- 1Department of Rehabilitation Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
- 2Research Institute of Rehabilitation Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
- 3Department of Speech-Language Pathology, Wonkwang Digital University, Seoul, Republic of Korea
- 4Research Institute for Future Medicine, Samsung Medical Center, Seoul, Republic of Korea
Background: Pneumonia is a serious complication of stroke, particularly in patients with dysphagia during inpatient rehabilitation, as it significantly increases morbidity, prolongs hospital stays, and impairs functional recovery. Early identification of patients at risk for pneumonia is crucial for improving outcomes and reducing post-stroke complications. This study aimed to develop a comprehensive algorithm for predicting post-stroke pneumonia risk by integrating clinical assessments of defense mechanisms against pneumonia.
Methods: This case-control study enrolled stroke patients at a single tertiary hospital and followed them for 4 weeks to assess pneumonia incidence. A total of 812 patients aged 20 years or older with ischemic or hemorrhagic stroke and signs of dysphagia were screened. Of these, 484 were excluded based on the following criteria: inability to maintain a sitting posture with back support, dyspnea requiring oxygen supplementation, concurrent aspiration pneumonia before enrollment, infectious diseases requiring isolation, and refusal to participate. Final cohort of 328 patients was enrolled. All participants underwent evaluations, including a videofluoroscopic swallowing study (VFSS), a modified cough reflex test (mCRT), and assessments of nutritional status (serum albumin) and cognitive function [Mini-Mental State Examination (MMSE)]. Pneumonia was diagnosed using the Mann criteria, and predictive factors were analyzed using univariate logistic regression and classification and regression tree (CART) analysis.
Results: Among 328 participants, 28 (8.5%) developed pneumonia. Significant predictors included tracheostomy status (OR 9.34), VFSS-confirmed aspiration (OR 8.21) and bilateral stroke lesions (OR 5.91). CART analysis revealed tracheostomy, VFSS-confirmed aspiration, cough frequency, albumin levels, and MMSE scores as key predictors. The algorithm demonstrated a predictive accuracy of 92.7% with an AUC of 0.89 (95% CI: 0.82–0.95).
Conclusion: This study developed a highly accurate predictive algorithm for post-stroke pneumonia, emphasizing the role of defense mechanisms against pneumonia. Implementing this algorithm in clinical practice could enable early preventive measures, reduce pneumonia incidence, and improve patient outcomes.
1 Introduction
Pneumonia is a serious complication following stroke, with an estimated incidence of 5%–30% (1, 2). Subsequent treatments for pneumonia significantly prolong hospital stays, increase medical costs, hinder functional recovery, and lead to poor outcomes (3–5). Therefore, early identification of patients at risk is essential not only for preventing post-stroke pneumonia but also for optimizing recovery.
Several risk scoring tools such as the A2DS2 score (6), acute ischemic stroke-associated pneumonia score (AIS-APS) (7), and independence pre-stroke, sex, age, National Institutes of Health Stroke Scale (ISAN) score (8) use clinical factors such as age, sex, stroke severity, and comorbidities to predict pneumonia risk. However, these tools primarily focus on pneumonia in the acute stage of stroke and do not directly assess the body’s defense mechanisms against infection. Instead, they rely on simplified combinations of demographic factors, stroke severity, and medical history, despite the fact that pneumonia develops when pathogens reach the alveoli and overwhelm host defenses due to the microorganism’s virulence or inoculum` size.
Understanding the complex defense mechanisms against pneumonia is crucial for accurately predicting post-stroke pneumonia risk. Emerging evidence suggests that multiple neuroanatomical (9) and physiological defense mechanisms–such as airway clearance (10), swallowing function (11), cognitive processing (12), and immunonutrition (13)–play critical roles in pneumonia prevention. These defense mechanisms function in an integrated manner rather than independently, yet their interconnections remain poorly understood. Therefore, we hypothesize that integrating standardized assessment tools that evaluate each level of the neurophysiologic defense system against pneumonia will improve the prediction of post-stroke pneumonia compared to existing models.
2 Materials and methods
2.1 Study participants and data collection
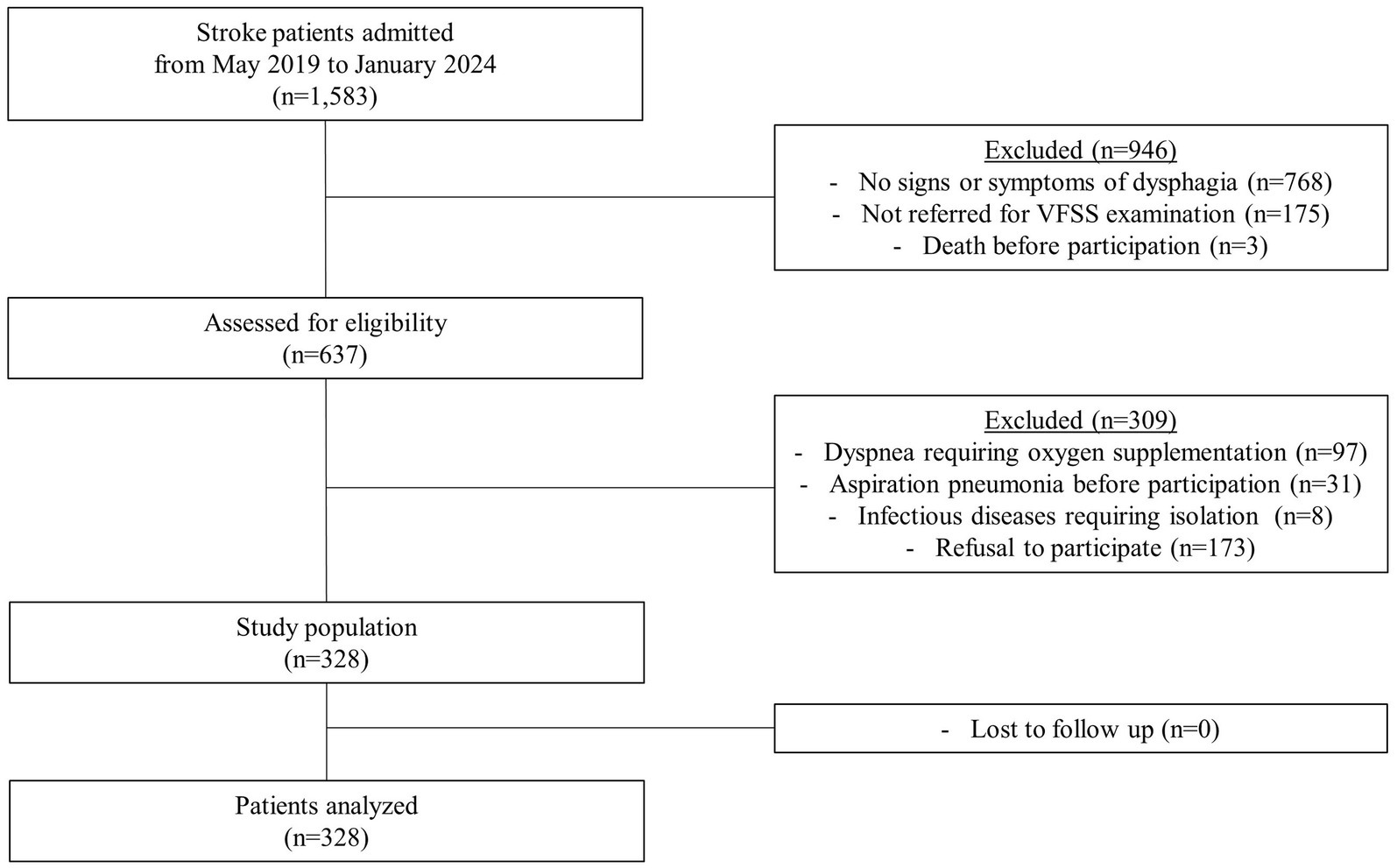
In this prospective case-control study, stroke patients who were admitted to a single tertiary hospital in Korea from 2019 to 2024 were screened based on the following inclusion criteria: (1) either ischemic or hemorrhagic stroke confirmed by computed tomography or magnetic resonance imaging, (2) age 20 years or older, and (3) presence of signs or symptoms of dysphagia, and (4) referred for videofluoroscopic swallowing study (VFSS). The exclusion criteria were as follows: (1) dyspnea requiring oxygen supplementation, (2) a prior diagnosis of aspiration pneumonia before enrollment, (3) a diagnosis of an infectious disease requiring isolation, and (4) refusal to participate. A total of 328 patients were enrolled, with no loss to follow-up (Figure 1).
