Abstract
Background:
Cerebellar transcranial direct current stimulation (ctDCS) has emerged as a promising non-invasive neuromodulatory approach for managing pain. Early evidence suggests beneficial effects on pain perception in both healthy individuals and patients with chronic pain. However, the underlying mechanisms and clinical efficacy remain unclear. This systematic review aimed to synthesize the current evidence on cerebellar involvement in pain processing and to evaluate the potential of ctDCS as a therapeutic intervention.
Methods:
A systematic search was conducted in PubMed, Embase, and the Cochrane Library, following PRISMA guidelines. MeSH and Emtree descriptors related to “Cerebellum,” “Pain,” and “tDCS” were used to identify relevant studies published up to December 11, 2024. Eligible studies were randomized controlled trials (RCTs) that investigated the effects of ctDCS on pain. Risk of bias was assessed using the Cochrane Risk of Bias Tool version 2 (RoB 2).
Results:
Of 819 records screened, five RCTs met the inclusion criteria. The primary methodological limitations included incomplete reporting of randomization procedures and inadequate blinding of outcome assessors. Two studies lacked key demographic and clinical details, while one showed a high risk of bias due to repeated same-day stimulation. Despite these issues, Across the included studies anodal ctDCS generally increased pain thresholds and enhanced endogenous pain inhibition, whereas cathodal ctDCS tended to reduce thresholds. Neurophysiological findings supported these behavioral results, with EEG data showing modulation of cortical activity related to pain processing.
Conclusion:
Preliminary findings suggest that ctDCS may modulate nociceptive pathways and enhance pain inhibition. However, the small number of studies and methodological heterogeneity limit the generalizability of current results. Further high-quality RCTs are needed to optimize stimulation protocols, assess long-term effects, and establish clinical benefits. This review supports the cerebellum as a relevant and underexplored target for neuromodulatory pain interventions.
1 Introduction
Chronic pain affects approximately 20% of the adult population in Western countries and represents a major public health challenge due to its multifactorial nature and significant impact on quality of life (1). Typically, it is defined as pain occurring on most days or every day for more than three months (2). Despite advances in both pharmacological and non-pharmacological approaches, effective management remains elusive. Recent U.S. data show that 24.3% of adults reported chronic pain in 2023, and 8.5% experienced high-impact chronic pain—pain that frequently limits daily or work activities—reflecting a worsening trend and emphasizing the need for more personalized and integrated therapeutic approaches (3–6).
In response to the growing burden of chronic pain, there has been renewed interest in innovative treatments targeting central pain modulation mechanisms. Non-invasive neuromodulation techniques have shown promise in enhancing pain outcomes. Among these, transcranial direct current stimulation (tDCS) has emerged as a compelling modality, demonstrating efficacy across a range of pain conditions, coupled with a favorable safety profile and low incidence of adverse effects (7, 8).
Most neuromodulation research to date has focused on cortical targets, particularly the dorsolateral prefrontal cortex (DLPFC) (9, 10) and the primary motor cortex (M1) (10, 11). However, increasing attention has recently turned to the cerebellum as a novel target for non-invasive stimulation. While traditionally associated with sensorimotor integration, the cerebellum is now recognized as a key modulator of pain through its role in integrating nociceptive input with motor and cognitive-affective processes, thereby influencing both pain perception and emotional reactivity (12).
Functional neuroimaging studies have further elucidated the cerebellum’s role in pain processing, revealing its interactions with key regions, including the anterior midcingulate cortex, supplementary motor area, and thalamus (13, 14). Although emerging studies involving both healthy individuals and patients with neuropathic pain offer promising insights (15–19), several critical gaps remain. These include uncertainties regarding optimal stimulation parameters, the precise mechanisms of action, long-term efficacy, and the potential benefits of multisite stimulation protocols targeting regions such as M1 and DLPFC.
To advance the field, this review offers a comprehensive analysis of cerebellar involvement in pain processing, drawing on anatomical, clinical, and experimental evidence. It explores the role of the cerebellum in pain modulation and evaluates the therapeutic potential of ctDCS. Key technical considerations, current limitations, and future directions for research and clinical application are also discussed.
2 Mapping cerebellar circuits: anatomical insights into pain processing
2.1 Cerebrocortical and cerebellar contributions to pain modulation
Pain is a multidimensional experience arising from the interplay of sensory, affective, cognitive, and social factors (20), processed through distributed brain networks that include the thalamus, brainstem nuclei, somatosensory cortices, and prefrontal regions (21). Among cortical targets, the M1 and DLPFC have been the most extensively investigated in neuromodulation. Stimulation of M1 enhances excitability within intracortical circuits, thalamic relays, and descending modulatory pathways, producing analgesic effects partly mediated by endogenous opioids (22). The DLPFC, in turn, contributes to top-down regulation of cognitive-affective processes, strengthening connectivity with the periaqueductal gray (PAG) and engaging in expectation-driven analgesia (23). Evidence from clinical and preclinical studies shows that modulation of these regions not only reduces pain intensity but also alleviates maladaptive cognitive-emotional factors such as catastrophizing and depressive symptoms (24–26).
Beyond these well-established cortical sites, increasing evidence highlights the cerebellum as a promising and integrative hub in pain processing. Far from being solely a motor structure, the cerebellum contributes to sensory, cognitive, and emotional dimensions of the pain experience (27, 28). Its activation has been reported across various pain states, including acute, chronic, and neuropathic pain (21, 29). Together, these findings position the cerebellum as a novel neuromodulatory target, with ctDCS offering a promising approach to influence distributed pain-related networks through cerebellar stimulation.
2.2 Anatomical and functional framework of the cerebellum in pain
The cerebellum constitutes only a small fraction of total brain volume, yet it contains more than half of all neurons in the central nervous system (27, 30). Traditionally regarded as a motor structure, converging evidence highlights its integrative role in cognitive and emotional processing (31, 32). Structurally, it comprises the vermis, paravermal zones, and lateral hemispheres, which form the anterior, posterior, and flocculonodular lobes (33). Functionally, it is classically divided into the vestibulocerebellum (balance and oculomotor control), spinocerebellum (muscle tone and proprioception), and cerebrocerebellum (motor planning and execution) (34).
At the microcircuit level, mossy and climbing fibers provide the main excitatory inputs to the cerebellar cortex, converging on Purkinje cells that exert inhibitory control over the deep cerebellar nuclei. These nuclei—fastigial, dentate, globose, and emboliform—constitute the principal output hubs, projecting to motor, sensory, limbic, and associative regions, thereby linking the cerebellum to both motor and non-motor functions (35–38). This highly organized architecture provides the substrate for cerebellar contributions to pain processing, enabling integration of sensory, affective, and cognitive information.
Topographical mapping further reveals pain-related specialization. Larsell’s classification divides the cerebellum into ten lobules (I–X), which display functional heterogeneity. Sensorimotor processing predominates in lobules I–IV and VIII (39), whereas lobules VI–IX support emotional and cognitive regulation (32), and lobules V–VII and IX–X contribute to vestibular and interoceptive integration (40, 41). Neuroimaging studies show that acute pain recruits lobules III–VI, VIIb, Crus II, and bilateral hemisphere VI (28), whereas chronic pain preferentially engages vermal lobules IV–V and hemispheric lobules V, VI, and Crus I—patterns consistent with sustained pain states and emotional dysregulation (28, 42). Distinct networks have also been described in visceral pain, with consistent activation of bilateral lobules V–VI, Crus I, lobule VIII, and vermal VI (43). In the context of somatic pain, sex-related differences have been observed: men exhibit increased activation of the lateral cerebellar cortex following muscle and cutaneous stimulation, whereas women display reduced or absent responses; this pattern may reflect variability in endogenous analgesic systems (44). Beyond the direct encoding of pain, anticipatory mechanisms have also been identified, with ipsilateral posterior cerebellar activation preceding noxious heat stimulation, suggesting a role in sensory prediction and pre-attentive processing (45). Collectively, these findings highlight the heterogeneity of cerebellar activation across pain modalities, as summarized in Figure 1.
Figure 1

Cerebellar activation patterns in different pain states. The diagram illustrates brain regions activated during acute pain (lobules III–VI, VIIb, Crus II), chronic pain (vermal IV–V, hemispheric V–VI, Crus I), visceral pain (bilateral V–VI, Crus I, VIII, vermal VI), and somatic pain (lateral cerebellar cortex with sex-related differences).
Taken together, these anatomical and functional features position the cerebellum as a dynamic hub for integrating sensorimotor, autonomic, and affective information in pain processing. This organizational scaffold forms the basis for the afferent and efferent cerebrocerebellar circuits described in the following sections.
2.3 Cerebrocerebellar afferent systems involved in pain processing
The cerebellum receives afferent input from diverse cerebral sources involved in motor, somatosensory, cognitive, affective, and reward processing. The convergence of these signals allows cerebellar circuits to integrate nociceptive information and modulate pain perception across multiple functional domains.
Excitatory input reaches the cerebellar cortex through two principal pathways. Mossy fibers, originating from various brainstem nuclei, form excitatory synapses with granule cells and golgi cells in the granular layer. The axons of these granule cells ascend into the molecular layer and bifurcate into parallel fibers that synapse with the dendrites of Purkinje cells, thereby activating them. This activation ultimately produces inhibitory output from Purkinje cells to the deep cerebellar nuclei, contributing to both motor and non-motor adaptive responses (33, 46).
In parallel, climbing fibers arising from the contralateral inferior olivary nucleus establish powerful excitatory synapses with Purkinje cells, inducing long-term depression (LTD) at parallel fiber–Purkinje cell synapses (47). These fibers also send collateral projections to the deep cerebellar nuclei and engage inhibitory interneurons, including basket and stellate cells, which promote lateral inhibition of neighboring Purkinje cells (48, 49). Through these mechanisms, climbing fibers convey precise timing and prediction-error signals essential for sensorimotor integration and pain modulation (50, 51). These connections between mossy- and climbing-fiber inputs and cerebellar cortical and nuclear elements are illustrated in the Figure 2.
Figure 2

Afferent convergence in the cerebellar cortex and deep nuclei. Mossy fibers from brainstem and spinal relays excite granule and Golgi cells in the granular layer; granule axons ascend as parallel fibers and contact Purkinje dendrites in the molecular layer. Climbing fibers from the inferior olive form powerful synapses on Purkinje dendrites and send collaterals to the deep cerebellar nuclei. The inhibitory output of Purkinje neurons to these nuclei, which relay to thalamic and brainstem targets, provides an anatomical substrate consistent with the integration of nociceptive, sensorimotor, and affective information.
