- 1Department of Neurosurgery, Jiangsu Province Hospital and the First Affiliated Hospital with Nanjing Medical University, Nanjing, Jiangsu, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, China
- 3Department of Neurosurgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
Background: Flow-related aneurysms (FAs) are more common and have an increased risk of rupture in posterior circulation arteriovenous malformations (pAVMs). However, the risk factors for FA rupture in pAVMs are understudied and warrant further investigation.
Objective: This study aimed to investigate the risk factors for FA rupture associated with pAVMs using a multi-centric database from 1 January 2020 to 31 December 2024.
Methods: Patients diagnosed with AVMs were selected from databases. Patients with ruptured FAs and those with unruptured FAs were compared. Independent Student’s t-test, Mann–Whitney U-test, Fisher’s exact test, and multivariable binary logistic regression were used for analysis.
Results: Among 284 patients, 37 patients with 22 ruptured FAs and 28 with unruptured FAs met the inclusion criteria. The results indicated that ruptured FAs were associated with smaller pAVMs (2.41 ± 1.02 cm vs. 3.93 ± 2.68 cm, p = 0.001) and Spetzler–Martin grades I to II (p = 0.013). Patients with ruptured FAs had greater aneurysm size [median size, 4.55 mm (IQR, 3.20–7.55 mm) vs. 3.00 mm (IQR, 2.40–5.00 mm); p = 0.009], larger size ratio [median, 2.72 (IQR, 1.95–4.12) vs. 2.23 (1.10–2.87); p = 0.021], and larger relative size ratio [median, 0.24 (IQR, 0.137–0.358) vs. 0.096 (IQR, 0.063–0.129); p < 0.001] than those with unruptured FAs. The multivariable binary logistic regression analysis indicated that only the AVM size [OR, 0.92 (95% CI, 0.84–0.99); p = 0.039] and the size ratio [OR, 1.66 (95% CI, 1.07–2.59); p = 0.024] remained associated with the risk of rupture.
Conclusion: The size of pAVMs and size ratio of FAs to average diameter of the parent vessel are independent risk factors for FA rupture associated with pAVMs.
Introduction
Intracranial aneurysms associated with brain arteriovenous malformations (AVMs) can be classified into three types: unrelated aneurysms (type I), flow-related aneurysms (type II), and intranidal aneurysms (type III) (1). Aneurysm rupture constitutes the primary cause of hemorrhage in AVMs with associated aneurysms, accounting for approximately 49.2% of all cases (2). Flow-related aneurysms (FAs), with an overall prevalence of 11.2—25%, significantly increase the hemorrhagic risk, particularly when located in the posterior circulation (1–5).
Multiple risk factors for intracranial aneurysm rupture have been established in previous studies, including hypertension, smoking, and morphological features such as irregular shape, aspect ratio ≥1.3, and elevated size ratio (6–10). Older age, smaller AVM size, and cerebellar location have been identified as risk factors for FA rupture in a retrospective study involving 69 FA cases (11). In contrast, another study of 101 FAs found that hypertension and high-grade AVMs (Spetzler–Martin grades III–V) were associated with bleeding risk in univariate analysis, with only hypertension remaining significant in multivariable analysis (12).
Despite this existing evidence, risk factors specifically for FA rupture remain poorly defined; however, understanding these factors are critical for informed clinical decision-making. In this study, we aim to identify the predictors of rupture in posterior circulation AVMs (pAVMs) with associated FAs, analyzing demographic, angioarchitectural, and morphological characteristics.
Methods
Patient population
This is a retrospective, observational study approved by the institutional review committee (2024-SR-777) and registered on ClinicalTrials.gov (NCT07050381). The study included patients diagnosed with pAVMs and associated FAs by digital subtraction angiography (DSA) at Jiangsu Province Hospital, The First Affiliated Hospital of Soochow University, and Southwest Hospital from 1 January 2020 to 31 December 2024. Patients were excluded from the study if they lacked clinical or DSA data or had a dissecting aneurysm.
