Abstract
Introduction:
Despite various recommended treatments, no effective therapy has been established for the satisfactory rehabilitation of subacute and chronic debilitating facial palsy. To address this, we evaluated the safety and efficacy of a novel therapeutic approach that combines repeated differential facial nerve blocks with facial nerve stimulation using a hypodermic needle.
Methods:
We retrospectively reviewed 47 patients (acute, n = 4; subacute, n = 3; chronic, n = 40) who were treated at a private pain clinic between January 2017 and December 2023. Patients with persistent facial palsy who were unresponsive to conventional therapies underwent repeated sessions of bilateral facial nerve block following hypodermic needle stimulation of the facial nerves and branches. Facial function was assessed using the House–Brackmann and Sunnybrook grading systems.
Results:
More than 85% of patients showed significant improvements in facial symmetry and function. In the chronic group, Sunnybrook scores improved from 42 to 78 (P < 0.01), and House-Brackmann grades improved from IV–V to I–II. In the acute and subacute groups, both grading scores showed significant improvement. Transient bruising was noted as a minor adverse event, and the clinical improvement remained stable after treatment.
Conclusions:
Our novel integrative treatment is demonstrated to be a safe and effective option for treating intractable subacute and chronic facial palsy.
1 Introduction
Bell's palsy, characterized by sudden unilateral facial nerve paresis or paralysis, predominantly has an idiopathic etiology. Several studies have identified herpes simplex virus infection as the principal causative factor of its pathogenesis (1–4). The incidence of Bell's palsy exhibits international variability, but typically ranges from 10 to 40.2 cases per 100,000 individuals annually (1, 2). Facial palsy is typically classified by lesion location (central or peripheral) (5) and temporally as acute (minutes to days), subacute (days to weeks), or chronic (lasting beyond several weeks), although precise definitions are lacking (5, 6).
Although generally self-limiting, approximately 16–30% of patients develop chronic and debilitating sequelae, including persistent paresis, synkinesis (involuntary movements concurrent with voluntary movements), hyperkinesis, oral dysfunction during eating and drinking, speech articulation challenges, facial pain, abnormal lacrimation, contracture, and decreased quality of life (1, 7, 8).
Current therapeutic approaches for Bell's palsy include corticosteroids, antivirals, combination therapy of corticosteroids and antivirals, physiotherapy, botulinum toxin injections, electrostimulation, acupuncture with or without thread lifting, surgical intervention, and photobiomodulation (2, 6, 9).
Nonetheless, major international guidelines, including those from the American Academy of Neurology (10), American Academy of Otolaryngology-Head and Neck Surgery (11), and Canadian medical authorities (1), strongly recommend the administration of oral corticosteroids (prednisone, 50–60 mg per day for 5 days followed by a 5-day taper) within 72 h as the first-line treatment for Bell's palsy (1, 10–13). High-dose corticosteroids (80–200 mg) have also shown favorable outcomes in recent studies (14, 15). Despite various proposed treatments and guidelines for complex facial palsy, no conclusive medical remedy has been established for subacute and chronic palsy (16).
Several studies have indicated that interventions such as botulinum toxin injections with or without thread lifting and selective surgical procedures can mitigate the long-term disability associated with chronic facial palsy (16–21). However, concerns remain regarding their efficacy, safety, and accessibility (1, 10, 11). Treatment guidelines offer no clear recommendations for managing chronic facial palsy, underscoring the need for individualized, multidisciplinary, and multimodal treatments to achieve safe and satisfactory outcomes (16).
Given the heterogeneous progression and multifactorial nature of facial palsy, there is a growing interest in integrative treatments that combine nerve stimulation, modulation, and structural support for optimal personalized results (16). However, evidence-based, comprehensive strategies that effectively address the diverse mechanisms of subacute and chronic facial palsy, including neuromuscular dysfunction and myofascial complications, remain insufficient.
Therefore, we developed a novel integrative treatment method that combined repeated differential facial nerve blocks with hypodermic needle-based stimulation of the facial nerve and its branches (sometimes corresponding to acupoints). This method also incorporates targeted myofascial traction to address fibrosis and contracture without relying on electrical or manual stimulation.
Our approach is based on the hypothesis that repeated facial nerve stimulation and an asymmetrical differential nerve block, which delivers greater anesthetic volume to the unaffected side than to the affected side, may transiently rebalance afferent input to the brain, thereby facilitating neuroplasticity in both the facial nerve and motor cortex. This non-electrical multimodal strategy aims to improve clinical outcomes by enhancing neuromodulation via peripheral and central mechanisms. This method also aims to alleviate myofascial fibrosis and contracture by incorporating targeted facial traction techniques into the injection and stimulation processes.
This retrospective study aimed to evaluate the efficacy, safety, and potential clinical benefits of this integrative treatment method in patients with acute, subacute, and chronic facial palsy who were unresponsive to standard therapies.
2 Materials and methods
This study was approved by the Wiltse Memorial Hospital's Joint Research Ethics Committee Institutional Review Board (2024-W13), was granted a waiver of written informed consent owing to its retrospective design, and adhered to the ethical standards of the Declaration of Helsinki.
Facial palsy was categorized into acute ( ≤ 3 wk), subacute (3–6 wk), and chronic (>6 wk), according to symptom duration. We reviewed the records of 47 patients treated at Sirh's Private Pain Clinic in Seoul from January 2, 2017, to December 31, 2023. Among them, 44 patients (acute, n = 3; subacute, n = 3; chronic, n = 38) met the inclusion criteria and completed the treatment. Three patients were excluded because they dropped out or met the exclusion criteria (Figure 1).
Figure 1
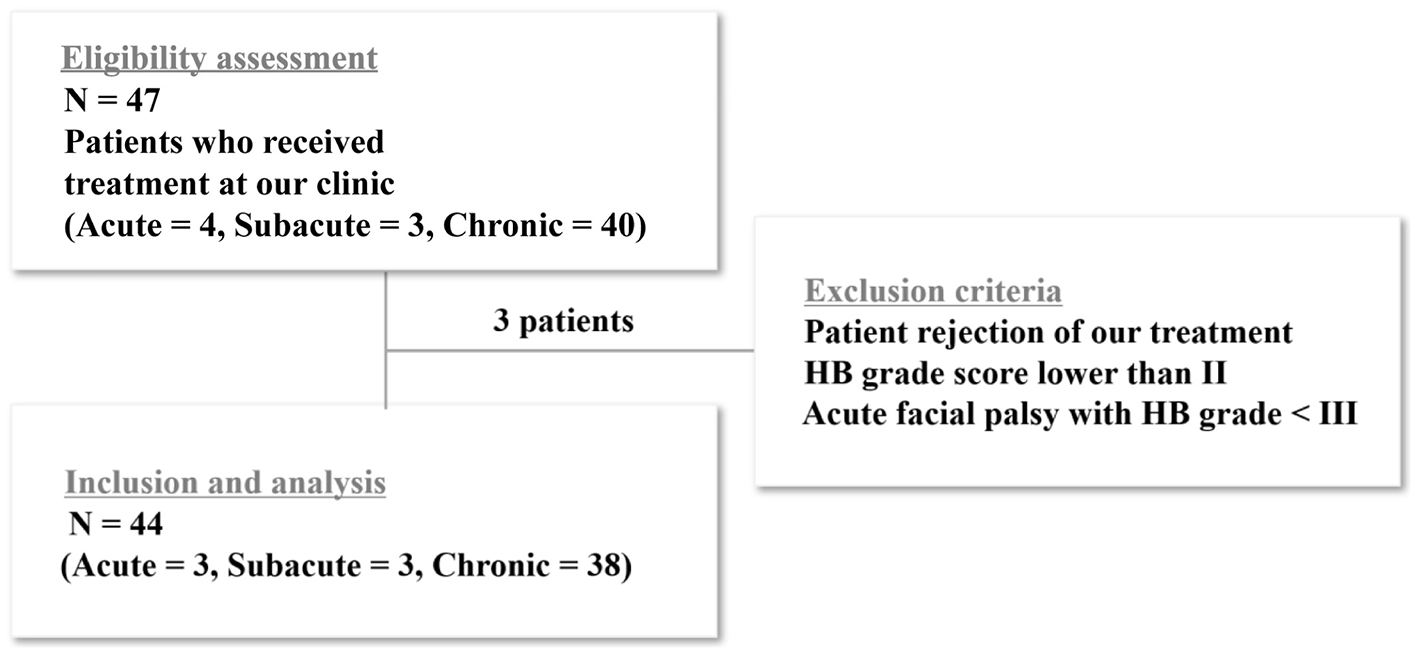
Flow chart of patient enrollment. Among the enrolled patients, 47 met the inclusion criteria and completed the study. Three patients were excluded because they either dropped out of the study or met the exclusion criteria. For the statistical analysis, the acute and subacute groups were combined and compared with the chronic group.
