Abstract
Introduction:
Research on the association between hemorrhoidal diseases (HDs) and dementia is limited. We explored this relationship in a population-based longitudinal cohort and proposed that individuals with HD may experience a higher incidence of dementia.
Methods:
Our study included 381,031 participants drawn from results from the South Korean health-screening cohort database, between 2005 and 2010. HD was identified based on at least two claims using the International Classification of Diseases, Tenth Revision (ICD-10) code I84. We used propensity score matching (PSM) to categorize the participants into two groups based on the presence or treatment of HD. The primary outcome was the incidence of all-cause dementia as determined by two or more claims with ICD-10 codes (F00-03, G30, and G31). Secondary outcomes included the occurrence of Alzheimer’s (F00 or G30) and vascular dementia (F01).
Results:
Over a median follow-up period of 15.49 years (interquartile range: 12.21–18.77 years), the cumulative incidence of all-cause dementia was 80,488 cases (22.47%). Multivariate analysis showed that the group with HD consistently had a higher incidence of all-cause dementia than the group without HD after PSM (hazard ratio [HR], 1.243; 95% confidence interval [CI], 1.199–1.288). Participants who underwent surgical procedures or treatment for HD revealed a significantly lower incidence of all-cause dementia after PSM (HR, 0.925; 95% CI, 0.872–0.981).
Discussion:
This study revealed a significantly higher incidence of all-cause dementia among participants with hemorrhoidal disease, suggesting that while hemorrhoidal disease may not directly cause dementia, it may serve as a marker of an underlying systemic condition that increases dementia risk.
1 Introduction
Hemorrhoids are naturally occurring vascular structures located in the lower rectum and anal canal. They are essential for maintaining continence (1). However, the term “hemorrhoids” commonly refers to a pathological condition involving the abnormal displacement of this tissue, known as hemorrhoidal disease (1). This condition affects nearly 40% of adults and can cause considerable discomfort, disrupt daily functioning, and negatively impact the quality of life (2, 3). Hemorrhoidal disease poses a significant clinical challenge and has notable socioeconomic consequences, exerting pressure on healthcare resources (4).
Dementia refers to a group of disorders characterized by persistent and worsening memory decline, cognitive dysfunction, and behavioral alterations that interfere with everyday functioning (5). Alzheimer’s disease (AD) and vascular dementia (VD) account for most dementia cases. Although VD is traditionally viewed as a consequence of stroke and vascular disease, increasing evidence indicates that vascular risk factors play a role in AD (5). The exact cause of dementia remains unclear; however, genetic predisposition, environmental factors, neuroinflammation, and vascular damage appear to contribute to its development (6). Further research is necessary to explore the associations and risk factors associated with dementia.
Although hemorrhoidal disease is relatively common, it may also be a clinical sign of compromised vascular integrity. In addition to other chronic venous conditions, hemorrhoidal diseases may be associated with an increased risk of cardiovascular diseases (7). Furthermore, damage to the cerebral venous integrity is a well-known pathological mechanism of dementia (8). Therefore, hemorrhoidal disease and dementia may share several risk factors, and are possibly related (8, 9). However, there has been limited research on the association between the presence of hemorrhoidal disease and the risk of dementia. We proposed that hemorrhoidal disease is associated with a heightened risk of developing dementia. This study aimed to explore the relationship between the occurrence and management of hemorrhoidal disease and the likelihood of dementia, using a nationwide longitudinal dataset from the general population.
2 Materials and methods
2.1 Data source
The Korean National Health Insurance System (NHIS) provides an extensive database containing information on demographics, socioeconomic status, medical diagnoses, and treatment methods. It also includes data from national health screening and healthcare facilities (10). According to the NHIS recommendations, individuals enrolled in the system are encouraged to undergo regular biennial health screening. This study made use of data from the NHIS-National Health Screening Cohort (NHIS-HEALS) (11, 12). The NHIS-HEALS cohort comprises of data from 10% of individuals, randomly selected, who underwent health examinations between 2002 and 2019. This cohort includes participants over the age of 40 who participated in the NHIS health-screening programs. From this cohort, we gathered data including demographic information, height, weight, household income, smoking and alcohol habits, physical activity, existing medical history, and health conditions. This study adhered to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Ewha Womans University Seoul Hospital (EUMC 2024-03-006). The requirement for informed consent was waived owing to the retrospective nature of the study and the minimal risk to the patients.
2.2 Study population
Using data from the NHIS-HEALS database, we selected 381,031 participants between 2005 and 2010, who were then followed up through to December 31, 2019. Those with a prior history of dementia, that is 1,678 individuals with International Classification of Diseases-10 (ICD-10) codes F00, F01, F02, F03, G30, or G31 were excluded based on records from January 1, 2002, to December 31, 2010. Additionally, 21,111 participants with missing or incomplete data, and 103 individuals with less than 30 days of follow-up, were excluded to minimize reverse causation. The final study population comprised of 358,139 individuals (Figure 1).
