- 1Department of Occupational Therapy, The University of Texas Medical Branch, Galveston, TX, United States
- 2Department of Clinical Research, Scottish Rite for Children, Dallas, TX, United States
- 3Department of Occupational Therapy, Medical University of South Carolina, Charleston, SC, United States
Background: Children with cerebral palsy often experience persistent upper extremity impairments that impact independence and participation in daily activities. Wearable neurotechnology devices offer a promising, non-invasive approach to enhance motor control, promote neuroplasticity, and extend neurorehabilitation beyond clinical settings. However, the development and application of such devices in pediatric populations remains poorly defined. This scoping review aimed to map the existing literature on wearable neurotechnology systems used for upper extremity rehabilitation in children with cerebral palsy and identify knowledge gaps to guide future research and clinical translation in pediatric neurorehabilitation.
Methods: This review followed the JBI Scoping Review Methodology and PRISMA-ScR guidelines. Four electronic database sources, MEDLINE, Scopus, CINAHL, and PsycINFO, were systematically searched to identify studies on wearable neurotechnology devices for upper extremity rehabilitation in children with cerebral palsy. Included studies consisted of journal articles published from January 2005 to June 2025, with full texts available in English and relevant gray literature sources. Data were extracted on neurotechnology characteristics, regulatory status, intervention protocols, and outcome measures.
Results: From the 2,892 articles screened, 21 met the eligibility criteria. Most devices were in early developmental stages, with only five receiving regulatory approval. Studies examined various systems, including electromyography-triggered stimulation, virtual reality, and robot-assisted devices with haptic or electrical stimulation, and wearable garments embedded with electrical or vibrotactile stimulators. Intervention protocols varied widely across studies in terms of treatment intensity, wear schedules, and co-interventions. Feasibility was generally positive across studies, with high adherence rates and minimal adverse events reported. Many studies reported improvements in motor outcomes, including enhanced grip strength, hand use, range of motion, grasp and release ability, and muscular recruitment.
Conclusions: Wearable neurotechnology shows potential to augment upper extremity rehabilitation in children with cerebral palsy, particularly through systems that support task-specific, feedback-driven practice. However, translation to clinical practice is limited by heterogeneity in device design, lack of standardized protocols, and limited high-quality evidence. Future research should prioritize standardization, clinician-centered implementation studies, and long-term outcomes to support integration into pediatric care.
Systematic review registration: https://osf.io/5qxpe.
1 Introduction
Cerebral palsy (CP) is the most common movement disorder among the pediatric population (1). Prevalence estimates vary, with global population-based studies reporting rates between 1 and 4 per 1,000 live births (1). A meta-analysis from the Global CP Prevalence Group provides a more refined estimate of ~1.6 per 1,000 live births in high-income settings, with higher rates of up to ~3.4 per 1,000 reported in some low- and middle-income regions (2). CP arises from congenital or acquired neurological insults during fetal or early infant brain development, resulting in a group of non-progressive neurological disorders characterized by impaired motor and postural control (3). Clinical presentation varies depending on the size and location of the brain lesion and may include neuromuscular deficits such as weakness, limited range of motion, spasticity, and the development of atypical fine and gross motor patterns (4). These motor impairments present challenges with reaching, grasping, bimanual coordination, and manipulation skills, thereby restricting the functional use of the upper extremities for participation in play and self-help activities (5).
Rehabilitation strategies for children with CP aim to improve motor control and functional independence, although many individuals experience lifelong impairments (6). Therapeutic interventions typically include task-oriented approaches such as Constraint-Induced Movement Therapy (CIMT) and Hand-Arm Bimanual Intensive Therapy (HABIT), both of which are designed to leverage principles of neuroplasticity, the brain's capacity to reorganize in response to experience, learning, or injury (7). These approaches have demonstrated efficacy in promoting motor recovery in children with CP; however, they rely on residual voluntary motor function and may be less suitable for children with more severe motor impairments (7).
Recent advancements in neuromodulation research have introduced new avenues for enhancing neuroplasticity through targeted stimulation of neural circuits (8). Neuromodulation refers to the process of altering neural activity via electrical, mechanical, or sensory input to influence brain function and behavior (8, 9). Parallel to these developments, the field of neurotechnology has expanded to encompass a range of devices that offer new strategies to address the persistent challenges of restoring motor functions following neurological injury (8, 9). In this context, neurotechnology refers to the use of technological systems that interact with the nervous system to restore, enhance, or modulate neural function (9). Neurotechnology can be broadly categorized into invasive systems, which involve surgical implantation (e.g., brain-computer interfaces), and non-invasive systems, which operate externally without surgical implantation or direct penetration of the skin (8–10). Non-invasive neurotechnology systems are of particular interest in pediatric populations due to their reduced risk and ease of use (9).
Wearable neurotechnology represents a subcategory of non-invasive systems characterized by their portability and ability to integrate into real-life contexts to support motor rehabilitation (11, 12). These devices often involve wearable garments embedded with surface electrodes and sensors that detect movement intention and deliver neuromodulatory inputs such as functional electrical stimulation (FES), neuromuscular electrical stimulation (NMES), or vibrotactile feedback (11–13). These neurotechnologies are frequently incorporated into electromechanical, robotic, and virtual reality-based systems that facilitate intensive, repetitive, and goal-directed training designed to drive motor learning (9). Their accessibility encourages at-home use, increasing rehabilitation opportunities through more frequent practice, and improving carryover into everyday activities (11).
Collectively, wearable neurotechnology devices converge on the goal of enhancing neurorehabilitation, defined as a multidisciplinary process aimed at restoring function and improving quality of life following neurological injury or disease (7). Neurorehabilitation often integrates therapeutic interventions with emerging technologies to promote adaptive neuroplastic changes and functional recovery (9, 10). For example, innovations such as closed-loop systems, which adjust stimulation parameters in real time based on physiological feedback, exemplify the potential for personalized, responsive treatment paradigms (12, 14).
While previous studies have shown wearable devices to improve upper extremity performance in adults with upper motor neuron injuries, there is limited synthesized research on the types of wearable neurotechnology available for children with CP and their effects on upper extremity outcomes (13, 15, 16). Furthermore, pediatric studies often lack clear and consolidated information regarding device specifications, training models, and outcomes relevant to clinical practice (10). These gaps complicate decision-making for clinicians seeking to adopt innovative approaches to neurorehabilitation in children with CP. Emerging wearable neurotechnology systems that facilitate movement with electrical stimulation, electromyography (EMG) biofeedback, or haptic feedback hold the potential to reshape pediatric neurorehabilitation and enhance volitional motor control. These devices could significantly impact the quality of life in children with CP by facilitating increased movement and use of the upper extremities, leading to increased independence and participation in meaningful occupations (17).
