Abstract
Background:
Migraine is a familial, episodic disorder characterized by complex sensory processing dysfunction, with headache serving as its hallmark feature. While numerous risk factors have been proposed, the causal nature of these associations often remains ambiguous. Mendelian randomization (MR) represents a robust epidemiological framework that leverages genetic variants to infer causal relationships, thereby overcoming limitations of observational studies. This study systematically reviews and meta-analyzes MR evidence to elucidate bidirectional causal relationships between migraine and systemic diseases, identify novel risk determinants, and highlight critical gaps for future mechanistic investigations.
Methods:
A comprehensive literature search was conducted across seven databases (PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure, WanFang Data Knowledge Service Platform, and VIP China Science and Technology Journal Database) using predefined search strategies and exclusion criteria. The search time limit was from the construction of the database to July 3, 2024. Study eligibility was independently assessed by two reviewers, with data extraction processes adhering to STROBE-MR guidelines. Included studies were evaluated for quality using validated criteria, and relevant data (study design, participant demographics, genetic instruments, analytical methods, and outcomes) were systematically extracted. Data synthesis involved meta-analytical pooling of effect estimates using Review Manager 5.4, with forest plots generated to visualize results. Causal relationships were interpreted according to the WHO ICD-11 disease classification system, with subgroup analyses performed for migraine with aura (MWA) and migraine without aura (MOA).
Results:
A total of 60 studies involving 331 MR analyses were included, revealing bidirectional causal relationships between migraine and multiple phenotypes: migraine was identified as a causal factor for 6 diseases (Alzheimer’s disease, cervical artery dissection, venous thromboembolism, coronary artery disease, angina, large artery stroke), 3 behavioral habits (delayed age at first sexual intercourse, maternal smoking, reduced physical activity), 1 dietary intakes (alcohol consumption), and 3 physiological indicators (elevated interleukin-2, increased Body Mass Index, higher serum vitamin D levels) (p < 0.05). Conversely, 6 diseases (venous thromboembolism, breast cancer, insomnia, difficulty awakening, major depressive disorder, depression), 5 behavioral factors (television watching, smoking initiation, delayed AFS, more schooling, reduced physical activity), 4 dietary determinants (coffee, alcohol, cheese, salad intake), 13 physiological parameters (hemostatic, cardiovascular, metabolic, and genetic markers), and 1 gut microbiota taxon (LachnospiraceaeUCG001) were causal determinants of migraine risk (p < 0.05). Subtype-specific analyses showed MOA was causally associated with 4 diseases (AD, CeAD, CAD, LAS) and delayed AFS as an exposure, and influenced by breast cancer, celiac disease, TV watching, delayed AFS, increased schooling, and physiological parameters (DBP, PP, serum calcium, IGF-1) as an outcome; MWA demonstrated causal relationships with CeAD and LAS as an exposure, and associations with VTE, SLE, MDD, delayed AFS, coffee intake, and hemostatic markers as an outcome (p < 0.05 for all).
Conclusion:
This systematic review provides robust genetic evidence supporting bidirectional causal relationships between migraine and multiple phenotypes, including systemic diseases, behavioral habits, dietary factors, and physiological parameters. Subtype-specific analyses highlight distinct causal pathways for MOA and MWA, underscoring the clinical heterogeneity of migraine. These findings advance our understanding of migraine pathogenesis and inform precision medicine approaches, while also identifying novel therapeutic targets for this disabling condition. More data will be needed in the future to obtain a more specific assessment.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD42025636141, Identifier CRD42025636141.
1 Introduction
Migraine represents the most prevalent neurological disorder in primary care settings, accounting for over 90% of headache-related consultations according to epidemiological studies (1, 2). As highlighted by the Global Burden of Disease study, it ranks as the second leading cause of disability globally and the primary cause among young women (3). With a prevalence of 18% in females and 6% in males, migraine imposes substantial societal and economic burdens, particularly chronic migraine, which affects 2% of the global population (4).
Clinically defined by severe throbbing, unilateral headache accompanied by nausea, photophobia, and vomiting, migraine pathogenesis involves trigeminovascular system activation modulated by genetic and environmental factors (5). The disorder is classified into two primary forms: migraine with aura (MWA) and migraine without aura (MOA), with further categorization into chronic or episodic subtypes. Hemiplegic migraine, a rare variant, manifests as transient unilateral weakness and sensory disturbances (6). Recent genome-wide association studies have identified 28 genetic loci associated with headache phenotypes, including 14 previously linked to migraine. Notably, these studies uncovered significant brain-specific genetic correlations (7).
The primary objective of migraine management is to mitigate attack severity and duration (8). Pharmacological interventions encompass a diverse range of agents, including acetaminophen, triptans, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen and diclofenac potassium, dihydroergotamine, non-opioid analgesics, NSAID-triptan combinations, and antiemetics (9).
Mendelian randomization (MR) leverages genetic variants as instrumental variables (IVs) to infer causal relationships between exposure and outcome traits (83, 84). By capitalizing on genetic epidemiology, MR circumvents limitations inherent in observational studies through the random allocation of genotypes during meiosis (10). Valid IVs must satisfy three key assumptions: (1) robust association with the exposure of interest, (2) independence from confounding variables, and (3) exclusive mediation of outcomes via the exposure. A repertoire of MR methods, including inverse variance weighting (IVW), MR-Egger, weighted median, and mode-based approaches, are employed to validate causal inferences (11).
This review systematically synthesizes evidence from MR studies on migraine to explore bidirectional causal relationships between migraine and other diseases. The objectives are twofold: (1) to characterize the causal associations between migraine and its subtypes with various phenotypes, and (2) to advance therapeutic target identification for migraine management. By integrating genetic evidence, this review aims to clarify migraine’s role as both a risk factor and potential outcome of systemic disorders, thereby informing mechanistic research and novel drug development.
2 Materials and methods
The study group conducted the systematic review following the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (12), and it was registered in the International Prospective Register of Systematic Reviews (PROSPERO), CRD42025636141, 9 January 2025.
2.1 Search strategy
To identify MR studies on migraine, the study group performed a systematic literature search in PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), WanFang Data Knowledge Service Platform (WanFang), and VIP China Science and Technology Journal Database (VIP). A combination of subject terms and free words was used for the search. The English search terms included Mendelian randomization, migraine, hemicrania, cephalalgia, headache, cephalodynia, cranial pain, and head pain, and the Chinese search terms included Mendelian Randomization and migraine. The system searched the literature in the above databases that used Mendelian randomization to study the correlation between migraine and disease phenotypes. The search time limit was from the construction of the database to July 3, 2024.
2.2 Criteria for inclusion and exclusion of literature
2.2.1 Inclusion criteria
-
(1) All published papers using MR to explore the causal relationship between migraine and multiple exposures or diseases, including unidirectional or bidirectional MR studies.
-
(2) Associations reported as Odds Ratios (OR) and 95%confidence intervals (95% CIs) using instrumental variable methods.
2.2.2 Exclusion criteria
-
(1) Articles unrelated to the topic were excluded.
-
(2) Any case reports, narrative reviews, letters, editorials, opinions, incomplete manuscripts, and conference abstracts were excluded.
-
(3) Articles in languages other than Chinese or English were excluded.
-
(4) Articles without extractable indicators were excluded.
-
(5) When there are multiple publications based on the same Genome-Wide Association Studies (GWAS) (same participants), only publications with the largest sample size and the latest published study (if the sample size is the same) are included, without sample size limitations.
2.3 Study selection and data extraction
Relevant article data were systematically retrieved from databases, downloaded, and imported into the reference management software Endnote X9. Duplicate references were automatically removed using the software. Two reviewers (XL and QL) independently screened the title and abstract of all retrieved articles after employing the search strategy. Studies included after screening were retrieved for full-text review. Disagreements were resolved by consulting a third reviewer (JN). The original author was contacted for incomplete literature if needed.
A data extraction form was created to extract the following information from each study: (1) general information about the article: title, author, publication year, abstract; (2) basic information about the research: research methods, exposure and outcome, study population, sample size, exposure GWAS data source, outcome GWAS data source, main causal effect estimation method; (3) outcomes: number of single nucleotide polymorphisms (SNPs), computed OR values, 95% CI, p-values; and (4) other information required by the Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR) guidelines, for example, sensitivity and additional analysis. Microsoft Excel 2019 was used to extract data. Two reviewers (XL and QL) independently extracted data from the studies included. Disagreements were resolved by consulting a third reviewer (SY). IVW is the traditional MR method for converting the composite ratio estimate for each SNP into an overall estimate, and it is also the method used to select the extraction results when extracting the data in this study.
2.4 Evaluation of literature quality
To evaluate the quality of the Mendelian randomization (MR) studies incorporated in the systematic review, the STROBE-MR guidelines (13, 14) were employed as a tool for literature quality assessment. This quality assessment guideline takes into consideration both the research methodology and reporting standards, enabling a fair evaluation of potentially biased studies. The guideline is structured into several sections: title and abstract (item 1), introduction (items 2–3), methods (items 4–9), results (items 10–13), discussion (items 14–17), and other information (items 18–20). Items 1 to 14 within these entries were adapted from the assessment entries in the study by Ibrahim et al. (15). These entries encompassed title and abstract, background, objectives, study design and data sources, statistical methods for main analyses, software and preregistration, descriptive data, main results, sensitivity analyses and additional analyses, key results, limitations, interpretation, generalizability, and the core assumptions of Mendelian randomization. Ibrahim et al. adjusted and utilized these quality assessment scores in MR meta-analyses and systematic reviews. They converted the scores into percentages, with scores below 80% indicating a high risk of bias, scores between 80 and 90% representing a medium risk of bias, and scores above 90% signifying a low risk of bias. Two reviewers (XL and HN) independently evaluated the risk of bias for the included studies and cross-checked the results. Any disagreements were resolved through discussion with a third reviewer (SY). The initial quality assessment scheme is provided in Supplementary Table 2.
2.5 Classification of research themes
The themes of studies included in the literature were categorized according to the International Classification of Diseases, 11th Revision (ICD-11) for Mortality and Morbidity Statistics issued by the World Health Organization (WHO) (16). Each disease was searched in ICD-11, and the largest submenu containing the disease was selected to represent the system where the disease is located. Categorize them into: mental, behavioral or neurodevelopmental disorders; diseases of the circulatory system; sleep–wake disorders; diseases of the digestive system; diseases of the skin; endocrine, nutritional or metabolic diseases; diseases of the nervous system; neoplasms; diseases of the immune system; diseases of the respiratory system; diseases of the ear or mastoid process; diseases of the genitourinary system; and other ICD-11 diseases, 13 categories in total. After categorizing the included literature according to the ICD-11 classification, there were still some non-disease studies that were difficult to organize, so the authors of this paper categorized them as “non-disease” and subdivided the “non-disease” factors into behavioral habits non-disease factors, dietary intake non-disease factors, physiologic non-disease factors, and other non-disease factors. When encountering the same indicator with different names in different literature, we combined them into one name (e.g., coffee intake and coffee consumption were merged into coffee consumption).
2.6 Statistical analysis
When two or more MR estimates could be obtained based on the same results from non-overlapping samples, a meta-analysis was performed by Review Manage 5.4 software to obtain a combined estimate, generate forest plots, and the choice of model was decided based on the value of I2. We used OR and 95% CI as our effect indicators. Heterogeneity was assessed using I2, with I2 values between 25 and 50% considered mild heterogeneity, I2 values between 50 and 75% moderate heterogeneity, and I2 values greater than 75% considered severe heterogeneity. A random-effects model was selected when I2 was greater than 50%, and a fixed-effects model was selected when it was less than or equal to 50%. p < 0.05 was considered a statistically significant difference.
3 Results
3.1 Results of the literature search
Based on the established literature search strategy, the search was conducted in the corresponding databases according to the search formula, and the search results are shown in Supplementary Table 1.
A total of 389 literature articles were retrieved through the above search formula to study migraine using MR methods, and after removing duplicate records through Endnote X9, 250 articles were left, and 60 articles were finally obtained by reading the titles and abstracts for initial screening, and then by reading the full text to screen out the unavailability of the full text/missing the screening of the literature, 60 literature were finally included. The literature screening process is shown in Figure 1.
Figure 1

PRISMA flow diagram of literature search. * CNKI, China National Knowledge Infrastructure; WanFang, WanFang Data Knowledge Service Platform; VIP, VIP China Science and Technology Journal Database; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.2 General information about the studies included
A total of 60 original journal articles were included, with publication years from the year of the library’s creation to July 3, 2024. The general information of the included literature is shown in Table 1.
Table 1
| Study (first author, year) | Method | Theme | Ethnicity of exposure | Ethnicity of outcome | Whether causality exists | ||
|---|---|---|---|---|---|---|---|
| Forward | Reverse | Subtypes | |||||
| Peter Yin, 2017 (56) | Two-sample unidirectional MR | Elevation of serum calcium levels by 1 mg/dL | European | European | No causal effect | Risk factor | Elevation of serum calcium levels by 1 mg/dL-MOA: risk factor |
| Johnsen, M. B, 2018 (57) | One-sample unidirectional MR | Smoking | Norway | Norway | No causal effect | / | / |
| Daghlas, I, 2020 (1) (28) | Two-sample unidirectional MR | AD, intelligence, brain volume | European | European | No causal effect | / | / |
| Daghlas, I, 2020 (2) (23) | Two-sample bidirectional MR | Insomnia, difficulty awakening | European | European | No causal effect | Risk factors | / |
| Daghlas, I, 2020 (3) (17) | Two-sample unidirectional MR | CAD, myocardial infarction, angina, AF | European | European | Protective factors: CAD, myocardial infarction, angina No causal effect: AF |
/ | MOA-CAD: protective factor MWA-CAD: no causal effect |
| Emmanuel O. Adewuyi, 2020 (38) | Two-sample bidirectional MR | Endometriosis | European (approximately 93%) and Japanese ancestries (from Australia, Belgium, Denmark, Iceland, Japan, the UK, and the USA) | European | No causal effect | No causal effect | / |
