Abstract
Background:
Acupuncture is a widely used complementary therapy; however, the central mechanisms underlying its effects, particularly how stimulation at different specific acupoints modulates brain function in distinct or common ways, remain poorly understood. This gap persists due to a lack of large-sample, systematic comparative studies under a unified experimental paradigm. Task-based and resting-state functional magnetic resonance imaging (fMRI) offer powerful tools to capture both the instant and sustained neural responses to acupuncture.
Methods:
We designed a randomized, single-blind, controlled trial. To achieve high statistical power and generalizability, 250 healthy participants will be enrolled. Each participant will undergo acupuncture at one of seven predefined acupoints (verum) and its corresponding non-acupoint (sham control) in two separate sessions, with a 1-week interval. Each session includes: (1) resting-state fMRI before and after needle manipulation, and (2) task-fMRI during the manipulation. The primary outcomes are fMRI-derived brain activity and functional connectivity patterns. Blinding assessment and the Modified Massachusetts General Hospital Acupuncture Sensation Scale-Chinese version (C-MMASS) will be collected to evaluate the credibility of sham control and the Deqi sensation.
Discussion:
This study is novel in its comprehensive approach to mapping the neural correlates of multiple acupoints within a single, rigorous design. We anticipate that our results will provide the first systematic characterization of the “acupoint-brain functional network” map, elucidating both common activation patterns across acupoints and acupoint-specific differential responses. This will significantly contribute to understanding the functional neuroanatomy of acupuncture and provide high-level evidence for its mechanism of action, ultimately helping to bridge the gap between traditional practice and modern neuroscience.
Conclusion:
The findings of this trial are expected to establish a robust empirical foundation for the neural basis of acupuncture, offering insights that could validate clinical practice and guide future target-specific acupuncture applications.
Clinical trial registration:
Identifier ITMCTR2025000066.
1 Introduction
Acupuncture, an ancient Chinese therapeutic practice dating back over 2,000 years (1), is a non-pharmacological therapy rooted in Traditional Chinese Medicine (TCM) and its Meridian Theory. It is believed to regulate bodily functions by stimulating vital energy (Qi) at specific acupoints, which then travels along meridians to treat various diseases. According to a recent review, acupuncture shows therapeutic potential for more than 70 conditions, with moderate to substantial evidence supporting its efficacy in at least 8 specific diseases (2). Nonetheless, its underlying mechanisms—particularly how stimulating distal acupoints (remote from the affected area) elicits therapeutic effects through meridian pathways—remain unclear.
The focus of research on these mechanisms has undergone a paradigm shift, moving from the periphery to the central nervous system (CNS). There is growing consensus that the insertion and manipulation of acupuncture needles generate complex somatic afferent signals, which are integrated and processed within the brain (3, 4). This central neuromodulation is crucial not only for mediating analgesia—through the regulation of pain-processing regions like the anterior cingulate cortex and periaqueductal gray (5, 6)—but also for influencing higher-order cognitive and affective processes. Emerging evidence indicates that acupuncture can significantly modulate functions such as attention, emotion regulation, and interoceptive awareness by altering activity in key brain networks (7, 8).
The advancement of multi-modal neuroimaging has been instrumental in delineating these neural mechanisms, with each technique offering unique insights: functional magnetic resonance imaging (fMRI), with its high spatial resolution, excels at mapping the brain’s hemodynamic response and has been pivotal in demonstrating that acupuncture modulates functional connectivity within and between large-scale networks (e.g., DMN, SN, executive control network) in various patient populations (9, 10). Electroencephalography (EEG), providing millisecond-level temporal resolution, captures the dynamic electrophysiological brain responses to acupuncture, such as the normalization of aberrant EEG microstates in patients with post-stroke depression (11, 12). Structural MRI (sMRI) has revealed that sustained acupuncture intervention can induce neuroplastic changes, evidenced by alterations in gray matter density or white matter integrity in specific pathways (9). While these complementary approaches collectively affirm that acupuncture’s CNS effects are robust and reproducible, a direct comparison highlights a critical gap: fMRI is uniquely positioned to bridge the spatial specificity of acupoint effects with the network-level dynamics that underlie cognitive changes, making it the ideal modality for investigating how acupoint stimulation influences organized brain networks.
Despite this progress, the neuroimaging literature on acupuncture is fraught with methodological inconsistencies that obscure a clear interpretation. Many previous fMRI studies employ combinations of acupoints (13, 14), making it impossible to attribute observed effects to any single acupoint. Furthermore, studies focusing on individual acupoints are often hampered by small sample sizes (15, 16) and inadequate sham-controlled designs (16, 17), thereby conflating specific neuromodulatory effects with non-specific placebo responses.
To address these limitations, the present study employs a rigorous, single-acupoint design under a well-controlled, task-based fMRI paradigm. We focus on a therapeutically significant and commonly used acupoint to precisely investigate its distinct impact on functional brain network dynamics. Our objectives are twofold: (1) to isolate the specific central effects of a defined acupoint from non-specific responses using a robust sham-control design, and (2) to interpret these findings within the established framework of CNS network modulation, thereby contributing to a more precise understanding of acupuncture’s neural mechanisms and its potential applications in cognitive regulation.
2 Methods
2.1 Study design
This prospective, single-center, randomized, single-blind, placebo-controlled study will enroll 250 healthy participants. Participants will undergo acupuncture in separate sessions at one of seven predefined acupoints (verum acupuncture) and its corresponding non-acupoint (sham acupuncture), with a 1-week interval between sessions (18) (Figure 1). All participants will undergo two identical fMRI scanning sessions, separated by a 1-week interval, which corresponds to the washout period between the two acupuncture interventions. Each session will include resting-state fMRI scans before and after acupuncture, as well as task-based fMRI scans during acupuncture (Figure 2). The sequence of verum and sham protocols will be randomized across all fMRI runs, and the order of presentation will be counterbalanced across subjects (18).
Figure 1

Flow chart.
