- 1Department of Radiology, Zhejiang Hospital, Hangzhou, China
- 2Institute of Research and Clinical Innovations, Neusoft Medical Systems Co., Ltd., Shanghai, China
- 3Yiruide Medical Equipment New Technology Co., Ltd., Wuhan, China
Introduction: The present study aims to investigate the effects of repetitive transcranial magnetic stimulation (rTMS) on smoking cessation and white matter (WM) structure related to the mesolimbic dopamine pathway using diffusion spectrum imaging (DSI).
Methods: The rTMS over the left dorsolateral prefrontal cortex (DLPFC) was repeated 10 times in 18 smokers. Quantitative anisotropy (QA) and fractional anisotropy (FA) were calculated for the anisotropy assessment, and mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) were determined for the diffusivity evaluation. Nicotine dependence, and craving and withdrawal symptoms were evaluated using the Fagerström Test for Nicotine Dependence (FTND), the short version of the Tobacco Craving Questionnaire (sTCQ), the visual analogue scale (VAS), and the Minnesota Nicotine Withdrawal Scale (MNWS).
Results: After 10 times of rTMS, the FTND, MNWS and VAS scores significantly decreased, when compared to baseline, and withdrawal symptoms were partially alleviated. Furthermore, cigarette consumption was significantly decreased by rTMS, and four participants completely stopped smoking after rTMS treatment. Importantly, the smokers only had a reduction of AD in the right nucleus accumbens (NAc) fibers after rTMS, and a strong positive correlation was observed between the change in cigarette consumption and change in AD values in the right NAc fibers after rTMS treatment.
Discussion: These results suggest that rTMS over the left DLPFC is a potential effective strategy for nicotine dependence and craving, which is probably due to the modulation of the right NAc fibers. The right NAc emerged as a region of interest that warrants further investigation as a potential therapeutic target.
1 Introduction
Smoking has been recognized as one of the most widespread and enduring forms of addiction, with its well-documented adverse health effects (1). China is presently the largest producer and consumer of tobacco in the world, and the number of tobacco-related deaths in China is proposed to increase from approximately one million in 2010 to two million annually by 2030 and three million by 2050, unless widespread smoking cessation occurs (2).
The nature of tobacco addiction primarily stems from the effects of nicotine on the central nervous system (CNS) (3). Similar to other addictive substances, nicotine activates the mesolimbic dopamine pathway which originates from the ventral tegmental area (VTA) and projects to reward-related regions, including the nucleus accumbens (NAc), subthalamic nucleus (STN), amygdala (AMY), prefrontal cortex (PFC), hippocampus (HIP), and other areas (4–7). Indeed, accumulating neuroimaging evidence has revealed abnormalities in these regions associated to chronic smoking and relapse (8, 9). However, studies that employed diffusion tensor imaging (DTI) to explore the effect of nicotine on the brain microstructure have yielded inconsistent findings. For instance, chronic nicotine consumption has been linked to both decreased fractional anisotropy (FA) (10, 11) and increased FA (12), along with decreased radial diffusivity (RD) (12) and increased RD (13) levels. In addition, a study on nicotine addiction indicated that smokers have decreased FA values in the left anterior and posterior insula cortex-NAc fiber tracts, and higher FA, and lower axial diffusivity (AD), RD, and mean diffusivity (MD) values in the right posterior insula cortex-NAc fiber tracts, when compared to non-smokers (14). Despite these advancements, studies that used neuroimaging for nicotine addiction diagnosis, prognosis and treatment remain scarce. This challenge may be partially attributed to the limited investigations conducted on brain white matter (WM), which constitutes >50% of the total mass of the human brain, and is essential for facilitating connections among various cortical regions (9).
Diffusion magnetic resonance imaging (dMRI) is the unique non-intrusive approach available for evaluating the structural integrity of WM (15). The predominant dMRI technique utilized for studying WM connectivity, and its associations with substance abuse and addictive behaviors is DTI, based on the Gaussian model (12, 16). However, fiber tracking based on DTI has limitations in revealing the crossing or branching fibers in a voxel (17, 18). Diffusion spectrum imaging (DSI) can reliably track the fibers along different directions within a voxel, thereby improving fiber reconstruction, and providing a more accurate reflection of the brain’s complex structures (19). For instance, a study revealed that DSI tractography displayed the lower maturity in the cingulum bundle when myelination was incomplete, while DTI tractography tended to terminate in such regions, possibly due to the presence of crossing fibers (20). Furthermore, DSI provides high-quality fiber tractography, reduces partial volume effects and false continuation artifacts, and has been utilized to reveal more comprehensive connectivity patterns and detailed anatomical structures (21–23). In addition, DSI imaging data can be employed to analyze elementary DTI indicators (24). Therefore, DSI demonstrates a powerful potential for enhancing the tractography of complex WM fibers, and has been increasingly utilized in studying a range of clinical conditions, including psychosis, stroke and epilepsy, among others (25–27). However, reports on the application of DSI in adult nicotine addiction remain rare.
In clinical practice, varenicline, bupropion and nicotine withdrawal management therapy are commonly prescribed for smoking cessation. Although these treatments are effective for a number of smokers, a substantial number of individuals do not respond to these treatments, presenting persistent challenges in addressing relapse (28). To date, alternative treatments with greater efficacy, such as neurostimulation techniques, including transcranial magnetic stimulation (TMS), have been developed for smoking discontinuation (29). Repetitive transcranial magnetic stimulation (rTMS) has been described as a non-invasive, medication-free therapeutic approach rooted in neural circuit modulation (30–32). Although TMS can only stimulate the cortical surface beneath the device’s magnetic coil (33), empirical evidence suggests that TMS can additionally influence the activity in the “downstream” brain regions (34). Furthermore, a recent study that combined functional magnetic resonance imaging (fMRI) and rTMS suggested that the dorsomedial PFC-extended AMY/basal forebrain circuit may be associated to nicotine addiction in schizophrenia and non-schizophrenia individuals (35). Other studies have also suggested the potential effect of rTMS on smoke cessation (36–38). However, the mechanism of rTMS in the treatment of smokers without psychosis has not been well-investigated.
Thus, DSI tractography was utilized to investigate the WM microstructure changes in the mesolimbic dopamine pathway-related regions (VTA, NAc, STN, AMY, PFC and HIP) in smokers who received rTMS treatment. Furthermore, it was hypothesized that rTMS can be beneficial for behavioral improvement, and modulate the WM microstructure in mesolimbic dopamine pathway-related regions. Moreover, a correlation may exist between changes in nicotine addiction behavior and alterations in WM microstructure.
