- 1Unité de Neurophysiologie Clinique, Hôpital Henri Mondor, AP-HP, Créteil, France
- 2EA4391 (ENT), Faculté de Santé, Université Paris Est Créteil, Créteil, France
New perspectives are opening up today in the management of diabetes thanks to the possibility of measuring, over long periods in daily life, different biomarkers likely to improve glycaemic control, such as continuous glucose monitoring and time-in-range assessment. This is part of personalized medicine. There is therefore a challenge to also benefit from specific biomarkers in the prevention and monitoring of polyneuropathy in diabetics, one of the most common type of peripheral nerve disorder worldwide. This is now possible with the development of connected tools, allowing for example to monitor at home the evolution of skin temperature or conductance at the level of the feet. In this article, the current use and recent advances in laboratory tools for the early diagnosis and objective monitoring of diabetic polyneuropathy and its progression will be presented. The follow-up of neuropathies will undoubtedly be significantly modified in clinical practice in the future, particularly in the context of diabetes, thanks to the use of connected tools and remote monitoring.
Introduction
Diabetic neuropathy (DN) affects millions of people worldwide, impairing quality of life and daily functioning (1, 2). DN is also associated with an increased relative risk of death, especially due to the dysfunction of the peripheral autonomic nervous system (3, 4). This highly morbid disorder is therefore the cause of major socio-economic problems and very significant annual health costs, even only considering the diabetic foot syndrome, a dramatic consequence leading to difficult-to-treat ulcers and amputations (5, 6). Diabetic foot syndrome is defined by the World Health Organization as an “ulceration of the foot (distally from the ankle and including the ankle) associated with neuropathy and different grades of ischemia and infection.”
Distal symmetric polyneuropathy (DPN) is the most common form of DN, characterized by the progressive damage and loss of various populations of nerve fibers in a symmetrical and length-dependent pattern, therefore starting at the feet (7–10). The clinical picture includes a variable mix of negative sensory signs and symptoms (hypoesthesia and numbness) and positive sensory signs and symptoms (non-painful paresthesias, such as tingling, or painful dysesthesias, whether spontaneous or evoked). These sensory features involve large-diameter A-beta nerve fibers and small-diameter A-delta and type C nerve fibers. Unmyelinated C fibers are also involved in the autonomic part of DPN, mainly at the origin of vasomotor or sudomotor dysfunction of the limb extremities (11, 12). In more advanced cases of DPN, this can result in ulcers, infections and amputations in the feet, as well as loss or dysfunction of larger-diameter nerve fibers involved in motor or proprioceptive function. Ultimately, the patients may show balance disorders and an increased risk of falls, unnoticed injuries, and fractures (13, 14).
Also, to avoid this deleterious evolution and to prevent morbidity and complications, there is an obvious need to develop laboratory tools allowing DPN to be diagnosed early, especially because of a frequent asymptomatic onset (15), and also to objectively monitor its evolution. These tools could be directed towards the detection of neurodegeneration, for example by measuring serum neurofilament light chains (sNfL) levels (16–18). However, the value of sNfL measurement has been shown to be neither sensitive (19) nor specific (with respect to the detection of central nervous system involvement) for the diagnosis of DPN (20). Thus, from a more neurophysiological perspective, these tools must be more specifically linked to the evaluation of a given type of nerve fibers at the level of the feet, repeatable, reproducible, and sensitive to alterations and early changes in nerve function.
Different assessment tools for different nerve fiber types
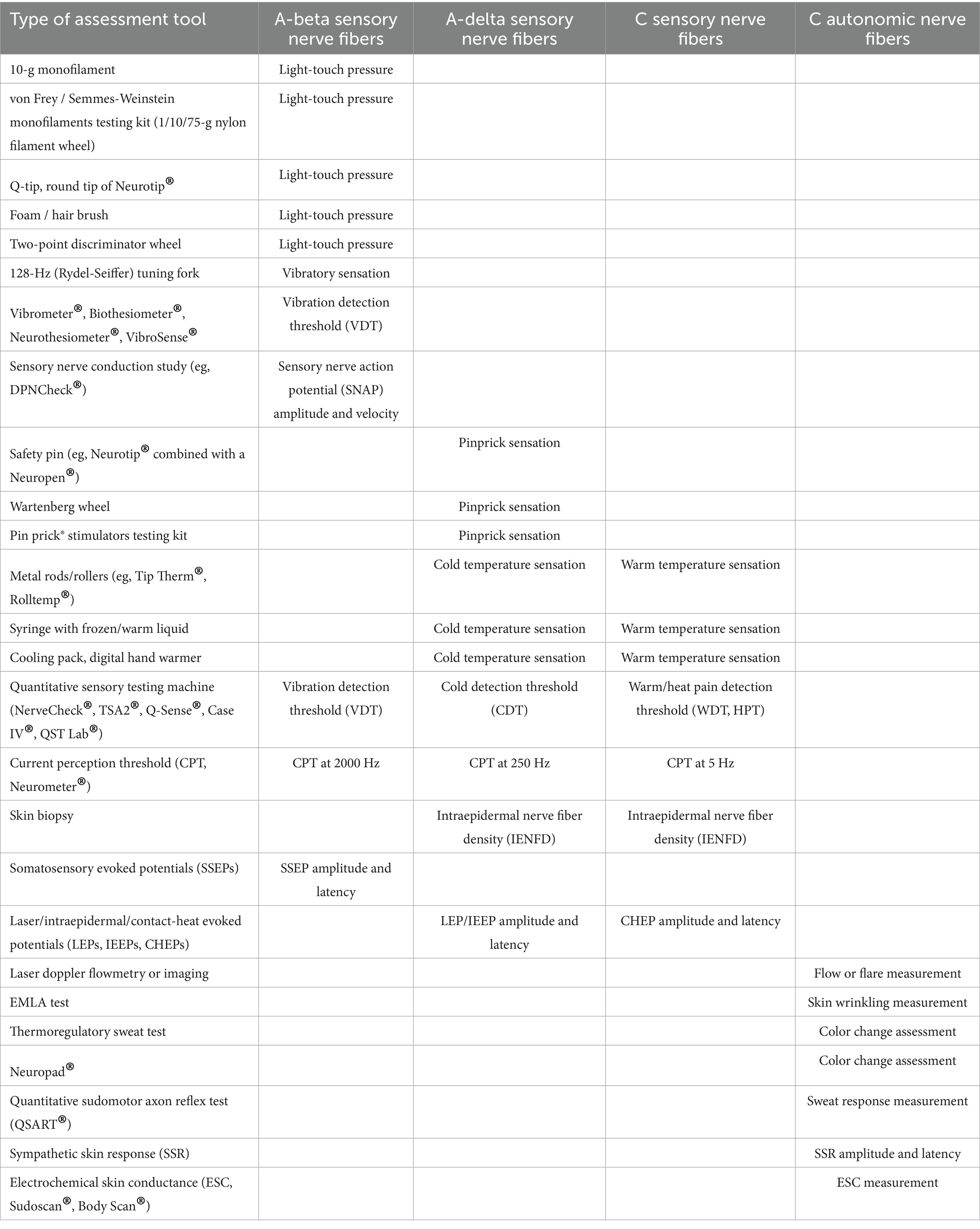
Mainly four types of nerve fibers must be assessed: A-beta sensory fibers, A-delta sensory fibers, type C sensory fibers, and type C autonomic fibers. The various tests that can be used in clinical practice to assess impairment of these different types of nerve fibers in the feet are presented in Table 1 and Figure 1.
