Abstract
Background and purpose:
Vertebrobasilar artery occlusion (VBAO) is a rare yet severe type of ischemic stroke, often leading to respiratory failure that necessitates invasive mechanical ventilation and is associated with high mortality rates. While endovascular treatment (EVT) has improved outcomes for VBAO, many patients still require prolonged mechanical ventilation (PMV) post-EVT, further increasing mortality and posing challenging decisions for families. Currently, no predictive model exists to identify VBAO patients at risk of needing PMV after EVT. This study aims to develop and validate a predictive score for PMV in this patient population following EVT.
Materials and methods:
The derivation cohort prospectively recruited VBAO patients undergoing EVT from four comprehensive stroke centers (CSCs) in China. PMV was defined as continuous mechanical ventilation lasting for ≥7 days. Multivariable logistic regression was conducted to develop a scoring system. The performance of the model was evaluated for discrimination, calibration, and clinical utility. Four hundred and fourteen patients from acute Posterior circulation ischemic Stroke registry were enrolled to externally validate the model. Sensitivity analysis redefined PMV as using mechanical ventilation last for ≥ 14 days to further validate the model.
Results:
The derivation cohort consisted of 419 patients from four CSCs, among whom 113 (27.0%) required PMV. The presence of malignant cerebellar edema, posterior circulation collateral status, symptomatic intracranial hemorrhage post-EVT, atrial fibrillation, intravenous thrombolsis, vasopressor therapy and Glasgow coma score classification are found to be independent predictors of PMV in logistic regression, then ‘AIRFLOW’ scoring system was created. The AIRFLOW score demonstrated good discrimination in derivation cohort (C-index, 0.85, 95% CI 0.81 to 0.89), as well as the validation cohort (C-index, 0.82, 95% CI 0.77 to 0.86). Calibration plots and decision curve analysis for AIRFLOW score indicated that the model accurately predicted the risk of PMV and had satisfactory net benefit across various thresholds. Similar results were found in sensitivity analysis.
Conclusion:
The AIRFLOW score may help predict PMV in VBAO patients after EVT.
Introduction
Vertebrobasilar artery occlusion (VBAO) is a rare but severe form of ischemic stroke, accounting for 1% of all strokes and 5% of large vessel occlusions (LVOs) (1, 2). Unlike anterior circulation LVOs, VBAO frequently necessitates invasive mechanical ventilation because it injures brainstem centers that regulate consciousness, central respiratory drive, and airway protective reflexes; posterior fossa complications (e.g., malignant cerebellar edema, progressive brainstem infarction) further predispose patients to central hypoventilation, severe neurogenic dysphagia, and aspiration pneumonia, thereby prolonging ventilation and contributing to higher disability and mortality (3, 4). Recent meta-analysis has demonstrated that combining endovascular treatment (EVT) with optimal medical care improves functional outcomes at 90 days compared to medical care alone (5). In a comprehensive, nationally representative cohort of hospitalized acute ischemic stroke patients in the US, approximately 16.7% of those undergoing EVT needed prolonged mechanical ventilation (PMV). This group had a significantly higher in-hospital mortality rate compared to those who did not require PMV (32.1% vs. 8.2%). It’s important to note that the study primarily focused on patients with anterior circulation LVOs (6). Additionally, when a patient require PMV, family members not only face high medical expenses but also the tough decision of whether to continue treatment in hopes of a good outcome.
Given the distinct divergence in MV requirements and outcomes between anterior and posterior circulation LVOs patients, coupled with the encouraging advancements in EVT for VBAO, there is a clear lack of a predictive model for identifying VBAO patients at risk of requiring PMV after EVT. Our study aims to develop and validate a risk model to predict PMV post-EVT for VBAO patients. We presented this article in accordance with TRIPOD Checklist.
Materials and methods
Derivation cohort
We enrolled VBAO patients who underwent EVT at four comprehensive stroke centers (First Affiliated Hospital of University of Science and Technology of China [USTC], Guangdong second Provincial General Hospital, the Second Affiliated Hospital of Fujian Medical University [FJMU], The Affiliated hospital of Yangzhou University [YZU]) between January 2019 and June 2022. Inclusion criteria were: (1) age ≥18 years; (2) imaging confirmation of acute symptomatic VBAO; and (3) EVT performed within 24 h of estimated occlusion. Patients with only posterior cerebral artery occlusion treated with EVT and a pre-stroke modified Rankin Scale (mRS) score >2 were excluded. Additionally, we excluded patients without MV information (n = 12), those who withdrew life-sustaining treatment (WLST) within 7 days after hospitalization (n = 74), as well as those who died within the first 7 days while on MV (n = 64).
External validation cohort
The acute Posterior circulation ischemic Stroke registry (PERSIST) database is a retrospective registry of patients with acute VBAO who underwent EVT within 24 h of estimated occlusion time (EOT) between December 2015 and December 2018 at 21 stroke centers in 13 provinces of China (http://www.chictr.org.cn/; unique identifier: ChiCTR2000033211). Details of the PERSIST study have been described previously (7–9). In this study, we excluded patients lacking MV data (n = 58), those who died within the first 7 days of initiating MV (n = 52), and patients with WLST during the first 7 days after hospitalization (n = 65).
