Abstract
Background:
Acute symptomatic seizures (ASS) occurring within 7 days after traumatic brain injury (TBI) may exacerbate secondary brain injury via excitotoxicity and elevated intracranial pressure. They are also risk factors for post-traumatic epilepsy (PTE). However, the optimal anti-seizure medication for preventing ASS remains unclear. This study aimed to compare the effectiveness of perampanel (PER) versus levetiracetam (LEV) for ASS prevention in patients with moderate to severe TBI.
Methods:
We conducted a retrospective cohort study including 32 patients with moderate to severe TBI who received either LEV (n = 19) or PER (n = 13) as prophylactic anti-seizure therapy. The primary outcome was the incidence of ASS within 7 days post-injury. Secondary outcomes included PTE development, psychiatric adverse events (PAEs), and functional outcomes assessed by the Glasgow Outcome Scale–Extended (GOS-E) at 3 months. Incidence rates were compared between groups using appropriate statistical tests.
Results:
The incidence of ASS was significantly lower in the PER group (7.7%) compared to the LEV group (42.1%) (OR 0.115, p = 0.050), despite a higher prevalence of cerebral contusions in the PER group. There were no significant differences in the incidence of PTE (23.1% vs. 26.3%, OR 0.84, p > 0.99), PAEs (23.1% vs. 26.3%, p > 0.99), or favorable GOS-E scores (38.5% vs. 26.3%, p = 0.707) between the PER and LEV groups.
Conclusion:
PER demonstrated a significant advantage over LEV in preventing ASS following moderate to severe TBI. Given its comparable psychiatric safety profile and functional outcomes, PER may be a promising therapeutic option for acute seizure prophylaxis in this population. However, further prospective studies with larger sample sizes are warranted to validate these findings.
1 Introduction
Traumatic brain injury (TBI) can lead to acute symptomatic seizures (ASS) and post-traumatic epilepsy (PTE), both of which significantly impact neurological outcomes (1, 2). ASS refers to seizures occurring within 7 days of TBI and is considered a predictive marker for the subsequent development of PTE. Therefore, preventing ASS may affect long-term prognosis (3, 4).
ASS in the context of TBI results from mitochondrial dysfunction, neuronal hyperexcitability, inflammation, and structural tissue damage (5, 6). It can exacerbate secondary brain injury by increasing cerebral metabolism and triggering glutamate-mediated excitotoxicity (7, 8). Therefore, suppressing seizures during the acute phase of TBI is not only a symptomatic intervention, but also has neuroprotective potential.
Among the current anti-seizure medications (ASMs) used for the prevention of ASS and PTE, phenytoin and levetiracetam (LEV) are most commonly administered (9); however, the evidence supporting their efficacy remains limited, and their ability to prevent either ASS or PTE are lacking (3, 4, 10–12).
In recent years, perampanel (PER) has gained attention as a selective non-competitive antagonist of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which suppress excitatory neurotransmission to control seizure activity (13, 14). Beyond its antiseizure effects, PER may also exert neuroprotective properties by inhibiting neuronal cell death (15). In brain tumors, PER may not only reduce epileptiform activity, but also suppress tumor growth (16). In cerebrovascular diseases, PER demonstrates seizure control activity and is also expected to reduce infarct volume (17, 18). In TBI models, PER attenuates neuronal cell death and improves neurological outcomes, further suggesting a neuroprotective role (15, 19). These results support the possibility that PER exerts similar protective effects against ASS in TBI; however, clinical evidence regarding the use of PER for the prevention of ASS or PTE in TBI patients remains scarce. The aim of this study was to determine whether PER can reduce the incidence of ASS in the acute phase following TBI.
2 Materials and methods
This retrospective study included patients with moderate to severe TBI, which was defined as a Glasgow Coma Scale (GCS) score of 3–13 on admission, or those who required surgical intervention, and who were treated at Osaka Medical and Pharmaceutical University Hospital between April 2023 and March 2024. Of these patients, only those who received prophylactic administration of either LEV or PER during the acute phase of injury were included. The choice of ASM limited to LEV or PER and was made at the discretion of the attending physician. Both LEV and PER were administered intravenously for 7 days as seizure prophylaxis, and discontinued if no seizures occurred. Patients who had taken ASM before the injury or who died within 24 h following the injury were excluded from the analysis.
Our institutional protocol recommends early intravenous ASM administration in patients with GCS ≤ 13, those with traumatic intracranial hemorrhage, or those requiring craniotomy. Both LEV and PER were administered intravenously for 7 days and discontinued if no seizures occurred.
2.1 Definition of acute symptomatic seizures
ASS was defined as seizures within 7 days of injury. Diagnosis was made by clinical observation by physicians and nurses. Electroencephalography (EEG) was performed selectively when the level of consciousness did not correlate with neuroimaging findings or when unexplained deterioration occurred. If EEG revealed epileptiform discharges consistent with status epilepticus, the patient was diagnosed as having ASS. Seizure types were not further sub-classified in our dataset.
2.2 Patient selection and clinical data collection
Patients who received LEV were categorized into the LEV group, whereas those who received PER were in the PER group. Clinical data were retrospectively collected from medical records and included age, sex, mechanism of injury, GCS, and mean arterial pressure upon admission, and history of psychiatric disorders (including depression, schizophrenia, and bipolar disorder), dementia, or cerebral stroke. Additional information on the clinical course, radiological findings [acute subdural hematoma (ASDH), contusion, traumatic subarachnoid hemorrhage (tSAH)], laboratory values, and neurological outcomes was also collected.