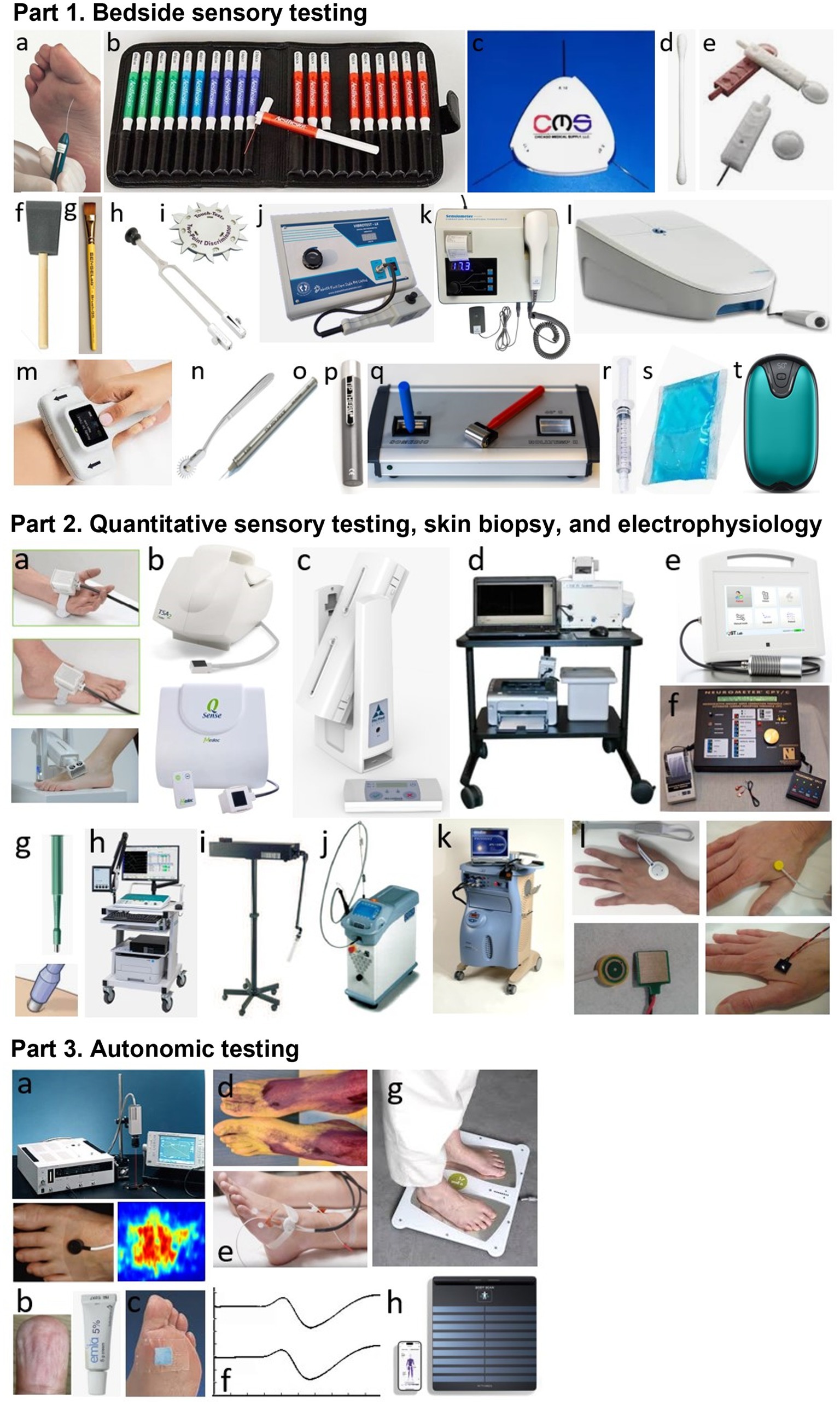
Figure 1. Part 1. Bedside sensory testing. a: 10-g monofilament, b: von Frey/Semmes-Weinstein monofilaments testing kit, c: 1/10/75-g nylon filament wheel, d: Q-tip, e: sharp and round tips of Neurotip®, f: foam brush, g: calibrated hair brush, h: 128-Hz (Rydel-Seiffer) tuning fork, i: two-point discriminator wheel, j: Vibrometer®, k: Biothesiometer®, l: VibroSense®, m: DPNCheck®, n: Wartenberg wheel, o: Pin prick® stimulator, p: Tip Therm®, q: Rolltemp®, r: filled syringe, s: cooling pack, t: digital hand warmer. Part 2. Quantitative sensory testing, skin biopsy, and electrophysiology. a: thermodes, b: TSA2® and Q-Sense®, c: NerveCheck®, d: Case IV®, e: QST. Lab®, f: Neurometer®, g: disposable skin biopsy punch, h; machine for performing nerve conduction study or evoked potentials, i: CO2 laser, j: Nd: YAP laser, k; contact-heat evoked potentials, l: different electrodes for performing intraepidermal evoked potentials. Part 3. Autonomic testing (at foot level). a: Laser doppler flowmetry or imaging, b: EMLA test, c: Neuropad®, d: thermoregulatory sweat test, e: QSART®, f: sympathetic skin response, g: Sudoscan®, h: Body Scan®.
In routine practice, the screening of DPN is based on the assessment of large A-beta fibers involved in light touch on three or four plantar sites with a 10-g monofilament and involved in vibration sense on the dorsal aspect of the great toe (interphalangeal joint) with a 128-Hz tuning fork (10). Other simple tools can be used for bedside sensory testing, such as the two-point discrimination test (21), which appears to measure sensory properties of the foot that differ from light touch assessed using monofilaments in diabetic patients (22). In addition, small A-delta fibers can be assessed for pinprick sensation with a safety pin (e.g., Neurotip® combined with a Neuropen®) (23) and for cold temperature sensation with a cold metal object (e.g., Tip Therm®) (24).
On the other hand, more complex quantitative sensory testing (QST) can be performed using computerized devices (Table 1; Figure 1). These devices allow sensory thresholds to be quantified as numerical values, more accurately than with conventional bedside testing, which is usually performed in a binary manner (stimulation perceived or not). Some tools are of intermediate use and combine portability (portable devices) with quantification of sensory thresholds. This is the case of the Biothesiometer® or Neurothesiometer® to assess vibration detection threshold (25, 26) or the NerveCheck®, which also assesses cold, warm, and heat pain detection thresholds with simple paradigms (27, 28).
Sensory nerve fibers can also be assessed using electrophysiological techniques of nerve conduction studies (29, 30). In the context of length-dependent diabetic polyneuropathy, sensory nerve action potentials (SNAPs) should be recorded distally in the lower limbs, particularly for the sural nerves. These recordings can be performed using a conventional EMG device, in conjunction with motor nerve conduction study in this case, or using dedicated devices, such as the DPNCheck®, which is limited to recording SNAPs from the sural nerve to the ankle (31, 32). The measurement of SNAPs is a particularly objective method of assessing large-diameter A-beta sensory nerve fibers in their distal segment, but provides no information on smaller-diameter sensory nerve fibers.
For small-diameter nerve fibers, electrophysiological tests can also be performed routinely, using stimulating devices capable of selectively stimulating this type of nerve fibers (33). The stimulation techniques that can be used for this purpose are based on thermal or electrical stimulation, while the recording of “evoked potentials” is performed using scalp electrodes and based on the averaging of electroencephalographic activities. Thermal stimulation can be radiant heating delivered by a laser or contact heating delivered by a thermode, allowing the recording of laser evoked potentials (LEPs) (34–36) or contact-heat evoked potentials (CHEPs) (37, 38), respectively. Electrical stimulation should aim to deliver a very focal current limited to the epidermis, where only the endings of small diameter nerve fibers are present. Different types of electrodes can be used for this purpose, allowing the recording of intraepidermal evoked potentials (IEEPs) (33). Usual somatosensory evoked potentials (SSEPs), obtained with a large bipolar stimulating electrode (as for SNAP recordings), engage subepidermal endings of large-diameter A-beta sensory fibers. The main limitation of using LEPs, CHEPs, IEEPs for the study of small-diameter A-delta or C fibers is that only brain responses can be recorded with these techniques, which precludes the assessment of a purely peripheral component (unlike SSEPs for large-diameter A-beta fibers) (33).
On the other hand, small-diameter sensory nerve endings can be assessed very specifically in the distal lower limbs by measuring intraepidermal nerve fiber density in a small skin biopsy (39–41). However, the representativeness of the measurement on a skin surface as small as a few mm2 is questionable. Furthermore, except in dedicated research studies (42), the repeatability of this invasive technique is limited for routine longitudinal monitoring of patients with DPN, particularly due to the increased risk of healing problems. Another technique to study small-diameter sensory innervation is corneal confocal microscopy, with the measurement of intracorneal nerve fiber density, fiber length, or branching density (43, 44). Although these measures may show significant correlations with the existence of more diffuse DPN (45–47), they do not directly assess innervation at the foot level and this technique is therefore less relevant than others for the specific assessment of diabetic foot syndrome.
