- 1School of Nursing, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Department of Neurology, The Second Affiliated Hospital of Zhejiang Chinese Medical University (The Xin Hua Hospital of Zhejiang Province), Hangzhou, Zhejiang, China
- 3Department of Rehabilitation, The Third Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 4Department of Neurosurgery, Tongde Hospital of Zhejiang Province, Hangzhou, Zhejiang, China
Introduction: Obstructive sleep apnea (OSA) is a highly prevalent but frequently undiagnosed sleep disorder among stroke patients. It is associated with increased risks of stroke recurrence, reduced rehabilitation effectiveness, and elevated mortality. Despite guideline recommendations for routine OSA screening in stroke care, implementation remains inconsistent in clinical practice. As a modifiable sleep-related risk factor with significant implications for neurological outcomes, better integration of OSA screening in post-stroke care is urgently needed. Thus, this scoping review protocol outlines a systematic approach to identifying barriers to and facilitators of OSA screening in stroke populations.
Methods and analysis: This scoping review will follow the methodological framework provided by the Joanna Briggs Institute (JBI) and will be reported using Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines. The search will be performed on CNKI, WanFang, SinoMed, PubMed, Embase, Web of Science, the Cochrane Library, CINAHL, ProQuest Dissertations, OpenGrey, and Google Scholar. Targeted searches of international organization websites will also be conducted. No restrictions will be imposed based on study design or year of publication. Data will be synthesized using the content analysis approach and mapped onto the Ottawa Model of Research Use (OMRU), including domains such as evidence-based innovation, potential adopters, practice environment, implementation process, and adoption outcomes.
Discussion: The findings are expected to inform future research and support the integration of sleep disorder screening into stroke care pathways. Ultimately, the review will improve stroke outcomes by addressing sleep health as a critical but often overlooked component of post-stroke management.
Scoping review registration: Open science framework (osf.io/tb7z8).
1 Introduction
Stroke is defined as a neurological deficit resulting from acute focal injury due to an interruption or reduction in cerebral blood flow (1). It is broadly classified into ischemic and hemorrhagic types (2), whereas its acute clinical manifestation exhibits significant heterogeneity. This complexity necessitates further etiological stratification, such as cardioembolic stroke, lacunar infarction, and atherothrombotic infarction for ischemic strokes, alongside intracerebral hemorrhage for hemorrhagic strokes (3). Accurate differentiation of stroke subtypes is clinically important, as they are associated with distinct risk profiles, initial severities, and prognostic trajectories, all of which influence management strategies (3). According to the 2019 Global Burden of Disease (GBD) Study, stroke remains the second leading cause of global deaths (4). Annually, approximately 12.2 million new stroke cases are reported. Moreover, 101 million individuals live with stroke-related conditions, and 6.55 million deaths are attributed to stroke (4).
Sleep-related breathing disorders (SRBDs), particularly obstructive sleep apnea (OSA), have been increasingly recognized as significant contributors to both the onset of first-ever ischemic stroke and the risk of recurrence. SRBDs are also associated with poorer clinical outcomes, including increased stroke severity, prolonged hospitalization, and reduced functional recovery (5). Among SRBDs, OSA is the most prevalent condition in stroke patients (5). Post-stroke neuromuscular dyscoordination, particularly involving the upper airway, intercostal, and diaphragmatic muscles, can predispose individuals to OSA (6). Notably, OSA is a disorder characterized by recurrent pharyngeal airway collapse during sleep, resulting in repeated arousals, fragmented sleep patterns, and pronounced daytime sleepiness (7). A meta-analysis reported that 72% of stroke patients exhibit an apnea-hypopnea index (AHI) greater than 5 (8) and a prevalence significantly higher than the 35% observed in the general population (9). Additionally, the prevalence of post-stroke OSA has recently shown a rising trend (10). This is particularly concerning given that comorbid OSA is associated with an increased rate of stroke recurrence and all-cause mortality (11). Consequently, a 10-year cohort study reported a 75% higher mortality risk in stroke patients with concomitant OSA compared to those without sleep apnea (12). In recognition of these risks, the American Heart Association and the American Stroke Association issued a 2021 guideline recommending that patients with ischemic stroke or transient ischemic attack (TIA) be considered for OSA evaluation (Class 2b, Level of Evidence B-NR) (13).
The prevalence of OSA varies across different stages of stroke recovery (14). Research shows that OSA is more common during the acute and subacute phases of stroke compared to the chronic phase, with reported prevalence rates of 68.4% in the acute phase, 71.3% in the subacute phase, and 60.6% in the chronic phase (15). These fluctuations likely reflect the time-dependent nature of post-stroke impairments in respiratory regulation, including abnormal breathing patterns and reduced respiratory muscle tone, which often stabilize after the subacute period (16).
