- 1Department of Neurology, First Affiliated Hospital, Kunming Medical University, Kunming, Yunnan, China
- 2Department of Rehabilitation, The Third People’s Hospital of Yunnan Province, Kunming, Yunnan, China
Objectives: This study aimed to evaluate the efficacy and safety of intermittent immunoadsorption (IA) in critically ill patients with refractory autoimmune neurological disorders.
Methods: We retrospectively reviewed 13 patients admitted to the neurocritical care unit with severe autoimmune encephalitis, Guillain–Barré syndrome, neuromyelitis optica spectrum disorders, or chronic inflammatory demyelinating polyneuropathy, all of whom had failed first-line immunotherapy (intravenous methylprednisolone and/or intravenous immunoglobulin). IA was administered intermittently, with schedules individualized based on clinical status.
Results: The modified Rankin Scale (mRS) improved significantly following IA (p = 0.02), while the Acute Physiology and Chronic Health Evaluation II scores (APACHE II) remained stable (p = 0.95). Serum IgG levels declined by a median of 55.6%. Pathogenic antibody negativity was achieved in 65% of plasma and 38% of cerebrospinal fluid samples. Although 92% experienced treatment interruptions (e.g., infection and hypotension), IA was generally well tolerated and not permanently discontinued.
Discussion: This study supports the feasibility and clinical utility of intermittent IA in critically ill patients with treatment-refractory neuroimmunological disorders. Despite frequent complications, flexible scheduling allowed continued therapy with sustained benefit. These findings highlight a potentially adaptable treatment strategy in a population often excluded from therapeutic interventions and suggest that IA warrants further study in neurocritical care settings.
Introduction