Figure 2
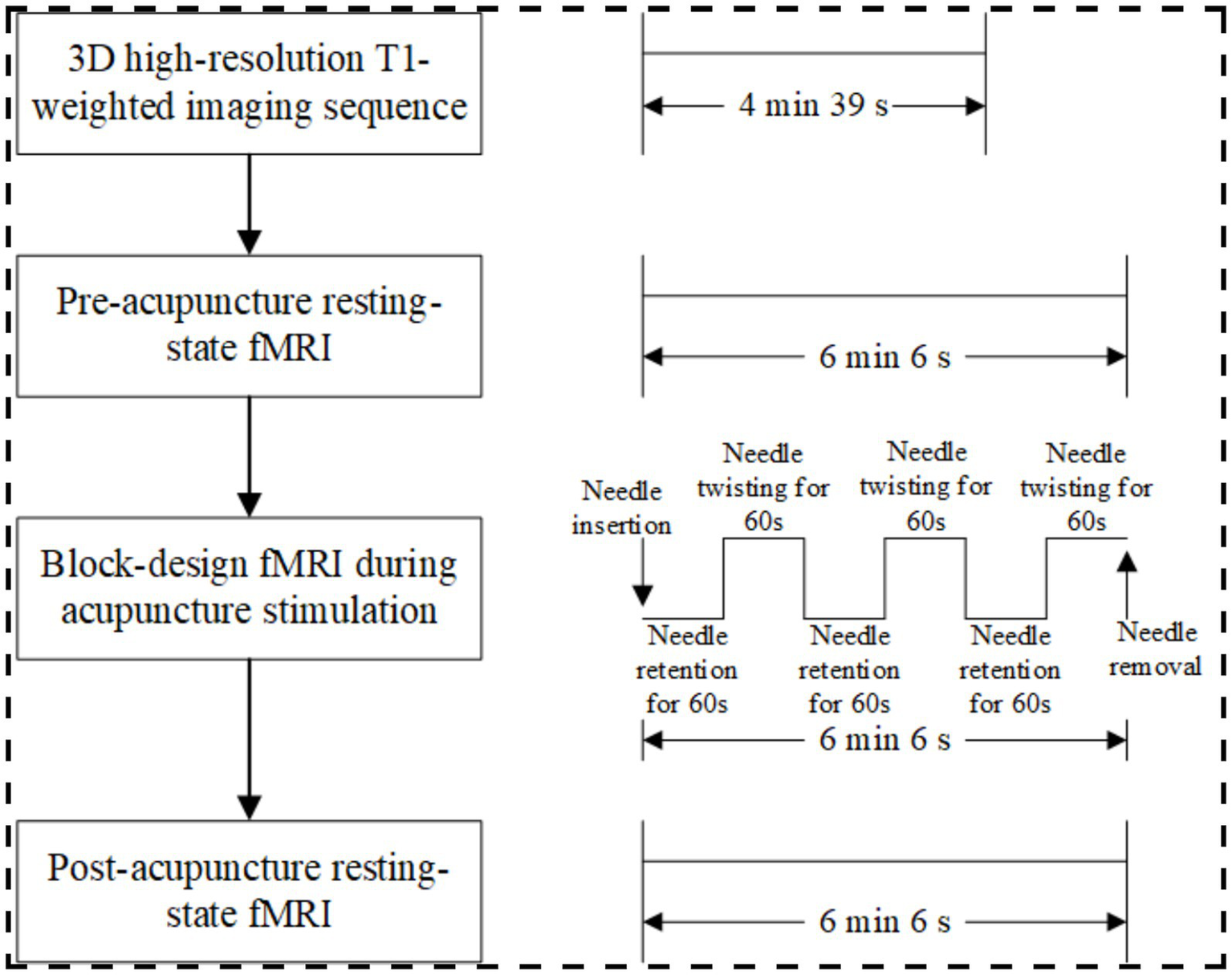
fMRI protocol.
2.2 Participants
Participants in this study will be recruited from Shenzhen Traditional Chinese Medicine Hospital. Written informed consent will be obtained from each individual prior to enrollment.
2.2.1 Inclusion criteria
-
(1) Aged 18–45 years, regardless of gender.
-
(2) Right-handed.
-
(3) No clinically significant history of cardiovascular, respiratory, or neurological diseases.
-
(4) No personal or familial history of psychiatric disorders.
-
(5) No acupuncture treatment within the past month.
-
(6) No fever or use of antipyretic analgesics within the past 3 months.
-
(7) Willing to provide informed consent and sign the consent form.
2.2.2 Exclusion criteria
-
(1) Pregnancy or lactation.
-
(2) Individuals who do not respond to acupuncture stimulation.
-
(3) Individuals with claustrophobia.
-
(4) Those with metallic implants or other contraindications for MRI.
2.2.3 Withdrawal criteria during the study
-
(1) Participants who fail to comply with the study procedures, thereby compromising data quality.
-
(2) Those who experience severe adverse events or emergencies during the trial.
-
(3) Participants unable to complete the study as scheduled.
-
(4) Those exhibiting excessive head motion during MRI scanning, leading to unusable data.
-
(5) Participants who receive other acupuncture treatments during the trial period.
2.3 Sample size
Currently, there are no standardized criteria for determining sample sizes in MRI studies, as they are often constrained by practical considerations such as scanning time and cost. Based on an efficiency analysis of sample size estimation in MRI research, a previous study indicated that approximately 12 participants are required to achieve 80% power at the single-voxel level for typical activation effects (19). In line with this reference and considering the study design, we set a sample size of 30 participants per acupuncture point. Each volunteer will undergo both verum and sham acupuncture at the same acupoint, resulting in a total of 210 experimental sessions. To account for potential data loss due to head motion or participant dropout, we plan to enroll a total of 250 subjects.
2.4 Randomization, allocation concealment, and blinding
In this study, participants will be randomly assigned to one of the seven acupoint groups. The randomization sequence is generated using SPSS 26.0 software, and the allocation scheme is concealed using opaque sealed envelopes. Upon participant enrollment, the allocation will be revealed by sequentially opening the envelopes to determine the assigned acupoint for acupuncture. The blinding procedure involves three aspects: first, participants are blinded to the specific acupoints used in the trial; they are only informed that the acupoints are located in the distal limbs. Second, radiologists, due to their professional constraints, are unaware of the specific acupoints and their significance, ensuring the objectivity of the scanning process. Third, image processors are blinded to the participants’ group assignments, ensuring the objectivity of the image processing.
2.5 Intervention
Based on a comprehensive review of fMRI studies exploring acupuncture mechanisms and clinical consensus on frequently used acupoints, this study selects the following acupoints: LI4 (Hegu), PC6 (Neiguan), ST36 (Zusanli), SP6 (Sanyinjiao), GB34 (Yanglingquan), KI3 (Taixi), and LR3 (Taichong) (Table 1 and Figure 3). These acupoints frequently employed in clinical practice to regulate the flow of Qi and blood, address imbalances in multiple organ systems (Zang-fu), and promote overall homeostasis. All acupoint localizations strictly adhere to the WHO Standard Acupuncture Point Locations in the Western Pacific Region (20).
Table 1
| Acupoints | Locations | Insert depth |
|---|---|---|
| Hegu (LI4) (unilateral) | On the dorsum of the hand, radial to the midpoint of the second metacarpal bone | 0.5–1 cun |
| Neiguan (PC6) (unilateral) | On the anterior aspect of the forearm, between the tendons of the palmaris longus and the flexor carpi radialis, 2 B-cun proximal to the palmar wrist crease | 0.5–1 cun |
| Zusanli (ST36) (unilateral) | On the anterior aspect of the leg, on the line connecting ST35 with ST41, 3 B-cun inferior to ST35 | 1–2 cun |
| Sanyinjiao (SP6) (unilateral) | On the tibial aspect of the leg, posterior to the medial border of the tibia, 3 B-cun superior to the prominence of the medial malleolus | 1–1.5 cun |
| Yanglingquan (GB34) (unilateral) | On the fibular aspect of the leg, in the depression anterior and distal to the head of the fibula | 1–1.5 cun |
| Taixi (KI3) (unilateral) | On the posteromedial aspect of the ankle, in the depression between the prominence of the medial malleolus and the calcaneal tendon | 0.5–0.8 cun |
| Taichong (LR3) (unilateral) | On the dorsum of the foot, between the first and second metatarsal bones, in the depression distal to the junction of the bases of the two bones, over the dorsalis pedis artery | 0.5–0.8 cun |
Locations of acupoints.