However, some studies have found no statistically significant differences in OSA prevalence between the acute and chronic phases (8). This discrepancy may arise from variations in diagnostic criteria, timing of assessment, or the natural resolution of symptoms in patients with milder cases (17). Despite these inconsistencies, OSA can persist and continue to negatively impact recovery throughout all phases of stroke rehabilitation– from acute to chronic. Therefore, early detection and timely intervention for OSA may be crucial in improving post-stroke recovery and reducing associated complications (18, 19).
Screening for OSA is critical in stroke care, as it can help prevent serious complications and optimize treatment strategies, as OSA is recognized as a predictor of poor post-stroke outcomes (20). Observational studies have indicated that untreated OSA following a stroke is linked to an increased risk of stroke recurrence (21), higher mortality rates (12, 22, 23), reduced effectiveness of rehabilitation, and prolonged hospitalization (24). Thus, early identification and management of post-stroke OSA may improve clinical outcomes. For example, screening for OSA and initiating treatment during the acute phase of a stroke may enhance recovery by preserving the ischemic penumbra and improving cerebral perfusion (25). Continuous positive airway pressure (CPAP) therapy is the first-line treatment for patients with moderate to severe OSA after a stroke (26). Moreover, evidence suggests that initiating CPAP therapy within the first 48 h of stroke onset leads to better neurological outcomes compared to delayed initiation (27, 28). Additionally, CPAP therapy has been shown to improve short-term outcomes, such as significant reductions in the AHI (22), improved sleep quality, decreased daytime sleepiness (21), reduced snoring, and enhanced cognitive function (29). Thus, the evidence strongly supports the incorporation of OSA screening into routine post-stroke management, with treatments tailored according to the severity of the condition and any comorbidities present (20, 30).
Despite its clinical significance, screening for OSA in post-stroke patients remains infrequent. One study found that 22% of stroke patients were diagnosed with OSA within 15 days of onset, yet many had not been screened for the condition (31). Additionally, research by Brown et al. (32) revealed that only 6% of stroke patients in the United States were tested for sleep apnea after experiencing a stroke. To address this issue, Johnson et al. (33) examined the ongoing debate surrounding the necessity of screening for this prevalent but treatable condition. They attributed the reluctance to trade-offs involving assessment time, the selection and validity of diagnostic tools, and uncertainties regarding the effectiveness of treatment. Nevertheless, given the growing evidence, OSA should be systematically screened for and managed as a routine component of post-stroke care.
A previous review by Swartz et al. (34) examined barriers to implementing screening and management strategies for depression, OSA, and cognitive impairment screening in post-stroke patients. The study proposed an innovative framework that conceptualizes the interconnectedness of post-stroke depression, OSA, and cognitive dysfunction as “DOC.” It systematically identified various multidimensional barriers to the implementation of these strategies in clinical practice. These included factors related to the screening tools, the practice environment, and the characteristics of potential adopters. However, the review primarily focused on the broader rationale and challenges associated with screening for DOC comorbidities as a group rather than delving into issues specific to OSA. Additionally, it did not address key challenges unique to OSA screening in stroke care, such as the absence of standardized screening guidelines. Furthermore, our comprehensive search of multiple databases did not identify any existing systematic or scoping reviews that specifically evaluate the barriers to and facilitators of OSA screening in post-stroke populations.
Existing evidence indicates that the evaluation of OSA signs and symptoms should be conducted in all stroke patients (33). However, OSA is frequently underrecognized as a modifiable risk factor for stroke recurrence (35), leading to inadequate post-stroke recovery outcomes. A comprehensive understanding of the facilitators and barriers to OSA screening in this population may aid the development of large-scale screening strategies as part of routine stroke care. Notably, such insights could also guide the design of more effective screening and diagnostic tools, ultimately improving the management of stroke-related OSA. Therefore, this scoping review, guided by the Joanna Briggs Institute’s methodological framework (36), aims to identify and synthesize the barriers to and facilitators of OSA screening in stroke patients. Ultimately, the goal is to promote the integration of evidence-based assessment practices into clinical care.
2 Review question
What are the barriers and facilitators to implementing screening for OSA in stroke patients?
3 Methods and analysis
3.1 Inclusion and exclusion criteria
To ensure scientific rigor, reproducibility, and applicability, this scoping review will follow the methodological framework established by the Joanna Briggs Institute (JBI) (36). The review will also adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist (37) (see Supplementary Additional File 1) to promote transparency and standardized reporting. Given the limited research available, the review will include a wide range of evidence sources, including original research studies (quantitative, qualitative, and mixed-methods), reviews, clinical practice guidelines, expert opinions, and conference abstracts or proceedings. No restrictions will be imposed based on study design or year of publication. The review will be guided by the Population, Concept, Context framework described below and aligned with the study’s objectives and research questions.