2 Methods
2.1 Participants
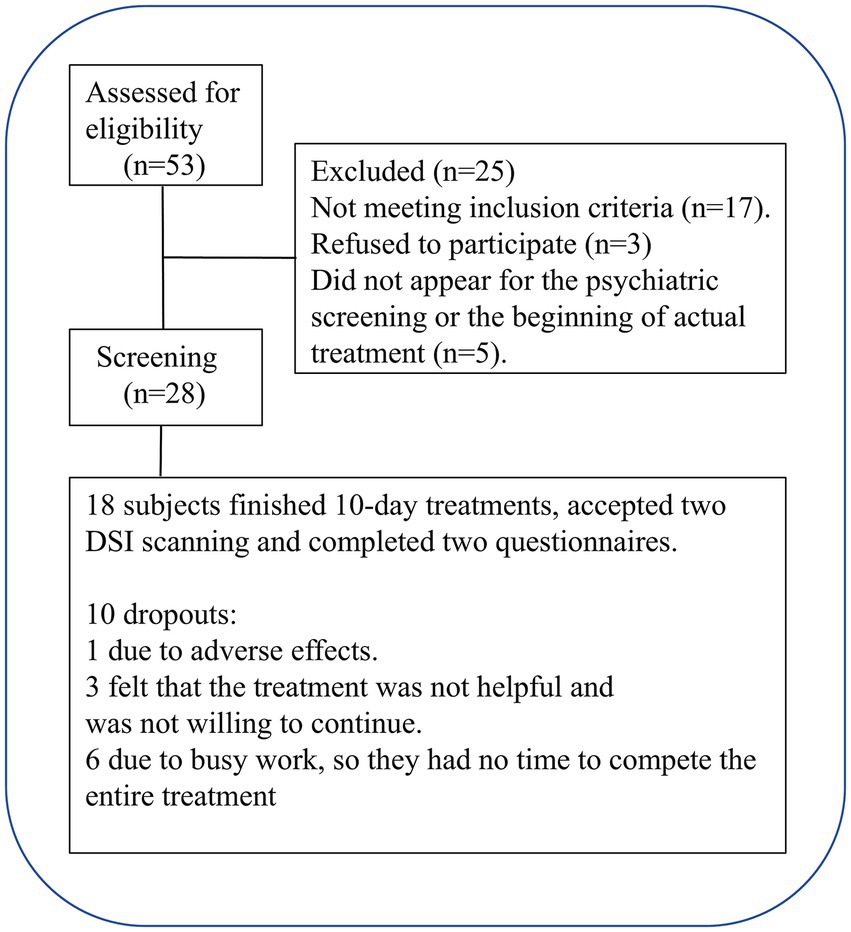
Fifty-three smokers were engaged via online postings and referrals through verbal communication. Initial screening was conducted through a brief telephone interview, followed by a detailed interview, according to the criteria for inclusion and exclusion (Table 1), and 20 smokers were excluded. Participants who met the inclusion criteria underwent further face-to-face screening, and five smokers failed to attend the psychiatric screening or the initial phase of the actual treatment. Finally, 18 participants finished all the assigned tasks and 10 rTMS treatments. The flowchart for participant recruitment and treatment is presented in Figure 1. The present study was approved by the Institutional Review Board of Zhejiang Hospital (2019 Pro-examination-34K No. -X2). All participants provided a signed consent form.
2.2 Assessment of cigarette consumption, nicotine dependence, and craving levels
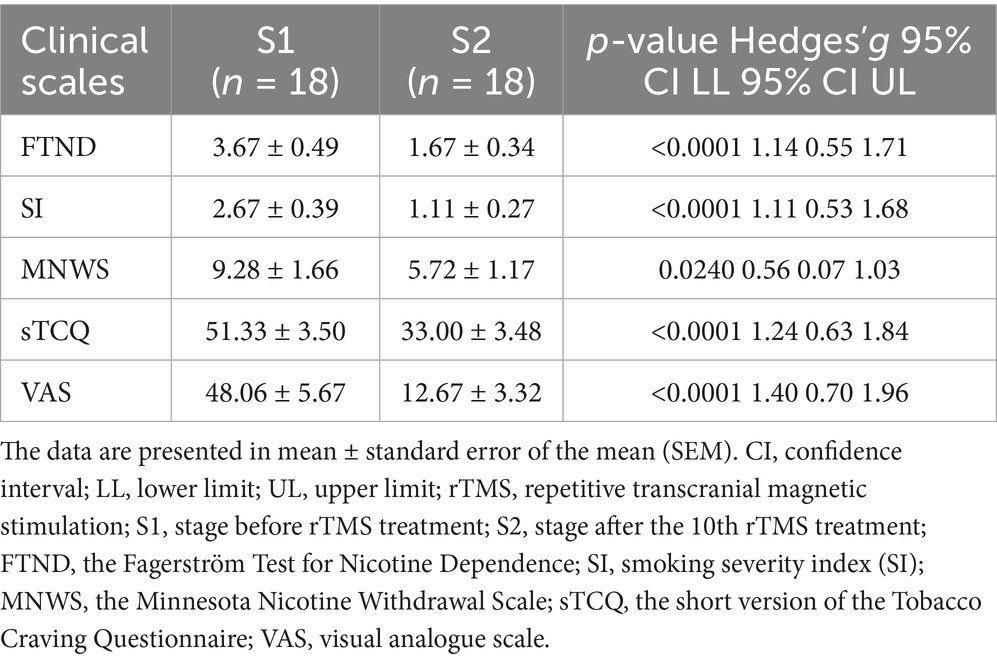
Daily cigarette consumption was self-reported by the participants, and nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND) (39) and smoking severity index (SI) (40). Symptoms related to nicotine withdrawal were evaluated using the Minnesota Nicotine Withdrawal Scale (MNWS) formulated in 1986 (41). Cravings were assessed using the short version of the Tobacco Craving Questionnaire (sTCQ) (42) and a subjective visual analogue scale (VAS) (43, 44). The FTND, SI, MNWS and sTCQ were measured twice: at baseline before rTMS (S1) and after the 10th rTMS session (S2). VAS was assessed before and after each rTMS session through the responses of the participants to the following question: “How much do you want to smoke right now?”
2.3 The rTMS procedure
The rTMS treatment was conducted according to prior studies (31, 45) by targeting the left dorsolateral prefrontal cortex (DLPFC) using a YRD CCY-II TMS (Yiruide, China) stimulator equipped with an O-coil design. The resting motor threshold (RMT) was established through motor strip activation, followed by the analysis of the resultant motor-evoked potential recorded from the right abductor pollicis brevis (APB). The stimulation site was identified using a TMS head cap. The stimulation lasted for 800 s, and delivered 1,800 pulses that comprised 36 trains of 50 pulses at 10 Hz, with an inter-train interval of 20 s, through an intensity set at 100% of the RMT. Each participant received 10 treatments over 2 weeks.
2.4 Imaging acquisition and processing
The magnetic resonance imaging (MRI) scan was conducted using a 3 T MRI scanner (Magnetom Skyra, Siemens, Germany) equipped with a 32-channel head coil. During the MRI, the participants were instructed to remain motionless, close their eyes, and avoid focusing on any specific thought. Motion artifacts were minimized by placing a foam pad between the head and coil. Three-dimensional (3D) T1-weighted images were acquired using the following settings: 176 sagittal slices, gap = 0 mm, field of view (FOV) = 256 × 256 mm, repetition time (TR) = 1,900 ms, echo time (TE) = 2.50 ms, inversion time (TI) = 900 ms, flip angle = 30°, and voxel size = 1.00 × 1.00 × 1.00 mm3. DSI scans were acquired using the following parameters: slice number = 60, TR = 8,700 ms, TE = 110.00 ms, FOV = 240 × 240 mm, voxel size = 1.10 × 1.10 × 2.50 mm3, phase encoding direction of anterior-to-posterior (A to P), maximum b-value = 3,000 s/mm2, q-space diffusion mode, q-space weightings = 3, and half q-space coverage.
The dMRI data was preprocessed by eddy current and motion correction using DSI Studio.1 Then, the diffusion data underwent reconstruction, utilizing the generalized q-sampling imaging (46), according to a previous study (12). The quantitative anisotropy (QA), FA, MD, AD, and RD values of each participant were computed using DSI Studio.
2.5 Regions of interest-based fiber tracking
The fiber clusters of six regions of interest (ROIs) related to nicotine addiction circuits (VTA, NAc, STN, AMY, PFC and HIP) were chosen from the Anatomical Automatic Labeling (AAL) template. Linear and non-linear transformations were applied to map the QA, FA, MD, AD and RD image coordinates of each participant’s brain onto the Montreal Neurological Institute’s (MNI) space. The working flowchart is presented in Figures 2A,B.
