Abstract
Background:
In Parkinson’s disease (PD) patients, the severity of motor symptoms is closely related to the degree of gait disorder and cognitive impairment; notably, the latter also exhibits a significant correlation with gait disorder. Evidently, there exists a complex relationship between motor symptoms, cognitive function, and gait characteristics.
Methods:
This study aims to conduct an in-depth analysis of the relationships among MDS-UPDRS III score, MoCA score, and gait parameters by constructing a mediation model.
Results:
We found that a higher MDS-UPDRS III score was associated with a smaller plantar dorsiflexion angle, slower velocity, and worse swing phase symmetry, and these associations were not mediated by the MoCA score. Age was a confounder in the relationship between MDS-UPDRS III score, MoCA, and velocity. A higher MDS-UPDRS III score was associated with shorter stride length, and this association was partially mediated by the MoCA score.
Conclusion:
The results indicated that, when analyzing plantar dorsiflexion angle and swing phase symmetry in PD patients, the influence of motor symptoms was dominant; when analyzing stride length, motor symptoms and cognitive function need to be considered simultaneously; when analyzing velocity, the influence of motor symptoms and age should be focused on.
1 Introduction
The motor symptoms of Parkinson’s disease (PD) are complex and diverse, with gait disorder being one of the important clinical manifestations (1). Typical motor symptoms such as bradykinesia and rigidity directly affect the normal gait pattern (2). The gait characteristics of PD usually include reduced walking speed, shortened step length, and gait instability (2). Moreover, with the progression of the disease, these gait problems will gradually worsen (3) and severely affect the patients’ daily activities and the quality of life (4). Therefore, an accurate, scientific, timely, and reliable gait monitoring system is of crucial significance for clinicians to achieve accurate diagnosis, optimize treatment plans, and effectively monitor the progression of the disease.
Based on these, research studies on the quantitative assessment of gait have achieved profound progress. Numerous studies analyzed the characteristics of gait disorder in PD patients by comparing them with healthy controls (5, 6). Some researchers focused on exploring gait characteristics in early-stage (7) and drug-naive patients (8). In addition, researchers also attempted to explore gait characteristics under different Hoehn and Yahr (H&Y) stages (9) and section III of Unified Parkinson’s Disease Rating Scale (UPDRS III) score (10). Based on disease phenotypes, the gait differences between tremor-dominant (TD) and postural instability and gait difficulty (PIGD) were compared (11).
It is worth noting that gait disorder is not unique in PD (12); it is also very common in the field of cognitive impairment. Ghoraani et al. divided participants into healthy individuals and individuals with mild cognitive impairment and Alzheimer’s disease, and they found that there was a significant positive correlation between the degree of cognitive impairment and the severity of gait disorder (13). Moreover, gait abnormalities are also very common among elderly people, primarily manifesting as slowed walking speed, reduced step length, increased step width, and prolonged double support phase (14), and as age increases, these gait changes became increasingly pronounced.
Among PD patients, the severity of motor symptoms is closely related to the degree of gait disorder (3) and cognitive impairment (15), and cognitive impairment also has a significant correlation with gait disorder (13). There is obviously a complex relationship among motor symptoms, cognitive function, and gait characteristics in PD patients. However, most current studies on the gait of PD patients have limitations. Participants were often limited to those with a Mini-Mental State Examination (MMSE) score of 24 or above (16), or their cognitive status was completely ignored. Although some researchers have noticed the relationship among the three, most of them divided PD patients into a cognitive impairment group and a cognitive normal group and conducted a comparative analysis of the gait differences between the two groups (17). Several studies investigated the associations between cognitive and gait disorders in individuals with PD during single-task and dual-task walking (18, 19) and demonstrated that PD increased reliance on cognition for gait control (20). Based on this finding, some investigations showed that reducing attentional costs (21) could improve gait function (22). However, these studies did not explore the relationships among the three factors in detail.
Mediation analysis is used when the researcher seeks to understand, explain, or test a hypothesis about how or by what process or mechanism a variable X transmits its effect on Y. A mediator variable M is causally located between X and Y and is the conduit through which X transmits its effect on Y. A mediator can be most anything—a psychological state, a cognitive or affective response, or a biological change (23). Some mediation models have been used in PD. Scholl et al. recently showed that cognitive function mediated the relationship between PD severity and freezing-of-gait severity (24). However, they used the freezing-of-gait questionnaire to assess the severity of freezing of gait, but did not involve specific quantitative evaluations of gait parameters such as gait speed and stride length. Another study used a mediation model to relate gait metrics, UPDRS, and outcomes (e.g., falls risk) (25), but they did not consider the impact of cognition. Dubbioso et al. investigated cognitive impairment, gait variability, and fall risk in amyotrophic lateral sclerosis and showed that a mild cognitive impairment is associated with exaggerated gait variability and predicts the occurrence and number of short-term falls, but their study was applied in amyotrophic lateral sclerosis, not in PD (26).
This study aims to conduct an in-depth analysis of the relationships among Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS III) score (27), the Montreal Cognitive Assessment (MoCA) score (28), and gait parameters by constructing a mediation model. The aim of this study is to reveal the mechanism among motor symptoms, cognitive function, and gait characteristics in PD.
2 Methods
2.1 Participants
A total of 28 patients with primary PD were included in this study, with 12 men and 16 women. The inclusion criteria were as follows: (1) meeting the diagnostic criteria for primary PD established by the Movement Disorder Society (MDS) in 2015 (29); (2) H&Y stage ranged from 1 to 3; (3) age ≥40 years; and (4) being able to complete the test independently without the assistance of others. The exclusion criteria included the following: (1) Atypical Parkinsonian syndrome; (2) severe systemic and other neurological diseases; and (3) uncorrected visual impairments or diseases that may change gait patterns. This study was approved by the Ethics Committee of Central Hospital of Dalian University of Technology (YN2022-039-57). Each participant signed a consent form before participating in this study.
2.2 Clinical assessment
This study collected the demographic and clinical information of the patients, including age, gender, height (cm), weight (kg), disease duration, and levodopa equivalent daily dose (LEDD) (30). All participants underwent a comprehensive neurological examination and clinical scale assessment by experts in movement disorders. MoCA was used to evaluate the cognitive function, and the test was completed during the “ON” state (i.e., the stage when anti-Parkinson drugs exert their full efficacy). The H&Y staging (31) and MDS-UPDRS III scales were used to assess the disease severity, and they were conducted during the “OFF” state (i.e., the stage at least 12 h without using any anti-Parkinson drugs).
