Abstract
Background:
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality worldwide, with outcomes influenced by age and comorbidities. This study aimed to compare the clinical characteristics, surgical management, and outcomes of elderly and younger TBI patients.
Methods:
Between 2017 and 2022, 1,260 TBI patients admitted to our hospital were included and categorized into younger (18–59 years) and elderly (≥60 years) groups. Demographic data, injury mechanisms, types of brain trauma, surgical interventions, and discharge outcomes were analyzed.
Results:
Elderly patients had higher rates of comorbidities, with traffic accidents as the leading cause of injury and falls predominating in those aged ≥75 years. They showed a higher proportion of subdural hemorrhages, higher preoperative GCS scores, and required more mechanical ventilation and tracheostomy but underwent fewer decompressive craniectomies. In-hospital mortality was slightly lower in the elderly group, whereas rates of vegetative state and moderate-to-severe disability were higher, reflecting age-related differences in clinical outcomes and surgical management.
Conclusions:
Age significantly influences the clinical presentation, management strategies, and functional outcomes of TBI patients. Tailored surgical and postoperative care are crucial for optimizing survival and quality of life in elderly patients.
Introduction
Traumatic brain injury (TBI) is induced by external mechanical forces, including direct impact, rapid acceleration–deceleration, or penetrating head injury, and represents a major global health concern (1). According to a global epidemiological study conducted in 2016, the annual incidence and prevalence of traumatic brain injury (TBI) in the United States were 333 and 605 per 100,000 population, respectively. In China, the corresponding figures were 313 and 742 per 100,000 population, highlighting the substantial burden of TBI in both countries (2). Moreover, due to its large population, the absolute number of TBI cases in China surpasses that of most other countries, resulting in a considerable social and familial burden (3).
Globally, population aging further amplifies this challenge. In 2019, the number of people aged 60 years or older reached one billion, and this figure is projected to rise to 1.4 billion by 2030 and 2.1 billion by 2050 (4). This unprecedented demographic transition, particularly pronounced in developing countries, is expected to be accompanied by a marked increase in injuries and injury-related mortality among the elderly.
At present, research on neurosurgical approaches and prognostic outcomes in elderly patients with TBI remains limited, particularly in developing regions (5). There is an urgent need for further investigations in this underexplored yet rapidly expanding demographic to guide clinical practice and ensure adequate healthcare resource allocation.
Linyi People's Hospital, a Class A tertiary care center in Shandong Province, functions as the primary neurosurgical referral institution for Linyi, the largest developing city in the province, with a population of more than 11 million (6). Against this backdrop, we designed a retrospective study to evaluate the clinical characteristics, surgical interventions, and in-hospital outcomes of elderly patients undergoing neurosurgical treatment for TBI at our institution in recent years.
Methods
Study material
The present study was a retrospective analysis of patients with acute traumatic brain injury (TBI) who underwent neurosurgical treatment at Linyi People's Hospital between 2017 and 2022. Eligible patients were those consecutively admitted within 24 h of injury, aged 18 years or older, with cerebral computed tomography (CT) demonstrating intracranial signs of trauma. Clinical data were extracted from the hospital's electronic medical records and included demographic variables (age, sex), injury-related factors (cause of injury, associated injuries), medical history (comorbidities, prior conditions), preoperative neurological status [Glasgow Coma Scale (GCS) score], treatment details, and outcomes assessed by the Glasgow Outcome Scale (GOS) at discharge.
Inclusion criteria: Patients who underwent neurosurgical treatment during hospitalization, including decompressive craniectomy (DC), craniotomy, skull burr holes/cranial trephination and drainage, intracranial pressure (ICP) catheter implantation, and skull fracture repair.
Exclusion criteria: (1) Patients who did not undergo cranial surgery during hospitalization; (2) Patients who died within 24 h of admission; (3) Patients younger than 18 years; (4) Patients with incomplete electronic clinical information.
In this study, ICP monitoring catheters included both intraventricular and intraparenchymal devices. Cranial trephination and drainage included external ventricular drainage (EVD) and evacuation/drainage of intracranial hematomas. Drainage catheters can be placed epidurally, subdurally, within a hematoma, or intraventricularly. Skull fracture repair procedures included reduction of skull fracture, cranioplasty, elevation and debridement, dural repair, and fixation.
