- 1Department of Radiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 2Department of Nuclear Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
- 3Department of Radiology, The People's Hospital of Yuhuan, Yuhuan, Zhejiang, China
- 4Key Laboratory of Novel Nuclide Technologies on Precision Diagnosis and Treatment & Clinical Transformation of Wenzhou City, Wenzhou, Zhejiang, China
Objective: The purpose of this study was to investigate the association between stroke onset time and prognosis after endovascular thrombectomy (EVT) in Acute Ischemic Stroke (AIS) patients at different ages.
Methods: The AIS patients who underwent endovascular thrombectomy (EVT) between August 2018 to June 2022 were collected retrospectively. The patients were divided into two onset time groups [day-onset (6:00 h−18:00 h) vs. night-onset (18:00 h−6:00 h)], and further divided into 4 onset age groups (<55 y, 55–64 y, 65–74 y and 75+ y). The primary outcome was discharge National Institutes of Health Stroke Scale (NIHSS) score and secondary outcomes were malignant brain edema, hemorrhagic transformation (HT), and early vascular recanalization (mTICI ≥ 2b). Mediation analyses were applied to explore how malignant brain edema, HT, and early vascular recanalization affect the relationship between onset time and outcome.
Results: A total of 470 AIS patients were enrolled, of whom 68 patients were younger than 55 years. After adjusting for confounders, younger (<55 y) day-onset AIS patients had worse discharge outcomes (discharge NIHSS≥16, OR = 0.136, 95%CI = 0.027–0.678, P = 0.015) and were more prone to neurological deterioration (ΔNIHSS ≥ 4, OR = 0.081, 95%CI = 0.012–0.544, P = 0.010), malignant brain edema (OR = 0.145, 95%CI = 0.027–0.798, P = 0.026), and HT (OR = 0.231, 95%CI = 0.057–0.946, P = 0.042), while early vascular recanalization was less likely to occur (OR = 0.118, 95%CI = 0.017–0.813, P = 0.007). Mediation analysis showed that stroke onset time → malignant brain edema → discharge NIHSS score pathway was significant (c' = −2.029, BC 95%CI = −6.217; −0.087).
Conclusions: The outcomes of night-onset young AIS patients treated with EVT were better than day-onset, which may be related to the diurnal difference of malignant brain edema. However, these results were related to a small subgroup of patients and given the methodological statistical limitations (multiple testing correction), these results are only driving hypothesis. Further research with larger patient sizes are required to validate these results and explore the underlying mechanisms.
1 Introduction
Circadian biology regulates almost every aspect of mammalian physiology, pathology, and the therapeutic efficacy of diseases. In stroke, circadian biology may impact disease susceptibility, injury degree, and therapy response (1). A recent study suggested that the challenges of translating neuroprotective treatments of stroke from the lab to clinical trials might be due to a mismatch between the circadian rhythms of animal models and patients in clinical trials (2). Compared to the failures of neuroprotective strategies, intravenous thrombolysis and endovascular thrombectomy (EVT) have been effective in clinical trials, suggesting that reperfusion is a valid treatment on both awake and non-awake strokes (2). Recently, EVT has been regarded as a standard therapy for vessel recanalization after stroke (3). However, clinical research on the correlation of stroke onset time and stroke outcome have been limited and inconsistent. Wang et al. (3) found that acute ischemic stroke (AIS) patients treated with EVT, whose onset time were between 00:00 h and 12:00 h, had a higher proportion of 3-months good outcomes. Ryu et al. (4) found that night-onset AIS patients presented higher neurological severity and worse outcomes. A recent study based on melatonin levels in stroke patients suggested that melatonin secretion levels affect stroke outcomes (5). Whereas Ding et al. (6) found that circadian rhythm had no association with the outcome of AIS patients treated with recombinant tissue plasminogen activator. Therefore, the relationship between stroke prognosis and circadian rhythm remains to be further investigated.
Stroke is considered a disease of the elderly in the prevailing view since published reports indicated that the majority of stroke cases occurred in the elderly (7). However, the global incidence of stroke is gradually becoming younger (8, 9), and the incidence of ischemic stroke in young patients has reached a significant increase in recent years. A previous England study compared people living in Oxford in 2002–2010 vs. 2010–2018 and found that there was a significant increase in stroke incidence among people under the age of 55 (10). The possible reason may be that lifestyle-related risk factors for stroke are more common in the younger population, such as excessive alcohol consumption, smoking, and illicit drug use (11). Stroke in the young imposes a greater financial burden on society than stroke in the elderly, as the disease cripples them during their most productive years. A previous study has shown that circadian rhythmic activities change markedly as advancing age (12), indicating that circadian rhythm is related to age. The amplitude of many rhythms weakened in the elderly relative to younger adults. A recent study published in Science examined the age and sex differences in the expression of genes related to circadian clocks. They found that younger people had stronger circadian rhythms than older people (13). Further studies on the relationship between age, circadian rhythm, and stroke prognosis are needed in order to provide better management for AIS patients.
Therefore, the purpose of this study was to investigate the association between stroke onset time and outcomes after EVT treatment in AIS patients at different ages. We hypothesized that circadian rhythm differences were more pronounced in younger stroke patients and were independently associated with outcomes.