Convergent nociceptive-related signals reach cerebellar circuits via both cerebrocerebellar and spino-olivocerebellar pathways. Among the most prominent are the corticopontocerebellar projections, which originate from M1, premotor cortices, DLPFC, and primary somatosensory cortex (S1), and relay through the pontine nuclei to reach the cerebellar cortex (52–56). Projections from M1 and S1 primarily target lobules I–V and VI, delivering motor efference copies and somatosensory feedback critical for fast adaptive responses during nociceptive events (57). The lateral hemisphere, especially Crus I, receives input from the DLPFC and parietal cortices, providing a substrate for integrating attention, salience, and pain expectation (50, 58). In addition, dopaminergic projections from the ventral tegmental area (VTA) terminate predominantly in Crus II and, to a lesser extent, in Crus I. This mesolimbic-cerebellar pathway forms a neuroanatomical interface between motivational-affective processing and cerebellar circuits, implicating the cerebellum in reinforcement learning and the drive for analgesia (59–61).
Complementing these cerebrocortical inputs, animal studies have shown that direct nociceptive information can reach the cerebellum via the spino-olivocerebellar pathway. Ekerot et al. (60) demonstrated that stimulation of A- and B-type cutaneous nociceptive fibers in animal models triggered climbing fiber-mediated activation of Purkinje cells in the anterior cerebellum. This effect was likely mediated by the spino-olivocerebellar tract, which ascends via the dorsal and dorsolateral funiculi to deliver nociceptive input directly to cerebellar circuits (28, 62). The inferior olive itself receives convergent input from midbrain structures involved in pain control, including the red nucleus, zona incerta, and the periaqueductal gray (PAG), a key hub of descending pain modulation (63). Figure 3 schematically illustrates the topographical and anatomical organization of these afferent systems.
Figure 3

Topographical mapping of afferent projections to the cerebellum from brain regions involved in motor, sensory, cognitive, affective, and reward processing. M1 – primary motor cortex; S1 – primary somatosensory cortex; DLPFC – dorsolateral prefrontal cortex; V1 – visual cortex; PAG – periaqueductal gray; VTA – ventral tegmental area. This schematic illustrates the multidimensional integration of nociceptive inputs by the cerebellum through mossy and climbing fibers, which contributes to the modulation of sensory-discriminative, affective, and cognitive components of pain.
2.4 Cerebrocerebellar efferent pathways modulating nociception
Among cerebellar efferent pathways, projections originating in lobules IV–VI and Crus I/II provide the structural substrate for top-down modulation of pain-related behaviors. Purkinje cells in these lobules send inhibitory outputs to the deep cerebellar nuclei, which in turn project excitatory fibers to the motor thalamic nuclei. These thalamic relays establish reciprocal circuits with the M1, forming a closed-loop circuit that integrates motor commands and nociceptive processing (53). Optogenetic stimulation of Crus I also modulates M1 via the dentate nucleus, influencing whisker movement and tactile responses in rodents and highlighting its role in sensorimotor integration (54). This circuitry is depicted in Figure 4.
Figure 4

Efferent cerebellar pathway potentially involved in pain modulation. Schematic illustration of the dentatothalamocortical tract, an efferent pathway projecting from the dentate nucleus of the cerebellum to the thalamus, and subsequently to cortical regions such as the primary motor cortex and prefrontal cortex. This tract is thought to contribute to the modulation of both sensorimotor and affective-cognitive components of pain processing. Abbreviations: M1 – primary motor cortex; S1 – primary somatosensory cortex; DLPFC – dorsolateral prefrontal cortex; MPFC – medial prefrontal cortex; ACC – anterior cingulate cortex; PAG – periaqueductal gray; VTA – ventral tegmental area.
The functional coupling between the cerebellum and M1 is particularly relevant for pain modulation, given that M1 stimulation induces analgesic effects in chronic pain conditions. Such effects are attributed to the antidromic recruitment of thalamocortical circuits and engagement of descending modulatory systems (22, 64–66). Moreover, cerebellar output pathways appear to influence not only sensorimotor dimensions but also affective–motivational components of pain, involving key limbic regions such as the ACC and anterior insula (22, 42, 67).
Beyond M1, cerebellar projections from lobules IV–VI and Crus I also target medial prefrontal areas. Specifically, lobules IV–V project to the ACC, while vermal lobule VI and Crus I are connected to the prelimbic and orbitofrontal cortices (68, 69). The medial prefrontal cortex is involved in emotion regulation, memory-guided decision-making, and higher-order behavioral control (70, 71). Its role in pain modulation—particularly in descending control, ruminative processing, and comorbid affective symptoms such as anxiety and depression—is well supported by both preclinical and clinical studies (72–74). Taken together, this evidence supports a role for cerebellar output in modulating both sensory-discriminative and cognitive-affective aspects of pain.
Pain- and motivation-related brain regions, such as the nucleus accumbens (NAc) and the VTA, can be modulated by cerebellar output. While direct anatomical projections from the cerebellum to the NAc have not been conclusively identified, studies of functional connectivity and evidence for polysynaptic relay circuits suggest that cerebellar activity can influence NAc function. In contrast, the modulatory pathway from Purkinje cells in Crus I to VTA neurons—mediated via the dentate nucleus—is more clearly established (68, 75). Both the NAc and VTA are key components of mesolimbic circuits responsible for encoding the salience and motivational valence of pain. Consistently, chemogenetic activation of Crus I Purkinje cells projecting to the VTA has been shown to attenuate depressive-like behaviors in chronically stressed mice, reinforcing the notion that cerebellar circuits contribute to the regulation of affective states and mood-related comorbidities commonly observed in chronic pain (75).
The cerebellum also communicates with the hippocampus, a region central to memory and contextual processing of pain. Vermal lobule VI and Crus I influence the dentate gyrus via fastigial and dentate nuclei through polysynaptic thalamic–septal relays (76). Optogenetic stimulation of vermal lobules IV–V and hemispheric lobule VI modulates hippocampal activity and alters performance on hippocampus-dependent spatial tasks (77). The hippocampus itself exhibits structural and functional abnormalities in both rodent models of neuropathic pain and patients with chronic low back pain and complex regional pain syndrome (78). Chronic pain impairs the extinction of contextual—but not cued—fear memories, indicating a selective disruption of hippocampus-dependent learning, associated with decreased neurogenesis and synaptic remodeling. These findings suggest that cerebellar modulation of hippocampal circuits may contribute to maladaptive emotional and mnemonic responses observed in persistent pain states.
3 Transcranial direct current stimulation -technical factors
3.1 Methods
The methodology of this systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. No protocol was registered or published in PROSPERO (International Prospective Register of Systematic Reviews) before the development of this study.
Relevant studies were identified through comprehensive searches of the Cochrane Library (from 1996), PubMed (from 1996), and Embase (from 1993). The MeSH terms and entry terms used, along with their combinations, were: [(“Cerebellum”) AND (“Pain” OR “tDCS”) AND (“tDCS”)] (search conducted up to December 11, 2024). Additionally, the reference lists of the included studies were manually reviewed to identify other potentially eligible articles.
3.2 Study selection and eligibility criteria
To be eligible, studies were required to meet the following inclusion criteria: (1) involve human participants; (2) be published in English, Portuguese, or Spanish. In addition to English, Portuguese and Spanish were included because all reviewers are fluent in these languages, which ensured accurate assessment and data extraction without introducing interpretation bias; and (3) be classified as clinical studies, randomized controlled trials, systematic reviews, meta-analyses, or book chapters. Eligible studies specifically investigated the application of ctDCS for pain modulation, either in individuals with clinical pain or in healthy participants undergoing experimental pain induction. No restrictions were placed on the year of publication.
Title and abstract screening were conducted independently by two reviewers. In cases of disagreement, a third reviewer was consulted to reach a consensus. Duplicate records were removed before screening. Studies involving animal models or not aligned with the review objectives were then excluded.
The initial selection targeted studies with titles containing key terms such as “pain” and “cerebellum,” “pain” and “cerebellar tDCS,” or simply “tDCS.” Articles using synonymous or related MeSH terms were also considered. Abstracts were reviewed to assess relevance to ctDCS as a therapeutic intervention for pain or to the cerebellum’s role in nociceptive processing. Full-text articles were then analyzed, and only those that fulfilled all inclusion criteria were retained.
For each included study, the following data were extracted: first author, year of publication, study design, number of participants, electrode montage and stimulation site, stimulation parameters, reported adverse events (if any), neurophysiological outcomes (when applicable), pain assessment methods, study objectives, and main findings.
3.3 Quality and bias risk assessment
The methodological quality of the included studies was independently assessed by two reviewers using the Cochrane Risk of Bias Tool for Randomized Trials (RoB 2). In cases of disagreement, a third reviewer was consulted to reach a consensus. The domains evaluated included the randomization process, potential bias arising from period and carryover effects (when applicable), deviations from intended interventions, missing outcome data, outcome measurement, and selection of the reported results.
Following the assessment, the overall risk of bias for each study was categorized as “low risk,” “some concerns,” or “high risk.” It is important to note that the specific domains assessed varied according to the study design, such as parallel group randomized controlled trials or randomized crossover trials.
4 Results
A summary of the search strategy is illustrated in Figure 5, following the PRISMA flowchart methodology. A total of 819 records were initially identified—808 through database searches and 11 through manual screening of bibliographic references. After removing duplicates and excluding studies involving animal models, 511 records remained for title and abstract screening. Studies that did not address the use of tDCS for pain management or the relationship between the cerebellum and pain processing were excluded. Subsequently, 18 full-text articles were assessed for eligibility, of which only five met the predefined inclusion criteria. These five studies specifically investigated the effects of ctDCS on pain modulation, either in healthy participants exposed to nociceptive stimuli or in patients with pain-related conditions.