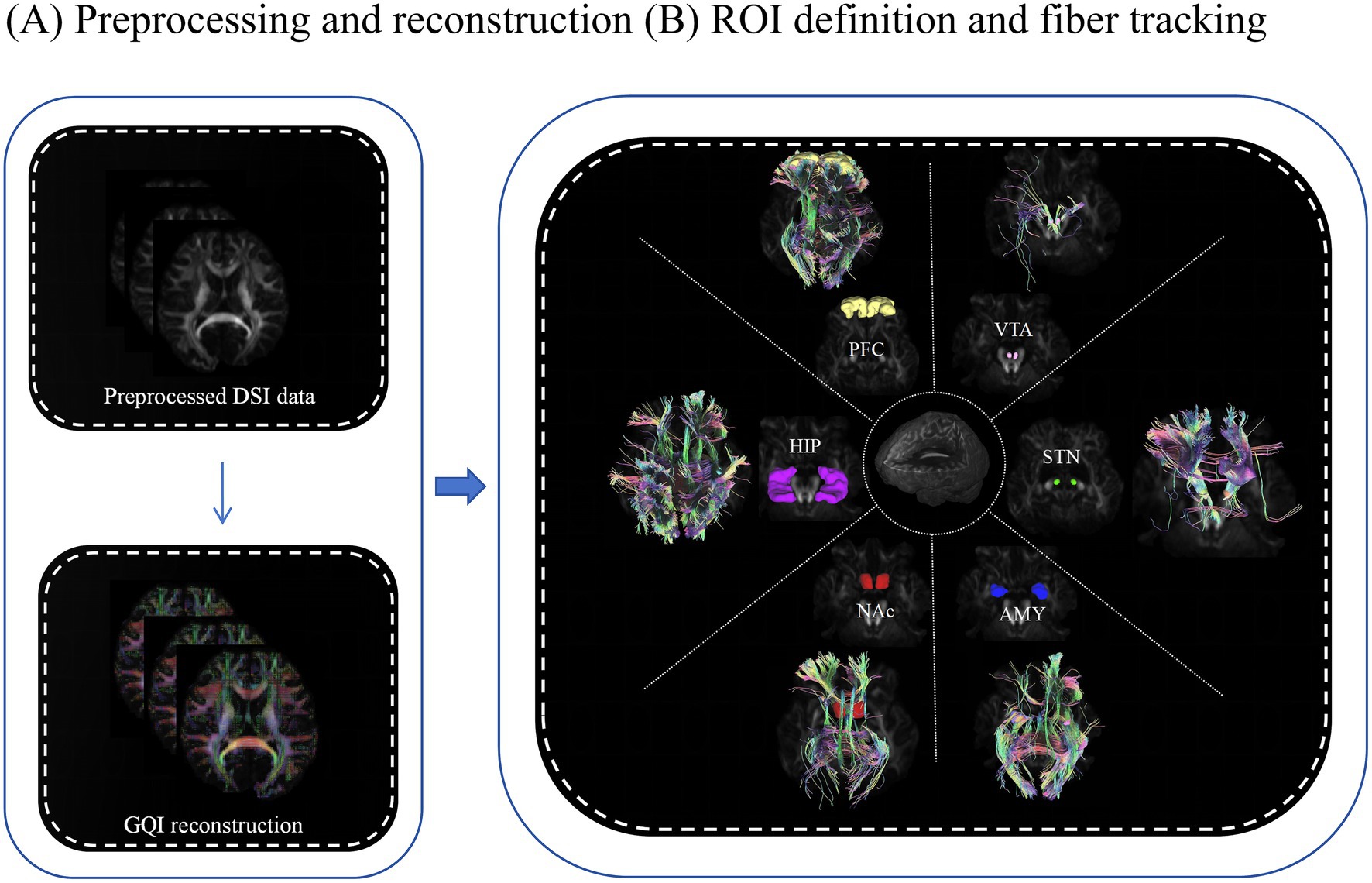
Figure 2. Working flowchart for image processing. (A) Preprocessing and reconstruction. (B) Definition of ROIs and tracking fibers using the DSI studio software. The above processes were prepared for collecting the diffusion measures for statistical analysis. ROI, region of interest; GQI, generalized q-sampling imaging; PFC, prefrontal cortex (yellow); HIP, hippocampus (purple); NAc, nucleus accumbens (red); AMY, amygdala (blue); STN, subthalamic nucleus (green); VTA, ventral tegmental area (pink).
2.6 Statistical analysis
The data was expressed in mean ± standard error of the mean (SEM), and analyzed using the Statistical Package for the Social Sciences (SPSS, version 31; IBM, Armonk, NY, United States). Paired t-test was conducted to assess continuous variables (outcomes), which included the FTND, SI, MNWS, sTCQ and VAS scores, and the diffusion metrics along the fiber tracts of ROIs between S1 and S2. Network-based statistics (NBS) (47) correction was adopted for the ROIs, according to the following steps: forming a set of supra-threshold edges using an initial threshold (p < 0.001) in the paired t-test matrix, and determining the statistical significance of each component’s size using non-parametric permutation testing (1,000 permutations), which provides strong control over the family-wise error rate (FWER). ROIs that exhibited significant changes in the diffusion metrics (QA, FA, MD, AD and RD) following the rTMS treatment were further analyzed for Pearson correlation with various tobacco addiction behavioral scales. Then, a multiple linear regression model was used to assess the association between the changes in these diffusion metrics and changes in tobacco addiction behavioral scales, controlling for the potential confounding effects of age. A p-value of <0.05 was set as the level of significance.
3 Results
3.1 Demographic characteristics
The present study included a total of 18 male smokers with an age of 39.56 ± 2.71 years old, education duration of 11.83 ± 0.85 years, and smoking age of 19.17 ± 2.28 years old. The ethnic groups of the participants included the Han population (n = 17) and other populations (n = 1).
3.2 Treatment effects of rTMS on nicotine dependence and craving
As shown in Table 2, the rTMS (S2) significantly decreased nicotine dependence at S2, as demonstrated by the significant decrease in FTND (p < 0.0001) and SI (p < 0.0001), when compared to that at S1. The MNWS scores indicated that the syndrome related to nicotine withdrawal significantly decreased between S1 and S2 (p = 0.024). In addition, the sTCQ (p < 0.0001) and VAS (p < 0.0001) scores indicated that the nicotine cravings significantly decreased after rTMS, when compared to S1. These results suggest that rTMS was potentially effective in smoking cessation, as supported by the self-reported cigarette consumption (Figure 3). Figure 3A shows that there was a decreasing trend of daily cigarette consumption following rTMS treatments, when compared to baseline (S1), and this trend reached a significantly different level after session 5. Figure 3B shows that there was a significant difference in cigarette consumption between S1 and S2. Among the 18 participants, four participants completely stopped smoking after 2 or 3 days during the study period, and all other participants presented with gradually decreased cigarette consumption.