2.3 Gait assessment
In this study, a self-developed measurement system was used to collect gait data. The structure of the gait data acquisition system was shown in our previous study (32). This system consisted of a handheld control terminal and two sensor nodes (the sampling frequency was 200 Hz). The sensor nodes integrated high-precision sensing and data processing modules, with the following specific configurations: (1) a three-axis accelerometer (dynamic range: ±18 g, sensitivity (/LSB): 0.833 mg, bandwidth (kHz): 330, and alignment error (deg): 0.2); (2) a three-axis gyroscope (dynamic range: ±1,000 deg./s, sensitivity (/LSB): 0.04 deg./s, bandwidth (kHz): 330, and alignment error (deg): 0.05); (3) a microcontroller; (4) a WIFI wireless communication module; and (5) a 450-mAh lithium battery. The sensor nodes were installed on the side of the lateral malleolus of each foot. The handheld terminal supported wireless command transmission and could regulate the working state of the sensors wirelessly. The collected data were uploaded through a wireless network for gait analysis.
The gait parameters collected in this study include plantar dorsiflexion angle (PDA) (left/right, L/R), stride length (L/R), velocity, cadence, stride time, support phase symmetry, and swing phase symmetry (Table 1). The calculation method for gait parameters had been described in a previous study (33). All data was collected during the “OFF” state of the drugs. The participants independently completed walking on a 10-m obstacle-free path at a natural and comfortable speed. Protective facilities were provided throughout the experiment to avoid falling. At the same time, a standardized operation process was adopted, such as avoiding the interference of repetitive verbal cues on the gait pattern, and the data of the first and last steps were excluded in order to ensure the accuracy of the analysis. The accuracy of the gait data acquisition system had been validated in a previous study (34); and compared to the gold standard (optical motion capture), the position estimation error is <1% with regard to three-dimensional motion.
Table 1
| Gait parameters | Definition |
|---|---|
| Plantar dorsiflexion angle (PDA) (°) | The absolute value of the angle between the foot and the ground at heel-strike moment. |
| Stride length (m) | Distance between two consecutive heel-strikes, that is, the distance between the landing points of the same feet. |
| Velocity (m/s) | Calculated by dividing stride length by stride time. |
| Cadence (steps/min) | Steps per minute. |
| Stride time (s) | Duration between two heel strikes of the same foot. |
| Support phase symmetry | , the smaller value indicates the better symmetry. |
| Swing phase symmetry | , the smaller value indicates the better symmetry. |
Specific definitions of gait parameters in this study.
L, left; R, right.
2.4 Statistical analysis
This study used SPSS 26.0 (IBM Corporation, Armonk, NY) for statistical analysis. Continuous variables with normal distributions were presented as means ± standard deviations (x̄ ± s). Continuous variables with non-normal distributions were presented as medians and interquartile distances [M (P25, P75)], and the count variables were described by frequency. The correlation between clinical assessment indicators and gait parameters was analyzed using Spearman’s correlation. A p-value of < 0.05 was considered statistically significant. The figures were configured using OriginPro 2021 (OriginLab Corporation, Northampton, Massachusetts, USA).
2.5 Mediation model
Considering that the MDS-UPDRS III score was correlated with the severity of gait disorder and MoCA score, the MoCA score was also correlated with the severity of gait disorder. This study was conducted to investigate the mediation effect of MoCA between MDS-UPDRS III score and gait parameters. Statistical analysis found that age had no significant correlation with MDS-UPDRS III score, but was associated with MoCA score, suggesting that age is a confounder. Based on this, this study included age as a covariate in the mediation model.
Mediation analysis was performed using the PROCESS macro for SPSS, which employed a regression-based approach to examine the indirect effects; specifically, we conducted a simple mediation analysis following Model 4 according to the Bootstrap method proposed by Hayes (35). This study selected the gait parameters that significantly correlated with the MDS-UPDRS III score, including PDA (L/R), stride length (L/R), velocity, and swing phase symmetry, and included them in the model. Among them, the MDS-UPDRS III score was taken as the independent variable (X), the MoCA score was taken as the mediator variable (M), each gait parameter was taken as the dependent variable (Y), and age was taken as a covariate variable in the mediation model (Figure 1). The significance of the total effect, direct effect, and indirect effect was tested using bootstrapping with 5,000 resamples, and a 95% confidence interval (CI) was reported. The effect was considered statistically significant if the 95%CI did not include 0.
Figure 1

Conceptual diagram of mediation model 4. It illustrating the relationship between X (MDS-UPDRS III score), M (MoCA), and Y (Gait parameters). Arrows indicate direction with “Std. Coef.” labels.
3 Results
3.1 Demographic and clinical characteristics
A total of 28 PD patients were included in this study, and their demographic and clinical characteristics were collected. Among them, there were 12 men and 16 women, with an average age of 68.71 ± 8.93 years, a height of 164.32 ± 7.72 cm, and a weight of 65.71 ± 9.26 kg, as shown in the first four lines of Table 2. In terms of clinical characteristics, the disease duration ranged from 1 to 8 years, the MoCA score was 21.14 ± 4.31 points, the H&Y stage ranged from stage 1 to stage 3, the MDS-UPDRS III score was 37.57 ± 15.54 points, and the LEDD was between 0 and 750 mg, as shown in lines 5–9 of Table 2. In addition, the gait parameters of PD patients are detailed in lines 10 to 18 of Table 2.
Table 2
| Variables | PD patients (n = 28) |
|---|---|
| Gender (male/female) | 12/16 |
| Age (years) | 68.71 ± 8.93 |
| Height (cm) | 164.32 ± 7.72 |
| Weight (kg) | 65.71 ± 9.26 |
| Disease duration (years) | 1–8 |
| MoCA (score) | 21.14 ± 4.31 |
| H&Y stage | 1–3 |
| MDS-UPDRS III (score) | 37.57 ± 15.54 |
| LEDD (mg) | 0–750 |
| PDA (L) (°) | 7.31 (2.23, 12.09) |
| PDA (R) (°) | 8.28 ± 5.60 |
| Stride length (L) (m) | 0.97 (0.73, 1.08) |
| Stride length (R) (m) | 0.85 ± 0.28 |
| Velocity (m/s) | 0.73 ± 0.27 |
| Cadence (steps/min) | 52.04 ± 6.91 |
| Stride time (s) | 1.12 (1.05, 1.23) |
| Support phase symmetry | 3.09 (1.64, 4.85) |
| Swing phase symmetry | 3.27 (1.59, 7.84) |
Demographic and clinical characteristics.
PD, Parkinson’s disease; MoCA, Montreal Cognitive Assessment; H&Y, Hoehn and Yahr; MDS-UPDRS III, Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale Part III; LEDD, Levodopa Equivalent Daily Dose; PDA, Plantar dorsiflexion angle; L, Left; R, Right.
3.2 Correlation between clinical assessment indicators and gait parameters
This study explored the correlations between clinical assessment indicators and gait parameters, and the results were visually presented through a correlation heatmap (Figure 2). The data showed that the MDS-UPDRS III score was negatively correlated with PDA (L/R), stride length (L/R), and velocity, and positively correlated with swing phase symmetry. There was no correlation between MDS-UPDRS III score and cadence, stride time, and support phase symmetry. MoCA score was positively correlated with PDA (L), stride length (L/R), and velocity, but there was no significant correlation between MoCA score and PDA (R), cadence, stride time, support phase symmetry, and swing phase symmetry. Additionally, age was negatively correlated with PDA (L), stride length (L/R), and velocity but had no correlations with other gait parameters.