Variable definition
Demographic and clinical information included age, sex, hypertension, and diabetes history. The initial diagnosis of FA rupture was conducted when a non-contrast CT scan at admission demonstrated isolated subarachnoid hemorrhage (SAH) without concurrent intracerebral hemorrhage (ICH) (Figure 1). For cases with a mixed pattern of hemorrhage on CT, the diagnosis was based on a comprehensive assessment based on two key aspects: the anatomical correlation between the hemorrhage location and the lesion and the characteristics of the AVMs and FAs observed on intraoperative 3D-DSA reconstruction. The radiographic variables of AVMs included their size, location, Spetzler–Martin grade, and the presence of deep venous drainage. FA size, location, aspect ratio (AR, aneurysm depth to aneurysm neck (13)), size ratio (SR, aneurysm maximum diameter to average parent vessel diameter (10)), and relative size ratio (RSR, aneurysm maximum diameter to AVM maximum diameter) were collected to assess the characteristics of FAs. Angioarchitectural characteristics were recorded using 3D-DSA. Additionally, the therapeutic approach and treatment sequence were recorded. Modified Rankin scale (mRS) was used to evaluate the functional status at presentation and follow-up.
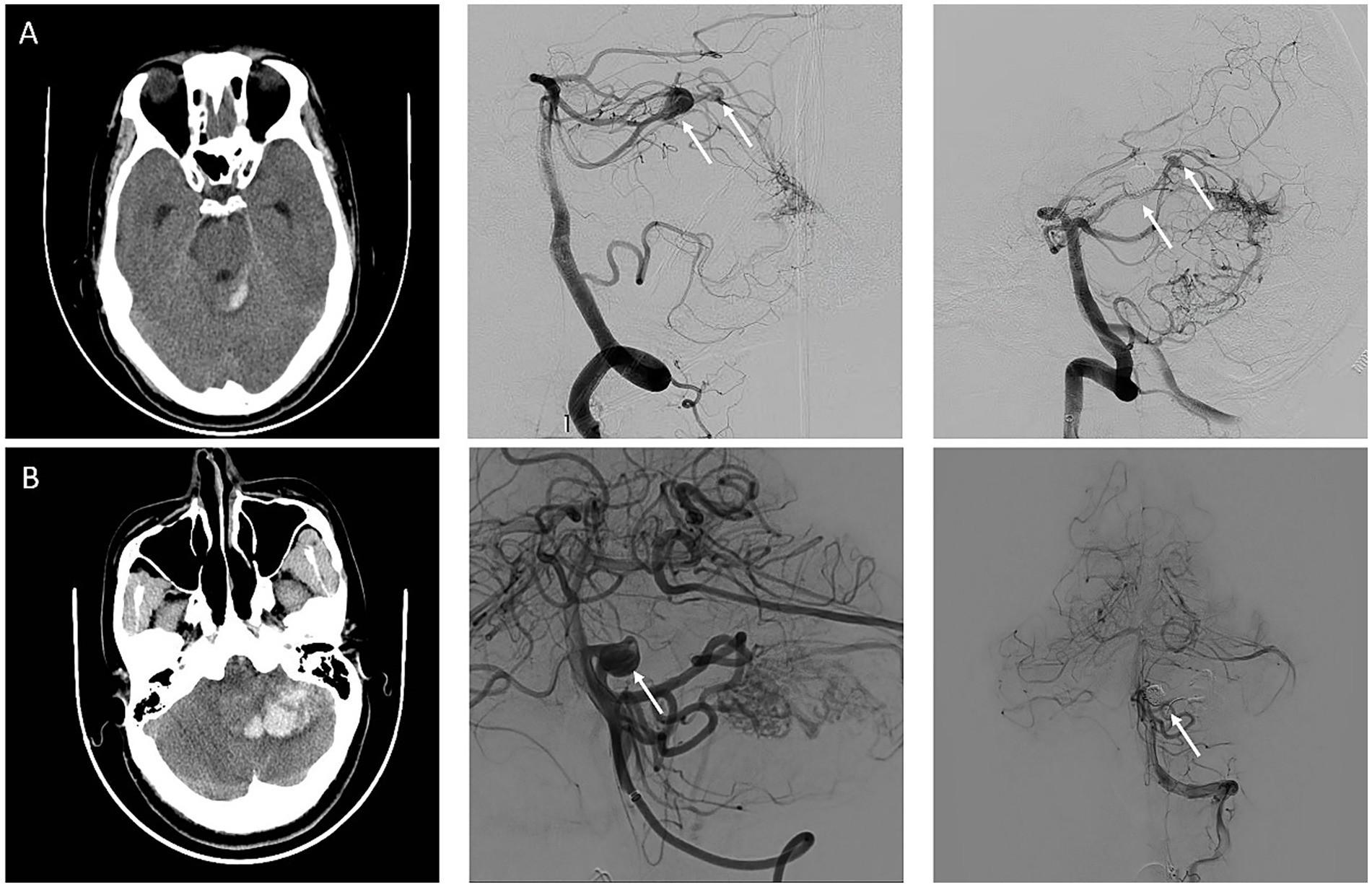
Figure 1. Representative cases of ruptured flow-related aneurysm and unruptured flow-related aneurysm. (A) An adult patient with a sudden headache was diagnosed with subarachnoid hemorrhage on a computed tomography (CT). Arteriovenous malformation fed by the left superior cerebellar artery and two flow-related aneurysms (white arrow) were detected on digital subtraction angiography. The larger FAs with a maximum diameter of 10 mm were the reason for hemorrhage and was treated with coils for the first time. (B) An adult patient diagnosed with intracerebral hemorrhage due to immediate headache and imbalance by CT. AVMs fed by the left anterior inferior cerebellar artery and posterior inferior cerebellar artery and FAs (white arrow) located in the anterior inferior cerebellar artery were detected in DSA. FAs and AVMs were treated with coils and onyx at the same time.