Weaning protocol
Generally, all patients were monitored in neurocritical intensive care unit for at least 24 h after EVT. Patients on MV were evaluated daily by trained respiratory therapists and physicians at each center with the aim of weaning them as soon as possible according to the predefined weaning criteria: (1) stable cardiovascular status (heart rate[HR] ≤ 140 beats/min, systolic blood pressure[SBP] 90–160 mmHg, and minimal or absence of catecholamine); (2) adequate oxygenation (oxygen saturation measured by pulse oximetry [Spo2] ≥ 90%, fractional inspired oxygen tension [Fio2] ≤ 40%, positive end-expiratory pressure ≤8 cm H2O, respiratory rate ≤35 breaths/min); (3) PaCO2 ≤ 50 mmHg; (4) temperature <38.5 °C; (5) pH > 7.35. When patients met the criteria above, a spontaneous breathing trial was performed with 30-min T-tube trial or ventilatory support level ≤ 7 to 8 cm H2O. Weaning test failure was defined by the development of any the following criteria within 30 min: respiratory rate >35 breaths/min with increased accessory muscle activity, Spo2 < 90% (on Fio2 = 0.4), HR > 140 beats/min, SBP < 90 mmHg or >180 mmHg, major dyspnea or agitation (10). The determination of weaning failure is to be made by trained respiratory therapists and physicians at each center.
Data collection
The data on patient characteristics, medical history, level of fasting blood glucose (FBG), stroke cause, pre-treatment imaging parameters, treatment-related factors, and clinical examination and radiographic results at follow-up were meticulously gathered. All patients underwent regular clinical examinations by experienced physicians at each center. The severity of strokes was evaluated using the National Institutes of Health Stroke Scale (NIHSS) score at admission. Consciousness was assessed with the Glasgow Coma Scale (GCS) score on admission, categorizing patients into a severe consciousness impairment group (3–8 points) and a moderate consciousness impairment group (9–15 points).
Imaging parameters were collected, including infarction core status, assessed using the posterior circulation Alberta Stroke Program Early CT Score (pc-ASPECTS) on CT or MRI (11); Posterior circulation collateral status evaluated through the Basilar artery on CT angiography (BATMAN) was categorized into two groups: less than 7 points indicating poor collateral and 7–10 points indicating good collateral (12); and reperfusion status determined according to modified Thrombolysis in Cerebral Infarction scale on final angiography, with scores of 2b or 3 indicating successful reperfusion (13). The occlusion site was categorized as either ‘basilar artery’ or ‘basilar artery plus vertebral artery’. The basilar artery plus vertebral artery occlusion was characterized as an occlusion of vertebral artery resulting in no flow to basilar artery (9).
Typically, patients underwent CT or MRI scans within 72 h post-EVT for radiographic assessment. Symptomatic intracranial hemorrhage (sICH) was defined as neuroimaging-identified intracranial hemorrhage coupled with ≥4-point increase in NIHSS score or an increase of >2 point for each item (14). Malignant cerebellar edema (MCE) was determined using the Jauss scale for neuroradiological mass effect, with a score of ≥4 indicating MCE (15, 16). All neuroimaging data were sent to the core laboratory of USTC and evaluated blindly by two experienced neuroradiologists. For instances of disagreement, decisions were made by a third experienced neuroradiologist.
Treatment-related factors such as the choice of first-line treatment, intravenous thrombolysis (IVT), time from EOT to puncture, time from puncture to reperfusion, type of anesthesia administered, and vasopressor therapy (defined as the use of vasoconstrictors like norepinephrine, epinephrine, aramine, and dopamine to manage shock caused by various underlying conditions during hospitalization) (17) were also documented.
Outcomes
Following a previous study (18), the development of PMV was defined as continuous invasive MV lasting for 7 days or more. Data was collected from anonymized electronic medical record system and hospital information system by two experienced physicians (S. Z and L. Y).
Statistical analysis
The data were presented as the median with interquartile range or numbers with percentages. Univariate analysis was performed using the χ2 test for dichotomous variables and Mann–Whitney U test for continuous variables to compare patients with and without PMV in derivation cohort. For the missing variables, multivariate imputation by chained equation was performed using the fully conditional specification approach.
To identify the independent predictors of PMV, we implemented a multivariable logistic regression analysis (forward stepwise method based on maximum likelihood estimation) including variables with a p-value <0.05 in univariate analysis of derivation cohort. Regression coefficients, odds ratios (OR), and 95% confidence intervals (95% CI) for each variable in the model were calculated. Based on the regression coefficients from the model, we calculated the corresponding points on the scale. We created a scoring system by rounding to the nearest whole number and assigning integer values (19).
To evaluate prediction accuracy, we used three methods: concordance index (C-index) with 95% CI to measure discrimination, calibration plot to measure model fit, and decision curve analysis (DCA) to measure clinical utility. Internal validation was performed by generating 1,000 bootstrap resamples from the derivation cohort to ensure robustness. External validation was conducted using data from the validation cohort. A clinically useful C-index was considered to be ≥ 0.70.
In sensitivity analysis, we redefined PMV as continuous invasive MV lasting for 14 days or more. Thus, we excluded patients who died within 14 days while on MV and those who had WLST within 14 days after hospitalization in both cohorts. The scoring system was then validated using the remaining patients from both cohorts.
The performance of the new scoring system was then compared with the previously published risk model (SET score, Supplementary Table S1) (20) using the DeLong method. All statistical analyses were performed with SAS version 9.4 and R version 4.3.1. A two-sided p-value <0.05 was considered statistically significant.
Results
The study included 593 VBAO patients who underwent EVT in the derivation cohort and 609 patients in the validation cohort. Of these, 419 patients (median age 64 years, 27.4% female) from the derivation cohort and 414 patients (median age 66 years, 25.8% female) from the validation cohort were eligible for analysis. Figure 1 presents the flowchart for patient selection, and Table 1 shows the characteristics of patients in both cohorts.
Figure 1

Flow chart of patient inclusion. VBAO, vertebrobasilar artery occlusion; EVT, endovascular treatment; CSC, comprehensive stroke centers; PERSIST, acute posterior circulation ischemic stroke registry; mRS, modified Rankin Scale; MV, mechanical ventilation; WLST, withdraw life-sustaining treatment.