3.1.1 Participants
This review will include studies involving populations relevant to the influence and implementation of OSA screening in stroke care. Eligible participants may include (i) stroke patients at any stage of recovery (acute, subacute, or chronic), regardless of age, gender, stroke type (ischemic or hemorrhagic), or sociodemographic characteristics; (ii) family members involved in decision-making regarding OSA screening for stroke patients; (iii) healthcare professionals directly engaged in the screening, assessment, or implementation of OSA screening in stroke care, such as neurologists, stroke nurses, sleep specialists, and primary care providers; and (iv) policymakers or healthcare administrators involved in the development, implementation, or evaluation of OSA screening policies or strategies within stroke care pathways.
Exclusion criteria include studies involving (i) individuals with sleep disorders other than OSA (e.g., narcolepsy); (ii) patients receiving only palliative, hospice, or comfort care; and (iii) healthcare professionals or policymakers not directly involved in stroke-related OSA screening or management.
3.1.2 Concept
This review aims to identify and synthesize barriers and facilitators of OSA screening in stroke patients and related populations involved in the screening process. The concept encompasses a wide range of factors that influence the implementation of screening, including but not limited to:
(i) The use and accessibility of screening tools (e.g., questionnaires, polysomnography);
(ii) Characteristics of the screening process (e.g., timing, integration into care pathways);
(iii) Healthcare provider’s knowledge, skills, and attitudes toward OSA screening;
(iv) Policy or institutional support for screening programs;
(v) Patient acceptance, engagement, and adherence to screening and follow-up;
(vi) Indirect influences such as perceived or actual treatment outcomes affecting screening uptake.
Studies that focus solely on OSA treatment modalities or the underlying pathophysiological mechanisms, without addressing issues related to screening implementation, will be excluded.
3.1.3 Context
The context of this review will include a range of healthcare settings where stroke patients may undergo screening for OSA, including hospitals, rehabilitation centers, outpatient clinics, and community health settings. Studies will be included if they report on OSA screening practices conducted during hospitalization, rehabilitation, or community-based stroke management. Studies without accessible full texts will be excluded.
3.1.4 Evidence sources
All types of studies will be included, including original research, reviews, guidelines, and conference proceedings.
3.2 Search strategy
To identify relevant published literature, a comprehensive search will be conducted across multiple electronic databases and platforms. Complete search strategies for PubMed, CINAHL, and CNKI are provided in Supplementary Additional File 2 and will serve as the basis for adapting searches for other databases, including WanFang, SinoMed, Embase (via Ovid), Web of Science, and the Cochrane Library. The search strategy was collaboratively developed and critically reviewed in consultation with a health sciences librarian with expertise in academic literature retrieval. The search will incorporate controlled vocabulary (e.g., MeSH terms) and free-text keywords related to the core concepts of stroke, obstructive sleep apnea, screening, and influencing factors. Boolean operators will be used to structure search queries tailored to each database’s indexing terms and syntax. No restrictions will be placed on the publication date or language.
In addition to database searches, gray literature sources will be consulted to identify unpublished or non-indexed studies. These sources will include ProQuest Dissertations, OpenGrey, and Google Scholar. Details of the Google Scholar search strategy, including search terms, number of records retrieved (limited to the first 200 results per query), and exclusion criteria, are provided in the Supplementary Additional File 2. Targeted searches of international organization websites will also be conducted to identify relevant policy documents, clinical guidelines, and reports. These will include materials from the World Stroke Organization, the American Heart Association/American Stroke Association, the American Academy of Sleep Medicine, the European Sleep Research Society, and the World Health Organization. Conference abstracts and proceedings will be included, drawing from both electronic databases and platforms in addition to Google Scholar. No date or language restrictions will be applied.
To minimize language bias, records published in languages other than English or Chinese will still be captured during the search process. Titles and abstracts will be screened using an automated translation tool (Google Translate). Full-text articles that meet the inclusion criteria will be translated with the assistance of professional translation services or qualified language experts.
Additionally, the reference lists of all included studies will be manually screened to capture any additional relevant literature that may not have been identified through the electronic searches. This multi-pronged approach is designed to ensure a comprehensive and systematic collection of all pertinent evidence.
3.3 Source of evidence selection
NoteExpress will be used to manage the retrieved literature and remove duplicates. Rayyan software (38) will be used to facilitate collaborative screening and streamline the review process. Two trained reviewers will independently conduct an initial screening of titles and abstracts, followed by the full-text screening of potentially relevant studies based on predefined inclusion and exclusion criteria. Discrepancies will be resolved through discussion, with the involvement of a third reviewer, if a consensus cannot be reached.
For each conference abstract or non-peer-reviewed record, efforts will be made to identify a corresponding full-text publication. All available information will be extracted, and the completeness of the data will be documented. If essential data are missing, the corresponding or presenting author will be contacted for clarification. If no response is received within 7 days, a follow-up email will be sent. Records with unresolved missing data after an additional 7 days will be excluded from the review.
The study selection process will be documented and reported following the PRISMA-ScR checklist and explanation (37) (see Supplementary Additional File 3). Prior to the formal screening, a pilot test will be conducted on a random sample of 200 records. During this phase, the review team will discuss any disagreements and refine the inclusion criteria and operational definitions as necessary. Formal screening will begin once ≥80% agreement between reviewers is achieved.