Small-diameter nerve fibers also include autonomic fibers. Many tests of the autonomic nervous system are applicable in clinical practice (48). However, some tests do not directly assess distal autonomic innervation at the feet, such as cardiac autonomic function tests (Ewing tests) (49). In contrast, other tests specifically assess distal autonomic nerve fibers, which is highly relevant in the context of DPN, and generally rely on the vasomotor or sudomotor aspects of autonomic innervation of the foot (50, 51). There are methods that are easy to implement, but which nevertheless require a fairly long examination time and provide only a semi-quantitative assessment, such as the visualization of local vasoconstriction produced by the cutaneous application of a eutectic mixture of local anesthetics (EMLA test) (52–55) or the Neuropad® plaster test for sudomotor function (56–60). A better quantified assessment of distal autonomic functions can be achieved using more complex, time-consuming, and expensive techniques, such as laser Doppler techniques measuring vasomotor-mediated axon reflexes in response to different types of local cutaneous stimuli using vasoactive drugs, electrical stimulation, or heating (61). Laser Doppler techniques include laser flowmetry (LDF) (62–70) and flare response imaging (LDI) (71–75), but LDF is characterized by high intra- and inter-individual measurement variability and LDI by the lack of standardized image analysis methods, thus limiting their use in clinical practice.
Regarding the assessment of sudomotor function in the limbs, the quantitative sudomotor axon reflex test (QSART), developed in 1983 (76), has been promoted by its inventors as the gold standard technique (77, 78). This technique is based on the measurement, by a sudorometer, of the sweat response to local acetylcholine iontophoresis. However, the QSART technique requires complex expertise, a temperature- and humidity-controlled environment, and a relatively long examination time. In addition, its diagnostic sensitivity is limited by the high variability and low reproducibility of measures performed in the lower limbs (79, 80). Also, another technique, called Sudoscan®, simpler and faster (examination time of 2–3 min) than the QSART, has attracted great interest for quantitatively assessing distal sudomotor autonomic innervation of the extremities in clinical practice. The Sudoscan® technique is based on the principle of chronoamperometry and reverse iontophoresis, with measurement of electrochemical skin conductance (ESC) in microSiemens (μS). The ESC measurement depends on the current induced by the release of chloride ions from the eccrine sweat glands following activation by a low constant current of the sympathetic C fibers innervating these glands (81, 82). The Sudoscan® test has demonstrated its validity in the diagnosis of distal autonomic C-fiber lesion associated with DPN (83–94) or distal polyneuropathies of other causes (95). This technique does not require complex operator training (96) and has completely replaced the recording of sympathetic skin responses (SSRs), which was previously the routine electrodiagnostic test for assessing distal autonomic innervation of the limbs (97, 98). Indeed, SSR recording is poorly reproducible (99, 100) and is not specific to distal innervation by sympathetic C-fibers, as it is influenced by large-fiber sensory afferents and central reflex processing.
Screening strategy for the early diagnosis of DPN
The risk of developing diabetic foot syndrome and therefore presenting with DPN must be assessed annually in primary care according to international recommendations (9, 101, 102). However, this recommendation faces several difficulties. The first is the absence of a sensitive, objective, and validated strategy for diagnosing early DPN. As stated previously, DPN is routinely screened by semi-objective methods assessing touch, pinprick, and temperature sensations. Binns-Hall et al. showed that the combination of distal investigation of large-diameter sensory fires using the DPNCheck® and small-diameter autonomic fires using the Sudoscan® could be sensitive (95%) and specific (82%) to distinguish between the absence and presence of DPN and risk for diabetic foot syndrome with a strong correlation with clinical questionnaires (103).
However, such a one-stop screening strategy requires a hospital setting and many diabetic patients may encounter difficulties accessing hospital structures due to a lack of supplies or specialized structures. This is the reason why a large-scale project was developed in France to perform Sudoscan® in community health structures, ie more than 400 pharmacies. The measurement of ESC at the feet was combined with the Michigan Neuropathy Screening Instrument (104) with the physical assessment completed by the pharmacist, who was also asked to take eight photographs of the patients’ feet from different angles. All these data (ESC values, MNSI scores, and pictures of the feet) were sent by remote transmission to reference diabetology units for analysis. This study showed that reduced ESC in the feet was highly predictive of diabetic foot syndrome, particularly in cases of asymmetric ESC values or ESC values below 50 μS (unpublished data). A similar project had already been proposed in Canada, but using sural neve conduction measurement with the DPNCheck® in community pharmacies, instead of the ESC as a biomarker of DPN (105). The objective is that the pharmacists use these test results to educate patients on preventing DPN through a better glycaemic control and lifestyle, and improving foot self-care to avoid diabetic foot syndrome.
New perspectives with connected devices and telemedicine
New perspectives for diabetes monitoring are now opening up thanks to the development of connected tools, also adapted in clinical practice as a means of therapeutic education. This is the case of recent innovations such as continuous glucose monitoring (CGM) and time in range (TIR), which are emerging clinical endpoints for improving glycaemic control (106–110).
A variety of approaches have been proposed and studied to improve the management of diabetes by telemedicine (111–116), including the transmission of biomarkers, such as glycaemia (117) or body mass index (118), or telecoaching to improve lifestyle and promote exercise (119–121), or both (122). A telemonitoring program has already been performed in France (EDUC@DOM study) (123, 124), which combined biomedical data measurement with connected objects used at home, including a scale with impedancemetry, actimeter and blood glucose meter, and interactive educational software programs (with artificial intelligence (AI) algorithms). Compared to standard care, the remote monitoring performed by diabetologists with this telemedicine program over one or 2 years tended to result into a greater reduction of HbA1c levels (123) and was significantly cost-saving on socio-economic grounds (124). However, this program did not provide tools or measures to specifically monitor DPN.
It is now possible to measure ESC at the feet using a connected body scale, called Body Scan®. The ESC measurements obtained with the Body Scan® in just 20 s are perfectly consistent with those obtained with the Sudoscan®, thus allowing to consider a similar sensitivity and specificity in the diagnosis of distal autonomic neuropathy (125). Moreover, compared to the Sudoscan®, the advantage of the Body Scan® is that it allows the recording of ESC on a daily basis, at home, by the patients themselves. The association of this connected tool, more specifically assessing DPN, with other connected tools for assessing glycaemic control, could prove interesting. Indeed, a reduction in TIR and an increase in glycaemic variability revealed by CGM have been associated with progression of DPN and reduced ESC values at the feet measured with the Sudoscan® (126, 127).
On the other hand, ESC asymmetry at the feet > 9.5% was found to have 80% sensitivity and 91% specificity to determine the risk of diabetic foot syndrome (128). Thus, including a valuable biomarker of foot innervation, such as ESC, could be a way to improve the detection and monitoring of DPN, more specifically than the telemedicine strategies previously described. It is therefore tempting to design a large-scale cohort study to determine the adherence to a program of at-home ESC measurements at the feet over a long period of time for the follow-up of diabetic patients and monitoring of DPN, in particular to confirm the predictive value of ESC asymmetry in the development of diabetic foot complication.
A concurrent approach is to monitor foot temperature at home, using an infrared thermometer, a sensor mat, or temperature measuring socks (129). Adherence to this type of monitoring was found to range between 56 and 86% and is even better for socks. When the temperature difference between the feet is greater than 2.2 °C (at the hot spot), the patients are recommended to reduce their daily steps by 50% and notify a healthcare professional or podiatrist as this indicates a significantly increased risk of foot ulcers. Constant monitoring of foot temperature could be combined with plantar pressure measurements using sensors embedded in a wearable insole (130). In one study, it was proposed that patients self-assess the plantar thermal images they took at home using smartphone-based thermography (131). Early detection of diabetic foot complication could benefit from AI for thermographic image analysis in future smartphone apps (132, 133).
Another home-based approach with smartphone-based self-photographs aims to assess the presence or extent of foot ulcers (134, 135) by allowing patients to photograph the plantar surface of their feet unassisted [“foot selfie,” (136)] and transmit these images to a remote server. Wound imaging systems with commercial portable devices have already demonstrated high accuracy (137, 138) and are expected to benefit even more from AI and machine-learning algorithms in the future (139–142) to prevent the development of diabetic foot ulcers.
Finally, a novel smartphone-based home monitoring approach to DPN has recently been reported, including patient self-assessment through large fiber sensory testing, including vibration perception and two-point discrimination assessed with 3D-printed accessories, combined with a clinical neuropathy assessment questionnaire (143). In the context of chemotherapy-induced peripheral neuropathy, another group also proposed a smartphone app for neuropathy monitoring, comprising clinical questionnaires and six functional assessments using smartphone sensors to provide information on neurological functions, such as walking, standing, and dexterity (144, 145). In any case, there are increasing perspectives for the use of smart wearable technologies and various types of sensors integrated into smartphones, socks, insoles, or shoes, for continuous or at-home health monitoring, prevention of diabetic foot ulcers or risk of falls, including AI solutions and deep learning models to improve data analysis (146–150).