| Guo, Y, 2020 (58) | Two-sample bidirectional MR | BP | European | European | No causal effect | Risk factors: SBP, DBP, PP | DBP-MOA: risk factors PP-MOA: risk factors |
| Chu, S, 2021 (24) | Two-sample bidirectional MR | Insomnia | European | European | No causal effect | Risk factor | / |
| Guo, Y, 2021 (59) | Two-sample bidirectional MR | Hemostatic profile | European | European | No causal effect | Risk factors: FVIII activity, vWF levels, phosphorylated fibrinopeptide A Protective factors: fibrinogen levels, APTT |
Fibrinogen levels-MWA: protective factor FVIII activity-MWA: risk factor vWF levels-MWA: risk factor APTT-MWA: protective factor Phosphorylated fibrinopeptide A-MWA: risk factor Fibrinogen levels-MOA: no causal effect |
| Brittany L Mitchell, 2022 (41) | Two-sample bidirectional MR | ICV | European | European | No causal effect | Protective factor | / |
| Chen, H, 2022 (60) | Two-sample unidirectional MR | Coffee consumption | British | European | No causal effect | / | No causal effect |
| Daghals, I, 2022 (21) | Two-sample unidirectional MR | CeAD, LAS | European | European | Risk factor: CeAD Protective factor: LAS |
/ | MOA-CeAD: risk factor MOA-LAS: protective factor MWA-CeAD: risk factor MWA-LAS: protective factor |
| Islam, M. R, 2022 (27) | Two-sample bidirectional MR | T2D | European | European | No causal effect | No causal effect | / |
| Keon-Joo Lee, 2022 (18) | Two-sample unidirectional MR | Stroke, ischemic stroke, hemorrhagic stroke | European | European | No causal effect | / | No causal effect |
| Mei-Jun Shu, 2022 (19) | Two-sample unidirectional MR | Ischemic stroke | European | European | No causal effect | / | / |
| Peng-Peng Niu, 2022 (42) | Two-sample bidirectional MR | Higher serum vitamin D levels | European | European | Risk factor | Protective factor | No causal effect |
| Reziya Abuduxukuer, 2022 (43) | Two-sample bidirectional MR | IGF-1 | European (94.3%) | European | No causal effect | Protective factor | IGF-1-MWA: no causal effect IGF1-MOA: protective factor |
| Shuai Yuan, 2022 (61) | Two-sample bidirectional MR | Alcohol consumption, coffee consumption, smoking initiation, smoking index | European | European | Protective factor: alcohol consumption | Risk factors: smoking initiation Protective factors: alcohol consumption, coffee consumption No causal effect: smoking index |
/ |
| Bi, Y, 2023 (71) | Two-sample unidirectional MR | Genetic instrumental variables for lipids and lipid modifying targets | N/A | European (92.55%) | / | Protective factor: APO-A1 | / |
| Chong Fu, 2023 (44) | Two-sample bidirectional MR | Inflammatory cytokines | Finnish descent | European | Migraine-IL-2: protective factor | HGF-migraine: risk factor | / |
| Fang, T, 2023 (35) | Two-sample unidirectional MR | Breast cancer | European | European | / | Risk factor | Breast cancer-MWA: no causal effect Breast cancer-MOA: risk factor |
| Guo, X, 2023 (62) | Two-sample bidirectional MR | Total cortical SA, average cortical thickness, GMV, WMH, HV | European | European | No causal effect | Protective factors: SA, HV | / |
| Huo, J, 2023 (45) | Two-sample bidirectional MR | WMLs | European | European | No causal effect | No causal effect | / |
| Horton, M. K, 2023 (29) | Two-sample unidirectional MR | MS | European | N/A | No causal effect | / | / |
| He, Q, 2023 (46) | Two-sample bidirectional MR | Gut microbiota | European | European | N/A | N/A | N/A |
| Hua Xue, 2023 (30) | Two-sample unidirectional MR | AD | European | European | No causal effect | / | / |
| Hui Zheng,2023 (63) | Two-sample unidirectional MR | More years of schooling | European | European | / | Protective factors | More years of schooling-MOA: protective factor |
| Jin, C, 2023 (81) | Two-sample unidirectional MR | Tea intake | European | European | / | No causal effect | No causal effect |
| Lei Zhao, 2023 (47) | Two-sample bidirectional MR | WM | European | European | Established | Established | / |
| Mengmeng Wang, 2023 (20) | Two-sample unidirectional MR | Ischemic stroke | European | European | No causal effect | / | No causal effect |
| Nike Zoe Welander, 2023 (25) | Two-sample bidirectional MR | IBD, celiac disease | European | European | No causal effect | No causal effect | Celiac disease-MOA: protective factor |
| Tao Wei, 2023 (64) | Two-sample unidirectional MR | Neuralized E3 ubiquitin-protein ligase 1 | European | European | / | Protective factor | / |
| Wenqiang Zhang, 2023 (39) | Two-sample bidirectional MR | CKD | European | European and Japanese ancestries | No causal effect | No causal effect | / |
| Xinhui Liu, 2023 (48) | Two-sample bidirectional MR | 83 dietary habits | European | European | Include only supported hypotheses | Include only supported hypotheses | / |
| Xiaofeng Lv, 2023 (51) | Two-sample bidirectional MR | MDD | European | European | No causal effect | Risk factor | MDD-MWA: risk factor |
| Zhen-Ni Zhao, 2023 (40) | Two-sample bidirectional MR | PD | European | European | No causal effect | No causal effect | / |
| Baranova, A, 2024 (31) | Two-sample unidirectional MR | AD | European | European | Risk factor | / | / |
| Chengfeng Xu, 2024 (32) | Two-sample unidirectional MR | AD | European | European | Risk factor | / | / |
| Chengcheng Zhang, 2024 (72) | Two-sample unidirectional MR | Blood cis-eQTL, brain cis-eQTL | European | European | / | / | |
| Danfeng Xu, 2024 (54) | Two-sample unidirectional MR | SLE | European | European | / | No causal effect | SLE-MWA: risk factor SLE-MOA: no causal effect |
| Geng, C, 2024 (33) | Two-sample bidirectional MR | AD | European | European | Risk factor | No causal effect | / |
| Guanglu Li, 2024 (26) | Two-sample bidirectional MR | Psoriasis, T1D, RA, SLE, AR, asthma | European | European | No causal effect | No causal effect | No causal effect |
| Guoliang Zhu, 2024 (65) | Two-sample bidirectional MR | Delayed AFS | European | European | Protective factor | Protective factor | MOA-AFS: protective factor AFS-MWA: protective factor AFS-MOA: protective factor |
| Hao Lv, 2024 (36) | Two-sample bidirectional MR | AR | European | European | No causal effect | No causal effect | No causal effect |
| Hong, P, 2024 (73) | Two-sample unidirectional MR | Lipid metabolism characteristics | European | European | / | / | / |
| Jianxiong Gui, 2024 (74) | Two-sample unidirectional MR | TWAS | N/A | European | / | Protective factor: REV1 Risk factor: SREBF2 |
/ |
| Jareebi, Mohammad A, 2024 (66) | Two-sample unidirectional MR | Smoking initiation, smoking intensity, maternal smoking, cheese intake, salad intake, coffee consumption, BMI, physical activity | European | European | / | Risk factor: maternal smoking Protective factors: coffee consumption, cheese intake, salad intake, BMI, physical activity |
/ |
| Jinjin Zhang, 2024 (67) | Two-sample unidirectional MR | Coffee intake | European | European | / | Protective factor | Coffee intake-MWA: protective factor Coffee intake-MOA: no causal effect |
| Kang Qu, 2024 (1) (49) | Two-sample bidirectional MR | Gut microbiota | European | European | No causal effect | Risk factor: LachnospiraceaeUCG001 | / |
| Kang Qu, 2024 (2) (68) | Two-sample unidirectional MR | LDL-C, APOB, TC | European | European | / | No causal effect | / |
| Kangjia Zhang, 2024 (37) | Two-sample bidirectional MR | MD | European | European | No causal effect | No causal effect | / |
| Lei Zhao, 2024 (34) | Two-sample unidirectional MR | AD, VaD, FTD, LBD | European | European | Migraine-AD: risk factor | / | MOA-AD: risk factor |
| Meixuan Ren, 2024 (55) | Two-sample unidirectional MR | SLE | European | European | / | / | SLE-MWA: risk factor SLE-MOA: no causal effect |
| Peihong Li, 2024 (69) | Two-sample unidirectional MR | SBs | European | European | / | Risk factor: watching TV | Watching TV-MOA: risk factor |
| Peng-Peng Niu, 2024 (70) | Two-sample unidirectional MR | LRP11, ITIH1, ADGRF5 | European | European | / | Protective factors: LRP11, ADGRF5 Risk factors: ITIH1 |
/ |
| Xiangyue Meng, 2024 (50) | Two-sample unidirectional MR | Gut microbiota | European (78%) | European | No causal effect | Risk factor: LachnospiraceaeUCG001 | / |
| Xu-Peng Wu, 2024 (53) | Two-sample bidirectional MR | VTE | European | European | Risk factor | Risk factors | VTE-MWA: risk factor |
| Ya Li, 2024 (82) | Two-sample unidirectional MR | Psoriasis | European | European | / | No causal effect | / |
| Yang Li, 2024 (52) | Two-sample unidirectional MR | Depression, MDD, insomnia, sleep duration, short sleep duration, daytime sleepiness, napping | European | European | / | Risk factors: depression, MDD, insomnia No causal effect: sleep duration, short sleep duration, daytime sleepiness, napping |
/ |
| Yang Wang, 2024 (22) | Two-sample bidirectional MR | VTE | European | European | Risk factors | Risk factors | / |
General information about the studies included.
* Directionality in this table: Forward: Indicates the causal effect of migraine on the specified disease. Reverse: Indicates the causal effect of the specified disease on migraine. ** N/A: Not Applicable. *** Established: A causal relationship is established. Since the data is complex, they will be shown later using descriptive language. AD, Alzheimer’s disease; CAD, coronary artery disease; AF, atrial fibrillation; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; FVIII, coagulation factor VIII; vWF, von Willebrand factor; APTT, activated partial thromboplastin time; ICV, intracranial volume; CeAD, cervical artery dissection; LAS, large artery stroke; T2D, type 2 Diabetes; IGF-1, insulin-like growth factor 1; APO-A1, Apolipoprotein A1; IL-2, Interleukin-2; HGF, hepatocyte growth factor; SA, surface area (cortical); GMV, gray matter volume; WMH, white matter hyperintensities; HV, Hippocampal volume; WMLs, white matter lesions; MS, Multiple sclerosis; WM, white matter; IBD, inflammatory bowel disease; CKD, chronic kidney disease; MDD, major depressive disorder; PD; periodontitis; eQTL, expression quantitative trait loci; SLE, systemic lupus erythematosus; T1D, type 1 diabetes; RA, rheumatoid arthritis; AR, allergic rhinitis; AFS, age at first sexual intercourse; TWAS, cross-tissue transcriptome association studies; BMI, body mass index; LDL-C, low-density lipoprotein cholesterol; APOB, apolipoprotein B; TC, total cholesterol; MD, Meniere’s disease; VaD, vascular dementia; FTD, frontotemporal dementia; LBD, Lewy body dementia; SBs, sedentary behaviors; LRP11, ITIH1, ADGRF5, proteins; VTE, Venous thromboembolism.
We also extracted the details of the included literature, including general information, exposure data, outcome data, and main analysis, the results are shown in Supplementary Table 4.
3.3 Results of the literature quality assessment
A total of 60 included literature were assessed by using the STROBE-MR checklist. Seventeen as low risk of bias, 31 as medium risk of bias, and 12 as high risk of bias after the quality assessment process. The specific assessment results of all the literature are shown in Supplementary Table 3.
3.4 Causal relationships between migraine and multiple diseases
3.4.1 Diseases of the circulatory system
Six studies (17–22) discussed the causal relationship between migraine and diseases of the circulatory system. Mei-Jun Shu et al. (19), Mengmeng Wang et al. (20), and Keon-Joo Lee et al. (18) have done an MR analysis on the causal relationship between migraine and ischemic stroke. Since the data sources of the three studies were different, the three sets of data were meta-analyzed first, and then a second meta-analysis was performed in this study. The MR analysis showed that there was no significant association between migraine and atrial fibrillation (AF) (OR = 1.00, 95% CI: 0.95–1.05), suggesting a possible negative correlation between migraine and angina (OR = 0.86, 95% CI: 0.75–0.99), and coronary artery disease (CAD) showed a similar protective effect (OR = 0.86, 95% CI: 0.76–0.97). Meta-analysis suggested that migraine was a risk factor for cervical artery dissection (CeAD) (OR = 1.69, 95% CI: 1.24–2.30); there was no significant relationship between migraine and hemorrhagic stroke (OR = 1.26, 95%CI: 0.84–1.89) or ischemic stroke (OR = 0.96, 95%CI: 0.90–1.02). The results suggested that there was a negative correlation between migraine and large artery stroke (LAS) (OR = 0.86, 95%CI: 0.76–0.97) and no significant relationship between migraine and myocardial infarction (OR = 0.86, 95%CI: 0.74–1.00) (Figure 2A). The meta-analysis suggested that migraine was a risk factor for venous thromboembolism (VTE) (OR = 96.16, 95% CI: 4.34–2129.67). This estimate was derived from 11 SNPs included in the VTE dataset, with individual SNP F-statistics ranging from 29.76 to 96.77 (all >10), indicating minimal risk of weak instrument bias. Cochran’s Q test for MR-Egger regression and IVW method yielded statistics of 5.610 and 5.973, respectively (both p > 0.05), suggesting no significant heterogeneity among SNPs. MR-Egger regression showed the intercept term was not statistically different from zero (p = 0.5617), indicating no evidence of genetic pleiotropy. Leave-one-out analysis further confirmed that excluding any single SNP did not substantially alter the causal effect estimate, supporting the robustness of this result. Pooled analysis showed no significant causal association between migraine and diseases of the circulatory system (OR = 0.95, 95% CI: 0.88–1.03, p = 0.22). I2 = 75%, suggesting severe heterogeneity. However, migraine is a highly associated risk factor for VTE, this result has been validated by methodology (Figure 2B). For details, see Figure 2.
Figure 2
![Forest plots A and B show odds ratios with 95% confidence intervals for various studies and conditions, including atrial fibrillation, angina, CAD, CeAD, hemorrhagic stroke, ischemic stroke, LAS, myocardial infarction, stroke, and venous thromboembolism. Plot A's scale ranges from 0.5 to 2, while plot B's scale ranges from 0.001 to 1000. The overall effect in both is 0.95 [0.88, 1.03], with significant heterogeneity noted.](https://www.frontiersin.org/files/Articles/1660995/xml-images/fneur-16-1660995-g002.webp)
Forward: causal relationship between migraine and diseases of the circulatory system (divided into two parts to show the effect size). (A) Narrow vertical axis spacing (to show more details). (B) Wide coordinate axis spacing (to show VTE detail). Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; CeAD, cervical artery dissection; LAS, large artery stroke.
3.4.2 Sleep–wake disorders
Two studies (23, 24) discussed the causal relationship between migraine and sleep–wake disorders. No significant causal relationship between migraine and insomnia was detected in the Chu, S et al. study (24) (OR = 1.01, 95% CI: 1.00–1.02, p = 0.159). There was no evidence in the Daghlas, I et al. (23) of a causal relationship between migraine susceptibility and difficulty awakening (β = 0.00, 95% CI: −0.01–0.01, p = 0.75) or insomnia (β = 0.02, 95% CI: −0.00–0.05, p = 0.09).
3.4.3 Diseases of the digestive system
One study (25) discussed the causal relationship between migraine and disease of digestive system. For all migraine, meta-analysis showed no significant causal relationship between migraine and celiac disease and inflammatory bowel disease (OR = 1.08, 95% CI: 0.97–1.21, p = 0.17). For MOA, meta-analysis showed no significant causal relationship between MOA and celiac disease and inflammatory bowel disease (OR = 1.06; 95% CI: 0.97–1.16; p = 0.23). Pooled analysis showed no significant causal relationship between migraine and disease of digestive system (OR = 1.07, 95% CI: 0.99–1.14, p = 0.07). I2 = 0%, no heterogeneity (see Figure 3).
Figure 3
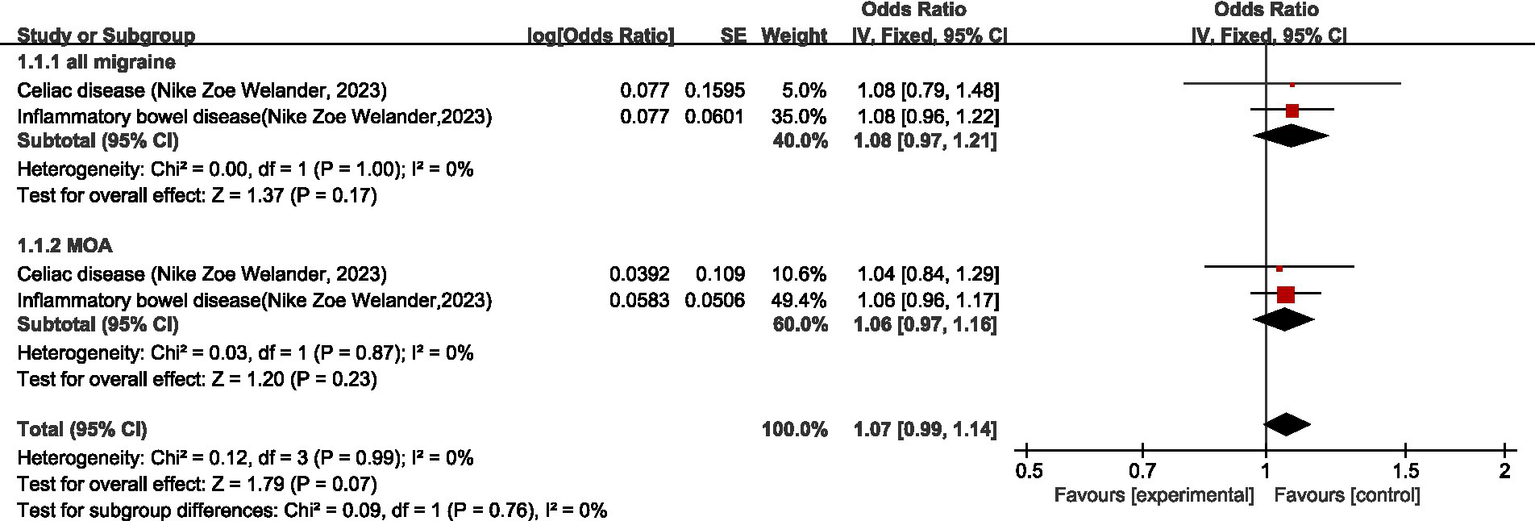
Forward: causal relationship between migraine and diseases of the digestive system.
3.4.4 Diseases of the skin
One study (26) discussed the causal relationship between migraine and the disease of the skin. Guanglu Li et al. (26) did not support a causal relationship between the risk of migraine and its subtypes and psoriasis (migraine-psoriasis: OR = 1.0033, 95% CI: 0.8831–1.1398, p = 0.76; MWA-psoriasis: OR = 1.0213, 95% CI: 0.9499–1.0980, p = 0.56; MOA-psoriasis: OR = 1.1057, 95% CI: 0.9938–1.2303, p = 0.06).
3.4.5 Endocrine, nutritional or metabolic diseases
Two studies (26, 27) discussed the causal relationship between migraine and endocrine, nutritional or metabolic diseases. Guanglu Li et al. (26) analyzed the MR data from three GWAS data sources (International Headache Genetic Consortium (IHGC), UK Biobank (UKB), and Finnish Genome Study (FinnGen)), respectively. In this paper, the data from these three data sources were meta-analyzed first before analyzing the data during the analysis process. For all migraine, meta-analysis showed that there was no significant causal relationship between migraine and type 1 diabetes (T1D) and type 2 diabetes (T2D) (OR = 0.97, 95% CI: 0.90–1.04, p = 0.35). For MOA and MWA, MR analysis showed that neither MOA nor MWA had a significant causal relationship with T1D (OR = 0.94, 95%CI: 0.81–1.09) (OR = 1.00, 95%CI: 0.92–1.09). Pooled analysis showed no significant causal relationship between migraine and endocrine, nutritional or metabolic diseases (OR = 0.98, 95% CI: 0.93–1.03, p = 0.37). I2 = 0%, no heterogeneity. See Figure 4 for details.