Immunoadsorption (IA) is a therapeutic apheresis technique designed to remove pathogenic antibodies from circulation with high specificity (1). It has proven efficacy in immune-mediated neurological diseases such as autoimmune encephalitis (AE), myasthenia gravis, neuromyelitis optica spectrum disorders (NMOSDs), and Guillain–Barré syndrome (GBS) (1). In less critically ill patients, IA is generally well tolerated and associated with favorable clinical outcomes (2). However, its use in critically ill patients remains underreported, limited by concerns about safety, immunosuppression, and procedural risk. This retrospective study evaluates the feasibility, tolerability, and clinical outcomes of intermittent IA in critically ill patients with refractory immune-mediated neurological diseases, aiming to address this therapeutic gap.
Methods
Design
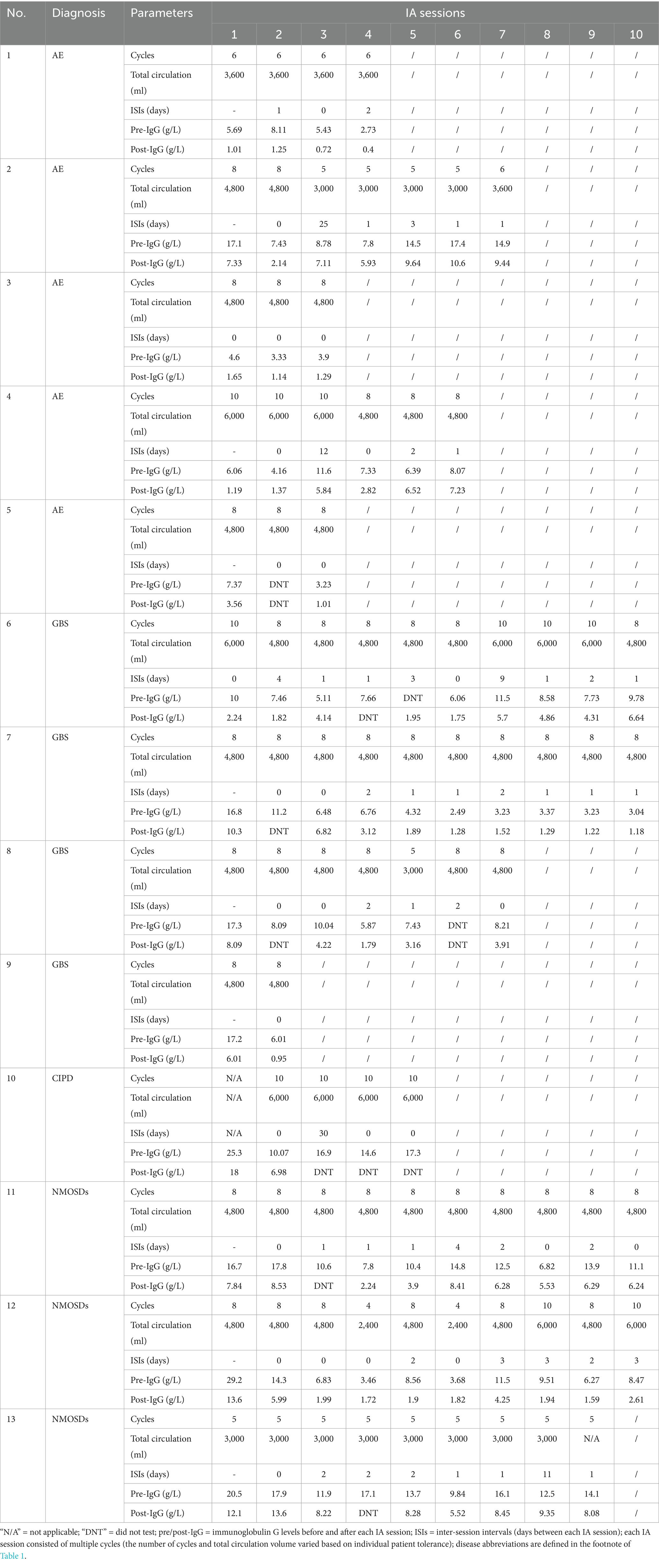
This retrospective observational study included 13 critically ill patients admitted to the neurocritical care unit (NCU) between January 2021 and July 2024 who received intermittent IA for refractory autoimmune neurological disease. Inclusion criteria included: (1) confirmed diagnosis, (2) failure of first-line treatment (intravenous methylprednisolone (IVMP) and/or intravenous immunoglobulin (IVIG)), and (3) clinical deterioration requiring intensive care (e.g., respiratory failure and severe motor deficits). Patients with mild disease (Acute Physiology and Chronic Health Evaluation II (APACHE II) < 5 or Modified Rankin Scale (mRS) < 2) were excluded. Both baseline scores were assessed upon admission to the NCU. All eligible patients during the study period were included consecutively to reduce selection bias. Standardized measures (mRS, APACHE II) and certified laboratory tests were used to minimize observer and information bias. No sample size calculation was performed due to the retrospective nature. IA