Abstract
Introduction:
Many military Veterans who experienced blast-related traumatic brain injuries (TBI) in the conflicts in Iraq and Afghanistan currently suffer from chronic cognitive and mental health problems including post-traumatic stress disorder (PTSD). Rats exposed to repetitive low level blast injury exhibit chronic PTSD-related behavioral traits. Inflammation has long been suspected of playing a role in blast-induced brain injury and rats exposed to repetitive low-level blast develop chronic inflammatory changes. Minocycline is a tetracycline antibiotic that besides having antibacterial properties has anti-inflammatory activity. The aim of this study was to determine whether minocycline could reverse PTSD related behavioral traits in rats exposed to repetitive low level blast exposure.
Methods:
Rats were exposed to three 74.5 kPa blast exposures administered one per day for three consecutive days. We tested two cohorts of blast-exposed rats at 8–8.5 months after blast exposure. Rats were tested in a novel object recognition (NOR) task, elevated zero maze (EZM) and cued fear learning paradigm. In one experiment rats were treated with five doses of minocycline over a 9-day period. In the second experiment blast-exposed rats were treated with a 4-week course of minocycline with the drug administered 11 times. After the second experiment blast-induced effects on expression of the serotonin receptor 2A (5-HT2AR) and the post-synaptic density protein-95 (PSD-95) were examined by Western blotting. Microglial morphology was examined by Iba1 immunostaining.
Results:
In both experiments, cognitive changes in NOR and anxiety in an EZM were reversed by minocycline. However, in neither experiment was exaggerated fear learning rescued. Minocycline did not reverse blast induced effects on expression of 5-HT2AR or PSD-95 although it did appear to modulate blast-induced effects on microglial morphology.
Conclusions:
These studies have implications for understanding the nature of blast-induced behavioral traits, some of which may be the direct result of inflammatory effects, while others may be independent of inflammation or if the result of inflammation, not reversible once downstream structural or neurochemical changes are established.
Introduction
Military-related traumatic brain injuries (TBIs) occur for many reasons, but certain types are relatively unique to military environments. The most prominent of these is blast exposure. In Iraq and Afghanistan, exposure to improvised explosive devices (IEDs) caused most TBIs in Service Members (1). Concern also exists over the possible effects of subclinical blast exposure (2). Military occupational blast exposure as it is now being referred to is common for many Service Members during training and military operations. Whether repetitive, low-level blast exposure causes health problems in later life is unclear, but cumulative low-level blast exposure over a Veteran's career has been associated with chronically worse brain health, more post-traumatic stress disorder (PTSD), and a greater risk for developing new symptoms after a later blast injury (3).
PTSD is a mental health disorder that develops after experiencing or witnessing a psychologically traumatizing event (4). Clinical features include re-experiencing phenomena (e.g., flashbacks), as well as hyperarousal symptoms including heightened acoustic startle and avoidance of places or events that serve as reminders of the psychological trauma. Changes in mood and cognition are associated features.
Rats exposed to repetitive low-level blast develop PTSD-related traits including anxiety, increased acoustic startle and changes in cognition that appear in a delayed manner and remain present for more than 1 year after exposure (5–10). Blast-exposed rats also exhibit exaggerated fear learning which has been extensively studied in rodents for its relevance to the neurobiology of PTSD (11, 12). We have referred to the condition in these animals as blast-induced PTSD (7) in the belief that it models a state which may be different pathophysiologically from PTSD following a psychological stressor. These animals thus represent a model to study the chronic neurobehavioral syndromes that often affect Veterans after blast exposure (13–15). The progressivity seen in this model is reminiscent of that seen in longitudinal studies of Service Members who suffered blast-related TBIs in Afghanistan which found that between 1 and 5 years of follow up, overall global functioning declined in >70% a decline nearly completely driven by worsening PTSD and depression (14).
Inflammation has long been suspected of playing a role in blast-induced brain injury (16, 17). In animal models, blast exposure causes increases in many inflammatory markers in blood and plasma (16, 17). Elevated pro-inflammatory cytokines and chemokines as well as microglial activation have been observed in multiple brain regions (reviewed in Ref. (16, 17)). Recently, a study in special forces personnel identified brain inflammation using a positron emission tomography ligand which recognizes activated microglia (18). The increases correlated with lifetime blast exposure (18).
Rats exposed to repetitive blast exposure in our model exhibit an early and selective vascular pathology with prominent gliovascular injury (17, 19, 20). Perivascular inflammation is a chronic feature of the disease (21) and associated with chronic vascular remodeling as well as increased levels of matrix metalloproteinases (MMP-2 and MMP-9), collagen IV loss, and microglial activation around affected vasculature. A transcriptomic study in our rat model (22) identified tumor necrosis factor α (TNFα) as a potential upstream regulator of a cluster of differentially expressed genes (DEGs) whose expression was affected by blast exposure across the time frame over which the neurocognitive phenotype appears.
Minocycline has broad anti-inflammatory and neuroprotective actions (23) with multiple studies documenting its ability to inhibit TNFα production (24–30). Minocycline has been widely studied for treatment of TBI in animals with Bergold et al. in their 2023 review identifying over 40 studies in mice and rats (31). These studies which have been conducted almost exclusively in the acute setting and in non-blast models suggest that minocycline has significant neuroprotective effects acutely (31). In one study using a blast model (32) rats were treated for four consecutive days with 50 mg/kg minocycline after a single blast injury. At 8 and 45 days after blast exposure, behavior in blast-exposed animals treated with minocycline was nearly identical to controls while several serum and tissue level markers of inflammatory, as well as vascular, neuronal, and glial markers of injury were normalized (32). Another study found that minocycline delivered via nanoparticles could ameliorate blast induced hearing loss in an animal model (33). In the present study, we tested whether minocycline could reverse established PTSD related behavioral traits in a well-characterized rat model of repetitive low level blast exposure.
Methods
Animals
A total of 73 adult male Long Evans hooded rats (250–350 g; 10 weeks of age; Charles River Laboratories International, Wilmington, MA, USA) were used. All studies involving animals were approved by the Institutional Animal Care and Use Committees of the Walter Reed Army Institute of Research (WRAIR)/Naval Medical Research Command (NMRC) and the James J. Peters VA Medical Center. Studies were conducted in compliance with the Public Health Service policy on the humane care and use of laboratory animals, the NIH Guide for the Care and Use of Laboratory Animals, and all applicable Federal regulations governing the protection of animals in research.
Blast overpressure exposure
Rats were exposed to overpressure injury using a shock tube at either the WRAIR/NMRC (cohort 1) or the James J. Peters VA Medical Center (cohort 2). Exposures using the WRAIR/NMRC shock tube have been described in detail in multiple prior studies (5–9, 19–22, 34–38). The shock tube at the James J. Peters VA Medical Center was designed and constructed by Baker Engineering and Risk Consultants (San Antonio, TX, USA). The instrument is approximately 6.4 m in length and comparable in design to other shock tubes in use that have been built by Baker Risk (39). The tube consists of a variable volume driver that permits control of the duration of the primary positive pressure wave independent of the peak overpressure. Pressurized air was used for all experiments. A dual diaphragm spooler holding two polyethylene terephthalate Mylar TM sheets (Du Pont, Wilmington, DE, USA) was used to control pressure differences between the driver and expansion section of the tube. To induce “detonation,” the pressure between the two diaphragms of the spooler was released via remotely controlled electronic valves causing both diaphragms to rupture simultaneously. The peak pressure at the end of the expansion chamber was determined with piezoresistive gauges specifically designed for pressure-time (impulse) measurements (Model 102M152, PCB, Piezotronics, Depew, NY, USA). All sensor data was collected and processed using Lab View2011 software (Austin, TX, USA). The end of the shock tube is fitted with an attenuator chamber that reduces ambient blast noise and suppresses reflected shock waves.
Individual rats were anesthetized using an isoflurane gas anesthesia system consisting of a vaporizer, gas lines and valves and an activated charcoal-scavenging system adapted for use with rodents. Rats were placed into a polycarbonate induction chamber, which was closed and immediately flushed with a 5% isoflurane mixture in air for 2 min. Rats were placed into a cone-shaped plastic restraint device and mounted on a square grid. Head and body movement was restricted by harnesses that affixed the animal to the grid and restricted movement during the blast overpressure exposure without restricting breathing. Rats were randomly assigned to sham or blast conditions and were placed in the shock tube lying prone with the plane representing a line from the tail to the nose of the body in line with the longitudinal axis of the shock tube and the head placed upstream facing the shock wave. The total length of time under anesthesia including placement in the shock tube and execution of the blast procedure was typically less than 3 min. Blast-exposed animals received 74.5 kPa peak overpressure exposures (equivalent to 10.8 psi, duration 4.8 ms, impulse 175.8 kPa*ms). These exposures closely mimic those used in prior studies which used the WRAIR/NMRC shock tube (40). Exposures were administered one exposure per day for three consecutive days.