The required sample size was determined using Power Analysis and Sample Size Software [PASS (version 12, NCSS, Kaysville, Utah, USA)], based on an estimation of two proportions. To assess the utility of the evaluation tools through regression analysis, with a significance level of 0.05, a power of 0.8, and accounting for a 10% dropout rate, the target sample size was calculated as 328.
All 328 patients underwent VFSS and modified cough reflex test (mCRT). Demographic information and baseline characteristics were collected at admission. Neurological and laboratory assessments, including the Mini-Mental State Examination (MMSE), serum albumin levels, white blood cell (WBC) count, total neutrophil count, neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein (CRP) level, were conducted within 24 h of admission. Written informed consent was obtained from all participants or their legally authorized representatives and the study was performed on accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board of our hospital (IRB No. 4-2019-0111).
2.2 Clinical assessment of dysphagia
Patients were assessed for dysphagia by certified rehabilitation specialists and trained speech-language pathologists. As documented in previous studies (14–16), dysphagia was defined by the presence of any of the following: impaired sensory or motor function of oral structures (jaw, lips, tongue, palate, or cheeks), cranial nerve dysfunction (trigeminal, facial, glossopharyngeal, vagus, or hypoglossal nerves), and voice changes (wet or gurgly voice) after swallowing, drooling, or apraxia.
2.3 Definition of pneumonia
Patients were monitored for 4 weeks following baseline assessments, and pneumonia was diagnosed based on the Mann criteria (17). A blinded physician confirmed the diagnosis if three or more of the following criteria were met: fever (>38 °C), productive cough with purulent sputum, abnormal respiratory findings (tachypnea >22/min, tachycardia, inspiratory crackles, or bronchial breathing), abnormal chest radiographic findings, arterial hypoxemia (PO₂ < 70 mmHg or SpO₂ < 94%), or identification of a relevant pathogen (positive Gram stain or culture).
Based on previous studies, pneumonia risk was classified into three categories. High risk was defined as a pneumonia incidence exceeding 25% (8, 18). No risk was assigned to incidences below 2%, a threshold lower than the lowest reported post-stroke pneumonia incidence (2.3%) in previous studies (18, 19). Incidences between 2% and 25% were categorized as low risk.
2.4 Videofluoroscopic swallowing study
VFSS, the gold standard for evaluating swallowing function and visualizing oral-pharyngeal anatomy, was used to detect aspiration (5). A real-time fluoroscopic video was recorded with patients seated upright in a chin-tuck position. A barium-impregnated bolus was prepared in both plain liquid and semisolid yogurt mixed with a liquid thickener. Boluses were administered in a fixed order, starting with semisolid followed by liquid, with each consistency tested at volumes of 5 and 15 mL. The test was discontinued if aspiration was observed or if the patient was unable to tolerate the procedure. All 328 participants completed the VFSS protocol.
Penetration or aspiration was assessed using the 8-point Penetration-Aspiration Scale (PAS) (20), which evaluates the depth of airway invasion and whether the material is expelled. A PAS score greater than 5 was considered indicative of aspiration, as supported by previous studies (21). VFSS recordings were reviewed and PAS scores were provided by rehabilitation specialists blinded to the patients’ clinical data.
2.5 Modified cough reflex test
A Sidestream nebulizer (Philips Respironics, Parsippany, NJ, USA) was modified to include a peak flow meter via a T-piece. Capsaicin (Sigma-Aldrich Korea, Seoul, Korea) was dissolved in polyoxyethylene sorbitan and ethanol, then diluted with normal saline to concentrations of 7.8 μM and 31.25 μM. Patients inhaled 7.8 μM capsaicin mist through the nebulizer for 15 s. For those with a tracheostomy tube, the mist was administered directly through the tracheostomy site. The total number of coughs and the peak cough flow (PCF) were recorded within 30 s after inhalation. The procedure was then repeated using the 31.25 μM capsaicin solution. Four of 328 participants (1.2%) were unable to complete the mCRT due to poor cooperation.
2.6 Statistical analysis
Numerical variables are presented as mean with standard deviation and categorical variables as count with percentage. The Mann–Whitney test was used to compare continuous variables, and the chi-square test was applied to compare categorical variables between the pneumonia and non-pneumonia groups. Univariate logistic regression was conducted to identify potential risk factors for pneumonia. Variables with p < 0.01 were included in the classification and regression tree (CART) analysis. CART analysis was performed using the “gini” index, with a maximum tree depth of 3 and complexity parameter of 0.001. Minimum split size and bucket size were set at 4 and 2, respectively. Ten-fold cross-validation was repeated twice to evaluate performance, and the area under the receiver operating characteristic curve (AUC) and overall accuracy were calculated. For analyses involving mCRT, four missing data were excluded. A p-value of less than 0.05 was considered statistically significant. All analyses were conducted using R statistical software (version 4.2.3, R Project for Statistical Computing, Vienna, Austria) and SPSS (version 25.0, IBM, Chicago, Illinois, USA).