2 Materials and methods
2.1 Patients selection
Data on patients with AIS admitted to The First Affiliated Hospital of Wenzhou Medical University from August 2018 to June 2022 were reviewed retrospectively. Inclusive criteria were as follow: (1) AIS of anterior or posterior circulatory large vessel occlusion confirmed by CTA or DSA; (2) No intracranial hemorrhage or cerebral edema observed on baseline CT; (3) Received EVT treatment within 24 h of onset; (4) Follow-up CT after surgery; (5) Complete laboratory and clinical data; (6) No deaths occurred during the hospitalization period. Thirty five patients were excluded for the following reasons: (1) Unrecorded operation time (n = 8); (2) Unknown medical history (n = 6); (3) Loss of baseline CT images (n = 6) and (4) Poor quality of CT images, causing difficulty to validate image characteristics such as brain edema and hemorrhagic transformation (n = 15). Finally, a total of 470 cases were enrolled. The patients were divided into two- groups [day-onset (6:00 h−18:00 h) vs. night-onset (18:00 h−6:00 h)] according to the onset time of stroke, or 4 age groups (<55 y, 55–64 y, 65–74 y and 75+ y) based on onset age (9). Stroke onset time was defined as time of first observed neurological deficits by patients or their relatives. The flowchart of the study was showed on Supplementary Figure 1. Our study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University. The written informed consent was waived due to the retrospective design.
2.2 Data collection
We retrieved demographic and baseline clinical information, including sex, age, stroke onset time, stroke onset season (Spring for March to May, Summer for June to August, Autumn for September to November, and Winter for December to February), and medical history (hypertension, diabetes mellitus, smoke, drink, atrial fibrillation, previous history of stroke and coronary heart disease). Laboratory data included four items of blood coagulation (prothrombin time, fibrinogen, activated partial thromboplastin time, and thrombin time) and D-Dimer. Imaging data included the Alberta Stroke Program Early CT Score (ASPECTS) or posterior circulation Alberta Stroke Program Early CT Score (pc-ASPECTS) assessed on initial CT and malignant brain edema and hemorrhagic transformation (HT) assessed on review CT. Clinical features included admission and discharge National Institutes of Health Stroke Scale (NIHSS) scores, change in NIHSS score (ΔNIHSS: discharge NIHSS score minus admission NIHSS score), stroke subtypes, time from onset to EVT, and early vascular recanalization. Stroke subtypes were estimated by an experienced neurologist and are classified into three categories (cardioembolic stroke, large-artery atherosclerosis stroke, and other types of stroke) according to the Trial of Org 10172 in Acute Stroke Treatment criteria (14).
2.3 Outcome assessments
We explored the following primary outcomes based on NIHSS score: (1) Discharge severity of stroke, as measured by discharge NIHSS score (continuous variable); (2) Poor discharge prognosis, defined as discharge NIHSS ≥ 16 points (3). (3) Neurological function change, as measured by ΔNIHSS (continuous variable). (4) Neurological function deterioration, defined as ΔNIHSS ≥ 4 (15). In addition, we explored the following three secondary outcomes: (1) Malignant brain edema, defined as the presence of large hypodensity lesions at hemisphere causing midline shift >5 mm on CT review or need for decompressive hemicraniectomy or death (16); (2) HT evaluated on the 24-h review CT scan image; (3) Early vascular recanalization, described as modified Thrombolysis in Cerebral Infarction Score (mTICI) ≥ 2b.
2.4 Statistical analysis
Continuous variables were expressed as mean ± SD or median (IQR) based on their distribution and compared using Student T-test or Mann–Whitney U-test. Categorical variables were summarized as count (percentage) and compared using the χ2 test or Fisher's exact test. To assess the association of stroke onset time [day-onset (6:00 h−18:00 h) vs. night-onset (18:00 h−6:00 h) groups], multivariate linear regression was used for continuous outcome variables (discharge NIHSS score and ΔNIHSS score), and multivariate logistic regression was used for binary outcome variables (discharge NIHSS ≥16, ΔNIHSS ≥4, malignant brain edema, hemorrhagic transformation, and early vascular recanalization). We conducted not only extensive assessments in the overall cohort, but also subgroup analyses in different age groups (<55 y, 55–64 y, 65–74 y, and 75+ y). Age, sex, hypertension, diabetes mellitus, smoking, alcohol consumption, atrial fibrillation, previous history of stroke, coronary heart disease, onset to surgery time, and stroke onset season were added into the models as covariates.
Point-Biserial Correlation analysis was performed to investigate the correlation between discharge NIHSS score and malignant brain edema, HT, and early vascular recanalization. In addition, mediation models were developed to explore the role of malignant brain edema, HT, and early vascular recanalization on the association between onset time and discharge NIHSS score. Indirect effects were estimated with bias-corrected (BC) bootstrapping using 1,000 iterations. All analyses were performed using SPSS (version 25.0; IBM, Armonk, NY, USA) and Amos (version 26.0.0; IBM, Armonk, NY, USA) software. P < 0.05 was considered statistically significant. The significance of the BC bootstrap estimate was indicated by confidence intervals (CI) that did not contain 0 (17).