Animal housing and handling
Animals were housed at a constant 21–22 °C temperature with rooms on a 12:12 hour light cycle with lights on at 7 a.m. All subjects were individually housed in standard clear plastic cages equipped with Bed-O'Cobs laboratory animal bedding (The Andersons, Maumee, OH, USA) and EnviroDri nesting paper (Sheppard Specialty Papers, Milford, NJ, USA). Access to food and water was ad libitum. Subjects were housed on racks in random order to prevent rack position effects. Prior to behavioral testing, animals were habituated to handling to reduce stress during subsequent experimental procedures. Subjects were gently removed from their home cages and allowed to freely explore an open area measuring 100 cm × 60 cm for 5 min, three times per week. Following this, researchers gradually introduced handling by gently restraining the animals to further acclimate them to human manipulation. Cages were coded to allow maintenance of blinding to groups during behavioral testing. Experimenters were blinded to experimental groups to prevent bias during handling and data collection.
Drug administration
Minocycline (Sigma, St. Louis MO, USA) was dissolved in saline. Blast exposed subjects were treated with 45 mg/kg per administration given intraperitoneally. Animals were divided into three experimental groups: (1) sham-exposed rats treated with vehicle (sham + vehicle) (2) blast-exposed rats treated with vehicle (blast + vehicle), and (3) blast-exposed rats treated with minocycline (blast + Mino). Two cohorts were studied. The timing of drug administration in relationship to blast exposure and behavioral testing is shown in Figure 1. Dose was chosen based on 45 mg/kg being a dose that is tolerated and has successfully improved outcomes in multiple rat models of TBI (31).
Figure 1

Experimental design. Minocycline (45 mg/kg) was administered to two cohorts of blast-exposed rats. Cohort 1 (n = 7 sham + vehicle, 10 blast + vehicle, 11 blast + minocycline) was treated at 8 months after blast exposure. Administrations of minocycline were given daily for three consecutive days. On day 4 and 5 rats were tested in an NOR task. Rats were given additional booster doses of minocycline on days 8 and 19. On day 22 they were retrained and tested in NOR. On day 24 they were tested in an elevated zero maze (EZM) and on days 25 and 26 trained and tested in a cued fear conditioning protocol. Cohort 2 (n = 15 per group, sham + vehicle, blast + vehicle and blast + minocycline) was treated at 8.5 months after blast exposure. Rats received three treatments per week with the first administration on a Wednesday and then continued Monday, Wednesday, Friday over a period of 4 weeks (11 administrations total over days 1–24). On days 27 and 28 they were tested in an EZM and then on days 29 and 30 in an NOR task. On day 31 they were given one additional booster dose of drug or vehicle. On days 34 and 35, they were trained and tested in a cued fear conditioning protocol.
Behavioral testing
The exact timing of testing of each cohort in relationship to blast exposure and drug administration is described in Figure 1.
Elevated zero maze (EZM)
The apparatus consisted of a circular black Plexiglas runway 121.92 cm in diameter and raised 76 cm off the floor (San Diego Instruments, San Diego, CA, USA). The textured runway itself was 5.08 cm across and divided equally into alternating quadrants of open runway enclosed only by a 1.27 cm lip and closed runway with smooth 15.24 cm walls. All subjects received a 5-min trial beginning in a closed arc of the runway. During each trial, subjects were allowed to move freely around the runway, with all movement tracked automatically by a video camera placed on the ceiling directly above the maze. Data were analyzed by ANYMAZE (San Diego Instruments) yielding measures of total movement time and distance for the entire maze, as well as time spent, and distance traveled in each of the individual quadrants. From the quadrant data, measures of total open and closed arc times, latency to enter an open arc, total open arm entries and latency to completely cross an open arc between two closed arcs were calculated. Subject position was determined by centroid location.
Novel object recognition (NOR)
Rats were habituated to the arena (90 cm length × 60 cm width × 40 cm height) for 20 min, 24 h before training. On the training day, two identical objects were placed on opposite ends of the empty arena, and the rat was allowed to explore the objects freely for 7 min. After a 1-h delay, during which the rat was held in its home cage, one of the two familiar objects was replaced with a novel one, and the rat was allowed to freely explore the familiar and novel object for 5 min to assess short-term memory (STM). After a 24-h delay, during which the rat was held in its home cage, the novel object from the STM testing was replaced with a second novel object different from the one used during STM testing but placed in the same location as the novel object in the STM testing. The rat was allowed to freely explore the familiar and novel object for 5 min to assess long-term memory (LTM). Raw exploration times for each object were expressed in seconds. Object exploration was defined as sniffing or touching the object with the vibrissae or when the animal's head was oriented toward the object with the nose placed at less than 2 cm from the object. All sessions were recorded by video camera (Sentech, Carrollton TX, USA) and analyzed with ANYMAZE software (San Diego Instruments). In addition, offline analysis by an investigator blind to the blast-exposed status of the animals was performed. Objects to be discriminated were of different size, shape and color and were made of plastic or metal material. The objects consisted of a 330 ml soda can, a metal box, a cup and a plastic tube. All objects were cleaned with 70% ethanol between trials.
Cued fear conditioning
Sound-attenuated isolation cubicles (Ugo Basile, Gemonio, (VA), Italy) were utilized. Each cubicle was equipped with a grid floor for delivery of the unconditioned stimulus (US) and overhead cameras. All aspects of the test were controlled and monitored by the Freeze Frame conditioning and video tracking system (Ethovision, Leesburg, VA, USA). During training the chambers were scented with almond extract, lined with white paper towels, had background noise generated by a small fan and were cleaned before and between trials with 70% ethanol. Each subject was placed inside the conditioning chamber for 2 min before the onset of a conditioned stimulus (CS; an 80 dB, 2 kHz tone), which lasted for 20 s with a co-terminating 2-s footshock [0.7 mA; unconditioned stimulus (US)]. A total of three tone/shock pairings were administered with the first/second and second/third separated by 1 min. Each rat remained in the chamber for an additional 40 s following the third CS-US pairing before being returned to its home cage. Freezing was defined as a lack of movement (except for respiration) in each 10-s interval. Minutes 0–2 during the training session were used to measure baseline freezing. Animals were returned to their home cage for another 24 h at which time cued fear response was tested. To create a new context with different properties, the chambers were free of background noise (fan turned off), lined with blue paper towels, scented with lemon extract and cleaned before and during all trials with isopropanol. Each subject was placed in this novel context for 2 min and baseline freezing was measured, followed by exposure to the CS (20-s tone) at 120, 260 and 420 s.
Tissue collection
For biochemical studies rats were euthanized by CO2 inhalation, the brain was removed and regionally dissected as previously described (5). For immunohistochemistry, rats were perfused with 4% paraformaldehyde (PFA) in phosphate buffer saline (PBS) and the brain dissected and post-fixed overnight in PFA and stored in PBS.
Western blot analysis
Tissues were homogenized in 0.1 M Tris HCl pH 7.6 containing 150 mM NaCl, 1% Triton, 0.1% and a protease and phosphatase inhibitors (Halt, Protease and phosphatase inhibitor cocktail, ThermoFisher, Waltham MA, USA) using Zirconia beads and the Fast prep tissue homogenizer (MP Biomedicals, Irvine, CA, USA). The lysates were centrifuged at 14,000 rpm for 20 min and the supernatant saved and frozen at −80 °C. Protein concentration was measured with the BCA assay (ThermoFisher). Protein (50 μg) was separated on SDS-PAGE gels, blotted onto Immobilon P membranes (Millipore) and blocked in a 5% non-fat dry milk in 20 mM Tris HCl, pH 7.5, 0.25 M NaCl, 0.1% Tween 20 (TBST). The membrane was incubated overnight at 4 °C with the primary antibody (Table 1) diluted in blocking solution, washed in TBST and incubated at room temperature 1.5 h with horseradish conjugated anti rabbit IgG (NA934, 1:7,500, Cytiva, Marlborough, MA, USA) diluted in blocking solution. The membrane was developed with ECL Prime western blotting detection reagent (Cytiva) and imaged with Amesham ImageQuant 800 imaging system. Membranes were stained with Ponceau Red S Stain (Sigma) to image total protein loading. Bands were quantitated with ImageQuant TL (Cytiva). Levels were expressed as ratio to glyceraldehyde 3- phosphate dehydrogenase (GAPDH) or to total protein loading derived from integrated intensity of the Ponceau S-stained bands. Stripping of blots was performed with Reblot Plus buffer (ThermoFisher) as recommended by the manufacturer.