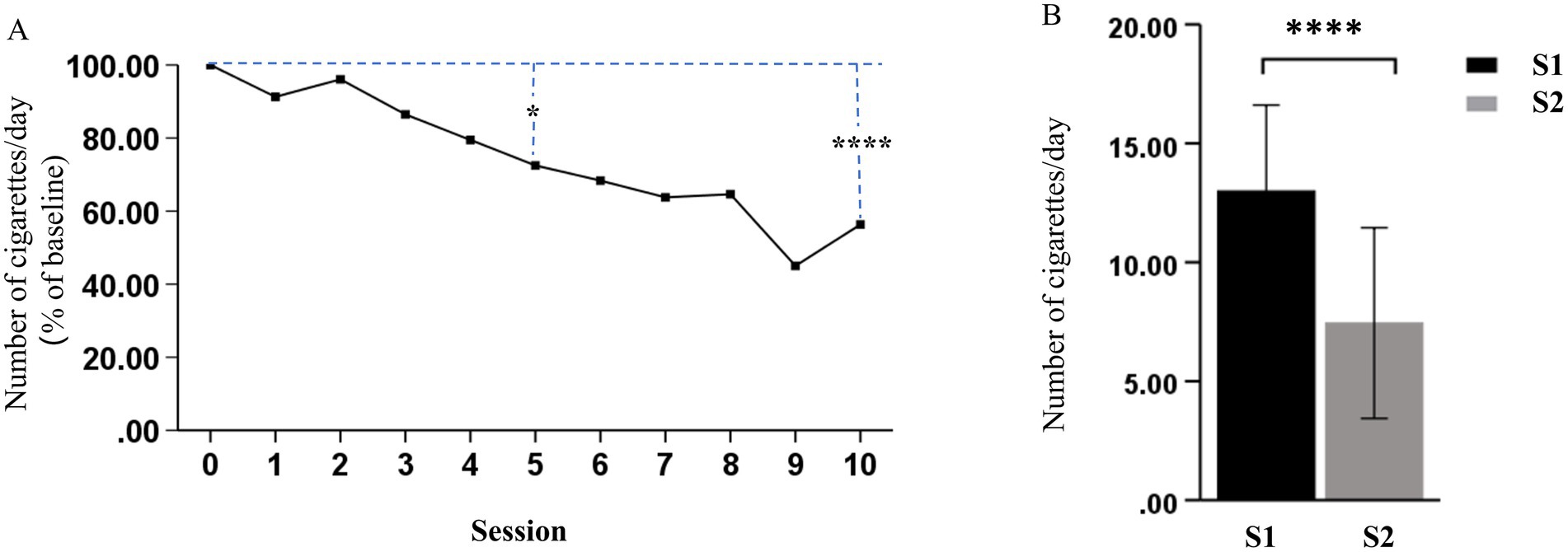
Figure 3. Effects of rTMS on cigarette consumption. (A) Daily change (% of baseline) in the number of cigarettes smoked for each treatment session. (B) The number of cigarettes smoked per day significantly decreased after the last treatment session (S2), when compared to before the first treatment session (S1) (*p < 0.05 and ****p < 0.0001).
3.3 Effects of rTMS on the dMRI metrics of smokers
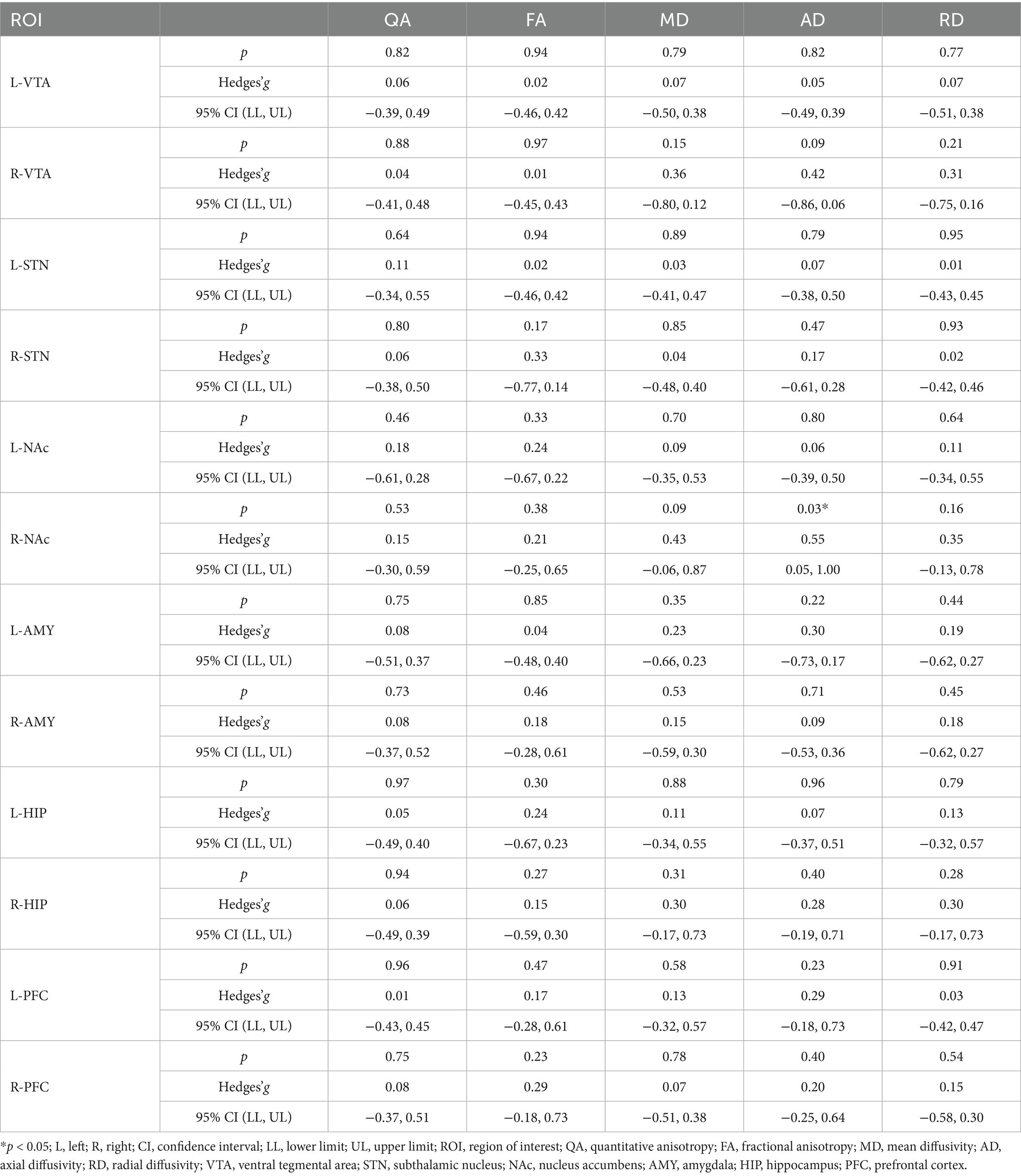
The analysis of the image metrics of MRI of smokers before and after rTMS treatment revealed a significant decrease in AD values in the right NAc fiber tracts after rTMS treatment (p = 0.03, Hedges’g = 0.55, Table 3). However, the other image metrics, including QA, FA, MD and RD, did not significantly change after rTMS in the right NAc fiber tracts. In addition, the other nicotine addiction-related ROIs, including VTA, STN, AMY, PFC and HIP, were not significantly affected by the rTMS treatment, when compared to those between S1 and S2 (all p > 0.05, Hedges’g < 0.5, Table 3).
3.4 Correlation and regression analysis of treatment outcomes
There was no significant correlation between AD values in the right NAc fibers and various tobacco addiction behavioral scales, either at pre- or post-treatment (all p > 0.05). However, a strong positive correlation (r = 0.621, p = 0.006) was observed between the change in cigarette consumption and change in AD values in the right NAc fibers before and after rTMS treatment (Figure 4). Furthermore, the multiple linear regression yielded a standardized model, in which the change in AD values of the right NAc fibers was primarily predicted by the change in cigarette consumption (β = 0.608, p = 0.009), with age making a minimal contribution (β = −0.089, p = 0.666): Z_ΔAD = (0.608 × Z_Δcigarette) + (−0.089 × Z_age).