Figure 4
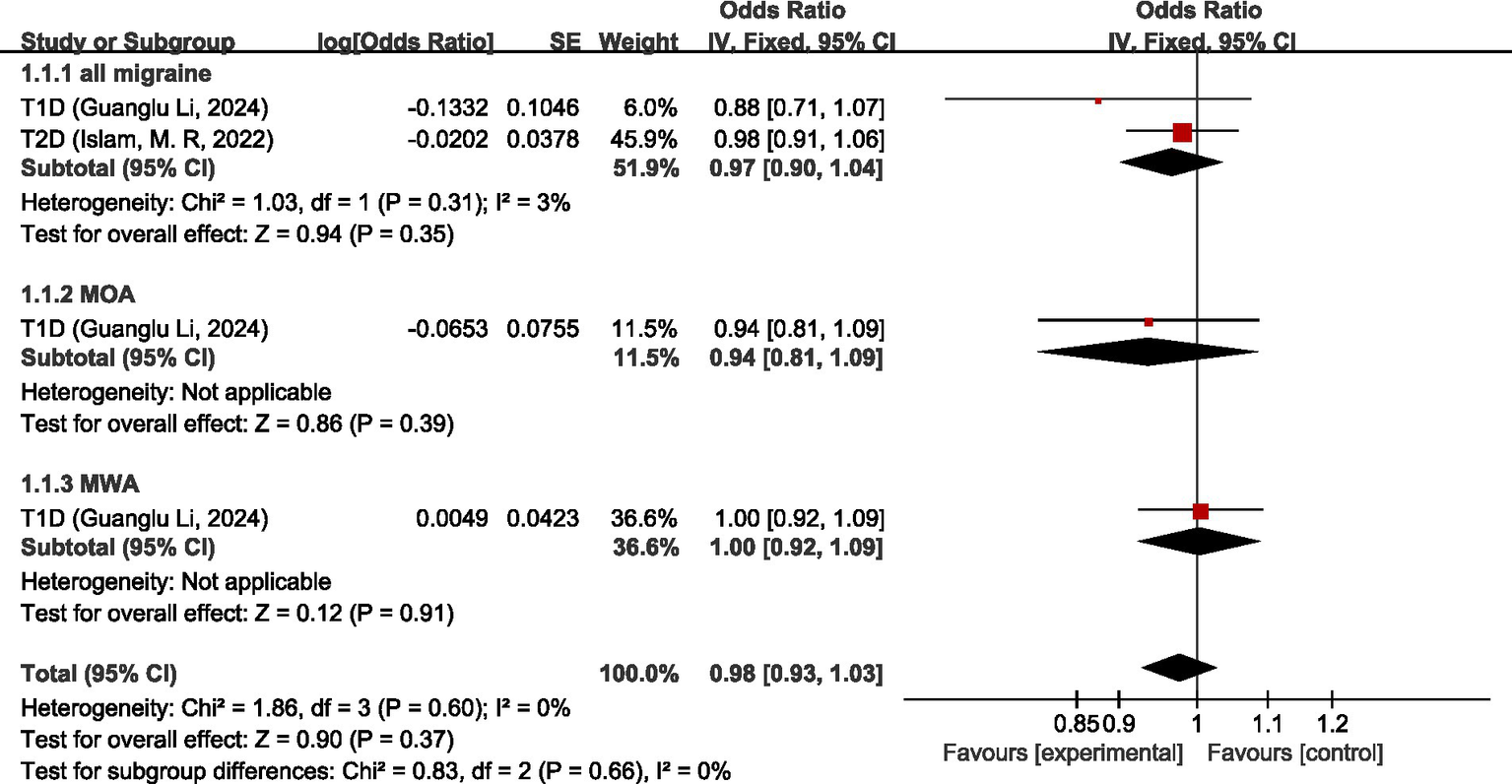
Forward: causal relationship between migraine and endocrine, nutritional or metabolic diseases. T1D, type 1 diabetes; T2D, type 2 diabetes.
3.4.6 Diseases of the nervous system
Seven studies (28–34) discussed the causal relationship between migraine and diseases of the nervous system. Chengfeng Xu et al. (32), Hua Xue et al. (30), Daghlas, I et al. (28), Geng, C et al. (33), Lei Zhao et al. (34), and Baranova, A. et al. (31) have studied the causal relationship between migraine and Alzheimer’s disease (AD), and since the GWAS data sources are the same, the newest and the study with the largest sample size, i.e., the study by Lei Zhao et al. (34), was selected for the analysis of our study. The results of the MR analysis showed that there was a significant positive correlation between migraine and AD (OR = 1.01; 95% CI: 1.04–1.16). There was no significant causal relationship between migraine and frontotemporal dementia, Lewy body dementia, multiple sclerosis (MS), or vascular dementia (OR = 0.87, 95% CI: 0.60–1.26) (OR = 0.96, 95% CI: 0.76–1.20) (OR = 1.09, 95% CI: 0.94–1.28) (OR = 0.85, 95% CI: 0.69–1.07). In the overall analysis, there was no significant causal relationship between migraine and disorders of the nervous system (OR = 1.07, 95% CI: 1.02–1.13, p = 0.004). I2 = 3%, suggesting mild heterogeneity. For details, see Figure 5.
Figure 5
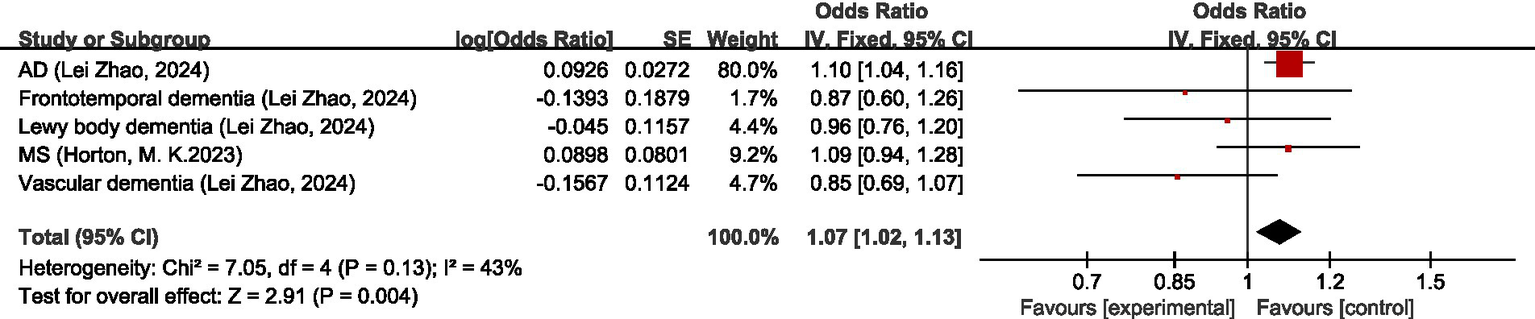
Forward: causal relationship between migraine and diseases of the nervous system. AD, Alzheimer’s disease; MS, multiple sclerosis.
3.4.7 Neoplasms
One study (35) discussed the causal relationship between migraine and neoplasms. MR in Fang, T et al. (35) showed that migraine was a risk factor for breast cancer (OR = 1.072, 95% CI: 1.035–1.110, p = 8.78 × 10−5); MOA was associated with an increase in breast cancer risk (OR = 1.042, 95% CI: 1.005–1.081, p = 0.0267); MWA was not causally associated with breast cancer (OR = 0.922, 95% CI: 0.840–1.103, p = 0.0919).
3.4.8 Diseases of the immune system
Two studies (26, 36) discussed the causal relationship between migraine and diseases of the immune system. Hao Lv et al. (36) and Guanglu Li et al. (26) both investigated the causal relationship between migraine/MOA/MWA and allergic rhinitis (AR). Due to the different data sources of GWAS, the original data were first meta-analyzed before being used for analysis in our study.
Regarding all migraine, MR analysis showed that there was no significant causal association between all migraine and AR, asthma, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE), and all migraine was not significantly causally associated with diseases of the immune system (OR = 1.00, 95% CI 0.99–1.01, p = 0.98). Regarding MWA, MR analysis showed that there was no significant causal association between MWA and AR, asthma, RA, and SLE, and there was no significant causal association between MWA and diseases of the immune system (OR = 1.00, 95% CI: 0.99–1.01, p = 0.86). Regarding MOA, MR analysis showed no significant causal association between MOA and AR, asthma, RA, and SLE, and no significant causal association between MOA and diseases of the immune system (OR = 0.99, 95% CI: 0.99–1.00, p = 0.05). Pooled analysis showed no causal relationship between migraine and its subtypes and disorders of the immune system (OR = 1.00, 95%CI: 0.99–1.00, p = 0.09); I2 = 0%, no heterogeneity. For details, see Figure 6.
Figure 6
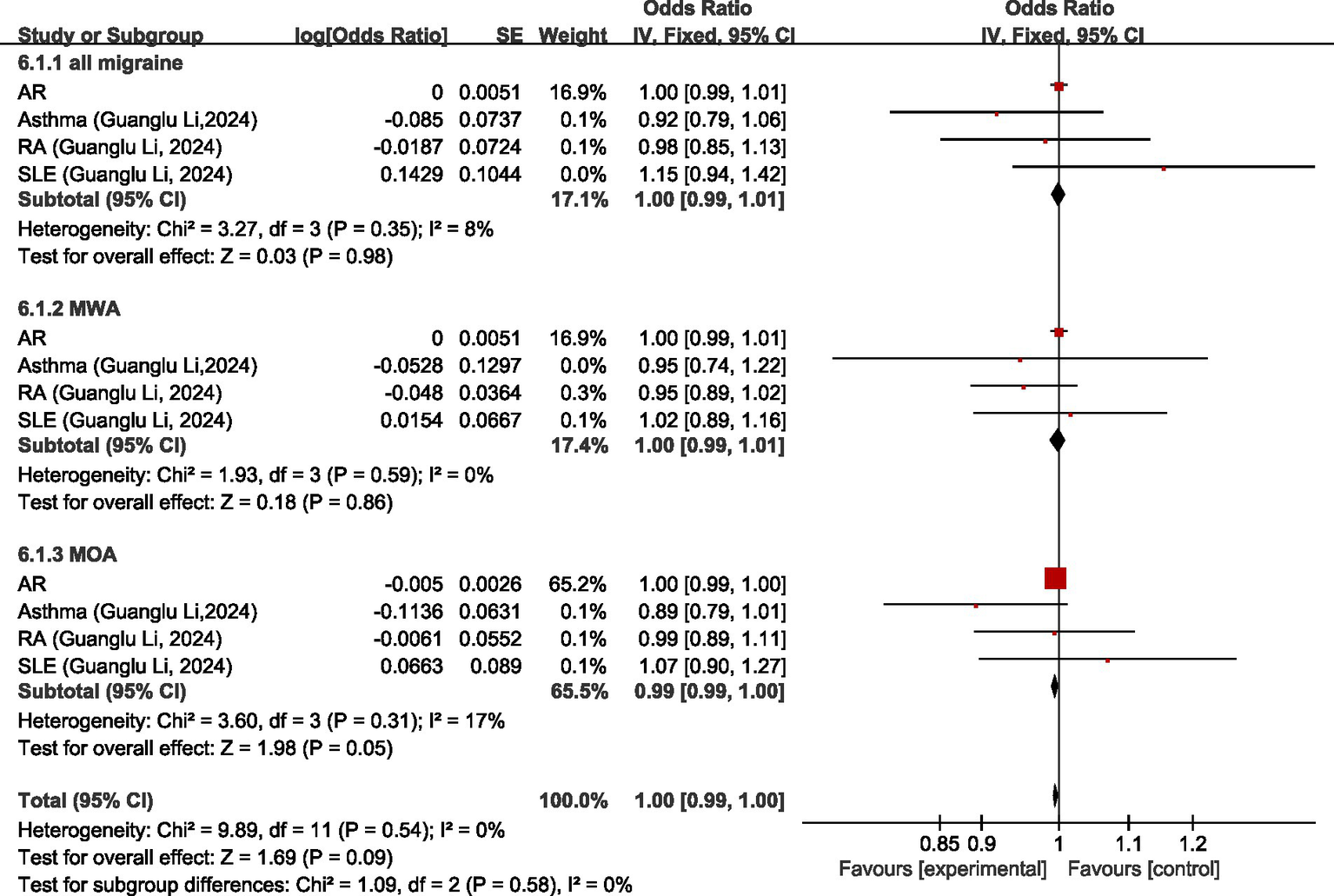
Forward: causal relationship between migraine and diseases of the immune system. AR, allergic rhinitis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
3.4.9 Diseases of the respiratory system
One study (26) discussed the causal relationship between migraine and diseases of the respiratory system. Guanglu Li et al. (26) did not find a significant causal relationship between migraine or its subtypes and asthma (all migraine-asthma: OR = 0.9185, 95% CI: 0.7949–1.0613, p = 0.22) (MOA-asthma: OR = 0.8926, 95% CI: 0.7888–1.0101, p = 0.07) (MWA-asthma: OR = 0.9486, 95% CI: 0.7357–1.2230, p = 0.68).
3.4.10 Diseases of the ear or mastoid process
One study (37) discussed the causal relationship between migraine and diseases of the ear or mastoid process. Kangjia Zhang et al. (37) demonstrated that there was no significant causal relationship between migraine and Meniere’s disease (MD) risk (p = 0.825).
3.4.11 Diseases of the genitourinary system
Two studies (38, 39) discussed the causal relationship between migraine and diseases of the genitourinary system. Emmanuel O. Adewuyi et al. (38) found no evidence of a causal relationship between migraine and endometriosis in their study (data not shown in the original article). Wenqiang Zhang et al. (39) showed that genetic susceptibility to migraine does not affect chronic kidney disease (CKD) risk (OR = 1.03, 95% CI = 0.98–1.09; p = 0.28).
3.4.12 Other ICD-11 classified diseases
One study (40) discussed the causal relationship between migraine and other ICD-11 classified diseases. Zhen-Ni Zhao et al. (40) did not find a causal relationship between migraine and periodontitis (PD) (OR = 1.00, 95%CI: 0.99–1.00, p = 0.65).
3.4.13 Non-disease
Eleven studies (28, 41–50) discussed the causal relationship between migraine and non-disease factors.
In terms of dietary intake non-disease factors, Xinhui Liu et al. (48) showed that migraine on overall alcohol intake (β = −0.0571, 95% CI: −0.07, −0.04) as a positive association.
Three studies (42–44) discussed the causal relationship between migraine and physiologic non-disease factors. MR analysis showed no significant causal relationship between migraine and insulin-like growth factor 1 (IGF-1) levels or higher serum vitamin D levels (OR = 1.00, 95% CI: 0.97–1.02) (OR = 0.98, 95% CI: 0.97–1.00); there is a heightened risk of migraines and diminished levels of interleukin-2 (IL-2) levels (OR = 0.01, 95% CI: 0.00–0.09) (Figure 7A). Overall, there was no significant causal relationship between migraine and physiologic non-disease factors (OR = 0.99, 95% CI: 0.92–1.05, p = 0.66) (Figure 7B). I2 = 89%, suggesting severe heterogeneity and was entirely attributable to the IL-2 data point, as evidenced by the elimination of heterogeneity upon its exclusion (Figure 7B). However, the overall effect remained non-significant after exclusion, as detailed in Figure 7.
Figure 7
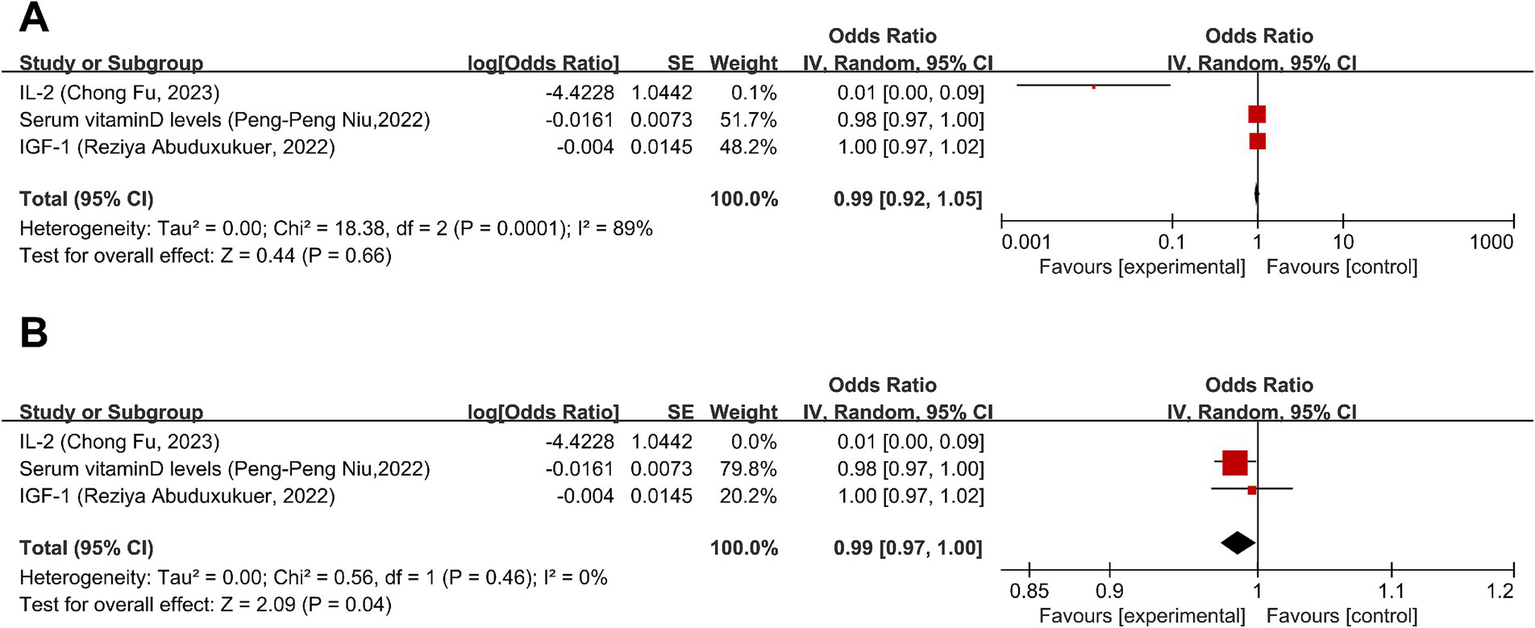
Forward: causal relationship between migraine and physiologic non-disease factors. (A) IL-2 included. (B) IL-2 eliminated. IGF-1, insulin-like growth factor 1; IL-2, interleukin-2.
