Abstract
Background:
The albumin-to-globulin ratio (AGR) is a biomarker reflecting both nutritional status and inflammation, which has recently been implicated in the development of ischemic stroke. However, its potential association with the occurrence of cerebrovascular stenosis remains unclear. This study aimed to investigate the relationship between AGR and the incidence of cerebral atherosclerotic stenosis.
Methods:
Data from 766 adult patients with acute ischemic stroke (AIS) were included in this cross-sectional analysis. Binary logistic regression was used to evaluate the independent association between AGR and the risk of various cerebrovascular stenosis, including anterior circulation stenosis, posterior circulation stenosis, intracranial and extracranial stenosis. To explore the potential non-linear relationship between AGR and these outcomes, restricted cubic spline models were employed to further clarify these associations. Stratified analyses by body mass index (BMI), age, and sex were additionally conducted to explore the correlation between AGR and cerebral atherosclerotic stenosis under different conditions.
Results:
Patients with cerebral atherosclerotic stenosis had lower AGR levels than those without corresponding vascular stenosis. After adjusting for multiple covariates, AGR levels were negatively associated with the presence of stenosis in the posterior circulations (OR = 0.59, 95%CI = 0.38 ~ 0.90, p = 0.015) and intracranial stenosis (Q4: OR = 0.55, 95% CI = 0.34 ~ 0.89, p = 0.015). This association was essentially unaffected by BMI, age, or sex. Furthermore, a negative linear relationship was observed between AGR levels and the occurrence of posterior circulation stenosis (p for overall = 0.001, p for non-linear = 0.228) and intracranial vascular stenosis (p for overall <0.001, p for non-linear = 0.440).
Conclusion:
Higher AGR is associated with a reduced risk of multiple cerebral atherosclerotic stenosis. AGR levels are significantly associated with the presence of specific stenosis subtypes and could be hypothesized as a marker for risk stratification; this utility requires validation in prospective cohorts.
Graphical Abstract
The association between serum albumin-to-globulin ratio and cerebral atherosclerotic stenosis in patients with acute ischemic stroke: a study stratified by body mass index, age, and sex.
Background
Stroke represents a critical global health challenge, with ischemic stroke accounting for 65.3% of all stroke cases and ranking as the third leading cause of mortality worldwide (1). Between 1990 and 2021, inadequate management of modifiable risk factors—including obesity, elevated systolic blood pressure, and hyperglycemia—has further contributed to the rising incidence and socioeconomic burden of stroke (1). Therefore, the continuous development and implementation of effective and accessible strategies for early detection and prevention of stroke are of paramount importance. Ischemic stroke is a multifaceted pathological condition driven by a complex interplay of multiple factors, including aberrant immune activation, chronic inflammatory cascades, dysregulated lipid metabolism, and synergistic effects of various risk factors. It can be categorized into several subtypes: large artery atherosclerosis (LAA), cardioembolism (CE), small artery occlusion (SAO), stroke of other determined etiology (SOE), and stroke of undetermined etiology (SUE) (2). Among these, LAA is the most prevalent subtype and is characterized as an immune-mediated chronic inflammatory process occurring within the arterial wall (3). Dysfunction in nutritional and metabolic pathways may disrupt immune homeostasis, promote chronic inflammation, and heighten susceptibility to immune-related disorders. According to anatomical location and vascular origin, cerebral vessels can be classified as intracranial, extracranial, anterior circulation and posterior circulation. Posterior circulation stroke, primarily resulting from lesions in the vertebrobasilar arterial system, is often insidious in onset and associated with poor clinical outcomes and high mortality rates (4, 5). In contrast to the more recognizable manifestations of anterior circulation stroke, such as hemiparesis or aphasia, early symptoms of posterior circulation stroke frequently present as dizziness, which may delay timely diagnosis and treatment. Therefore, the early identification and monitoring of vascular stenosis across intracranial and extracranial regions, as well as within both anterior and posterior circulations, are essential for improving outcomes.
Albumin-to-globulin ratio (AGR) is a metabolic biomarker that reflect nutritional and immune status. BMI is a globally recognized screening index for obesity and serves as an established risk factor for ischemic stroke (6, 7). Serum albumin is commonly used as a biochemical marker for evaluating nutritional status; reduced albumin levels may indicate malnutrition or compromised health. Globulin levels are closely associated with the degree of systemic inflammation. In addition to the metabolic burden caused by the inflammatory response itself, increased capillary permeability during inflammation can facilitate the extravasation of serum albumin into interstitial spaces, resulting in hypoproteinemia, which has been linked to adverse clinical outcomes (8). Compared with individual measurements of serum albumin or globulin, AGR demonstrates greater diagnostic and prognostic value (9). Emerging evidence suggests that AGR is associated with depression (10), frailty (11), prognosis in malignant tumors (12), chronic disease progression, and the risk of postoperative infections. A prospective cohort study demonstrated that, among patients with acute ischemic stroke, a lower serum albumin-to-globulin ratio was independently associated with an increased risk of poor functional outcome and all-cause mortality at both 3-month and 1-year follow-up (13). The study by Han et al. (14) demonstrated that a lower albumin-to-globulin ratio is associated with more pronounced carotid atherosclerosis; in acute ischemic stroke, the albumin-to-globulin ratio serves as a superior predictor of “vascular aging” compared with globulin alone. A large-scale population study based on NHANES further confirmed that individuals with lower AGR levels exhibit increased incidence of both hemorrhagic and ischemic stroke (15).
However, it remains unclear whether AGR has a direct association with the risk of cerebral atherosclerotic stenosis. To address this gap, the present study employed digital subtraction angiography (DSA) to investigate the potential relationship between serum AGR levels and cerebral atherosclerotic stenosis, including both intracranial and extracranial involvement, as well as lesions located in the anterior or posterior circulation. Stratified analyses were further conducted based on BMI, age, and sex, aiming to provide evidence that may inform future research on the risk stratification and etiology of cerebrovascular stenosis.
Methods
Study population
This retrospective observational study enrolled 1,189 adult patients diagnosed with acute ischemic stroke (AIS) who underwent DSA in the First Hospital of Jilin University between March 2024 and June 2025. The exclusion criteria were strictly defined as follows: (1) age <18 years; (2) presence of concurrent hemorrhagic stroke, cerebral aneurysm, or intracranial space-occupying lesions; (3) cardiogenic cerebral embolism, vascular malformations (including arteriovenous malformations or arteriovenous fistulas), or vasculitis; (4) severe hepatic or renal dysfunction, or active systemic infection; (5) coexisting hematological disorders, history of malignancy, or tuberculosis; (6) autoimmune diseases, immunodeficiency conditions, or ongoing immunosuppressive therapy (including glucocorticoids); (7) incomplete clinical records, including missing data on height, weight, plasma albumin, or globulin levels. The study protocol was reviewed and approved by the Medical Ethics Committee of the First Hospital of Jilin University (approval number: 2025-480).
Clinical and laboratory data collection
Baseline data encompassed demographic characteristics, clinical parameters, and laboratory measurements. Specifically, the following variables were collected: age, sex, BMI (kg/m2), systolic blood pressure (SBP), diastolic blood pressure (DBP), history of comorbidities (including hypertension, diabetes mellitus, coronary heart disease (CHD), and stroke), smoking status, alcohol consumption history, and medication use (such as antihypertensive agents, hypoglycemic agents, statins, and antiplatelet drugs). Fasting venous blood samples were collected from all participants within 24 h of hospital admission. Laboratory assessments included white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), platelet count (PLT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, globulin, fasting blood glucose, total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), homocysteine, creatinine, uric acid, and urea. The AGR was defined as the ratio of serum albumin (g/L) to globulin (g/L). Serum albumin was measured using the Bromocresol Green method, and serum globulin level was quantitatively determined by immunoturbidimetry. Rigorous quality control procedures were implemented, including daily internal quality control (with a coefficient of variation < 5% for all assays) and regular participation in external quality assessment programs.
Imaging data acquisition and analysis
Cerebral angiography data were independently interpreted and subjected to quality control by two experienced neurointerventional specialists, each with over 5 years of diagnostic experience in DSA. Any disagreements in the quantitative stenosis measurement (defined as an interrater difference >10%) were adjudicated by a third senior neurointerventionalist who was blinded to the initial assessments. The vascular evaluation encompassed the following anatomical regions:
Intracranial arteries: M1–M2 segments of the middle cerebral artery, C6–C7 segments of the internal carotid artery, A1–A2 segments of the anterior cerebral artery, the entire basilar artery, V4 segment of the vertebral artery, and P1–P2 segments of the posterior cerebral artery; Extracranial arteries: the full length of the common carotid artery, C1–C5 segments of the internal carotid artery, V1–V3 segments of the vertebral artery, and the proximal portion of the subclavian artery; Anterior circulation: the common carotid artery – internal carotid artery – anterior/middle cerebral artery system; Posterior circulation: the vertebral artery – basilar artery – posterior cerebral artery system, including the origin of the subclavian artery.