2.3 Primary and secondary outcomes
The primary outcome was the incidence of ASS, which was defined as seizures occurring within 7 days post-injury. Secondary outcomes included the incidence of PTE, the incidence of psychiatric adverse effects (PAEs), and the Glasgow Outcome Scale-Extended (GOS-E) score at 3 months post-injury. A GOS-E score of 5–8 was considered a favorable outcome, whereas a score of 1–4 was considered an unfavorable outcome.
2.4 Statistical analysis
Statistical analyses were performed using SPSS software (version 30.0; SPSS Inc., Chicago, IL, United States). Quantitative variables were reported as the mean ± standard deviation or as median values, depending on the distribution. Categorical variables were compared using Pearson’s chi-square test, t-test, or two-tailed Fisher’s exact test, where appropriate. For continuous variables, the Mann–Whitney U test was used to compare differences between groups based on data distribution. Cumulative seizure-free survival was assessed using the Kaplan–Meier method, and comparisons between groups were made using the log-rank test. A p-value <0.05 was considered statistically significant.
This study was approved by the Institutional Ethics Committee of Osaka Medical and Pharmaceutical University (2841-4). The requirement for informed consent was waived because of the retrospective nature of the study. All procedures were conducted in accordance with the Declaration of Helsinki.
3 Results
3.1 Characteristics of participants
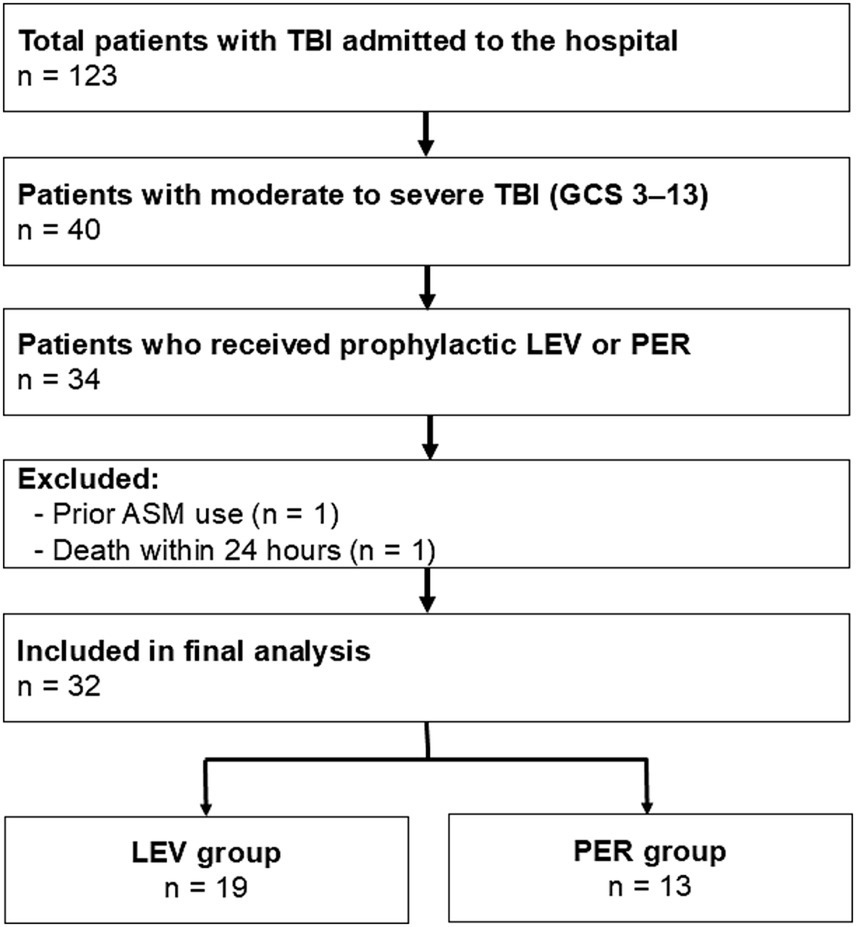
Patients (n = 123) were admitted to our hospital for TBI. Forty presented with a GCS score of 3–13, and 34 received prophylactic ASM treatment during the acute phase. One patient who had been taking ASMs before injury and one patient who died within 24 h of admission were excluded. A total of 32 patients were included in the final analysis (Figure 1), with 19 receiving LEV (LEV group) and 13 receiving PER (PER group).