2.1 Inclusion and exclusion criteria
The inclusion criteria were as follows:
1. acute, subacute, and chronic facial palsy or paralysis;
2. consent to undergo integrative treatment;
3. persistent or recurrent symptoms;
4. no satisfactory response to standard therapies including corticosteroids, botulinum toxin, acupuncture, or selective surgical procedures;
5. either unilateral or bilateral presentation.
The exclusion criteria were as follows:
1. Patients who prematurely stopped treatment for any reason;
2. Patients with House–Brackmann (HB) score ≤ II or acute-phase facial palsy with HB grade < III. These cases were excluded because they generally demonstrate favorable outcomes with oral corticosteroids and are often managed during the early stages with acupuncture in Korea;
3. Patients in the acute stage suspected of central facial palsy who present with facial weakness sparing the forehead.
These patients were excluded because of minimal symptom-related distress and limited willingness to undergo our treatment protocol, which is often attributed to a low perceived need for intervention or concerns regarding treatment-related costs and time commitment.
2.2 Procedure
2.2.1 Newly designed 30G, 1.9–3.8 cm thin hypodermic needles
Innovative 30G hypodermic needles (1.9–3.8 cm in length) with transparent plastic hubs and caps (Figure 2) were used in the management of acute, subacute, and chronic intractable facial palsy, as well as associated tinnitus, motor-sensory dysfunction, and pain syndromes refractory to conventional medical, surgical, traditional acupuncture, or neuromodulatory approaches.
Figure 2
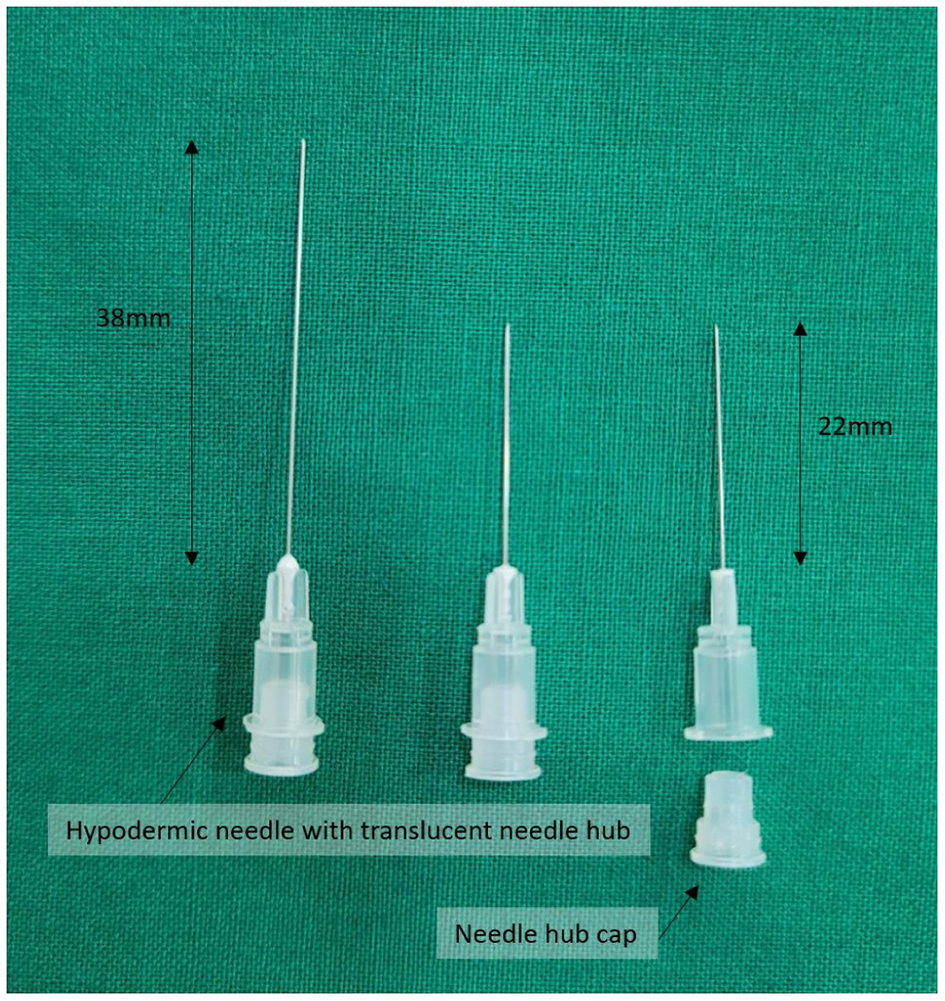
Innovative 30G hypodermic needles (length: 1.9–3.8 cm) with transparent plastic hubs and caps, designed to enhance procedural safety by enabling visual confirmation of blood or cerebrospinal fluid during needle placement. The device was developed by Dr. Hun Man Sirh and has been officially registered as a utility model with the Korean Intellectual Property Office (Registration No. 0399639).
These needles are designed to reduce the risk of inadvertent nerve or vascular injury during needle placement or anesthetic administration by allowing direct visual confirmation of blood or cerebrospinal fluid, thereby eliminating the need for aspiration.
The needle design was officially registered with the Korean Intellectual Property Office as a utility model (Registration No. 0399639) and was originally developed by Dr. Hun Man Sirh.
2.2.2 Facial nerve approach
The entry site was the area where the skin was indented upon finger palpation (usually using the fifth fingertip) between the posterior border of the mandibular ramus and the anterior border of the mastoid process, above the lowest point of the earlobe (Figure 3).
Figure 3
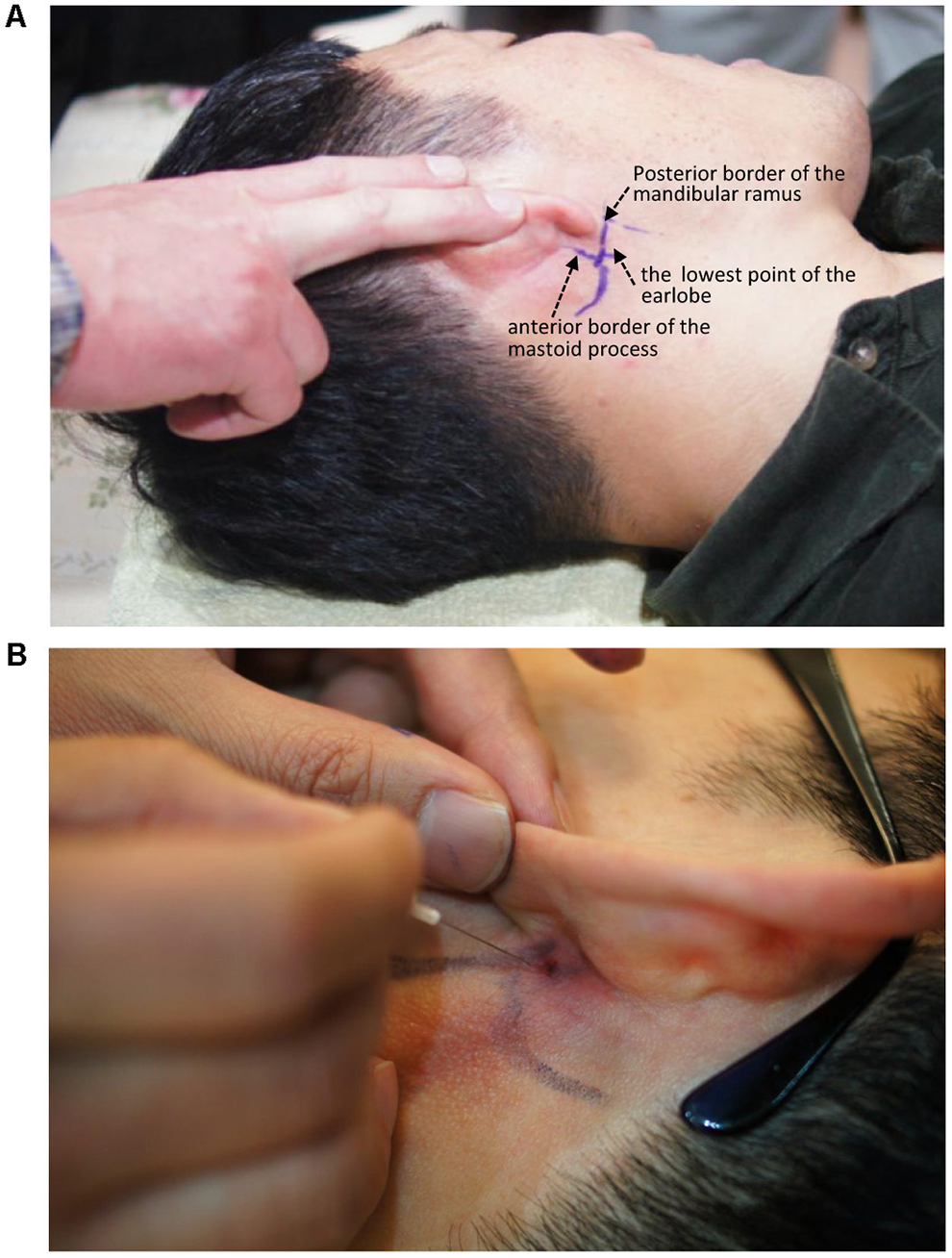
Needle entry point for administering a facial nerve block. (A) Entry point. (B) Needling direction.