Table 1
| Derivation cohort | Validation cohort | P | |
|---|---|---|---|
| N = 419 | N = 414 | ||
| Age, years | 64(55–73) | 66(56–73) | 0.463 |
| Sex | 0.601 | ||
| Male | 304(72.6) | 307(74.2) | |
| Female | 115(27.4) | 107(25.8) | |
| Medical history | |||
| Hypertension | 279(66.6) | 288(69.6) | 0.357 |
| Diabetes mellitus | 93(22.2) | 100(24.2) | 0.503 |
| Hyperlipidemia | 159(37.9) | 143(34.5) | 0.307 |
| Atrial fibrillation | 96(22.9) | 85(20.5) | 0.405 |
| Previous stroke | 81(19.3) | 94(22.7) | 0.232 |
| Coronary artery disease | 40(9.5) | 42(10.1) | 0.702 |
| Current smoking | 136(32.5) | 124(30.0) | 0.435 |
| Drink | 90(21.5) | 87(21.0) | 0.870 |
| Fasting blood glucose, mmol/L | 7.3(6.0–9.6) | 6.7(5.8–8.4) | <0.001 |
| Clinical examination | |||
| NIHSS | 22(13–28) | 22(10–28) | 0.348 |
| GCS classification | <0.001 | ||
| Moderate impairment | 197(47.0) | 252(60.9) | |
| Severe impairment | 222(53.0) | 162(39.1) | |
| Stroke cause | 0.560 | ||
| Large artery atherosclerosis | 273(65.2) | 255(61.6) | |
| Cardioembolism | 88(21.0) | 95(22.9) | |
| Other | 21(5.0) | 18(4.3) | |
| Undetermined | 37(8.8) | 46(11.1) | |
| Pre-treatment imaging parameters | |||
| pc-ASPECTS | 9(8–10) | 8(7–10) | <0.001 |
| Collateral circulation status | <0.001 | ||
| Good | 93(22.2) | 160(38.6) | |
| Poor | 326(77.8) | 254(61.4) | |
| Site of occlusion | 0.791 | ||
| Basilar artery | 295(70.4) | 288(69.6) | |
| Basilar artery + vertebral artery | 124(29.6) | 126(30.4) | |
| Treatment-related factors | |||
| Intravenous thrombolysis | 87(20.8) | 113(27.3) | 0.027 |
| Estimated occlusion to puncture time, minutes | 300(215–455) | 210(90–366) | <0.001 |
| Puncture to reperfusion time, minutes | 105(69–148) | 110(75–150) | 0.483 |
| Type of anesthesia | <0.001 | ||
| General anesthesia | 101(24.2) | 191(46.1) | |
| Sedative | 317(75.8) | 223(53.9) | |
| First-line treatment | <0.001 | ||
| Stent retriever | 272(64.9) | 344(83.1) | |
| Aspiration | 83(19.8) | 25(6.0) | |
| Balloon angioplasty or stenting | 54(12.9) | 41(9.9) | |
| Intra-arterial thrombolysis | 10(2.4) | 4(1.0) | |
| Rescue therapy | 196(46.8) | 120(29.3) | <0.001 |
| Successful reperfusion | 377(90.0) | 381(92.0) | 0.301 |
| Vasopressor therapy | 80(19.1) | 48(11.6) | 0.003 |
| Radiographic results at follow-up | |||
| sICH | 26(6.2) | 23(5.6) | 0.690 |
| Malignant cerebellar edema | 56(13.4) | 49(11.8) | 0.506 |
Baseline characteristics of the two cohorts.
Data are present as n (%). Continuous variables are expressed as median (interquartile range). GCS, Glasgow coma scale; NIHSS, National Institutes of Health Stroke Scale; pc-ASPECTS, posterior circulation-Alberta Stroke Program Early CT score; sICH, symptomatic intracranial hemorrhage. The bold number indicate statistical significance.
In the derivation cohort, 113 patients (27.0%) required PMV for a median of 12 days (IQR, 8.5–16 days). In the validation cohort, 102 (24.6%) needed PMV for a median of 10 days (IQR, 9–16 days). The distribution of mRS scores at 90 days based on PMV status (Supplementary Figure S1) and the survival probability of patients with PMV in the two cohorts are shown in Supplementary Figure S2.
Model development
A comparison of baseline characteristics, medical history, clinical examination, treatment-related factors, and imaging parameters between non-PMV and PMV in derivation cohort is shown in Table 2. Compared with non-PMV patients, PMV patients were significantly less likely to be female (p = 0.048), have atrial fibrillation (AF) (p = 0.039), receive IVT (p < 0.001), and have good collateral (p < 0.001). They were more likely to have severe consciousness impairment and higher NIHSS score (both p < 0.001) and higher FBG level (p = 0.007). They also had a higher proportion of vasopressor therapy, presence of sICH, and MCE at follow-up imaging (all p < 0.001).