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD) or median (interquartile range, IQR) based on distribution normality. Categorical variables are shown in numbers (percentages). Independent Student’s t-test and Mann–Whitney U-test were used for analyzing normally and non-normally distributed variables, respectively. Fisher’s exact test was used to analyze categorical variables. Multicollinearity was assessed via variance inflation factors (VIFs) (14). Variables with VIF ≥10 were excluded from the multivariable model. Multivariable binary logistic regression analysis was performed to assess the correlation of multiple factors influencing FA rupture. A two-sided p-value of <0.05 is considered statistically significant. All data were analyzed using SPSS (version 26.0; IBM).
Results
Baseline demographic and clinical information
A total of 299 patients diagnosed with AVMs were selected from the database. Of these, 284 patients had complete DSA and clinical data, while 115 (40.5%) patients were diagnosed with pAVMs. Moreover, 76 patients did not have FAs, and 2 had dissecting aneurysms. Ultimately, 37 (32.7%) patients with associated FAs were included for analysis, and 22 (59.4%) patients with hemorrhagic presentations experienced these due to ruptured FAs (Figure 2). Baseline demographics are shown in Table 1. FAs are more likely seen in men (78.4%), and the mean age was 47.35 ± 15.62 years. No significant differences were found in terms of age (49.18 ± 14.04 vs. 44.67 ± 17.85, p = 0.396), sex (p = 0.690), hypertension (36.4% vs. 33.3%, p > 0.999), or diabetes (9.1% vs. 13.3%, p > 0.999) between patients with pAVMs who had ruptured versus unruptured FAs.

Table 1. Demographic and clinical information of patients with ruptured and unruptured flow-related aneurysms.
Characteristics of pAVMs and FAs
Characteristics of pAVMs and FAs are shown in Table 2. The average pAVM nidus size in maximum diameter was 2.41 ± 1.02 cm and 3.93 ± 2.68 cm for ruptured FAs and unruptured FAs, respectively (p = 0.001). The AVMs with ruptured FAs tend to have a lower Spetzler–Martin grade (grade 1 and 2) with a significant difference (p = 0.013). The eloquence location (18.2% vs. 40%, p = 0.258), deep venous drainage (45.5% vs. 46.7%, p > 0.999), and AVM location (p = 0.334) were found to be similar between pAVMs with ruptured FAs and unruptured FAs.