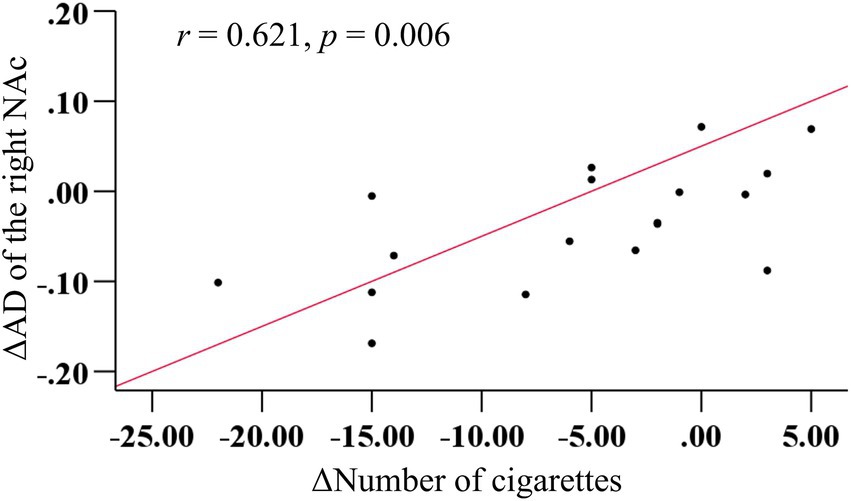
Figure 4. More decrease in cigarettes consumption predicted more reduction in AD in the right NAc fibers after rTMS treatment.
3.5 Power analysis
A post-hoc power analysis using the AD values of the right NAc fibers before and after TMS treatment in smokers was conducted, with the α error probability set at 0.05. The achieved statistical power (1 − β) was calculated via the G*Power software, and the result was 0.6. This result indicates that the statistical power of the present study only reached a moderate level. Subsequently, a priori power analysis was performed using the same effect size (d = 0.554, derived from the pre- and post-treatment AD values of the right NAc fibers), with the α error probability set to 0.05, and the desired power (1 − β) set to 0.8. The analysis indicated that a minimum sample size of 28 participants would be required to achieve adequate power in future studies.
4 Discussion
Smoking and nicotine addiction are worldwide problems (1–3). Recent studies have indicated that nicotine addiction can lead to abnormalities in the brain, including its structures and functions (9, 48), as detected by advanced imaging techniques, such as fMRI, DTI and DSI (11, 12, 24, 35). However, there is no satisfactory strategy for smoking cessation. rTMS is a novel non-invasive technique, which has been used in multiple diseases (24–26). The present study delved in determining whether rTMS can influence the WM microstructure in mesolimbic dopamine pathway-related regions in individuals with nicotine dependence. The findings revealed that cigarette consumption and the questionnaire scores decreased after rTMS treatment, suggesting that rTMS treatment is a potential effective intervention for reducing nicotine dependence and craving in smokers, and can partially alleviate the anxiety and depression induced by nicotine withdrawal. Furthermore, the present study revealed that AD decreased in the right NAc fiber tracts after short-term rTMS treatment, suggesting that rTMS may have the potential to change the WM microstructure, and thereby decrease nicotine addiction, although there were no significant changes in QA, FA, MD and RD in NAc and other dopamine pathway related regions.
Previous studies have indicated that chronic nicotine exposure diminishes communication throughout the brain, and increases local connectivity between specific network nodes (49). The NAc receives direct glutamatergic projections from the AMY, HIP, thalamus and PFC, and indirect mesolimbic dopaminergic projections from the VTA and substantia nigra (50). Furthermore, the NAc plays a critical role in neural mechanisms of addiction, primarily due to dopamine release, and the activation of multiple receptors within the NAc (50). Emerging evidence suggests that structural and functional changes can occur in the NAc of subjects with substance use disorders. For example, previous studies have reported the decrease in structural volume of the left NAc (51–53), and the increase in thickness of the right VTA-NAc (53) in subjects with heroin addiction. Similarly, the present study indicated that rTMS significantly decreased AD in the right NAc fiber tracts in smokers with lower cigarette consumption, suggesting that the right NAc is involved in nicotine addiction.
It has generally been considered that AD primarily reflects axonal integrity, with decreased AD indicating a reduction in axonal volume, and the beginning stages of axonal damage (54, 55). However, the increase in volume of glial cells may also be correlated to lower AD (56), and nicotine exposure may increase the number and density of glial cells (57). In addition, a decrease in AD may indicate early neural immune activation or the initial phases of intracellular inflammation and edema (55). Therefore, this phenomenon was attributed to the fact that the majority of participants (14 of 18 participants) continued smoking throughout the treatment period, despite the reduction in the number of cigarettes smoked, and merely four participants ceased smoking completely in the last 2 or 3 days of the study period. A previous study suggested that long-term smoking can compromise the integrity of WM in the brain (58). However, there is no definitive consensus on the exact duration or number of days required for this effect to manifest (49, 59). The present study indirectly demonstrated that nicotine intake over a period of approximately 2 weeks may initially influence the WM microstructure of the right NAc fibers. However, several studies have reported that the WM of the left hemisphere may be more vulnerable to the neurotoxic effects of nicotine, when compared to that of the right hemisphere (14, 58). These results were inconsistent with the present results, in which the right NAc fibers may be more vulnerable to nicotine. In addition, the Pearson correlation analysis indicated that the greater the reduction in cigarette consumption, the greater the decrease in AD values in the right NAc fibers after rTMS treatment. Nonetheless, the results may not be fully aligned with the previous explanation of the investigators. If the reduction in AD values was attributed entirely to the fact that most subjects continued to smoke during the treatment period, the more the cigarette consumption decreased, the less the AD values should have decreased. Therefore, the investigators proposed a hypothesis that rTMS may alter the microstructure of the right NAc fibers and modulate dopamine release within the NAc, thereby reducing nicotine dependence and craving in smokers. A study reported that the reduction in dopamine release in the NAc decreased the nicotine-taking behavior (60). In contrast, a decrease in AD does not necessarily indicate the presence of axonal injury, and reductions in AD have been observed in healthy brain maturation (9). Meanwhile, the age-related reduction of AD in a number of brain regions have been reported, which may be correlated to the growth of neurofibrils, such as microtubules and neurofilaments (61, 62). However, the results of the multiple linear regression analysis indicated that age had a minimal contribution to the reduction of AD in the right NAc fibers in the present study. This aligns with the perspective that axon coherence, which is associated to AD, does not serve as a major contributing factor to the developmental changes observed in numerous studies (63). Hence, the significant reduction in AD values in the right NAc fiber tracts in smokers after rTMS treatment may indicate that rTMS may facilitate the growth of neurofibrils, and modulate the microstructure of the right NAc fiber tracts, excluding the influence of age. The previous study conducted by the investigators on functional MRI revealed that rTMS can increase the functional connectivity of the right NAc with several visual and sensory processing regions in smokers, and that the right NAc appeared to play a central role in the adaptive reorganization of brain networks during the cessation of smoking through rTMS treatment (64). Therefore, the changes in AD are likely to reflect the complex interactions among multiple biological factors that influence its progression in various directions (56). The present study may provide crucial new insights into the neural mechanisms of rTMS in treating smoking addiction. In future studies, the role of NAc WM laterality, particularly in its relation to smoking and rTMS, warrants further investigation.