Conclusion
In conclusion, DN, including DPN, remains a major health problem, with serious consequences such as diabetic foot syndrome. Early and accurate detection of DPN, particularly through specific and sensitive tools targeting different nerve fiber types, is essential for its prevention and improvement of outcomes. Technological advances, notably through connected devices specifically assessing foot innervation by conductance or temperature measurements for example, offer promising perspectives for continuous home monitoring of nerve function in large cohorts of patients. Combined with connected glucose control measures, telemedicine, and patient education, these innovations could significantly transform the management of DPN by improving early diagnosis, disease monitoring, and overall patient care, which could prevent serious complications such as foot ulcers and amputations, reduce healthcare costs, and improve the quality of life of diabetic patients worldwide.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
J-PL: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declares that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sloan, G, Selvarajah, D, and Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat Rev Endocrinol. (2021) 17:400–20. doi: 10.1038/s41574-021-00496-z
2. Ziegler, D, Gries, FA, Spüler, M, and Lessmann, F. The epidemiology of diabetic neuropathy DiaCAN Multicenter Study Group. Diabet Med. (1993) 10:82S–6S.
3. Fang, M, Hu, J, Jeon, Y, Matsushita, K, Selvin, E, and Hicks, CW. Diabetic foot disease and the risk of major clinical outcomes. Diabetes Res Clin Pract. (2023) 202:110778. doi: 10.1016/j.diabres.2023.110778
4. Gill, G, and Moulik, P. Mortality and diabetic neuropathy. Diabet Med. (2005) 22:1289. doi: 10.1111/j.1464-5491.2005.01729.x
5. Kerr, M, Barron, E, Chadwick, P, Evans, T, Kong, WM, Rayman, G, et al. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet Med. (2019) 36:995–1002. doi: 10.1111/dme.13973
6. Sorber, R, and Abularrage, CJ. Diabetic foot ulcers: epidemiology and the role of multidisciplinary care teams. Semin Vasc Surg. (2021) 34:47–53. doi: 10.1053/j.semvascsurg.2021.02.006
7. Ang, L, Mizokami-Stout, K, Eid, SA, Elafros, M, Callaghan, B, Feldman, EL, et al. The conundrum of diabetic neuropathies-past, present, and future. J Diabetes Complicat. (2022) 36:108334. doi: 10.1016/j.jdiacomp.2022.108334
8. Bondar, A, Popa, AR, Papanas, N, Popoviciu, M, Vesa, CM, Sabau, M, et al. Diabetic neuropathy: a narrative review of risk factors, classification, screening and current pathogenic treatment options (review). Exp Ther Med. (2021) 22:690. doi: 10.3892/etm.2021.10122
9. Pop-Busui, R, Boulton, AJ, Feldman, EL, Bril, V, Freeman, R, Malik, RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. (2017) 40:136–54. doi: 10.2337/dc16-2042
10. Ziegler, D, Tesfaye, S, Spallone, V, Gurieva, I, Al Kaabi, J, Mankovsky, B, et al. Screening, diagnosis and management of diabetic sensorimotor polyneuropathy in clinical practice: international expert consensus recommendations. Diabetes Res Clin Pract. (2022) 186:109063. doi: 10.1016/j.diabres.2021.109063
11. Boulton, AJ. Diabetic neuropathy and foot complications. Handb Clin Neurol. (2014) 126:97–107. doi: 10.1016/B978-0-444-53480-4.00008-4
12. Kim, J. The pathophysiology of diabetic foot: a narrative review. J Yeungnam Med Sci. (2023) 40:328–34. doi: 10.12701/jyms.2023.00731
13. Neville, RF, Kayssi, A, Buescher, T, and Stempel, MS. The diabetic foot. Curr Probl Surg. (2016) 53:408–37. doi: 10.1067/j.cpsurg.2016.07.003
14. Allen, L, Powell-Cope, G, Mbah, A, Bulat, T, and Njoh, E. A retrospective review of adverse events related to diabetic foot ulcers. Ostomy Wound Manage. (2017) 63:30–3.
15. Boulton, AJ, Vinik, AI, Arezzo, JC, Bril, V, Feldman, EL, Freeman, R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. (2005) 28:956–62. doi: 10.2337/diacare.28.4.956
16. Kender, Z, Jende, JME, Kurz, FT, Tsilingiris, D, Schimpfle, L, Sulaj, A, et al. Sciatic nerve fractional anisotropy and neurofilament light chain protein are related to sensorimotor deficit of the upper and lower limbs in patients with type 2 diabetes. Front Endocrinol. (2023) 14:1046690. doi: 10.3389/fendo.2023.1046690
17. Maalmi, H, Nguyen, PBH, Strom, A, Bönhof, GJ, Rathmann, W, Ziegler, D, et al. Prediction model for polyneuropathy in recent-onset diabetes based on serum neurofilament light chain, fibroblast growth Factor-19 and standard anthropometric and clinical variables. Diabetes Metab Res Rev. (2024) 40:e70009. doi: 10.1002/dmrr.70009
18. Määttä, LL, Andersen, ST, Parkner, T, Hviid, CVB, Bjerg, L, Kural, MA, et al. Longitudinal change in serum neurofilament light chain in type 2 diabetes and early diabetic polyneuropathy: ADDITION-Denmark. Diabetes Care. (2024) 47:986–94. doi: 10.2337/dc23-2208
19. Thrysøe, M, Parkner, T, Tankisi, H, Nyengaard, JR, Vestergaard, ET, Kristensen, K, et al. Biochemical use of neurofilament light polypeptide and vitamin B12 in relation to diabetic polyneuropathy in Danish adolescents with type 1 diabetes: a cross-sectional study. BMJ Open. (2025) 15:e085749. doi: 10.1136/bmjopen-2024-085749
20. Ciardullo, S, Muraca, E, Bianconi, E, Cannistraci, R, Perra, S, Zerbini, F, et al. Diabetes mellitus is associated with higher serum neurofilament light chain levels in the general US population. J Clin Endocrinol Metab. (2023) 108:361–7. doi: 10.1210/clinem/dgac580
21. Eryilmaz, M, Koçer, A, Kocaman, G, and Dikici, S. Two-point discrimination in diabetic patients. J Diabetes. (2013) 5:442–8. doi: 10.1111/1753-0407.12055
22. Periyasamy, R, Manivannan, M, and Narayanamurthy, VB. Correlation between two-point discrimination with other measures of sensory loss in diabetes mellitus patients. Int J Diabetes Dev Ctries. (2008) 28:71–8. doi: 10.4103/0973-3930.44076
23. Paisley, A, Abbott, C, van Schie, C, and Boulton, A. A comparison of the Neuropen against standard quantitative sensory-threshold measures for assessing peripheral nerve function. Diabet Med. (2002) 19:400–5. doi: 10.1046/j.1464-5491.2002.00706.x
24. Viswanathan, V, Snehalatha, C, Seena, R, and Ramachandran, A. Early recognition of diabetic neuropathy: evaluation of a simple outpatient procedure using thermal perception. Postgrad Med J. (2002) 78:541–2. doi: 10.1136/pmj.78.923.541
25. Bloom, S, Till, S, Sönksen, P, and Smith, S. Use of a biothesiometer to measure individual vibration thresholds and their variation in 519 non-diabetic subjects. Br Med J. (1984) 288:1793–5. doi: 10.1136/bmj.288.6433.1793
26. Young, MJ, Every, N, and Boulton, AJ. A comparison of the neurothesiometer and biothesiometer for measuring vibration perception in diabetic patients. Diabetes Res Clin Pract. (1993) 20:129–31. doi: 10.1016/0168-8227(93)90006-Q
27. Ponirakis, G, Odriozola, MN, Odriozola, S, Petropoulos, IN, Azmi, S, Fadavi, H, et al. NerveCheck: an inexpensive quantitative sensory testing device for patients with diabetic neuropathy. Diabetes Res Clin Pract. (2016) 113:101–7. doi: 10.1016/j.diabres.2015.12.023
28. Ponirakis, G, Odriozola, MN, Odriozola, S, Petropoulos, IN, Azmi, S, Ferdousi, M, et al. NerveCheck for the detection of sensory loss and neuropathic pain in diabetes. Diabetes Technol Ther. (2016) 18:800–5. doi: 10.1089/dia.2016.0279
29. Perkins, B, and Bril, V. Electrophysiologic testing in diabetic neuropathy. Handb Clin Neurol. (2014) 126:235–48. doi: 10.1016/B978-0-444-53480-4.00018-7
