Abstract
Background:
Migraine is linked to circadian rhythm disruptions, with morning attack peaks, circadian variations in trigeminal pain sensitivity, anterior hypothalamus involvement, and core circadian clock gene activity. Irregular night shift work, affecting up to 50% of the population, including new parents and students, causes significant circadian disruption. We hypothesize that irregular night shifts increase migraine prevalence compared to fixed schedules.
Methods:
A systematic review and meta-analysis of observational studies up to March 27, 2025, assessed migraine prevalence in irregular versus fixed night shift workers, searching Web of Science and PubMed with terms like “shift work” and “migraine” (PRISMA/MOOSE-compliant, PROSPERO: CRD420250654865). Study quality was evaluated using the Newcastle–Ottawa Scale (NOS). A random-effects meta-analysis calculated weighted odds ratios (ORs) for migraine prevalence.
Results:
From 203 records, 13 high-quality cross-sectional studies (N = 38,798,271, 77% female, NOS 9–10) showed irregular night shifts significantly increased migraine odds (OR = 1.61, 95% CI: 1.27–2.04, p < 0.0001, I2 = 73%), with females at higher odds (OR = 2.02–4.21). Meta-regression linked higher female representation to increased migraine odds (β = 0.70, p = 0.0003, R2 = 50%). Irregular night shifts showed no association with tension-type headache (OR = 0.79, 95% CI: 0.43–1.45).
Conclusion:
Irregular night shifts disrupt circadian rhythms, elevating migraine odds but not tension-type headache, suggesting fixed schedules may reduce the burden. Chronobiology-informed management, including slow-rotating schedules (≥5 days with rest days), delay-directed rotations, timed light exposure, and ambient temperature regulation, needs testing to prevent ‘Shift Work Migraine Disorder,’ a proposed distinct migraine subgroup.
Systematic review registration:
PROSPERO, CRD420250654865.
Introduction
Migraine is a complex and debilitating neurological disorder characterized by recurrent episodes of severe headaches, often accompanied by sensitivity to light, sound, and nausea (1). The pathophysiology of migraine is multifaceted, involving the interplay of various neural systems, including the trigeminal nerve, the brainstem, and the cerebral cortex (2). Recent studies have highlighted the critical role of circadian rhythms in migraine pathophysiology, suggesting a significant link between disruptions in internal biological processes and migraine attacks (3–9).
The observed circadian rhythmicity in migraine attacks exhibits distinct patterns, including matutinal/morning peaks in attack frequency, as well as circaseptan (weekly) and infradian (longer than 24 h) patterns (4, 7). Furthermore, circadian variations in trigeminal pain sensitivity have been observed, suggesting a complex interplay between circadian rhythms and migraine pathophysiology (10). The involvement of the anterior hypothalamus (11), home to the suprachiasmatic nucleus (SCN), the “master clock” regulating circadian rhythms, and the expression of circadian-related genes such as CK1δ, PER2, and RORα, provide additional evidence supporting the link between circadian rhythms and migraine (4, 12, 13).
The circadian system is a highly conserved and hardwired biological system that regulates various physiological processes, including pain sensitivity (14, 15), sleep–wake cycles (16), neurotransmitter and hormone secretion (17, 18), and metabolism (17, 19). Disruptions to this intricate system, whether due to lifestyle factors, environmental influences, or genetic predispositions, can have far-reaching detrimental consequences, contributing to the development of various chronic diseases, such as cancer (20–24), diabetes (19, 25–27), chronic pain (14, 28), cardiovascular disease (29), and neurological disorders such as Alzheimer’s disease (30–32).
Shift work involves organizing 24-h operations into two or three distinct shifts, with start and end times varying based on shift length (33). According to the US National Institute for Occupational Safety and Health (NIOSH), day shift typically runs from 5–8 a.m. to 2–6 p.m., evening shift from 2–6 p.m. to 10 p.m.–2 a.m., and night shift (colloquially known as “graveyard shift”) from 10 p.m.–2 a.m. to 5–8 a.m. (33). The International Labor Organization (ILO) (34) and International Agency for Research on Cancer (IARC) (35) define shift work as any work schedule outside conventional daytime hours, typically spanning 7:00 a.m. or 8:00 a.m. to 5:00 p.m. or 6:00 p.m., such as evening or night shifts. The ILO defines night work as any work performed during a period of at least seven consecutive hours, including midnight to 5 a.m., and a night worker as someone whose job involves a substantial amount of such hours exceeding a specified threshold (34). The European Union (EU) Working Time Directive (WTD) adopts the same definition of “night time” as the ILO and defines a “night worker” as an individual who regularly works at least 3 h of their daily shift during this period (36). Globally, approximately 20% of the workforce engages in shift work, with regional variations, such as 12% in Europe (up to 58% in evening work) (37) and 15% in Chile (38), while in the U.S., shift work prevalence is highest in service industries like protective services (50.4%) and food preparation (49.4%) (38). In other regions, night work affects 7.6% of workers in Brazil (39), 16% in Australia (40), 17.5% in China (41), 20% in Senegal (42, 43), 21.8% in Japan (44), and 28% in Canada (45), with significant prevalence in sectors like healthcare, hospitality, manufacturing, and transportation. Approximately 27% of the U.S. workforce reports engaging in evening or night shift work, with 7.4% specifically reporting frequent night shift work, defined as working between 1:00 a.m. and 5:00 a.m. for 6 to 30 days within the preceding 30-day period (46).
Migraine shows significant disparities in prevalence and severity across age groups (47, 48), sexes (48–50), and races (51–56). The specific impact of night shift work as a contributing factor to these disparities remains underexplored, warranting focused investigation.
-
Age and night work prevalence: According to the NIOSH, the prevalence of frequent night work demonstrates a clear age-related pattern, with the highest rates observed in the youngest demographic: 18–29 years (8.61%). This prevalence gradually decreases with increasing age: 30–44 years (7.78%), 45–64 years (6.93%), and is lowest in individuals aged 65 and older (3.68%) (46). Similarly, as per the US Department of Labor (DOL) report, young workers (aged 15–24) are more likely to work non-daytime schedules, including evening, night, rotating, or irregular shifts, with 31.9% on such shifts compared to 16.4% of the total workforce (57).
-
Sex differences: Females (5.57%) engaged in less night shift work compared to males (9.11%) (46). Studies show that females experience a migraine burden three times greater than males (48–50). This increased susceptibility in females is further exacerbated by a higher prevalence of psychological comorbidities, such as anxiety and depression, and shorter free-running circadian cycles (58). These factors may contribute to increased circadian disruption when subjected to night shift schedules, potentially amplifying the adverse effects on migraine.
-
Racial disparities: Racial disparities in night work prevalence are important. In the NIOSH report, Black populations demonstrate the highest rates of night shift work (10.5%), followed by White populations (7.07%) and other racial groups (6.48%) (46). The DOL report shows that Black workers are more likely to work non-daytime schedules, with 24.1% on such shifts compared to 15.2% of White workers and 16.4% of the total workforce (57). Notably, research indicates that Black individuals tend to have shorter free-running circadian cycles compared to White individuals (58–60). This physiological difference may further exacerbate the detrimental effects of circadian disruption associated with night shift work, potentially intensifying health disparities. Black populations also have limited healthcare access, lower treatment rates, and greater functional disability due to more severe migraine pain, intensifying migraine burden and disparities (51–53, 56, 61, 62).
-
Intersection of demographics, night shift work, and migraine: The demographic profiles of younger females and Black workers align with populations already identified as having a higher prevalence and burden of migraine. Previous studies have consistently documented elevated migraine burden, increased severity, and pronounced disparities within these groups (48, 50, 53, 55, 61). We hypothesize that frequent night work, with its inherent disruption of circadian rhythms, to be a significant trigger for both migraine exacerbation and de novo onset. Beyond ‘traditional’ shift work, students facing academic pressure and new parents experiencing fragmented sleep due to newborn care are particularly vulnerable to night shifting and its associated circadian rhythm disruption (63, 64). Further in-depth investigation into the impact of night work on migraine prevalence and severity across all affected populations is warranted to develop targeted interventions and mitigation strategies that benefit diverse demographic groups.
Rotating or irregular night shift work, characterized by unpredictable changes in shift timing, is linked to greater circadian disruption, long sleep, depression, anxiety, and fatigue compared to fixed night shift work, likely due to increased recovery needs (65–71). Rotating night shifts also significantly elevate psychological distress and impair sleep quality, posing substantial health risks (65, 67). Given the established link between circadian disruptions and migraine, we hypothesize that irregular or rotating night shift work, which is associated with increased circadian disruption, psychological distress, and reduced sleep quality compared to fixed night shift work, may correlate with a higher prevalence of migraine. Our objective is to investigate this relationship to inform evidence-based interventions aimed at reducing migraine burden in this vulnerable population.
Methods
Research question
Our research question followed the PECO (Population, Exposure, Comparison, Outcome) (19) format: general working population (P); irregular night shift work or rotating night shifts with ≥ 5 nights/month (E), defined by not remaining on the same schedule for ≥2 weeks; regular non-rotating night/evening or permanent night shifts with <5 nights/month (C); and migraine/headache prevalence (O), assessed via odds ratios from observational studies.
Eligibility criteria
Studies were eligible if they included adult workers (≥18 years) from general populations (any occupation/industry; excluding clinical/non-working groups like patients/retirees). Exposure required frequent irregular/rotating night/evening shifts with schedule changes (34, 36); comparisons needed fixed non-rotating night/evening schedules. Evening/night shifts were grouped due to temporal overlap (e.g., 9:00 p.m.–midnight) and shared health effects.
While there’s overlap in the hours [9:00 p.m. to midnight are included in both evening and night shifts (33, 46, 57)], both evening and night shifts are categorized together in these studies due to shared characteristics and health implications. Both shift types occur during peak melatonin production and sleep propensity, contributing to similar health risks (72). Including both captures the broader impact of night shift work schedules on circadian disruption, despite overlapping hours. Outcomes of interest were the prevalence of migraine or severe headache, defined by clinical criteria (e.g., International Classification of Headache Disorders/ICHD-based questionnaire for migraine), medical interview, or self-report. We included studies reporting migraine prevalence or associations with shift work, even if they encompassed other headache subtypes [e.g., tension-type headache (TTH), chronic daily headache (CDH), or medication-overuse headache (MOH)]; we retained those with extractable migraine-specific data but also included severe headache or CDH-focused studies without explicit migraine subgroups as proxies for high-burden migraine-like presentations.
Studies were required to provide odds ratios (ORs) to quantify the association between shift work and migraine or headache prevalence, either directly reported ORs or data allowing for OR calculation (e.g., prevalence rates, contingency tables). Eligible study designs included observational studies, specifically cross-sectional studies, case–control studies, and cohort studies (prospective or retrospective), published in peer-reviewed journals or grey literature (e.g., theses, conference proceedings) with sufficient data, in any language (with translation available if needed), and with no date restrictions, given continuous knowledge updates. The 2015 NHIS report (46) was included in our systematic review as it provided relevant data on night shift work and migraine, aligning with our study’s focus.
