.8 (1.3, 2.6) | 0.001** |
| Preoperative CSF IL-1 (pg/mL) | 19.9 (12.3, 28.7) | 21.7 (11.8, 32.1) | 0.688 |
| Preoperative CSF IL-6 (pg/mL) | 16.1 (11.9, 20.8) | 19.2 (16.7, 21.5) | 0.031* |
Comparison of LCN2 concentration in plasma and CSF between the POD group and non-POD group patients, as well as comparison of IL-1 and IL-6 in CSF between the two groups of patients.
Non-normaldata are expressed as median (25th percentile, 75th percentile). CSF, cerebrospinal cfluid; LCN2, lipocalin-2; IL-1, interleukin-1; IL-6, interleukin-6; POD, postoperative delirium. *p < 0.05, **p < 0.01.
Figure 2
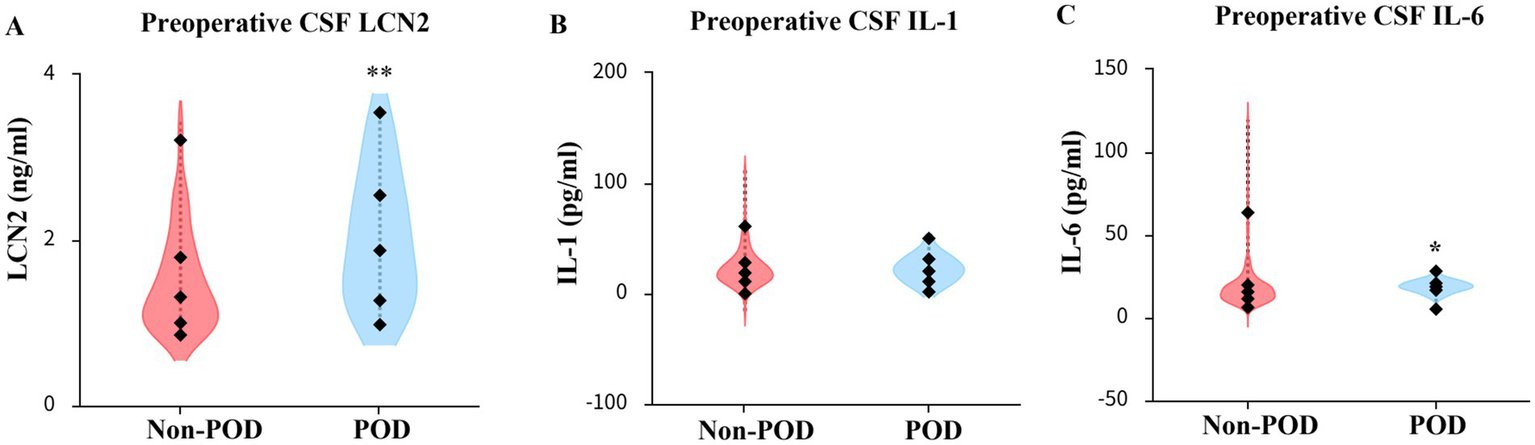
Comparison of LCN2, IL-1, IL-6 concentration in CSF between POD group and non-POD group patients. (A) Comparison of reoperative CSF LCN2 concentration between POD group and non-POD group patients. (B) Comparison of preoperative CSF IL-1 concentration between POD group and non-POD group patients. (C) Comparison of preoperative CSF IL-6 concentration between POD group and non-POD group patients. CSF, cerebrospinal fluid; LCN2, lipocalin-2; IL-1, interleukin-1; IL-6, Interleukin-6; POD, postoperative delirium. *p < 0.05, **p < 0.01.
3.3 Plasma concentrations of LCN2
There was no statistically significant difference in preoperative and postoperative plasma LCN2 concentrations between the POD group and the non-POD group (Table 2; Figure 3). To investigate the systemic effects of surgery on LCN2, we compared the plasma LCN2 levels before and after surgery. There was a highly significant increase in postoperative plasma LCN2 concentration (mean: preoperative 93.72 ng/mL vs. postoperative 132.9 ng/mL, p < 0.01) among total patients. A significant increase in postoperative concentration was observed in both the non-POD group (p < 0.01) and the POD group (p < 0.01). We further compared the D-value in LCN2 concentration in postoperative and preoperative plasma between non-POD and POD groups, and found no difference (Table 2; Figure 3).