This scoping review aims to (i) map existing evidence on wearable, non-invasive neurotechnology devices used to improve upper extremity function in children with CP; (ii) define wearable, non-invasive neurotechnology in the context of motor rehabilitation for children with CP; and (iii) identify gaps to guide future research and clinical translation in pediatric neurorehabilitation.
2 Methods
2.1 Study design
This review followed the Joanna Briggs Institute (JBI) Scoping Review Methodology and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines (18, 19). A protocol was prospectively registered with the Open Science Framework (https://osf.io/5qxpe).
2.2 Search strategy
A comprehensive search strategy was developed in collaboration with a medical librarian and applied across four databases: MEDLINE, Scopus, CINAHL, and PsycINFO, with formatting tailored to each database. These databases were selected to capture studies related to neurological disorders, pediatrics, rehabilitation, and technology. Search terms included combinations of keywords such as “cerebral palsy,” “neurotechnology,” “rehabilitation,” and “children.” The full list of search terms and strategies is provided in Supplementary material 1. To ensure an exhaustive search, an additional literature search was conducted by hand searching in Google Scholar and PubMed, using the same search terms. Reference lists of included studies and relevant systematic reviews were also screened for potentially eligible studies. To supplement peer-reviewed literature and address potential publication bias, gray literature sources were also searched by reviewing conference proceedings, organizational reports, and market research related to neurotechnology development and commercially available devices. This involved targeted searches of neurotechnology devices, companies, researchers, and manufacturers identified in the included studies and related systematic reviews. The literature search was completed on May 8, 2025.
2.3 Study selection
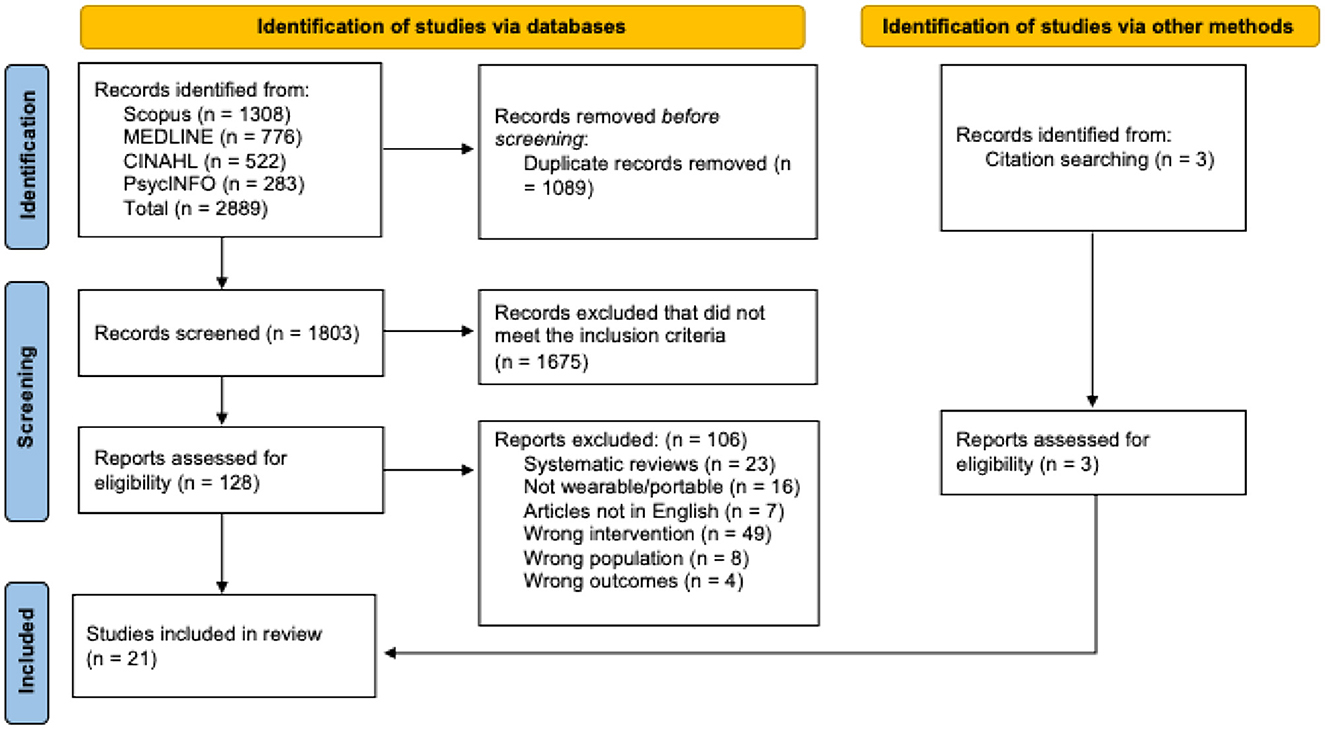
Articles were compiled into Covidence Systematic Review Software (Veritas Health Innovation, Melbourne, Australia), where duplicates were removed. Articles were then screened in two phases and checked for reliability by the research team. Two independent reviewers screened article titles and abstracts for eligibility and assessed the remaining articles' full texts for eligibility criteria detailed in Table 1. A third reviewer resolved any disputes through blind voting and a consensus discussion. Reasons for exclusion were documented during the full text phase and included in the PRISMA flow diagram in Figure 1 (20).
Included studies consisted of peer-reviewed journal articles published from January 2005 to May 2025, with full text available in English. Considering the limited literature available, multiple study designs were included for synthesis, such as randomized controlled trials, controlled trials, longitudinal studies, case series, and case studies. Feasibility studies were included if they provided relevant information on the safety and usability of these devices for children with CP. Additionally, systematic and scoping reviews were excluded; however, relevant reviews were scanned for references that fit within our inclusion criteria.