The definition of a symptomatic stenosis was predicated on the occurrence of a transient ischemic attack or ischemic stroke within the relevant vascular territory in the month prior to DSA, with diagnoses adhering to ICD-11 standards (16). The degree of arterial stenosis was quantitatively assessed on DSA images using the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria. Briefly, the stenosis percentage was calculated as: [1 – (minimal residual lumen diameter/normal distal internal carotid artery diameter)] × 100%. A stenosis was considered hemodynamically significant if the lumen reduction was ≥50%. Utilizing this metric, cases were categorized: a lumen reduction of ≥30% indicated stenosis, a reduction of 70–99% or occlusion indicated severe stenosis.
Statistical analyses
All statistical analyses were conducted using R software (version 4.3.3), SPSS (version 26.0), and GraphPad Prism (version 9.0.0). Statistical significance was defined as a two-tailed p-value < 0.05.
The study population characteristics were divided into four groups based on AGR quartiles (Q1–Q4). The basic characteristics of the participants are presented as mean ± standard deviation or medians (25th–75th percentiles) for continuous variables and counts (percentages) for categorical variables. Comparisons among the four groups were conducted via analysis of variance (ANOVA) or the Kruskal–Wallis test for continuous variables and the chi-square (χ2) test for categorical variables. Multivariate logistic regression was used to analyze the associations between the AGR and the risk of various cerebrovascular stenosis, including anterior circulation stenosis, posterior circulation stenosis, intracranial stenosis and extracranial stenosis. In the quartile analyses, the p-value for trend was assessed by assigning the median value of the AGR to each quartile and treating this median as a continuous variable in the regression model. Model 1 was unadjusted; Model 2 was adjusted for age and sex; and Model 3 was further adjusted for age, sex, BMI, hypertension, diabetes mellitus, coronary heart disease, stroke, smoking and drinking. Multicollinearity among the covariates in the multivariate logistic regression models was evaluated using the variance inflation factor (VIF). A VIF value of less than 5 was considered to indicate no substantial multicollinearity. For each of the three models, p-values from all comparisons were adjusted for multiple testing using the Benjamini–Hochberg procedure to control the false discovery rate (FDR). Furthermore, restricted cubic spline (RCS) analyses with three knots was employed to examine potential non-linear relationships between AGR and different types of cerebrovascular stenosis risk. The location of three knots is based on clinical cut-offs. According to BMI status, the population was classified into two categories: individuals with a BMI ranging from 18.5 to 23.9 were defined as “normal weight,” those with a BMI of 24 or higher were defined as “overweight/obese.” We further stratified all participants by BMI, age (65 was the cutoff) and sex to explore the association between AGR and risk of cerebral stenosis in different states.
Results
Baseline characteristics of the study participants
This study enrolled 1,189 patients with acute ischemic stroke (AIS) who underwent digital subtraction angiography (DSA), and 766 cases completed the data analysis finally (enrollment process is shown in Figure 1).
Figure 1

Flowchart of participants’ selection. DSA, digital subtraction angiography.
Patients were divided into four groups according to AGR levels: Q1 group (AGR ≤ 1.34), Q2 group (1.34 < AGR ≤ 1.49), Q3 group (1.49 < AGR ≤ 1.64), and Q4 group (AGR > 1.64). Baseline characteristics are shown in Table 1: the mean age of patients was 63.6 years, and 50.1% were male. Hypertension was present in 514 cases (67.1%). Among all patients, 587 cases (76.6%) had anterior circulation stenosis, 440 cases (57.4%) had posterior circulation stenosis, 585 cases (76.4%) had intracranial vascular stenosis, and 479 cases (62.5%) had extracranial cerebrovascular stenosis. Additionally, the incidences of posterior circulation stenosis and intracranial stenosis were lower in the Q4 group.
Table 1
| Variables | Overall | Q1 | Q2 | Q3 | Q4 | p-value |
|---|---|---|---|---|---|---|
| (n = 766) | (n = 197) | (n = 189) | (n = 189) | (n = 191) | ||
| Age (years) | 63.6 ± 11.6 | 65.3 ± 11.2 | 65.7 ± 14.1 | 61.9 ± 10.2 | 61.5 ± 9.6 | <0.001*** |
| sex (Male) | 384 (50.1) | 84 (42.6) | 81 (42.9) | 100 (52.9) | 119 (62.3) | <0.001*** |
| BMI (kg/m2) | 24.9 ± 3.9 | 24.9 ± 4.3 | 25.2 ± 4.2 | 25.0 ± 3.8 | 24.7 ± 3.4 | 0.609 |
| SBP | 146.9 ± 20.2 | 149.3 ± 22.5 | 146.9 ± 19.7 | 146.7 ± 19.1 | 144.6 ± 19.2 | 0.149 |
| DBP | 85.7 ± 12.6 | 87.6 ± 14.0 | 84.8 ± 12.3 | 85.2 ± 10.6 | 85.5 ± 13.0 | 0.115 |
| Medical history | ||||||
| Hypertension | 514 (67.1) | 136 (69.0) | 129 (68.3) | 122 (64.6) | 127 (66.5) | 0.792 |
| DM | 223 (29.1) | 58 (29.4) | 51 (27.0) | 51 (27.0) | 63 (33.0) | 0.527 |
| CHD | 157 (20.5) | 44 (22.3) | 38 (20.1) | 42 (22.2) | 33 (17.3) | 0.575 |
| Stroke | 239 (31.2) | 66 (33.5) | 62 (32.8) | 59 (31.2) | 52 (27.2) | 0.548 |
| Smoking | 170 (22.2) | 44 (22.3) | 37 (19.6) | 44 (23.3) | 45 (23.6) | 0.780 |
| Drinking | 60 (7.8) | 21 (10.7) | 10 (5.3) | 13 (6.9) | 16 (8.4) | 0.242 |
| Medication | ||||||
| Antihypertensive drugs | 444 (58.0) | 119 (60.4) | 115 (60.8) | 104 (55.0) | 106 (55.5) | 0.518 |
| Antidiabetic drugs | 204 (26.6) | 54 (27.4) | 45 (23.8) | 48 (25.4) | 57 (29.8) | 0.575 |
| Statins | 171 (22.3) | 47 (23.9) | 48 (25.4) | 41 (21.7) | 35 (18.3) | 0.376 |
| Antiplatelet aggregation drugs | 292 (38.1) | 76 (38.6) | 78 (41.3) | 75 (39.7) | 63 (33.0) | 0.370 |
| Laboratory tests | ||||||
| WBC (×109/L) | 6.2 (5.2, 7.8) | 6.4 (5.2, 8.0) | 6.0 (5.3, 8.0) | 6.2 (5.2, 7.8) | 6.1 (5.3, 7.6) | 0.796 |