2.2.3 Integrative treatment method and course
All patients who met the inclusion criteria began treatment immediately after providing their informed consent. Each session used a custom 30G hypodermic needle (1.9–3.8 cm) with a transparent plastic hub. The needle was inserted bilaterally near the stylomastoid foramen to target the main trunk of the facial nerve and into the affected facial regions to target symptomatic peripheral branches (Figure 4A).
Figure 4
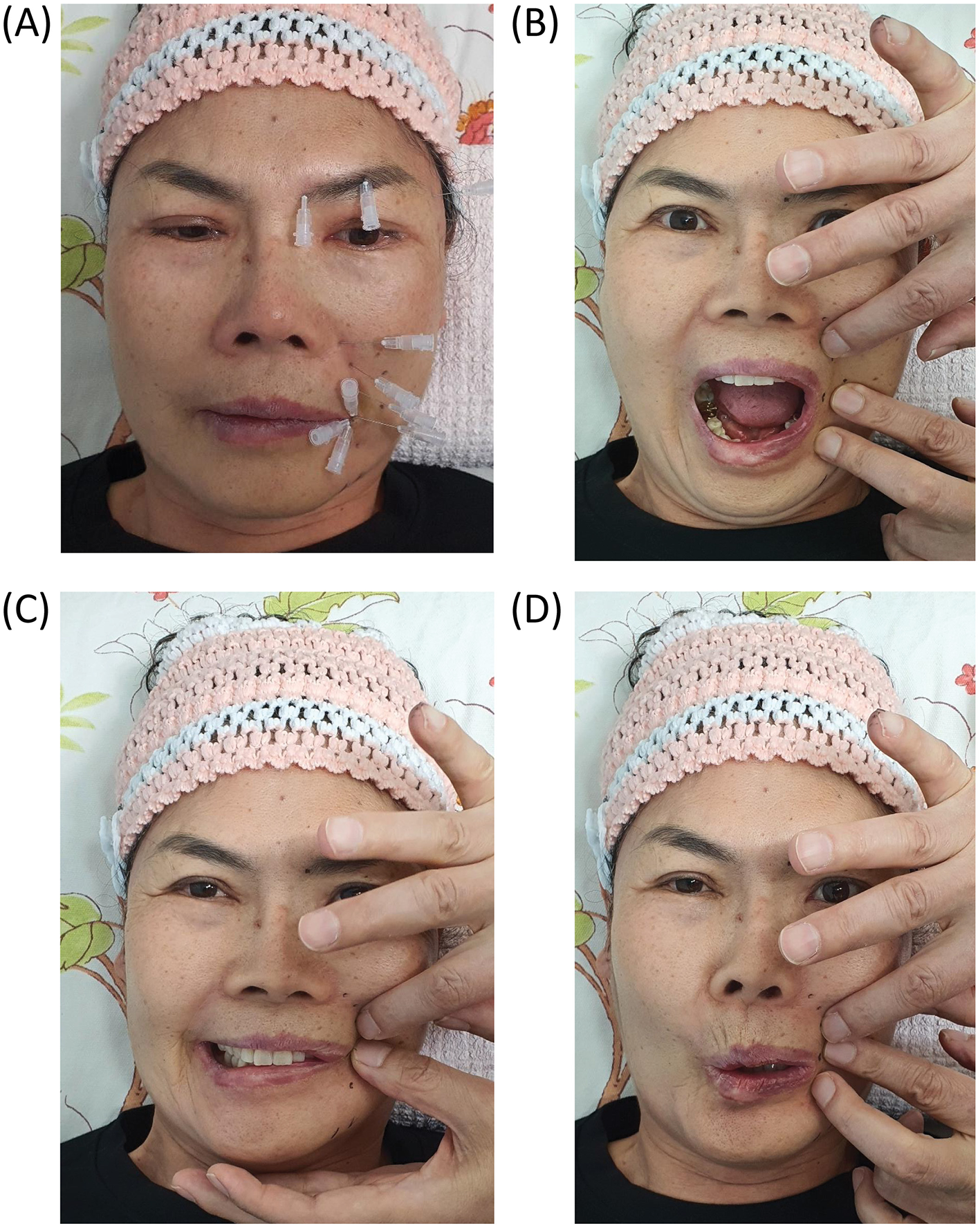
Representative clinical photographs showing key components of the integrative treatment protocol for subacute and chronic facial palsy. (A) Hypodermic needle insertion targeting multiple peripheral branches of the facial nerve on the affected hemiface. Needle placement was guided by palpation and anatomical landmarks to deliver localized mechanical stimulation and pharmacologic modulation. (B–D) Dynamic assessment of facial symmetry during phoneme articulation (“A,” “E,” and “O”) using finger traction maneuvers to determine the most symmetrical bilateral entry points for intervention. Patient consent was obtained for clinical photography and publication.
The therapeutic regimen was tailored to manage facial muscle hyperfunction and weakness by employing finger traction maneuvers (e.g., lifting, pulling, and multidirectional skin tension) for differential facial nerve block and needle steering. The operator identified the most symmetrical point bilaterally using finger traction to manipulate the facial soft tissue. This site was selected as the entry point for needle insertion, and the needle was advanced horizontally along the fascial plane where the facial nerve typically resides (Figures 4B–D).
These techniques are aimed at enhancing facial symmetry, reducing muscular hypercontraction, and attenuating synkinesis. No local anesthesia was employed during the hypodermic needle insertion and stimulation procedures. Mechanical needle stimulation was applied for 20–30 min (acute/subacute) or 30–40 min (chronic). Differential facial nerve block was performed after stimulation. A total of 0.5% lidocaine was administered near the stylomastoid foramen, with 1–2.5 cc injected on the unaffected side and 0.5 cc on the affected side, corresponding to a 1:2 to 1:5 ratio, with higher ratios applied in cases of more severe facial paralysis. In rare cases, a combination of 0.5% lidocaine on the affected side and 1% lidocaine on the unaffected side was administered at a ratio of 1:2 to 1:3. Additional injections of 0.2 to 0.3 cc of 0.5% lidocaine were administered directly to the peripheral branches of the facial nerve on the affected side.
These blocks were conducted after a 20–40-min period of needle-induced nerve stimulation and myofascial traction using a hypodermic needle, which paralleled the initial finger traction maneuver.
Our approach sought to temporarily equalize the afferent nerve signals from both sides of the face. Our differential nerve block involved adjusting the dosage or concentration of the anesthetic injection according to the severity of facial paralysis, as well as the desired duration of temporary paralysis on the unaffected side. The overall procedure typically lasted 50–60 min.