Figure 1
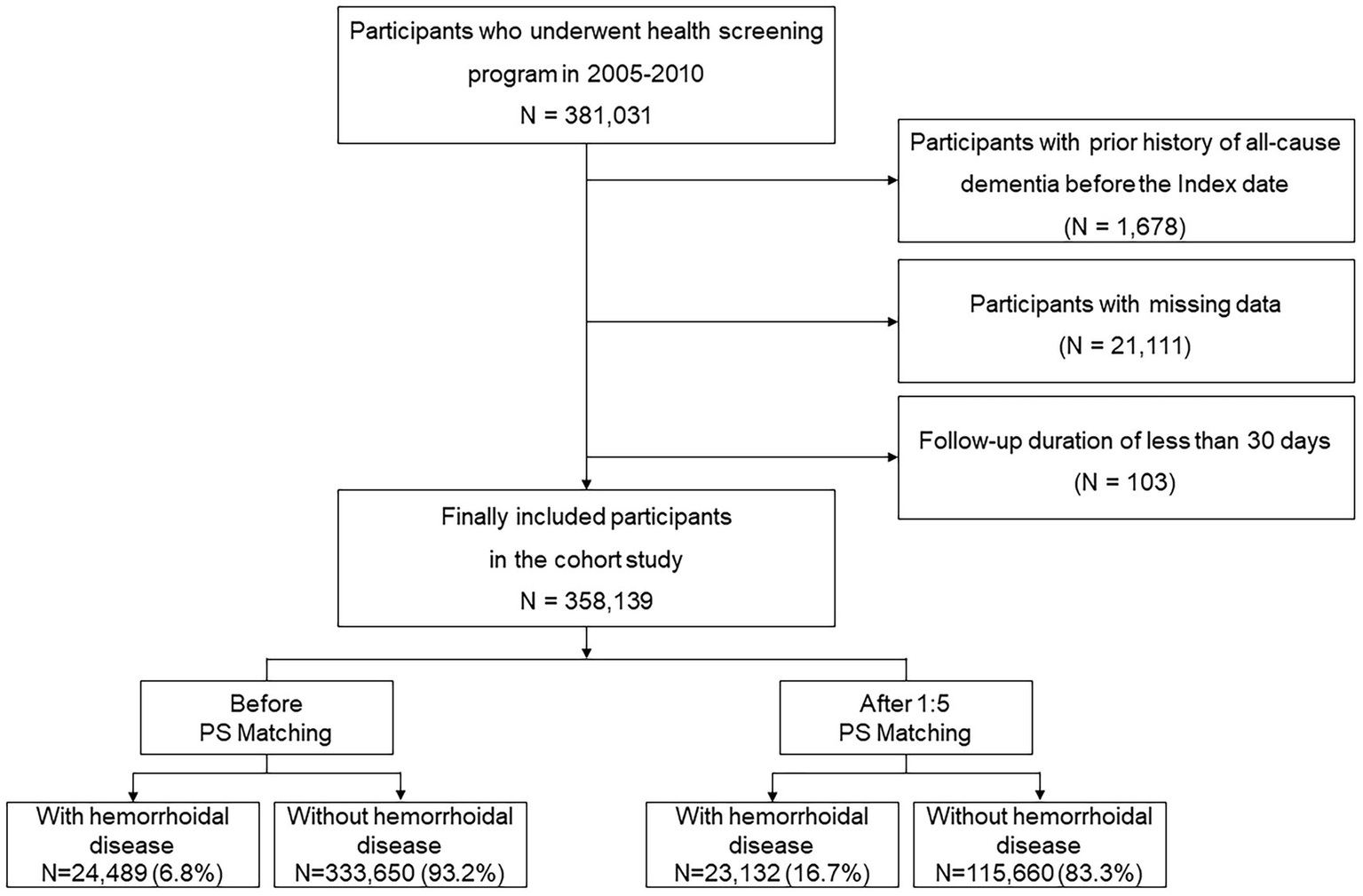
Flow chart of inclusion and exclusion criteria.
2.3 Definition of hemorrhoidal disease
The presence of hemorrhoidal disease was identified when participants had at least two separate medical claims with ICD-10 code I84, following the criteria of a previous study (13). Surgical and procedural treatments for hemorrhoids were identified using the following procedure codes: Q3012 for thrombosed hemorrhoid surgery, Q3013 for hemorrhoidectomy, Q3014 for surgery on strangulated circumferential hemorrhoids, Q3015 for thrombectomy or excision of hemorrhoidal skin tags, Q3016 for treatments including coagulation, cauterization, sclerotherapy, or rubber band ligation, and Q3017 for circular stapled hemorrhoidectomy (13).
2.4 Definition of outcomes
The main outcome of this study was the occurrence of all-cause dementia, including AD, VD, and other forms of dementia, as identified by the ICD-10 diagnostic codes F00-F03, G30, and G31. An event was considered an outcome when it corresponded to these ICD-10 codes, and there were records of prescribed anti-dementia medications such as donepezil, galantamine, rivastigmine, and memantine. This algorithm for defining dementia cases is acceptable because of its high level of accuracy, as evidenced by a 94.7% positive predictive value (14). Secondary outcomes were AD (ICD-10 codes F00 or G30) and VD (ICD-10 code F01). Follow-up was performed until either death, the first incidence of all-cause dementia or December 31, 2019.
2.5 Definition of covariates
Age, sex, body mass index (BMI), household income, smoking status, alcohol consumption, regular physical activity, hypertension, diabetes mellitus, dyslipidemia, stroke, myocardial infarction, chronic obstructive pulmonary disease (COPD), renal disease, liver disease, cancer, social determinants of health (SDoH), inflammatory bowel disease (IBD), and Charlson Comorbidity Index (CCI) were investigated as covariates. SDoH was defined using low-income status and claims of ICD-10 codes, including depression, anxiety-, and stress-related disorders. Detailed definitions of these covariates are provided in Supplementary Methods 1 (15–18).
2.6 Statistical analysis
A comparison of the baseline characteristics between the participants in the hemorrhoidal disease group and those in the non-hemorrhoidal disease group was performed using an independent t-test for continuous variables and a chi-squared test (or Fisher’s exact test) for categorical variables. To ensure balanced baseline characteristics and minimize potential confounding between the two groups, a 1:5 propensity score matching (PSM) approach was employed (13, 19). Propensity scores were estimated using a multivariate logistic regression model including demographic, lifestyle, and clinical variables (age, sex, BMI, household income, smoking status, alcohol consumption, regular physical activity, hypertension, diabetes mellitus, dyslipidemia, stroke, myocardial infarction, COPD, renal disease, liver disease, cancer, and CCI) Supplementary Methods 2. The effectiveness of matching was evaluated using the standardized mean difference (SMD), with an absolute SMD value below 0.1 indicating an acceptable level of balance (20, 21). The calculation of the SMD for a categorical variable is described in Supplementary Methods 3.
To analyze the incidence risk of all-cause dementia, AD, and VD, we used Kaplan–Meier survival curves and tested the differences between the hemorrhoidal and non-hemorrhoidal disease groups using log-rank tests. Cox proportional hazards models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs). We used the Grambsch and Therneau test based on Schoenfeld residuals and confirmed that the proportional hazards assumption was reasonably satisfied in our model (22). Additionally, we analyzed the Wald Chi-square test results from the Cox proportional hazards model, including hemorrhoid treatment status as a covariate, based on the cohort before and after PSM. These were visualized using forest plots.
Further subgroup analyses were performed to assess the association between the presence of hemorrhoidal disease and all-cause dementia according to demographic data and covariates, with p-values for interaction. Additionally, we performed Wald chi-square statistics on the Cox model before and after PSM. To assess the relationship between the severity and treatment of hemorrhoidal disease and the risk of developing dementia, a sensitivity analysis was conducted to examine whether surgical intervention or other treatments for hemorrhoidal disease were associated with the incidence of all-cause dementia, AD, and VD. This analysis was conducted using Cox regression, with pre- and post- 1:1 PSM. Furthermore, we conducted a mediation analysis based on the Cox proportional hazards model to evaluate whether the effect of hemorrhoidal disease on dementia is mediated through a selected dementia risk factor considering the SDoH variable (Supplementary Methods 4 and Supplementary Figure 1) (23). Statistical analyses were conducted using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA) and R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value of less than 0.05 was considered statistically significant for all tests.