| RBC (×1012/L) | 4.4 (3.7, 4.9) | 4.3 (3.8, 5.0) | 4.3 (3.6, 4.9) | 4.4 (3.6, 5.0) | 4.4 (3.8, 4.9) | 0.397 |
| HGB (g/L) | 140.0 ± 14.6 | 140.2 ± 13.5 | 138.7 ± 15.4 | 139.4 ± 14.9 | 141.5 ± 14.7 | 0.282 |
| PLT (×109/L) | 224.1 ± 51.9 | 224.1 ± 50.1 | 224.6 ± 50.2 | 223.4 ± 53.5 | 224.0 ± 54.1 | 0.997 |
| ALT (U/L) | 19.6 (16.8, 24.8) | 20.9 (16.3, 25.2) | 19.6 (17.0, 25.6) | 19.6 (17.0, 24.3) | 19.1 (16.3, 24.0) | 0.604 |
| AST (U/L) | 17.5 (14.0, 22.4) | 17.5 (13.0, 22.4) | 17.5 (14.3, 19.8) | 17.1 (13.7, 21.6) | 18.2 (15.1, 23.9) | 0.204 |
| Albumin (g/L) | 40.1 (37.6, 43.1) | 38.8 (35.4, 41.2) | 39.5 (37.3, 43.0) | 40.7 (38.7, 43.9) | 41.5 (39.5, 44.7) | <0.001*** |
| Glucose (mmol/L) | 5.9 (5.2, 7.1) | 5.9 (5.3, 7.2) | 5.8 (5.2, 6.9) | 5.9 (5.2, 7.1) | 5.8 (5.1, 7.5) | 0.354 |
| TG (mmol/L) | 1.4 (1.0, 1.8) | 1.4 (1.1, 2.0) | 1.4 (1.1, 1.7) | 1.3 (1.0, 1.7) | 1.3 (0.9, 1.8) | 0.081 |
| TC (mmol/L) | 4.0 (3.4, 4.9) | 4.3 (3.6, 5.2) | 4.1 (3.4, 5.0) | 3.9 (3.3, 4.8) | 4.0 (3.3, 4.6) | <0.001*** |
| HDL-C (mmol/L) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.2) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.0 (0.9, 1.2) | 0.923 |
| LDL-C (mmol/L) | 2.5 (2.0, 3.1) | 2.8 (2.2, 3.3) | 2.6 (2.0, 3.1) | 2.3 (1.8, 3.0) | 2.4 (1.9, 2.9) | <0.001*** |
| Homocysteine (μmmol/L) | 11.5 (9.2, 14.7) | 12.2 (9.3, 15.6) | 11.6 (9.3, 14.4) | 11.1 (8.7, 13.9) | 11.3 (9.5, 14.7) | 0.084 |
| Uric acid (μmol/L) | 307.1 ± 81.5 | 306.9 ± 89.6 | 306.6 ± 75.4 | 308.1 ± 84.6 | 306.7 ± 76.0 | 0.998 |
| Urea (mmol/L) | 6.2 (5.2, 7.7) | 6.0 (5.2, 7.6) | 6.1 (5.2, 7.6) | 6.4 (5.2, 7.7) | 6.4 (5.4, 7.8) | 0.567 |
| Creatinine (μmol/L) | 66.5 (56.2, 78.5) | 67.1 (57.6, 77.5) | 66.1 (56.1, 81.6) | 65.8 (54.7, 79.4) | 66.6 (56.1, 78.4) | 0.943 |
| Stenosis status | ||||||
| No stenosis | 46 (6.0) | 8 (4.1) | 9 (4.8) | 13 (6.9) | 16 (8.4) | 0.262 |
| Intracranial stenosis | 585 (76.4) | 159 (80.7) | 151 (79.9) | 142 (75.1) | 133 (69.6) | 0.040* |
| Extracranial stenosis | 479 (62.5) | 129 (65.5) | 116 (61.4) | 115 (60.8) | 119 (62.3) | 0.784 |
| Anterior circulation stenosis | 587 (76.6) | 153 (77.7) | 143 (75.7) | 143 (75.7) | 148 (77.5) | 0.941 |
| Posterior circulation stenosis | 440 (57.4) | 130 (66.0) | 109 (57.7) | 99 (52.4) | 102 (53.4) | 0.027* |
Baseline characteristics of participants categorized by AGR quartiles.
AGR, Albumin/Globulin Ratio; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; DM, diabetes mellitus; CHD, coronary heart disease; WBC, white blood cell count; RBC, red blood cell count; HGB, Hemoglobin; PLT, platelet count; ALT, alanine aminotransferase; AST, aspartate transaminase; TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. Smoking status: Patients were categorized as ‘Yes’ (smokers) if they were current smokers (defined as having smoked cigarettes continuously or cumulatively for ≥6 months and were smoking at the time of or within 1 month prior to admission). All other patients were classified as ‘No’ (non-smokers); Alcohol use: Patients were categorized as ‘Yes’ (drinkers) if they were current alcohol drinkers (defined as consuming alcohol at least once per week for a continuous period of ≥6 months prior to admission). All other patients were classified as ‘No’ (non-drinkers); Stroke history: A history of stroke was confirmed by a combination of self-report and verification through medical records, requiring documented clinical evidence (e.g., neurological deficit) supported by compatible neuroimaging findings (CT or MRI). *p < 0.05, ***p < 0.001.
Violin plots showed differences in AGR distribution among patients with different stenosis statuses. AGR levels in patients with anterior circulation stenosis, posterior circulation stenosis, intracranial vascular stenosis, and extracranial cerebrovascular stenosis were all lower than those in participants without corresponding vascular stenosis (Figure 2).
Figure 2
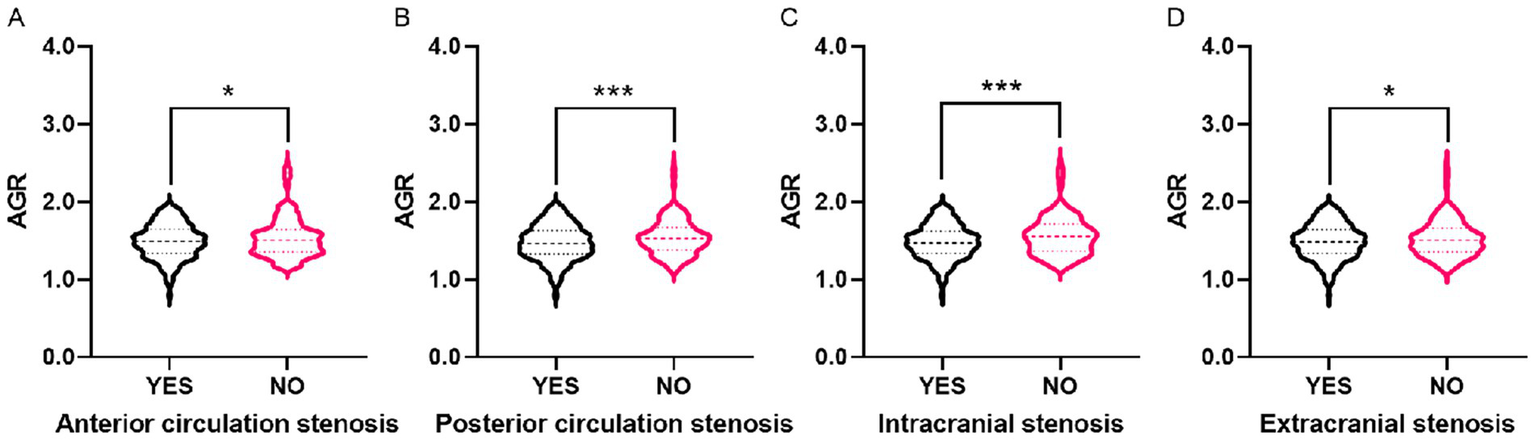
The violin plots demonstrating the distribution of the AGR among participants in different groups. (A) With and without anterior circulation stenosis. (B) With and without posterior circulation stenosis. (C) With and without intracranial stenosis. (D) With and without extracranial stenosis. The AGR is a dimensionless quantitative variable. The demarcation values used throughout the analysis are as follows: Q1 (≤1.34), Q2 (1.34 < AGR ≤ 1.49), Q3 (1.49 < AGR ≤ 1.64), Q4 (>1.64). AGR, Albumin/Globulin Ratio. *p < 0.05, ***p < 0.001.
The association of AGR with anterior and posterior circulation stenosis
As shown in Table 2, after adjusting for covariates (age, sex, BMI, hypertension, diabetes mellitus, CHD, stroke history, smoking, and drinking), no significant association was observed between AGR and anterior circulation stenosis when AGR was analyzed as either a continuous or categorical variable (all p > 0.05). However, after adjusting for the same covariates, a negative association was observed between AGR and the occurrence of posterior circulation stenosis when AGR was analyzed as a continuous variable (OR = 0.27, 95% CI = 0.14 ~ 0.53, p < 0.001). When AGR was analyzed as a categorical variable, compared with patients in the lowest AGR group (Q1), those in the higher AGR groups (Q3: OR = 0.60, 95% CI = 0.39 ~ 0.92, p = 0.019; Q4: OR = 0.59, 95% CI = 0.38 ~ 0.90, p = 0.015) had a significantly lower incidence of posterior circulation stenosis. Furthermore, it is noteworthy that AGR quartiles were significantly associated with a trend in posterior circulation stenosis (p for trend < 0.05). We assessed multicollinearity among all covariates included in the multivariate logistic regression models (Model 3) by calculating the VIF. The results confirm that no significant multicollinearity was present (all VIF values < 5) (Supplementary Table S1). Then, we applied the Benjamini-Hochberg correction for multiple testing to adjust the p-values for all comparisons across the three models (Supplementary Table S2).