To optimize patient comfort during and after the therapeutic procedure, we employed a carefully controlled method to temporarily suppress normal nerve function on the healthy side for a specified duration (typically 10–30 min). Treatment was initially administered two to three times per week. The frequency and total number of sessions were individualized according to clinical response, particularly improvements in HB and Sunnybrook facial grading system (SFGS) scores, with adjustments ranging from one to three times weekly and a total of approximately 10 to 20 sessions, rather than being based on a fixed protocol.
We describe a representative case for illustrative purposes. A 64-year-old woman with no prior medical history experienced a sudden-onset facial palsy on July 23, 2022. She was immediately admitted to a traditional medicine hospital where she received daily acupuncture and massage; however, her symptoms progressively worsened. Subsequently, she was transferred to a university hospital where she underwent high-dose corticosteroid therapy for 3 wk. Brain computed tomography and magnetic resonance imaging (MRI) revealed unremarkable findings. However, electroneurography revealed degeneration of approximately 63% of facial nerves. Despite continued acupuncture and massage therapy during hospitalization, the patient showed minimal clinical improvement. She presented to Sirh's Clinic on October 4, 2022, approximately 10 wk after symptom onset, with HB grade V facial palsy. After 15 sessions of integrative treatment, substantial functional improvement was achieved. Thereafter, the treatment frequency was gradually tapered to once or twice per week. Repetitive sessions were continued until the patient reached a satisfactory level of recovery, at which point treatment was concluded (Figure 5).
Figure 5

Representative facial expressions before and after integrative treatment in a 64-year-old woman with chronic facial palsy. Frontal photographs were taken at the initial visit (top row) and after 15–20 treatment sessions (bottom row) administered 2–3 times per week at Sirh's Clinic. The images demonstrate a notable improvement in facial symmetry and muscle activation. Expressions include the neutral position, phonation of “A,” “E,” “O,” “U,” eye closed, and forehead elevation. The patient initially presented with House–Brackmann grade V facial palsy approximately 10 wk after onset.
2.3 Outcome measures
To assess facial motor recovery, we used the HB and SFGS scores (Supplementary Figure 1) as outcome measures to assess the efficacy of our treatment before treatment initiation and at the final treatment session (22). These two scales are frequently used to evaluate facial nerve function, with the SFGS scale offering higher sensitivity to treatment-induced changes. According to Kanerva et al. (23), HB scores can be approximately converted to SFGS scores as follows: HB I ≈ SFGS 100, HB II ≈ SFGS 70–99, HB III ≈ SFGS 43–69, HB IV ≈ SFGS 26–42, HB V ≈ SFGS 13–25, and HB VI ≈ SFGS 0–12. This conversion allowed us to capture subtle improvements more precisely during post-treatment evaluation. In this study, unsatisfactory outcomes were defined as HB scores >3 or equivalent and SFGS scores below a predetermined threshold of 80.
The primary outcome was the degree of functional recovery evaluated by changes in the HB and SFGS scores between baseline and the final session (3–12 mo).
The secondary outcomes included motor synkinesis, autonomic dysfunction (lacrimation and salivation), and muscular contraction at the end of the study.
2.4 Statistical analysis
This study aimed to assess the therapeutic outcomes in patients with acute, subacute, and chronic facial palsy, and to discern the differential effects based on the duration of symptoms. Changes in the HB and SFGS scores were compared before and at the end of treatment. Owing to the small number of patients who presented within 6 wk of onset, the acute and subacute groups were combined into a single group for statistical analysis and compared with the chronic group. This limited sample size is primarily attributable to the fact that patients in the acute or subacute phase often experience spontaneous recovery within 1 month and are more likely to meet the exclusion criteria than those in the chronic phase.
Multiple regression analysis was conducted to identify variables that influenced treatment outcomes. The independent variables included age, sex, number of treatment sessions, disease duration (months), affected side (left or right), and steroid treatment history. Statistical analyses were performed using Stata software (version 17.0; StataCorp LLC, College Station, TX, USA). Descriptive analysis, paired sample t-tests, and multiple regression analysis were employed to detect significant differences in the HB and SFGS scores between the two groups. Statistical significance was set at P < 0.05. Because this was a retrospective study without a control group, treatment outcomes were evaluated using within-subject pre- and post-treatment comparisons. We chose not to employ traditional nerve blocks, high-dose steroid therapy, combined corticosteroid-antiviral regimens, or acupuncture as a control owing to ethical considerations and their limited evidence of efficacy in treating subacute or chronic facial palsy, as indicated by the current literature guidelines (13).
3 Results
3.1 Clinical outcomes
Satisfactory recovery was defined as an HB score of II or lower and an SFGS score exceeding 80 at treatment completion. All patients (n = 44) met the criteria for satisfactory recovery by the final session.
Minor adverse effects have been reported in a small number of patients. Two patients experienced transient injection-site bruising that resolved spontaneously within 48 h. No cases of nausea, dyspepsia, constipation, or allergic reactions were reported.
Secondary outcome improvements included the resolution or significant reduction in motor synkinesis, lacrimation, and facial tightness. These symptoms were observed in 27 of 38 chronic cases at baseline and improved in 21 cases after treatment. All patients with preexisting myofascial stiffness reported subjective improvements in facial relaxation and symmetry.
3.2 Demographic characteristics
Demographic data, baseline HB and SFGS scores, and clinical characteristics of the patients are summarized in Tables 1, 2. Patients were grouped based on symptom duration: the acute/subacute group (n = 6, ≤ 6 wk) and the chronic group (n = 38, >6 wk).
Table 1
| Age/Sex Duration1, laterality | HBS before treatment | HBS after treatment | SFGS score before treatment | SFGS score after treatment | Total number of treatments | Medical history | Co-morbid symptoms | Treatment history | History of steroid use | Degree of improvement at treatment initiation compared to the peak severity of palsy (subjective description) |
|---|---|---|---|---|---|---|---|---|---|---|
| 56/F 1 wk, Rt. | 3 | 1 | 61 | 100 | 15 | Bilateral facial tremor, ear fullness | Medication | O | No improvement | |
| 38/M 6 wk, Rt. | 6 | 2 | 11 | 96 | 16 | Herpes zoster, 4 d prior R/O Ramsay-Hunt syndrome |
Sudden deafness (90% recovered) | Medication | O | 30% improvement |
| 60/M 4 wk, Lt. | 4 | 2 | 37 | 96 | 6 | Hypertension | Medication, acupuncture | O | N-C | |
| 47/F 6 wk, Lt. | 3 | 1 | 76 | 100 | 6 | Medication, manual therapy | O | 80% improvement | ||
| 27/M 10 d, Lt. | 3 | 1 | 35 | 100 | 13 | Medication | O | No improvement | ||
| 55/F 11 d, Rt. | 3 | 1 | 67 | 100 | 17 | Eye irritation and tearing, rash around the ear. Complete resolution of tearing after 9 treatments. | Medication | O | No improvement |
Patients with acute and subacute facial palsy: demographic data, House–Brackmann and Sunnybrook Facial Grading System scores, and clinical findings.
HBS, House–Brackmann score; SFGS, Sunnybrook Facial Grading System score; N-C, not-checked; R/O, rule out.
1The treatment duration was computed starting from the first day of their visit to our clinic.
