- 1Department of Immunity and Inflammation, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, United States
- 2St. Francis Hospital and Medical Center, University of Connecticut School of Medicine, Hartford, CT, United States
- 3Mellen Center for Multiple Sclerosis, Cleveland Clinic, Cleveland, OH, United States
- 4Neuromuscular Center, Cleveland Clinic, Cleveland, OH, United States
Recent advances in the understanding of immune-mediated neurological disorders have led to a paradigm shift toward pathophysiology-directed therapies. Central to this progress is a deeper appreciation of the complement system, a key component of innate immunity, and its role in neuroinflammation. Complement activation, while essential for host defense and tissue homeostasis, has been implicated increasingly in a spectrum of central and peripheral neurological disorders where complement dysregulation contributes to inflammation, cellular damage, and disease progression. Breakthroughs in conditions such as myasthenia gravis and aquaporin-4 IgG-positive neuromyelitis optica spectrum disorder underscore the therapeutic potential of targeting complement pathways to improve patient outcomes. In this review, we provide a comprehensive overview of complement activation pathways, regulatory controls, and their involvement in various autoimmune neurological diseases. We also highlight current and emerging complement-targeted therapies, many of which are now completing or entering clinical trials. Together, these insights offer a holistic perspective on the complement system as both a contributor to and a target for intervention in neurological diseases.
1 Introduction
Over the past several years, our understanding of immune-mediated neurological disorders has evolved dramatically, catalyzing a new era of pathophysiology-directed therapies. Advances in complement-targeted therapeutics have been particularly striking in diseases such as myasthenia gravis (MG) and aquaporin-4 IgG-positive neuromyelitis optica spectrum disorder (AQP4 + NMOSD). These diseases now serve as a blueprint for the therapeutic potential of complement modulation to yield significant improvements in function and quality of life.
The complement system is an important component of innate immunity and consists of a cascade of proteolytic events, collectively defined as complement activation, that lead to the opsonization of pathogens, recruitment of immune cells, and direct lysis of target cells (1, 2). While its primary function is to protect the host from infections and to clear apoptotic cells, dysregulation of the complement system has been implicated in a range of neuroinflammatory and autoimmune neurological disorders, where excessive or misdirected complement activation contributes to inflammation, cellular damage, and progressive neurological impairment.
This review provides a comprehensive view of complement systems in selected autoimmune neurological diseases. We examine complement activation pathways, regulatory mechanisms, and pathological roles in various nervous system disorders. We highlight the most promising complement-targeted therapies—ranging from monoclonal antibodies to fusion proteins and cyclic peptides—many of which have already gained regulatory approval or are currently in pivotal clinical trials. By providing a holistic view of complement biology in the nervous system, we aim to clarify its role as both a mediator of disease and a therapeutic target in neurological disorders.
2 Overview of the complement system
2.1 Three complement activation pathways
Complement activation proceeds through three distinct pathways: classical, lectin, and the alternative pathways (Figure 1). The primary distinction among these pathways lies in their initiation mechanisms. The classical and lectin pathways require specific pattern recognition molecules for their initiation. In contrast, the alternative pathway does not depend on external recognition but instead perpetuates its activity through spontaneous hydrolysis and self-amplification. Once activated, these pathways converge on a shared cascade involving the cleavage and activation of several complement proteins, many of which are produced by various cell types. Although the majority of complement proteins are synthesized by hepatocytes (3–5), other cell types, including monocytes (6), fibroblasts (7), endothelial cells (8), epithelial cells (9), and glial cells (10, 11), are also capable of producing complement components. Most complement proteins function as acute-phase proteins, and their expression can be upregulated by pro-inflammatory cytokines (8, 12), enabling a rapid response during acute inflammatory states (13, 14).
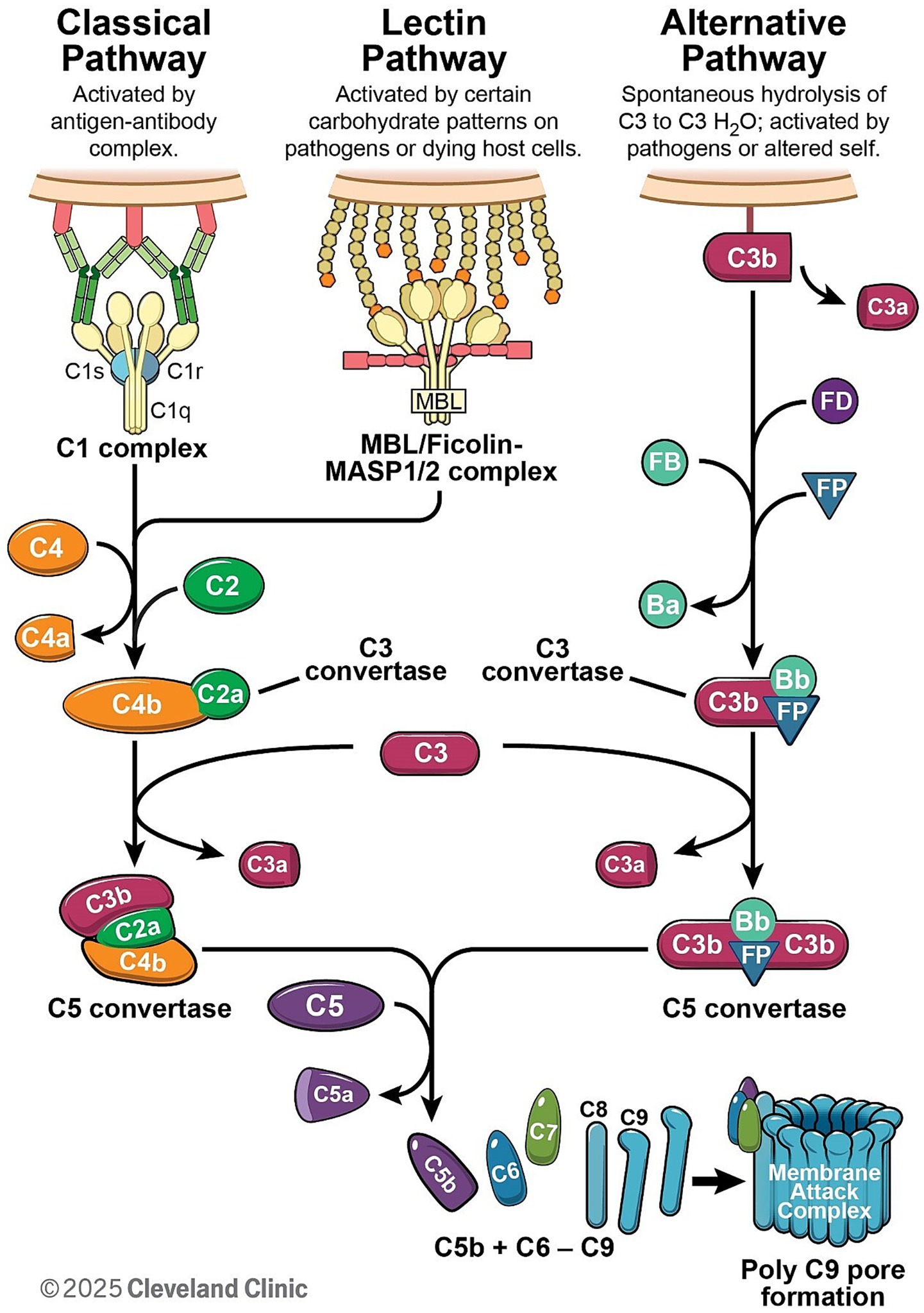
Figure 1. The complement cascade can be initiated through one of three pathways: the classical, lectin, or alternative pathway. The classical pathway is initiated when the C1 complex binds to antibody–antigen complexes, whereas the lectin pathway is activated through the recognition of pathogen-associated molecular patterns. Both pathways culminate in the formation of the C3 convertase C4b2a. In contrast, the alternative pathway is spontaneously activated and forms the C3 convertase C3bBb with the assistance of Factor B (FB) and Factor D (FD), and its stability is further enhanced by properdin (FP). All three pathways converge at the cleavage of complement component C3. The cleavage of C5 facilitates the formation of membrane attack complex.
The classical pathway (CP) is initiated by antigen–antibody immune complexes (Figure 1). C1q is the recognition molecule that binds to the Fc portion of IgG or IgM within the immune complexes and triggers CP activation. C1q binding triggers activation of the C1 complex (C1qC1r2C1s2), which then cleaves downstream C4 and C2 to generate the CP C3 convertase C4b2a, leading to C3 cleavage (Figure 1). Notably, complement recruitment and activation is highly subclass dependent and is determined mainly by their affinity for C1q, which follows the order IgG3 > IgG1 > IgG2 > IgG4 (15). The hinge also regulates the Fab–Fc flexibility, which is IgG3 > IgG1 > IgG4 > IgG2 (16) and, in turn, affects immune complex formation and C1q engagement (17). As a result, IgG1 and IgG3 are potent activators of complement, IgG2 is weak, and IgG4 has no or little capacity to activate complement.
The lectin pathway is triggered by mannose-binding lectin (MBL) or ficolins binding to pathogen-associated molecular patterns (Figure 1). For example, MBL recognizes specific carbohydrate patterns that are rarely present on host cells but are expressed frequently on pathogens and dying cells (18). Ficolins bind to acetyl groups such as N-acetylglucosamine (GlcNAc) and N-acetylgalactosamine (GalNAc) found on the surface of bacteria (19, 20). As a result, MBL/Ficolins activate MASP1 or MASP2 in the blood, resulting in cleavage of C4 and C2 and assembly of the C3 convertase C4b2a, which promotes cleavage of C3 (Figure 1).
Uniquely, the alternative pathway is spontaneously activated via low-level hydrolysis of C3 into C3(H2O) (so called complement “tickover”), which occurs naturally in the blood at a low rate. The hydrolysis of C3 to C3(H2O) can be accelerated through interactions with various biological and artificial interfaces, such as biomaterial surfaces, lipid membranes, and molecular complexes (21). A low level of spontaneous activation of C3 also allows Factor B and Factor D to form a C3 convertase C3(H2O)Bb, which cleaves further C3 into active fragments C3a and C3b. C3b can then bind to local cell surfaces and amplify the cascade through the AP convertase C3bBb (Figure 1). MASP-1/3 have also been proposed to have a role in regulating basal Factor D activity (22, 23). More importantly, the alternative pathway serves as the primary amplification mechanism within the complement system, amplifying immune responses via C3b generation. This amplification can be initiated not only by the alternative pathway itself, but also through C3b produced via the classical or lectin pathways.
2.2 Consequences of initial complement activation
Upon activation, the classical and lectin pathways generate C4b2a convertase, whereas the alternative pathway forms C3bBb convertase (Figure 1). These convertases cleave C3, a plasma protein of high concentration (24), leading to the formation of C5 convertase (C4b2aC3b for classical and lectin pathway, and C3bBbC3b for alternative pathway). These C5 convertases drive the assembly of the membrane attack complex (MAC; C5b-9), which disrupts cellular membranes, altering ion homeostasis (Figure 1). MAC is widely recognized as a potent driver of cellular function disruptions, inflammatory responses, and progressive tissue damage, such as demyelination (25–27) and axon degeneration in neurons (28, 29).
During complement activation, potent anaphylatoxins such as C3a and C5a are released during C3 and C5 cleavage (Figure 1), also triggering inflammatory responses via C3a or C5a receptors, which are G protein-coupled receptors. These receptors are widely expressed on cells of the myeloid lineage, such as neutrophils, basophils, eosinophils, mast cells, macrophages and microglia (30), and are also found on astrocytes (30), neurons (31), and endothelial cells (32, 33). In central nervous system (CNS) disorders, C3a- and C5a-mediated signaling promotes microglial activation (34), leading to phagocytosis of synapses (as seen in neurodegenerative diseases) and destruction of oligodendrocytes (35). In peripheral nervous system (PNS) diseases such as Guillain-Barre syndrome (GBS), antibodies against gangliosides activate the complement system. The resultant complement deposition on the surface of Schwann cells and axons leads not only to direct injury, but also to recruitment of macrophages. These macrophages then invade the myelin and axons, causing further injury and propagating the immune response (29). Although C4a is also produced during complement activation, its anaphylatoxin activity is significantly weaker than that of C3a and C5a (36) and is less studied in neurological disorders.
3 Regulation of complement activation and dysregulation implications
Complement activation is tightly regulated by regulatory proteins to prevent excessive activation that may result damage of healthy host tissues. Deficiencies or mutations in these regulatory proteins can result in uncontrolled complement activity, contributing to the pathogenesis of various neuroinflammatory diseases.
3.1 Regulation at the initiation phase
At the initiation of the complement cascade, C1 esterase inhibitor (C1-INH) is a key regulator of the classical pathway. C1-INH inactivates C1r and C1s by forming covalent complexes (37). While C1-INH deficiency is associated classically with hereditary angioedema (38), no neurological disorders have been reported in association with C1-INH deficiency (39).
For the lectin pathway, regulatory proteins including Map44 (40) and Map19 (41) inhibit the binding of MASP1 and MASP2 to MBL and ficolins. While the role of these regulatory proteins in neurological disorders remains under investigation, lectin pathway dysregulation has been implicated in cerebral ischemia and neurovascular inflammation (42).
3.2 Regulation at the activation phase
Complement activation is tightly regulated by a variety of proteins that function either in the fluid phase or on cell surfaces. Key fluid-phase regulators include C4-binding protein, Factor H (FH) and Factor H elated proteins, Factor I, clusterin, and vitronectin. Factor I inactivates C3b and C4b, and its deficiency has been associated with fulminant CNS disorder involving the brain and spine cord because of unchecked complement overactivation (43, 44). Clusterin and vitronectin act downstream to inhibit MAC formation on host membranes. Clusterin modulates amyloid beta aggregation and clearance in the brain (45). Genetic studies have identified clusterin as a major risk factor for Alzheimer’s disease, linking complement dysregulation to neurodegeneration (46).
On the cell surface, complement regulatory proteins such as membrane cofactor protein (MCP; CD46), decay-accelerating factor (DAF; CD55), and complement receptor 1 (CR1; CD35) prevent excessive complement activation by reducing the C3/C5 convertases activity. CD59 (protectin) prevents C9 from polymerizing and inhibits MAC assembly. Mutations in CD59 are associated with a rare inherited form of demyelinating neuropathy (47, 48). Notably, AQP4 is expressed on many peripheral organs (i.e., kidney, stomach, skeletal muscle) that are exposed to circulating AQP4-IgG. These tissues are relatively spared in AQP4 + NMOSD. The CNS-selective vulnerability may be attributed to the limited expression of complement regulatory proteins (CD46, CD55, and CD59) in the CNS, which renders astrocytes particularly susceptible to AQP4-IgG–induced complement-mediated damage (49, 50).
3.3 Regulation on effectors
Complement effector functions are also regulated to limit inflammation within the nervous system. Carboxypeptidase N inactivates the potent inflammatory mediators C3a and C5a by converting them into desArg forms (51). This enzymatic conversion limits prolonged inflammation, helps maintain homeostasis, and is particularly important in the context of chronic or relapsing neuroinflammatory disorders.