Table 2
| Univariable | Multivariable logistic | |||||
|---|---|---|---|---|---|---|
| PMV | Non-PMV | P | β | OR (95% CI) | P | |
| n = 113 | n = 306 | |||||
| Age, years | 65(55–73) | 64(55–73) | 0.717 | |||
| Sex | 0.048 | |||||
| Male | 90(79.6) | 214(69.9) | ||||
| Female | 23(20.4) | 92(30.1) | ||||
| Hypertension | 80(70.8) | 199(65.0) | 0.267 | |||
| Diabetes mellitus | 25(22.1) | 68(22.2) | 0.983 | |||
| Hyperlipidemia | 43(38.1) | 116(37.9) | 0.978 | |||
| Atrial fibrillation | 18(15.9) | 78(25.5) | 0.039 | −0.88 | 0.42(0.21–0.84) | 0.014 |
| Previous stroke | 21(18.6) | 60(19.6) | 0.814 | |||
| Coronary artery disease | 13(11.5) | 27(8.8) | 0.407 | |||
| Current smoking | 32(28.3) | 104(34.0) | 0.271 | |||
| Drink | 20(17.7) | 70(22.9) | 0.252 | |||
| Fasting blood glucose, mmol/L | 8.0(6.3–11.0) | 7.3(5.9–9.1) | 0.007 | |||
| National Institutes of Health Stroke Scale | 27(19–31) | 19(11–28) | <0.001 | |||
| Glasgow coma score classification | <0.001 | 0.009 | ||||
| Moderate impairment | 30(26.5) | 167(54.6) | - | reference | – | |
| Severe impairment | 83(73.5) | 139(45.4) | 0.74 | 2.10(1.21–3.66) | ||
| Stroke cause | 0.682 | |||||
| Large artery atherosclerosis | 72(63.7) | 201(65.7) | ||||
| Cardioembolism | 22(19.5) | 66(21.6) | ||||
| Other | 6(5.3) | 15(4.9) | ||||
| Undetermined | 13(11.5) | 24(7.8) | ||||
| pc-ASPECTS | 9(7–10) | 9(8–10) | 0.567 | |||
| Collateral circulation status | <0.001 | 0.001 | ||||
| Good | 6(5.3) | 87(28.4) | – | reference | ||
| Poor | 107(94.7) | 219(71.6) | 1.61 | 5.03(1.99–12.78) | ||
| Site of occlusion | 0.537 | |||||
| Basilar artery | 77(68.1) | 218(71.2) | ||||
| Basilar artery + vertebral artery | 36(31.9) | 88(28.8) | ||||
| Intravenous thrombolysis | 9(8.0) | 78(25.5) | <0.001 | −1.26 | 0.29(0.12–0.67) | 0.004 |
| Estimated occlusion to puncture time, minutes | 270(199–444) | 310(222–455) | 0.373 | |||
| Puncture to reperfusion time, minutes | 105(70–145) | 105(67–150) | 0.710 | |||
| Type of anesthesia | 0.310 | |||||
| General anesthesia | 31(27.7) | 70(22.9) | ||||
| Sedative | 81(72.3) | 236(77.1) | ||||
| First-line treatment | 0.832 | |||||
| Stent retriever | 77(68.1) | 195(63.7) | ||||
| Aspiration | 20(17.7) | 63(20.6) | ||||
| Angioplasty or stenting | 14(12.4) | 40(13.1) | ||||
| Intra-artery thrombolysis | 2(1.8) | 8(2.6) | ||||
| Rescue therapy | 59(52.2) | 137(44.8) | 0.175 | |||
| Successful reperfusion | 97(85.8) | 280(91.5) | 0.087 | |||
| Vasopressor therapy | 47(41.6) | 33(10.8) | <0.001 | 1.59 | 4.90 (2.68–8.96) | <0.001 |
| Symptomatic intracranial hemorrhage | 16(14.2) | 10(3.3) | <0.001 | 1.19 | 3.27(1.24–8.67) | 0.017 |
| Malignant cerebellar edema | 34(30.1) | 22(7.2) | <0.001 | 1.50 | 4.48(2.19–9.17) | <0.001 |
Results of the univariable and multivariable logistic regression for PMV after EVT in the derivation cohort.
Data are present as n (%). Continuous variables are expressed as median (interquartile range). EVT, endovascular treatment; PMV, prolonged mechanical ventilator; pc-ASPECTS, posterior circulation-Alberta Stroke Program Early CT score. The bold number indicate statistical significance.
Multivariate logistic analysis found that malignant cerebellar edema at follow-up imaging, posterior circulation collateral status, symptomatic intracranial hemorrhage post-EVT, atrial fibrillation, intravenous thrombolysis, vasopressor therapy and Glasgow coma score classification was independently associated with PMV (Table 2). The ‘AIRFLOW’ scores were then developed based on the β coefficients of individual variables (Table 3).
Table 3
| Items | Categories | Points |
|---|---|---|
| Malignant cerebellar edema | Yes | 2 |
| No | 0 | |
| posterior collateral circulation status | Poor | 2 |
| Good | 0 | |
| Symptomatic intracranial hemorrhage | Yes | 2 |
| No | 0 | |
| Atrial fibrillation | No | 1 |
| Yes | 0 | |
| Intravenous thrombolysis | No | 2 |
| Yes | 0 | |
| Vasopressor therapy | Yes | 2 |
| No | 0 | |
| Glasgow coma score classification | Severe impairment | 1 |
| Moderate impairment | 0 | |
| Total scores: | ||
Components of the AIRFLOW grading scale.
The C-index for the AIRFLOW score was 0.85 (95% CI 0.81 to 0.89) in derivation cohort. The internal validation yielded a bootstrap-corrected C-index of 0.85 (95% CI 0.81 to 0.89). Calibration plots of the AIRFLOW score in both the derivation and internal validation (Figure 2A) demonstrated that the predicted probabilities of PMV were close to the observed PMV.