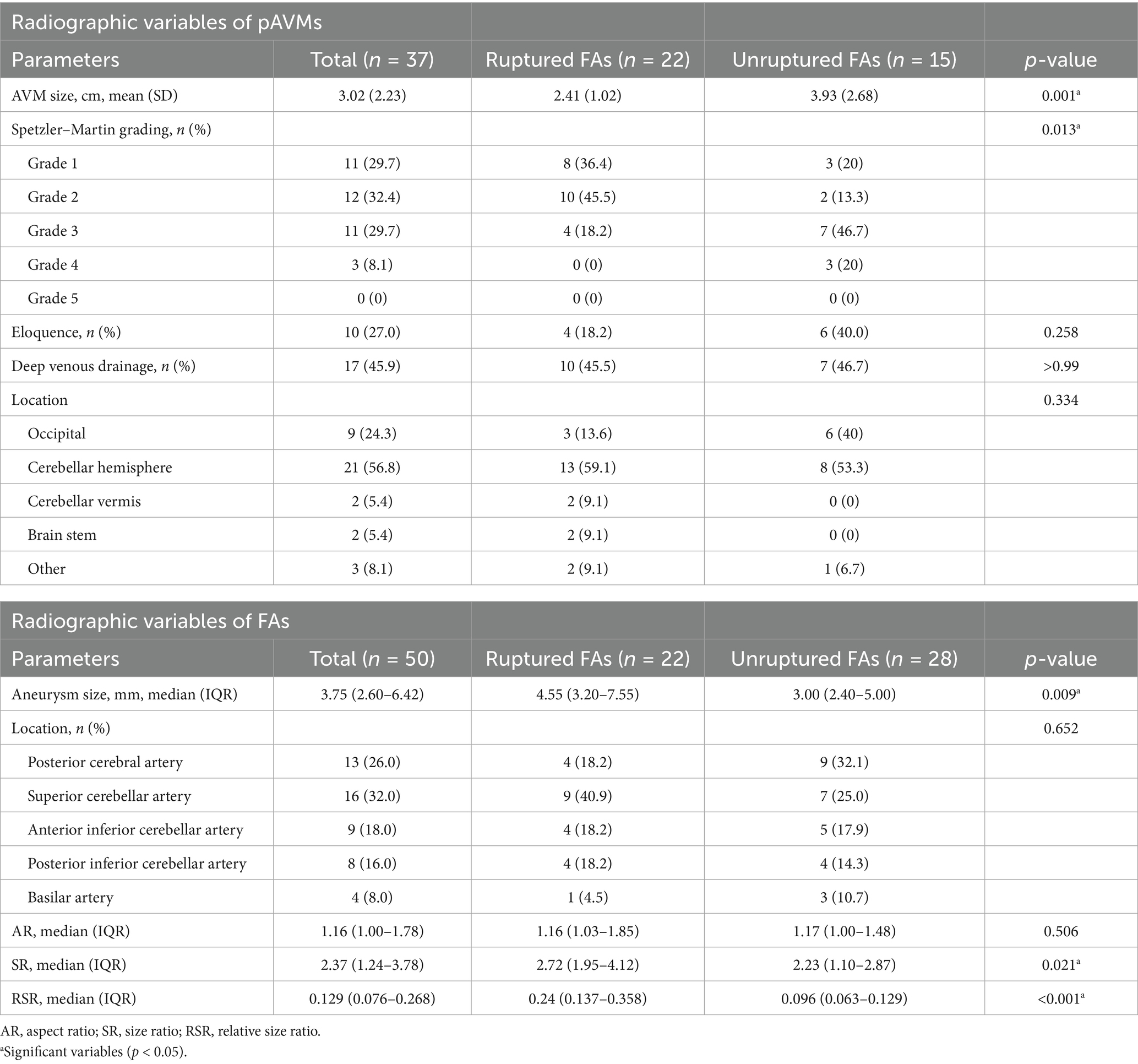
Table 2. Radiographic variables of posterior circulation arteriovenous malformations and flow-related aneurysms.
A total of 50 FAs were detected in 37 patients. Larger aneurysm size was correlated with FA rupture [median size, 4.55 mm (IQR, 3.20–7.55 mm) vs. 3.00 mm (IQR, 2.40–5.00 mm); p = 0.009]. The ruptured group had a bigger SR than the unruptured group [median, 2.72 (IQR, 1.95–4.12) vs. 2.23 (1.10–2.87); p = 0.021]. A larger RSR was also associated with FA rupture [median, 0.24 (IQR, 0.137–0.358) vs. 0.096 (IQR, 0.063–0.129); p < 0.001]. The most common location of FAs was the superior cerebellar artery (32%), while the least common location was the basilar artery branches (8%). The distribution of aneurysm locations was similar between these two groups. The aspect ratio did not show a significant difference [median, 1.16 (IQR, 1.03–1.85) vs. 1.17 (IQR, 1.00–1.48); p = 0.506].