Figure 5
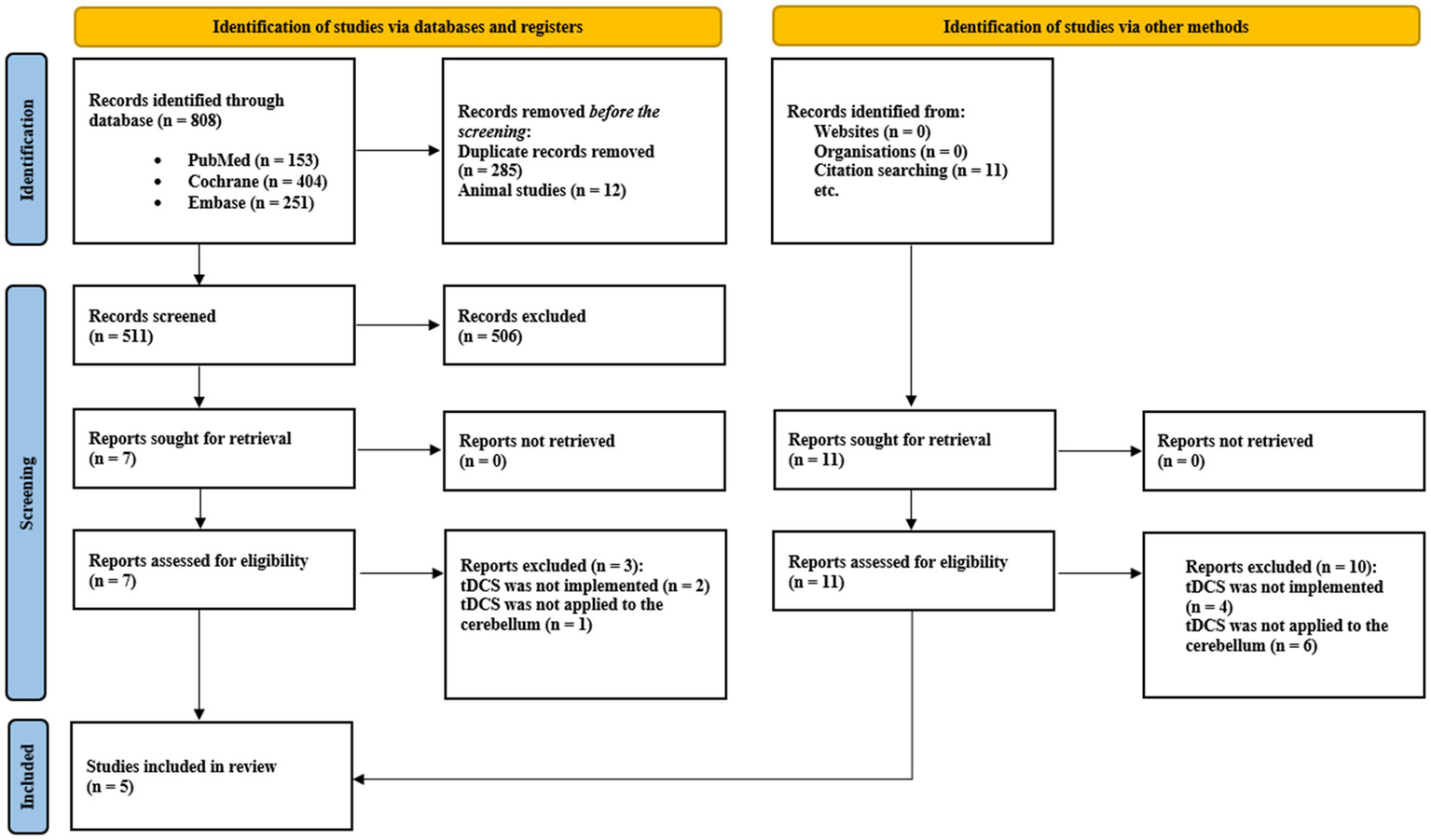
PRISMA fowchart of the included studies.
4.1 Demographic characteristics of the patients included
The five randomized clinical trials included a total of 80 participants. Of these, 66 were healthy individuals enrolled across four studies, while 14 were patients with unilateral upper limb amputation (eight with left-sided and six with right-sided amputation), assessed in a single study (17). Four studies employed a crossover randomized controlled trial (RCT) design (15, 17–19), and one utilized a parallel-arm RCT design, including 16 healthy participants as controls (16). All participants in the active intervention arms were adults, with a mean age of approximately 28.5 years (SD = 7.0). A total of 47 women were included, representing a slight difference compared to men across both studies involving healthy individuals and those involving participants with chronic pain.
4.2 Stimulation protocol
The stimulation protocols employed across the studies typically positioned the target electrode (anode, cathode, or sham) along the midline, 2 cm below the inion, with the lateral edges placed approximately 1 cm medial to the mastoid processes. The placement of the reference electrode varied among studies: in three investigations, it was positioned on the right shoulder (16, 17, 19); in the remaining two, it was placed on the lateral aspect of the upper arm near the deltoid region (18) and the right buccinator area (15), respectively. The electrodes used had surface areas of either 35 cm2 (17, 18) or 25 cm2 (15, 16, 19).
All studies applied a stimulation intensity of 2 mA, and sham stimulation being discontinued after the ramp-up phase. Session duration and frequency varied. Four studies delivered a single session, whereas one study (17) implemented five consecutive daily sessions. Regarding duration, three studies (17–19) used 20-min sessions; one used 15-min (16) and one 5-min (15) protocols.
4.3 Neurophysiological tools and pain outcome measures
In three studies (16, 17, 19), laser stimulation combined with EEG was employed to assess perceptual thresholds, pain perception, and laser-evoked potentials (LEPs). Additionally, two studies incorporated peripheral electrical stimulation with surface electrodes into their protocols. Pereira et al. used cutaneous stimulation to quantify sensory and pain thresholds (15), whereas Stacheneder et al. (18) applied electrical sural-nerve stimulation and EEG to record spinal and cortical responses, as well as subjective pain ratings. In the same study, thermal stimuli and the conditioned pain modulation (CPM) paradigm were applied to evaluate endogenous pain modulation and to determine individual heat pain thresholds (18).
Subjective pain intensity was assessed using two different scales: the Visual Analog Scale (VAS) was employed in three studies (15, 17, 19), while the Numerical Rating Scale (NRS) was used in two studies (16, 18).
4.4 Adverse effects
In general, the studies included in this review did not report any adverse effects associated with ctDCS. An exception was observed in the study by Stacheneder et al., which described mild and transient side effects, including itching at the electrode site and post-session headaches (18).
4.5 Overview of study outcomes
The evidence gathered from the reviewed studies consistently supports the efficacy of ctDCS in modulating pain processing in both healthy individuals and patients with chronic pain conditions. Three studies reported polarity-dependent effects of ctDCS (16, 18, 19). Anodal stimulation was associated with increased pain thresholds, reduced scores on the VAS, and enhanced endogenous pain inhibition. In contrast, cathodal stimulation tended to decrease pain thresholds and impair inhibitory responses. Neurophysiological data supported these behavioral findings. Anodal ctDCS resulted in reduced amplitudes and prolonged latencies of the N1 and N2/P2 components in EEG recordings, whereas cathodal stimulation led to increased amplitudes and shortened latencies of the same components.
Additional support for the analgesic potential of anodal ctDCS was provided by Bocci et al. (17) and Pereira et al. (15). In patients with phantom limb pain, anodal stimulation reduced the frequency of paroxysmal pain episodes and alleviated non-painful phantom sensations (17). In healthy participants, it consistently increased pain thresholds, suggesting a facilitative effect on endogenous pain modulation mechanisms (15). Table 1 summarizes the demographic characteristics of the participants, the study designs, the main specifications of the stimulation protocols, and the reported outcomes across the included studies.
Table 1
| Reference | Study design | Active group (n) | Sham group (n) | Anode location | Cathode location | tDCS protocol | Adverse effects | Neurophysiological measurements | Pain outcome used | Notes (aim, results) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bocci et al. (19) | RCT, crossover design | 15 (7/8 M) | In a crossover study, participants underwent three interventions—anodal, cathodal, and sham stimulation—with 1- week interval between sessions | For anodal stimulation, the electrode was centered on the median line, 2 cm below the inion, with its lateral borders positioned 1 cm medially to the mastoid apophysis. For cathodal stimulation, the electrode was placed on the right shoulder |
For cathodal stimulation, the electrode was centered on the median line, 2 cm below the inion, with its lateral borders positioned 1 cm medially to the mastoid apophysis. For anodal stimulation, the electrode was placed on the right shoulder |
2 mA, 20 min, 1 session of each stimulation with intervals of 1 week Cathodal, 2 mA, 20 min Anodal, 2 mA, 20 min Sham, current on only for the initial 30 s |
Not informed | Laser stimulation was applied to the dorsum of the left hand. The PTh was quantified, and nociceptive laser stimuli were applied at two intensities (VAS1 and VAS2). N1 and N2/P2 components were captured using EEG RMT was assessed pre- and post-intervention using TMS |
VAS | The study aimed to evaluate the effect of ctDCS on perceptive threshold, pain intensity, and laser-evoked potentials Result: Cathodal stimulation decreased the perceptive threshold, increased the VAS score, enhanced N1 and N2/P2 amplitudes, and reduced their latencies, while anodal stimulation had the opposite effect. Motor thresholds were not affected by the intervention |
| Bocci et al. (16) | RCT | 16 highly hypnotizable volunteers (9/7 M) were recruited | There was no sham group; the control group consisted of 16 healthy subjects matched for age and gender, not selected based on hypnotizability. | For anodal stimulation, the electrode was centered on the median line, 2 cm below the inion, with its lateral borders positioned 1 cm medially to the mastoid apophysis For cathodal stimulation, the electrode was placed on the right shoulder |
For cathodal stimulation, the electrode was centered on the median line, 2 cm below the inion, with its lateral borders positioned 1 cm medially to the mastoid apophysis For anodal stimulation, the electrode was placed on the right shoulder |
2 mA, 15 min, 1 session of each stimulation was applied Cathodal, 2 mA, 15 min Anodal, 2 mA, 15 min |
Not informed | Laser stimulation was applied to the dorsum of the left hand. The PTh was quantified, followed by eight nociceptive laser stimuli, with subjects rating the perceived pain. N1 and N2/P2 components were recorded using EEG | NRS | The study aimed to evaluate the effect of ctDCS on pain perception and LEPs in highly hypnotizable individuals compared to controls Result: In highly hypnotizable individuals, anodal stimulation increased N2/P2 amplitude without affecting perceived pain, while cathodal stimulation had no significant effects. In contrast, control participants reported decreased pain and reduced N1 and N2/P2 amplitudes after anodal stimulation, with the opposite pattern observed following cathodal stimulation. |
| Pereira et al. (15) | RCT, crossover design | 14 (7/7 M) | In a crossover study, participants underwent three interventions—anodal, cathodal, and sham stimulation—with at least 5 h interval between sessions | For anodal stimulation, the electrode was centered on the median line, 2 cm below the inion, with its lateral borders positioned 1 cm medially to the mastoid apophysis For cathodal stimulation, the electrode was placed over the right buccinator area |
For cathodal stimulation, the electrode was centered on the median line, 2 cm below the inion, with its lateral borders positioned 1 cm medially to the mastoid apophysis For anodal stimulation, the electrode was placed over the right buccinator area |
2 mA, 5 min, 1 session of each stimulation with intervals separated by at least 5 h. Cathodal, 2 mA, 5 min Anodal, 2 mA, 5 min Sham, turned off after the ramp-up phase |
Not informed | Electrical stimulation was applied to the proximal third of the right medial tibia. The ST was defined as a slight sensation in the leg (VAS 1), and the PThres as barely painful (VAS 4) | VAS | The study aimed to evaluate the effect of ctDCS on lower extremity sensory and pain thresholds. Result: Anodal stimulation increased the pain threshold, while cathodal and sham stimulations had no significant effect. No changes in sensory threshold were observed with any stimulation. |
| Bocci et al. (19) | RCT, crossover design | 14 (8/6 M) with unilateral upper limb amputation | In a crossover study, participants underwent two interventions—anodal, and cathodal stimulation—with a 3-month interval between sessions | For anodal stimulation, the electrode was centered on the median line, 2 cm below the inion, with its lateral borders positioned 1 cm medially to the mastoid apophysis For cathodal stimulation, the electrode was placed on the right shoulder |
For cathodal stimulation, the electrode was centered on the median line, 2 cm below the inion, with its lateral borders positioned 1 cm medially to the mastoid apophysis For anodal stimulation, the electrode was placed on the right shoulder |
2 mA, 20-min sessions were applied to each type of stimulation—anodal, and cathodal—over five consecutive days, with three-month intervals separating each stimulation type. Cathodal, 2 mA, 20 min Anodal, 2 mA, 20 min |
Not informed | Laser stimulation was applied to the stump to determine the PT, followed by twenty nociceptive laser stimuli, during which subjects rated their perceived pain. EEG was used to record the N1 and N2/P2 components Clinical evaluations were conducted at baseline (T0), at the end of the ctDCS week (T1), and at 2 weeks (T2) and 4 weeks (T3) post-intervention. The variables assessed included PLP intensity, pain paroxysms, stump pain, non-painful phantom limb sensations, and phantom limb movements |