3 Results
3.1 Baseline characteristics of the study population
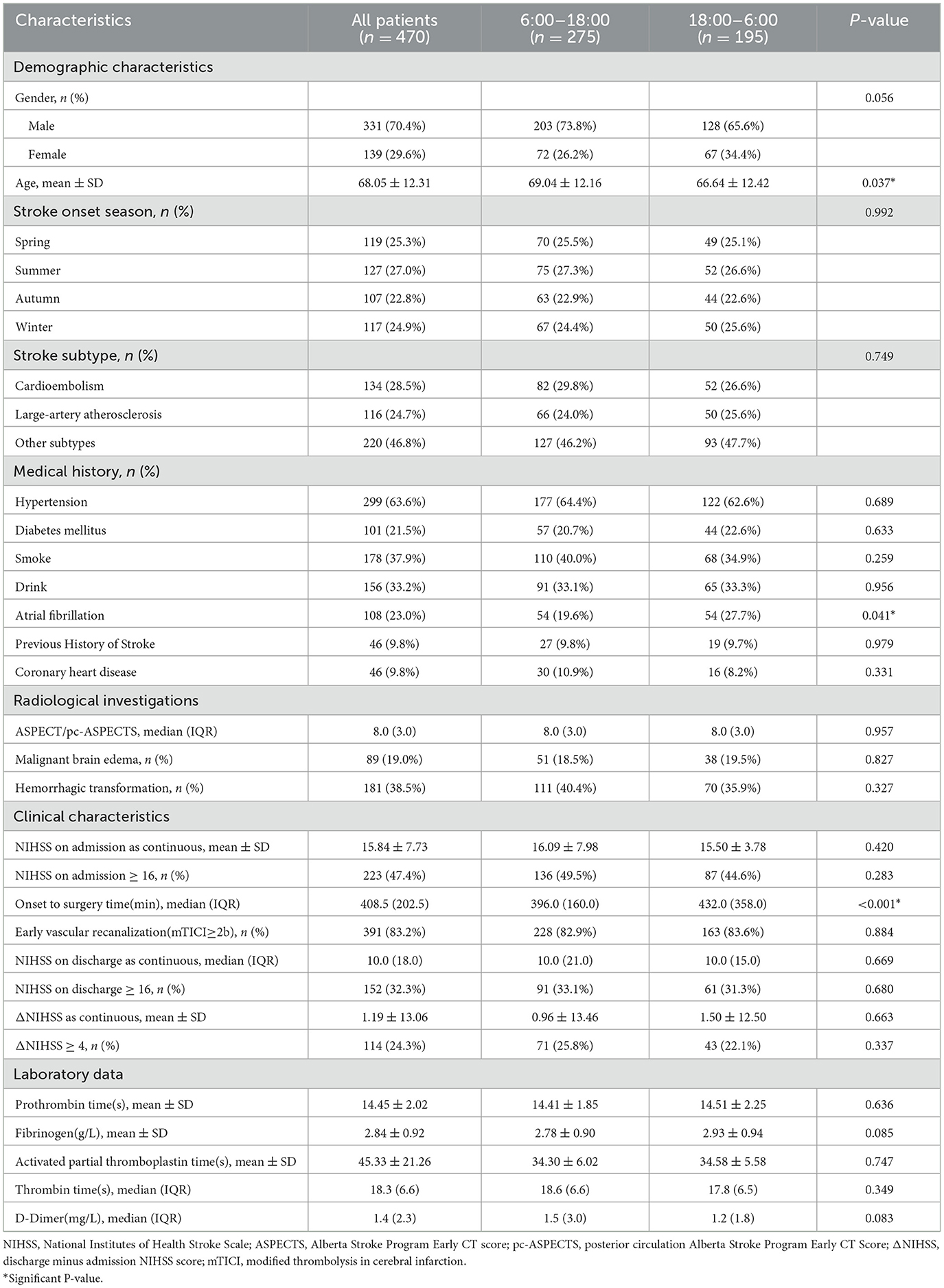
Table 1 summarizes the demographic and clinical characteristics of the overall cohort divided into day and night groups. The mean (±SD) age of stroke patients was 68.05 (±12.31) years, and 331 cases were men (70.4%). Patients who occurred stroke at night (18:00 h−6:00 h) were younger (P = 0.037), had a higher proportion of a history of atrial fibrillation (P = 0.041), and had longer onset to EVT time (P < 0.001). Other baseline variables were not significantly associated with stroke onset time. Here, we observed a circadian dependence in the stroke onset age. Although the age difference between the day-onset and night-onset groups was statistically significant, the absolute difference of 2.4 years is still of uncertain clinical relevance in the context of stroke outcomes.
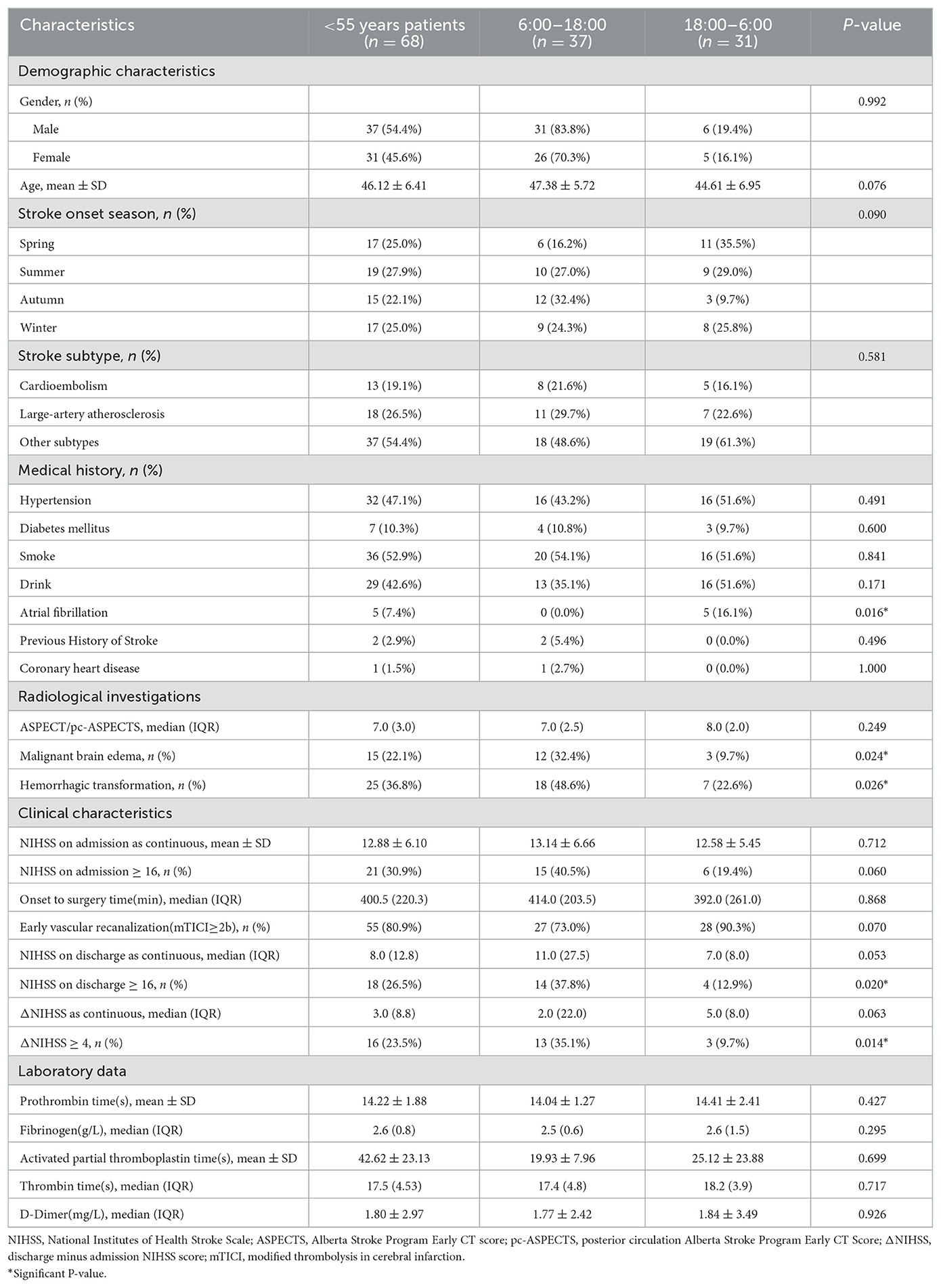
Thereupon, we performed a subgroup analysis dividing patients into four groups based on stroke onset age: <55 y (n = 68), 55-64 y (n = 90), 65-74 y (n = 156), and 75+ y (n = 156). For each age group, we further divided day and night stroke onset time groups and conducted univariate analysis. Except for AIS patients younger than 55 years of age, the age of patients presenting with nighttime strokes is significantly different than those presenting with daytime strokes across the other three groups (Supplementary Tables 1–3). In patients aged 55–64, 65–74, and 75+ y, there were more males than females, while in the group aged <55 y, there were relatively equal numbers of males and females (Supplementary Figure 2). There were 68 patients younger than 55 years old, of which 37 (54.41%) were day-onset cases and 31 (45.59%) were night-onset. Patients who develop stroke at night were more likely to have a history of atrial fibrillation (P = 0.016). Among the day-onset patients, 12 cases (32.4%) had malignant brain edema (P = 0.024), 18 cases (48.6%) had HT (P = 0.026), 14 cases (37.8%) had discharge NIHSS score ≥16 (P = 0.020), and 13 cases (35.1%) had ΔNIHSS ≥ 4 (P = 0.014) (Table 2), suggesting that day-onset stroke patients younger than 55 years of age showed notable circadian rhythms in discharge outcomes, neurological deterioration, malignant brain edema, and HT. Whereas in the other three age groups, there was no significant difference (Supplementary Tables 1–3).
3.2 Associations of stroke onset time with discharge outcomes and neurological deterioration in patients younger than 55 years of age
We performed multivariable linear and logistic regression analysis for patients in different age groups. After adjusting for age, sex, hypertension, diabetes mellitus, smoking, alcohol consumption, atrial fibrillation, previous history of stroke, coronary heart disease, onset to EVT time, and stroke onset season, stroke onset time was independently associated with discharge NIHSS ≥ 16 (adjusted OR = 0.136, 95%CI = 0.027–0.678, P = 0.015), ΔNIHSS ≥ 4 (adjusted OR = 0.081, 95%CI = 0.012–0.544, P = 0.010), malignant brain edema (adjusted OR = 0.145, 95%CI = 0.027–0.798, P = 0.026), HT (adjusted OR = 0.231, 95%CI = 0.057–0.946, P = 0.042), early vascular recanalization (adjusted OR = 0.118, 95%CI = 0.017–0.813, P = 0.007) (Table 3) and discharge NIHSS score (standard β = −0.334, 95%CI: −0.021, −0.005, P = 0.003), ΔNIHSS score (standard β = 0.343, 95%CI = 0.005–0.022, P = 0.002) (Table 4). These results suggested that night-onset stroke had a better discharge prognosis and was less likely to suffer from neurological deterioration, malignant brain edema, and HT, and more likely to occur with early vascular recanalization.
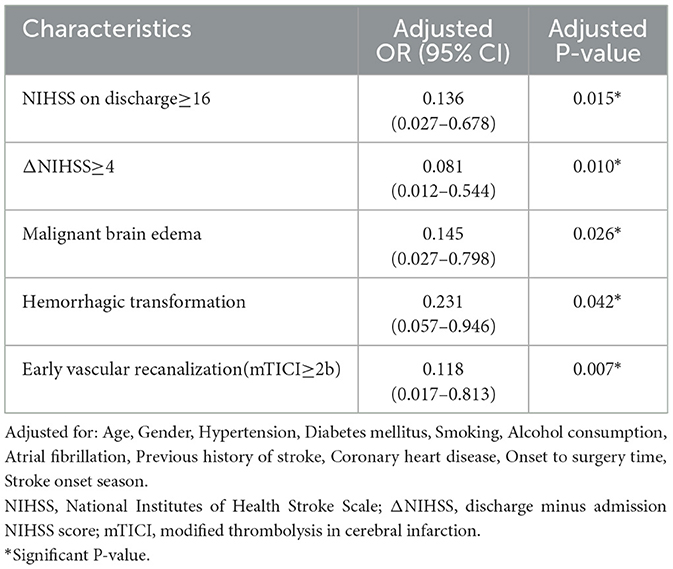
Table 3. Multivariable logistic regression for associations between outcomes and stroke onset time of patients with age <55 years.