Additionally, both Daghlas, I et al. (28) and Brittany L Mitchel et al. (41) studied the relationship between migraine and intracranial volume (ICV); as the GWAS data source was the same, the most recent and largest study containing sample size was selected, i.e., the Brittany L Mitchel et al. (41) to conduct the study analyzed herein, which did not find a significant causal relationship between migraine and ICV (OR: 0.95, 95% CI: 0.89–1.02, p = 0.16). Lei Zhao et al. (47) demonstrated that migraine exhibited significant causal effects on two white matter (WM) imaging-derived phenotypes (IDPs) (both the mode of anisotropy of the right uncinate fasciculus and the orientation dispersion index of the left superior cerebellar peduncle decreased) (p < 3.29 × 10−4). Huo, J et al. (45) found no association between migraine and white matter lesions (p > 0.05). Xiangyue Meng et al. (50), He, Q et al. (46), and Kang Qu et al. (49) all investigated the causal relationship between migraine and gut microbiota. Since the GWAS data sources were the same, the most recent study with the largest sample size, Kang Qu et al. (49), was chosen for the analysis of our study. The study did not find a significant causal relationship between migraine and gut microbiota.
3.5 Causal relationship between multiple diseases and migraine
3.5.1 Mental, behavioral or neurodevelopmental disorders
Two studies (51, 52) discussed the causal relationship between mental, behavioral or neurodevelopmental disorders and migraine. MR analysis showed a significant causal relationship between depression and migraine (OR = 1.32, 95% CI: 1.18–1.47). Yang Li et al. (52) and Xiaofeng Lv et al. (51) both investigated the causal relationship between major depressive disorders (MDD) and migraine. Since the GWAS data source was the same, the most recent study with the largest sample size, i.e., Yang Li et al. (52), was chosen for the analysis of our study. The results of the MR analysis showed that there was also a significant causal relationship between major depressive disorder and migraine (OR = 1.26, 95% CI: 1.11–1.43). Overall, there was a positive correlation between mental, behavioral or neurodevelopmental disorders and migraine (OR = 1.29, 95% CI: 1.19–1.47, p < 0.05). I2 = 0%, no heterogeneity. For details, see Figure 8.
Figure 8
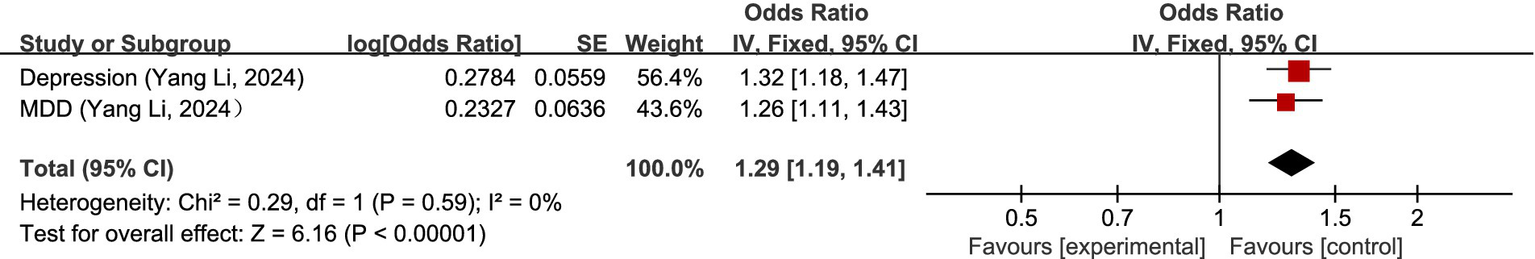
Reverse: causal relationship between mental, behavioral or neurodevelopmental disorders and migraine. MDD, major depressive disorder.
3.5.2 Disease of the circulatory system
Two studies (22, 53) discussed the causal relationship between disorders of the circulatory system and migraine. Xu-Peng Wu et al. (53) showed that VTE was associated with an increased risk of MWA (OR = 1.137, 95% CI: 1.062–1.218, p = 2.47 × 10−4). Yang Wang et al. (22) showed that VTE was a risk factor for migraine (OR = 1.002, 95% CI: 1.000–1.004, p = 0.016).
3.5.3 Sleep–wake disorders
Three studies (23, 24, 52) discussed the causal relationship between sleep–wake disorders and migraine. Yang Li et al. (52) and Chu S et al. (24) both did MR on the causal relationship between insomnia and migraine. Due to the difference in data sources between the two studies, the original data were meta-analyzed first before the meta-analysis of our study. MR analysis showed that there was a positive correlation between difficulty awakening and migraine (OR = 1.37, 95%CI: 1.12–1.68); meta-analysis showed insomnia had no significant causal relationship with migraine (OR = 1.38, 95%CI: 1.00–1.90). Pooled analysis showed a positive correlation between sleep–wake disorders and migraine (OR = 1.37, 95% CI: 1.16–1.63, p < 0.05). I2 = 0%, no heterogeneity. See Figure 9 for details.
Figure 9
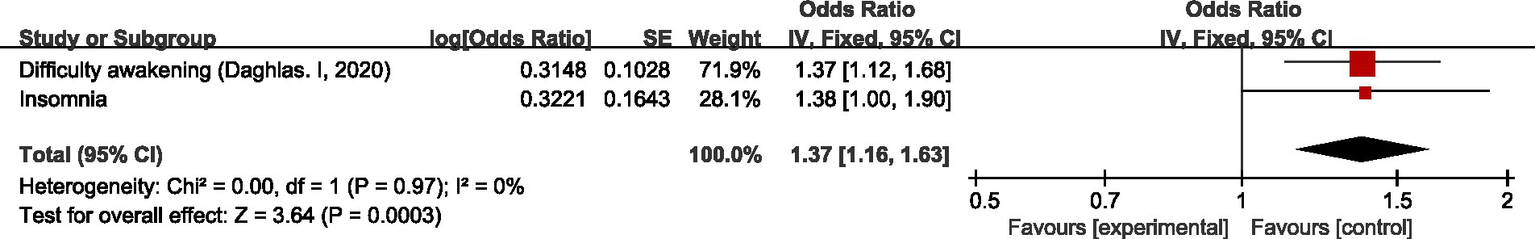
Reverse: causal relationship between sleep–wake disorders and migraine.
3.5.4 Diseases of the digestive system
One study (25) discussed the causal relationship between diseases of the digestive system and migraine. Regarding all migraine, meta-analysis showed that there was no significant causal relationship between diseases of the digestive system and all migraine (OR = 1.00, 95% CI: 0.99–1.01). Regarding MOA, MR analysis showed a negative association between celiac disease and MOA (OR = 0.95, 95% CI: 0.92–0.98); no significant causal association between inflammatory bowel disease and MOA (OR = 1.01, 95% CI: 0.97–1.05); and no significant causal relationship between diseases of the digestive system and MOA (OR = 0.98, 95% CI: 0.92–1.04, p = 0.47). Regarding MWA, MR analysis showed a positive association between celiac disease and MWA (OR = 1.04, 95% CI: 1.00–1.08); there was no significant causal association between inflammatory bowel disease and MWA (OR = 0.99, 95% CI: 0.95–1.03), and the association between the digestive system and MWA was not significant (OR = 0.98, 95% CI: 0.92–1.04). In the combined analysis, there was no significant causal relationship between diseases of the digestive system and migraine (OR = 1.00, 95% CI: 0.98–1.01, p = 0.72). I2 = 64%, moderate heterogeneity. See Figure 10 for details.
Figure 10
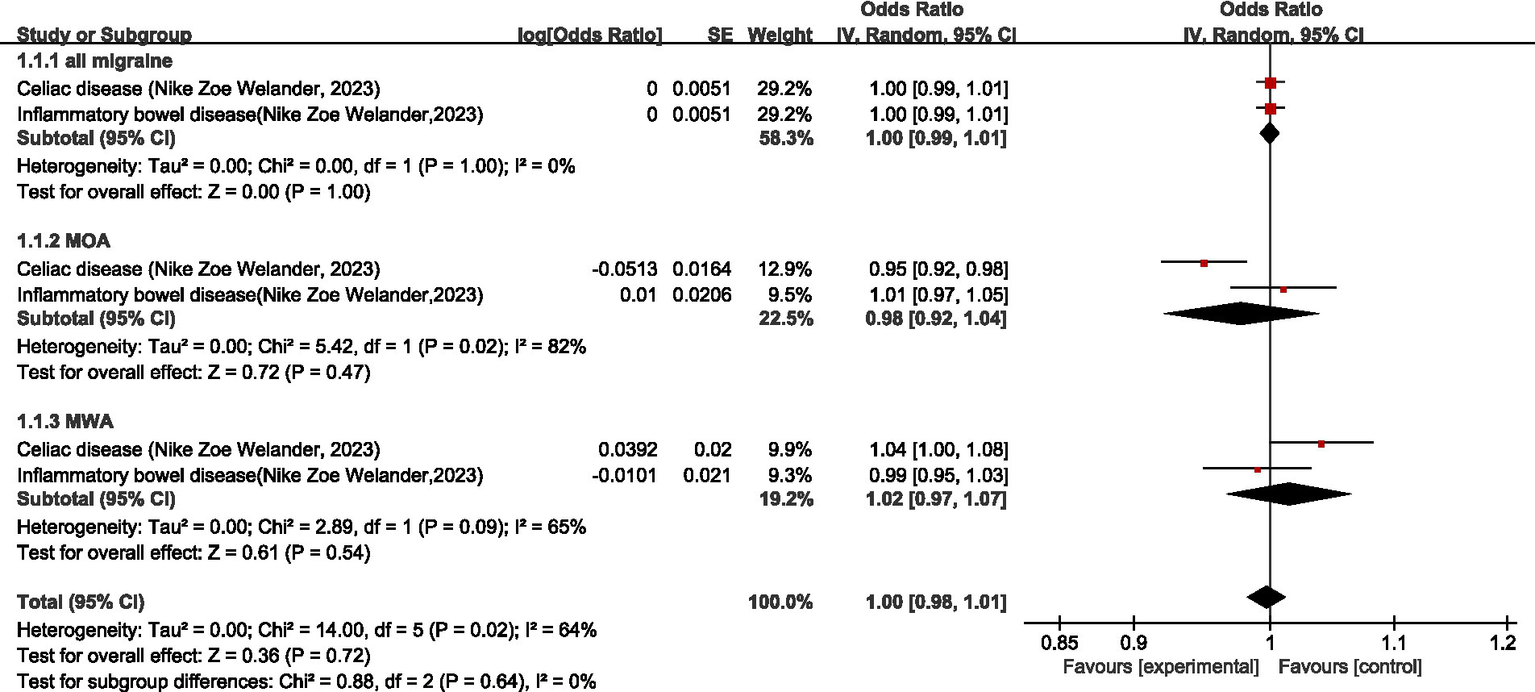
Reverse: causal relationship between diseases of the digestive system and migraine.
3.5.5 Diseases of the skin
One study (26) discussed the causal relationship between diseases of the skin and migraine. In the study by Guanglu Li et al. (26), MR analysis was performed on data from three GWAS data sources (IHGC, UKB, FinnGen), respectively. We meta-analyzed the data from these three GWAS data sources before analyzing the data in our study. The results of MR analysis showed that, regarding all migraine, there was no significant relationship between psoriasis and all migraine (OR = 0.98, 95% CI: 0.96–1.00, p = 0.11). Regarding MOA and MWA, there was no significant causal relationship between psoriasis and both MOA and MWA (OR = 1.00, 95% CI: 0.97–1.04, p = 0.85) (OR = 0.96, 95% CI: 0.90–1.01, p = 0.13). Pooled analyses showed there was no significant association between diseases of the skin and migraine (OR = 0.99, 95% CI: 0.97–1.00, p = 0.10). I2 = 7.9%, mild heterogeneity. See Figure 11 for details.
Figure 11
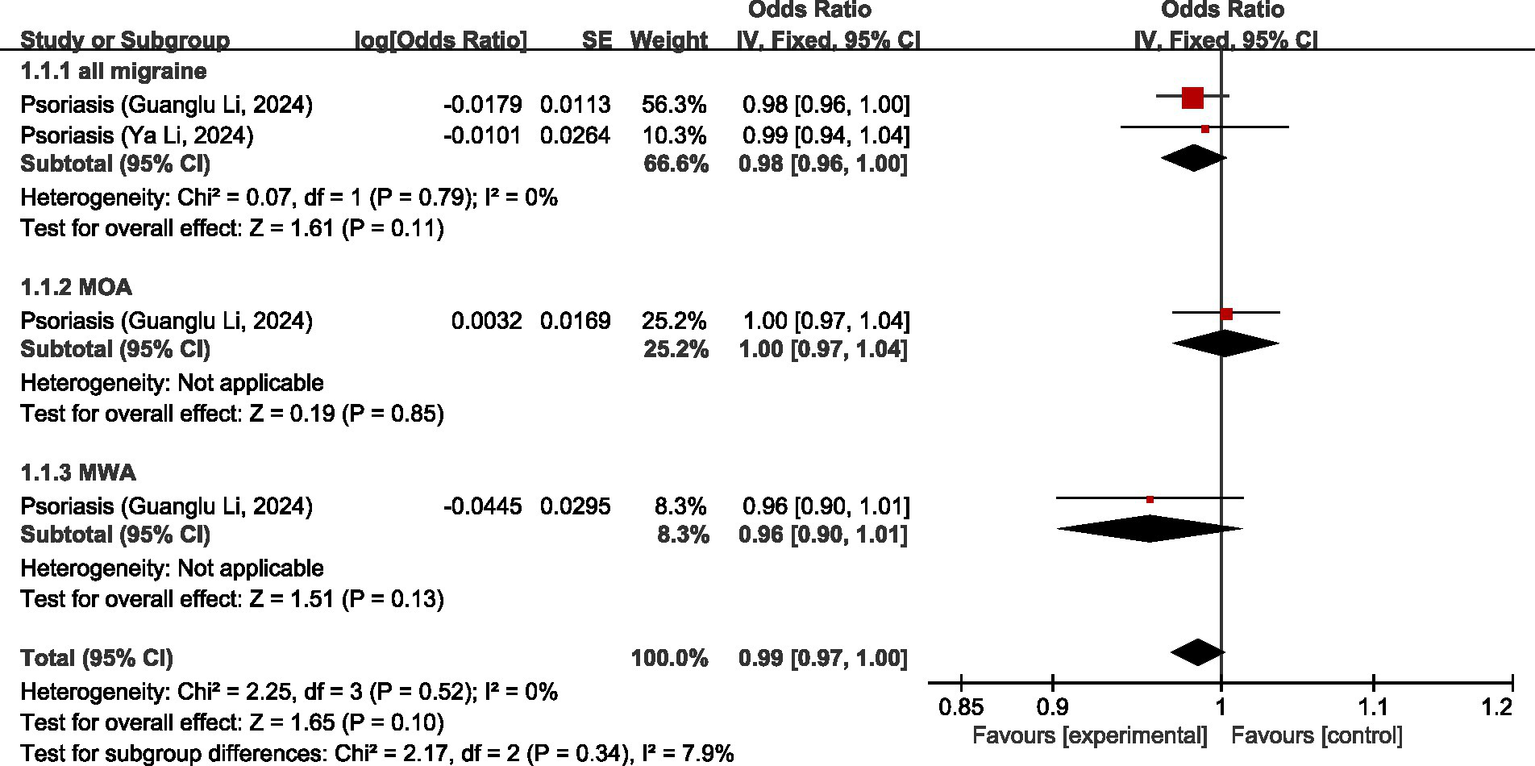
Reverse: causal relationship between diseases of the skin and migraine.
3.5.6 Endocrine, nutritional or metabolic diseases
Two studies (26, 27) discussed the causal relationship between endocrine, nutritional or metabolic diseases and migraine. In the study by Guanglu Li et al. (26), MR analysis was performed on data from three GWAS data sources (IHGC, UKB, FinnGen), respectively. We meta-analyzed the data from these three GWAS data sources before analyzing the data in our study. For all migraine, MR analysis showed that there was no significant causal relationship between T1D and T2D (OR = 0.99, 95% CI: 0.97–1.01, p = 0.44). For MOA and MWA, MR analysis showed that there was no significant causal relationship between T1D and both MOA and MWA (OR = 0.99, 95%CI: 0.97–1.01) (OR = 1.00, 95%CI: 0.98–1.01). Pooled analysis showed no significant causal association between endocrine, nutritional or metabolic diseases and migraine (OR = 1.00, 95% CI: 0.99–1.00, p = 0.26). I2 = 42%, mild heterogeneity. See Figure 12 for details.