Exclusion criteria were applied to maintain focus and quality. Studies were excluded if they exclusively involved non-workers (e.g., students, unemployed individuals, retirees), were limited to pediatric populations (<18 years) or specific clinical cohorts (e.g., only migraine patients without a working context), lacked clear definitions of night shift work (e.g., no mention of timing or irregularity), did not differentiate shift work (e.g., combining day and night shifts without separate analysis), or focused solely on day shift work without night shift comparison. Studies without a comparison group (e.g., case series or descriptive studies with no control) or those comparing night shift work to irrelevant groups (e.g., unemployed individuals) rather than the specified comparators were also excluded. Additionally, studies were excluded if they did not report migraine or headaches as outcomes (e.g., focusing only on sleep disorders or fatigue) reported only headache subtypes other than migraine (e.g., tension-type or cluster headaches), unless headaches were non-subtyped, or provided only qualitative outcomes (e.g., no prevalence or OR data). Studies not providing odds ratios or sufficient data to derive them (e.g., only p-values or narrative results), using experimental or non-associational metrics (e.g., means without prevalence), or employing non-observational designs (e.g., randomized controlled trials, laboratory-based experiments) were excluded. Reviews, editorials, or opinion pieces without original data were also excluded, though their reference lists were screened for eligible studies. Studies with insufficient detail on shift work patterns (e.g., no frequency or quick return data) were excluded. Including studies where ORs could be calculated from prevalence or contingency tables (not just reported) broadened the pool of eligible studies without sacrificing rigor. All reviewers used these criteria to screen titles and abstracts, then full texts, resolving discrepancies through consensus. The criteria ensured that studies aligned with the question’s focus on the impact of irregular night shifts on migraine and headaches, while excluding irrelevant or low-quality data.
Search strategy and study selection
The process for identifying, screening, and selecting studies followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The search strategy was tailored to the research question and the inclusion/exclusion criteria, specifying the databases, keywords, and time frame used to identify observational studies examining the association between irregular or rotating night shift work and the prevalence of migraine or non-subtyped headaches among workers, as measured by odds ratios or calculable odds ratios (e.g., from prevalence or contingency tables). The databases searched included PubMed and Web of Science. The search used a combination of controlled vocabulary (e.g., MeSH terms in PubMed) and free-text keywords to capture the population, exposure, comparison, outcome, and study design elements, grouped by concept and combined using Boolean operators (AND, OR, NOT). For the population (workers), terms included controlled terms like “Workers” [MeSH], “Occupational Groups” [MeSH], “Employment” [MeSH], and free-text terms such as worker*, employee*, “working population,” “labor force,” occupation*, staff, and personnel. For exposure (irregular or rotating night shift work), controlled terms included “Shift Work Schedule” [MeSH], “Work Schedule Tolerance” [MeSH], “Circadian Rhythm” [MeSH], and free-text terms like “night shift*,” “shift work,” “rotating shift*,” “irregular shift*,” “quick return*,” “short recovery,” “frequent shift*,” “shift change*,” and “night work.” Comparison terms (less frequent shifts, regular shifts, non-night work) included free-text terms such as “regular shift*,” “fixed shift*,” “non-rotating shift*,” “day shift*,” “permanent schedule*,” “non-night shift*,” and “standard work hours.” For the outcome (migraine and non-subtyped headaches), controlled terms were “Migraine Disorders” [MeSH], “Headache” [MeSH], “Headache Disorders” [MeSH], and free-text terms included migraine*, headache*, cephalalgia, “head pain,” and “cranial pain,” with a note that subtypes like “tension-type” or “cluster” would be excluded unless part of broader headache data. Measurement and study design terms (odds ratios, relative risks, observational studies) included controlled terms like “Odds Ratio” [MeSH], “Relative Risk” [MeSH], “Observational Study” [MeSH], “Cross-Sectional Studies” [MeSH], “Case–Control Studies” [MeSH], “Cohort Studies” [MeSH], and free-text terms such as “odds ratio*,” OR, prevalence, association, “cross-sectional,” “case–control,” cohort, observational, and epidemiology*.
An example search string for PubMed was as follows: (“Workers” [MeSH Terms] OR “Occupational Groups” [MeSH Terms] OR “Employment” [MeSH Terms] OR worker* OR employee* OR “working population” OR occupation*) AND (“Shift Work Schedule” [MeSH Terms] OR “Work Schedule Tolerance” [MeSH Terms] OR “night shift*” OR “shift work” OR “rotating shift*” OR “irregular shift*” OR “quick return*” OR “night work”) AND (“Migraine Disorders” [MeSH Terms] OR “Headache” [MeSH Terms] OR migraine* OR headache* OR cephalalgia) AND (“Odds Ratio” [MeSH Terms] OR “relative risk*” OR “hazard ratio*” OR “Observational Study” [MeSH Terms] OR “Cross-Sectional Studies” [MeSH Terms] OR “Case–Control Studies” [MeSH Terms] OR “Cohort Studies” [MeSH Terms] OR “odds ratio*” OR prevalence OR “cross-sectional” OR cohort OR observational). A similar structure was adapted for Web of Science: TS = ((worker* OR employee* OR “working population” OR occupation*) AND (“night shift*” OR “shift work” OR “rotating shift*” OR “irregular shift*” OR “quick return*” OR “night work”) AND (migraine* OR headache* OR cephalalgia) AND (“odds ratio*” OR “relative risk*” OR “hazard ratio*” OR prevalence OR “cross-sectional” OR “case–control” OR cohort OR observational)). Filters in PubMed included study type (Observational Study, Cross-Sectional Studies, Case–Control Studies, Cohort Studies where applicable), language (no restriction, translations sought if needed), and species (humans), while Web of Science filters included document type (Article, Conference Proceeding, Early Access), research area (Medicine, Public Environmental Occupational Health, Neurosciences, Epidemiology if applicable), and language (no restriction). There was no date restriction, with searches including all available records up to the current date (March 27, 2025), reflecting the lack of a cutoff in the inclusion criteria and the continuous knowledge update capability, which ensures comprehensive coverage of both historical and recent studies. Search strings, run dates, and result counts were recorded for transparency (Figure 1, PRISMA flow diagram). Reference lists of included studies and relevant reviews were manually searched for additional eligible studies. This strategy strikes a balance between sensitivity (capturing relevant studies) and specificity (focusing on the question’s scope). We prioritized ORs for consistency across study designs, although cohort studies often report relative risks or hazard ratios. Therefore, we included terms for these metrics in our search and calculated ORs where possible.
Figure 1

PRISMA flow diagram of study selection.
Data extraction
The following data were extracted: first author, year of publication, country, sample size, male to female ratio, study design, type of comparison (rotating vs. non-rotating night shiftwork, frequent vs. infrequent irregular night shift work), and number of people with migraine or headache (where headache was not phenotyped) in the compared groups. Six authors participated in data extraction.
Quality assessment
The Newcastle–Ottawa Scale (73) (by YW and MF) was used to assess the quality of each included article in the following three domains: selection of study groups, comparability of groups, and ascertainment of exposure or outcome.
Statistical analysis
A random-effects model was employed for the meta-analysis to account for between-study heterogeneity, which arises from differences in study settings, populations, and sample sizes. This model provides a more conservative estimate of the overall effect compared to a fixed-effects model. The inverse variance method with the DerSimonian-Laird estimator was used to weight studies based on their precision, accommodating the wide range of study sizes and heterogeneity effectively. In cases of sparse events, the robustness of this approach was considered, with the Mantel–Haenszel method as a potential alternative for sensitivity analyses to ensure stability in estimates. Odds ratios (ORs) were used as the effect measure instead of relative risk, as ORs provide consistent and comparable associations across observational studies, particularly cross-sectional designs. ORs are less sensitive to variations in baseline risks and are well-suited for studies where temporality or causality cannot be established. Data were synthesized using the online platform1 (74) to calculate effect sizes and assess heterogeneity. Meta-Essentials software (75) was utilized to perform statistical analyses, generate forest plots, conduct meta-regression, and evaluate publication bias. All procedures adhered to standard meta-analysis guidelines to ensure robust and reliable results. The study protocol was registered on PROSPERO (CRD420250654865) on 24 February 2025. Inter-rater reliability checks and duplicate screening were conducted by YW and EH to enhance methodological rigor.
Results
Study selection: Figure 1 presents the PRISMA flow diagram detailing the study selection process. A total of (n = 203) records were identified from PubMed [(n = 75)] and Web of Science [(n = 127)], as well as government dataset (NHIS). After removing (n = 69) duplicates, 59 records were screened by title and abstract, excluding (n = 36) as irrelevant. Of 23 full-text articles assessed for eligibility, 10 were excluded for reasons including insufficient shift work data (n = 6) or no odds ratios or calculable data (n = 4). Ultimately, 13 studies (46, 76–87) were included in quantitative synthesis.
Study characteristics: The study analyzed a combined sample size of 38,798,271 participants, comprising 77% female participants. The participants originated from eight countries: China (3), Denmark (2), Saudi Arabia (2), Norway (1), United Kingdom (1), Singapore (1), Canada (1), United States (2). All included studies involved a cross-sectional design.
Migraine and headache diagnosis: Of the 13 included studies, eight employed migraine-specific diagnoses, while the remaining five used broader criteria for severe or chronic headache (Table 1). Five studies employed validated migraine diagnoses (e.g., ICHD-3 or physician interview/medical record review), while nine [including the NHIS 2015 (46)] relied on self-report measures.
Table 1
| Selection | Comparability | Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Q1 | Q2 | Q3 | Q4 | Q5a | Q5b | Q6 | Q7 | Total | Diagnosis | |
| 1 | Affatato et al. (87) | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 10 | Validated by physician diagnosis (migraine) |
| 2 | Al Maqwashi et al. (86) | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 10 | Self-administered questionnaire (migraine) |
| 3 | Bjorvatn et al. (84) | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 10 | ICHD-3b validated self-administered questionnaire (migraine, TTH, MOH) |
| 4 | Chan et al. (83) | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 10 | Self-administered questionnaire (headache > once/week) |
| 5 | Jakobsen et al. (82) | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 10 | Self-administered questionnaire (migraine) |
| 6 | Jensen et al. (81) | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 1 | 9 | Self-administered questionnaire (headache) |
| 7 | Lees et al. (80) | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 1 | 9 | Medical record review (headache) |
| 8 | Tasto et al. (78) | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 1 | 9 | Self-administered questionnaire (CDH) |
| 9 | Wang et al. (77) | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 10 | ICHD-3b validated self-administered questionnaire (migraine, TTH, CDH) |
| 10 | Xie et al. (76) | 1 | 0 | 1 | 2 | 1 | 1 | 2 | 1 | 9 | ICDH-3, neurologist interview validated, self-administered questionnaire (migraine, TTH) |
| 11 | Alturaiki et al. (85) | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 9 | Self-administered questionnaire (migraine, TTH) |
| 12 | Liu et al. (79) | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 10 | Self-administered questionnaire (headache) |
| 13 | National Institute for Occupational Safety and Health (46) | 1 | 1 | 1 | 2 | 1 | 0 | 2 | 1 | 9 | Interview (migraine or severe headache) |
| 14 | Molarius et al. (88) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 | Self-administered questionnaire (migraine/recurrent headache) |
The risk of bias assessment shows Newcastle-Ottawa Scale (NOS) scores ranging from 9 to 10, indicating a high methodological quality.