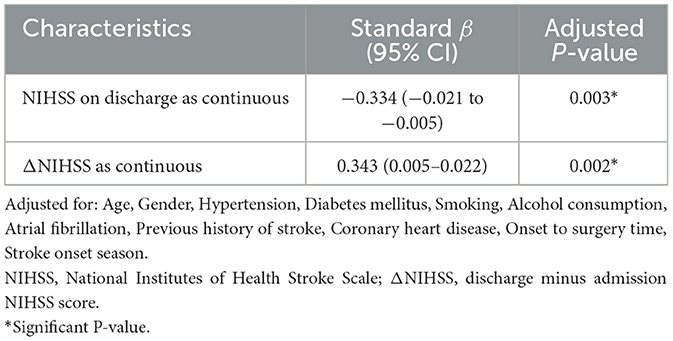
Table 4. Multivariable linear regression for associations between continuous outcomes and stroke onset time of patients with age <55 years.
Figure 1 shows the comparison of multivariate linear and logistic regression results of different outcome variables between different age groups. As illustrated, only patients with age <55 y showed circadian difference in functional outcomes, while in the other three age groups there was no independent correlation between functional outcomes and stroke onset time.

Figure 1. Multivariable and linear logistic regression results of outcomes in different age groups. (a–e) respectively represent multivariable logistic regression results of NIHSS on discharge≥16, ΔNIHSS≥4, malignant brain edema, hemorrhagic transformation and early vascular recanalization in different age groups. (f, g) Respectively represent multivariable linear regression results of NIHSS on discharge as continuous and ΔNIHSS as continuous. Adjusted for: Age, Sex, Hypertension, Diabetes mellitus, Smoking, Alcohol consumption, Atrial fibrillation, Previous history of stroke, Coronary heart disease, Onset to EVT time, Stroke onset season. NIHSS, National Institutes of Health Stroke Scale; ΔNIHSS, discharge minus admission NIHSS score. *Significant P-value.
3.3 Point-Biserial Correlation analysis and mediation analysis in patients younger than 55 years of age
The results of Point-Biserial Correlation analysis between discharge NIHSS score and malignant brain edema, HT, and early vascular recanalization are shown in Table 5. Discharge NIHSS score was positively associated with malignant brain edema (r = 0.358, P = 0.003) and HT (r = 0.336, P = 0.005), while there was no significant correlation between discharge NIHSS score and early vascular recanalization.
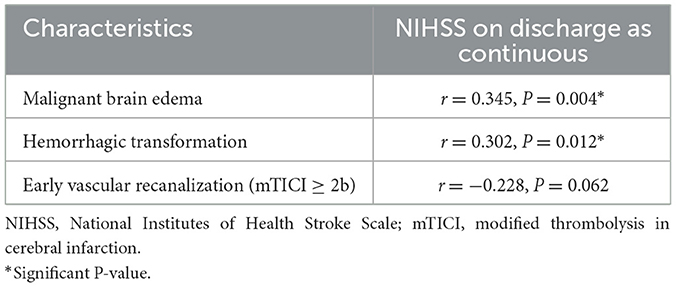
Table 5. Point-Biserial Correlation analysis of NIHSS on discharge as continuous with Malignant brain edema, Hemorrhagic transformation and Early vascular recanalization (mTICI ≥ 2b) of patients with age <55 years.
Mediation model was established to investigate whether the association between stroke onset time and discharge NIHSS score was mediated by malignant brain edema, HT, or early vascular recanalization. With stroke onset time as the independent variable, discharge NIHSS score as the dependent variable, and malignant brain edema, HT, and early vascular recanalization as mediators, we found that the mediation effect was significant (c' = −3.221, BC 95%CI = −7.690; −0.464), in which only the pathway stroke onset time → malignant brain edema → discharge NIHSS score showed significant result (Figure 2). Therefore, HT and early vascular recanalization were removed from the model, and we established a simple mediation model of stroke onset time → malignant brain edema → discharge NIHSS score. The result showed that the indirect effect of malignant brain edema was significant (c' = −2.029, BC 95%CI = −6.217; −0.087) (Figure 2), implying that the correlation of stroke onset time and stroke prognosis was mediated by malignant brain edema.
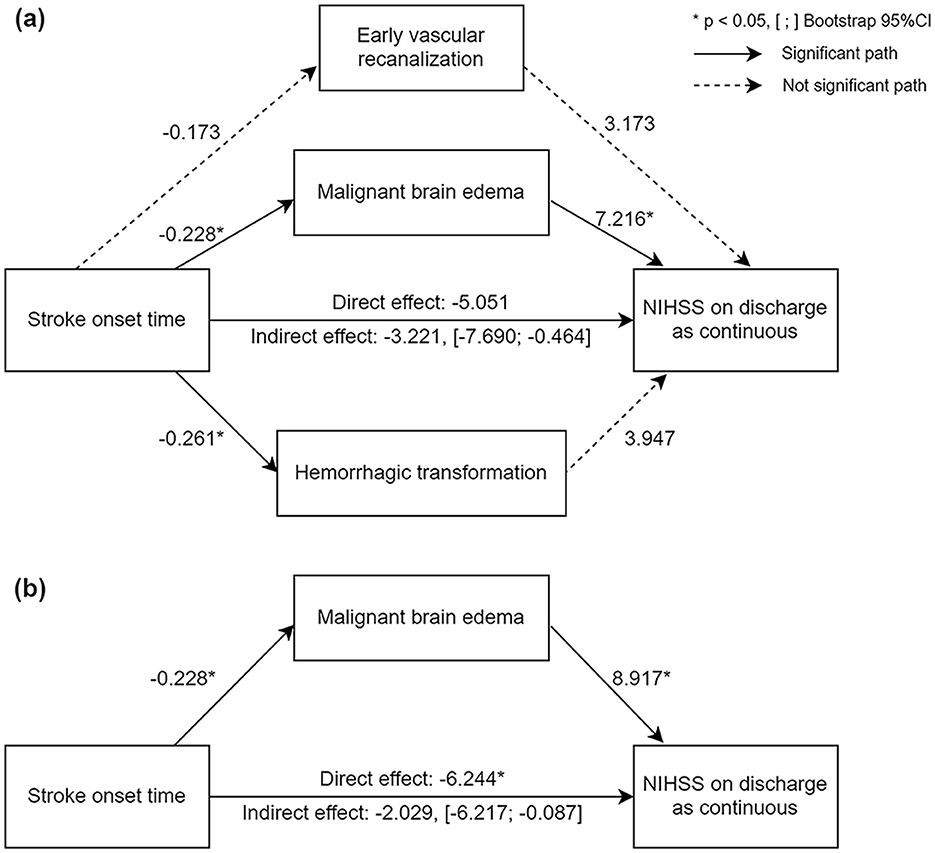
Figure 2. Diagram of mediation analyses with pathways from stroke onset time to discharge NIHSS score. (a) Parallel mediation model; (b) simple mediation model. The number represents the regression coefficient for each path. Solid lines represent significant pathway and dotted lines represent not significant pathways. *Significant P-value. [;], The 95%CI of indirect effect validated by bootstrap test.