Figure 3
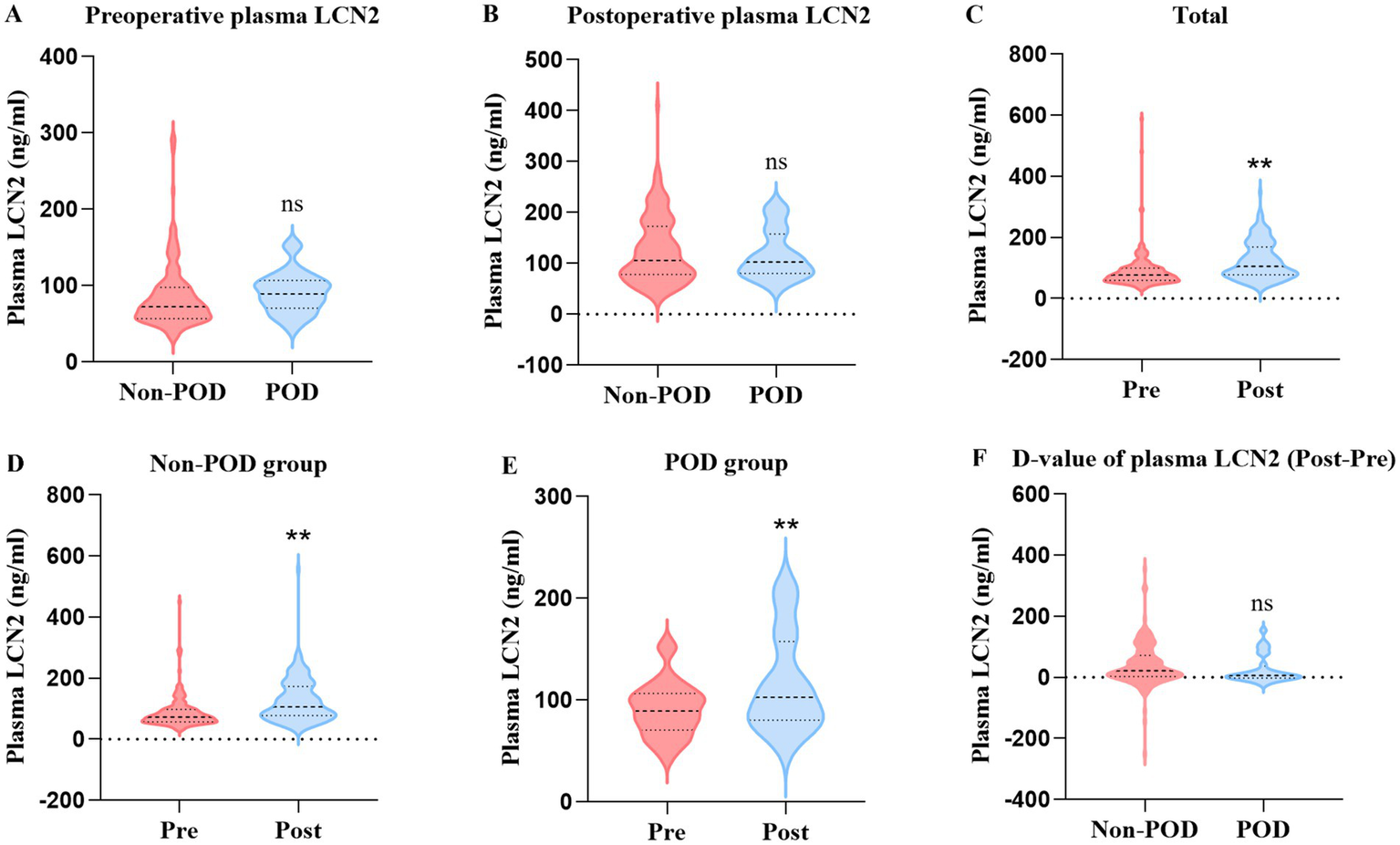
Comparison of preoperative and postoperative plasma LCN2 among total patients, POD group patients, and non-POD group patients. (A) Comparison of preoperative plasma LCN2 concentration between POD group and non-POD group patients. (B) Comparison of postoperative plasma LCN2 concentration between POD group and non-POD group patients. (C) Comparison of LCN2 concentration in postoperative and preoperative plasma in total patients. (D) Comparison of LCN2 concentration in postoperative and preoperative plasma in non-POD group patients. (E) Comparison of LCN2 concentration in postoperative and preoperative plasma in POD group patients. (F) Comparison of D-value of postoperative and preoperative plasma LCN2 concentration between the POD group and non-POD group patients. LCN2, lipocalin-2; POD, postoperative delirium. **p < 0.01.
3.4 Binary logistic regression analysis
To determine the independent predictive factors for POD occurrence, binary logistic analysis was conducted. Items with p < 0.05 were included in the model analysis (because the ACCI score included whether there was diabetes, we used ACCI instead of “diabetes” and “using insulin/diabetic drugs”). The variables included in the model include age, MMSE score, ACCI score, preoperative CSF LCN2, and preoperative CSF IL-6. We found that lower preoperative MMSE scores were a risk factor for POD (OR 0.297, 95% CI 0.167–0.257; p < 0.001), whereas higher preoperative CSF LCN2 concentrations were an independent risk factor for POD (OR 2.546, 95% CI 1.345–4.822; p = 0.004) (Table 3). A forest plot displays the OR and 95% CI of the multivariate logistic regression analysis used to predict POD (Figure 4).
Table 3
| Variable | Regression coefficient | p-value | OR value | 95% CI |
|---|---|---|---|---|
| Age | −0.007 | 0.841 | 0.994 | 0.932 ~ 1.059 |
| MMSE | −1.216 | <0.001 | 0.297 | 0.167 ~ 0.527 |
| ACCI | 0.305 | 0.155 | 1.357 | 0.891 ~ 2.065 |
| Preoperative CSF LCN2 | 0.935 | 0.004 | 2.546 | 1.345 ~ 4.822 |
| Preoperative CSF IL-6 | 0.013 | 0.529 | 1.013 | 0.973 ~ 1.055 |
Logistic regression analysis of factors of POD.
OR, odds ratio; CI, confidence interval; MMSE, Mini-Mental State Examination; ACCI, Age-adjusted Charlson Comorbidity Index; LCN2, lipocalin-2; IL-6, interleukin-6.