3 Results
3.1 Baseline characteristics of participants
Table 1 shows the baseline characteristics based on the presence of hemorrhoidal disease. The average age of the participants was 54.4 ± 9.7 years, with males comprising 53.7% of the population. Patients diagnosed with hemorrhoidal disease tended to be younger and of male gender. In addition, they were less likely to have a history of smoking, heavy alcohol consumption, hypertension, or diabetes mellitus. Conversely, individuals with hemorrhoidal disease more commonly exhibited comorbid conditions such as dyslipidemia, stroke, myocardial infarction, COPD, kidney disease, liver disease, cancer, SDoH, and IBD, and had higher CCI scores (Table 1). Following PSM, the groups with and without hemorrhoidal disease were well matched across the covariates (Table 1).
Table 1
| Variable | Before PSM, N = 358,139 | After 1:5 PSM, N = 138,792 | |||||
|---|---|---|---|---|---|---|---|
| Total | Hemorrhoidal disease (−) | Hemorrhoidal disease (+) | p-value | Hemorrhoidal disease (−) | Hemorrhoidal disease (+) | SMD* | |
| Mean ± SD, N (%) | Mean ± SD, N (%) | Mean ± SD, N (%) | Mean ± SD, N (%) | ||||
| Number | 358,139 | 333,650 | 24,489 | 115,660 | 23,132 | ||
| Age, years | 54.4 ± 9.7 | 54.4 ± 9.7 | 53.8 ± 8.8 | <0.001 | 53.7 ± 9.5 | 53.8 ± 8.9 | −0.013 |
| Sex | <0.001 | −0.001 | |||||
| Female | 165,815 (46.3) | 155,832 (46.7) | 9,983 (40.8) | 48,675 (42.1) | 9,726 (42.1) | ||
| Male | 192,324 (53.7) | 177,818 (53.3) | 14,506 (59.2) | 66,985 (57.9) | 13,406 (57.9) | ||
| Body mass index (kg/m2) | 24.0 ± 3.0 | 24.0 ± 3.0 | 24.0 ± 2.8 | 0.620 | 23.9 ± 2.9 | 23.9 ± 2.8 | −0.002 |
| Household income | <0.001 | 0.001 | |||||
| Low | 108,030 (30.2) | 101,970 (30.6) | 6,060 (24.8) | 29,333 (25.4) | 5,859 (25.3) | ||
| Middle | 130,145 (36.3) | 121,297 (36.4) | 8,848 (36.1) | 41,791 (36.1) | 8,374 (36.2) | ||
| High | 119,964 (33.5) | 110,383 (33.0) | 9,581 (39.1) | 44,536 (38.5) | 8,899 (38.5) | ||
| Smoking status | <0.001 | 0.001 | |||||
| Never | 244,775 (68.4) | 227,579 (68.2) | 17,196 (70.2) | 81,459 (70.4) | 16,278 (70.4) | ||
| Former | 30,609 (8.6) | 27,939 (8.4) | 2,670 (10.9) | 12,179 (10.5) | 2,426 (10.5) | ||
| Current | 82,755 (23.0) | 78,132 (23.4) | 4,623 (18.9) | 22,022 (19.1) | 4,428 (19.1) | ||
| Alcohol consumption (days/week) | <0.001 | −0.001 | |||||
| None | 208,935 (58.3) | 194,922 (58.4) | 14,013 (57.2) | 66,739 (57.7) | 13,361 (57.8) | ||
| 1–2 times | 109,414 (30.6) | 101,275 (30.4) | 8,139 (33.2) | 37,964 (32.8) | 7,569 (32.7) | ||
| 3–4 times | 24,188 (6.8) | 22,648 (6.8) | 1,540 (6.3) | 7,231 (6.4) | 1,437 (6.2) | ||
| ≥5 times | 15,602 (4.3) | 14,805 (4.4) | 797 (3.3) | 3,726 (3.1) | 765 (3.3) | ||
| Regular physical activity (days/week) | <0.001 | 0.001 | |||||
| None | 198,854 (55.5) | 186,968 (56.0) | 11,886 (48.5) | 57,249 (49.5) | 11,434 (49.4) | ||
| 1–4 days | 123,151 (34.4) | 113,154 (33.9) | 9,997 (40.8) | 46,180 (39.9) | 9,223 (39.9) | ||
| ≥5 days | 36,134 (10.1) | 33,528 (10.1) | 2,606 (10.7) | 12,231 (10.6) | 2,475 (10.7) | ||
| Comorbidities | |||||||
| Hypertension | 88,684 (24.8) | 83,396 (25.0) | 5,288 (21.6) | <0.001 | 25,058 (21.7) | 5,032 (21.8) | −0.003 |
| Diabetes mellitus | 37,488 (10.5) | 35,636 (10.7) | 1,852 (7.6) | <0.001 | 8,944 (7.7) | 1,814 (7.8) | −0.004 |
| Dyslipidemia | 50,625 (14.1) | 44,907 (13.5) | 5,718 (23.4) | <0.001 | 24,169 (20.9) | 4,887 (21.1) | −0.006 |
| Stroke | 766 (0.2) | 686 (0.2) | 80 (0.3) | <0.001 | 313 (0.3) | 61 (0.3) | 0.002 |
| Myocardial infarction | 389 (0.1) | 358 (0.1) | 31 (0.1) | 0.376 | 149 (0.1) | 29 (0.1) | 0.000 |
| COPD | 62,902 (17.6) | 56,455 (16.9) | 6,447 (26.3) | <0.001 | 28,622 (24.8) | 5,703 (24.7) | 0.002 |
| Renal disease | 7,464 (2.1) | 6,759 (2.0) | 705 (2.9) | <0.001 | 3,027 (2.6) | 629 (2.7) | −0.006 |
| Liver disease | 43,674 (12.2) | 38,299 (11.5) | 5,375 (22.0) | <0.001 | 22,247 (19.2) | 4,506 (19.5) | −0.006 |
| Cancer | 10,943 (3.1) | 9,676 (2.9) | 1,267 (5.2) | <0.001 | 4,999 (4.3) | 1,042 (4.5) | −0.009 |
| SDoH | 4,762 (1.3) | 4,215 (1.3) | 547 (2.2) | <0.001 | 1,453 (1.3) | 522 (2.3) | −0.088 |
| IBD | 2,529 (0.7) | 2,137 (0.6) | 392 (1.6) | <0.001 | 889 (0.8) | 372 (1.6) | −0.090 |
| Charlson comorbidity index | <0.001 | 0.004 | |||||
| 0 | 313,901 (93.1) | 291,415 (93.1) | 22,486 (93.2) | 107,875 (93.3) | 21,557 (93.2) | ||
| 1 | 20,783 (6.2) | 19,276 (6.2) | 1,507 (6.2) | 7,120 (6.2) | 1,443 (6.2) | ||
| ≥2 | 2,223 (0.7) | 2,089 (0.7) | 134 (0.6) | 665 (0.5) | 132 (0.6) | ||
Baseline characteristics of study participants.