Table 1
| Antibody target | Type | Source | Catalog number | Dilution |
|---|---|---|---|---|
| PSD-95 | Rabbit monoclonal | Cell Signaling Technology | 3409S | 1:1,200 |
| GAPDH | Rabbit monoclonal | Cell Signaling Technology | 5174S | 1:1,000 |
| 5-HT2AR | Mouse monoclonal | Santa Cruz | sc-166775 | 1:600 |
Antibodies used for western blotting.
Immunohistochemistry
Coronal sections (50 μm thickness) were prepared with a VT1000S Vibratome (Leica Biosystems, Buffalo Grove, IL, USA). Floating sections were blocked with 10% normal goat serum in 50 mM Tris HCl, pH 7.6, 0.15 M NaCl, 0.3% Triton-X-100 for 2 h and incubated overnight with rabbit anti-ionized calcium-binding adaptor molecule 1 polyclonal antibody (Iba1, 019-19741, RRID:AB_839504, Fujifilm Wako Pure Chemical, Osaka, Japan) diluted 1:300 in blocking solution at room temperature. After washing with PBS (six times for 10 min each), sections were incubated with an Alexa Fluor 647-conjugated anti-rabbit secondary antibody (1:300, ThermoFisher) in blocking solution for 2 h. To visualize nuclei, sections were incubated in 0.1 mg/ml DAPI 4′,6-diamidine-2′-phenylindole dihydrochloride) in PBS. After washing with PBS (six times for 10 min each), the sections were mounted with Fluorogel mounting medium (Electron Microscopy Sciences, Hatfield, PA, USA). Immunostained sections were imaged with a laser scanning confocal microscope Zeiss LSM 980 with Airyscan 2 (Carl Zeiss Microscopy, White Plains, NY, USA).
Statistical analysis
Data sets were examined for assumptions of parametric tests (normality, homogeneity of variance). Values are expressed as mean ± SEM. Comparisons were performed using univariate ANOVA, repeated-measures ANOVA, or unpaired t-tests. When three groups were being compared if the ANOVA was significant (p ≤ 0.05), Fisher's LSD was used to determine between group differences. When repeated-measures ANOVA was used the Greenhouse-Geisser correction was utilized. Statistical tests were performed using the program GraphPad Prism 10.6.1 (GraphPad Software, San Diego, CA, USA) or SPSS v30 (IBM, Chicago IL, USA).
Results
Short term treatment with minocycline partially reverses blast induced neurobehavioral deficits
To determine whether minocycline might reverse blast-induced effects on behavior we treated blast-exposed rats at 8 months after blast exposure with 45 mg/kg of minocycline for 5 days over a 9-day period (Figure 1, cohort 1). A three-group design was used in which sham-exposed rats were treated with vehicle alone while groups of blast-exposed rats were treated with vehicle or minocycline. Active drug or vehicle were administered intraperitoneally. In this design comparison of sham- and blast-treated with vehicle served as a positive control for the presence of the blast-induced behavioral phenotype while comparison of blast-exposed treated with minocycline vs. blast-treated with vehicle allowed effect of minocycline to be determined. The weights for cohort 1 before treatment and at the end of behavioral testing are shown in Figure 2. There were no between group differences (F2, 25 = 2.537, p = 0.099) and there was no mortality during the study.
Figure 2

Weights for cohort 1. Shown are the weights for the rats used in cohort 1, obtained before treatment and at the end of behavioral testing. A repeated measures ANOVA showed that rats gained weight from before treatment to after behavioral testing (F1, 25 = 7.535, p = 0.011) but there was no interaction effect of group (F2, 25= 0.024, p = 0.976) and no between group differences (F2, 25 = 2.537, p = 0.099). Error bars indicate the standard error of the mean (SEM).
After an initial 3 days of treatment, we tested rats on an NOR task (Figures 3A–C). In the short-term memory (STM) and long-term memory (LTM) testing at 1 h or 24 h after training the sham exposed rats spent more time exploring the novel object (NO) than the familiar object (FO). By contrast the blast-exposed rats treated with vehicle or minocycline spent an equal amount of time exploring the FO and NO indicating that blast-induced deficits in NOR were not rescued by minocycline.
Figure 3

Testing of cohort 1 in novel object recognition (NOR) and elevated zero maze (EZM). We treated blast-exposed rats at 8 months after blast exposure with 45 mg/kg of minocycline on days 1, 2, and 3 of the protocol shown in Figure 1. NOR testing was performed on days 4 and 5. Shown is time spent exploring object 1 (Ob1) or object 2 (Ob2) during the training (A), short term memory testing (STM) at 1 h after training (B) or long-term memory testing (LTM) at 24 h after training (C). Time spent exploring the familiar (FO) and novel (NO) objects were compared using unpaired t-tests (**p < 0.01). On days 8 and 19 of the protocol rats were administered additional doses of minocycline. On days 22 and 23 rats were tested again in an NOR task (round 2). Shown is training (D), total exploration time during the training session (E), testing for STM (F) and LTM (G) memory and a discrimination index (DI) calculated for the LTM testing (H). Asterisks in (B), (F) and (G) indicate comparisons using unpaired t-tests (*p < 0.05, **p < 0.01). For total exploration time (E), a one-way ANOVA was significant (F2, 24 = 7.7, p = 0.0025). Asterisks indicate significant differences between groups (**p < 0.01, Fisher's LSD). On day 24 EZM testing was performed. Panel (I) shows the latency to enter an open arm. A one-way ANOVA was significant (F2, 21 = 3.8, p = 0.0374, *p < 0.05, Fisher's LSD). Error bars indicate the SEM in all panels.
Reasoning that the treatment had not been long enough, we treated these same rats with two additional doses of minocycline on days 8 and 19 (Figure 1). On day 22 of the protocol, rats were trained and tested again in an NOR task, on day 24 an EZM, and on days 25 and 26 trained and tested for cued fear learning. As shown in Figure 3D, in NOR testing all groups spent equal amounts of time exploring the objects in the training phase although the blast-treated with minocycline spent less total time exploring the objects (Figure 3E). When recognition memory was tested 1 h later (Figure 3F), sham-treated with vehicle explored the NO more than the FO (p < 0.05) while blast-exposed treated with vehicle did not explore the NO more than the FO. Blast-exposed treated with minocycline explored the NO more than the FO although this did not reach statistical significance (p = 0.08). However, when recognition memory was tested 24 h later (Figure 3G), the blast-exposed treated with minocycline explored the NO more than the FO (p < 0.05) while the sham- and blast-treated with vehicle explored them equally. When a discrimination index (DI), which measures the relative preference for the NO vs. FO, was calculated (Figure 3H), the sham-treated with saline preferred the NO to the FO more than the blast-exposed treated with vehicle. Preference for the NO was restored to that of sham + vehicle in blast-treated with minocycline. During the second round of behavioral testing, compared to controls, minocycline also reduced the latency of blast-exposed rats to enter an open arm in the EZM (Figure 3I).
When rats were tested in a cued fear learning protocol, all groups trained similarly exhibiting freezing in response to pairing the tone and the shock (Figure 4A). However, in the cued testing (Figure 4B), the blast-exposed groups, whether treated with vehicle or minocycline, froze more the sham + vehicle, and there was no difference between blast-treated with minocycline and blast-treated with vehicle (p = 0.213). Thus, while a course of minocycline given five times over 9-day period improved blast-induced deficits in object recognition memory and anxiety, exaggerated fear leaning was not improved.