Statistical analysis
The distribution of variables was assessed using the Kolmogorov–Smirnov test. Continuous variables that did not follow a normal distribution were analyzed using the Mann–Whitney U test and presented as medians with interquartile ranges (IQR). Categorical variables were expressed as absolute numbers and percentages, and comparisons were made using the χ2 test when no inherent order was present. A p-value of < 0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA).
Results
As shown in Figure 1, from 2017 to 2022, a total of 5,112 TBI patients were admitted to our hospital. After applying exclusion criteria, 1,260 patients were included in the analysis. Patients were divided into two age groups: the Younger Group (18 ≤ age < 60) and the Elderly Group (age ≥ 60). Glasgow Outcome Scale (GOS) scores at discharge were recorded for all patients. Demographic characteristics and clinical management for the two groups are summarized in Table 1. Compared with younger patients, the elderly group had a lower proportion of male patients but a significantly higher prevalence of diabetes, hypertension, and prior stroke.
Figure 1

Patient inclusion and exclusion flowchart.
Table 1
| Variables | Younger group (n = 832) | Elderly group (n = 428) | p-Value |
|---|---|---|---|
| Gender (male), n (%) | 643 (77.3) | 304 (71.0) | 0.015 |
| Diabetes, n (%) | 37 (4.4) | 42 (9.8) | < 0.001 |
| Hypertension, n (%) | 71 (8.5) | 95 (22.2) | < 0.001 |
| Cerebral stroke, n (%) | 13 (1.6) | 16 (3.7) | 0.015 |
| Aetiology, n (%) | < 0.001 | ||
| Road traffic injuries | 367 (44.1) | 237 (55.4) | |
| Fall | 410 (49.3) | 178 (41.6) | |
| Violence | 55 (6.6) | 13 (3.0) | |
| TBI subtype, n(%) | |||
| CC | 703 (84.5) | 340 (79.4) | 0.024 |
| SDH | 664 (79.8) | 383 (89.5) | < 0.001 |
| SAH | 656 (78.8) | 324 (75.7) | 0.203 |
| EDH | 415 (49.9) | 82 (19.2) | < 0.001 |
| SF | 352 (42.3) | 110 (25.7) | < 0.001 |
| Traumatic epilepsy, n (%) | 38 (4.6) | 23 (5.4) | 0.528 |
| Preoperation GCS | 6 (5.9) | 8 (6.10) | 0.012 |
| Cerebral herniation, n (%) | 157 (18.9) | 73 (17.1) | 0.43 |
| Systemic hypotension, n (%) | 45 (5.4) | 31 (7.2) | 0.063 |
| Hypoxia, n (%) | 106 (12.7) | 68 (15.9) | 0.034 |
| Cardiopulmonary resuscitation, n (%) | 14 (1.7) | 14 (3.3) | 0.07 |
| Concomitant injuries, n(%) | |||
| Chest | 257 (30.9) | 141 (32.9) | 0.457 |
| Abdomen | 14 (1.7) | 7 (1.6) | 0.951 |
| Limbs | 50 (6.0) | 18 (4.2) | 0.18 |
| Spine | 115 (13.8) | 58 (13.6) | 0.895 |
| Neurosurgical treatment, n(%) | |||
| DC | 430 (51.7) | 190 (44.4) | 0.014 |
| ICP monitoring | 498 (59.9) | 265 (61.9) | 0.479 |
| Cranial trephination and drainage | 314 (37.7) | 224 (52.3) | < 0.001 |
| Craniotomy | 295 (35.5) | 128 (29.9) | 0.059 |
| Skull fracture repair surgery | 217 (26.1) | 67 (15.7) | < 0.001 |
| Unplanned second surgery | 52 (6.2) | 26 (6.1) | 0.521 |
| Ventilator, n (%) | 27 (3.2) | 26 (6.1) | 0.035 |
| Tracheotomy, n (%) | 30 (3.6) | 30 (7.0) | 0.007 |
| Hospital stay, days | 29 (25,30) | 28 (24.29) | 0.874 |
| In-hospital mortality, n (%) | 141 (16.9) | 60 (14.1) | 0.039 |
| Hospital cost, ¥ | |||
| Medical care fees | 62,880.840 (38,078.943, 84,775.105) | 59,431.845 (38,574.280, 66,840.705) | 0.71 |
| Medicine expenses | 47,278.685 (27,691.293, 59,220.273) | 41,976.510 (28,653.140, 57,743.735) | 0.339 |
| Surgery fee | 28,546.155 (21,586.573, 37,982.695) | 26,850.605 (16,165.480, 35,694.715) | < 0.001 |
| Cost of anti-infective drugs | 6,024.095 (2,556.635, 12,578.475) | 6,556.570 (2,152.333, 102,77.755) | 0.744 |
Demographic characteristics and clinical treatment of the two groups.