COPD, chronic obstructive pulmonary disease; IBD, inflammatory bowel disease; N, number; PSM, propensity score matching; SD, standard deviation; SDoH, social determinants of health; SMD, standardized mean difference.
All standardized mean difference values were <0.1 in the propensity score matched cohort.
3.2 Association of presence of hemorrhoidal disease with dementia
During a median follow-up period of 15.49 years (interquartile range: 12.21–18.77 years), the cumulative incidence of all-cause dementia was 80,488 (22.47%), including 38,094 cases of AD (10.64%) and 15,198 cases of VD (4.24%). The Kaplan–Meier survival curves for the occurrence of dementia according to the presence of hemorrhoidal disease are shown in Figure 2. Participants had an increased risk of all-cause dementia according to the presence of hemorrhoidal disease (log-rank test, p < 0.001) regardless of PSM. In the multivariate Cox regression analysis, the hemorrhoidal disease group consistently showed an increased risk of all-cause dementia compared with the group without hemorrhoidal disease before PSM (HR: 1.228; 95% CI: 1.194–1.263; p < 0.001) and after PSM (HR: 1.243; 95% CI: 1.199–1.288; p < 0.001; Table 2 and Supplementary Table 1). In the Wald Chi-square test, the global tests for the full model (including 26 covariates) were highly significant for all outcomes before PSM (Wald χ2 = 79,669.454 for all-cause dementia, p < 0.001) and after PSM (Wald χ2 = 32,255.940 for all-cause dementia, p < 0.001; Supplementary Tables 2, 3). Additionally, these positive associations were consistently noted in AD before PSM (HR: 1.073; 95% CI: 1.028–1.121; p < 0.001) and after PSM (HR: 1.075; 95% CI: 1.012–1.142; p < 0.001), and VD before PSM (HR: 1.130; 95% CI: 1.103–1.157; p < 0.001) and after PSM (HR: 1.086; 95% CI: 1.020–1.152; p < 0.001; Table 2, Supplementary Tables 4, 5). Furthermore, strong global significance was noted in AD before PSM (Wald χ2 = 57,133.027, df = 26, p < 0.001) and after PSM (Wald χ2 = 22,102.189, df = 26, p < 0.001), and VD before PSM (Wald χ2 = 16,841.816, df = 26, p < 0.001) and after PSM (Wald χ2 = 6,655.687, df = 26, p < 0.001; Supplementary Tables 2, 3). In the subgroup analysis, the link between hemorrhoidal disease and an elevated risk of all-cause dementia remained consistent across all covariate categories (Figure 3, Supplementary Tables 6, 7).
Figure 2
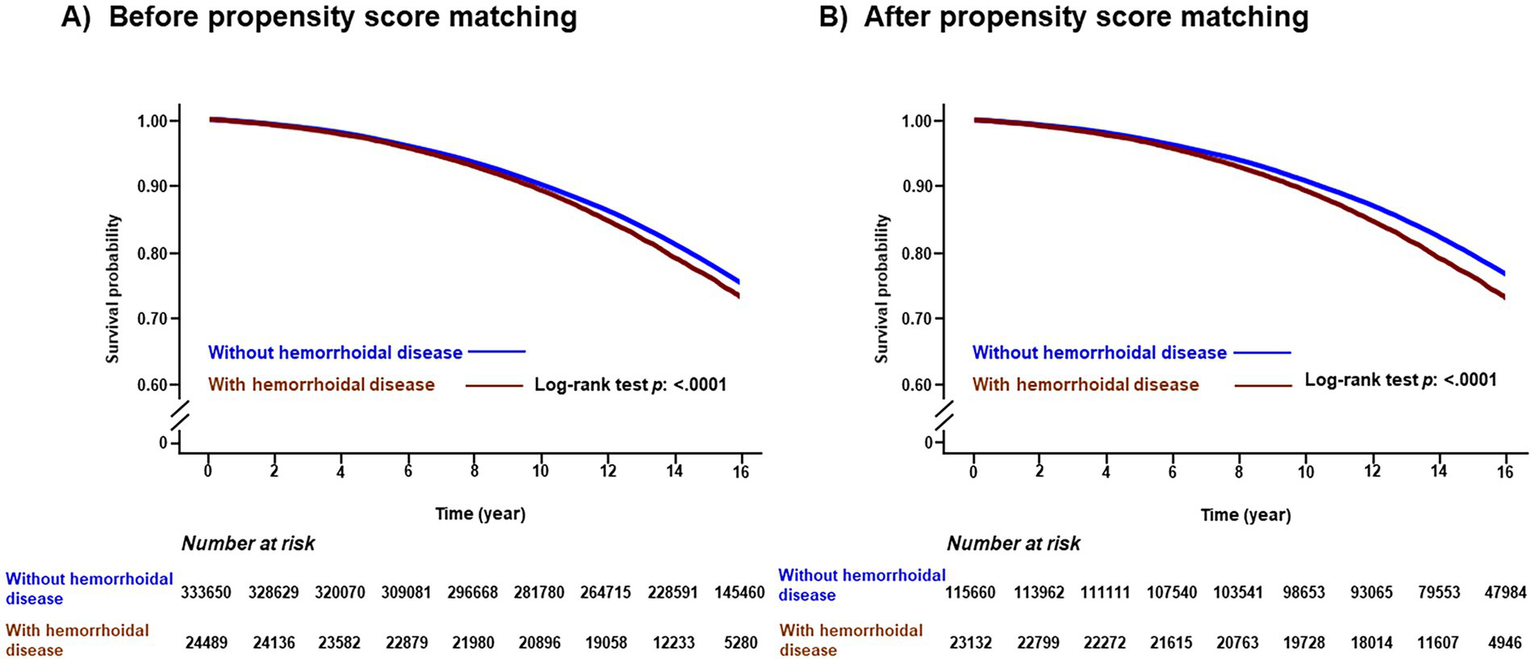
Kaplan-Meier survival curves of all-cause dementia according to presence of hemorrhoidal disease before propensity score matching (A) and after propensity score matching (B).