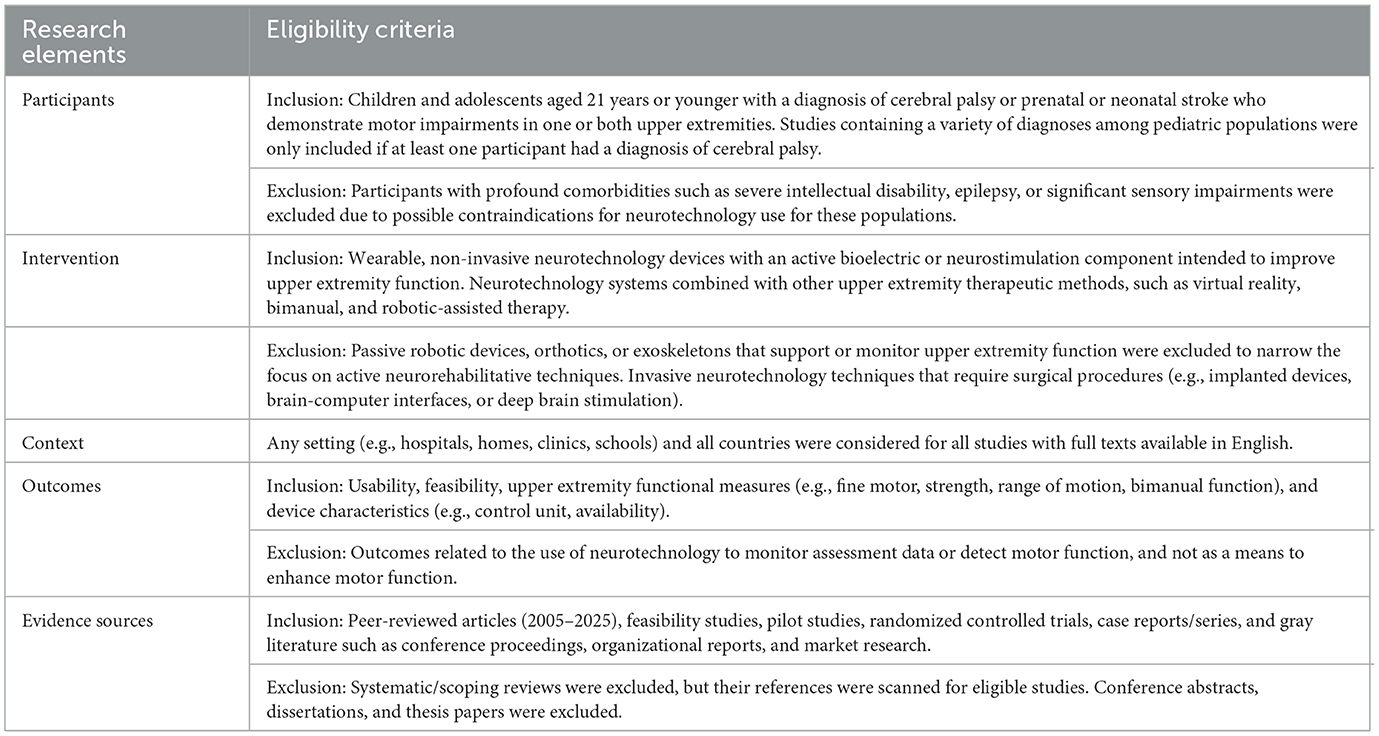
The specific inclusion criteria for neurotechnology devices involved: (i) Wearable non-invasive neurotechnology devices for upper extremity rehabilitation, (ii) Devices that include electrical stimulation, haptic, or vibro-tactile biofeedback components applied to the skin or muscle bellies to enhance motor activation, and/or (iii) neurotechnology devices combined with other upper extremity therapeutic methods, such as virtual reality, bimanual therapy, and robotic-assisted therapy. Exclusion criteria included: (i) studies that only investigate passive robotic devices, orthotics, or exoskeletons that support the upper extremity, (ii) Invasive neurotechnology techniques that require surgical procedures, such as implanted devices, brain-computer interfaces, or deep brain stimulation, (iii) neurotechnologies that do not have a wearable component but are used to enhance upper extremity function, such as transcranial magnetic stimulation, peripheral magnetic stimulation, transcranial direct current stimulation, or other non-invasive brain stimulation techniques, (iv) Studies that only utilize wearable technology components to monitor assessment data and not as a means to facilitate upper extremity outcomes.
Although this search strategy was intentionally broad, the number of studies meeting the inclusion criteria remained limited. This highlights the early stage of research in this area and underscores the need for further investigation into wearable neurotechnology for upper extremity rehabilitation in children with CP.
2.4 Data extraction
Two independent reviewers extracted data from the eligible articles and organized relevant information into a detailed charting system that aligns with the research questions. Extracted data is presented in a comprehensive table that includes information about the author(s), year of publication, study design, population demographics, sample size, setting, interventions and dosage, control conditions, and any additional therapeutic techniques provided to participants. Outcome measures related to feasibility and upper extremity rehabilitation were also reported. Specific details about the types of neurotechnology systems used, how they are controlled, whether the devices are FDA approved, the price range and availability of the technology, and whether the study includes company-sponsored research were also extracted. Study protocols and gray literature that include data from websites and organizations that develop neurotechnology devices and market research regarding commercially available neurotechnology devices were used to locate supplementary information. The tertiary reviewer resolved disputes involving data extraction methods among reviewers through discussion.
2.5 Data synthesis
The results of data extraction were synthesized using descriptive analysis. The data were first organized into a comparative chart to facilitate cross-study evaluation of key variables and outcomes. A narrative synthesis was developed based on descriptive and thematic patterns related to neurotechnology characteristics, availability, upper extremity outcomes, and feasibility. The synthesis was conducted collaboratively among reviewers to identify strengths, limitations, and gaps in the use of wearable neurotechnology for upper extremity rehabilitation for children with CP.
3 Results
3.1 Literature search results
A total of 2,889 articles were identified across the databases searched, with an additional 3 articles located through citation searching. After removing 1,089 duplicates, 1,803 articles remained for title and abstract screening. Of these, 1,675 were excluded for not meeting the eligibility criteria. The remaining 131 articles underwent full-text review, resulting in 21 studies that met the inclusion criteria and were included in the synthesis (Figure 1).
Many studies were excluded during the initial screening phase due to the invasive nature of the neurotechnology designs or the use of electrical stimulation without a wearable component. Additional articles investigating brain stimulation methods or pharmaceutical interventions for spasticity reduction were excluded because they did not meet the eligibility criteria of this review. During full-text review, several studies were also excluded for lacking a bioelectric or feedback component within the technology system, such as those using handheld gaming controllers or virtual reality platforms alone. These exclusion criteria substantially narrowed the evidence base, contributing to the final inclusion of only 21 studies and highlighting the limited scope of current research in this area.
The included studies were published between 2009 and 2025 (Supplementary material 2). Study designs included three randomized controlled trials (21–23), one pre-test-post-test study (24), one cross-sectional study (25), three feasibility studies (26–28), four pilot studies (29–32), one longitudinal retrospective cohort study (33), four case series (34–37), and four case reports (38–41). Geographically, studies were conducted in the United States (n = 7), Switzerland (n = 4), Italy (n = 4), Japan (n = 2), and one each in Egypt, Taiwan, Romania, and Canada. Settings included rehabilitation centers, hospitals, outpatient clinics, and home or community environments.