4 Discussion
In this study, we observed that: (1) Younger patients showed more significant diurnal-night differences in the outcomes than the elderly; (2) There were diurnal-night differences in discharge prognosis in younger (< 55 y) patients, the prognosis of night-onset was better than that of day-onset; (3) The circadian difference of stroke prognosis may be related to the diurnal difference of malignant brain edema. This is basically consistent with our original assumption.
In a recent study, Benali et al. (18) revealed this phenomenon: based on the findings that AIS patients treated with EVT at night were more likely to have a clinically favorable 3-month outcome, the author further compared the characteristics of patients treated at night with favorable vs. unfavorable outcomes and found no significant differences in other medical history characteristics other than age. Age is the most considerable risk factor for AIS, as it influence the outcome among AIS patients and can't be modified (19). Jayaraman et al. (20) quantified the relationship between age and prognosis after EVT and found that among recanalized patients, modified Rankin Scale (mRS) worsened with the increase of age. In our study, no circadian rhythm difference was found for AIS outcomes across the whole cohort. However, we also detected significant age differences between day-onset and night-onset patients. Similar to the previous study (18), we found that night-onset stroke patients were slightly younger than those with day-onset stroke. Therefore, we further performed a subgroup analysis based on stroke onset age. In addition to AIS patients younger than 55 y, there also existed a significant difference in the stroke onset age in the other three groups. We found that there were diurnal-night differences in discharge prognosis in younger (< 55 y) patients, the prognosis of night-onset was better than that of day-onset, while no circadian rhythm difference was found in the elderly. This suggested that age of onset may influence circadian differences in the outcomes of AIS patients treated with EVT.
The possible reason was that circadian rhythm is dynamic across lifetimes, with younger people having stronger circadian rhythms than older people. Recent animal studies have shown that glial expression of the clock protein PERIOD decreases with age in fly models (21) and drpr mRNA showed a rhythmic profile only in young flies compared with the older (22). Similarly, a recent human study showed that rhythmic programs of mRNA generally dampened with age in most tissues, which means that younger people have stronger rhythmic programs than older people (23). In addition, age-related changes in circadian rhythm may cause pathological changes, such as chronic inflammation and metabolic disorders (24). As a result, no diurnal difference in stroke prognosis was observed in older patients in our study. More attention should be paid to the effect of patient age on circadian rhythm in future stroke treatment and management. Moreover, the majority of animal studies on stroke used young-adult rodents, though stroke occurs mainly in the aged human population (25). The findings of this study provided a certain reference for the selection of animals for research purposes. Age may have been neglected in rodent models of stroke, which might be the reason why the translation of rodent models to human stroke was found difficult.
Moreover, we found that the circadian difference in stroke outcomes in young stroke patients may be related to the diurnal difference in malignant brain edema, HT, and early vascular recanalization. Among them, malignant brain edema mediated the association of circadian rhythm and the outcome of young stroke patients. Brain edema is one of the severe complications closely related to poor outcomes of stroke (26). Despite no circadian rhythm differences have been found in previously published studies (3, 6, 27), in this study, we observed circadian rhythm differences in malignant brain edema in young patients, which was more likely to occur during the day. We speculated that the underlying reasons might be related to the rhythmic changes in the blood-brain barrier (BBB) permeability and the glymphatic function. A recent animal study showed increasing BBB permeability during the active phase and decreasing BBB permeability during the resting phase of mice (28). Hence, we infer that the BBB permeability increased during diurnal active period, which ultimately increased the incidence of malignant brain edema after stroke. Whereas, the BBB permeability decreased during the night when the BBB rhythm is more stable, and therefore the probability of malignant brain edema during the night was lower. The glymphatic system also plays a vital role during the formation of brain edema, promoting the clearance of vascular edema in the late stage (29). A previous study showed that glymphatic clearance exhibit endogenous circadian rhythms, which was enhanced during sleep and inhibited during wakefulness period (30). This was consistent with our result that malignant brain edema was more likely to occur during the day. In contrast, in older adults, the nocturnal circadian rhythms were more erratic, hence no difference was observed. This speculation needs to be verified by further clinical and experimental studies in the future.