30. Shabeeb, D, Najafi, M, Hasanzadeh, G, Hadian, MR, Musa, AE, and Shirazi, A. Electrophysiological measurements of diabetic peripheral neuropathy: a systematic review. Diabetes Metab Syndr. (2018) 12:591–600. doi: 10.1016/j.dsx.2018.03.026
31. Chatzikosma, G, Pafili, K, Demetriou, M, Vadikolias, K, Maltezos, E, and Papanas, N. Evaluation of sural nerve automated nerve conduction study in the diagnosis of peripheral neuropathy in patients with type 2 diabetes mellitus. Arch Med Sci. (2016) 12:390–3. doi: 10.5114/aoms.2016.59265
32. Shibata, Y, Himeno, T, Kamiya, T, Tani, H, Nakayama, T, Kojima, C, et al. Validity and reliability of a point-of-care nerve conduction device in diabetes patients. J Diabetes Investig. (2019) 10:1291–8. doi: 10.1111/jdi.13007
33. Lefaucheur, JP. Clinical neurophysiology of pain. Handb Clin Neurol. (2019) 161:121–48. doi: 10.1016/B978-0-444-64142-7.00045-X
34. Agostino, R, Cruccu, G, Romaniello, A, Innocenti, P, Inghilleri, M, and Manfredi, M. Dysfunction of small myelinated afferents in diabetic polyneuropathy, as assessed by laser evoked potentials. Clin Neurophysiol. (2000) 111:270–6. doi: 10.1016/S1388-2457(99)00247-3
35. Ragé, M, Van Acker, N, Knaapen, MW, Timmers, M, Streffer, J, Hermans, MP, et al. Asymptomatic small fiber neuropathy in diabetes mellitus: investigations with intraepidermal nerve fiber density, quantitative sensory testing and laser-evoked potentials. J Neurol. (2011) 258:1852–64. doi: 10.1007/s00415-011-6031-z
36. Di Stefano, G, La Cesa, S, Leone, C, Pepe, A, Galosi, E, Fiorelli, M, et al. Diagnostic accuracy of laser-evoked potentials in diabetic neuropathy. Pain. (2017) 158:1100–7. doi: 10.1097/j.pain.0000000000000889
37. Chao, CC, Tseng, MT, Lin, YJ, Yang, WS, Hsieh, SC, Lin, YH, et al. Pathophysiology of neuropathic pain in type 2 diabetes: skin denervation and contact heat-evoked potentials. Diabetes Care. (2010) 33:2654–9. doi: 10.2337/dc10-1135
38. Wong, MC, and Chung, JW. Feasibility of contact heat evoked potentials for detection of diabetic neuropathy. Muscle Nerve. (2011) 44:902–6. doi: 10.1002/mus.22192
39. Beiswenger, KK, Calcutt, NA, and Mizisin, AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. (2008) 110:351–62. doi: 10.1016/j.acthis.2007.12.004
40. Lauria, G, Hsieh, ST, Johansson, O, Kennedy, WR, Leger, JM, Mellgren, SI, et al. European Federation of Neurological Societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the peripheral nerve society. Eur J Neurol. (2010) 17:e44–9. doi: 10.1111/j.1468-1331.2010.03023.x
41. Mellgren, SI, Nolano, M, and Sommer, C. The cutaneous nerve biopsy: technical aspects, indications, and contribution. Handb Clin Neurol. (2013) 115:171–88. doi: 10.1016/B978-0-444-52902-2.00010-2
42. Khoshnoodi, MA, Truelove, S, Burakgazi, A, Hoke, A, Mammen, AL, and Polydefkis, M. Longitudinal assessment of small Fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol. (2016) 73:684–90. doi: 10.1001/jamaneurol.2016.0057
43. Petropoulos, IN, Bitirgen, G, Ferdousi, M, Kalteniece, A, Azmi, S, D'Onofrio, L, et al. Corneal confocal microscopy to image small nerve Fiber degeneration: ophthalmology meets neurology. Front Pain Res. (2021) 2:725363. doi: 10.3389/fpain.2021.725363
44. Lukashenko, MV, Gavrilova, NY, Bregovskaya, AV, Soprun, LA, Churilov, LP, Petropoulos, IN, et al. Corneal confocal microscopy in the diagnosis of small fiber neuropathy: faster, easier, and more efficient than skin biopsy? Pathophysiology. (2021) 29:1–8. doi: 10.3390/pathophysiology29010001
45. Malik, RA, Kallinikos, P, Abbott, CA, van Schie, CH, Morgan, P, Efron, N, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. (2003) 46:683–8. doi: 10.1007/s00125-003-1086-8
46. Tavakoli, M, Petropoulos, IN, and Malik, RA. Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. J Diabetes Sci Technol. (2013) 7:1179–89. doi: 10.1177/193229681300700509
47. Gad, H, Petropoulos, IN, Khan, A, Ponirakis, G, MacDonald, R, Alam, U, et al. Corneal confocal microscopy for the diagnosis of diabetic peripheral neuropathy: a systematic review and meta-analysis. J Diabetes Investig. (2022) 13:134–47. doi: 10.1111/jdi.13643
48. Illigens, BMW, and Gibbons, CH. Autonomic testing, methods and techniques. Handb Clin Neurol. (2019) 160:419–33. doi: 10.1016/B978-0-444-64032-1.00028-X
49. Fisher, VL, and Tahrani, AA. Cardiac autonomic neuropathy in patients with diabetes mellitus: current perspectives. Diabetes Metab Syndr Obes. (2017) 10:419–34. doi: 10.2147/DMSO.S129797
50. Lefaucheur, JP. Assessment of autonomic nervous system dysfunction associated with peripheral neuropathies in the context of clinical neurophysiology practice. Neurophysiol Clin. (2023) 53:102858. doi: 10.1016/j.neucli.2023.102858
51. Zouari, HG, Ng Wing Tin, S, Wahab, A, Damy, T, and Lefaucheur, JP. Assessment of autonomic innervation of the foot in familial amyloid polyneuropathy. Eur J Neurol. (2019) 26:94–e10. doi: 10.1111/ene.13774
52. Wilder-Smith, E, and Chow, A. Water immersion and EMLA cause similar digit skin wrinkling and vasoconstriction. Microvasc Res. (2003) 66:68–72. doi: 10.1016/S0026-2862(03)00020-7
53. Teoh, HL, Chow, A, and Wilder-Smith, EP. Skin wrinkling for diagnosing small fibre neuropathy: comparison with epidermal nerve density and sympathetic skin response. J Neurol Neurosurg Psychiatry. (2008) 79:835–7. doi: 10.1136/jnnp.2007.140947
54. Ping Ng, KW, Ong, JJ, Nyein Nyein, TD, Liang, S, Chan, YC, Lee, KO, et al. EMLA-induced skin wrinkling for the detection of diabetic neuropathy. Front Neurol. (2013) 4:126. doi: 10.3389/fneur.2013.00126
55. Wilder-Smith, EP. Stimulated skin wrinkling as an indicator of limb sympathetic function. Clin Neurophysiol. (2015) 126:10–6. doi: 10.1016/j.clinph.2014.08.007
56. Papanas, N, Papatheodorou, K, Christakidis, D, Papazoglou, D, Giassakis, G, Piperidou, H, et al. Evaluation of a new indicator test for sudomotor function (Neuropad) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes. (2005) 113:195–8. doi: 10.1055/s-2005-837735
57. Quattrini, C, Jeziorska, M, Tavakoli, M, Begum, P, Boulton, AJ, and Malik, RA. The neuropad test: a visual indicator test for human diabetic neuropathy. Diabetologia. (2008) 51:1046–50. doi: 10.1007/s00125-008-0987-y
58. Spallone, V, Morganti, R, Siampli, M, Fedele, T, D'Amato, C, Cacciotti, L, et al. Neuropad as a diagnostic tool for diabetic autonomic and sensorimotor neuropathy. Diabet Med. (2009) 26:686–92. doi: 10.1111/j.1464-5491.2009.02760.x
59. Papanas, N, Boulton, AJ, Malik, RA, Manes, C, Schnell, O, Spallone, V, et al. A simple new non-invasive sweat indicator test for the diagnosis of diabetic neuropathy. Diabet Med. (2013) 30:525–34. doi: 10.1111/dme.12000
60. Ponirakis, G, Petropoulos, IN, Fadavi, H, Alam, U, Asghar, O, Marshall, A, et al. The diagnostic accuracy of Neuropad for assessing large and small fibre diabetic neuropathy. Diabet Med. (2014) 31:1673–80. doi: 10.1111/dme.12536
61. Kubasch, ML, Kubasch, AS, Torres Pacheco, J, Buchmann, SJ, Illigens, BM, Barlinn, K, et al. Laser doppler assessment of vasomotor axon reflex responsiveness to evaluate neurovascular function. Front Neurol. (2017) 8:370. doi: 10.3389/fneur.2017.00370
62. Parkhouse, N, Le, P, and Quesne, M. Impaired neurogenic vascular response in patients with diabetes and neuropathic foot lesions. N Engl J Med. (1988) 318:1306–9. doi: 10.1056/NEJM198805193182005
