Abstract
Background:
Parkinson's disease (PD) is a common neurodegenerative disorder that primarily affects individuals over the age of 60. Impaired limb balance, cognitive decline, and emotional disturbances are core symptoms of PD, significantly impacting patients' quality of life. While medication can alleviate motor symptoms, its effectiveness in improving non-motor symptoms (such as cognitive and emotional disturbances) is limited, and long-term use may lead to adverse effects. In recent years, exercise therapy has garnered increasing attention due to its safety, accessibility, and potential to offer both motor and non-motor benefits, making it an important direction in PD rehabilitation research. This study systematically evaluated nine exercise rehabilitation interventions to provide evidence-based non-pharmacological alternatives for PD management.
Methods:
A systematic search of six major databases was conducted, and 55 randomized controlled trials involving 4,417 patients with Parkinson's disease were included. The outcome measures were evaluations of balance, cognition, Emotional Functions, and quality of life-related indicators. Stata 17.0 was used to perform a net meta-analysis to assess the relative effectiveness of each intervention and to test the consistency of direct and indirect evidence.
Results:
Exoskeletal Training (ET) was the most effective intervention for improving balance (SMD = −2.52, 95% CI [−3.38, −1.67], p < 0.0001), resistance training (RT) provided the greatest benefit for reducing Emotional Functions (SMD = 1.02, 95% CI [0.67, 1.38], p < 0.0001). In terms of enhancing cognitive function, mind-body exercise (MBE) emerged as the optimal choice (SMD = −1.42, 95% CI [−2.01, −0.84], p < 0.0001), while resistance training (RT) was most effective in improving quality of life (SMD = 1.83, 95% CI [0.41, 4.07], p < 0.0001).
Conclusion:
Dance (DA) is the most effective intervention for improving balance, while aquatic training (ABT) and resistance training (RT) are most effective for emotional regulation. Mind-body exercise (MBE) demonstrates exceptional efficacy in cognitive function, while resistance training has the greatest impact on improving quality of life. These findings provide evidence-based guidance for optimizing exercise-based rehabilitation for Parkinson's disease, supporting tailored interventions targeting specific symptom domains. Future research should focus on refining protocols to maximize treatment efficacy.
1 Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra (1). Its core clinical symptoms include resting tremor, muscle rigidity, bradykinesia, and postural instability, often accompanied by non-motor symptoms such as cognitive impairment, depression, anxiety, and sleep disorders (2). According to data from the Global Burden of Disease Study, the global PD patient population has exceeded 6 million, with incidence rates significantly increasing with age, reaching approximately 1–2% in individuals aged 65 and older (3). The pathophysiological basis of the disease primarily involves the selective loss of dopaminergic neurons in the substantia nigra pars compacta (4), leading to a significant decrease in dopamine levels in the striatum and disrupting the functional balance of the basal ganglia motor regulation circuit (5). As the disease progresses, approximately 40% of patients develop Parkinson’s disease dementia (6), and the prevalence of depressive symptoms reaches as high as 40% (7), significantly impairing patients’ quality of life (8). Current clinical interventions primarily focus on slowing disease progression and alleviating symptoms (9).
Mainstream treatment approaches for Parkinson’s disease primarily include pharmacological therapy and surgical intervention (10, 11). However, both have significant limitations. Pharmacological treatment centers on levodopa-based medications (12), which effectively alleviate motor symptoms but may lead to end-of-dose phenomena and dyskinesia upon long-term use (13). Surgical interventions such as deep brain stimulation can significantly improve symptoms in moderate-to-severe patients (14), but they carry surgical risks, are costly, and may result in complications such as infections (15).
Physical therapy, including mind–body exercises, resistance training, and aquatic exercises, can slow neurodegeneration through mechanisms such as regulating brain-derived neurotrophic factor (BDNF) (16). For Parkinson’s patients, exercise therapy has unique advantages (17). However, most studies have focused on patients in the early to mid-stages of the disease, and there is a lack of evidence-based guidelines for elderly PD patients (18); secondly, the optimal combination of different types of exercise (e.g., aerobic vs. resistance training) remains unclear (19); furthermore, the mechanisms by which exercise interventions affect non-motor symptoms (e.g., cognition, depression) in elderly PD patients remain controversial (20). Currently, the optimal exercise regimen and individualized protocols require further evidence-based research, particularly in elderly patients (21). Overall, exercise therapy is a safe, non-pharmacological method that can improve the quality of life of Parkinson’s patients by enhancing motor function and non-motor symptoms such as cognition and mood (22).
This study used a network meta-analysis to compare the effects of nine exercise therapies on balance, mood, and other functional outcomes (23). It analyzed multiple dimensions, including physiological aspects, cognitive levels, and emotional functions, ultimately aiming to improve patients’ overall quality of life. This study provides valuable insights for offering safe and effective non-pharmacological options for Parkinson’s patients, reducing long-term medical burdens, and guiding clinical rehabilitation practices.
2 Methods
This study was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for network meta-analyses (NMAs10) and the Cochrane Handbook for Systematic Reviews of Interventions. Registration number: CRD420251059397.
2.1 Data sources
We conducted a systematic search on PubMed, Embase, Web of Science Cochrane, EBSCO, and China National Knowledge Infrastructure (CNKI1), with two researchers independently selecting studies for inclusion. In PubMed and Cochrane, we used terms from the Medical Subject Headings (MeSH) database for the search. In Embase, we used terms from the Emtree database, and in CNKI, we combined subject terms with free-text keywords for the search. Additionally, we manually screened the reference lists of relevant articles to identify other studies that might meet the inclusion criteria. The search period ranged from January 2000 to June 2025, and only human studies published in Chinese or English were included, with Chinese studies limited to core journals.
The search strategy followed the evidence-based medicine PICOS principles: (P) Population: elderly Parkinson’s disease patients; (I) Intervention: 1. Regular Aerobic Training (RAT); 2. Gait Stability Training (GST); 3. Mind–Body Exercise (MBE); 4. Sensory Stimulation Training (SST); 5. VR Games Training (VRGT); 6. Exoskeletal Training (ET); 7. Resistance Training (RT); 8. Aqua-Based Training (ABT); 9. Dance (DA); (C) Control group: Control group is other exercise therapies; (O) Outcomes: Physical function was assessed using the BA (balance) index, measurement using scales (e.g., Balance Pull Test, BBS, Mini-BES Test, ABC); cognitive function using the COG (cognitive) index, measure using scales (e.g., TMT-B, reaction time, PDCRS total score): emotional regulation using the EF (Emotional Function) index, measurement using scales (e.g., HADS-anxiety, HADS-depression, BDI, GDS-15, PDQ-39, Emotional well-being, SDS); and quality of life using the QOL (quality of life) index, measurement using scales (e.g., Impairment in daily life, PDQ39, PDQ39_Total).(S) Study type: RCTs.
2.2 Study selection
Medical search terms were used to conduct searches in PubMed and Cochrane. Additionally, to ensure the comprehensiveness and accuracy of the literature, the reference lists of relevant articles were manually screened to identify other studies that may meet the criteria. It was ensured that literature related to exercise therapy and Parkinson’s disease was retrieved.
After obtaining the initial literature, a rigorous screening process was conducted on these documents. First, EndNote software was used to perform an automatic duplicate literature check to eliminate duplicate records that may have appeared in the database due to differences in search strategies or data sources. Subsequently, duplicate items not automatically identified during the screening process were further removed through manual review of titles and abstracts to ensure the uniqueness and representativeness of the screened literature. For the remaining literature, a more rigorous review was conducted. The following types of studies were primarily excluded: studies targeting non-Parkinson’s patient populations, studies that did not assess relevant indicators, and studies that did not use exercise therapy interventions. Additionally, review articles, conference abstracts, animal studies, research protocols, case reports, retrospective studies, and book chapters were excluded because they typically lack sufficient original data or scientific validity to provide specific analyses and conclusions regarding the effectiveness of interventions. By adopting these stringent literature screening criteria, the final included studies provide high-quality evidence to support this research, further enhancing its scientific validity and credibility.
2.3 Eligibility criteria
We included randomized clinical trials in patients with diagnosed Parkinson’s disease (Includes randomized clinical trials for patients with), comparing the effects of different treatments.
Studies meeting the following criteria were eligible for inclusion: (1) were RCTs; (2) involved patients with Parkinson’s disease; (3) Complete outcome measure data; (4) The experimental group received any of the following unconventional movement therapies: RAT, GST, MBE, SST, VRGT, ET, RT, ABT, or DA, while the control group received other movement therapies; (5) At least one of the following measures was assessed: BA (balance), COG (cognitive), EF (Emotional Function), or QOL (quality of life).(6)The age range for the elderly is 60 to 90 years old.
Studies were excluded if they met the following criteria: (1) non-RCTs; (2) animal studies, review articles, conference reports, case reports, letters, or duplicated publications; (3) full-text unavailable; (4) incomplete experimental results or inability to extract data indicators; (5) Failure to report relevant indicators of interest to this study; (6) Patients with other neurological disorders in addition to Parkinson’s disease; (7) Non-core journal articles published in Chinese.
2.4 Data collection
Two researchers imported the collected literature into EndNote 20 software according to the search strategy and screened the obtained literature. First, duplicate documents were excluded, followed by a preliminary screening based on titles and abstracts. Full-text articles were then read in detail according to the inclusion and exclusion criteria to further screen the remaining literature. Subsequently, two researchers cross-checked their respective screening results. If they agreed, the literature was included in the study; if there were any discrepancies, a third researcher was consulted, and after discussion and consensus, the literature was finally included.
For eligible trials, two trained researchers independently extracted data from the included literature using standardized data extraction forms and summarized the risk of bias. The extracted data primarily included: (1) basic information of the included literature (first author, publication year, country, etc.); (2) demographic characteristics of the participants (number, age, and gender of the experimental and control groups); (3) detailed information about the intervention (type, intensity, duration, and frequency of the intervention); (4) Outcome measures (mean and standard deviation; primary outcome measures selected included those assessing physical function, cognitive function, emotional regulation, and quality of life; secondary outcome measures selected were used to assess risk of bias). Selected primary outcome measures include those scoring physical function and those scoring cognitive and emotional aspects; selected secondary outcome measures include those rating quality of life. For studies presenting results graphically without numerical summaries, numerical data were extracted using a validated graph digitization tool (GetData 2.22) for analysis. When necessary, we contacted the article authors to obtain information.
2.5 Data risk of bias of the systematic review
Using the Cochrane 5.1 version bias risk assessment tool (which includes seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases), two researchers assessed the risk of bias (ROB) for all eligible studies. Risk assessment analysis was conducted using Review Manager 5.3 (Scandinavian Cochrane, Denmark), with each domain assessed as unclear, low risk, or high risk. Based on these assessments, we classified the overall risk of bias for each study as follows: (1) Low ROB: No domains assessed as high risk, and possibly some assessed as unclear but fewer than three; (2) Moderate ROB: One domain assessed as high risk, but no more than one; or no high-risk domains, but more than three domains assessed as unclear; (3) High ROB: All other cases not falling under the above categories were classified as high risk.
2.6 Statistical analysis
In this study, META analyzed the data using STATA 17.0 software (Stata Corp LLC, College Station, TX, United States), with the outcome measures being continuous variables. The NMA integrated the pre- and post-intervention changes in both the experimental and control groups to systematically assess the effects of exercise therapy on balance, cognition, Emotional Functions, and quality of life indicators in Parkinson’s patients. To accurately assess the effectiveness of these interventions, the standardized mean difference (SMD) and its 95% confidence intervals (CI) were calculated for each indicator. The baseline was uniformly adjusted to α = 0.05, and effect estimates were synthesized using a random-effects model to account for heterogeneity among studies in terms of participant characteristics and intervention methods. We used the following method to calculate the standard deviation of change scores based on the Cochrane Handbook guidelines: For studies that reported baseline and endpoint SD values but lacked SD values for change scores, we used the following formula: *SD_change = √(SD_pre2 + SD_post2–2 × r × SD_pre × SD_post). Here, r is the correlation coefficient between baseline and post-treatment scores. When r was not reported, we used the conservative default value recommended by the Cochrane guidelines (r = 0.8) based on similar randomized controlled trials (RCTs) in the analysis. Heterogeneity was quantified using the I2 statistic and Cochran Q test. The relationships between different non-invasive methods were visualized using a network diagram, where lines connecting nodes represent direct comparisons between different non-invasive methods. The size of nodes and the thickness of connecting lines are proportional to the number of studies including that comparison, and the diagram visually represents the relative strength of interventions and their position within the network. Additionally, the drawn network contributions further quantify the contribution of each direct comparison to the entire network, aiding in analyzing the impact of each intervention on the entire network. Furthermore, to assess publication bias in the studies, a corrected comparison funnel plot was used to analyze publication bias for the primary outcome measures. Finally, the cumulative ranking curve under the curve (SUCRA) method was used to calculate the probability of an intervention being the best.
3 Result
3.1 Study selection
A total of 5,647 potentially relevant documents were identified through the search. After removing 2,269 duplicate documents, 3,378 articles remained for screening. Through screening of titles and abstracts, 1,739 documents were excluded. According to the inclusion criteria, a further full-text review was conducted, and 1,586 documents that did not meet the criteria were excluded. Ultimately, 53 articles were included. The document selection flowchart is shown in Figure 1.
Figure 1
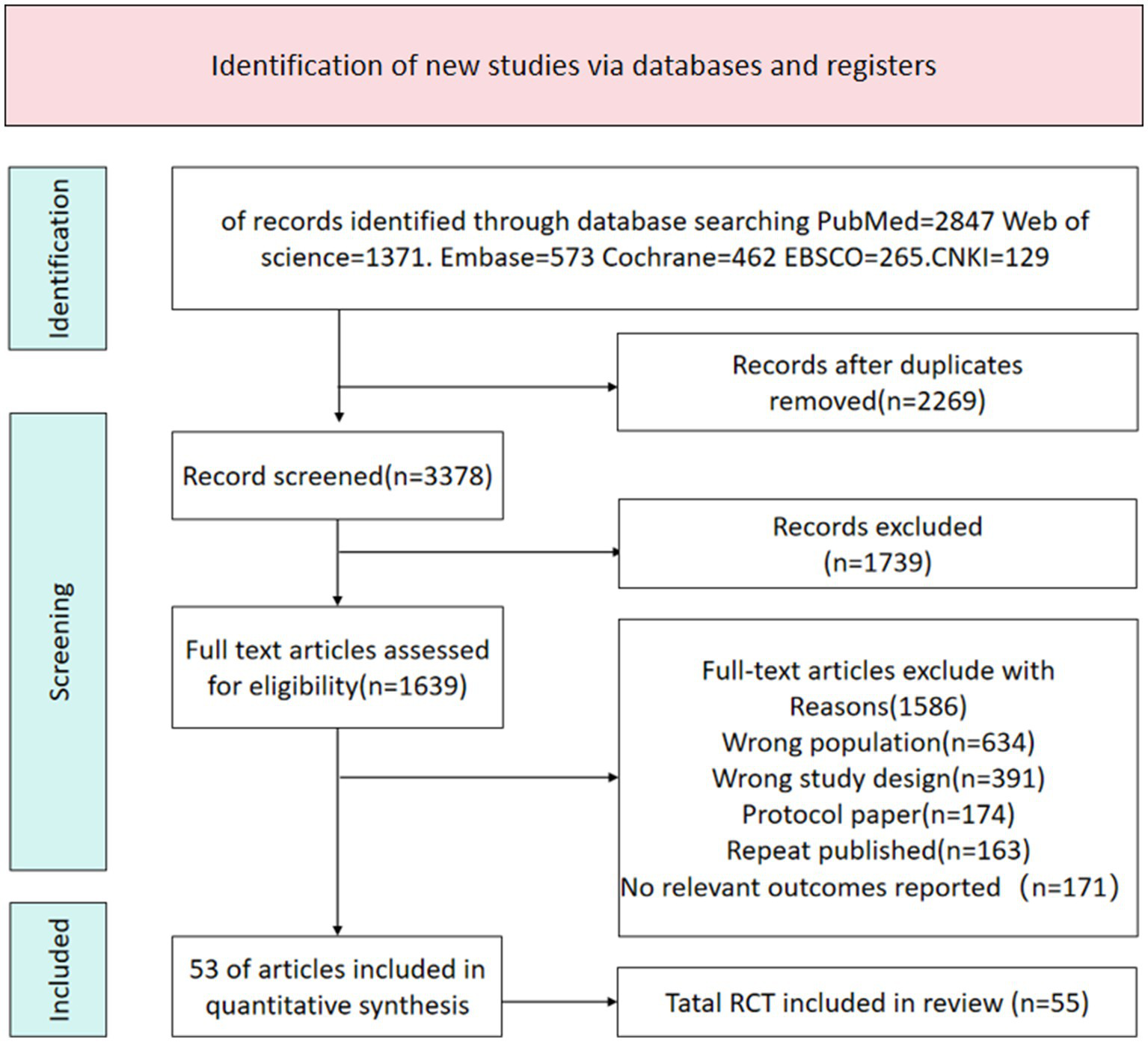
Literature search flowchart.