Figure 2
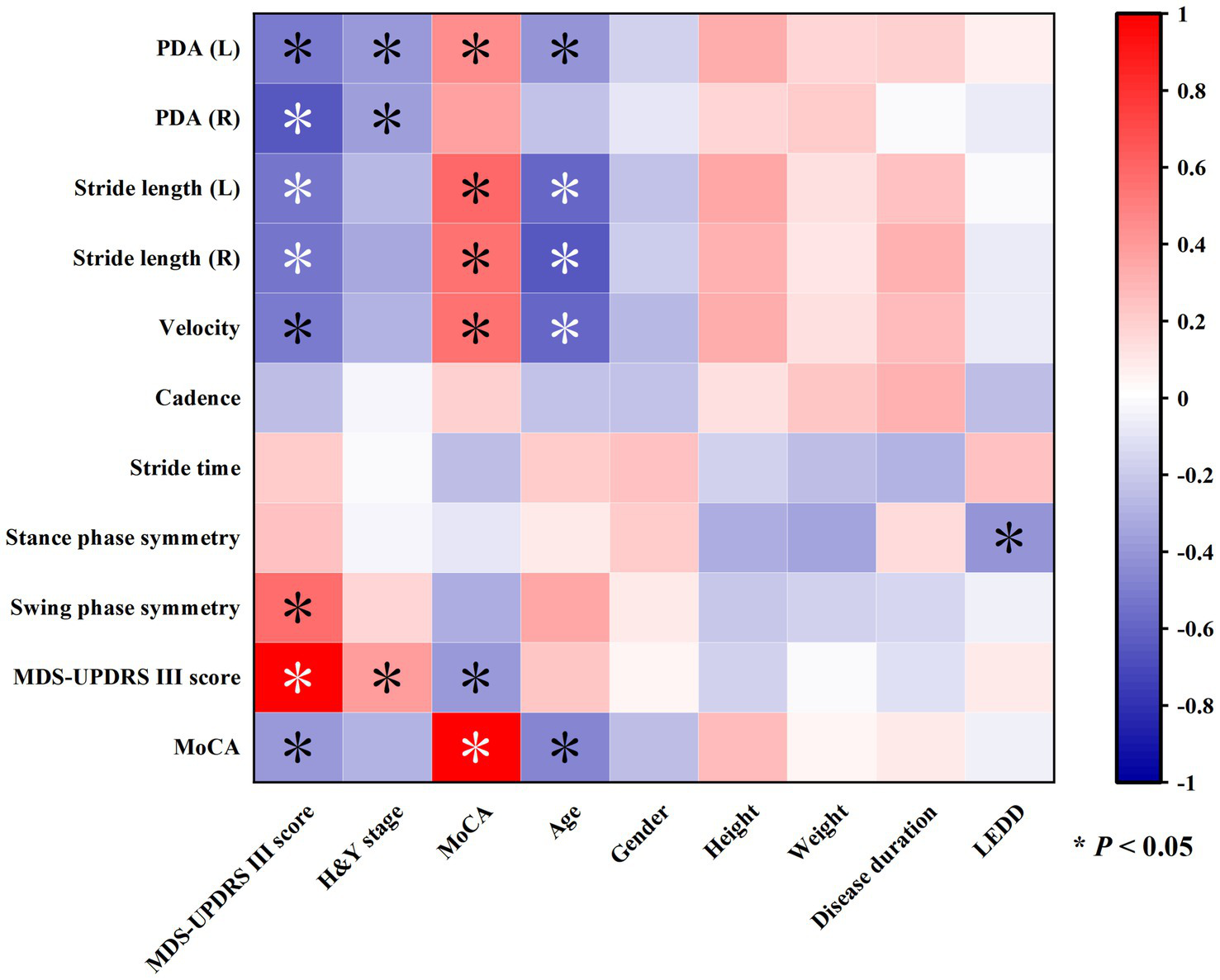
Heatmap showing correlations among various gait and clinical parameters. Colors range from red to blue, indicating positive and negative correlations, respectively. Asterisks mark significant correlations with p-values less than 0.05.
Notably, the MDS-UPDRS III score was negatively correlated with the MoCA score but not with age, and the MoCA score was negatively correlated with age.
Further analysis of the relationships between gait parameters and demographic characteristics (gender, height, weight) revealed no significant correlations, suggesting that these factors may have limited influence on gait parameters in this study.
3.3 Mediation model
3.3.1 The mediation effect of MoCA in the relationship between MDS-UPDRS III score and PDA (L/R)
The diagram of the mediation effect of MoCA in the relationship between MDS-UPDRS III score and PDA (L/R) are shown in Figures 3A,B, and the regression analysis among the variables are shown in Table 3, indicating that MDS-UPDRS III score had a negative relationship with PDA (L/R) and MoCA, but MoCA had no relationship with PDA (L/R).