3.4 Data extraction
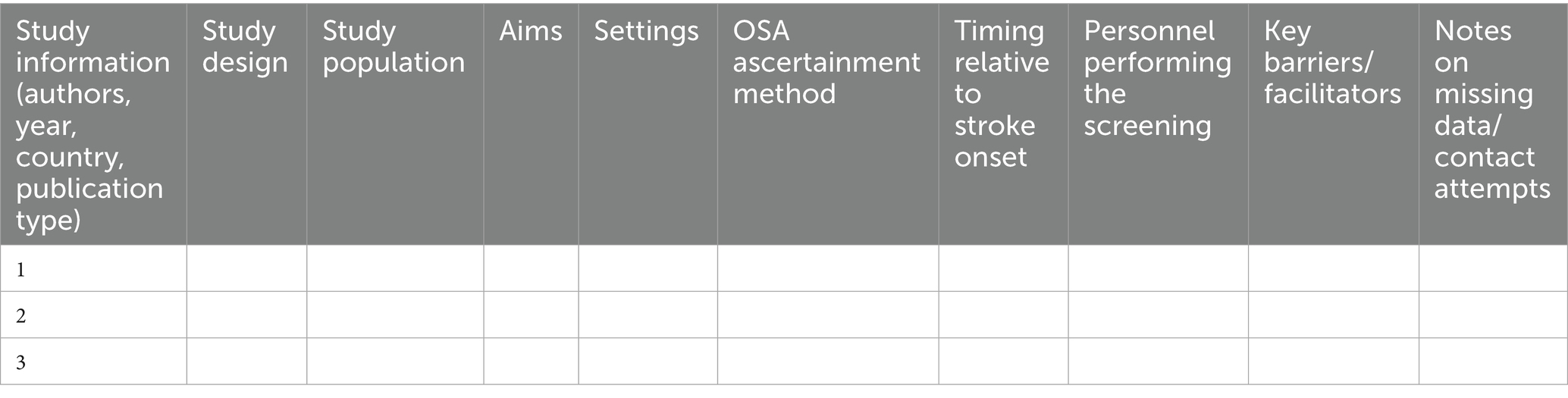
A standardized data extraction form will be used to systematically organize the included literature and ensure completeness and comparability of the data. The extracted information consists of basic study characteristics (Table 1), such as authorship, year, country, publication type, study design, population, aims, settings (stroke unit, rehabilitation, outpatient, etc.), OSA ascertainment method (screening questionnaire, portable monitoring, or polysomnography), timing of screening relative to stroke onset (acute/subacute/chronic; days/weeks), personnel performing the screening (e.g., nurse, physician, sleep specialist, or research staff), key barriers/facilitators, and notes on missing data or contact attempts with study authors. Factors related to barriers and facilitators will be extracted descriptively during the data extraction phase. These factors will later be categorized and mapped to the OMRU domains during the analysis phase (see Table 2).
To ensure reliability, the data extraction form will be piloted on 10% of the included studies. If frequent discrepancies are identified, the extraction instructions and definitions will be refined before full data extraction begins. Data will be extracted independently by two reviewers. Any disagreements will be resolved through discussion; if consensus cannot be reached, a third reviewer will be consulted for arbitration.
A methodological quality appraisal of the included studies will not be conducted, in accordance with established scoping review methodology. The primary aim of this review is to map the existing evidence and identify barriers and facilitators of OSA screening, rather than to assess the risk of bias in individual studies (36). This approach aligns with the latest JBI guidance and other published methodological recommendations for conducting scoping (36, 39).
3.5 Data analysis and presentation
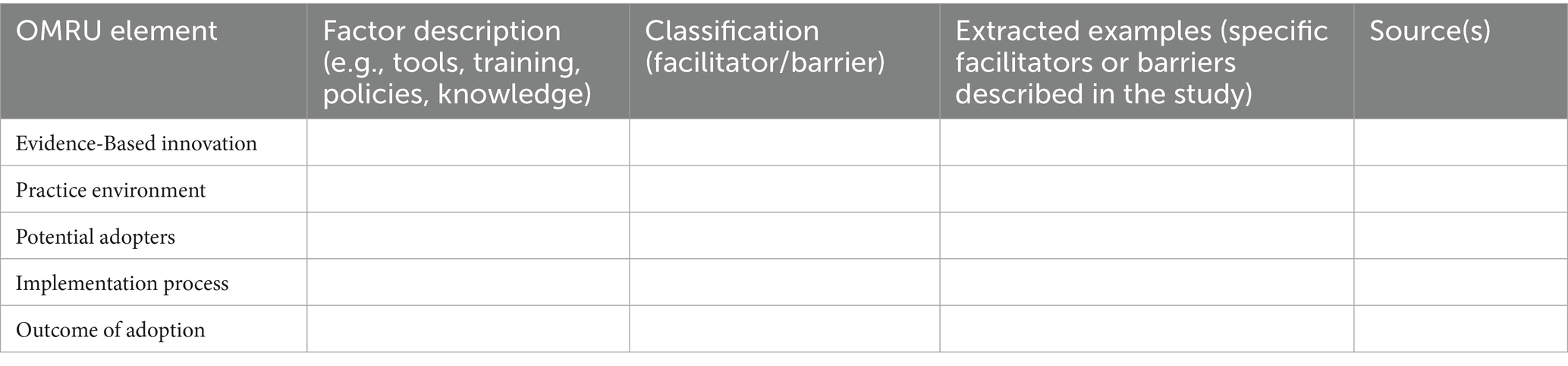
Following data extraction, the analysis and synthesis will be guided by the OMRU (40), a widely applied framework for identifying and categorizing barriers and facilitators in healthcare implementation research (41). Importantly, OMRU provides a structured, multi-level approach to examining factors that influence the implementation of post-stroke OSA screening. As such, this encompasses dimensions such as research evidence, potential adopters, practice environment, implementation processes, and adoption outcomes.