3.2 Literature characterization
A total of 53 articles were included in the analysis, with the basic characteristics of all included studies presented in Table 1. This systematic review and network meta-analysis encompassed studies published between 2007 and 2024, involving multiple countries including Brazil, Italy, China, Canada, Spain, India, Germany, Turkey, Australia, the United States, Japan, Portugal, and Pakistan. The experimental group comprised 2,389 participants who underwent nine types of exercise therapy (RAT, GST, MBE, SST, VRGT, ET, RT, ABT, DA), while the control group included 2,028 participants. In total, 4,417 Parkinson’s disease patients were included, with ages ranging from 60.2 to 77.7 years and an average disease duration of 6.8 years. The duration of exercise intervention ranged from 2 weeks to 12 months, with a median of 8 weeks; each session lasted 20–120 min, with a median of 50 min; sessions were conducted 1–7 times per week, with 3 times being the most common. SST was the most widely used (35.2%), followed by GST (24.1%) and DA control (22.2%). For details, see Table 1.
Table 1
| Study | Country | Group | Sample size (M/F) | Age (mean ± SD) | Intervention duration | Intervention frequency | 认知 | PD病程 (年) |
|---|---|---|---|---|---|---|---|---|
| Vanbellingen 2017 (45) | Brazil | RT | 52 (18/34) | 67.15 ± 7.94 | 4w | 30 min, 5 times/week | 26.65 ± 1.78 | 6.12 ± 3.52 |
| RT | 51 (22/29) | 68.16 ± 7.38 | 30 min, 5 times/week | 26.59 ± 2.30 | 6.35 ± 3.99 | |||
| Agosta 2017 (46) | Italy | SST | 13 (8/5) | 69.0 ± 8.0 | 4w | 60 min,’3 times/week | ||
| DA | 12 (10/2) | 64.0 ± 7.0 | 60 min, 3 times/week | |||||
| Sarasso 2021 (47) | Italy | SST | 13 (5/8) | 66.41 ± 9.23 | 6w | 60 min, 3 times/week | 8.08 ± 4.13 | |
| SST | 12 (4/8) | 63.86 ± 13.82 | 60 min, 3 times/week | 7.92 ± 3.53 | ||||
| Yang 2016 (48) | China | VRGT | 11 (4/7) | 72.5 ± 8.4 | 6w | 50 min, twice/week | (MMSE) 27.5 ± 4.0 | 9.4 ± 3.6 |
| GST | 12 (5/7) | 75.4 ± 6.3 | 50 min, twice/week | 27.2 ± 2.5 | 8.3 ± 4.1 | |||
| Lin 2024 (49) | China | SST | 8 (4/4) | 62.63 ± 5.32 | 8w | 25-45 min, twice/week | 28.13 ± 0.99 | 5.38 ± 3.16 |
| DA | 8 (7/1) | 63.63 ± 6.48 | 25-45 min, twice/week | 27.50 ± 1.51 | 5.13 ± 1.55 | |||
| Nadeau 2014 (50) | Canada | SST | 12 (4/8) | 64.0 ± 6.6 | 24w | 60 min, 3 times/week | 27.8 ± 2.2 | |
| DA | 11 (2/9) | 64.3 ± 5.6 | 60 min, twice/week | (MMSE) 28.6 ± 1.1 | ||||
| Ferraz 2018 (51) | Brazil | CRT | 22 (6/16) | 71 ± 6.4 | 8w | 50 min, 3 times/week | 27.00 ± 1.1 | 4 ± 3 |
| DA | 20 (9/11) | 67 ± 4.4 | 50 min, 3 times/week | 27.00 ± 1.1 | 6 ± 4 | |||
| San 2020 (52) | Spain | SST | 23 (12/11) | 66.38 ± 7.06 | 10w | 60 min, twice/week | ||
| GST | 17 (5/12) | 64.75 ± 8.77 | 60 min, twice/week | |||||
| Alagumoorthi 2022 (53) | India | VRGT | 96 (45/51) | 69.7 ± 10 | 12w | 30-40 min, 3 times/week | 5.37 ± 3.57 | |
| GST | 96 (33/63) | 68.5 ± 9.8 | 30-40 min, 3 times/week | 5.34 ± 3 | ||||
| Morris 2009 (54) | Australia | SST | 14 | 68 ± 9.2 | 2w | 45 min, 7 times/week | ||
| DA | 14 | 66 ± 8.6 | 45 min, 7 times/week | |||||
| Spina 2021 (55) | Italy | ET | 11 (5/6) | 68.0 ± 6.9 | 4w | 45 min, 5 times/week | 6.0 ± 1.7 | |
| GST | 11 (4/7) | 67.3 ± 4.85 | 45 min, 5 times/week | 5.0 ± 2.3 | ||||
| Capecci 2019 (56) | Italy | ET | 48 (29/19) | 68.1 ± 9.8 | 4w | 45 min, 5 times/week | MMSE 26.1 ± 2.0 | 8.9 ± 5.3 |
| GST | 48 (24/24) | 67.0 ± 7.6 | 45 min, 5 times/week | 26.8 ± 2.1 | 8.9 ± 4.3 | |||
| Poier 2019 (57) | Germany | ART | 14 (5/9) | 68.50 ± 8.07 | 10w | 60 min, once/week | ||
| MBE | 15 (12/3) | 68.87 ± 10.96 | 60 min, once/week | |||||
| Zhu 2020 (58) | China | MBE | 19 (7/12) | 68.53 ± 1.90 | 12w | Tai Chi: 40-50 min, 3 times/week regular exercise:40-50 min, twice/week |
4.68 ± 0.43 | |
| DA | 22 (9/13) | 67.77 ± 1.72 | 40-50 min, 5 times/week | 4.00 ± 0.39 | ||||
| Kurt 2018 (59) | Turkey | ABT | 20 (9/11) | 62.41 ± 6.76 | 5w | 60 min, 5 times/week | ||
| DA | 20 (7/13) | 63.61 ± 7.18 | 60 min, 5 times/week | |||||
| Volpe 2014 (60) | Italy | ABT | 17 | 66 ± 8 | 2 m | 60 min, 5 times/week | 7.6 ± 4.63 | |
| DA | 17 | 68 ± 7 | 60 min, 5 times/week | 7.5 ± 5.1 | ||||
| Gandolfi 2017 (61) | Italy | VRGT | 38 (15/23) | 67.45 ± 7.18 | 7w | 50 min, 3 times/week | MMSE 26.77 ± 1.48 | 6.16 ± 3.81 |
| GST | 38 (10/28) | 69.84 ± 9.41 | 50 min, 3 times/week | 28.64 ± 6.96 | 7.47 ± 3.90 | |||
| Kwok 2019 (30) | China | MBE | 71 (34/37) | 63.7 ± 8.2 | 8w | 90 min, once/week | ||
| RT | 67 (39/28) | 63.5 ± 9.3 | 90 min, once/week | |||||
| Pompeu 2012 (62) | Brazil | SST | 12 | 67.4 ± 8.1 | 7w | 60 min, twice/week | ||
| GST | 12 | 67.4 ± 8.1 | 60 min, twice/week | |||||
| Zhou 2024 (63) | China | SST | 14 (6/8) | 62.29 ± 5.37 | 8w | 90 min, twice/week | (MMSE) 29.00 ± 1.57 | 6.41 ± 5.14 |
| DA | 14 (5/9) | 63.14 ± 9.39 | 90 min, twice/week | 28.86 ± 1.79 | 4.09 ± 3.92 | |||
| Wong 2024 (64) | China | GST | 11 (5/6) | 65.86 ± 5.60 | 6w | 40 min, twice/week | (MMSE) 29 ± 1.1 | 5.0 ± 1.6 |
| SST | 11 (4/7) | 67.63 ± 6.92 | 40 min, twice/week | 28 ± 1 | 3.0 ± 1.0 | |||
| Silva 2021 (65) | Brazil | SST | 5 | 64 ± 11.9 | 8w | twice/week | 4.8 ± 1.9 | |
| DA | 5 | 63 ± 12.7 | twice/week | 3.4 ± 2.9 | ||||
| Fernandes 2015 (66) | Portugal | SST | 8 (3/5) | 63.4 ± 9.5 | 6w | 60 min, twice/week | 7.7 ± 7.5 | |
| GST | 7 (1/6) | 62.3 ± 12.9 | 8.8 ± 4.3 | |||||
| Shen 2012 (67) | China | SST | 14 (5/9) | 63.0 ± 8.5 | 4w | 45 min, 3 times/week | 7.1 ± 3.2 | |
| RT | 14 (7/7) | 66.5 ± 8.6 | 60 min, 3 times/week | 5.8 ± 2.2 | ||||
| Bekkers 2020 (68) | Belgium, Israel, UK, Italy, Netherlands | VRGT | 62 (25/37) | 71.06 ± 6.3 | 6w | 45 min, 3 times/week | MMSE 27.76 ± 1.7 | 9.05 ± 5.5 |
| DA | 59 (22/37) | 70.86 ± 6.0 | 45 min, 3 times/week | 28.34 ± 1.5 | 9.55 ± 7.2 | |||
| Chang 2024 (49) | China | MBE | 16 (9/7) | 66.31 ± 6.54 | 12w | 60 min, 3 times/week | 26.75 ± 3.53 | 6.75 ± 5.49 |
| DA | 14 (9/5) | 64.43 ± 7.37 | 30 min, 3 times/week | 27.36 ± 3.07 | 6.57 ± 7.92 | |||
| Li 2024 (69) | China | MBE | 32 (15/17) | 62.7 ± 5.51 | 12 m | 60 min, twice/week | MMSE 28.80 ± 1.19 | 13.60 ± 2.71 |
| DA | 31 (9/22) | 61.5 ± 5.53 | 60 min, twice/week | 29.00 ± 1.27 | 12.9 ± 2.51 | |||
| Cruz 2018 (70) | Spain | ABT | 14 (9/5) | 65.87 ± 7.090 | 11w | 45 min, twice/week | ||
| DA | 15 (8/7) | 66.44 ± 5.726 | 45 min, twice/week | |||||
| Hashimoto 2015 (93) | Japan | ART | 15 (12/3) | 67.9 ± 7.0 | 12w | 60 min, once/week | MMSE 28.2 ± 2.0 | 6.3 ± 4.6 |
| DA | 17 (15/2) | 62.7 ± 14.9 | 60 min, once/week | 28.5 ± 2.0 | 7.8 ± 6.2 | |||
| Bezerra 2022 (71) | Brazil | SST | 21 | 64.6 ± 9.3 | 4w | 60 min, 3 times/week | 21.0 ± 2.5 | |
| GST | 18 | 60.7 ± 6.8 | 60 min, 3 times/week | 23.0 ± 2.5 | ||||
| Pelosin 2018 (68) | Italy | SST | 32 (18/14) | 70.4 ± 4.5 | 5w | 45 min, twice/week | (MMSE) 27.3 ± 2.1 | 10.7 ± 3.9 |
| DA | 32 (17/15) | 72.8 ± 3.1 | 45 min, twice/week | 28.2 ± 1.7 | 9.5 ± 4.2 | |||
| Kashif 2024 (72) | Pakistan | VRGT | 20 (8/12) | 63.20 ± 4.85 | 12w | 60 min, 3 times/week | (MMSE) 26.50 ± 0.68 | 6.65 ± 1.59 |
| SST | 20 (10/10) | 64.85 ± 5.10 | everyday | 26.40 ± 1.14 | 6.40 ± 2.50 | |||
| Landers 2016 (73) | USA | SST | 10 (6/4) | 72.2 ± 4.4 | 4w | 45 min, 3 times/week | (MMSE) 27.6 ± 1.1 | |
| GST | 10 (7/3) | 70.1 ± 9.5 | 45 min, 3 times/week | 29.6 ± 0.5 | ||||
| Wong-Yu 2015 (74) | China | GST | 32 (13/19) | 60.2 ± 9.0 | 8w | 120 min, once/week | 7.3 ± 4.6 | |
| RT | 36 (16/20) | 61.9 ± 8.5 | 120 min, once/week | 5.4 ± 3.6 | ||||
| Capato 2020 (75) | Brazil | SST | 56 (27/29) | 74 ± 8 | 5w | 45 min, twice/week | 26 ± 3 | 5 ± 3 |
| CNT | 48 (19/29) | 73 ± 10 | 24 ± 3 | 8 ± 3 | ||||
| Steib 2017 (76) | Germany | GST | 18 (7/11) | 67.6 ± 8.2 | 8w | 40 min, twice/week | 7.9 ± 4.0 | |
| DA | 19 (4/15) | 62.5 ± 7.9 | 40 min, twice/week | 7.3 ± 4.4 | ||||
| Gaßner 2019 (77) | Germany | GST | 18 (7/11) | 67.6 ± 8.2 | 8w | 30 min, twice/week | 25.8 ± 3.7 | 7.9 ± 4.0 |
| DA | 20 (4/16) | 62.5 ± 7.9 | 30 min, twice/week | 25.6 ± 3.9 | 7.3 ± 4.4 | |||
| Cheng 2016 (63) | China | GST | 12 (4/8) | 66.4 ± 7.8 | 4-6w | 40 min, 3 times/week | (MMSE) 28.1 ± 1.8 | 6.5 ± 2.4 |
| DA | 12 (3/9) | 65.8 ± 11.5 | 40 min, 3 times/week | 27.7 ± 1.3 | 6.1 ± 4.1 | |||
| Cheng 2016 (63) | Brazil | DA | 5 (1/4) | 64.8 ± 11.9 | 12w | twice/week | (MMSE) 24.6 ± 4.0 | 6.6 ± 1.5 |
| RT | 8 (2/6) | 64.1 ± 9.9 | twice/week | 25.8 ± 3.2 | 6.0 ± 2.6 | |||
| Calabrò 2019 (78) | Italy | SST | 25 (9/11) | 70 ± 8 | 8w | 30 min, 5 times/week | MMSE 26 ± 3 | 10 ± 3 |
| DA | 25 (6/14) | 73 ± 8 | 30 min, 5 times/week | 25 ± 3 | 9.3 ± 3 | |||
| Gandolfi 2017 (61) | Italy | VRGT | 38 (15/23) | 67.45 ± 7.18 | 7w | 50 min, 3 times/week | MMSE 26.77 ± 1.48 | 6.16 ± 3.81 |
| GST | 38 (10/28) | 69.84 ± 9.41 | 50 min, 3 times/week | 28.64 ± 6.96 | 7.47 ± 3.90 | |||
| Kim 2022 (79) | South Korea | ET | 22 (16/6) | 68.7 ± 6.9 | 4w | 45 min, 3 times/week | MMSE 28.4 ± 1.3 | 9 ± 2 |
| DA | 22 (15/7) | 67.5 ± 9.3 | 45 min, 3 times/week | 28.1 ± 1.8 | 104.6 ± 53.4 | |||
| Picelli 2015 (80) | Italy | ET | 33 (11/22) | 68.2 ± 9.2 | 4w | 45 min, 3 times/week | 7.5 ± 5.6 | |
| GST | 33 (7/26) | 69.7 ± 7.2 | 45 min, 3 times/week | 8.3 ± 4.1 | ||||
| Picelli 2013 (81) | Italy | ET | 20 (11/9) | 68.50 ± 10.10 | 4w | 45 min, 3 times/week | 6.52 ± 5.30 | |
| DA | 20 (14/6) | 68.80 ± 7.72 | 45 min, 3 times/week | 6.99 ± 6.17 | ||||
| Picelli 2012 (82) | Italy | ET | 17 | 68.3 ± 7.5 | 4w | 40 min, 3 times/week | ||
| CRT | 17 | 68.3 ± 7.5 | 40 min, 3 times/week | |||||
| Li 2022 (83) | China | MBE | 32 (15/17) | 62.7 ± 5.51 | 12 m | 60 min, 3 times/week | 5.91 ± 4.01 | |
| DA | 31 (9/22) | 61.9 ± 5.64 | 60 min, 3 times/week | 3.82 ± 1.87 | ||||
| Zhang 2015 (84) | China | MBE | 20 (7/13) | 66.00 ± 11.80 | 12w | 60 min, twice/week | 27.40 ± 2.33 | 6.80 ± 5.43 |
| DA | 20 (9/11) | 64.35 ± 10.53 | 60 min, twice/week | 26.35 ± 2.39 | 4.85 ± 3.72 | |||
| Xiao 2016 (85) | China | MBE | 45 (14/31) | 68.17 ± 2.27 | 6 m | 45 min, 4 times/week | MMSE 28.08 ± 1.87 | 5.45 ± 3.61 |
| DA | 44 (13/31) | 66.52 ± 2.13 | 30 min, 7 times/week | 27.9 ± 1.49 | 6.15 ± 2.63 | |||
| Cruz 2017 (31) | Spain | ABT | 15 | 66.8 ± 5.267 | 10w | 45 min, twice/week | 6.2 ± 2.541 | |
| DA | 15 | 67.53 ± 9.89 | 45 min, twice/week | 6.7 ± 3.225 | ||||
| Vivas 2011 (86) | Spain | ABT | 6 (3/3) | 65.67 3.67 | 4w | 45 min, twice/week | 27.83 ± 2.23 | 4.17 1.60 |
| DA | 6 (2/4) | 68.33 6.92 | 45 min, twice/week | 27.5 ± 2.17 | 7.83 3.92 | |||
| Hackney 2007 (87) | USA | ART | 9 (3/6) | 72.6 2.20 | 13w | 60 min, twice/week | 6.2 ± 1.5 | |
| RT | 10 (4/6) | 69.6 2.1 | 60 min, twice/week | 3.3 ± 0.5 | ||||
| Ni 2016 (88) | USA | CNT | 10 (6/4) | 74.9 ± 8.3 | 60 min, once/month | 5.9 ± 6.2 | ||
| MBE | ||||||||
| Cherup 2021 (89) | USA | MBE | 15 (5/10) | 69.8 ± 7.3 | 12w | 45 min, twice/week | ||
| GST | 18 (7/11) | 71.4 ± 12.1 | 45 min, twice/week | |||||
| Furnari 2017 (90) | Italy | ET | 19 (8/11) | 71.5 ± 11.7 | 4w | 60 min, 6 times/week | ||
| GST | 19 (9/10) | 77.7 ± 8.3 | 60 min, 6 times/week | |||||
| Carda 2012 (91) | Italy | ET | 15 | 67.87 ± 7.05 | 4w | 30 min, 3 times/week | MMSE 25.94 ± 2.04 | |
| GST | 15 | 66.93 ± 5.13 | 30 min, 3 times/week | 25.52 ± 2.19 |
Basic features of the included.