VAS | The study aimed to evaluate the effect of ctDCS on modulating nociceptive processing and pain perception in patients with painful and non-painful phantom limb sensations Result: Anodal ctDCS reduced paroxysmal pain, non-painful phantom limb sensations, and phantom limb movements |
| Stacheneder et al. (18) | RCT, crossover design | 21 (16/6 M) | In a crossover study, participants underwent three interventions—anodal, cathodal, and sham stimulation—with a 5-day interval between sessions | For anodal stimulation, the electrode was centered on the median line, 1-2 cm below the inion For cathodal stimulation, the electrode was placed on the lateral upper arm |
For cathodal stimulation, the electrode was centered on the median line, 1- 2 cm below the inion For anodal stimulation, the electrode was placed on the lateral upper arm |
2 mA, 20 min, 1 session of each stimulation with intervals of 1 week Cathodal, 2 mA, 20 min Anodal, 2 mA, 20 min Sham, current on only for the initial 15 s |
Itching under the electrode and headache following the procedure | The study recorded spinal and cortical responses to sural nerve stimulation, assessing the RIII reflex from the biceps femoris and SEPs from the vertex referenced to the forehead. Each 2-min cycle included 12 stimuli, and pain intensity was rated using an NRS after each cycle. Thermal stimuli were employed to assess pain perception and modulation mechanisms. This protocol determined heat pain intensity and offset analgesia CPM was evaluated using a test stimulus (a 30-s heat application) and a conditioning stimulus (a 60-s cold water immersion) |
NRS | The study aimed to assess the impact of ctDCS on nociceptive processing and endogenous pain modulation. Result: Findings indicated that cathodal ctDCS increased pain perception and reduced endogenous pain inhibition, while anodal ctDCS enhanced endogenous pain inhibition |
Demographic characteristics and protocol specifications (n = 80).
RCT Randomized Clinical Trial, PTh Perceptive Threshold, EEG Electroencephalogram, VAS Visual Analogue Scale, RMT Resting Motor Threshold, TMS Transcranial Magnetic Stimulation, ctDCS Cerebellar Transcranial Direct Current Stimulation, NRS Numerical Rating Scale, LEPs Laser Evoked Potentials, ST Sensory Threshold, PThres Pain Threshold, PLP Phantom Limb Pain, SEPs Somatosensory Evoked Potentials, CPM Conditioned Pain Modulation.
4.6 Bias risk assessment
The risk of bias was assessed using the RoB 2 tool, as recommended by the Cochrane Collaboration, and is illustrated in Figures 6, 7. Overall, the main concerns identified across the studies included insufficient reporting of the randomization process (Domain D1 of RoB 2) and inadequate information regarding blinding of outcome assessors (Domain D2). Although not classified as a high risk, the studies by Pereira et al. (15) and Bocci et al. (19) lacked important details—such as demographic and clinical characteristics of the participants—that would have allowed for a more comprehensive characterization of the intervention groups.
Figure 6

Risk of bias of studies included. Crossover trials (including the DS variable) and randomized clinical trials were conducted according to the authors’ interpretations and methodologies.
Figure 7

Risk of bias—authors’ judgments on each risk of bias item are presented as percentages across all included trials, assessed using the Cochrane risk of bias tool.
In the study by Pereira et al. (15), a high risk of bias was identified due to potential carryover effects (Domain DS of RoB 2). Although the interval between sessions may vary among studies, conducting multiple sessions on the same day can lead to cumulative effects, which may confound the outcomes (15).
5 Discussion
Chronic pain remains a major clinical and societal burden, often refractory to conventional treatments and associated with significant adverse effects. In this context, ctDCS has emerged as a promising neuromodulatory technique. This systematic review provides an integrative synthesis of the stimulation protocols employed, the clinical characteristics of the populations studied, and the potential therapeutic effects of ctDCS in pain management. Despite preliminary findings suggesting polarity-dependent analgesic effects, the overall evidence remains limited, highlighting the need for more rigorous and standardized investigations.
5.1 Cerebellar tDCS and pain perception: mechanisms and effects
Evidence from the studies included in this review supports the role of ctDCS in modulating nociceptive processing through polarity-dependent mechanisms. In healthy volunteers, anodal stimulation consistently increased pain thresholds and reduced pain-related evoked potentials, whereas cathodal stimulation either enhanced pain sensitivity or produced no change (15, 16, 19). In phantom limb pain, anodal ctDCS reduced paroxysmal pain and phantom sensations (16), while Stacheneder et al. (18) showed that anodal stimulation enhanced inhibitory control and cathodal stimulation impaired descending inhibition, reinforcing polarity-dependent effects.
Mechanistically, it has been proposed that nociceptive inputs may converge on Purkinje cells, which exert inhibitory control over the dentate nucleus. Through these circuits, the cerebellum can influence thalamic and brainstem nociceptive relays that project to cortical regions involved in pain processing (12, 28, 42). The analgesic effects of ctDCS may rely on modulation of the cerebello–thalamo–cortical pathway, whose inhibitory influence has been consistently demonstrated via TMS-based cerebellar–brain inhibition (CBI) (79). Supporting this, Galea et al. (80) showed that anodal ctDCS increases Purkinje cell excitability, thereby reinforcing the inhibitory influence over the dentate nucleus and reducing thalamocortical drive. In contrast, cathodal stimulation produces the opposite effect, confirming the polarity-specific nature of cerebellar neuromodulation (80). Such bidirectional control becomes particularly relevant in chronic pain, where thalamic plasticity alters connectivity, excitability, and rhythmicity, leading to thalamocortical dysrhythmia that amplifies nociceptive salience and sustains persistent pain (81, 82). It is therefore plausible that ctDCS restores inhibitory control within the dentate–thalamo–cortical pathway, counteracting pathological oscillatory activity. This framework parallels the rationale in dystonia, where reduced cerebellar inhibition justifies anodal stimulation (83), and in tremor disorders, where abnormal olivocerebellar rhythmicity supports the use of cathodal interventions (84).
A complementary mechanism involves the anti-inflammatory and neuroprotective actions of tDCS. In experimental neuropathic pain models, repeated stimulation has been shown to downregulate pro-inflammatory cytokines (e.g., spinal IL-1β, hippocampal TNF-α), upregulate IL-10, and suppress microglial and astrocytic activation, thereby fostering adaptive plasticity and reducing hypersensitivity (85, 86). Although clinical evidence remains limited, these preclinical findings, when integrated with the polarity-dependent modulation of cerebellar circuits, suggest a dual potential of ctDCS: rebalancing dysfunctional thalamocortical activity and attenuating neuroinflammatory processes. Collectively, these mechanisms strengthen the conceptualization of the cerebellum as a central modulatory hub in pain pathophysiology and support ctDCS as a mechanism-based strategy for chronic pain management.
5.2 Determinants of interindividual variability in tDCS response
Interindividual variability in response to ctDCS is a critical challenge that limits the generalizability of its clinical effects. Beyond the stimulation parameters, factors such as neurochemical balance, inflammatory states, and genetic predispositions can substantially modulate outcomes, shaping both the magnitude and direction of neuromodulatory effects.
At the neurochemical level, anodal stimulation can reduce cortical GABA concentrations, as shown by spectroscopy (87). Its after-effects are also sensitive to the dopaminergic state, with evidence that receptor function/availability modulates the magnitude and direction of plasticity (88–90). Moreover, tDCS-induced plasticity critically depends on NMDA receptor mechanisms: antagonists abolish and partial agonists enhance these effects, indicating that departures from an optimal glutamatergic balance can dampen these neurophysiological changes (91, 92). In neuropathic conditions, reduced GABAergic tone and disruption of opioid signaling further compromise responsiveness (93). Consistent with this, PET imaging demonstrated that anodal tDCS over M1 enhances endogenous opioid release and increases μ-opioid receptor binding, highlighting the role of opioid-mediated mechanisms to analgesia (94, 95). Neuroinflammation also emerges as a critical determinant: elevated cytokines such as TNF-α, IL-1β, and IL-6 destabilize synaptic homeostasis, impair inhibitory neurotransmission within descending pain pathways, and exacerbate central sensitization, ultimately limiting the therapeutic impact of tDCS (96–98). Genetic polymorphisms further contribute to variability. The BDNF Val66Met variant affects activity-dependent plasticity and functional connectivity, influencing both pain modulation and responsiveness to stimulation (99, 100). Similarly, COMT Val158Met, which alters dopaminergic tone, has been linked to differences in cognitive flexibility and neuromodulatory outcomes (101–103).
In sum, ctDCS responsiveness appears intrinsically state-dependent: the neurochemical milieu, inflammatory activity, and genetic background gate both the sign and magnitude of induced plasticity, offering a coherent explanation for the between- and within-subject heterogeneity observed to date.
5.3 Clinical characteristics of the study population
In the present review, most included ctDCS studies were conducted in healthy young adults, which restricts the applicability of their findings to clinical pain populations. Only one trial specifically investigated patients—upper-limb amputees with phantom limb pain—reporting that anodal ctDCS reduced paroxysmal pain and phantom sensations (17). Among studies with healthy participants, polarity-dependent effects on pain perception were observed only in low-hypnotizable individuals, suggesting that cognitive traits may modulate cerebellar–cortical interactions and shape the neuromodulatory impact of ctDCS (16).