For the right insula-NAc fiber, a study reported lower FA values in individuals with heroin addiction, when compared to healthy controls (65). Furthermore, DTI studies have revealed reduced FA in tracts projecting from the bilateral NAc to the frontal cortex in individuals with nicotine addiction, when compared to healthy controls (66, 67). Compared to non-smokers, smokers exhibit an increased integration coefficient between the frontoparietal network (FPN) and basal ganglia network (BGN), and a reduced integration coefficient between the medial frontal network (MFN) and FPN. Furthermore, the analysis of variance revealed that rTMS treatment can reduce the integration coefficient between the FPN and BGN, and improve the recruitment coefficient of the FPN (68). These studies indicate that nicotine addiction involves other brain regions. However, the present study did not detect significant changes in QA, FA, MD and RD in the right NAc, and all metrics in other brain regions, which was inconsistent with the expectations of the investigators. This discordance may have been primarily derived from the following reasons: (1) the different indicators exhibited varying sensitivities to different diseases (19); (2) there was a possibility that nicotine did not damage the relevant fibers; (3) the rTMS treatment required more repetitions to demonstrate its effect. These factors may also account for the mismatch between robust behavioral improvements and limited imaging changes. Furthermore, the placebo effect could have contributed to this phenomenon. Therefore, sham stimulation experiments should be conducted (31, 35).
5 Limitations
There were some limitations in the present study that require clarification. First, the sample size was small. Thus, a large sample study is required to validate the present findings. Second, there was no control group, such as sham treatment and non-smokers. Third, further research that involves female smokers is necessary to ensure the applicability of the present findings across genders, although it is difficult to recruit female smokers in China. Fourth, the absence of objective biochemical validation limited the precision of the present experimental findings. Incorporating established biochemical markers in future studies, such as CO/cotinine (14, 35), would enhance the accuracy and reliability of the research outcomes. Fifth, the participants should be stratified by age, since a study reported that smokers are generally associated to lower FA in adults, and higher FA in young adults (≤30 years old) (69). Sixth, more repetitions of the rTMS treatment and follow-up may be helpful to verify the present results. Finally, other regions should be carefully investigated for whole-brain tractography, since the NAc receives projections from the medial PFC, anterior insula, AMY and VTA, contributing to its intricate anatomical connections within addiction circuits (50).
6 Conclusion
The present study was the first attempt to determine how rTMS affects the brain microstructure in individuals with nicotine dependence using DSI. The results indicated that the decreased AD values within the right NAc fiber tracts could be the “result” of nicotine addiction, or directly attributable to HF-rTMS. These results suggest that the right NAc emerged as a region of interest that warrants further investigation as a potential therapeutic target.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Zhejiang Hospital (2019 Pro-examination-34K No. -X4). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DC: Formal analysis, Investigation, Visualization, Writing – original draft. ZL: Formal analysis, Investigation, Writing – review & editing. MX: Data curation, Investigation, Supervision, Writing – review & editing. TW: Software, Writing – review & editing. RL: Software, Writing – review & editing. YC: Conceptualization, Writing – review & editing. SL: Conceptualization, Writing – review & editing. QZ: Conceptualization, Writing – review & editing. YL: Writing – review & editing. XL: Writing – review & editing. HM: Supervision, Writing – review & editing. LZ: Supervision, Writing – review & editing. JZ: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Zhejiang Provincial Medical and Health Research Fund Project (Nos. 2022KY011 and 2021KY427), and the Zhejiang Provincial Co-construction Project (No. WKJ-ZJ-2015).
Conflict of interest
YL and XL were employed by Neusoft Medical Systems Co., Ltd. HM was employed by Yiruide Medical Equipment New Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Ng, M, Freeman, MK, Fleming, TD, Robinson, M, Dwyer-Lindgren, L, Thomson, B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. (2014) 311:183–92. doi: 10.1001/jama.2013.284692
2. Chen, Z, Peto, R, Zhou, M, Iona, A, Smith, M, Yang, L, et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. (2015) 386:1447–56. doi: 10.1016/S0140-6736(15)00340-2
3. Jarvik, ME, Madsen, DC, Olmstead, RE, Iwamoto-Schaap, PN, Elins, JL, and Benowitz, NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacol Biochem Behav. (2000) 66:553–8. doi: 10.1016/s0091-3057(00)00261-6
4. Brody, AL, Olmstead, RE, London, ED, Farahi, J, Meyer, JH, Grossman, P, et al. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. (2004) 161:1211–8. doi: 10.1176/appi.ajp.161.7.1211
5. Volkow, ND, Wang, GJ, Fowler, JS, Tomasi, D, and Telang, F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci USA. (2011) 108:15037–42. doi: 10.1073/pnas.1010654108
6. Ghahremani, DG, Faulkner, P, Cox, CM, and London, ED. Behavioral and neural markers of cigarette-craving regulation in young-adult smokers during abstinence and after smoking. Neuropsychopharmacology. (2018) 43:1616–22. doi: 10.1038/s41386-018-0019-7
7. Volkow, ND, and Morales, M. The brain on drugs: from reward to addiction. Cell. (2015) 162:712–25. doi: 10.1016/j.cell.2015.07.046
8. Yuan, K, Zhao, M, Yu, D, Manza, P, Volkow, ND, Wang, GJ, et al. Striato-cortical tracts predict 12-h abstinence-induced lapse in smokers. Neuropsychopharmacology. (2018) 43:2452–8. doi: 10.1038/s41386-018-0182-x
9. Hampton, WH, Hanik, IM, and Olson, IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. (2019) 197:288–98. doi: 10.1016/j.drugalcdep.2019.02.005
10. Yuce, I, Kantarci, M, Keles, P, Yesilyurt, H, Ogul, H, Yuce, H, et al. Diffusion tensor imaging of the hippocampus in chronic cigarette smokers. Eur J Radiol. (2016) 85:1538–44. doi: 10.1016/j.ejrad.2016.06.003
11. Baeza-Loya, S, Velasquez, KM, Molfese, DL, Viswanath, H, Curtis, KN, Thompson-Lake, DGY, et al. Anterior cingulum white matter is altered in tobacco smokers. Am J Addict. (2016) 25:210–4. doi: 10.1111/ajad.12362
12. Wang, S, Zuo, L, Jiang, T, Peng, P, Chu, S, and Xiao, D. Abnormal white matter microstructure among early adulthood smokers: a tract-based spatial statistics study. Neurol Res. (2017) 39:1094–102. doi: 10.1080/01616412.2017.1379277