Most studies (7 of 13) scored a perfect 10 in selection, comparability, and outcomes (Q1–Q7). The other six studies scored 9, with minor deductions mainly in Q2 or Q6, reflecting slight variations in representativeness or exposure/outcome ascertainment. Molarius et al. (88) was included solely for direct OR comparison of female vs. male night-shift migraine risk (n = 10,503; 45% female), as it is one of only two studies providing disaggregated data. Of the 13 included studies, eight employed migraine-specific diagnoses, while the remaining five used broader criteria for severe or chronic headache. Five studies employed validated migraine diagnoses (e.g., ICHD-3 or physician interview/medical record review), while nine [including the National Institute for Occupational Safety and Health (46)] relied on self-report measures. Q stands for Question Number.
Quality assessment (Table 1): The Newcastle–Ottawa Scale (NOS) (20) scores for the listed studies range from 9 to 10, indicating high methodological quality across the board. The majority of studies (7 out of 13) scored a perfect score of 10, excelling in selection, comparability, and outcome criteria (Q1-Q7). The remaining six studies scored 9, with minor deductions primarily in Q2 or Q6, suggesting slight variations in representativeness or ascertainment of exposure and outcome.
Quantitative synthesis: Altogether, 13 studies were analyzed. Based on the analysis performed using a random effects model with the inverse variance method to compare the OR, a statistical difference was observed; the summarized OR was 1.61 with a 95% confidence interval/CI of 1.27–2.04 (see Figure 2). The test for overall effect showed significance at p < 0.0001. The I2 value indicated a 73% inter-study heterogeneity.
Figure 2
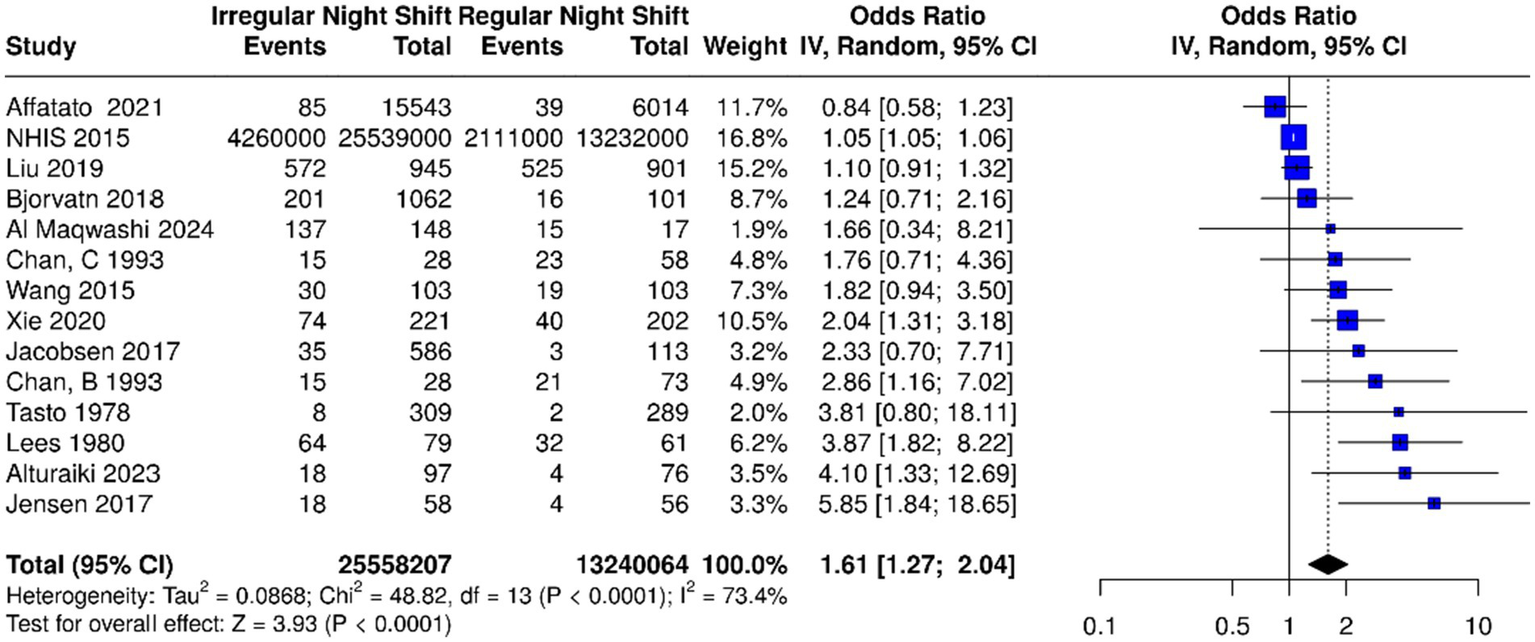
Based on the analysis performed using a random effects model with the inverse variance method to compare the odds ratio (OR), there was a statistically significant association between irregular night shift work and migraine prevalence; the summarized OR was 1.61 with a 95% confidence interval (CI) of 1.27–2.04 among the 13 studies [14 datasets including factories B and C from Chan et al. (83)]. Individual study estimates are depicted as squares (size proportional to weight), with horizontal lines for 95% CIs; the diamond summarizes the overall pooled OR and CI. IV, inverse variance; dF, degrees of freedom. Events represent the number of cases of migraine.
Sensitivity analysis: Sensitivity analysis by diagnosis type yielded pooled ORs of 1.64 (95% CI: 1.02–2.69) for the five validated studies and 1.54 (95% CI: 1.16–2.05) for the eight self-report studies, with no significant subgroup difference (p = 0.83). Sensitivity analysis by specificity of diagnosis yielded pooled ORs of 1.40 (95% CI: 1.04–1.89) for the eight migraine-specific studies and 2.53 (95% CI: 1.30–4.93) for the five severe/recurrent daily headache studies, with a significant subgroup difference (p = 0.002). Excluding the NHIS study (which had a large sample size) resulted in a pooled OR of 1.88 (95% CI: 1.37–2.60) from the other 12 studies. Including it did not significantly change the results (p = 0.45).
Among the 13 studies reviewed, sex-specific data were available in only one study (87). An additional study, the only other in the literature providing disaggregated sex-specific data on night-shift migraine risk (88) was included outside the primary 13 studies, resulting in 10,503 participants (45% female) across these two studies. The OR for females was 2.02 (95% CI: 1.71–2.39) (88) in one study and 4.21 (95% CI: 2.09–8.46) (87) in the other, suggesting that females had approximately two to four times higher odds of migraine associated with night shift work compared to males. A meta-regression across 12 studies, which provided male-to-female percentage data, reinforced this finding. The meta-regression analysis revealed a significant association between the proportion of females and increased migraine odds in irregular versus regular night shift workers, with a β (standardized beta) of 0.70 (p = 0.0003), accounting for 50% of the variance (Figure 3).
Figure 3

Meta-regression of 12 studies reporting male-to-female percentages, with bubble sizes indicating study weight and numbers corresponding to studies, showing a significant association between higher female proportion and increased migraine odds in irregular versus regular night shift workers (standardized β = 0.70, p = 0.0003), explaining 50% of the variance. Studies and their variance percentage contribution: 1, Al Maqwashi et al. (0.87%) (86); 2, Tasto et al. (0.92%) (78); 3, Jakobsen et al. (1.52%) (82); 4, Jensen et al. (1.62%) (81); 5, Alturaiki et al. (1.70%) (85); 6, Chan et al. (2.61%) (83); 7, Wang et al. (4.59%) (77), 8, Bjorvatn et al. (6.05%) (84); 9, Xie et al. (8.56%) (76); 10, Affatato et al. (10.68%) (87); 11, Liu et al. (21.94%) (79); 12, National Institute for Occupational Safety and Health (32.80%) (46).
A meta-analysis of four studies with available tension-type headache (TTH) data, conducted using a random-effects model with the inverse variance method, evaluated the association between irregular night shift work and TTH prevalence. The pooled OR was 0.79 (95% CI: 0.43–1.45), indicating no statistically significant association (p > 0.05 for overall effect, I2 = 80%; Figure 4). Subgroup analysis showed a significant effect for migraine-only (subgroup 1) and all headaches (including non-phenotyped, subgroup 2). No significant differences were observed between migraine-only and all headaches subgroups (p = 0.25), with migraine appearing as the primary driver of the overall effect.
Figure 4
![Forest plot displaying odds ratios with 95% confidence intervals for four studies: Alturaiki 2023, Bjorvatn 2018, Xie 2020, and Wang 2015. The combined odds ratio is 0.79 [0.43; 1.45], with high heterogeneity (I² = 80.4%). Each study's result is represented by a red square, proportional to its weight, with a diamond representing the overall effect.](https://www.frontiersin.org/files/Articles/1684169/xml-images/fneur-16-1684169-g004.webp)
Meta-analysis of four studies using a random-effects model with inverse variance method to compare odds ratios (OR) of tension-type headache prevalence associated with irregular night shift work. The summarized OR was 0.79 (95% CI: 0.43–1.45), indicating no statistically significant association (test for overall effect, p > 0.05).
Meta-regression, using log-transformed (log10) sample size as a covariate to account for its wide range, showed no significant effect of sample size on the outcome (β = −0.31, p = 0.215).
Sleep disturbances in included studies: Among the eight studies reporting sleep data, significant disturbances were prevalent in shift workers, including insomnia [31.7% (84)], trouble initiating sleep [58% (81)], and overall sleep problems [~50% (79)]. These were associated with elevated migraine risk (OR: 1.43–2.64) and served as triggers [54.6% disturbances, 57.1% insufficient sleep (86)]. Short/long sleep durations further heightened odds [OR: 1.49–1.54 (82)]. No studies reported on sleep-affecting medications.
Discussion
Night shift work, a necessity in many professions, disrupts the delicate balance of the circadian system. This disruption leads to physiological stress, irregular sleep patterns, and heightened sensitivity to migraine triggers. Our meta-analytic evidence suggests a robust association between irregular or rotational shift work and a greater prevalence of migraine. Our findings confirm that night shift work, particularly its irregular scheduling, is associated with an increased migraine burden. This discussion elaborates on the implications of fixed versus rotating shift schedules, female susceptibility to night shift effects, introduces the concept of “Shift Work Migraine Disorder” (SWMD) as a potential subgroup within the migraine spectrum, necessitating tailored interventions, and proposes strategies to reduce migraine exacerbation.
The comparable pooled ORs in self-report studies (1.54, 95% CI: 1.16–2.05) versus validated (1.64, 95% CI: 1.02–2.69; p = 0.83) affirm methodological consistency, indicating that self-report measures, while less rigorous, yield reliable estimates of shift work-migraine associations in population-based research. Self-report measures show a high level of agreement (~87%) with ICHD criteria in analogous population-based studies (89, 90). Sensitivity analysis excluding the NHIS study with the largest sample size yielded a pooled OR of 1.88 (95% CI: 1.37–2.60) from the remaining 12 studies, with no significant difference upon its inclusion (p = 0.45 for heterogeneity), underscoring the robustness of the overall estimate. The higher pooled OR in severe/recurrent daily headache studies (2.53, 95% CI: 1.30–4.93) versus migraine-specific ones (1.40, 95% CI: 1.04–1.89; p = 0.002) likely reflects inclusion of undiagnosed severe migraine cases—more vulnerable to circadian disruption—rather than TTH, which showed no shift work association, underscoring SWMD’s migraine-centric etiology and a dose–response gradient where broader criteria amplify observed effects.