A directed content analysis will be employed to map the extracted data onto the key domains of the OMRU framework (42). This approach is well-suited to implementation research, as it enables the systematic classification of barriers and facilitators using established theoretical constructs, while also allowing for the identification of novel factors that may emerge from the data.
Evidence-based innovation: Evaluation of various OSA screening tools’ validity, reliability, and clinical applicability.
Potential adopters: Assessment of healthcare providers’ awareness, attitudes, and competencies in OSA screening, as well as patient acceptance and adherence to screening recommendations.
Practice environment: Analysis of institutional and organizational factors, including resource availability, hospital policies, and interdisciplinary collaboration, that influence the feasibility of screening.
Implementation process: Identification of barriers and facilitators related to workflow integration, timing of screening, and coordination among care teams within stroke services.
Outcomes of adoption: Examination of screening uptake, influence on clinical decision-making, and potential long-term impacts on patient outcomes.
For qualitative data, two trained reviewers (coders) will independently code the extracted information using NVivo software. An initial coding framework will be developed deductively based on OMRU domains. During the coding process, open coding will also be applied inductively to capture any emerging themes that are not encompassed within the predefined OMRU domains.
Before full coding begins, a pilot coding phase will be conducted on approximately 15–20% of the data to refine the coding guidelines and ensure a shared understanding of coding rules. During this phase, inter-coder agreement will be assessed, with a target of at least 80% agreement between coders. To address discrepancies, coders will meet regularly to review differences, and unresolved issues will be resolved through discussion and consensus, with a third senior coder available for adjudication if needed. This process will be maintained throughout both the pilot and main coding phases to ensure consistent application of final codes. All key decisions and modifications to the coding framework will be documented to ensure transparency and methodological rigor.
Quantitative indicators, such as reported screening rates, adherence statistics, and institutional policy metrics, will be summarized descriptively using measures such as the median, interquartile range, and range. Where conceptually aligned, these quantitative findings will be mapped to OMRU domains and integrated with qualitative data to provide a comprehensive overview. As a final step, the synthesis will provide a structured summary of barriers and facilitators by OMRU domain, supported by exemplar quotes or data points, and will highlight priority areas for future research.
3.6 Stakeholder consultation
In accordance with JBI methodology (36), stakeholder consultation is considered an optional stage in scoping reviews. While such engagement can enrich the interpretation of findings and enhance their relevance, this review will not include a formal stakeholder consultation due to practical constraints. These include limited time and resources, as well as the geographic dispersion of key stakeholders across institutions. Conducting a meaningful consultation would require careful planning, recruitment of diverse participants (e.g., clinicians, patients, policymakers), and prior ethical approval, all of which fall outside the scope of the current study. Future research will prioritize stakeholder involvement through interviews or focus groups to complement the literature-based findings and address real-world implementation challenges not captured in published sources.
4 Patient and public involvement
No patients or members of the public will be involved in the design, conduct, reporting, or dissemination of this scoping review.
5 Ethics and dissemination
Ethical approval is not required for this scoping review, as it analyzes data from publicly available sources and does not involve human participants. The findings will be disseminated through multiple targeted strategies. In addition to publication in peer-reviewed journals and presentations at academic conferences, results will be shared with stroke care networks, sleep medicine societies, and professional organizations involved in neurological rehabilitation to promote awareness and support the implementation of OSA screening. Policy briefs and practical checklists summarizing key barriers and facilitators will be developed for stroke units and hospital administrators to facilitate the integration of evidence into clinical practice. Potential end-users of this work include clinicians, nurse specialists, rehabilitation teams, and health policy makers involved in post-stroke care.
6 Discussion
Establishing a detailed and pre-defined protocol for this scoping review enhances methodological transparency and rigor, which helps minimize the risk of reporting bias. To the best of our knowledge, this will be the first scoping review to systematically map the literature on barriers to and facilitators of OSA screening in stroke populations, utilizing the OMRU framework as a guiding reference. By applying a multidimensional conceptual model, the review aims to capture the complex factors that influence the screening practices in real-world clinical settings.