Table 2
| Variable | Before PSM N = 358,139 |
After 1:5 PSM N = 138,792 |
||||
|---|---|---|---|---|---|---|
| Incidence rate (per 100,000 person-years) | Crude HR (95%CI) | Adjusted HR (95%CI) | Incidence rate (per 100,000 person-years) | Crude HR (95%CI) | Adjusted HR (95%CI) | |
| All-cause dementia | 1,633.404 | 1.111 (1.081–1.142) | 1.228 (1.194–1.263) | 1,557.404 | 1.188 (1.152–1.226) | 1.243 (1.199–1.288) |
| Alzheimer’s disease | 747.482 | 1.115 (1.076–1.155) | 1.073 (1.028–1.121) | 671.973 | 1.038 (0.990–1.088) | 1.075 (1.012–1.142) |
| Vascular dementia | 292.398 | 1.053 (1.023–1.084) | 1.130 (1.103–1.157) | 268.584 | 1.045 (0.972–1.124) | 1.086 (1.020–1.152) |
Results of Cox regression analysis for the association of hemorrhoidal disease with incidence risk of dementia.
CI, confidence interval; HR, hazard ratio; N, number; PSM, propensity score matching. Values from the multivariable Cox regression models were adjusted for age, sex, body mass index, household income, smoking status, alcohol consumption, regular physical activity, comorbidities, social determinants of health, inflammatory bowel disease and Charlson comorbidity index.
Figure 3
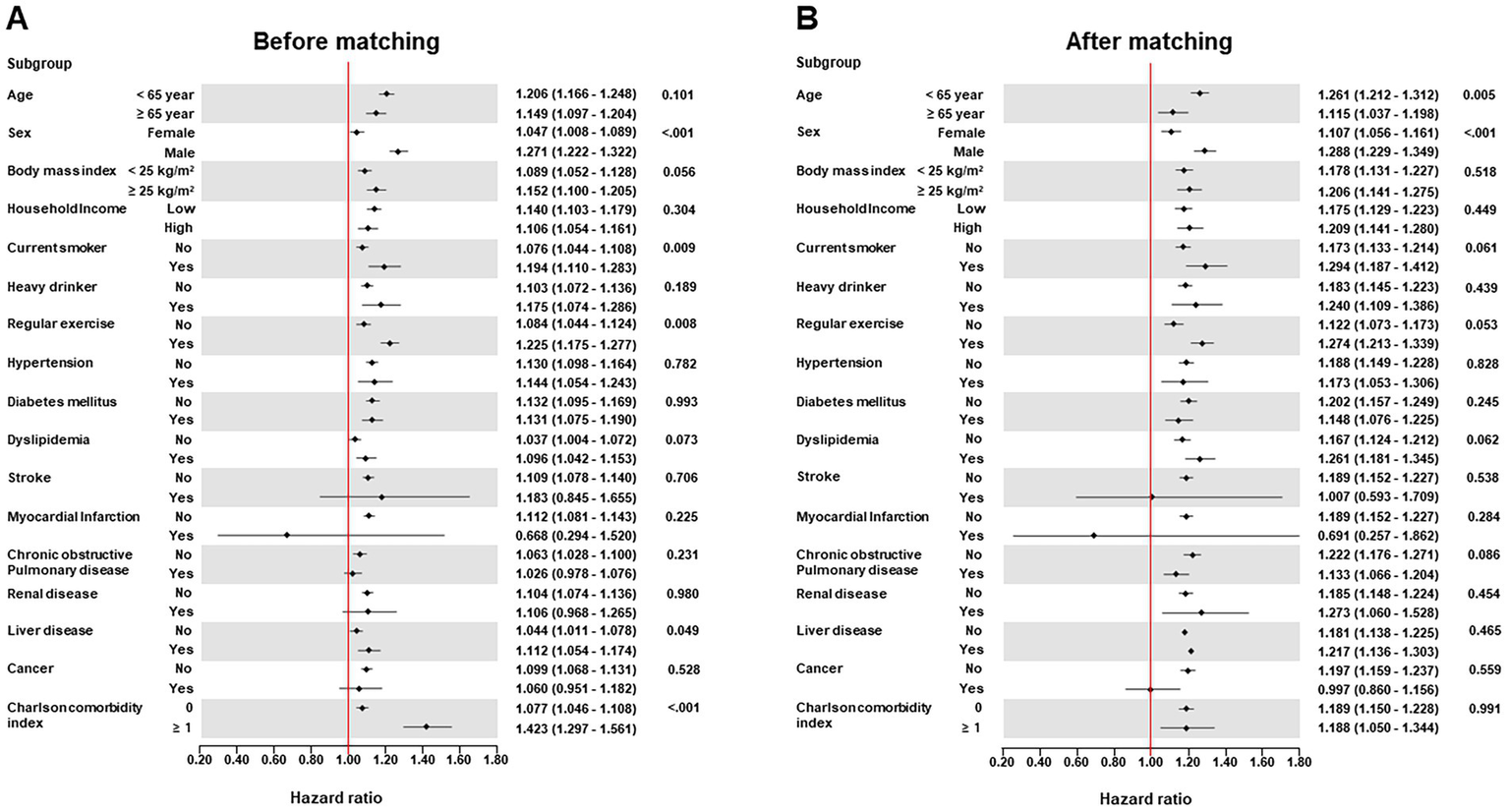
Forest plots of subgroup analysis according to demographic data and comorbidities for the association of hemorrhoidal disease with incidence risk of all-cause dementia before propensity score matching (A) and after propensity score matching (B).
3.3 Association of treatment/procedure of hemorrhoidal disease with dementia
Supplementary Table 8 outlines the clinical characteristics before and after PSM based on whether the participants underwent surgical procedures or treatments for hemorrhoidal disease. The specific frequencies of these procedures and treatments are shown in Supplementary Table 9. Notably, participants who underwent surgical intervention or treatment for hemorrhoidal disease exhibited a significantly reduced risk of developing all-cause dementia (HR: 0.925; 95% CI: 0.872–0.981; p = 0.012), AD (HR: 0.901; 95% CI: 0.823–0.987; p = 0.007), and VD (HR: 0.848; 95% CI: 0.736–0.976; p = 0.003), even after PSM in multivariate Cox regression analysis (Table 3, Supplementary Tables 10–12). In addition, the Wald Chi-square test results showed that hemorrhoid treatment status was a significant predictor of dementia, both before and after PSM (Supplementary Tables 13, 14). Moreover, in the mediation analysis, our findings were significant, even when considering SDoH as a mediator (Supplementary Tables 15, 16). There was no significant multicollinearity in the association between hemorrhoidal disease and dementia among the covariates (Supplementary Table 17).