3.2 Population demographics
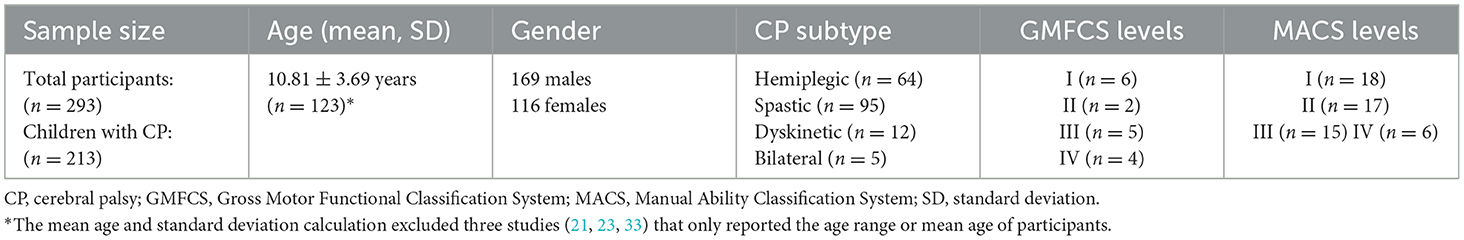
Across the 21 included studies, a total of 293 participants were reported. This sample comprised 213 children with CP (ages 4–19) and 13 healthy controls. One study included both children and adults with CP (ages 9–38) (33), while another enrolled adults as healthy controls (26). In addition, eight studies included participants with other diagnoses, which contributed to the overall sample size (22, 24–27, 30, 33, 36). Gender was reported for most participants, with 169 males (59.3%) and 116 females (40.7%). Thirteen studies reported CP subtypes, including hemiplegic CP (n = 64), spastic CP (n = 95), dyskinetic CP (n = 12), and bilateral CP (n = 5) (21, 23, 24, 28–32, 35, 37–41). Several studies also reported motor impairment severity using the Manual Ability Classification System (MACS) and Gross Motor Function Classification System (GMFCS), with distributions summarized in Table 2.
3.3 Neurotechnology characteristics
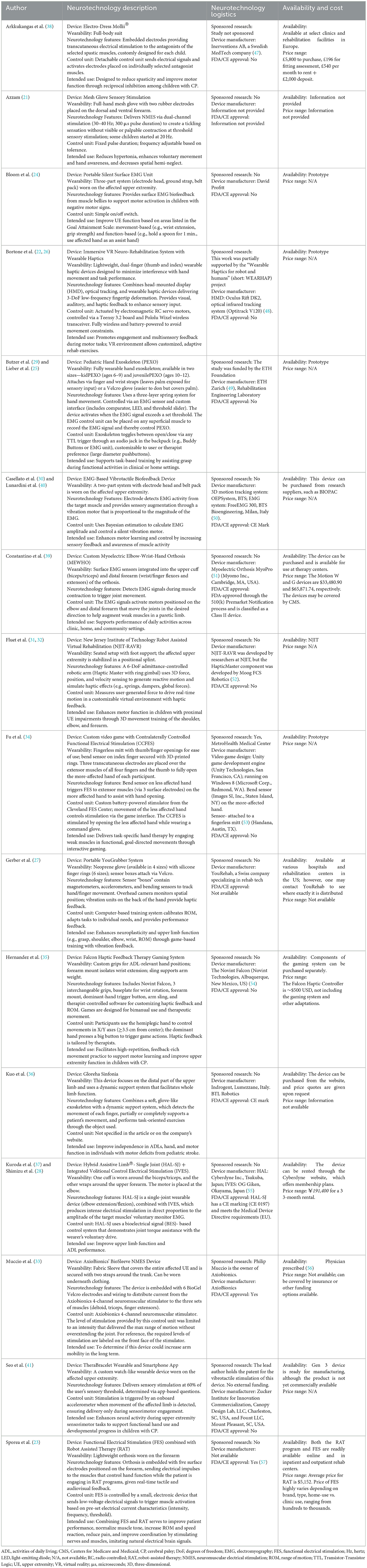
The included studies investigated a wide variety of wearable neurotechnology systems designed to facilitate upper extremity rehabilitation in children with CP (Table 3). Despite their variety, several common characteristics and thematic groupings emerged based on design features, control mechanisms, wearability, and modes of stimulation. These categories included: (i) surface EMG-triggered stimulation systems (38.1%), (ii) game-based or virtual reality (VR) platforms with integrated haptic feedback or electrical stimulation (23.8%), (iii) robot-assisted devices incorporating haptic feedback or stimulation (19.05%), and (iv) wearable garments embedded with electrical or vibrotactile stimulators (19.05%).
Devices in this category leveraged surface EMG technology to detect volitional muscle activity and trigger electrical or vibrotactile stimulation. Examples included the Hybrid Assistive Limb®-Single Joint (HAL-SJ) system with Integrated Volitional Control Electrical Stimulation (IVES) (28, 37), the EMG-Based Vibrotactile Biofeedback Device (30, 40), the Pediatric Hand Exoskeleton (PEXO) (25, 29), a Portable Silent Surface EMG Unit (24), and a custom Myoelectric Elbow-Wrist-Hand Orthosis (MEWHO) (39). These systems often operated as closed-loop feedback mechanisms, using real-time bioelectrical signals to modulate stimulation parameters and enhance movement control. This integration of neuromuscular feedback aimed to reinforce motor learning by aligning external stimulation with voluntary effort.
Virtual reality and gamified platforms, often combined with haptic feedback and/or electrical stimulation to the upper extremity, were designed to create engaging, task-specific environments that promote motivation and repetitive practice. Systems such as the Immersive VR Neuro-Rehabilitation System with Wearable Haptics (22, 26), the YouGrabber® glove-based home exergame (27), a contralaterally controlled functional electrical stimulation (CCFES) video game interface (34), and the Falcon haptic-feedback gaming system (35) enabled interactive upper limb tasks. These technology systems aimed to enhance motivation and participation by embedding therapeutic goals into immersive and enjoyable activities.
Robot-assisted devices provided guided movements, gravity compensation, and joint-specific support, often paired with haptic feedback, EMG control, or functional electrical stimulation (FES). These included the Gloreha Sinfonia (36), a dynamic hand exoskeleton; the Robot-Assisted Virtual Rehabilitation (RAVR) system with a six-degree-of-freedom robotic arm (31, 32); and a combined FES orthosis with robot-assisted therapy (RAT) (23). These systems aimed to facilitate neuroplasticity through precise, repetitive motion training while incorporating sensorimotor feedback.
Several studies evaluated garments embedded with stimulatory components to reduce spasticity and enhance neuromuscular activation. Examples included the Mollii® suit, a full-body transcutaneous electrical stimulation (TENS) system tailored to individual spasticity profiles (38); AxioBionics' BioSleeve NMES Device (33); the Mesh Glove Sensory Stimulation device for localized hand stimulation (21); and the TheraBracelet, a wrist-worn vibratory stimulator linked to movement and paired with a mobile app (41). These devices prioritized ease of wear and consistent stimulation, particularly for home-based or long-duration use.