*B-cun: *proportional bone (skeletal) cun.
Figure 3
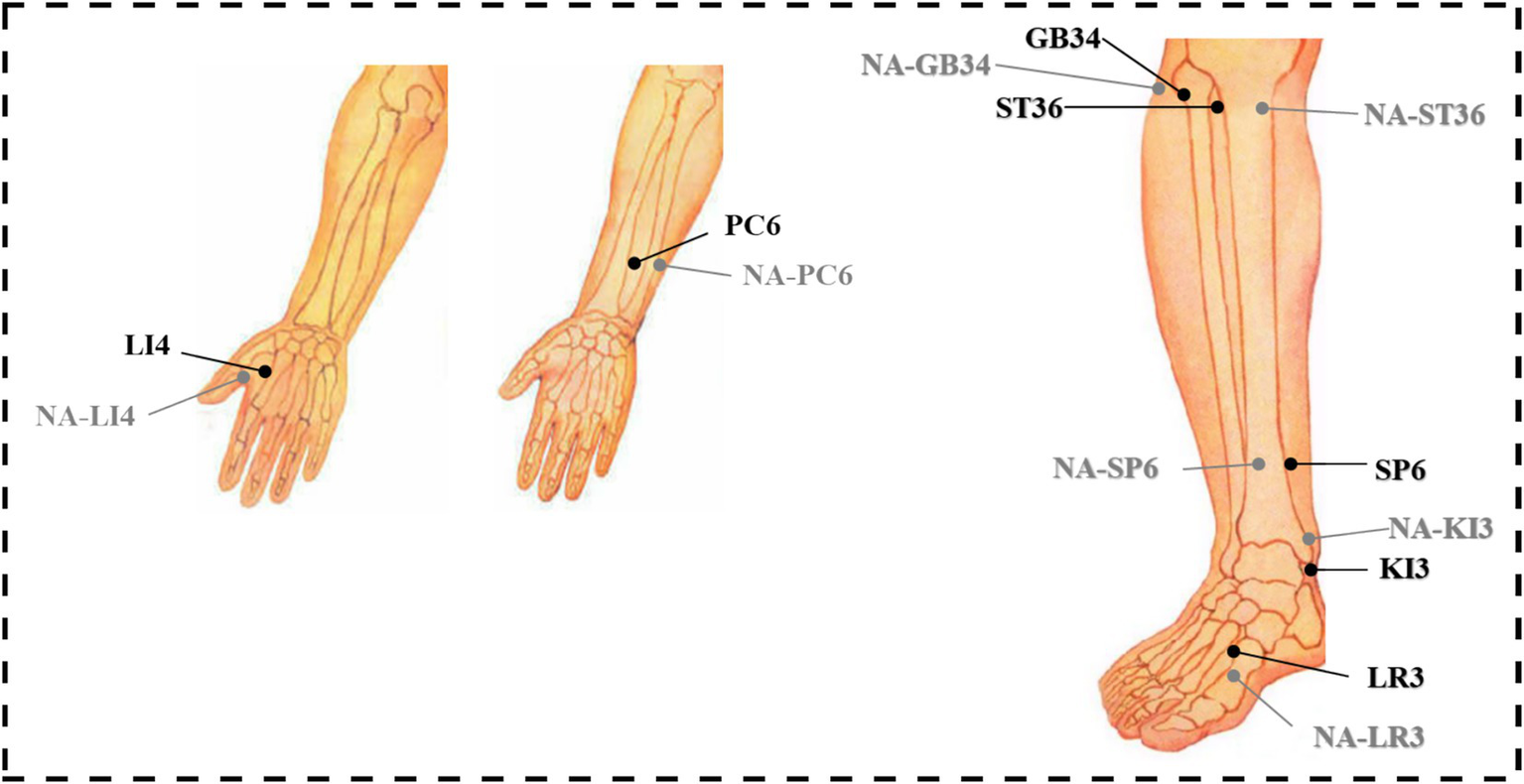
Location of acupoints and non-acupoints in the trial.
2.5.1 Verum acupuncture group
Following skin disinfection, an opaque, non-metallic sponge pad will be positioned on the skin surrounding the target area. Non-magnetic acupuncture needles (Suzhou Acupuncture & Moxibustion Appliance Co., Ltd., China; 0.25 × 40 mm) will be inserted to 2 cm deep into the designated acupoints on the right limbs (Table 1). After Deqi (A special sensation of acupuncture characterized by pain, soreness, distension, heaviness, or numbness), twisting (90–180°, 60–90 times/min), and lifting-thrusting (0.3–0.5 cm, 60–90 times/min) were performed for 1 min. During task-based fMRI scanning, the same twisting manipulation will be performed for 60 s at 60-s intervals (synchronized with scan initiation), continuing until completion of the 6-min 6-s protocol, followed by needle removal.
2.5.2 Sham acupuncture group
Non-acupoints are localized at 1.5 cm lateral to the corresponding verum acupoints (Table 1), outside meridian trajectories and neurovascular bundles, avoiding tender points and hair follicles (Figure 3). Identical opaque, non-metallic sponge pads, as used in the verum group, will be placed on the skin surrounding the target area after disinfection. Blunt-tipped placebo needles (Suzhou Acupuncture & Moxibustion Appliance Co., Ltd., China; 0.25 × 30 mm) will be vertically placed on the skin surface without penetration, maintaining slight tactile pressure. Hence, the Deqi experience of the participants will not be overemphasized. Except locations of acupoints and needle penetration, the manipulation procedures and course will be the same with the verum acupuncture group (Figure 4).
Figure 4
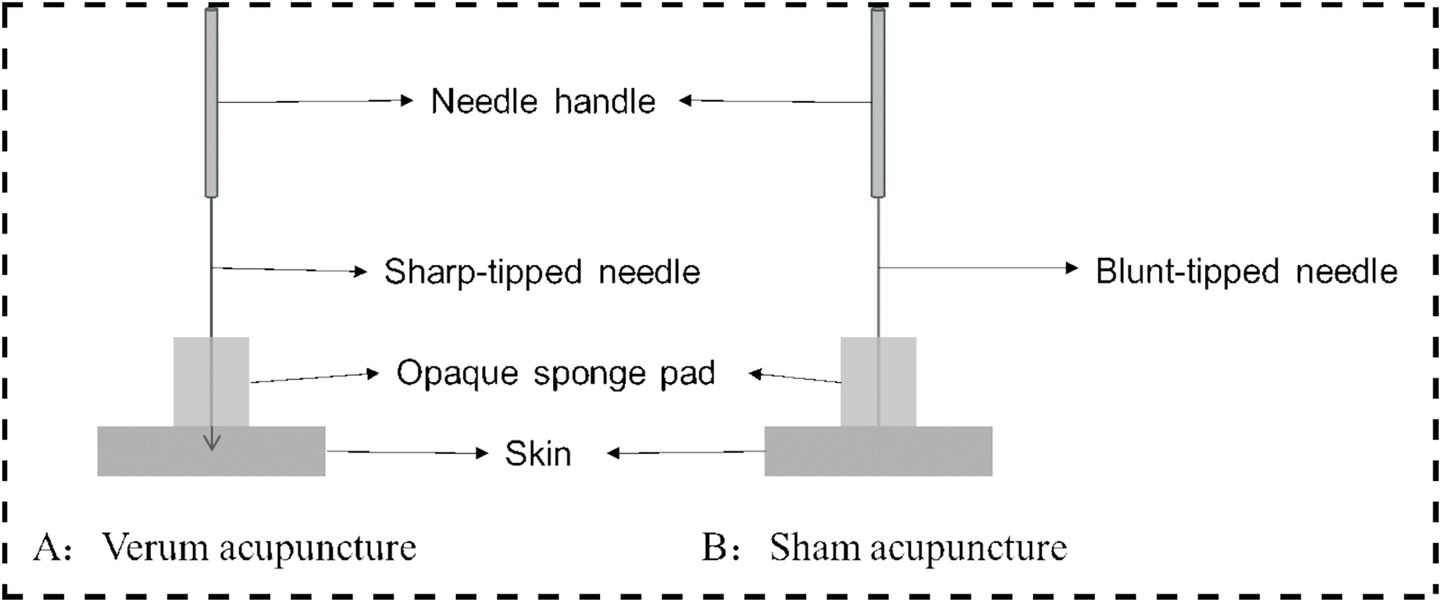
Verum and sham acupuncture.