3.3 Risk of bias assessment
Table 2 and Figure 2 provide detailed information on the ROB assessment for each study. Among the 53 articles, 53 mentioned random allocation, with 16 describing the method of random allocation and 4 describing allocation concealment; 46 reported blinding; 49 reported outcome assessment blinding; and 51 studies showed a low risk of selective reporting; No other biases were identified in any of the articles. In summary,37 articles were judged to have low ROB, 1 article has been judged to have high ROB.
Table 2
| Inclusion of literature | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Agosta 2017 (46) | L | L | L | L | L | L | L |
| Alagumoorthi 2022 (53) | L | U | L | L | L | L | L |
| Bekkers 2020 (68) | L | U | L | L | L | L | L |
| Bezerra 2022 (71) | L | L | L | L | L | L | L |
| Calabrò 2019 (78) | L | U | L | L | L | L | L |
| Capato 2020 (75) | L | L | L | L | L | L | L |
| Capecci 2019 (56) | L | U | L | U | L | L | L |
| Carda 2012 (91) | L | L | L | U | L | L | L |
| Carvalho 2015 (92) | L | U | L | L | L | L | L |
| Chang 2024 (49) | L | L | L | L | L | L | L |
| Cheng 2016 (63) | L | U | L | L | L | L | L |
| Cherup 2021 (89) | L | U | L | L | L | L | L |
| Cruz 2017 (31) | L | U | L | L | L | L | L |
| Cruz 2018 (70) | L | U | U | L | L | L | L |
| Fernandes 2015 (66) | L | U | L | U | L | L | L |
| Ferraz 2018 (51) | L | U | L | L | L | L | L |
| Furnari 2017 (90) | L | U | L | L | L | L | L |
| Gandolfi 2017 (61) | L | U | L | L | L | L | L |
| Gaßner 2019 (77) | L | U | L | L | L | L | L |
| Hackney 2007 (87) | L | U | L | L | L | L | L |
| Kashif 2024 (72) | L | L | L | L | L | L | L |
| Kim 2022 (79) | L | U | L | L | L | L | L |
| Kurt 2018 (59) | L | U | U | L | L | L | L |
| Kwok 2019 (30) | L | L | L | L | L | L | L |
| Landers 2016 (73) | L | U | L | U | L | L | L |
| Li 2022 (83) | L | U | U | L | L | L | L |
| Li 2024 (69) | L | U | L | L | U | L | L |
| Lin 2024 (49) | L | U | L | L | L | L | L |
| Morris 2009 (54) | L | L | L | L | L | L | L |
| Nadeau 2014 (50) | L | L | L | L | L | L | L |
| Ni 2016 (88) | L | L | L | L | L | L | L |
| Pelosin 2018 (68) | L | L | L | L | L | L | L |
| Picelli 2012 (82) | L | L | L | L | L | L | L |
| Picelli 2013 (81) | L | U | L | L | L | L | L |
| Picelli 2015 (80) | L | U | L | L | L | L | L |
| Poier 2019 (57) | L | U | U | L | L | L | L |
| Pompeu 2012 (62) | L | U | L | L | L | L | L |
| San 2020 (52) | L | U | L | L | L | L | L |
| Sarasso 2021 (47) | L | L | L | L | L | L | L |
| Shen 2012 (67) | L | U | L | L | L | L | L |
| Silva 2021 (65) | L | U | U | L | L | L | L |
| Spina 2021 (55) | L | U | L | L | L | L | L |
| Steib 2017 (76) | L | L | L | L | L | L | L |
| Vanbellingen 2017 (45) | L | L | L | L | L | L | L |
| Vivas 2011 (86) | L | U | L | L | L | H | L |
| Volpe 2014 (60) | L | L | L | L | L | L | L |
| Wong 2024 (64) | L | U | L | L | L | L | L |
| Wong-Yu 2015 (74) | L | U | L | L | L | L | L |
| Xiao 2016 (85) | L | U | L | L | L | L | L |
| Yang 2016 (48) | L | U | L | L | L | L | L |
| Zhang 2015 (84) | L | U | L | L | L | L | L |
| Zhou 2024 (63) | L | U | L | L | L | L | L |
| Zhu 2020 (58) | L | U | L | L | L | U | L |
Evaluation results of literature quality risk bias of included articles.
Figure 2
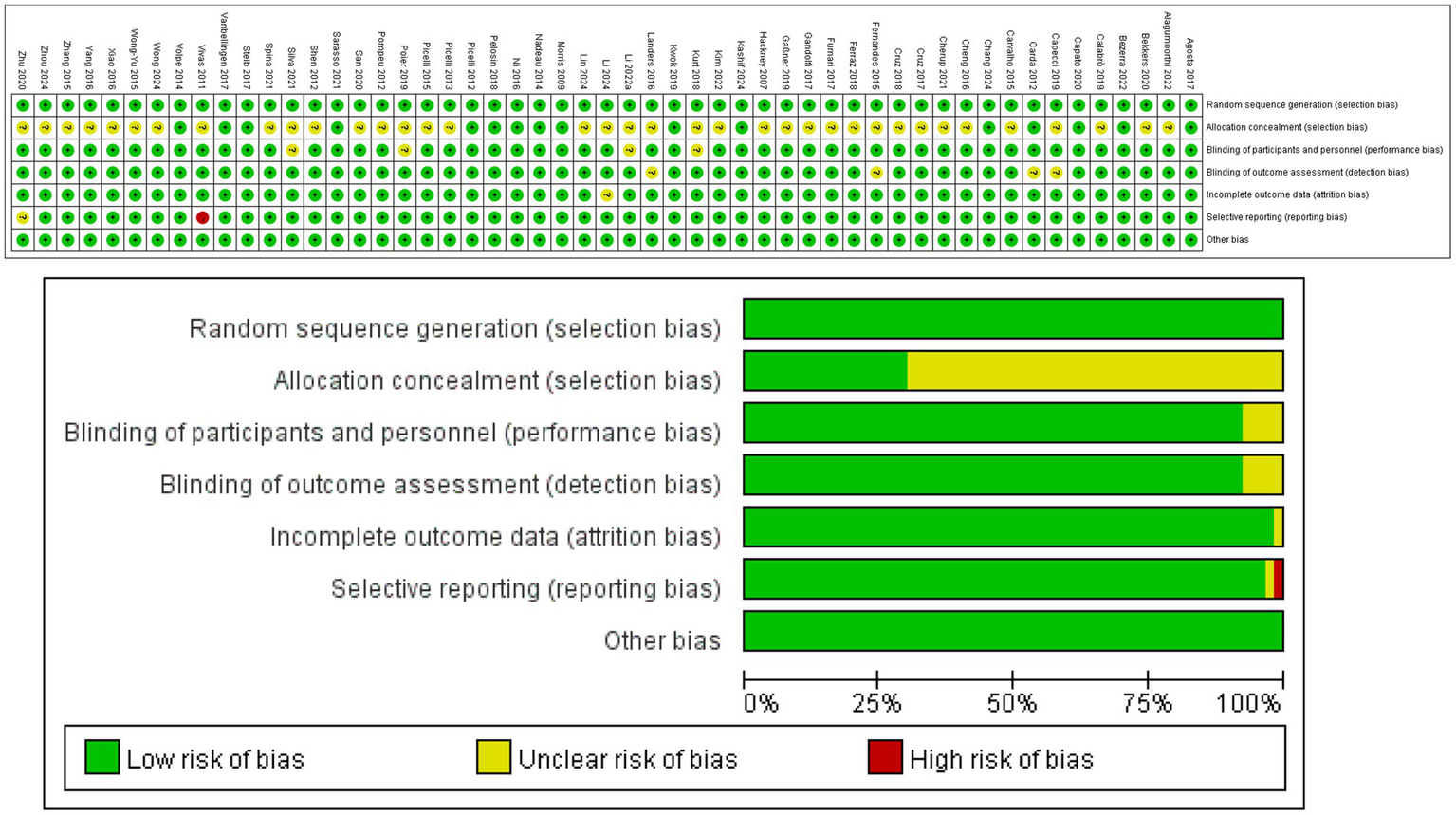
Risk assessment results.
3.4 Direct pairwise meta-analyses (primary outcome)
First, the effects of various intervention measures on balance outcomes were analyzed among 1,977 participants. The forest plot for balance is shown in Figure 3. The study compared the effects of various intervention measures with the control group (primarily RAT and GST) on improving balance function using forest plot analysis. The results showed that exoskeleton training (ET) demonstrated the most significant therapeutic advantage among all intervention measures. Compared with RAT, the mean difference for ET was −2.53 (95% CI: −3.38, −1.67, p < 0.0001), with the largest effect size and high statistical significance, indicating that ET can significantly improve balance function. In contrast, other interventions such as dance (DA) showed moderate improvements. Compared with RAT, the mean difference for DA was −0.88 (95% CI: −1.52, −0.15, p = 0.02), although the effect was significant, its effect size was notably smaller than that of ET. This suggests that the effects of ART may vary significantly across different studies. ART (mean difference −1.03, p < 0.01) had some effect, but the effect size was small, and ART exhibited moderate heterogeneity (I2 = 74%). GST, VRGT, and MBE showed no significant differences compared to the control group (p > 0.05), with MBE exhibiting extremely high heterogeneity (I2 = 96%). This may be related to methodological differences or variations in participant characteristics across studies. In summary, ET is the optimal intervention for improving balance function based on current evidence, with clear and stable efficacy when compared to RAT. DA and ART also showed some improvement effects, but the effect sizes were small or there were issues of heterogeneity. VRGT, MBE, and other interventions did not show significant advantages in this analysis. These findings provide important evidence for selecting balance function improvement strategies in clinical practice and also suggest that future studies need to further explore the sources of effect differences in interventions with high heterogeneity. See Figure 3 for details.
Figure 3
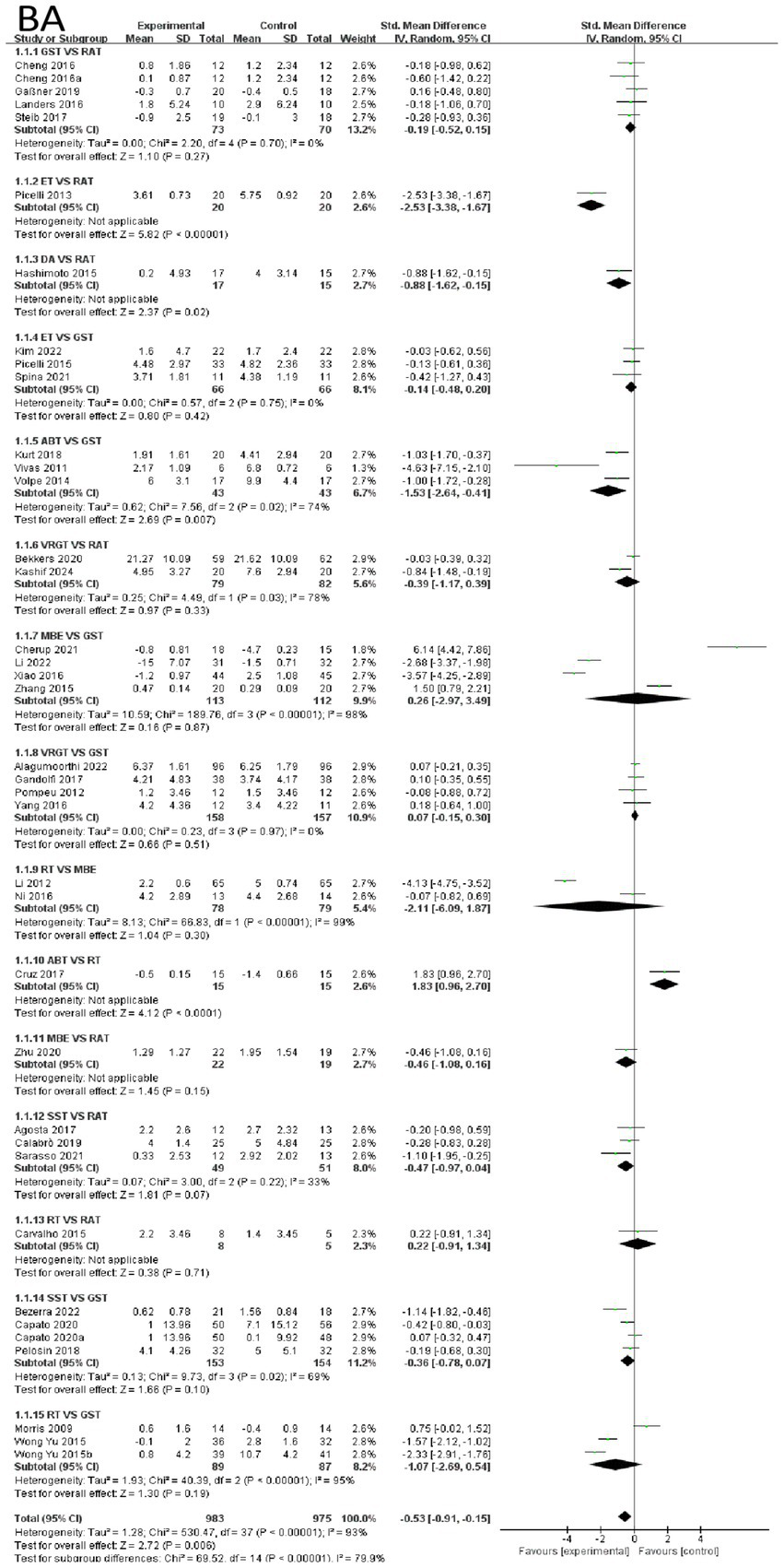
Forest of balance.