Figure 2
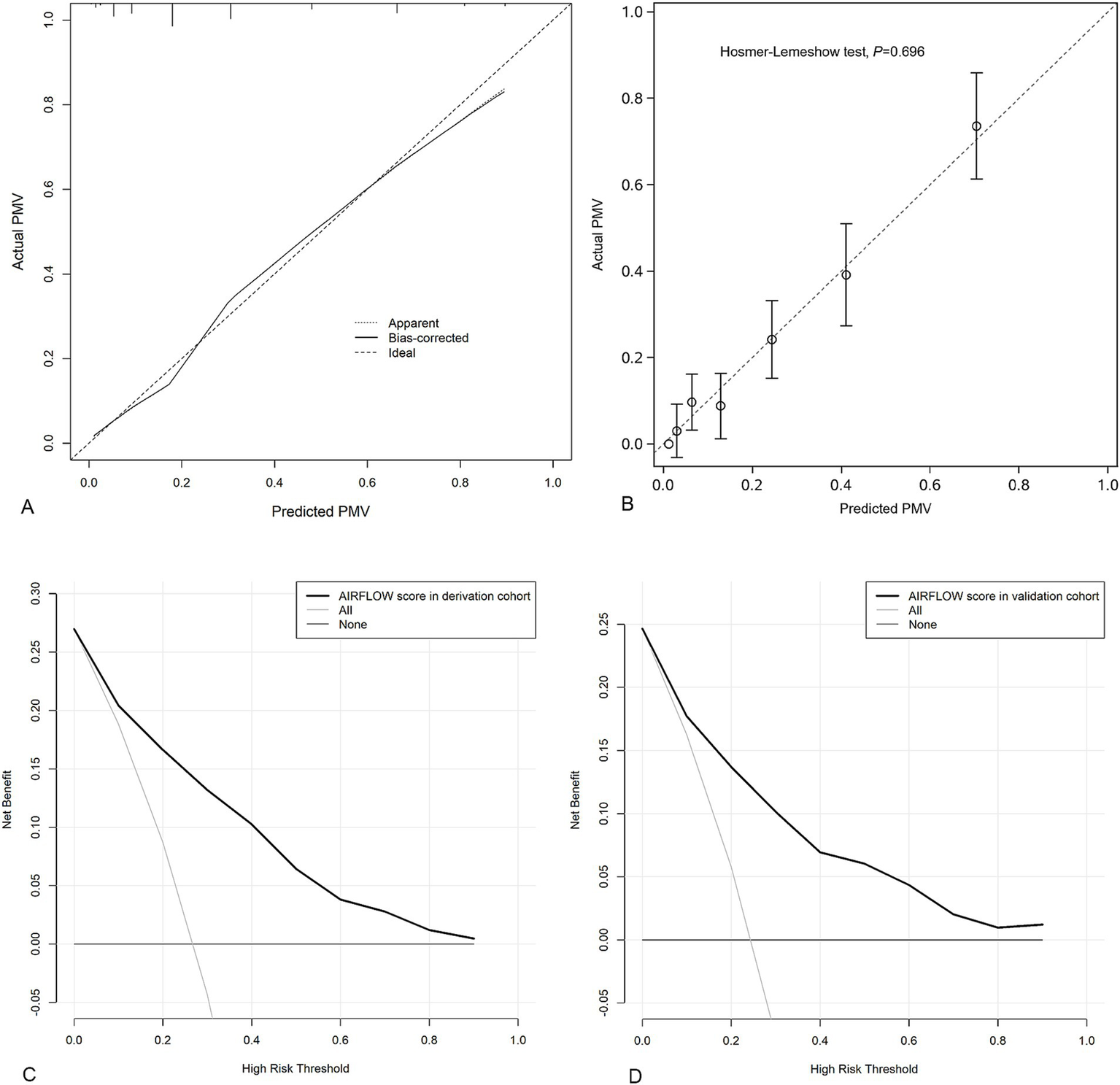
Calibration plots to assess model internal validation with 1,000 bootstrap resamples; The dotted line represents the performance of the AIRFLOW score. The solid line corrects for any bias in the AIRFLOW score. The dashed line is the reference line where an ideal AIRFLOW score would lie (A). Calibration plots of external validation for the AIRFLOW score (B). Decision curve analysis demonstrating the net benefit associated with the use of the AIRFLOW score for predicting PMV in derivation cohort (C) and validation cohort (D). PMV prolonged mechanical ventilation.
Model validation
In the external validation, the AIRFLOW score had a C-index of 0.82 (95% CI 0.77 to 0.86). Calibration plots of the AIRFLOW score indicated that the model accurately predicted the risk of PMV (Figure 2B).
Clinical utility
Based on DCA in derivation cohort the AIRFLOW scores to predict PMV provided more net benefit than either a “treat-all-patients scheme” or a “treat-none scheme” (Figure 2C). These findings were consistent in the validation cohort as well (Figure 2D).
Risk stratification
In the derivation cohort, the maximum sum AIRFLOW score was 12 points. A patient with a score of 0 has PPMV = 0.3%, and a patient with a full score of 12 has PPMV = 98.6%. As the sum AIRFLOW scores increased, the predicted probability of PMV also increased gradually (Figure 3 and Supplementary Table S2).
Figure 3

The change of sensitivity (solid line), specificity (dashed line) and predicted probability (bars) of requiring prolonged mechanical ventilation as AIRFLOW score increases.
A sum AIRFLOW score of ≤5 points indicated a low risk (0–25%) for PMV, 6–7 points indicated an intermediate risk (26–50%), and ≥8 points indicated a high risk (>50%) for PMV. The association between three risk stratification of sum AIRFLOW score and actual PMV is displayed Supplementary Figure S3.
Sensitivity analysis
When PMV was redefined as using continuous invasive MV last for ≥14 days, the AIRFLOW score was used to predict the risk of PMV. A C-index of 0.84 (95% CI 0.79–0.88) was obtained from remaining patients in derivation cohort and of 0.82 (95% CI 0.76–0.87) in validation cohort. Both calibration plots of the AIRFLOW score indicated that the model accurately predicted the risk of PMV (Supplementary Figures S4A,B). Moreover, based on DCA, the AIRFLOW score to predict PMV would provide more net benefit than either a “treat-all-patients scheme” or a “treat-none scheme” in both cohorts (Supplementary Figures S4C,D).