Given the increasing recognition of sleep disorders as critical yet underdiagnosed contributors to neurological outcomes, this review will provide timely insight into how sleep-related conditions, particularly OSA, are addressed in the stroke care continuum. Thus, a better understanding of the challenges and factors that facilitate implementation may clarify the role of sleep health in post-stroke recovery and long-term prognosis. The findings are expected to identify gaps between evidence-based screening recommendations and current clinical practices, informing future research and practical strategies to improve screening uptake.
Beyond the scope of this review, future research should investigate the comparative effectiveness of various OSA screening tools in stroke populations, as well as strategies for integrating screening into multidisciplinary stroke care pathways. Such efforts are essential to generate evidence for developing standardized and scalable screening protocols. Additionally, future studies should examine how clinical heterogeneity among stroke subtypes may influence the relationship between stroke and OSA. Notably, given the distinct pathophysiological mechanisms, prognostic implications, and clinical characteristics of lacunar versus non-lacunar ischemic strokes, it is critical to assess whether these differences affect the prevalence, clinical presentation, and management of post-stroke OSA (43). Ultimately, this review may contribute to more integrated and responsive models of post-stroke care, where the identification and management of sleep-disordered breathing are recognized as essential components of secondary prevention and neurorehabilitation.
7 Strengths and limitations
This scoping review will follow the methodological framework established by the JBI and adhere to the PRISMA-ScR guidelines, thereby ensuring methodological rigor, transparency, and reproducibility. A key strength of this review is the application of a well-established implementation science framework – the OMRU – which will guide the categorization of barriers and facilitators, thereby enhancing the practical relevance of the findings in both clinical and policy contexts. Additionally, a comprehensive search strategy will be developed in collaboration with an experienced research librarian to maximize sensitivity and minimize the risk of missing relevant studies. However, this review has several limitations. While scoping reviews are designed to map the breadth of existing evidence rather than critically appraise study quality, the heterogeneity of study designs and variability in reporting completeness may limit the ability to draw firm conclusions regarding the relative importance of specific barriers and facilitators. Additionally, certain influential factors, such as implicit biases in clinical decision-making or subtle systemic barriers, may not be explicitly addressed or well-documented in the literature. As a result, critical but underreported determinants of OSA screening implementation may be overlooked. The absence of stakeholder consultation further limits the ability to capture context-specific or practical implementation challenges that are not reflected in published studies. These limitations should be taken into account when interpreting the findings.
Author contributions
YL: Writing – original draft, Writing – review & editing. JZ: Data curation, Writing – review & editing. WX: Methodology, Writing – review & editing. FD: Methodology, Writing – review & editing. ZF: Methodology, Writing – review & editing. LG: Methodology, Writing – review & editing. SL: Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Zhejiang Provincial Medical and Health Science and Technology Project (Grant no. 2021RC095).
Acknowledgments
The authors would like to express their sincere gratitude to Chunjian Hu, the Academic Services Librarian at Zhejiang Chinese Medical University, for her invaluable support in developing the search strategy. The authors also sincerely thank AiMi Academic Services (www.aimieditor.com) for their professional English language editing and review services of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1690372/full#supplementary-material
Abbreviations
OSA, obstructive sleep apnea; JBI, Joanna Briggs Institute; PRISMA-ScR, Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews; OMRU, Ottawa Model of Research Use; GBD, Global Burden of Disease; AHI, apnea–hypopnea index; TIA, transient ischemic attack; CPAP, continuous positive airway pressure; SRBDs, sleep-related breathing disorders.