was performed using a protein A adsorber (KONPIA®; Guangzhou Koncen Bioscience Co., Ltd., Guangzhou, China) and a hemoperfusion device (DTB-100A, Chongqing Duotai Medical Device Co., Ltd., Chongqing, China). Sessions were adapted based on patient tolerance, infection status, and immune function. The standard operating procedure for IA used at our center is provided as Supplementary materials.
Measurements
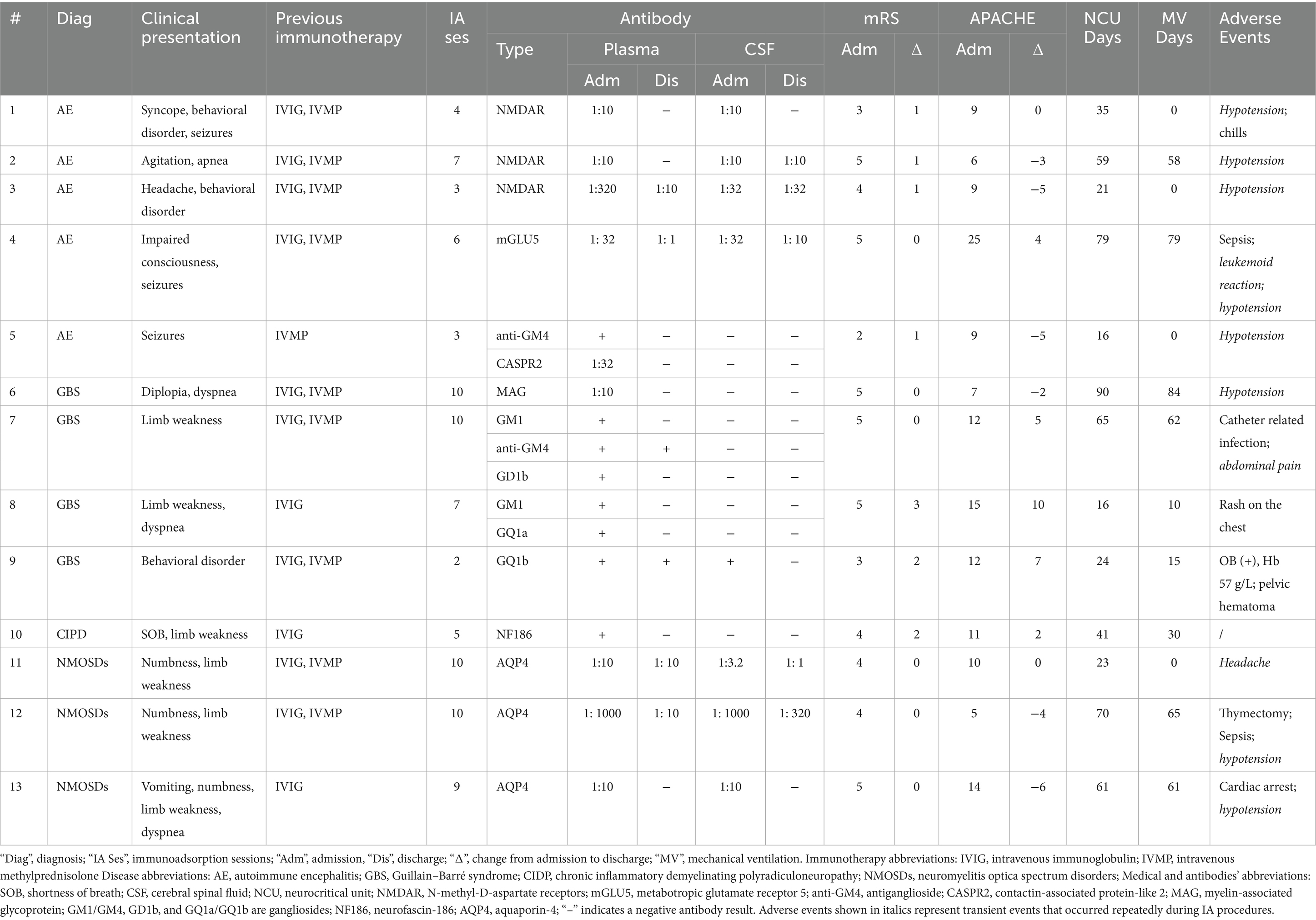
Demographic and clinical data were derived from the review of medical records (Table 1), including neurological and disease severity assessments, as well as IA-related parameters (Table 2). General outcomes included the mRS scale and APACHE II score at admission and discharge, respectively. The mRS is a clinically validated functional assessment scale (0–6), where 0 = no symptoms, 1–2 = mild disability, 3–4 = moderate to severe disability, 5 = severe disability requiring constant care, and 6 = death. A change of ±1 point was considered deterioration/improvement (3). Disease severity was assessed using the APACHE II score (4), which ranges from 0 to 71, with higher scores indicating greater physiological derangement and increased mortality risk. IA treatment outcomes included serum IgG levels pre- and post-each IA session and pathogenic antibody alterations in plasma and cerebrospinal fluid (CSF), classified as negative conversion (undetectable after treatment), reduction (decrease in antibody titer but still detectable), and no change (persistent antibody detection).
Statistical analysis
Data were analyzed using GraphPad Prism version 10.0 or later. Variables were presented as median and interquartile range (IQR). Changes in mRS and APACHE II were analyzed using the Wilcoxon test; changes in IgG were analyzed using multiple Wilcoxon tests. A p-value of < 0.05 was considered statistically significant.