2.5.3 Acupuncturist qualification
To ensure consistency, licensed acupuncturist will be trained to conduct the acupuncture operations.
2.6 Outcome measurements
2.6.1 Demographic and basic clinical information collection
The demographic information (age, gender, education, marital status and occupation) and time of enrollment will be obtained at baseline.
2.6.2 MRI scanning protocol
The fMRI data will be acquired with the Siemens Prisma 3.0T MRI (Siemens, Munich, Germany) at the Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, China. High-resolution T1-weighted structural images will be acquired using a magnetization-prepared rapid gradient-echo (MPRAGE) sequence with the following parameters: repetition time (TR) = 2,200 ms, echo time (TE) = 2.45 ms, field of view (FOV) = 256 mm × 256 mm, isotropic voxel resolution = 1 × 1 × 1 mm3, 175 slices with no interslice gap, and a flip angle (FA) of 8°. For resting-state and task-based functional scans, the parameters will be as follows: TR = 2,000 ms, TE = 30 ms, 37 slices, in-plane resolution = 64 × 64, voxel resolution = 3.75 × 3.75 × 4 mm3, and 240 volumes acquired per run. Before the scanning session, participants will be positioned supine with their heads immobilized using a pillow. They will be instructed to keep their eyes closed, remain relaxed, avoid deliberate mental activity, and breathe naturally. Foam earplugs are provided to minimize scanner noise. Scanning commences after participants have acclimated to the environment.
Both verum and sham acupuncture protocols will follow the procedure below (Figure 2). After 20 min of quiet rest, the subject will first undergo a 14-s locator scan to determine the imaging range. This will be followed by a 4-min and 39-s 3D high-resolution T1-weighted imaging sequence to acquire anatomical structural information. A pre-acupuncture resting-state fMRI scan lasting 6 min and 6 s will then be performed. Subsequently, a licensed acupuncturist will enter the MRI scanning room to administer acupuncture on the right limb using a block paradigm: three 60-s cycles of twisting needle manipulation will alternate with three 60-s cycles of needle-retaining baseline states. Concurrently, task-based fMRI scanning will be conducted for 6 min and 6 s. Following needle removal, a 6-min and 6-s post-acupuncture resting-state fMRI scan will be acquired.
2.6.3 Blinding assessment
When recruiting and screening eligible participants, subjects will be informed that they will receive both verum and sham acupuncture stimulation while the orders of presentation remain blinded to them. Following the completion of two fMRI scanning sessions, participants will be asked to guess the type of acupuncture they received during each session to assess the blinding effectiveness. The question posed to participants will be: “Which type of acupuncture do you believe you received during the two sessions?” Participants will be provided with three response options: traditional acupuncture, sham acupuncture, or uncertain.
2.6.4 Deqi sensation assessment
Following the completion of both trials, participants will be assessed for the types and intensity of Deqi sensations using the Modified Massachusetts General Hospital Acupuncture Sensation Scale-Chinese version (C-MMASS) (21). The C-MMASS evaluates 12 specific types of sensations: soreness, pain, pressure, heaviness, distension, tingling, numbness, dull ache, warmth, cold, throbbing, and other sensations. The intensity of each sensation will be rated on a scale from 0 to 10, where 0 indicates no sensation, 1–3 represents mild intensity, 4–6 moderate intensity, 7–9 severe intensity, and 10 signifies the maximum tolerable intensity.
2.7 Data management and statistical analysis
2.7.1 Data preprocessing
The preprocessing of magnetic resonance imaging (MRI) data will involve format conversion using the dcm2niigui software, followed by subsequent preprocessing steps performed with the Statistical Parametric Mapping (SPM12) toolbox on the MATLAB R2021A platform. The workflow will include the following procedures: (1) To ensure data stability, images from the first five time points will be excluded during preprocessing. (2) All remaining scanned images will then be aligned to the middle image and corrected for head motion. (3) During motion correction, volumes exhibiting head displacement with 3D translation of >1.5 mm or 3D rotation >1.5° will be deleted (22). (4) For spatial registration, each patient’s T1-weighted images will be co-registered with their corresponding functional images. (5) The images will then be uniformly segmented and resampled. The retained data will undergo spatial normalization into Montreal Neurological Institute (MNI) space. (6) Finally, spatial smoothing will be applied using a Gaussian kernel with a full width at half maximum of 6 mm.
2.7.2 Functional connectivity and network analysis
Resting-state functional connectivity (FC) analysis was employed to investigate the synchronized activity between brain regions (23, 24). Specifically, we utilized seed-based correlation analysis, a widely used method to quantify FC between specific regions of interest (25). Following standard resting-state fMRI preprocessing, the mean time series will be extracted from a predefined seed region of interest (ROI). Whole-brain connectivity maps will be generated by computing Pearson’s correlation coefficients between the seed time series and the time series of every other voxel in the brain. The resulting correlation coefficients (r-values) will be converted to Z-scores using Fisher’s Z-transformation to normalize their distribution for subsequent group-level statistical analysis.
The resulting connectivity matrices were then used to construct brain networks where nodes were defined according to the Dosenbach-160 atlas (26), and edges represented the significant FC between node pairs. Subsequently, graph-theoretical analysis was performed to characterize the topological properties of these networks using measures such as clustering coefficient and characteristic path length (27, 28). All analyses were carried out using the GRETNA toolbox (29). Using these nodes and the Fisher’s Z-transformed connectivity strengths as weighted edges, we will construct individual functional brain networks for each subject and time point. Key graph-theoretic metrics, including global efficiency (quantifying global integration), local efficiency (quantifying local specialization and fault tolerance), and nodal degree/strength (quantifying regional influence), will be computed from these networks across a range of connection densities to characterize their topological organization robustly.