GCS, Glasgow Coma Scale; CC, cerebral contusion; SAH, traumatic subarachnoid hemorrhage; SDH, acute subdural hematoma; SF, skull fractures (including the base of skull fractures); EDH, acute epidural hematoma; DC, decompressive craniectomy; ICP, intracranial pressure.
Traffic accidents were the leading cause of injury in the elderly group (55.4%), followed by falls (41.6%). To further examine injury mechanisms, the elderly group was subdivided into four age subgroups, as shown in Table 2. Notably, falls were the primary cause of injury only in patients aged 75 years or older.
Table 2
| Variables | 60–64 years old (n = 143) | 65–69 years old (n = 140) | 70–74 years old (n = 76) | ≥75 years old (n = 69) | p-Value |
|---|---|---|---|---|---|
| Aetiology, n(%) | |||||
| Road traffic injuries | 73 (51) | 83 (59.3) | 48 (63.2) | 33 (47.8) | 0.292 |
| Fall | 63 (44.1) | 54 (38.6) | 27 (35.5) | 34 (49.3) | |
| Violence | 7 (4.9) | 3 (2.1) | 1 (1.3) | 2 (2.9) | |
Comparison of etiology of TBI in different age groups.
Regarding the types of brain trauma, the elderly group exhibited lower rates of brain contusions, epidural hematomas, and skull/skull base fractures, but a higher proportion of subdural hemorrhages compared with the younger group (Table 1). The comparison of traumatic brain injury types among different age groups is presented in Figure 2. Preoperatively, elderly patients had higher median GCS scores (median 8). No significant differences were observed between the groups in rates of brain herniation or post-traumatic epilepsy. However, the elderly group had higher rates of hypoxemia (15.9 vs. 12.7%) and mechanical ventilation (6.1 vs. 3.2%). Rates of cardiopulmonary resuscitation and hypotension were also higher in the elderly group, though these differences were not statistically significant.
Figure 2

Comparison of brain trauma types in different age groups.
There were no significant differences in concomitant injuries between the two groups. Regarding surgical management, the elderly group had lower rates of decompressive craniectomy (DC) and skull fracture repair procedures (44.4 vs. 51.7%; 15.7 vs. 26.1%) but a higher rate of cranial trephination and drainage (52.3 vs. 37.7%), with statistically significant differences. No significant differences were observed in ICP monitoring, craniotomy, or unplanned secondary surgeries.
In-hospital mortality was lower in the elderly group than in the younger group (14.1 vs. 16.9%), whereas the rate of tracheostomy was higher (7 vs. 3.6%). Length of hospital stay was similar between the groups. Surgical costs were lower in the elderly group, while other hospitalization costs (including medical care, medications, and anti-infective drugs) were comparable.
Surgical approaches were tailored to patient conditions, such as DC combined with ICP catheter implantation or DC combined with cranial trephination and drainage. The comparative surgical characteristics between the two groups are shown in Figure 3.
Figure 3

Selection and combination of cranial surgery types in the two groups. UpSet plots were used to display cranial surgery types. Four primary cranial surgery types about TBI lesion (DC decompressive craniectomy; ICP, intracranial pressure monitoring; Drainage, cranial trephination and drainage; craniotomy).
Prognostic comparisons based on discharge GOS scores are shown in Figure 4. The elderly group exhibited lower mortality and complete recovery rates than the younger group, whereas the proportion of patients in a vegetative state or with moderate-to-severe disability was higher in the elderly group.