Figure 4
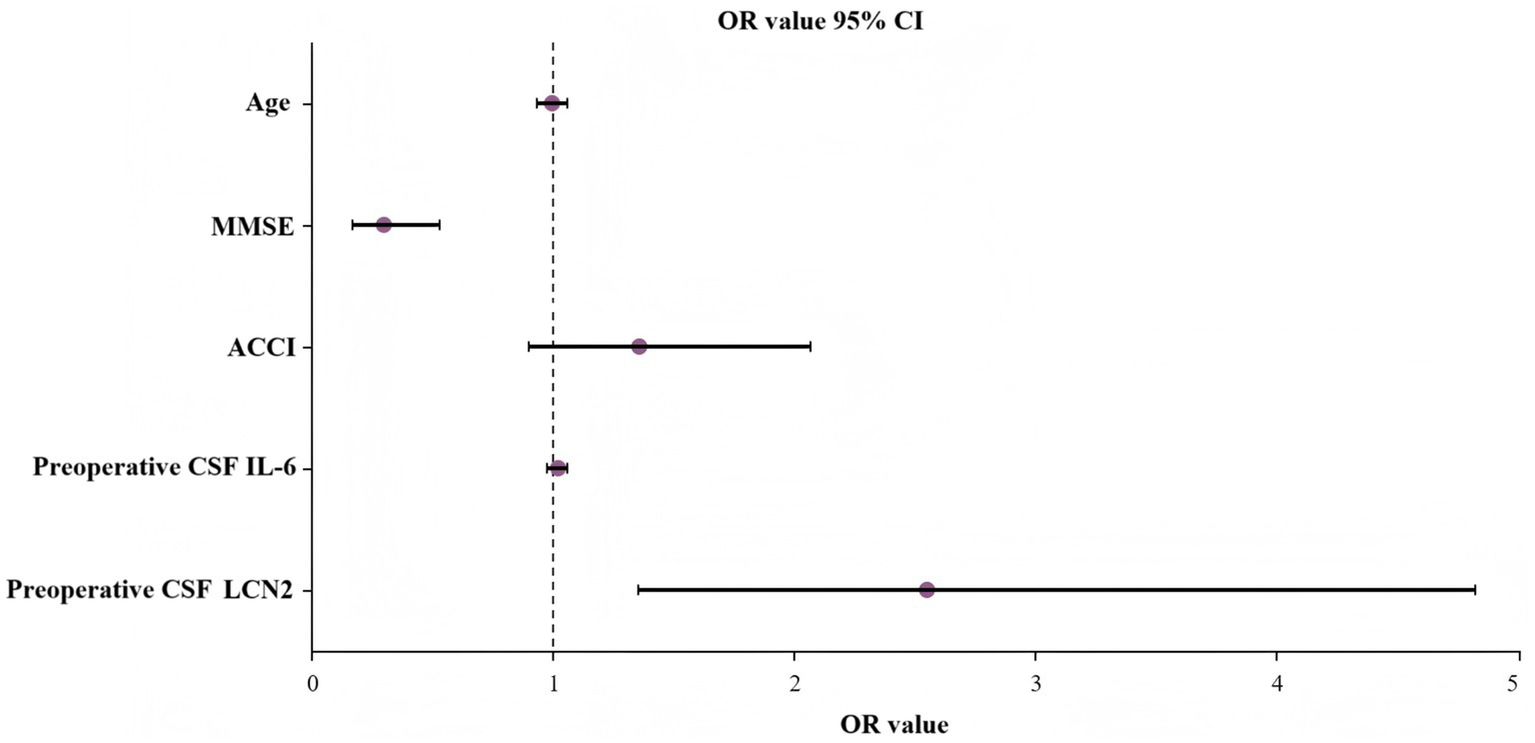
Predictors of POD with OR and 95% CI. MMSE, Mini-Mental State Examination; ACCI, Age-adjusted Charlson Comorbidity Index; CSF, cerebrospinal fluid; IL-6, interleukin-6; POD, postoperative delirium.
3.5 Correlation of preoperative CSF LCN2 with CSF IL-6 and MDAS scores
To explore the potential mechanisms linking LCN2 to POD, we performed correlation analyses within the POD group (n = 29).
First, we investigated the relationship between CSF LCN2 and a key neuroinflammatory cytokine, IL-6. As shown in Figure 5A, a moderate but significant positive correlation was found between preoperative CSF LCN2 concentrations and preoperative CSF IL-6 concentrations (rs = 0.379, p = 0.043) (Supplementary Table 1). This finding suggests that elevated LCN2 levels are associated with a heightened baseline neuroinflammatory state.
Figure 5
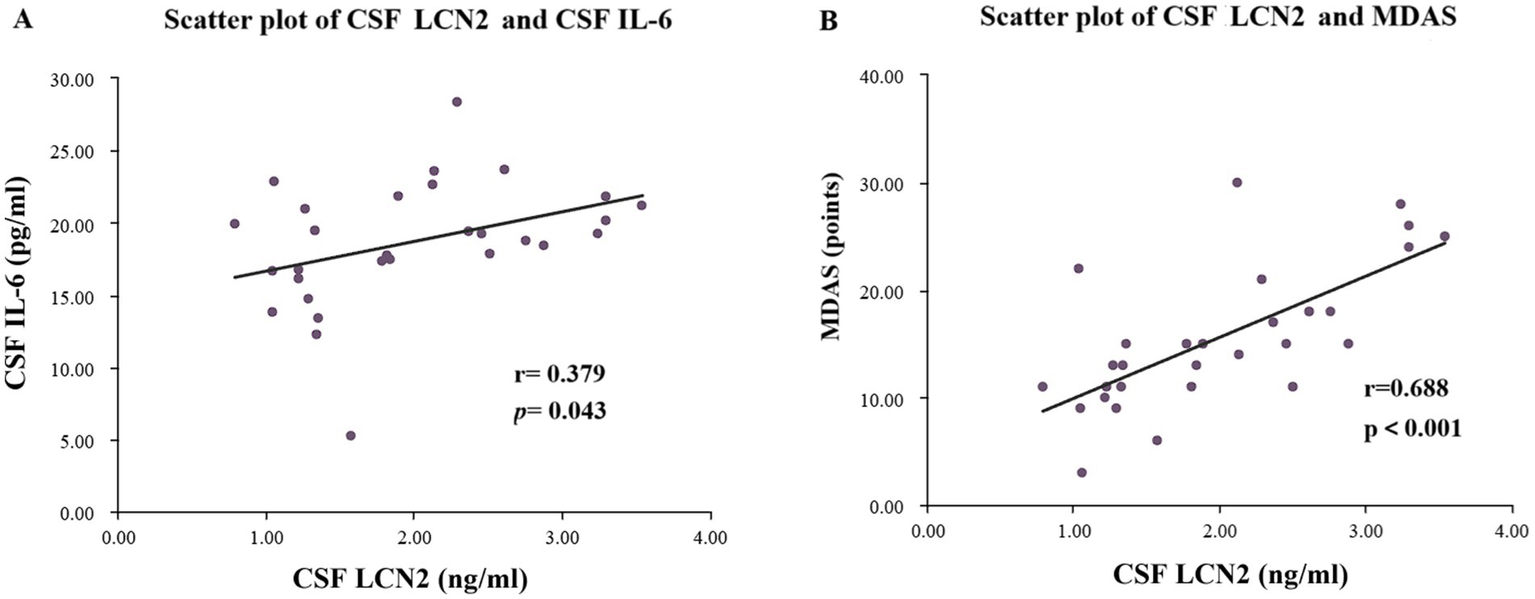
Scatter plots of LCN2 content and IL-6 content in CSF of the POD group patients, as well as scatter plots of LCN2 content in CSF and MDAS score. (A) Scatter plot of preoperative CSF LCN2 concentration and preoperative CSF IL-6 concentration. (B) Scatter plot of preoperative CSF LCN2 concentration and MDAS scores. POD, postoperative delirium; CSF, cerebrospinal fluid; LCN2, lipocalin-2; MDAS, Memorial Delirium Assessment Scale.