Model comparison
In the derivation cohort with complete data on items of SET score, the AIRFLOW score demonstrated superior discriminative performance compare to the SET score, reflected by a higher C-index (0.85, 95%CI 0.81–0.89, vs. 0.67, 95%CI 0.61–0.74, p < 0.001). This superiority was corroborated in the external validation cohort with complete data on items of SET score, where the AIRFLOW score maintained a higher C-index (0.82, 95%CI 0.77–0.87, vs. 0.72, 95%CI 0.66–0.78, p < 0.001, Supplementary Figures S5A,B) compared to the SET score. Furthermore, sensitivity analysis with complete data on items of SET score also underscored the AIRFFLOW score’s enhanced performance over the SET score across both cohorts (Supplementary Figures S5C,D).
Discussion
This study demonstrated that prolonged mechanical ventilation (PMV) is commonly needed in vertebrobasilar artery occlusion (VBAO) patients treated with endovascular treatment (EVT). Seven readily accessible variables were identified as potential predictors for PMV: the presence of malignant cerebellar edema on follow-up imaging, posterior circulation collateral status, symptomatic intracranial hemorrhage after EVT, atrial fibrillation, intravenous thrombolysis, vasopressor therapy and Glasgow coma score classification. Utilizing these metrics, the ‘AIRFLOW’ scoring system was devised, showing strong discriminative capabilities and a good model fit. Its validity was confirmed through internal validation using 1,000 bootstrap resamples, along with external validation procedures.
In patients with VBAO undergoing EVT who require PMV, poor outcomes are prevalent. Specifically, our study reveals that less than 10% of patients achieve favorable outcomes, with more than 60% mortality within 90 days. It is crucial to highlight that, in addition to severe impairment in brainstem areas controlling consciousness, respiration, and circulation, which contribute to premature in-hospital mortality, the withdrawal of life-sustaining therapy (WLST) during hospitalization stands out as a pivotal determinant of patient outcomes. Prior research also indicates that severe central nervous system injury is a primary factor in WLST decisions in intensive care settings (21).
In our study, PMV was defined as mechanical ventilation for 7 days or more, deviating from the standard threshold of ≥21 days (22) to enhance inclusivity, considering the high rate of in hospital mortality or WLST before reaching 21 days. A supplementary sensitivity analysis was conducted, redefined PMV as mechanical ventilation lasting 14 days or more. Despite this adjustment, the AIRFLOW scoring system retained its predictive accuracy and reliability. On the contrary, according to the definition of PMV, we cannot include patients who die within 7 days of using MV in the analysis. In clinical practice, when a severe stroke requires MV, physicians and some family members often agree to a time-limited trial to see if the patient’s condition improves or deteriorates (23). Therefore, predicting the use of MV within 7 days might not be crucial.
Mechanism of associations
The presence of MCE and sICH after EVT contributes to obstructive hydrocephalus and cerebellar tonsillar herniation. These conditions can cause secondary injury to the brainstem due to mass effect, increasing the risk of impaired consciousness and failure of respiratory or circulatory functions, both of which are radiographically linked to PMV (20).
Collateral circulation status, as evaluated by BATMAN score, plays a pivotal role in the pathophysiology of cerebral ischemia. An unfavorable BATMAN score (less than 7 points) significantly correlates with a poor outcome (12), likely due to accelerated progression of the ischemic penumbra into infarction. This leads to a large infarct affecting brainstem respiratory or circulatory centers.
Interestingly, a history of AF emerged as a protective factor against PMV in VBAO patients post-EVT. This may be because the cardioembolic mechanism often underlying VBAO in AF patients is linked to higher rates of successful recanalization compared to atherosclerotic mechanisms (24, 25). Consequently, this might reduce damage to critical brainstem centers.
The EVT with IVT first was associated with better functional outcomes in patients with VBAO treated within 24 h of onset (26). Potential advantages of bridging therapy include early thrombus fragmentation, microvascular reperfusion and enhanced recanalization (27, 28). In clinical practice, patients with acute ischemic stroke presenting within 4.5 h of symptom onset are prioritized for IVT. This suggests that patients receiving IVT present earlier medically than those who do not, resulting in shorter onset-to-treatment times and improved outcome (29). These effects help salvage significant amounts of cerebral tissue, potentially reducing damage to critical brainstem centers.
Vasopressor therapy emerged as the strong predictor for PMV in the AIRFLOW score. Patients requiring vasopressor therapy often struggle to meet the weaning criteria due to unstable cardiovascular status. Additionally, vasopressor therapy may signify impairment of the cardiovascular regulating center in the medulla, potentially caused by infarction or intracranial hypertension, thereby increasing the risk of cardiac arrest (1).
Finally, severe consciousness impairment (Glasgow coma score of 8 points or less), which reduces protection reflexes and increases the risk for aspiration, is associated with a high need for tracheostomy or a higher rate of extubation failure (20, 30).
Clinical implications
To our best knowledge, the AIRFLOW scoring system is the first designed specifically to predict the risk of PMV in patients with VBAO treated with EVT. Compared to the SET score, the AIRFLOW score requires fewer items and offers superior discriminatory performance.
The AIRFLOW score serves as a clinical tool that helps stratify patients into distinct risk categories for PMV. It assists clinicians in making informed decisions, communicating effectively with patients’ relatives, and optimizing patient care. For patients with a low risk who are currently on MV, this scoring system predicts a good chance of eventually independent breathing. This provides peace of mind for patients and their families helps avoid premature decisions to WLST. For high-risk patients using MV, considering a time-limited trial is advisable while respecting their wishes and values. If needed, moving toward comfort care afterward is a logical next step. Especially when the AIRFLOW score exceeds 9, the specificity reaches 100% (false positive rate of zero).
Notably, the AIRFLOW scoring system includes a unique adjustable factor—the administration of IVT before EVT. This introduces a specific strategy for intervention in high-risk groups. Increasing the frequency of IVT may reduce the occurrence of PMV after EVT, thereby substantially improving patient outcomes.