Multivariable analysis
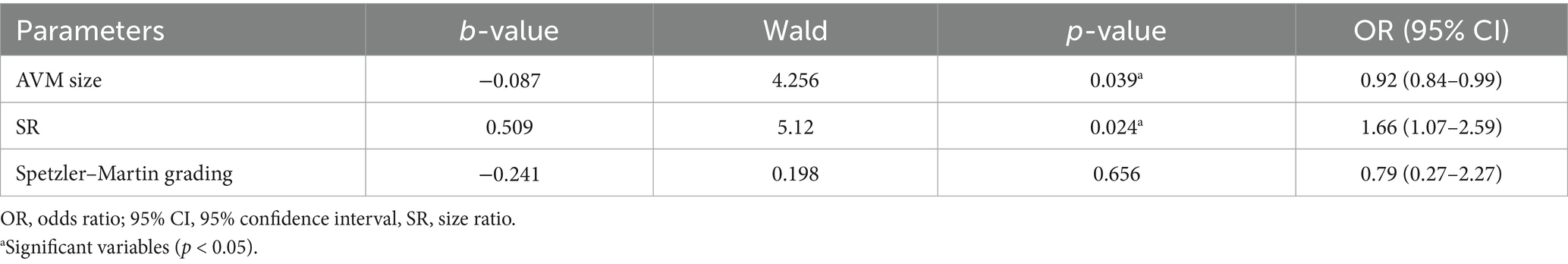
In univariable analysis, five variables, namely AVM size, Spetzler–Martin grade, aneurysm size, SR, and RSR, that demonstrated potential significance were considered for inclusion in the multivariable logistic regression model. To avoid substantial collinearity between these variables, collinearity analysis was conducted (Supplementary Table 1). Variables such as aneurysm size (VIF = 32.76) and RSR (VIF = 33.23) were removed from subsequent multivariable analysis due to concerns about multicollinearity. AVM nidus size [OR, 0.92 (95% CI, 0.84–0.99); p = 0.039] and SR [OR, 1.66 (95% CI, 1.07–2.59); p = 0.024] remain significant risk factors for FA rupture (Table 3).
Treatment and outcome
Out of 37 patients, 27 (64.9%) received treatment for concurrent lesions during the initial therapy, while the remaining were treated for the ruptured lesion first. The average follow-up duration was 27.97 ± 17.03 months. Both treatment strategies yielded similar clinical outcomes. One patient had a hemorrhage, and another patient had an acute ischemic stroke immediately after AVM embolization, both which were highly related to the surgery. No novel hemorrhagic or infarct foci were found during follow-up (Table 4).
Discussion
A comparative analysis of demographic and clinical information and angioarchitectural and morphological characteristics in pAVMs with associated ruptured or unruptured FAs was conducted in this study. The overall incidence of FAs in pAVMs in this study was 32.7% (37/113), which is modestly higher than the previously reported range of 11.2–25% (4, 15). No significant difference was observed in the mean age at presentation between the groups. Sex, hypertension, and diabetes were not associated with FA rupture. AVM nidus size [OR, 0.92 (95% CI, 0.84–0.99); p = 0.039] and SR [OR, 1.66 (95% CI, 1.07–2.59); p = 0.024] are independent risk factors for FA rupture in multivariable logistic regression. Although the Spetzler–Martin grade of AVMs, aneurysm size, and RSR did not retain statistical significance in the multivariable regression analysis, numerically significant differences were observed between the groups. Further studies are warranted to elucidate their pathophysiological roles in FA rupture.
FAs are more common in pAVMs than those located in the anterior circulation (11). Contrary to the associated venous aneurysm, which is considered a protective factor against AVM rupture, concurrence of FAs increases the risk of hemorrhage, and FA rupture is the main reason for hemorrhage, approximately accounting for 49.2% of cases (2, 16). AVMs located in the cerebellar vermis and hemispheres are more likely to accompany FAs (62.2%). Previous studies reported that FAs in smaller AVMs tend to rupture; this conclusion aligns with our study (11, 17). Smaller AVMs appear to have elevated blood pressure in the feeding artery, which may be the reason for FA rupture (16). Although Zhang et al. (16) demonstrated a significant correlation between smaller AVMs and hemorrhagic incidents, the ruptured AVM, which is contained in the unruptured FA group, is larger in our study. This discrepancy may be attributed to two factors: first, the previous studies included AVMs supplied by both anterior and posterior circulations; second, there was no significant difference in the distribution of intranidal or flow-related aneurysms between the ruptured and unruptured AVM groups, which led to their exclusion from model construction. Soldozy et al. (18) demonstrated that ruptured aneurysms tended to have higher wall shear stress (WSS) and wall shear stress gradient associated with lower oscillatory shear index, which represented faster, impinging flow. In coronary artery diseases, several parameters, such as mean time average peak velocity and mean diastolic/systolic velocity ratio, are used to evaluate pre- and post-procedural variations using Doppler guidewires (19). In contrast, the field of cerebrovascular disease predominantly relies on transcranial Doppler ultrasound, with very few reports on the use of intravascular Doppler ultrasound; therefore, further investigation is needed to explore the links between intravascular pressure changes, blood flow velocity parameters, and hemodynamic parameters in the cerebrovascular system.