Figure 4
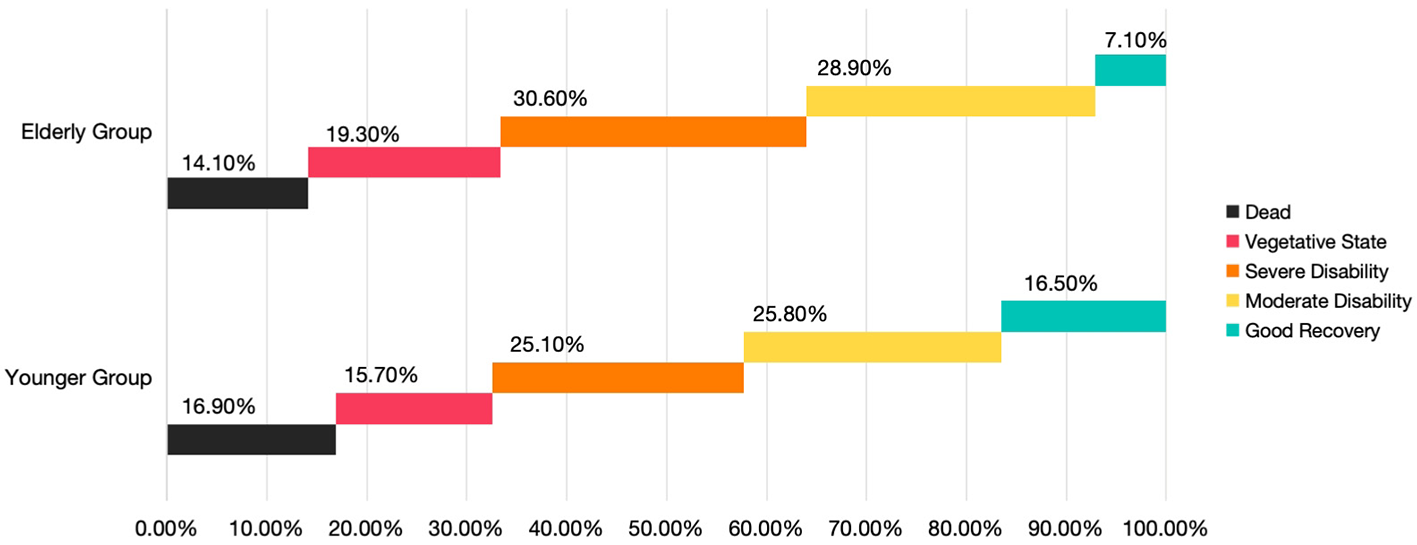
Comparison of percentage of patients between the two groups based on GOS class at hospital discharge.
Discussion
Our study analyzed the demographic characteristics, surgical approaches, and prognostic outcomes of elderly patients with acute TBI who underwent neurosurgical intervention. We found that elderly patients were more likely to receive minimally invasive procedures of shorter duration. Although their short-term mortality rate was lower, these patients exhibited a higher likelihood of moderate to severe postoperative disability, necessitating intensive care and prolonged rehabilitation. These findings provide real-world, evidence-based insights to inform future management strategies for elderly patients with TBI.
In our cohort, the main reason for injury among elderly patients was road traffic accidents. By contrast, a study conducted in the United Kingdom by Kehoe et al. (7) reported that more than 80% of elderly patients (≥65 years) sustained TBIs as a result of falls. Similarly, a large epidemiological survey in China indicated that incidental falls (n = 1,044, 43.23%) were the leading cause of TBI in old patients (8). These observations highlight a distinct difference in the predominant mechanisms of injury between developed and developing regions, underscoring the need for region-specific prevention and intervention strategies.
In this study, the incidence of subduralhemorrhage among elderly TBI patients increased with advancing age. This high frequency is thought to be related to age-associated brain atrophy, which enlarges the subdural space and predisposes elderly individuals to such lesions (9).
Elderly individuals frequently present with preexisting conditions such as hypertension and diabetes, making them more vulnerable to post-TBI complications including asphyxia, hypoxia, and shock. It is well-recognized that aging reduces physiological reserve, and the presence of comorbidities—as well as their ongoing treatments—can significantly influence the disease course and clinical outcomes (10). Moreover, the management of blood pressure in elderly hypertensive patients following TBI remains a subject of debate (11, 12). In particular, the optimal systolic blood pressure (SBP) threshold has recently been questioned, especially in developing countries where advanced monitoring tools such as intracranial perfusion pressure and cerebral blood flow measurements are not routinely available (13).