Figure 12
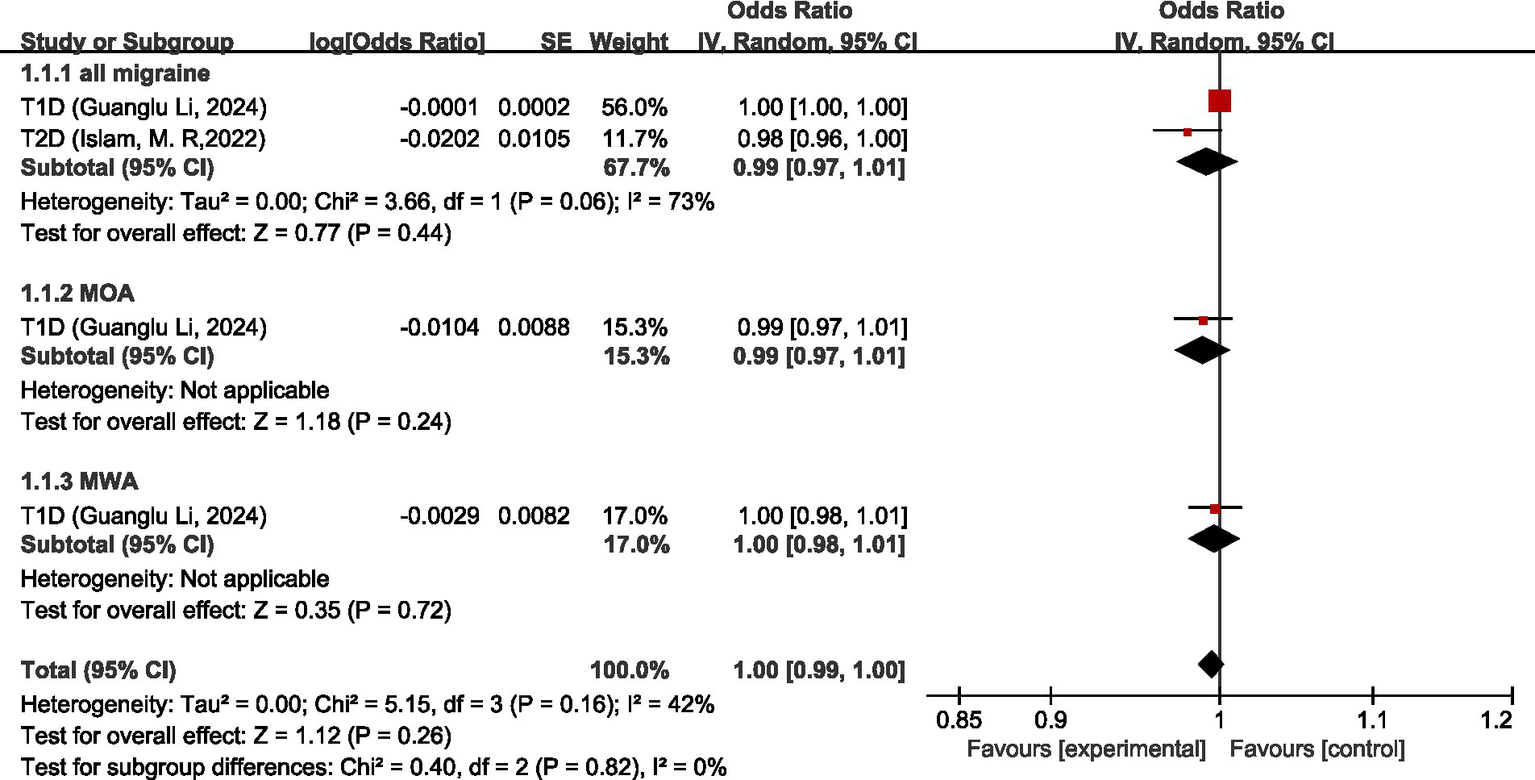
Reverse: causal relationship between endocrine, nutritional or metabolic diseases and migraine. T1D, type 1 diabetes; T2D, type 2 diabetes.
3.5.7 Diseases of the nervous system
One study (33) discussed the causal relationship between disorders of the nervous system and migraine. Geng, C et al. (33) showed no significant causal relationship between AD and migraine (OR = 1.000, 95%CI: 0.999–1.006, p = 0.971).
3.5.8 Diseases of the immune system
Four studies (26, 36, 54, 55) discussed the causal relationship between diseases of the immune system and migraine. Both Guanglu Li et al. (26) and Danfeng Xu et al. (54) investigated the causal relationship between SLE and migraine, MOA, or MWA; and Meixuan Ren et al. (55) investigated the causal relationship of SLE with MOA or MWA. Due to the different GWAS data sources, the original data were meta-analyzed before analyzing in our study (for all migraine, meta-analyzed Guanglu Li et al. (26) and Danfeng Xu et al. (54); for MOA and MWA, meta-analyzed Guanglu Li et al. (26), Danfeng Xu et al. (54), and Meixuan Ren et al. (55)). Both Guanglu Li et al. (26) and Hao Lv et al. (36) investigated the causal relationship between AR and migraine and its subtypes, and because of the different data sources of GWAS, the original data were meta-analyzed first and then analyzed in our study.
Regarding all migraine, MR analysis showed that AR, RA, and SLE had no significant causal relationship with migraine, asthma was a protective factor for all migraine (OR = 0.92, 95% CI: 0.88–0.97); meta-analysis showed diseases of the immune system were not significantly causally related to all migraine (OR = 0.99, 95% CI: 0.97–1.01, p = 0.51). Regarding MWA, MR analysis showed that AR, asthma, and RA had no significant causal relationship with MWA; SLE was a risk factor for MWA (OR = 1.02, 95%CI: 1.01–1.03); meta-analysis showed that diseases of the immune system had a positive correlation with MWA (OR = 1.02, 95%CI: 1.01–1.03, p < 0.05). Regarding MOA, MR analysis showed that AR, asthma, RA, and SLE had no significant causal association with MOA, and diseases of the immune system had no significant causal relationship with MOA (OR = 1.00, 95% CI: 0.99–1.01, p = 0.69). Comprehensive meta-analysis showed no significant causal relationship between diseases of the immune system and migraine (OR = 1.00, 95% CI: 0.99–1.01, p = 0.88). I2 = 61%, moderate heterogeneity. See Figure 13 for details.
Figure 13
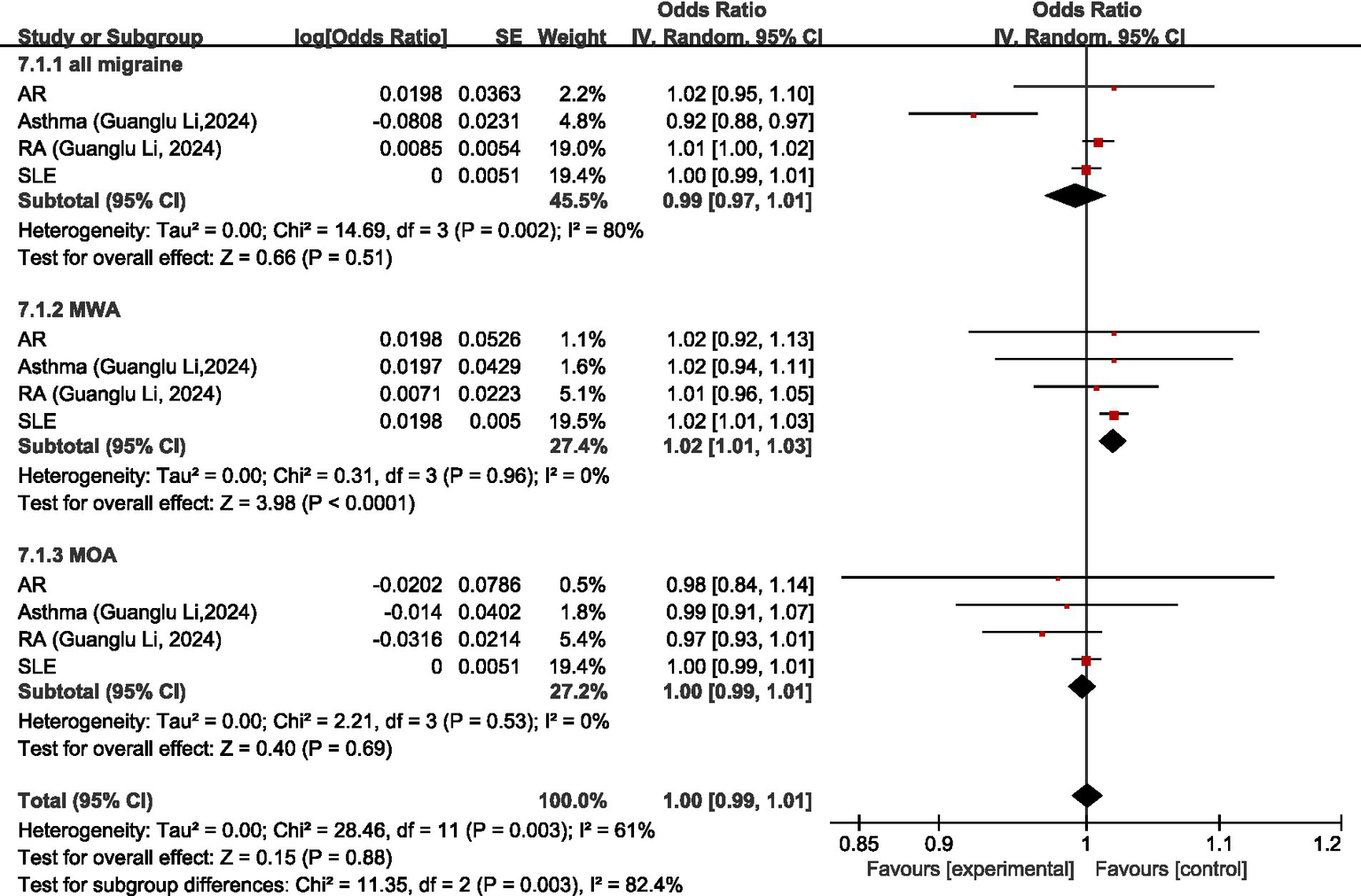
Reverse: causal relationship between diseases of the immune system and migraine. AR, allergic rhinitis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
3.5.9 Diseases of the respiratory system
One study (26) discussed the causal relationship between disorders of the respiratory system and migraine. Guanglu Li et al. (26) did not find a significant causal relationship between asthma and migraine and its subtypes (asthma-all migraine: OR = 0.9224, 95%CI: 0.8816–1.0175, p = 0.16) (asthma-MOA: OR = 0.9861, 95%CI: 0.9113–1.0671, p = 0.72) (asthma-MWA: OR = 1.0199, 95% CI: 0.9376–1.1093, p = 0.64).
3.5.10 Diseases of the ear or mastoid process
One study (37) discussed the causal relationship between diseases of the ear or mastoid process and migraine. Kangjia Zhang et al. (37) demonstrated no significant causal relationship between migraine and MD risk (OR = 0.999, p = 0.020).
3.5.11 Diseases of the genitourinary system
Two studies (38, 39) discussed the causal relationship between diseases of the genitourinary system and migraine. MR analysis showed that there was no significant causal relationship between CKD and migraine (OR = 1.03, 95% CI: 0.99–1.07), or endometriosis and migraine (OR = 0.98, 95% CI: 0.89–1.08). Pooled meta-analysis showed no significant causal relationship between diseases of the genitourinary system and migraine (OR = 1.02, 95%CI: 0.99–1.06, p = 0.23). I2 = 0%, no heterogeneity. For details, see Figure 14.
Figure 14
![Forest plot comparing the odds ratios for CKD (chronic kidney disease) by Wenqiang Zhang, 2023, and Endometriosis by Emmanuel O. Adewuyi, 2020. CKD shows an odds ratio of 1.03 [0.99, 1.07] with 85.5% weight, and Endometriosis shows an odds ratio of 0.98 [0.89, 1.08] with 14.5% weight. The total pooled odds ratio is 1.02 [0.99, 1.06]. Heterogeneity indicated by Chi² = 0.88, I² = 0%. Overall test effect Z = 1.20 (P = 0.23).](https://www.frontiersin.org/files/Articles/1660995/xml-images/fneur-16-1660995-g014.webp)
Reverse: causal relationship between diseases of the genitourinary system and migraine. CKD, chronic kidney disease.
3.5.12 Other ICD-11 classified diseases
Zhen-Ni Zhao et al. (40) found no significant causal relationship between PD and migraine (OR = 1.000, 95% CI: 0.99–1.00, p = 0.65).
3.5.13 Non-disease
Twenty-six studies (41–50, 52, 56–70) discussed the causal relationship between non-disease factors and migraine.
For behavioral habits non-disease factors, MR analysis showed that the four sleep habits of daytime sleeping, napping, short sleep duration, and sleep duration had no significant causal relationship with migraine; maternal smoking was a risk factor for migraine (OR = 1.02, 95% CI: 1.01–1.03), smoking index had no significant causal relationship with migraine (OR = 1.27, 95%CI: 0.98–1.65), smoking initiation was a risk factor for migraine (OR = 1.24, 95%CI: 1.04–1.48); physical activity had no significant causal relationship with migraine (OR = 0.95, 95% CI: 0.91–1.00); watching TV, a sedentary behavior (SBs), was a risk factor for migraine (OR = 1.63, 95% CI: 1.25–2.13). Pooled MR analysis showed that behavioral habits non-disease factors were not significantly causally associated with migraine (OR = 1.07, 95%CI: 0.99–1.16, p = 0.07), I2 = 72%, moderate heterogeneity. See Figure 15 for details.
Figure 15

Reverse: causal relationship between behavioral habits non-disease factors and migraine. SBs, sedentary behaviors.
Regarding dietary intake non-disease factors, Shuai Yuan et al. (61), Chen, H et al. (60), Jareebi et al. (66), and Jinjin Zhang et al. (67) studied the causal relationship between coffee consumption and all migraine. Since the data sources were the same, the most recent study with the largest sample size, i.e., Jinjin Zhang et al. (67), was chosen for the analysis in our study; Chen, H et al. (60) and Jinjin Zhang et al. (67) both studied the causal relationship between coffee intake and MWA. Because of the same data source, the most recent study with the largest sample size, Jinjin Zhang et al. (67), was chosen for the analysis of our study.
MR analysis showed that for all migraine, tea intake was not significantly causally associated with all migraines (OR = 0.94, 95% CI: 0.70–1.26), and higher alcohol consumption, cheese intake, coffee consumption, and salad intake were all protective factors for all migraines (OR = 0.58, 95% CI: 0.35–0.96), (OR = 0.76, 95% CI: 0.61–9.85), (OR = 0.53, 95% CI: 0.34–0.82), and (OR = 0.47, 95% CI: 0.26–0.85). Overall, these dietary intake non-disease factors were protective factors for all migraines (OR = 0.69, 95% CI: 0.54–0.87, p < 0.05). For MWA, tea intake was not significantly causally associated with MWA (OR = 0.93, 95% CI: 0.51–1.70), and higher coffee consumption was a protective factor for MWA (OR = 0.37, 95% CI: 0.21–0.67); these dietary intake non-disease factors have no association with MWA (OR = 0.59, 95% CI: 0.24–1.44, p = 0.24). For MOA, both coffee consumption and tea intake were not significantly causally associated with MOA (OR = 0.97, 95%CI: 0.71–1.33) (OR = 0.90, 95%CI: 0.52–1.56); these dietary intake non-disease factors were not significantly causally associated with MOA (OR = 0.95, 95%CI: 0.73–1.25, p = 0.72). Pooled MR analysis showed no significant causal relationship between dietary intake non-disease factors and migraine (OR = 0.72, 95%CI: 0.59–0.88, p = 0.001). I2 = 53%, suggesting moderate heterogeneity. For details, see Figure 16.
Figure 16
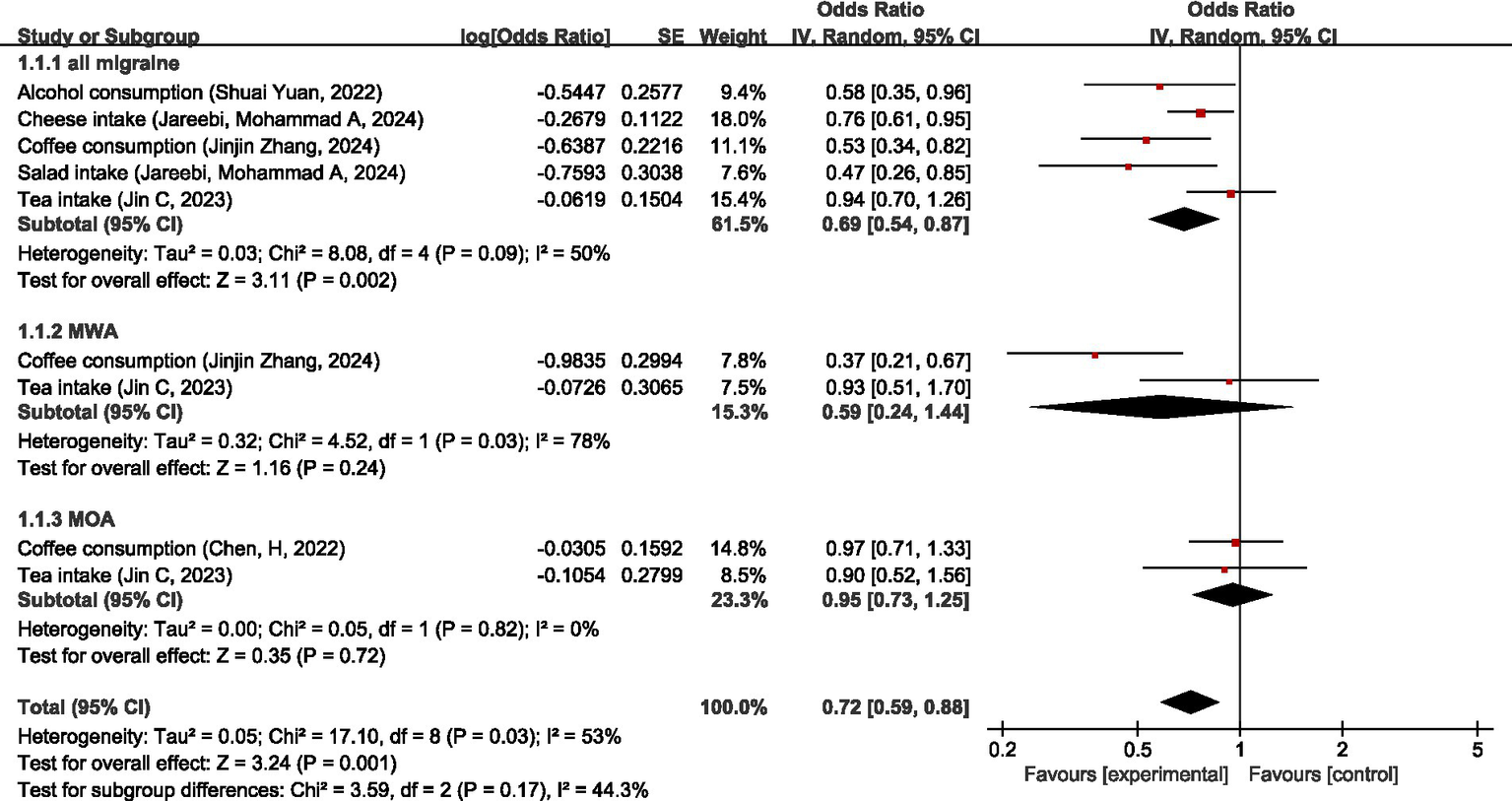
Reverse: causal relationship between dietary intake non-disease factors and migraine.