63. Newrick, PG, Cochrane, T, Betts, RP, Ward, JD, and Boulton, AJ. Reduced hyperaemic response under the diabetic neuropathic foot. Diabet Med. (1988) 5:570–3. doi: 10.1111/j.1464-5491.1988.tb01053.x
64. Flynn, MD, Edmonds, ME, Tooke, JE, and Watkins, PJ. Direct measurement of capillary blood flow in the diabetic neuropathic foot. Diabetologia. (1988) 31:652–6. doi: 10.1007/BF00278747
65. Obeid, AN, Barnett, NJ, Dougherty, G, and Ward, G. A critical review of laser doppler flowmetry. J Med Eng Technol. (1990) 14:178–81. doi: 10.3109/03091909009009955
66. Wilson, SB, Jennings, PE, and Belch, JJ. Detection of microvascular impairment in type I diabetics by laser doppler flowmetry. Clin Physiol. (1992) 12:195–208. doi: 10.1111/j.1475-097X.1992.tb00306.x
67. Forst, T, Pfützner, A, Kunt, T, Pohlmann, T, Schenk, U, Bauersachs, R, et al. Skin microcirculation in patients with type I diabetes with and without neuropathy after neurovascular stimulation. Clin Sci (Lond). (1998) 94:255–61. doi: 10.1042/cs0940255
68. Quattrini, C, Harris, ND, Malik, RA, and Tesfaye, S. Impaired skin microvascular reactivity in painful diabetic neuropathy. Diabetes Care. (2007) 30:655–9. doi: 10.2337/dc06-2154
69. Au, M, and Rattigan, S. Barriers to the management of diabetes mellitus - is there a future role for laser doppler flowmetry? Australas Med J. (2012) 5:627–32. doi: 10.4066/AMJ.2012.1526
70. Lal, C, and Unni, SN. Correlation analysis of laser doppler flowmetry signals: a potential non-invasive tool to assess microcirculatory changes in diabetes mellitus. Med Biol Eng Comput. (2015) 53:557–66. doi: 10.1007/s11517-015-1266-y
71. Bickel, A, Krämer, HH, Hilz, MJ, Birklein, F, Neundörfer, B, and Schmelz, M. Assessment of the neurogenic flare reaction in small-fiber neuropathies. Neurology. (2002) 59:917–9. doi: 10.1212/WNL.59.6.917
72. Krämer, HH, Schmelz, M, Birklein, F, and Bickel, A. Electrically stimulated axon reflexes are diminished in diabetic small fiber neuropathies. Diabetes. (2004) 53:769–74. doi: 10.2337/diabetes.53.3.769
73. Krishnan, ST, and Rayman, G. The ldiflare: a novel test of C-fiber function demonstrates early neuropathy in type 2 diabetes. Diabetes Care. (2004) 27:2930–5. doi: 10.2337/diacare.27.12.2930
74. Baker, N, Green, A, Krishnan, S, and Rayman, G. Microvascular and C-fiber function in diabetic charcot neuroarthropathy and diabetic peripheral neuropathy. Diabetes Care. (2007) 30:3077–9. doi: 10.2337/dc07-1063
75. Illigens, BM, Siepmann, T, Roofeh, J, and Gibbons, CH. Laser doppler imaging in the detection of peripheral neuropathy. Auton Neurosci. (2013) 177:286–90. doi: 10.1016/j.autneu.2013.06.006
76. Low, PA, Caskey, PE, Tuck, RR, Fealey, RD, and Dyck, PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Ann Neurol. (1983) 14:573–80. doi: 10.1002/ana.410140513
77. Low, PA. Autonomic nervous system function. J Clin Neurophysiol. (1993) 10:14–27. doi: 10.1097/00004691-199301000-00003
78. Low, PA, Tomalia, VA, and Park, KJ. Autonomic function tests: some clinical applications. J Clin Neurol. (2013) 9:1–8. doi: 10.3988/jcn.2013.9.1.1
79. Peltier, A, Smith, AG, Russell, JW, Sheikh, K, Bixby, B, Howard, J, et al. Reliability of quantitative sudomotor axon reflex testing and quantitative sensory testing in neuropathy of impaired glucose regulation. Muscle Nerve. (2009) 39:529–35. doi: 10.1002/mus.21210
80. Berger, MJ, and Kimpinski, K. Test-retest reliability of quantitative sudomotor axon reflex testing. J Clin Neurophysiol. (2013) 30:308–12. doi: 10.1097/WNP.0b013e3182873254
81. Novak, P. Electrochemical skin conductance: a systematic review. Clin Auton Res. (2019) 29:17–29. doi: 10.1007/s10286-017-0467-x
82. Vittrant, B, Ayoub, H, and Brunswick, P. From Sudoscan to bedside: theory, modalities, and application of electrochemical skin conductance in medical diagnostics. Front Neuroanat. (2024) 18:1454095. doi: 10.3389/fnana.2024.1454095
83. Mayaudon, H, Miloche, PO, and Bauduceau, B. A new simple method for assessing sudomotor function: relevance in type 2 diabetes. Diabetes Metab. (2010) 36:450–4. doi: 10.1016/j.diabet.2010.05.004
84. Gin, H, Baudoin, R, Raffaitin, CH, Rigalleau, V, and Gonzalez, C. Non-invasive and quantitative assessment of sudomotor function for peripheral diabetic neuropathy evaluation. Diabetes Metab. (2011) 37:527–32. doi: 10.1016/j.diabet.2011.05.003
85. Yajnik, CS, Kantikar, VV, Pande, AJ, and Deslypere, JP. Quick and simple evaluation of sudomotor function for screening of diabetic neuropathy. ISRN Endocrinol. (2012) 2012:103714. doi: 10.5402/2012/103714
86. Calvet, JH, Dupin, J, Winiecki, H, and Schwarz, PE. Assessment of small fiber neuropathy through a quick, simple and non invasive method in a German diabetes outpatient clinic. Exp Clin Endocrinol Diabetes. (2013) 121:80–3. doi: 10.1055/s-0032-1323777
87. Casellini, CM, Parson, HK, Richardson, MS, Nevoret, ML, and Vinik, AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther. (2013) 15:948–53. doi: 10.1089/dia.2013.0129
88. Smith, AG, Lessard, M, Reyna, S, Doudova, M, and Singleton, JR. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complicat. (2014) 28:511–6. doi: 10.1016/j.jdiacomp.2014.02.013
89. Selvarajah, D, Cash, T, Davies, J, Sankar, A, Rao, G, Grieg, M, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One. (2015) 10:e0138224. doi: 10.1371/journal.pone.0138224
90. Mao, F, Liu, S, Qiao, X, Zheng, H, Xiong, Q, Wen, J, et al. Sudoscan is an effective screening method for asymptomatic diabetic neuropathy in Chinese type 2 diabetes mellitus patients. J Diabetes Investig. (2017) 8:363–8. doi: 10.1111/jdi.12575
91. Jin, J, Wang, W, Gu, T, Chen, W, Lu, J, Bi, Y, et al. The application of SUDOSCAN for screening diabetic peripheral neuropathy in Chinese population. Exp Clin Endocrinol Diabetes. (2018) 126:472–7. doi: 10.1055/s-0043-116673
92. Carbajal-Ramírez, A, Hernández-Domínguez, JA, Molina-Ayala, MA, Rojas-Uribe, MM, and Chávez-Negrete, A. Early identification of peripheral neuropathy based on sudomotor dysfunction in Mexican patients with type 2 diabetes. BMC Neurol. (2019) 19:109. doi: 10.1186/s12883-019-1332-4
93. Veloso, DLC, Nascimento, RCG, Leite, EB, de Avila Santana, L, and Amato, AA. Predictors of sudomotor dysfunction in patients with type 1 diabetes without clinical evidence of peripheral neuropathy. Diabetes Res Clin Pract. (2020) 170:108500. doi: 10.1016/j.diabres.2020.108500
94. Gautier, JF, Riveline, JP, Potier, L, Bourron, O, Bordier, L, Vittrant, B, et al. Electrochemical skin conductance: a tool for risk stratification and early anticipation of diabetic foot ulcers. Front Endocrinol. (2025) 16:1437858. doi: 10.3389/fendo.2025.1437858
95. Lefaucheur, JP. The value of electrochemical skin conductance measurement by Sudoscan® for assessing autonomic dysfunction in peripheral neuropathies beyond diabetes. Neurophysiol Clin. (2023) 53:102859. doi: 10.1016/j.neucli.2023.102859
96. Bordier, L, Dolz, M, Monteiro, L, Névoret, ML, Calvet, JH, and Bauduceau, B. Accuracy of a rapid and non-invasive method for the assessment of small Fiber neuropathy based on measurement of electrochemical skin Conductances. Front Endocrinol. (2016) 7:18. doi: 10.3389/fendo.2016.00018
97. Shahani, BT, Halperin, JJ, Boulu, P, and Cohen, J. Sympathetic skin response--a method of assessing unmyelinated axon dysfunction in peripheral neuropathies. J Neurol Neurosurg Psychiatry. (1984) 47:536–42. doi: 10.1136/jnnp.47.5.536