Demographic variables are also relevant. The predominance of young female samples introduces potential bias, as sex-related anatomical factors—such as greater skull thickness in men—may reduce current penetration (104). In addition, age-related gray matter atrophy and increased cerebrospinal fluid volume can alter current flow and target engagement (105). Hormonal fluctuations—particularly in estrogen and cortisol—may further modulate excitability and plasticity, adding variance to ctDCS outcomes (106). Medications and commonly used substances also represent important confounders. Anticonvulsants, benzodiazepines, and other GABAergic agents may attenuate stimulation-induced plasticity, whereas SSRIs may prolong facilitatory after-effects (107–109). Caffeine and nicotine have also been shown to modulate cortical excitability in a dose-dependent manner (110, 111).
Viewed across studies, sample composition is a first-order determinant of observed effects: clinical diagnosis, cognitive traits, sex/age/hormonal status, and medication/substance exposure shape current delivery and plasticity, and thereby the size and stability of ctDCS outcomes.
5.4 Anatomical and technical determinants of ctDCS efficacy
Despite the use of standardized protocols—typically positioning the active electrode 1–2 cm below the inion and 1 cm medial to the mastoids to target the cerebellar vermis—reported outcomes remain inconsistent: some studies demonstrate robust neuromodulatory effects, whereas others do not detect significant changes. One major factor contributing to this variability is interindividual anatomical diversity, which shapes both the magnitude and spatial distribution of the electric field within cerebellar structures. Computational modeling studies have shown that the distance between the scalp and cerebellum is the most influential anatomical variable, accounting for up to 60% of the variance in intracerebellar electric field strength (112–114). Additional morphometric features—such as the angulation of the cerebellar and pontine fossae—can further shape the trajectory and depth of current penetration (115). These anatomical variations imply that even under standardized electrode placement, the resulting neural engagement can differ substantially across individuals. Emerging evidence suggests that variability in local electric field strength has functional consequences. Studies have reported that differences in field intensity are associated with changes in GABA concentrations, modulation of motor-evoked potentials, and alterations in cerebello-cortical connectivity (90, 116–118). Together, these findings indicate that anatomical features not only affect current delivery but also determine the neurophysiological and behavioral outcomes of ctDCS, underscoring the importance of individualized targeting strategies.
In addition to anatomical variability, the electrode montage plays a decisive role in shaping current distribution. Cephalic montages, in which the reference electrode is placed over frontal or supraorbital regions, can generate stronger overall fields but with greater spatial dispersion, potentially engaging adjacent cortical structures such as the prefrontal and occipital cortices (112, 119). In contrast, extracephalic montages—where the reference electrode is positioned on the shoulder or buccinator muscle—tend to produce more focal stimulation of cerebellar targets while reducing unintended spread to supratentorial regions (114, 120). Despite these well-documented biophysical differences, current evidence does not clearly demonstrate whether one montage translates into superior clinical efficacy.
Stimulation parameters are another critical determinant of ctDCS efficacy. Most protocols apply 2 mA for 15–25 min across multiple sessions interspersed with rest days, a regimen considered the current standard. Shorter applications, such as 5 min, can induce transient changes in cortical excitability (9, 121), but their ability to produce sustained modulation of cerebellar circuits remains uncertain. To ensure effective engagement, Habas et al. recommend using at least 1.5 mA (36), whereas Workman et al. (122) demonstrated that 4 mA is safe and well tolerated in patients with Parkinson’s disease. Importantly, posterior-fossa morphometrics—such as scalp-to-cerebellum distance and the cerebellopontine angle—further determine how stimulation intensity translates into effective current delivery (114). The effects of 2 mA protocols, however, must be interpreted with caution. Several studies included in this review found that anodal ctDCS increased pain thresholds and reduced pain perception, whereas cathodal stimulation lowered thresholds and enhanced pain perception (16, 18, 19). Nonetheless, evidence from non-cerebellar paradigms highlights important inconsistencies. Batsikadze et al. (123) reported that cathodal tDCS over M1 at 2 mA paradoxically enhanced excitability, producing facilitatory effects similar to anodal stimulation. In line with this, Vimolratana et al. (124) showed that both anodal and cathodal stimulation at 2 mA increased muscle strength in healthy participants, while cathodal stimulation at 1–1.5 mA produced the expected inhibitory effects. Dyke et al. (125) added further nuance by showing that 2 mA stimulation yields highly variable outcomes: while anodal stimulation increased excitability at the group level, its effects were poorly reliable within individuals, and cathodal stimulation failed to produce consistent changes either within or between subjects. Overall, the evidence suggests that, outside the cerebellum, 2 mA stimulation may not reliably induce polarity-specific responses; instead, observed changes may reflect non-linear or state-dependent physiology rather than genuine polarity-dependent modulation. By contrast, in the cerebellum, polarity-dependent effects of ctDCS have been demonstrated: anodal tends to strengthen, whereas cathodal tends to reduce, the cerebellar inhibitory influence on cortical excitability (80). Importantly, any apparent polarity effects should be interpreted in the context of effective intracerebellar field strength, montage-dependent current distribution, and individual posterior fossa morphology, which together determine target engagement and downstream effects.
From a translational standpoint, montage selection and stimulation parameters should be aligned with the intended neuromodulatory goal. Future progress will depend on integrating high-resolution imaging, individualized electric-field modeling, and systematic evaluation of stimulation intensity and dosing schedules to consolidate polarity-sensitive interventions and establish the therapeutic potential of ctDCS for chronic pain.
6 Future directions
Recent evidence supports a paradigm shift in pain neuroscience, highlighting the cerebellum not merely as a motor structure but as a central hub that integrates sensory, affective, and cognitive dimensions of pain processing. In this context, ctDCS emerges as a promising technique to modulate distributed pain-related networks.
One important gap in the current literature is the limited use of inflammatory and neuroimmune biomarkers as outcome measures. Experimental studies in neuropathic pain models suggest that tDCS exerts anti-inflammatory and neuroprotective effects, including the down-regulation of pro-inflammatory cytokines (e.g., TNF-α, IL-1β), the up-regulation of IL-10, and the suppression of microglial and astrocytic activation, thereby fostering adaptive plasticity and reducing hypersensitivity (85, 86). Incorporating such biomarkers in clinical trials may provide a translational link between molecular pathways and behavioral outcomes, clarifying whether modulation of neuroinflammatory cascades contributes to the analgesic effects of tDCS, tACS, or rTMS in chronic, drug-resistant pain syndromes.
Another promising avenue is the use of multi-target stimulation protocols. Preliminary evidence suggests that concurrent anodal stimulation of M1 and the cerebellum can induce synergistic plasticity, producing greater facilitatory effects than single-site stimulation (126). Extending this rationale, combined cerebellar–spinal tDCS may enhance descending modulatory control. Advances in computational modeling and “deep NIBS” approaches also offer the possibility of predicting and optimizing electric field distribution within deep cerebellar nuclei and subcortical structures, providing novel, non-invasive ways to engage circuits traditionally considered inaccessible (127).
Finally, the integration of neurophysiological and imaging techniques (e.g., EEG, fMRI, TMS) remains essential to clarify the mechanisms of ctDCS and identify biomarkers predictive of treatment response. Such multimodal approaches will not only improve mechanistic understanding but also guide the development of personalized and network-oriented stimulation strategies.
Overall, future research should prioritize biomarker-based validation, multi-target stimulation designs, and multimodal neurophysiological assessments. This integrative approach may enhance reproducibility, reduce variability, and consolidate ctDCS as a precision tool for mechanism-based interventions in chronic pain.
7 Limitations
When interpreting the findings of this review, several limitations must be acknowledged. First, most studies included small sample sizes predominantly composed of healthy young female participants, which limits statistical power and reduces the generalizability of results to clinical populations. Second, the widespread use of single-session ctDCS protocols prevents meaningful conclusions about cumulative or long-term effects. As tDCS-induced plasticity relies on activity-dependent mechanisms, both the number and frequency of sessions are likely to influence clinical outcomes. Therefore, longitudinal studies involving repeated ctDCS applications are needed to evaluate the durability and therapeutic relevance of its effects over time.
Third, another important limitation is the absence of advanced neuroimaging techniques—such as functional near-infrared spectroscopy and functional magnetic resonance imaging—which hinders mechanistic interpretation. Without such tools, it remains unclear whether observed changes in pain perception result from direct cerebellar modulation or downstream effects on broader cortical networks. Integrating multimodal neuroimaging in future research could enhance mechanistic understanding and support the development of more targeted and effective interventions.
Fourth, a further limitation relates to the prevalent use of 2 mA stimulation. Evidence from M1 tDCS indicates that cathodal stimulation at 2 mA may fail to produce the canonical inhibitory effect and can even yield facilitatory changes; although this has not been demonstrated directly for ctDCS, several studies in this review using 2 mA have reported polarity-dependent analgesic effects. Given target-specific differences between M1 and the cerebellum, interpretations of cathodal ctDCS at 2 mA should be made with caution. Future trials should include systematic dose–response comparisons (e.g., 1–1.5 mA vs. 2–4 mA), immediate and delayed outcome assessments, and repeated-session designs to clarify intensity–polarity interactions.
Fifth, heterogeneity in stimulation parameters—spanning electrode montage, current polarity, session duration, and outcome measures—complicates cross-study comparisons. Standardized reporting and methodological harmonization are essential for improved reproducibility and the identification of optimal stimulation protocols.
Sixth, only a small number of studies included clinical populations, and those that did focused primarily on individuals with phantom limb pain—a condition characterized predominantly by neuropathic mechanisms. As a result, it remains uncertain whether similar effects would be observed in other pain phenotypes, such as nociplastic pain (e.g., fibromyalgia), nociceptive pain (e.g., osteoarthritis), or other neuropathic conditions (e.g., diabetic neuropathy). These conditions differ in their underlying neurobiology, including degrees of central sensitization, descending pain inhibition, and ongoing peripheral input, all of which may influence responsiveness to ctDCS. Future studies should explicitly address these pain subtypes to clarify the broader applicability of cerebellar stimulation.
Seventh and lastly, the influence of individual psychological and biological characteristics—such as anxiety, pain catastrophizing, pain sensitivity, and placebo responsiveness—was rarely considered in the reviewed studies. Incorporating psychometric and biological markers in future protocols will be essential for elucidating interindividual variability in response to ctDCS and for guiding the development of personalized neuromodulatory interventions.
8 Conclusion
The ctDCS has emerged as a promising non-invasive technique for modulating nociceptive processing and may provide therapeutic benefits for individuals with chronic pain—even after a single session. However, its clinical efficacy is shaped by a complex interplay of anatomical, neurochemical, cognitive, and psychological factors that influence individual responsiveness to neuromodulation.