Figure 3
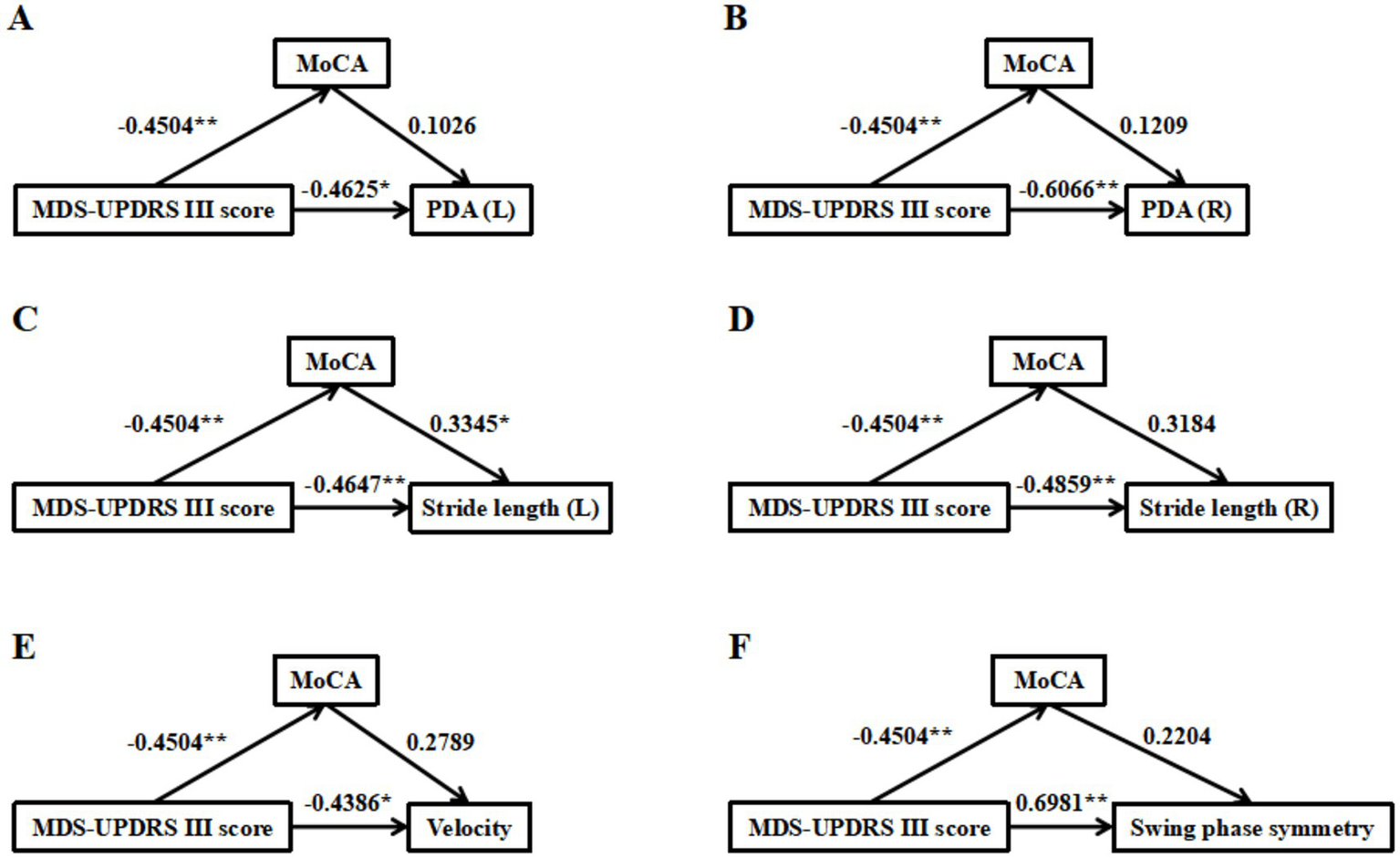
Mediation effect of MoCA in the relationship of MDS-UPDRS III score and gait parameters (labeled A–F, including PDA (left, right), stride length (left, right), velocity and swing phase symmetry). Values on arrows indicate correlation strengths, with significance levels marked by asterisks. *p < 0.05, **p < 0.01, ***p < 0.001.
Table 3
| Model | Dependent variable | Independent variable | R 2 | F | Coef. | Bootstrap 95% CI | Std. Coef. | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| LLCI | ULCI | ||||||||
| MDS-UPDRS III score→ MoCA→PDA (L) | PDA (L) | MDS-UPDRS III score | 0.3410 | 6.4669 | −0.2339 | −0.3925 | −0.0754 | −0.5087 | 0.0055 |
| MoCA | MDS-UPDRS III score | 0.4527 | 10.3403 | −0.1249 | −0.2120 | −0.0378 | −0.4504 | 0.0068 | |
| PDA (L) | MDS-UPDRS III score | 0.3467 | 4.2458 | −0.2127 | −0.4002 | −0.0252 | −0.4625 | 0.0279 | |
| MoCA | 0.1701 | −0.5931 | 0.9333 | 0.1026 | 0.6497 | ||||
| MDS-UPDRS III score→ MoCA→PDA (R) | PDA (R) | MDS-UPDRS III score | 0.4304 | 9.4456 | −0.2382 | −0.3537 | −0.1227 | −0.6610 | 0.0003 |
| MoCA | MDS-UPDRS III score | 0.4527 | 10.3403 | −0.1249 | −0.2120 | −0.0378 | −0.4504 | 0.0068 | |
| PDA (R) | MDS-UPDRS III score | 0.4384 | 6.2453 | −0.2186 | −0.3548 | −0.0823 | −0.6066 | 0.0029 | |
| MoCA | 0.1571 | −0.3974 | 0.7116 | 0.1209 | 0.5641 | ||||
| MDS-UPDRS III score→ MoCA→ Stride length (L) | Stride length (L) | MDS-UPDRS III score | 0.6203 | 20.4233 | −0.0114 | −0.0163 | −0.0066 | −0.6153 | 0.0001 |
| MoCA | MDS-UPDRS III score | 0.4527 | 10.3403 | −0.1249 | −0.2120 | −0.0378 | −0.4504 | 0.0068 | |
| Stride length (L) | MDS-UPDRS III score | 0.6816 | 17.1219 | −0.0086 | −0.0139 | −0.0033 | −0.4647 | 0.0025 | |
| MoCA | 0.0224 | 0.0009 | 0.0439 | 0.3345 | 0.0420 | ||||
| MDS-UPDRS III score→ MoCA→ Stride length (R) | Stride length (R) | MDS-UPDRS III score | 0.6295 | 21.2416 | −0.0114 | −0.0161 | −0.0067 | −0.6293 | < 0.0001 |
| MoCA | MDS-UPDRS III score | 0.4527 | 10.3403 | −0.1249 | −0.2120 | −0.0378 | −0.4504 | 0.0068 | |
| Stride length (R) | MDS-UPDRS III score | 0.6850 | 17.3994 | −0.0088 | −0.0139 | −0.0037 | −0.4859 | 0.0017 | |
| MoCA | 0.0208 | −0.0001 | 0.0417 | 0.3184 | 0.0508 | ||||
| MDS-UPDRS III score→ MoCA→ Velocity | Velocity | MDS-UPDRS III score | 0.5274 | 13.9488 | −0.0098 | −0.0149 | −0.0047 | −0.5643 | 0.0005 |
| MoCA | MDS-UPDRS III score | 0.4527 | 10.3403 | −0.1249 | −0.2120 | −0.0378 | −0.4504 | 0.0068 | |
| Velocity | MDS-UPDRS III score | 0.5700 | 10.6034 | −0.0076 | −0.0134 | −0.0019 | −0.4386 | 0.0115 | |
| MoCA | 0.0175 | −0.0059 | 0.0409 | 0.2789 | 0.1363 | ||||
| MDS-UPDRS III score→ MoCA→ Swing phase symmetry | Swing phase symmetry | MDS-UPDRS III score | 0.3374 | 6.3643 | 0.2376 | 0.1004 | 0.3748 | 0.5988 | 0.0015 |
| MoCA | MDS-UPDRS III score | 0.4527 | 10.3403 | −0.1249 | −0.2120 | −0.0378 | −0.4504 | 0.0068 | |
| Swing phase symmetry | MDS-UPDRS III score | 0.3640 | 4.5778 | 0.2770 | 0.1174 | 0.4367 | 0.6981 | 0.0015 | |
| MoCA | 0.3154 | −0.3345 | 0.9653 | 0.2204 | 0.7923 | ||||
Regression analysis of variable relationships.
MDS-UPDRS III, Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale Part III; MoCA, Montreal Cognitive Assessment; PDA, Plantar dorsiflexion angle; L, Left; R, Right; Coef. = Coefficients; Std. Coef. = Standardized coefficients. P value < 0.05 were shown in bold.