Fixed versus irregular shift schedules
Our results align with prior evidence that fixed night shift schedules are preferable to irregular or rotating schedules for minimizing circadian disruption (70). Irregular shifts, particularly those with rapid rotations [changing night shift schedules every 1–4 days (91)], quick returns [less than 11 h of rest between shifts (92)], and consecutive night shifts (>3 nights) (93, 94) desynchronize the SCN, the master circadian pacemaker, from peripheral clocks in organs such as the liver and gut (95). This desynchronization manifests as irregular sleep–wake cycles, and gastrointestinal disturbances, and may amplify sensory processing (3, 9, 96, 97) to migraine triggers such as bright light, loud sound, and dietary irregularities, though direct evidence is needed. Fixed schedules, by contrast, allow for gradual circadian adaptation for workers transitioning from day to night shifts, involving a 12-h schedule change, with the biological clock adjusting by approximately 1–2 h per day (60, 98). Based on this adaptation rate, we recommend maintaining consistent shift schedules for at least 12–14 days to allow sufficient time for entrainment of the 12-h shift, potentially reducing physiological stress and migraine risk, though further research is needed to confirm this effect.
Rotating shifts, when unavoidable, should follow a delay direction (morning → evening → night) rather than an advance direction (night → morning) (99–101). This aligns with the natural tendency of the human circadian clock to delay rather than advance, as demonstrated in a meta-analysis, which found lower rates of sleep disruption and mood disturbances with delay-rotated schedules (99–101). Additionally, minimizing consecutive night shifts—ideally to one per cycle—reduces cumulative sleep debt and cortisol dysregulation, both of which are implicated in migraine pathophysiology (102–106).
Avoiding quick returns (shifts with <11 h of rest) reduces fatigue and sleep disturbances, as short inter-shift intervals exacerbate circadian misalignment (92, 107). However, Katsifaraki et al. (108) found no association between sleep duration and headaches in shift workers (OR = 1.00, 95% CI: 0.97–1.02), suggesting headache triggers may involve circadian factors rather than sleep loss.
Female susceptibility to night shift effects
Our meta-regression and subgroup meta-analysis of 10,503 individuals (45% female) reveal a significant sex disparity in night shift migraine risk, with females facing over twice the odds versus males. This aligns with women’s higher migraine prevalence, amplified by circadian disruption (50). Despite lower night shift participation (15.2% vs. 17.6% for males) (46), females bear greater shift work-migraine burden, underscoring the need to probe underlying mechanisms. This sex disparity may stem from inherent circadian differences, with females exhibiting a shorter free-running circadian period (24.2 vs. 24.5 h in males) (58, 109–111) that heightens vulnerability to night-shift desynchronization. Hormonal fluctuations (menstrual cycles, pregnancy, or menopause) exacerbate misalignment effects (112), while females show greater sensitivity to sleep deprivation and stress (113, 114), further compounded by caregiving duties (115, 116) and circadian-timed stress responses, as seen in female mice with exaggerated active-phase disruptions in clock genes (113, 114).
Shift work migraine disorder: a proposed subgroup
Our study links irregular night shifts to higher migraine burden, proposing “shift work migraine disorder” (SWMD) as a novel chronobiological subtype warranting validation. We hypothesize chronic circadian misalignment amplifies trigeminovascular activation (117) via neuroinflammation and oxidative stress (118–120), evidenced by higher migraine prevalence in night vs. day workers and irregular vs. fixed night workers. SWMD likely increases susceptibility to circadian triggers, necessitating chronobiology-focused management. SWMD’s migraine specificity (vs TTH) aligns with greater sleep disturbances in migraine (121, 122); the absent TTH link underscores circadian disruption’s preferential migraine impact. Longitudinal studies should validate SWMD’s pathophysiology for potential ICHD inclusion, with proposed criteria:
-
A) Meets ICHD-31 migraine criteria.
-
B) ≥3 months irregular night shifts [rotating shifts (91), quick returns (92), >3 consecutive night shifts (93, 94)].
-
C) Migraine onset/exacerbation temporally linked to shifts (during/within 24 h of changes).
-
D) Circadian evidence (actigraphy irregularities or daytime sleepiness).
-
E Not better explained by other disorders, including ruling out obstructive sleep apnea or periodic limb movements via polysomnography if clinically indicated.
These criteria distinguish SWMD from subtypes (e.g., chronic or menstrual migraine) by emphasizing circadian triggers, akin to shift work sleep disorder (SWSD) (123), a recognized circadian rhythm sleep disorder. Though sleep disruption triggers migraine (106, 124), SWMD uniquely ties attacks to shift transitions, differing from temporary jet lag disorder (125) and mirroring SWSD’s chronicity (Table 2). Unique factors include occupational context and melatonin suppression (72, 126–128), supporting targeted interventions and policies. Fixed schedules (e.g., 14-day consistency) and chronotherapy could aid alignment, unlike easier jet lag recovery, but require testing. In a study of 2,762 participants, social jet lag affected 18.9%, more in headache sufferers (21% vs. 17%, p = 0.006) (129), but not migraine-specific (22.4% vs. 20.8%, p = 0.651) (129) – indicating broad circadian effects without migraine distinction. Conversely, occupational SWMD merits separate recognition. Longitudinal validation and targeted interventions are essential; the Phase 3 solriamfetol trial for SWSD [NCT06568367 (130)] – a wake-promoting agent targeting TAAR-1, dopamine, and norepinephrine pathways overlapping with migraine mechanisms—may offer translational insights for SWMD due to shared circadian disruptions, but only as a hypothesis pending dedicated, migraine-specific efficacy trials.
Table 2
| Study | Key sleep findings | Association with migraine/headache | Notes on medications/caffeine |
|---|---|---|---|
| Affatato et al. (87) | Sleep disorders were exclusion criteria. | N/A | No information on sleep medications. |
| Al Maqwashi et al. (86) | Sleep disturbances reported as a migraine trigger in 54.6% of participants; insufficient sleep in 57.1%. | High prevalence as triggers in migraineurs. | No information on sleep medications. |
| Alturaiki et al. (85) | No explicit sleep disorder data. | N/A | No information on sleep disorders or medications; caffeine use noted in 0.9% of migraine cases. |
| Bjorvatn et al. (84) | Insomnia prevalence: 31.7%. | OR = 1.55 (95% CI: 1.18–2.02) for migraine; OR = 1.01 (95% CI: 0.79–1.29) for tension-type headache. | No information on sleep medications. |
| Jakobsen et al. (82) | Short sleep (≤6 h: OR = 1.49, 95% CI: 1.21–1.85) and long sleep (≥9 h: OR = 1.54, 95% CI: 0.99–2.39) associated with higher migraine occurrence. | Associations persisted after adjustment for shift work. | No information on sleep medications. |
| Jensen et al. (81) | 58% of nurses reported trouble falling asleep during night shifts. | Linked to shift work challenges. | No information on sleep medications. |
| Liu et al. (79) | ~50% reported sleep problems; positive association with night shifts (OR = 1.43, 95% CI: 1.21–1.69). | OR = 2.64 (95% CI: 2.27–3.07) for headache. | No information on sleep medications. |
| Chan et al. (83) | Night shift workers had shorter sleep duration; rotating shifts had higher rates of poor sleep quality vs. controls. | Elevated poor sleep in rotating shift group. | No information on sleep medications. |
Summary of sleep disturbances in shift workers from included studies.
OR, odds ratio; CI, confidence interval; TTH, tension-type headache. Data is limited to 8/13 studies reporting sleep metrics; there are no reports on sleep-affecting medications.
Recent within-person data from the 1,001 Nights cohort (131) bolster our SWMD proposal as a distinct chronobiological subtype, showing 31% higher headache prevalence on night shifts (aPR/adjusted prevalence ratio 1.31, 95% CI 1.13–1.52) persisting after adjustments for sleep, psychosocial stressors, and demands—echoing our meta-analysis OR of 1.61 for irregular shifts and emphasizing circadian misalignment over sleep disruption. Diary-based sleep controls (naps/latency, quality) uphold the association (aPR 1.31 from 1.33), affirming non-sleep-mediated hypothalamic-trigeminal disruption. Second-night peaks (aPR 1.54 vs. days, 95% CI 1.28–1.84) pre-adaptation refine phenotyping for early irregular exacerbations, aligning with our irregular/fixed split, endorsing slow rotations (≥5 days), and urging validation. Its female-dominant sample (n = 522, 14% migraine) improves our female predominance meta-regression (β = 0.70, p = 0.0003) generalizability via within-person design, advancing chronotherapy and ICHD-3 candidacy for preventable SWMD.
Proposed strategies: how to work night shift and mitigate migraine burden
Beyond shift scheduling, our findings support multifaceted interventions, including chronotherapy (e.g., timed light) and policies such as mandatory recovery days, alongside sleep hygiene education (cool/dark environment, stimulus control, and sleep restriction) – proven effective in a meta-analysis for shift workers (132). Further research is needed on circadian-migraine links to refine strategies (Table 3).
-
A. Photic circadian entrainment
Table 3
| Feature | Jet lag disorder | Shift work sleep disorder (SWSD) | Social jet lag | Shift work migraine disorder (SWMD) |
|---|---|---|---|---|
| Cause | Time zone travel (more severe on traveling eastward) (125) | Night or rotating shifts (71, 123) | Misalignment between social and biological clocks (e.g., work vs. non-work sleep schedules) (129, 159) | Irregular night shift schedules |
| Duration | Temporary (125) | Long-term (123) | Chronic or recurrent (159, 160) | Chronic or recurrent |
| Symptoms | Insomnia, sleepiness, fatigue (125) | Insomnia, sleepiness, reduced alertness (123) | Sleep disruption, fatigue, mood changes (159, 160) | New onset or worsening migraine, characterized by increased frequency, severity, and heightened sensitivity to triggers (e.g., light, noise), associated with night shift work |
| Prevalence | Common in travelers (125) | 27% (up to 49%) (123, 161–163) prevalence among night and rotating shift workers | 21% in headache sufferers, similar in migraine vs. non-migraine headache sufferers (22.4% vs. 20.8%, p = 0.651) (129) | 6 to 12% in evening and night shift workers (84); elevated migraine odds in irregular night shift workers (OR = 1.61), not TTH (OR = 0.79) |
| Severity | Mild to moderate (125) | Moderate to severe (123) | Mild to moderate (129, 159, 160) | Moderate to severe |
| Adaptation | Circadian alignment restores with time zone adjustment (125) | Circadian alignment difficult, requiring consistent schedules or interventions (123) | Persists without alignment of social and biological schedules (159, 160) | Circadian alignment challenging, may improve with fixed schedules (e.g., 14-day consistency) |
| Proposed treatment strategies | Timed light exposure, sleep schedules, melatonin supplementation (125, 164) | Fixed shifts, chronotherapy (e.g., light therapy), clockwise/delayed rotation, cool sleep environments, sleep hygiene (123, 165) | Consistent sleep schedules, limited weekend sleep variability (159, 160) | Fixed schedules (e.g., 14-day consistency), chronotherapy (e.g., timed light exposure), recovery days, clockwise/delayed rotation, cooling sleep environment; requires further study |
Comparison of shift work related conditions: Jet lag disorder, shift work sleep disorder (SWSD), social jet lag, and proposed shift work migraine disorder (SWMD).