In our study, elderly patients underwent fewer decompressive craniectomies (DC) and craniotomies compared with younger patients. For this population, surgeons appeared to favor shorter, less invasive procedures aimed at rapidly alleviating life-threatening conditions. These findings reflect current trends in surgical decision-making. DC is generally considered a life-saving intervention for TBI patients with pronounced intracranial mass effect and refractory intracranial hypertension (14, 15). However, evidence regarding its long-term benefit remains mixed. In the CENTER-TBI study, comparative effectiveness analyses in patients with acute subdural hematoma demonstrated that institutional preference for an early surgical strategy, compared with initial conservative management, was not significantly associated with improved outcomes (OR 0.92, 95% CI: 0.77–1.09) (16). Similarly, results from the TRACK-TBI trials reported unfavorable long-term neurological outcomes in patients undergoing DC (17).
In addition, the indications for DC in geriatric patients remain undefined. In cases of epidural hematoma (EDH), emergency trephination—creating a small burr hole in the skull—can serve as a life-saving measure by providing temporary decompression of the hematoma (18, 19). Craniotomy remains the standard treatment for acute subdural hematoma (SDH), and placement of a subdural drain postoperatively facilitates removal of residual blood or cerebrospinal fluid (CSF), thereby preventing dangerous elevations in intracranial pressure (20). For selected SDH patients, particularly elderly individuals or those with unstable vital signs, preoperative trephination and drainage performed in the emergency setting may help reduce intracranial pressure, shorten operative duration, and ultimately decrease mortality (17, 21).
In current craniocerebral trauma surgery, ICP monitoring is often initiated first. Guided by ICP readings, elevated intracranial pressure can be gradually and controlledly reduced. Consequently, the incidence of acute encephalocele and contralateral delayed epidural hematoma in patients undergoing stepwise decompression is significantly lower than in those receiving conventional decompression (22). Moreover, this controlled decompression approach can also reduce the rates of vegetative state and 6-month postoperative mortality (23).
Beyond the increasing adoption of ICP monitoring, clinicians are employing more precise techniques to optimize the placement of external ventricular drain (EVD) tubes (24, 25). Minimally invasive strategies are also gaining traction; for instance, initiating a needle craniotomy for extradural hematoma using an EZ-IO device is feasible, allowing more rapid relief of intracranial pressure and providing additional time for definitive neurosurgical intervention (26). According to the Monro-Kellie doctrine, fluid removal decreases intracranial pressure, thereby improving cerebral perfusion and facilitating the delivery of osmotic agents, further supporting medical resuscitation efforts (27).
In the analysis of surgical characteristics, a higher proportion of elderly patients underwent cranial trephination and drainage, with or without ICP catheter implantation. Although a detailed subgroup analysis has not yet been performed, there is reason to believe that minimally invasive surgery may be more appropriate for elderly patients under close ICP monitoring. First, due to age-related brain atrophy, elderly patients generally have greater tolerance to intracranial hypertension and cerebral edema, and the need for decompressive craniectomy (DC) is therefore less urgent than in younger individuals. Second, elderly patients often present with multiple comorbidities, and the surgical trauma and physiological stress associated with conventional craniotomy may exacerbate their pre-existing conditions. Shen et al. (28) reported that patients aged ≥75 years had poor prognoses following traditional surgical interventions.
Wong et al. (29) prospectively collected data on consecutive trauma patients between 2001 and 2008 and demonstrated that advancing age was an unfavorable prognostic factor among patients with multiple trauma requiring neurosurgical intervention. In contrast, Lau et al. (30) prospectively enrolled consecutive patients with head trauma from 2006 to 2009 and found no statistically significant differences between age groups in 30-day mortality or recovery to baseline functional status. In the present study, which included patients admitted between 2017 and 2022, the relatively low in-hospital mortality observed among elderly patients with TBI may be explained by the increasing adoption of ICP monitoring and advanced life-support techniques, including mechanical ventilation. These advances have enabled elderly patients to better withstand the acute critical phase following injury (31).