Figure 1
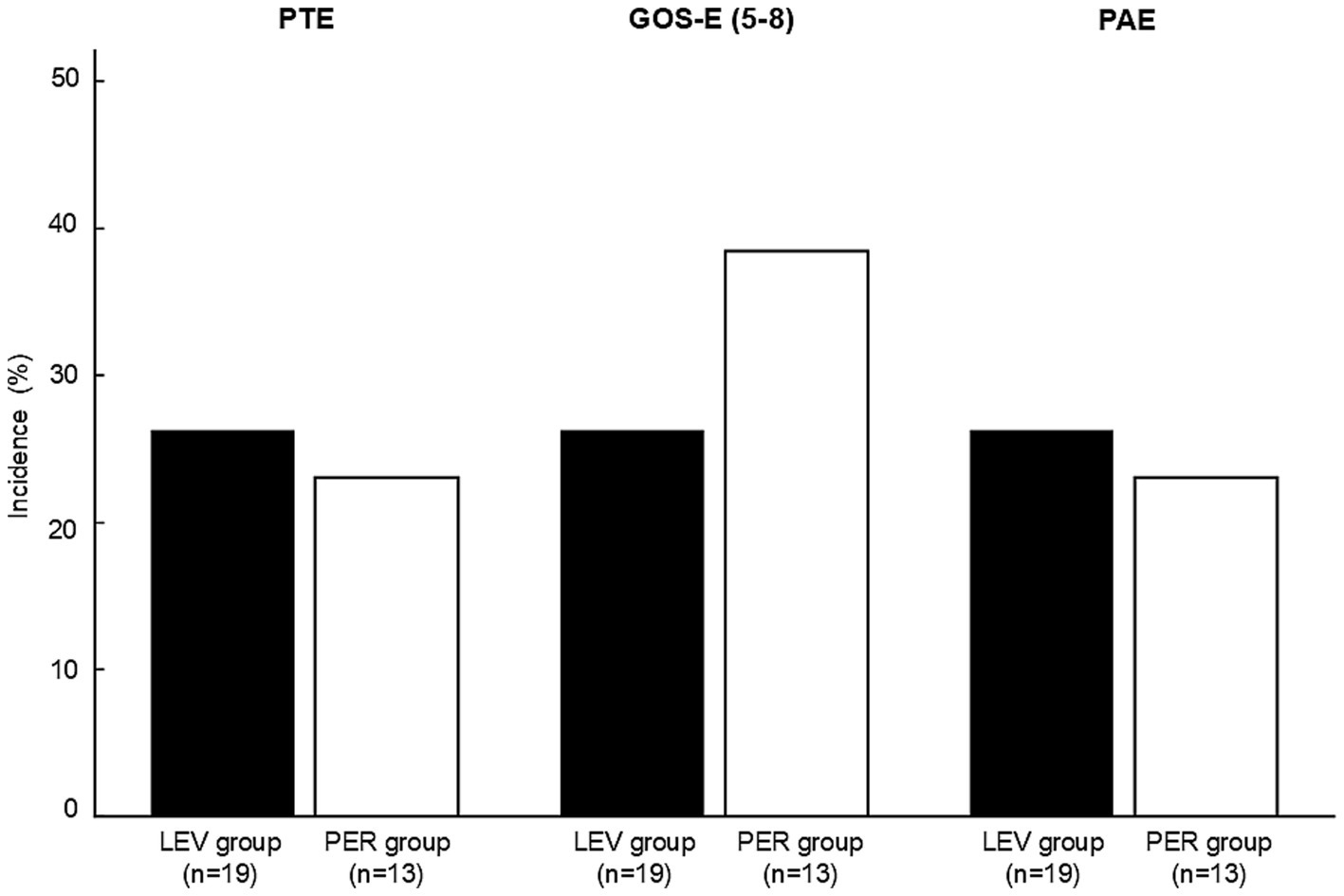
Incidence of post-traumatic epilepsy, favorable outcome (GOS-E score 5–8), and psychiatric adverse effects in the LEV and PER groups. No statistically significant differences were observed between the two groups for any outcome (PTE: p > 0.99, GOS-E: p = 0.71, PAEs: p > 0.99). Statistical analysis was performed using Fisher’s exact test. PTE: post-traumatic epilepsy, GOS-E; Glasgow outcome scale-extended, PAE; psychiatric adverse effects, LEV: levetiracetam, PER: perampanel.
3.2 Comparison of baseline characteristics
Baseline characteristics, including age, sex, GCS on admission, past medical history, and type of injury, were comparable between the two groups, with no statistically significant differences (Table 1); however, the PER group had a significantly higher incidence of cerebral contusions compared with the LEV group (n = 12, 92.3% vs. n = 11, 57.9%, p < 0.05). The prevalence of ASDH and tSAH was not significantly different between the two groups (p = 0.06 and p = 0.70). The location of the contusion also did not significantly differ between groups. Except for significantly lower hemoglobin levels in the LEV group, there were no significant differences in other laboratory values between the two groups (Table 1). Overall, 29.7% of patients underwent EEG, with 36.8% in the LEV group and 30.8% in the PER group, with no significant difference between groups (p > 0.99).