Table 2
| Age/Sex Duration1, laterality | HBS before treatment | HBS after treatment | SFGS before treatment | SFGS after treatment | Total number of treatments | Medical history | Co-morbid symptoms | Treatment history | History of steroid use | Degree of improvement at treatment initiation compared to the peak severity of palsy (subjective description) |
|---|---|---|---|---|---|---|---|---|---|---|
| 65/M 3 mo, Lt. | 5 | 2 | 13 | 92 | 30 | Liver cirrhosis | Acupuncture | N-C | ||
| 59/F 4 y, Rt. | 4 | 2 | 31 | 92 | 10 | Otalgia | Medication, acupuncture, Botox injection | O | 50% improvement after acupuncture, Botox treatment | |
| 36/F 5 y, Rt. | 3 | 1 | 59 | 100 | 20 | Facial tremor | Acupuncture | N-C | ||
| 49/F 10 y, Lt. | 4 | 2 | 31 | 96 | 12 | Acupuncture | 50% improvement after acupuncture | |||
| 60/F 8 y, Rt. | 4 | 2 | 22 | 90 | 12 | Dizziness, fibrotic change of facial muscles | Medication, Botox, decompressive craniotomy operation, ptosis operation | O | N-C | |
| 66/F 1.5 y, Lt. |
4 | 2 | 24 | 86 | 12 | Synkinesis (eye), periorbital pain | Medication, acupuncture | 60% improvement | ||
| 65/F 2.5 y, Lt. |
3 | 2 | 56 | 91 | 19 | Synkinesis, tearing | Acupuncture, stem cell therapy | O | 50% improvement | |
| 70/M 1.5 y, Rt. |
4 | 2 | 22 | 99 | 17 | Medication, acupuncture | O | N-C | ||
| 64/M 8 y, Lt. | 4 | 2 | 22 | 91 | 3 | Medication | N-C | |||
| 56/M 6 mo, Rt. | 4 | 2 | 26 | 90 | 12 | Herpes zoster, 10 d prior, R/O Ramsay-Hunt Syndrome | Medication | O | N-C | |
| 43/M 60 mo, Rt. | 3 | 2 | 55 | 95 | 17 | Right side facial spasm, synkinesis, ptosis | Medication, acupuncture | O | N-C | |
| 43/M 3 y, Rt. | 3 | 2 | 43 | 96 | 13 | Hypesthesia on the right V2 area and neck, Fibrotic change of facial muscles Complete recovery of sensation after treatment | Acupuncture | N-C | ||
| 58/F 1.8 y, Rt. |
3 | 2 | 57 | 96 | 3 | Medication, acupuncture | O | N-C | ||
| 43/M 3 mo, Lt. | 3 | 1 | 69 | 100 | 9 | Left-sided facial spasm | Medication, acupuncture | N-C | ||
| 53/M 5 y, Lt. | 3 | 2 | 56 | 95 | 15 | Microvascular decompression operation, 2 y prior | Left side facial spasm, ptosis (aggravated with walking) | Medication | O | N-C |
| 46/M 3 y, Rt. | 3 | 1 | 57 | 100 | 4 | Tearing and rhinorrhea during eating | Acupuncture, physical therapy, manual therapy | 50% improvement | ||
| 30/F 2 y, Rt. | 3 | 1 | 77 | 100 | 15 | Zygoma fracture operation, 2 y prior | Synkinesis, otalgia | Acupuncture | No improvement | |
| 32/M 4 mo, Lt. | 4 | 2 | 29 | 91 | 6 | Lt. facial palsy at 13-year-old |
Acupuncture | 50% improvement | ||
| 55/M 2 y, Rt. | 4 | 2 | 35 | 86 | 7 | Craniotomy, vestibular schwannoma operation | Tearing while eating | Acupuncture, physical therapy, manual therapy | O | 30% improvement |
| 50/M 10 y, Rt. | 5 | 2 | 17 | 70 | 35 | Synkinesis, fibrotic change of facial muscles | Medication, acupuncture | O | N-C | |
| 60/F 3 y, Lt. | 4 | 2 | 27 | 86 | 10 | Left-sided facial spasm, facial pain | Medication, acupuncture | 50% improvement | ||
| 45/M 2 y, Rt. | 4 | 1 | 36 | 100 | 8 | Left-sided tinnitus | Medication | N/C | ||
| 58/M 8 y, Lt. | 4 | 2 | 33 | 86 | 10 | Drooling, synkinesis | Medication, ptosis operation | O | N-C | |
| 69/M 4 mo, Lt. | 5 | 2 | 17 | 91 | 23 | Fibrotic change of facial muscles | Medication, acupuncture | O | 30% improvement | |
| 48/M 10 mo, Lt. | 3 | 2 | 44 | 95 | 8 | Acupuncture | 60% improvement | |||
| 74/F 1.8 y, Rt. |
3 | 2 | 46 | 96 | 7 | Vestibular neuritis | Dizziness | Medication, rehabilitation training | O | 50% improvement |
| 50/M 4 y, Lt. | 3 | 1 | 49 | 100 | 7 | Vestibular schwannoma, Lt. | Left-sided deafness, facial tremor, synkinesis | Medication, tumor removal operation, Botox injection | O | 40% improvement |
| 57/M 3 y, Rt. | 4 | 2 | 39 | 82 | 30 | Herpes zoster | Facial tremor, bilateral hearing difficulty, fibrotic change of facial muscles | Medication | O | 60% improvement |
| 43/F 2 y, Rt. | 3 | 1 | 48 | 100 | 20 | Synkinesis, hypesthesia of lips | Medication | O | 70% improvement | |
| 47/M 10 y, Lt. | 3 | 2 | 42 | 95 | 15 | Acupuncture | 30% improvement | |||
| 18/M 4 mo, Rt. | 3 | 1 | 63 | 100 | 6 | Medication, acupuncture | O | 50% improvement | ||
| 38/M 8 mo, Rt. | 4 | 2 | 40 | 90 | 58 | Tearing, tongue numbness, Synkinesis 98% degeneration ratio on ENoG | Medication, stellate ganglion block, physiotherapy | O | N-C | |
| 65/M 9 mo, Lt. | 3 | 1 | 75 | 100 | 15 | Lt. facial palsy, 30 y prior | Pain on posterior auricular area, left side facial numbness | Medication | O | 30% improvement |
| 42/F 2 y, Rt. | 4 | 1 | 36 | 100 | 7 | Masticatory discomfort, Eye irritation | Medication | O | N-C | |
| 41/M 1 y, Rt. | 3 | 1 | 74 | 100 | 16 | Synkinesis (complete recovery after the 7th treatment) | Medication, acupuncture | O | N-C | |
| 24/M 9 mo, Lt. | 3 | 1 | 44 | 100 | 18 | Synkinesis (complete recovery after the 8th treatment), masticatory discomfort, tearing, dysesthesia on the V2 area | Medication, Botox injection (Masseter muscle) | O | N-C | |
| 64/F 2.5 mo, Lt. |
5 | 2 | 17 | 88 | 30 | Synkinesis, facial discomfort 63% degeneration ratio on ENoG | Medication, acupuncture, manual therapy | O | N-C | |
| 36/M 2 mo, Lt. | 3 | 1 | 45 | 100 | 22 | Altered taste sensation, facial discomfort 95% degeneration ratio on ENoG | Medication, acupuncture, physiotherapy | O | N-C |
Patients with chronic facial palsy: demographic data, House–Brackmann and Sunnybrook Facial Grading System scores, and clinical findings.
HBS, House–Brackmann score; SFGS, Sunnybrook Facial Grading System score; ENoG, electroneurography; N-C, Not checked.
1The treatment duration was computed starting from the first day of their visit to our clinic.
The mean age of the participants was 50.1 y (SD = 13.3; range: 18–74). The study cohort consisted of 28 male and 16 female patients. Facial palsy affected the left and right sides in 21 and 23 patients, respectively. The mean number of treatment sessions was 14.9 (SD = 10.1; range: 3–58). The average duration of facial palsy at baseline was 31.3 mo (SD = 35.5; range: 0.25–120 mo).
3.3 Treatment effects
The treatment outcomes are shown in Tables 1, 2. In the acute/subacute group, the mean HB score improved from 3.67 to 1.34 (P < 0.001), and the SFGS score increased from 47.83 to 98.67 (P < 0.001). In the chronic group, the HB score improved from 3.53 to 1.66 (P < 0.001), and the SFGS grading system score increased from 41.47 to 93.82 (P < 0.001). There was no statistically significant difference between the groups in the degree of change from pre- to post-treatment in either the HB or SFGS scores.
None of the patients required a surgical intervention. After treatment, in the acute/subacute group, four patients achieved complete recovery with an HB score of I, and two patients achieved an HB score of II. In the chronic group, 13 patients achieved HB score I, and 25 patients recovered to HB score II.
3.4 Regression analysis
Multiple linear regression analysis was performed to investigate predictors of treatment outcomes, with the final HB and SFGS scores as dependent variables. The analysis examined the effects of age, sex, number of treatment sessions, disease duration (months), laterality of symptoms (left/right), history of previous steroid use, past medical history, and presence of accompanying symptoms (e.g., synkinesis and contracture). Table 3 presents the results of the multiple regression analyses.