3 Results
3.1 Patient characteristics
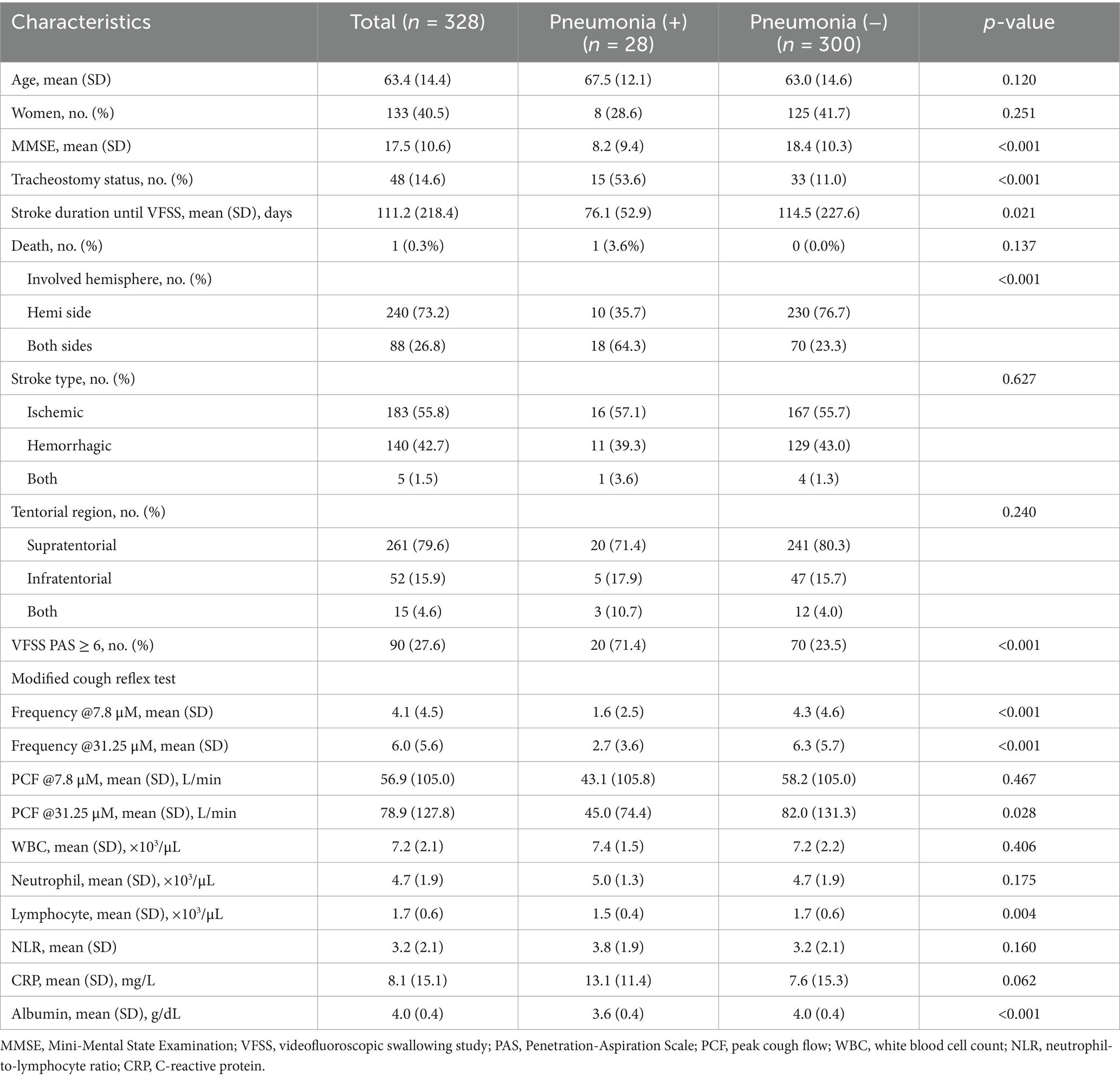
Among the 328 participants, 28 (8.5%) were diagnosed with pneumonia within 4 weeks (Table 1). The age of the participants was 63.4(14.4) years, and 40.5% were female. The pneumonia group had a significantly higher proportion of patients with tracheostomy (53.6% vs. 11.0%), aspiration on VFSS (71.4% vs. 23.5%), and stroke lesions affecting both brain hemispheres (64.3% vs. 23.3%) than the non-pneumonia group (p < 0.01). In mCRT, the pneumonia group exhibited significantly lower cough frequency at both 7.8 μM [1.6 (2.5) vs. 4.3 (4.6)] and 31.25 μM [2.7 (3.6) vs. 6.3 (5.7)], as well as lower PCF at 31.25 μM [45.0 (74.4) L/min vs. 82.0 (131.3) L/min] (p < 0.01). The pneumonia group also had significantly lower albumin levels [3.6 (0.4) g/dL vs. 4.0 (0.4) g/dL], MMSE scores [8.2 (9.4) vs. 18.4 (10.3)], and total lymphocyte counts [1.5 (0.4) × 103/μL vs. 1.7 (0.6) × 103/μL] (p < 0.01). However, no significant differences were observed between the two groups in stroke types, lesion locations, or biomarkers such as WBC, neutrophil counts, NLR, and CRP.
3.2 Clinical predictors of post-stroke pneumonia
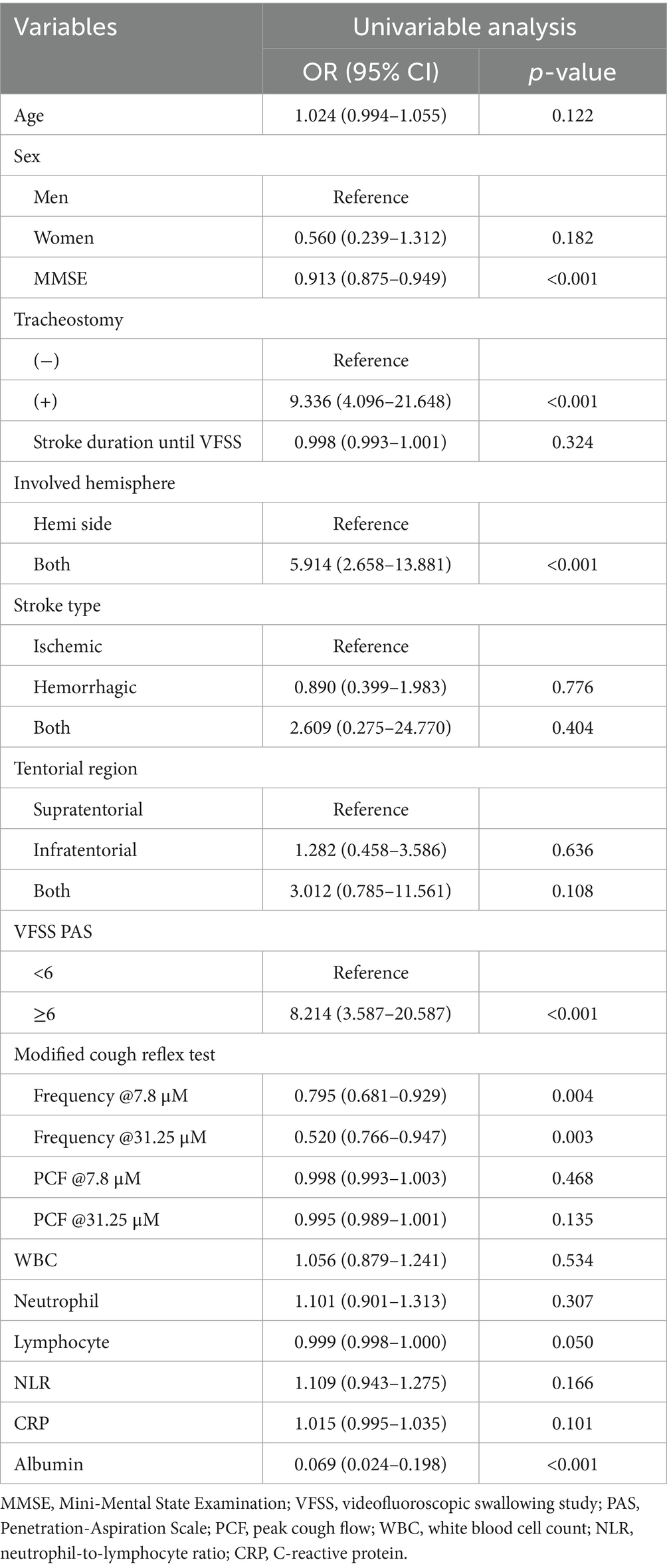
Univariate logistic regression analysis identified several significant predictors of post-stroke pneumonia (Table 2, p < 0.01). Tracheostomy status had the highest odds ratio (OR) at 9.34 (95% CI: 4.10–21.65), followed by aspiration on VFSS (OR: 8.21) and bilateral stroke involvement (OR: 5.91). MMSE scores, cough frequency at both 7.8 μM and 31.25 μM mCRT, and albumin levels also showed statistically significant associations. However, age and sex were not significantly associated with post-stroke pneumonia, and PCF at both 7.8 μM and 31.25 μM mCRT did not demonstrate significant ORs.