To enhance its translational potential, ctDCS should be integrated into personalized, network-based strategies, ideally in combination with stimulation of other cortical regions. Optimizing protocols—through tailored selection of stimulation parameters, electrode montage, and dosing schedules—requires mechanistic insight and careful consideration of patient-specific features. Incorporating neurophysiological and neuroimaging assessments within a biopsychosocial framework will be essential for refining interventions and identifying biomarkers of treatment response.
Collectively, the available evidence indicates that the effects of ctDCS in chronic pain are modulated by neurochemical, inflammatory, and genetic factors. Addressing these dimensions through precision neuromodulation approaches will be key to improving clinical outcomes and establishing ctDCS as a viable therapeutic tool in pain management.
Statements
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author contributions
DB: Writing – original draft, Writing – review & editing, Conceptualization, Formal analysis, Investigation. MT: Formal analysis, Writing – review & editing. MO: Data curation, Writing – review & editing. SP: Data curation, Writing – review & editing. IT: Writing – review & editing. FF: Writing – review & editing. WC: Conceptualization, Formal analysis, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Program for Academic Excellence (PROEX) and the Postgraduate Research Group at the Hospital de Clínicas de Porto Alegre (FIPE-HCPA, project no. 2022-0504). The trial was sponsored by the National Council for Scientific and Technological Development (CNPq) in partnership with the Health Secretary of the State of Rio Grande do Sul (SEARS), under the PPSUS program (Call 08/2020, no. 21/2551-0000129-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. Portions of this manuscript were prepared with the assistance of ChatGPT (OpenAI, GPT-4o, accessed in 2025), which was used under human supervision to support writing clarity and language refinement. All content was reviewed and validated by the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Rikard SM Strahan AE Schmit KM Guy GP . Chronic pain among adults—United States, 2019-2021. MMWR Morb Mortal Wkly Rep. (2023) 72:379–85. doi: 10.15585/mmwr.mm7215a1
2.
Zelaya CE Dahlhamer JM Lucas JW Connor EM . Chronic Pain and High-impact Chronic Pain Among U.S. Adults, 2019. NCHS Data Brief. (2020) 1–8.
3.
Coninx S . A multidimensional phenomenal space for pain: structure, primitiveness, and utility. Phenomenol Cogn Sci. (2022) 21:223–43. doi: 10.1007/s11097-021-09727-0
4.
Hadi MA GA MH Closs SJ . Impact of chronic pain on patients’ quality of life: a comparative mixed-methods study. J Patient Exp. (2018) 6:133–41. doi: 10.1177/2374373518786013
5.
Raja SN Carr DB Cohen M Finnerup NB Flor H Gibson S et al The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. (2020);161. Available online at: https://journals.lww.com/pain/fulltext/2020/09000/the_revised_international_association_for_the.6.aspx
6.
Lucas JW Sohi I . Chronic pain and high-impact chronic pain in U.S. adults, 2023. NCHS Data Brief. (2024) 518:CS355235. doi: 10.15620/cdc/169630
7.
Pacheco-Barrios K Alejandra CR Aurore T Beatriz C Isadora F Wolnei C et al . Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expert Rev Med Devices. (2020) 17:879–98. doi: 10.1080/17434440.2020.1816168
8.
Moshfeghinia R Shekouh D Mostafavi S Hosseinzadeh M Bahadori AR Abdollahifard S et al . The effects of transcranial direct-current stimulation (tDCS) on pain intensity of patients with fibromyalgia: a systematic review and meta-analysis. BMC Neurol. (2023) 23:395. doi: 10.1186/s12883-023-03445-7
9.
Boggio PS Zaghi S Lopes M Fregni F . Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. (2008) 15:1124–30. doi: 10.1111/j.1468-1331.2008.02270.x
10.
Caumo W Lopes Ramos R Vicuña Serrano P da Silveira Alves CF Medeiros L Ramalho L et al . Efficacy of home-based transcranial direct current stimulation over the primary motor cortex and dorsolateral prefrontal cortex in the disability due to pain in fibromyalgia: A factorial sham-randomized clinical study. J Pain. (2024) 25:376–92. doi: 10.1016/j.jpain.2023.09.001
11.
Zortea M Ramalho L Alves RL Alves CF d S Braulio G Torres IL d S et al . Transcranial direct current stimulation to improve the dysfunction of descending pain modulatory system related to opioids in chronic non-cancer pain: an integrative review of neurobiology and meta-analysis. Front Neurosci. (2019) 13:1218. doi: 10.3389/fnins.2019.01218
12.
Saab CY Willis WD . The cerebellum: organization, functions and its role in nociception. Brain Res Rev. (2003);42:85–95. Available online at: https://www.sciencedirect.com/science/article/pii/S0165017303001516
13.
Coombes SA Misra G . Pain and motor processing in the human cerebellum. Pain (2016);157. Available online at: https://journals.lww.com/pain/fulltext/2016/01000/pain_and_motor_processing_in_the_human_cerebellum.12.aspx
14.
Helmchen C Mohr C Erdmann C Petersen D Nitschke MF . Differential cerebellar activation related to perceived pain intensity during noxious thermal stimulation in humans: a functional magnetic resonance imaging study. Neurosci Lett. (2003) 335:202–6. doi: 10.1016/S0304-3940(02)01164-3
15.
Pereira Manuel Rafiq Basil Chowdhury Einul Babayev Jacqueline Boo HyunJi Metwaly Rowan et al Anodal cerebellar tDCS modulates lower extremity pain perception. NeuroRehabilitation. (2017);40:195–200. Available online at: https://journals.sagepub.com/action/showAbstract
16.
Bocci T Barloscio D Parenti L Sartucci F Carli G Santarcangelo EL . High hypnotizability impairs the cerebellar control of pain. Cerebellum. (2017) 16:55–61. doi: 10.1007/s12311-016-0764-2
17.
Bocci T De Carolis G Ferrucci R Paroli M Mansani F Priori A et al . Cerebellar transcranial direct current stimulation (ctDCS) ameliorates phantom limb pain and non-painful phantom limb sensations. The Cerebellum. (2019) 18:527–35. doi: 10.1007/s12311-019-01020-w
18.
Stacheneder R Alt L Straube A Ruscheweyh R . Effects of transcranial direct current stimulation (t-DCS) of the cerebellum on pain perception and endogenous pain modulation: a randomized, monocentric, double-blind, sham-controlled crossover study. Cerebellum. (2023) 22:1234–42. doi: 10.1007/s12311-022-01498-x
19.
Bocci T Santarcangelo E Vannini B Torzini A Carli G Ferrucci R et al . Cerebellar direct current stimulation modulates pain perception in humans. Restor Neurol Neurosci. (2015) 33:597–609. doi: 10.3233/RNN-140453
20.
Boggero IA Carlson CR . Somatosensory and affective contributions to emotional, social, and daily functioning in chronic pain patients. Pain Med. (2015) 16:341–7. doi: 10.1111/pme.12598
21.
Lanz S Seifert F Maihöfner C . Brain activity associated with pain, hyperalgesia and allodynia: an ALE meta-analysis. J Neural Transm. (2011) 118:1139–54. doi: 10.1007/s00702-011-0606-9
22.
DosSantos MF Ferreira N Toback RL Carvalho AC DaSilva AF . Potential mechanisms supporting the value of motor cortex stimulation to treat chronic pain syndromes. Front Neurosci. (2016) 10:18. doi: 10.3389/fnins.2016.00018
23.
Sankarasubramanian V Cunningham DA Potter-Baker KA Beall EB Roelle SM Varnerin NM et al . Transcranial direct current stimulation targeting primary motor versus dorsolateral prefrontal cortices: proof-of-concept study investigating functional connectivity of Thalamocortical networks specific to sensory-affective information processing. Brain Connect abril de. (2017) 7:182–96. doi: 10.1089/brain.2016.0440
24.
Etkin A Egner T Kalisch R . Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. (2011) 15:85–93. doi: 10.1016/j.tics.2010.11.004
25.
Cardenas VA Bhat JV Horwege AM Ehrlich TJ Lavacot J Mathalon DH et al . Anatomical and fMRI-network comparison of multiple DLPFC targeting strategies for repetitive transcranial magnetic stimulation treatment of depression. Brain Stimul. (2022) 15:63–72. doi: 10.1016/j.brs.2021.11.008
26.
Caumo W Franca BR Orzechowski R Bueno G França A Dos Santos da Silva JV et al . Home-based transcranial direct current stimulation vs placebo for fibromyalgia: A randomized clinical trial. JAMA Netw Open. (2025) 8:e2514262. doi: 10.1001/jamanetworkopen.2025.14262
27.
Schmahmann JD . The cerebellum and cognition. Neurosci Lett. (2019) 688:62–75. doi: 10.1016/j.neulet.2018.07.005
28.
Moulton EA Schmahmann JD Becerra L Borsook D . The cerebellum and pain: passive integrator or active participator?Brain Res Rev. (2010) 65:14–27. doi: 10.1016/j.brainresrev.2010.05.005
29.
Jensen KB Regenbogen C Ohse MC Frasnelli J Freiherr J Lundström JN . Brain activations during pain: a neuroimaging meta-analysis of patients with pain and healthy controls. Pain. (2016) 157. doi: 10.1097/j.pain.0000000000000517
30.
Buckner RL . The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. (2013) 80:807–15. doi: 10.1016/j.neuron.2013.10.044
31.
Stoodley CJ Schmahmann JD . Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. (2009) 44:489–501. doi: 10.1016/j.neuroimage.2008.08.039
32.
Melka N Pszczolinska A Klejbor I Moryś J . The cerebellum: the ‘little’ brain and its big role. Folia Morphol (Warsz). (2024) 83:497–508. doi: 10.5603/fm.93251
33.
Voogd J Glickstein M . The anatomy of the cerebellum. Trends Neurosci. (1998) 21:370–5. doi: 10.1016/S0166-2236(98)01318-6
34.
Singh R . Cerebellum: its anatomy, functions and diseases In: TunalıNE, editor. Neurodegenerative Diseases. Rijeka: IntechOpen (2021)
35.
Albus JS . A theory of cerebellar function. Math Biosci (1971);10:25–61. Available online at: https://www.sciencedirect.com/science/article/pii/0025556471900514
36.
Habas C van Dun K Manto M Mariën P . Chapter 13—Deep Cerebellar Nuclei (DCN) and Language. Em: MariënPMantoM. The linguistic cerebellum. San Diego: Academic Press; (2016). 337–353. Available online at: https://www.sciencedirect.com/science/article/pii/B978012801608400013X
37.
D’Angelo E . Physiology of the cerebellum. Handb Clin Neurol. (2018) 154:85–108. doi: 10.1016/B978-0-444-63956-1.00006-0
38.