Next, we assessed whether CSF LCN2 levels correlated with MDAS scores. As depicted in Figure 5B, we observed a strong and highly significant positive correlation between preoperative CSF LCN2 levels and MDAS scores (rs = 0.688, p < 0.001) (Supplementary Table 1). These correlations indicate that higher preoperative central LCN2 levels are not only predictive of POD occurrence but are also linked to a more severe clinical presentation of delirium.
3.6 Prediction performance of preoperative CSF LCN2 for POD
We evaluated the predictive ability of preoperative CSF LCN2 for POD in older adults with hip fractures using ROC curve analysis. The results showed that preoperative CSF LCN2 had moderate predictive accuracy, with an AUC of 0.713 (95% CI 0.615–0.810; p < 0.001). According to the Youden index, the optimal cutoff value for CSF LCN2 was determined to be 1.769 ng/mL. At this threshold, the sensitivity and specificity of this biomarker for predicting POD were 58.6 and 75.0%, respectively (Figure 6; Table 4).
Figure 6
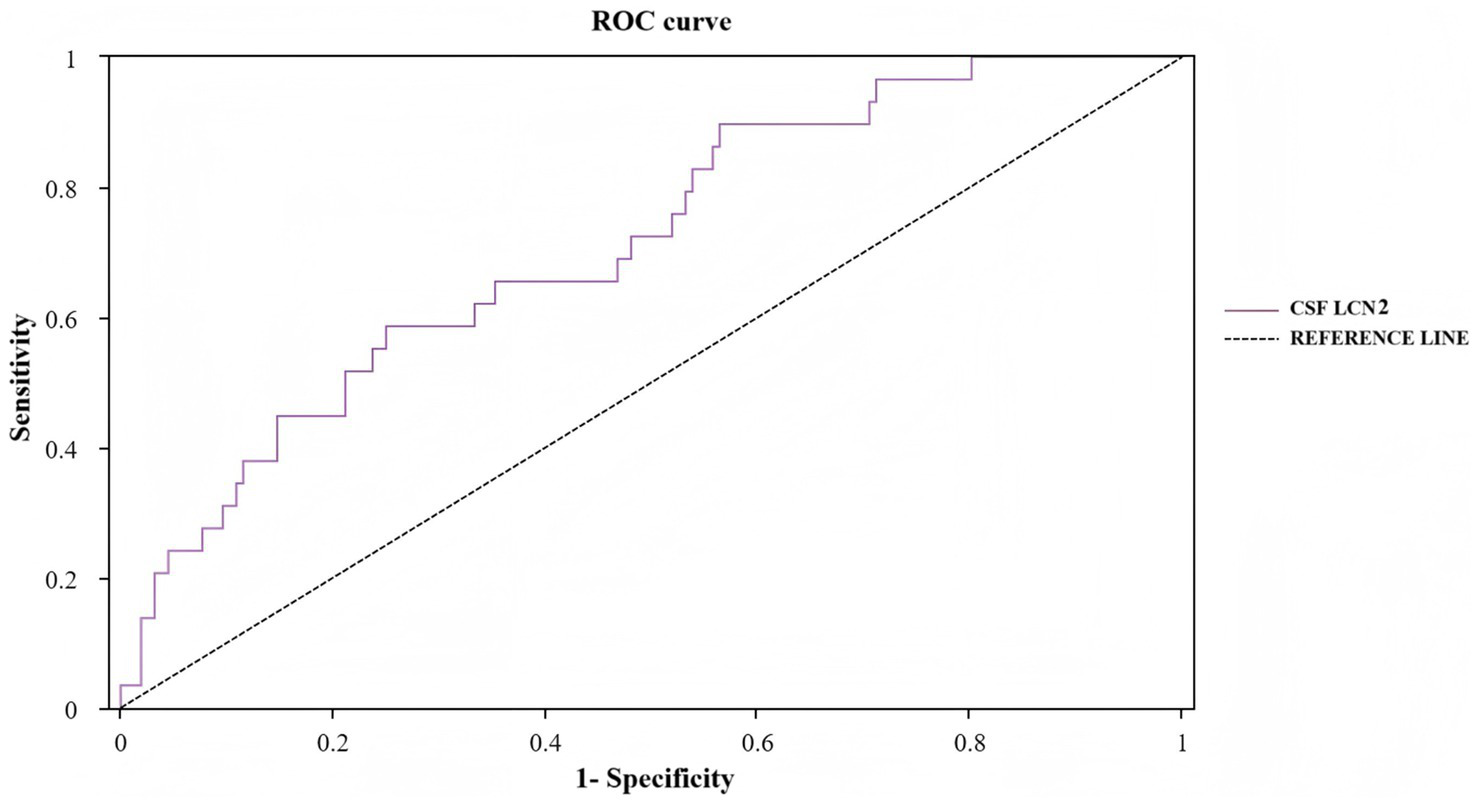
ROC curve for preoperative CSF LCN2 in POD. ROC, receiver operating characteristic; LCN2, lipocalin-2; POD, postoperative delirium.
Table 4
| Variable | AUC | Sensitivity+ Specificity-1 | Sensitivity | Specificity | Cut-off | Std. error | p | 95% CI for AUC |
|---|---|---|---|---|---|---|---|---|
| Preoperative CSF LCN2 | 0.713 | 0.336 | 0.586 | 0.750 | 1.769 | 0.050 | <0.001 | 0.615 ~ 0.810 |
The predicted values of preoperative CSF LCN2 for POD.
AUC, area under the curve; CI, confidence interval; CSF, cerebrospinal fluid; LCN2, lipocalin-2; POD, postoperative delirium.