4 Current complement-targeted therapies
Recent advances in complement-targeted therapies have revolutionized the treatment of many complement-mediated diseases. These therapies aim to inhibit specific pathways or complement effectors, either by supplementing complement regulation, or inhibiting effector functions in one or multiple pathways. This review focuses on the inhibition of complement activation.
4.1 Overview of complement-targeted therapeutics across pathways
Therapeutic strategies targeting the complement system have offered multiple points of intervention across its activation pathways. Table 1 summarizes key complement targeting drugs in clinical use or development, their mechanisms of action, targeted pathways, and clinical indications. The specific use/development of these drugs in PNS and CNS disorders will be discussed in later sections.
Inhibiting C3 and C5—central nodes of the complement cascade—remains a cornerstone. C5 inhibitors, such as eculizumab and ravulizumab (52, 53), block C5 cleavage and prevent MAC formation, with ravulizumab offering extended half-life (54). Zilucoplan, a macrocyclic peptide, works similarly but shows activity against eculizumab-resistant C5 protein variants (55). While C5 inhibition blocks terminal complement activation, it does not fully prevent upstream activity in the complement activation cascade, allowing continued deposition of C3 fragments and immune-mediated damage in some patients. This is particularly evident in disorders such as paroxysmal nocturnal hemoglobinuria (PNH), where residual extravascular hemolysis (typically in the liver and spleen) persists despite C5 blockade (56, 57). As a result, targeting C3 offers a broader approach by intercepting the cascade earlier and preventing both amplification and generation of downstream effectors like C3a, C5a, C3b/iC3b-mediated opsonization and MAC-induced cellular damage. Clinical evidence in PNH showed that C3 inhibition can more effectively control disease activity in cases where C5-targeted therapies fall short (58). For example, C3 inhibition can prevent both intravascular and extravascular hemolysis, and may provide a more comprehensive disease control in complement-mediated hemolytic disorders.
Rather than inhibiting the entire complement system, selectively targeting one or two of the pathways has emerged as promising therapeutic targets in immune-mediated disorders with improved safety. Molecules [e.g., plasma derived C1-INH (59)] and antibodies [e.g., sutimlimab (60) and ANX005 (61)] targeting at the CP offers selective modulation of complement activation in GBS and NMOSD (62–64).
Several inhibitors targeting the lectin pathway or both the classical and the lectin pathways are under active development. Narsoplimab (65), a humanized monoclonal antibody, selectively inhibits the lectin pathway effector MASP-2 without affecting the classical or alternative pathways, and has demonstrated clinical efficacy in hematopoietic stem cell transplantation-associated thrombotic microangiopathy (HSCT-TMA) (66). To modulate both the classical and lectin pathways, C2-targeting agents such as empasiprubart (formerly ARGX-117) (67) have been developed. Empasiprubart binds to the C2b fragment and is being investigated in multifocal motor neuropathy (MMN), a disease characterized by antibody-mediated complement activation at the nodes of Ranvier (57, 68). More recently, a nanobody-based inhibitor, Nab1B10, has been developed to target the C2a protease domain, exhibiting enhanced inhibitory potency compared to empasiprubart in preclinical models (69).
The alternative pathway has also gained attraction as a therapeutic focus, with Factor B, Factor D, and MASP-3 inhibitors showing promise in PNH (70) and lupus nephritis (71), along with age-related macular degeneration, a neurodegenerative retinal condition (72, 73). Although the trial that targets Factor D did not meet efficacy endpoints in geographic atrophy (GA) secondary to age-related macular degeneration (73), it demonstrated the feasibility of targeting alternative pathway proteins in retina. Together, these agents illustrate the growing interest in expanding the therapeutic reach beyond traditional C3 and C5 inhibition.
In addition to central and pathway-specific interventions, targeting complement-derived effector molecules such as the anaphylatoxins C3a and C5a has emerged as a promising strategy to modulate inflammation while preserving upstream immune surveillance. As mentioned earlier, these small peptides are potent pro-inflammatory mediators in the pathophysiology of numerous autoimmune, infectious, and neurodegenerative diseases. Among them, the C5a–C5aR1 axis has received particular attention due to its involvement in leukocyte recruitment, blood–brain barrier disruption, and neuroinflammation. Avacopan, the first oral C5aR1 inhibitor approved for anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, has paved the way for additional agents such as ACT-1014-6,470, ALS-205, and avdoralimab, which are currently under development for ANCA-associated vasculitis, post-traumatic epilepsy, and severe infections such as COVID-19 (74–77). Vilobelimab selectively inhibits C5a, but it’s in vivo specificity has been questioned—particularly in light of the high dosing regimen (800 mg) (78–80). Considering the naturally low circulating level of C5a, such high therapeutic doses raise the possibility of cross-reactivity with intact C5, which may partly account for its pharmacological effects. Beyond C5a and its receptor, the terminal pathway components C6 and C7 have also emerged as potential intervention points to block MAC (81, 82) and may be especially relevant in diseases where MAC-driven damage is a key pathogenic mechanism, such as AQP4 + NMOSD and autoimmune neuropathies.
4.2 Integrating complement regulators with targeted therapeutic strategies
In addition to inhibiting complement activation/effector proteins, supplementing and correcting the deficiency of complement regulators is another promising therapeutic strategy. For instance, exogenous FH delivery has been shown to restore complement homeostasis in FH-deficient mice (83). Engineered constructs such as mini-FH containing regulatory domains (CCP 1–5), binding domains (CCP 19–20), and CR2-FH, which uses CR2 to localize the therapeutic payload to complement activation sites, improve pharmacokinetics and tissue targeting (84). In murine models of collagen antibody-induced arthritis (85) and choroidal neovascularization (86), CR2-FH showed targeted inhibition of complement activation. More recently, AAV-mediated delivery of CR2-FH has been tested as a gene therapy for choroidal neovascularization, setting the stage for potential applications in retinal neurodegeneration and CNS demyelinating disorders (87).
Other approaches fuse antibodies against complement fragments, like C3d (88), with regulatory domains (FH or CR1) to provide localized complement inhibition (88–90). These approaches harness natural complement inhibitors and direct them precisely to inflamed tissues, enhancing therapeutic efficacy while minimizing systemic immunosuppression. Groups also attempt to conjugate CD55 with an antibody that recognizes the alpha subunit of the acetylcholine receptor. This targeted approach seems to improve the clinical score in experimentally acquired MG rat models (91). Chimeric fusion proteins combining regulatory or localization domains from CD55, FH, and CR1 further enhance regulation by accelerating convertase decay, promoting Factor I–mediated inactivation, and selectively targeting inflamed tissues (92).
5 Complement in PNS disorders
Complement activation represents a critical pathogenic element in numerous autoimmune PNS disorders, including MG, chronic inflammatory demyelinating polyneuropathy (CIDP), GBS, MMN, and dermatomyositis (DM). The susceptibility of the PNS to complement-mediated immune attack is increased by the lack of a protective barrier equivalent to the blood–brain barrier in the CNS. This section provides an overview of the complement system’s role in many autoimmune PNS conditions and uses current and emerging complement-targeting therapies to highlight disease pathophysiology. Complement-targeting therapies are summarized in Table 2.
5.1 Immune mediated neuropathies
5.1.1 Guillain-Barré syndrome
Guillain-Barré syndrome encompasses a spectrum of acute autoimmune polyradiculoneuropathies primarily triggered by antecedent infections through molecular mimicry. Pathogenic autoantibodies of IgG1/IgG3 subclass, generated against microbial antigens (e.g., campylobacter jejuni lipo-oligosaccharides), cross-react with specific peripheral nerve gangliosides, such as GM1 and GD1a in the axonal form (acute motor axonal neuropathy (AMAN)) or myelin proteins/glycolipids in the demyelinating form (acute inflammatory demyelinating polyradiculoneuropathy (AIDP)) (93). Antibody binding triggers the CP and MAC formation, which leads ultimately to demyelination (in AIDP) or axonal degeneration (in AMAN). Pathological studies confirm deposition of activated complement components, including MAC, at sites of nerve damage in GBS patients (94, 95).
Intravenous immunoglobulin (IVIg) is an established treatment for patients exhibiting significant neurological disability for both CNS and PNS disorders. IVIg targets the immune system by multiple mechanisms, with complement inhibition being the predominant (96). Clinical trials targeting complement activation in GBS have yielded inconsistent results. Studies with eculizumab, when added to standard IVIg therapy, did not demonstrate significant clinical improvement over IVIg alone in Phase II and III trials (97–99). However, recent results from a Phase III study employing ANX005 (Tanruprubart), an anti-C1q antibody, showed significant clinical benefits in GBS patients (100). One of the reasons for the difference in efficacy seems to be from the site of action of these agents. C1q inhibition by Tanruprubart locks the CP at its origin, preventing the generation of not only MAC and C5a, but also the upstream anaphylatoxin C3a and the opsonin C3b. If C3-mediated inflammation and opsonization play a significant role in the pathogenesis of GBS (particularly in AIDP), C1q inhibition might offer a broader therapeutic benefit when compared to selective C5 inhibition by eculizumab (101).
5.1.2 Chronic inflammatory demyelinating polyradiculoneuropathy
CIDP represents a spectrum of acquired immune-mediated PNS disorders characterized by progressive or relapsing–remitting demyelination. In a significant portion of CIDP cases mediated by IgG1/IgG3 subclasses, the complement system is a major effector pathway. Antibody binding to nerve targets initiates the CP via C1q leading to MAC formation and myelin breakdown (102). Studies have shown that levels of complement activation products C3a, C5a, and soluble MAC in the serum and the CSF remained unchanged in individuals who responded to IVIg therapy, which suggests other immunomodulatory mechanisms of IVIg in addition to complement inhibition (103). Preliminary results of a Phase II trial of Riliprubart in 25 CIDP patients showed favorable benefit–risk profiles and clinical improvement (104). A Phase III trial is ongoing for another C1s inhibitor, DNTH103 (NCT06858579), and is planned for the C2 inhibitor Empasiprubart (NCT06920004).
5.1.3 Multifocal motor neuropathy
Multifocal motor neuropathy is an autoimmune disorder almost exclusively affecting motor nerves (105). Around 30–48% of patients with MMN have antibodies against GM1, a crucial ganglioside found in nerve myelin, particularly at the node of Ranvier (106). Anti-GM1 antibodies have been shown to activate complement and cause MAC deposition at the node of Ranvier in animal models, and complement activity seems correlating with MMN severity (105, 107–110).
Eculizumab was tested in an open label study in 13 MMN patients for 14 weeks (10 of whom were concomitantly receiving IVIg). The use of eculizumab did not change IVIg dosing, but there was some improvement in patient-rated subjective scores and clinical/electrophysiological measurements (111). TNT005 (Riliprubart), an investigational humanized C1s monoclonal antibody, was able to reverse complement deposition and functional deficits in a human-on-chip model of MMN (112). One study showed that IgM antibodies from MMN patients could bind motor neurons and activate complement cascade in an iPSC-derived motor neuron model. The study also showed efficacy of empasiprubart, a C2 inhibitory antibody, in blocking complement activation (113). Interim results of a Phase II trial in MMN patients showed that empasiprubart was well tolerated, reduced the risk of IVIg retreatment by 91% compared to placebo, and improved grip/muscle strength (114). A Phase III trial is ongoing (115).
5.1.4 Myelin-associated glycoprotein neuropathy
Anti-Myelin-Associated Glycoprotein (MAG) IgM paraprotein-related neuropathy (anti-MAG PN) is a common chronic demyelinating neuropathy causing progressive imbalance, ataxia, sensory issues, and tremor (116, 117). Strong evidence implicates the role of anti-MAG antibodies, which bind to sulfoglucuronyl glycosphingolipid in peripheral nerves. In anti-MAG neuropathy, IgM and complement (including MAC) deposition on nerve myelin occurs, causing structural damage such as myelin widening and axonal atrophy. Several studies have found a correlation between the amount of IgM and complement deposition and the extent of myelin morphological changes (118, 119). While complement activation is observed, studies have not consistently linked anti-MAG antibody levels or complement activation directly to clinical disease severity. Therefore, current evidence does not provide strong support using complement inhibitors for this condition (120, 121).
5.2 Myasthenia gravis
Myasthenia Gravis is an autoimmune disorder characterized by impaired neuromuscular transmission due to autoantibody-mediated dysfunction at the neuromuscular junction (NMJ) (Figure 2). Approximately 80% of MG patients have antibodies against acetylcholine receptor (AChR). Of the remaining 20% of MG patients, antibodies directed against muscle-specific kinase (MuSK) and low-density lipoprotein receptor-related protein- 4 (LRP4) were detected in the majority of patients (122).
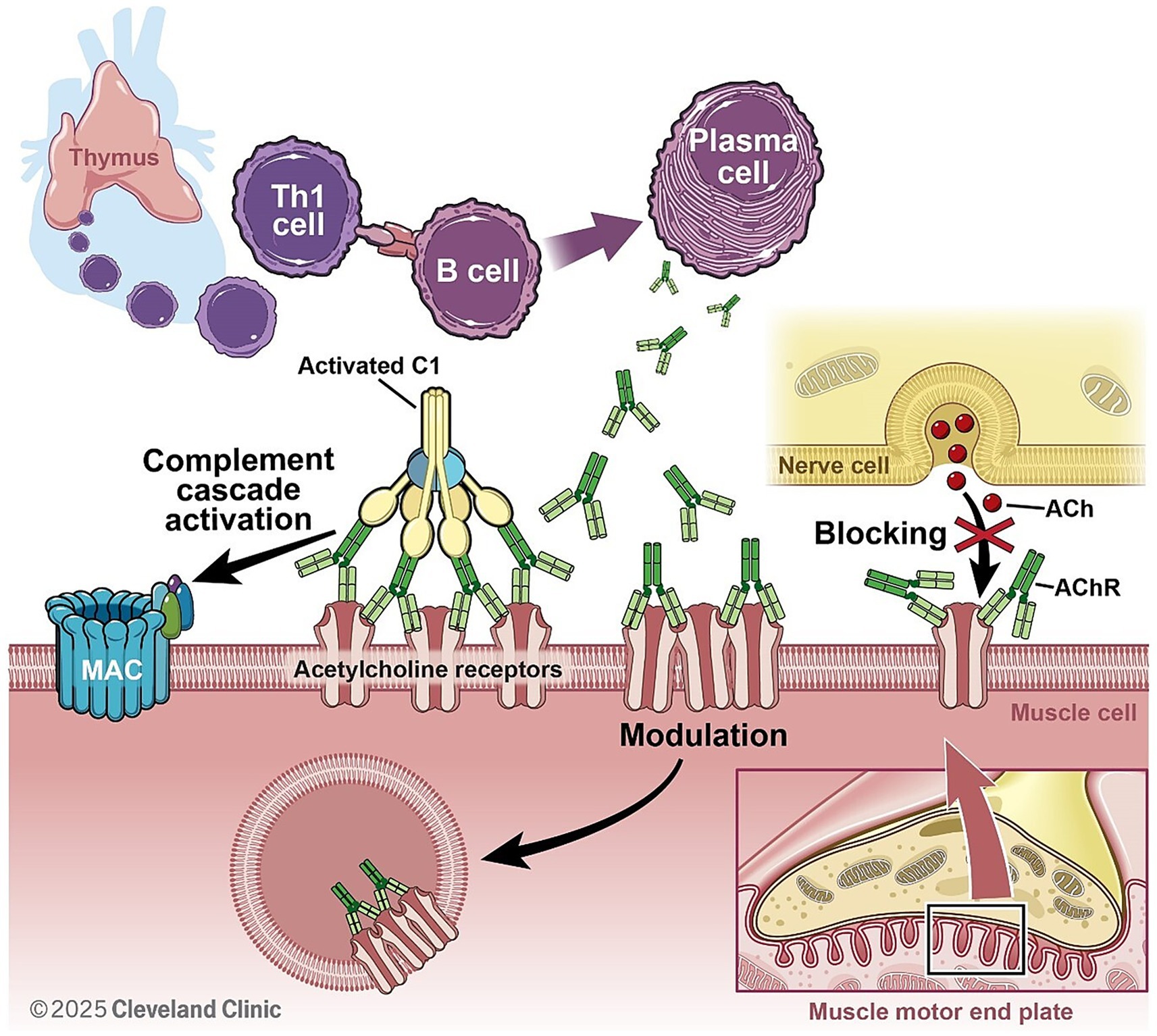
Figure 2. In AchR + MG, autoantibodies (produced by B cells with support from Th1 cells) are mainly of IgG1 subclass and activate complement cascade after binding to post synaptic acetylcholine receptors. This ultimately leads to MAC formation, pore formation on post synaptic membrane and internalization of AchR, reducing efficacy of neuromuscular junction transmission.