2.7.3 Statistical analysis
Neuroimaging analyses will be conducted using the SPM12 toolbox on the MATLAB platform. First, one-sample t-tests will be performed to identify brain activation patterns associated with verum acupuncture (at true acupoints) and sham acupuncture (at non-acupoints). Subsequently, repeated-measures analysis of variance (ANOVA) will be applied. If statistically significant effects are detected, paired-sample t-tests will be conducted between the two groups to identify differential brain activation patterns between true acupoints and non-acupoints. Additionally, paired-sample t-tests will be performed to compare: (1) Resting-state functional activity pre- versus post-acupuncture; (2) Resting-state activity (pre-acupuncture) versus task-based activity (during acupuncture). A voxel-wise threshold of p < 0.005 with a false discovery rate-corrected cluster level of p < 0.05 was applied in the task fMRI analysis. Significant activation clusters will be visualized and localized using the xjView, MRIcron, and BrainNet Viewer toolboxes within the MATLAB platform.
2.7.4 Common brain effects of acupoints, pain-related brain regions, and differential brain effects
Analyses of common brain effects elicited by acupoint stimulation will be performed using GingerALE software (Version 3.0.2, http://brainmap.org/ale) with cluster-level family-wise error (FWE) correction. The following parameters will be applied: 5,000 permutations for statistical testing, a cluster-forming threshold of p < 0.001, and a statistical significance threshold of p < 0.05 (cluster-level FWE corrected) (30). Additionally, a predefined pain-related brain map (derived from the Neurosynth database, https://neurosynth.org) will be used to evaluate spatial overlap between acupoint-induced activation clusters and pain-associated brain regions. The overlap analysis will adopt a statistical significance threshold of p < 0.05.
2.7.5 Functional decoding
To determine the functional roles of acupoint-induced activation clusters, forward inference and reverse inference analyses will be conducted using the BrainMap database.1 Forward inference will decode the behavioral domains (BD) and paradigm classes (PC) associated with the identified brain regions. Behavioral domains are hierarchically categorized into five major classes (behavioral, cognitive, emotional, interoceptive, and perceptual) and 51 subclasses. For forward inference, the functional profile of a brain region will be defined by the probability of its involvement in specific behavioral or cognitive tasks based on task-classification labels. Reverse inference will identify the most likely behavioral domains and paradigm classes linked to the functional connectivity patterns of activation clusters using Bayesian probability.2 This analysis will quantify the posterior probability that a specific brain region is engaged during a particular task or behavioral context.
The significance of forward and reverse inference results will be assessed using likelihood ratio tests and chi-square tests, respectively. A false discovery rate (FDR) correction for multiple comparisons will be applied to both analyses, with a significance threshold of p < 0.05.
2.7.6 Statistical analysis of baseline information and C-MMASS
Statistical analyses of participants’ baseline characteristics and inter-group comparisons of C-MMASS scores will be performed using SPSS software (version 26.0). Categorical variables between groups will be analyzed with the chi-square test, while continuous variables will be assessed using independent samples t-tests. Within-group comparisons of categorical variables (pre- vs. post-intervention) will employ the chi-square test, and continuous variables will be evaluated with paired samples t-tests. All quantitative data will be presented as mean ± standard deviation. A threshold of p > 0.05 will indicate no statistically significant difference.
2.8 Safety measurements
All adverse events must be documented in detail in the Case Report Form (CRF), including symptom characteristics, severity, onset/duration, management measures, and outcomes. An objective assessment of the causality between the event and the acupuncture intervention is required. The attending physician may discontinue a participant’s involvement based on clinical judgment; such decisions require the signature and date from the researcher.
Regarding MRI examinations, participants may experience discomfort from the prolonged scanning time and acoustic noise. Headphones will be provided to reduce noise exposure. If a participant remains unable to tolerate the procedure, the scan may be terminated by mutual agreement, and this will be recorded as a study withdrawal. The decision to resume scanning after a rest period will be based on the physician’s assessment of the participant’s readiness.
3 Results
3.1 Immediate brain responses during task fMRI
3.1.1 Common brain effects
Both verum and sham acupuncture may commonly activate a brain network involving the anterior cingulate cortex, anterior insula, primary/secondary somatosensory cortices, and prefrontal cortex. This network is responsible for processing stimulus salience, bodily sensations, and cognitive appraisal, reflecting the non-specific effects of acupuncture. That is, this brain activity largely stems from shared components such as attention, sensation, and expectation elicited by the skin penetration itself, overlapping with the neural substrates of placebo effects.
3.1.2 Differential brain effects
Verum acupuncture is hypothesized to produce more robust and widespread brain modulation than sham acupuncture, engaging distinct neural mechanisms. Specifically, it activates subcortical centers (e.g., hypothalamus, brainstem) and limbic regions (e.g., amygdala, hippocampus), implicating its role in autonomic, affective, and homeostatic regulation. In parallel, it more strongly suppresses the core default mode network (DMN), including the posterior cingulate and medial prefrontal cortices. This pronounced DMN deactivation may reflect an enhanced redirection of attention toward bodily stimuli, aligning with the concept of “regulating the spirit” and distinguishing the holistic action of verum acupuncture.
3.2 Resting-state fMRI: functional connectivity and network analysis
This aspect may yield the most intriguing findings, as it reflects the sustained after-effects of acupuncture, rather than immediate responses.
3.2.1 Pre- vs. post-acupuncture changes
Following verum acupuncture, significant reorganization of resting-state functional connectivity occurs compared to sham acupuncture. This includes weakened connectivity within the default mode network (DMN), suggesting that the brain may remain less prone to mind-wandering even at rest; enhanced coupling between the salience network (SN) and the central executive network (CEN), indicating a potential improvement in the brain’s ability to flexibly switch between internal Deqi sensation and external tasks; and reduced hyper-connectivity among pain-related regions such as S1, S2, thalamus, and anterior insula, which may underlie its long-term analgesic effects.
3.2.2 Graph theory network metrics
Verum acupuncture may promote a more optimal topological organization of the brain’s functional network.
3.3 Correlation between subjective sensations (Deqi—C-MMASS) and objective brain activity
The intensity and multidimensional characteristics of the “Deqi” sensation will correlate strongly with specific patterns of brain activity. The composite intensity of sensations like soreness, numbness, distension, and heaviness may positively correlate with activation intensity in the insula and ACC.
3.4 Functional decoding analysis
Decoding via databases like BrainMap will reveal that the brain clusters activated by verum acupuncture are associated with functional domains far beyond mere “sensory processing.” major behavioral domains (BD) and paradigm classes (PC) include areas such as sensation, perception, and interoception.
3.5 Blinding assessment and placebo effects
The blinding assessment may show that a portion of participants cannot accurately distinguish between verum and sham acupuncture, with a potentially high proportion selecting “uncertain.”
If the subjective guesses between groups do not differ significantly, then the differences in brain responses are more likely attributable to the biological effects of acupuncture itself rather than participant expectation. This would enhance the credibility of the findings. Even if placebo effects are present, this study is well-designed to dissociate them (common effects) from the specific effects (differential effects) using neuroimaging.
4 Discussion
In this study, we will examine whether acupoints engage both shared and distinct brain regions during neural regulation, with the specificity of these regions potentially underlying their unique therapeutic functions.