Figure 4
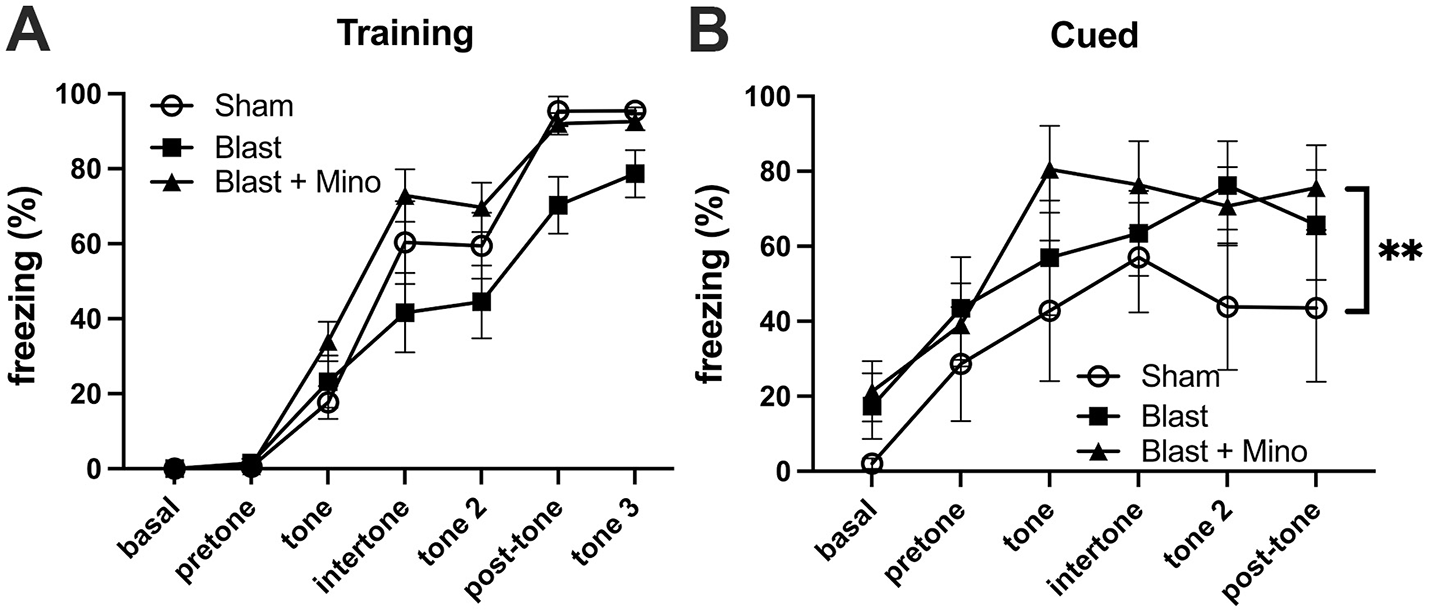
Testing of cohort 1 in fear learning. Panel (A) shows training. Basal and pretone represent the first and second minute respectively before the first presentation of the tone + shock. A repeated measures ANOVA showed that all groups responded with increasing freezing following pairing of the tone and shock (F2.684, 53.681 = 143.973, p < 0.001) with no interaction effect of group (F5.368, 53.681 = 2.176, p = 0.066) and no between group differences (F2, 20 = 2.084, p = 0.151). During the cued testing session (B), all groups responded with freezing following presentation of the tone (repeated measures ANOVA: F2.353, 35.295 = 14.088, p < 0.001) with no significant interaction effect of group (F4.706, 97.328 = 0.821, p = 0.537). A comparison of between subject effects found significant group effects (F2, 15 = 4.544, p = 0.03; **p < 0.01, Fisher's LSD) but there was no difference between blast-treated with minocycline and blast-treated with vehicle (p = 0.213). Error bars indicate the SEM in all panels.
A 4-week course of minocycline reverses blast induced anxiety and cognitive deficits but not exaggerated fear learning
Reasoning that treatment for 5 days over a 9-day period might be sufficient to rescue some traits but not others, we designed a treatment protocol in which rats received three treatments per week with the first administration on a Wednesday and then continued Monday, Wednesday, and Friday over a period of 4 weeks (11 administrations total, Figure 1). The weights for cohort 2 before treatment and during the protocol are shown in Figure 5. There were no between group differences (F2, 25 = 2.537, p = 0.099) and there was no mortality during the study. At the end of treatment rats were tested in an EZM, NOR and cued fear learning. Rats received one additional booster dose of drug or vehicle between NOR and cued fear testing (Figure 1).
Figure 5

Weights for cohort 2. Shown are the weights for the rats used in cohort 2, obtained before and at the end of treatment. A repeated measures ANOVA showed that rats lost weight comparing before to after treatment (F1.303, 48.226 = 5.265, p = 0.018) but there was no interaction effect of group (F2.607, 48.226 = 1.182, p = 0.323) and no between group differences (F2, 37 = 2.723, p = 0.079). Error bars indicate the SEM.
EZM was tested on two consecutive days (Figure 6). Unfortunately, the anxiety phenotype was not as strong in the blast-exposed treated with vehicle as has been observed in many prior studies (5, 6, 8, 9, 38, 41). However, the two measurements that were abnormal when comparing blast-treated with vehicle to sham treated with vehicle (latency to an open arm day 1, cross latency day 2) were both reversed by minocycline.
Figure 6

Testing of cohort 2 in EZM. Blast-exposed and control rats were tested for 5 min on two consecutive days in an EMZ. Shown is total distance moved (A, G), time in motion (B, H), mean speed (C, I). latency to enter an open arm (D, J), time spent in an open arm (E, K) and the latency to cross between two open arms (F, L, cross latency) on days 1 and 2. Error bars indicate the SEM in all panels. One-way ANOVAs were significant for open arm latency on day 1 (F2, 27 = 8.295, p = 0.0016) and cross arm latency on day 2 (F2, 38 = 3.795, p = 0.0.0314). Asterisks indicate values significantly different between groups (*p < 0.05, **p < 0.01, Fisher's LSD).
In NOR testing of cohort 2 during training all groups spent equal amounts of time exploring the objects (Figure 7A). In STM testing blast + minocycline explored the NO more than the FO while sham and blast + vehicle explored them equally (Figure 7B). In LTM testing, sham + vehicle and blast + minocycline explored the NO more than the FO while blast + vehicle explored them equally (Figure 7C). When a DI was calculated for the STM (Figure 7D) and LTM (Figure 7E) testing, deficits in object recognition in the blast + vehicle were rescued by minocycline in the blast-exposed in both. Blast +vehicle also spent less total time exploring the objects, an effect that was restored in blast + minocycline (Figure 7F). All groups spent equal total time exploring the objects in the STM (Figure 7G) and LTM (Figure 7H) testing.
Figure 7

Testing of cohort 2 in NOR. Shown is time spent exploring object 1 (Ob1) or object 2 (Ob2) during the training (A), short-term memory testing (STM) at 1 h after training (B) or long-term memory testing (LTM) at 24 h after training (C). Time spent exploring the familiar (FO) and novel (NO) objects were compared using unpaired t-tests (*p < 0.05). Panels D and E show discrimination indices (DI) calculated for the STM (D) and LTM (E) testing. Panels (F–H) show total exploration time during the training (F), STM (G) and LTM (H) testing. One-way ANOVAs were significant for STM DI (F2, 36 = 7.117, p = 0.0025), LTM DI (F2, 38 = 7.112, p = 0.0024) and exploration during training (F2, 38 = 4.168, p = 0.0231). Asterisks in panels (D–H) indicate significant differences between groups (*p < 0.05, **p < 0.01, ***p < 0.001 Fisher's LSD). Error bars indicate the SEM in all panels.
When cohort 2 was tested in fear learning (Figure 8), as in the first cohort, all groups responded during training (Figure 8A) with increasing freezing following pairing of the tone and shock and there were no differences between groups. However, also as in cohort 1 in cued fear testing the blast-exposed groups whether treated with minocycline or not, froze more than the sham when the tone was presented without the shock and this effect was not rescued by minocycline (Figures 8B, C). Thus, while a 4-week course of minocycline rescued blast-induced deficits in object recognition and anxiety, as in the first experiment it did not rescue exaggerated fear learning.
Figure 8

Testing of cohort 2 in fear learning. Panel (A) shows training. A repeated measures ANOVA showed that all groups responded with increasing freezing following pairing of the tone and shock (F4.300, 159.098 = 159.098, p < 0.001) with no interaction effect of group (F8.600, 159.098 = 1.705, p = 0.095) and no between group differences (F2, 37 = 0.721, p = 0.493). Panel (B) shows a comparison of freezing to the first tone during the cued phase testing. A one-way ANOVA was significant (F2, 32 = 6.8, p = 0.0034). p Values are indicated (**p < 0.01; ns, not significant). During the cued testing session (C), all groups responded with freezing following presentation of the tone (repeated measures ANOVA; F2.949, 97.328 = 18.994, p < 0.001) with no significant interaction effect of group (F5.899, 97.328 = 0.455, p = 0.837). A comparison of between subject effects found that blast froze more than sham (p = 0.05) but there was no difference between blast-treated with minocycline and blast-treated with vehicle (p = 0.646). Error bars indicate the SEM in all panels.
Minocycline does not alter blast-induced effects on serotonin 2A receptor (5-HT2AR) or post-synaptic density protein 95 (PSD-95) expression
To begin to explore the underlying mechanisms of minocycline's effects on at least some blast induced behavioral traits we examined expression of 5-HT2AR and PSD-95 which have both been found to be decreased after blast exposure in this model (42, 43). In studies on animals from cohort 2, while 5-HT2AR (Figure 9) and PSD-95 (Figure 10), were decreased following blast exposure minocycline did not reverse these changes.