In AChR, as well as LRP4, antibody-positive MG complement activation is a key pathogenic mechanism contributing to synaptic dysfunction. Pathogenic IgG1 and IgG3 autoantibodies trigger the classical complement cascade by engaging C1q, ultimately leading to opsonization, immune complex deposition, and the formation of MAC. This process culminates in reduced efficiency of neuromuscular transmission (123–126).
Research in the 1950s hinted at complement’s role in MG due to altered protein levels in patient sera (127–129). Subsequently, complement components (i.e., C3a and MAC) were observed directly at the NMJ of MG patients (130, 131). Furthermore, MG patient sera could induce complement-mediated destruction of cultured muscle cells. More recently, lower levels of properdin, a complement positive regulator, have been linked to more severe MG (132).
Complement mediated therapies have become established care in patients with AChR antibody positive MG. In 2017, eculizumab became the first complement inhibitor to gain regulatory approval for the therapy of AChR antibody positive generalized MG (gMG) on the basis of the REGAIN trial results (133). Based on positive results from the subsequent CHAMPION (134) and RAISE (135) trials, Ravulizumab and Zilucoplan were also approved as treatment of AChR positive gMG patients. Topline results from a recently completed trial accessing efficacy and safety profile of gefurulimab in AchR ab positive gMG patients showed significant and sustained improvement in MG ADL and Mg WMG scores (136). Trial outcomes, dosages, and emerging agents in closed and on-going trials are summarized in Table 2.
It should be noted that MuSK antibodies are mostly of the IgG4 subtype, which does not activate complement. Thus, complement inhibition therapy is ineffective in MuSK antibody-positive MG (137, 138).
5.3 Idiopathic inflammatory myopathies
5.3.1 Dermatomyositis
Dermatomyositis is a systemic disease targeting skeletal muscle and skin (139). Approximately 60% of patients have myositis specific antibodies (i.e., anti-MDA-5, -NXP-2, −Mi-2, -TIF1-γ, -SAE-1) (140). An essential feature in the immunopathogenesis of classic DM is a complement-mediated microvasculopathy in the muscle, leading to capillary dropout, muscle ischemia, and anaphylatoxin-mediated muscle fiber damage (139, 141–145).
IVIg has been used as an effective treatment for DM (146–148). Novel complement-targeting agents have not demonstrated clear efficacy in the treatment of DM in clinical trials. A few case reports have shown efficacy of eculizimab in refractory dermatomyositis (149). A Phase II trial of ravulizumab for DM was terminated due to lack of efficacy (NCT04999020). A Phase II study has been initiated for empasiprubart, a C2 inhibitor, for the treatment of DM (NCT06284954). Ruxoprubart is a selective blocker of the alternative complement pathway that targets Factor B, leaving the CP intact. A Phase II efficacy trial of Ruxoprubart (NM8074) has been initiated for DM.
5.3.2 Immune-mediated necrotizing myopathy
Immune-mediated necrotizing myopathy (IMNM) features significant muscle fiber damage with sparse inflammation, differing from DM and polymyositis. IMNM is linked to signal recognition particle or 3-hydroxy-3-methylglutarylcoenzyme A reductase autoantibodies. In IMNM, the complement system directly attacks muscle fibers. Pathogenic IgG autoantibodies bind to antigens on the muscle fiber surface (sarcolemma), activate the classical complement pathway, and lead to MAC formation and, finally, muscle fiber necrosis (150–153).
A Phase II trial investigated zilucoplan in adult IMNM assuming complement activation being the primary driver for disease pathophysiology. Although the drug effectively and safely inhibited the complement pathway, it failed to improve muscle enzyme (CK) levels or clinical symptoms. This lack of efficacy may contradict the prevailing theory that complement-driven MAC deposition is the main driver of IMNM. These results, supported by preclinical models (where therapeutic administration of zilucoplan following disease onset failed to significantly restore muscle strength in humanized mice model), suggest that IMNM autoantibodies may cause muscle damage through complement-independent mechanisms (i.e., antibody mediated muscle fiber dysfunction) (154, 155).
5.3.3 Inclusion body myositis
Inclusion body myositis (IBM) is a common acquired muscle disease in individuals over 50 years of age. Autoantibodies against cytoplasmic 5′-nucleotidase (cN1A) have been found in 35–80% of IBM patients, however, their presence does not correlate clearly with disease severity or survival (156, 157). Recent immunological findings have intensified the debate regarding inflammation as the primary pathogenic driver in IBM. Passive transfer experiments have demonstrated that serum from anti-NT5C1A seropositive IBM patients can induce disease-specific pathology in both myotube cultures and mouse models, supporting an antibody-mediated inflammatory component (158). One study has shown upregulation of MAC (C5b-9) in muscle biopsies from 17 of 20 patients with IBM (159). These findings suggest a potential role of complement activation in IBM.
To date, no immunosuppressive or complement-targeting therapies have demonstrated efficacy in the treatment of IBM. A clinical trial investigating the efficacy and safety of Pozelimab and Cemdisiran combination therapy in IBM is ongoing (NCT06479863).
5.4 Immune check point-related neuromuscular disorders
Neurologic immune-related adverse events develop in approximately 2–4% of patients with neuromuscular conditions receiving immune checkpoint inhibitors (ICIs), which constitutes the majority of cases (160). Proposed mechanisms for immune-related adverse events involve primarily T-cell dysfunction, potentially including enhanced T-cell activity against antigens shared by tumors and healthy tissues, coupled with elevated inflammatory cytokines (161, 162). There is also some evidence suggesting a role for B cells, indicated by the development of autoantibodies after ICI treatment (163). Additionally, the complement system appears to be involved. Enhanced complement-mediated inflammation may occur due to direct antibody binding [e.g., CTLA4-IgG (164)] or due to increased levels of pre-existing antibodies. Although not studied extensively, one report documented complement deposition around muscle fibers and capillaries in a patient with ICI-related myasthenia-myositis-myocarditis-overlap syndrome (165). Furthermore, successful treatment of ICI-associated MG with eculizumab and ravulizumab has been reported, suggesting the relevance of complement activation in these ICI related disorders (133, 164, 165).
6 Complement activation and treatment in the CNS
Complement activation is involved in the pathogenesis of multiple autoimmune CNS disorders and serves as a key driver of pathogenicity in AQP4 + NMOSD. Complement inhibitors have been explored as treatment options in multiple PNS disorders and are approved and undergoing clinical trials for AQP4 + NMOSD in the CNS. This section provides an overview of the complement system’s role in many CNS conditions, focusing on proposed pathophysiological mechanisms. Current and emerging complement-targeting therapies are summarized briefly in Table 3.
6.1 Aquaporin-4 IgG-positive neuromyelitis optica spectrum disorder
Aquaporin-4 IgG-positive Neuromyelitis Optica Spectrum Disorder is an inflammatory CNS disorder defined by the presence of pathogenic autoantibodies to the water channel AQP4 (166). Core clinical inflammatory syndromes occur in areas with highest AQP4 expression and blood brain barrier (BBB) permeability, including the optic nerve, spinal cord, area postrema, diencephalon, and cerebrum.
Unknown immune tolerance checkpoint defects enable AQP4 IgG to be produced by autoreactive plasmablasts differentiated from immature B cells in the peripheral blood (167–169). Peripheral AQP4 IgG breaches the BBB by unclear mechanisms and binds AQP4 located on the end feet of astrocytes lining the BBB. The pathogenic M23 isoform of AQP4 assembles orthogonal arrays of particles, which facilitate AQP4 IgG clustering on the cell membrane and enable optimal C1q binding. AQP4 IgG binding triggers an immunologic cascade resulting in further BBB disruption, complement activation, astrocyte injury, secondary demyelination, and neuronal cell death (170). Destruction is mediated by complement-mediated cytotoxicity via MAC generation, as well as additional inflammatory mechanisms generated through production of the anaphylatoxin C5a (171). Lesions characteristically demonstrate extensive demyelination, edema, axonal injury, perivascular complement deposition, and necrosis (27, 170, 172, 173). Multiple in vivo and ex vivo studies have shown that both AQP4 IgG and complement are required to reproduce typical AQP4 + NMOSD lesions (27, 173, 174). Additionally, C5a levels are increased in the CSF of AQP4 + NMOSD compared to MS and other CNS disorders and correlate with severity of exacerbations (171, 175).
The role of complement in NMSOD pathophysiology was further confirmed by two Phase III clinical trials, PREVENT and CHAMPION-NMOSD, showing that both eculizumab and ravulizumab significantly decrease relapses in patients with AQP4 + NMOSD (53, 176). In 2019, eculizumab became the first FDA-approved maintenance treatment for AQP4 + NMOSD followed by ravulizumab in 2024. A Phase Ib trial showed that treating acute NMOSD relapses with the C1-esterase inhibitor Cinryze and steroids was safe but efficacy data was limited (62). The Phase II trial, EASE-NMO (NCT06673394), aims to explore the addition of eculizumab to steroids in acute relapses is ongoing.
6.2 Multiple sclerosis
Multiple sclerosis is a chronic CNS disorder characterized by inflammation, demyelination, and neuronal degeneration (177). Evidence is growing for the role of B cells, antibodies, and the innate immune system in pathogenesis, supported by the success of B-cell-depleting treatments and Bruton’s tyrosine kinase inhibitors (i.e., tolebrutinib) (178, 179). Additionally, evidence exists to support varying degrees of complement activation in MS. Histopathological studies showed evidence of complement deposition along the MS disease spectrum with complement becoming increasingly prominent as the disease progresses (180, 181). In progressive MS, complement markers are present in chronic active lesions, chronic inactive lesions, and normal appearing white matter despite the absence of other inflammatory components. Inflammation relies on innate (i.e., macrophages and CNS-resident microglia) rather than adaptive mechanisms (182–184).
Although there is evidence of complement involvement in MS pathogenesis, the source and the trigger of complement are unclear. Systemic complement was initially thought to enter the CNS through BBB disruption similar to other CNS autoimmune conditions. However, complement can also be produced by CNS cells, and compartmentalized intrinsic activation of complement by degenerating neurons independent of cell-mediated demyelination may contribute to pathogenesis, including synaptic pruning, in progressive diseases such as MS. In addition, complement may be activated initially to promote the clearance of degradation products and other debris, but the ineffective clearance of complement-targeted products by senescent microglia may lead to aberrant activation with demyelination and synapse loss (185, 186).
There is currently no clinical trial data investigating the use of complement inhibitors in MS. Preclinical data has shown complement inhibitors have some benefit in experimental autoimmune encephalomyelitis mouse models (187–189). In case reports, relapsing and progressive MS patients treated with eculizumab for other indications continued to demonstrate a clinical and radiographical deterioration of their MS (190, 191). The complex pathophysiology around MS, along with the fact that it is not an antibody-associated disorder, makes any conclusive statements difficult. Whether complement activation represents a primary pathogenesis or is secondary to other inflammatory events remains uncertain.
6.3 Myelin oligodendrocyte glycoprotein antibody-associated disease
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is defined by the presence of myelin oligodendrocyte glycoprotein (MOG)-IgG. However, MOG-IgG has been seen in a small proportion of patients with other neurologic diseases and is less specific at low titers, raising questions on its pathogenicity, especially in contrast to AQP + NMOSD (192–196). MOGAD histopathology is characterized by inflammatory demyelination, particularly in perivascular regions, without astrocyte destruction. Areas of demyelination show varying loss of MOG staining and mainly consist of macrophages and T-cells with CD4 + dominancy but also show some evidence of complement-mediated destruction (195).
Similar to AQP4 IgG, MOG-IgG is predominantly IgG1 subtype and capable of fixing complement (197). However, the extent of complement activation and overall role of complement in MOGAD pathophysiology is not completely understood. MOG protein constitutes a relatively minor component of the myelin sheath, making it difficult for antibodies to cluster. MOG-IgG also requires bivalent binding, which poorly binds C1q, diminishing subsequent complement activation (198). Compared to patients with AQP4 + NMOSD, patients with MOGAD have significantly lower levels of MAC but similarly increased levels of the anaphylatoxins C3a and C5a in CSF, suggesting that complement activation is present, but may generate destruction through mechanisms other than MAC-mediated cytotoxicity (199). Additionally, a recent study evaluated serum and CSF complement levels and showed that combinations of various complement factors have diagnostic and prognostic value in MOGAD patients. Combination of serum levels of C3a, C4a, and the C3a/C3 ratio were able to discriminate MOGAD from MS and NMOSD with MOGAD demonstrating higher levels of C3a and C4a compared to other pathologies. In the CSF, lower C4 levels were associated with a higher number of relapses during follow up, and a high C4a/4 ratio was associated with increased risk of second relapse during the first year and shorter time to second relapse. Further, elevated baseline levels of the MAC SC5b9 in the CSF were associated with increased risk of disability on the Expanded Disability Status Scale, suggesting complement inhibition could have therapeutic benefit in this disease (200).