Our study has several limitations. Firstly, we lacked complete data on the SET score items, many of which could be potential predictors for PMV. Missing key variables means the model might not capture all factors affecting the outcomes, reducing prediction accuracy. Secondly, we excluded patients on MV who died or had life-sustain treatment withdrawn within 7 days. This exclusion could lead to survivorship bias, making model overly optimistic by ignoring patients who died or ceased treatment quickly due to severe conditions. Thirdly, our dataset lacked the Full Outline of UnResponsiveness (FOUR) score. FOUR captures brainstem reflexes and respiratory patterns and may be more sensitive than GCS for posterior circulation stroke, but its required variables were not collected—especially in the validation cohort—and cannot be reconstructed retrospectively. We therefore used GCS, which was consistently available and clinically relevant to airway protection and ventilator weaning. This gap may underrepresent brainstem dysfunction; prospective studies should record both GCS and FOUR to enable direct comparison and potentially improve PMV prediction. Lastly, the high rate of catastrophic outcomes in patients need PMV may partly result from premature WLST decision. Further external validation of the AIRFLOW score and PMV outcomes is necessary.
Conclusion
In this study, we identified seven variables linked to PMV in VABO patients that can be easily assessed after EVT. We propose the AIRFLOW score to estimate the likelihood of PMV. These findings can assist patient families and caregivers in better understand the treatment path. Further validation of the AIRFLOW score’s effectiveness is necessary.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by Institutional Review Boards (IRB) of the first affiliated hospital of USTC (IRB2020-40), Guangdong Second Provincial General Hospital (IRB2020-KY-KZ-100-03), the Second Affiliated Hospital of FMU (IRB2020-339) and The Affiliated hospital of YZU (IRB2020-153). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Informed consent from the derivation cohort was not sought because IRB at each center approved our use of anonymized patient information.
Author contributions
QC: Formal analysis, Writing – original draft, Conceptualization, Writing – review & editing, Funding acquisition. MH: Conceptualization, Data curation, Writing – review & editing, Writing – original draft, Formal analysis. YX: Writing – review & editing, Investigation, Resources, Data curation. SZ: Resources, Writing – review & editing, Data curation, Methodology. ZH: Data curation, Funding acquisition, Resources, Writing – review & editing. PX: Writing – review & editing, Data curation, Project administration, Resources. CC: Data curation, Writing – review & editing, Resources, Supervision. JC: Writing – review & editing, Investigation, Data curation, Resources. LY: Resources, Conceptualization, Supervision, Writing – review & editing. WS: Formal analysis, Funding acquisition, Supervision, Resources, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Startup Fund for scientific research of Fujian Medical University (XJ2021014001 and No2020QH1106).
Acknowledgments
The authors would like to thank Shufen Liu and Zhengping Huang at The Second Affiliated Hospital of Fujian Medical University for their expert input and assistance in the review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1673616/full#supplementary-material
References
1.
Mattle HP Arnold M Lindsberg PJ Schonewille WJ Schroth G . Basilar artery occlusion. Lancet Neurol. (2011) 10:1002–14. doi: 10.1016/s1474-4422(11)70229-0
2.
Schonewille WJ Wijman CA Michel P Rueckert CM Weimar C Mattle HP et al . Treatment and outcomes of acute basilar artery occlusion in the basilar artery international cooperation study (BASICS): a prospective registry study. Lancet Neurol. (2009) 8:724–30. doi: 10.1016/s1474-4422(09)70173-5
3.
Bösel J . Use and timing of tracheostomy after severe stroke. Stroke. (2017) 48:2638–43. doi: 10.1161/strokeaha.117.017794
4.
Wijdicks EF Scott JP . Outcome in patients with acute basilar artery occlusion requiring mechanical ventilation. Stroke. (1996) 27:1301–3. doi: 10.1161/01.str.27.8.1301
5.
Nogueira RG Jovin TG Liu X Hu W Langezaal LCM Li C et al . Endovascular therapy for acute vertebrobasilar occlusion (VERITAS): a systematic review and individual patient data meta-analysis. Lancet. (2025) 405:61–9. doi: 10.1016/s0140-6736(24)01820-8
6.
Saber H Palla M Kazemlou S Navi BB Yoo AJ Simonsen CZ et al . Prevalence, predictors, and outcomes of prolonged mechanical ventilation after endovascular stroke therapy. Neurocrit Care. (2021) 34:1009–16. doi: 10.1007/s12028-020-01125-9
7.
Xiao L Gu M Lu Y Xu P Wang J Lan W et al . Influence of renal impairment on clinical outcomes after endovascular recanalization in vertebrobasilar artery occlusions. J Neurointerv Surg. (2022) 14:1077–83. doi: 10.1136/neurintsurg-2021-018003
8.
Sun W Duan Z Xu P Xiao L Wang J Gui W et al . The safety and effectiveness of endovascular treatment for patients with vertebrobasilar artery occlusions: according to the BEST and BASICS criteria. Ther Adv Neurol Disord. (2022) 15:17562864221114627. doi: 10.1177/17562864221114627
9.
Zhang P Xu P Duan Z Zhang F Fang Y Yan D et al . Effects of admission systemic inflammatory indicators on clinical outcomes in patients with vertebrobasilar artery occlusion: insight from the PERSIST registry. J Neurointerv Surg. (2023) 15:e270–6. doi: 10.1136/jnis-2022-019437
10.
Boles JM Bion J Connors A Herridge M Marsh B Melot C et al . Weaning from mechanical ventilation. Eur Respir J. (2007) 29:1033–56. doi: 10.1183/09031936.00010206
11.
Puetz V Sylaja PN Coutts SB Hill MD Dzialowski I Mueller P et al . Extent of hypoattenuation on CT angiography source images predicts functional outcome in patients with basilar artery occlusion. Stroke. (2008) 39:2485–90. doi: 10.1161/strokeaha.107.511162
12.