Table 3
| Model | (1) Dependent variable: Sunnybrook facial grading scores (SFGS) | (2) Dependent variable: House–Brackmann scores (HB) |
|---|---|---|
| Age | −0.177 (−2.56)** | 0.020 (3.90)*** |
| Sex | 1.583 (0.84) | −0.152 (−1.11) |
| Number of treatment sessions | −0.231 (−2.67)** | 0.011 (1.80)* |
| Duration of facial palsy (mo) | −0.068 (−2.75)*** | 0.004 (2.04)** |
| Laterality of symptom | −0.170 (−0.09) | −0.069 (−0.53) |
| History of steroid usage | 0.012 (0.01) | −0.041 (−0.30) |
| Past medical history (present or absent) | −0.624 (−0.33) | 0.021 (0.15) |
| Accompanying symptoms | −0.547 (−0.27) | −0.295 (−2.00)* |
| Cons | 107.441 (24.53)*** | 0.816 (2.55)** |
| F-stats | 3.33*** | 4.18*** |
| Adjusted R2 | 0.303 | 0.372 |
| N | 44 | 44 |
Multiple regression results.
Corresponding t-statistics are reported in parentheses. ***, **, and * represent significance at 1%, 5%, and 10% levels, respectively.
All models were statistically significant (F-statistics, P < 0.01) with adjusted R2 values of 0.303 (SFGS score) and 0.372 (HB score), indicating that the models adequately explained a substantial portion of the variance in treatment outcomes. Multicollinearity was not detected (Variance Inflation Factor < 2 for all variables).
Significant predictors were as follows:
-
Age: Older age was associated with poorer SFGS scores (β = −0.177, P < 0.05).
-
Number of treatment sessions: More sessions were associated with lower SFGS scores (β = −0.231, P < 0.05), indicating slower recovery in these patients.
-
Duration of facial palsy (months): Longer disease duration negatively affected both SFGS (β = −0.068, P < 0.01) and HB scores (β = 0.004, P < 0.05).
-
Accompanying symptoms: Presence of synkinesis or other symptoms correlated with higher HB scores (β = −0.295, P < 0.05).
These findings underscore the importance of early intervention and suggest that age and chronicity are critical prognostic factors.
4 Discussion
This retrospective analysis demonstrated that our integrative treatment, which combined hypodermic needle-induced facial nerve stimulation with modified bilateral facial nerve blocks, resulted in substantial functional recovery in patients with acute/subacute and chronic facial palsy who were unresponsive to conventional medical, surgical, or acupuncture-based interventions. Most patients in the cohort achieved satisfactory recovery, supported by statistically significant improvements in both HB and SFGS scores. Regression analysis further identified younger age, shorter disease duration, and fewer required treatment sessions as favorable prognostic indicators, underscoring the importance of early intervention following onset; treatment-resistant facial nerve maladaptive neuroplasticity generally emerges 1.5–2 mo or more after onset (24–27). These findings emphasize that early interventions are critical for optimal recovery. Delayed treatment may allow the establishment of maladaptive neuroplasticity and fixed synkinetic or fibrotic changes, which can affect the facial and amygdalo-motor systems, including facial nerve degeneration and psychoemotional pathology. These compensatory processes are particularly likely to emerge beyond the 1.5-mo post-onset window (24–27). Our data support the notion that prompt initiation of neuromodulatory therapy before the entrenchment of chronic patterns may improve prognosis and reduce the need for adjunctive interventions.
Additionally, meaningful improvements were noted in secondary outcomes such as motor synkinesis and myofascial symptoms, even in chronic-stage patients, despite the lack of statistical testing for these variables. Functional recovery is typically achieved after 10–20 treatment sessions, and early responses strongly correlate with long-term outcomes. Notably, even in chronic cases, sustained improvements in facial relaxation and reductions in synkinetic movements have been reported. Transient complete or significant improvements observed in initial treatments often lead to excellent outcomes. However, the optimal minimum number of treatments required remains unknown. These results highlight the need for a comprehensive investigation into advanced therapies, including integrative needle treatments, electrical or magnetic neuromodulation, and neurostimulation.
We hypothesized that our method would promote functional and mechanical rebalance through both peripheral and central mechanisms. The facial nerve consists of both motor (efferent) and sensory (afferent) roots, including the nervus intermedius, which connects to the autonomic (sympathetic via interconnections and parasympathetic) nervous system. Somatic sensory pathways play a crucial role in the treatment of facial palsy (28). By stimulating both the trunk and peripheral branches (related acupoints) of the facial nerve using fine hypodermic needles in a controlled, directional manner, and by adjusting pharmacologic denervation asymmetrically, our treatment appears to modulate afferent input to cortical and subcortical motor regions. This is likely involved in adaptive neuroplastic processes, as observed in other neurorehabilitation models.
We further posit that repeated needle stimulation induces changes within the facial motor nucleus, corticobulbar pathways, amygdalo-motor circuits, supplementary motor areas, and networks involved in both voluntary and emotional facial expressions. These expressions are generated through the coordinated activity of brain regions, including the amygdala and cortical and subcortical motor areas, which integrate sensory and motor signals to modulate facial output (29). This dual modulation may explain not only the improvements in facial symmetry and voluntary motor control but also the normalization of autonomic symptoms such as lacrimation and salivation. Although our clinical findings suggest improvements in both voluntary and autonomic facial functions, these effects may reflect a broader modulation of facial affect circuits. However, in the absence of direct neurophysiological or imaging evidence, the proposed central mechanisms remain unclear and require further investigations. Furthermore, we hypothesized that needle-guided mechanical stimulation, when applied along the anatomical fascial plane and aligned with the physiological trajectory of the nerve, could reduce aberrant axonal misdirection. In chronic facial palsy with synkinesis, transversely aligned needling may serve as a nondestructive strategy to facilitate accurate axonal reinnervation and prevent maladaptive sprouting. We observed repeated clinical improvements in facial synkinesis cases with fascia-aligned needle retention, potentially mediated by Schwann cell orientation and regeneration guidance (30).
Although various treatments exist for Bell's palsy, current clinical guidelines predominantly recommend high-dose corticosteroids, either alone or in combination with antiviral agents, as the first-line therapy for Bell's palsy (1, 10, 11). However, no consensus exists for treatment beyond the acute phase. Although botulinum toxin, electrostimulation, acupuncture, and selective surgery have been explored, their efficacy, access, and safety remain limited. Studies on electro-acupuncture and manual acupuncture suggest mixed efficacies, and high-quality trials are lacking (31–39). Furthermore, these techniques rarely account for the myofascial or fascial adhesion components in chronic dysfunction.
Conversely, evidence from vagus nerve stimulation (40–44) and contralateral botulinum toxin studies (29, 45) shows that asymmetric modulation of afferent input can enhance cortical adaptive plasticity and recovery. Vagus nerve stimulation-dependent plasticity in the corticospinal, corticorubral, and propriospinal networks likely drives functional recovery improvements (40–44). Similarly, we believe that our treatment, with or without facial motor training, operates through a comparable mechanism, with the added advantages of anatomical precision and non-electrical application. Therefore, it may serve as a safer and more targeted alternative for modulating facial motor plasticity.
Unlike conventional methods, our approach is purely mechanical and pharmacological, and avoids electrical or thermal nerve injury. It incorporates several novel features: (1) bilateral, asymmetrical differential nerve blocks; (2) needle-based peripheral nerve stimulation without electrical current; (3) dynamic myofascial traction and tissue steering guided by palpation; and (4) targeting of both motor and autonomic fibers of the facial nerve. This method was designed to be safe, even for patients on anticoagulants, resulting in minimal complications. Several cases of mild complications have been reported. Some patients experienced transient discomfort during mouth opening, likely owing to a temporary muscle imbalance following a facial nerve block near the stylomastoid foramen. Post-injection pain lasting 1–2 days, along with minimal bleeding or bruising at the needle insertion site, was also observed. To minimize such complications, we consistently used 0.5% lidocaine instead of higher concentrations or long-acting anesthetics.
Myofascial traction, a key component of our protocol, may relieve fascial restriction, reduce synkinesis, and allow more precise needle steering into appropriate nerve planes. This combination of mechanical, pharmacological, and neurofunctional targeting sets our technique apart from existing modalities.