However, MRI T2 lesions in MOGAD show complete resolution more frequently compared to AQP4 + NMOSD and MS, which raises questions regarding the underlying pathophysiology as complement activation has been shown to be highly destructive in AQP4 + NMOSD (201). Overall, given varying levels of complement activity demonstrated in multiple studies, the role of complement activation in MOGAD pathophysiology is unclear. Although IVIg has been shown to be effective in preventing MOGAD attacks, there are currently no ongoing clinical trials investigating complement inhibitors (202, 203).
6.4 Autoimmune encephalitis & related disorders
The diagnosis of antibody associated autoimmune encephalitis (AE) is suggested by the subacute onset of cognitive or neuropsychiatric symptoms along with a CNS inflammation usually detected on MRI or CSF studies (204). Clinical phenotypes can be further defined by the presence of a corresponding antibody, which can target intracellular or cell surface/synaptic receptors leading to diverse pathophysiology. Complement has been investigated in the pathogenesis of AE with variable results.
The role of complement is most studied in AE related to N-methyl D-aspartate (NMDA) antibodies. NMDA antibodies are IgG1 subclass and able to activate complement in vitro. However, when examined at autopsy, cases of NMDA encephalitis showed prominent microgliosis and deposits of IgG with rare inflammatory infiltrates and no complement deposits, suggesting complement may not play a role in vivo (205–208).
A study of 52 patients with glycine antibody positive patients found that glycine antibodies are mainly of the IgG1 subclass and were capable of activating complement in vitro (209). Histology from patients with contactin-associated protein-like 2 (CASPR2) antibodies who underwent temporal lobe resections for seizures showed the presence of T cells, B cells, and microglia, but complement deposition was identified in only a few neurons despite strong leakage of IgG through the BBB (210). In patients with glial fibrillary acidic protein (GFAP) antibodies, histopathology showed prominent immunoreactivity of astrocytes with the complement activation product C4d, but did not detect the deposition of IgG, C1q, and terminal complement complex on astrocytes (211). Another study examined hippocampal tissue from patients with glutamic acid decarboxylase (GAD) antibodies and found increased transcriptional levels for genes encoding complement proteins. Hippocampal C3d deposition was visualized in 5 of 7 patients (212). Although these studies suggest complement may play a role in autoimmune encephalitis, more investigation is needed to determine the specific mechanisms for each antibody.
IVIg is used routinely to treat a variety of AEs with varying efficacies, likely supporting a role for complement activation (213, 214). The best evidence for IVIg is within the GAD antibody–spectrum disorders. One double-blind, placebo-controlled trial with IVIg in stiff person syndrome showed significant improvements in mobility and spasms, which translated into improved activities of daily living. This is one of the few disorders that has clear, prospective evidence (Level 1a) for IVIg usage (215, 216). In case reports of glycine antibody-associated stiff person syndrome, one patient had significant improvement on eculizumab, while another patient did not experience improvement and was quickly taken off eculizumab due to the associated complication of meningitis (217).
6.5 Primary angiitis of the central nervous system
Primary angiitis of the CNS (PACNS) is a rare form of vasculitis limited to the brain, spinal cord, and leptomeninges that leads frequently to cerebral ischemia and hemorrhage. Histopathology shows granulomatous inflammation, lymphocytic infiltration, and acute necrotizing vasculitis, but the exact pathogenesis remains unknown (218). The role of complement activation has been explored in patients with PACNS with conflicting results. A study evaluating the CSF from 8 biopsy-proven cases of PACNS suggests that the complement cascade is a significant feature of the disease. Complement proteins in the alternative pathway and terminal complement were significantly upregulated, and the MAC inhibitor CD59 was significantly downregulated in PACNS compared to noninflammatory controls (219, 220). Another study evaluating the serum and CSF in 20 patients with PACNS found that levels of complement activation including components specific to the classical and the alternative pathways were unchanged in PACNS patients when compared to those from noninflammatory controls (221). There are currently no ongoing clinical trials investigating complement inhibitors in PACNS.
7 Future directions
7.1 Balancing between complement activation control and host defense
While all pathway inhibition at C3/C5 levels can control complement overactivation effectively, long-term complement inhibition increases the susceptibility to infections, particularly those caused by certain bacteria, such as meningococcal species. This risk is highlighted by the FDA black box warnings issued to all complement inhibitors (222), requiring vaccinations prior to initiating these complement-targeting therapies to mitigate infection risks. The latest Center for Disease Control recommendation for adult patients with complement deficiencies including the usage of complement inhibitor therapies includes a series of vaccinations for meningococcal serogroups A, C, W, and Y along with serogroup B. The complete series takes 6 months to complete and vaccination for meningococcal must remain up to date while on therapy. Antimicrobial prophylaxis is required, but the duration of antimicrobial prophylaxis is currently at clinicians’ discretion. At our centers, we typically will also include pneumococcal 20-valent and haemophilus b conjugate vaccines if not previously administered.
Breakthrough infections can still occur even after full vaccination, especially in immunocompromised patients. For example, the Meningococcal B vaccine targets Neisseria meningitidis serogroup B (MenB), a leading cause of bacterial meningitis across all age groups in many industrialized countries. However, it is not fully protective, with a range between approximately 76 and 83% (223, 224). Real-world data and post-marketing surveillance have also revealed additional non-meningococcal infections, including pneumonia, bacteremia, sepsis and septic shock, urinary tract infections, staphylococcal infections, and opportunistic viral infections (225). These findings underscore the need for ongoing infection monitoring, booster vaccinations, and patient education, particularly in vulnerable populations (e.g., those with neuro-immunological comorbidities).
Beyond concerns of acute safety events, prolonged complement suppression may impair immune surveillance, a function critical to the clearance of apoptotic and early neoplastic cells (226). Recent studies have revealed that complement plays a dual role in cancer (227). On one hand, complement enhances antibody-mediated cytotoxicity through CP activation and MAC formation. On the other hand, aberrant complement activation in the tumor microenvironment can foster tumor progression by sustaining chronic inflammation, promoting angiogenesis, and recruiting myeloid-derived suppressor cells (MDSCs) that suppresses T cell responses. In neurological cancers and metastases affecting the CNS, complement components such as C3a and C5a may also disrupt the blood–CSF barrier, further facilitating tumor spreading (32, 228). This complex, context-dependent interplay highlights the importance of maintaining a tenuous balance of proper complement activation to avoid tipping from protective immunosurveillance toward tumor-supportive inflammation.
Unlike pan-complement blockade, which eliminates the formation of opsonins (C3b, iC3b) and MAC, pathway-specific inhibition allows complement activity to remain partially intact. This targeted approach may reduce the risk of severe infections, minimize immune dysregulation and off-target tissue damage, and avoid undesired immunosuppression–factors especially important for pediatric, elderly, or immunocompromised patients (229). In diseases where autoantibody-mediated activation of the CP drives pathology, such as AQP4 + NMOSD, GBS, and MG, selective inhibition of C1s or C1q can reduce immune complex-driven inflammation without compromising the alternative or lectin pathways. Sutimlimab (229) and ANX005 (230) exemplify this strategy, demonstrating the ability to dampen neuroinflammation leading to meaningful clinical improvement while minimizing typical safety concerns associated with broader complement blockade.
The alternative pathway serves as an amplification loop for all complement activation, but in many diseases its overactivity alone is sufficient to result in tissue damage. Targeted inhibitors of Factor B (Iptacopan) and Factor D (Danicopan, Vemircopan) can attenuate this amplification precisely. This may be especially beneficial in chronic neurological disorders, where localized complement dysregulation contributes to glial activation, synapse loss (231), and axon degeneration (232).
7.2 Precision-targeted and combination therapeutic strategies
Next,-generation complement therapies are moving toward context-sensitive modulation and synergistic combination regimen to maximize efficacy while minimizing systemic immunosuppression. For instance, CR2-targeted inhibitors as mentioned in section 4.2 are engineered to localize specifically to sites of complement activation by binding deposited C3b/iC3b fragments, ensuring focused inhibition at diseased tissues.
Complement inhibitors can also be deployed in combination with other immunomodulatory agents to enhance therapeutic outcomes, particularly in antibody-mediated neurological disorders, as exemplified by recent strategies in oncology and ophthalmology. A notable clinical-stage example is the Phase II trial (NCT04919629) evaluating APL-2, a C3 inhibitor, in combination with pembrolizumab (anti-PD-1) and/or bevacizumab (anti-VEGF). This combination strategy aims to reduce immunosuppressive C5a signaling in the tumor microenvironment, where C5a recruits myeloid-derived suppressor cells and inhibits CD8+ T cell function (233), thereby potentiating the efficacy of ICIs. In a separate domain, bispecific fusion proteins, efdamrofusp alfa (234) and KNP-301 (235, 236), which bind both C3b and VEGF, demonstrated selective inhibition of the alternative pathway and anti-angiogenic effects in preclinical models of neovascular and dry age-related macular degeneration.
Together, these examples highlight the promise of dual-targeted or combination-based strategies that modulate both complement and other disease-relevant pathways as a blueprint for treating complex autoimmune neurological disorders, where complement is only one component of a multifaceted pathological network.
Gene therapies are also being explored to restore function in cells lacking surface complement regulators. For instance, GT005 (237), an AAV-based therapy encoding Factor I, and HMR59, encoding a soluble form of CD59 (NCT03144999), are being evaluated for their ability to reinstate complement regulation in target tissues in age-related macular degeneration. Additionally, nano-vesicular delivery systems incorporating GPI-anchored complement regulators offer a novel means of protection against complement-mediated cell lysis. In studies using biomimetic proteolipid vesicles, human membrane proteins were embedded in synthetic phospholipid bilayers to reconstitute missing GPI-anchored regulators on the surface of complement-vulnerable cells. These vesicles significantly enhanced resistance to complement attack in both healthy and patient-derived blood cells (238). Both healthy and PNH peripheral blood mononuclear blood cells treated with these biomimetic lipid vesicles exhibited increased resistance to complement-mediated lysis.
8 Conclusion
Over the past decade, complement-related therapeutic advances, including those targeting central components such as C3 and C5 or specific pathways, have transformed the clinical landscape for many immune-mediated neurological disorders diseases. Ongoing therapeutic strategies into context-specific complement regulation, effector-targeted strategies, and gene-based delivery platforms promises to further refine these approaches. Yet, challenges remain to balance complement activation with therapeutic suppression, to manage patient heterogeneity, and to identify biomarkers that predict complement-driven pathology. As our mechanistic understanding deepens and precision therapies evolve, we will develop superior therapies with various modifying strategies that are both safer and more effective.
Author contributions
JC: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. NS: Conceptualization, Writing – original draft, Writing – review & editing. KP: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. YL: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. FL: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. JA: Conceptualization, Formal analysis, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
John Fredieu PhD is a Scientific Medical Writer and reviewed the article for clarity and we appreciate his input.
Conflict of interest
YL served on scientific advisory board for Argenx, Amgen, Johnson & Johnson and Vertex. JA received research support from Amgen. Served on scientific advisory board for Amgen, TG therapeutics, EMD Serono and Genentech. Serve as an editorial board member of Frontiers, at the time of submission.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Merle, NS, Church, SE, Fremeaux-Bacchi, V, and Roumenina, LT. Complement system part I–molecular mechanisms of activation and regulation. Front Immunol. (2015) 6:262. doi: 10.3389/fimmu.2015.00262
2. Merle, NS, Noe, R, Halbwachs-Mecarelli, L, Fremeaux-Bacchi, V, and Roumenina, LT. Complement system part ii: role in immunity. Front Immunol. (2015) 6:257. doi: 10.3389/fimmu.2015.00257
3. Torisu, M, Yokoyama, T, Kohler, P, Durst, A, Martineau, G, Schroter, G, et al. Serum complement after Orthotopic transplantation of the human liver. Clin Exp Immunol. (1972) 12:21–30.