13. Lin, F, Wu, G, Zhu, L, and Lei, H. Heavy smokers show abnormal microstructural integrity in the anterior corpus callosum: a diffusion tensor imaging study with tract-based spatial statistics. Drug Alcohol Depend. (2013) 129:82–7. doi: 10.1016/j.drugalcdep.2012.09.013
14. Wang, C, Wang, S, Huang, P, Shen, Z, Qian, W, Luo, X, et al. Abnormal white matter tracts of insula in smokers. Brain Imaging Behav. (2021) 15:1955–65. doi: 10.1007/s11682-020-00389-1
15. Denis, LB, and Mami, I. Diffusion magnetic resonance imaging: what water tells us about biological tissues. PLoS Biol. (2015) 13:e1002203. doi: 10.1371/journal.pbio.1002203
16. Epstein, KA, and Kumra, S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: a naturalistic diffusion tensor imaging study. Psychiatry Res. (2015) 232:34–41. doi: 10.1016/j.pscychresns.2014.10.010
17. Jeurissen, B, Leemans, A, Tournier, JD, Jones, DK, and Sijbers, J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. (2013) 34:2747–66. doi: 10.1002/hbm.22099
18. Raffelt, DA, Smith, RE, Ridgway, GR, Tournier, JD, Vaughan, DN, Rose, S, et al. Connectivity-based fixel enhancement: whole-brain statistical analysis of diffusion MRI measures in the presence of crossing fibres. NeuroImage. (2015) 117:40–55. doi: 10.1016/j.neuroimage.2015.05.039
19. Sun, F, Huang, Y, Wang, J, Hong, W, and Zhao, Z. Research progress in diffusion spectrum imaging. Brain Sci. (2023) 13:1497. doi: 10.3390/brainsci13101497
20. Dai, G, Das, A, Hayashi, E, Chen, Q, and Takahashi, E. Regional variation of white matter development in the cat brain revealed by ex vivo diffusion MR tractography. Int J Dev Neurosci. (2016) 54:32–8. doi: 10.1016/j.ijdevneu.2016.08.004
21. Wu, Y, Sun, D, Wang, Y, and Wang, Y. Subcomponents and connectivity of the inferior fronto-occipital fasciculus revealed by diffusion spectrum imaging fiber tracking. Front Neuroanat. (2016) 10:88. doi: 10.3389/fnana.2016.00088
22. Suo, X, Guo, L, Fu, D, Ding, H, Li, Y, and Qin, W. A comparative study of diffusion fiber reconstruction models for pyramidal tract branches. Front Neurosci. (2021) 15:777377. doi: 10.3389/fnins.2021.777377
23. Liu, X, Kinoshita, M, Shinohara, H, Hori, O, Ozaki, N, and Nakada, M. A fiber dissection study of the anterior commissure: correlations with diffusion spectrum imaging tractography and clinical relevance in gliomas. Brain Topogr. (2022) 35:232–40. doi: 10.1007/s10548-021-00879-5
24. Zhang, H, He, WJ, Liang, LH, Zhang, HW, Zhang, XJ, Zeng, L, et al. Diffusion spectrum imaging of corticospinal tracts in idiopathic normal pressure hydrocephalus. Front Neurol. (2021) 12:636518. doi: 10.3389/fneur.2021.636518
25. Tang, PF, Ko, YH, Luo, ZA, Yeh, FC, Chen, SH, and Tseng, WY. Tract-specific and region of interest analysis of corticospinal tract integrity in subcortical ischemic stroke: reliability and correlation with motor function of affected lower extremity. AJNR Am J Neuroradiol. (2010) 31:1023–30. doi: 10.3174/ajnr.A1981
26. Griffa, A, Baumann, PS, Ferrari, C, Do, KQ, Conus, P, Thiran, JP, et al. Characterizing the connectome in schizophrenia with diffusion spectrum imaging. Hum Brain Mapp. (2015) 36:354–66. doi: 10.1002/hbm.22633
27. Wang, ZM, Wei, PH, Zhang, M, Wu, C, Shan, Y, Yeh, FC, et al. Diffusion spectrum imaging predicts hippocampal sclerosis in mesial temporal lobe epilepsy patients. Ann Clin Transl Neurol. (2022) 9:242–52. doi: 10.1002/acn3.51503
28. Babb, S, Malarcher, A, Schauer, G, Asman, K, and Jamal, A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. (2017) 65:1457–64. doi: 10.15585/mmwr.mm6552a1
29. Hauer, L, Scarano, GI, Brigo, F, Golaszewski, S, Lochner, P, Trinka, E, et al. Effects of repetitive transcranial magnetic stimulation on nicotine consumption and craving: a systematic review. Psychiatry Res. (2019) 281:112562. doi: 10.1016/j.psychres.2019.112562
30. Orru, G, Baroni, M, Conversano, C, and Gemignani, A. Exploring the therapeutic potential of tDCS, TMS and DBS in overcoming tobacco use disorder: an umbrella review. AIMS Neurosci. (2024) 11:449–67. doi: 10.3934/Neuroscience.2024027
31. Li, X, Hartwell, KJ, Henderson, S, Badran, BW, Brady, KT, and George, MS. Two weeks of image-guided left dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation improves smoking cessation: a double-blind, sham-controlled, randomized clinical trial. Brain Stimul. (2020) 13:1271–9. doi: 10.1016/j.brs.2020.06.007
32. Dinur-Klein, L, Dannon, P, Hadar, A, Rosenberg, O, Roth, Y, Kotler, M, et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry. (2014) 76:742–9. doi: 10.1016/j.biopsych.2014.05.020
33. Allen, EA, Pasley, BN, Duong, T, and Freeman, RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science. (2007) 317:1918–21. doi: 10.1126/science.1146426
34. Sydnor, VJ, Cieslak, M, Duprat, R, Deluisi, J, Flounders, MW, Long, H, et al. Cortical-subcortical structural connections support transcranial magnetic stimulation engagement of the amygdala. Sci Adv. (2022) 8:eabn5803. doi: 10.1126/sciadv.abn5803
35. Du, X, Choa, FS, Chiappelli, J, Bruce, H, Kvarta, M, Summerfelt, A, et al. Combining neuroimaging and brain stimulation to test alternative causal pathways for nicotine addiction in schizophrenia. Brain Stimul. (2024) 17:324–32. doi: 10.1016/j.brs.2024.02.020
36. Ibrahim, C, Tang, VM, Blumberger, DM, Malik, S, Tyndale, RF, Trevizol, AP, et al. Efficacy of insula deep repetitive transcranial magnetic stimulation combined with varenicline for smoking cessation: a randomized, double-blind, sham controlled trial. Brain Stimul. (2023) 16:1501–9. doi: 10.1016/j.brs.2023.10.002
37. Ibrahim, C, Tang, VM, Blumberger, DM, and Le Foll, B. Repetitive transcranial magnetic stimulation for smoking cessation. CMAJ. (2024) 196:E187–90. doi: 10.1503/cmaj.230806
38. Zangen, A, Moshe, H, Martinez, D, Barnea-Ygael, N, Vapnik, T, Bystritsky, A, et al. Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry. (2021) 20:397–404. doi: 10.1002/wps.20905
39. Heatherton, TF, Kozlowski, LT, Frecker, RC, and Fagerström, KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. (1991) 86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x
40. Heatherton, TF, Kozlowski, LT, Frecker, RC, Rickert, W, and Robinson, J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. (1989) 84:791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x
41. Hughes, JR, and Hatsukami, D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. (1986) 43:289–94. doi: 10.1001/archpsyc.1986.01800030107013
42. Heishman, SJ, Singleton, EG, and Moolchan, ET. Tobacco Craving Questionnaire: reliability and validity of a new multifactorial instrument. Nicotine Tob Res. (2003) 5:645–54. doi: 10.1080/1462220031000158681
43. Dallery, J, Houtsmuller, EJ, Pickworth, WB, and Stitzer, ML. Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharmacology. (2003) 165:172–80. doi: 10.1007/s00213-002-1242-8