3.3 Construction of decision tree with classification and regression tree analysis
Significant clinical factors associated with post-stroke pneumonia in the logistic regression analysis were included in the CART analysis. Tracheostomy status, aspiration on VFSS, cough frequency at 7.8 μM mCRT, albumin levels, and MMSE scores were identified as significant predictors of post-stroke pneumonia (Figure 2). However, cough frequency at 31.25 μM mCRT and bilateral hemispheric lesions were not utilized in constructing the decision tree.
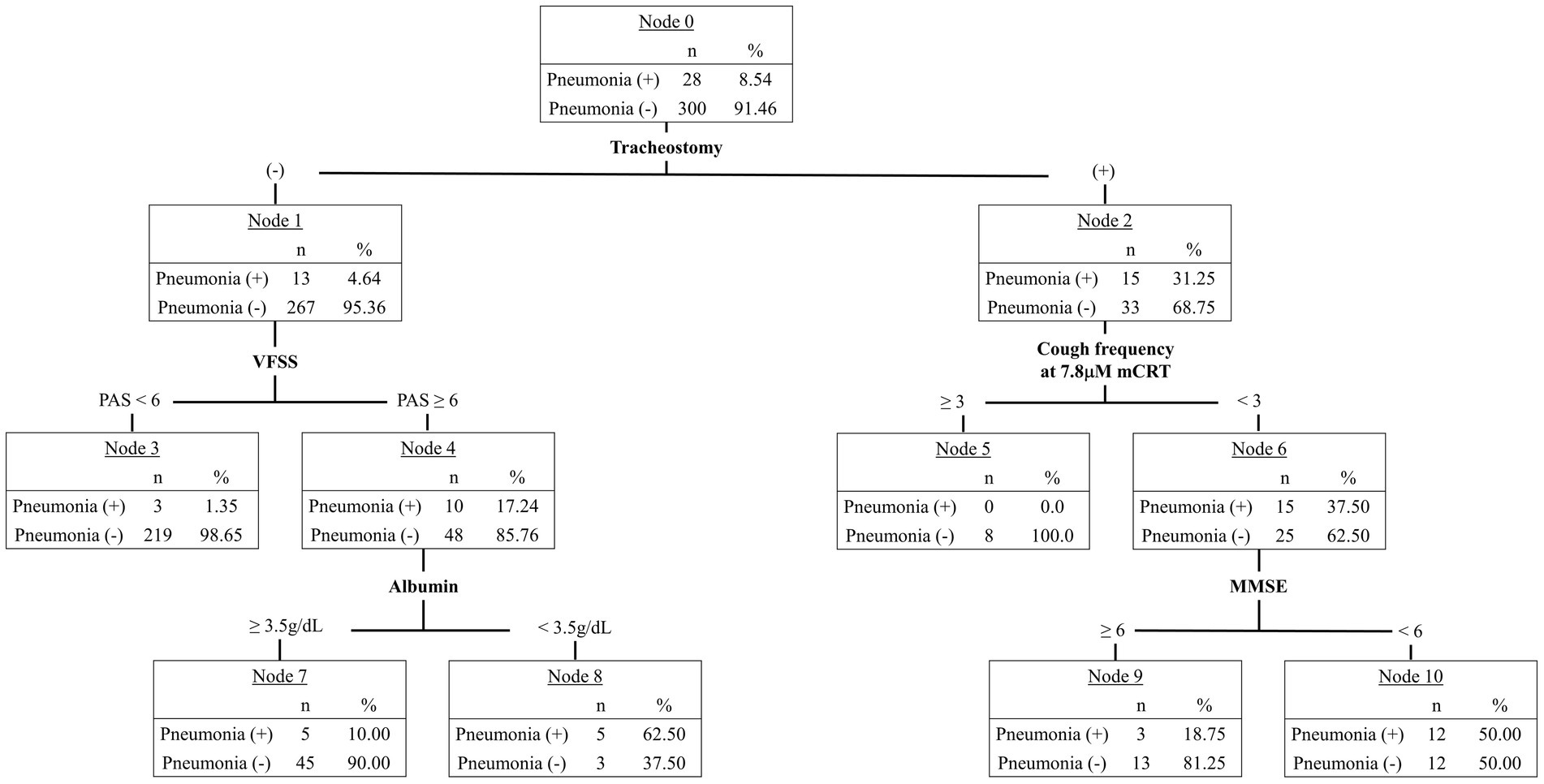
Figure 2. Classification and regression tree (CART) analysis identifying factors predicting post-stroke pneumonia.
Tracheostomy status was the primary predictor in the decision tree, with 31.25% (n = 15) of patients with tracheostomy developing pneumonia, compared with 4.64% (n = 13) of those without. Among patients without tracheostomy, a PAS score below 6 on VFSS indicated a pneumonia incidence of 1.35% (n = 3). If aspiration was confirmed on VFSS, low nutritional status—defined by an albumin level below 3.5 g/dL—was associated with a 62.50% (n = 5) pneumonia risk, whereas an albumin level of 3.5 g/dL or higher indicated a 10.0% (n = 5) risk. In patients with tracheostomy, a cough frequency of 3 or higher at 7.8 μM mCRT was associated with no pneumonia risk. However, if cough frequency was below 3, an MMSE score of less than 6 resulted in a 50.0% (n = 12) pneumonia incidence, while a score of 6 or higher indicated an 18.75% (n = 3) risk.