Our aim is to establish a bridge between traditional medicine and modern medicine through this research. Based on traditional Chinese medicine theory, LI4 (Hegu) is primarily used for pain relief and disorders of the head and face, with strong regulatory effects on Qi and blood. PC6 (Neiguan) harmonizes the stomach, alleviates nausea and vomiting, calms the Shen, and addresses cardiac and chest discomfort. ST36 (Zusanli) tonifies the whole body, strengthens the spleen and stomach, improves digestion and energy, and supports immunity. SP6 (Sanyinjiao), where the spleen, liver, and kidney meridians converge, is key in treating gynecological and urogenital disorders and nourishing blood and Yin. GB34 (Yanglingquan), the influential point for tendons, is essential for musculoskeletal conditions and lateral body pain. KI3 (Taixi), the kidney source point, tonifies kidney essence (Jing) and addresses deficiencies in both kidney Yin and Yang, which are foundational in aging and chronic disease. LR3 (Taichong), the liver source point, soothes the liver, promotes Qi flow, alleviates stress and emotional constraint, and treats disorders related to liver Qi stagnation. For instance, we anticipate that acupuncture at ST36 will significantly modulate the DMN and the salience network. Notably, DMN activity is closely linked to self-referential processing, introspection, and impairments of the gut-brain axis in gastrointestinal diseases (31, 32). Thus, we expect that our results may provide a neuroscientific basis for the action of ST36: by regulating the DMN and visceral sensory regions, ST36 could help restore homeostasis of the gut-brain axis, offering a modern biological correlate to its traditional role in “harmonizing the stomach and spleen.”
In terms of the mechanism of fMRI, previous review demonstrated that GB34, an acupoint mainly used in stroke and Parkinson’s disease, could activate brain response in the premotor cortex, the supplementary motor area, and the supramarginal gyrus (33). And Liu et al. (34) found that there is signal synchronization change in ReHo in different brain regions including cognitive, motor, default network, limbic system and other parts of the encephalic region following acupuncture at GB34. Our meta-analysis also found that ST36 could activate the left cerebellum, the bilateral rolandic operculum, the right supramarginal gyrus, and the right cerebellum, which are mainly associated with action and perception (35). Acupuncture at PC6 could provoke extensive signal attenuations in the cerebrocerebellar and subcortical area, and selectively evoke neural responses of the insula, hypothalamus, and flocculonodular lobe of cerebellum (nodulus and uvula) (36). The limbic/paralimbic-cerebellum and subcortical areas showed extensive causal interactions following acupuncture at PC6 (37). Acupuncture at SP6 could activate the default mode network, descending pain modulation pathway and visual cortices. He et al. (38) found that the decreased ReHo after acupuncture at KI3 was concentrated in the left postcentral, right paracentral lobule, and right SMA, which are important for processing sensory information and motor control. Furthermore, acupuncture at KI3 has a specific effect on certain brain regions associated with perception, body movement, spirit, and association (22). The latest meta-analysis (39) confirmed that acupuncture at the LR3 could activate regions such as the right postcentral gyrus, left thalamus, left middle frontal gyrus, and right superior frontal gyrus. Those activated regions align with the basal ganglia network, auditory network, left executive control network, posterior salience network, right executive control network, and sensorimotor networks, which are related to pain perception, emotional processing, and linguistic functions. While our analysis of fMRI activation clusters evoked by individual acupoints may demonstrate spatial convergence with those existing reports, this study will advance the field by decoding the functional significance of these regions. Specifically, we will employ forward and reverse inference approaches to elucidate whether the observed activations map onto established neural networks—transcending purely descriptive localization toward mechanistic interpretation.
This study is designed to have substantial translational potential. First, the single-acupoint fMRI paradigm we establish will serve as a standardized control for future research, aiding in the identification of acupoint-specific neural signatures. For example, comparing cerebral responses between ST36 (which strengthens the spleen) and LV3 (Taichong, which soothes the liver) may reveal differences in brain network representations of TCM functional systems corresponding to the liver and spleen. Second, specific brain network response patterns—such as the extent of DMN suppression—will be evaluated as potential objective biomarkers for predicting treatment response, thereby advancing acupuncture toward “precision medicine.” Future studies will recruit clinical populations (e.g., patients with irritable bowel syndrome) to undergo fMRI before and after treatment, with the aim of validating these biomarkers and exploring underlying molecular mechanisms.
The selection of healthy participants is primarily motivated by the need to eliminate confounding effects arising from underlying pathological states on brain functional responses. To begin with, patients, especially those with neurological disorders, often exhibit abnormal regional brain activation, brain network dysfunction and abnormal functional connectivity within the brain (40–43). Consequently, fMRI signal changes observed in patient cohorts become ambiguous, making it challenging to definitively attribute those alterations to the acupuncture intervention itself or the inherent neuropathological features of the underlying disease. Secondly, the responses to acupuncture in patients may differ from those in healthy individuals (44–46). Therefore, in this trial, we will recruit healthy subjects rather than patients.
Currently, there are two major paradigms for task-based fMRI study: non-repeated event-related (NRER) design and block design. NRER designs effectively capture transient neural responses to discrete stimuli (e.g., instantaneous needle insertion) (47). In contrast, our block design paradigm better reflects real-world clinical acupuncture practice which comprises four sequential phases: needle insertion, needle manipulations (lifting-thrusting and rotating) to elicit Deqi, needle retention and needle withdrawal. Acupuncture elicits prolonged neural and hemodynamic responses rather than transient bursts of activity. Block design, with its alternating periods of stimulation and rest (e.g., 60 s needle manipulation followed by 60 s rest), optimally captures these sustained effects. Furthermore, as the most commonly applied and the most efficient paradigm (46, 47), block design has the advantages of a relatively large blood oxygen level dependent (BOLD) signal change relative to baseline (48), superior signal-to-noise ratio (SNR) and increased statistical power (47, 49).
However, there are some limitations in this study. Firstly, due to resource constraints, this study will be unable to encompass all acupoints. Future work will be needed to construct a comprehensive brain mapping encompassing all acupoints to better inform clinical practice. Secondly, considering the characteristics of acupuncture, it is difficult to design a practitioner-blinded trial. Thirdly, the study will be limited to manual acupuncture, which may introduce variability due to subtle differences in needle manipulation techniques. Furthermore, different acupuncture modalities (e.g., electroacupuncture vs. manual acupuncture) may induce different effects (46, 50, 51). Future studies should compare outcomes across different acupuncture modalities to determine modality-specific neural correlates. Finally, although a sham acupuncture control will be employed, this approach may not eliminate the influence of placebo effects inherent in acupuncture trials.