Figure 9

Western blot analysis of 5-HT2AR in rats from cohort 2. 5-HT2AR expression was analyzed in the anterior cortex of control rats treated with saline (n = 5), and blast-exposed rats treated with saline or minocycline (n = 6). Top panel: 5-HT2AR; middle panel GAPDH; bottom panel: Ponceau S staining. The targets were sequentially analyzed on the same blot in the same order as indicated in the Figure. The bar graphs show 5-HT2AR levels expressed as a ratio to GAPDH or total protein load calculated from the Ponceau S staining. Error bars indicate the SEM in all panels. Asterisks indicate significant differences (Fisher's LSD after a significant one-way ANOVA, *p < 0.05, **p < 0.01).
Figure 10
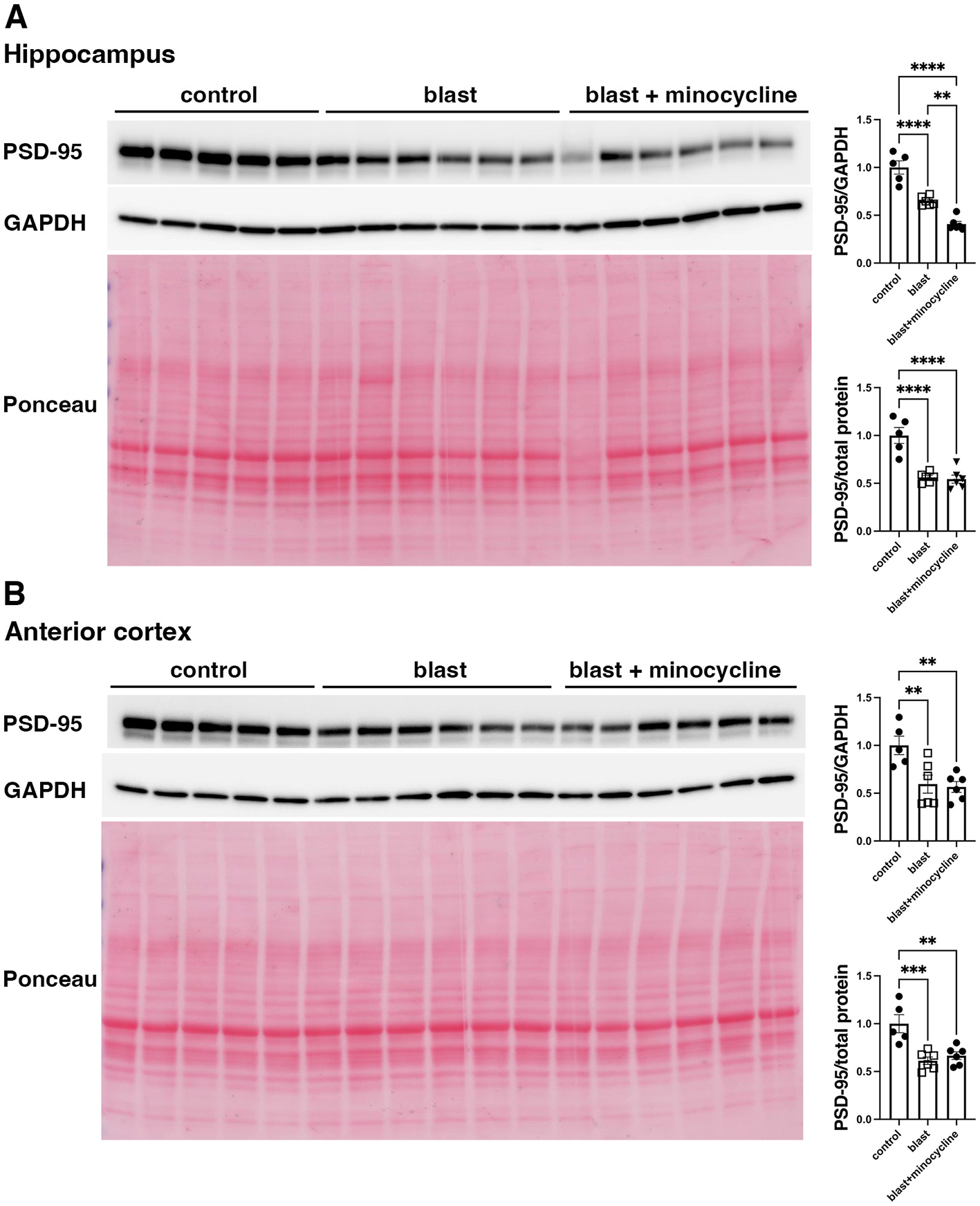
Western blot analysis of PSD-95 in rats from cohort 2. PSD-95 was analyzed in hippocampus (A) and anterior cortex (B) of control rats treated with saline (n = 5), and blast exposed rats treated with saline (blast) or minocycline (n = 6). Top panel: PSD-95; middle panel: GAPDH and bottom panel Ponceau S stain. The targets were sequentially analyzed on the same blot in the same order as indicated in the Figure. The bar graphs show PSD-95 levels expressed as a ratio to GAPDH or total protein load calculated from Ponceau S staining. Error bars indicate the SEM. Asterisks indicate significant differences (Fisher's LSD after a significant one-way ANOVA, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Minocycline modulates microglial morphology
To determine whether minocycline might be modulating microglial activation state, we stained tissue sections from cohort 2 with Iba1. As shown in Figure 11, compared to the branched morphology of microglia in sham animals (Figures 11A, D), blast exposed without treatment frequently exhibited an activated ameboid transition with an enlarged soma and decreased branching (Figures 11B, E). By contrast microglia in blast-exposed rats treated with minocycline (Figures 11C, F) exhibited a more branched morphology (quantitated in panel D).
Figure 11

Minocycline effects on microglial morphology. Shown are high and low magnification 3D images of microglia (Iba1+, magenta) in the somatosensory cortex of control treated with saline (A and D), blast-exposed treated with saline (B and E) and blast-exposed treated with minocycline (C and F). Inset in panel (D) shows the percentage of Iba1+ microglia which were branched (as in panels A and C) vs. unbranched (panel B). Cells were scored by an observer blinded to the condition of the animal (n = 2 sham, n = 3 blast; n = 3 blast + minocycline). A mean of 48.6 ± 4.2 (SEM) Iba1 positive cells were scored per animal. Error bars indicate the SEM (*p < 0.05). Animals are from cohort 2. Scale bar: A–C, 10 μm; D–F, 50 μm.
Discussion
We used a well-established animal model which employs male rats and mimics the type of open field low-level blast exposure associated with human mild traumatic brain injury (mTBI) or subclinical blast exposure in humans (5–9, 19–22, 34–38). The pressure level used (74.5 kPa, 10.8 psi) is transmitted to brain (44) but does not cause gross neuropathological effects nor lung injury (40). Because blast-related TBI may involve a combination of injuries related to effects of the primary blast wave as well as damage from rotational/acceleration injury (45, 46), during the overpressure exposures head motion is restricted to minimize rotation/acceleration injury. A three-exposure paradigm was chosen to mimic the multiple blast exposures that are commonly experienced by Service Members in modern military environments (47, 48).
Inflammation has long been suspected of playing a role in blast-induced brain injury (16, 17). The purpose of this study was to determine whether minocycline, an anti-inflammatory and neuroprotective agent, could reverse blast induced behavioral traits after they are established by administering minocycline to male rats at 8–8.5 months after blast exposure when the behavioral phenotype is established.
In an initial study minocycline was given five times over a 9-day period. This regimen improved blast induced deficits in object recognition memory and anxiety but did not rescue exaggerated fear leaning. Reasoning that treatment for 5 days over a 9-day period might be insufficient to rescue all traits, we conducted a second study in which rats received a total of 11 administrations of minocycline over a period of 4 weeks. The results of this study were similar to the original; object recognition memory and anxiety were rescued but exaggerated fear leaning was not. Thus, in two studies aspects of the behavioral phenotype were readily reversed while others were not.
The rapid reversal of the cognitive and anxiety related phenotype in comparison to the resistance of the fear leaning phenotype suggests that different mechanisms may be driving the two phenotypes. Rapid reversal of cognitive and anxiety related traits suggests that these are being driven by levels or combinations of cytokines/inflammatory factors which can be modulated by minocycline. By contrast, traits such as exaggerated fear learning may be independent of inflammation or if the result of inflammation, associated with chronic structural changes that are not readily reversible once established.