The effects of various intervention measures on Emotional Function indicators were examined in a study involving 742 participants. The forest plot for Emotional Functions is shown in Figure 4. The results showed that the overall effect size of the study was 0.51 (95% CI: 0.08–0.94), Z = 2.33 (p = 0.02), indicating that the interventions had a statistically significant effect on improving Emotional Function overall. However, it is important to note that there was high heterogeneity (I2 = 86%, Tau2 = 0.48), suggesting significant differences between studies. In subgroup analyses, RT and MBE showed extremely high effect sizes of 1.02 (95% CI: 0.67–1.38), with significant effects (z = 5.63, p < 0.0001), indicating that RT has a significant advantage over MBE in improving Emotional Function. Additionally, the comparison effect size between VRGT and GST was 0.70 (95% CI: 0.41–0.99, z = 4.72, p < 0.0001), also indicating a significant effect of the intervention group. The effect size for the comparison between GST and RAT was 0.48 (95% CI: −0.03–0.98, Z = 1.85, p = 0.06), which was close to significance but did not reach statistical significance. In contrast, other intervention groups such as ABT and MBE also showed some effects, but these were relatively small, and some groups exhibited high heterogeneity. In summary, RT was most effective in improving emotional function, while the effects of other interventions varied. Emotional Function were not associated with other categorical variables (e.g., age, study region, study year). Compared to shorter interventions, longer exercise interventions (8 weeks or longer) were significantly associated with reduced levels of Emotional Function indicators. See Figure 4 for details.
Figure 4
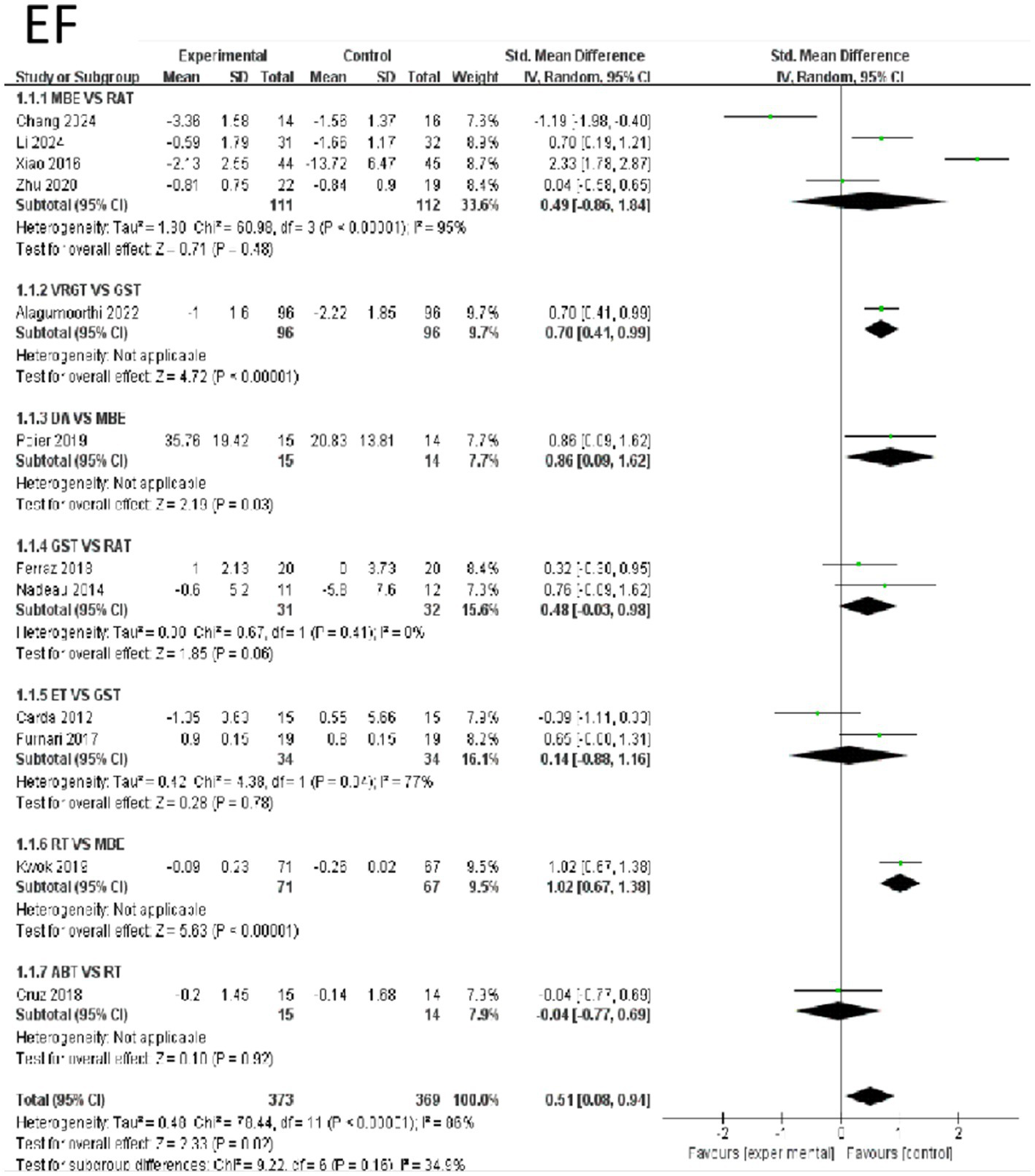
Forest of emotional function.
The effects of 8 intervention measures on cognitive indicators among 698 participants. The overall analysis showed that the pooled standardized mean difference (SMD) for all interventions was not statistically significant (SMD = −0.19, 95% CI [−0.47, 0.09], p = 0.19), but subgroup analysis indicated significant heterogeneity in the effects of different interventions (I2 = 81%, p < 0.0001). Specifically, movement-based interventions (MBE) had the most significant effect on improving cognitive outcomes: the SMD for MBE vs. RAT was −0.79 (95% CI [−1.25, −0.33], p = 0.0009), and MBE vs. GST had an SMD of −1.20 (95% CI [−1.57, −0.83], p < 0.0001). Additionally, ABT vs. RT (SMD = −0.78, 95% CI [−1.52, −0.04], p = 0.04) and DA vs. RAT (SMD = −1.54, 95% CI [−2.82, −0.26], p = 0.02) also showed significant advantages, but the sample sizes were small. Differences between other interventions (e.g., VRGT, SST) and the control group were not statistically significant (p > 0.05). In summary, MBE is the most effective in improving cognitive indicators. Aerobic exercise and coordination training can increase the secretion of brain-derived neurotrophic factor (BDNF), promote hippocampal neurogenesis and synaptic plasticity, thereby enhancing learning, memory, and executive function. The evidence strength is high (large sample size and extremely low p-value), making it a priority intervention strategy. See Figure 5 for details.
Figure 5
![Forest plot showing a meta-analysis of various studies grouped into subcategories on cognitive measures (COG). Each subgroup displays experimental versus control data with standard mean differences, confidence intervals, and weights. Diamonds represent overall effects, with heterogeneity and significance levels noted. The total effect size is shown at the bottom, indicating favor towards the control group with SMD of -0.25 [-0.63, 0.12] and a significant p-value of 0.04.](https://www.frontiersin.org/files/Articles/1666552/xml-images/fneur-16-1666552-g005.webp)
Forest of cognitive.
First, the effects of various intervention measures on quality-of-life indicators were analyzed in a study involving 1,478 participants. The results showed that the overall effect size (mean difference) exhibited high heterogeneity (I2 = 93%, p < 0.0001). The pooled effect size indicated that exercise therapy significantly improved quality of life (SMD = 0.69, 95% CI [0.26, 1.12], p = 0.002), but there were significant differences between different intervention measures (subgroup heterogeneity I2 = 96.6%, p < 0.0001). Subgroup analysis and regression analysis are detailed in Appendix 2. In subgroup analysis, the comparison between RT and MBE showed the largest effect size (SMD = 10.81, 95% CI [9.47, 12.14], p < 0.0001), indicating that RT was significantly superior to MBE. Additionally, ABT demonstrated a significant advantage over GST (SMD = 1.11, 95% CI [0.62, 1.61], p < 0.0001). The comparison between VRGT and GST also showed a moderate effect size (SMD = 0.36, 95% CI [0.03, 0.68], p = 0.03), while the comparison between SST and GST was also significant (SMD = 0.37, 95% CI [0.04, 0.71], p = 0.03). In contrast, the comparative effects of RAT with other interventions were weaker, such as with GST (SMD = 0.43, 95% CI [−0.02, 0.89], p = 0.06) and MBE (SMD = 0.16, 95% CI [−0.46, 0.77], p = 0.61). In summary, RT and ABT are the most effective interventions for improving quality of life, followed by VRGT and SST. The effects of RAT and MBE are relatively limited. These results suggest that interventions based on strength (e.g., RT, ABT) and technology-assisted approaches (e.g., VRGT) may be more suitable for enhancing quality of life. See Figure 6 for details.
Figure 6
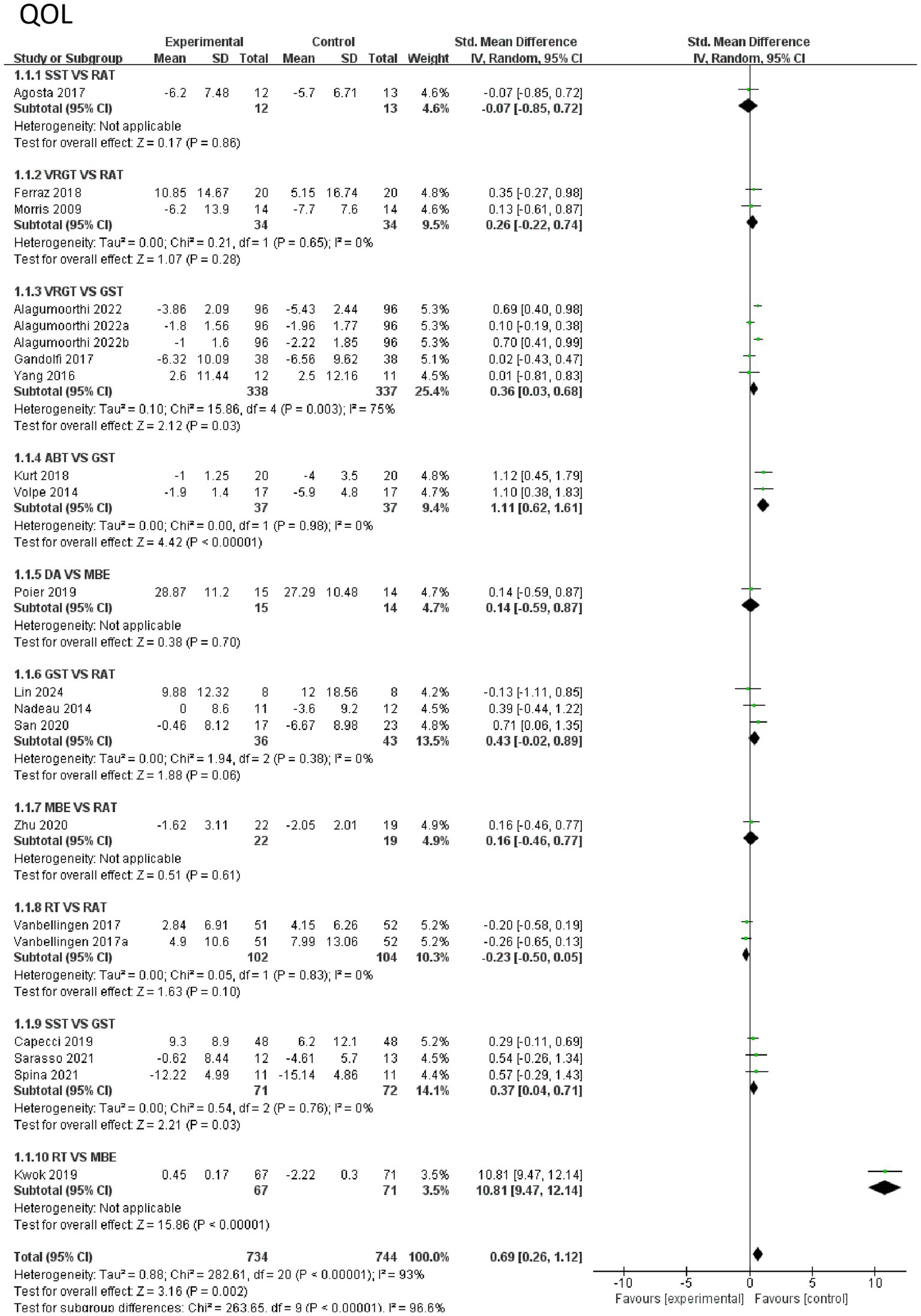
Forest of quality of life.
3.5 Network meta-analysis
3.5.1 Network diagram of included studies
Figure 7 shows the NMA diagram for nine types of exercise therapy. This diagram evaluates the effectiveness of nine types of exercise therapy. The size of the nodes in the chart reflects the sample size of each type of exercise therapy, and the thickness of the lines between the nodes indicates the number of studies comparing these interventions. GST is the most widely used intervention, while DA and ET have been studied less. The network diagram of the outcome measures is shown in detail in Figure 7. The results of consistency test and local inconsistency test are detailed in Appendix 1.