References
1. Sacco, RL, Kasner, SE, Broderick, JP, Caplan, LR, Connors, JJ, Culebras, A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
2. Coupland, AP, Thapar, A, Qureshi, MI, Jenkins, H, and Davies, AH. The definition of stroke. J R Soc Med. (2017) 110:9–12. doi: 10.1177/0141076816680121
3. Gasull, T, and Arboix, A. Molecular mechanisms and pathophysiology of acute stroke: emphasis on biomarkers in the different stroke subtypes. Int J Mol Sci. (2022) 23:9476. doi: 10.3390/ijms23169476
4. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
5. Uscamaita, K, Parra Ordaz, O, Yazbeck Morell, I, Pla, MG, Sánchez-López, M, and Arboix, A. From molecular to clinical implications of sleep-related breathing disorders on the treatment and recovery of acute stroke: a scoping review. Curr Issues Mol Biol. (2025) 47:138. doi: 10.3390/cimb47030138
6. Alexiev, F, Brill, AK, Ott, SR, Duss, S, Schmidt, M, and Bassetti, CL. Sleep-disordered breathing and stroke: chicken or egg? J Thorac Dis. (2018) 10:S4244–52. doi: 10.21037/jtd.2018.12.66
7. Jordan, AS, McSharry, DG, and Malhotra, A. Adult obstructive sleep apnoea. Lancet. (2014) 383:736–47. doi: 10.1016/S0140-6736(13)60734-5
8. Johnson, KG, and Johnson, DC. Frequency of sleep apnea in stroke and tia patients: a meta-analysis. J Clin Sleep Med. (2010) 6:131–7. doi: 10.5664/jcsm.27760
9. Ghavami, T, Kazeminia, M, Ahmadi, N, and Rajati, F. Global prevalence of obstructive sleep apnea in the elderly and related factors: a systematic review and meta-analysis study. J Perianesth Nurs. (2023) 38:865–75. doi: 10.1016/j.jopan.2023.01.018
10. Brown, DL, Gibbs, R, Shi, X, Case, E, Chervin, R, and Lisabeth, LD. Abstract P597: growing prevalence of post-stroke sleep-disordered breathing. Stroke. (2021) 52:AP597. doi: 10.1161/str.52.suppl_1.P597
11. Hermann, DM, and Bassetti, CL. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology. (2016) 87:1407–16. doi: 10.1212/WNL.0000000000003037
12. Sahlin, C, Sandberg, O, Gustafson, Y, Bucht, G, Carlberg, B, Stenlund, H, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. (2008) 168:297–301. doi: 10.1001/archinternmed.2007.70
13. Kleindorfer, DO, Towfighi, A, Chaturvedi, S, Cockroft, KM, Gutierrez, J, Lombardi-Hill, D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the american heart association/american stroke association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
14. Hasan, F, Gordon, C, Wu, D, Huang, HC, Yuliana, LT, Susatia, B, et al. Dynamic prevalence of sleep disorders following stroke or transient ischemic attack: systematic review and meta-analysis. Stroke. (2021) 52:655–63. doi: 10.1161/STROKEAHA.120.029847
15. Liu, X, Lam, DC, Chan, K, Chan, HY, Ip, MS, and Lau, KK. Prevalence and determinants of sleep apnea in patients with stroke: a meta-analysis. J Stroke Cerebrovasc Dis. (2021) 30:106129. doi: 10.1016/j.jstrokecerebrovasdis.2021.106129
16. Grefkes, C, and Fink, GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract. (2020) 2:17. doi: 10.1186/s42466-020-00060-6
17. Su, X, Liu, S, Wang, C, Cai, Y, Li, Y, Wang, D, et al. Prevalence, incidence, and the time trends of sleep-disordered breathing among patients with stroke: a systematic review and meta-analysis. Front Neurol. (2024) 15:1432085. doi: 10.3389/fneur.2024.1432085
18. Han, S, Park, K, Kim, YS, Kim, J, and Lee, S. Effect of obstructive sleep apnea on the functional outcome of acute ischemic stroke. J Sleep Med. (2019) 16:88–94. doi: 10.13078/jsm.190035
19. Ott, SR, Fanfulla, F, Miano, S, Horvath, T, Seiler, A, Bernasconi, C, et al. Sas care 1: sleep-disordered breathing in acute stroke an transient ischaemic attack-prevalence, evolution and association with functional outcome at 3 months, a prospective observational polysomnography study. Erj Open Res. (2020) 6:334–2019. doi: 10.1183/23120541.00334-2019
20. Baillieul, S, Dekkers, M, Brill, AK, Schmidt, MH, Detante, O, Pepin, JL, et al. Sleep apnoea and ischaemic stroke: current knowledge and future directions. Lancet Neurol. (2022) 21:78–88. doi: 10.1016/S1474-4422(21)00321-5
21. Ryan, CM, Bayley, M, Green, R, Murray, BJ, and Bradley, TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. (2011) 42:1062–7. doi: 10.1161/STROKEAHA.110.597468
22. Gupta, A, Shukla, G, Afsar, M, Poornima, S, Pandey, RM, Goyal, V, et al. Role of positive airway pressure therapy for obstructive sleep apnea in patients with stroke: a randomized controlled trial. J Clin Sleep Med. (2018) 14:511–21. doi: 10.5664/jcsm.7034
23. Martinez-Garcia, MA, Soler-Cataluna, JJ, Ejarque-Martinez, L, Soriano, Y, Roman-Sanchez, P, Illa, FB, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. (2009) 180:36–41. doi: 10.1164/rccm.200808-1341OC