4. Alper, CA, Johnson, AM, Birtch, AG, and Moore, FD. Human C′ 3: evidence for the liver as the primary site of synthesis. Science. (1969) 163:286–8. doi: 10.1126/science.163.3864.286
5. Morris, KM, Aden, DP, Knowles, BB, and Colten, H. Complement biosynthesis by the human hepatoma-derived cell line Hepg2. J Clin Invest. (1982) 70:906–13. doi: 10.1172/JCI110687
6. Whaley, K. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J Exp Med. (1980) 151:501–16. doi: 10.1084/jem.151.3.501
7. Reid, KB, and Solomon, E. Biosynthesis of the first component of complement by human fibroblasts. Biochem J. (1977) 167:647–60. doi: 10.1042/bj1670647
8. Kulics, J, Circolo, A, Strunk, R, and Colten, H. Regulation of synthesis of complement protein C4 in human fibroblasts: cell-and gene-specific effects of cytokines and lipopolysaccharide. Immunology. (1994) 82:509–15. doi: 10.1016/0161-5890(93)90262-A
9. Strunk, RC, Eidlen, DM, and Mason, RJ. Pulmonary alveolar type ii epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest. (1988) 81:1419–26. doi: 10.1172/JCI113472
10. Gasque, P, Fontaine, M, and Morgan, BP. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J Immunol (Baltimore, Md: 1950). (1995) 154:4726–33. doi: 10.4049/jimmunol.154.9.4726
11. Koski, CL, Estep, AE, Sawant-Mane, S, Shin, ML, Highbarger, L, and Hansch, GM. Complement regulatory molecules on human myelin and glial cells: differential expression affects the deposition of activated complement proteins. J Neurochem. (1996) 66:303–12. doi: 10.1046/j.1471-4159.1996.66010303.x
12. Katz, Y, Revel, M, and Strunk, RC. Interleukin 6 stimulates synthesis of complement proteins factor B and C3 in human skin fibroblasts. Eur J Immunol. (1989) 19:983–8. doi: 10.1002/eji.1830190605
13. Petry, F, Botto, M, Holtappels, R, Walport, MJ, and Loos, M. Reconstitution of the complement function in C1q-deficient (C1qa−/−) mice with wild-type bone marrow cells. J Immunol. (2001) 167:4033–7. doi: 10.4049/jimmunol.167.7.4033
14. Fonseca, MI, Chu, S-H, Hernandez, MX, Fang, MJ, Modarresi, L, Selvan, P, et al. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflammation. (2017) 14:1–15. doi: 10.1186/s12974-017-0814-9
15. Schumaker, VN, Calcott, MA, Spiegelberg, HL, and Muller-Eberhard, HJ. Ultracentifuge studies of the binding of igg of different subclasses to the Clq subunit of the first component of complement. Biochemistry. (1976) 15:5175–81. doi: 10.1021/bi00668a035
16. Vidarsson, G, Dekkers, G, and Rispens, T. Igg subclasses and Allotypes: from structure to effector functions. Front Immunol. (2014) 5:520. doi: 10.3389/fimmu.2014.00520
17. Frischauf, N, Strasser, J, Borg, EGF, Labrijn, AF, Beurskens, FJ, and Preiner, J. Complement activation by igg subclasses is governed by their ability to Oligomerize upon antigen binding. Proc Natl Acad Sci USA. (2024) 121:e2406192121. doi: 10.1073/pnas.2406192121
18. Sævarsdóttir, S, Vikingsdottir, T, and Valdimarsson, H. The potential role of Mannan-binding lectin in the clearance of self-components including immune complexes. Scand J Immunol. (2004) 60:23–9. doi: 10.1111/j.0300-9475.2004.01437.x
19. Frederiksen, PD, Thiel, S, Larsen, CB, and Jensenius, JC. M-Ficolin, an innate immune Defence molecule, binds patterns of acetyl groups and activates complement. Scand J Immunol. (2005) 62:462–73. doi: 10.1111/j.1365-3083.2005.01685.x
20. Krarup, A, Thiel, S, Hansen, A, Fujita, T, and Jensenius, JC. L-Ficolin is a pattern recognition molecule specific for acetyl groups. J Biol Chem. (2004) 279:47513–9. doi: 10.1074/jbc.M407161200
21. Nilsson, B, and Ekdahl, KN. The tick-over theory revisited: is C3 a contact-activated protein? Immunobiology. (2012) 217:1106–10. doi: 10.1016/j.imbio.2012.07.008
22. Iwaki, D, Kanno, K, Takahashi, M, Endo, Y, Matsushita, M, and Fujita, T. The role of mannose-binding lectin-associated serine Protease-3 in activation of the alternative complement pathway. J Immunol. (2011) 187:3751–8. doi: 10.4049/jimmunol.1100280
23. Takahashi, M, Ishida, Y, Iwaki, D, Kanno, K, Suzuki, T, Endo, Y, et al. Essential role of mannose-binding lectin-associated serine Protease-1 in activation of the complement factor D. J Exp Med. (2010) 207:29–37. doi: 10.1084/jem.20090633
24. Gaya da Costa, M, Poppelaars, F, Van Kooten, C, Mollnes, TE, Tedesco, F, Würzner, R, et al. Age and sex-associated changes of complement activity and complement levels in a healthy Caucasian population. Front Immunol. (2018) 9:2664. doi: 10.3389/fimmu.2018.02664
25. Mead, RJ, Singhrao, SK, Neal, JW, Lassmann, H, and Morgan, BP. The membrane attack complex of complement causes severe demyelination associated with acute axonal injury. J Immunol. (2002) 168:458–65. doi: 10.4049/jimmunol.168.1.458
26. Hinson, SR, Romero, MF, Popescu, BFG, Lucchinetti, CF, Fryer, JP, Wolburg, H, et al. Molecular outcomes of Neuromyelitis Optica (Nmo)-igg binding to Aquaporin-4 in astrocytes. Proc Natl Acad Sci. (2012) 109:1245–50. doi: 10.1073/pnas.1109980108
27. Saadoun, S, Waters, P, Bell, BA, Vincent, A, Verkman, A, and Papadopoulos, MC. Intra-cerebral injection of Neuromyelitis Optica immunoglobulin G and human complement produces Neuromyelitis Optica lesions in mice. Brain. (2010) 133:349–61. doi: 10.1093/brain/awp309
28. Ramaglia, V, King, RHM, Nourallah, M, Wolterman, R, de Jonge, R, Ramkema, M, et al. The membrane attack complex of the complement system is essential for rapid Wallerian degeneration. J Neurosci. (2007) 27:7663–72. doi: 10.1523/JNEUROSCI.5623-06.2007
29. Van den Berg, B, Walgaard, C, Drenthen, J, Fokke, C, Jacobs, BC, and Van Doorn, PA. Guillain–Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. (2014) 10:469–82. doi: 10.1038/nrneurol.2014.121
30. Veerhuis, R, Nielsen, HM, and Tenner, AJ. Complement in the brain. Mol Immunol. (2011) 48:1592–603. doi: 10.1016/j.molimm.2011.04.003
31. Humayun, S, Gohar, M, Volkening, K, Moisse, K, Leystra-Lantz, C, Mepham, J, et al. The complement factor C5a receptor is upregulated in Nfl−/− mouse motor neurons. J Neuroimmunol. (2009) 210:52–62. doi: 10.1016/j.jneuroim.2009.01.028
32. Propson, NE, Roy, ER, Litvinchuk, A, Köhl, J, and Zheng, H. Endothelial C3a receptor mediates vascular inflammation and blood-brain barrier permeability during aging. J Clin Invest. (2021) 131:e140966. doi: 10.1172/JCI140966
33. Gasque, P, Singhrao, SK, Neal, JW, Götze, O, and Morgan, BP. Expression of the receptor for complement C5a (Cd88) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am J Pathol. (1997) 150:31–41.
34. Möller, T, Nolte, C, Burger, R, Verkhratsky, A, and Kettenmann, H. Mechanisms of C5a and C3a complement fragment-induced [Ca2+] I signaling in mouse microglia. J Neurosci. (1997) 17:615–24. doi: 10.1523/JNEUROSCI.17-02-00615.1997
35. Duan, T, Smith, AJ, and Verkman, AS. Complement-independent bystander injury in Aqp4-igg seropositive Neuromyelitis Optica produced by antibody-dependent cellular cytotoxicity. Acta Neuropathol Commun. (2019) 7:1–16. doi: 10.1186/s40478-019-0766-7
36. Barnum, SR. C4a: an anaphylatoxin in name only. J Innate Immun. (2015) 7:333–9. doi: 10.1159/000371423
37. Harpel, P, and Cooper, N. Studies on human plasma C1 Inactivator-enzyme interactions. I. Mechanisms of interaction with C1s, plasmin, and trypsin. J Clin Invest. (1975) 55:593–604. doi: 10.1172/JCI107967
38. Schmaier, AH. The hereditary angioedema syndromes. J Clin Invest. (2019) 129:66–8. doi: 10.1172/JCI125378
39. Huang, YF, Briggs, CM, Gokhale, S, and Punga, AR. Elevated C1s/C1-Inh in serum and plasma of myasthenia gravis patients. J Neuroimmunol. (2024) 396:578447. doi: 10.1016/j.jneuroim.2024.578447
40. Degn, SE, Hansen, AG, Steffensen, R, Jacobsen, C, Jensenius, JC, and Thiel, S. Map44, a human protein associated with pattern recognition molecules of the complement system and regulating the lectin pathway of complement activation. J Immunol. (2009) 183:7371–8. doi: 10.4049/jimmunol.0902388
41. Degn, SE, Thiel, S, Nielsen, O, Hansen, AG, Steffensen, R, and Jensenius, JC. Map19, the alternative splice product of the Masp2 gene. J Immunol Methods. (2011) 373:89–101. doi: 10.1016/j.jim.2011.08.006
42. De Blasio, D, Fumagalli, S, Orsini, F, Neglia, L, Perego, C, Ortolano, F, et al. Human brain trauma severity is associated with lectin complement pathway activation. J Cereb Blood Flow Metab. (2019) 39:794–807. doi: 10.1177/0271678X18758881
43. Massey, V, Nguyen, C-TE, François, T, De Bruycker, JJ, Bonnefoy, A, Lapeyraque, A-L, et al. Cns inflammation as the first sign of complement factor I deficiency: A severe myelitis treated with intense immunotherapy and Eculizumab. Neurol Neuroimmunol Neuroinflamm. (2023) 11:e200191. doi: 10.1212/NXI.0000000000200191
44. Altmann, T, Torvell, M, Owens, S, Mitra, D, Sheerin, NS, Morgan, BP, et al. Complement factor I deficiency: A potentially treatable cause of fulminant cerebral inflammation. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e689. doi: 10.1212/NXI.0000000000000689
45. Nelson, AR, Sagare, AP, and Zlokovic, BV. Role of Clusterin in the brain vascular clearance of amyloid-Beta. Proc Natl Acad Sci USA. (2017) 114:8681–2. doi: 10.1073/pnas.1711357114
46. Foster, EM, Dangla-Valls, A, Lovestone, S, Ribe, EM, and Buckley, NJ. Clusterin in Alzheimer’s disease: mechanisms, genetics, and lessons from other pathologies. Front Neurosci. (2019) 13:164. doi: 10.3389/fnins.2019.00164
47. Mevorach, D, Reiner, I, Grau, A, Ilan, U, Berkun, Y, Ta-Shma, A, et al. Therapy with Eculizumab for patients with Cd59 P. Cys89tyr mutation. Ann Neurol. (2016) 80:708–17. doi: 10.1002/ana.24770
48. Karbian, N, Eshed-Eisenbach, Y, Tabib, A, Hoizman, H, Morgan, BP, Schueler-Furman, O, et al. Molecular pathogenesis of human Cd59 deficiency. Neurol Genet. (2018) 4:e280. doi: 10.1212/NXG.0000000000000280
49. Yao, X, and Verkman, AS. Complement regulator Cd59 prevents peripheral organ injury in rats made seropositive for Neuromyelitis Optica immunoglobulin G. Acta Neuropathol Commun. (2017) 5:57. doi: 10.1186/s40478-017-0462-4
50. Saadoun, S, and Papadopoulos, MC. Role of membrane complement regulators in Neuromyelitis Optica. Mult Scler. (2015) 21:1644–54. doi: 10.1177/1352458515571446
51. Bokisch, VA, and Müller-Eberhard, HJ. Anaphylatoxin Inactivator of human plasma: its isolation and characterization as a carboxypeptidase. J Clin Invest. (1970) 49:2427–36. doi: 10.1172/JCI106462
52. Rother, RP, Rollins, SA, Mojcik, CF, Brodsky, RA, and Bell, L. Discovery and development of the complement inhibitor Eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. (2007) 25:1256–64. doi: 10.1038/nbt1344
53. Pittock, SJ, Berthele, A, Fujihara, K, Kim, HJ, Levy, M, Palace, J, et al. Eculizumab in Aquaporin-4–positive Neuromyelitis Optica Spectrum disorder. N Engl J Med. (2019) 381:614–25. doi: 10.1056/NEJMoa1900866
54. Sheridan, D, Yu, Z-X, Zhang, Y, Patel, R, Sun, F, Lasaro, MA, et al. Design and preclinical characterization of Alxn1210: a novel anti-C5 antibody with extended duration of action. PLoS One. (2018) 13:e0195909. doi: 10.1371/journal.pone.0195909
55. Tang, G-Q, Tang, Y, Dhamnaskar, K, Hoarty, MD, Vyasamneni, R, Vadysirisack, DD, et al. Zilucoplan, a macrocyclic peptide inhibitor of human complement component 5, uses a dual mode of action to prevent terminal complement pathway activation. Front Immunol. (2023) 14:1213920. doi: 10.3389/fimmu.2023.1213920
56. Hill, A, Rother, RP, Arnold, L, Kelly, R, Cullen, MJ, Richards, SJ, et al. Eculizumab prevents intravascular hemolysis in patients with paroxysmal nocturnal hemoglobinuria and unmasks low-level extravascular hemolysis occurring through C3 Opsonization. Haematologica. (2010) 95:567–73. doi: 10.3324/haematol.2009.007229
57. Allen, J, Nobile-Orazio, E, Peric, S, Katzberg, H, Cadour, S, Van de Walle, I, et al. Safety, efficacy, and pharmacokinetics of Argx-117 in adults with multifocal motor neuropathy: a global, multicenter, placebo controlled phase 2 study (Arda). Neurology. (2022) 99:S40–1. doi: 10.1212/01.wnl.0000903320.23411.f9
58. Hillmen, P, Szer, J, Weitz, I, Röth, A, Höchsmann, B, Panse, J, et al. Pegcetacoplan Versus Eculizumab in Paroxysmal Nocturnal Hemoglobinuria. N Engl J Med. (2021) 384:1028–37. doi: 10.1056/NEJMoa2029073
59. Gadek, JE, Hosea, SW, Gelfand, JA, Santaella, M, Wickerhauser, M, Triantaphyllopoulos, D, et al. Replacement therapy in hereditary angioedema: successful treatment of acute episodes of angioedema with partly purified C1 inhibitor. N Engl J Med. (1980) 302:542–6. doi: 10.1056/NEJM198003063021002
60. Pruzanski, W, and Shumak, KH. Biologic activity of cold-reacting autoantibodies: (first of two parts). N Engl J Med. (1977) 297:538–42. doi: 10.1056/NEJM197709082971005
61. Gertz, MA, Qiu, H, Kendall, L, Saltarelli, M, Yednock, T, and Sankaranarayanan, S. Anx0005, an inhibitory antibody against C1q, blocks complement activation triggered by cold agglutinins in human disease. Blood. (2016) 128:1265. doi: 10.1182/blood.V128.22.1265.1265
62. Levy, M, and Mealy, MA. Purified human C1-esterase inhibitor is safe in acute relapses of Neuromyelitis Optica. Neurol Neuroimmunol Neuroinflamm. (2014) 1:e5. doi: 10.1212/NXI.0000000000000005