Statements
Ethics statement
The studies involving humans were approved by Shenzhen Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XL: Resources, Conceptualization, Project administration, Writing – review & editing, Formal analysis, Validation, Visualization, Funding acquisition, Methodology, Supervision, Writing – original draft, Investigation, Data curation, Software. JO: Methodology, Investigation, Writing – review & editing, Project administration, Writing – original draft. LD: Resources, Writing – original draft, Project administration, Formal analysis, Writing – review & editing, Supervision. WD: Visualization, Writing – review & editing, Writing – original draft, Resources, Software. YL: Data curation, Validation, Conceptualization, Writing – review & editing, Project administration, Resources, Software, Writing – original draft, Methodology, Supervision, Formal analysis, Investigation, Visualization. JZ: Resources, Funding acquisition, Project administration, Writing – original draft, Formal analysis, Validation, Data curation, Visualization, Supervision, Conceptualization, Writing – review & editing, Software, Methodology, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82305388), Shenzhen Excellent Scientific and Technological Innovation Talent Training Program (RCBS20231211090814025), Sanming Project of Medicine in Shenzhen (SZZYSM202311002), Construction Project of Guangdong Famous Traditional Chinese Medicine Inheritance Studio [Document No. 108 (2023) issued by the Guangdong Traditional Chinese Medicine Administration], Program of the Guangdong Provincial Administration of Traditional Chinese Medicine (No. 20251316).
Acknowledgments
The authors acknowledge all participants in this study, as well as all acupuncturists who will participate in this study but are not on the list of authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ma Y Dong M Zhou K Mita C Liu J Wayne PM . Publication trends in acupuncture research: a 20-year bibliometric analysis based on PubMed. PLoS One. (2016) 11:e0168123. doi: 10.1371/journal.pone.0168123
2.
Lu L Zhang Y Tang X Ge S Wen H Zeng J et al . Evidence on acupuncture therapies is underused in clinical practice and health policy. BMJ. (2022) 376:e067475. doi: 10.1136/bmj-2021-067475
3.
Xiao LY Wang XR Ye Y Yang JW Cao Y Ma SM et al . Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation. (2018) 21:762–76. doi: 10.1111/ner.12724
4.
Li WR Ren LL Zhao TT Dai DQ Gao XF Liang HZ et al . Multidimensional analgesia of acupuncture by increasing expression of MD2 in central nervous system. Chin J Integr Med. (2024) 30:1035–44. doi: 10.1007/s11655-024-4106-9
5.
Wei XY Wang X Shi GX Tu JF Yang JW Ren MM et al . Acupuncture modulation of chronic neuropathic pain and its association with brain functional properties. J Pain. (2024) 25:104645. doi: 10.1016/j.jpain.2024.104645
6.
Wei XY Wang ZY Shi GX Zhang N Li JL Ren MM et al . Effect of acupuncture treatment for patients with knee osteoarthritis on brain fluctuation amplitude and functional connectivity: a randomized three-armed fMRI study. BMC Complement Med Ther. (2025) 25:244. doi: 10.1186/s12906-025-04985-w
7.
Jung C Kim J Park K . Cognitive and affective interaction with somatosensory afference in acupuncture—a specific brain response to compound stimulus. Front Hum Neurosci. (2023) 17:1105703. doi: 10.3389/fnhum.2023.1105703
8.
Liu Q Xiao K Wan P Zou Q . Resting-state fMRI reveals the neural correlates of acupuncture in the treatment of vascular cognitive impairment. Clin Interv Aging. (2025) 20:1191–204. doi: 10.2147/CIA.S529416
9.
Wang ZN Ding JR Li X Shi L Yin B Bai GH et al . Acupuncture improves MRI brain microstructure with postconcussion symptoms in mild TBI: a randomized controlled trial. Radiology. (2025) 316:e250315. doi: 10.1148/radiol.250315
10.
Jiang TF Chen ZY Liu J Yin XJ Tan ZJ Wang GL et al . Acupuncture modulates emotional network resting-state functional connectivity in patients with insomnia disorder: a randomized controlled trial and fMRI study. BMC Complement Med Ther. (2024) 24:311. doi: 10.1186/s12906-024-04612-0
11.
Yu H Li F Liu J Liu D Guo H Wang J et al . Evaluation of acupuncture efficacy in modulating brain activity with periodic-aperiodic EEG measurements. IEEE Trans Neural Syst Rehabil Eng. (2024) 32:2450–9. doi: 10.1109/TNSRE.2024.3421648
12.
Wei C Yang Q Chen J Rao X Li Q Luo J . EEG microstate as a biomarker of post-stroke depression with acupuncture treatment. Front Neurol. (2024) 15:1452243. doi: 10.3389/fneur.2024.1452243
13.
Usichenko TI Wesolowski T Lotze M . Verum and sham acupuncture exert distinct cerebral activation in pain processing areas: a crossover fMRI investigation in healthy volunteers. Brain Imaging Behav. (2015) 9:236–44. doi: 10.1007/s11682-014-9301-4
14.
Li H Wang Z Yu H Pang R Ni H Li C-SR et al . The long-term effects of acupuncture on hippocampal functional connectivity in aMCI with hippocampal atrophy: a randomized longitudinal fMRI study. Neural Plast. (2020) 2020:6389368. doi: 10.1155/2020/6389368
15.
Wu MT Hsieh JC Xiong J Yang CF Pan HB Chen YCI et al . Central nervous pathway for acupuncture stimulation: localization of processing with functional MR imaging of the brain—preliminary experience. Radiology. (1999) 212:133–41. doi: 10.1148/radiology.212.1.r99jl04133
16.
Zhong C Bai L Dai R Xue T Wang H Feng Y et al . Modulatory effects of acupuncture on resting-state networks: a functional MRI study combining independent component analysis and multivariate granger causality analysis. J Magn Reson Imaging. (2012) 35:572–81. doi: 10.1002/jmri.22887
17.
Claunch JD Chan ST Nixon EE Qiu WQ Sporko T Dunn JP et al . Commonality and specificity of acupuncture action at three acupoints as evidenced by fMRI. Am J Chin Med. (2012) 40:695–712. doi: 10.1142/S0192415X12500528
18.
Liu J Qin W Guo Q Sun J Yuan K Dong M et al . Divergent neural processes specific to the acute and sustained phases of verum and SHAM acupuncture. J Magn Reson Imaging. (2011) 33:33–40. doi: 10.1002/jmri.22393
19.
Desmond JE Glover GH . Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. (2002) 118:115–28. doi: 10.1016/S0165-0270(02)00121-8
20.
Lim S . WHO standard acupuncture point locations. Evid Based Complement Alternat Med. (2010) 7:167–8. doi: 10.1093/ecam/nep006
21.
Yu DT Jones AY Pang MY . Development and validation of the Chinese version of the Massachusetts General Hospital Acupuncture Sensation Scale: an exploratory and methodological study. Acupunct Med. (2012) 30:214–21. doi: 10.1136/acupmed-2012-010145
22.
Zhu B Wang Y Zhang G Ouyang H Zhang J Zheng Y et al . Acupuncture at KI3 in healthy volunteers induces specific cortical functional activity: an fMRI study. BMC Complement Altern Med. (2015) 15:361. doi: 10.1186/s12906-015-0881-3
23.
Biswal B Zerrin Yetkin F Haughton VM Hyde JS . Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. (1995) 34:537–41. doi: 10.1002/mrm.1910340409
24.
van den Heuvel MP Hulshoff Pol HE . Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. (2010) 20:519–34. doi: 10.1016/j.euroneuro.2010.03.008
25.
Fox MD Raichle ME . Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. (2007) 8:700–11. doi: 10.1038/nrn2201
26.
Dosenbach NU Nardos B Cohen A Fair D Power J Church J et al . Prediction of individual brain maturity using fMRI. Science. (2010) 329:1358–61. doi: 10.1126/science.1194144
27.