Hypertension, smoking, alcohol consumption, and being female have been reported as risk factors for intracranial aneurysm rupture (8, 20). However, there were no significant differences in our study. Several predictors of intracranial aneurysm rupture, including AR, SR, bifurcation angle, and WSS, have been demonstrated in previous studies (10, 13, 21). We found that ruptured FAs had a larger SR and aneurysm size compared to unruptured FAs [median, 2.72 (IQR, 1.95–4.12) vs. 2.23 (1.10–2.87); median size, 4.55 mm (IQR, 3.20–7.55 mm) vs. 3.00 mm (IQR, 2.40–5.00 mm), respectively]. However, the AR did not differ significantly [median, 1.16 (IQR, 1.03–1.85) vs. 1.17 (IQR, 1.00–1.48)]. Aneurysm wall inflammation is positively related to morphological characteristics. Specifically, intracranial aneurysms with larger size and size ratio exhibit increased expression of CD68 and NFKB1, which reflect acute inflammation and the activation of inflammatory pathways, respectively. Elevated levels of inflammation may lead to aneurysm wall remodeling, which is associated with rupture (22).
It is generally agreed that the ruptured lesion should be prioritized for treatment in cases involving hemorrhagic complications (2). However, the timing for treating a concurrent lesion is still debated. Redekop et al. (1) and Andereggen et al. (23) reported spontaneous regression of FAs following the treatment of associated AVMs. Świątnicki et al. (24), meanwhile, reported the growth of FAs after AVM occlusion. In our study, the majority of patients were treated for concurrent lesions at initial therapy, while others were treated for the ruptured lesion first. Only two patients experienced surgery-related hemorrhagic or ischemic events. The outcomes of both strategies during follow-up were similar. Considering the risk factors related to FA rupture, FAs in smaller AVMs or with a larger SR should be treated early.
There exist several limitations in this retrospective study. One of the limitations of this study is that only 37 patients were ultimately included in the analysis, which is insufficient to accurately elucidate the relationship between disease characteristics and aneurysms. Subsequent studies should include cases from multiple centers to validate the findings presented here and to enhance the generalizability of the results. In addition, the retrospective nature of this article makes it challenging to draw unequivocal conclusions regarding the rupture risk. Previous reports demonstrated that hemodynamic characteristics are also associated with aneurysm status. In this study, we focus on demographic, angioarchitectural, and morphological characteristics, and further research is needed to clarify the relationship between FA rupture and hemodynamic characteristics.
Conclusion
Risk factors for FAs associated with pAVMs remain largely unknown. In this study, we focus on the demographic, angioarchitectural, and morphological characteristics of pAVMs and FAs. Our findings indicate that a smaller AVM size in maximum diameter and a larger size ratio are independent risk factors for FA rupture.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Jiangsu Province Hospital Ethics Committee (Approval Number: 2024-SR-777). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin due to the retrospective nature of the study.
Author contributions
WY: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. LG: Formal Analysis, Writing – review & editing. LChe: Validation, Visualization, Writing – review & editing. CS: Validation, Visualization, Writing – review & editing. CL: Validation, Visualization, Writing – review & editing. CH: Methodology, Software, Writing – review & editing. LH: Data curation, Writing – review & editing. LW: Data curation, Writing – review & editing. LZ: Methodology, Supervision, Writing – review & editing. LCha: Methodology, Supervision, Writing – review & editing. XX: Methodology, Supervision, Writing – review & editing. CZ: Resources, Writing – review & editing, Supervision. LW: Resources, Writing – review & editing, Supervision. LH: Resources, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. ChatGPT 4.0 was used to check and correct spelling and grammar.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1685261/full#supplementary-material
Abbreviations
AVM, Arteriovenous malformation; AR, Aspect ratio; DSA, Digital subtraction angiography; FA, Flow-related aneurysm; ICH, Intracerebral hemorrhage; IQR, Interquartile range; mRS, Modified Rankin Scale; pAVM, Posterior circulation arteriovenous malformation; RSR, Relative size ratio; SD, Standard deviation; SR, Size ratio; VIF, Variance inflation factor.