Table 3
| Variable | Before PSM N = 24,489 |
After 1:1 PSM N = 18,330 |
||||
|---|---|---|---|---|---|---|
| Incidence rate (per 100,000 person-years) | Crude HR (95%CI) | Adjusted HR (95%CI) | Incidence rate (per 100,000 person-years) | Crude HR (95%CI) | Adjusted HR (95%CI) | |
| All-cause dementia | 1,692.232 | 0.660 (0.626–0.696) | 0.904 (0.856–0.954) | 1,876.238 | 0.939 (0.886–0.996) | 0.925 (0.872–0.981) |
| Alzheimer’s disease | 671.663 | 0.570 (0.525–0.620) | 0.911 (0.837–0.992) | 757.833 | 0.905 (0.826–0.990) | 0.901 (0.823–0.987) |
| Vascular dementia | 275.762 | 0.578 (0.508–0.657) | 0.865 (0.758–0.988) | 306.753 | 0.846 (0.734–0.974) | 0.848 (0.736–0.976) |
Results of Cox regression analysis for the association of surgical procedure/treatment for hemorrhoidal disease with incidence risk of dementia.
CI, confidence interval; HR, hazard ratio; N, number; PSM, propensity score matching. Values from the multivariable Cox regression models were adjusted for age, sex, body mass index, household income, smoking status, alcohol consumption, regular physical activity, comorbidities, social determinants of health, inflammatory bowel disease and Charlson comorbidity index.
4 Discussion
Our study revealed that in a longitudinal setting, the presence of hemorrhoidal disease was associated with the incidence of all-cause dementia, AD, and VD, regardless of PSM. Moreover, our study has several strengths that distinguish it from previous studies. To reduce bias, we used a 1:5 PSM approach for the general population in a longitudinal setting. Our findings confirmed that the presence of hemorrhoidal disease was significantly associated with an increased risk of all-cause dementia, including AD and VD. Furthermore, surgical procedures or treatments for hemorrhoidal diseases may be associated with a lower risk of all-cause dementia, AD, and VD.
There is increasing focus on the role of hemorrhoidal diseases and their association with neuropsychiatric disorders, cardiovascular diseases, and risk factors. Previous studies have suggested that older age, current smoking status, and the presence of hypertension, are associated with hemorrhoidal disease (9). Moreover, increased incidences of peripheral artery occlusive disease and coronary artery disease have been observed in patients with hemorrhoidal disease compared to those without hemorrhoidal disease (7, 24). Furthermore, obesity, abdominal obesity, and depression may be risk factors for hemorrhoidal disease (25). Hemorrhoidal disease is genetically associated with psychiatric symptoms such as depression, bipolar disorder, anxiety disorders, and schizophrenia. However, no studies have examined hemorrhoidal disease and cognitive decline, including AD and VD (26). In our study, we found that the presence of hemorrhoidal disease was associated with an increased risk of dementia, with consistent results across subtypes, including AD and VD. These results provide valuable insights that contribute to the growing body of evidence on the relationship between hemorrhoidal diseases and other health conditions.
A direct relationship between hemorrhoidal diseases and dementia has not yet been clearly established. However, several potential pathophysiologies may be related to these two diseases. First, hemorrhoidal diseases and dementia are associated with several risk factors. According to previous studies, obesity and history of pregnancy are associated with hemorrhoidal disease (25, 27). Additionally, diabetes treatment with metformin reduces the risk of hemorrhoids (28). All-cause dementia is associated with obesity (29, 30), diabetes mellitus (31), and pregnancy (32). The overlap of these risk factors supports the conclusion that an association exists between these two diseases.
Second, the development of dementia may occur through a pro-inflammatory and dysregulated gut–brain axis process. Hemorrhoidal diseases typically cause localized inflammation, but in severe cases it may serve as a source of chronic inflammatory mediators that can enter the systemic circulation through the gut–brain axis, thereby inducing systemic inflammation (33–35). In the past decade, a persistent inflammatory response in the brain has been recognized as a pathological feature of dementia (36). Previous research in both humans and animals indicates that systemic inflammation occurring outside the central nervous system, may contribute significantly to neurodegeneration, the pathology of AD, and cognitive decline in older adults (37). Therefore, severe hemorrhoidal disease can trigger a systemic inflammatory response, including the inflammation of the central nervous system, which may increase the risk of dementia (38). Moreover, hemorrhoidal diseases are associated with gut microbiota dysbiosis, which may contribute to increased intestinal permeability and systemic inflammatory responses (39–41). Such systemic inflammation is known to promote the development of dementia (42). A recent study suggested a causal relationship between gut microflora and the occurrence of dementia and its subtypes, including AD and VD (43). Thus, rather than being a direct cause of dementia, hemorrhoidal diseases may be a sign of a systemic state that causes dementia.
In our study, population who underwent surgical intervention for hemorrhoidal disease—potentially representing a cohort with more severe disease—exhibited a lower risk of developing dementia compared to those who did not receive treatment. While this finding may suggest that proactive management of hemorrhoidal disease could mitigate dementia risk, it is more plausibly attributable to the health-conscious behaviors of individuals who seek surgical care. These individuals may have better access to healthcare resources and engage in healthier lifestyles, factors that are not fully captured by the measured covariates. Thus, the observed association may reflect residual confounding by behavioral characteristics rather than a direct effect of the surgical intervention. That is, because the possibility of healthy user bias cannot be entirely excluded in our analysis, warranting cautious interpretation of the findings.
Our study has several limitations. First, our findings may be affected by ethnic bias, which could limit their applicability to other demographic groups. Therefore, further studies involving diverse racial and ethnic populations are essential to enhance generalizability. Second, despite conducting a sub-analysis of participants with hemorrhoidal disease who underwent surgical procedures or treatment, reliance on claims data limited our ability to verify imaging findings or precisely categorize the severity and type of hemorrhoidal disease. Therefore, we were unable to assess whether dementia risk varies according to the severity of hemorrhoidal disease in a dose–response manner. Additionally, although our study provides evidence that hemorrhoidal disease is associated with dementia, given that the effect size is relatively small, caution is warranted in interpreting this finding. Third, because the sample size was reasonably large, small or even trivial effects may be statistically significant. Although the associations between hemorrhoidal disease and AD or VD, were statistically significant, the magnitudes of these associations, such as hazard ratios, were not large. In particular, the effectiveness of treatments for hemorrhoidal diseases in reducing the risk of dementia is limited. Therefore, the results of this study should be interpreted with caution. Finally, although this was a nationwide cohort study, its retrospective nature presented challenges in establishing clear cause-and-effect relationships.
In conclusion, this study revealed a significantly higher incidence of all-cause dementia among participants with hemorrhoidal disease. This finding suggests that hemorrhoidal disease may be a sign of a systemic state that causes dementia.