Rubinov M Sporns O . Complex network measures of brain connectivity: uses and interpretations. NeuroImage. (2010) 52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003
28.
Bullmore E Sporns O . Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. (2009) 10:186–98. doi: 10.1038/nrn2575
29.
Wang J Wang X Xia M Liao X Evans A He Y . GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. (2015) 9:386. doi: 10.3389/fnhum.2015.00386
30.
Lancaster JL Tordesillas-Gutiérrez D Martinez M Salinas F Evans A Zilles K et al . Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. (2007) 28:1194–205. doi: 10.1002/hbm.20345
31.
Knyazev GG Savostyanov AN Bocharov AV Levin EA Rudych PD . Intrinsic connectivity networks in the self- and other-referential processing. Front Hum Neurosci. (2020) 14:579703. doi: 10.3389/fnhum.2020.579703
32.
Ding Y Ou Y Yan H Fu X Yan M Li H et al . Disrupted cerebellar-default mode network functional connectivity in major depressive disorder with gastrointestinal symptoms. Front Cell Neurosci. (2022) 16:833592. doi: 10.3389/fncel.2022.833592
33.
Zhang J Li Z Li Z Li J Hu Q Xu J et al . Progress of acupuncture therapy in diseases based on magnetic resonance image studies: a literature review. Front Hum Neurosci. (2021) 15:694919. doi: 10.3389/fnhum.2021.694919
34.
Liu L Chen S Zeng D Li H Shi C Zhang L . Cerebral activation effects of acupuncture at Yanglinquan (GB34) point acquired using resting-state fMRI. Comput Med Imaging Graph. (2018) 67:55–8. doi: 10.1016/j.compmedimag.2018.04.004
35.
Zhang J Liu Y Li Z Hu Q Huang X Lv H et al . Functional magnetic resonance imaging studies of acupuncture at ST36: a coordinate-based meta-analysis. Front Neurosci. (2023) 17:1180434. doi: 10.3389/fnins.2023.1180434
36.
Bai L Yan H Li L Qin W Chen P Liu P et al . Neural specificity of acupuncture stimulation at pericardium 6: evidence from an fMRI study. J Magn Reson Imaging. (2010) 31:71–7. doi: 10.1002/jmri.22006
37.
Feng Y Bai L Zhang W Xue T Ren Y Zhong C et al . Investigation of acupoint specificity by multivariate granger causality analysis from functional MRI data. J Magn Reson Imaging. (2011) 34:31–42. doi: 10.1002/jmri.22585
38.
He L Chen G Zheng R Hu Y Chen X Ruan J . Heterogeneous acupuncture effects of Taixi (KI3) on functional connectivity in healthy youth and elder: a functional MRI study using regional homogeneity and large-scale functional connectivity analysis. Neural Plast. (2020) 2020:8884318. doi: 10.1155/2020/8884318
39.
Rao Y Ge L Wu J . A systematic review and coordinate-based meta-analysis of fMRI studies on acupuncture at LR 3. Front Neurosci. (2024) 18:1341567. doi: 10.3389/fnins.2024.1341567
40.
Wei XY Luo SL Chen H Liu SS Gong ZG Zhan SH . Functional connectivity changes during migraine treatment with electroacupuncture at Shuaigu (GB8). J Integr Med. (2022) 20:237–43. doi: 10.1016/j.joim.2022.01.009
41.
Li HJ Hou XH Liu HH Yue CL He Y Zuo XN . Toward systems neuroscience in mild cognitive impairment and Alzheimer’s disease: a meta-analysis of 75 fMRI studies. Hum Brain Mapp. (2015) 36:1217–32. doi: 10.1002/hbm.22689
42.
Yeo S Lim S Choe IH Choi YG Chung KC Jahng GH et al . Acupuncture stimulation on GB34 activates neural responses associated with Parkinson’s disease. CNS Neurosci Ther. (2012) 18:781–90. doi: 10.1111/j.1755-5949.2012.00363.x
43.
Zheng W Su Z Liu X Zhang H Han Y Song H et al . Modulation of functional activity and connectivity by acupuncture in patients with Alzheimer disease as measured by resting-state fMRI. PLoS One. (2018) 13:e0196933. doi: 10.1371/journal.pone.0196933
44.
Napadow V Kettner N Liu J Li M Kwong KK Vangel M et al . Hypothalamus and amygdala response to acupuncture stimuli in carpal tunnel syndrome. Pain. (2007) 130:254–66. doi: 10.1016/j.pain.2006.12.003
45.
Cho SY Kim M Sun JJ Jahng GH Kim HJ Park SU et al . A comparison of brain activity between healthy subjects and stroke patients on fMRI by acupuncture stimulation. Chin J Integr Med. (2013) 19:269–76. doi: 10.1007/s11655-013-1436-4
46.
Huang W Pach D Napadow V Park K Long X Neumann J et al . Characterizing acupuncture stimuli using brain imaging with fMRI—a systematic review and meta-analysis of the literature. PLoS One. (2012) 7:e32960. doi: 10.1371/journal.pone.0032960
47.
Friston KJ Zarahn E Josephs O Henson RNA Dale AM . Stochastic designs in event-related fMRI. NeuroImage. (1999) 10:607–19. doi: 10.1006/nimg.1999.0498
48.
Buxton RB Wong EC Frank LR . Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. (1998) 39:855–64. doi: 10.1002/mrm.1910390602
49.
Friston KJ Holmes AP Price CJ Büchel C Worsley KJ . Multisubject fMRI studies and conjunction analyses. NeuroImage. (1999) 10:385–96. doi: 10.1006/nimg.1999.0484
50.
Napadow V Makris N Liu J Kettner NW Kwong KK Hui KKS . Effects of electroacupuncture versus manual acupuncture on the human brain as measured by fMRI. Hum Brain Mapp. (2005) 24:193–205. doi: 10.1002/hbm.20081
51.
Liu JP Li YY Yang KZ Shi SF Gong Y Tao Z et al . Electroacupuncture and manual acupuncture at LR3 and ST36 have attenuating effects on hypertension and subsequent cognitive dysfunction in spontaneously hypertensive rats: a preliminary resting-state functional magnetic resonance imaging study. Front Neurosci. (2023) 17:1129688. doi: 10.3389/fnins.2023.1129688
Summary
Keywords
acupuncture, healthy subjects, functional magnetic resonance imaging, randomized controlled trial, protocol
Citation
Lu X, Ou J, Ding L, Dang W, Liu Y and Zhang J (2025) Brain activities responding to acupuncture at acupoint in healthy subjects: a study protocol based on task-based fMRI. Front. Neurol. 16:1655478. doi: 10.3389/fneur.2025.1655478
Received
18 July 2025
Accepted
29 September 2025
Published
13 October 2025
Volume
16 - 2025
Edited by
Catarina Rua, Antaros Medical, Sweden
Reviewed by
Haitao Yu, Tianjin University, China
Yilei Chen, Shanghai University of Traditional Chinese Medicine, China
Updates
Copyright
© 2025 Lu, Ou, Ding, Dang, Liu and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongfeng Liu, 2934808419@qq.com; Jinhuan Zhang, zjh3424@gzucm.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.