As shown in Table 4, the upper and lower limits of the 95% CI of the total effect and direct effect did not include 0, but the upper and lower limits of the 95% CI of the indirect effect included 0. This finding indicated that a higher MDS-UPDRS III score was associated with smaller PDA (direct effect L: 90.94%, direct effect R: 91.77%), and this association was not mediated by MoCA score.
Table 4
| Association | Model effect | Effect size | SE | Bootstrap 95% CI | P-value | Effect proportion | |
|---|---|---|---|---|---|---|---|
| LLCI | ULCI | ||||||
| MDS-UPDRS III score→MoCA→PDA (L) | Total effect | −0.2339 | 0.0770 | −0.3925 | −0.0754 | 0.0055 | 100% |
| Direct effect | −0.2127 | 0.0908 | −0.4002 | −0.0252 | 0.0279 | 90.94% | |
| Indirect effect | −0.0212 | 0.0473 | −0.1249 | 0.0680 | > 0.05 | N/A | |
| MDS-UPDRS III score→MoCA→PDA (R) | Total effect | −0.2382 | 0.0561 | −0.3537 | −0.1227 | 0.0003 | 100% |
| Direct effect | −0.2186 | 0.0660 | −0.3548 | −0.0823 | 0.0029 | 91.77% | |
| Indirect effect | −0.0196 | 0.0329 | −0.0898 | 0.0416 | > 0.05 | N/A | |
| MDS-UPDRS III score→MoCA→Stride length (L) | Total effect | −0.0114 | 0.0024 | −0.0163 | −0.0066 | 0.0001 | 100% |
| Direct effect | −0.0086 | 0.0026 | −0.0139 | −0.0033 | 0.0025 | 75.44% | |
| Indirect effect | −0.0028 | 0.0013 | −0.0058 | −0.0005 | < 0.05 | 24.56% | |
| MDS-UPDRS III score→MoCA→Stride length (R) | Total effect | −0.0114 | 0.0023 | −0.0161 | −0.0067 | < 0.0001 | 100% |
| Direct effect | −0.0088 | 0.0025 | −0.0139 | −0.0037 | 0.0017 | 77.19% | |
| Indirect effect | −0.0026 | 0.0013 | −0.0054 | −0.0003 | < 0.05 | 22.81% | |
| MDS-UPDRS III score→MoCA→Velocity | Total effect | −0.0098 | 0.0025 | −0.0149 | −0.0047 | 0.0005 | 100% |
| Direct effect | −0.0076 | 0.0028 | −0.0134 | −0.0019 | 0.0115 | 77.55% | |
| Indirect effect | −0.0022 | 0.0013 | −0.0052 | 0.0001 | > 0.05 | N/A | |
| MDS-UPDRS III score→MoCA→Swing phase symmetry | Total effect | 0.2376 | 0.0666 | 0.1004 | 0.3748 | 0.0015 | 100% |
| Direct effect | 0.2770 | 0.0774 | 0.1174 | 0.4367 | 0.0015 | 116.58% | |
| Indirect effect | −0.0394 | 0.0404 | −0.1313 | 0.0262 | > 0.05 | N/A | |
Decomposition of total effects, direct effects, and indirect effects.
MDS-UPDRS III, Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale Part III; MoCA, Montreal Cognitive Assessment; PDA, Plantar dorsiflexion angle; L, Left; R, Right; SE, Standard error; CI, Confidence interval; LLCI, Lower limits of the 95% confidence interval; ULCI, Upper limits of the 95% confidence interval; N/A, not applicable.
3.3.2 The mediation effect of MoCA in the relationship between MDS-UPDRS III score and stride length (L/R)
The diagram of the mediation effect of MoCA in the relationship between MDS-UPDRS III score and stride length (L/R) are shown in Figures 3C,D, and the regression analysis among the variables are shown in Table 3, which indicated that the MDS-UPDRS III score had a negative relationship with stride length (L/R) and MoCA, and MoCA had a positive relationship with stride length (L), but had no relationship with stride length (R) (p = 0.0508).
As shown in Table 4, the upper and lower limits of the 95% CI of the total effect, direct effect, and indirect effect did not include 0. This finding indicated that a higher MDS-UPDRS III score was associated with shorter stride length (direct effect L: 75.44%, direct effect R: 77.19%), and this association was partially mediated by the MoCA score (indirect effect L: 24.56%, indirect effect R: 22.81%).
The mediation effect of MoCA in the relationship between MDS-UPDRS III score and stride length (L/R) was significant before and after including age as a covariate variable, suggesting that the confounding effect of age was limited, and the association among MDS-UPDRS III score, MoCA, and stride length (L/R) had strong stability.
3.3.3 The mediation effect of MoCA in the relationship between MDS-UPDRS III score and velocity
The diagram of the mediation effect of MoCA in the relationship between MDS-UPDRS III score and velocity is shown in Figure 3E, and the regression analysis among the variables is shown in Table 3, indicating that MDS-UPDRS III score had a negative relationship with velocity and MoCA, while MoCA had no relationship with velocity.
According to Table 4, the upper and lower limits of the 95% CI of the total effect and direct effect did not include 0, but the upper and lower limits of the 95% CI of the indirect effect included 0. This finding indicated that a higher MDS-UPDRS III score was associated with lower velocity (direct effect: 77.55%), and this association was not mediated by MoCA score.
Interestingly, the mediation effect of MoCA was significant before including age as a covariate variable, but it became non-significant after including age, indicating a notable confounding effect of age. Further comparison of the R2 values (Table 5) revealed that, when predicting MoCA with MDS-UPDRS III score alone, the R2 value increased from 0.3006 to 0.4527 (ΔR2 = 0.1521) after including age, reflecting the additional explanatory power of age on MoCA. When predicting velocity with both MDS-UPDRS III score and MoCA, the R2 value increased from 0.5318 to 0.5700 (ΔR2 = 0.0382) after including age, reflecting the additional explanatory power of age on velocity. The larger increase in R2 for MoCA (0.1521 > 0.0382) suggested that age had a stronger influence on MoCA, and the suppression of the mediation effect was attributed to the confounder of age.
Table 5
| Dependent variable | Independent variable | R 2 before including age | R 2 after including age | Difference (ΔR2) |
|---|---|---|---|---|
| MoCA | MDS-UPDRS III score | 0.3006 | 0.4527 | 0.1521 |
| Velocity | MDS-UPDRS III score | 0.5318 | 0.5700 | 0.0382 |
| MoCA |
Comparison of R2 values before and after including age as a covariate variable for the association among MDS-UPDRS III score, MoCA and velocity.
MDS-UPDRS III, Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale Part III; MoCA, Montreal Cognitive Assessment.
3.3.4 The mediation effect of MoCA in the relationship between MDS-UPDRS III score and swing phase symmetry
The diagram of the mediation effect of MoCA in the relationship between MDS-UPDRS III score and swing phase symmetry is shown in Figure 3F, and the regression analysis among the variables is shown in Table 3, indicating that MDS-UPDRS III score had a positive relationship with swing phase symmetry and had a negative relationship with MoCA, but MoCA had no relationship with swing phase symmetry.