Results
Thirteen patients (9 women, 4 men, median age 46 years, IQR: 14–58, median body weight 70 kg, IQR: 40–80) met the inclusion criteria. Diagnoses include AE (n = 5), GBS (n = 4), neuromyelitis optica spectrum disorders (NMOSDs; n = 3), and chronic inflammatory demyelinating polyneuropathy (CIDP; n = 1). Although the median mRS score at both admission and discharge was 4 (IQR: 2–5 at admission and 1–5 at discharge), the distribution of individual changes showed a statistically significant improvement (p = 0.02) (Figure 1A). Specifically, 7 out of 13 patients demonstrated a ≥ 1 point reduction in mRS by discharge (Table 1), indicating meaningful functional recovery. The median APACHE II score was 10 (IQR: 5–25) on admission and 9 (IQR: 5–21) on discharge (p = 0.95), indicating a stable systemic condition (Figure 1B). The median duration of mechanical ventilation was 30 days (IQR: 0–62), and the median NCU stay was 41 (IQR: 23–65) days.
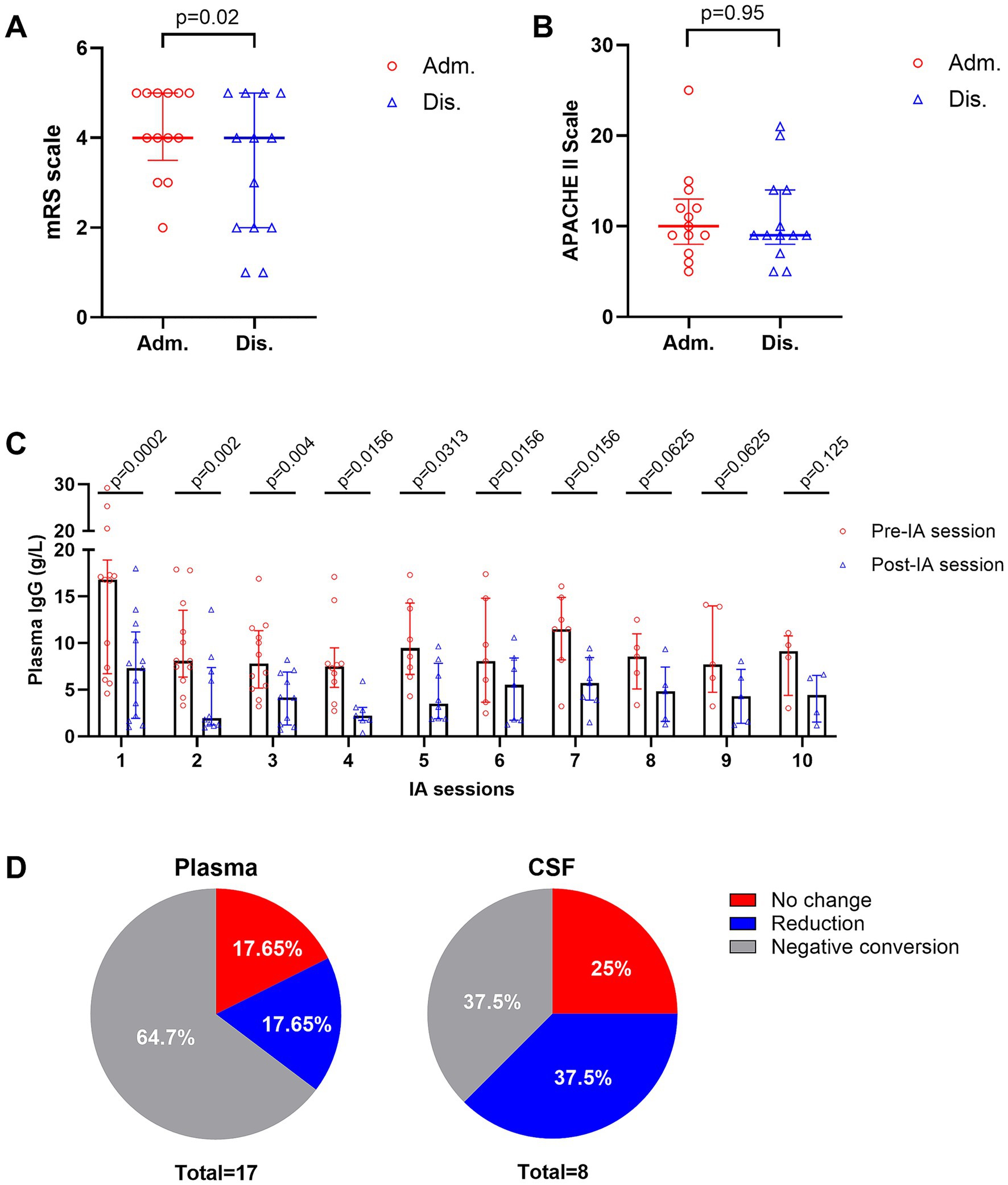
Figure 1. Clinical and laboratory outcomes of intermittent immunoadsorption (IA). (A) Individual patients’ mRS scores at admission and discharge. (B) APACHE II scores admission and discharge. (C) Changes in serum IgG levels before and after each IA session. (D) Pathogenic antibody responses in plasma (left) and CSF (right). Data are presented as medians with interquartile ranges.