3.4 Neurotechnology availability and cost
Information regarding device availability and cost was limited across the included studies and was supplemented through searches of company websites and publicly available market data (Table 3). Among the wearable neurotechnology systems, only the Gloreha Sinfonia (36), Axiobionics' Biosleeve (33), and Cyberdyne's Hybrid Assistive Limb® (28, 37) are currently commercially available to the public on company websites. Other devices, such as the Electro-Dress Mollii® (38), EMG-Based Vibrotactile Biofeedback Device (30, 40), MEWHO (39), NJIT-RAVR (31, 32), YouGrabber (27), and FES + RAT (23), are only accessible through medical institutions, rehabilitation centers, or collaborative research programs. Several systems remained in prototype stages, including the Immersive VR Neuro-Rehabilitation System (22, 26), PEXO (25, 29), the Portable Silent Surface EMG unit (24), the CCFES-video game system (34), and the TheraBracelet (41). Price data was also limited and revealed substantial cost variation among neurotechnology systems (Table 3). Only five devices had obtained FDA or CE regulatory approval (23, 28, 30, 36, 37, 39, 40), highlighting the novelty of wearable neurotechnology devices and limited availability for the pediatric population.
3.5 Interventions and protocols
Intervention protocols varied widely across studies in terms of treatment intensity, wear schedules, and co-interventions (Supplementary material 2). Most interventions were delivered over multiple weeks, with wear times ranging from 15 min to 8 h per day, and frequencies of 1–7 sessions per week. In contrast, some feasibility and pilot studies occurred over a single session (25, 26, 29).
Three studies explored the use of wearable devices exclusively in home or community settings (24, 27, 41). Five of the studies' protocols included both clinical interventions and home-based programs (21, 33, 34, 37, 39), while the remaining studies utilized the devices solely during therapist-led sessions conducted in hospital or outpatient clinic settings. All studies, except those focused exclusively on home or community use, included therapist-guided upper extremity rehabilitation with wearable neurotechnology devices. Although specific intervention protocols differed across studies, many incorporated conventional upper extremity rehabilitation strategies, including graduated exercises, facilitation and inhibition techniques, constraint-induced movement therapy, bimanual training, grasp and reach tasks, and guided functional task practice. Key characteristics of each study's interventions are summarized in Supplementary material 2.
3.6 Study outcomes
Feasibility and usability were assessed in many studies through measures such as device tolerance, time worn, ease of donning, and participant satisfaction via logs or interviews. Feasibility was generally positive across studies, with high adherence rates and minimal adverse events reported (24–29, 36–39, 41). However, usability challenges were noted, including device bulk, fatigue, need for therapist assistance, and sensory discomfort from device weight, warmth, or skin irritation in some cases. Additionally, a few studies reported technical issues with neurotechnology systems, particularly at-home systems, such as the Portable YouGrabber System (27), TheraBracelet (41), and the Portable Silent Surface EMG unit (24). Social and psychological barriers also emerged, with some participants expressing reluctance to wear devices in school or public settings due to self-consciousness or fear of damaging the equipment, even when perceiving physical benefits (39, 41).
A broad range of outcome measures were used to assess motor and functional performance, including standardized tools such as the Assisting Hand Assessment (AHA), Box and Blocks Test (BBT), ABILHAND-kids, Action Research Arm Test (ARAT), Modified Ashworth Scale (MAS), and Functional Independence Measure (FIM), as well as EMG-based muscle activity analysis. Many studies reported improvements in motor outcomes, including grip strength, hand use, range of motion, grasp and release, and muscular recruitment. However, heterogeneity in study designs and outcome measures limited direct comparisons. Only a subset of studies used standardized assessments, and long-term follow-up data were largely unavailable.
4 Discussion
This scoping review identified 21 studies investigating 16 wearable neurotechnology systems designed to support upper extremity rehabilitation in children with CP. Despite considerable heterogeneity in device design, study methodologies, intervention protocols, and outcome measures, the findings indicate that many of these technologies are feasible, well-tolerated, and demonstrate promising potential for improving motor function when integrated with task-specific, repetitive training.
Across studies, wearable neurotechnology devices were associated with improvements in various domains of upper extremity function, including grip strength, range of motion, muscle activation, coordination, and bimanual task performance. Systems that incorporated gamification, virtual reality, or home-based integration frequently reported enhanced engagement and usability, which are critical factors for adherence and functional carryover in pediatric rehabilitation (42). Additionally, several wearable systems employed closed-loop control mechanisms, such as EMG-triggered stimulation or movement-activated sensory feedback. These technologies closely align with established principles of motor learning and neuroplasticity, which emphasize active engagement, self-initiation of movement, and real-time feedback (10). Such features are believed to facilitate cortical reorganization and enhance functional recovery (8, 43, 44). Notably, devices such as BioSleeve, HAL-SJ, and MEWHO orthosis exemplify systems that support task-specific training with potential applications in daily routines (28, 33, 37, 39). Their design reflects a shift toward portable, personalized neurorehabilitation strategies that accommodate real-world use (11, 12).
Beyond device function, implementation context emerged as a notable consideration. Home-based systems offer the possibility of distributed, intensive practice, addressing limitations in access to pediatric therapy and intensive programs (11, 14). Many studies revealed the importance of motivation, autonomy, and adaptability, especially for children navigating social and environmental challenges. This echoes findings from previous neurorehabilitation research, where motivation and autonomy, often enhanced through biofeedback and gamified systems, were key drivers of positive outcomes (42).
While the adult neurorehabilitation literature has extensively documented the benefits of robotic and sensor-based technologies for stroke and spinal cord injury, studies targeting children with CP remain sparse (13, 15, 16). This review adds to the limited but growing body of work emphasizing wearable, non-invasive neurotechnology specifically tailored to the pediatric population. These devices stand out for their affordability, portability, and scalability, positioning them well for home and community integration, a priority in pediatric rehabilitation models (12).
Nevertheless, implementation challenges remain. Many devices are early-stage prototypes, lacking regulatory approval and formal clinical guidelines. Few studies offered detailed implementation guidelines, creating uncertainty for therapists and families regarding setup, customization, safety, and integration into existing care models (9, 10). These findings are consistent with previous calls for more standardized outcome measures and more rigorous study designs in pediatric neurotechnology research (10, 45, 46). Practical concerns, including device bulkiness, donning difficulty, and sensory discomfort, were common. Social stigma associated with wearing conspicuous technology in public settings also emerged as a barrier, underscoring the need for discreet, child-friendly designs that balance therapeutic benefits with usability.
4.1 Strengths and limitations
A strength of this review is its comprehensive scope and inclusion of diverse study designs, technologies, and intervention approaches, supported by a broad search strategy across multiple databases and gray literature sources. The focus on children with CP fills an important gap in the literature, as most prior reviews have focused on adult populations or non-wearable technologies.