Table 2
| Variables | Model 1 | p-value | Model 2 | p-value | Model 3 | p-value |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Anterior circulation stenosis | ||||||
| AGR | 0.46 (0.22 ~ 0.94) | 0.034* | 0.51 (0.25 ~ 1.07) | 0.076 | 0.49 (0.23 ~ 1.23) | 0.060 |
| AGR quartiles | ||||||
| Q1 | Reference | |||||
| Q2 | 0.89 (0.56 ~ 1.43) | 0.642 | 0.89 (0.55 ~ 1.43) | 0.630 | 0.89 (0.55 ~ 1.43) | 0.628 |
| Q3 | 0.89 (0.56 ~ 1.43) | 0.642 | 0.95 (0.59 ~ 1.53) | 0.835 | 0.94 (0.58 ~ 1.52) | 0.799 |
| Q4 | 0.99 (0.61 ~ 1.60) | 0.966 | 1.08 (0.66 ~ 1.76) | 0.758 | 1.06 (0.65 ~ 1.72) | 0.832 |
| P for trend | 0.998 | 0.682 | 0.738 | |||
| Posterior circulation stenosis | ||||||
| AGR | 0.28 (0.15 ~ 0.53) | <0.001*** | 0.28 (0.14 ~ 0.55) | <0.001*** | 0.27 (0.14 ~ 0.53) | <0.001*** |
| AGR quartiles | ||||||
| Q1 | Reference | |||||
| Q2 | 0.70 (0.47 ~ 1.06) | 0.093 | 0.70 (0.46 ~ 1.06) | 0.090 | 0.71 (0.47 ~ 1.09) | 0.115 |
| Q3 | 0.57 (0.38 ~ 0.86) | 0.007** | 0.59 (0.39 ~ 0.89) | 0.013* | 0.60 (0.39 ~ 0.92) | 0.019* |
| Q4 | 0.59 (0.39 ~ 0.89) | 0.012* | 0.60 (0.39 ~ 0.91) | 0.016* | 0.59 (0.38 ~ 0.90) | 0.015* |
| P for trend | 0.010* | 0.016* | 0.016* | |||
Association of AGR with anterior and posterior circulation stenosis.
Model 1: unadjusted; Model 2:adjusted for age and sex; Model 3:adjusted for age, sex, BMI, hypertension, diabetes mellitus, coronary heart disease, stroke, smoking and drinking. AGR, Albumin/Globulin Ratio; BMI, body mass index; OR, odds ratio; CI, confidence interval. *p < 0.05, **p < 0.01, ***p < 0.001.
Multivariable-adjusted restricted cubic spline (RCS) analysis showed a linear association between AGR and posterior circulation stenosis (p for overall = 0.001, p for non-linear = 0.228) (Figure 3).
Figure 3
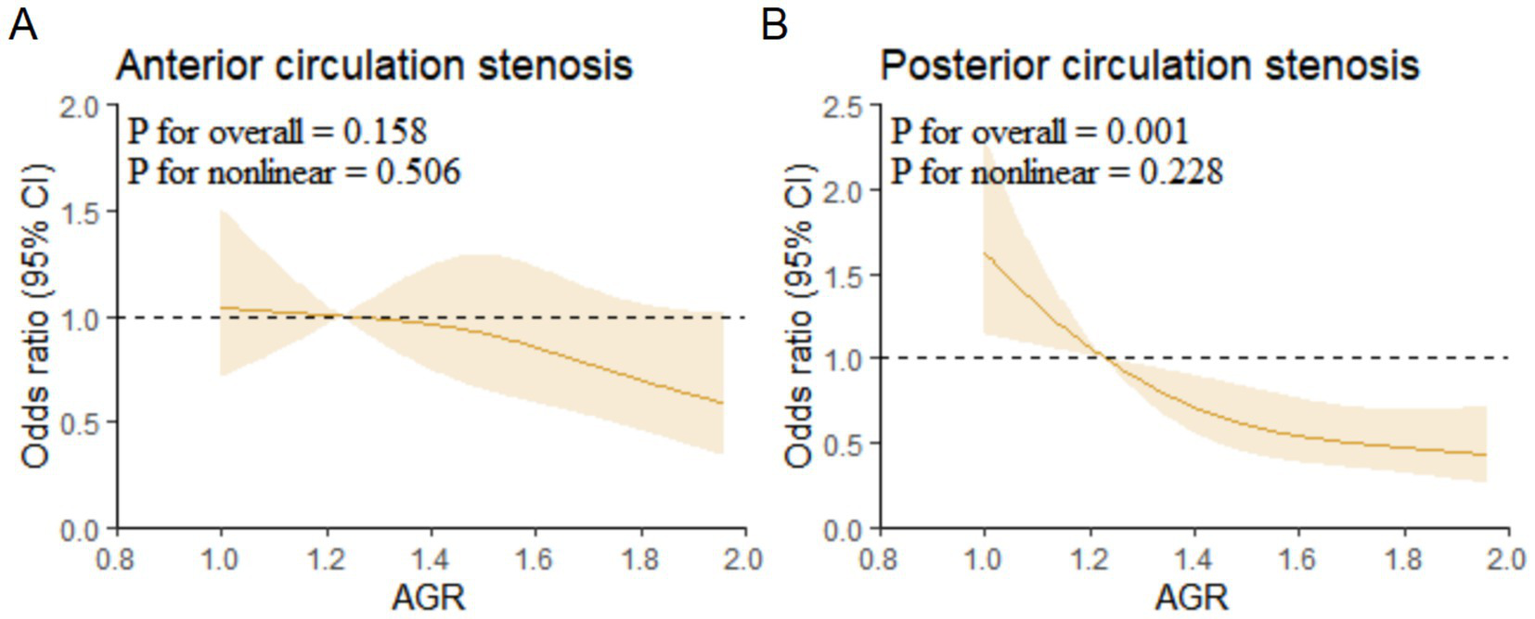
Restricted cubic spline regression analysis for association of AGR with anterior and posterior circulation stenosis. (A) Anterior circulation stenosis. (B) Posterior circulation stenosis. AGR, Albumin/Globulin Ratio.
The association of AGR with intracranial and extracranial stenosis
After adjusting for covariates, when AGR was analyzed as a continuous variable, a negative association was observed between AGR and intracranial stenosis (OR = 0.20, 95% CI = 0.09 ~ 0.42, p < 0.001), as well as extracranial stenosis (OR = 0.47, 95% CI = 0.24 ~ 0.93, p = 0.029). When AGR was analyzed as a categorical variable, compared with patients in the lowest AGR group (Q1), those in the higher AGR groups (Q4: OR = 0.55, 95% CI = 0.34 ~ 0.89, p = 0.015) had a significantly lower incidence of intracranial circulation stenosis. Moreover, it is noteworthy that AGR quartiles were significantly associated with a trend in intracranial stenosis (p for trend < 0.05). However, when AGR was analyzed as a categorical variable, no association was observed between AGR and extracranial stenosis (Table 3).
Table 3
| Variables | Model 1 | p-value | Model 2 | p-value | Model 3 | p-value |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Intracranial stenosis | ||||||
| AGR | 0.21 (0.10 ~ 0.43) | <0.001*** | 0.20 (0.09 ~ 0.43) | <0.001*** | 0.20 (0.09 ~ 0.42) | <0.001*** |
| AGR quartiles | ||||||
| Q1 | Reference | |||||
| Q2 | 0.95 (0.58 ~ 1.57) | 0.840 | 0.96 (0.58 ~ 1.58) | 0.859 | 0.93 (0.56 ~ 1.54) | 0.774 |
| Q3 | 0.72 (0.45 ~ 1.17) | 0.187 | 0.72 (0.44 ~ 1.17) | 0.178 | 0.70 (0.43 ~ 1.15) | 0.162 |
| Q4 | 0.55 (0.34 ~ 0.88) | 0.012* | 0.55 (0.34 ~ 0.86) | 0.014* | 0.55 (0.34 ~ 0.89) | 0.015* |
| P for trend | 0.005** | 0.007** | 0.006** | |||
| Extracranial stenosis | ||||||
| AGR | 0.48 (0.25 ~ 0.91) | 0.024* | 0.48 (0.25 ~ 0.94) | 0.033* | 0.47 (0.24 ~ 0.93) | 0.029* |
| AGR quartiles | ||||||
| Q1 | Reference | |||||
| Q2 | 0.84 (0.55 ~ 1.27) | 0.402 | 0.84 (0.55 ~ 1.28) | 0.411 | 0.87 (0.56 ~ 1.33) | 0.508 |
| Q3 | 0.82 (0.54 ~ 1.24) | 0.345 | 0.87 (0.57 ~ 1.33) | 0.520 | 0.88 (0.57 ~ 1.36) | 0.565 |
| Q4 | 0.87 (0.58 ~ 1.32) | 0.515 | 0.89 (0.58 ~ 1.37) | 0.586 | 0.88 (0.57 ~ 1.35) | 0.550 |
| P for trend | 0.551 | 0.663 | 0.715 | |||
Association of AGR with intracranial and extracranial stenosis.