The predictive accuracy of the algorithm (Figure 3) was 92.7%, with an AUC of 0.89 (95% CI: 0.82–0.95). In the no-risk group, the algorithm underestimated pneumonia risk for three patients who were later diagnosed with pneumonia. However, overall accuracy for the no-risk group remained high at 98.7% (227/230).
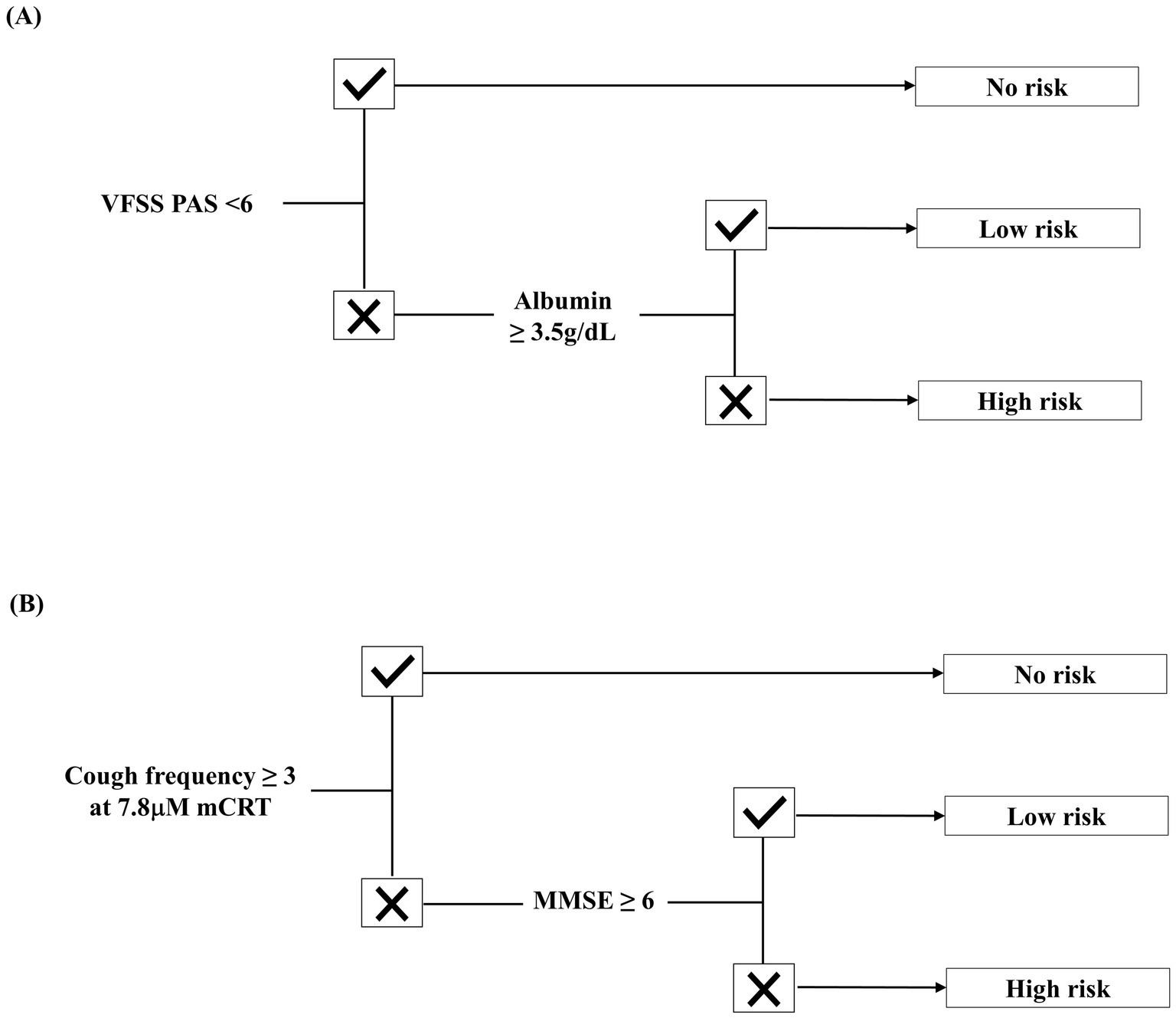
Figure 3. Predictive algorithm for aspiration pneumonia in post-stroke patients with dysphagia. (A) Algorithm for patients without tracheostomy. (B) Algorithm for patients with tracheostomy.
4 Discussion
This study is the first to identify predictors and develop an algorithm for forecasting pneumonia risk in stroke patients undergoing inpatient rehabilitation using defense mechanism-specific assessment tools. The key predictors incorporated into the algorithm include tracheostomy status, aspiration on VFSS, cough frequency, albumin levels, and MMSE scores. This integrated algorithm demonstrated a predictive accuracy of 92.7%, with an AUC of 0.89. Notably, this AUC tended to be higher than that of previously developed predictive models, such as the A2DS2 (AUC 0.84), AIS-APIS (AUC 0.78), and ISAN scores (AUC 0.79) (6–8), which primarily rely on general clinical characteristics.
Understanding the multifaceted defense mechanisms against pneumonia is critical to advancing predictive models. First, anatomic protection against aspiration is ensured through anatomic integrity, glottic closure and activation of oropharyngeal muscles (11). Second, the voluntary cough reflex clears foreign material from the airway and serves as a primary line of defense (10). Third, nutritional status plays a role in immune competence, with malnutrition associated with systemic inflammation and impaired host defense (13). Fourth, the act of swallowing involves complex cortical and subcortical processing, underscoring the cognitive contribution to airway protection (12). This study’s findings support the notion that a multi-dimensional evaluation of these defense layers can significantly enhance predictive capability.
In this study, tracheostomy status emerged as the most influential predictor in the decision tree and the factor with the highest OR. Aspiration rates in tracheostomized patients have been reported to range from 50% to 83% (22, 23). This finding aligns with previous study results showing tracheostomy as a major contributor to increased post-stroke pneumonia risk (24, 25). Dai et al. (24) demonstrated that prolonged tracheostomy duration is associated with a higher risk of pneumonia in stroke patients with both tracheostomy and dysphagia. Despite its role in secretion management, tracheostomy negatively affects airway protection mechanisms. The tracheostomy tube anchors the larynx to surrounding neck tissues, restricting laryngeal elevation (25), reducing protective laryngeal reflexes, and causing uncoordinated laryngeal closure responses (26). Moreover, it shortens the duration of vocal cord adduction/abduction (27), leading to disuse atrophy of laryngeal muscles (28). Additionally, an open tracheostomy reduces subglottic air pressure during swallowing, further compromising airway protection (29). These previous findings, along with the present study, reinforce tracheostomy as a critical risk factor for post-stroke pneumonia.