4 Discussion
Our investigation reveals that elevated preoperative CSF LCN-2 levels are associated with the development of postoperative delirium in older adults undergoing hip fracture surgery. Furthermore, within the POD cohort, preoperative CSF LCN2 concentrations correlated positively with both delirium severity, as measured by the MDAS score, and levels of the proinflammatory cytokine IL-6 in the CSF.
We found that 29 of 186 older adults with hip fractures developed POD, with a POD incidence of 15.6%, which is consistent with previous studies (46, 51, 52). The results of the present study showed that preoperative low MMSE scores, preoperative high IL-6 levels in CSF, and high ACCI scores were associated with the occurrence of POD. These results are also consistent with those of previous studies (10, 53–57). Neuroinflammation is considered one of the important mechanisms underlying the occurrence of POD (58). The significantly high levels of LCN2 and IL-6 in preoperative CSF strongly indicate that some individuals have a pre-existing, subclinical neuroinflammatory state upon admission. This state may reflect age-related cellular aging, early neurodegenerative processes, or the accumulation of chronic low-grade inflammation. Under multiple impacts such as fracture trauma and surgical anesthesia, peripheral inflammation cascades and amplifies to the CNS in the “fragile brain” population, leading to postoperative delirium.
It is also instructive to consider why some established risk factors, such as a history of stroke and intraoperative blood loss, were not significantly associated with POD in our multivariable analysis. This apparent lack of association may be attributed to several factors. For a history of stroke, its effect may be primarily mediated through variables already in our model, such as a lower baseline MMSE score or a higher ACCI score; once these powerful predictors were accounted for, the independent contribution of stroke history may have become attenuated. The nearly identical volumes of blood loss between groups (Table 1) likely reflect a highly standardized and effective surgical and anesthetic management, which minimized its variability and thus its potential as a risk differentiator in this cohort. Although we cannot exclude that a larger sample size might reveal a significant association (a Type II error), the lack of significance of these factors in our model serves to highlight the potent and independent predictive capacity of the preoperative neuroinflammatory state, as marked explicitly by CSF LCN2.
LCN2 has a role in various pathophysiological processes throughout the body, including inflammatory response and cognitive function (31, 39). Toll-like receptors activate downstream inflammatory cascades during the inflammatory response, which have been shown to upregulate LCN2 (17). The LCN2 promoter has a common site for NF-κB, a transcription factor activated by various inflammatory cytokines (59). LCN2 seems to be associated with many neurodegenerative diseases, and there is evidence to suggest that LCN2 can participate in the pathophysiology of neurodegenerative diseases by affecting pathways such as inflammation, cell death/survival signaling, and iron metabolism (60). A meta-analysis showed that the peripheral blood LCN2 concentration in AD patients was significantly elevated compared with that in the control group. Peripheral blood LCN2 levels are also elevated in patients with mild cognitive impairment (35). Changes in LCN2 are associated with decreased executive ability in AD patients (61). A positive correlation between LCN2 and amyloid β-42 in CSF has been recognized, especially in MCI patients. LCN2 in CSF may serve as a predictive biomarker for the transition from MCI to AD dementia (38). Studies have shown that elevated plasma LCN2 levels are associated with non-motor symptoms in patients with Parkinson disease, and their mediated neuroinflammation is associated with cognitive impairment in patients with Parkinson disease (62).
The role of LCN2 in postoperative cognitive impairment remains to be elucidated. In rat models of POD, peripheral and central LCN2 concentrations increase after cardiac and abdominal surgery (41). Correlation analysis shows that spatial learning ability is correlated with plasma and hippocampal LCN2 levels (40). A prospective cohort study showed that the difference between postoperative and preoperative plasma LCN2 levels was associated with the occurrence of POD, with a greater difference observed in the POD group (42). We found that in both POD group and non-POD group patients, postoperative CSF LCN2 levels were significantly higher than preoperative levels. However, contrary to previous findings, we found no significant difference in the postoperative-to-preoperative change in LCN2 levels between patients with and without POD. This discrepancy might be attributable to differences in the patient populations; our cohort was comprised exclusively of older adults with hip fractures, who endure greater preoperative pain and stress. Differing anesthetic techniques and surgical durations may also have contributed to the discrepancy.
Strengths of the present study include its prospective design, which minimizes recall and selection bias. By focusing on a specific, homogenous population of older adults with hip fracture, we minimized baseline variability. Crucially, the exclusive use of spinal anesthesia for all participants is a major strength because it effectively eliminated the significant confounding influence that different anesthetic techniques (i.e., general vs. regional anesthesia) can have on POD outcomes. This rigorous standardization enhances the internal validity of the observed association between CSF LCN2 and POD.
Several limitations should be acknowledged. First, because this was a single-center study, the generalizability of our findings may be limited. Second, the relatively small sample size may have limited the statistical power to detect smaller effects. Therefore, multicenter, large-sample prospective studies are warranted to validate our findings. Third, although we controlled for key confounders, the observational nature of the study precludes definitive conclusions about causality, and residual confounding may exist. Fourth, the invasive nature of CSF collection limits the direct clinical translatability of CSF LCN2 as a routine screening tool. Finally, our panel of inflammatory markers was limited, and we did not measure plasma cytokines. Future research to address these limitations is warranted, including relevant basic research to further verify and expand on the findings. Animal models of hip fracture could be employed to mechanistically dissect how LCN2 contributes to neuroinflammation and delirium-like behaviors, providing a crucial bridge from clinical observation to molecular pathophysiology.