Furthermore, Xinhui Liu et al. (48) focused on 83 dietary habits; their study showed that more cups of coffee, oily fish, and cheese intake were significantly negatively associated with the risk of migraine (OR = 0.71, 95%CI: 0.59–0.86) (OR = 0.73, 95%CI: 0.59–0.89) (OR = 0.78, 95%CI: 0.63–0.95). This is consistent with the results of this study. They also found that there was an insufficiency of evidence of negative associations between more vegetables (OR = 0.72, 95% CI: 0.57–0.92) as well as wholemeal/wholegrain bread type (OR = 0.76, 95% CI: 0.63–0.92) and migraine. Additionally, they found weak evidence that drinks with meals (OR = 0.61, 95% CI: 0.47–0.80), more red wine (OR = 0.65, 95% CI: 0.51–0.82), and ore alcohol (OR = 0.74, 95% CI: 0.62–0.88) were associated with a decrease in risk of migraine; taking muesli was negatively associated with migraine (OR = 0.65, 95% CI: 0.48–0.89), while cornflakes/frosties were positively associated with migraine (OR = 1.53, 95% CI: 1.14–2.05); more white bread was associated with an increase in risk of migraine; poultry intake was positively associated with migraine (OR = 1.70, 95% CI: 1.19–2.43). Hui Zheng et al. (63) suggested that higher vitamin B12 intake was a protective factor for MWA (OR = 0.49, 95% CI: 0.24–0.99, p = 0.046).
Regarding physiologic non-disease factors, MR analysis showed that for all migraine, activated partial thromboplastin time (APTT), average thickness, gray matter volume (GMV), hepatocyte growth factor (HGF), white matter hyperintensities (WMH) had no significant causal relationship with migraine; higher diastolic blood pressure (DBP), pulse pressure (PP), systolic blood pressure (SBP), elevation of serum calcium levels by 1 mg/dL, higher coagulation factor VIII (FVIII) activity, phosphorylated fibrinopeptid A level, and von Willebrand factor (vWF) levels were associated with the increased risk of migraine; higher fibrinogen levels, Hippocampal volume (HV), IGF-1 level, neuralized E3 ubiquitin-protein ligase 1, surface area (cortical) (SA), serum vitamin D levels, and pulse pressure (PP) were associated with the decreased risk of migraine. Overall, there was no significant causal relationship between physiologic non-disease factors and all migraine (OR = 1.02, 95% CI: 0.99–1.04, p = 0.29).
For MWA, serum vitamin D levels did not have a significant causal relationship with MWA; FVIII activity, phosphorylated fibrinopeptide A, and vWF levels were risk factors for MWA; higher APTT, fibrinogen level, and IGF-1 were protective factors for MWA. Overall, there was no significant causal relationship between physiologic non-disease factors and MWA (OR = 1.00, 95% CI: 0.91–1.10, p = 0.98).
For MOA, IGF-1, and serum vitamin D levels had no significant causal relationship with MOA; higher DBP, PP, and elevation of serum calcium levels by 1 mg/dL was associated with the increased risk of MOA. Overall, there was no significant causal relationship between physiologic non-disease factors and MOA (OR = 1.15, 95% CI: 0.94–1.40, p = 0.18).
Overall, there is no causal relationship between physiologic non-disease factors and migraine (OR = 1.02, 95% CI: 1.00–1.05, p = 0.09), see Figure 17.
Figure 17
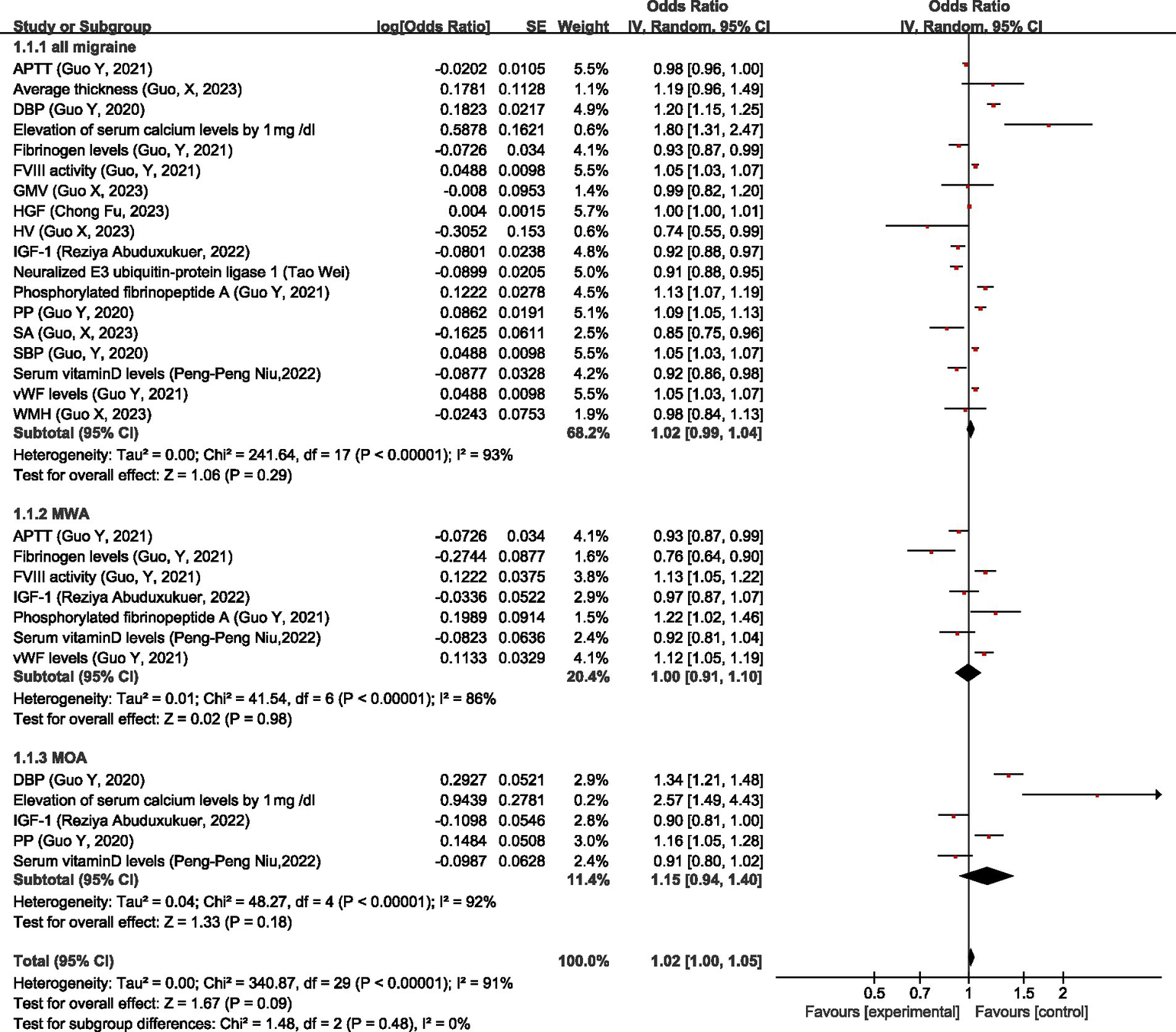
Reverse: causal relationship between physiologic non-disease factors and migraine. APTT, activated partial thromboplastin time; DBP, diastolic blood pressure; FVIII, coagulation factor VIII; GMV, gray matter volume; HGF, hepatocyte growth factor; HV, Hippocampal volume; IGF-1: insulin-like growth factor 1; PP, pulse pressure; SA, surface area (cortical); SBP, systolic blood pressure; vWF, von Willebrand factor; WMH, white matter hyperintensities.
Additionally, Brittany L. Mitchel et al. (41) found that there was a negative effect of larger ICV on migraine risk (OR = 0.91, 95%CI: 0.85–0.97, p = 0.006). Lei Zhao et al. (47) identified two WM IDPs that exhibited significant causal effects on migraine in the MR analysis (p < 3.29e × 10−4). Huo, J et al. (45) did not find any effect of white matter lesions (WMLs) on migraine. Xiangyue Meng et al. (50), He, Q et al. (46), and Kang Qu et al. (49) all investigated the relationship between gut microbiota and migraine. Since the GWAS data sources are the same, the most recent study with the largest sample size, i.e., Kang Qu et al. (49), was chosen for the analysis of this study. It was found that only the genus LachnospiraceaeUCG001 remained significantly associated with migraine (OR = 1.12, 95% CI: 1.05–1.20, p = 3.65 × 10−4). Kang Qu et al. (68) showed no association between low-density lipoprotein cholesterol (LDL-C), Apolipoprotein B (APOB), total cholesterol (TC) and migraine. Peng-Peng Niu et al. (70) showed that LRP11 (a protein) was significantly associated with the risk of any migraine (OR = 0.968, 95% CI: 0.955–0.981, p = 1.27 × 10−6) and significantly associated with migraine subtypes. ITIH1 (a protein) was significantly associated with the risk of migraine (OR = 1.044, 95% CI = 1.024–1.065, p = 1.08 × 10−5). ADGRF5 (a protein) was significantly associated with the risk of migraine (OR = 0.964, 95% CI: 0.946–0.982, p = 8.74 × 10−5) and suggestively associated with MWA.
Hui Zheng et al. (63) suggested that more years of schooling was negatively associated with MOA (OR = 0.57, 95%CI: 0.44–0.75, p < 0.0001), and eicosapentaenoic acid status level (OR = 2.54, 95%CI: 1.03–6.26, p = 0.043) was a risk factor for MWA. Guoliang Zhu et al. (65) found there was a causal relationship between delayed AFS and risk for migraine (OR = 0.73, 95% CI: 0.61–0.86), both for MWA (OR = 0.72, 95% CI: 0.58-0.89) and MOA (OR = 0.66, 95% CI:0.51-0.86). Daghlas, I et al. (28) found that genetic liability to migraine was not associated with intelligence (standardized beta = 0.01, 95% CI: 0.00–0.02, p = 0.13).
3.6 Overview of the establishment of a causal relationship between migraine and multiple factors
Overall, regarding migraine, migraine was a risk factor for 3 diseases (AD, CeAD, and VTE) and a protective factor for 3 diseases (CAD, angina, and LAS), 3 behavioral habits factors (delayed AFS, more physical activity, and maternal smoking), 1 dietary intake factors (more alcohol consumption), 3 physiologic factors (higher IL-2, BMI, and serum vitamin D level). Migraine had no association with 24 diseases (AF, hemorrhagic, ischemic stroke, stroke, myocardial infarction, insomnia, difficulty awakening, celiac disease, inflammatory bowel disease, psoriasis, T1D, T2D, frontotemporal dementia, Lewy body dementia, MS, vascular dementia, AR, asthma, RA, SLE, MD, and PD), 5 physiologic factors (IGF-1, higher serum vitamin D levels, ICV, WMLs, and gut microbiota). Six diseases (VTE, breast cancer, insomnia, difficulty awakening, MDD, and depression), 2 behavioral habits factors (watching TV, smoking initiation), and 11 physiologic factors (higher FVIII activity, vWF levels, phosphorylated fibrinopeptide A level, HGF, SBP, DBP, PP, elevation of calcium level by 1 mg/dL, ITIH1, SREBF2, and LachnospiraceaeUCG001) were risk factors of migraine. Three behavioral habits factors (delayed AFS, more years of schooling, and physical activity), 4 dietary intake factors (more alcohol consumption, coffee consumption, cheese intake, and salad intake), and 13 physiologic factors (higher fibrinogen levels, APTT, serum vitamin D level, IGF-1, SA, HV, neuralized E3 ubiquitin-protein ligase 1, LRP11, ADGRF5, APO-A1, REV1, ICV, and BMI) were protective factors of migraine. Fifteen diseases (insomnia, celiac disease, inflammatory bowel disease, psoriasis, T1D, T2D, AD, AR, RA, SLE, asthma, MD, chronic kidney disease, endometriosis, and PD), 6 behavioral habits factors (daytime sleeping, napping, short sleep duration, sleep duration, smoking index, and physical activity), 1 dietary intake factor (more tea intake), and 8 physiologic factors (APTT, average thickness, GMV, HGF, WMH, LDL-C, APOB, TC) had no association with migraine.
Regarding MOA, MOA was a risk factor for 2 diseases (AD and CeAD), a protective factor for 2 diseases (CAD and LAS), and 1 behavioral habits factor (delayed AFS). MOA had no association with 8 diseases (celiac disease, inflammatory bowel disease, psoriasis, T1D, asthma, AR, SLE, and RA). One disease (breast cancer), 1 behavioral habits factor (watching TV), and 3 physiologic factors (higher DBP, PP, and elevation of serum calcium level by 1 mg/dL) were risk factors of MOA. One disease (celiac disease), 2 behavioral habits factors (delayed AFS and more years of schooling), and 1 physiologic factor (IGF-1) were protective factors of MOA. Seven diseases (inflammatory bowel disease, psoriasis, T1D, AR, asthma, RA, and SLE), 2 dietary intake factors (more coffee consumption and tea intake), and 2 physiologic factors (IGF-1and serum vitamin D level) showed no association with MOA.
Regarding MWA, MWA was a risk factor of 1 disease (CeAD) and a protective factor of 1 disease (LAS). MWA had no association with 7 diseases (psoriasis, T1D, breast cancer, asthma, AR, SLE, and RA). Three diseases (VTE, SLE, and MDD) and 3 physiologic factors (higher FVIII activity, vWF levels, and phosphorylated fibrinopeptide A) were risk factors of MWA; 1 behavioral habits factor (delayed AFS), 1 dietary intake factor (more coffee consumption), and 2 physiologic factors (fibrinogen levels and APTT) were protective factors of MWA. Six diseases (inflammatory bowel disease, psoriasis, T1D, AR, asthma, and RA), 1 dietary intake factor (more tea intake), and 1 physiologic factor (higher serum vitamin D levels) showed no association with MWA (see Figure 18).
Figure 18
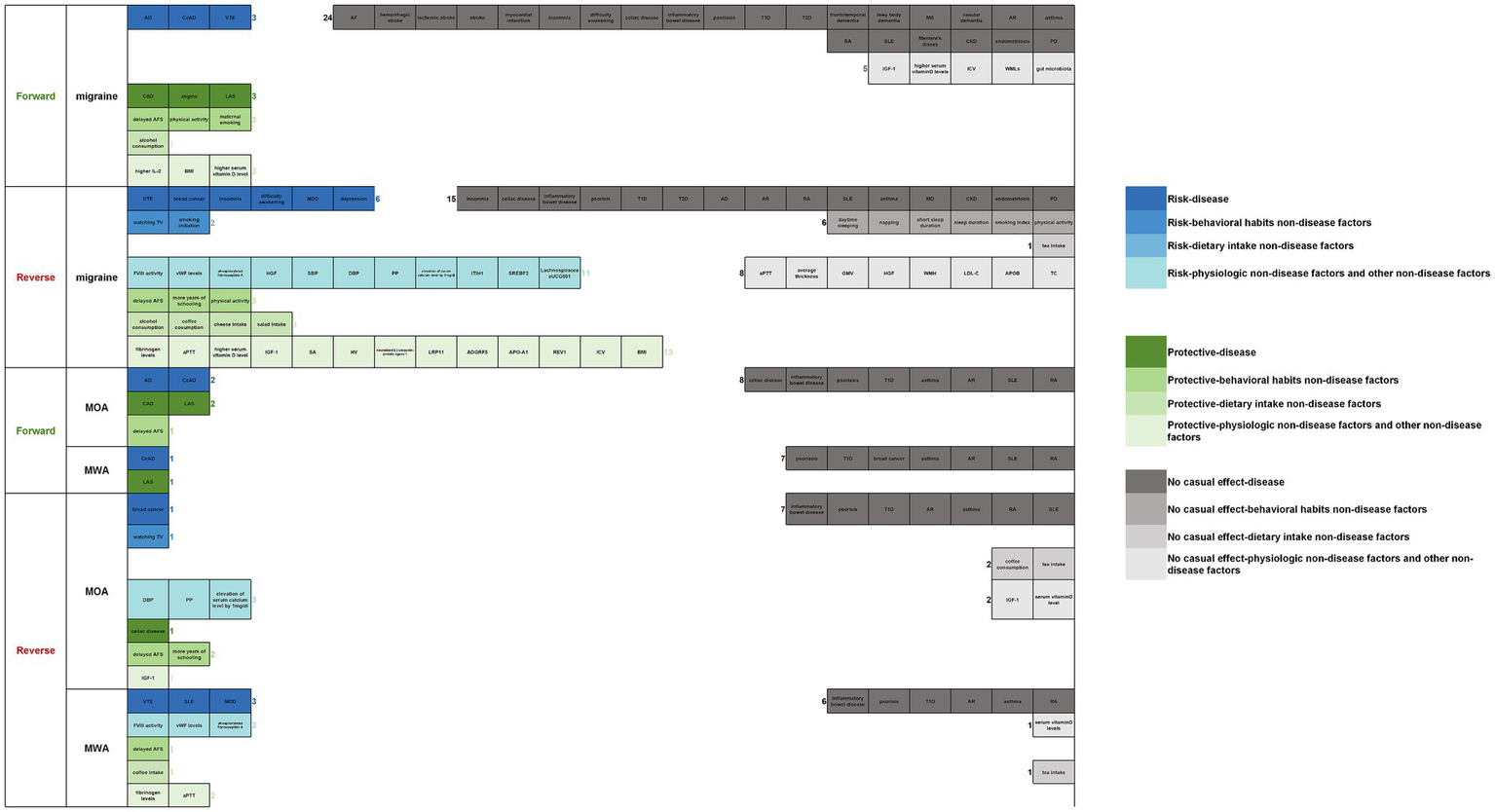
Overview of the establishment of a causal relationship between migraine and multiple factors (forward/reverse).