Marr D . A theory of cerebellar cortex. J Physiol. (1969) 202:437–70. doi: 10.1113/jphysiol.1969.sp008820
39.
Cakar ME Okada NJ Cummings KK Jung J Bookheimer SY Dapretto M et al . Functional connectivity of the sensorimotor cerebellum in autism: associations with sensory over-responsivity. Front Psych. (2024) 15:1337921. doi: 10.3389/fpsyt.2024.1337921
40.
Manto M. The underpinnings of cerebellar ataxias. Clin Neurophysiol Pract. (2022) 7:372–87. doi: 10.1016/j.cnp.2022.11.002
41.
Apps R Hawkes R . Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. (2009) 10:670–81. doi: 10.1038/nrn2698
42.
Li CN Keay KA Henderson LA Mychasiuk R . Re-examining the mysterious role of the cerebellum in pain. J Neurosci. (2024) 44. doi: 10.1523/JNEUROSCI.1538-23.2024
43.
Claassen J Koenen LR Ernst TM Labrenz F Theysohn N Forsting M et al . Cerebellum is more concerned about visceral than somatic pain. J Neurol Neurosurg Psychiatry. (2020) 91:218–9. doi: 10.1136/jnnp-2019-321025
44.
Henderson LA Gandevia SC Macefield VG . Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. NeuroImage. (2008) 39:1867–76. doi: 10.1016/j.neuroimage.2007.10.045
45.
Manda O Hadjivassiliou M Varrassi G Zavridis P Zis P . Exploring the role of the cerebellum in pain perception: a narrative review. Pain Ther. (2025) 14:803–16. doi: 10.1007/s40122-025-00724-8
46.
Gao Z van Beugen BJ De Zeeuw CI . Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. (2012) 13:619–35. doi: 10.1038/nrn3312
47.
Lev-Ram V Makings LR Keitz PF Kao JP Tsien RY . Long-term depression in cerebellar Purkinje neurons results from coincidence of nitric oxide and depolarization-induced Ca2+ transients. Neuron. (1995) 15:407–15. doi: 10.1016/0896-6273(95)90044-6
48.
Ito M . Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. (2008) 9:304–13. doi: 10.1038/nrn2332
49.
Llinas RR . The olivo-cerebellar system: a key to understanding the functional significance of intrinsic oscillatory brain properties. Front Neural Circuits. (2014) 7:96. doi: 10.3389/fncir.2013.00096
50.
Amino Y Kyuhou S Matsuzaki R Gemba H . Cerebello-thalamo-cortical projections to the posterior parietal cortex in the macaque monkey. Neurosci Lett. (2001) 309:29–32. doi: 10.1016/S0304-3940(01)02018-3
51.
Armstrong DM . Functional significance of connections of the inferior olive. Physiol Rev. (1974) 54:358–417. doi: 10.1152/physrev.1974.54.2.358
52.
Dum RP Strick PL . An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. (2003) 89:634–9. doi: 10.1152/jn.00626.2002
53.
Kelly RM Strick PL . Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. (2003) 23:8432–44. doi: 10.1523/JNEUROSCI.23-23-08432.2003
54.
Proville RD Spolidoro M Guyon N Dugué GP Selimi F Isope P et al . Cerebellum involvement in cortical sensorimotor circuits for the control of voluntary movements. Nat Neurosci. (2014) 17:1233–9. doi: 10.1038/nn.3773
55.
Schmahmann JD . From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. (1996) 4:174–98. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO
56.
Watson TC Becker N Apps R Jones MW . Back to front: cerebellar connections and interactions with the prefrontal cortex. Front Syst Neurosci. (2014) 8:4. doi: 10.3389/fnsys.2014.00004
57.
Kuypers HG Lawrence DG . Cortical projections to the red nucleus and the brain stem in the Rhesus monkey. Brain Res. (1967) 4:151–88. doi: 10.1016/0006-8993(67)90004-2
58.
Ichinohe N Mori F Shoumura K . A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. (2000) 880:191–7. doi: 10.1016/S0006-8993(00)02744-X
59.
Carta I Chen CH Schott AL Dorizan S Khodakhah K . Cerebellar modulation of the reward circuitry and social behavior. Science. (2019) 363:eaav0581. doi: 10.1126/science.aav0581
60.
Xiao L Bornmann C Hatstatt-Burklé L Scheiffele P . Regulation of striatal cells and goal-directed behavior by cerebellar outputs. Nat Commun. (2018) 9:3133. doi: 10.1038/s41467-018-05565-y
61.
Wagner MJ Kim TH Savall J Schnitzer MJ Luo L . Cerebellar granule cells encode the expectation of reward. Nature. (2017) 544:96–100. doi: 10.1038/nature21726
62.
Ekerot CF Gustavsson P Oscarsson O Schouenborg J . Climbing fibres projecting to cat cerebellar anterior lobe activated by cutaneous A and C fibres. J Physiol. (1987) 386:529–38. doi: 10.1113/jphysiol.1987.sp016549
63.
Fields HL . Chapter 18—pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. (2000) 122: 245–53.
64.
Peyron R Faillenot I Mertens P Laurent B Garcia-Larrea L . Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. NeuroImage. (2007) 34:310–21. doi: 10.1016/j.neuroimage.2006.08.037
65.
Nguyen JP Nizard J Keravel Y Lefaucheur JP . Invasive brain stimulation for the treatment of neuropathic pain. Nat Rev Neurol. (2011) 7:699–709. doi: 10.1038/nrneurol.2011.138
66.
Antal A Terney D Kühnl S Paulus W . Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. J Pain Symptom Manag. (2010) 39:890–903. doi: 10.1016/j.jpainsymman.2009.09.023
67.
Picarelli H Teixeira MJ de Andra DC Myczkowski ML Luvisotto TB Yeng LT et al . Repetitive transcranial magnetic stimulation is efficacious as an add-on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. J Pain. (2010) 11:1203–10. doi: 10.1016/j.jpain.2010.02.006
68.
Pisano TJ Dhanerawala ZM Kislin M Bakshinskaya D Engel EA Hansen EJ et al . Homologous organization of cerebellar pathways to sensory, motor, and associative forebrain. Cell Rep. (2021) 36:109721. doi: 10.1016/j.celrep.2021.109721
69.
Kelly C Toro R Di Martino A Cox CL Bellec P Castellanos FX et al . A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage. (2012) 61:1129–42. doi: 10.1016/j.neuroimage.2012.03.021
70.
Euston DR Gruber AJ McNaughton BL . The role of medial prefrontal cortex in memory and decision making. Neuron. (2012) 76:1057–70. doi: 10.1016/j.neuron.2012.12.002
71.
Etkin A Büchel C Gross JJ . The neural bases of emotion regulation. Nat Rev Neurosci. (2015) 16:693–700. doi: 10.1038/nrn4044
72.
Crawford LS Mills EP Peek A Macefield VG Keay KA Henderson LA . Function and biochemistry of the dorsolateral prefrontal cortex during placebo analgesia: how the certainty of prior experiences shapes endogenous pain relief. Cereb Cortex. (2023) 33:9822–34. doi: 10.1093/cercor/bhad247
73.
Kummer KK Mitrić M Kalpachidou T Kress M . The medial prefrontal cortex as a central hub for mental comorbidities associated with chronic pain. Int J Mol Sci. (2020) 21:3440. doi: 10.3390/ijms21103440
74.
Kucyi A Moayedi M Weissman-Fogel I Goldberg MB Freeman BV Tenenbaum HC et al . Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. (2014) 34:3969–75. doi: 10.1523/JNEUROSCI.5055-13.2014
75.
Baek SJ Park JS Kim J Yamamoto Y Tanaka-Yamamoto K . VTA-projecting cerebellar neurons mediate stress-dependent depression-like behaviors. eLife. (2022) 11:e72981. doi: 10.7554/eLife.72981
76.
Watson TC Obiang P Torres-Herraez A Watilliaux A Coulon P Rochefort C et al . Anatomical and physiological foundations of cerebello-hippocampal interaction. eLife. (2019) 8:e41896. doi: 10.7554/eLife.41896
77.
Zeidler Z Hoffmann K Krook-Magnuson E . HippoBellum: acute cerebellar modulation alters hippocampal dynamics and function. J Neurosci. (2020) 40:6910–26. doi: 10.1523/JNEUROSCI.0763-20.2020
78.
Mutso AA Radzicki D Baliki MN Huang L Banisadr G Centeno MV et al . Abnormalities in hippocampal functioning with persistent pain. J Neurosci. (2012) 32:5747–56. doi: 10.1523/JNEUROSCI.0587-12.2012
79.
Ugawa Y Uesaka Y Terao Y Hanajima R Kanazawa I . Magnetic stimulation over the cerebellum in humans. Ann Neurol. (1995) 37:703–13. doi: 10.1002/ana.410370603
80.
Galea JM Jayaram G Ajagbe L Celnik P . Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. (2009) 29:9115–22. doi: 10.1523/JNEUROSCI.2184-09.2009
81.
Llinás RR Ribary U Jeanmonod D Kronberg E Mitra PP . Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA. (1999) 96:15222–7. doi: 10.1073/pnas.96.26.15222
82.
Borsook D . Pain and motor system plasticity. Pain. (2007) 132:8–9. doi: 10.1016/j.pain.2007.09.006
83.
Bleton JP Cossé C Caloc’h T Suarez Moreno A Diverres E Derkinderen P et al . Combination of anodal tDCS of the cerebellum with a goal-oriented motor training to treat cervical dystonia: a pilot case series. Front Neurol. (2024) 15:1381390. doi: 10.3389/fneur.2024.1381390
84.
Lamy JC Varriale P Apartis E Mehdi S Blancher-Meinadier A Kosutzka Z et al . Trans-spinal direct current stimulation for managing primary orthostatic tremor. Mov Disord. (2021) 36:1835–42. doi: 10.1002/mds.28581
85.
Ethridge VT Gargas NM Sonner MJ Moore RJ Romer SH Hatcher-Solis C et al . Effects of transcranial direct current stimulation on brain cytokine levels in rats. Front Neurosci. (2022) 16:1069484. doi: 10.3389/fnins.2022.1069484
86.
Zhang H Zheng X Zhang B . Anti-inflammatory pathways mediating tDCS’s effects on neuropathic pain. Biology (Basel). (2025) 14:892. doi: 10.3390/biology14070892
87.
Stagg CJ Best JG Stephenson MC O’Shea J Wylezinska M Kineses ZT et al . Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. (2009) 29:5202–6. doi: 10.1523/JNEUROSCI.4432-08.2009
88.
Bunai T Hirosawa T Kikuchi M Fukai M Yokokura M Ito S et al . TDCS-induced modulation of GABA concentration and dopamine release in the human brain: a combination study of magnetic resonance spectroscopy and positron emission tomography. Brain Stimul. (2021) 14:154–60. doi: 10.1016/j.brs.2020.12.010
89.