Table 1
| Variable | LEV group | PER group | p-value |
|---|---|---|---|
| n | 19 | 13 | |
| Age [IQR] | 74 [54–78] | 64 [43–74] | 0.36 |
| Male n, (%) | 11 (57.9) | 9 (69.2) | 0.71 |
| MAP on admission (mmHg) | 106.2 ± 27.6 | 100.4 ± 14.6 | 0.44 |
| GCS [IQR] | 7 [6–9] | 10 [6–13] | 0.18 |
| Past history | |||
| Psychiatric disorders n, (%) | 3 (15.8) | 2 (15.4) | >0.99 |
| Dementia n, (%) | 2 (10.5) | 1 (7.7) | >0.99 |
| Cerebral stroke n, (%) | 4 (21.1) | 1 (7.7) | 0.63 |
| Oral antithrombotic agent use n, (%) | 3(15.8) | 2(15.4) | >0.99 |
| Radiological finding | |||
| Contusion n, (%) | 11 (57.9%) | 12 (92.3%) | <0.05 |
| ASDH n, (%) | 13 (68.4%) | 11 (84.6%) | 0.06 |
| tSAH n, (%) | 11 (57.9%) | 8 (61.5%) | 0.70 |
| Contusion location | |||
| Frontal lobe n, (%) | 4 (36.4) | 6 (50.0) | 0.14 |
| Temporal lobe n, (%) | 6 (54.5) | 6 (50.0) | 0.47 |
| Insula n, (%) | 1 (9.1) | 0 | >0.99 |
| Parietal lobe n, (%) | 0 | 0 | |
| Occipital lobe n, (%) | 0 | 0 | |
| Laboratory values | |||
| White blood cell (/μL) | 11041.6 ± 496.8 | 11789.2 ± 3802.2 | 0.40 |
| Hemoglobin (g/dL) | 11.7 ± 2.4 | 13.3 ± 1.5 | <0.05 |
| C-reactive protein (mg/dL) | 1.1 ± 2.2 | 0.4 ± 1.4 | 0.16 |
| Fibrinogen (mg/dL) | 306.9 ± 82.1 | 270.1 ± 50.8 | 0.18 |
| D-dimer (μg/mL) | 20.6 ± 15.4 | 34.3 ± 34.9 | 0.38 |
| Albumin (g/dL) | 3.7 ± 0.5 | 4.0 ± 0.5 | 0.14 |
| Glucose (mg/dL) | 161.4 ± 52.2 | 156.0 ± 40.3 | 0.70 |
| Sodium (mEq/L) | 140.5 ± 5.2 | 138.5 ± 3.8 | 0.15 |
| Calcium (mg/dL) | 8.3 ± 1.6 | 8.9 ± 0.4 | 0.38 |
Baseline characteristics, radiological findings, and laboratory values.
Baseline characteristics, radiological findings and laboratory values of patients in the LEV and PER groups. Psychiatric disorders included depression, schizophrenia, and bipolar disorder. Continuous variables are presented as mean ± standard error or as median (interquartile range) for non-normally distributed data. Categorical variables are shown as counts and percentages. Comparisons between groups were performed using Fisher’s exact test or Mann–Whitney U test as appropriate. p < 0.05 was considered statistically significant. GCS: Glasgow Coma Scale, LEV: levetiracetam, PER: perampanel, MAP: mean arterial pressure. GCS: Glasgow Coma Scale. ASDH: acute subdural hematoma. tSAH: traumatic subarachnoid hemorrhage.
3.3 Incidence of acute symptomatic seizures and post-traumatic epilepsy
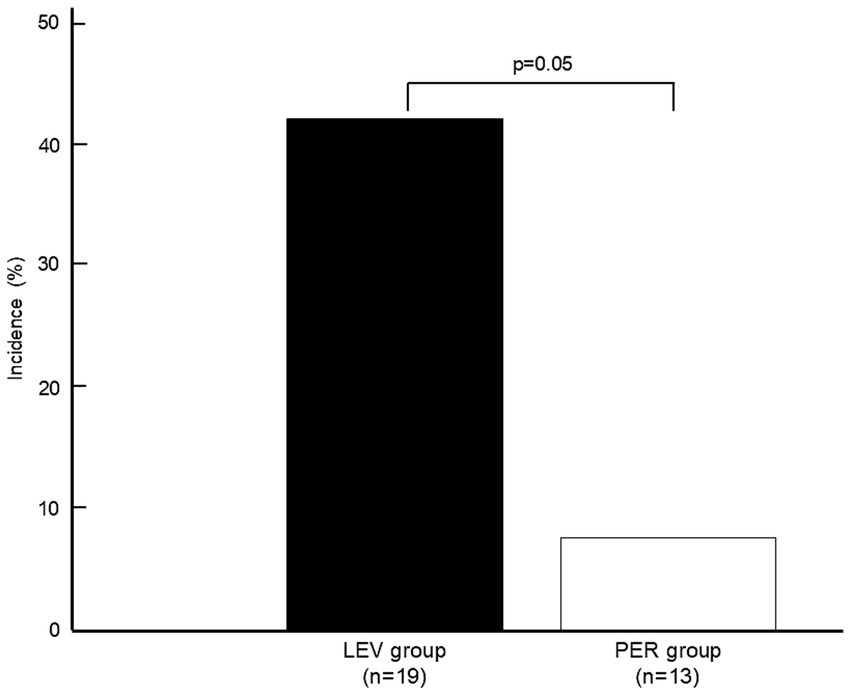
ASS occurred in one patient (n = 1, 7.7%) in the PER group compared with eight patients (n = 8, 42.1%) in the LEV group (OR 0.115, p = 0.050) (Figure 2).
Figure 2
Flow diagram of the included patients. Flow diagram showing the inclusion and exclusion criteria for patients with moderate to severe TBI (GCS 3-13) who received prophylactic AED treatment. Thirty-two patients were included in the final analysis. Patients who received LEV were categorized into the LEV group, whereas those who received PER were in the PER group. TBI: Traumatic brain injury, GCS: Glasgow Coma Scale, ASM: anti-seizure medication, LEV: levetiracetam, PER: perampanel.