98. Claus, D, and Schondorf, R. Sympathetic skin response. Electroencephalogr Clin Neurophysiol. (1999) 52:277–82.
99. Hoeldtke, RD, Davis, KM, Hshieh, PB, Gaspar, SR, and Dworkin, GE. Autonomic surface potential analysis: assessment of reproducibility and sensitivity. Muscle Nerve. (1992) 15:926–31. doi: 10.1002/mus.880150810
100. Toyokura, M, and Murakami, K. Reproducibility of sympathetic skin response. Muscle Nerve. (1996) 19:1481. doi: 10.1002/(SICI)1097-4598(199611)19:11<>3.0.CO;2-W
101. Tesfaye, S, Boulton, AJ, Dyck, PJ, Freeman, R, Horowitz, M, Kempler, P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. (2010) 33:2285–93. doi: 10.2337/dc10-1303
102. Bril, V, Perkins, B, and Toth, CCanadian Diabetes Association Clinical Practice Guidelines Expert Committee. Neuropathy. Can J Diabetes. (2013) 37:S142–4. doi: 10.1016/j.jcjd.2013.01.039
103. Binns-Hall, O, Selvarajah, D, Sanger, D, Walker, J, Scott, A, and Tesfaye, S. One-stop microvascular screening service: an effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabet Med. (2018) 35:887–94. doi: 10.1111/dme.13630
104. Feldman, EL, Stevens, MJ, Thomas, PK, Brown, MB, Canal, N, and Greene, DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. (1994) 17:1281–9. doi: 10.2337/diacare.17.11.1281
105. Poulose, S, Cheriyan, E, Poulose, A, Cheriyan, R, Vadakkanezath, B, and Ziemer, P. Usefulness of the NC-stat DPNCheck nerve conduction test in a community pharmacy as an educational tool for patients with diabetes. Can Pharm J. (2015) 148:17–20. doi: 10.1177/1715163514561055
106. American Diabetes Association. Glycemic targets: standards of medical care in Diabetes-2020. Diabetes Care. (2020) 43:S66–76. doi: 10.2337/dc20-S006
107. Azizi, F, Diehl, K, Enser, L, and Heacock, S. Achievement of time in range goals among patients with diabetes using continuous glucose monitoring. J Am Pharm Assoc. (2025) 8:102924. doi: 10.1016/j.japh.2025.102924
108. Alfadli, SF, Alotaibi, YS, Aqdi, MJ, Almozan, LA, Alzubaidi, ZB, Altemani, HA, et al. Effectiveness of continuous glucose monitoring systems on glycemic control in adults with type 1 diabetes: a systematic review and meta-analysis. Metabol Open. (2025) 27:100382. doi: 10.1016/j.metop.2025.100382
109. Savoy, A, Barboi, C, Thomas, MR, and Weiner, M. Usability of continuous glucose monitoring for older adults with type 2 diabetes: a systematic review. Diabetes Technol Ther. (2025) in press). doi: 10.1177/15209156251369021
110. Maiorino, MI, Di Martino, N, Angelino, S, Maio, A, Caruso, P, Petrizzo, M, et al. The therapeutic efficacy of continuous glucose monitoring in diabetes: an updated meta-analysis with meta-regression. Endocrine. (2025). doi: 10.1007/s12020-025-04405-6
111. Holtz, B, and Lauckner, C. Diabetes management via mobile phones: a systematic review. Telemed J E Health. (2012) 18:175–84. doi: 10.1089/tmj.2011.0119
112. Siriwardena, LS, Wickramasinghe, WA, Perera, KL, Marasinghe, RB, Katulanda, P, and Hewapathirana, R. A review of telemedicine interventions in diabetes care. J Telemed Telecare. (2012) 18:164–8. doi: 10.1258/jtt.2012.SFT110
113. Marcolino, MS, Maia, JX, Alkmim, MB, Boersma, E, and Ribeiro, AL. Telemedicine application in the care of diabetes patients: systematic review and meta-analysis. PLoS One. (2013) 8:e79246. doi: 10.1371/journal.pone.0079246
114. Fu, H, McMahon, SK, Gross, CR, Adam, TJ, and Wyman, JF. Usability and clinical efficacy of diabetes mobile applications for adults with type 2 diabetes: a systematic review. Diabetes Res Clin Pract. (2017) 131:70–81. doi: 10.1016/j.diabres.2017.06.016
115. Lee, SWH, Chan, CKY, Chua, SS, and Chaiyakunapruk, N. Comparative effectiveness of telemedicine strategies on type 2 diabetes management: a systematic review and network meta-analysis. Sci Rep. (2017) 7:12680. doi: 10.1038/s41598-017-12987-z
116. Wang, G, Zhang, Z, Feng, Y, Sun, L, Xiao, X, Wang, G, et al. Telemedicine in the Management of Type 2 diabetes mellitus. Am J Med Sci. (2017) 353:1–5. doi: 10.1016/j.amjms.2016.10.008
117. Lee, PA, Greenfield, G, and Pappas, Y. The impact of telehealth remote patient monitoring on glycemic control in type 2 diabetes: a systematic review and meta-analysis of systematic reviews of randomised controlled trials. BMC Health Serv Res. (2018) 18:495. doi: 10.1186/s12913-018-3274-8
118. Izquierdo, R, Lagua, CT, Meyer, S, Ploutz-Snyder, RJ, Palmas, W, Eimicke, JP, et al. Telemedicine intervention effects on waist circumference and body mass index in the IDEATel project. Diabetes Technol Ther. (2010) 12:213–20. doi: 10.1089/dia.2009.0102
119. Holmen, H, Torbjørnsen, A, Wahl, AK, Jenum, AK, Småstuen, MC, Arsand, E, et al. A Mobile health intervention for self-management and lifestyle change for persons with type 2 diabetes, part 2: one-year results from the Norwegian randomized controlled trial RENEWING HEALTH. JMIR Mhealth Uhealth. (2014) 2:e57. doi: 10.2196/mhealth.3882
120. de Vasconcelos, HCA, Lira Neto, JCG, de Araújo, MFM, Carvalho, GCN, de Souza Teixeira, CR, de Freitas, RWJF, et al. Telecoaching programme for type 2 diabetes control: a randomised clinical trial. Br J Nurs. (2018) 27:1115–20. doi: 10.12968/bjon.2018.27.19.1115
121. Ng Wing Tin, S, Zouari, HG, Ayache, SS, Tropeano, AI, Ajzenberg, C, Xhaxho, J, et al. Coaching of lifestyle recommendations improves sensory neurophysiological parameters in neuropathies related to glycemic disorder or metabolic syndrome. A pilot study. Neurophysiol Clin. (2019) 49:59–67. doi: 10.1016/j.neucli.2018.12.004
122. Bollyky, JB, Bravata, D, Yang, J, Williamson, M, and Schneider, J. Remote lifestyle coaching plus a connected glucose meter with certified diabetes educator support improves glucose and weight loss for people with type 2 diabetes. J Diabetes Res. (2018) 2018:3961730. doi: 10.1155/2018/3961730
123. Turnin, MC, Gourdy, P, Martini, J, Buisson, JC, Chauchard, MC, Delaunay, J, et al. Impact of a remote monitoring programme including lifestyle education software in type 2 diabetes: results of the Educ@dom randomised multicentre study. Diabetes Ther. (2021) 12:2059–75. doi: 10.1007/s13300-021-01095-x
124. Mounié, M, Costa, N, Gourdy, P, Latorre, C, Schirr-Bonnans, S, Lagarrigue, JM, et al. Cost-effectiveness evaluation of a remote monitoring programme including lifestyle education software in type 2 diabetes: results of the Educ@dom study. Diabetes Ther. (2022) 13:693–708. doi: 10.1007/s13300-022-01207-1