The results of this study also indicate a higher prevalence of elderly TBI patients in a vegetative state or with severe disability, which aligns with findings from previous studies. While aggressive intensive care unit (ICU) management can improve survival in geriatric TBI patients, it is associated with an increased likelihood of discharge with severe disability (32). Elderly patients often require longer hospital stays, more home health services, and additional support during the first year post-injury (8, 33). In real-world settings, the rehabilitation and care of patients with severe disabilities or vegetative states present substantial challenges. Researchers such as Sveen et al. (34) have recommended a stronger clinical focus on older TBI patients, emphasizing the importance of continuity of care.
Several limitations should be acknowledged. First, potential selection bias may exist due to the relatively small sample size and the single-center study design. Second, the long-term prognosis of elderly patients with TBI was not evaluated. In addition, this study lacks further in-depth analyses, including the assessment of the strengths and limitations of different surgical techniques, the incorporation of the time interval between injury and surgery, and the evaluation of risk factors among patients who developed a vegetative state or severe disability. These aspects warrant further investigation.
In future research, we plan to compare patients with similar injury locations and hemorrhage types who undergo different surgical procedures, incorporate the precise interval between injury and surgery, and perform long-term follow-up to obtain a more comprehensive understanding and generate more robust conclusions. For elderly patients, future outcomes should emphasize not only survival but also changes in quality of life, which may serve as an indirect indicator of the effectiveness of local rehabilitation systems.
In summary, our study found that the in-hospital mortality rate of elderly patients with TBI decreased after surgical treatment, but there were still many patients with severe disability and vegetative state. We concluded that minimally invasive surgical procedures (such as cranial trephination and drainage) and life-support interventions (including mechanical ventilation) are crucial measures for reducing in-hospital mortality among patients with TBI. However, severe disability and a vegetative state remain conditions that require intensive care and long-term rehabilitation. For this specific patient population, greater efforts are needed to minimize disability and promote postoperative neurological recovery.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Linyi People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because As this study was a retrospective study and did not involve the leakage of subject information, the ethics committee approved the exemption of informed consent.
Author contributions
X-tF: Investigation, Validation, Methodology, Resources, Supervision, Writing – original draft. H-yZ: Writing – review & editing, Data curation, Formal analysis, Resources. C-fW: Writing – original draft, Software, Methodology, Validation, Visualization. B-xG: Resources, Writing – original draft, Supervision, Investigation, Data curation. JL: Writing – review & editing, Investigation, Resources, Data curation, Project administration. YX: Project administration, Investigation, Resources, Writing – original draft, Visualization, Formal analysis. Y-gL: Resources, Funding acquisition, Writing – review & editing, Validation, Supervision. S-jW: Visualization, Resources, Data curation, Writing – review & editing, Formal analysis, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Keelan RE Mahoney EJ Sherer M Hart T Giacino J Bodien YG et al . Neuropsychological characteristics of the confusional state following traumatic brain injury. J Int Neuropsychol Soc. (2019) 25:302–13. doi: 10.1017/S1355617718001157
2.
GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators . Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. (2019) 18:56–87. doi: 10.1016/S1474-4422(21)00383-5
3.
Jiang JY Gao GY Feng JF Mao Q Chen LG Yang XF et al . Traumatic brain injury in China. Lancet Neurol. (2019) 18:286–95. doi: 10.1016/S1474-4422(18)30469-1
4.
The World Health Organization (WHO) . Ageing. Available online at: http://www.who.int/health-topics/ageing#tab=tab_1 (Accessed August 5, 2025).
5.
Hume CH Wright BJ Kinsella GJ . Systematic review and meta-analysis of outcome after mild traumatic brain injury in older people. J Int Neuropsychol Soc. (2022) 28:736–55. doi: 10.1017/S1355617721000795
6.
Wang SJ Cheng ZX Fan XT Lian YG . Development of an optimized risk score to predict short-term death among acute myocardial infarction patients in rural China. Clin Cardiol. (2021) 44:699–707. doi: 10.1002/clc.23598
7.
Kehoe A Smith JE Bouamra O Edwards A Yates D . Older patients with traumatic brain injury present with a higher GCS score than younger patients for a given severity of injury. Emerg Med J. (2016) 33:381–5. doi: 10.1136/emermed-2015-205180
8.
Yang C Lang L He Z Hui J Jiang J Gao G et al . Epidemiological characteristics of older patients with traumatic brain injury in China. J Neurotrauma. (2022) 39:850–9. doi: 10.1089/neu.2021.0275
9.