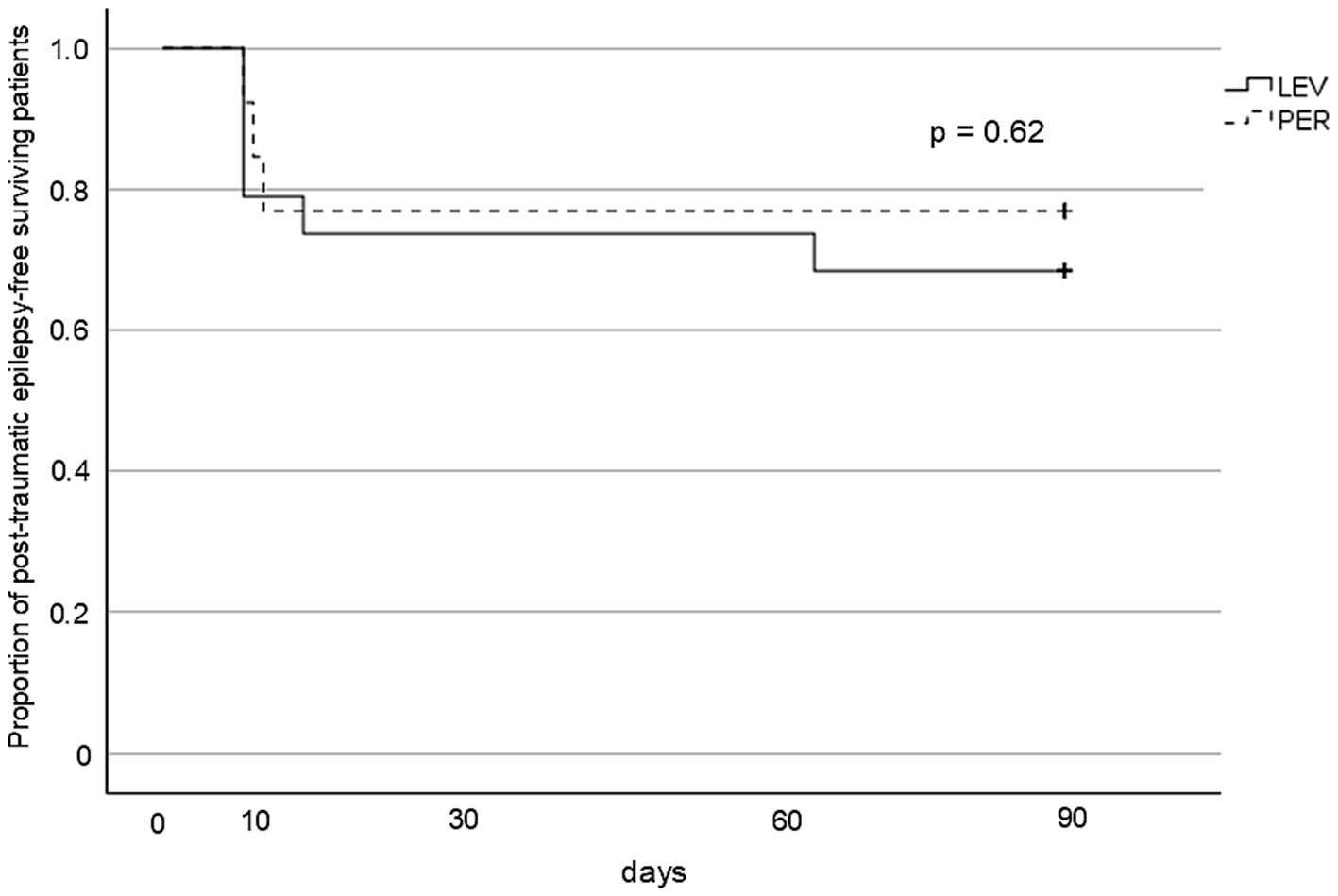
PTE occurred in five patients (n = 5, 26.3%) in the LEV group and three patients (n = 3, 23.1%) in the PER group, with no significant difference between the groups (OR 0.84, p > 0.99) (Figure 3). Kaplan–Meier analysis showed no significant difference in PTE-free survival within three months post-injury (p = 0.62) (Figure 4).
Figure 3
Kaplan–Meier survival curves for PTE-free survival. The cumulative incidence of PTE over 3 months from injury. No significant difference was observed between the groups (log-rank p = 0.62). PTE: post-traumatic epilepsy, LEV: levetiracetam, PER: perampanel.
Figure 4
Incidence of acute symptomatic seizures. Comparison of ASS incidence between the LEV and PER groups. The PER group exhibited lower ASS rates (7.7% vs. 42.1%, p = 0.050). A statistical comparison was performed using Fisher’s exact test. ASS: acute symptomatic seizures, LEV: levetiracetam, PER: perampanel.
3.4 Functional outcomes and psychiatric adverse events
There were no significant differences between the groups in either functional outcomes [favorable GOS-E: 26.3% (the LEV group) vs. 38.5% (the PER group), p = 0.71] or the incidence of PAEs [26.3% (the LEV group) vs. 23.1% (the PER group), p > 0.99] (Figure 3).