63. Tradtrantip, L, Asavapanumas, N, Phuan, P-W, and Verkman, A. Potential therapeutic benefit of C1-esterase inhibitor in Neuromyelitis Optica evaluated in vitro and in an experimental rat model. PLoS One. (2014) 9:e106824. doi: 10.1371/journal.pone.0106824
64. Mohammad, QD, Islam, Z, Papri, N, Hayat, S, Jahan, I, Azad, KAK, et al. Results from a phase 1 study evaluating the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of Anx0005, a C1q inhibitor, in patients with Guillain–Barré syndrome. J Peripher Nerv Syst. (2025) 30:e70009. doi: 10.1111/jns.70009
65. Dudler, T, Yaseen, S, and Cummings, WJ. Development and characterization of Narsoplimab, a selective Masp-2 inhibitor, for the treatment of lectin-pathway–mediated disorders. Front Immunol. (2023) 14:1297352. doi: 10.3389/fimmu.2023.1297352
66. Khaled, SK, Claes, K, Goh, YT, Kwong, YL, Leung, N, Mendrek, W, et al. Narsoplimab, a Mannan-binding lectin-associated serine Protease-2 inhibitor, for the treatment of adult hematopoietic stem-cell transplantation–associated thrombotic Microangiopathy. J Clin Oncol. (2022) 40:2447–57. doi: 10.1200/JCO.21.02389
67. Van de Walle, I, Silence, K, Budding, K, Van de Ven, L, Dijkxhoorn, K, de Zeeuw, E, et al. Argx-117, a therapeutic complement inhibiting antibody targeting C2. J Allergy Clin Immunol. (2021) 147:1420–1429.e7. doi: 10.1016/j.jaci.2020.08.028
68. Budding, K, Johansen, LE, Van de Walle, I, Dijkxhoorn, K, de Zeeuw, E, Bloemenkamp, LM, et al. Anti-C2 antibody Argx-117 inhibits complement in a disease model for multifocal motor neuropathy. Neurol Neuroimmunol Neuroinflamm. (2021) 9:e1107. doi: 10.1212/NXI.0000000000001107
69. Chen, JY, Zhang, L, Luo, L, Yang, M, Chen, Y, and Lin, F. A Nanobody-based complement inhibitor targeting complement component 2 reduces hemolysis in a complement humanized mouse model of autoimmune hemolytic Anemia. Clin Immunol. (2023) 253:109678. doi: 10.1016/j.clim.2023.109678
70. Peffault de Latour, R, Röth, A, Kulasekararaj, AG, Han, B, Scheinberg, P, Maciejewski, JP, et al. Oral Iptacopan monotherapy in paroxysmal nocturnal hemoglobinuria. N Engl J Med. (2024) 390:994–1008. doi: 10.1056/NEJMoa2308695
72. Katschke, KJ, Wu, P, Ganesan, R, Kelley, RF, Mathieu, MA, Hass, PE, et al. Inhibiting alternative pathway complement activation by targeting the factor D exosite. J Biol Chem. (2012) 287:12886–92. doi: 10.1074/jbc.M112.345082
73. Holz, FG, Sadda, SR, Busbee, B, Chew, EY, Mitchell, P, Tufail, A, et al. Efficacy and safety of Lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri phase 3 randomized clinical trials. JAMA Ophthalmol. (2018) 136:666–77. doi: 10.1001/jamaophthalmol.2018.1544
74. Jayne, DR, Merkel, PA, Schall, TJ, and Bekker, P. Avacopan for the treatment of Anca-associated Vasculitis. N Engl J Med. (2021) 384:599–609. doi: 10.1056/NEJMoa2023386
75. Anliker-Ort, M, Dingemanse, J, Farine, H, Groenen, P, Kornberger, R, van den Anker, J, et al. Multiple-ascending doses of Act-1014-6470, an Oral complement factor 5a receptor 1 (C5a1 receptor) antagonist: tolerability, pharmacokinetics and target engagement. Br J Clin Pharmacol. (2023) 89:380–9. doi: 10.1111/bcp.15508
76. Chen, M, Edwards, SR, and Reutens, DC. Complement in the development of post-traumatic epilepsy: prospects for drug repurposing. J Neurotrauma. (2020) 37:692–705. doi: 10.1089/neu.2019.6942
77. Carvelli, J, Meziani, F, Dellamonica, J, Cordier, P-Y, Allardet-Servent, J, Fraisse, M, et al. Avdoralimab (anti-C5ar1 Mab) versus placebo in patients with severe Covid-19: results from a randomized controlled trial (for Covid elimination [Force]). Crit Care Med. (2022) 50:1788–98. doi: 10.1097/CCM.0000000000005683
78. Vlaar, AP, de Bruin, S, Busch, M, Timmermans, SA, van Zeggeren, IE, Koning, R, et al. Anti-C5a antibody Ifx-1 (Vilobelimab) treatment versus Best supportive Care for Patients with severe Covid-19 (Panamo): an exploratory, open-label, phase 2 randomised controlled trial. Lancet Rheumatol. (2020) 2:e764–73. doi: 10.1016/S2665-9913(20)30341-6
79. Bauer, M, Weyland, A, Marx, G, Bloos, F, Weber, S, Weiler, N, et al. Efficacy and safety of Vilobelimab (Ifx-1), a novel monoclonal anti-C5a antibody, in patients with early severe Sepsis or septic shock—a randomized, placebo-controlled, double-blind, multicenter, phase Iia trial (Sciens study). Crit Care Explor. (2021) 3:e0577. doi: 10.1097/CCE.0000000000000577
80. Pittaluga, A, Torre, V, Olivero, G, Rosenwasser, N, and Taddeucci, A. Non-canonical roles of complement in the Cns: from synaptic organizer to presynaptic modulator of glutamate transmission. Curr Neuropharmacol. (2025) 23:820–34. doi: 10.2174/011570159X327960240823065729
81. Lin, K, Zhang, L, Kong, M, Yang, M, Chen, Y, Poptic, E, et al. Development of an anti-human complement C6 monoclonal antibody that inhibits the assembly of membrane attack complexes. Blood Adv. (2020) 4:2049–57. doi: 10.1182/bloodadvances.2020001690
82. Lekova, E, Zelek, WM, Gower, D, Spitzfaden, C, Osuch, IH, John-Morris, E, et al. Discovery of functionally distinct anti-C7 monoclonal antibodies and stratification of anti-nicotinic Achr positive myasthenia gravis patients. Front Immunol. (2022) 13:968206. doi: 10.3389/fimmu.2022.968206
83. Tschongov, T, Konwar, S, Kleindienst, J, Dabrowska-Schlepp, P, Busch, A, Schaaf, A, et al. Effective Long-term treatment with Moss-produced factor H by overcoming the antibody response in a mouse model of C3g. Front Immunol. (2025) 16:1535547. doi: 10.3389/fimmu.2025.1535547
84. Schmidt, CQ, Bai, H, Lin, Z, Risitano, AM, Barlow, PN, Ricklin, D, et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J Immunol. (2013) 190:5712–21. doi: 10.4049/jimmunol.1203548
85. Banda, NK, Levitt, B, Glogowska, MJ, Thurman, JM, Takahashi, K, Stahl, GL, et al. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J Immunol. (2009) 183:5928–37. doi: 10.4049/jimmunol.0901826
86. Rohrer, B, Long, Q, Coughlin, B, Wilson, RB, Huang, Y, Qiao, F, et al. A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest Ophthalmol Vis Sci. (2009) 50:3056–64. doi: 10.1167/iovs.08-2222
87. Schnabolk, G, Parsons, N, Obert, E, Annamalai, B, Nasarre, C, Tomlinson, S, et al. Delivery of Cr2-Fh using Aav vector therapy as treatment strategy in the mouse model of choroidal neovascularization. Mol Ther Methods Clin Dev. (2018) 9:1–11. doi: 10.1016/j.omtm.2017.11.003
88. Thurman, JM, Kulik, L, Orth, H, Wong, M, Renner, B, Sargsyan, SA, et al. Detection of complement activation using monoclonal antibodies against C3d. J Clin Invest. (2013) 123:2218–30. doi: 10.1172/JCI65861
89. Liu, F, Ryan, ST, Fahnoe, KC, Morgan, JG, Cheung, AE, Storek, MJ, et al. C3d-targeted factor H inhibits tissue complement in disease models and reduces glomerular injury without affecting circulating complement. Mol Ther. (2024) 32:1061–79. doi: 10.1016/j.ymthe.2024.02.001
90. Fahnoe, KC, Liu, F, Morgan, JG, Ryan, ST, Storek, M, Stark, EG, et al. Development and optimization of bifunctional fusion proteins to locally modulate complement activation in diseased tissue. Front Immunol. (2022) 13:869725. doi: 10.3389/fimmu.2022.869725
91. Kusner, LL, Satija, N, Cheng, G, and Kaminski, HJ. Targeting therapy to the neuromuscular junction: proof of concept. Muscle Nerve. (2014) 49:749–56. doi: 10.1002/mus.24057
92. Sonnentag, SJ, Dopler, A, Kleiner, K, Garg, BK, Mannes, M, Späth, N, et al. Triple-fusion protein (Trifu): a potent, targeted, enzyme-like inhibitor of all three complement activation pathways. J Biol Chem. (2024) 300:105784. doi: 10.1016/j.jbc.2024.105784
93. Yuki, N, and Hartung, HP. Guillain-Barré Syndrome. N Engl J Med. (2012) 366:2294–304. doi: 10.1056/NEJMra1114525
94. Kuwabara, S, and Yuki, N. Axonal Guillain-Barré syndrome: concepts and controversies. Lancet Neurol. (2013) 12:1180–8. doi: 10.1016/s1474-4422(13)70215-1
95. Hafer-Macko, CE, Sheikh, KA, Li, CY, Ho, TW, Cornblath, DR, McKhann, GM, et al. Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann Neurol. (1996) 39:625–35. doi: 10.1002/ana.410390512
96. Basta, M. Activation and inhibition of complement by immunoglobulins In: J Szebeni, editor. The complement system: Novel roles in health and disease. Boston, MA: Springer US (2004). 517–29.
97. Misawa, S, Kuwabara, S, Sato, Y, Yamaguchi, N, Nagashima, K, Katayama, K, et al. Safety and efficacy of Eculizumab in Guillain-Barré syndrome: a multicentre, double-blind, randomised phase 2 trial. Lancet Neurol. (2018) 17:519–29. doi: 10.1016/s1474-4422(18)30114-5
98. Davidson, AI, Halstead, SK, Goodfellow, JA, Chavada, G, Mallik, A, Overell, J, et al. Inhibition of complement in Guillain-Barré syndrome: the Ica-Gbs study. J Peripher Nerv Syst. (2017) 22:4–12. doi: 10.1111/jns.12194
99. Kuwabara, S, Kusunoki, S, Kuwahara, M, Yamano, Y, Nishida, Y, Ishida, H, et al. Efficacy and safety of Eculizumab in Guillain-Barré syndrome: a phase 3, multicenter, double-blind, randomized, placebo-controlled clinical trial. J Peripher Nerv Syst. (2024) 29:339–49. doi: 10.1111/jns.12646
100. Kroon, H-A, Islam, Z, Collins, P, Hoehn, B, Humphriss, E, Lin, P, et al. Efficacy and safety of targeted immunotherapy with Anx0005 in treating Guillain-Barré syndrome: a phase 3 multicenter study (Pl5.006). Neurology. (2025) 104:4954. doi: 10.1212/WNL.0000000000212033
101. McGonigal, R, Cunningham, ME, Yao, D, Barrie, JA, Sankaranarayanan, S, Fewou, SN, et al. C1q-targeted inhibition of the classical complement pathway prevents injury in a novel mouse model of acute motor axonal neuropathy. Acta Neuropathol Commun. (2016) 4:23. doi: 10.1186/s40478-016-0291-x
102. Querol, LA, Hartung, HP, Lewis, RA, van Doorn, PA, Hammond, TR, Atassi, N, et al. The role of the complement system in chronic inflammatory demyelinating polyneuropathy: implications for complement-targeted therapies. Neurotherapeutics. (2022) 19:864–73. doi: 10.1007/s13311-022-01221-y
103. Keller, CW, Quast, I, Dalakas, MC, and Lünemann, JD. Ivig efficacy in Cidp patients is not associated with terminal complement inhibition. J Neuroimmunol. (2019) 330:23–7. doi: 10.1016/j.jneuroim.2019.02.001
104. Querol, L, Lewis, R, Hartung, H-P, Van Doorn, P, Wallstroem, E, Luo, X, et al. Preliminary efficacy and safety data from the phase 2 trial of Riliprubart (Sar445088), a humanized monoclonal antibody targeting complement C1s, in chronic inflammatory demyelinating polyneuropathy (Cidp) (S15.008). Neurology. (2024) 102:2552. doi: 10.1212/WNL.0000000000204596
105. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on Management of Multifocal Motor Neuropathy. Report of a joint task Force of the European Federation of Neurological Societies and the peripheral nerve society--first revision. J Peripher Nerv Syst. (2010) 15:295–301. doi: 10.1111/j.1529-8027.2010.00290.x
106. Nobile-Orazio, E, Giannotta, C, Musset, L, Messina, P, and Léger, JM. Sensitivity and predictive value of anti-Gm1/Galactocerebroside Igm antibodies in multifocal motor neuropathy. J Neurol Neurosurg Psychiatry. (2014) 85:754–8. doi: 10.1136/jnnp-2013-305755
107. Willison, HJ, and Yuki, N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. (2002) 125:2591–625. doi: 10.1093/brain/awf272
108. Vlam, L, Cats, EA, Harschnitz, O, Jansen, MD, Piepers, S, Veldink, JH, et al. Complement activity is associated with disease severity in multifocal motor neuropathy. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e119. doi: 10.1212/nxi.0000000000000119
109. Thomas, FP, Trojaborg, W, Nagy, C, Santoro, M, Sadiq, SA, Latov, N, et al. Experimental autoimmune neuropathy with anti-Gm1 antibodies and immunoglobulin deposits at the nodes of Ranvier. Acta Neuropathol. (1991) 82:378–83. doi: 10.1007/bf00296548
110. Harvey, GK, Toyka, KV, Zielasek, J, Kiefer, R, Simonis, C, and Hartung, HP. Failure of anti-Gm1 igg or Igm to induce conduction block following Intraneural transfer. Muscle Nerve. (1995) 18:388–94. doi: 10.1002/mus.880180404
111. Fitzpatrick, AM, Mann, CA, Barry, S, Brennan, K, Overell, JR, and Willison, HJ. An open label clinical trial of complement inhibition in multifocal motor neuropathy. J Peripher Nerv Syst. (2011) 16:84–91. doi: 10.1111/j.1529-8027.2011.00328.x
112. Rumsey, JW, Lorance, C, Jackson, M, Sasserath, T, McAleer, CW, Long, CJ, et al. Classical complement pathway inhibition in a “human-on-a-Chip” model of autoimmune demyelinating neuropathies. Adv Ther. (2022) 5:2200030. doi: 10.1002/adtp.202200030
113. Budding, K, Johansen, LE, Van de Walle, I, Dijkxhoorn, K, de Zeeuw, E, Bloemenkamp, LM, et al. Anti-C2 antibody Argx-117 inhibits complement in a disease model for multifocal motor neuropathy. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e1107. doi: 10.1212/NXI.0000000000001107
114. van der Pol, W. Ludo. Available online at: https://argenxmedical.com/content/dam/medical-affairs/congress-posters/nuerology/mmn/pns/Empasiprubart%20%28ARGX-117%29%20in%20Multifocal%20Motor%20Neuropathy%20Initial%20Safety%20and%20Efficacy%20Data%20of%20the%20Phase%202%20ARDA%20Study.pdf (Accessed july 1, 2025).