Statements
Data availability statement
The datasets presented in this article are not readily available because the data used in this study are available from the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) database. However, restrictions apply to the public availability of the data used under license for the current study. Requests for access to NHIS data can be made through the National Health Insurance Sharing Service homepage (http://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do). To access the database, a completed application form, research proposal, and application for approval from the Institutional Review Board should be submitted to the Inquiry Committee of Research Support at the NHIS for review. Requests to access the datasets should be directed to http://nhiss.nhis.or.kr/bd/ab/bdaba021eng.do. Our dataset number is NHIS-2024-10-2-145.
Ethics statement
The studies involving humans were approved by this study adhered to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of the Ewha Womans University Seoul Hospital (EUMC 2024-03-006). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HW: Methodology, Data curation, Writing – review & editing, Supervision, Software, Writing – original draft, Investigation, Formal analysis, Validation, Resources, Conceptualization, Visualization, Project administration. M-SP: Resources, Visualization, Data curation, Formal analysis, Project administration, Validation, Software, Methodology, Writing – review & editing, Supervision, Investigation, Writing – original draft, Conceptualization. J-YP: Resources, Investigation, Supervision, Conceptualization, Writing – review & editing, Data curation, Project administration, Software, Writing – original draft, Formal analysis, Validation, Methodology, Visualization. HC: Writing – review & editing, Project administration, Formal analysis, Writing – original draft, Resources, Data curation, Methodology, Supervision, Visualization, Conceptualization, Investigation, Software, Validation. T-JS: Resources, Investigation, Writing – original draft, Software, Funding acquisition, Visualization, Validation, Formal analysis, Data curation, Conceptualization, Writing – review & editing, Methodology, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korean government (MSIT) (No. 2022-0-00621, RS-2022-II220621, Development of artificial intelligence technology that provides dialog-based multimodal explainability). This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: RS-2023-00262087 to T-JS). This research was supported by the BK21 Fostering Outstanding Universities for Research (FOUR) funded by the Ministry of Education (MOE, Korea) and the National Research Foundation of Korea (NRF-5199990614253, Education Research Center for 4IR-Based Health Care). The funding source had no role in the design, conduct, or reporting of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1655944/full#supplementary-material
References
1.
Abramowitz L Benabderrahmane M Pospait D Philip J Laouénan C . The prevalence of proctological symptoms amongst patients who see general practitioners in France. Eur J Gen Pract. (2014) 20:301–6. doi: 10.3109/13814788.2014.899578
2.
Riss S Weiser FA Schwameis K Riss T Mittlböck M Steiner G et al . The prevalence of hemorrhoids in adults. Int J Color Dis. (2012) 27:215–20. doi: 10.1007/s00384-011-1316-3
3.
Senagore AJ . Surgical management of hemorrhoids. J Gastrointest Surg. (2002) 6:295–8. doi: 10.1016/S1091-255X(01)00082-8
4.
Yang JY Peery AF Lund JL Pate V Sandler RS . Burden and cost of outpatient hemorrhoids in the United States employer-insured population, 2014. Am J Gastroenterol. (2019) 114:798–803. doi: 10.14309/ajg.0000000000000143
5.
Arvanitakis Z Shah RC Bennett DA . Diagnosis and management of dementia: review. JAMA. (2019) 322:1589–99. doi: 10.1001/jama.2019.4782
6.
Bir SC Khan MW Javalkar V Toledo EG Kelley RE . Emerging concepts in vascular dementia: a review. J Stroke Cerebrovasc Dis. (2021) 30:105864. doi: 10.1016/j.jstrokecerebrovasdis.2021.105864
7.
Chang SS Sung FC Lin CL Hu WS . Association between hemorrhoid and risk of coronary heart disease: a nationwide population-based cohort study. Medicine. (2017) 96:e7662. doi: 10.1097/MD.0000000000007662
8.
Pansieri J Hadley G Lockhart A Pisa M DeLuca GC . Regional contribution of vascular dysfunction in white matter dementia: clinical and neuropathological insights. Front Neurol. (2023) 14:1199491. doi: 10.3389/fneur.2023.1199491
9.
Hong YS Jung KU Rampal S Zhao D Guallar E Ryu S et al . Risk factors for hemorrhoidal disease among healthy young and middle-aged Korean adults. Sci Rep. (2022) 12:129. doi: 10.1038/s41598-021-03838-z
10.
Kim MK Han K Koh ES Kim HS Kwon HS Park YM et al . Variability in total cholesterol is associated with the risk of end-stage renal disease: a nationwide population-based study. Arterioscler Thromb Vasc Biol. (2017) 37:1963–70. doi: 10.1161/ATVBAHA.117.309803
11.
Song SO Jung CH Song YD Park CY Kwon HS Cha BS et al . Background and data configuration process of a nationwide population-based study using the Korean national health insurance system. Diabetes Metab J. (2014) 38:395–403. doi: 10.4093/dmj.2014.38.5.395
12.
Seong SC Kim YY Park SK Khang YH Kim HC Park JH et al . Cohort profile: the national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open. (2017) 7:e016640. doi: 10.1136/bmjopen-2017-016640
13.
Hong J Kim I Song J Ahn BK . Socio-demographic factors and lifestyle associated with symptomatic hemorrhoids: big data analysis using the national health insurance service-national health screening cohort (NHIS-HEALS) database in Korea. Asian J Surg. (2022) 45:353–9. doi: 10.1016/j.asjsur.2021.06.020
14.
Kim D Yang PS Yu HT Kim TH Jang E Sung JH et al . Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J. (2019) 40:2313–23. doi: 10.1093/eurheartj/ehz386
15.
Chang Y Woo HG Lee JS Song TJ . Better oral hygiene is associated with lower risk of stroke. J Periodontol. (2021) 92:87–94. doi: 10.1002/JPER.20-0053
16.
Lee K Lee JS Kim J Lee H Chang Y Woo HG et al . Oral health and gastrointestinal cancer: a nationwide cohort study. J Clin Periodontol. (2020) 47:796–808. doi: 10.1111/jcpe.13304
17.
Song TJ Kim JW Kim J . Oral health and changes in lipid profile: a nationwide cohort study. J Clin Periodontol. (2020) 47:1437–45. doi: 10.1111/jcpe.13373
18.
Lee H Park MS Kang MK Song TJ . Association between proteinuria status and risk of hypertension: a nationwide population-based cohort study. J Pers Med. (2023) 13:1414. doi: 10.3390/jpm13091414
19.
Yoo J Kim JH Jeon J Kim J Song TJ . Risk of COVID-19 infection and of severe complications among people with epilepsy: a nationwide cohort study. Neurology. (2022) 98:e1886–92. doi: 10.1212/WNL.0000000000200195
20.