Model 1: unadjusted; Model 2:adjusted for age and sex; Model 3:adjusted for age, sex, BMI, hypertension, diabetes mellitus, coronary heart disease, stroke, smoking and drinking. AGR, Albumin/Globulin Ratio; BMI, body mass index; OR, odds ratio; CI, confidence interval. *p < 0.05, **p < 0.01, ***p < 0.001.
Multivariable-adjusted RCS analysis showed a linear association between AGR and intracranial vascular stenosis (p for overall < 0.001, p for non-linear = 0.440) (Figure 4).
Figure 4
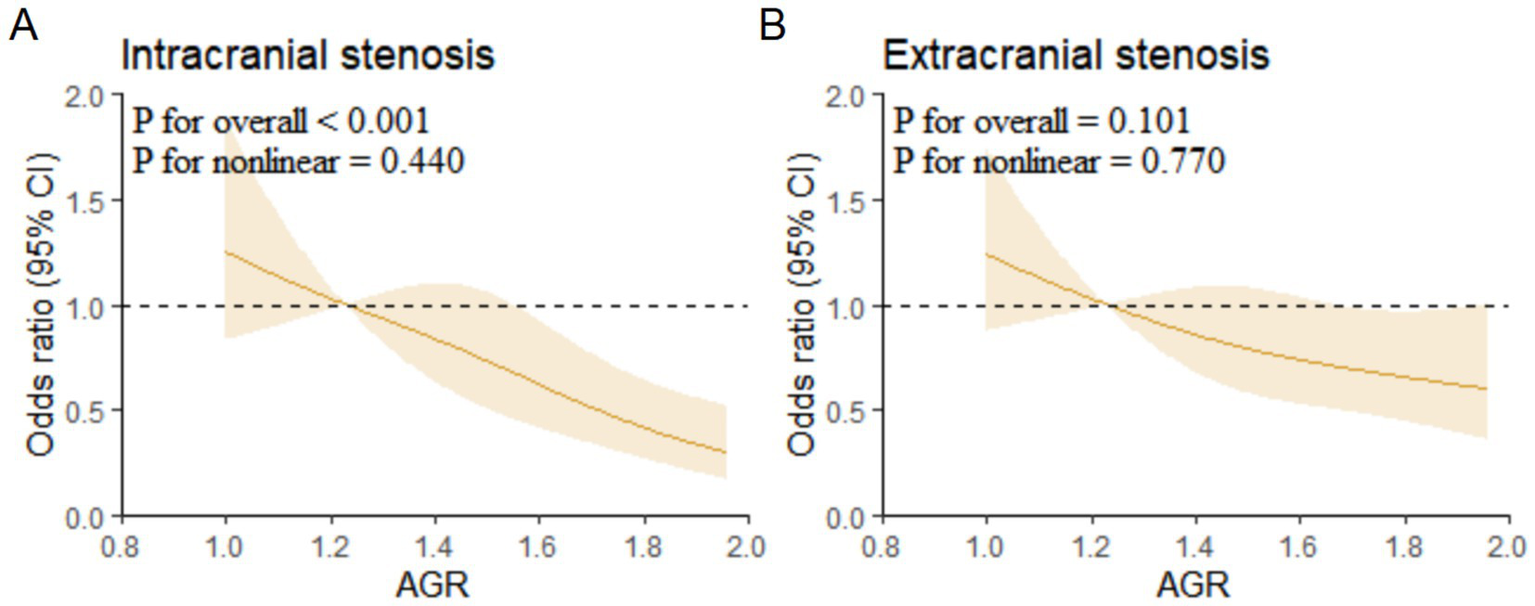
Restricted cubic spline regression analysis for association of AGR with intracranial and extracranial stenosis. (A) Intracranial stenosis. (B) Extracranial stenosis. AGR, Albumin/Globulin Ratio.
The association between AGR and vascular stenosis stratified by BMI
We further performed stratified analysis by BMI status to explore the association between AGR and cerebrovascular stenosis (Figure 5). Firstly, when AGR was treated as a continuous variable, AGR was negatively associated with the occurrence of posterior circulation stenosis (normal weight: OR = 0.23, 95%CI = 0.08 ~ 0.65, p = 0.005; overweight/obese: OR = 0.29, 95%CI = 0.12 ~ 0.73, p = 0.008) and intracranial vascular stenosis (normal weight: OR = 0.21, 95%CI = 0.07 ~ 0.66, p = 0.008; overweight/obese: OR = 0.19, 95%CI = 0.07 ~ 0.56, p = 0.003) in both normal weight and overweight/obese groups.
Figure 5
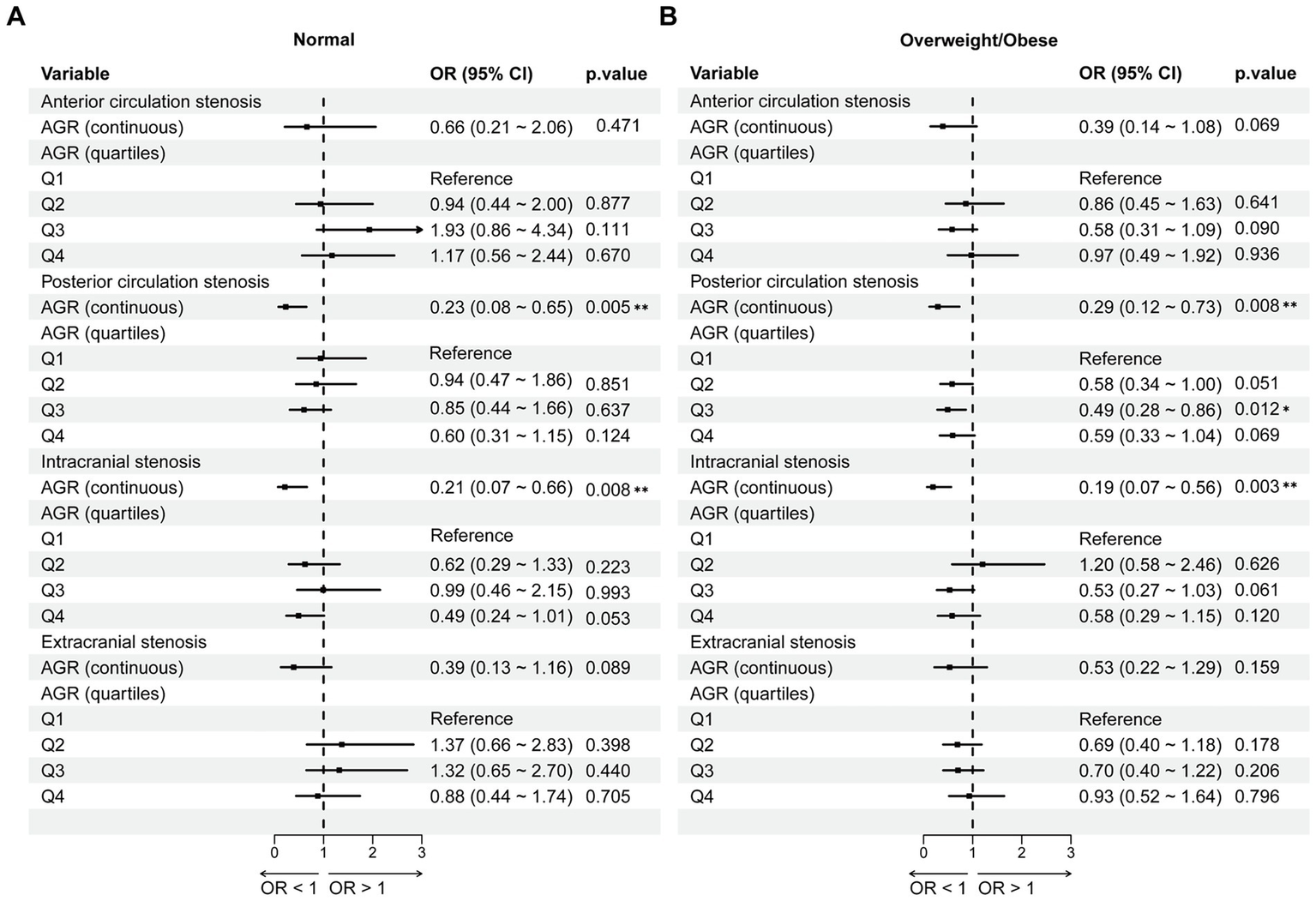
The association between AGR and vascular stenosis stratified by BMI. (A) Normal weight group. (B) Overweight/Obese group. Adjusted for age, sex, hypertension, diabetes mellitus; coronary heart disease, stroke, smoking and drinking. AGR, Albumin/Globulin Ratio; OR, odds ratio; CI, confidence interval. *p < 0.05, **p < 0.01.