5 Conclusion
Elevated preoperative CSF LCN2 concentrations are associated with an increased risk of POD in older adults undergoing hip fracture surgery. Moreover, in patients with POD, the preoperative CSF LCN2 level is positively correlated with preoperative CSF IL-6 concentration and with MDAS scores.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Jishuitan Hospital (Approval number: JLKS202204-08). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NK: Formal analysis, Writing – original draft, Data curation, Visualization, Investigation, Software. XH: Writing – review & editing, Software, Validation, Data curation, Resources. TL: Visualization, Funding acquisition, Formal analysis, Supervision, Writing – review & editing. JH: Writing – review & editing, Methodology, Data curation, Visualization. ZhuL: Visualization, Methodology, Writing – review & editing, Formal analysis. ZheL: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. YY: Visualization, Formal analysis, Writing – review & editing, Supervision. YS: Supervision, Funding acquisition, Writing – review & editing, Resources, Methodology. NY: Conceptualization, Funding acquisition, Writing – review & editing, Supervision, Methodology. XG: Conceptualization, Investigation, Funding acquisition, Project administration, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82271289, 82271222, 81901095, 82101265 and 82201336), Research Fund for Young Anesthesiologists, Anesthesiology Branch, Chinese Medical Association (Z-2017-24-2421), Beijing Natural Science Foundation (L252119) and Beijing Hospitals Authority Clinical Medicine Development of special funding support (YGLX202320).
Acknowledgments
We thank Robin James Storer, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1653407/full#supplementary-material
References
1.
Chen FP Shyu YC Fu TS Sun CC Chao AS Tsai TL et al . Secular trends in incidence and recurrence rates of hip fracture: a nationwide population-based study. Osteoporos Int. (2017) 28:811–8. doi: 10.1007/s00198-016-3820-3
2.
Chen Y Liang S Wu H Deng S Wang F Lunzhu C et al . Postoperative delirium in geriatric patients with hip fractures. Front Aging Neurosci. (2022) 14:1068278. doi: 10.3389/fnagi.2022.1068278
3.
Jin Z Hu J Ma D . Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. (2020) 125:492–504. doi: 10.1016/j.bja.2020.06.063
4.
Migirov A Chahar P Maheshwari K . Postoperative delirium and neurocognitive disorders. Curr Opin Crit Care. (2021) 27:686–93. doi: 10.1097/MCC.0000000000000882
5.
Austin CA O'Gorman T Stern E Emmett D Stürmer T Carson S et al . Association between postoperative delirium and long-term cognitive function after major nonemergent surgery. JAMA Surg. (2019) 154:328–34. doi: 10.1001/jamasurg.2018.5093
6.
Maldonado JR . Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. (2018) 33:1428–57. doi: 10.1002/gps.4823
7.
Maldonado JR . Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. (2013) 21:1190–222. doi: 10.1016/j.jagp.2013.09.005
8.
Alam A Hana Z Jin Z Suen KC Ma D . Surgery, neuroinflammation and cognitive impairment. EBioMedicine. (2018) 37:547–56. doi: 10.1016/j.ebiom.2018.10.021
9.
Wang Y Shen X . Postoperative delirium in the elderly: the potential neuropathogenesis. Aging Clin Exp Res. (2018) 30:1287–95. doi: 10.1007/s40520-018-1008-8
10.
Lin X Tang J Liu C Li X Cao X Wang B et al . Cerebrospinal fluid cholinergic biomarkers are associated with postoperative delirium in elderly patients undergoing Total hip/knee replacement: a prospective cohort study. BMC Anesthesiol. (2020) 20:246. doi: 10.1186/s12871-020-01166-9
11.
Neerland BE Hall RJ Seljeflot I Frihagen F MacLullich AMJ Ræder J et al . Associations between delirium and preoperative cerebrospinal fluid C-reactive protein, Interleukin-6, and Interleukin-6 receptor in individuals with acute hip fracture. J Am Geriatr Soc. (2016) 64:1456–63. doi: 10.1111/jgs.14238
12.
Kjeldsen L Johnsen AH Sengeløv H Borregaard N . Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. (1993) 268:10425–32. doi: 10.1016/S0021-9258(18)82217-7
13.
Liu F Yang H Chen H Zhang M Ma Q . High expression of neutrophil gelatinase-associated lipocalin (NGAL) in the kidney proximal tubules of diabetic rats. Adv Med Sci. (2015) 60:133–8. doi: 10.1016/j.advms.2015.01.001
14.
Jaberi SA Cohen A D'Souza C Abdulrazzaq YM Ojha S Bastaki S et al . Lipocalin-2: structure, function, distribution and role in metabolic disorders. Biomed Pharmacother. (2021) 142:112002. doi: 10.1016/j.biopha.2021.112002
15.
Latouche C El Moghrabi S Messaoudi S Cat AND Hernandez-Diaz I de la Rosa DA et al . Neutrophil gelatinase-associated lipocalin is a novel mineralocorticoid target in the cardiovascular system. Hypertension. (2012) 59:966–72. doi: 10.1161/HYPERTENSIONAHA.111.187872
16.
Eilenberg W Stojkovic S Piechota-Polanczyk A Kaun C Rauscher S Gröger M et al . Neutrophil gelatinase-associated Lipocalin (NGAL) is associated with symptomatic carotid atherosclerosis and drives pro-inflammatory state in vitro. Eur J Vasc Endovasc Surg. (2016) 51:623–31. doi: 10.1016/j.ejvs.2016.01.009
17.
Flo TH Smith KD Sato S Rodriguez DJ Holmes MA Strong RK et al . Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. (2004) 432:917–21. doi: 10.1038/nature03104
18.
Devarajan P . Neutrophil gelatinase-associated lipocalin--an emerging troponin for kidney injury. Nephrol Dial Transplant. (2008) 23:3737–43. doi: 10.1093/ndt/gfn531
19.
Chen JJ Lee TH Lee CC Chang CH . Using lipocalin as a prognostic biomarker in acute kidney injury. Expert Rev Mol Diagn. (2021) 21:455–64. doi: 10.1080/14737159.2021.1917384
20.
Romejko K Markowska M Niemczyk S . The review of current knowledge on neutrophil gelatinase-associated Lipocalin (NGAL). Int J Mol Sci. (2023) 24:10470. doi: 10.3390/ijms241310470
21.
Sciarretta F Ceci V Tiberi M Zaccaria F Li H Zhou ZY et al . Lipocalin-2 promotes adipose-macrophage interactions to shape peripheral and central inflammatory responses in experimental autoimmune encephalomyelitis. Mol Metab. (2023) 76:101783. doi: 10.1016/j.molmet.2023.101783
22.