Dynamic lighting mimicking day-night cycles (high melanopic lux 250–300 during shifts, dim <50 lux post-shift) reduces fatigue/mood issues (133), potentially stabilizing rhythms to cut migraine frequency. Blue-blockers post-shift reduce melatonin suppression (128); early-shift bright melanopic light (460-480 nm) boosts alertness (128, 134). Light therapy glasses (461 nm) cut sleepiness post-first shift (p = 0.012) (135), blue-enriched light (17,000 K) improves subjective alertness (136), and meta-analyses confirm benefits (134). Pre-Tmin bright light (7,000–12,000 lux, 2 h pre-wake) accelerate phase delays (133), while post-Tmin darkness (e.g., blue-blockers on commutes) aid adaptation and could reduce migraine – subject to verification (133, 137).
-
B. Non-photic circadian entrainment
Evening/nocturnal exercise (e.g., 19:00–22:00 or 00:30, moderate-high intensity) delays melatonin for better alertness/sleep (138–140). Time-restricted eating (10 h window post-shift) syncs metabolism, enhances cognition, cuts cardiometabolic risks (141–144). Cooler ambient temps (~23 °C) boost alertness, reduce discomfort, and adapt melatonin (145). Social interactions during/post-shift anchor rhythms (146–149), amplified by light strategies (150). Judicious caffeine (100-200 mg at onset) combats sleepiness (151) but risks daytime sleep disruption (152–154). Melatonin (0.5-3 mg, 1-2 h pre-sleep) aids re-entrainment (155, 156); a 2024 meta-analysis favors 4 mg 3 h pre-bed for optimization, with timing for phase shifts (morning delay, evening advance) (156, 157).
Sleep disturbances in 8 studies tie chronodisruption—through circadian misalignment—to migraine exacerbation, as irregular sleep rhythms heighten trigeminovascular activation, reinforcing our SWMD proposal. Incomplete sleep adjustments may still pose residual confounding in shift work-migraine associations, despite high NOS comparability (mean 9.6/10) indicating robust quality. The 1,001 Nights cohort (131) resolves this by confirming sleep non-mediation, with associations persisting (aPR 1.31) post-rigorous controls for sleep duration, quality, and timing. Chronic circadian misalignment from irregular night shifts in SWMD is hypothesized to disrupt the hypothalamic-trigeminovascular pathway, amplifying neurogenic inflammation and cortical spreading depression susceptibility, independent of sleep disruption—as evidenced by persistent associations post-sleep adjustment in cohort studies (131).
Limitations
The moderate heterogeneity observed in our meta-analysis is scientifically reasonable, given the diversity of the included studies, i.e., different working populations, countries, settings, and time periods. A consistent direction of effect across most studies (as seen in the forest plot, with the majority to the right) supports the observed trend. Future trials should assess objective sleep assessments (158) to refine interventions. A key limitation is the heterogeneity in migraine diagnostic classifications across included studies—from rigorous ICHD-3 criteria or physician validation (n = 5) to unvalidated self-report (n = 9)—which may introduce misclassification bias by over-including non-migraine headaches or under-detecting subclinical cases, potentially yielding conservative pooled ORs. However, sensitivity analyses by validation type (OR 1.64 validated vs. 1.54 self-report, p = 0.83) and specificity (OR 1.40 migraine-specific vs. 2.53 severe headache, p = 0.002) confirm robustness, with the elevated severe headache estimate likely reflecting undiagnosed high-burden migraine cases more susceptible to circadian disruption, further supported by subgroup analyses showing significant shift work associations only for migraine (OR 1.40, 95% CI: 1.04–1.89) versus null for tension-type headache (OR 0.92, 95% CI: 0.71–1.19; p = 0.01 for difference), reinforcing the hypothalamic-trigeminovascular pathway’s selectivity.
Conclusion
Night shift work poses a formidable challenge to the general population, potentially triggering migraine or exacerbating pre-existing ones. Evidence-based scheduling [clockwise rotations: morning–afternoon–night, favoring phase delays for better adaptation and longer rest (99, 100)] and circadian strategies, including sleep/light interventions, could optimize adaptation but require migraine-specific testing. With growing links between circadian health and chronic conditions like migraine, recognizing ‘shift work migraine disorder’ (SWMD) as a clinical entity is timely, warranting further study to enhance health for millions of night shift workers (99, 100).
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AR: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. EH: Data curation, Investigation, Methodology, Writing – review & editing. RC: Data curation, Investigation, Methodology, Writing – review & editing. MF: Data curation, Methodology, Writing – review & editing. EJ: Data curation, Investigation, Methodology, Writing – review & editing. MW: Data curation, Investigation, Methodology, Writing – review & editing. JZ: Conceptualization, Investigation, Methodology, Writing – review & editing. CM: Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National Institute of Neurological Diseases and Stroke, National Institutes of Health, grant number K01NS124911 to YW. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1.
Headache Classification Committee of the International Headache Society (IHS) . The international classification of headache disorders3rd. Cephalalgia (2018) 38: 1–211. doi: 10.1177/0333102417738202,
2.
Burstein R Noseda R Borsook D . Migraine: multiple processes, complex pathophysiology. J Neurosci. (2015) 35:6619–29. doi: 10.1523/JNEUROSCI.0373-15.2015,
3.
Ong JC Taylor HL Park M Burgess HJ Fox RS Snyder S et al . Can circadian dysregulation exacerbate migraines?Headache. (2018) 58:1040–51. doi: 10.1111/head.13310,
4.
Benkli B Kim SY Koike N Han C Tran CK Silva E et al . Circadian features of cluster headache and migraine. Neurology. (2023) 100:e2224–36. doi: 10.1212/WNL.0000000000207240,
5.
Woldeamanuel YW Palesh O Cowan RP . Time it right! The feasibility, Acceptability & Preliminary Efficacy of a circadian-based migraine intervention. Ann Neurol. (2023) 94:S152–2.
6.
Woldeamanuel YW Xia C Ding S Fonteh A Arakaki X (2025). RNA-seq reveals transcriptomic differences in circadian-related genes of the choroid plexus in a preclinical chronic migraine model. bioRxiv.
7.
Gonzalez-Martinez A Ray JC Haghdoost F Ashraf U Cerrahoglu Sirin T Dantes MC et al . Time and headache: insights into timing processes in primary headache disorders for diagnosis, underlying pathophysiology and treatment implications. Cephalalgia. (2024) 44:3331024241297652. doi: 10.1177/03331024241297652,
8.
Yang M-Y Wu C-N Lin Y-T Tsai M-H Hwang C-F Yang C-H . Dissecting the circadian clock and toll-like receptor gene alterations in Meniere’s disease and vestibular migraine. Otolaryngol Head Neck Surg. (2025) 172:999–1005. doi: 10.1002/ohn.1085,
9.
Poulsen AH Younis S Thuraiaiyah J Ashina M . The chronobiology of migraine: a systematic review. J Headache Pain. (2021) 22:76. doi: 10.1186/s10194-021-01276-w,
10.
Shirakawa Y Ohno SN Yamagata KA Kuramoto E Oda Y Nakamura TJ et al . Circadian rhythm of PERIOD2::LUCIFERASE expression in the trigeminal ganglion of mice. Front Neurosci. (2023) 17:1142785. doi: 10.3389/fnins.2023.1142785,
11.
Schulte LH May A . The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. (2016) 139:1987–93. doi: 10.1093/brain/aww097,
12.
Brennan KC Bates EA Shapiro RE Zyuzin J Hallows WC Huang Y et al . Casein kinase iδ mutations in familial migraine and advanced sleep phase. Sci Transl Med. (2013) 5:1–11. doi: 10.1126/scitranslmed.3005784
13.
Imai N . Molecular and cellular neurobiology of circadian and circannual rhythms in migraine: a narrative review. Int J Mol Sci. (2023) 24:10092. doi: 10.3390/ijms241210092,
14.
Bumgarner JR Walker WH Nelson RJ . Circadian rhythms and pain. Neurosci Biobehav Rev. (2021) 129:296–306. doi: 10.1016/j.neubiorev.2021.08.004,
15.
Daguet I Raverot V Bouhassira D Gronfier C . Circadian rhythmicity of pain sensitivity in humans. Brain. (2022) 145:3225–35. doi: 10.1093/brain/awac147,
16.
Potter GDM Skene DJ Arendt J Cade JE Grant PJ Hardie LJ . Circadian rhythm and sleep disruption: causes, metabolic consequences, and countermeasures. Endocr Rev. (2016) 37:584–608. doi: 10.1210/er.2016-1083,
17.
Fishbein AB Knutson KL Zee PC . Circadian disruption and human health. J Clin Invest. (2021) 131:e148286. doi: 10.1172/JCI148286,
18.
Kiehn J-T Faltraco F Palm D Thome J Oster H . Circadian clocks in the regulation of neurotransmitter systems. Pharmacopsychiatry. (2023) 56:108–17. doi: 10.1055/a-1027-7055,
19.
Reinke H Asher G . Crosstalk between metabolism and circadian clocks. Nat Rev Mol Cell Biol. (2019) 20:227–41. doi: 10.1038/s41580-018-0096-9,
20.
Innominato PF Roche VP Palesh OG Ulusakarya A Spiegel D Lévi FA . The circadian timing system in clinical oncology. Ann Med. (2014) 46:191–207. doi: 10.3109/07853890.2014.916990,
21.
Innominato PF Komarzynski S Palesh OG Dallmann R Bjarnason GA Giacchetti S et al . Circadian rest-activity rhythm as an objective biomarker of patient-reported outcomes in patients with advanced cancer. Cancer Med. (2018) 7:4396–405. doi: 10.1002/cam4.1711,
22.
IARC Monographs Vol 124 group . Carcinogenicity of night shift work. Lancet Oncol. (2019) 20:1058–9. doi: 10.1016/S1470-2045(19)30455-3,
23.
Amidi A Wu LM . Circadian disruption and cancer- and treatment-related symptoms. Front Oncol. (2022) 12:1009064. doi: 10.3389/fonc.2022.1009064,
24.
Hadadi E Taylor W Li X-M Aslan Y Villote M Rivière J et al . Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat Commun. (2020) 11:3193. doi: 10.1038/s41467-020-16890-6,
25.
Zhang C Tait C Minacapelli CD Bhurwal A Gupta K Amin R et al . The role of race, sex, and age in circadian disruption and metabolic disorders. Gastro Hep Adv. (2022) 1:471–9. doi: 10.1016/j.gastha.2022.02.015,
26.
Xu Y Su S McCall WV Isales C Snieder H Wang X . Rest-activity circadian rhythm and impaired glucose tolerance in adults: an analysis of NHANES 2011–2014. BMJ Open Diabetes Res Care. (2022) 10:e002632. doi: 10.1136/bmjdrc-2021-002632,
27.