4 Discussion
In the present study, we found that the incidence of ASS was lower in the PER group compared with the LEV group, despite the PER group including a significantly higher proportion of patients with cerebral contusion, which is a known risk factor for ASS (2, 20). Although the reduction in ASS did not reach statistical significance (p = 0.05), the observed difference between the PER and LEV groups suggests a potential clinical benefit worthy of further study. ASS exacerbates secondary brain injury through increased metabolic demand, excitotoxicity, and elevated intracranial pressure (6). Excitotoxicity through glutamate release and AMPA/NMDA receptor activation is a well-known mechanism of secondary brain injury following TBI. By inhibiting AMPA receptors, PER may mitigate these processes and reduce the occurrence of ASS (21, 22). Following TBI, alterations in the expression and function of N-methyl-D-aspartate (NMDA) and AMPA receptors have been reported (23, 24). In particular, excess activation of NMDA receptors and the resulting Ca2+ influx, observed shortly after the primary injury, have been implicated as pathophysiological mechanisms that drive excitotoxicity (25). The activation of NMDA receptors subsequently induces receptor phosphorylation, subunit modification, and further activation of AMPA receptors (23).
By contrast, LEV exerts its antiseizure effects through modulation of the synaptic vesicle protein SV2A, thereby regulating neurotransmitter release rather than directly targeting excitatory glutamatergic pathways (26). This mechanistic difference suggests that PER may provide unique benefits in mitigating glutamate-driven excitotoxicity that underlies ASS in the acute phase of TBI, whereas LEV provides broader synaptic stabilization. Furthermore, in a rat TBI model, PER attenuated neuronal cell death and increased anti-inflammatory cytokine expression, suggesting potential neuroprotective and anti-inflammatory effects beyond seizure prevention (27). Nevertheless, it remains uncertain whether these advantages translate into clinically meaningful superiority in acute TBI, and further prospective studies are warranted.
Because ASS is considered a downstream consequence of secondary brain injury processes, suppressing ASS with PER may not only reduce the risk of PTE, but also mitigate the extent of the secondary brain injury. Therefore, the prevention of ASS is an important component in the acute management of TBI. From this perspective, our results suggest that PER may be an effective prophylactic option for seizure control in the acute phase of TBI, particularly among patients at high risk for ASS.
Although PER appeared to effectively suppress ASS, it did not result in a significant reduction in the incidence of PTE compared with LEV (Figure 3). This discrepancy likely reflects the fundamental differences in the pathophysiology between ASS and PTE. PTE may arise from chronic epileptogenic processes that evolve over weeks to months. These include axonal sprouting, synaptic reorganization, sustained neuroinflammation, and reactive gliosis, all of which contribute to long-term changes in neuronal excitability and network structure (28, 29). Because these changes extend beyond the temporal scope of early pharmacological prophylaxis, PTE may develop despite the effective suppression of ASS.
Interestingly, several cases of PTE in the present study developed within a relatively short timeframe (Figure 4). In cases of severe TBI, prolonged inflammatory responses and persistent excitotoxicity lower the seizure threshold for ASS development (30). Moreover, such pathological neurochemical environments can last beyond 8 days post-injury (31). Therefore, in severe TBI, it may be reasonable to consider extending the risk window for ASS beyond the conventional 7-day period.
PAEs are an important concern associated with ASM. In the present study, approximately 15% of patients in the LEV and PER groups had a prior history of psychiatric disorders (Table 1). Regardless of psychiatric history, the incidence of PAEs in the PER group was comparable to that in the LEV group (Figure 3). Because of the high prevalence of psychiatric symptoms after TBI and the known psychiatric side effects of ASM (32–35), comparable PAE incidence between PER and LEV suggests that PER is a clinically acceptable alternative in this regard.
5 Limitations
This study had several limitations. First, the follow-up period was limited to three months, which may not adequately capture the development of late-onset PTE or long-term outcomes. Second, because of the small sample size, we were unable to perform multivariate analyses to adjust for baseline differences or potential confounders. EEG was not systematically performed in all patients, and available data only indicated whether EEG was conducted. Therefore, non-convulsive seizures may have been under detected. Third, ASM selection was based on physician discretion, which may have potentially introduced selection bias. As the choice was not randomized or blinded, residual bias cannot be excluded. Future prospective randomized and blinded studies will be required to validate our findings. Finally, this study did not assess the cost-effectiveness of each ASM, which may affect the generalizability of our findings in different healthcare settings.
6 Conclusion
In this retrospective study of patients with moderate to severe TBI, PER demonstrated an advantage over LEV in suppressing ASS; however, there were no significant differences between the two groups in terms of PTE, 3-month outcomes, or PAEs. Because of the established role of ASS in exacerbating secondary brain injury through excitotoxicity and increased intracranial pressure, suppression of ASS with PER may contribute to the mitigation of secondary injury processes. From this perspective, PER is a clinically viable option for seizure prophylaxis in the acute phase of TBI, particularly in patients at high risk for ASS, with psychiatric tolerability comparable to that of LEV.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Osaka Medical and Pharmaceutical University (2841-4). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this study was conducted using the opt-out method, written informed consent was not required. The study protocol was approved by the institutional review board, and information regarding the study was made publicly available to allow patients the opportunity to decline participation.