When AGR was categorized, compared with the Q1 group, only AGR in the Q3 group of the overweight/obese group was negatively associated with posterior circulation stenosis (OR = 0.49, 95%CI = 0.28 ~ 0.86, p = 0.012). Overall, the association between AGR and cerebrovascular stenosis was hardly affected by BMI status.
The association between AGR and vascular stenosis stratified by age and sex
Subgroup analyses were further performed to evaluate the predictive value of AGR for cerebrovascular stenosis in different populations. Specifically, when stratified by age (cutoff at 65 years), as a continuous variable, AGR was negatively associated with posterior circulation stenosis (OR = 0.39, 95% CI = 0.16 ~ 0.96, p = 0.041) and intracranial stenosis (OR = 0.23, 95% CI = 0.08 ~ 0.66, p = 0.006) in participants <65 years, and similarly with posterior circulation stenosis (OR = 0.17, 95% CI = 0.06 ~ 0.48, p = 0.001) and intracranial stenosis (OR = 0.14, 95% CI = 0.05 ~ 0.41, p < 0.001) in those ≥65 years. As a categorical variable, in participants ≥65 years, compared with the Q1 group, the Q4 group showed a negative association between AGR and posterior circulation stenosis (OR = 0.52, 95% CI = 0.27 ~ 0.99, p = 0.047), as well as intracranial stenosis (OR = 0.32, 95% CI = 0.16 ~ 0.65, p = 0.002) (Supplementary Table S3).
When stratified by sex, as a continuous variable, AGR was negatively associated with posterior circulation stenosis (OR = 0.35, 95% CI = 0.14 ~ 0.88, p = 0.025) and intracranial stenosis (OR = 0.16, 95% CI = 0.06 ~ 0.45, p = 0.001) in males, and with posterior circulation stenosis (OR = 0.22, 95% CI = 0.08 ~ 0.60, p = 0.003) and intracranial stenosis (OR = 0.22, 95% CI = 0.07 ~ 0.71, p = 0.011) in females (Supplementary Table S4). Overall, the results of age- and sex-stratified analyses were consistent with those in the overall population.
Discussion
In this study, we observed that the AGR was negatively associated with the presence of cerebral atherosclerotic stenosis, including both anterior and posterior circulation stenosis as well as intracranial and extracranial stenosis. This association remained consistent across subgroups stratified by BMI, age, and sex. Furthermore, our findings revealed, for the first time, a linear relationship between AGR levels and the severity of posterior circulation and intracranial stenosis. These results suggest that AGR may serve as a novel biomarker for cerebral artery stenosis and could potentially offer enhanced predictive value for stenosis risk in specific vascular territories.
Albumin is a plasma protein synthesized by the hepatic tissue and serves as a critical component in maintaining colloid osmotic pressure and facilitating the transport of various substances within the circulatory system. It exerts multiple physiological functions, including modulation of immune responses, mitigation of inflammatory injury, and scavenging of free radicals (17). Globulin, predominantly synthesized by lymphoid organs, is primarily secreted by plasma cells. As a central element of humoral immunity, globulin collaborates with albumin to preserve plasma oncotic pressure, thereby preventing tissue edema. Additionally, it plays an essential role in complement activation and inflammatory processes. Under conditions of trauma, inflammation, or systemic stress, serum albumin levels are typically diminished. A reduction in albumin concentration (<35 g/L) reflects impaired hepatic synthetic capacity and compromised nutritional status. Consequently, several studies have proposed that serum albumin may serve as a potential biomarker for assessing systemic inflammatory states (15, 18).
Clinical observational studies have demonstrated that persistent hypoalbuminemia is associated with adverse outcomes in patients with sepsis, whereas normalization of serum albumin levels may indicate clinical improvement (19). Accumulating evidence indicates that serum albumin levels significantly influence clinical outcomes in individuals with ischemic stroke (20). A nationwide registry study involving 13,618 patients revealed that elevated admission albumin levels were independently linked to improved functional recovery and reduced mortality, with consistent associations observed at both 3-month and 1-year follow-up periods (21). Similarly, a meta-analysis encompassing 23,597 patients confirmed that low admission albumin levels were significantly correlated with increased long-term mortality following stroke (22). Nonetheless, the therapeutic use of albumin infusion during the acute phase of ischemic stroke remains controversial. Some studies suggest that such treatment may elevate the risk of pulmonary edema and intracranial hemorrhage (23), although these effects appear to be influenced by the dosage and timing of administration.
AGR represents a novel biomarker reflecting both nutritional status and immune function, which has demonstrated significant clinical relevance in the pathogenesis and progression of various diseases. Compared to individual measurements of albumin or globulin levels, AGR exhibits superior prognostic performance, likely due to its integrated assessment of both nutritional and inflammatory components that influence disease outcomes. Currently, AGR is recognized as a valuable prognostic indicator in multiple malignancies. A meta-analysis summarizing 18 studies on solid tumors over the past decade reported that AGR was significantly associated with at least one prognostic outcome in each cancer type examined, supporting its role as a cost-effective and clinically meaningful predictive marker (12). When AGR is less than 1.1, it is associated with a shorter overall survival period in patients with renal cell carcinoma (24). Moreover, emerging evidence suggests that AGR serves as an effective diagnostic biomarker for periprosthetic joint infection following prosthetic implantation (25). In critically ill ICU patients, reduced AGR levels are independently associated with increased risks of pneumonia and 28-day mortality (26). A cross-sectional study involving 8,381 participants revealed that long-term regular aerobic exercise (≥2 sessions per week for more than 1 year) significantly elevates AGR levels, potentially contributing to the inhibition of atherosclerotic progression. Furthermore, higher AGR values were correlated with reduced systemic inflammation (27). Han et al. (14) reported a significant association between AGR and common carotid artery intima-media thickness among ischemic stroke patients, suggesting a potential role of AGR in modulating atherosclerosis progression. Specifically, elevated AGR levels were associated with a 59% reduction in the risk of abnormal intima-media thickness. However, the mere presence of atherosclerosis does not necessarily lead to hemodynamically significant stenosis, as this progression depends on plaque characteristics, spatial distribution, and vascular compensatory mechanisms. In the present study, we provide the first evidence demonstrating a direct negative correlation between AGR levels and the presence of cerebral atherosclerotic stenosis, encompassing both anterior and posterior circulation as well as intracranial and extracranial involvement. These findings suggest that individuals with low AGR may benefit from earlier vascular evaluation and intensified therapeutic interventions to prevent irreversible stenotic changes.
It is well known that being overweight and obese are risk factors for atherosclerosis. The immune response in atherosclerosis involves various immune cells, along with their associated cytokines and adhesion molecules. Cholesterol in the brain accounts for approximately 25% of the body’s total cholesterol, with the majority located in the myelin sheath (28). Cholesterol is also a key component of synaptic vesicles, playing essential roles in maintaining cell membrane integrity, myelin formation, synaptic development, neural repair and remodeling, and signaling pathways critical to neurodevelopment (29). However, when cholesterol becomes overloaded, it can aggregate into crystals that activate the NLRP3 inflammasome, a crucial driver of atherosclerosis (30, 31). Elevated circulating cholesterol levels could influence T-cell activation and reprogramming, promoting the differentiation of CD4 + T cells toward an inflammatory Th1 phenotype, as observed in mouse models (32). For individuals with a BMI indicating overweight status, early implementation of weight management strategies and active weight reduction interventions have been demonstrated to significantly lower the risk of ischemic stroke (6). Notably, an elevated BMI during adolescence in males has been linked to an increased risk of early-onset atrial fibrillation, which in turn is associated with worse long-term outcomes following ischemic stroke (7).