Peng X Fan R Xie L Shi X Dong K Zhang S et al . A growing link between circadian rhythms, type 2 diabetes mellitus and Alzheimer’s disease. Int J Mol Sci. (2022) 23:504. doi: 10.3390/ijms23010504,
28.
Warfield AE Prather JF Todd WD . Systems and circuits linking chronic pain and circadian rhythms. Front Neurosci. (2021) 15:15. doi: 10.3389/fnins.2021.705173,
29.
Chellappa SL Vujovic N Williams JS Scheer FAJL . Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. (2019) 30:767–79. doi: 10.1016/j.tem.2019.07.008,
30.
Hoyt KR Obrietan K . Circadian clocks, cognition, and Alzheimer’s disease: synaptic mechanisms, signaling effectors, and chronotherapeutics. Mol Neurodegener. (2022) 17:35. doi: 10.1186/s13024-022-00537-9,
31.
Musiek ES Bhimasani M Zangrilli MA Morris JC Holtzman DM Ju Y-ES . Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol. (2018) 75:582–90. doi: 10.1001/jamaneurol.2017.4719,
32.
Ahmad F Sachdeva P Sarkar J Izhaar R . Circadian dysfunction and Alzheimer’s disease - an updated review. Aging Med. (2023) 6:71–81. doi: 10.1002/agm2.12221,
33.
Plain language about shiftwork. RosaRoger RudolphColliganMichael: National Institute for Occupational Safety and Health. Department of Health and Human Services. Division of biomedical and Behavioral science; education and information division. Available online at: https://stacks.cdc.gov/view/cdc/5177
34.
International Labour Organization (1990). Convention C171 - night work convention, 1990 (no. 171). Available online at: https://normlex.ilo.org/dyn/nrmlx/en/f?p=NORMLEXPUB:12100:0::NO:12100:P12100_INSTRUMENT_ID:312316:NO (Accessed 16 July 2025).
35.
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Painting, firefighting, and shiftwork. Lyon: International Agency for Research on Cancer (2010). Definition and occurrence of exposure. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK326824/
36.
European Union (2003). Directive 2003/88/EC of the European Parliament and of the council of 4 November 2003 concerning certain aspects of the organisation of working time. Available online at: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003L0088 (Accessed July 16, 2025)
37.
Eurostat (2025). Employed Persons Working at Night by Sex, Age and Professional Status. Brussels: European Commission. Available online at: https://ec.europa.eu/eurostat/databrowser/view/lfsa_ewpnig/default/line?lang=en&category=qoe.qoe_woli.qoe_wta (Accessed July 16, 2025).
38.
Chile Echeverría M . Labour organization and time in Chile. ILO conditions of work and employment programme unpublished report (2002)
39.
PNAD (2016). Síntese de indicadores. Coordenação de Trabalho e Rendimento. Rio de Janeiro, Brazil: Pesquisa Nacional por Amostra de Domicílios, Instituto Brasileiro de Geografia e Estatística (IBGE). [Portuguese].
40.
Australian Bureau of Statistics (2012). Working time arrangements. Australia. November 2012. Canberra: Australian Bureau of Statistics. Available online at: https://www.abs.gov.au.
41.
Zeng X Liang LU Idris SU . Working time in transition: The dual task of standardization and flexibilization in China. Geneva: International Labour Office (2005).
42.
Lee S McCann D Messenger JC . Working time around the world. Trends in working hours, laws and policies in a global comparative perspective. Geneva: International Labour Organization (2007).
43.
Ndiaye A . Étude sur le temps de travail et l’organisation du travail au Sénégal. Conditions of Work and Employment Programme Series No. 13. Geneva: International Labour Office (2006).
44.
Kubo T . Estimate of the number of night shift Workers in Japan. J UOEH. (2014) 36:273–6. doi: 10.7888/juoeh.36.273,
45.
Williams C. (2008). Work-life balance of shift workers. Available online at: https://www150.statcan.gc.ca/n1/en/pub/75-001-x/2008108/pdf/10677-eng.pdf?st=nnrihcKZ (Accessed July 29, 2025).
46.
National Institute for Occupational Safety and Health . Unadjusted prevalence of work organization characteristics (NHIS 2015) among workers (NHIS-OHS). Ctr Dis Control Prev (2019). Available online at: https://wwwn.cdc.gov/NIOSH-WHC/chart/ohs-workorg?T=OU&OU=*&V=R&chk_codes=False (Accessed July 15, 2025)
47.
Stewart WF Roy J Lipton RB . Migraine prevalence, socioeconomic status, and social causation. Neurology. (2013) 81:948–55. doi: 10.1212/WNL.0b013e3182a43b32,
48.
Lipton RB Bigal ME Diamond M Freitag F Reed ML Stewart WF . Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. (2007) 68:343–9. doi: 10.1212/01.wnl.0000252808.97649.21,
49.
Victor T Hu X Campbell J Buse D Lipton R . Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. (2010) 30:1065–72. doi: 10.1177/0333102409355601,
50.
Woldeamanuel YW Cowan RP . Migraine affects 1 in 10 people worldwide featuring recent rise: a systematic review and meta-analysis of community-based studies involving 6 million participants. J Neurol Sci. (2017) 372:307–15. doi: 10.1016/j.jns.2016.11.071,
51.
Bazargan M Comini J Kibe LW Assari S Cobb S . Association between migraine and quality of life, mental health, sleeping disorders, and health care utilization among older African American adults. J Racial Ethn Health Disparities. (2024) 11:1530–40. doi: 10.1007/s40615-023-01629-y,
52.
Charleston L . Headache disparities in African-Americans in the United States: a narrative review. J Natl Med Assoc. (2021) 113:223–9. doi: 10.1016/j.jnma.2020.09.148,
53.
Nicholson RA Rooney M Vo K O’Laughlin E Gordon M . Migraine care among different ethnicities: do disparities exist?Headache. (2006) 46:754–65. doi: 10.1111/j.1526-4610.2006.00453.x,
54.
Heckman BD Holroyd KA O’Donnell FJ et al . Race differences in adherence to headache treatment appointments in persons with headache disorders. J Natl Med Assoc. (2008) 100:247–55. doi: 10.1016/S0027-9684(15)31213-X,
55.
Heckman BD Merrill JC Anderson T . Race, psychiatric comorbidity, and headache characteristics in patients in headache subspecialty treatment clinics. Ethn Health. (2013) 18:34–52. doi: 10.1080/13557858.2012.682219,
56.
Getz M Charleston L Armand CE Willis AW Seng E . Perceived discrimination and migraine-specific quality of life: a cross-sectional survey study in a black/African American sample. Headache. (2025) 2:1–11 doi: 10.1111/head.14970,
57.
U.S. Bureau of Labor Statistics . Job flexibilities and work schedules -- 2017-2018 data from the American time use survey. Washington, DC: U.S. Department of Labor (2019).
58.
Eastman CI Tomaka VA Crowley SJ . Sex and ancestry determine the free-running circadian period. J Sleep Res. (2017) 26:547–50. doi: 10.1111/jsr.12521,
59.
Eastman CI Molina TA Dziepak ME Smith MR . Blacks (African Americans) have shorter free-running circadian periods than whites (Caucasian Americans). Chronobiol Int. (2012) 29:1072–7. doi: 10.3109/07420528.2012.700670,
60.
Eastman CI Suh C Tomaka VA Crowley SJ . Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci Rep. (2015) 5:8381. doi: 10.1038/srep08381,
61.
Heckman BD Britton AJ . Headache in African Americans: an overlooked disparity. J Natl Med Assoc. (2015) 107:39–45. doi: 10.1016/S0027-9684(15)30023-7,
62.
Heckman BD Ellis G . Preventive medication adherence in African American and Caucasian headache patients. Headache. (2011) 51:520–32. doi: 10.1111/j.1526-4610.2011.01866.x,
63.
Åkerstedt T Narusyte J Svedberg P . Night work, mortality, and the link to occupational group and sex. Scand J Work Environ Health. (2020) 46:508–15. doi: 10.5271/sjweh.3892,
64.
Boivin DB Boudreau P . Impacts of shift work on sleep and circadian rhythms. Pathol Biol. (2014) 62:292–301. doi: 10.1016/j.patbio.2014.08.001,
65.
Härmä M Karhula K Puttonen S Ropponen A Koskinen A Ojajärvi A et al . Shift work with and without night work as a risk factor for fatigue and changes in sleep length: a cohort study with linkage to records on daily working hours. J Sleep Res. (2019) 28:e12658. doi: 10.1111/jsr.12658,
66.
Czeisler CA Moore-Ede MC Coleman RH . Rotating shift work schedules that disrupt sleep are improved by applying circadian principles. Science. (1982) 217:460–3. doi: 10.1126/science.7089576,
67.
Schneider D Harknett K . Consequences of routine work-schedule instability for worker health and well-being. Am Sociol Rev. (2019) 84:82–114. doi: 10.1177/0003122418823184,
68.
Vangelova K . The effect of shift rotation on variations of cortisol, fatigue and sleep in sound engineers. Ind Health. (2008) 46:490–3. doi: 10.2486/indhealth.46.490,
69.
Lin P-C Chen C-H Pan S-M Chen YM Pan CH Hung HC et al . The association between rotating shift work and increased occupational stress in nurses. J Occup Health. (2015) 57:307–15. doi: 10.1539/joh.13-0284-OA,
70.
Khan WAA Jackson ML Kennedy GA Conduit R . A field investigation of the relationship between rotating shifts, sleep, mental health and physical activity of Australian paramedics. Sci Rep. (2021) 11:866. doi: 10.1038/s41598-020-79093-5,
71.
Kalmbach DA Pillai V Cheng P Arnedt JT Drake CL . Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity. Sleep Med. (2015) 16:1532–8. doi: 10.1016/j.sleep.2015.09.007,
72.
Zeitzer JM Dijk DJ Kronauer R Brown E Czeisler C . Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. (2000) 526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x,
73.
Wells G. A. Shea B. O’Connell D. Peterson J. Welch V. Losos M. et al . (2000). The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hosp Res Inst. Available online at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed July 24, 2025)
74.
Fekete JT Győrffy B . MetaAnalysisOnline.com: Web-Based Tool for the Rapid Meta-Analysis of Clinical and Epidemiological Studies. J Med Internet Res. (2025) 27:e64016. doi: 10.2196/64016,
75.
Suurmond R van Rhee H Hak T . Introduction, comparison, and validation of Meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods. (2017) 8:537–53. doi: 10.1002/jrsm.1260,
76.
Xie W Li R He M Cui F Sun T Xiong J et al . Prevalence and risk factors associated with headache amongst medical staff in South China. J Headache Pain. (2020) 21:5. doi: 10.1186/s10194-020-1075-z,
77.
Wang Y Xie J Yang F Wu S Wang H Zhang X et al . The prevalence of primary headache disorders and their associated factors among nursing staff in North China. J Headache Pain. (2015) 16:4. doi: 10.1186/1129-2377-16-4,
78.
Tasto DL Colligan MJ Skjei EW Polly SJ . Health consequences of shift work. Report no. PB80176563. National Inst Occup. Saf. Health. (1978) 1–150.
79.