Author contributions
JN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft. HK: Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing. KoY: Data curation, Writing – review & editing. AK: Data curation, Writing – review & editing. RK: Data curation, Writing – review & editing. KH: Data curation, Writing – review & editing. YA: Conceptualization, Writing – review & editing. JH: Data curation, Writing – review & editing. KS: Writing – review & editing. KaY: Conceptualization, Resources, Visualization, Writing – review & editing. SK: Writing – review & editing. MW: Supervision, Visualization, Writing – review & editing. AT: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by JSPS KAKENHI Grant-in-Aid for Early-Career Scientists (grant number JP24K19519).
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Annegers JF Hauser WA Coan SP Rocca WA . A population-based study of seizures after traumatic brain injuries. N Engl J Med. (1998) 338:20–4. doi: 10.1056/NEJM199801013380104
2.
Gunawardane N Fields M . Acute symptomatic seizures and provoked seizures: to treat or not to treat?Curr Treat Options Neurol. (2018) 20:41. doi: 10.1007/s11940-018-0525-2
3.
Frontera JA Gilmore EJ Johnson EL Olson DW Rayi A Tesoro E et al . Guidelines for seizure prophylaxis in adults hospitalized with moderate-severe traumatic brain injury: a clinical practice guideline for health care professionals from the neurocritical care society. Neurocrit Care. (2024) 40:819–44. doi: 10.1007/s12028-023-01907-x
4.
Lucke-Wold BP Nguyen L Turner RC Logsdon AF Chen YW Smith KE et al . Traumatic brain injury and epilepsy: underlying mechanisms leading to seizure. Seizure. (2015) 33:13–23. doi: 10.1016/j.seizure.2015.10.002
5.
Yardi R Vasireddy RP Galovic M Punia V . Antiseizure medication use in acute symptomatic seizures: a narrative review. Epilepsia. (2025) 66:955–69. doi: 10.1111/epi.18275
6.
Reinert M Khaldi A Zauner A Doppenberg E Choi S Bullock R . High extracellular potassium and its correlates after severe head injury: relationship to high intracranial pressure. Neurosurg Focus. (2000) 8:e10. doi: 10.3171/foc.2000.8.1.2027
7.
Temkin NR . Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. (2001) 42:515–24. doi: 10.1046/j.1528-1157.2001.28900.x
8.
Agrawal A Timothy J Pandit L Manju M . Post-traumatic epilepsy: an overview. Clin Neurol Neurosurg. (2006) 108:433–9. doi: 10.1016/j.clineuro.2005.09.001
9.
Beghi E . Overview of studies to prevent posttraumatic epilepsy. Epilepsia. (2003) 44:21–6. doi: 10.1046/j.1528-1157.44.s10.1.x
10.
Kharatishvili I Pitkänen A . Posttraumatic epilepsy. Curr Opin Neurol. (2010) 23:183–8. doi: 10.1097/WCO.0b013e32833749e4
11.
Pease M Mittal A Merkaj S Okonkwo DO Gonzalez-Martinez JA Elmer J et al . Early seizure prophylaxis in mild and moderate traumatic brain injury: a systematic review and meta-analysis. JAMA Neurol. (2024) 81:507–14. doi: 10.1001/jamaneurol.2024.0689
12.
Jones KE Puccio AM Harshman KJ Falcione B Benedict N Jankowitz BT et al . Levetiracetam versus phenytoin for seizure prophylaxis in severe traumatic brain injury. Neurosurg Focus. (2008) 25:E3. doi: 10.3171/FOC.2008.25.10.E3
13.
Hanada T Hashizume Y Tokuhara N Takenaka O Kohmura N Ogasawara A et al . Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. (2011) 52:1331–40. doi: 10.1111/j.1528-1167.2011.03109.x
14.
Lavu A Aboulatta L Abou-Setta AM Aloud B Askin N Rabbani R et al . Efficacy and safety of perampanel in epilepsy: a systematic review and meta-analysis of randomised controlled trials. Seizure. (2022) 102:54–60. doi: 10.1016/j.seizure.2022.09.020
15.
Alqahtani F Assiri MA Mohany M Imran I Javaid S Rasool MF et al . Coadministration of ketamine and perampanel improves behavioral function and reduces inflammation in acute traumatic brain injury mouse model. Biomed Res Int. (2020) 2020:3193725. doi: 10.1155/2020/3193725
16.
Tabaee Damavandi P Pasini F Fanella G Cereda GS Mainini G DiFrancesco JC et al . Perampanel in brain tumor-related epilepsy: a systematic review. Brain Sci. (2023) 13:326. doi: 10.3390/brainsci13020326
17.
Pascarella A Manzo L Gasparini S Marsico O Abelardo D Torino C et al . Perampanel in post-stroke epilepsy: clinical practice data from the PERampanel as only concomitant antiseizure medication (PEROC) study. J Neurol Sci. (2024) 462:123106. doi: 10.1016/j.jns.2024.123106
18.
Shin HJ Lee KY Kang JW Choi SG Kim DW Yi YY . Perampanel reduces brain damage via induction of M2 microglia in a neonatal rat stroke model. Int J Nanomedicine. (2022) 17:2791–804. doi: 10.2147/IJN.S361377
19.
Aida V Niedzielko TL Szaflarski JP Floyd CL . Acute administration of perampanel, an AMPA receptor antagonist, reduces cognitive impairments after traumatic brain injury in rats. Exp Neurol. (2020) 327:113222. doi: 10.1016/j.expneurol.2020.113222
20.