Alemseged F Shah DG Diomedi M Sallustio F Bivard A Sharma G et al . The basilar artery on computed tomography angiography prognostic score for basilar artery occlusion. Stroke. (2017) 48:631–7. doi: 10.1161/strokeaha.116.015492
13.
Higashida RT Furlan AJ Roberts H Tomsick T Connors B Barr J et al . Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. (2003) 34:e109–37. doi: 10.1161/01.Str.0000082721.62796.09
14.
von Kummer R Broderick JP Campbell BC Demchuk A Goyal M Hill MD et al . The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/strokeaha.115.010049
15.
Broocks G Elsayed S Kniep H Kemmling A Flottmann F Bechstein M et al . Early prediction of malignant cerebellar edema in posterior circulation stroke using quantitative lesion water uptake. Neurosurgery. (2021) 88:531–7. doi: 10.1093/neuros/nyaa438
16.
Jüttler E Schweickert S Ringleb PA Huttner HB Köhrmann M Aschoff A . Long-term outcome after surgical treatment for space-occupying cerebellar infarction: experience in 56 patients. Stroke. (2009) 40:3060–6. doi: 10.1161/strokeaha.109.550913
17.
Russell JA . Vasopressor therapy in critically ill patients with shock. Intensive Care Med. (2019) 45:1503–17. doi: 10.1007/s00134-019-05801-z
18.
Rass V Ianosi BA Lindlbauer M Lindner A Kofler M Schiefecker AJ . Factors associated with prolonged mechanical ventilation in patients with subarachnoid hemorrhage-the RAISE score. Crit Care Med. (2022) 50:103–13. doi: 10.1097/ccm.0000000000005189
19.
Sullivan LM Massaro JM D'Agostino RB . Presentation of multivariate data for clinical use: the Framingham study risk score functions. Stat Med. (2004) 23:1631–60. doi: 10.1002/sim.1742
20.
Schönenberger S Al-Suwaidan F Kieser M Uhlmann L Bösel J . The SET score to predict tracheostomy need in cerebrovascular Neurocritical care patients. Neurocrit Care. (2016) 25:94–104. doi: 10.1007/s12028-015-0235-5
21.
Verkade MA Epker JL Nieuwenhoff MD Bakker J Kompanje EJ . Withdrawal of life-sustaining treatment in a mixed intensive care unit: most common in patients with catastropic brain injury. Neurocrit Care. (2012) 16:130–5. doi: 10.1007/s12028-011-9567-y
22.
MacIntyre NR Epstein SK Carson S Scheinhorn D Christopher K Muldoon S . Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. (2005) 128:3937–54. doi: 10.1378/chest.128.6.3937
23.
Quill TE Holloway R . Time-limited trials near the end of life. JAMA. (2011) 306:1483–4. doi: 10.1001/jama.2011.1413
24.
Sun X Raynald Tong X Gao F Deng Y Ma G et al . Analysis of treatment outcome after endovascular treatment in different pathological subtypes of basilar artery occlusion: a single center experience. Transl Stroke Res. (2021) 12:230–8. doi: 10.1007/s12975-020-00833-w
25.
Lee WJ Jung KH Ryu YJ Kim JM Lee ST Chu K et al . Impact of stroke mechanism in acute basilar occlusion with reperfusion therapy. Ann Clin Transl Neurol. (2018) 5:357–68. doi: 10.1002/acn3.536
26.
Nie X Wang D Pu Y Wei Y Lu Q Yan H et al . Endovascular treatment with or without intravenous alteplase for acute ischaemic stroke due to basilar artery occlusion. Stroke Vasc Neurol. (2022) 7:190–9. doi: 10.1136/svn-2021-001242
27.
Desilles JP Loyau S Syvannarath V Gonzalez-Valcarcel J Cantier M Louedec L et al . Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. (2015) 46:3241–8. doi: 10.1161/strokeaha.115.010721
28.
Mueller L Pult F Meisterernst J Heldner MR Mono ML Kurmann R et al . Impact of intravenous thrombolysis on recanalization rates in patients with stroke treated with bridging therapy. Eur J Neurol. (2017) 24:1016–21. doi: 10.1111/ene.13330
29.
Joundi RA Sun JL Xian Y Alhanti B Nogueira RG Bhatt DL et al . Association between endovascular therapy time to treatment and outcomes in patients with basilar artery occlusion. Circulation. (2022) 145:896–905. doi: 10.1161/circulationaha.121.056554
30.
Asehnoune K Seguin P Lasocki S Roquilly A Delater A Gros A et al . Extubation success prediction in a multicentric cohort of patients with severe brain injury. Anesthesiology. (2017) 127:338–46. doi: 10.1097/aln.0000000000001725
Summary
Keywords
mechanical ventilation, vertebrobasilar artery, endovascular treatment, predictors, stroke
Citation
Cai Q, Hong M, Xu Y, Zhang S, Huang Z, Xu P, Chen C, Chen J, Ye L and Sun W (2025) Predicting prolonged mechanical ventilation after endovascular treatment for acute vertebrobasilar artery occlusion: AIRFLOW score. Front. Neurol. 16:1673616. doi: 10.3389/fneur.2025.1673616
Received
15 August 2025
Accepted
27 October 2025
Published
14 November 2025
Volume
16 - 2025
Edited by
Alba Scerrati, University of Ferrara, Italy
Reviewed by
Nour Shaheen, Alexandria University, Egypt
Mingjun Pu, Mianyang Central Hospital, China
Updates
Copyright
© 2025 Cai, Hong, Xu, Zhang, Huang, Xu, Chen, Chen, Ye and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiankun Cai, caiqiankun@126.comWen Sun, sunwen_medneuro@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.