Liu H Liu J Chen M Tan X Zheng T Kang Z et al . Sleep problems of healthcare workers in tertiary hospital and influencing factors identified through a multilevel analysis: a cross-sectional study in China. BMJ Open. (2019) 9:e032239. doi: 10.1136/bmjopen-2019-032239,
80.
Lees RE Romeril CS Wetherall LD . A study of stress indicators in workers exposed to industrial noise. Can J Public Health. (1980) 71:261–5.
81.
Jensen HI Larsen JW Thomsen TD . The impact of shift work on intensive care nurses’ lives outside work: a cross-sectional study. J Clin Nurs. (2018) 27:e703–9. doi: 10.1111/jocn.14197,
82.
Jakobsen GS Timm AM Hansen ÅM Garde AH Nabe-Nielsen K . The association between shift work and treatment-seeking migraine in Denmark. Ergonomics. (2017) 60:1207–17. doi: 10.1080/00140139.2016.1278463,
83.
Chan OY Gan SL Yeo MH . Study on the health of female electronics workers on 12 hour shifts. Occup Med. (1993) 43:143–8. doi: 10.1093/occmed/43.3.143,
84.
Bjorvatn B Pallesen S Moen BE Waage S Kristoffersen ES . Migraine, tension-type headache and medication-overuse headache in a large population of shift working nurses: a cross-sectional study in Norway. BMJ Open. (2018) 8:e022403. doi: 10.1136/bmjopen-2018-022403,
85.
Alturaiki HM Aldawood MA Alghirash F Alhajji AM Almubarak A al Boesa S et al . Headache characteristics and risk factors among healthcare providers in Al-Ahsa, Saudi Arabia. Cureus. (2023) 15:e45377. doi: 10.7759/cureus.45377,
86.
Al Maqwashi LS Sufyani AM Bichara MM Rajikhan YT Albishri M Hamood NA et al . The association between shift work and migraine attacks among healthcare Workers in the Kingdom of Saudi Arabia. Cureus. (2024) 16:e53315. doi: 10.7759/cureus.53315,
87.
Affatato O Miguet M Schiöth HB Mwinyi J . Major sex differences in migraine prevalence among occupational categories: a cross-sectional study using UK biobank. J Headache Pain. (2021) 22:145. doi: 10.1186/s10194-021-01356-x,
88.
Molarius A Tegelberg Å Öhrvik J . Socio-economic factors, lifestyle, and headache disorders — a population-based study in Sweden. Headache: J Head Face Pain. (2008) 48:1426–37. doi: 10.1111/j.1526-4610.2008.01178.x,
89.
Schürks M Buring J Kurth T . Agreement of self-reported migraine with ICHD-II criteria in the women’s health study. Cephalalgia. (2009) 29:1086–90. doi: 10.1111/j.1468-2982.2008.01835.x,
90.
Overeem LH Ulrich M Fitzek MP Lange KS Hong JB Reuter U et al . Consistency between headache diagnoses and ICHD-3 criteria across different levels of care. J Headache Pain. (2025) 26:6. doi: 10.1186/s10194-024-01937-6,
91.
Pilcher JJ Lambert BJ Huffcutt AI . Differential effects of permanent and rotating shifts on self-report sleep length: a meta-analytic review. Sleep. (2000) 23:155–63. doi: 10.1093/sleep/23.2.1b,
92.
Eldevik MF Flo E Moen BE Pallesen S Bjorvatn B . Insomnia, excessive sleepiness, excessive fatigue, anxiety, depression and shift work disorder in nurses having less than 11 hours in-between shifts. PLoS One. (2013) 8:e70882. doi: 10.1371/journal.pone.0070882,
93.
James L Elkins-Brown N Wilson M James SM Dotson E Edwards CD et al . The effects of three consecutive 12-hour shifts on cognition, sleepiness, and domains of nursing performance in day and night shift nurses: a quasi-experimental study. Int J Nurs Stud. (2021) 123:104041. doi: 10.1016/j.ijnurstu.2021.104041,
94.
Sim J Yun B-Y Lee J Kim SK Lee S Cho A et al . The association between the number of consecutive night shifts and insomnia among shift workers: a multi-Center study. Front Public Health. (2021) 9:9. doi: 10.3389/fpubh.2021.761279,
95.
Boivin DB Boudreau P Kosmadopoulos A . Disturbance of the circadian system in shift work and its health impact. J Biol Rhythm. (2022) 37:3–28. doi: 10.1177/07487304211064218,
96.
Daguet I Bouhassira D Demarquay G Gronfier C . Visual discomfort and pain in migraine: homeostatic or circadian origin? (2019). Available online at: https://theses.hal.science/tel-04977136v1/file/TH2019DAGUETINES.pdf (Accessed October 10, 2025).
97.
Strother L Ptacek L Goadsby P Holland P . Environmental and genetic circadian disruption increases migraine-associated phenotypes in mice (2700). Neurology. (2020) 94:2700. doi: 10.1212/WNL.94.15_supplement.2700
98.
Monk TH Buysse DJ Billy BD DeGrazia JM . Using nine 2-h delays to achieve a 6-h advance disrupts sleep, alertness, and circadian rhythm. Aviat Space Environ Med. (2004) 75:1049–57.
99.
Lavie P Tzischinsky O Epstein R Zomer J . Sleep-wake cycle in shift workers on a “clockwise” and “counter-clockwise” rotation system. Isr J Med Sci. (1992) 28:636–44.
100.
Shiffer D Minonzio M Dipaola F Bertola M Zamuner AR Dalla Vecchia LA et al . Effects of clockwise and counterclockwise job shift work rotation on sleep and work-life balance on hospital nurses. Int J Environ Res Public Health. (2018) 15:2038. doi: 10.3390/ijerph15092038,
101.
Neil-Sztramko SE Pahwa M Demers PA Gotay CC . Health-related interventions among night shift workers: a critical review of the literature. Scand J Work Environ Health. (2014) 40:543–56. doi: 10.5271/sjweh.3445,
102.
Vetter C Fischer D Matera JL Roenneberg T . Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr Biol. (2015) 25:907–11. doi: 10.1016/j.cub.2015.01.064,
103.
Woldeamanuel YW Sanjanwala BM Cowan RP . Endogenous glucocorticoids may serve as biomarkers for migraine chronification. Ther Adv Chronic Dis. (2020) 11:204062232093979. doi: 10.1177/2040622320939793,
104.
Mackus M Kraneveld A Garssen J Verster J . 1161 migraine and sleep: a bi-directional association. Sleep. (2017) 40:A433–3. doi: 10.1093/sleepj/zsx050.1160
105.
Lin Y-K Lin G-Y Lee J-T Lee MS Tsai CK Hsu YW et al . Associations between sleep quality and migraine frequency: a cross-sectional case-control study. Medicine. (2016) 95:e3554. doi: 10.1097/MD.0000000000003554,
106.
Kelman L Rains JC . Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. (2005) 45:904–10. doi: 10.1111/j.1526-4610.2005.05159.x,
107.
Akerstedt T Wright KP . Sleep loss and fatigue in shift work and shift work disorder. Sleep Med Clin. (2009) 4:257–71. doi: 10.1016/j.jsmc.2009.03.001,
108.
Katsifaraki M Nilsen KB Christensen JO Wærsted M Knardahl S Bjorvatn B et al . Sleep duration mediates abdominal and lower-extremity pain after night work in nurses. Int Arch Occup Environ Health. (2019) 92:415–22. doi: 10.1007/s00420-018-1373-9,
109.
Crowley SJ Eastman CI . Free-running circadian period in adolescents and adults. J Sleep Res. (2018) 27:e12678. doi: 10.1111/jsr.12678,
110.
Crowley SJ Acebo C Carskadon MA . Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. (2007) 8:602–12. doi: 10.1016/j.sleep.2006.12.002,
111.
Duffy JF Cain SW Chang A-M Phillips AJK Münch MY Gronfier C et al . Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci. (2011) 108:15602–8. doi: 10.1073/pnas.1010666108,
112.
Chau YM West S Mapedzahama V . Night work and the reproductive health of women: an integrated literature review. J Midwifery Women’s Health. (2014) 59:113–26. doi: 10.1111/jmwh.12052,
113.
Mingardi J Giovenzana M Nicosia N Misztak P Ieraci A Musazzi L . Sex and circadian rhythm dependent Behavioral effects of chronic stress in mice and modulation of clock genes in the prefrontal cortex. Int J Mol Sci. (2025) 26:6410. doi: 10.3390/ijms26136410,
114.
Verma P Hellemans KGC Choi FY Yu W Weinberg J . Circadian phase and sex effects on depressive/anxiety-like behaviors and HPA axis responses to acute stress. Physiol Behav. (2010) 99:276–85. doi: 10.1016/j.physbeh.2009.11.002,
115.
DePasquale N Sliwinski MJ Zarit SH Buxton OM Almeida DM . Unpaid caregiving roles and sleep among women working in nursing homes: a longitudinal study. Gerontologist. (2019) 59:474–85. doi: 10.1093/geront/gnx185,
116.
Korompeli A Chara T Chrysoula L Sourtzi P . Sleep disturbance in nursing personnel working shifts. Nurs Forum. (2013) 48:45–53. doi: 10.1111/nuf.12005,
117.
Ashina M Hansen JM Do TP Melo-Carrillo A Burstein R Moskowitz MA . Migraine and the trigeminovascular system—40 years and counting. Lancet Neurol. (2019) 18:795–804. doi: 10.1016/S1474-4422(19)30185-1,
118.
Chen Q-W Meng R-T Ko C-Y . Modulating oxidative stress and neurogenic inflammation: the role of topiramate in migraine treatment. Front Aging Neurosci. (2024) 16:1455858. doi: 10.3389/fnagi.2024.1455858,
119.
Borkum JM . Migraine triggers and oxidative stress: a narrative review and synthesis. Headache: J Head Face Pain. (2016) 56:12–35. doi: 10.1111/head.12725,
120.
Han C Lim JY Koike N Kim SY Ono K Tran CK et al . Regulation of headache response and transcriptomic network by the trigeminal ganglion clock. Headache. (2024) 64:195–210. doi: 10.1111/head.14670,
121.
Fernández-de-Las-Peñas C Fernández-Muñoz JJ Palacios-Ceña M Parás-Bravo P Cigarán-Méndez M Navarro-Pardo E . Sleep disturbances in tension-type headache and migraine. Ther Adv Neurol Disord. (2018) 11:1756285617745444. doi: 10.1177/1756285617745444,
122.
Hamamcı M Dumanlıdağ S . Sleep disorders accompanying migraine and tension headaches. J Turk Sleep Med. (2020) 7:57–64. doi: 10.4274/jtsm.galenos.2020.10820
123.
Sachdeva A Goldstein C . Shift Work Sleep Disorder In: Circadian rhythm sleep-wake disorders. Cham: Springer International Publishing (2020). 149–82. doi: 10.1007/978-3-030-43803-6_11
124.
Woldeamanuel YW Cowan RP . The impact of regular lifestyle behavior in migraine: a prevalence case–referent study. J Neurol. (2016) 263:669–76. doi: 10.1007/s00415-016-8031-5,
125.
Avidan A Colwell C . Jet lag syndrome: circadian organization, pathophysiology, and management strategies. Nat Sci Sleep. (2010) 2:187–98. doi: 10.2147/NSS.S6683,
126.