Despite these promising results, further validation is required. Large-scale randomized controlled trials are needed to compare the efficacies of botulinum toxin, electrostimulation, and manual acupuncture. Our treatment approach, which avoids electrical or mechanical damage to the cranial nerves, notably enhances the effects of therapeutic stimulation on the peripheral and central nervous systems, as well as nerve block in chronic and subacute facial palsy cases. Electrical or manual stimulation near large nerves poses risks. To date, no studies have specifically investigated the effects of innocuous mechanical nerve stimulation using needle placement without electrical or manual intervention. Given that recovery from facial palsy appears to be influenced by the duration of needle placement, the therapeutic mechanism warrants further exploration. These outcomes suggest the need for extensive research on integrative needle treatments. Finally, as this treatment involves both peripheral and central systems, future studies should explore its application in other cranial neuropathies and functional somatic disorders involving autonomic dysregulation.
4.1 Limitations
This study had certain limitations. As this was a retrospective chart review, randomization and blinding were not performed. Most patients have previously experienced suboptimal outcomes from high-dose corticosteroids, botulinum toxin injections, surgery, acupuncture, or a combination of conventional and traditional therapies. Owing to their psychological distress and refusal to accept randomization, conducting controlled trials with a placebo or sham arm is ethically and practically unfeasible.
Neurophysiological tests, such as electromyography and nerve conduction velocity studies, were not conducted, limiting the objective quantification of nerve recovery. Although composite scores from both HB and SFGS have been used, they are semi-subjective and may not fully capture subtle clinical changes. Although an approximate correlation exists between the two, contemporary research suggests the need for a more precise and standardized facial grading system (23).
Imaging, including brain MRI, was not routinely performed in all patients, particularly those without suspected central lesions. Therefore, only a small number of patients with possible brain lesions were included. Although routine imaging is not universally recommended for idiopathic facial palsy (1, 6, 10, 46), the selective use of MRI and cerebrospinal fluid analysis may be important in cases with suspected otogenic or neoplastic etiologies (46, 47).
Another limitation was the relatively small sample size, particularly in the acute and subacute groups, which restricts the generalizability of the statistical conclusions. Additionally, the cost of treatment was the responsibility of the patients, supplemented in part by health insurance. Future research should include larger prospective randomized controlled trials focusing specifically on repeated facial nerve blocks or needle-based facial nerve stimulation. Objective neurophysiological outcome measures and imaging-based validation will help establish the efficacy and mechanisms of this novel integrative approach.
5 Conclusions
Our findings demonstrate that repeated hypodermic needle-guided facial nerve stimulation and differential facial nerve blocks effectively mitigate the persistent sequelae of facial palsy, including facial asymmetry, muscular hypercontraction, synkinesis, and hyperkinesis. This therapeutic strategy represents a novel and promising option for managing subacute and chronic facial palsy, with the potential to guide future research on facial nerve rehabilitation and motor neuron recovery.
Although the subacute group included a limited number of patients, the consistency of improvements across the entire cohort supports the potential clinical utility of this approach. Further studies with larger and more balanced samples are required to validate these findings. Moreover, this strategy could serve as a foundation for future integrative interventions aimed at promoting neurofunctional recovery and neuromuscular rebalancing in complex treatment-resistant facial nerve disorders. Its therapeutic effects may be further enhanced by combining this approach with regenerative techniques such as nerve conduits, stem cell therapy, neurotrophic factor delivery, and other emerging therapeutics.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Wiltse Memorial Hospital's Joint Research Ethics Committee Institutional Review Board (Approval number: 2024-W13). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin due to the retrospective nature of the study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HYM: Formal analysis, Investigation, Methodology, Visualization, Writing – original draft. SWS: Data curation, Project administration, Resources, Writing – review & editing. HMS: Conceptualization, Supervision, Writing – review & editing. SJS: Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to thank Young Sik Kim for their assistance with statistical analyses and interpretation of data. We would like to thank Editage (http://www.editage.co.kr) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. Generative AI (ChatGPT by OpenAI) was used to assist in improving the clarity, grammar, and academic tone of certain passages under the supervision of the author(s). All scientific content, data interpretation, and final decisions were made by the author(s).
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1655894/full#supplementary-material
References
1.
de Almeida JR Guyatt GH Sud S Dorion J Hill MD Kolber MR et al . Management of Bell palsy: clinical practice guideline. CMAJ. (2014) 186:917–22. 10.1503/cmaj.131801
2.
Eviston TJ Croxson GR Kennedy PGE Hadlock T Krishnan AV . Bell's palsy: aetiology, clinical features and multidisciplinary care. J Neurol Neurosurg Psychiatry. (2015) 86:1356–61. 10.1136/jnnp-2014-309563
3.
McCormick DP . Herpes-simplex virus as a cause of Bell's palsy. Lancet. (1972) 1:937–9. 10.1016/S0140-6736(72)91499-7
4.
Kennedy PG . Herpes simplex virus type 1 and Bell's palsy-a current assessment of the controversy. J Neurovirol. (2010) 16:1–5. 10.3109/13550280903552446
5.
Rath B Gidudu JF Anyoti H Bollweg B Caubel P Chen YH et al . Facial nerve palsy including Bell's palsy: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. (2017) 35:1972–83. 10.1016/j.vaccine.2016.05.023
6.
Kim SJ Lee HY . Acute peripheral facial palsy: recent guidelines and a systematic review of the literature. J Korean Med Sci. (2020) 35:e245. 10.3346/jkms.2020.35.e245
7.
Bylund N Hultcrantz M Jonsson L Marsk E . Quality of life in Bell's palsy: correlation with sunnybrook and house-brackmann over time. Laryngoscope. (2021) 131:E612–8. 10.1002/lary.28751
8.
Peitersen E . Bell's palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Oto-Laryngol Suppl. (2002) (549):4–30. 10.1080/000164802760370736
9.
Uysal SC Özden F Özkeskin M . Current physiotherapy approaches in patients with facial palsy. In:BennettGGoodallE, editors. The Palgrave Encyclopedia of Disability. Cham: Springer Nature Switzerland (2024). p. 1–11. 10.1007/978-3-031-40858-8_137-1
10.
Gronseth GS Paduga R . American Academy of Neurology. Evidence-based guideline update: steroids and antivirals for Bell palsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. (2012) 79:2209–13. 10.1212/WNL.0b013e318275978c
11.
Baugh RF Basura GJ Ishii LE Schwartz SR Drumheller CM Burkholder R et al . Clinical practice guideline: Bell's palsy. Otolaryngol Head Neck Surg. (2013). 149(3 Suppl):S1–27. 10.1177/0194599813506835
12.
Dalrymple SN Row JH Gazewood J . Bell palsy: rapid evidence review. Am Fam Physician. (2023) 107:415–20.
13.
Van Haesendonck GV Jorissen C Lammers M Ocak I Menovsky T Ní Dhubhghaill S et al . Guidelines for the initial management of acute facial nerve palsy. B-ENT. (2022) 18:67–72. 10.5152/B-ENT.2022.21770
14.
Gupta KK Balai E Tang HT Ahmed AA Doshi JR . Comparing the use of high dose to standard-dose corticosteroids for the treatment of Bell's palsy in adults-A systematic review and meta-analysis. Otol Neurotol. (2023) 44:310–6. 10.1097/MAO.0000000000003823
15.
Fujiwara T Namekawa M Kuriyama A Tamaki H . High-dose corticosteroids for adult Bell's Palsy: Systematic Review and Meta-analysis. Otol Neurotol. (2019) 40:1101–8. 10.1097/MAO.0000000000002317
16.
Lapidus JB Lu JC Santosa KB Yaeger LH Stoll C Colditz GA et al . Too much or too little? A systematic review of postparetic synkinesis treatment. J Plast Reconstr Aesthet Surg. (2020) 73:443–52. 10.1016/j.bjps.2019.10.006
17.
Choe WJ Kim HD Han BH Kim J . Thread lifting: a minimally invasive surgical technique for long-standing facial paralysis. HNO. (2017) 65:910–5. 10.1007/s00106-017-0367-3
18.
Mehta RP . Surgical treatment of facial paralysis. Clin Exp Orl. (2009) 2:1–5. 10.3342/ceo.2009.2.1.1
19.