The median number of IA sessions per treatment course was 7 (IQR: 4–10), with inter-session intervals (ISIs) ranging from 1 to 30 days. Interruptions occurred in 92% of patients due to infection, hypotension, leukemoid reaction, or procedural complications (e.g., cardiac arrest and pelvic hematoma). Median IgG level decreased from 8.56 g/L (IQR: 6.17–14) to 4.18 g/L (IQR: 1.78–7.14), representing a median 55.6% reduction (Figure 1C, Table 2). In some cases, at our center, IVIG was administered during ISIs when serum IgG levels fell below 4 g/L, with or without concomitantly low IgA levels (~0.8 g/L), to support immunity (5). Pathogenic antibody analysis revealed negative conversion in 64.7% (plasma) and 37.5% (CSF), partial reduction in 17.65% (plasma) and 37.5% (CSF), and no change in 17.65% (plasma) and 25% (CSF) (Figure 1D).
Discussion
Our findings suggest that intermittent IA treatment is both feasible and effective in critically ill patients with immune-mediated neurological disorders who fail standard first-line therapies. The flexible schedule allows clinicians to pause IA during critical complications and restart it when stable. In our center, decisions to suspend or prolong ISIs were based on multiple factors, including overall patient tolerance and the occurrence of serious adverse events (e.g., hypotension requiring vasopressor support, sepsis, or catheter-related infection). Among these, infectious complications typically result in substantially longer ISIs. This adaptive model may improve safety without compromising efficacy.
Notably, neurological function, as assessed by mRS, improved in most patients, whereas systemic disease severity, as measured by the APACHE II score, remained stable despite clinical complications. Given that the APACHE II score incorporates parameters from multiple organ systems, it provides an objective measure of overall physiological derangement (4). These findings suggest that intermittent IA treatment is a safe treatment option for critically ill patients with autoimmune neurological disorders.
Minor complications commonly associated with apheresis and vascular access (e.g., transient hypotension and hematoma) were observed in our cohort, although absent in some prior reports (6), and were clinically manageable. Leukemoid reactions were transient and self-limiting, while catheter-related infections and sepsis were successfully treated and did not necessitate permanent discontinuation of IA therapy. One patient experienced cardiac arrest during IA but was successfully resuscitated with cardiopulmonary resuscitation and completed the planned treatment course. In contrast, the reported adverse events in the intensive care unit during plasma exchange included anaphylactoid reactions, severe hypotension, catheter-related infections, pneumothorax, local bleeding, hypocalcemia, and paresthesia (7). These complications, largely related to plasma substitution and citrate anticoagulation, were uncommon in our IA cohort (Table 1), suggesting that IA may offer a favorable safety profile in critically ill neurologic patients, although confirmation in larger prospective studies is warranted.
While our observed IgG reduction was slightly lower than previously reported in general populations (55.6% vs. 62–93%) (1), this may reflect lower starting IgG levels or treatment interruptions. Despite concerns about IA-related immunosuppression, no patient in our cohort experienced fatal infection or irreversible deterioration. This supports the safe use of IA in selected patients with marginal IgG values, provided monitoring and IVIG support are available.
IA remains underutilized, partly due to high costs and limited guidelines for ICU settings. In Germany, a session costs ~€ 1,200 (8); in the U. S., five sessions may cost ~$ 58,952 (9). At our center, a typical course costs ~30,000 RMB (~3,000 per session). Costs vary widely based on location, condition, and institutional procurement, but early use in high-risk patients may reduce long-term ICU burden.