However, the current evidence base remains preliminary, with most studies comprising small sample sizes and low levels of evidence, such as feasibility studies and case series. Only three randomized controlled trials were identified, only one study included long-term follow-up, and no studies included direct measures of cortical change. Many studies involved co-interventions, making it difficult to isolate the effects of the neurotechnology alone. Furthermore, intervention protocols varied widely across studies in terms of duration, intensity, frequency, and task specificity, which may introduce bias and limit the comparability and generalizability of reported outcomes. Variability in outcome measures further restricted cross-study comparability. As with all scoping reviews, no formal quality appraisal or meta-analysis was conducted, and results should be interpreted accordingly.
4.2 Future directions
Future research should follow a structured roadmap to advance the clinical translation of wearable neurotechnology for upper extremity rehabilitation in children with cerebral palsy. The first priority is the systematic validation of emerging devices through regulatory pathways to ensure safety, usability, and efficacy. Depending on device readiness, this process may range from benchmark and safety testing to full-scale regulatory approval (e.g., FDA or CE certification). Large-scale efficacy trials are then warranted to evaluate device performance across varied rehabilitation contexts, including home-based and community settings. Establishing an international working group to identify standardized and clinically meaningful outcome measures, encompassing functional performance, participation, and patient-reported experiences, will be essential to enhance data comparability and enable meta-analytic synthesis.
Subsequent implementation research should focus on integrating wearable systems into routine pediatric rehabilitation practice while addressing barriers to clinician and family adoption. Studies should examine training requirements, customization needs, and interdisciplinary coordination to optimize usability and adherence. Incorporating neurophysiological and neuroimaging assessments (e.g., electroencephalogram (EEG) and functional magnetic resonance imaging (fMRI) within these studies could provide mechanistic insight into neuroplastic changes associated with device use. Finally, long-term follow-up and real-world effectiveness studies are needed to evaluate sustained outcomes, user satisfaction, and scalability. Economic analyses addressing cost-effectiveness, accessibility, and reimbursement potential will be critical to inform sustainable integration into pediatric rehabilitation services. Collectively, these steps represent a progressive trajectory, from regulatory validation to implementation and long-term impact, that will guide the responsible advancement of wearable neurotechnology toward widespread clinical adoption.
5 Conclusion
This scoping review provides an overview of the current landscape of wearable, non-invasive neurotechnology systems used to support upper extremity rehabilitation in children with CP. The findings demonstrate an increasing interest in leveraging wearable neurotechnology devices, such as EMG-triggered stimulators, virtual reality systems, robotic interfaces, and sensor-embedded garments that provide sensory feedback or electrical stimulation, to enhance motor function, promote neuroplasticity, and increase access to high-frequency, task-specific practice. While the studies reviewed suggest wearable neurotechnology devices are generally feasible, well-tolerated, and promising in improving motor outcomes, the literature remains early in development, with limited high-level evidence and inconsistent outcome reporting. Wearable neurotechnology offers an exciting frontier in pediatric neurorehabilitation. With continued development, collaborative design, and translational research, these systems may play a critical role in supporting functional independence and improving quality of life for children with CP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SB: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing, Data curation, Project administration, Visualization. AS: Conceptualization, Methodology, Supervision, Writing – review & editing, Project administration, Resources, Validation. CT: Investigation, Methodology, Writing – review & editing, Data curation. RB: Writing – review & editing, Project administration, Resources, Supervision, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors would like to acknowledge Alison Hansen from the library of The University of Texas Medical Branch for her work on the literature search strategy for this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. Generative AI was used during the preparation of this manuscript. The author(s) used ChatGPT-4.5 to improve readability and grammar. After utilizing these tools, the author(s) thoroughly reviewed and edited the content as needed, taking full responsibility for the final version of the published article.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1663596/full#supplementary-material
References
1. Centers for Disease Control and Prevention. Data and Statistics for Cerebral Palsy. U.S. Department of Health and Human Services (2022). Available online at: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/ncbddd/cp/data.html (Accessed March 15, 2025).
2. McIntyre S, Goldsmith S, Webb A, Ehlinger V, Julsen Hollung S, McConnell K, et al. Global prevalence of cerebral palsy: a systematic analysis. Dev Med Child Neurol. (2022) 64:1494–506. doi: 10.1111/dmcn.15346
3. Graham HK, Rosenbaum P, Paneth N, Dan B, Lin JP, Damiano DL, et al. Cerebral palsy. Nat Rev Dis Primers. (2016) 2:15082. doi: 10.1038/nrdp.2015.82
4. Pingel J, Potts C, Petersen TW, Nielsen JB. Cerebral palsy and stroke: early and late brain lesion present differences in systemic biomarkers and gene expression related to muscle contractures. World J Neurosci. (2021) 11:34–47. doi: 10.4236/wjns.2021.111005
5. Walker C, Shierk A, Roberts H. Constraint induced movement therapy in infants and toddlers with hemiplegic cerebral palsy: a scoping review. Occup Ther Health Care. (2022) 36:29–45. doi: 10.1080/07380577.2021.1953206
6. Bondoc S, Goodrich B, Gitlow L, Smith RO. Assistive technology and occupational performance. Am J Occup Ther. (2016) 70(Suppl. 2):7012410030p1. doi: 10.5014/ajot.2016.706S02