Kim JH Michiko N Choi IS Kim Y Jeong JY Lee MG et al . Aberrant activation of hippocampal astrocytes causes neuroinflammation and cognitive decline in mice. PLoS Biol. (2024) 22:e3002687. doi: 10.1371/journal.pbio.3002687
23.
Jung BK Park Y Yoon B Bae JS Han SW Heo JE et al . Reduced secretion of LCN2 (lipocalin 2) from reactive astrocytes through autophagic and proteasomal regulation alleviates inflammatory stress and neuronal damage. Autophagy. (2023) 19:2296–317. doi: 10.1080/15548627.2023.2180202
24.
Xiang X Tang X Yu Y Xie S Liu L Chen ML et al . Role of lipocalin-2 in surgery-induced cognitive decline in mice: a signal from neuron to microglia. J Neuroinflammation. (2022) 19:92. doi: 10.1186/s12974-022-02455-5
25.
Chen Y Zheng D Wang H Zhang S Zhou Y Ke X et al . Lipocalin 2 in the paraventricular thalamic nucleus contributes to DSS-induced depressive-like behaviors. Neurosci Bull. (2023) 39:1263–77. doi: 10.1007/s12264-023-01047-4
26.
Jin M Kim JH Jang E Lee YM Han HS Woo DK et al . Lipocalin-2 deficiency attenuates neuroinflammation and brain injury after transient middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. (2014) 34:1306–14. doi: 10.1038/jcbfm.2014.83
27.
Mosialou I Shikhel S Liu JM Maurizi A Luo N He Z et al . MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. (2017) 543:385–90. doi: 10.1038/nature21697
28.
Kang SS Ren Y Liu CC Kurti A Baker KE Bu G et al . Lipocalin-2 protects the brain during inflammatory conditions. Mol Psychiatry. (2018) 23:344–50. doi: 10.1038/mp.2016.243
29.
Du Y Li W Lin L Lo EH Xing C . Effects of lipocalin-2 on brain endothelial adhesion and permeability. PLoS One. (2019) 14:e0218965. doi: 10.1371/journal.pone.0218965
30.
Jha MK Lee S Park DH Kook H Park KG Lee IK et al . Diverse functional roles of lipocalin-2 in the central nervous system. Neurosci Biobehav Rev. (2015) 49:135–56. doi: 10.1016/j.neubiorev.2014.12.006
31.
Xiao X Yeoh BS Vijay-Kumar M . Lipocalin 2: an emerging player in Iron homeostasis and inflammation. Annu Rev Nutr. (2017) 37:103–30. doi: 10.1146/annurev-nutr-071816-064559
32.
Doliwa M Kuzniewska B Nader K Reniewicz P Kaczmarek L Michaluk P et al . Astrocyte-secreted Lcn2 modulates dendritic spine morphology. Cells. (2025) 14:159. doi: 10.3390/cells14030159
33.
Chen X Qiu F Zhao X Lu J Tan X Xu J et al . Astrocyte-derived Lipocalin-2 is involved in mitochondrion-related neuronal apoptosis induced by methamphetamine. ACS Chem Neurosci. (2020) 11:1102–16. doi: 10.1021/acschemneuro.9b00559
34.
Li J Xu P Hong Y Xie Y Peng M Sun R et al . Lipocalin-2-mediated astrocyte pyroptosis promotes neuroinflammatory injury via NLRP3 inflammasome activation in cerebral ischemia/reperfusion injury. J Neuroinflammation. (2023) 20:148. doi: 10.1186/s12974-023-02819-5
35.
Wu CY Bawa KK Ouk M Leung N Yu D Lanctôt KL et al . Neutrophil activation in Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis of protein markers in blood and cerebrospinal fluid. Ageing Res Rev. (2020) 62:101130. doi: 10.1016/j.arr.2020.101130
36.
Doroszkiewicz J Kulczyńska-Przybik A Dulewicz M Mroczko J Borawska R Słowik A et al . Associations between microglia and astrocytic proteins and tau biomarkers across the continuum of Alzheimer's disease. Int J Mol Sci. (2024) 25:25 (14). doi: 10.3390/ijms25147543
37.
Kim BW Jeong KH Kim JH Jin M Kim JH Lee MG et al . Pathogenic upregulation of glial Lipocalin-2 in the parkinsonian dopaminergic system. J Neurosci. (2016) 36:5608–22. doi: 10.1523/JNEUROSCI.4261-15.2016
38.
Naudé PJW Ramakers I van der Flier WM Jiskoot LC Reesink FE Claassen JAHR et al . Serum and cerebrospinal fluid neutrophil gelatinase-associated lipocalin (NGAL) levels as biomarkers for the conversion from mild cognitive impairment to Alzheimer's disease dementia. Neurobiol Aging. (2021) 107:1–10. doi: 10.1016/j.neurobiolaging.2021.07.001
39.
Li X Wang X Guo L Wu K Wang L Rao L et al . Association between lipocalin-2 and mild cognitive impairment or dementia: a systematic review and meta-analysis of population-based evidence. Ageing Res Rev. (2023) 89:101984. doi: 10.1016/j.arr.2023.101984
40.
Gouweleeuw L Hovens IB van Leeuwen BL Schoemaker RG . Neutrophil gelatinase-associated lipocalin and microglial activity are associated with distinct postoperative behavioral changes in rats. Behav Brain Res. (2017) 319:104–9. doi: 10.1016/j.bbr.2016.11.023
41.
Hovens IB van Leeuwen BL Mariani MA Kraneveld AD Schoemaker RG . Postoperative cognitive dysfunction and neuroinflammation; cardiac surgery and abdominal surgery are not the same. Brain Behav Immun. (2016) 54:178–93. doi: 10.1016/j.bbi.2016.02.003
42.
Brattinga B Plas M Spikman JM Rutgers A de Haan JJ Absalom AR et al . The association between the inflammatory response following surgery and post-operative delirium in older oncological patients: a prospective cohort study. Age Ageing. (2022) 51:afab237. doi: 10.1093/ageing/afab237
43.
Cuschieri S . The STROBE guidelines. Saudi J Anaesth. (2019) 13:S31–s34. doi: 10.4103/sja.SJA_543_18
44.