3.7 Relationships between genes associated with migraine or their expression products and drug targets
Seven studies (57, 68, 70–74) have discussed the relationship between genes or their expression products and drug targets associated with migraine. SNP rs1051730 is by far the strongest genetic variant associated with smoking behavior found in genome-wide studies (75, 76). Johnsen, M. B et al. (57) indicated that no association was found between the rs1051703T allele and migraine (all participants: OR = 0.98, 95%CI: 0.95–1.02, p = 0.38; never smokers: OR = 0.99, 95%CI: 0.93–1.04, p = 0.63; ever smokers: OR = 0.97, 95%CI: 0.92–1.03, p = 0.32). Bi Y et al. (71) found that 3-Hydroxy-3-Methylglutaryl-CoA Reductase (HMGCR) inhibition, corresponding to the reduction in LDL-C, was significantly causally associated with a lower risk of migraine (OR = 0.73, 95% CI: 0.60–0.89, p = 0.0016). Lipoprotein lipase (LPL) enhancement was significantly causally associated with a lower risk of migraine (OR = 0.89, 95% CI: 0.83–0.96, p = 0.0016). This suggests that LPL and HMGCR have the potential to serve as candidate drug targets for the treatment or prevention of migraine. Chengcheng Zhang et al. (72) identified 21 druggable genes significantly associated with migraine (BRPF3, CBFB, CDK4, CHD4, DDIT4, EP300, EPHA5, FGFRL1, FXN, HMGCR, HVCN1, KCNK5, MRGPRE, NLGN2, NR1D1, PLXNB1, TGFB1, TGFB3, THRA, TLN1, and TP53), two of which were significant in both blood and brain (HMGCR and TGFB3). TGFB3 was mainly associated with IGF-1 levels, and HMGCR was highly correlated with LDL- C. Hong, P et al. (73) demonstrated that genotypes of HMGCR related to higher LDL-C level might increase the risk of migraine (OR = 1.46, 95% CI: 1.03–2.07, p = 0.035) and MWA (OR = 2.03, 95% CI: 1.20–3.42, p = 0.008), genotypes of APOB related to higher LDL-C level might decrease the risk of MOA (OR = 0.62, 95% CI: 0.47–0.81, p = 0.000), and genotypes of PCSK9 related to higher LDL-C level might decrease the risk of migraine (OR = 0.75, 95% CI: 0.64–0.89, p = 0.001) and MWA (OR = 0.69, 95% CI: 0.54–0.89, p = 0.004). Kang Qu et al. (68) indicated that HMGCR expression (OR = 1.55, 95% CI: 1.30–1.84, p = 6.87 × 10−7) and the circulating levels of three lipids (LDL-C, APOB, and TC) adjusted by HMGCR expression (OR = 1.55, 95% CI: 1.30–1.84, p = 6.87 × 10−7) were significantly associated with an increased risk of migraine (LDL-C: OR = 1.51, 95% CI 1.21–1.88, p = 2.50 × 10−4; APOB: OR = 2.12, 95% CI: 1.56–2.87, p = 1.35 × 10−6; TC: OR = 1.63, 95% CI: 1.30–2.06, p = 2.93 × 10−5). Four groups of studies (68, 71–73) on the relationship between HMGCR and migraine reached consistent conclusions, and these findings indicate a correlation between HMGCR and migraine. Future investment in research on HMGCR inhibitors may provide a new approach to migraine prevention.
Jianxiong Gui et al. (74) showed that REV1 may reduce the migraine risk by regulating DNA damage repair, while SREBF2 may increase the risk of migraine by regulating cholesterol metabolism. The REV1 gene is located on chromosome 2q11.2, and MR analysis confirmed a causal relationship between REV1 and migraine (p < 0.05). MR analysis of testicular tissue confirmed a significant causal relationship between the SREBF2 gene and migraine (OR = 1.10, 95% CI: 1.01–1.19, p < 0.05). Pengpeng Niu et al. (70) found that LRP11 was significantly associated with the risk of migraine (OR = 0.968, 95% CI: 0.955–0.981, p = 1.27 × 10−6). ITIH1 was significantly associated with the risk of migraine (OR = 1.044, 95% CI: 1.024–1.065, p = 1.08 × 10−5). ADGRF5 was significantly associated with the risk of migraine (OR = 0.964, 95% CI: 0.946–0.982, p = 8.74 × 10−5).
4 Discussion
Migraine is a chronic, progressive neurological disorder (77). So far, a large number of studies have been conducted on migraine and its causal factors, but almost no one has yet integrated these results. This is a systematic review focusing on evidence concerning factors contributing to migraine from Mendelian randomization studies.
A review of the pathogenesis of migraine found that three theories—cortical spreading depression (CSD), trigeminal vascular theory, and genetics—are widely accepted in academia. The review by Pleș H et al. further highlighted the key role of calcitonin gene-related peptide (CGRP) signaling in migraine pathophysiology (78). However, despite significant research, the precise biological mechanisms linking these established pathways (CSD, trigeminovascular activation, CGRP) and emerging factors to migraine onset and progression remain incompletely understood. This mechanistic uncertainty poses major challenges for treatment, often resulting in suboptimal outcomes, recurrence, and significant impacts on patients’ socialization and quality of life. In this study, we comprehensively integrated evidence of causal associations between migraine and multidimensional exposure factors (including disease, non-disease, and genetic factors, both forward and reverse) through a Mendelian randomization systematic review. Crucially, these MR-derived causal relationships provide new avenues to explore the underlying biological mechanisms. By identifying robust causal factors, this work generates specific hypotheses for how these factors might interact with or modulate known pathways (like CGRP signaling) or implicate novel biological processes in migraine pathogenesis, thereby offering direction for future mechanistic research and therapeutic development.
This systematic review included a total of 60 MR studies, comprising 331 MR analyses. Migraine was a risk factor for 3 diseases and a protective factor for 3 diseases, 3 behavioral habits factors, 1 dietary intake factors, and 3 physiologic factors. Migraine had no association with 24 diseases and 5 physiologic factors. Six diseases, 2 behavioral habits factors, and 11 physiologic factors were risk factors for migraine. Three behavioral habits factors, 4 dietary intake factors, and 13 physiologic factors were protective factors of migraine. Fifteen diseases, 6 behavioral habits factors, 1 dietary intake factor, and 8 physiologic factors had no association with migraine. In subtype analysis, we found MOA was a risk factor for 2 diseases, a protective factor for 2 diseases, and 1 behavioral habit factor. MOA had no association with 8 diseases. 1 disease, 1 behavioral habit factor, and 3 physiologic factors were risk factors of MOA. One disease, 2 behavioral habits factors, and 1 physiologic factor were protective factors of MOA. Seven diseases, 2 dietary intake factors, and 2 physiologic factors had no association with MOA. MWA was a risk factor for 1 disease and a protective factor for 1 disease. MWA had no association with 7 diseases. Three diseases and 3 physiologic factors were risk factors of MWA. One behavioral habit factor, 1 dietary intake factor, and 2 physiologic factors were protective factors of MWA. Six diseases, 1 dietary intake factor, and 1 physiologic factor had no association with MWA.
This study also integrated 29 migraine-associated drug targets, including genetic variants linked to smoking behavior, lipid metabolism-related genes, and their expressed proteins. Their expression levels were found to be associated with migraine risk, providing novel insights into the genetic architecture of migraine. However, the pharmacological effects on these targets demonstrate considerable promiscuity and many off-target effects cannot be adequately explored through MR analysis. Further fundamental research and clinical investigations are required to elucidate the potential bioactivities of these significant signals and achieve a more comprehensive understanding.
Some recent studies have revealed that the impact of certain factors on migraine is not direct but rather indirect. For instance, Zhonghua Xiong et al. discovered that atrophy in the subthalamic nucleus subregion plays a critical role in increasing migraine risk, and this effect is partially mediated through alterations in the gut microbiome composition (79). Similarly, Zixiong Shen et al. identified a positive causal relationship between gastroesophageal reflux disease (GERD) and migraine, highlighting the mediating role of depression in increasing migraine risk induced by GERD (80). These findings align to some extent with the results of the present study, yet these researchers delved deeper into the mediating relationships among these factors. This suggests that the migraine-related factors identified in existing research may arise through more complex cascades of mechanisms. Future studies should focus on the interconnections among multiple factors to derive more comprehensive conclusions.
This study has several limitations. First, the analytical framework may lack generalizability across populations with diverse ethnicities or geographical distributions, as the majority of included studies exclusively involved individuals of European ancestry—a constraint inherent to the original data sources. Subsequent data collection and analyses are warranted to validate the universality of findings. Second, the absence of disease severity stratification in case classification precludes assessment of potential associations between migraine progression and clinical severity gradients. Third, there may exist a minimal number of MR investigations utilizing non-overlapping GWAS sources that were not subjected to meta-analysis consolidation. Fourth, our screening protocol did not impose restrictions on the number of SNPs employed in MR analyses, nor did it exclude studies utilizing limited genetic instruments (<10 SNPs). This methodological heterogeneity could introduce estimation biases when integrating such studies with adequately powered investigations during meta-analysis. As MR methodologies continue to evolve, incorporating advanced statistical approaches such as multivariable MR and mediation analysis may help mitigate confounding effects, thereby enhancing the precision of causal inference. Since some included MR studies did not report multiple testing correction, the synthesized results may carry an inflated risk of type I error. This highlights the need for future primary MR studies to adhere to relevant reporting standards and implement comprehensive multiple testing corrections to enhance the reliability of findings.
In addition, some results in this study exhibited substantial heterogeneity. Specifically, high heterogeneity was observed in the analysis investigating the impact of migraine on circulatory system diseases. After removing the outlier (VTE), the I2 value remained high at 71%. We propose that residual heterogeneity may stem from the use of identical genetic instruments/data alongside the combination of outcomes with distinct biological mechanisms. Regarding migraine and non-disease physiological factors, the severe heterogeneity was entirely attributable to the IL-2 data point, as evidenced by the elimination of heterogeneity upon its exclusion. However, the overall effect remained non-significant after exclusion, indicating no causal relationship between migraine and non-disease physiological factors. This heterogeneity likely originated from fundamental differences in exposure characteristics or variations in study design. This finding underscores that combining exposures with divergent biological mechanisms may distort causal inference. Both primary and subgroup analyses of non-disease physiological factors and migraine showed significant heterogeneity (Figure 17). Leave-one-out analysis revealed no substantial changes in results upon sequential removal of individual studies. Potential sources include (1) the inclusion of diverse physiological indicators linked to distinct biological pathways of disease pathogenesis and (2) substantial methodological variations across studies that were difficult to standardize.
Comprehensive analysis indicates that high heterogeneity primarily emerged in the non-disease physiological factors category—a supplementary classification for exposures not covered by ICD-11 disease codes. Although we subcategorized these factors into dietary intake, behavioral habits, and physiological indicators, the latter remains an excessively broad domain encompassing countless physiological mechanisms, rendering the classification inadequate. Current limitations in data richness further impede finer subcategorization. Future studies should therefore conduct systematic and granular stratified analyses of physiological indicators based on underlying biological mechanisms to better elucidate bidirectional causal relationships between physiological markers and migraine.
5 Conclusion
This systematic review summarizes the potential causal relationships between migraine and various factors derived from MR studies and also analyzes migraine subtypes. We discuss risk factors and protective factors associated with migraine, both forward and reverse. Moreover, we summarize the genes associated with migraine and their downstream signaling and drug targets, which will provide ideas and directions for future migraine research and bring value to the prevention and treatment of migraine.
Statements
Author’s note
This study conducted the systematic review following the criteria of the PRISMA guidelines and checked against the PRISMA 2020 checklist, which is attached as Supplementary material 3.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
XL: Visualization, Validation, Writing – review & editing, Formal analysis, Data curation, Writing – original draft. QL: Investigation, Writing – review & editing, Writing – original draft, Data curation, Formal analysis, Methodology. HN: Writing – original draft, Investigation, Writing – review & editing, Validation. JN: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing, Funding acquisition, Resources, Project administration, Writing – original draft. SY: Investigation, Software, Conceptualization, Writing – original draft, Resources, Writing – review & editing, Validation, Project administration, Supervision. JJ: Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the State Scholarship Fund of China [No. 72374151], Fundamental Research Funds for the Central Universities (YJ202339), Sichuan University Research Funds for Graduate Education and Teaching Reform (GSSCU2023120), the Traditional Chinese Medicine Science and Technology Industry Innovation Team of Sichuan Province and the Multi-dimensional Evaluation and Product Development Innovation Team of Characteristic Traditional Chinese Medicine Resources (No. 2022C001), the Sichuan Science and Technology Program (2025JDKP0108), and Sichuan University Research Funds for Basic Medicine Research and University of Macau (UMDF-TISF/2025/001/FHS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1660995/full#supplementary-material
- MWA
Migraine with aura
- MOA
Migraine without aura
- NSAID
Non-Steroidal Anti-Inflammatory Drug
- MR
Mendelian randomization
- IVs
Instrumental variables
- IVW
Inverse Variance Weighted
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- CNKI
China National Knowledge Infrastructure
- WanFang
Wanfang Data Knowledge Service Platform
- VIP
VIP China Science and Technology Journal Database
- OR
Odds Ratio
- 95%CI
95% Confidence Interval
- GWAS
Genome-Wide Association Studies
- SNP
Single-nucleotide polymorphism
- STROBE-MR
Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization
- ICD-11
International Classification of Diseases, 11th Revision
- WHO
World Health Organization
- I2
I-squared statistic
- AF
Atrial fibrillation
- CAD
Coronary artery disease
- CeAD
Cervical artery dissection
- LAS
Large artery stroke
- VTE
Venous thromboembolism
- IHGC
International Headache Genetic Consortium
- UKB
UK Biobank
- FinnGen
Finnish Genome Study
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- AD
Alzheimer’s disease
- MS
Multiple sclerosis
- AR
Allergic rhinitis
- RA
Rheumatoid arthritis
- SLE
Systemic lupus erythematosus
- MD
Meniere’s disease
- CKD
Chronic kidney disease
- PD
Periodontitis
- IGF-1
Insulin-like growth factor 1
- IL-2
Interleukin-2
- ICV
Intracranial volume
- WM
White matter
- IDPs
Imaging-derived phenotypes
- MDD
Major depressive disorder
- SBs
Sedentary behaviors
- APTT
Activated partial thromboplastin time
- GMV
Gray matter volume
- HGF
Hepatocyte growth factor
- WMH
White matter hyperintensities
- DBP
Diastolic blood pressure
- PP
Pulse pressure
- SBP
Systolic blood pressure
- FVIII
Coagulation factor VIII
- vWF
von Willebrand factor
- HV
Hippocampal volume
- SA
Surface area (cortical)
- WMLs
White matter lesions
- LDL-C
Low-density lipoprotein cholesterol
- APOB
Apolipoprotein B
- TC
Total cholesterol
- HMGCR-3
Hydroxy-3-Methylglutaryl-CoA Reductase
- LPL
Lipoprotein lipase
- CSD
Cortical spreading depression
- CGRP
Calcitonin gene-related peptide
- GERD
gastroesophageal reflux disease
Glossary
References
1.
Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
2.
Tepper SJ Dahlof CG Dowson A Newman L Mansbach H Jones M et al . Prevalence and diagnosis of migraine in patients consulting their physician with a complaint of headache: data from the landmark study. Headache. (2004) 44:856–64. doi: 10.1111/j.1526-4610.2004.04167.x
3.
Steiner TJ Stovner LJ Jensen R Uluduz D Katsarava Z . Migraine remains second among the world's causes of disability, and first among young women: findings from GBD 2019. J Headache Pain. (2020) 21:137. doi: 10.1186/s10194-020-01208-0
4.
Goadsby PJ Holland PR Martins-Oliveira M Hoffmann J Schankin C Akerman S . Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. (2017) 97:553–622. doi: 10.1152/physrev.00034.2015
5.
Burstein R Noseda R Borsook D . Migraine: multiple processes, complex pathophysiology. J Neurosci. (2015) 35:6619–29. doi: 10.1523/JNEUROSCI.0373-15.2015
6.
Olesen J . International classification of headache disorders. Lancet Neurol. (2018) 17:396–7. doi: 10.1016/S1474-4422(18)30085-1
7.
Meng W Adams MJ Hebert HL Deary IJ McIntosh AM Smith BH . A genome-wide association study finds genetic associations with broadly-defined headache in UK biobank (n=223, 773). EBioMedicine. (2018) 28:180–6. doi: 10.1016/j.ebiom.2018.01.023
8.
Marmura MJ Silberstein SD Schwedt TJ . The acute treatment of migraine in adults: the American headache society evidence assessment of migraine pharmacotherapies. Headache. (2015) 55:3–20. doi: 10.1111/head.12499
9.
Khan J Asoom LIA Sunni AA Rafique N Latif R Saif SA et al . Genetics, pathophysiology, diagnosis, treatment, management, and prevention of migraine. Biomed Pharmacother. (2021) 139:111557. doi: 10.1016/j.biopha.2021.111557
10.
Emdin CA Khera AV Kathiresan S . Mendelian randomization. JAMA. (2017) 318:1925–6. doi: 10.1001/jama.2017.17219
11.
Abidin FNZ Wells HRR Altmann A Dawson SJ . Hearing difficulty is linked to Alzheimer's disease by common genetic vulnerability, not shared genetic architecture. NPJ Aging Mech Dis. (2021) 7:17. doi: 10.1038/s41514-021-00069-4
12.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
13.
Skrivankova VW Richmond RC Woolf BA Woolf BAR Davies NM Swanson SA et al . Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. (2021) 375:n2233. doi: 10.1136/bmj.n2233
14.
Smith GD Davies NM Dimou N Egger M Gallo V Golub R et al . Strobe-MR: guidelines for strengthening the reporting of Mendelian randomization studies. PeerJ. (2019) 7:e27857v1. doi: 10.7287/peerj.preprints.27857v1
15.