Wei T Li C Heng Y Gao X Zhang G Wang H et al . Association between night-shift work and level of melatonin: systematic review and meta-analysis. Sleep Med. (2020) 75:502–9. doi: 10.1016/j.sleep.2020.09.018,
127.
Hunter CM Figueiro MG . Measuring light at night and melatonin levels in shift workers: a review of the literature. Biol Res Nurs. (2017) 19:365–74. doi: 10.1177/1099800417714069,
128.
Lowden A Åkerstedt T Wibom R . Suppression of sleepiness and melatonin by bright light exposure during breaks in night work. J Sleep Res. (2004) 13:37–43. doi: 10.1046/j.1365-2869.2003.00381.x,
129.
Chu M Cho S Joo E Kim J . Social jetlag is prevalent among headache sufferers: a population-based study. Headache. (2015) 55:171.
130.
Axsome Therapeutics, Inc. (2025). Studying solriamfetol modulation of TAAR-1, dopamine, and norepinephrine in shift work disorder (SUSTAIN) (NCT06568367). Available online at: https://clinicaltrials.gov/study/NCT06568367 (Accessed: July 29, 2025)
131.
Harmsen R Hansen JM Matre D Arup AESF Garde AH Nabe-Nielsen K . The acute effect of night work-related circadian misalignment on headache episodes: results from the 1001 nights-cohort. Headache. (2025) 65:1554–64. doi: 10.1111/head.15054,
132.
Jeon BM Kim SH Shin SH . Effectiveness of sleep interventions for rotating night shift workers: a systematic review and meta-analysis. Front Public Health. (2023) 11:11. doi: 10.3389/fpubh.2023.1187382,
133.
Nie J Zhou T Chen Z Dang W Jiao F Zhan J et al . The effects of dynamic daylight-like light on the rhythm, cognition, and mood of irregular shift workers in closed environment. Sci Rep. (2021) 11:13059. doi: 10.1038/s41598-021-92438-y,
134.
Czeisler CA Johnson MP Duffy JF Brown EN Ronda JM Kronauer RE . Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. (1990) 322:1253–9. doi: 10.1056/NEJM199005033221801,
135.
Aarts MPJ Hartmeyer SL Morsink K Kort HSM de Kort YAW . Can special light glasses reduce sleepiness and improve sleep of nightshift workers? A placebo-controlled explorative field study. Clocks Sleep. (2020) 2:225–45. doi: 10.3390/clockssleep2020018,
136.
Sletten TL Ftouni S Nicholas CL Magee M Grunstein RR Ferguson S et al . Randomised controlled trial of the efficacy of a blue-enriched light intervention to improve alertness and performance in night shift workers. Occup Environ Med. (2017) 74:792–801. doi: 10.1136/oemed-2016-103818,
137.
Guyett A Lovato N Manners J Stuart N Toson B Lechat B et al . A circadian-informed lighting intervention accelerates circadian adjustment to a night work schedule in a submarine lighting environment. Sleep. (2024) 47:zsae146. doi: 10.1093/sleep/zsae146,
138.
Buxton OM Lee CW L’Hermite-Balériaux M Turek FW Van Cauter E . Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. Am J Phys Regul Integr Comp Phys. (2003) 284:R714–24. doi: 10.1152/ajpregu.00355.2002,
139.
Youngstedt SD Elliott JA Kripke DF . Human circadian phase-response curves for exercise. J Physiol. (2019) 597:2253–68. doi: 10.1113/JP276943,
140.
Youngstedt SD Kline CE Elliott JA Zielinski MR Devlin TM Moore TA . Circadian phase-shifting effects of bright light, exercise, and bright light + exercise. J Circadian Rhythms. (2016) 14:2. doi: 10.5334/jcr.137,
141.
Gupta CC Dorrian J Grant CL Pajcin M Coates AM Kennaway DJ et al . It’s not just what you eat but when: the impact of eating a meal during simulated shift work on driving performance. Chronobiol Int. (2017) 34:66–77. doi: 10.1080/07420528.2016.1237520,
142.
Gupta CC Centofanti S Dorrian J Coates A Stepien JM Kennaway D et al . Altering meal timing to improve cognitive performance during simulated nightshifts. Chronobiol Int. (2019) 36:1691–713. doi: 10.1080/07420528.2019.1676256,
143.
Gupta CC Centofanti S Dorrian J Coates AM Stepien JM Kennaway D et al . The impact of a meal, snack, or not eating during the night shift on simulated driving performance post-shift. Scand J Work Environ Health. (2021) 47:78–84. doi: 10.5271/sjweh.3934,
144.
Manoogian ENC Zadourian A Lo HC Gutierrez NR Shoghi A Rosander A et al . Feasibility of time-restricted eating and impacts on cardiometabolic health in 24-h shift workers: the healthy heroes randomized control trial. Cell Metab. (2022) 34:1442–1456.e7. doi: 10.1016/j.cmet.2022.08.018,
145.
Kim JH Song Y . The effects of indoor ambient temperature at work on physiological adaptation in night shift nurses. J Nurs Manag. (2020) 28:1098–103. doi: 10.1111/jonm.13052,
146.
Aschoff J Fatranska M Giedke H Doerr P Stamm D Wisser H . Human circadian rhythms in continuous darkness: entrainment by social cues. Science. (1971) 171:213–5. doi: 10.1126/science.171.3967.213,
147.
Easton DF Gupta CC Vincent GE Ferguson SA . Move the night way: how can physical activity facilitate adaptation to shift work?Commun Biol. (2024) 7:259. doi: 10.1038/s42003-024-05962-8,
148.
Elmore SK Betrus PA Burr R . Light, social zeitgebers, and the sleep–wake cycle in the entrainment of human circadian rhythms. Res Nurs Health. (1994) 17:471–8. doi: 10.1002/nur.4770170610,
149.
Mistlberger RE Skene DJ . Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev. (2004) 79:533–56. doi: 10.1017/S1464793103006353,
150.
Mistlberger RE Skene DJ . Nonphotic entrainment in humans?J Biol Rhythm. (2005) 20:339–52. doi: 10.1177/0748730405277982,
151.
Carrier J Fernandez-Bolanos M Robillard R Dumont M Paquet J Selmaoui B et al . Effects of caffeine are more marked on daytime recovery sleep than on nocturnal sleep. Neuropsychopharmacology. (2007) 32:964–72. doi: 10.1038/sj.npp.1301198,
152.
Walsh JK Muehlbach MJ Humm TM Stokes Dickins Q Sugerman JL Schweitzer PK . Effect of caffeine on physiological sleep tendency and ability to sustain wakefulness at night. Psychopharmacology. (1990) 101:271–3. doi: 10.1007/BF02244139,
153.
McHill AW Smith BJ Wright KP . Effects of caffeine on skin and Core temperatures, alertness, and recovery sleep during circadian misalignment. J Biol Rhythm. (2014) 29:131–43. doi: 10.1177/0748730414523078,
154.
Institute of Medicine (US) Committee on Military Nutrition Research In: MarriottBM, editor. Food components to enhance performance: An evaluation of potential performance-enhancing food components for operational rations. Washington, DC: National Academies Press (US) (1994)
155.
Hannemann J Laing A Middleton B Schwedhelm E Marx N Federici M et al . Effect of oral melatonin treatment on insulin resistance and diurnal blood pressure variability in night shift workers. A double-blind, randomized, placebo-controlled study. Pharmacol Res. (2024) 199:107011. doi: 10.1016/j.phrs.2023.107011,
156.
Carriedo-Diez B Tosoratto-Venturi JL Cantón-Manzano C Wanden-Berghe C Sanz-Valero J . The effects of the exogenous melatonin on shift work sleep disorder in health personnel: a systematic review. Int J Environ Res Public Health. (2022) 19:10199. doi: 10.3390/ijerph191610199,
157.
Cruz-Sanabria F Bruno S Crippa A Frumento P Scarselli M Skene DJ et al . Optimizing the time and dose of melatonin as a sleep-promoting drug: a systematic review of randomized controlled trials and dose−response Meta-analysis. J Pineal Res. (2024) 76:e12985. doi: 10.1111/jpi.12985,
158.
Błaszczyk B Martynowicz H Niemiec P Przegrałek J Staszkiewicz M Wojakowska A et al . Sleep bruxism and obstructive sleep Apnea are not risk factors for tension-type headache (TTH): a polysomnographic study. J Clin Med. (2024) 13:3835. doi: 10.3390/jcm13133835,
159.
Wittmann M Dinich J Merrow M Roenneberg T . Social jetlag: misalignment of biological and social time. Chronobiol Int. (2006) 23:497–509. doi: 10.1080/07420520500545979,
160.
Caliandro R Streng AA van Kerkhof LWM van der Horst GTJ Chaves I . Social jetlag and related risks for human health: a timely review. Nutrients. (2021) 13:4543. doi: 10.3390/nu13124543,
161.
Li Y Lv X Li R Wang Y Guan X Li L et al . Predictors of shift work sleep disorder among nurses during the COVID-19 pandemic: a Multicenter cross-sectional study. Front Public Health. (2021) 9:9. doi: 10.3389/fpubh.2021.785518,
162.
Pallesen S Bjorvatn B Waage S Harris A Sagoe D . Prevalence of shift work disorder: a systematic review and meta-analysis. Front Psychol. (2021) 12:12. doi: 10.3389/fpsyg.2021.638252
163.
Drake CL Roehrs T Richardson G Walsh JK Roth T . Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. (2004) 27:1453–62. doi: 10.1093/sleep/27.8.1453,
164.
Eastman CI Burgess HJ . How to travel the world without jet lag. Sleep Med Clin. (2009) 4:241–55. doi: 10.1016/j.jsmc.2009.02.006,
165.
Drake CL Wright KP . Chapter 71 - shift work, shift-work disorder, and jet lag In: KrygerMHRothTDementWC, editors. Principles and practice of sleep medicine. 5th ed. Philadelphia, PA: W.B. Saunders (2011). 784–98. doi: 10.1016/B978-1-4160-6645-3.00071-2
Summary
Keywords
migraine, circadian, shiftwork, meta-analysis, systematic review, headache, chronobiology
Citation
Woldeamanuel YW, Rahman A, Hyimanot ET, Chirravuri R, Fani M, Javaheri ED, Welch M, Zhuang J and Mun CJ (2025) Disrupting the clock: meta-analysis of irregular night shifts and migraine, proposing shift work migraine disorder with chronobiology strategies. Front. Neurol. 16:1684169. doi: 10.3389/fneur.2025.1684169
Received
12 August 2025
Revised
29 October 2025
Accepted
07 November 2025
Published
09 December 2025
Volume
16 - 2025
Edited by
Christofer Lundqvist, University of Oslo, Norway
Reviewed by
Marta Waliszewska-Prosół, Wroclaw Medical University, Poland
Sulthan Al Rashid, Saveetha Medical College & Hospital, India
Harald Hrubos-Strøm, Akershus University Hospital, Norway
Updates
Copyright
© 2025 Woldeamanuel, Rahman, Hyimanot, Chirravuri, Fani, Javaheri, Welch, Zhuang and Mun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yohannes W. Woldeamanuel, Woldeamanuel.YohannesWoubishet@mayo.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.