24. Aaronson, JA, van Bennekom, CA, Hofman, WF, van den Bezeij, T, Aardweg, JG, Groet, E, et al. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. Sleep. (2015) 38:1431–7. doi: 10.5665/sleep.4984
25. Filchenko, I, Duss, SB, Salzmann, S, Brill, AK, Korostovtseva, L, Amelina, V, et al. Early sleep apnea treatment in stroke (esatis) – a multicentre, randomised controlled, rater-blinded, clinical trial: the association of post-stroke cognition with sleep-disordered breathing and its treatment. J Sleep Res. (2024) 34:e14296. doi: 10.1111/jsr.14296
26. Dharmakulaseelan, L, and Boulos, MI. Sleep apnea and stroke. Chest. (2024) 166:857–66. doi: 10.1016/j.chest.2024.04.028
27. Boulos, MI, Dharmakulaseelan, L, Brown, DL, and Swartz, RH. Trials in sleep apnea and stroke: learning from the past to direct future approaches. Stroke. (2021) 52:366–72. doi: 10.1161/STROKEAHA.120.031709
28. Minnerup, J, Ritter, MA, Wersching, H, Kemmling, A, Okegwo, A, Schmidt, A, et al. Continuous positive airway pressure ventilation for acute ischemic stroke: a randomized feasibility study. Stroke. (2012) 43:1137–9. doi: 10.1161/STROKEAHA.111.637611
29. Kim, H, Im, S, Park, JI, Kim, Y, Sohn, MK, and Jee, S. Improvement of cognitive function after continuous positive airway pressure treatment for subacute stroke patients with obstructive sleep apnea: a randomized controlled trial. Brain Sci. (2019) 9:252. doi: 10.3390/brainsci9100252
30. Plomaritis, P, Theodorou, A, Lourentzos, K, Stefanou, MI, Palaiodimou, L, Papagiannopoulou, G, et al. Sleep-disordered breathing in acute stroke: a single-center, prospective, longitudinal study. J Clin Med. (2023) 12:986. doi: 10.3390/jcm12030986
31. Klingman, KJ, Billinger, SA, Britton-Carpenter, A, Bartsch, B, Duncan, PW, and Fulk, GD. Prevalence and detection of obstructive sleep apnea early after stroke. [Epubh ahead of preprint]. (2024). doi: 10.1101/2024.06.16.24309011v1
32. Brown, DL, Jiang, X, Li, C, Case, E, Sozener, CB, Chervin, RD, et al. Sleep apnea screening is uncommon after stroke. Sleep Med. (2019) 59:90–3. doi: 10.1016/j.sleep.2018.09.009
33. Johnson, KG, and Johnson, DC. When will it be time? Evaluation of Osa in stroke and tia patients. Sleep Med. (2019) 59:94–5. doi: 10.1016/j.sleep.2018.10.016
34. Swartz, RH, Bayley, M, Lanctot, KL, Murray, BJ, Cayley, ML, Lien, K, et al. Post-stroke depression, obstructive sleep apnea, and cognitive impairment: rationale for, and barriers to, routine screening. Int J Stroke. (2016) 11:509–18. doi: 10.1177/1747493016641968
35. Hale, E, Gottlieb, E, Usseglio, J, and Shechter, A. Post-stroke sleep disturbance and recurrent cardiovascular and cerebrovascular events: a systematic review and meta-analysis. Sleep Med. (2023) 104:29–41. doi: 10.1016/j.sleep.2023.02.019
36. Joanna Briggs Institute. JBI manual for evidence synthesis. (2024). Available online at: https://synthesismanual.jbi.global. (accessed March 27, 2025)
37. Tricco, AC, Lillie, E, Zarin, W, O'Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA Extension for Scoping Reviews PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
38. Ouzzani, M, Hammady, H, Fedorowicz, Z, and Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
39. Tricco, AC, Lillie, E, Zarin, W, O'Brien, K, Colquhoun, H, Kastner, M, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. (2016) 16:15. doi: 10.1186/s12874-016-0116-4
40. Graham, ID, and Logan, J. Innovations in knowledge transfer and continuity of care. Can J Nurs Res. (2004) 36:89–103.
41. Boland, L, Graham, ID, Legare, F, Lewis, K, Jull, J, Shephard, A, et al. Barriers and facilitators of pediatric shared decision-making: a systematic review. Implement Sci. (2019) 14:7. doi: 10.1186/s13012-018-0851-5
42. Hsieh, HF, and Shannon, SE. Three approaches to qualitative content analysis. Qual Health Res. (2005) 15:1277–88. doi: 10.1177/1049732305276687
Keywords: obstructive sleep apnea, stroke, sleep disorders screening, barriers and facilitators, scoping review protocol, ottawa model of research use
Citation: Lin Y, Zhao J, Xiao W, Ding F, Fang Z, Guo L and Lou S (2025) Barriers to and facilitators of implementing obstructive sleep apnea screening in stroke patients: a scoping review protocol. Front. Neurol. 16:1690372. doi: 10.3389/fneur.2025.1690372
Edited by:
Cristina Tiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Adria Arboix, Sacred Heart University Hospital, SpainKamalesh Tayade, All India Institute of Medical Sciences, India
Copyright © 2025 Lin, Zhao, Xiao, Ding, Fang, Guo and Lou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhui Lou, bG91c2hoQHpjbXUuZWR1LmNu
†ORCID: Shuhui Lou, orcid.org/0000-0002-1282-7764
 Yashi Lin
Yashi Lin Jiali Zhao1
Jiali Zhao1 Shuhui Lou
Shuhui Lou