125. Riveline, JP, Mallone, R, Tiercelin, C, Yaker, F, Alexandre-Heymann, L, Khelifaoui, L, et al. Validation of the body scan®, a new device to detect small fiber neuropathy by assessment of the sudomotor function. Agreement with the Sudoscan®. Front Neurol. (2023) 14:1256984. doi: 10.3389/fneur.2023.1256984
126. Feng, ZQ, Guo, QY, Wang, W, Yuan, YY, Jin, XG, Zhou, H, et al. Time in range, especially overnight time in range, is associated with sudomotor dysfunction in patients with type 1 diabetes. Diabetol Metab Syndr. (2021) 13:119. doi: 10.1186/s13098-021-00739-z
127. Guo, QY, Lu, B, Guo, ZH, Feng, ZQ, Yuan, YY, Jin, XG, et al. Continuous glucose monitoring defined time-in-range is associated with sudomotor dysfunction in type 2 diabetes. World J Diabetes. (2020) 11:489–500. doi: 10.4239/wjd.v11.i11.489
128. Gatev, T, Gateva, A, Assyov, Y, Nacheva, S, Petrova, J, Poromanski, I, et al. The role of Sudoscan feet asymmetry in the diabetic foot. Prim Care Diabetes. (2020) 14:47–52. doi: 10.1016/j.pcd.2019.05.003
129. Jones, PJ, Lavery, L, Davies, MJ, Webb, D, and Rowlands, AV. Hotspots: adherence in home foot temperature monitoring interventions for at-risk feet with diabetes-a narrative review. Diabet Med. (2023) 40:e15189. doi: 10.1111/dme.15189
130. Khandakar, A, Mahmud, S, Chowdhury, MEH, Reaz, MBI, Kiranyaz, S, Mahbub, ZB, et al. Design and implementation of a smart insole system to measure plantar pressure and temperature. Sensors. (2022) 22:7599. doi: 10.3390/s22197599
131. Qin, Q, Nakagami, G, Ohashi, Y, Dai, M, Sanada, H, and Oe, M. Development of a self-monitoring tool for diabetic foot prevention using smartphone-based thermography: plantar thermal pattern changes and usability in the home environment. Drug Discov Ther. (2022) 16:169–76. doi: 10.5582/ddt.2022.01050
132. Wartakusumah, R, Yamada, A, Noguchi, H, and Oe, M. Analysis of foot thermography images of diabetic patients using artificial intelligence: a scoping review. Diabetes Res Clin Pract. (2025) 228:112446. doi: 10.1016/j.diabres.2025.112446
133. Khandakar, A, Chowdhury, MEH, Ibne Reaz, MB, Md Ali, SH, Hasan, MA, Kiranyaz, S, et al. A machine learning model for early detection of diabetic foot using thermogram images. Comput Biol Med. (2021) 137:104838. doi: 10.1016/j.compbiomed.2021.104838
134. Kuang, B, Pena, G, Szpak, Z, Edwards, S, Battersby, R, Cowled, P, et al. Assessment of a smartphone-based application for diabetic foot ulcer measurement. Wound Repair Regen. (2021) 29:460–5. doi: 10.1111/wrr.12905
135. Pak, C, In Jeon, J, Kim, H, Kim, J, Park, S, Ahn, KH, et al. A smartphone-based teleconsultation system for the management of chronic pressure injuries. Wound Repair Regen. (2018) 26:S19–26. doi: 10.1111/wrr.2
136. Swerdlow, M, Shin, L, D'Huyvetter, K, and Mack, WJ. Armstrong Armstrong, DG. Initial clinical experience with a simple, home system for early detection and monitoring of diabetic foot ulcers: the foot selfie. J Diabetes Sci Technol. (2023) 17:79–88. doi: 10.1177/19322968211053348
137. Chan, KS, and Lo, ZJ. Wound assessment, imaging and monitoring systems in diabetic foot ulcers: a systematic review. Int Wound J. (2020) 17:1909–23. doi: 10.1111/iwj.13481
138. Kairys, A, Pauliukiene, R, Raudonis, V, and Ceponis, J. Towards home-based diabetic foot ulcer monitoring: a systematic review. Sensors (Basel). (2023) 23:3618. doi: 10.3390/s23073618
139. Zoppo, G, Marrone, F, Pittarello, M, Farina, M, Uberti, A, Demarchi, D, et al. AI technology for remote clinical assessment and monitoring. J Wound Care. (2020) 29:692–706. doi: 10.12968/jowc.2020.29.12.692
140. Lucas, Y, Niri, R, Treuillet, S, Douzi, H, and Castaneda, B. Wound size imaging: ready for smart assessment and monitoring. Adv Wound Care. (2021) 10:641–61. doi: 10.1089/wound.2018.0937
141. Chan, KS, Chan, YM, Tan, AHM, Liang, S, Cho, YT, Hong, Q, et al. Clinical validation of an artificial intelligence-enabled wound imaging mobile application in diabetic foot ulcers. Int Wound J. (2022) 19:114–24. doi: 10.1111/iwj.13603
142. Cassidy, B, Hoon Yap, M, Pappachan, JM, Ahmad, N, Haycocks, S, O'Shea, C, et al. Artificial intelligence for automated detection of diabetic foot ulcers: a real-world proof-of-concept clinical evaluation. Diabetes Res Clin Pract. (2023) 205:110951. doi: 10.1016/j.diabres.2023.110951
143. Piaggio, D, Castaldo, R, Garibizzo, G, Iadanza, E, and Pecchia, L. A smartphone-based tool for screening diabetic neuropathies: a mHealth and 3D printing approach. Biomed Signal Process Control. (2024) 97:106719. doi: 10.1016/j.bspc.2023.105807
144. Chen, CS, Kim, J, Garg, N, Guntupalli, H, Jagsi, R, Griggs, JJ, et al. Chemotherapy-induced peripheral neuropathy detection via a smartphone app: cross-sectional pilot study. JMIR Mhealth Uhealth. (2021) 9:e27502. doi: 10.2196/27502
145. Chen, CS, Dorsch, MP, Alsomairy, S, Griggs, JJ, Jagsi, R, Sabel, M, et al. Remote monitoring of chemotherapy-induced peripheral neuropathy by the NeuroDetect iOS app: observational cohort study of patients with Cancer. J Med Internet Res. (2025) 27:e65615. doi: 10.2196/65615
146. Sendilraj, V, Pilcher, W, Choi, D, Bhasin, A, Bhadada, A, Bhadadaa, SK, et al. DFUCare: deep learning platform for diabetic foot ulcer detection, analysis, and monitoring. Front Endocrinol. (2024) 15:1386613. doi: 10.3389/fendo.2024.1386613
147. Rukmini, PG, Hegde, RB, Basavarajappa, BK, Bhat, AK, Pujari, AN, Gargiulo, GD, et al. Recent innovations in footwear and the role of smart footwear in healthcare-a survey. Sensors. (2024) 24:4301. doi: 10.3390/s24134301
148. Zhao, X, Zhang, Y, Huang, Z, Wu, X, and Lin, J. Innovative therapies for diabetic foot ulcers: application and prospects of smart dressings. Biomed Pharmacother. (2025) 191:118498. doi: 10.1016/j.biopha.2025.118498
149. Kosaji, D, Awad, MI, Katmah, R, Jelinek, HF, Domingues, MF, Baguneid, M, et al. Diabetic foot prevention, assessment, and management using innovative smart wearable technology: a systematic review. J Neuroeng Rehabil. (2025) 22:168. doi: 10.1186/s12984-025-01695-9
Keywords: autonomic neuropathy, diabetes, diagnosis, monitoring, polyneuropathy, small fiber neuropathy, smartphone, telemedicine
Citation: Lefaucheur J-P (2025) Screening and monitoring of diabetic polyneuropathy in clinical practice: present and future with connected devices. Front. Neurol. 16:1679277. doi: 10.3389/fneur.2025.1679277
Edited by:
Mamede De Carvalho, University of Lisbon, PortugalReviewed by:
Miguel Santos, Santa Maria Hospital, PortugalCopyright © 2025 Lefaucheur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Pascal Lefaucheur, amVhbi1wYXNjYWwubGVmYXVjaGV1ckBobW4uYXBocC5mcg==
 Jean-Pascal Lefaucheur
Jean-Pascal Lefaucheur