As shown in Table 4, the upper and lower limits of the 95% CI of the total effect and direct effect did not include 0, but the upper and lower limits of the 95% CI of the indirect effect included 0. This finding indicated that a higher UPDRS III score was associated with worse swing phase symmetry (direct effect: 116.58%), and this association was not mediated by MoCA. Notably, the correlation coefficient was greater than 100%, indicating a suppression effect, but it was not statistically significant.
4 Discussion
Gait control is a complex process that involves the integration of multiple systems, such as the motor, perceptual, and cognitive processes (36, 37). Once there is dysfunction in these systems, gait abnormalities emerge. In patients with PD, rigidity symptom limits the range of joint movement, and stride length is closely related to the torque of the knee and ankle joints (38). PDA is closely related to the tibialis anterior muscle (39), so PD patients exhibit shortened stride length and reduced PDA. In addition, bradykinesia symptoms in PD patients can directly lead to a decrease in velocity (40). Moreover, the symptoms of PD are mostly left–right asymmetric (41), further exacerbating the degree of asymmetry of gait. Our research findings were consistent with the above findings.
We found that a higher MDS-UPDRS III score was associated with smaller PDA (L/R) and worse swing phase symmetry, and these associations were not mediated by the MoCA score. A higher MDS-UPDRS III score was associated with shorter stride length, and this association was partially mediated by MoCA score, and the mediation effect had strong stability and was less affected by age. A higher MDS-UPDRS III score was associated with slower velocity, and this association was not mediated by MoCA score. The mediation effect held when the age covariate was not included but failed to hold when age was included.
It has been demonstrated that, as PD progresses, gait and cognition deteriorate (42). Associations between cognitive and gait disorders have been consistently demonstrated in individuals with PD during single- and dual-task walking (18, 19) to compensate for declined gait performance, and individuals increase reliance on cognition to control gait (20). Importantly though, cognitive decline may not be a coincidental impairment that accompanies gait deficits but may rather stem from a pathology linked closely to the mechanisms that affect gait in PD (43).
From the neuroanatomical perspective, cognitive function and gait control share the same neural regulatory basis. The prefrontal cortex not only plays an important role in cognitive function but is also a key component of the motor execution pathway (44, 45). This means that damage to cognitive function may interfere with the prefrontal cortex in task situations. Studies have shown that, when the cognitive resources decreased, the gait patterns showed abnormalities (46).
Functional magnetic resonance imaging (fMRI) is used in studies of motor imagery. Notably, compared with healthy controls, PD patients exhibited increased prefrontal activation during gait imagery tasks (47, 48), and this observation reflected heightened cognitive effort, even during the gait preparation phase (2).
Recently, electroencephalography (EEG) and functional near-infrared spectroscopy (fNIRS) have emerged as promising tools for investigating brain function during walking. These dynamic imaging techniques facilitate a better understanding of neural activation patterns throughout the walking process. In PD, motor abnormalities were associated with reduced power in low-frequency bands. This neural feature was also linked to attention and executive function in cognitive processes. Specifically, changes in β activity correlated with freezing of gait, while alterations in γ activity were associated with motor execution and gait. Additionally, variations in θ activity were related to motor preparation (2). Findings from fNIRS studies further indicated that, compared with healthy controls, PD patients showed increased prefrontal activation even during simple walking tasks. This phenomenon suggested that PD patients required greater engagement of cognitive resources to maintain walking function (49). Kang et al. demonstrated the association between attention, frontal-executive function, and gait in patients with PD (50).
Age constitutes another key factor. With advancing age, the total prefrontal volume decreases, including white matter volume and gray matter volume, and the decline of white matter volume is disproportionately greater than the decline of gray matter volume (51). Age-related alterations in synaptic structure and function of the prefrontal cortex, as well as the remodeling of neuronal networks, exhibited a close correlation with age-dependent cognitive changes (52). Compared with younger adults, older individuals relied on the engagement of broader brain regions during motor control, with the prefrontal cortex being a particularly pivotal area (53). Importantly, these prefrontal regions were intricately linked to both cognitive function and gait regulation. This heightened and overlapping neural demand, coupled with age-related structural and functional declines in the prefrontal cortex, ultimately contributed to the development of age-associated gait disorder and cognitive impairment in older adults.
Collectively, these findings enhanced our understanding of the interplay between cognitive function, gait, age, and neural activity in PD, laying a foundation for future research. Moving forward, integrating larger-sample longitudinal designs, more precise cognitive domain-specific assessment tools, and advanced dynamic neuroimaging techniques could further elucidate the causal relationships and shared mechanisms underlying cognitive function and gait disorder in PD. Such advancements may ultimately inform the development of targeted interventions to mitigate these disabling symptoms and improve the quality of life for PD patients. For example, for patients with abnormal gait parameters identified by wearable devices, rehabilitation therapists could prioritize targeted training (e.g., for PDA and swing phase symmetry, motor symptoms should be the primary consideration; for stride length, motor symptoms and cognition should be targeted; and for velocity, motor symptoms and age should be considered) or adjust the intensity/duration of gait training to match the patients’ cognitive reserve and age, thereby improving the efficiency of rehabilitation.
This study had the following limitations. Our study is a cross-sectional study; although we inferred the relationships among the severity of PD, cognition, and gait based on the characteristics of PD and previous literature, our manuscript lacked longitudinal observational data to directly verify these causal relationships. The sample size of this study was small, with a potential risk of false negatives where “latent associations may not be detected.” The potential residual confounding, such as gender, height, weight, education level, different disease phenotypes (such as TD/PIGD), and freezing of gait status, was not included in the mediation model. This study used the MoCA scale to assess cognitive function but did not distinguish specific cognitive domains. For future studies, it is recommended to include a larger sample size, more variables, and adopt scales that cover detailed cognitive domains, which will help provide more comprehensive information.
5 Conclusion
This study explored the relationships between motor symptoms (MDS-UPDRS III), cognitive function (MoCA), and gait parameters in PD patients. Key findings included (1) higher MDS-UPDRS III score correlated with smaller PDA, slower velocity, and worse swing phase symmetry, and the association was not mediated by MoCA; (2) higher MDS-UPDRS III score correlated with shorter stride length, and the association was partially mediated by MoCA; (3) the MDS-UPDRS III → MoCA→stride length association demonstrated strong stability and less influenced by age; and (4) the MDS-UPDRS III → MoCA→velocity association was unstable and could be suppressed by age.
Theoretically, these results support a mediation effect of MoCA on stride length. Practically, they guide targeted interventions: motor-focused training for PDA/velocity/swing phase symmetry abnormalities, combined motor-cognitive training for stride length reduction, and age-aware assessment for velocity issues in PD patients.
Limitations include a small sample size (n = 28) and a cross-sectional design. Future research should prioritize large-scale longitudinal studies and domain-specific cognitive tests to validate findings.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Central Hospital of Dalian University of Technology (YN2022-039-57). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WY: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HG: Methodology, Writing – review & editing. RL: Formal analysis, Writing – review & editing. CS: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. CW: Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Institutional Research Fund (2022ZZ220).