Our study has several limitations. First, its retrospective design introduces inherent biases, including a small sample size and the absence of a control group. Additionally, the study was not powered to detect differences across subgroups of immune-mediated neurological diseases. The absence of certain severe conditions, such as myasthenic crisis, further limits the generalizability of our findings. Future research should focus on prospective, controlled studies with multiple centers and large cohorts to validate our observations and explore the optimal IA treatment protocols for diverse neuroimmunological conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Kunming Medical University [(2025)-L − 35]. Written informed consent was waived due to the retrospective observational nature of the study, which involved analysis of anonymized clinical data without any identifiable patient information.
Author contributions
QW: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Writing – original draft, Writing – review & editing. SD: Conceptualization, Writing – review & editing, Writing – original draft. JX: Validation, Writing – original draft, Writing – review & editing. DG: Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (82160260), the Yunnan Applied Basic Research Projects (202201AY070001-083), the Yunnan Health Training Project of High-Level Talents (D-2024037), the Yunnan Academic and Technical Leaders Program (202205AC160019), and the 535 Talent Program of First Affiliated Hospital of Kunming Medical University (2023535D04).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1666042/full#supplementary-material
References
1. Fuchs, K, Rummler, S, Ries, W, Helmschrott, M, Selbach, J, Ernst, F, et al. Performance, clinical effectiveness, and safety of immunoadsorption in a wide range of indications. Ther Apher Dial. (2022) 26:229–41. doi: 10.1111/1744-9987.13663
2. Heine, J, Ly, LT, Lieker, I, Slowinski, T, Finke, C, Pruss, H, et al. Immunoadsorption or plasma exchange in the treatment of autoimmune encephalitis: a pilot study. J Neurol. (2016) 263:2395–402. doi: 10.1007/s00415-016-8277-y
3. Dogan Onugoren, M, Golombeck, KS, Bien, C, Abu-Tair, M, Brand, M, Bulla-Hellwig, M, et al. Immunoadsorption therapy in autoimmune encephalitides. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e207. doi: 10.1212/NXI.0000000000000207
4. Bouch, DC, and Thompson, JP. Severity scoring systems in the critically ill. Contin Educ Anaesth Crit Care Pain. (2008) 8:181–5. doi: 10.1093/bjaceaccp/mkn033
5. Jolles, S, Chapel, H, and Litzman, J. When to initiate immunoglobulin replacement therapy (Igrt) in antibody deficiency: a practical approach. Clin Exp Immunol. (2017) 188:333–41. doi: 10.1111/cei.12915
6. Kohler, W, Ehrlich, S, Dohmen, C, Haubitz, M, Hoffmann, F, Schmidt, S, et al. Tryptophan immunoadsorption for the treatment of autoimmune encephalitis. Eur J Neurol. (2015) 22:203–6. doi: 10.1111/ene.12389
7. Bauer, PR, Ostermann, M, Russell, L, Robba, C, David, S, Ferreyro, BL, et al. Plasma exchange in the intensive care unit: a narrative review. Intensive Care Med. (2022) 48:1382–96. doi: 10.1007/s00134-022-06793-z
8. Rothenburg, J, Rink-Baron, S, Mueller, L, Ostermann, PN, Fischer, J, Stegbauer, J, et al. Covid-19 antibody donation using immunoadsorption: report of two cases. Transfus Apher Sci. (2021) 60:103193. doi: 10.1016/j.transci.2021.103193
Keywords: immunoadsorption, neurocritical care, autoimmune neurological disorders, therapeutic apheresis, intensive care unit
Citation: Wu Q, Zhu L, Dai S, Xu J and Ge D (2025) Intermittent immunoadsorption in critically ill patients with neuroimmunological disorders: a retrospective study. Front. Neurol. 16:1666042. doi: 10.3389/fneur.2025.1666042
Edited by:
Filipe Palavra, Centro Hospitalar e Universitário de Coimbra, PortugalReviewed by:
João J. Cerqueira, University of Minho, PortugalVictor M. Rivera, Baylor College of Medicine, United States
Copyright © 2025 Wu, Zhu, Dai, Xu and Ge. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Wu, cWlhbnd1QHlkeXkuY24=; d3Fsb2FsZWlAMTYzLmNvbQ==
†These authors share first authorship
 Qian Wu
Qian Wu Lin Zhu
Lin Zhu Shujuan Dai1†
Shujuan Dai1†