115. Querol, L. Autoimmune Nodopathies: treatable neuropathies beyond traditional classifications. J Neurol Neurosurg Psychiatry. (2021) 92:1025. doi: 10.1136/jnnp-2021-326676
116. Svahn, J, Petiot, P, Antoine, JC, Vial, C, Delmont, E, Viala, K, et al. Anti-mag antibodies in 202 patients: Clinicopathological and therapeutic features. J Neurol Neurosurg Psychiatry. (2018) 89:499–505. doi: 10.1136/jnnp-2017-316715
117. Smith, IS. The natural history of chronic demyelinating neuropathy associated with benign Igm Paraproteinaemia. A clinical and neurophysiological study. Brain. (1994) 117:949–57. doi: 10.1093/brain/117.5.949
118. Monaco, S, Bonetti, B, Ferrari, S, Moretto, G, Nardelli, E, Tedesco, F, et al. Complement-mediated demyelination in patients with Igm monoclonal Gammopathy and polyneuropathy. N Engl J Med. (1990) 322:649–52. doi: 10.1056/nejm199003083221002
119. Kawagashira, Y, Koike, H, Tomita, M, Morozumi, S, Iijima, M, Nakamura, T, et al. Morphological progression of myelin abnormalities in Igm-monoclonal Gammopathy of undetermined significance anti-myelin-associated glycoprotein neuropathy. J Neuropathol Exp Neurol. (2010) 69:1143–57. doi: 10.1097/NEN.0b013e3181fa44af
120. Amaador, K, Wieske, L, Koel-Simmelink, MJA, Kamp, A, Jongerius, I, de Heer, K, et al. Serum Neurofilament light chain, Contactin-1 and complement activation in anti-mag Igm Paraprotein-related peripheral neuropathy. J Neurol. (2022) 269:3700–5. doi: 10.1007/s00415-022-10993-4
121. van de Mortel, JPM, Budding, K, Dijkxhoorn, K, Minnema, MC, Vrancken, A, Notermans, NC, et al. The role of complement activation in Igm M-protein-associated neuropathies. Neurol Neuroimmunol Neuroinflamm. (2025) 12:e200339. doi: 10.1212/nxi.0000000000200339
122. Vincent, A, Huda, S, Cao, M, Cetin, H, Koneczny, I, Rodriguez Cruz, PM, et al. Serological and experimental studies in different forms of myasthenia gravis. Ann N Y Acad Sci. (2018) 1413:143–53. doi: 10.1111/nyas.13592
123. Conti-Fine, BM, Milani, M, and Kaminski, HJ. Myasthenia gravis: past, present, and future. J Clin Invest. (2006) 116:2843–54. doi: 10.1172/jci29894
124. Howard, JF Jr. Myasthenia gravis: the role of complement at the neuromuscular junction. Ann N Y Acad Sci. (2018) 1412:113–28. doi: 10.1111/nyas.13522
125. Engel, AG, and Fumagalli, G. Mechanisms of acetylcholine receptor loss from the neuromuscular junction. Ciba Found Symp. (1982) 90:197–224. doi: 10.1002/9780470720721.ch12
126. Dalakas, MC. Role of complement, anti-complement therapeutics, and other targeted immunotherapies in myasthenia gravis. Expert Rev Clin Immunol. (2022) 18:691–701. doi: 10.1080/1744666X.2022.2082946
127. Nastuk, WL, Plescia, OJ, and Osserman, KE. Changes in serum complement activity in patients with myasthenia gravis. Proc Soc Exp Biol Med. (1960) 105:177–84. doi: 10.3181/00379727-105-26050
128. Strauss, AJ, van der Geld, HW, Kemp, PG Jr, Exum, ED, and Goodman, HC. Immunological concomitants of myasthenia gravis. Ann N Y Acad Sci. (1965) 124:744–66. doi: 10.1111/j.1749-6632.1965.tb18999.x
129. Nastuk, WL, Strauss, AJL, and Osserman, KE. Search for a neuromuscular blocking agent in the blood of patients with myasthenia gravis. Am J Med. (1959) 26:394–409. doi: 10.1016/0002-9343(59)90248-7
130. Engel, AG, Lambert, EH, and Howard, FM. Immune complexes (igg and C3) at the motor end-plate in myasthenia gravis: ultrastructural and light microscopic localization and Electrophysiologic correlations. Mayo Clin Proc. (1977) 52:267–80.
131. Nakano, S, and Engel, AG. Myasthenia gravis: quantitative Immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology. (1993) 43:1167–72. doi: 10.1212/wnl.43.6.1167
132. Ozawa, Y, Uzawa, A, Yasuda, M, Kojima, Y, Oda, F, Himuro, K, et al. Changes in serum complements and their regulators in generalized myasthenia gravis. Eur J Neurol. (2021) 28:314–22. doi: 10.1111/ene.14500
133. Howard, JF Jr, Utsugisawa, K, Benatar, M, Murai, H, Barohn, RJ, Illa, I, et al. Safety and efficacy of Eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (Regain): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. (2017) 16:976–86. doi: 10.1016/S1474-4422(17)30369-1
134. Meisel, A, Annane, D, Vu, T, Mantegazza, R, Katsuno, M, Aguzzi, R, et al. Long-term efficacy and safety of Ravulizumab in adults with anti-acetylcholine receptor antibody-positive generalized myasthenia gravis: results from the phase 3 Champion Mg open-label extension. J Neurol. (2023) 270:3862–75. doi: 10.1007/s00415-023-11699-x
135. Howard, JF Jr, Bresch, S, Genge, A, Hewamadduma, C, Hinton, J, Hussain, Y, et al. Safety and efficacy of Zilucoplan in patients with generalised myasthenia gravis (raise): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Neurol. (2023) 22:395–406. doi: 10.1016/S1474-4422(23)00080-7
136. Gwathmey, K ed. Efficacy and safety of subcutaneous self-administered Gefurulimab in generalized myasthenia gravis: Topline results from a phase 3, randomized, double-blind, placebo-controlled study (Prevail). San Francisco, CA: Myasthenia Gravis Foundation of America Scientific Session (2025).
137. Huijbers, MG, Zhang, W, Klooster, R, Niks, EH, Friese, MB, Straasheijm, KR, et al. Musk Igg4 autoantibodies cause myasthenia gravis by inhibiting binding between Musk and Lrp4. Proc Natl Acad Sci USA. (2013) 110:20783–8. doi: 10.1073/pnas.1313944110
138. Kawakami, Y, Ito, M, Hirayama, M, Sahashi, K, Ohkawara, B, Masuda, A, et al. Anti-Musk autoantibodies block binding of collagen Q to Musk. Neurology. (2011) 77:1819–26. doi: 10.1212/WNL.0b013e318237f660
139. Dalakas, MC. Inflammatory muscle diseases. N Engl J Med. (2015) 372:1734–47. doi: 10.1056/NEJMra1402225
140. Lundberg, IE, Fujimoto, M, Vencovsky, J, Aggarwal, R, Holmqvist, M, Christopher-Stine, L, et al. Idiopathic inflammatory myopathies. Nat Rev Dis Prim. (2021) 7:86. doi: 10.1038/s41572-021-00321-x
141. Kissel, JT, Mendell, JR, and Rammohan, KW. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med. (1986) 314:329–34. doi: 10.1056/nejm198602063140601
142. Oldroyd, A, Lilleker, J, and Chinoy, H. Idiopathic inflammatory myopathies - a guide to subtypes, diagnostic approach and treatment. Clin Med (Lond). (2017) 17:322–8. doi: 10.7861/clinmedicine.17-4-322
143. Emslie-Smith, AM, and Engel, AG. Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol. (1990) 27:343–56. doi: 10.1002/ana.410270402
144. Greenberg, SA. Proposed immunologic models of the inflammatory myopathies and potential therapeutic implications. Neurology. (2007) 69:2008–19. doi: 10.1212/01.WNL.0000291619.17160.b8
145. Pachman, LM, and Khojah, AM. Advances in juvenile dermatomyositis: myositis specific antibodies aid in understanding disease heterogeneity. J Pediatr. (2018) 195:16–27. doi: 10.1016/j.jpeds.2017.12.053
146. Kamperman, RG, van der Kooi, AJ, de Visser, M, Aronica, E, and Raaphorst, J. Pathophysiological mechanisms and treatment of dermatomyositis and immune mediated necrotizing myopathies: a focused review. Int J Mol Sci. (2022) 23:4301. doi: 10.3390/ijms23084301
147. Basta, M, and Dalakas, MC. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking Endomysial deposition of activated complement fragments. J Clin Invest. (1994) 94:1729–35. doi: 10.1172/jci117520
148. Dalakas, MC, Illa, I, Dambrosia, JM, Soueidan, SA, Stein, DP, Otero, C, et al. A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med. (1993) 329:1993–2000. doi: 10.1056/nejm199312303292704
149. Faguer, S, Belliere, J, and Ribes, D. Complement C5-blocking agent in refractory dermatomyositis. J Rheumatol. (2018) 45:1710–1. doi: 10.3899/jrheum.180060
150. Bergua, C, Chiavelli, H, Allenbach, Y, Arouche-Delaperche, L, Arnoult, C, Bourdenet, G, et al. In vivo pathogenicity of igg from patients with anti-Srp or anti-Hmgcr autoantibodies in immune-mediated Necrotising myopathy. Ann Rheum Dis. (2019) 78:131–9. doi: 10.1136/annrheumdis-2018-213518
151. Pinal-Fernandez, I, Casal-Dominguez, M, and Mammen, AL. Immune-mediated necrotizing myopathy. Curr Rheumatol Rep. (2018) 20:21. doi: 10.1007/s11926-018-0732-6
152. Allenbach, Y, Benveniste, O, Stenzel, W, and Boyer, O. Immune-mediated necrotizing myopathy: clinical features and pathogenesis. Nat Rev Rheumatol. (2020) 16:689–701. doi: 10.1038/s41584-020-00515-9
153. Ma, X, and Bu, BT. Anti-Srp immune-mediated necrotizing myopathy: a critical review of current concepts. Front Immunol. (2022) 13:1019972. doi: 10.3389/fimmu.2022.1019972
154. Mammen, AL, Amato, AA, Dimachkie, MM, Chinoy, H, Hussain, Y, Lilleker, JB, et al. Zilucoplan in immune-mediated Necrotising myopathy: a phase 2, randomised, double-blind, placebo-controlled, multicentre trial. Lancet Rheumatol. (2023) 5:e67–76. doi: 10.1016/s2665-9913(23)00003-6
155. Julien, S, Vadysirisack, D, Sayegh, C, Ragunathan, S, Tang, Y, Briand, E, et al. Prevention of anti-Hmgcr immune-mediated Necrotising myopathy by C5 complement inhibition in a humanised mouse model. Biomedicine. (2022) 10:2036. doi: 10.3390/biomedicines10082036
156. Paul, P, Liewluck, T, Ernste, FC, Mandrekar, J, and Milone, M. Anti-Cn1a antibodies do not correlate with specific clinical, Electromyographic, or pathological findings in sporadic inclusion body myositis. Muscle Nerve. (2021) 63:490–6. doi: 10.1002/mus.27157
157. Amlani, A, Choi, MY, Tarnopolsky, M, Brady, L, Clarke, AE, Garcia-De La Torre, I, et al. Anti-Nt5c1a autoantibodies as biomarkers in inclusion body myositis. Front Immunol. (2019) 10:745. doi: 10.3389/fimmu.2019.00745
158. Tawara, N, Yamashita, S, Zhang, X, Korogi, M, Zhang, Z, Doki, T, et al. Pathomechanisms of anti-cytosolic 5'-Nucleotidase 1a autoantibodies in sporadic inclusion body myositis. Ann Neurol. (2017) 81:512–25. doi: 10.1002/ana.24919
159. Syed, A, Hannah, M, Marisol, G, Husham, H, Abdulwahhab, A, and Yessar, H.2023 Annual Meeting Abstracts Guide. Muscle Nerve. (2023) 68:476–686. doi: 10.1002/mus.27968
160. Farooq, MZ, Aqeel, SB, Lingamaneni, P, Pichardo, RC, Jawed, A, Khalid, S, et al. Association of Immune Checkpoint Inhibitors with neurologic adverse events: a systematic review and Meta-analysis. JAMA Netw Open. (2022) 5:e227722. doi: 10.1001/jamanetworkopen.2022.7722
161. Beecher, G, Pinal-Fernandez, I, Mammen, AL, and Liewluck, T. Immune checkpoint inhibitor myopathy. Neurology. (2024) 103:e210031. doi: 10.1212/WNL.0000000000210031
162. Postow, MA, Sidlow, R, and Hellmann, MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. (2018) 378:158–68. doi: 10.1056/NEJMra1703481
163. de Moel, EC, Rozeman, EA, Kapiteijn, EH, Verdegaal, EME, Grummels, A, Bakker, JA, et al. Autoantibody development under treatment with immune-checkpoint inhibitors. Cancer Immunol Res. (2019) 7:6–11. doi: 10.1158/2326-6066.Cir-18-0245
164. Zadeh, S, Price, H, Drews, R, Bouffard, MA, Young, LH, and Narayanaswami, P. Novel uses of complement inhibitors in myasthenia gravis-two case reports. Muscle Nerve. (2024) 69:368–72. doi: 10.1002/mus.28037
165. Nelke, C, Pawlitzki, M, Kerkhoff, R, Schroeter, CB, Aktas, O, Neuen-Jacob, E, et al. Immune checkpoint inhibition–related myasthenia-myositis-myocarditis responsive to complement blockade. Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200177. doi: 10.1212/NXI.0000000000200177
166. Lennon, VA, Wingerchuk, DM, Kryzer, TJ, Pittock, SJ, Lucchinetti, CF, Fujihara, K, et al. A serum autoantibody marker of Neuromyelitis Optica: distinction from multiple sclerosis. Lancet. (2004) 364:2106–12. doi: 10.1016/S0140-6736(04)17551-X
167. Cotzomi, E, Stathopoulos, P, Lee, CS, Ritchie, AM, Soltys, JN, Delmotte, FR, et al. Early B cell tolerance defects in Neuromyelitis Optica favour anti-Aqp4 autoantibody production. Brain. (2019) 142:1598–615. doi: 10.1093/brain/awz106
168. Jarius, S, Franciotta, D, Paul, F, Ruprecht, K, Bergamaschi, R, Rommer, PS, et al. Cerebrospinal fluid antibodies to Aquaporin-4 in Neuromyelitis Optica and related disorders: frequency, origin, and diagnostic relevance. J Neuroinflammation. (2010) 7:52. doi: 10.1186/1742-2094-7-52
169. Akaishi, T, Takahashi, T, Misu, T, Kaneko, K, Takai, Y, Nishiyama, S, et al. Difference in the source of anti-Aqp4-igg and anti-Mog-igg antibodies in Csf in patients with Neuromyelitis Optica Spectrum disorder. Neurology. (2021) 97:e1–e12. doi: 10.1212/WNL.0000000000012175
170. Guo, Y, Weigand, SD, Popescu, BF, Lennon, VA, Parisi, JE, Pittock, SJ, et al. Pathogenic implications of cerebrospinal fluid barrier pathology in Neuromyelitis Optica. Acta Neuropathol. (2017) 133:597–612. doi: 10.1007/s00401-017-1682-1
171. Piatek, P, Domowicz, M, Lewkowicz, N, Przygodzka, P, Matysiak, M, Dzitko, K, et al. C5a-Preactivated neutrophils are critical for autoimmune-induced astrocyte dysregulation in Neuromyelitis Optica Spectrum disorder. Front Immunol. (2018) 9:1694. doi: 10.3389/fimmu.2018.01694