Leow L A Marcos A Nielsen E Sewell D Ballard T Dux PE et al Dopamine alters the effect of brain stimulation on decision-making. J Neurosci. (2023) 43:6909–19. doi: 10.1523/JNEUROSCI.1140-23.2023
90.
Nandi T Puonti O Clarke WT Nettekoven C Barron HC Kolasinski J et al . tDCS induced GABA change is associated with the simulated electric field in M1, an effect mediated by grey matter volume in the MRS voxel. Brain Stimul. (2022) 15:1153–62. doi: 10.1016/j.brs.2022.07.049
91.
Nitsche MA Fricke K Henschke U Schlitterlau A Liebetanz D Lang N et al . Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. (2003) 553:293–301. doi: 10.1113/jphysiol.2003.049916
92.
Liebetanz D Nitsche MA Tergau F Paulus W . Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain. (2002) 125:2238–47. doi: 10.1093/brain/awf238
93.
Costigan M Scholz J Woolf CJ . Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. (2009) 32:1–32. doi: 10.1146/annurev.neuro.051508.135531
94.
DosSantos MF Love TM Martikainen IK Nascimento TD Fregni F Cummiford C et al . Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front Psych. (2012) 3:39. doi: 10.3389/fpsyt.2012.00093
95.
Li L Chen J Li YQ . The downregulation of opioid receptors and neuropathic pain. Int J Mol Sci. (2023) 24:5981. doi: 10.3390/ijms24065981
96.
Hiraga S i Itokazu T Nishibe M Yamashita T . Neuroplasticity related to chronic pain and its modulation by microglia. Inflamm Regen. (2022) 42:15. doi: 10.1186/s41232-022-00199-6
97.
Vanderwall AG Milligan ED . Cytokines in pain: harnessing endogenous anti-inflammatory signaling for improved pain management. Front Immunol. (2019) 10:3009. doi: 10.3389/fimmu.2019.03009
98.
Boadas-Vaello P Castany S Homs J Álvarez-Pérez B Deulofeu M Verdú E . Neuroplasticity of ascending and descending pathways after somatosensory system injury: reviewing knowledge to identify neuropathic pain therapeutic targets. Spinal Cord. (2016) 54:330–40. doi: 10.1038/sc.2015.225
99.
Egan MF Kojima M Callicott JH Goldberg TE Kolachana BS Bertolino A et al . The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. (2003) 112:257–69. doi: 10.1016/S0092-8674(03)00035-7
100.
Hariri AR Goldberg TE Mattay VS Kolachana BS Callicott JH Egan MF et al . Brain-derived neurotrophic factor val<sup>66</sup>met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. (2003) 23:6690–4. doi: 10.1523/JNEUROSCI.23-17-06690.2003
101.
Hayek D Antonenko D Witte AV Lehnerer SM Meinzer M Külzow N et al Impact of COMT val158met on tDCS-induced cognitive enhancement in older adults. Behav Brain Res. (2021) 401:113081. doi: 10.1016/j.bbr.2020.113081
102.
Plewnia C Zwissler B Längst I Maurer B Giel K Krüger R . Effects of transcranial direct current stimulation (tDCS) on executive functions: influence of COMT Val/met polymorphism. Cortex. (2013) 49:1801–7. doi: 10.1016/j.cortex.2012.11.002
103.
Stephens JA Jones KT Berryhill ME . Task demands, tDCS intensity, and the COMT val158met polymorphism impact tDCS-linked working memory training gains. Sci Rep. (2017) 7:13463. doi: 10.1038/s41598-017-14030-7
104.
Van Hoornweder S Geraerts M Verstraelen S Nuyts M Caulfield KA Meesen R . Differences in scalp-to-cortex tissues across age groups, sexes and brain regions: implications for neuroimaging and brain stimulation techniques. Neurobiol Aging (2024) 138:45–62. doi: 10.1016/j.neurobiolaging.2024.02.011
105.
Bhattacharjee S Kashyap R Goodwill AM O’Brien BA Rapp B Oishi K et al . Sex difference in tDCS current mediated by changes in cortical anatomy: A study across young, middle and older adults. Brain Stimul. (2022) 15:125–40. doi: 10.1016/j.brs.2021.11.018
106.
Vergallito A Feroldi S Pisoni A Romero Lauro LJ . Inter-individual variability in tDCS effects: a narrative review on the contribution of stable, variable, and contextual factors. Brain Sci. (2022) 12:522.
107.
McLaren ME Nissim NR Woods AJ . The effects of medication use in transcranial direct current stimulation: a brief review. Brain Stimul. (2018) 11:52–8. doi: 10.1016/j.brs.2017.10.006
108.
Nitsche MA Liebetanz D Schlitterlau A Henschke U Fricke K Frommann K et al . GABAergic modulation of DC stimulation-induced motor cortex excitability shifts in humans. Eur J Neurosci. (2004) 19:2720–6. doi: 10.1111/j.0953-816X.2004.03398.x
109.
Melo L Beaupain MC Ghanavati E Kuo MF Nitsche MA . Neurochemical mechanisms underlying serotonergic modulation of neuroplasticity in humans. Brain Stimul. (2024) 17:421–30. doi: 10.1016/j.brs.2024.04.001
110.
McLellan TM Caldwell JA Lieberman HR . A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. (2016) 71:294–312. doi: 10.1016/j.neubiorev.2016.09.001
111.
Grundey J Thirugnanasambandam N Kaminsky K Drees A Skwirba AC Lang N et al . Rapid effect of nicotine intake on neuroplasticity in non-smoking humans. Front Pharmacol. (2012) 3:186. doi: 10.3389/fphar.2012.00186
112.
Gomez-Tames J Asai A Mikkonen M Laakso I Tanaka S Uehara S et al . Group-level and functional-region analysis of electric-field shape during cerebellar transcranial direct current stimulation with different electrode montages. J Neural Eng. (2019) 16:036001. doi: 10.1088/1741-2552/ab0ac5
113.
Klaus J Schutter DJLG . Electrode montage-dependent intracranial variability in electric fields induced by cerebellar transcranial direct current stimulation. Sci Rep. (2021) 11:22183. doi: 10.1038/s41598-021-01755-9
114.
Maas RPPWM Faber J van de Warrenburg BPC Schutter DJLG . Interindividual differences in posterior fossa morphometry affect cerebellar tDCS-induced electric field strength. Clin Neurophysiol. (2023) 153:52–165. doi: 10.1016/j.clinph.2023.06.019
115.
Rezaee Z Dutta A . Lobule-specific dosage considerations for cerebellar transcranial direct current stimulation during healthy aging: a computational modeling study using age-specific magnetic resonance imaging templates. Neuromodulation, (2020) 23:341–65. doi: 10.1111/ner.13098
116.
Antonenko D Thielscher A Saturnino GB Aydin S Ittermann B Grittner U et al Towards precise brain stimulation: is electric field simulation related to neuromodulation? Brain Stimul. (2019) 12:1159–68. doi: 10.1016/j.brs.2019.03.072
117.
Laakso I Mikkonen M Koyama S Hirata A Tanaka S . Can electric fields explain inter-individual variability in transcranial direct current stimulation of the motor cortex?Sci Rep. (2019) 9:626. doi: 10.1038/s41598-018-37226-x
118.
Mosayebi-Samani M Jamil A Salvador R Ruffini G Haueisen J Nitsche MA . The impact of individual electrical fields and anatomical factors on the neurophysiological outcomes of tDCS: a TMS-MEP and MRI study. Brain Stimul. (2021) 14:316–26. doi: 10.1016/j.brs.2021.01.016
119.
van Dun K Bodranghien FCAA Mariën P Manto MU . Tdcs of the cerebellum: where do we stand in 2016? Technical issues and critical review of the literature. Front Hum Neurosci. (2016) 10:199. doi: 10.3389/fnhum.2016.00199
120.
Grimaldi G Manto M . Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann Biomed Eng. (2013) 41:2437–47. doi: 10.1007/s10439-013-0846-y
121.
Nitsche MA Paulus W . Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. (2001) 57:1899–901. doi: 10.1212/WNL.57.10.1899
122.
Workman CD Fietsam AC Uc EY Rudroff T . Cerebellar transcranial direct current stimulation in people with Parkinson’s disease: a pilot study. Brain Sci. (2020) 10:96. doi: 10.3390/brainsci10020096
123.
Batsikadze G Moliadze V Paulus W Kuo MF Nitsche MA . Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. (2013) 591:1987–2000. doi: 10.1113/jphysiol.2012.249730
124.
Vimolratana O Aneksan B Siripornpanich V Hiengkaew V Prathum T Jeungprasopsuk W et al . Effects of anodal tDCS on resting state EEG power and motor function in acute stroke: a randomized controlled trial. J Neuroeng Rehabil. (2024) 21:6. doi: 10.1186/s12984-023-01300-x
125.
Dyke K Kim S Jackson GM Jackson SR . Intra-subject consistency and reliability of response following 2 ma transcranial direct current stimulation. Brain Stimul. (2016) 9:819–25. doi: 10.1016/j.brs.2016.06.052
126.
Behrangrad S Zoghi M Kidgell D Mansouri F Jaberzadeh S . The effects of concurrent bilateral anodal tDCS of primary motor cortex and cerebellum on corticospinal excitability: a randomized, double-blind sham-controlled study. Brain Struct Funct. (2022) 227:2395–408. doi: 10.1007/s00429-022-02533-7
127.
Guidetti M Maria Bianchi A Parazzini M Maiorana N Bonato M Ferrara R et al Monopolar tDCS might affect brainstem reflexes: a computational and neurophysiological study. Clin Neurophysiol. (2023) 155:44–54. doi: 10.1016/j.clinph.2023.08.011
Summary
Keywords
cerebellar tDCS, pain modulation, chronic pain, neuromodulation, systematic review
Citation
Betancur DFA, Tarragó MdGL, Oliveira MEL, Peres SM, Torres ILS, Fregni F and Caumo W (2025) Cerebellar tDCS and pain modulation: a critical integrative and systematic review. Front. Neurol. 16:1681853. doi: 10.3389/fneur.2025.1681853
Received
08 August 2025
Accepted
22 September 2025
Published
31 October 2025
Volume
16 - 2025
Edited by
Michael D. Staudt, University Hospitals Cleveland Medical Center, United States
Reviewed by
Tommaso Bocci, University of Milan, Italy
Jean Paul Buu Cuong Nguyen, Centre Hospitalier Universitaire (CHU) de Nantes, France
Updates
Copyright
© 2025 Betancur, Tarragó, Oliveira, Peres, Torres, Fregni and Caumo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wolnei Caumo, wcaumo@hcpa.edu.br
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.