Acknowledgments
The authors acknowledge the support received from the Key Laboratory of Intelligent Control and Optimization for Industrial Equipment of the Ministry of Education and the School of Control Science and Engineering, Dalian University of Technology, Dalian, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Martinez-Ramirez D Rodriguez-Violante M Ramirez-Zamora A . Gait in Parkinson's disease. Parkinson's Disease. (2019) 2019:1962123. doi: 10.1155/2019/1962123
2.
Mirelman A Bonato P Camicioli R Ellis TD Giladi N Hamilton JL et al . Gait impairments in Parkinson’s disease. Lancet Neurol. (2019) 18:697–708. doi: 10.1016/S1474-4422(19)30044-4
3.
Dewey DC Miocinovic S Bernstein I Khemani P Dewey RB Querry R et al . Automated gait and balance parameters diagnose and correlate with severity in Parkinson disease. J Neurol Sci. (2014) 345:131–8. doi: 10.1016/j.jns.2014.07.026
4.
Schrag A Jahanshahi M Quinn N . What contributes to quality of life in patients with Parkinson's disease?J Neurol Neurosur Ps. (2000) 69:308–12. doi: 10.1136/jnnp.69.3.308
5.
Liu PP Yu NB Yang YC Yu Y Sun XY Yu H et al . Quantitative assessment of gait characteristics in patients with Parkinson’s disease using 2D video. Parkinsonism Relat Disord. (2022) 101:49–56. doi: 10.1016/j.parkreldis.2022.06.012
6.
Zanardi APJ da Silva ES Costa RR Passos-Monteiro E dos Santos IO Kruel LFM et al . Gait parameters of Parkinson's disease compared with healthy controls a systematic review and meta-analysis. Sci Rep. (2021) 11:752. doi: 10.1038/s41598-020-80768-2
7.
Shin KJ Park J Ha S Park KM Kim SE Lee BI et al . Decreased foot height may be a subclinical shuffling gait in early stage of Parkinson’s disease: a study of three-dimensional motion analysis. Gait Posture. (2020) 76:64–7. doi: 10.1016/j.gaitpost.2019.11.005
8.
Grajic M Stankovic I Radovanovic S Kostic V . Gait in drug naive patients with de novo Parkinson's disease - altered but symmetric. Neurol Res. (2015) 37:712–6. doi: 10.1179/1743132815Y.0000000043
9.
Yin WC Zhu WC Gao H Niu XH Shen CX Fan XM et al . Gait analysis in the early stage of Parkinson’s disease with a machine learning approach. Front Neurol. (2024) 15:1472956. doi: 10.3389/fneur.2024.1472956
10.
Schlachetzki JCM Barth J Marxreiter F Gossler J Kohl Z Reinfelder S et al . Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS One. (2017) 12:e0183989. doi: 10.1371/journal.pone.0183989
11.
Herman T Weiss A Brozgol M Giladi N Hausdorff JM . Gait and balance in Parkinson's disease subtypes: objective measures and classification considerations. J Neurol. (2014) 261:2401–10. doi: 10.1007/s00415-014-7513-6
12.
Allan LM Ballard CG Burn DJ Kenny RA . Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J Am Geriatr Soc. (2005) 53:1681–7. doi: 10.1111/j.1532-5415.2005.53552.x
13.
Ghoraani B Boettcher LN Hssayeni MD Rosenfeld A Tolea MI Galvin JE . Detection of mild cognitive impairment and Alzheimer’s disease using dual-task gait assessments and machine learning. Biomed Signal Process Control. (2021) 64:102249. doi: 10.1016/j.bspc.2020.102249
14.
Aboutorabi A Arazpour M Bahramizadeh M Hutchins SW Fadayevatan R . The effect of aging on gait parameters in able-bodied older subjects: a literature review. Aging Clin Exp Res. (2016) 28:393–405. doi: 10.1007/s40520-015-0420-6
15.
Williams LN Seignourel P Crucian GP Okun MS Rodriguez RL Skidmore FM et al . Laterality, region, and type of motor dysfunction correlate with cognitive impairment in Parkinson’s disease. Mov Disord. (2007) 22:141–5. doi: 10.1002/mds.21220
16.
Zhang MM Gan YW Wang XM Wang Z Feng T Zhang YM . Gait performance and non-motor symptoms burden during dual-task condition in Parkinson’s disease. Neurol Sci. (2023) 44:181–90. doi: 10.1007/s10072-022-06411-2
17.
Amboni M Barone P Iuppariello L Lista I Tranfaglia R Fasano A et al . Gait patterns in parkinsonian patients with or without mild cognitive impairment. Movement Disord. (2012) 27:1536–43. doi: 10.1002/mds.25165
18.
Lord S Rochester L Hetherington V Allcock LM Burn D . Executive dysfunction and attention contribute to gait interference in “off” state Parkinson’s disease. Gait Posture. (2010) 31:169–74. doi: 10.1016/j.gaitpost.2009.09.019
19.
Pieruccini-Faria F Jones JA Almeida QJ . Motor planning in Parkinson’s disease patients experiencing freezing of gait: the influence of cognitive load when approaching obstacles. Brain Cogn. (2014) 87:76–85. doi: 10.1016/j.bandc.2014.03.005
20.
Iansek R Danoudis M Bradfield N . Gait and cognition in Parkinson’s disease: implications for rehabilitation. Rev Neurosci. (2013) 24:293–300. doi: 10.1515/revneuro-2013-0006
21.
Young W Rodger M Craig CM . Perceiving and reenacting spatiotemporal characteristics of walking sounds. J Exp Psychol Hum Percept Perform. (2013) 39:464–76. doi: 10.1037/a0029402
22.
Young WR Shreve L Quinn EJ Craig C Bronte-Stewart H . Auditory cueing in Parkinson's patients with freezing of gait. What matters most: action-relevance or cue-continuity?Neuropsychologia. (2016) 87:54–62. doi: 10.1016/j.neuropsychologia.2016.04.034
23.
Igartua JJ Hayes AF . Mediation, moderation, and conditional process analysis: concepts, computations, and some common confusions. Span J Psychol. (2021) 24:e49. doi: 10.1017/SJP.2021.46
24.
Scholl JL Espinoza AI Rai W Leedom M Baugh LA Berg-Poppe P et al . Relationships between freezing of gait severity and cognitive deficits in Parkinson’s disease. Brain Sci. (2021) 11:1496. doi: 10.3390/brainsci11111496
25.
Lai YR Lien CY Huang CC Lin WC Chen YS Yu CC et al . Clinical disease severity mediates the relationship between stride length and speed and the risk of falling in Parkinson's disease. J Pers Med. (2022) 12:192. doi: 10.3390/jpm12020192
26.
Dubbioso R Spisto M Hausdorff JM Aceto G Iuzzolino VV Senerchia G . Cognitive impairment is associated with gait variability and fall risk in amyotrophic lateral sclerosis. Eur J Neurol. (2023) 30:3056–67. doi: 10.1111/ene.15936
27.