In this study, aspiration detected in VFSS was the second strongest predictor of pneumonia, aligning with findings from Martino et al. (30), who reported an 11-fold increased risk of pneumonia in stroke patients with confirmed aspiration. However, in the proposed algorithm, VFSS-confirmed aspiration was not used for patients with tracheostomy. This is consistent with the findings of Dai et al. (24), who found that in post-stroke patients with tracheostomy, only total tracheostomy duration, cough reflex dysfunction, and oropharyngeal phase dysfunction were significant pneumonia risk factors in logistic regression analysis, despite significant differences in PAS scores in descriptive statistics. In this study, aspiration in VFSS was observed in 66.7% (32/48) of tracheostomized patients, suggesting that its high incidence may limit the predictive value of VFSS-confirmed aspiration in this population.
Cough frequency also played a significant role in predicting pneumonia risk. A cough frequency of three or more was associated with a lower risk of pneumonia in tracheostomized patients. Previous studies have reported that cough frequency in the cough reflex test has a sensitivity of 0.70 to 0.87 for detecting silent aspiration in stroke patients (31, 32). Pekacka-Egli et al. (33) speculated that low cough frequency may indicate diminished cough sensitivity and reduced respiratory tract protection, increasing pneumonia risk due to silent aspiration. Further, Trimble et al. (34) and Miles et al. (35) proposed that fewer than two coughs in the cough reflex test could serve as a criterion for screening silent aspiration. Perry et al. (36) recommended assessing cough frequency as an initial screening measure before proceeding with oral intake trials to mitigate aspiration pneumonia risk in stroke patients with dysphagia. Consistent with prior study results, findings from this study underscore the importance of cough frequency in predicting pneumonia risk, particularly in tracheostomized patients who are highly susceptible to silent aspiration.
However, PCF did not show a significant association with pneumonia risk in this study. Kulnik et al. (37) reported that a strong cough protects against aspiration-related pneumonia and that stroke patients with a PCF below 400 L/min have a three-fold increased risk of pneumonia. Min et al. (38) and Bianchi et al. (10) suggested PCF as a useful prognostic factor for aspiration risk in patients with dysphagia. However, in this study, PCF at both 7.8 μM and 31.25 μM mCRT did not significantly predict post-stroke pneumonia. This discrepancy may be due to challenges in accurately measuring PCF in patients with tracheostomy and cognitive impairments, which may have led to generally low PCF values.
In this study, severe cognitive impairment was associated with a higher risk of pneumonia in tracheostomized patients. While earlier studies focused on the laterality (16) or locations (39) of brain lesions affecting dysphagia, recent research has emphasized the degree (40) and neuropsychological profiles (12) of cognitive impairment. Davenport et al. (41) suggested that sensory recognition of airway irritation requires cognitive awareness, enabling compensatory responses when the cough reflex is impaired. The algorithm in Figure 3B aligns with this rationale, incorporating cognitive function as a secondary defense mechanism when the cough reflex is compromised.
Malnutrition was another key factor associated with increased pneumonia risk, consistent with previous findings (13, 42–44). Dysphagia in stroke patients is closely associated with malnutrition due to reduced nutrient intake and increased metabolic demands during the recovery phase of stroke (42, 45). Furthermore, malnutrition weakens immune function and is strongly associated with infection, particularly post-stroke pneumonia, independent of aspiration (43, 44). The FOOD trial demonstrated that pneumonia was more common in undernourished stroke patients due to the detrimental effects of poor nutrition on immune function (13). More recently, a meta-analysis by Chen et al. found that proper nutrition in stroke patients may reduce systemic inflammation, enhance immune response, and subsequently lower the risk of stroke-associated infections (46). Figure 3A supports these findings, emphasizing the role of nutrition in pneumonia prevention, even in the presence of aspiration.
This integrative algorithm underscores the importance of evaluating multiple protective mechanisms against pneumonia—anatomical protection, swallowing reflex, cough reflex, cognition, and immunonutrition—to enhance predictive accuracy. A key distinction between this algorithm and previous predictive models is the lack of significance of age and sex in predicting post-stroke pneumonia. While earlier models (6–8) incorporated these demographic factors, a recent meta-analysis by Guo et al. (47) suggested that gender does not influence pneumonia risk, and many risk scoring tools (7, 48, 49) have excluded sex from their scoring systems. Although age is consistently identified as a risk factor, the arbitrary cutoff points used across different scoring systems limit its reliability. Consistent with recent studies, neither age nor sex demonstrated significant predictive value in this study.
This study has several strengths. First, it employs highly specific assessment tools to evaluate defense mechanisms against pneumonia. Second, the predictive accuracy of this algorithm (AUC 0.89) tended to be higher than that of previous demographic- and clinically based models (AUC 0.79–0.84) (6–8). Third, most clinical factors included in the algorithm are routinely obtainable and easy to assess, making it practical and accessible for widespread implementation.
Several limitations should be acknowledged. First, despite VFSS is the gold standard for detecting aspiration, not all patients can undergo this test because of patient’s condition or the limited availability of VFSS at many institutions. Incorporating simplified bedside screening tests, such as the water swallow test, could improve accessibility in future studies. Second, the incidence of pneumonia (n = 28) and the number of tracheostomized patients (n = 48) was relatively small. Moreover, higher-risk populations, such as patients requiring supplemental oxygen or with active pneumonia, were excluded. Future multicenter studies with larger sample size that include these higher-risk cohorts will be needed to validate it. Third, while dysphagia was clinically assessed by certified rehabilitation specialists and trained speech-language pathologists, standardized dysphagia scales–such as the Gugging Swallowing Screen (GUSS) (50) and the Mann Assessment of Swallowing Ability (MASA) (51)–were not applied. Adopting such validated tools would enable more consistent and reproducible assessment of swallowing, facilitate comparability across studies, and strengthen the clinical applicability of the predictive model. Fourth, this model was not compared head-to-head in the same cohort and use different case mixes and predictor sets with previously developed predicting models. Although higher AUC in this model is encouraging, prospective head-to-head comparison and external validation with same cohort is warranted. Finally, oral hygiene may be a potential predictor of post-stroke pneumonia, as bacterial colonization in saliva contributes to aspiration pneumonia. However, this data was not collected due to the lack of standardized assessment tools.