Figure 7
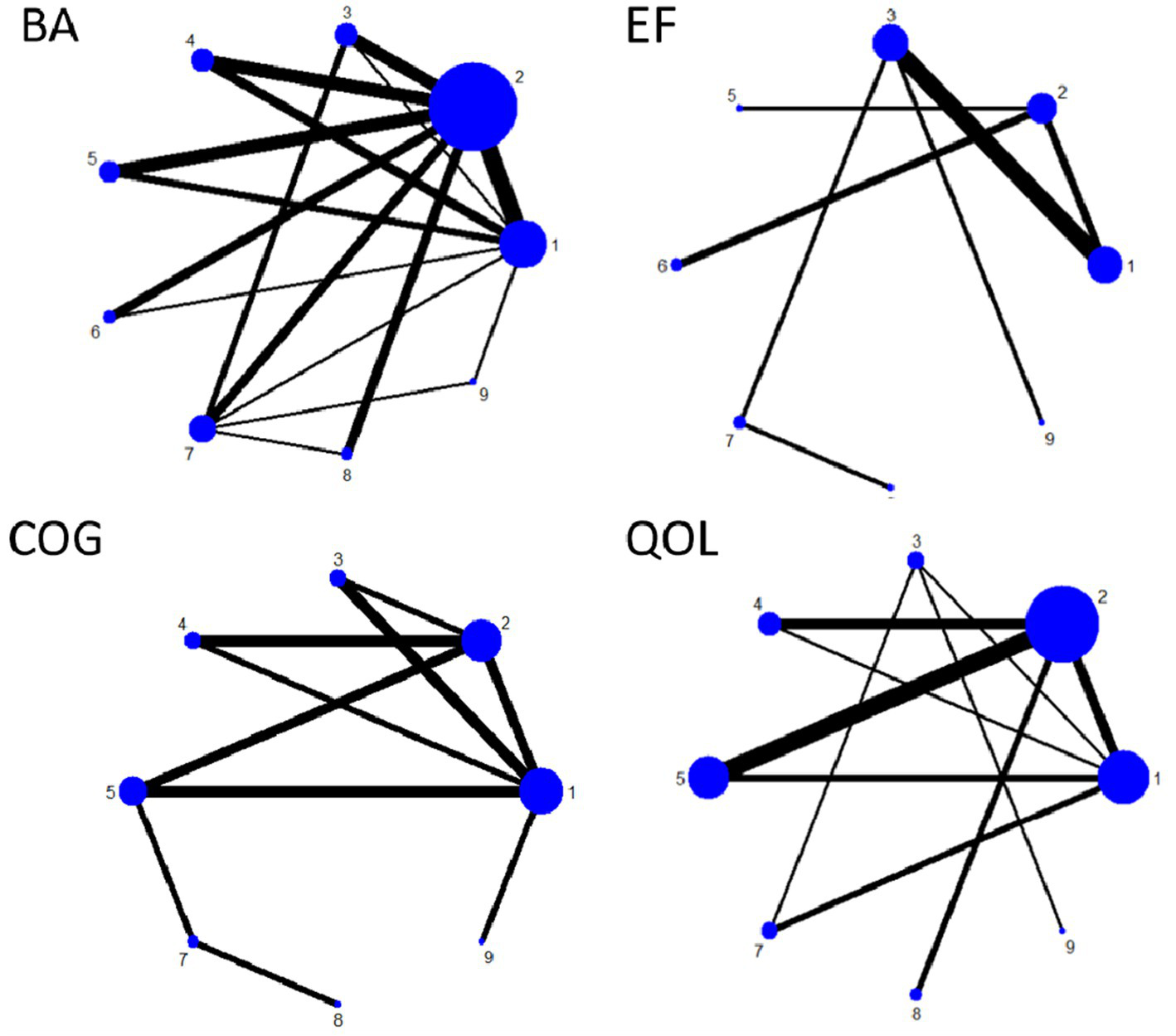
Network plot of outcome indicators.
3.5.2 Ranking of intervention effectiveness of nine exercise therapy
BA indicator: Effective ranking of 14 types of exercise therapy on balance in elderly Parkinson’s patients.
RAT ([SUCRA] = 25.4), GST ([SUCRA] = 41.6), MBE ([SUCRA] = 57.2), SST ([SUCRA] = 55.0), VRGT ([SUCRA] = 42.1), ET ([SUCRA] = 67.4), RT ([SUCRA] = 13.1), ABT ([SUCRA] = 66.1), DA ([SUCRA] = 82.1). See Figure 8 and Tables 3, 4.
Figure 8
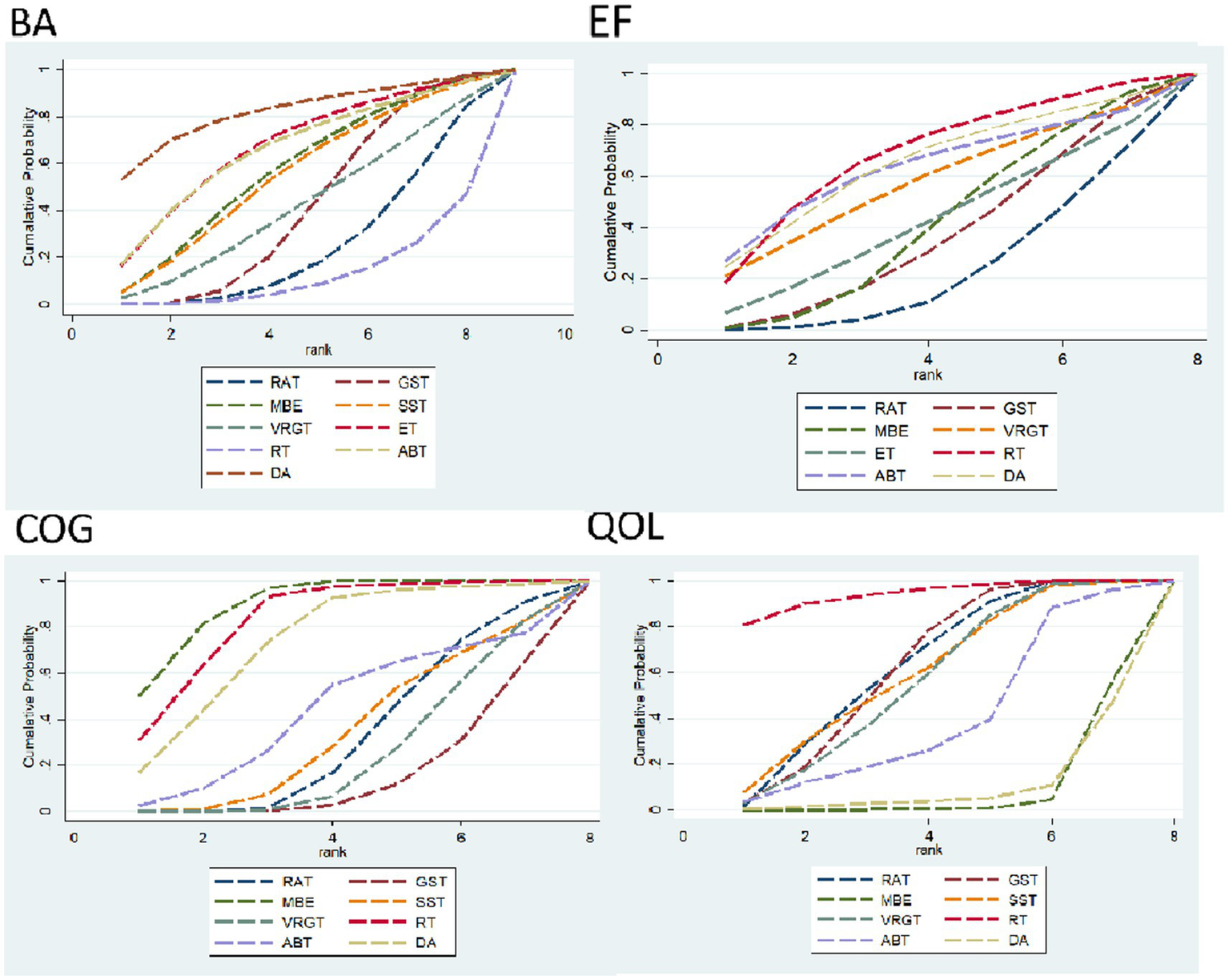
Area under the curve for cumulative ranking probability.
Table 3
| Treatment | Balance | Treatment | Emotional function | Treatment | Cognitive | Treatment | Quality of life | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SUCRA (%) | Rank | SUCRA (%) | Rank | SUCRA (%) | Rank | SUCRA (%) | Rank | ||||
| RAT | 25.4 | 8 | RAT | 23.5 | 8 | RAT | 33.0 | 6 | RAT | 63.8 | 2 |
| GST | 41.6 | 7 | GST | 37.1 | 7 | GST | 15.9 | 8 | GST | 63.5 | 3 |
| MBE | 57.2 | 4 | MBE | 41.9 | 6 | MBE | 89.7 | 1 | MBE | 8,9 | 8 |
| SST | 55.0 | 5 | VRGT | 57.7 | 4 | SST | 34.7 | 5 | SST | 61.3 | 4 |
| VRGT | 42.1 | 6 | ET | 42.8 | 5 | VRGT | 25.1 | 7 | VRGT | 57.3 | 5 |
| ET | 67.4 | 2 | RT | 68.6 | 1 | RT | 83.3 | 2 | RT | 94.3 | 1 |
| RT | 13.1 | 9 | ABT | 63.4 | 3 | ABT | 44.1 | 4 | ABT | 40.7 | 6 |
| ABT | 66.1 | 3 | DA | 64.9 | 2 | DA | 74.3 | 3 | DA | 10.3 | 7 |
| DA | 82.1 | 1 | |||||||||
Ranking the probability of nine exercise therapy.
Table 4
| Balance | ||||||||
| DA | −0.77 (−3.79, 2.24) | −0.78 (−3.82, 2.26) | −1.08 (−3.86, 1.69) | −1.14 (−3.93, 1.65) | −1.47 (−4.31, 1.38) | −1.45 (−4.03, 1.13) | −1.80 (−4.31, 0.72) | −2.20 (−4.75, 0.35) |
| 0.77 (−2.24, 3.79) | ET | −0.01 (−2.43, 2.42) | −0.31 (−2.42, 1.80) | −0.36 (−2.43, 1.71) | −0.69 (−2.82, 1.43) | −0.67 (−2.34, 0.99) | −1.02 (−2.84, 0.79) | −1.43 (−3.52, 0.67) |
| 0.78 (−2.26, 3.82) | 0.01 (−2.42, 2.43) | ABT | −0.30 (−2.46, 1.86) | −0.36 (−2.55, 1.83) | −0.69 (−2.92, 1.55) | −0.67 (−2.44, 1.10) | −1.02 (−3.02, 0.99) | −1.42 (−3.41, 0.56) |
| 1.08 (−1.69, 3.86) | 0.31 (−1.80, 2.42) | 0.30 (−1.86, 2.46) | MBE | −0.06 (−1.88, 1.76) | −0.38 (−2.27, 1.50) | −0.37 (−1.70, 0.97) | −0.71 (−2.25, 0.82) | −1.12 (−2.68, 0.44) |
| 1.14 (−1.65, 3.93) | 0.36 (−1.71, 2.43) | 0.36 (−1.83, 2.55) | 0.06 (−1.76, 1.88) | SST | −0.33 (−2.15, 1.50) | −0.31 (−1.62, 1.00) | −0.66 (−2.03, 0.71) | −1.06 (−2.86, 0.73) |
| 1.47 (−1.38, 4.31) | 0.69 (−1.43, 2.82) | 0.69 (−1.55, 2.92) | 0.38 (−1.50, 2.27) | 0.33 (−1.50, 2.15) | VRGT | 0.02 (−1.36, 1.40) | −0.33 (−1.82, 1.17) | −0.73 (−2.59, 1.12) |
| 1.45 (−1.13, 4.03) | 0.67 (−0.99, 2.34) | 0.67 (−1.10, 2.44) | 0.37 (−0.97, 1.70) | 0.31 (−1.00, 1.62) | −0.02 (−1.40, 1.36) | GST | −0.35 (−1.36, 0.66) | −0.75 (−2.06, 0.56) |
| 1.80 (−0.72, 4.31) | 1.02 (−0.79, 2.84) | 1.02 (−0.99, 3.02) | 0.71 (−0.82, 2.25) | 0.66 (−0.71, 2.03) | 0.33 (−1.17, 1.82) | 0.35 (−0.66, 1.36) | RAT | −0.40 (−1.89, 1.08) |
| 2.20 (−0.35, 4.75) | 1.43 (−0.67, 3.52) | 1.42 (−0.56, 3.41) | 1.12 (−0.44, 2.68) | 1.06 (−0.73, 2.86) | 0.73 (−1.12, 2.59) | 0.75 (−0.56, 2.06) | 0.40 (−1.08, 1.89) | RT |
| Emotional function | ||||||||
| RT | 0.16 (−3.16, 3.49) | 0.04 (−2.35, 2.43) | 0.47 (−3.37, 4.32) | 1.04 (−2.48, 4.55) | 1.02 (−1.28, 3.32) | 1.18 (−1.91, 4.26) | 1.51 (−1.07, 4.09) | |
| −0.16 (−3.49, 3.16) | DA | −0.12 (−4.22, 3.97) | 0.31 (−3.60, 4.22) | 0.88 (−2.70, 4.46) | 0.85 (−1.55, 3.25) | 1.01 (−2.15, 4.18) | 1.35 (−1.33, 4.02) | |
| −0.04 (−2.43, 2.35) | 0.12 (−3.97, 4.22) | ABT | 0.43 (−4.09, 4.96) | 1.00 (−3.25, 5.25) | 0.98 (−2.34, 4.29) | 1.14 (−2.77, 5.04) | 1.47 (−2.05, 4.99) | |
| −0.47 (−4.32, 3.37) | −0.31 (−4.22, 3.60) | −0.43 (−4.96, 4.09) | VRGT | 0.57 (−2.28, 3.41) | 0.54 (−2.54, 3.63) | 0.70 (−1.59, 3.00) | 1.04 (−1.81, 3.88) | |
| −1.04 (−4.55, 2.48) | −0.88 (−4.46, 2.70) | −1.00 (−5.25, 3.25) | −0.57 (−3.41, 2.28) | ET | −0.02 (−2.68, 2.64) | 0.14 (−1.54, 1.82) | 0.47 (−1.91, 2.85) | |
| −1.02 (−3.32, 1.28) | −0.85 (−3.25, 1.55) | −0.98 (−4.29, 2.34) | −0.54 (−3.63, 2.54) | 0.02 (−2.64, 2.68) | MBE | 0.16 (−1.90, 2.22) | 0.49 (−0.69, 1.67) | |
| −1.18 (−4.26, 1.91) | −1.01 (−4.18, 2.15) | −1.14 (−5.04, 2.77) | −0.70 (−3.00, 1.59) | −0.14 (−1.82, 1.54) | −0.16 (−2.22, 1.90) | GST | 0.33 (−1.36, 2.02) | |
| −1.51 (−4.09, 1.07) | −1.35 (−4.02, 1.33) | −1.47 (−4.99, 2.05) | −1.04 (−3.88, 1.81) | −0.47 (−2.85, 1.91) | −0.49 (−1.67, 0.69) | −0.33 (−2.02, 1.36) | RAT | |
| Cognitive | ||||||||
| MBE | −0.19 (−1.38, 0.99) | −0.38 (−1.46, 0.69) | −0.99 (−2.51, 0.53) | −1.21 (−2.04, −0.39) | −1.25 (−1.79, −0.71) | −1.33 (−1.98, −0.69) | −1.42 (−2.01, −0.84) | |
| 0.19 (−0.99, 1.38) | RT | −0.19 (−1.62, 1.24) | −0.80 (−1.75, 0.16) | −1.02 (−2.26, 0.21) | −1.06 (−2.15, 0.03) | −1.14 (−2.13, −0.15) | −1.23 (−2.32, −0.15) | |
| 0.38 (−0.69, 1.46) | 0.19 (−1.24, 1.62) | DA | −0.60 (−2.32, 1.12) | −0.83 (−2.00, 0.34) | −0.87 (−1.80, 0.06) | −0.95 (−1.98, 0.08) | −1.04 (−2.07, −0.01) | |
| 0.99 (−0.53, 2.51) | 0.80 (−0.16, 1.75) | 0.60 (−1.12, 2.32) | ABT | −0.23 (−1.79, 1.34) | −0.26 (−1.71, 1.19) | −0.34 (−1.72, 1.03) | −0.44 (−1.88, 1.01) | |
| 1.21 (0.39, 2.04) | 1.02 (−0.21, 2.26) | 0.83 (−0.34, 2.00) | 0.23 (−1.34, 1.79) | SST | −0.04 (−0.75, 0.68) | −0.12 (−0.86, 0.62) | −0.21 (−0.84, 0.41) | |
| 1.25 (0.71, 1.79) | 1.06 (−0.03, 2.15) | 0.87 (−0.06, 1.80) | 0.26 (−1.19, 1.71) | 0.04 (−0.68, 0.75) | RAT | −0.08 (−0.53, 0.37) | −0.17 (−0.62, 0.28) | |
| 1.33 (0.69, 1.98) | 1.14 (0.15, 2.13) | 0.95 (−0.08, 1.98) | 0.34 (−1.03, 1.72) | 0.12 (−0.62, 0.86) | 0.08 (−0.37, 0.53) | VRGT | −0.09 (−0.54, 0.35) | |
| 1.42 (0.84, 2.01) | 1.23 (0.15, 2.32) | 1.04 (0.01, 2.07) | 0.44 (−1.01, 1.88) | 0.21 (−0.41, 0.84) | 0.17 (−0.28, 0.62) | 0.09 (−0.35, 0.54) | GST | |
| Quality of life | ||||||||
| RT | −1.83 (−4.07, 0.41) | −1.87 (−4.61, 0.87) | −1.92 (−5.02, 1.17) | −2.05 (−4.89, 0.80) | −2.98 (−6.70, 0.74) | −6.29 (−10.85, −1.73) | −6.15 (−8.99, −3.31) | |
| 1.83 (−0.41, 4.07) | RAT | −0.04 (−1.61, 1.53) | −0.10 (−2.24, 2.04) | −0.22 (−1.97, 1.53) | −1.15 (−4.12, 1.82) | −4.46 (−8.99, 0.07) | −4.33 (−7.11, −1.54) | |
| 1.87 (−0.87, 4.61) | 0.04 (−1.53, 1.61) | GST | −0.05 (−1.88, 1.78) | −0.18 (−1.58, 1.23) | −1.11 (−3.63, 1.41) | −4.41 (−9.21, 0.38) | −4.28 (−7.48, −1.08) | |
| 1.92 (−1.17, 5.02) | 0.10 (−2.04, 2.24) | 0.05 (−1.78, 1.88) | SST | −0.12 (−2.35, 2.11) | −1.05 (−4.17, 2.06) | −4.36 (−9.37, 0.64) | −4.23 (−7.74, −0.72) | |
| 2.05 (−0.80, 4.89) | 0.22 (−1.53, 1.97) | 0.18 (−1.23, 1.58) | 0.12 (−2.11, 2.35) | VRGT | −0.93 (−3.82, 1.96) | −4.24 (−9.09, 0.61) | −4.11 (−7.39, −0.82) | |
| 2.98 (−0.74, 6.70) | 1.15 (−1.82, 4.12) | 1.11 (−1.41, 3.63) | 1.05 (−2.06, 4.17) | 0.93 (−1.96, 3.82) | ABT | −3.31 (−8.72, 2.10) | −3.17 (−7.25, 0.90) | |
| 6.29 (1.73, 10.85) | 4.46 (−0.07, 8.99) | 4.41 (−0.38, 9.21) | 4.36 (−0.64, 9.37) | 4.24 (−0.61, 9.09) | 3.31 (−2.10, 8.72) | DA | 0.13 (−3.44, 3.71) | |
| 6.15 (3.31, 8.99) | 4.33 (1.54, 7.11) | 4.28 (1.08, 7.48) | 4.23 (0.72, 7.74) | 4.11 (0.82, 7.39) | 3.17 (−0.90, 7.25) | −0.13 (−3.71, 3.44) | MBE | |
Network meta-analysis matrix of outcome.