We acknowledged some limitations of our study. First, the proportion of patients in the younger age group (< 55 y) was relatively small, which may cause experimental occasion. Second, this is a retrospective study based on EVT, which may lead to possible selection bias. Third, due to the lack of data, we used the NIHSS score as the outcome measure instead of the mRS Score, which may be more representative. Last but not least, a key finding of our univariate analysis was that patients with night-onset stroke were significantly younger than those with day-onset stroke. However, this difference did not survive correction for multiple testing (Bonferroni correction). This highlights an important limitation of our study. The observed differences in Table 1, while intriguing and potentially clinically relevant (e.g., the 2.4-year age difference), must be interpreted with extreme caution as they may represent false positive findings. They should be considered as generating hypotheses rather than confirming associations. To address these limitations, future studies with larger sample sizes and a priori hypotheses are needed to validate whether the conclusions.
In conclusion, the onset age could influence the circadian difference in the outcome of AIS patients treated with EVT. The prognosis of young stroke patients (< 55 y) treated with EVT showed diurnal difference, night-onset patients showed better outcomes compared with day-onset patients, which may be related to the diurnal difference in malignant brain edema. However, these results were related to a small subgroup of patients and given the methodological statistical limitations (multiple testing correction), these results are only driving hypothesis. Further research with larger patient sizes are required to validate these results and explore the underlying mechanisms. More attention should be paid to the patient's age on the effect of circadian rhythm in future stroke treatment and management.
Data availability statement
The datasets presented in this article are not readily available because they are private data. Requests to access the datasets should be directed to d3l5eWZza3huekAxNjMuY29t.
Ethics statement
The studies involving humans were approved by The First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective nature of the study.
Author contributions
MY: Visualization, Writing – original draft, Writing – review & editing. JL: Writing – original draft, Writing – review & editing. AD: Data curation, Formal analysis, Writing – original draft. KL: Data curation, Formal analysis, Writing – review & editing. ML: Formal analysis, Investigation, Writing – original draft. RL: Data curation, Investigation, Writing – review & editing. HXu: Conceptualization, Data curation, Writing – review & editing. MZ: Investigation, Writing – review & editing. YZ: Investigation, Writing – review & editing. FY: Investigation, Writing – review & editing. KZ: Data curation, Writing – review & editing. YH: Investigation, Writing – review & editing. HXi: Investigation, Writing – review & editing. YY: Conceptualization, Funding acquisition, Project administration, Writing – review & editing. NX: Conceptualization, Formal analysis, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by The Specialty Subspecialty (S Discipline) Support Program of The First Affiliated Hospital of Wenzhou Medical University; The Summit Advancement Disciplines of Zhejiang Province (Wenzhou Medical University—Pharmaceutics); The Key Laboratory of Novel Nuclide Technologies on Precision Diagnosis and Treatment & Clinical Transformation of Wenzhou City [Grant No. 2023HZSY0012]; The Social Development Category Science and Technology Projects of Yuhuan City [Grant No. 2024028]; and the Traditional Chinese Medicine Science and Technology Plan Project of Zhejiang [Grant No. 2022ZB213].
Acknowledgments
Thanks to all the coworkers who made a contribution to this research and to all the subjects who made this research possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1655646/full#supplementary-material
Abbreviations
AIS, Acute Ischemic Stroke; EVT, Endovascular Thrombectomy; HT, Hemorrhagic Transformation; ASPECTS, Alberta Stroke Program Early CT Score; pc-ASPECTS, posterior circulation Alberta Stroke Program Early CT Score; NIHSS, National Institutes of Health Stroke Scale; mTICI, modified Thrombolysis in Cerebral Infarction Score; mRS, modified Rankin Scale.
References
1. Lo EH, Albers GW, Dichgans M, Donnan G, Esposito E, Foster R, et al. Circadian biology and stroke. Stroke. (2021) 52:2180–90. doi: 10.1161/STROKEAHA.120.031742
2. Esposito E, Li W, Mandeville TE, Park J-H, Sencan I, Guo S, et al. Potential circadian effects on translational failure for neuroprotection. Nature. (2020) 582:395–8. doi: 10.1038/s41586-020-2348-z
3. Wang X, Wang XY, Ma J, Jia ML, Wu LF, Li WL, et al. Association between the time of day at stroke onset and functional outcome of acute ischemic stroke patients treated with endovascular therapy. J Cerebral Blood Flow Metab. (2022) 42:2191–200. doi: 10.1177/0271678X221111852
4. Ryu WS, Hong KS, Jeong SW, Park JE, Kim BJ, Kim JT, et al. Association of ischemic stroke onset time with presenting severity, acute progression, and long-term outcome: a cohort study. PLoS Med. (2022) 19:e1003910. doi: 10.1371/journal.pmed.1003910
5. Yu S-Y, Sun Q, Chen S-N, Wang F, Chen R, Chen J, et al. Circadian rhythm disturbance in acute ischemic stroke patients and its effect on prognosis. Cerebrovasc. Dis. (2024) 53, 14–27. doi: 10.1159/000528724
6. Ding J, Bai Z, Zhou D, Li X, Rajah GB, Ding Y, et al. Circadian rhythms may not influence the outcomes of thrombolysis in patients with ischemic stroke: A study from China. Chronobiol Int. (2018) 35:1533–42. doi: 10.1080/07420528.2018.1494602
7. Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
8. Smajlovic D. Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag. (2015) 11:157–64. doi: 10.2147/VHRM.S53203
9. Bejot Y, Daubail B, Jacquin A, Durier J, Osseby GV, Rouaud O, et al. Trends in the incidence of ischaemic stroke in young adults between 1985 and 2011: the Dijon Stroke Registry. J Neurol Neurosurg Psychiatry. (2014) 85:509–13. doi: 10.1136/jnnp-2013-306203
10. Li L, Scott CA, Rothwell PM. Association of younger vs older ages with changes in incidence of stroke and other vascular events, 2002–2018. JAMA. (2022) 328:563–74. doi: 10.1001/jama.2022.12759
11. Ohya Y, Matsuo R, Sato N, Irie F, Nakamura K, Wakisaka Y, et al. Causes of ischemic stroke in young adults vs. non-young adults: A multicenter hospital-based observational study. PLoS ONE. (2022) 17:e0268481. doi: 10.1371/journal.pone.0268481
12. Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest. (2017) 127:437–46. doi: 10.1172/JCI90328
13. Talamanca L, Gobet C, Naef F. Sex-dimorphic and age-dependent organization of 24-hour gene expression rhythms in humans. Science. (2023) 379:478–83. doi: 10.1126/science.add0846
14. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke. JAMA. (1998) 279, 1265–1272. doi: 10.1001/jama.279.16.1265
15. Saleem Y, Nogueira RG, Rodrigues GM, Kim S, Sharashidze V, Frankel M, et al. Acute neurological deterioration in large vessel occlusions and mild symptoms managed medically. Stroke. (2020) 51:1428–34. doi: 10.1161/STROKEAHA.119.027011
16. Ong CJ, Gluckstein J, Laurido-Soto O, Yan Y, Dhar R, Lee J-M, et al. Enhanced detection of Edema in Malignant Anterior Circulation Stroke (EDEMA) score. Stroke. (2017) 48, 1969–72. doi: 10.1161/STROKEAHA.117.016733
17. Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. (2002) 7:422–45. doi: 10.1037/1082-989X.7.4.422
18. Benali A, Moynier M, Dargazanli C, Deverdun J, Cagnazzo F, Mourand I, et al. Mechanical thrombectomy in nighttime hours: is there a difference in 90-day clinical outcome for patients with ischemic stroke? AJNR Am J Neuroradiol. (2021) 42:530–7. doi: 10.3174/ajnr.A6997
19. Roy-O'Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology. (2018) 159:3120–31. doi: 10.1210/en.2018-00465
20. Jayaraman MV, Kishkovich T, Baird GL, Hemendinger ML, Tung EL, Yaghi S, et al. Association between age and outcomes following thrombectomy for anterior circulation emergent large vessel occlusion is determined by degree of recanalisation. J Neurointerv Surg. (2019) 11:114–8. doi: 10.1136/neurintsurg-2018-013964
21. Long DM, Giebultowicz JM. Age-related changes in the expression of the circadian clock protein PERIOD in drosophila glial cells. Front Physiol. (2017) 8:1131. doi: 10.3389/fphys.2017.01131
22. Kuintzle RC, Chow ES, Westby TN, Gvakharia BO, Giebultowicz JM, Hendrix DA, et al. Circadian deep sequencing reveals stress-response genes that adopt robust rhythmic expression during aging. Nat Commun. (2017) 8:14529. doi: 10.1038/ncomms14529
23. Barth E, Srivastava A, Wengerodt D, Stojiljkovic M, Axer H, Witte OW, et al. Age-dependent expression changes of circadian system-related genes reveal a potentially conserved link to aging. Aging. (2021) 13:25694–716. doi: 10.18632/aging.203788
24. Khan S, Siddique R, Liu Y, Yong VW, Xue M. Towards improving the prognosis of stroke through targeting the circadian clock system. Int J Biol Sci. (2024) 20:403–13. doi: 10.7150/ijbs.88370
25. Zhang H, Lin S, Chen X, Gu L, Zhu X, Zhang Y, et al. The effect of age, sex and strains on the performance and outcome in animal models of stroke. Neurochem Int. (2019) 127:2–11. doi: 10.1016/j.neuint.2018.10.005
26. Chen S, Shao L, Ma L. Cerebral edema formation after stroke: emphasis on blood–brain barrier and the lymphatic drainage system of the brain. Front Cell Neurosci. (2021) 15:716825. doi: 10.3389/fncel.2021.716825
27. Lorenzano S, Ahmed N, Tatlisumak T, Gomis M, Dávalos A, Mikulik R, et al. Within-day and weekly variations of thrombolysis in acute Ischemic stroke. Stroke. (2014) 45:176–84. doi: 10.1161/STROKEAHA.113.002133
28. Zhang SL, Lahens NF, Yue Z, Arnold DM, Pakstis PP, Schwarz JE, et al. A circadian clock regulates efflux by the blood-brain barrier in mice and human cells. Nat Commun. (2021) 12:617. doi: 10.1038/s41467-020-20795-9
29. Li W, Chen D, Liu N, Luan Y, Zhu S, Wang H, et al. Modulation of lymphatic transport in the central nervous system. Theranostics. (2022) 12:1117–31. doi: 10.7150/thno.66026
Keywords: Ischemic stroke, endovascular thrombectomy, circadian rhythm, malignant brain edema, young age, outcome
Citation: Yu M, Lin J, Debora A, Lin K, Lin M, Lin R, Xu H, Zheng M, Zhou Y, Yao F, Zheng K, Huang Y, Xia H, Yang Y and Xia N (2025) Stroke onset time affected outcomes in the young acute ischemic stroke patients treated with endovascular thrombectomy. Front. Neurol. 16:1655646. doi: 10.3389/fneur.2025.1655646
Received: 14 July 2025; Accepted: 08 October 2025;
Published: 23 October 2025.
Edited by:
Stephan Meckel, University of Freiburg Medical Center, GermanyReviewed by:
Dongwei Sun, University of California, Riverside, United StatesSharon Yeatts, Medical University of South Carolina, United States
Copyright © 2025 Yu, Lin, Debora, Lin, Lin, Lin, Xu, Zheng, Zhou, Yao, Zheng, Huang, Xia, Yang and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nengzhi Xia, d3l5eWZza3huekAxNjMuY29t
†These authors share first authorship
 Mengying Yu
Mengying Yu Jie Lin2†
Jie Lin2† Asta Debora
Asta Debora Mengqi Lin
Mengqi Lin Haoli Xu
Haoli Xu Yingbao Huang
Yingbao Huang Yunjun Yang
Yunjun Yang Nengzhi Xia
Nengzhi Xia