References
1. Redekop, G, TerBrugge, K, Montanera, W, and Willinsky, R. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. (1998) 89:539–46. doi: 10.3171/jns.1998.89.4.0539
2. Cagnazzo, F, Brinjikji, W, and Lanzino, G. Arterial aneurysms associated with arteriovenous malformations of the brain: classification, incidence, risk of hemorrhage, and treatment—a systematic review. Acta Neurochir. (2016) 158:2095–104. doi: 10.1007/s00701-016-2957-3
3. Lv, X, Wu, Z, Jiang, C, Yang, X, Li, Y, Sun, Y, et al. Angioarchitectural characteristics of brain arteriovenous malformations with and without hemorrhage. World Neurosurg. (2011) 76:95–9. doi: 10.1016/j.wneu.2011.01.044
4. Stein, K-P, Wanke, I, Forsting, M, Zhu, Y, Moldovan, AS, Dammann, P, et al. Associated aneurysms in supratentorial arteriovenous malformations: impact of aneurysm size on haemorrhage. Cerebrovasc Dis. (2015) 39:122–9. doi: 10.1159/000369958
5. Orning, J, Amin-Hanjani, S, Hamade, YJ, Du, X, Hage, ZA, Aletich, V, et al. Increased prevalence and rupture status of feeder vessel aneurysms in posterior fossa arteriovenous malformations. J Neurointerv Surg. (2016) 8:1021–4. doi: 10.1136/neurintsurg-2015-012005
6. Backes, D, Vergouwen, MDI, Velthuis, BK, van der Schaaf, IC, Bor, ASE, Algra, A, et al. Difference in aneurysm characteristics between ruptured and unruptured aneurysms in patients with multiple intracranial aneurysms. Stroke. (2014) 45:1299–303. doi: 10.1161/STROKEAHA.113.004421
7. Juvela, S, and Korja, M. Intracranial aneurysm parameters for predicting a future subarachnoid hemorrhage: a long-term follow-up study. Neurosurgery. (2017) 81:432–40. doi: 10.1093/neuros/nyw049
8. Kleinloog, R, de Mul, N, Verweij, BH, Post, JA, Rinkel, GJE, and Ruigrok, YM. Risk factors for intracranial aneurysm rupture: a systematic review. Neurosurgery. (2018) 82:431–40. doi: 10.1093/neuros/nyx238
9. Han, P, Jin, D, Wei, W, Song, C, Leng, X, Liu, L, et al. The prognostic effects of hemodynamic parameters on rupture of intracranial aneurysm: a systematic review and meta-analysis. Int J Surg. (2021) 86:15–23. doi: 10.1016/j.ijsu.2020.12.012
10. Rahman, M, Smietana, J, Hauck, E, Hoh, B, Hopkins, N, Siddiqui, A, et al. Size ratio correlates with intracranial aneurysm rupture status: a prospective study. Stroke. (2010) 41:916–20. doi: 10.1161/STROKEAHA.109.574244
11. Hung, AL, Yang, W, Jiang, B, Garzon-Muvdi, T, Caplan, JM, Colby, GP, et al. The effect of flow-related aneurysms on hemorrhagic risk of intracranial arteriovenous malformations. Neurosurgery. (2019) 85:466–75. doi: 10.1093/neuros/nyy360
12. Lasica, N, Gull, HH, Sure, U, Vulekovic, P, Djilvesi, D, Andjelic, D, et al. Risk factors for bleeding in patients with arteriovenous malformations associated with intracranial aneurysms. Neurosurg Rev. (2025) 48:313. doi: 10.1007/s10143-025-03468-3
13. Ujiie, H, Tamano, Y, Sasaki, K, and Hori, T. Is the aspect ratio a reliable index for predicting the rupture of a saccular aneurysm? Neurosurgery. (2001) 48:495–502. doi: 10.1097/00006123-200103000-00007
14. Yoo, W, Mayberry, R, Bae, S, Singh, K, Peter He, Q, and Lillard, JW. A study of effects of multicollinearity in the multivariable analysis. Int J Appl Sci Technol. (2014) 4:9–19.