7. Babu JH, M Raja Srinivas, Kowshik, P Datta Sai. Effectiveness of Constraint-Induced Movement Therapy compared to Hand Arm Bimanual Intensive Therapy on quality of upper extremity function in hemiplegic cerebral palsy children: an experimental study. Neuro Quantol. (2023) 21:888–925. doi: 10.48047/nq.2023.21.01.NQ20069
8. Peng X, Hickman JL, Bowles SG, Donegan DC, Welle CG. Innovations in electrical stimulation harness neural plasticity to restore motor function. Bioelectron Med. (2018) 1:251–63. doi: 10.2217/bem-2019-0002
9. Palanivel V, Grove T, Burrough M. Revolutionizing paediatric neurorehabilitation: integrating innovation and contemporary practice. Paediatr Child Health. (2025) 35:134–9. doi: 10.1016/j.paed.2025.02.002
10. Gandolfi M, Valè N, Posteraro F, Morone G, Dell'orco A, Botticelli A, et al. Italian Consensus Conference on Robotics in Neurorehabilitation (CICERONE). State of the art and challenges for the classification of studies on electromechanical and robotic devices in neurorehabilitation: a scoping review. Eur J Phys Rehabil Med. (2021) 57:831–40. doi: 10.23736/S1973-9087.21.06922-7
11. Brown R, Pearse JE, Nappey T, Jackson D, Edmonds G, Guan Y, et al. Wrist-worn devices to encourage affected upper limb movement in unilateral cerebral palsy: participatory design workshops. Front Rehabil Sci. (2022) 3:1021760. doi: 10.3389/fresc.2022.1021760
12. Go G, Lee Y, Seo D, Lee T. Organic neuroelectronics: from neural interfaces to neuroprosthetics. Adv Mater. (2022) 34:e2201864. doi: 10.1002/adma.202201864
13. Meyers EC, Gabrieli D, Tacca N, Wengerd L, Darrow M, Schlink BR, et al. Decoding hand and wrist movement intention from chronic stroke survivors with hemiparesis using a user-friendly, wearable EMG-based neural interface. J Neuroeng Rehabil. (2024) 21:7. doi: 10.1186/s12984-023-01301-w
14. Peters HT, Page SJ, Persch A. Giving them a hand: wearing a myoelectric elbow-wrist-hand orthosis reduces upper extremity impairment in chronic stroke. Arch Phys Med Rehabil. (2017) 98:1821–7. doi: 10.1016/j.apmr.2016.12.016
15. Bouton CE, Shaikhouni A, Annetta NV, Bockbrader MA, Friedenberg DA, Nielson DM, et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature. (2016) 533:247. doi: 10.1038/nature17435
16. Conforto AB, dos Anjos SM, Bernardo WM, Silva AA da, Conti J, Machado AG, et al. Review of repetitive peripheral sensory stimulation and upper limb performance in stroke: a systematic review and meta-analysis. Neurorehabil Neural Repair. (2018) 32:863–71. doi: 10.1177/1545968318798943
17. Floreani ED, Rowley D, Kelly D, Kinney-Lang E, Kirton A. On the feasibility of simple brain-computer interface systems for enabling children with severe physical disabilities to explore independent movement. Front Hum Neurosci. (2022) 16:1007199. doi: 10.3389/fnhum.2022.1007199
18. Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalil H. Scoping reviews. In: JBI Manual for Evidence Synthesis. Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, , editors. JBI (2024) 417–76. doi: 10.46658/JBIMES-24-09
19. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
21. Azzam AM. Efficacy of mesh glove sensory stimulation on spasticity control in hemiplegic C.P. Indian J Physiother Occup Ther. (2012) 6:13–7.
22. Bortone I, Barsotti M, Leonardis D, Crecchi A, Tozzini A, Bonfiglio L, et al. Immersive virtual environments and wearable haptic devices in rehabilitation of children with neuromotor impairments: a single-blind randomized controlled crossover pilot study. J Neuroeng Rehabil. (2020) 17:144. doi: 10.1186/s12984-020-00771-6
23. Sporea C, Ferechide D. Benefits of upper limb functional electrical stimulation in children with spastic cerebral palsy. Med Modernă (Bucur). (2021) 28:201–7. doi: 10.31689/rmm.2021.28.2.201
24. Bloom R, Przekop A, Sanger TD. Prolonged electromyogram biofeedback improves upper extremity function in children with cerebral palsy. J Child Neurol. (2010) 25:1480–4. doi: 10.1177/0883073810369704
25. Lieber J, Dittli J, Lambercy O, Gassert R, Meyer-Heim A, van Hedel HJA. Clinical utility of a pediatric hand exoskeleton: identifying users, practicability, and acceptance, and recommendations for design improvement. J Neuroeng Rehabil. (2022) 19:17. doi: 10.1186/s12984-022-00994-9
26. Bortone I, Leonardis D, Solazzi M, Procopio C, Crecchi A, Bonfiglio L, et al. Integration of serious games and wearable haptic interfaces for neuro rehabilitation of children with movement disorders: a feasibility study. In: 2017 International Conference on Rehabilitation Robotics (ICORR). London: Institute of Electrical and Electronics Engineers (IEEE) (2017). p. 1094–9. doi: 10.1109/ICORR.2017.8009395
27. Gerber CN, Kunz B, van Hedel HJA. Preparing a neuropediatric upper limb exergame rehabilitation system for home-use: a feasibility study. J Neuroeng Rehabil. (2016) 13:33. doi: 10.1186/s12984-016-0141-x
28. Shimizu Y, Kadone H, Kubota S, Ueno T, Sankai Y, Hada Y, et al. Voluntary elbow extension-flexion using single joint hybrid assistive limb (HAL) for patients of spastic cerebral palsy: two cases report. Front Neurol. (2019) 10:2. doi: 10.3389/fneur.2019.00002
29. Butzer T, Dittli J, Lieber J, van Hedel HJA, Meyer-Heim A, Lambercy O, et al. PEXO - a pediatric whole hand exoskeleton for grasping assistance in task-oriented training. In: 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR). Toronto, ON: Institute of Electrical and Electronics Engineers (IEEE) (2019). p. 108–14. doi: 10.1109/ICORR.2019.8779489
30. Casellato C, Ambrosini E, Galbiati A, Biffi E, Cesareo A, Beretta E, et al. EMG-based vibro-tactile biofeedback training: effective learning accelerator for children and adolescents with dystonia? A pilot crossover trial. J Neuroeng Rehabil. (2019) 16:150. doi: 10.1186/s12984-019-0620-y
31. Fluet GG, Qiu Q, Saleh S, Ramirez D, Adamovich S, Kelly D, et al. Robot-assisted virtual rehabilitation (NJIT-RAVR) system for children with upper extremity hemiplegia. In: 2009 Virtual Rehabilitation International Conference. Haifa: Institute of Electrical and Electronics Engineers (IEEE) (2009). p. 189–92. doi: 10.1109/ICVR.2009.5174230
32. Fluet GG, Qiu Q, Kelly D, Parikh HD, Ramirez D, Saleh S, et al. Interfacing a haptic robotic system with complex virtual environments to treat impaired upper extremity motor function in children with cerebral palsy. Dev Neurorehabil. (2010) 13:335–45. doi: 10.3109/17518423.2010.501362