Emerging evidence also suggests that BMI may influence stroke incidence through interactive effects with the triglyceride-glucose index (TyG), highlighting the complex interplay between metabolic dysregulation and cerebrovascular events (33). Importantly, our findings reveal that the association between AGR and atherosclerotic vascular stenosis remains robust and consistently independent of confounding variables such as body mass index, age, or gender. This observation underscores the potential role of AGR as a stable and reliable biomarker across diverse patient subgroups. These results further imply that, while traditional risk factor modification remains crucial, clinicians should also monitor albumin and globulin levels in young individuals with normal body weight. Maintaining adequate intake of high-quality proteins to prevent hypoproteinemia may serve as a complementary strategy for reducing the risk of adverse clinical outcomes.
Furthermore, our study revealed a linear association between AGR levels and the presence of posterior circulation stenosis as well as intracranial vascular stenosis. This intriguing discrepancy may be attributed to a confluence of anatomical, hemodynamic, and pathological factors. Firstly, from an anatomical perspective, this phenomenon may be attributed to anatomical and physiological differences between intracranial and extracranial arteries, particularly in terms of nutrient vessel density and antioxidant capacity, which limit lipid deposition and render intracranial arteries more vulnerable to inflammatory insults, and intracranial arteries reside within the confined space of the skull and lack an external elastic lamina and vasa vasorum, rendering them more vulnerable to systemic metabolic and inflammatory insults reflected by AGR, compared to the more resilient extracranial arteries (34). Secondly, hemodynamic characteristics differ significantly; the posterior circulation, with its more tortuous anatomy and unique flow patterns (e.g., in the vertebrobasilar system), might experience altered shear stress that modulates the localization and progression of inflammation-driven atherosclerosis, to which AGR is intrinsically linked (13, 27). Lastly, posterior and anterior circulation arteries originate from distinct embryonic vascular systems, leading to inherent structural disparities in their vessel walls and more tortuous anatomical pathways in the posterior circulation (35). These morphological features directly influence cerebral hemodynamics (36) and may render posterior circulation vessels more sensitive to systemic nutritional status and localized arterial wall inflammation. High-Resolution Vessel Wall Imaging has further demonstrated marked differences in plaque morphology between anterior and posterior circulation arteries. Specifically, posterior circulation plaques exhibit larger surface areas and stronger contrast enhancement, likely due to increased inflammatory activity and neovascularization, thereby elevating the risk of plaque rupture (37). Compared with anterior circulation stroke, posterior circulation involvement is typically associated with more severe clinical presentations, worse functional outcomes, and higher mortality rates. The novel association between AGR and posterior circulation stenosis identified in this study may serve as a cost-effective and accessible parameter associated with the presence of posterior circulation stenosis. When integrated with conventional risk factors, AGR may aid in identifying asymptomatic high-risk individuals, thereby contributing to our understanding of the disease etiology and informing hypotheses for future interventional studies.
With the rapid advancement of multi-omics technologies, high-throughput analytical tools, and artificial intelligence, research into stroke biomarkers is transitioning from single-molecule approaches toward multidimensional and dynamic integration models. This paradigm shift presents both new opportunities and challenges for the precision diagnosis and tailored management of stroke. However, several limitations must be acknowledged. First, as a single-center investigation, our findings may be subject to selection bias and may not fully represent broader patient populations. Second, the current observational design does not allow for causal inference regarding the relationship between AGR and cerebral vascular stenosis. Further mechanistic studies and large-scale, multicenter prospective trials are warranted to validate these findings and assess their clinical utility. While our findings suggest the potential value of AGR in risk assessment, it is crucial to interpret these results within the context of our study population—individuals who have already experienced an acute ischemic stroke. Therefore, our results primarily illuminate the role of AGR in the pathophysiology of established cerebral atherosclerosis rather than its predictive power for first-ever events in asymptomatic individuals. The promising clinical outlook of using AGR for identifying asymptomatic high-risk individuals remains a hypothesis that must be rigorously validated in future large-scale, prospective cohort studies conducted in community-based populations.
Conclusion
This study demonstrates a significant association between AGR levels and the presence of posterior circulation stenosis as well as intracranial vascular stenosis. As a readily accessible and cost-effective biomarker, AGR holds potential for the early identification of cerebral atherosclerotic stenosis. Incorporating AGR into clinical practice may facilitate timely risk factor management and contribute to more informed decision-making in the diagnosis and treatment of stroke.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the First Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the research employs medical records and biological specimens collected from prior clinical diagnoses and treatments; therefore, a request for exemption from informed consent has been submitted.
Author contributions
Y-xF: Methodology, Formal analysis, Data curation, Software, Writing – review & editing, Investigation, Writing – original draft. JC: Conceptualization, Investigation, Methodology, Writing – review & editing, Data curation. XY: Writing – review & editing, Data curation, Visualization. YL: Data curation, Writing – review & editing, Visualization. T-fS: Data curation, Visualization, Writing – review & editing. LC: Project administration, Writing – review & editing, Supervision, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China [grant numbers 82071351, 82301538, and 82371371].
Acknowledgments
We would like to acknowledge the support of the National Natural Science Foundation of China for this research project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1666940/full#supplementary-material
- AGR
Albumin-to-Globulin Ratio
- AIS
Acute Ischemic Stroke
- AST
Aspartate Transaminase
- ALT
Alanine Aminotransferase
- ANOVA
Analysis of Variance
- BMI
Body Mass Index
- CHD
Coronary Heart Disease
- CE
Cardioembolism
- CI
Confidence Interval
- DM
Diabetes Mellitus
- DBP
Diastolic Blood Pressure
- HDL-C
High-Density Lipoprotein Cholesterol
- HGB
Hemoglobin
- LAA
Large Artery Atherosclerosis
- LDL-C
Low-Density Lipoprotein Cholesterol
- OR
Odds Ratio
- PLT
Platelet Count
- RCS
Restricted Cubic Spline
- RBC
Red Blood Cell Count
- SAO
Small Artery Occlusion
- SOE
Stroke of Other Determined Etiology
- SUE
Stroke of Undetermined Etiology
- SBP
Systolic Blood Pressure
- TG
Triglyceride
- TC
Total Cholesterol
- TyG
Triglyceride-Glucose Index
- WBC
White Blood Cell Count
Glossary
References
1.
GBD 2021. Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S1474-4422(24)00369-7
2.
Chen P-H Gao S Wang Y-J Xu A-D Li Y-S Wang D . Classifying ischemic stroke, from TOAST to CISS. CNS Neurosci Ther. (2012) 18:452–6. doi: 10.1111/j.1755-5949.2011.00292.x
3.
Ludewig B Zinkernagel RM Hengartner H . Arterial inflammation and atherosclerosis. Trends Cardiovasc Med. (2002) 12:154–9. doi: 10.1016/s1050-1738(01)00166-9
4.
Lee S-H Han JH Jung I Jung J-M . Do thrombolysis outcomes differ between anterior circulation stroke and posterior circulation stroke? A systematic review and meta-analysis. Int J Stroke. (2020) 15:849–57. doi: 10.1177/1747493020909634
5.
Huo X Raynald Gao F Ma N Mo D Sun X et al . Characteristic and prognosis of acute large vessel occlusion in anterior and posterior circulation after endovascular treatment: the ANGEL registry real world experience. J Thromb Thrombolysis. (2020) 49:527–32. doi: 10.1007/s11239-020-02054-2.
6.
Horn JW Feng T Mørkedal B Aune D Strand LB Horn J et al . Body mass index measured repeatedly over 42 years as a risk factor for ischemic stroke: the HUNT study. Nutrients. (2023) 15:1232. doi: 10.3390/nu15051232
7.
Djekic D Lindgren M Åberg ND Åberg M Fengsrud E Poci D et al . Body mass index in adolescence and long-term risk of early incident atrial fibrillation and subsequent mortality, heart failure, and ischemic stroke. J Am Heart Assoc. (2022) 11:e025984. doi: 10.1161/JAHA.121.025984
8.
Soeters PB Wolfe RR Shenkin A . Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. (2018) 43:181–93. doi: 10.1002/jpen.1451
9.
Yang D Shen J Huang H Wang J Sun F Zeng T et al . Elevated albumin to globulin ratio on day 7 is associated with improved function outcomes in acute ischemic stroke patients with intravenous thrombolysis. J Inflamm Res. (2022) 15:2695–705. doi: 10.2147/JIR.S347026
10.