Lee H-J Kim J-S Youn K-H Lee J Kim H-J . Ultrasound-guided botulinum neurotoxin Type A injection for correcting asymmetrical smiles. Aesthetic Surg J. (2018) 38:NP130–4. 10.1093/asj/sjy128
20.
Markey JD Loyo M . Latest advances in the management of facial synkinesis. Curr Opin Otolaryngol Head Neck Surg. (2017) 25:265–72. 10.1097/MOO.0000000000000376
21.
Steinhäuser J Volk GF Thielker J Geitner M Kuttenreich AM Klingner CM et al . Multidisciplinary care of patients with facial palsy: treatment of 1220 patients in a German facial nerve center. J Clin Med. (2022) 11:427. 10.3390/jcm11020427
22.
Mat Lazim N Ismail H Abdul Halim S Nik Othman NA Haron A . Comparison of 3 grading systems (House-Brackmann, Sunnybrook, Sydney) for the assessment of facial nerve paralysis and prediction of neural recovery. Medeni Med J. (2023) 38:111–9. 10.4274/MMJ.galenos.2023.42383
23.
Kanerva M Jonsson L Berg T Axelsson S Stjernquist-Desatnik A Engström M et al . Sunnybrook and House-Brackmann systems in 5397 facial gradings. Otolaryngol Head Neck Surg. (2011) 144:570–4. 10.1177/0194599810397497
24.
Song W Dai M Xuan L Cao Z Zhou S Lang C et al . Sensorimotor Cortical Neuroplasticity in the Early Stage of Bell's Palsy. Neural Plast. (2017) 2017:8796239. 10.1155/2017/8796239
25.
Meyers EC Kasliwal N. Solorzano BR Lai E Bendale G Berry A et al . Enhancing plasticity in central networks improves motor and sensory recovery after nerve damage. Nat Commun. (2019) 10:5782. 10.1038/s41467-019-13695-0
26.
Sun J Wang R Chen X Wang J Liu D Sai N et al . Surgical management and the prognosis of iatrogenic facial nerve injury in middle ear surgery: a 20-year experience. Head Face Med. (2023) 19:31. 10.1186/s13005-023-00377-y
27.
Hong CH Volk GF Guntinas-Lichius O . Treatment of acute and chronic facial palsy in children and adolescents: prognostic factors for the outcomes. Int J Pediatr Otorhinolaryngol. (2025) 190:112277. 10.1016/j.ijporl.2025.112277
28.
Sirh SJ Sirh SW Mun HY Sirh HM . Integrative treatment for tinnitus combining repeated facial and auriculotemporal nerve blocks with stimulation of auditory and non-auditory nerves. Front Neurosci. (2022) 16:758575. 10.3389/fnins.2022.758575
29.
Guntinas-Lichius O Glowka TR Angelov DN Irintchev A Neiss WF . Improved functional recovery after facial nerve reconstruction by temporary denervation of the contralateral mimic musculature with botulinum toxin in rats. Neurorehabil Neural Repair. (2011) 25:15–23. 10.1177/1545968310376058
30.
Zhai X Wang Y . Physical modulation and peripheral nerve regeneration: a literature review. Cell Regen. (2024) 13:32. 10.1186/s13619-024-00215-9
31.
Langevin HM Schnyer R MacPherson H Davis R Harris RE Napadow V et al . Manual and electrical needle stimulation in acupuncture research: pitfalls and challenges of heterogeneity. J Altern Complement Med. (2015) 21:113–28. 10.1089/acm.2014.0186
32.
Li P Qiu T Qin C . Efficacy of acupuncture for Bell's palsy: a systematic review and meta-analysis of randomized controlled trials. PLoS One. (2015) 10:e0121880. 10.1371/journal.pone.0121880
33.
Zhang R Wu T Wang R Wang D Liu Q . Compare the efficacy of acupuncture with drugs in the treatment of Bell's palsy: a systematic review and meta-analysis of RCTs. Med (Baltim). (2019) 98:e15566. 10.1097/MD.0000000000015566
34.
Yu G Luo S Zhu C Chen L Huang H Nie B et al . Global trends and performances of acupuncture therapy on Bell's palsy from 2000 to 2023: a bibliometric analysis. J Pain Res. (2023) 16:2155–69. 10.2147/JPR.S401086
35.
Lee B Kwon CY Lee HW Nielsen A Wieland LS Kim TH et al . Needling point Location Used in sham Acupuncture for chronic nonspecific low back pain: a systematic review and network meta-analysis. JAMA Netw Open. (2023) 6:e2332452. 10.1001/jamanetworkopen.2023.32452
36.
Xu S Yu L Luo X Wang M Chen G Zhang Q et al . Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: multicentre, randomised clinical trial. BMJ. (2020) 368:m697. 10.1136/bmj.m697
37.
Pu JK Wong SC So KH Tsang AC Li LF . Acupuncture as part of iatrogenic facial nerve palsy rehabilitation-first report. World Neurosurg. (2020) 140:e343–7. 10.1016/j.wneu.2020.05.079
38.
Davis ME Greene JJ . Advances and future directions in the care of patients with facial paralysis. Oper Tech Otolayngol Head Neck Surg. (2022) 33:60–71. 10.1016/j.otot.2022.02.010
39.
Hershman DL Unger JM Greenlee H Capodice J Lew DL Darke A et al . Comparison of acupuncture vs sham acupuncture or waiting list control in the treatment of aromatase inhibitor–related joint pain: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2241720. 10.1001/jamanetworkopen.2022.41720
40.
Morrison RA Hulsey DR Adcock KS Rennaker RL Kilgard MP Hays SA . Vagus nerve stimulation intensity influences motor cortex plasticity. Brain Stimul. (2019) 12:256–62. 10.1016/j.brs.2018.10.017
41.
Hays SA Rennaker RL Kilgard MP . Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog Brain Res. (2013) 207:275–99. 10.1016/B978-0-444-63327-9.00010-2
42.
Pruitt DT Schmid AN Kim LJ Abe CM Trieu JL Choua C et al . Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J Neurotrauma. (2016) 33:871–9. 10.1089/neu.2015.3972
43.
Ganzer PD Darrow MJ Meyers EC Solorzano BR Ruiz AD Robertson NM et al . Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury. Elife. (2018) 7:e32058. 10.7554/eLife.32058.032
44.
Gothard KM . The amygdalo-motor pathways and the control of facial expressions. Front Neurosci. (2014) 8:43. 10.3389/fnins.2014.00043
45.
Salles AG da Costa EF Ferreira MC . do Nascimento Remigio AF, Moraes LB, Gemperli R. Epidemiologic overview of synkinesis in 353 patients with longstanding facial paralysis under treatment with botulinum toxin for 11 years. Plast Reconstr Surg. (2015) 136:1289–98. 10.1097/PRS.0000000000001802
46.
Zimmermann J Jesse S Kassubek J Pinkhardt E Ludolph AC . Differential diagnosis of peripheral facial nerve palsy: a retrospective clinical, MRI and CSF-based study. J Neurol. (2019) 266:2488–94. 10.1007/s00415-019-09387-w
47.
Savary T Fieux M Douplat M Tournegros R Daubie S Pavie D et al . Incidence of underlying abnormal findings on routine magnetic resonance imaging for Bell palsy. JAMA Netw Open. (2023) 6:e239158. 10.1001/jamanetworkopen.2023.9158
Summary
Keywords
facial paralysis, facial nerve block, facial nerve stimulation, House–Brackmann grading system, Sunnybrook facial grading system, neuroplasticity
Citation
Mun HY, Sirh SW, Sirh HM and Sirh SJ (2025) Integrative therapy for acute, subacute and chronic facial palsy: repeated differential facial nerve blocks combined with hypodermic needle-based facial nerve stimulation. Front. Neurol. 16:1655894. doi: 10.3389/fneur.2025.1655894
Received
28 June 2025
Accepted
06 October 2025
Published
29 October 2025
Volume
16 - 2025
Edited by
Kathrin Machetanz, Tübingen University Hospital, Germany
Reviewed by
Jeevani Dahanayake, University of Colombo, Sri Lanka
Ülkü Sabuncu, Ankara Bilkent City Hospital University, Türkiye
Updates
Copyright
© 2025 Mun, Sirh, Sirh and Sirh.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soo Ji Sirh sujiezzb@hanmail.net
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.