Owen AR Amundson AW Fruth KM Duncan CM Smith HM Johnson RL et al . Spinal versus general anesthesia in contemporary revision total hip arthroplasties. J Arthroplast. (2023) 38:S184–S188.e181. doi: 10.1016/j.arth.2023.03.013
45.
Owen AR Amundson AW Fruth KM Duncan CM Smith HM Johnson RL et al . Spinal compared with general anesthesia in contemporary primary Total hip arthroplasties. J Bone Joint Surg Am. (2022) 104:1542–7. doi: 10.2106/JBJS.22.00280
46.
Song Y Liu Y Yuan Y Jia X Zhang W Wang G et al . Effects of general versus subarachnoid anaesthesia on circadian melatonin rhythm and postoperative delirium in elderly patients undergoing hip fracture surgery: a prospective cohort clinical trial. EBioMedicine. (2021) 70:103490. doi: 10.1016/j.ebiom.2021.103490
47.
Marcantonio ER Ngo LH O'Connor M Jones RN Crane PK Metzger ED et al . 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. (2014) 161:554–61. doi: 10.7326/M14-0865
48.
Marcantonio E Ta T Duthie E Resnick NM . Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. (2002) 50:850–7. doi: 10.1046/j.1532-5415.2002.50210.x
49.
Müller M Kuiperij HB Claassen JA Küsters B Verbeek MM . Micro RNAs in Alzheimer's disease: differential expression in hippocampus and cell-free cerebrospinal fluid. Neurobiol Aging. (2014) 35:152–8. doi: 10.1016/j.neurobiolaging.2013.07.005
50.
Peduzzi P Concato J Kemper E Holford TR Feinstein AR . A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. (1996) 49:1373–9. doi: 10.1016/S0895-4356(96)00236-3
51.
Kang N Yang N Zhao K Li Z Zhang W Han Y et al . Preoperative plasma visfatin may have a dual effect on the occurrence of postoperative delirium. Front Med. (2022) 9:1024942. doi: 10.3389/fmed.2022.1024942
52.
He R Wang F Shen H Zeng Y Zhang L . Association between increased neutrophil-to-lymphocyte ratio and postoperative delirium in elderly patients with total hip arthroplasty for hip fracture. BMC Psychiatry. (2020) 20:496. doi: 10.1186/s12888-020-02908-2
53.
Zhao J Liang G Hong K Pan J Luo M Liu J et al . Risk factors for postoperative delirium following total hip or knee arthroplasty: a meta-analysis. Front Psychol. (2022) 13:993136. doi: 10.3389/fpsyg.2022.993136
54.
Segernäs A Skoog J Ahlgren Andersson E Almerud Österberg S Thulesius H Zachrisson H . Prediction of postoperative delirium after cardiac surgery with a quick test of cognitive speed, Mini-mental state examination and hospital anxiety and depression scale. Clin Interv Aging. (2022) 17:359–68. doi: 10.2147/CIA.S350195
55.
Hirsch J Vacas S Terrando N Yuan M Sands LP Kramer J et al . Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. (2016) 13:211. doi: 10.1186/s12974-016-0681-9
56.
Liu K Song Y Yuan Y et al . Type 2 diabetes mellitus with tight glucose control and poor pre-injury stair climbing capacity may predict postoperative delirium: a secondary analysis. Brain Sci. (2022) 12:951. doi: 10.3390/brainsci12070951
57.
Liu J Li J He J Zhang H Liu M Rong J . The age-adjusted Charlson comorbidity index predicts post-operative delirium in the elderly following thoracic and abdominal surgery: a prospective observational cohort study. Front Aging Neurosci. (2022) 14:979119. doi: 10.3389/fnagi.2022.979119
58.
Eckenhoff RG Eckenhoff MF . Perioperative neurocognitive disorder: reply. Anesthesiology. (2020) 133:243–4. doi: 10.1097/ALN.0000000000003363
59.
Wang Y Jia M Yan X Cao L Barnes PJ Adcock IM et al . Increased neutrophil gelatinase-associated lipocalin (NGAL) promotes airway remodelling in chronic obstructive pulmonary disease. Clin Sci. (2017) 131:1147–59. doi: 10.1042/CS20170096
60.
Ferreira AC S DM Sousa JC Correia-Neves M Sousa N Palha JA et al . From the periphery to the brain: Lipocalin-2, a friend or foe?Prog Neurobiol. (2015) 131:120–36. doi: 10.1016/j.pneurobio.2015.06.005
61.
Bawa KK Krance SH Herrmann N Cogo-Moreira H Ouk M Yu D et al . A peripheral neutrophil-related inflammatory factor predicts a decline in executive function in mild Alzheimer's disease. J Neuroinflammation. (2020) 17:84. doi: 10.1186/s12974-020-01750-3
62.
Fan Y Li X Ma J Yang D Liang K Shen Y et al . Increased plasma lipocalin-2 levels are associated with nonmotor symptoms and neuroimaging features in patients with Parkinson's disease. J Neurosci Res. (2024) 102:e25303. doi: 10.1002/jnr.25303
Summary
Keywords
hip fracture, elderly patient, lipocalin-2, postoperative delirium, interleukin-6
Citation
Kang N, Han X, Liu T, Huang J, Li Z, Li Z, Yuan Y, Song Y, Yang N and Guo X (2025) Lipocalin-2 in preoperative cerebrospinal fluid is a biomarker for postoperative delirium after hip fracture surgery in older adults: a prospective cohort study. Front. Neurol. 16:1653407. doi: 10.3389/fneur.2025.1653407
Received
25 June 2025
Accepted
25 August 2025
Published
12 September 2025
Volume
16 - 2025
Edited by
Daniele Corbo, University of Brescia, Italy
Reviewed by
Penghuan Wu, Shaoguan First People’s Hospital, China
Maokai Xu, Fujian Provincial Hospital, China
Updates
Copyright
© 2025 Kang, Han, Liu, Huang, Li, Li, Yuan, Song, Yang and Guo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Yang, yangning@bjmu.edu.cn; Xiangyang Guo, puthmzk@hsc.pku.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.