Goetz CG Tilley BC Shaftman SR Stebbins GT Fahn S Martinez-Martin P et al . Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
28.
Nasreddine ZS Phillips NA Bedirian V Charbonneau S Whitehead V Collin I et al . The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
29.
Postuma R Berg D Stern M Poewe W Olanow C Oertel W et al . MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
30.
Schade S Mollenhauer B Trenkwalder C . Levodopa equivalent dose conversion factors: an updated proposal including Opicapone and safinamide. Mov Disord Clin Pract. (2020) 7:343–5. doi: 10.1002/mdc3.12921
31.
Hoehn MM Yahr MD . Parkinsonism: onset, progression, and mortality. Neurology. (1967) 17:427–42. doi: 10.1212/WNL.17.5.427
32.
Yin WC Gao H Liang BC Liu RC Liu Y Shen CX et al . Quantitative analysis of gait parameters in Parkinson’s disease and the clinical significance. Front Neurol. (2025) 16:1527020. doi: 10.3389/fneur.2025.1527020
33.
Liu RC Wang ZL Qiu S Zhao HY Wang C Shi X et al . A wearable gait analysis and recognition method for Parkinson’s disease based on error state Kalman filter. IEEE J Biomed Health Inform. (2022) 26:4165–75. doi: 10.1109/JBHI.2022.3174249
34.
Qiu S Wang ZL Zhao HY . Using distributed wearable sensors to measure and evaluate human lower limb motions. IEEE Trans Instrum Meas. (2016) 65:939–50. doi: 10.1109/TIM.2015.2504078
35.
Hayes AF . Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Publications (2013).
36.
Peel NM Alapatt LJ Jones LV Hubbard RE . The association between gait speed and cognitive status in community-dwelling older people: a systematic review and meta-analysis. J. Gerontology Series A. (2019) 74:943–8. doi: 10.1093/gerona/gly140
37.
Lena F Modugno N Greco G Torre M Cesarano S Santilli M et al . Rehabilitation interventions for improving balance in Parkinson's disease: a narrative review. Am J Phys Med Rehabil. (2023) 102:270–4. doi: 10.1097/PHM.0000000000002077
38.
Allet L Ijzerman H Meijer K Willems P Savelberg H . The influence of stride-length on plantar foot-pressures and joint moments. Gait Posture. (2011) 34:300–6. doi: 10.1016/j.gaitpost.2011.05.013
39.
Chen M Wu B Lou XX Zhao T Li JH Xu ZS et al . A self-adaptive foot-drop corrector using functional electrical stimulation (FES) modulated by tibialis anterior electromyography (EMG) dataset. Med Eng Phys. (2013) 35:195–204. doi: 10.1016/j.medengphy.2012.04.016
40.
Morris ME Iansek R Matyas TA Summers JJ . The pathogenesis of gait hypokinesia in Parkinson's disease. Brain. (1994) 117:1169–81. doi: 10.1093/brain/117.5.1169
41.
Hobson DE . Asymmetry in parkinsonism, spreading pathogens and the nose. Parkinsonism Relat Disord. (2012) 18:1–9. doi: 10.1016/j.parkreldis.2011.06.011
42.
Maetzler W Liepelt I Berg D . Progression of Parkinson’s disease in the clinical phase: potential markers. Lancet Neurol. (2009) 8:1158–71. doi: 10.1016/S1474-4422(09)70291-1
43.
Intzandt B Beck EN Silveira CRA . The effects of exercise on cognition and gait in Parkinson’s disease: a scoping review. Neurosci Biobehav Rev. (2018) 95:136–69. doi: 10.1016/j.neubiorev.2018.09.018
44.
Cohen JA Verghese J Zwerling JL . Cognition and gait in older people. Maturitas. (2016) 93:73–7. doi: 10.1016/j.maturitas.2016.05.005
45.
Hamacher D Herold F Wiegel P Hamacher D Schega L . Brain activity during walking: a systematic review. Neurosci Biobehav Rev. (2015) 57:310–27. doi: 10.1016/j.neubiorev.2015.08.002
46.
Weng WH Yang YR Yeh NC Ku PH Wang PS Liao YY et al . Gait performance and prefrontal cortex activation during single and dual task walking in older adults with different cognitive levels. Front Aging Neurosci. (2023) 15:1177082. doi: 10.3389/fnagi.2023.1177082
47.
Maidan I Rosenberg-Katz K Jacob Y Giladi N Deutsch JE Hausdorff JM et al . Altered brain activation in complex walking conditions in patients with Parkinson's disease. Parkinsonism Relat Disord. (2016) 25:91–6. doi: 10.1016/j.parkreldis.2016.01.025
48.
Peterson DS Pickett KA Duncan RP Perlmutter JS Earhart GM . Brain activity during complex imagined gait tasks in Parkinson disease. Clin Neurophysiol. (2015) 125:995–1005. doi: 10.1016/j.clinph.2013.10.008
49.
Maidan I Nieuwhof F Bernad-Elazari H Reelick MF Bloem BR Giladi N et al . The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: an fNIRS study. Neurorehabil Neural Repair. (2016) 30:963–71. doi: 10.1177/1545968316650426
50.
Kang SH Kim J Lee JY Koh SB . Mild cognitive impairment is associated with poor gait performance in patients with Parkinson's disease. Front Aging Neurosci. (2022) 14:1003595. doi: 10.3389/fnagi.2022.1003595
51.
Salat DH Kaye JA Janowsky JS . Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. (1999) 56:338–44. doi: 10.1001/archneur.56.3.338
52.
Morrison JH Baxter MG . The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. (2012) 13:240–50. doi: 10.1038/nrn3200
53.
Seidler RD Bernard JA Burutolu TB Fling BW Gordon MT Gwin JT et al . Motor control and aging links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. (2010) 34:721–33. doi: 10.1016/j.neubiorev.2009.10.005
Summary
Keywords
Parkinson’s disease, motor symptom, cognitive function, gait characteristic, mediation effect
Citation
Yin W, Gao H, Liu R, Shen C, Liu Y and Wang C (2025) Mediation analysis for integrating motor symptoms of Parkinson’s disease, cognitive function, and gait characteristics. Front. Neurol. 16:1659581. doi: 10.3389/fneur.2025.1659581
Received
04 July 2025
Accepted
18 September 2025
Published
13 October 2025
Volume
16 - 2025
Edited by
Marios Spanakis, University of Crete, Greece
Reviewed by
Myriam Spisto, University of Campania Luigi Vanvitelli, Italy
Francesco Lena, Mediterranean Neurological Institute Neuromed (IRCCS), Italy
Danial Kazemi, Isfahan University of Medical Sciences, Iran
Srishti Banerjee, LJ Institute of Physiotherapy, India
Updates
Copyright
© 2025 Yin, Gao, Liu, Shen, Liu and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Gao, hgao@dlmu.edu.cnCui Wang, sjnsktg@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.