In an initial series of studies designed to probe the effects of minocycline on blast related injury we did not find any rescue of 5-HT2AR or PSD-95 expression which have been found to be decreased after blast exposure in this model (42, 43). Minocycline has been frequently reported to inhibit microglial activation and we found very similar changes reported in other studies (49–51) in terms of minocycline's effects on microglial morphology. Blast exposed rats without treatment frequently exhibited a proinflammatory ameboid morphology which was partially restored to the branched morphology of controls by 4 weeks of minocycline treatment. Due to the small sample size and variability in the minocycline treated animals, these results must be interpreted with some caution and future studies will need to be performed on larger sample sizes. However, they provide some support for the notion that whatever minocycline's beneficial effects are they are at least in part being mediated through actions on microglia. How this observation would explain minocycline's effect on cognition but not fear learning is unclear. Future studies will be needed to understand minocycline's effects on inflammatory cytokines and other factors modulated by microglial inflammatory state.
TNFα has been elevated in many studies following blast injury in rodents (16, 52–54), Soldiers exposed to moderate blasts during training exercises show transient increases in blood TNFα levels (55). Human studies in experienced breachers exposed to high numbers of career blast exposures also show dysregulation of genes associated with chronic inflammation in blood (56) and have identified elevated TNFα levels in neuronal-derived extracellular vesicles isolated from serum (57). TNFα is a therapeutic target in many human inflammatory conditions and has revolutionized treatment of some (58).
A prior transcriptomic study in our rat model identified TNFα as a potential upstream regulator of a cluster of differentially expressed genes whose expression was affected by blast exposure (22). TNFα is of special interest in that minocycline has broad anti-inflammatory and neuroprotective actions (23) with multiple studies documenting its ability to inhibit TNFα production (24–30). In brain, TNFα has both homeostatic and pathological roles. It is produced mainly by microglia but also by neurons and astrocytes (59). Under physiological conditions, constitutive TNFα expression plays roles in synaptic plasticity, learning and memory (59). Under pathological conditions, microglia and astrocytes release TNFα which is a crucial mediator of chronic inflammation and secondary brain injury (60). TNFα in brain also has biological activities independent of its role in inflammation, being implicated in PTSD-related hyperarousal states and regulating fear learning (17).
Thus, a variety of evidence supports a potential role for TNFα signaling in the neurobiological basis of blast effects on behavior through inflammatory and non-inflammatory mechanisms although in this study minocycline did not reverse blast effects on fear learning. Exaggerated fear responses in this model have been reversed by mGluR2/3 antagonists and hydroxynorketamine (9, 22, 38), agents that would be expected to act on already established neurochemical abnormalities and likely to act independent of inflammation. Future studies will be needed to determine the extent to which minocycline's effects on TNFα activity may underlie its partial effectiveness on the blast-related behavioral phenotype.
However, it should be noted that while minocycline has TNFα inhibiting activity, it also has broader anti-inflammatory and non-inflammatory effects, including affecting calcium regulation, mitochondrial stabilization and reducing oxygen radical toxicity (61). This model develops a chronic tau pathology (34) and minocycline reduced tau pathology in a rat model of neuroinflammation (62). Chronic alterations in perivascular microglial and astrocytes are seen in the current model (20, 21, 43, 63). These cells might also be targets for minocycline's actions independent of any TNFα effects.
Several limitations of the current study should be mentioned. One is the lack of inclusion of female rats. Sex differences in TBI outcomes are well-known (64) with studies in female Veterans suggesting that they are more likely to report persisting neurobehavioral symptoms and use more outpatient services than their male counterparts (65). In experimental animals, sex differences in response to blast has been little studied, although several reports have suggested that blast responses in female rats and mice differ (66–68). With the increasing number of female Veterans these studies assume a high importance. Another limitation is the lack of insight these studies provide at the molecular level into how minocycline is reversing some behavioral deficits but not others. Future studies will be needed to address other mechanisms.
Conclusion
Our findings demonstrate that minocycline treatment can rapidly reverse cognitive changes and anxiety but not exaggerated fear learning. These studies have implications for understanding the nature of blast-induced behavioral traits, some of which may result directly from an inflammatory environment that can be rapidly reversed while others may be independent of inflammation, or not reversible once structural or neurochemical changes are established. Future prevention studies seem warranted to determine whether initiating anti-inflammatory therapy before symptom onset can block the development of the full behavioral phenotype. In summary, while inflammation appears to play a significant role in the pathogenesis of blast-induced behavioral changes, it is likely only one component of a complex and multifaceted process.
Statements
Author’s note
The views expressed in this article reflect the results of research conducted by the authors and do not necessarily reflect the official policy or position of the of The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the Department of the Navy, or the Department of Defense (DoD). Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government. The study protocol was reviewed and approved by the Institutional Animal Care and Use committees of the Walter Reed Army Institute of Research/Naval Medical Research Command and the James J. Peters VA Medical Center. Studies were conducted in compliance with all applicable Federal regulations governing the protection of animals in research and in compliance with the Animal Welfare Act and per the principles set forth in the “Guide for Care and Use of Laboratory Animals,” Institute of Laboratory Animals Resources, National Research Council, National Academy Press, 2011. Some of the authors are military Service Members or federal/contracted employees of the United States Government. This work was prepared as part of their official duties. Title 17 U.S.C. § 105 provides that Copyright protection under this title is not available for any work of the United States Government. Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military Service Member or employee of the U.S. Government as part of that person's official duties.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committees of the Walter Reed Army Institute of Research (WRAIR)/Naval Medical Research Command (NMRC) and the James J. Peters VA Medical Center. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
GPG: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Visualization, Writing – review & editing. GP: Investigation, Writing – review & editing. RD: Investigation, Writing – review & editing. MG: Investigation, Writing – review & editing. RA: Writing – review & editing. UK: Writing – review & editing, Investigation. CZ: Writing – review & editing, Formal analysis. CT: Writing – review & editing. PH: Writing – review & editing. SA: Writing – review & editing, Funding acquisition, Methodology, Supervision. GE: Funding acquisition, Supervision, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service awards 1I01RX003846 (GE) and Department of Veterans Affairs Office of Research and Development Medical Research Service award 1I01BX005882 (GE), and by Department of Defense work unit number 0000B999.0000.000.A1503 (STA) and NIA P30 AG066514 (PRH, CWZ).
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Elder GA Ehrlich ME Gandy S . Relationship of traumatic brain injury to chronic mental health problems and dementia in military veterans. Neurosci Lett. (2019) 707:134294. doi: 10.1016/j.neulet.2019.134294
2.
Engel C Hoch E Simmons M . The Neurological Effects of Repeated Exposure to Military Occupational Blast: Implications for Prevention and Health: Proceedings, Findings, and Expert Recommendations from the Seventh Department of Defense State-of-the-Science Meeting, Rand Corporation. Arlington: VA (2019). doi: 10.7249/CF380.1
3.
Bailie JM Lippa SM Hungerford L French LM Brickell TA Lange RT . Cumulative blast exposure during a military career negatively impacts recovery from traumatic brain injury. J Neurotrauma. (2024) 41:604–12. doi: 10.1089/neu.2022.0192
4.
Bryant RA . Post-traumatic stress disorder: a state-of-the-art review of evidence and challenges. World Psychiatry. (2019) 18:259–69. doi: 10.1002/wps.20656
5.
Elder GA Dorr NP De Gasperi R Gama Sosa MA Shaughness MC Maudlin-Jeronimo E et al . Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J Neurotrauma. (2012) 29:2564–75. doi: 10.1089/neu.2012.2510
6.
Perez-Garcia G Gama Sosa MA De Gasperi R Lashof-Sullivan M Maudlin-Jeronimo E Stone JR et al . Chronic post-traumatic stress disorder-related traits in a rat model of low-level blast exposure. Behav Brain Res. (2018) 340:117–25. doi: 10.1016/j.bbr.2016.09.061
7.
Perez-Garcia G Gama Sosa MA De Gasperi R Tschiffely AE McCarron RM Hof PR et al . Blast-induced “PTSD”: evidence from an animal model. Neuropharmacology. (2019) 145:220–9. doi: 10.1016/j.neuropharm.2018.09.023
8.
Perez Garcia G Perez GM De Gasperi R Gama Sosa MA Otero-Pagan A Pryor D et al . Progressive cognitive and post-traumatic stress disorder-related behavioral traits in rats exposed to repetitive low-level blast. J Neurotrauma. (2021) 38:2030–45. doi: 10.1089/neu.2020.7398
9.
Perez-Garcia G De Gasperi R Gama Sosa MA Perez GM Otero-Pagan A Tschiffely A . PTSD-related behavioral traits in a rat model of blast-induced mTBI are reversed by the mGluR2/3 receptor antagonist BCI-838. eNeuro. (2018) 5:ENEURO.0357-17.2018. doi: 10.1523/ENEURO.0357-17.2018
10.