Karibe H Hayashi T Narisawa A Kameyama M Nakagawa A Tominaga T . Clinical characteristics and outcome in elderly patients with traumatic brain injury: for establishment of management strategy. Neurol Med Chir. (2017) 57:418–25. doi: 10.2176/nmc.st.2017-0058
10.
Böhm JK Güting H Thorn S Schäfer N Rambach V Schöchl H et al . Global characterisation of coagulopathy in isolated traumatic brain injury (iTBI): a center-TBI analysis. Neurocrit Care. (2021) 35:184–96. doi: 10.1007/s12028-020-01151-7
11.
Robinson CP . Moderate and severe traumatic brain injury. Continuum. (2021) 27:1278–300. doi: 10.1212/CON.0000000000001036
12.
Maas AIR Menon DK Manley GT Abrams M Åkerlund C Andelic N et al . Traumatic brain injury: progress and challenges in prevention, clinical care, and research. Lancet Neurol. (2022) 21:1004–60. doi: 10.1016/S1474-4422(22)00307-6
13.
Huijben JA Wiegers EJA Lingsma HF Citerio G Maas AIR Menon DK et al . Changing care pathways and between-center practice variations in intensive care for traumatic brain injury across Europe: a CENTER-TBI analysis. Intensive Care Med. (2020) 46:995–1004. doi: 10.1007/s00134-020-05965-z
14.
Hutchinson PJ Kolias AG Timofeev IS Corteen EA Czosnyka M Timothy J et al . Trial of decompressive craniectomy for traumatic intracranial hypertension. N Engl J Med. (2016) 375:1119–30. doi: 10.1056/NEJMoa1605215
15.
Geeraerts T Velly L Abdennour L Asehnoune K Audibert G Bouzat P et al . Management of severe traumatic brain injury (first 24 hours). Anaesth Crit Care Pain Med. (2018) 37:171–86. doi: 10.1016/j.accpm.2017.12.001
16.
van Essen TA van Erp IAM Lingsma HF Pisică D Yue JK Singh RD et al . Comparative effectiveness of decompressive craniectomy versus craniotomy for traumatic acute subdural hematoma (CENTER-TBI): an observational cohort study. EClinicalMedicine. (2023) 63:102161. doi: 10.1016/j.eclinm.2023.102161
17.
Yue JK Kanter JH Barber JK Huang MC van Essen TA Elguindy MM et al . Clinical profile of patients with acute traumatic brain injury undergoing cranial surgery in the United States: report from the 18-centre TRACK-TBI cohort study. Lancet Reg Health Am. (2024) 39:100915. doi: 10.1016/j.lana.2024.100915
18.
Smith SW Clark M Nelson J Heegaard W Lufkin KC Ruiz E . Emergency department skull trephination for epidural hematoma in patients who are awake but deteriorate rapidly. J Emerg Med. (2010) 39:377–83. doi: 10.1016/j.jemermed.2009.04.062
19.
Ma H Cui Q Wang B Chen J Wei Z . Comparison of burr hole drainage and craniotomy for acute liquid epidural hematoma in pediatric patients. Childs Nerv Syst. (2024) 40:1471–6. doi: 10.1007/s00381-023-06258-8
20.
Hutchinson PJ Adams H Mohan M Devi BI Uff C Hasan S et al . Decompressive Craniectomy versus craniotomy for acute subdural hematoma. New Engl J Med. (2023) 388:2219–29. doi: 10.1056/NEJMoa2214172
21.
Lu T Guan J . Preoperative trepanation and drainage for acute subdural hematoma: two case reports. Exp Ther Med. (2015) 10:225–30. doi: 10.3892/etm.2015.2456
22.
Martínez-Palacios K Vásquez-García S Fariyike OA Robba C Rubiano AM Noninvasive ICP Monitoring International Consensus Group . Using optic nerve sheath diameter for intracranial pressure (ICP) monitoring in traumatic brain injury: a scoping review. Neurocrit Care. (2024) 40:1193–212. doi: 10.1007/s12028-023-01884-1
23.
Sun G Shi L Pan T Li X Zhang S . Technique of ICP monitored stepwise intracranial decompression effectively reduces postoperative complications of severe bifrontal contusion. Front Neurol. (2016) 7:56. doi: 10.3389/fneur.2016.00056
24.