Riascos D Buriticá E Jiménez E Castro O Guzmán F Palacios M et al . Neurodegenerative diversity in human cortical contusion: histological analysis of tissue derived from decompressive craniectomy. Brain Res. (2013) 1537:86–99. doi: 10.1016/j.brainres.2013.09.016
21.
Gruenbaum BF Zlotnik A Fleidervish I Frenkel A Boyko M . Glutamate neurotoxicity and destruction of the blood-brain barrier: key pathways for the development of neuropsychiatric consequences of TBI and their potential treatment strategies. Int J Mol Sci. (2022) 23:9628. doi: 10.3390/ijms23179628
22.
Hoffe B Holahan MR . Hyperacute excitotoxic mechanisms and synaptic dysfunction involved in traumatic brain injury. Front Mol Neurosci. (2022) 15:831825. doi: 10.3389/fnmol.2022.831825
23.
Spaethling JM Klein DM Singh P Meaney DF . Calcium-permeable AMPA receptors appear in cortical neurons after traumatic mechanical injury and contribute to neuronal fate. J Neurotrauma. (2008) 25:1207–16. doi: 10.1089/neu.2008.0532
24.
Sta Maria NS Reger ML Cai Y Baquing MAT Buen F Ponnaluri A et al . D-Cycloserine restores experience-dependent neuroplasticity after traumatic brain injury in the developing rat brain. J Neurotrauma. (2017) 34:1692–702. doi: 10.1089/neu.2016.4747
25.
Kalia LV Kalia SK Salter MW . NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. (2008) 7:742–55. doi: 10.1016/S1474-4422(08)70165-0
26.
Yamagata A Ito K Suzuki T Dohmae N Terada T Shirouzu M . Structural basis for antiepileptic drugs and botulinum neurotoxin recognition of SV2A. Nat Commun. (2024) 15:3027. doi: 10.1038/s41467-024-47322-4
27.
Chen T Dai SH Jiang ZQ Luo P Jiang XF Fei Z et al . The AMPAR antagonist Perampanel attenuates traumatic brain injury through anti-oxidative and anti-inflammatory activity. Cell Mol Neurobiol. (2017) 37:43–52. doi: 10.1007/s10571-016-0341-8
28.
Fang M Liu W Tuo J Liu M Li F Zhang L et al . Advances in understanding the pathogenesis of post-traumatic epilepsy: a literature review. Front Neurol. (2023) 14:1141434. doi: 10.3389/fneur.2023.1141434
29.
Algattas H Huang JH . Traumatic brain injury pathophysiology and treatments: early, intermediate, and late phases post-injury. Int J Mol Sci. (2013) 15:309–41. doi: 10.3390/ijms15010309
30.
Liesemer K Bratton SL Zebrack CM Brockmeyer D Statler KD . Early post-traumatic seizures in moderate to severe pediatric traumatic brain injury: rates, risk factors, and clinical features. J Neurotrauma. (2011) 28:755–62. doi: 10.1089/neu.2010.1518
31.
Kovesdi E Kamnaksh A Wingo D Ahmed F Grunberg NE Long JB et al . Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front Neurol. (2012) 3:111. doi: 10.3389/fneur.2012.00111
32.
Kureshi N Clarke DB Feng C . Association between traumatic brain injury and mental health care utilization: evidence from the Canadian community health survey. Inj Epidemiol. (2023) 10:16. doi: 10.1186/s40621-023-00424-x
33.
Howlett JR Nelson LD Stein MB . Mental health consequences of traumatic brain injury. Biol Psychiatry. (2022) 91:413–20. doi: 10.1016/j.biopsych.2021.09.024
34.
Perucca P Mula M . Antiepileptic drug effects on mood and behavior: molecular targets. Epilepsy Behav. (2013) 26:440–9. doi: 10.1016/j.yebeh.2012.09.018
35.
Fong YO Huang P Hsu CY Yang YH . Effects of perampanel on seizure control, cognition, behavior, and psychological status in patients with epilepsy: a systematic review. J Clin Neurol. (2022) 18:653–62. doi: 10.3988/jcn.2022.18.6.653
Summary
Keywords
AMPA receptor blockade, neuroprotection, head trauma, secondary brain injury, seizure prophylaxis
Citation
Nakao J, Kashiwagi H, Yoshimura K, Kambara A, Kotera R, Honda K, Amemiya Y, Hatakeyama J, Sakakibara K, Yamakawa K, Kawabata S, Wanibuchi M and Takasu A (2025) Acute symptomatic seizure prevention with perampanel in moderate and severe traumatic brain injury: a retrospective comparison with levetiracetam. Front. Neurol. 16:1665997. doi: 10.3389/fneur.2025.1665997
Received
14 July 2025
Accepted
25 September 2025
Published
16 October 2025
Volume
16 - 2025
Edited by
Sérgio Brasil, University of São Paulo, Brazil
Reviewed by
Nitish Chourasia, University of Tennessee Health Science Center (UTHSC), United States
Aman Shrivastava, Institute of Professional Studies College of Pharmacy, India
Updates
Copyright
© 2025 Nakao, Kashiwagi, Yoshimura, Kambara, Kotera, Honda, Amemiya, Hatakeyama, Sakakibara, Yamakawa, Kawabata, Wanibuchi and Takasu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junzo Nakao, junzo.nakao@ompu.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.