EF indicator: The effective ranking of 14 types of exercise therapy for alleviating Emotional Function in elderly Parkinson’s patients, based on SUCRA values from highest to lowest, is as follows: RT ([SUCRA] = 68.6), DA ([SUCRA] = 64.9), ABT ([SUCRA] = 63.4), VRGT ([SUCRA] = 57.7), ET ([SUCRA] = 42.8), MBE ([SUCRA] = 41.9), GST ([SUCRA] = 37.1), RAT ([SUCRA] = 23.5). See Figure 8 and Tables 3, 4.
COG indicators: The effective ranking of eight types of exercise therapy on cognitive function in elderly Parkinson’s disease patients, MBE ([SUCRA] = 89.7), RT ([SUCRA] = 83.3), DA ([SUCRA] = 74.3), ABT ([SUCRA] = 44.1), SST ([SUCRA] = 34.7), RAT ([SUCRA] = 33.0), VRGT ([SUCRA] = 25.1), and GST ([SUCRA] = 15.9). For details, see Figure 8 and Tables 3, 4.
QOL indicators: The effective ranking of eight types of exercise therapy on the quality of life of elderly Parkinson’s patients: RT ([SUCRA] = 94.3), RAT ([SUCRA] = 63.8), GST ([SUCRA] = 63.5), SST ([SUCRA] = 61.3), VRGT ([SUCRA] = 57.3), ABT ([SUCRA] = 40.7), DA ([SUCRA] = 10.3), MBE ([SUCRA] = 8.9), see Figure 8 and Tables 3, 4.
3.6 Ranking of intervention effects for outcome indicators
In terms of balance, the DA group (SUCRA = 82.1%, ranked 1st), ET group (SUCRA = 67.4%, ranked 2nd), and ABT group (SUCRA = 66.1%, ranked 3rd) all showed significant improvements compared to the control group (RAT). Among these, the improvement effect of DA was the most prominent (SMD = 0.2, 95% CI [4.93, 3.14], p = 0.02), followed by ET (SMD = 0.61, 95% CI [0.73, 0.92], p < 0.0001). The efficacy of the MBE group was moderate (SUCRA = 57.2%, ranked 4th), with no significant difference from the control group (SMD = 1.29, 95% CI [1.27, 1.54], p = 0.87). The RT group had the weakest efficacy (SUCRA = 13.1%, rank 9), with a significant but poor effect compared to the control group (SMD = −0.5, 95% CI [0.15, 0.66]). See Table 3 for details.
In terms of emotional function, The RT group (SUCRA = 68.6%, ranked 1st), DA group (SUCRA = 64.9%, ranked 2nd), and ABT group (SUCRA = 63.4%, ranked 3rd) all showed significant improvement compared to the control group (RAT). Among these, the RT group showed the most significant effect (SMD = 1.02, 95% CI [0.67, 1.38], p < 0.0001), followed by the DA group (SMD = 0.85, 95% CI [−1.55, 3.25], p = 0.06) and the ABT group (SMD = 0.98, 95% CI [−2.34, 4.29], p = 0.56). The VRGT group (SUCRA = 57.7%, ranked 4th) showed a moderate effect (SMD = 0.70, 95% CI [−1.59, 3.00], p = 0.55), while the ET group (SUCRA = 42.8%, ranked 5th) and MBE group (SUCRA = 41.9%, ranked 6th) showed weaker but still significant effects (ET: SMD = 0.47, 95% CI [−1.91, 2.85], p = 0.70; MBE: SMD = 0.49, 95% CI [−0.69, 1.67], p = 0.42). The GST group (SUCRA = 37.1%, ranked 7th) and RAT group (SUCRA = 23.5%, ranked 8th) showed the smallest improvement and no significant difference from the control group (GST: SMD = 0.33, 95% CI [−1.36, 2.02], p = 0.70; RAT: SMD = 0.22, 95% CI [−1.53, 1.97], p = 0.81). See Table 3 for details. For a sensitivity analysis of quality of life, please refer to Appendices 3, 4.
In terms of cognition, the MBE group (SUCRA = 89.7%, ranked 1st), RT group (SUCRA = 83.3%, ranked 2nd), and DA group (SUCRA = 74.3%, ranked 3rd) all showed significant improvement compared to the control group (RAT). Among these, MBE showed the most prominent improvement (SMD = 1.29, 95% CI [1.27, 1.54], p < 0.0001), followed by RT (SMD = −0.5, 95% CI [0.15, 0.66], p = 0.006). The efficacy of the DA group was significant (SMD = 1.5, 95% CI [1.5, 3.1], p = 0.02). The efficacy of the ABT group was moderate (SUCRA = 44.1%, ranked 4th) and significantly different from the control group (SMD = −0.78, 95% CI [0.02, 1.34], p = 0.04). The GST group had the weakest efficacy (SUCRA = 15.9%, rank 8), with no significant difference from the control group (SMD = −0.08, 95% CI [−0.86, 0.71]). See Table 3 for details.
In terms of quality of life, the RT group (SUCRA = 94.3%, ranked 1st), ART group (SUCRA = 63.8%, ranked 2nd), and GST group (SUCRA = 63.5%, ranked 3rd) all showed varying degrees of improvement compared to the control group (RAT). Among these, RT showed the most significant improvement (SMD = 10.81, 95% CI [9.47, 12.14], p < 0.0001), followed by ABT (SMD = 1.11, 95% CI [0.62, 1.61], p < 0.0001). The efficacy of the SST group was moderate (SUCRA = 61.3%, ranked 4th) and significantly different from the control group (SMD = 0.37, 95% CI [0.04, 0.71], p = 0.03). The efficacy of the VRGT group was weak (SUCRA = 57.3%, rank 5), with no significant difference from the control group (SMD = 0.26, 95% CI [−0.22, 0.74], p = 0.28). The DA group had the worst efficacy (SUCRA = 10.3%, ranked 8th), with no significant difference from the control group (SMD = 0.14, 95% CI [−0.59, 0.87]). See Table 3 for details.
3.7 Publication bias and small sample size tests
For studies included in the network META analysis, corrected comparison funnel plots were used for small-sample effect estimation and publication bias testing. The included studies were largely symmetrical (see Figure 8 for details). For details on the Egger test, please refer to Appendix 5.
4 Discussion
In terms of balance, DA and ET are the most effective interventions for improving balance in elderly patients with Parkinson’s disease, with dance ranking highest (SUCRA = 82.1%) (SMD = −0.88, 95% CI [−1.52, −0.15]) (Figure 9). This finding is consistent with previous studies (24), as dance significantly improves balance function in Parkinson’s disease patients by combining rhythmic movements and cognitive tasks (25). DA outperforms traditional RAT and GST, possibly because it emphasizes both motor and cognitive engagement, meaning that interventions combining motor and cognitive elements can more effectively activate the basal ganglia-cortical loop (26). However, this study found that the efficacy of GST (SUCRA = 41.6%) was lower than expected, which may be due to the heterogeneity of the included studies (I2 = 74%) or the fact that some studies did not strictly standardize the intensity of gait training (27). In contrast, the efficacy of resistance training (RT) was limited (SUCRA = 13.1%), consistent with previous findings (28), which indicated that isolated strength training has limited effects on improving dynamic balance (SMD = 0.21, 95% CI [−0.05, 0.47]). These findings not only validate the advantages of DA and ET as multimodal interventions but also provide more precise evidence-based guidance for selecting balance rehabilitation programs in clinical practice (29).
Figure 9
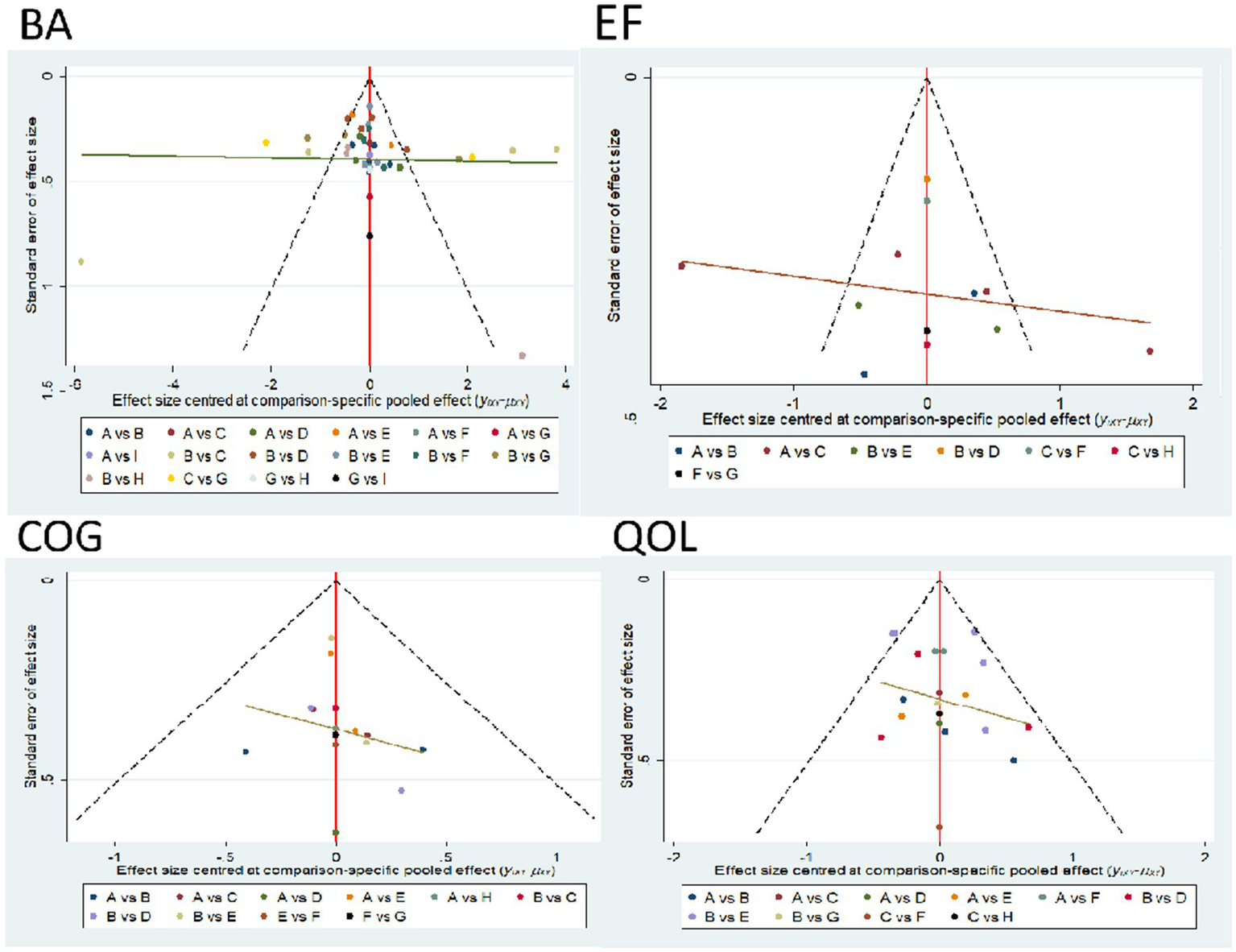
Comparison funnel plot for outcome indicators, A = RAT; B = GST; C = MBE; D = SST; E = VRGT; F = ET; G = RT; H = ABT.
In terms of emotional function, our research indicates that resistance training (RT) is the most effective intervention (SUCRA = 68.6%, SMD = 1.02, 95% CI [0.67, 1.38]), consistent with previous studies suggesting that RT reduces emotional disorders by regulating neurotrophic factors and enhancing psychological resilience (30). The significant benefits of dance (DA, SUCRA = 64.9%) and aquatic training (ABT, SUCRA = 63.4%) further support the role of multimodal exercise in emotional regulation, consistent with findings that rhythmic and aquatic activities alleviate anxiety and depression symptoms in Parkinson’s disease (PD) patients (31). However, the effects of mind–body exercise (MBE, SUCRA = 41.9%) were weaker than expected, contrasting with studies emphasizing the efficacy of MBE in reducing depressive symptoms through mindfulness and stress reduction (32). This discrepancy may stem from differences in intervention protocols, such as shorter training durations or insufficient emphasis on mindfulness components in the trials. Similarly, the limited impact of gait stability training (GST, SUCRA = 37.1%) and routine aerobic training (RAT, SUCRA = 23.5%) suggests that isolated movement-focused interventions may lack the cognitive-emotional engagement required to achieve significant emotional improvements. These findings highlight the superiority of RT and integrative approaches (e.g., DA, ABT) in PD emotional regulation, while emphasizing the need to develop standardized MBE protocols to maximize therapeutic potential (33). Future research should explore dose–response relationships and cultural adaptability to optimize emotional treatment outcomes.