Perez-Garcia G Gama Sosa MA De Gasperi R Lashof-Sullivan M Maudlin-Jeronimo E Stone JR et al . Exposure to a predator scent induces chronic behavioral changes in rats previously exposed to low-level blast: implications for the relationship of blast-related TBI to PTSD. Front Neurol. (2016) 7:176. doi: 10.3389/fneur.2016.00176
11.
Bouton ME Maren S McNally GP . Behavioral and neurobiological mechanisms of pavlovian and instrumental extinction learning. Physiol Rev. (2021) 101:611–81. doi: 10.1152/physrev.00016.2020
12.
Maddox SA Hartmann J Ross RA Ressler KJ . Deconstructing the gestalt: mechanisms of fear, threat, and trauma memory encoding. Neuron. (2019) 102:60–74. doi: 10.1016/j.neuron.2019.03.017
13.
Lange RT Brickell TA Ivins B Vanderploeg RD . French LM. Variable, not always persistent, postconcussion symptoms after mild TBI in US military service members: a five-year cross-sectional outcome study. J Neurotrauma. (2013) 30:958–69. doi: 10.1089/neu.2012.2743
14.
Mac Donald CL J Barber M Jordan AM Johnson S Dikmen JR Fann et al . Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA Neurol. (2017) 74:821–9. doi: 10.1001/jamaneurol.2017.0143
15.
Mac Donald CL Barber J Patterson J Johnson AM Dikmen S Fann JR et al . Association between 5-year clinical outcome in patients with nonmedically evacuated mild blast traumatic brain injury and clinical measures collected within 7 days postinjury in combat. JAMA Netw Open. (2019) 2:e186676. doi: 10.1001/jamanetworkopen.2018.6676
16.
Elder GA Gama Sosa MA De Gasperi R Stone JR Dickstein DL Haghighi F et al . Vascular and inflammatory factors in the pathophysiology of blast-induced brain injury. Front Neurol. (2015) 6:48. doi: 10.3389/fneur.2015.00048
17.
Elder GA Gama Sosa MA De Gasperi R Perez Garcia G Perez GM Abutarboush R et al . The neurovascular unit as a locus of injury in low-level blast-induced neurotrauma. Int J Mol Sci. (2024) 251:1150. doi: 10.3390/ijms25021150
18.
Stone JR Avants BB Tustison NJ Gill J Wilde EA Neumann KD et al . Neurological effects of repeated blast exposure in special operations personnel. J Neurotrauma. (2024) 41:942–56. doi: 10.1089/neu.2023.0309
19.
Gama Sosa MA De Gasperi R Janssen PL Yuk FJ Anazodo PC Pricop PE et al . Selective vulnerability of the cerebral vasculature to blast injury in a rat model of mild traumatic brain injury. Acta Neuropathol Commun. (2014) 2:67. doi: 10.1186/2051-5960-2-67
20.
Gama Sosa MA De Gasperi R Perez Garcia GS Perez GM Searcy C Vargas D et al . Low-level blast exposure disrupts gliovascular and neurovascular connections and induces a chronic vascular pathology in rat brain. Acta Neuropathol Commun. (2019) 7:6. doi: 10.1186/s40478-018-0647-5
21.
Gama Sosa MA De Gasperi R Pryor D Perez Garcia GS Perez GM Abutarboush R et al . Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation. Acta Neuropathol Commun. (2021) 9:167. doi: 10.1186/s40478-021-01269-5
22.
De Gasperi R Gama Sosa MA Perez Garcia GS Perez GM Abutarboush R Kawoos U et al . Progressive transcriptional changes in the amygdala implicate neuroinflammation in the effects of repetitive low-level blast exposure in male rats. J Neurotrauma. (2023) 40:561–77. doi: 10.1089/neu.2022.0282
23.
Li J Qin Y Zhao C Zhang Z Zhou Z . Tetracycline antibiotics: potential anticancer drugs. Eur J Pharmacol. (2023) 956:175949. doi: 10.1016/j.ejphar.2023.175949
24.
Gong K Zou X Fuchs PN Lin Q . Minocycline inhibits neurogenic inflammation by blocking the effects of tumor necrosis factor-alpha. Clin Exp Pharmacol Physiol. (2015) 42:940–9. doi: 10.1111/1440-1681.12444
25.
Xie ST Fan WC Zhao XS Ma XY Li ZL Zhao YR et al . Proinflammatory activation of microglia in the cerebellum hyperexcites Purkinje cells to trigger ataxia. Pharmacol Res. (2023) 191:106773. doi: 10.1016/j.phrs.2023.106773
26.
Pawletko K Jedrzejowska-Szypulka H Bogus K Pascale A Fahmideh F Marchesi N et al . After ischemic stroke, minocycline promotes a protective response in neurons via the RNA-binding protein HuR, with a positive impact on motor performance. Int J Mol Sci. (2023) 24:9446. doi: 10.3390/ijms24119446
27.
Matias ME Radulski DR Rodrigues da . Silva T, Raymundi AM, Stern CAJ, Zampronio AR. Involvement of cannabinoid receptors and neuroinflammation in early sepsis: implications for posttraumatic stress disorder. Int Immunopharmacol. (2023) 123:110745. doi: 10.1016/j.intimp.2023.110745
28.
Long Y Wang Y Shen Y Huang J Li Y Wu R et al . Minocycline and antipsychotics inhibit inflammatory responses in BV-2 microglia activated by LPS via regulating the MAPKs/JAK-STAT signaling pathway. BMC Psychiatry. (2023) 23:514. doi: 10.1186/s12888-023-05014-1
29.
Liang X Zhang R . Effects of minocycline on cognitive impairment, hippocampal inflammatory response, and hippocampal Alzheimer's related proteins in aged rats after propofol anesthesia. Dis Markers. (2022) 2022:4709019. doi: 10.1155/2022/4709019
30.
Mishra S Rajput C Singh MP . Cypermethrin induces the activation of rat primary microglia and expression of inflammatory proteins. J Mol Neurosci. (2021) 71:1275–83. doi: 10.1007/s12031-020-01753-y
31.
Bergold PJ Furhang R Lawless S . Treating traumatic brain injury with minocycline. Neurotherapeutics. (2023) 20:1546–64. doi: 10.1007/s13311-023-01426-9
32.
Kovesdi E Kamnaksh A Wingo D Ahmed F Grunberg NE Long JB et al . Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front Neurol. (2012) 3:111. doi: 10.3389/fneur.2012.00111
33.
Perumal V Ravula AR Shao N Chandra N . Effect of minocycline and its nano-formulation on central auditory system in blast-induced hearing loss rat model. J Otol. (2023) 18:38–48. doi: 10.1016/j.joto.2022.09.002
34.
Dickstein DL De Gasperi R Gama Sosa MA Perez-Garcia G Short JA Sosa H et al . Brain and blood biomarkers of tauopathy and neuronal injury in humans and rats with neurobehavioral syndromes following blast exposure. Mol Psychiatry. (2021) 26:5940–54. doi: 10.1038/s41380-020-0674-z
35.
Gama Sosa MA De Gasperi R Perez Garcia GS Sosa H Searcy C Vargas D et al . Lack of chronic neuroinflammation in the absence of focal hemorrhage in a rat model of low-energy blast-induced TBI. Acta Neuropathol Commun. (2017) 5:80. doi: 10.1186/s40478-017-0483-z
36.
Perez Garcia G De Gasperi R Gama Sosa MA Perez GM Otero-Pagan A Pryor D et al . Laterality and region-specific tau phosphorylation correlate with PTSD-related behavioral traits in rats exposed to repetitive low-level blast. Acta Neuropathol Commun. (2021) 9:33. doi: 10.1186/s40478-021-01128-3
37.
Gama Sosa MA De Gasperi R Paulino AJ Pricop PE Shaughness MC Maudlin-Jeronimo E et al . Blast overpressure induces shear-related injuries in the brain of rats exposed to a mild traumatic brain injury. Acta Neuropathol Commun. (2013) 1:51. doi: 10.1186/2051-5960-1-51
38.
Perez Garcia G Perez GM Gasperi R Sosa MAG Otero-Pagan A Abutarboush R et al . (2R,6R)-Hydroxynorketamine treatment of rats exposed to repetitive low-level blast injury. Neurotrauma Rep. (2023) 4:197–217. doi: 10.1089/neur.2022.0088
39.
Huber BR Meabon JS Martin TJ Mourad PD Bennett R Kraemer BC et al . Blast exposure causes early and persistent aberrant phospho- and cleaved-tau expression in a murine model of mild blast-induced traumatic brain injury. J Alzheimers Dis. (2013) 37:309–23. doi: 10.3233/JAD-130182
40.