33. Muccio P, Salama R, Chopra N, Schueller J, Durrant D, Dabrowski E. Longitudinal retrospective study of a wearable NMES system to determine the effects on arm usage in hemiparetic and hemiplegic patients. J Prosthet Orthot. (2025) 37:53–63. doi: 10.1097/JPO.0000000000000508
34. Fu MJ, Curby A, Suder R, Katholi B, Knutson JS. Home-based functional electrical stimulation-assisted hand therapy video games for children with hemiplegia: development and proof-of-concept. IEEE Trans Neural Syst Rehabil Eng. (2020) 28:1461–70. doi: 10.1109/TNSRE.2020.2992036
35. Hernandez H, Poitras I, Fay L, Khan A, Roy J-S, Biddiss E. A gaming system with haptic feedback to improve upper extremity function: a prospective case series. Technol Disabil. (2021) 33:195–206. doi: 10.3233/TAD-200319
36. Kuo FL, Lee HC, Hsiao HY, Lin JC. Robotic-assisted hand therapy for improvement of hand function in children with cerebral palsy: a case series study. Eur J Phys Rehabil Med. (2020) 56:237–42. doi: 10.23736/S1973-9087.20.05926-2
37. Kuroda MM, Iwasaki N, Yoshikawa K, Takeuchi R, Mataki Y, Nakayama T, et al. Voluntary-assisted upper limb training for severe cerebral palsy using robotics devices and neuromuscular electrical stimulation: three case reports. Prog Rehabil Med. (2022) 7:20220050. doi: 10.2490/prm.20220050
38. Arkkukangas M, Hedberg Graff J, Denison E. Evaluation of the electro-dress Mollii® to affect spasticity and motor function in children with cerebral palsy: seven experimental single-case studies with an ABAB design. Cogent Eng. (2022) 9:2064587. doi: 10.1080/23311916.2022.2064587
39. Constantino C, May E, Flanagan A, Altiok H, Harris G. Myoelectric elbow-wrist-hand orthosis for an adolescent with hemiparesis: a case report. J Prosthet Orthot. (2022) 34:e99–102. doi: 10.1097/JPO.0000000000000330
40. Lunardini F, Cesareo A, Biffi E, Casellato C, Pedrocchi A, Sanger TD. EMG-based vibro-tactile biofeedback improves motor control in children with secondary dystonia: two case reports. Neuropsychiatry. (2016) 6:337–43. doi: 10.4172/Neuropsychiatry.1000158
41. Seo NJ, Brinkhoff M, Fredendall S, Coker-Bolt P, McGloon K, Humanitzki E. The use of TheraBracelet upper extremity vibrotactile stimulation in a child with cerebral palsy—a case report. Electronics (Basel). (2024) 13:3147. doi: 10.3390/electronics13163147
42. Maggio MG, Valeri MC, De Luca R, Di Iulio F, Ciancarelli I, De Francesco M, et al. The role of immersive virtual reality interventions in pediatric cerebral palsy: a systematic review across motor and cognitive domains. Brain Sci. (2024) 14:490. doi: 10.3390/brainsci14050490
43. Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci. (2017) 18:86–100. doi: 10.1038/nrn.2016.164
44. Yoo JW, Lee DR, Cha YJ, You SH. Augmented effects of EMG biofeedback interfaced with virtual reality on neuromuscular control and movement coordination during reaching in children with cerebral palsy. Neuro Rehabil. (2017) 40:175–85. doi: 10.3233/NRE-161402
45. Santos CA, de Moura RCF, Lazzari RD, Dumont AJL, Braun LAF, Oliveira CS. Upper limb function evaluation scales for individuals with cerebral palsy: a systematic review. J. Phys. Ther. Sci. (2015) 27:1617–20. doi: 10.1589/jpts.27.1617
46. Reid LB, Rose SE, Boyd RN, Ziviani J. Rehabilitation and neuroplasticity in children with unilateral cerebral palsy. Nat Rev Neurol. (2021) 17:205–19. doi: 10.1038/nrneurol.2015.97
47. Otto Bock SE & Co. KGaA. Exopulse Mollii Suit – Personal Neurostimulation Suit. Duderstadt: Otto Bock SE & Co. KGaA. Available online at: https://www.ottobock.com/en-ex/exopulse-b2c (Accessed Jul 10, 2025).
48. Leonardis D, Solazzi M, Bortone I, Frisoli A. A 3-RSR haptic wearable device for rendering fingertip contact forces. IEEE Trans Haptics. (2017) 10:305–16. doi: 10.1109/TOH.2016.2640291
49. ETH Zurich. Robotic Hand Orthosis for Therapy and Assistance in Activities of Daily Living. Zurich: ETH Zurich ReLab. Available online at: https://relab.ethz.ch/research/current-research-projects/robotic-hand-orthosis-for-therapy-and-assistance-in-activities-of-daily-living.html (Accessed Jul 10, 2025).
50. BTS Bioengineering. Free-Lab. Garbagnate Milanese: BTS Bioengineering. Available online at: https://www.btsbioengineering.com/products/freelab/ (Accessed Jul 10, 2025).
51. Myomo Inc. What is a MyoPro® orthosis?. Cambridge, MA: Myomo Inc. Available online at: https://myomo.com/what-is-a-myopro-orthosis/ (Accessed Jul 10, 2025).
52. Delft Haptics Lab. HapticMaster. Delft: Delft Haptics Lab. Available online at: https://delfthapticslab.nl/device/hapticmaster/ (Accessed Jul 10, 2025).
53. Cleveland Functional Electrical Stimulation Center. CCFES Pediatric – Hand Therapy Video Games. Cleveland, OH: Cleveland FES Center (2019). Available online a: https://fescenter.org/wp-content/uploads/2019/07/CCFES-Pediatric.pdf (Accessed Jul 10, 2025).
54. HapticsHouse.com. Novint's* Falcon Haptic Device. Richardson, TX: HapticsHouse.com. Available online at: https://hapticshouse.com/pages/novints-falcon-haptic-device/ (Accessed Jul 10, 2025).
55. Cyberdyne Care Robotics GmbH. HAL Single Joint. Bochum: Cyberdyne Care Robotics GmbH. Available online at: https://www.cyberdyne.eu/en/products/medical-device/hal-joint/ (Accessed Jul 10, 2025).
56. Axiobionics. Pediatric Rehab. Irvine, CA: Axiobionics. Available online at: https://www.axiobionics.com/pediatric-rehab/ (Accessed Jul 10, 2025).
Keywords: cerebral palsy, wearable neurotechnology, neurorehabilitation, pediatrics, upper extremity rehabilitation, occupational therapy
Citation: Burchfield SJ, Shierk A, Truong C and Blankenship R (2025) Wearable neurotechnology systems for upper extremity rehabilitation in children with cerebral palsy: a scoping review. Front. Neurol. 16:1663596. doi: 10.3389/fneur.2025.1663596
Received: 10 July 2025; Accepted: 14 October 2025;
Published: 05 November 2025.
Edited by:
Yu Liu, University of Minnesota Medical School, United StatesReviewed by:
Daniel Leal Pinheiro, Swiss Federal Institute of Technology Lausanne, SwitzerlandNinad Saraf, Integrated Rehabtech Systems Private Limited, India
Copyright © 2025 Burchfield, Shierk, Truong and Blankenship. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Jo Burchfield, c2pidXJjaGZAdXRtYi5lZHU=
 Sara Jo Burchfield
Sara Jo Burchfield Angela Shierk
Angela Shierk Cindy Truong1,2
Cindy Truong1,2 Regan Blankenship
Regan Blankenship