In terms of cognitive function, our study shows that MBE is the most effective intervention (SUCRA = 89.7%) (SMD = −1.42, 95% CI [−2.01, −0.84]), consistent with previous studies. Mind–body exercises (such as tai chi and yoga) significantly improve executive function in Parkinson’s patients through dual-task training and neuroplasticity mechanisms (34). The cognitive improvement effects of RT (SUCRA = 83.3%) (SMD = −1.23, 95% CI [−2.32, −0.15]) are also consistent with previous research findings, which confirmed that resistance training enhances hippocampal function by upregulating BDNF (35). However, our study found that traditional aerobic training (RAT, SUCRA = 33.0%) had limited effects, which partially contradicts previous findings suggesting that long-term aerobic exercise has cognitive benefits for Parkinson’s patients (36). This discrepancy may stem from the lack of cognitive engagement components in the RAT interventions included in this study, suggesting that aerobic exercise alone may be insufficient to improve Parkinson’s-specific cognitive impairments (37). Overall, our results support the superiority of exercise-cognition integration interventions, aligning with current recommendations in Parkinson’s disease rehabilitation guidelines (38). However, the differences in effect sizes across specific exercise modalities require further validation through standardized intervention protocols.
In terms of quality of life, our study showed that RT was the most effective intervention (SUCRA = 94.3%, SMD = 1.83, 95% CI [0.41, 4.07]), which is highly consistent with previous research findings (39), confirming that resistance training significantly improves QOL by enhancing muscle strength and functional independence (40). The moderate effects of RAT (SUCRA = 63.8%) and GST (SUCRA = 63.5%) align with previous conclusions, supporting the baseline improvement effects of aerobic and gait training on quality of life (41, 42). However, we found that MBE (SUCRA = 8.9%) had a significantly lower effect size, inconsistent with previously reported data. This discrepancy may stem from the shorter duration of the MBE program in this study and the absence of home-based training components (43). Similarly, the low effect size of DA (SUCRA = 10.3%) contradicts previous studies, potentially reflecting differences in cultural adaptability and training intensity of dance interventions (44). These results emphasize that function-oriented resistance training may offer greater clinical advantages than traditional mind–body exercises or art therapy for improving quality of life in Parkinson’s disease, but treatment protocols should be optimized based on individual patient characteristics.
5 Limitations
This study has several limitations that should be acknowledged. The high heterogeneity observed in this study primarily stems from systemic differences across multiple levels: at the design level, the 55 RCTs exhibited significant variations in exercise type, intervention dosage, frequency, duration, and control group settings. For example, the design of dance and mind–body exercise programs may yield differing outcomes due to variations in cultural context or training standards. Additionally, the control groups included multiple cross-comparisons, leading to natural dispersion of effect sizes. In terms of patient characteristics, the study population ranged in age from 25 to 80 years, with disease duration varying from 1 to 13 years. Significant differences in exercise tolerance, disease stage, and cognitive reserve further amplified variability in intervention responses. However, this study ensured the robustness of the results by conducting a rigorous network meta-analysis (NMA) to integrate direct and indirect evidence, using a random-effects model to correct for heterogeneity, and providing relative effectiveness rankings of interventions via SUCRA rankings. Heterogeneity primarily reflects the true diversity of clinical practice and patient populations rather than flaws in the analytical methods. We assessed the risk of bias and data quality of these studies and found their impact on overall results to be limited. Second, due to limited literature, the lack of direct comparisons between certain interventions may weaken the robustness of network meta-analysis results. Third, restricting the analysis scope to Chinese and English literature may introduce language bias, potentially overlooking relevant studies in other languages, thereby limiting the generalizability of the findings. Fourth, The notably large standardized mean difference (SMD) observed in this study is uncommon in behavioral and clinical research, which may reflect outlier effects driven by specific sample characteristics or intervention dynamics (e.g., RT’s more direct targeting of quality-of-life factors compared to MBE). Generalizing these findings to other populations should be approached with caution until replication studies confirm the effect size. These factors highlight the need for future studies to adopt more standardized research protocols and expand the scope of literature inclusion. It is also necessary to explore whether RT can continue to bring about such significant improvements in quality of life in the long term compared to MBE.
6 Conclusion
This study demonstrates that exercise therapy can significantly improve limb balance, emotional function, cognitive function, and quality of life (QOL) in patients with Parkinson’s disease (PD). Among them, DA and ET have a significant effect on improving balance function., RT and DA perform optimally in regulating emotional function, MBE has the greatest advantage in enhancing cognitive function, and RT has the most significant effect on improving quality of life. These findings provide evidence-based guidance for rehabilitation practices in Parkinson’s disease, aiding in the development of individualized exercise programs tailored to different symptom domains and offering patients safe and effective non-pharmacological intervention options. By clarifying the relative efficacy of various exercise therapies, this study provides important reference for clinical decision-making, promoting the optimization of Parkinson’s disease management, thereby improving patient outcomes and reducing the disease burden. Future research should further optimize intervention protocols to maximize these benefits.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JM: Data curation, Writing – original draft, Writing – review & editing. YX: Formal analysis, Methodology, Writing – original draft. YH: Methodology, Software, Writing – review & editing. XY: Conceptualization, Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1666552/full#supplementary-material
Abbreviations
PD, Parkinson’s disease; RAT, Regular Aerobic Training; GST, Gait Stability Training; SST, Sensory stimulation Training; VRGT, Games Training; ET, Exoskeletal Training; RT, Resistance training; ABT, Aqua-based training; DA, Dance; BA, Balance; COG, Cognitive; EF, Emotional Function; QOL, Quality of life; NMA, Network meta-analysis; RCTs, Randomized controlled trials; SUCRA, Surface Under the Cumulative Ranking Curve Analysis.
References
1.
Kalia LV Lang AE . Parkinson's disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
2.
Beitz JM . Parkinson's disease: a review. Front Biosci. (2014) 6:65–74. doi: 10.2741/s415
3.
Samii A Nutt JG Ransom BR . Parkinson's disease. Lancet. (2004) 363:1783–93. doi: 10.1016/S0140-6736(04)16305-8
4.
Funayama M Furukawa Y . Brain and nerve. Shinkei kenkyu no shinpo. (2025) 77:448–54. doi: 10.11477/mf.188160960770050448
5.
Marsili L Bologna M Chen LY Espay AJ . Treatment of motor symptoms of Parkinson's disease. Neurol Clin. (2025) 43:341–63. doi: 10.1016/j.ncl.2024.12.010
6.
Goldman JG Sieg E . Cognitive impairment and dementia in Parkinson disease. Clin Geriatr Med. (2020) 36:365–77. doi: 10.1016/j.cger.2020.01.001
7.
Niccolini F Diamantopoulos K Kiosses S Politis M . Be vigilant for dementia in Parkinson’s disease. Practitioner. (2017) 261:11–5. PMID:
8.
Zhao N Yang Y Zhang L Zhang Q Balbuena L Ungvari GS et al . Quality of life in Parkinson's disease: a systematic review and meta-analysis of comparative studies. CNS Neurosci Ther. (2021) 27:270–9. doi: 10.1111/cns.13549
9.
Cabreira V Massano J . Doença de Parkinson: Revisão Clínica e Atualização [Parkinson's Disease: Clinical Review and Update]. Acta Med Portuguesa. (2019) 32:661–70. doi: 10.20344/amp.11978
10.
Lozano AM Mahant N . Deep brain stimulation surgery for Parkinson's disease: mechanisms and consequences. Parkinsonism Relat Disord. (2004) 10:S49–57. doi: 10.1016/j.parkreldis.2003.12.006
11.
Church FC . Treatment options for motor and non-motor symptoms of Parkinson's disease. Biomolecules. (2021) 11:612. doi: 10.3390/biom11040612
12.
LeWitt PA . Levodopa therapy for Parkinson's disease: pharmacokinetics and pharmacodynamics. Movement Disord. (2015) 30:64–72. doi: 10.1002/mds.26082
13.
Colosimo C Merello M Hughes AJ Sieradzan K Lees AJ . Motor response to acute dopaminergic challenge with apomorphine and levodopa in Parkinson's disease: implications for the pathogenesis of the on-off phenomenon. J Neurol Neurosurg Psychiatry. (1996) 60:634–7. doi: 10.1136/jnnp.60.6.634
14.
Beudel M Brown P . Adaptive deep brain stimulation in Parkinson's disease. Parkinsonism Relat Disord. (2016) 22:S123–6. doi: 10.1016/j.parkreldis.2015.09.028
15.
Lim EC Quek AM Seet RC . Deep brain stimulation in Parkinson's disease: looking back, looking forward. Ann Acad Med Singap. (2024) 53:468–70. doi: 10.47102/annals-acadmedsg.2024260
16.
Xu X Fu Z Le W . Exercise and Parkinson's disease. Int Rev Neurobiol. (2019) 147:45–74. doi: 10.1016/bs.irn.2019.06.003
17.
Schootemeijer S van der Kolk NM Bloem BR de Vries NM . Current perspectives on aerobic exercise in people with Parkinson's disease. Neurotherapeutics. (2020) 17:1418–33. doi: 10.1007/s13311-020-00904-8
18.
Riboldi GM Frattini E Monfrini E Frucht SJ Di Fonzo A . A practical approach to early-onset parkinsonism. J Parkinsons Dis. (2022) 12:1–26. doi: 10.3233/JPD-212815
19.
Kim Y Lai B Mehta T Thirumalai M Padalabalanarayanan S Rimmer JH et al . Exercise training guidelines for multiple sclerosis, stroke, and Parkinson disease: rapid review and synthesis. Am J Phys Med Rehabil. (2019) 98:613–21. doi: 10.1097/PHM.0000000000001174
20.
Petzinger GM Fisher BE McEwen S Beeler JA Walsh JP Jakowec MW . Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. (2013) 12:716–26. doi: 10.1016/S1474-4422(13)70123-6
21.
Stuckenschneider T Askew CD Menêses AL Baake R Weber J Schneider S . The effect of different exercise modes on domain-specific cognitive function in patients suffering from Parkinson's disease: a systematic review of randomized controlled trials. J Parkinsons Dis. (2019) 9:73–95. doi: 10.3233/JPD-181484
22.
Gaßner H Trutt E Seifferth S Friedrich J Zucker D Salhani Z et al . Treadmill training and physiotherapy similarly improve dual task gait performance: a randomized-controlled trial in Parkinson's disease. J Neural Transmiss. (2022) 129:1189–200. doi: 10.1007/s00702-022-02514-4
23.
Lee YH . An overview of meta-analysis for clinicians. Korean J Intern Med. (2018) 33:277–83. doi: 10.3904/kjim.2016.195
24.
Michels K Dubaz O Hornthal E Bega D . "dance therapy" as a psychotherapeutic movement intervention in Parkinson's disease. Complement Ther Med. (2018) 40:248–52. doi: 10.1016/j.ctim.2018.07.005
25.
Yang Y Wang G Zhang S Wang H Zhou W Ren F et al . Efficacy and evaluation of therapeutic exercises on adults with Parkinson's disease: a systematic review and network meta-analysis. BMC Geriatr. (2022) 22:813. doi: 10.1186/s12877-022-03510-9
26.
Duarte JDS Alcantara WA Brito JS Barbosa LCS Machado IPR Furtado VKT et al . Physical activity based on dance movements as complementary therapy for Parkinson's disease: effects on movement, executive functions, depressive symptoms, and quality of life. PLoS One. (2023) 18:e0281204. doi: 10.1371/journal.pone.0281204
27.
Gulcan K Guclu-Gunduz A Yasar E Ar U Sucullu Karadag Y Saygili F . The effects of augmented and virtual reality gait training on balance and gait in patients with Parkinson's disease. Acta Neurol Belg. (2023) 123:1917–25. doi: 10.1007/s13760-022-02147-0
28.
Corcos DM Robichaud JA David FJ Leurgans SE Vaillancourt DE Poon C et al . A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Movement Disord. (2013) 28:1230–40. doi: 10.1002/mds.25380
29.
Feng H Li C Liu J Wang L Ma J Li G et al . Virtual reality rehabilitation versus conventional physical therapy for improving balance and gait in Parkinson's disease patients: a randomized controlled trial. Med Sci Monit. (2019) 25:4186–92. doi: 10.12659/MSM.916455
30.
Kwok JYY Kwan JCY Auyeung M Mok VCT Lau CKY Choi KC et al . Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. (2019) 76:755–63. doi: 10.1001/jamaneurol.2019.0534
31.
Pérez S . Effectiveness of aquatic therapy for the control of pain and increased functionality in people with Parkinson's disease: a randomized clinical trial. Eur J Phys Rehabil Med. (2017) 53:825–32. doi: 10.23736/S1973-9087.17.04647-0
32.
Mustafaoglu R Ahmed I Pang MYC . Which type of mind-body exercise is most effective in improving functional performance and quality of life in patients with Parkinson's disease? A systematic review with network meta-analysis. Acta Neurol Belg. (2022) 122:1433–46. doi: 10.1007/s13760-022-02070-4
33.
Yang X Wang Z . Effectiveness of progressive resistance training in Parkinson's disease: a systematic review and meta-analysis. Eur Neurol. (2023) 86:25–33. doi: 10.1159/000527029
34.
Jin X Wang L Liu S Zhu L Loprinzi PD Fan X . The impact of mind-body exercises on motor function, depressive symptoms, and quality of life in Parkinson's disease: a systematic review and meta-analysis. Int J Environ Res Public Health. (2019) 17:31. doi: 10.3390/ijerph17010031
35.
Hortobágyi T Granacher U Fernandez-Del-Olmo M Howatson G Manca A Deriu F et al . Functional relevance of resistance training-induced neuroplasticity in health and disease. Neurosci Biobehav Rev. (2021) 122:79–91. doi: 10.1016/j.neubiorev.2020.12.019
36.
Johansson ME Cameron IGM Van der Kolk NM de Vries NM Klimars E Toni I et al . Aerobic exercise alters brain function and structure in Parkinson's disease: a randomized controlled trial. Ann Neurol. (2022) 91:203–16. doi: 10.1002/ana.26291
37.
Alberts JL Rosenfeldt AB . The universal prescription for Parkinson's disease: exercise. J Parkinsons Dis. (2020) 10:S21–7. doi: 10.3233/JPD-202100
38.
Abbruzzese G Marchese R Avanzino L Pelosin E . Rehabilitation for Parkinson's disease: current outlook and future challenges. Parkinsonism Relat Disord. (2016) 22:S60–4. doi: 10.1016/j.parkreldis.2015.09.005
39.
Santos SM da Silva RA Terra MB Almeida IA de Melo LB Ferraz HB . Balance versus resistance training on postural control in patients with Parkinson's disease: a randomized controlled trial. Eur J Phys Rehabil Med. (2017) 53:173–83. doi: 10.23736/S1973-9087.16.04313-6
40.
Dibble LE Hale TF Marcus RL Gerber JP LaStayo PC . High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson's disease: a preliminary study. Parkinsonism Relat Disord. (2009) 15:752–7. doi: 10.1016/j.parkreldis.2009.04.009
41.
van der Kolk NM de Vries NM Kessels RPC Joosten H Zwinderman AH Post B et al . Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson's disease: a double-blind, randomised controlled trial. Lancet Neurol. (2019) 18:998–1008. doi: 10.1016/S1474-4422(19)30285-6
42.