5 Conclusion
This study was the first prospective study to develop an algorithm for predicting post-stroke pneumonia incidence using defense mechanism-specific assessment tools during inpatient rehabilitation. Among the various factors associated with aspiration pneumonia, tracheostomy status, aspiration in VFSS, cough frequency, albumin levels, and cognitive function were identified as the key predictors. This algorithm offers a comprehensive framework for post-stroke pneumonia screening and may facilitate early preventive interventions for at-risk patients. Future studies with larger, more diverse samples-including higher-risk cohorts-and external multicenter validation will be needed before wider clinical implementation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Severance Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants or their legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JL: Formal analysis, Validation, Writing – original draft. H-JL: Investigation, Writing – review & editing. HJ: Investigation, Writing – review & editing. YY: Investigation, Writing – review & editing. DK: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Badve, MS, Zhou, Z, van de Beek, D, Anderson, CS, and Hackett, ML. Frequency of post-stroke pneumonia: systematic review and meta-analysis of observational studies. Int J Stroke. (2019) 14:125–36. doi: 10.1177/1747493018806196
2. Westendorp, WF, Nederkoorn, PJ, Vermeij, JD, Dijkgraaf, MG, and van de Beek, D. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. (2011) 11:110. doi: 10.1186/1471-2377-11-110
3. Kumar, S, Selim, MH, and Caplan, LR. Medical complications after stroke. Lancet Neurol. (2010) 9:105–18. doi: 10.1016/S1474-4422(09)70266-2
4. Smithard, DG, O’Neill, PA, Parks, C, and Morris, J. Complications and outcome after acute stroke. Does dysphagia matter? Stroke. (1996) 27:1200–4. doi: 10.1161/01.str.27.7.1200
5. Nakamori, M, Imamura, E, Kuwabara, M, Ayukawa, T, Tachiyama, K, Kamimura, T, et al. Simplified cough test can predict the risk for pneumonia in patients with acute stroke. PLoS One. (2020) 15:e0239590. doi: 10.1371/journal.pone.0239590
6. Hoffmann, S, Malzahn, U, Harms, H, Koennecke, HC, Berger, K, Kalic, M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. (2012) 43:2617–23. doi: 10.1161/STROKEAHA.112.653055
7. Ji, R, Shen, H, Pan, Y, Wang, P, Liu, G, Wang, Y, et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke. (2013) 44:1303–9. doi: 10.1161/STROKEAHA.111.000598
8. Smith, CJ, Bray, BD, Hoffman, A, Meisel, A, Heuschmann, PU, Wolfe, CD, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc. (2015) 4:e001307. doi: 10.1161/JAHA.114.001307
9. Quinton, LJ, Walkey, AJ, and Mizgerd, JP. Integrative physiology of pneumonia. Physiol Rev. (2018) 98:1417–64. doi: 10.1152/physrev.00032.2017
10. Bianchi, C, Baiardi, P, Khirani, S, and Cantarella, G. Cough peak flow as a predictor of pulmonary morbidity in patients with dysphagia. Am J Phys Med Rehabil. (2012) 91:783–8. doi: 10.1097/PHM.0b013e3182556701
11. Valenzano, TJ, Guida, BT, Peladeau-Pigeon, M, and Steele, CM. Respiratory-swallow coordination in healthy adults during drinking of thin to extremely thick liquids: a research note. J Speech Lang Hear Res. (2020) 63:702–9. doi: 10.1044/2019_JSLHR-19-00163
12. Jo, SY, Hwang, JW, and Pyun, SB. Relationship between cognitive function and dysphagia after stroke. Ann Rehabil Med. (2017) 41:564–72. doi: 10.5535/arm.2017.41.4.564
13. FOOD Trial Collaboration. Poor nutritional status on admission predicts poor outcomes after stroke: observational data from the FOOD trial. Stroke. (2003) 34:1450–6. doi: 10.1161/01.STR.0000074037.49197.8C
14. Florie, M, Pilz, W, Dijkman, RH, Kremer, B, Wiersma, A, Winkens, B, et al. The effect of cranial nerve stimulation on swallowing: a systematic review. Dysphagia. (2021) 36:216–30. doi: 10.1007/s00455-020-10126-x
15. Logemann, JA, Veis, S, and Colangelo, L. A screening procedure for oropharyngeal dysphagia. Dysphagia. (1999) 14:44–51. doi: 10.1007/PL00009583
16. Robbins, J, Levine, RL, Maser, A, Rosenbek, JC, and Kempster, GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. (1993) 74:1295–300. doi: 10.1016/0003-9993(93)90082-L
17. Mann, G, Hankey, GJ, and Cameron, D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. (1999) 30:744–8. doi: 10.1161/01.str.30.4.744
18. Cugy, E, and Sibon, I. Stroke-associated pneumonia risk score: validity in a French stroke unit. J Stroke Cerebrovasc Dis. (2017) 26:225–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.015
19. Grube, MM, Koennecke, HC, Walter, G, Meisel, A, Sobesky, J, Nolte, CH, et al. Influence of acute complications on outcome 3 months after ischemic stroke. PLoS One. (2013) 8:e75719. doi: 10.1371/journal.pone.0075719
20. Rosenbek, JC, Robbins, JA, Roecker, EB, Coyle, JL, and Wood, JL. A penetration-aspiration scale. Dysphagia. (1996) 11:93–8. doi: 10.1007/BF00417897
21. Martin-Harris, B, Brodsky, MB, Michel, Y, Castell, DO, Schleicher, M, Sandidge, J, et al. MBS measurement tool for swallow impairment–MBSImp: establishing a standard. Dysphagia. (2008) 23:392–405. doi: 10.1007/s00455-008-9185-9
22. Elpern, EH, Scott, MG, Petro, L, and Ries, MH. Pulmonary aspiration in mechanically ventilated patients with tracheostomies. Chest. (1994) 105:563–6. doi: 10.1378/chest.105.2.563
23. Tolep, K, Getch, CL, and Criner, GJ. Swallowing dysfunction in patients receiving prolonged mechanical ventilation. Chest. (1996) 109:167–72. doi: 10.1378/chest.109.1.167
24. Dai, Y, Qiao, J, Ye, QP, Li, XY, Hu, JH, and Dou, ZL. Exploring the influence of dysphagia and tracheostomy on pneumonia in patients with stroke: a retrospective cohort study. Brain Sci. (2022) 12:1664. doi: 10.3390/brainsci12121664
25. Jung, SJ, Kim, DY, Kim, YW, Koh, YW, Joo, SY, and Kim, ES. Effect of decannulation on pharyngeal and laryngeal movement in post-stroke tracheostomized patients. Ann Rehabil Med. (2012) 36:356–64. doi: 10.5535/arm.2012.36.3.356
26. Buckwalter, JA, and Sasaki, CT. Effect of tracheotomy on laryngeal function. Otolaryngol Clin N Am. (1984) 17:41–8. doi: 10.1016/S0030-6665(20)31992-7
27. Shaker, R, Milbrath, M, Ren, J, Campbell, B, Toohill, R, and Hogan, W. Deglutitive aspiration in patients with tracheostomy: effect of tracheostomy on the duration of vocal cord closure. Gastroenterology. (1995) 108:1357–60. doi: 10.1016/0016-5085(95)90682-7