Stuart MJ Mehigan B Colbran RE Withers TK Ng W . Orthogonal external ventricular drain (EVD) trajectory from burr holes sited by junior neurosurgical staff is superior to freehand placement: an in-silico model. J Clin Neurosci. (2021) 94:65–9. doi: 10.1016/j.jocn.2021.09.041
25.
Huang K Zhan X Xue T Chen T Liao Z Luo J et al . Insights from using biplanar intersection for freehand frontal ventriculostomy: a retrospective case-control study with virtual simulation. Quant Imaging Med Surg. (2025) 15:1669–78. doi: 10.21037/qims-24-1381
26.
McClung CD Anshus JS Anshus AJ Baker SR . Bedside craniostomy and serial aspiration with an intraosseous drill/needle to temporize an acute epidural hemorrhage with mass effect. World Neurosurg. (2020) 142:218–21. doi: 10.1016/j.wneu.2020.06.215
27.
Wu JW Wang YF Hseu SS Chen ST Chen YL Wu YT et al . Brain volume changes in spontaneous intracranial hypotension: revisiting the Monro-Kellie doctrine. Cephalalgia. (2021) 41:58–68. doi: 10.1177/0333102420950385
28.
Shen H Liu H He J Wei L Wang S . Risk factors of prognosis in older patients with severe brain injury after surgical intervention. Eur J Med Res. (2023) 28:479. doi: 10.1186/s40001-023-01473-0
29.
Wong GK Graham CA Ng E Yeung JH Rainer TH Poon WS et al . Neurological outcomes of neurosurgical operations for multiple trauma elderly patients in Hong Kong. J Emerg Trauma Shock. (2011) 4:346–50. doi: 10.4103/0974-2700.83861
30.
Lau D El-Sayed AM Ziewacz JE Jayachandran P Huq FS Zamora-Berridi GJ et al . Postoperative outcomes following closed head injury and craniotomy for evacuation of hematoma in patients older than 80 years. J Neurosurg. (2012) 116:234–45. doi: 10.3171/2011.7.JNS11396
31.
Skaansar O Tverdal C Rønning PA Skogen K Brommeland T Røise O et al . Traumatic brain injury-the effects of patient age on treatment intensity and mortality. BMC Neurol. (2020) 20:376. doi: 10.1186/s12883-020-01943-6
32.
Hawryluk GWJ Citerio G Hutchinson P Kolias A Meyfroidt G Robba C et al . Intracranial pressure: current perspectives on physiology and monitoring. Intensive Care Med. (2022) 48:1471–81. doi: 10.1007/s00134-022-06786-y
33.
Stein DM Kozar RA Livingston DH Luchette F Adams SD Agrawal V et al . Geriatric traumatic brain injury-what we know and what we don't. J Trauma Acute Care Surg. (2018) 85:788–98. doi: 10.1097/TA.0000000000001910
34.
Sveen U Guldager R Soberg HL Andreassen TA Egerod I Poulsen I et al . Rehabilitation interventions after traumatic brain injury: a scoping review. Disabil Rehabil. (2022) 44:653–60. doi: 10.1080/09638288.2020.1773940
Summary
Keywords
traumatic brain injury, head injury, neurosurgery, clinical outcome, geriatric patients, elderly
Citation
Fan X-t, Zhao H-y, Wu C-f, Gao B-x, Li J, Xu Y, Lian Y-g and Wang S-j (2025) Neurosurgical management of geriatric patients with traumatic brain injury in a medium-developed Chinese city: a recent-years overview. Front. Neurol. 16:1691924. doi: 10.3389/fneur.2025.1691924
Received
24 August 2025
Accepted
17 October 2025
Published
07 November 2025
Volume
16 - 2025
Edited by
Hamid Ferdosi, George Washington University, United States
Reviewed by
Nenad Koruga, Osijek Clinical Hospital Center, Croatia
Pukovisa Prawiroharjo, Dr. Cipto Mangunkusumo National Central Public Hospital, Indonesia
Updates
Copyright
© 2025 Fan, Zhao, Wu, Gao, Li, Xu, Lian and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-gang Lian, lyg3143@163.comSheng-ji Wang, 18954959359@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.