44. Schuh, KJ, and Stitzer, ML. Desire to smoke during spaced smoking intervals. Psychopharmacology. (1995) 120:289–95. doi: 10.1007/bf02311176
45. Antonelli, M, Fattore, L, Sestito, L, Giuda, DD, Diana, M, and Addolorato, G. Transcranial magnetic stimulation: a review about its efficacy in the treatment of alcohol, tobacco and cocaine addiction. Addict Behav. (2021) 114:106760. doi: 10.1016/j.addbeh.2020.106760
46. Matochik, JA, London, ED, Eldreth, DA, Cadet, JL, and Bolla, KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. NeuroImage. (2003) 19:1095–102. doi: 10.1016/s1053-8119(03)00244-1
47. Zalesky, A, Fornito, A, and Bullmore, ET. Network-based statistic: identifying differences in brain networks. NeuroImage. (2010) 53:1197–207. doi: 10.1016/j.neuroimage.2010.06.041
48. Lin, F, Wu, G, Zhu, L, and Lei, H. Altered brain functional networks in heavy smokers. Addict Biol. (2015) 20:809–19. doi: 10.1111/adb.12155
49. Zhou, M, Hu, Y, Huang, R, Zhou, Y, Xie, X, Zhang, S, et al. Right arcuate fasciculus and left uncinate fasciculus abnormalities in young smoker. Addict Biol. (2022) 27:e13132. doi: 10.1111/adb.13132
50. Tisdall, L, MacNiven, KH, Padula, CB, Leong, JK, and Knutson, B. Brain tract structure predicts relapse to stimulant drug use. Proc Natl Acad Sci USA. (2022) 119:e2116703119. doi: 10.1073/pnas.2116703119
51. Seifert, CL, Magon, S, Sprenger, T, Lang, UE, Huber, CG, Denier, N, et al. Reduced volume of the nucleus accumbens in heroin addiction. Eur Arch Psychiatry Clin Neurosci. (2015) 265:637–45. doi: 10.1007/s00406-014-0564-y
52. Ceceli, AO, Huang, Y, Kronberg, G, Malaker, P, Miller, P, King, SG, et al. Common and distinct fronto-striatal volumetric changes in heroin and cocaine use disorders. Brain. (2023) 146:1662–71. doi: 10.1093/brain/awac366
53. Xu, Y, Wang, S, Chen, L, Shao, Z, Zhang, M, Liu, S, et al. Reduced midbrain functional connectivity and recovery in abstinent heroin users. J Psychiatr Res. (2021) 144:168–76. doi: 10.1016/j.jpsychires.2021.10.011
54. Jakabek, D, Yucel, M, Lorenzetti, V, and Solowij, N. An MRI study of white matter tract integrity in regular cannabis users: effects of cannabis use and age. Psychopharmacology. (2016) 233:3627–37. doi: 10.1007/s00213-016-4398-3
55. Baker, SK, and Claus, ED. White matter differences in early-stage alcohol use disorder: diffusion tensor and neurite orientation dispersion and density measures of structural integrity. Addict Biol. (2025) 30:e70019. doi: 10.1111/adb.70019
56. Jiang, L, Han, X, Wang, Y, Ding, W, Sun, Y, Zhou, Y, et al. Anterior and posterior cerebral white matter show different patterns of microstructural alterations in young adult smokers. Brain Imaging Behav. (2025) 19:195–203. doi: 10.1007/s11682-024-00963-x
57. Takarada, T, Nakamichi, N, Kawagoe, H, Ogura, M, Fukumori, R, Nakazato, R, et al. Possible neuroprotective property of nicotinic acetylcholine receptors in association with predominant upregulation of glial cell line-derived neurotrophic factor in astrocytes. J Neurosci Res. (2012) 90:2074–85. doi: 10.1002/jnr.23101
58. Ottino-Gonzalez, J, Uhlmann, A, Hahn, S, Cao, Z, Cupertino, RB, Schwab, N, et al. White matter microstructure differences in individuals with dependence on cocaine, methamphetamine, and nicotine: findings from the ENIGMA-addiction working group. Drug Alcohol Depend. (2022) 230:109185. doi: 10.1016/j.drugalcdep.2021.109185
59. Durazzo, TC, Meyerhoff, DJ, Yoder, KK, and Murray, DE. Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug Alcohol Depend. (2017) 177:228–36. doi: 10.1016/j.drugalcdep.2017.04.012
60. Jeon, KO, Yorgason, JT, Ford, L, Woolley, JT, Park, SH, Lee, BS, et al. The superoxide dismutase mimetic TEMPOL modulates nicotine-induced hyperlocomotor activity and nicotine-taking behavior in male rats. Sci Rep. (2025) 15:5531. doi: 10.1038/s41598-025-88667-0
61. Tamnes, CK, Ostby, Y, Fjell, AM, Westlye, LT, Due-Tonnessen, P, and Walhovd, KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. (2010) 20:534–48. doi: 10.1093/cercor/bhp118
62. Qiu, D, Tan, LH, Zhou, K, and Khong, PL. Diffusion tensor imaging of normal white matter maturation from late childhood to young adulthood: voxel-wise evaluation of mean diffusivity, fractional anisotropy, radial and axial diffusivities, and correlation with reading development. NeuroImage. (2008) 41:223–32. doi: 10.1016/j.neuroimage.2008.02.023
63. Lebel, C, and Deoni, S. The development of brain white matter microstructure. NeuroImage. (2018) 182:207–18. doi: 10.1016/j.neuroimage.2017.12.097
64. Wang, T, Li, R, Chen, D, Xie, M, Li, Z, Mao, H, et al. Modulation of high-frequency rTMS on reward circuitry in individuals with nicotine dependence: a preliminary fMRI study. Neural Plast. (2024) 2024:5673579. doi: 10.1155/2024/5673579
65. Lu, L, Yang, W, Zhao, D, Wen, X, Liu, J, Liu, J, et al. Brain recovery of the NAc fibers and prediction of craving changes in person with heroin addiction: a longitudinal study. Drug Alcohol Depend. (2023) 243:109749. doi: 10.1016/j.drugalcdep.2022.109749
66. Savjani, RR, Velasquez, KM, Thompson-Lake, DG, Baldwin, PR, Eagleman, DM, De La Garza, R 2nd, et al. Characterizing white matter changes in cigarette smokers via diffusion tensor imaging. Drug Alcohol Depend. (2014) 145:134–42. doi: 10.1016/j.drugalcdep.2014.10.006
67. Yuan, K, Yu, D, Zhao, M, Li, M, Wang, R, Li, Y, et al. Abnormal frontostriatal tracts in young male tobacco smokers. NeuroImage. (2018) 183:346–55. doi: 10.1016/j.neuroimage.2018.08.046
68. Li, S, Zhang, Z, Jiang, A, Ma, X, Wang, M, Ni, H, et al. Repetitive transcranial magnetic stimulation reshaped the dynamic reconfiguration of the executive and reward networks in individuals with tobacco use disorder. J Affect Disord. (2024) 365:427–36. doi: 10.1016/j.jad.2024.08.120
Keywords: repetitive transcranial magnetic stimulation, nicotine, smoking cessation, white matter, diffusion spectrum imaging
Citation: Chen D, Li Z, Xie M, Wang T, Li R, Chen Y, Li S, Zhang Q, Ling Y, Liang X, Mao H, Zhai L and Zhang J (2025) Repetitive transcranial magnetic stimulation reduces nicotine dependence and potentially modulates white matter microstructure in smokers: a pilot study by diffusion spectrum imaging. Front. Neurol. 16:1653926. doi: 10.3389/fneur.2025.1653926
Edited by:
Elisa Kallioniemi, New Jersey Institute of Technology, United StatesReviewed by:
Jalil Rasgado Toledo, National Autonomous University of Mexico, MexicoMatthew Gunn, University of Illinois Chicago, United States
Copyright © 2025 Chen, Li, Xie, Wang, Li, Chen, Li, Zhang, Ling, Liang, Mao, Zhai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianjun Zhang, emhhbmd5cDMxMTEzQDE2My5jb20=
†ORCID: Jianjun Zhang, orcid.org/0000-0001-9822-3832
 Dongyan Chen1
Dongyan Chen1 Zhiqiang Li
Zhiqiang Li Tao Wang
Tao Wang Xiaoyun Liang
Xiaoyun Liang Jianjun Zhang
Jianjun Zhang