28. DeVita, MA, and Spierer-Rundback, L. Swallowing disorders in patients with prolonged orotracheal intubation or tracheostomy tubes. Crit Care Med. (1990) 18:1328–30. doi: 10.1097/00003246-199012000-00004
29. Eibling, DE, and Gross, RD. Subglottic air pressure: a key component of swallowing efficiency. Ann Otol Rhinol Laryngol. (1996) 105:253–8. doi: 10.1177/000348949610500401
30. Martino, R, Foley, N, Bhogal, S, Diamant, N, Speechley, M, and Teasell, R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. (2005) 36:2756–63. doi: 10.1161/01.STR.0000190056.76543.eb
31. Wakasugi, Y, Tohara, H, Hattori, F, Motohashi, Y, Nakane, A, Goto, S, et al. Screening test for silent aspiration at the bedside. Dysphagia. (2008) 23:364–70. doi: 10.1007/s00455-008-9150-7
32. Miles, A, Moore, S, McFarlane, M, Lee, F, Allen, J, and Huckabee, ML. Comparison of cough reflex test against instrumental assessment of aspiration. Physiol Behav. (2013) 118:25–31. doi: 10.1016/j.physbeh.2013.05.004
33. Pekacka-Egli, AM, Kazmierski, R, Lutz, D, Kulnik, ST, Pekacka-Falkowska, K, Maszczyk, A, et al. Predictive value of cough frequency in addition to aspiration risk for increased risk of pneumonia in Dysphagic stroke survivors: a clinical pilot study. Brain Sci. (2021) 11:847. doi: 10.3390/brainsci11070847
34. Trimble, J, Patterson, JM, Wilson, JA, Dixit, AK, and Drinnan, M. Screening for silent aspiration in hyperacute stroke: a feasibility study of clinical swallowing examination and cough reflex testing. Int J Lang Commun Disord. (2023) 58:1657–67. doi: 10.1111/1460-6984.12893
35. Miles, A, Zeng, IS, McLauchlan, H, and Huckabee, ML. Cough reflex testing in dysphagia following stroke: a randomized controlled trial. J Clin Med Res. (2013) 5:222–33. doi: 10.4021/jocmr1340w
36. Perry, SE, Miles, A, Fink, JN, and Huckabee, ML. The dysphagia in stroke protocol reduces aspiration pneumonia in patients with dysphagia following acute stroke: a clinical audit. Transl Stroke Res. (2019) 10:36–43. doi: 10.1007/s12975-018-0625-z
37. Kulnik, ST, Birring, SS, Hodsoll, J, Moxham, J, Rafferty, GF, and Kalra, L. Higher cough flow is associated with lower risk of pneumonia in acute stroke. Thorax. (2016) 71:474–5. doi: 10.1136/thoraxjnl-2015-207810
38. Min, SW, Oh, SH, Kim, GC, Sim, YJ, Kim, DK, and Jeong, HJ. Clinical importance of peak cough flow in dysphagia evaluation of patients diagnosed with ischemic stroke. Ann Rehabil Med. (2018) 42:798–803. doi: 10.5535/arm.2018.42.6.798
39. Steinhagen, V, Grossmann, A, Benecke, R, and Walter, U. Swallowing disturbance pattern relates to brain lesion location in acute stroke patients. Stroke. (2009) 40:1903–6. doi: 10.1161/STROKEAHA.108.535468
40. Moon, HI, Pyun, SB, and Kwon, HK. Correlation between location of brain lesion and cognitive function and findings of Videofluoroscopic swallowing study. Ann Rehabil Med. (2012) 36:347–55. doi: 10.5535/arm.2012.36.3.347
41. Davenport, PW, Bolser, DC, Vickroy, T, Berry, RB, Martin, AD, Hey, JA, et al. The effect of codeine on the urge-to-cough response to inhaled capsaicin. Pulm Pharmacol Ther. (2007) 20:338–46. doi: 10.1016/j.pupt.2006.10.012
42. Scrutinio, D, Lanzillo, B, Guida, P, Passantino, A, Spaccavento, S, and Battista, P. Association between malnutrition and outcomes in patients with severe ischemic stroke undergoing rehabilitation. Arch Phys Med Rehabil. (2020) 101:852–60. doi: 10.1016/j.apmr.2019.11.012
43. Sabbouh, T, and Torbey, MT. Malnutrition in stroke patients: risk factors, assessment, and management. Neurocrit Care. (2018) 29:374–84. doi: 10.1007/s12028-017-0436-1
44. Dávalos, A, Ricart, W, Gonzalez-Huix, F, Soler, S, Marrugat, J, Molins, A, et al. Effect of malnutrition after acute stroke on clinical outcome. Stroke. (1996) 27:1028–32. doi: 10.1161/01.STR.27.6.1028
45. Labeit, B, Michou, E, Hamdy, S, Trapl-Grundschober, M, Suntrup-Krueger, S, Muhle, P, et al. The assessment of dysphagia after stroke: state of the art and future directions. Lancet Neurol. (2023) 22:858–70. doi: 10.1016/S1474-4422(23)00153-9
46. Chen, X, Hu, Y, Yuan, X, Yang, J, and Ka, L. Effect of early enteral nutrition combined with probiotics in patients with stroke: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. (2022) 76:592–603. doi: 10.1038/s41430-021-00986-3
47. Guo, T, Dou, L, and Zhou, X. Risk factors of stroke complicated with hospital-acquired pneumonia: a systematic review and meta-analysis of cohort studies. Ann Palliat Med. (2021) 10:12381–9. doi: 10.21037/apm-21-3278
48. Chumbler, NR, Williams, LS, Wells, CK, Lo, AC, Nadeau, S, Peixoto, AJ, et al. Derivation and validation of a clinical system for predicting pneumonia in acute stroke. Neuroepidemiology. (2010) 34:193–9. doi: 10.1159/000289350
49. Harms, H, Prass, K, Meisel, C, Klehmet, J, Rogge, W, Drenckhahn, C, et al. Preventive antibacterial therapy in acute ischemic stroke: a randomized controlled trial. PLoS One. (2008) 3:e2158. doi: 10.1371/journal.pone.0002158
50. Trapl, M, Enderle, P, Nowotny, M, Teuschl, Y, Matz, K, Dachenhausen, A, et al. Dysphagia bedside screening for acute-stroke patients: the Gugging swallowing screen. Stroke. (2007) 38:2948–52. doi: 10.1161/STROKEAHA.107.483933
51. Antonios, N, Carnaby-Mann, G, Crary, M, Miller, L, Hubbard, H, Hood, K, et al. Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: the modified Mann assessment of swallowing ability. J Stroke Cerebrovasc Dis. (2010) 19:49–57. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.007
Keywords: stroke, pneumonia, aspiration, cough, deglutition disorders
Citation: Lee JW, Lee HJ, Jang HJ, Yun YS and Kim DY (2025) Predicting pneumonia algorithm in stroke patients. Front. Neurol. 16:1690049. doi: 10.3389/fneur.2025.1690049
Edited by:
Francesco Corea, Azienda USL Umbria 2, ItalyReviewed by:
Zihao Liu, Shandong Provincial Hospital, ChinaFrancesco Palmerini, Fondazione Poliambulanza Istituto Ospedaliero, Italy
Copyright © 2025 Lee, Lee, Jang, Yun and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deog Young Kim, a2ltZHlAeXVocy5hYw==
 Jong Weon Lee
Jong Weon Lee Hyun-Joung Lee3
Hyun-Joung Lee3 Hyeon Ju Jang
Hyeon Ju Jang Deog Young Kim
Deog Young Kim