Park J Shin JI Kim DH Park J Jeon J Kim J et al . Association of atrial fibrillation with infectivity and severe complications of COVID-19: a nationwide cohort study. J Med Virol. (2022) 94:2422–30. doi: 10.1002/jmv.27647
21.
Chung SJ Chang Y Jeon J Shin JI Song TJ Kim J . Association of Alzheimer's disease with COVID-19 susceptibility and severe complications: a nationwide cohort study. J Alzheimer's Dis. (2022) 87:701–10. doi: 10.3233/JAD-220031
22.
Grambsch PM Therneau TM . Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. (1994) 81:515–26. doi: 10.1093/biomet/81.3.515
23.
Steen J Loeys T Moerkerke B Vansteelandt S . Medflex: an R package for flexible mediation analysis using natural effect models. J Stat Softw. (2017) 76:1–46. doi: 10.18637/jss.v076.i11
24.
Hu W-S Lin C-L . Hemorrhoid is associated with increased risk of peripheral artery occlusive disease: a nationwide cohort study. J Epidemiol. (2017) 27:574–7. doi: 10.1016/j.je.2016.12.015
25.
Lee J-H Kim H-E Kang J-H Shin J-Y Song Y-M . Factors associated with hemorrhoids in Korean adults: Korean national health and nutrition examination survey. Korean J Fam Med. (2014) 35:227–36. doi: 10.4082/kjfm.2014.35.5.227
26.
Huang Z Huang J Leung CK Zhang CJP Akinwunmi B Ming W-K . Hemorrhoidal disease and its genetic association with depression, bipolar disorder, anxiety disorders, and schizophrenia: a bidirectional Mendelian randomization study. Hum Genomics. (2024) 18:27. doi: 10.1186/s40246-024-00588-7
27.
Avsar AF Keskin HL . Haemorrhoids during pregnancy. J Obstet Gynaecol. (2010) 30:231–7. doi: 10.3109/01443610903439242
28.
Tseng C-H . Chronic metformin therapy is associated with a lower risk of hemorrhoid in patients with type 2 diabetes mellitus. Front Pharmacol. (2021) 11:578831. doi: 10.3389/fphar.2020.578831
29.
Ma Y Ajnakina O Steptoe A Cadar D . Higher risk of dementia in English older individuals who are overweight or obese. Int J Epidemiol. (2020) 49:1353–65. doi: 10.1093/ije/dyaa099
30.
Nianogo RA Rosenwohl-Mack A Yaffe K Carrasco A Hoffmann CM Barnes DE . Risk factors associated with Alzheimer disease and related dementias by sex and race and ethnicity in the US. JAMA Neurol. (2022) 79:584–91. doi: 10.1001/jamaneurol.2022.0976
31.
Cholerton B Baker LD Montine TJ Craft S . Type 2 diabetes, cognition, and dementia in older adults: toward a precision health approach. Diabet Spectr. (2016) 29:210–9. doi: 10.2337/ds16-0041
32.
Jang H Bae JB Dardiotis E Scarmeas N Sachdev PS Lipnicki DM et al . Differential effects of completed and incomplete pregnancies on the risk of Alzheimer disease. Neurology. (2018) 91:e643–51. doi: 10.1212/WNL.0000000000006000
33.
Wang Y Xie D Ma S Shao N Zhang X Wang X . Exploring the common mechanism of vascular dementia and inflammatory bowel disease: a bioinformatics-based study. Front Immunol. (2024) 15:1347415. doi: 10.3389/fimmu.2024.1347415
34.
Asby D Boche D Allan S Love S Miners JS . Systemic infection exacerbates cerebrovascular dysfunction in Alzheimer's disease. Brain. (2021) 144:1869–83. doi: 10.1093/brain/awab094
35.
Morgado PJ Suárez JA Gómez LG Morgado PJ Jr . Histoclinical basis for a new classification of hemorrhoidal disease. Dis Colon Rectum. (1988) 31:474–80. PMID:
36.
Kinney JW Bemiller SM Murtishaw AS Leisgang AM Salazar AM Lamb BT . Inflammation as a central mechanism in Alzheimer's disease. Alzheimer's Dementia (New York, N Y). (2018) 4:575–90. doi: 10.1016/j.trci.2018.06.014
37.
Walker KA Ficek BN Westbrook R . Understanding the role of systemic inflammation in Alzheimer's disease. ACS Chem Neurosci. (2019) 10:3340–2. doi: 10.1021/acschemneuro.9b00333
38.
Berkel AE Witte ME Koop R Hendrix MG Klaase JM . Brain abscess after transanal hemorrhoidal dearterialization: a case report. Case Rep Gastroenterol. (2013) 7:208–13. doi: 10.1159/000351817
39.
Yang F Lan Z Chen H He R . Causal associations between human gut microbiota and hemorrhoidal disease: a two-sample Mendelian randomization study. Medicine. (2024) 103:e37599. doi: 10.1097/MD.0000000000037599
40.
Palumbo VD Tutino R Messina M Santarelli M Nigro C Lo Secco G et al . Altered gut microbic flora and haemorrhoids: could they have a possible relationship?J Clin Med. (2023) 12:2198. doi: 10.3390/jcm12062198
41.
Escalante J Artaiz O Diwakarla S McQuade RM . Leaky gut in systemic inflammation: exploring the link between gastrointestinal disorders and age-related diseases. Geroscience. (2025) 47:1–22. doi: 10.1007/s11357-024-01451-2
42.
Grammas P . Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer's disease. J Neuroinflammation. (2011) 8:26. doi: 10.1186/1742-2094-8-26
43.
Fu J Qin Y Xiao L Dai X . Causal relationship between gut microflora and dementia: a Mendelian randomization study. Front Microbiol. (2023) 14:1306048. doi: 10.3389/fmicb.2023.1306048
Summary
Keywords
hemorrhoids, dementia, epidemiology, veins, treatment
Citation
Woo HG, Park M-S, Park J-Y, Chun H and Song T-J (2025) Association of hemorrhoidal disease with dementia risk: a nationwide cohort study. Front. Neurol. 16:1655944. doi: 10.3389/fneur.2025.1655944
Received
29 June 2025
Accepted
19 September 2025
Published
02 October 2025
Volume
16 - 2025
Edited by
Lu Zhang, Indiana University Indianapolis, United States
Reviewed by
Xiaowei Yu, University of Texas at Arlington, United States
Saiyang Na, The University of Texas at Arlington, United States
Updates
Copyright
© 2025 Woo, Park, Park, Chun and Song.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tae-Jin Song, knstar@ewha.ac.kr
†ORCID: Tae-Jin Song, orcid.org/0000-0002-9937-762X
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.