Debû B De Oliveira Godeiro C Lino JC Moro E . Managing gait, balance, and posture in Parkinson's disease. Curr Neurol Neurosci Rep. (2018) 18:23. doi: 10.1007/s11910-018-0828-4
43.
Deuel LM Seeberger LC . Complementary therapies in Parkinson disease: a review of acupuncture, tai chi, qi gong, yoga, and cannabis. Neurotherapeutics. (2020) 17:1434–55. doi: 10.1007/s13311-020-00900-y
44.
Kalyani HHN Sullivan KA Moyle G Brauer S Jeffrey ER Kerr GK . Impacts of dance on cognition, psychological symptoms and quality of life in Parkinson's disease. NeuroRehabilitation. (2019) 45:273–83. doi: 10.3233/NRE-192788
45.
Vanbellingen T Nyffeler T Nigg J Janssens J Hoppe J Nef T et al . Home based training for dexterity in Parkinson's disease: a randomized controlled trial. Parkinsonism Relat Disord. (2017) 41:92–8. doi: 10.1016/j.parkreldis.2017.05.021
46.
Agosta F Gatti R Sarasso E Volonté MA Canu E Meani A et al . Brain plasticity in Parkinson's disease with freezing of gait induced by action observation training. J Neurol. (2017) 264:88–101. doi: 10.1007/s00415-016-8309-7
47.
Sarasso E Agosta F Piramide N Gardoni A Canu E Leocadi M et al . Action observation and motor imagery improve dual task in Parkinson's disease: a clinical/fMRI study. Movement Disord. (2021) 36:2569–82. doi: 10.1002/mds.28717
48.
Yang WC Wang HK Wu RM Lo CS Lin KH . Home-based virtual reality balance training and conventional balance training in Parkinson's disease: a randomized controlled trial. Journal of the Formosan medical association =. Taiwan yi zhi. (2016) 115:734–43. doi: 10.1016/j.jfma.2015.07.012
49.
Lin YP Lin II Chiou WD Chang HC Chen RS Lu CS et al . Optimizing rehabilitation strategies in Parkinson's disease: a comparison of dual cognitive-walking treadmill training and single treadmill training. Sci Rep. (2024) 14:25210. doi: 10.1038/s41598-024-75422-0
50.
Nadeau A Pourcher E Corbeil P . Effects of 24 wk of treadmill training on gait performance in Parkinson's disease. Med Sci Sports Exerc. (2014) 46:645–55. doi: 10.1249/MSS.0000000000000144
51.
Ferraz DD Trippo KV Duarte GP Neto MG Bernardes Santos KO Filho JO . The effects of functional training, bicycle exercise, and exergaming on walking capacity of elderly patients with Parkinson disease: a pilot randomized controlled single-blinded trial. Arch Phys Med Rehabil. (2018) 99:826–33. doi: 10.1016/j.apmr.2017.12.014
52.
Valenzuela SM Moscardó LD López-Pascual J Serra-Añó P Tomás JM . Effects of dual-task group training on gait, cognitive executive function, and quality of life in people with Parkinson disease: results of randomized controlled DUALGAIT trial. Arch Phys Med Rehabil. (2020) 101:1849–1856.e1. doi: 10.1016/j.apmr.2020.07.008
53.
Alagumoorthi G Beulah JD Thirunavukarasu S . Effectiveness of Wii sports-based strategy training in reducing risk of falling, falls and improving quality of life in adults with idiopathic Parkinson's disease—a randomized comparative trial. Clin Rehabil. (2022) 36:1097–109. doi: 10.1177/02692155221089030
54.
Morris ME Iansek R Kirkwood B . A randomized controlled trial of movement strategies compared with exercise for people with Parkinson's disease. Movement Disord. (2009) 24:64–71. doi: 10.1002/mds.22295
55.
Spina S Facciorusso S Cinone N Armiento R Picelli A Avvantaggiato C et al . Effectiveness of robotic balance training on postural instability in patients with mild Parkinson's disease: a pilot, single blind, randomized controlled trial. J Rehabil Med. (2021) 53:jrm00154. doi: 10.2340/16501977-2793
56.
Capecci M Pournajaf S Galafate D Sale P Le Pera D Goffredo M et al . Clinical effects of robot-assisted gait training and treadmill training for Parkinson's disease. A randomized controlled trial. Ann Phys Rehabil Med. (2019) 62:303–12. doi: 10.1016/j.rehab.2019.06.016
57.
Poier D Rodrigues Recchia D Ostermann T Büssing A . A randomized controlled trial to investigate the impact of tango Argentino versus tai chi on quality of life in patients with Parkinson disease: a short report. Eine randomisierte kontrollierte Studie zur Untersuchung des Einflusses von tango Argentino versus tai-chi auf die Lebensqualität von Patienten mit Parkinson-Erkrankung: Ein kurzer Bericht. Complement Med Res. (2019) 26:398–403. doi: 10.1159/000500070
58.
Zhu M Zhang Y Pan J Fu C Wang Y . Effect of simplified tai chi exercise on relieving symptoms of patients with mild to moderate Parkinson's disease. J Sports Med Phys Fitness. (2020) 60:282–8. doi: 10.23736/S0022-4707.19.10104-1
59.
Kurt EE Büyükturan B Büyükturan Ö Erdem HR Tuncay F . Effects of Ai chi on balance, quality of life, functional mobility, and motor impairment in patients with Parkinson's disease<sup/>. Disabil Rehabil. (2018) 40:791–7. doi: 10.1080/09638288.2016.1276972
60.
Volpe D Giantin MG Maestri R Frazzitta G . Comparing the effects of hydrotherapy and land-based therapy on balance in patients with Parkinson's disease: a randomized controlled pilot study. Clin Rehabil. (2014) 28:1210–7. doi: 10.1177/0269215514536060
61.
Gandolfi M Geroin C Dimitrova E Boldrini P Waldner A Bonadiman S et al . Virtual reality Telerehabilitation for postural instability in Parkinson's disease: a multicenter, single-blind, randomized, Controlled Trial. BioMed Res Int. (2017) 2017:7962826. doi: 10.1155/2017/7962826
62.
Pompeu JE Mendes FA Silva KG Lobo AM Oliveira T Zomignani A et al . Effect of Nintendo Wii™-based motor and cognitive training on activities of daily living in patients with Parkinson's disease: a randomised clinical trial. Physiotherapy. (2012) 98:196–204. doi: 10.1016/j.physio.2012.06.004
63.
Zhou JH Wang RY Liu YT Cheng SJ Liu HH Yang YR . Improving executive function and dual-task cost in Parkinson disease: a randomized controlled trial. J Neurol Phys Therapy. (2024) 48:188–97. doi: 10.1097/NPT.0000000000000489
64.
Wong PL Hung CW Yang YR Yeh NC Cheng SJ Liao YY et al . Effects of motor and cognitive complex training on obstacle walking and brain activity in people with Parkinson's disease: a randomized controlled trial. Eur J Phys Rehabil Med. (2024) 60:611–20. doi: 10.23736/S1973-9087.24.08261-3
65.
Silva RDN Afonso SV Felipe LR Oliveira RA Patrizzi Martins LJ Sande P et al . Dual-task intervention based on trail making test: effects on Parkinson's disease. J Bodyw Mov Ther. (2021) 27:628–33. doi: 10.1016/j.jbmt.2021.04.013
66.
Fernandes  Rocha N Santos R Tavares JM . Effects of dual-task training on balance and executive functions in Parkinson's disease: a pilot study. Somatosens Mot Res. (2015) 32:122–7. doi: 10.3109/08990220.2014.1002605
67.
Shen X Mak MK . Repetitive step training with preparatory signals improves stability limits in patients with Parkinson's disease. J Rehabil Med. (2012) 44:944–9. doi: 10.2340/16501977-1056
68.
Bekkers EMJ Mirelman A Alcock L Rochester L Nieuwhof F Bloem BR et al . Do patients with Parkinson's disease with freezing of gait respond differently than those without to treadmill training augmented by virtual reality?Neurorehabil Neural Repair. (2020) 34:440–9. doi: 10.1177/1545968320912756
69.
Li G Huang P Cui S He Y Jiang Q Li B et al . Tai chi improves non-motor symptoms of Parkinson's disease: one-year randomized controlled study with the investigation of mechanisms. Parkinsonism Relat Disord. (2024) 120:105978. doi: 10.1016/j.parkreldis.2023.105978
70.
Pérez-de la Cruz S . A bicentric controlled study on the effects of aquatic Ai chi in Parkinson disease. Complement Ther Med. (2018) 36:147–53. doi: 10.1016/j.ctim.2017.12.001
71.
Bezerra PT Santiago LM Silva IA Souza AA Pegado CL Damascena CM et al . Action observation and motor imagery have no effect on balance and freezing of gait in Parkinson's disease: a randomized controlled trial. Eur J Phys Rehabil Med. (2022) 58:715–22. doi: 10.23736/S1973-9087.22.07313-0
72.
Kashif M Albalwi AA Zulfiqar A Bashir K Alharbi AA Zaidi S . Effects of virtual reality versus motor imagery versus routine physical therapy in patients with parkinson's disease: a randomized controlled trial. BMC Geriatr. (2024) 24:229. doi: 10.1186/s12877-024-04845-1
73.
Landers MR Hatlevig RM Davis AD Richards AR Rosenlof LE . Does attentional focus during balance training in people with Parkinson's disease affect outcome? A randomised controlled clinical trial. Clin Rehabil. (2016) 30:53–63. doi: 10.1177/0269215515570377
74.
Wong-Yu IS Mak MK . Task- and context-specific balance training program enhances dynamic balance and functional performance in parkinsonian nonfallers: a randomized controlled trial with six-month follow-up. Arch Phys Med Rehabil. (2015) 96:2103–11. doi: 10.1016/j.apmr.2015.08.409
75.
Capato TTC de Vries NM IntHout J Barbosa ER Nonnekes J Bloem BR . Multimodal balance training supported by rhythmical auditory stimuli in Parkinson's disease: a randomized clinical trial. J Parkinsons Dis. (2020) 10:333–46. doi: 10.3233/JPD-191752
76.
Steib S Klamroth S Gaßner H Pasluosta C Eskofier B Winkler J et al . Perturbation during treadmill training improves dynamic balance and gait in Parkinson's disease: a single-blind randomized controlled pilot trial. Neurorehabil Neural Repair. (2017) 31:758–68. doi: 10.1177/1545968317721976
77.
Gaßner H Steib S Klamroth S Pasluosta CF Adler W Eskofier BM et al . Perturbation treadmill training improves clinical characteristics of gait and balance in Parkinson's disease. J Parkinsons Dis. (2019) 9:413–26. doi: 10.3233/JPD-181534
78.
Calabrò RS Naro A Filoni S Pullia M Billeri L Tomasello P et al . Walking to your right music: a randomized controlled trial on the novel use of treadmill plus music in Parkinson's disease. J Neuroeng Rehabil. (2019) 16:68. doi: 10.1186/s12984-019-0533-9
79.
Kim H Kim E Yun SJ Kang MG Shin HI Oh BM et al . Robot-assisted gait training with auditory and visual cues in Parkinson's disease: a randomized controlled trial. Ann Phys Rehabil Med. (2022) 65:101620. doi: 10.1016/j.rehab.2021.101620
80.
Picelli A Melotti C Origano F Neri R Verzè E Gandolfi M et al . Robot-assisted gait training is not superior to balance training for improving postural instability in patients with mild to moderate Parkinson's disease: a single-blind randomized controlled trial. Clin Rehabil. (2015) 29:339–47. doi: 10.1177/0269215514544041
81.
Picelli A Melotti C Origano F Neri R Waldner A Smania N . Robot-assisted gait training versus equal intensity treadmill training in patients with mild to moderate Parkinson's disease: a randomized controlled trial. Parkinsonism Relat Disord. (2013) 19:605–10. doi: 10.1016/j.parkreldis.2013.02.010
82.
Picelli A Melotti C Origano F Waldner A Gimigliano R Smania N . Does robotic gait training improve balance in Parkinson's disease? A randomized controlled trial. Parkinsonism Relat Disord. (2012) 18:990–3. doi: 10.1016/j.parkreldis.2012.05.010
83.
Li G Huang P Cui SS Tan YY He YC Shen X et al . Mechanisms of motor symptom improvement by long-term tai chi training in Parkinson's disease patients. Transl Neurodegener. (2022) 11:6. doi: 10.1186/s40035-022-00280-7
84.
Zhang TY Hu Y Nie ZY Jin RX Chen F Guan Q et al . Effects of tai chi and Multimodal exercise training on movement and balance function in mild to moderate idiopathic Parkinson disease. Am J Phys Med Rehabil. (2015) 94:921–9. doi: 10.1097/PHM.0000000000000351
85.
Xiao CM Zhuang YC . Effect of health Baduanjin qigong for mild to moderate Parkinson's disease. Geriatr Gerontol Int. (2016) 16:911–9. doi: 10.1111/ggi.12571
86.
Vivas J Arias P Cudeiro J . Aquatic therapy versus conventional land-based therapy for Parkinson's disease: an open-label pilot study. Arch Phys Med Rehabil. (2011) 92:1202–10. doi: 10.1016/j.apmr.2011.03.017
87.
Hackney ME Kantorovich S Levin R Earhart GM . Effects of tango on functional mobility in Parkinson's disease: a preliminary study. J Neurol Phys Therapy. (2007) 31:173–9. doi: 10.1097/NPT.0b013e31815ce78b
88.
Ni M Signorile JF Mooney K Balachandran A Potiaumpai M Luca C et al . Comparative effect of power training and high-speed yoga on motor function in older patients with Parkinson disease. Arch Phys Med Rehabil. (2016) 97:345–354.e15. doi: 10.1016/j.apmr.2015.10.095
89.
Cherup NP Strand KL Lucchi L Wooten SV Luca C Signorile JF . Yoga meditation enhances proprioception and balance in individuals diagnosed with Parkinson's disease. Percept Mot Skills. (2021) 128:304–23. doi: 10.1177/0031512520945085
90.
Furnari A Calabrò RS De Cola MC Bartolo M Castelli A Mapelli A et al . Robotic-assisted gait training in Parkinson's disease: a three-month follow-up randomized clinical trial. Int J Neurosci. (2017) 127:996–1004. doi: 10.1080/00207454.2017.1288623
91.
Carda S Invernizzi M Baricich A Comi C Croquelois A Cisari C . Robotic gait training is not superior to conventional treadmill training in parkinson disease: a single-blind randomized controlled trial. Neurorehabil Neural Repair. (2012) 26:1027–34. doi: 10.1177/1545968312446753
92.
Carvalho A Barbirato D Araujo N Martins JV Cavalcanti JL Santos TM et al . Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for Parkinson's disease: pilot study. Clin Interv Aging. (2015) 10:183–91. doi: 10.2147/CIA.S68779
93.
Hashimoto H Takabatake S Miyaguchi H Nakanishi H Naitou Y . Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson’s disease: a quasi-randomized pilot trial. Complement Ther Med. (2015) 23:210–219. doi: 10.1016/j.ctim.2015.01.010
Summary
Keywords
Parkinson’s disease, exercise therapy, elderly patient, network meta-analysis, balance, cognitive emotions
Citation
Mao J, Xia Y, Hu Y and Yao X (2025) The effects of nine types of exercise rehabilitation therapies on improving limb balance, cognitive and emotional function, and quality of life in elderly patients with Parkinson’s disease: a network meta-analysis of 55 RCTs. Front. Neurol. 16:1666552. doi: 10.3389/fneur.2025.1666552
Received
15 July 2025
Accepted
05 August 2025
Published
26 August 2025
Volume
16 - 2025
Edited by
Joan Cabestany, Universitat Politecnica de Catalunya, Spain
Reviewed by
Zhan (John) Liu, Springfield College, United States
Guotuan Wang, Henan University, China
Updates
Copyright
© 2025 Mao, Xia, Hu and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuewu Yao, xuewusus@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.