15. Stapf, C, Mohr, JP, Pile-Spellman, J, Sciacca, RR, Hartmann, A, Schumacher, HC, et al. Concurrent arterial aneurysms in brain arteriovenous malformations with haemorrhagic presentation. J Neurol Neurosurg Psychiatry. (2002) 73:294–8. doi: 10.1136/jnnp.73.3.294
16. Zhang, Y, Zhu, H, Cao, T, Zhang, L, Chang, Y, Liang, S, et al. Rupture-related features of cerebral arteriovenous malformations and their utility in predicting hemorrhage. Stroke. (2024) 55:1339–48. doi: 10.1161/STROKEAHA.123.045456
17. Abou-Mrad, T, McGuire, LS, Theiss, P, Hossa, J, Bram, R, Pearce, C, et al. Feeder artery aneurysms in cerebral arteriovenous malformations: demographic, clinical, and morphological associations. Neurosurgery. (2025). doi: 10.1227/neu.0000000000003568
18. Soldozy, S, Norat, P, Elsarrag, M, Chatrath, A, Costello, JS, Sokolowski, JD, et al. The biophysical role of hemodynamics in the pathogenesis of cerebral aneurysm formation and rupture. Neurosurg Focus. (2019) 47:E11. doi: 10.3171/2019.4.FOCUS19232
19. Segal, J, Kern, MJ, Scott, NA, King, SB, Doucette, JW, Heuser, RR, et al. Alterations of phasic coronary artery flow velocity in humans during percutaneous coronary angioplasty. J Am Coll Cardiol. (1992) 20:276–86. doi: 10.1016/0735-1097(92)90091-z
20. Vlak, MH, Algra, A, Brandenburg, R, and Rinkel, GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. (2011) 10:626–36. doi: 10.1016/S1474-4422(11)70109-0
21. Rashad, S, Sugiyama, S, Niizuma, K, Sato, K, Endo, H, Omodaka, S, et al. Impact of bifurcation angle and inflow coefficient on the rupture risk of bifurcation type basilar artery tip aneurysms. J Neurosurg. (2017) 128:723–30. doi: 10.3171/2016.10.JNS161695
22. Liu, Q, Zhang, Y, Yang, J, Yang, Y, Li, M, Chen, S, et al. The relationship of morphological-hemodynamic characteristics, inflammation, and remodeling of aneurysm wall in unruptured intracranial aneurysms. Transl Stroke Res. (2022) 13:88–99. doi: 10.1007/s12975-021-00917-1
23. Andereggen, L, Gruber, P, Anon, J, Tortora, A, Steiger, H-J, Schubert, GA, et al. Spontaneous regression of multiple flow-related aneurysms following treatment of an associated brain arteriovenous malformation: a case report. Front Surg. (2022) 9:860416. doi: 10.3389/fsurg.2022.860416
Keywords: intracranial aneurysm, arteriovenous malformation, posterior circulation, subarachnoid hemorrhage, flow-related aneurysm
Citation: Yifei W, Guangxi L, Chenyuan L, Shu C, Leocardia CR, Hanxiao C, Han L, Wenyan L, Zheng L, Chao L, Xiupeng X, Zhi C, Wen L and Hua L (2025) Risk factors for flow-related aneurysm rupture associated with posterior circulation arteriovenous malformation: a multicenter retrospective study. Front. Neurol. 16:1685261. doi: 10.3389/fneur.2025.1685261
Edited by:
Lanfranco Pellesi, University of Southern Denmark, DenmarkReviewed by:
Nebojsa Lasica, University of Novi Sad, SerbiaJulio César López-Valdés, National Autonomous University of Mexico, Mexico
Copyright © 2025 Yifei, Guangxi, Chenyuan, Shu, Leocardia, Hanxiao, Han, Wenyan, Zheng, Chao, Xiupeng, Zhi, Wen and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Zhi, emhpY2hlbkB0bW11LmVkdS5jbg==; Li Wen, bGl3ZW5ndWFueXVuQDE2My5jb20=; Lu Hua, bHVodWFAbmptdS5lZHUuY24=
 Wang Yifei
Wang Yifei Li Guangxi1
Li Guangxi1 Chekera Ropafadzo Leocardia
Chekera Ropafadzo Leocardia Chang Hanxiao
Chang Hanxiao Li Wenyan
Li Wenyan Lin Chao
Lin Chao Xu Xiupeng
Xu Xiupeng Chen Zhi
Chen Zhi Li Wen
Li Wen Lu Hua
Lu Hua