Ahlers ST Vasserman-Stokes E Shaughness MC Hall AA Shear DA Chavko M et al . Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Front Neurol. (2012) 3:32. doi: 10.3389/fneur.2012.00032
41.
Perez Garcia G Perez GM Otero-Pagan A Abutarboush R Kawoos U De Gasperi R et al . Transcranial laser therapy does not improve cognitive and post-traumatic stress disorder-related behavioral traits in rats exposed to repetitive low-level blast injury. Neurotrauma Rep. (2021) 2:548–63. doi: 10.1089/neur.2021.0005
42.
De Gasperi R Perez Garcia G Gama Sosa MA Perez GM Abutarboush R Kawoos U et al . Serotonin 5-HT2A receptor expression is chronically decreased in the anterior cerebral cortex of male rats following repetitive low-level blast exposure. Front Neurol. (2025) 16:1594335. doi: 10.3389/fneur.2025.1594335
43.
Gama Sosa MA De Gasperi R Pryor D Perez Garcia GS Perez GM Abutarboush R et al . Late chronic local inflammation, synaptic alterations, vascular remodeling and arteriovenous malformations in the brains of male rats exposed to repetitive low-level blast overpressures. Acta Neuropathol Commun. (2023) 11:81. doi: 10.1186/s40478-023-01553-6
44.
Chavko M Koller WA Prusaczyk WK McCarron RM . Measurement of blast wave by a miniature fiber optic pressure transducer in the rat brain. J Neurosci Methods. (2007) 159:277–81. doi: 10.1016/j.jneumeth.2006.07.018
45.
Cernak I Stein DG Elder GA Ahlers S Curley K DePalma RG et al . Preclinical modelling of militarily relevant traumatic brain injuries: challenges and recommendations for future directions. Brain Inj. (2017) 31:1168–76. doi: 10.1080/02699052.2016.1274779
46.
Song H Cui J Simonyi A Johnson CE Hubler GK DePalma RG et al . Linking blast physics to biological outcomes in mild traumatic brain injury: narrative review and preliminary report of an open-field blast model. Behav Brain Res. (2018) 340:147–58. doi: 10.1016/j.bbr.2016.08.037
47.
Hoge CW McGurk D Thomas JL Cox AL Engel CC . Castro CA. Mild traumatic brain injury in US Soldiers returning from Iraq. N Engl J Med. (2008) 358:453–63. doi: 10.1056/NEJMoa072972
48.
Elder GA Mitsis EM Ahlers ST Cristian A . Blast-induced mild traumatic brain injury. Psychiatr Clin North Am. (2010) 33:757–81. doi: 10.1016/j.psc.2010.08.001
49.
Krady JK Basu A Allen CM Xu Y LaNoue KF Gardner TW et al . Minocycline reduces proinflammatory cytokine expression, microglial activation, and caspase-3 activation in a rodent model of diabetic retinopathy. Diabetes. (2005) 54:1559–65. doi: 10.2337/diabetes.54.5.1559
50.
Scholz R Sobotka M Caramoy A Stempfl T Moehle C Langmann T . Minocycline counter-regulates pro-inflammatory microglia responses in the retina and protects from degeneration. J Neuroinflammation. (2015) 12:209. doi: 10.1186/s12974-015-0431-4
51.
Yrjanheikki J Keinanen R Pellikka M Hokfelt T Koistinaho J . Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. (1998) 95:15769–74. doi: 10.1073/pnas.95.26.15769
52.
Hamacher J Hadizamani Y Huwer H Moehrlen U Bally L Stammberger U et al . Characteristics of inflammatory response and repair after experimental blast lung injury in rats. PLoS ONE. (2023) 18:e0281446. doi: 10.1371/journal.pone.0281446
53.
Heyburn L Batuure A Wilder D Long J Sajja VS . Neuroinflammation profiling of brain cytokines following repeated blast exposure. Int J Mol Sci. (2023) 24:12564. doi: 10.3390/ijms241612564
54.
Li Y Yang Z Liu B Valdez C Chavko M Cancio LC . Low-level primary blast induces neuroinflammation and neurodegeneration in rats. Mil Med. (2019) 184:265–72. doi: 10.1093/milmed/usy330
55.
Gill J Motamedi V Osier N Dell K Arcurio L Carr W et al . Moderate blast exposure results in increased IL-6 and TNFalpha in peripheral blood. Brain Behav Immun. (2017) 65:90–4. doi: 10.1016/j.bbi.2017.02.015
56.
Vorn R Edwards KA Hentig J Yun S Kim HS Lai C et al . A pilot study of whole-blood transcriptomic analysis to identify genes associated with repetitive low-level blast exposure in career breachers. Biomedicines. (2022) 10:690. doi: 10.3390/biomedicines10030690
57.
Edwards KA Leete JJ Smith EG Quick A Modica CM Wassermann EM et al . Elevations in tumor necrosis factor alpha and interleukin 6 from neuronal-derived extracellular vesicles in repeated low-level blast exposed personnel. Front Neurol. (2022) 13:723923. doi: 10.3389/fneur.2022.723923
58.
Scott LJ . Etanercept: a review of its use in autoimmune inflammatory diseases. Drugs. (2014) 74:1379–410. doi: 10.1007/s40265-014-0258-9
59.
Olmos G Llado J . Tumor necrosis factor alpha: a link between neuroinflammation and excitotoxicity. Mediators Inflamm. (2014) 2014:861231. doi: 10.1155/2014/861231
60.
Probert L . TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience. (2015) 302:2–22. doi: 10.1016/j.neuroscience.2015.06.038
61.
Shultz RB Zhong Y . Minocycline targets multiple secondary injury mechanisms in traumatic spinal cord injury. Neural Regen Res. (2017) 12:702–13. doi: 10.4103/1673-5374.206633
62.
Abdo Qaid EY Abdullah Z Zakaria R Long I . Minocycline mitigates tau pathology via modulating the TLR-4/NF-small ka, Cyrillicbeta signalling pathway in the hippocampus of neuroinflammation rat model. Neurol Res. (2024) 46:261–71. doi: 10.1080/01616412.2023.2296754
63.
Gama Sosa MA De Gasperi R Lind RH Pryor D Vargas DC Perez Garcia GS et al . Intramural hematomas and astrocytic infiltration precede perivascular inflammation in a rat model of repetitive low-level blast injury. J Neuropathol Exp Neurol. (2025) 84:337–52. doi: 10.1093/jnen/nlaf003
64.
Gupte R Brooks W Vukas R Pierce J Harris J . Sex differences in traumatic brain injury: what we know and what we should know. J Neurotrauma. (2019) 36:3063–91. doi: 10.1089/neu.2018.6171
65.
Cogan AM McCaughey VK Scholten J . Gender differences in outcomes after traumatic brain injury among service members and veterans. PM R. (2020) 12:301–4. doi: 10.1002/pmrj.12237
66.
Hubbard WB Velmurugan GV Brown EP Sullivan PG . Resilience of females to acute blood-brain barrier damage and anxiety behavior following mild blast traumatic brain injury. Acta Neuropathol Commun. (2022) 10:93. doi: 10.1186/s40478-022-01395-8
67.
McNamara EH Tucker LB Liu J Fu AH Kim Y Vu PA et al . Limbic responses following shock wave exposure in male and female mice. Front Behav Neurosci. (2022) 16:863195. doi: 10.3389/fnbeh.2022.863195
68.
Baskin BM Logsdon AF Janet Lee S Foresi BD Peskind E Banks WA et al . Timing matters: sex differences in inflammatory and behavioral outcomes following repetitive blast mild traumatic brain injury. Brain Behav Immun. (2023) 110:222–36. doi: 10.1016/j.bbi.2023.03.003
Summary
Keywords
blast, minocycline, post-traumatic stress disorder, rat, traumatic brain injury
Citation
Pérez Garcia G, Perez GM, De Gasperi R, Gama Sosa MA, Abutarboush R, Kawoos U, Zhu C, Toro CA, Hof PR, Ahlers ST and Elder GA (2025) Minocycline partially reverses established PTSD-related behavioral traits in rats exposed to repetitive low-level blast injury. Front. Neurol. 16:1666737. doi: 10.3389/fneur.2025.1666737
Received
15 July 2025
Accepted
03 November 2025
Published
02 December 2025
Volume
16 - 2025
Edited by
Dipa Natarajan, Kcat Enzymatic Pvt Ltd, India
Reviewed by
Luke R. Johnson, University of Tasmania, Australia
Mehrbod Mohammadian, Harvard Medical School, United States
Updates
Copyright
© 2025 Pérez Garcia, Perez, De Gasperi, Gama Sosa, Abutarboush, Kawoos, Zhu, Toro, Hof, Ahlers and Elder.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregory A. Elder, gregory.elder@va.gov
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.