Ibrahim M Thanigaimani S Singh TP Morris D Golledge J . Systematic review and meta-analysis of Mendelian randomisation analyses of abdominal aortic aneurysms. Int J Cardiol Heart Vasc. (2021) 35:100836. doi: 10.1016/j.ijcha.2021.100836
16.
World Health Organization . International Classification of Diseases Eleventh Revision (ICD-11). Geneva: World Health Organization (2022).
17.
Daghlas I Guo Y Chasman DI . Effect of genetic liability to migraine on coronary artery disease and atrial fibrillation: a Mendelian randomization study. Eur J Neurol. (2020) 27:550–6. doi: 10.1111/ene.14111
18.
Lee KJ Lee SJ Bae HJ Sung J . Exploring the causal inference of migraine on stroke: a Mendelian randomization study. Eur J Neurol. (2022) 29:335–8. doi: 10.1111/ene.15101
19.
Shu MJ Li JR Zhu YC Shen H . Migraine and ischemic stroke: a Mendelian randomization study. Neurol Ther. (2022) 11:237–46. doi: 10.1007/s40120-021-00310-y
20.
Wang M Daghlas I Zhang Z Ye D Li S Liu D . Genetic liability to migraine and functional outcome after ischemic stroke. Eur Stroke J. (2023) 8:517–21. doi: 10.1177/23969873231164728
21.
Daghals I Sargurupremraj M Danning R Gormley P Malik R Amouyel P et al . Migraine, stroke, and cervical arterial dissection: shared genetics for a triad of brain disorders with vascular involvement. Neurol Genet. (2022) 8:e653. doi: 10.1212/NXG.0000000000000653
22.
Wang Y Hu X Wang X Li L Lou P Liu Z . Exploring the two-way link between migraines and venous thromboembolism: a bidirectional two-sample Mendelian randomization study. Thromb Haemost. (2024) 124:1053–60. doi: 10.1055/a-2313-0311
23.
Daghlas I Vgontzas A Guo Y Chasman DI International Headache Genetics Consortium Saxena R . Habitual sleep disturbances and migraine: a Mendelian randomization study. Ann Clin Transl Neurol. (2020) 7:2370–80. doi: 10.1002/acn3.51228
24.
Chu S Wu Z Wu Z Wu J Qian Y . Association between insomnia and migraine risk: a case-control and bidirectional Mendelian randomization study. Pharmgenomics Pers Med. (2021) 14:971–6. doi: 10.2147/PGPM.S305780
25.
Welander NZ Rukh G Rask-Andersen M Harder AVE International Headache Genetics Consortium van den Maagdenberg AMJM et al . Migraine, inflammatory bowel disease and celiac disease: a Mendelian randomization study. Headache. (2023) 63:642–51. doi: 10.1111/head.14470
26.
Li G Duan S International Headache Genetics Consortium (IHGC) Zheng T Zhu T Qu B et al . Genetic causal relationship between immune diseases and migraine: a Mendelian randomization study. Front Immunol. (2024) 15:1376698. doi: 10.3389/fimmu.2024.1376698
27.
Islam MR The International Headache Genetics Consortium Ihgc Nyholt DR . Genetic overlap analysis identifies a shared etiology between migraine and headache with type 2 diabetes. Genes (Basel). (2022) 13:1845. doi: 10.3390/genes13101845
28.
Daghlas I Rist PM Chasman DI . Effect of genetic liability to migraine on cognition and brain volume: a Mendelian randomization study. Cephalalgia. (2020) 40:998–1002. doi: 10.1177/0333102420916751
29.
Horton MK Robinson SC Shao X Quach H Quach D Choudhary V et al . Cross-trait Mendelian randomization study to investigate whether migraine is a risk factor for multiple sclerosis. Neurology. (2023) 100:e1353–62. doi: 10.1212/WNL.0000000000206791
30.
Xue H Zeng L Liu S . Unraveling the link: exploring the causal relationship between diabetes, multiple sclerosis, migraine, and Alzheimer's disease through Mendelian randomization. Front Neurosci. (2023) 17:1233601. doi: 10.3389/fnins.2023.1233601
31.
Baranova A Zhao Q Cao H Chandhoke V Zhang F . Causal influences of neuropsychiatric disorders on Alzheimer's disease. Transl Psychiatry. (2024) 14:114. doi: 10.1038/s41398-024-02822-1
32.
Xu C Wu W Fan Y Zhu S . Independent causal effect of migraines on Alzheimer's disease risk: a multivariate Mendelian randomization study. Front Neurol. (2024) 15:1401880. doi: 10.3389/fneur.2024.1401880
33.
Geng C Chen C . Migraine association with Alzheimer's disease risk: evidence from the UK biobank cohort study and mendelian randomization. Can J Neurol Sci. (2024) 52:44–52. doi: 10.1017/cjn.2024.35
34.
Zhao L Tang Y Tu Y Cao J . Genetic evidence for the causal relationships between migraine, dementia, and longitudinal brain atrophy. J Headache Pain. (2024) 25:93. doi: 10.1186/s10194-024-01801-7
35.
Fang T Zhang Z Zhou H Wu W Ji F Zou L . Effect of genetic liability to migraine and its subtypes on breast cancer: a mendelian randomization study. BMC Cancer. (2023) 23:887. doi: 10.1186/s12885-023-11337-9
36.
Lv H Liu K Xie Y Wang Y Chen S Liu P et al . No causal association between allergic rhinitis and migraine: a Mendelian randomization study. Eur J Med Res. (2024) 29:78. doi: 10.1186/s40001-024-01682-1
37.
Zhang K Zhang Y Wu W Lai R . Mendelian randomization analysis does not reveal a causal association between migraine and Meniere's disease. Front Neurol. (2024) 15:1367428. doi: 10.3389/fneur.2024.1367428
38.
Adewuyi EO Sapkota Y International Endogene Consortium Iec Me Research Team International Headache Genetics Consortium Ihgc Auta A et al . Shared molecular genetic mechanisms underlie endometriosis and migraine comorbidity. Genes (Basel). (2020) 11:268. doi: 10.3390/genes11030268
39.
Zhang W Zhang L Yang L Xiao C Wu X Yan P et al . Migraine, chronic kidney disease and kidney function: observational and genetic analyses. Hum Genet. (2023) 142:1185–200. doi: 10.1007/s00439-023-02575-9
40.
Zhao ZN Zhang ZQ Wang QQ Zhao BL Wang H Ge XJ et al . Genetic predisposition to periodontitis and risk of migraine: a two-sample Mendelian randomization study. Neurol Ther. (2023) 12:1159–69. doi: 10.1007/s40120-023-00484-7
41.
Mitchell BL Diaz-Torres S Bivol S Cuellar-Partida G International Headache Genetics Consortium Gerring ZF et al . Elucidating the relationship between migraine risk and brain structure using genetic data. Brain. (2022) 145:3214–24. doi: 10.1093/brain/awac105
42.
Niu PP Wang X Xu YM . Higher circulating vitamin D levels are associated with decreased migraine risk: a Mendelian randomization study. Front Nutr. (2022) 9:907789. doi: 10.3389/fnut.2022.907789
43.
Abuduxukuer R Niu PP Guo ZN Xu YM Yang Y . Circulating insulin-like growth factor 1 levels and migraine risk: a Mendelian randomization study. Neurol Ther. (2022) 11:1677–89. doi: 10.1007/s40120-022-00398-w
44.
Fu C Chen Y Xu W Zhang Y . Exploring the causal relationship between inflammatory cytokines and migraine: a bidirectional, two-sample Mendelian randomization study. Sci Rep. (2023) 13:19394. doi: 10.1038/s41598-023-46797-3
45.
Huo J Zhang G Wang W Cao W Wan M Huang T et al . Migraine and white matter lesions: a mendelian randomization study. Sci Rep. (2023) 13:10984. doi: 10.1038/s41598-023-38182-x
46.
He Q Wang W Xiong Y Tao C Ma L Ma J et al . A causal effects of gut microbiota in the development of migraine. J Headache Pain. (2023) 24:90. doi: 10.1186/s10194-023-01609-x
47.
Zhao L Zhao W International Headache Genetics Consortium (IHGC) Cao J Tu Y . Causal relationships between migraine and microstructural white matter: a mendelian randomization study. J Headache Pain. (2023) 24:10. doi: 10.1186/s10194-023-01550-z
48.
Liu X Yu Y Hou L Yu Y Wu Y Wu S et al . Association between dietary habits and the risk of migraine: a Mendelian randomization study. Front Nutr. (2023) 10:1123657. doi: 10.3389/fnut.2023.1123657
49.
Qu K Li MX Gan L Cui ZT Li JJ Yang R et al . To analyze the relationship between gut microbiota, metabolites and migraine: a two-sample Mendelian randomization study. Front Microbiol. (2024) 15:1325047. doi: 10.3389/fmicb.2024.1325047
50.
Meng X Li Q Wang D Li J Cui Y Sun Z et al . Exploring the role of gut microbiota in migraine risk: a two-sample Mendelian randomization study. Scand J Gastroenterol. (2024) 59:411–8. doi: 10.1080/00365521.2023.2298370
51.
Lv X Xu B Tang X Liu S Qian JH Guo J et al . The relationship between major depression and migraine: a bidirectional two-sample Mendelian randomization study. Front Neurol. (2023) 14:1143060. doi: 10.3389/fneur.2023.1143060
52.
Li Y Luo G Zhou L Wang X Liu H Zhang Y et al . Mediators of the association between depression and migraine: a mendelian randomization study. Front Genet. (2024) 15:1326817. doi: 10.3389/fgene.2024.1326817
53.
Wu XP Niu PP Liu H . Association between migraine and venous thromboembolism: a Mendelian randomization and genetic correlation study. Front Genet. (2024) 15:1272599. doi: 10.3389/fgene.2024.1272599
54.
Xu D Wu B . Investigating the causal association between systemic lupus erythematosus and migraine using Mendelian randomization analysis. Headache. (2024) 64:624–31. doi: 10.1111/head.14723
55.
Ren M Yu H Xiao B Zhao Y Yan J Liu J . Causal association between systemic lupus erythematosus and the risk of migraine: a Mendelian randomization study. Brain Behav. (2024) 14:e3417. doi: 10.1002/brb3.3417
56.
Yin P Anttila V Siewert KM Palotie A Davey Smith G Voight BF . Serum calcium and risk of migraine: a Mendelian randomization study. Hum Mol Genet. (2017) 26:820–8. doi: 10.1093/hmg/ddw416
57.
Johnsen MB Winsvold BS Børte S Vie GÅ Pedersen LM Storheim K et al . The causal role of smoking on the risk of headache. A Mendelian randomization analysis in the HUNT study. Eur J Neurol. (2018) 25:1148–e102. doi: 10.1111/ene.13675
58.
Guo Y Rist PM Daghlas I Giulianini F International Headache Genetics Consortium 23andMe Research Team et al . A genome-wide cross-phenotype meta-analysis of the association of blood pressure with migraine. Nat Commun. (2020) 11:3368. doi: 10.1038/s41467-020-17002-0
59.
Guo Y Rist PM Sabater-Lleal M de Vries P Smith N Ridker PM et al . Association between hemostatic profile and migraine: a mendelian randomization analysis. Neurology. (2021) 96:e2481–7. doi: 10.1212/WNL.0000000000011931
60.
Chen H Zhang H Zheng L . No causal association between coffee consumption and risk of migraine: a Mendelian randomization study. Front Genet. (2022) 13:792313. doi: 10.3389/fgene.2022.792313
61.
Yuan S Daghlas I Larsson SC . Alcohol, coffee consumption, and smoking in relation to migraine: a bidirectional Mendelian randomization study. Pain. (2022) 163:e342–8. doi: 10.1097/j.pain.0000000000002360
62.
Guo X Wang D Ying C Hong Y . Association between brain structures and migraine: a bidirectional Mendelian randomization study. Front Neurosci. (2023) 17:1148458. doi: 10.3389/fnins.2023.1148458
63.
Zheng H Shi YZ Liang JT Lu LL Chen M . Modifiable factors for migraine prophylaxis: a mendelian randomization analysis. Front Pharmacol. (2023) 14:1010996. doi: 10.3389/fphar.2023.1010996
64.
Wei T Shi X Sun W Song W Zhou S Zhao Y et al . Identifying suitable targets for Alzheimer's disease and other eight common neurological disorders using the human plasma proteome: a Mendelian randomization study. J Alzheimers Dis Rep. (2023) 7:811–22. doi: 10.3233/ADR-230058
65.
Zhu G Wang M Wang Y Kong F . Assessing the association between age at first sexual intercourse and migraine: a Mendelian randomization study. Front Neurol. (2024) 15:1347831. doi: 10.3389/fneur.2024.1347831
66.
Jareebi MA Lyall DM Gharawi NF Shami MO Dahas N Alfaifi RF et al . Causal associations of modifiable risk factors with migraine: evidence from Mendelian randomization analysis. Cureus. (2024) 16:e53448. doi: 10.7759/cureus.53448
67.
Zhang J Liu Y Xu G Cao X Wang W Zhang D et al . Causal relationship between coffee intake and neurological diseases: a Mendelian randomization study. Eur J Clin Nutr. (2024) 78:114–9. doi: 10.1038/s41430-023-01355-y
68.
Qu K Li MX Yu P International Headache Genetics Consortium Wu BH Shi M et al . HMG-CoA reductase is a potential therapeutic target for migraine: a mendelian randomization study. Sci Rep. (2024) 14:12094. doi: 10.1038/s41598-024-61628-9
69.
Li P Li J Zhu H Sheng D Xiao Z Liu W et al . Causal effects of sedentary behaviours on the risk of migraine: a univariable and multivariable Mendelian randomization study. Eur J Pain. (2024) 28:1585–95. doi: 10.1002/ejp.2296
70.
Niu PP Zhang R Zhang C Li S Li YS . Identifying novel proteins for migraine by integrating proteomes from blood and CSF with genome-wide association data. CNS Neurosci Ther. (2024) 30:e14817. doi: 10.1111/cns.14817
71.
Bi Y Zhu Y Tang S Huang Y . Lipids, lipid-modifying drug target genes and migraine: a Mendelian randomization study. J Headache Pain. (2023) 24:112. doi: 10.1186/s10194-023-01633-x
72.
Zhang C He Y Liu L . Identifying therapeutic target genes for migraine by systematic druggable genome-wide Mendelian randomization. J Headache Pain. (2024) 25:100. doi: 10.1186/s10194-024-01805-3
73.
Hong P Han L Wan Y . Mendelian randomization study of lipid metabolism characteristics and migraine risk. Eur J Pain. (2024) 28:978–86. doi: 10.1002/ejp.2235
74.
Gui J Yang X Tan C Wang L Meng L Han Z et al . A cross-tissue transcriptome-wide association study reveals novel susceptibility genes for migraine. J Headache Pain. (2024) 25:94. doi: 10.1186/s10194-024-01802-6
75.
Liu JZ Tozzi F Waterworth DM Pillai SG Muglia P Middleton L et al . Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. (2010) 42:436–40. doi: 10.1038/ng.572
76.
Thorgeirsson TE Geller F Sulem P Rafnar T Wiste A Magnusson KP et al . A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. (2008) 452:638–42. doi: 10.1038/nature06846
77.
Andreou AP Edvinsson L . Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. (2019) 20:117. doi: 10.1186/s10194-019-1066-0
78.
Pleș H Florian IA Timis TL Covache-Busuioc RA Glavan LA Dumitrascu DI et al . Migraine: advances in the pathogenesis and treatment. Neurol Int. (2023) 15:1052–105. doi: 10.3390/neurolint15030067
79.
Xiong Z Zhao L Liu G Qiu D Mei Y Li X et al . Atrophy of hypothalamic subregions increases migraine risk: cross-sectional study and mendelian randomization analysis. J Headache Pain. (2025) 26:178. doi: 10.1186/s10194-025-02119-8
80.
Shen Z Bian Y Huang Y Zhou W Chen H Zhou X et al . Migraine and gastroesophageal reflux disease: disentangling the complex connection with depression as a mediator. PLoS One. (2024) 19:e0304370. doi: 10.1371/journal.pone.0304370
81.
Jin C Tu S Sun S Zhang Z Wang X . Noncausal effects between tea intake and migraine risk: a Mendelian randomization study. Sci Rep. (2023) 13:12898. doi: 10.1038/s41598-023-40171-z
82.
Li Y Cai J Chong H . Psoriasis and risk of central neurological disorders in European populations: a mendelian randomization analysis. Heliyon. (2024) 10:e24774. doi: 10.1016/j.heliyon.2024.e24774
83.
Verbanck M Chen CY Neale B Do R . Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7. Erratum in: Nat Genet. (2018) 50:1196. doi: 10.1038/s41588-018-0164-2
84.
Zhu H Ni H Yang Q Ni J Ji J Yang S et al . Evaluating the Bidirectional Causal Effects of Alzheimer’s Disease Across Multiple Conditions: A Systematic Review and Meta-Analysis of Mendelian Randomization Studies. Int J Mol Sci. (2025) 26:3589. doi: 10.3390/ijms26083589
Summary
Keywords
migraine, Mendelian randomization, causal relationship, systematic review, meta-analysis
Citation
Li X, Liu Q, Ni H, Ni J, Yang S and Ji J (2025) Causal factors for migraine in Mendelian randomization studies: a systematic review and meta-analysis. Front. Neurol. 16:1660995. doi: 10.3389/fneur.2025.1660995
Received
07 July 2025
Accepted
14 August 2025
Published
09 September 2025
Volume
16 - 2025
Edited by
Kengo Hamamura, Kyushu University, Japan
Reviewed by
Tao Zheng, China-Japan Friendship Hospital, China
Zixiong Shen, Cardio Pulmonary Institute (CPI), Germany
Updates
Copyright
© 2025 Li, Liu, Ni, Ni, Yang and Ji.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaqi Ni, jiaqini007@163.com; Shu Yang, yangshu1106@scu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.