Xu Q Wang J Li H Chen X . A study investigating how the albumin-globulin ratio relates to depression risk within U.S. adults: a cross-sectional analysis. Front Nutr. (2024) 11:1453044. doi: 10.3389/fnut.2024.1453044
11.
Sun L Li C Feng B-B Liu Y-Y Ma R-W Zhang Y-X et al . The association between albumin, globulin, albumin-to-globulin ratio, and frailty in middle-aged and older adults: evidence from NHANES 2013-2014. Curr Pharm Des. (2025) 31:3303–11. doi: 10.2174/0113816128362350250423113437
12.
Roberts WS Delladio W Price S Murawski A Nguyen H . The efficacy of albumin-globulin ratio to predict prognosis in cancer patients. Int J Clin Oncol. (2023) 28:1101–11. doi: 10.1007/s10147-023-02380-4
13.
Wang A Zhang Y Xia G Tian X Zuo Y Chen P et al . Association of serum albumin to globulin ratio with outcomes in acute ischemic stroke. CNS Neurosci Ther. (2023) 29:1357–67. doi: 10.1111/cns.14108
14.
Han Q You S Miao M Zheng D Du H Sun Y et al . Albumin-globulin ratio and common carotid artery intima-media thickness in patients with ischemic stroke. Curr Neurovasc Res. (2023). doi: 10.2174/0115672026276085231122053601 [Epub ahead of print].
15.
Tan X Lv C Lu C Luo Y Mei Z-G . Association between serum a/G ratio and stroke: data from NHANES 2009-2020. Front Nutr. (2025) 12:1512165. doi: 10.3389/fnut.2025.1512165
16.
Chimowitz MI Lynn MJ Derdeyn CP Turan TN Fiorella D Lane BF et al . Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
17.
Wu N Liu T Tian M Liu C Ma S Cao H et al . Albumin, an interesting and functionally diverse protein, varies from 'native' to 'effective' (review). Mol Med Rep. (2023) 29:24. doi: 10.3892/mmr.2023.13147
18.
Cheng L-T Tang L-J Chen H-M Tang W Wang T . Relationship between serum albumin and pulse wave velocity in patients on continuous ambulatory peritoneal dialysis. Vasc Health Risk Manag. (2008) 4:871–6. doi: 10.2147/vhrm.s1864
19.
Hu J Lv C Hu X Liu JY . Effect of hypoproteinemia on the mortality of sepsis patients in the ICU: a retrospective cohort study. Sci Rep. (2021) 11:24379. doi: 10.1038/s41598-021-03865-w
20.
Abubakar S Sabir A Ndakotsu M Imam M Tasiu M . Low admission serum albumin as prognostic determinant of 30-day case fatality and adverse functional outcome following acute ischemic stroke. Pan Afr Med J. (2013) 14:53. doi: 10.11604/pamj.2013.14.53.1941
21.
Zhou H Wang A Meng X Lin J Jiang Y Jing J et al . Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol. (2021) 6:458–66. doi: 10.1136/svn-2020-000676
22.
Thuemmler RJ Pana TA Carter B Mahmood R Bettencourt-Silva JH Metcalf AK et al . Serum albumin and post-stroke outcomes: analysis of UK regional registry data, systematic review, and Meta-analysis. Nutrients. (2024) 16. doi: 10.3390/nu16101486
23.
Hill MD Martin RH Palesch YY Moy CS Tamariz D Ryckborst KJ et al . Albumin Administration in Acute Ischemic Stroke: safety analysis of the ALIAS part 2 multicenter trial. PLoS One. (2015) 10:e0131390. doi: 10.1371/journal.pone.0131390
24.
Tobing E Tansol C Tania C . Albumin-globulin ratio (AGR) as independent predictor of poor survival in renal cell carcinoma: a systematic review and meta-analysis. Arab J Urol. (2024) 22:219–26. doi: 10.1080/20905998.2024.2352954
25.
Choe H Kamono E Abe K Hieda Y Ike H Kumagai K et al . Accuracy of albumin, globulin, and albumin-globulin ratio for diagnosing Periprosthetic joint infection: a systematic review and Meta-analysis. J Clin Med. (2023) 12:7512. doi: 10.3390/jcm12247512
26.
Liu B Xiao K Yan P Sun T Wang J Xie F et al . Albumin-globulin ratio is an independent determinant of 28-day mortality in patients with critical illness. Dis Markers. (2021) 2021:1–5. doi: 10.1155/2021/9965124
27.
Tani S Imatake K Suzuki Y Yagi T Takahashi A . Association of aerobic exercise habits with higher albumin-globulin ratio and lower cellular immune-inflammatory markers: implication of the preventive effect of aerobic exercise on atherosclerotic cardiovascular disease. Heart Vessel. (2024) 40:509–22. doi: 10.1007/s00380-024-02490-7
28.
Cantuti-Castelvetri L Fitzner D Bosch-Queralt M Weil M-T Su M Sen P et al . Defective cholesterol clearance limits remyelination in the aged central nervous system. Science. (2018) 359:684–8. doi: 10.1126/science.aan4183
29.
Passarella D Ronci M Di Liberto V Zuccarini M Mudò G Porcile C et al . Bidirectional control between cholesterol shuttle and purine signal at the central nervous system. Int J Mol Sci. (2022) 23:8683. doi: 10.3390/ijms23158683
30.
Guo C Chi Z Jiang D Xu T Yu W Wang Z et al . Cholesterol homeostatic regulator SCAP-SREBP2 integrates NLRP3 Inflammasome activation and cholesterol biosynthetic signaling in macrophages. Immunity. (2018) 49:842–856.e7. doi: 10.1016/j.immuni.2018.08.021
31.
Candelario-Jalil E Dijkhuizen RM Magnus T . Neuroinflammation, stroke, blood-brain barrier dysfunction, and imaging modalities. Stroke. (2022) 53:1473–86. doi: 10.1161/STROKEAHA.122.036946
32.
Proto JD Doran AC Subramanian M Wang H Zhang M Sozen E et al . Hypercholesterolemia induces T cell expansion in humanized immune mice. J Clin Invest. (2018) 128:2370–5. doi: 10.1172/JCI97785
33.
Huo R-R Liao Q Zhai L You X-M Zuo Y-L . Interacting and joint effects of triglyceride-glucose index (TyG) and body mass index on stroke risk and the mediating role of TyG in middle-aged and older Chinese adults: a nationwide prospective cohort study. Cardiovasc Diabetol. (2024) 23:30. doi: 10.1186/s12933-024-02122-4
34.
Xu J Lu X Shi G-P . Vasa vasorum in atherosclerosis and clinical significance. Int J Mol Sci. (2015) 16:11574–608. doi: 10.3390/ijms160511574
35.
Boyajian RA Schwend RB Wolfe MM Bickerton RE Otis SM . Measurement of anterior and posterior circulation flow contributions to cerebral blood flow. An ultrasound-derived volumetric flow analysis. J Neuroimaging. (1995) 5:1–3. doi: 10.1111/jon1995511
36.
Zarrinkoob L Ambarki K Wåhlin A Birgander R Eklund A Malm J . Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. (2015) 35:648–54. doi: 10.1038/jcbfm.2014.241
37.
Zheng L Li J Yang W Lam H-CC Wong KSL Chu W et al . Patterns and implications of intracranial atherosclerosis in anterior and posterior circulation identified by high-resolution vessel wall imaging. Cerebrovasc Dis. (2023) 53:403–10. doi: 10.1159/000534822
Summary
Keywords
albumin-to-globulin ratio, acute ischemic stroke, cerebral atherosclerotic stenosis, posterior circulation stenosis, intracranial vascular stenosis
Citation
Fu Y-x, Cao J, Yin X, Lang Y, Su T-f and Cui L (2025) Association between serum albumin-to-globulin ratio and subtypes of cerebral atherosclerotic stenosis in acute ischemic stroke. Front. Neurol. 16:1666940. doi: 10.3389/fneur.2025.1666940
Received
29 July 2025
Accepted
26 September 2025
Published
16 October 2025
Volume
16 - 2025
Edited by
Hujin Xie, Queensland University of Technology, Australia
Reviewed by
Jun Yan, Guangxi Medical University Cancer Hospital, China
Ying Liu, Huzhou Central Hospital, China
Updates
Copyright
© 2025 Fu, Cao, Yin, Lang, Su and Cui.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Cui, lcui@jlu.edu.cn
†ORCID: Li Cui, orcid.org/0000-0002-4254-6736
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.