172. Lucchinetti, CF, Mandler, RN, McGavern, D, Bruck, W, Gleich, G, Ransohoff, RM, et al. A role for humoral mechanisms in the pathogenesis of Devic's Neuromyelitis Optica. Brain. (2002) 125:1450–61. doi: 10.1093/brain/awf151
173. Zhang, H, Bennett, JL, and Verkman, AS. Ex vivo spinal cord slice model of Neuromyelitis Optica reveals novel Immunopathogenic mechanisms. Ann Neurol. (2011) 70:943–54. doi: 10.1002/ana.22551
174. Hinson, SR, Roemer, SF, Lucchinetti, CF, Fryer, JP, Kryzer, TJ, Chamberlain, JL, et al. Aquaporin-4-binding autoantibodies in patients with Neuromyelitis Optica impair glutamate transport by down-regulating Eaat2. J Exp Med. (2008) 205:2473–81. doi: 10.1084/jem.20081241
175. Kuroda, H, Fujihara, K, Takano, R, Takai, Y, Takahashi, T, Misu, T, et al. Increase of complement fragment C5a in cerebrospinal fluid during exacerbation of Neuromyelitis Optica. J Neuroimmunol. (2013) 254:178–82. doi: 10.1016/j.jneuroim.2012.09.002
176. Pittock, SJ, Barnett, M, Bennett, JL, Berthele, A, de Seze, J, Levy, M, et al. Ravulizumab in Aquaporin-4-positive Neuromyelitis Optica Spectrum disorder. Ann Neurol. (2023) 93:1053–68. doi: 10.1002/ana.26626
177. Krieger, S, Cook, K, and Hersh, CM. Understanding multiple sclerosis as a disease Spectrum: above and below the clinical threshold. Curr Opin Neurol. (2024) 37:189–201. doi: 10.1097/WCO.0000000000001262
178. Fox, RJ, Bar-Or, A, Traboulsee, A, Oreja-Guevara, C, Giovannoni, G, Vermersch, P, et al. Tolebrutinib in nonrelapsing secondary progressive multiple sclerosis. N Engl J Med. (2025) 392:1883–92. doi: 10.1056/NEJMoa2415988
179. Greenfield, AL, and Hauser, SL. B-cell therapy for multiple sclerosis: entering an era. Ann Neurol. (2018) 83:13–26. doi: 10.1002/ana.25119
180. Lucchinetti, C, Bruck, W, Parisi, J, Scheithauer, B, Rodriguez, M, and Lassmann, H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. (2000) 47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q
181. Schwab, C, and McGeer, PL. Complement activated C4d Immunoreactive oligodendrocytes delineate small cortical plaques in multiple sclerosis. Exp Neurol. (2002) 174:81–8. doi: 10.1006/exnr.2001.7851
182. Barnett, MH, Parratt, JD, Cho, ES, and Prineas, JW. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann Neurol. (2009) 65:32–46. doi: 10.1002/ana.21524
183. Ingram, G, Loveless, S, Howell, OW, Hakobyan, S, Dancey, B, Harris, CL, et al. Complement activation in multiple sclerosis plaques: an Immunohistochemical analysis. Acta Neuropathol Commun. (2014) 2:53. doi: 10.1186/2051-5960-2-53
184. Prineas, JW, Kwon, EE, Cho, ES, Sharer, LR, Barnett, MH, Oleszak, EL, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. (2001) 50:646–57. doi: 10.1002/ana.1255
185. Morgan, BP, Gommerman, JL, and Ramaglia, V. An "outside-in" and "inside-out" consideration of complement in the multiple sclerosis brain: lessons from development and neurodegenerative diseases. Front Cell Neurosci. (2020) 14:600656. doi: 10.3389/fncel.2020.600656
186. Saez-Calveras, N, and Stuve, O. The role of the complement system in multiple sclerosis: a review. Front Immunol. (2022) 13:970486. doi: 10.3389/fimmu.2022.970486
187. Hu, X, Holers, VM, Thurman, JM, Schoeb, TR, Ramos, TN, and Barnum, SR. Therapeutic inhibition of the alternative complement pathway attenuates chronic Eae. Mol Immunol. (2013) 54:302–8. doi: 10.1016/j.molimm.2012.12.018
188. Hu, X, Tomlinson, S, and Barnum, SR. Targeted inhibition of complement using complement receptor 2-conjugated inhibitors attenuates Eae. Neurosci Lett. (2012) 531:35–9. doi: 10.1016/j.neulet.2012.10.012
189. Li, Q, Nacion, K, Bu, H, and Lin, F. The complement inhibitor Fut-175 suppresses T cell autoreactivity in experimental autoimmune encephalomyelitis. Am J Pathol. (2009) 175:661–7. doi: 10.2353/ajpath.2009.081093
190. Kelly, H, and Levy, M. Eculizumab therapy in a patient with secondary progressive multiple sclerosis. Neuroimmunol Rep. (2022) 2:100111. doi: 10.1016/j.nerep.2022.100111
191. Allinovi, M, Bellinvia, A, Pesce, F, Milan Manani, S, Razzolini, L, Brezzi, B, et al. Safety and efficacy of Eculizumab therapy in multiple sclerosis: a case series. Brain Sci. (2021) 11:1341. doi: 10.3390/brainsci11101341
192. Held, F, Kalluri, SR, Berthele, A, Klein, AK, Reindl, M, and Hemmer, B. Frequency of myelin oligodendrocyte glycoprotein antibodies in a large cohort of neurological patients. Mult Scler J Exp Transl Clin. (2021) 7:20552173211022767. doi: 10.1177/20552173211022767
193. Sechi, E, Buciuc, M, Pittock, SJ, Chen, JJ, Fryer, JP, Jenkins, SM, et al. Positive predictive value of myelin oligodendrocyte glycoprotein autoantibody testing. JAMA Neurol. (2021) 78:741–6. doi: 10.1001/jamaneurol.2021.0912
194. Lerch, M, Bauer, A, and Reindl, M. The potential pathogenicity of myelin oligodendrocyte glycoprotein antibodies in the optic pathway. J Neuroophthalmol. (2023) 43:5–16. doi: 10.1097/WNO.0000000000001772
195. Moseley, CE, Virupakshaiah, A, Forsthuber, TG, Steinman, L, Waubant, E, and Zamvil, SS. Mog Cns autoimmunity and Mogad. Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200275. doi: 10.1212/NXI.0000000000200275
196. Huda, S, Whittam, D, Jackson, R, Karthikeayan, V, Kelly, P, Linaker, S, et al. Predictors of relapse in Mog antibody associated disease: a cohort study. BMJ Open. (2021) 11:e055392. doi: 10.1136/bmjopen-2021-055392
197. Keller, CW, Lopez, JA, Wendel, EM, Ramanathan, S, Gross, CC, Klotz, L, et al. Complement activation is a prominent feature of Mogad. Ann Neurol. (2021) 90:976–82. doi: 10.1002/ana.26226
198. Macrini, C, Gerhards, R, Winklmeier, S, Bergmann, L, Mader, S, Spadaro, M, et al. Features of Mog required for recognition by patients with Mog antibody-associated disorders. Brain. (2021) 144:2375–89. doi: 10.1093/brain/awab105
199. Kaneko, K, Kuroda, H, Matsumoto, Y, Sakamoto, N, Yamazaki, N, Yamamoto, N, et al. Different complement activation patterns following C5 cleavage in Mogad and Aqp4-igg+Nmosd. Neurol Neuroimmunol Neuroinflamm. (2024) 11:e200293. doi: 10.1212/NXI.0000000000200293
200. Villacieros-Alvarez, J, Lunemann, JD, Sepulveda, M, Valls-Carbo, A, Dinoto, A, Fernandez, V, et al. Complement activation profiles predict clinical outcomes in myelin oligodendrocyte glycoprotein antibody-associated disease. Neurol Neuroimmunol Neuroinflamm. (2025) 12:e200340. doi: 10.1212/NXI.0000000000200340
201. Sechi, E, Krecke, KN, Messina, SA, Buciuc, M, Pittock, SJ, Chen, JJ, et al. Comparison of Mri lesion evolution in different central nervous system demyelinating disorders. Neurology. (2021) 97:e1097–109. doi: 10.1212/WNL.0000000000012467
202. Chen, JJ, Flanagan, EP, Bhatti, MT, Jitprapaikulsan, J, Dubey, D, Lopez Chiriboga, ASS, et al. Steroid-sparing maintenance immunotherapy for Mog-igg associated disorder. Neurology. (2020) 95:e111–20. doi: 10.1212/WNL.0000000000009758
203. Chen, JJ, Huda, S, Hacohen, Y, Levy, M, Lotan, I, Wilf-Yarkoni, A, et al. Association of Maintenance Intravenous Immunoglobulin with prevention of relapse in adult myelin oligodendrocyte glycoprotein antibody-associated disease. JAMA Neurol. (2022) 79:518–25. doi: 10.1001/jamaneurol.2022.0489
204. Graus, F, Titulaer, MJ, Balu, R, Benseler, S, Bien, CG, Cellucci, T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
205. Tuzun, E, Zhou, L, Baehring, JM, Bannykh, S, Rosenfeld, MR, and Dalmau, J. Evidence for antibody-mediated pathogenesis in anti-Nmdar encephalitis associated with ovarian Teratoma. Acta Neuropathol. (2009) 118:737–43. doi: 10.1007/s00401-009-0582-4
206. Martinez-Hernandez, E, Horvath, J, Shiloh-Malawsky, Y, Sangha, N, Martinez-Lage, M, and Dalmau, J. Analysis of complement and plasma cells in the brain of patients with anti-Nmdar encephalitis. Neurology. (2011) 77:589–93. doi: 10.1212/WNL.0b013e318228c136
207. Bien, CG, Vincent, A, Barnett, MH, Becker, AJ, Blumcke, I, Graus, F, et al. Immunopathology of autoantibody-associated Encephalitides: clues for pathogenesis. Brain. (2012) 135:1622–38. doi: 10.1093/brain/aws082
208. Irani, SR, Bera, K, Waters, P, Zuliani, L, Maxwell, S, Zandi, MS, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and Paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. (2010) 133:1655–67. doi: 10.1093/brain/awq113
209. Carvajal-Gonzalez, A, Leite, MI, Waters, P, Woodhall, M, Coutinho, E, Balint, B, et al. Glycine receptor antibodies in perm and related syndromes: characteristics, clinical features and outcomes. Brain. (2014) 137:2178–92. doi: 10.1093/brain/awu142
210. Kortvelyessy, P, Bauer, J, Stoppel, CM, Bruck, W, Gerth, I, Vielhaber, S, et al. Complement-associated neuronal loss in a patient with Caspr2 antibody-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e75. doi: 10.1212/NXI.0000000000000075
211. Guo, Y, Endmayr, V, Zekeridou, A, McKeon, A, Leypoldt, F, Hess, K, et al. New insights into neuropathology and pathogenesis of autoimmune glial fibrillary acidic protein Meningoencephalomyelitis. Acta Neuropathol. (2024) 147:31. doi: 10.1007/s00401-023-02678-7
212. Chuquisana, O, Strippel, C, Troscher, AM, Baumgartner, T, Racz, A, Keller, CW, et al. Complement activation contributes to gad antibody-associated encephalitis. Acta Neuropathol. (2022) 144:381–3. doi: 10.1007/s00401-022-02448-x
213. Rittel, JC, Hudasch, D, Doppler, K, Bergh, FT, Lesser, M, Aktas, O, et al. Intravenous immunoglobulin as first-line acute treatment in adults with autoimmune encephalitis caused by antibodies to Nmdar, Lgi1 and Caspr2. J Neurol. (2025) 272:287. doi: 10.1007/s00415-025-13032-0
214. Dalmau, J, and Graus, F. Antibody-mediated encephalitis. N Engl J Med. (2018) 378:840–51. doi: 10.1056/NEJMra1708712
215. Dalakas, MC, Fujii, M, Li, M, Lutfi, B, Kyhos, J, and McElroy, B. High-dose intravenous immune globulin for stiff-person syndrome. N Engl J Med. (2001) 345:1870–6. doi: 10.1056/NEJMoa01167
216. Dubey, D, Britton, J, McKeon, A, Gadoth, A, Zekeridou, A, Lopez Chiriboga, SA, et al. Randomized placebo-controlled trial of intravenous immunoglobulin in autoimmune Lgi1/Caspr2 epilepsy. Ann Neurol. (2020) 87:313–23. doi: 10.1002/ana.25655
217. McCombe, JA, Klassen, BT, Flanagan, EP, Teener, JW, Zekeridou, A, Pittock, SJ, et al. Eculizumab for the treatment of Glycine receptor antibody associated stiff-person syndrome. J Neurol. (2023) 270:4555–7. doi: 10.1007/s00415-023-11777-0
218. Beuker, C, Strunk, D, Rawal, R, Schmidt-Pogoda, A, Werring, N, Milles, L, et al. Primary Angiitis of the Cns: a systematic review and Meta-analysis. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1093. doi: 10.1212/NXI.0000000000001093
219. Mandel-Brehm, C, Retallack, H, Knudsen, GM, Yamana, A, Hajj-Ali, RA, Calabrese, LH, et al. Exploratory proteomic analysis implicates the alternative complement Cascade in primary Cns Vasculitis. Neurology. (2019) 93:e433–44. doi: 10.1212/WNL.0000000000007850
220. Deb-Chatterji, M, Keller, CW, Koch, S, Wiendl, H, Gerloff, C, Magnus, T, et al. Profiling complement system components in primary Cns Vasculitis. Cells. (2021) 10:1139. doi: 10.3390/cells10051139
221. Nishida, H, Kumada, S, Komori, T, Takai, K, Mori, H, Morino, M, et al. Ivig in childhood primary Angiitis of the central nervous system: a case report. Brain Dev. (2020) 42:675–9. doi: 10.1016/j.braindev.2020.06.007
222. Crew, PE, Abara, WE, McCulley, L, Waldron, PE, Kirkcaldy, RD, Weston, EJ, et al. Disseminated gonococcal infections in patients receiving Eculizumab: a case series. Clin Infect Dis. (2019) 69:596–600. doi: 10.1093/cid/ciy958
223. Castilla, J, Garcia Cenoz, M, Abad, R, Sanchez-Cambronero, L, Lorusso, N, Izquierdo, C, et al. Effectiveness of a meningococcal group B vaccine (4cmenb) in children. N Engl J Med. (2023) 388:427–38. doi: 10.1056/NEJMoa2206433
224. Rappuoli, R, Pizza, M, Masignani, V, and Vadivelu, K. Meningococcal B vaccine (4cmenb): the journey from research to real world experience. Expert Rev Vaccines. (2018) 17:1111–21. doi: 10.1080/14760584.2018.1547637
225. Socié, G, Caby-Tosi, MP, Marantz, JL, Cole, A, Bedrosian, CL, Gasteyger, C, et al. Eculizumab in paroxysmal nocturnal Haemoglobinuria and atypical Haemolytic Uraemic syndrome: 10-year pharmacovigilance analysis. Br J Haematol. (2019) 185:297–310. doi: 10.1111/bjh.15790
226. Flierman, R, and Daha, MR. The clearance of apoptotic cells by complement. Immunobiology. (2007) 212:363–70. doi: 10.1016/j.imbio.2006.11.005
227. Reis, ES, Mastellos, DC, Ricklin, D, Mantovani, A, and Lambris, JD. Complement in Cancer: untangling an intricate relationship. Nat Rev Immunol. (2018) 18:5–18. doi: 10.1038/nri.2017.97
228. Jacob, A, Hack, B, Chiang, E, Garcia, JG, Quigg, RJ, and Alexander, JJ. C5a alters blood-brain barrier integrity in experimental lupus. FASEB J. (2010) 24:1682–8. doi: 10.1096/fj.09-138834
229. Dhillon, S. Sutimlimab: First Approval. Drugs. (2022) 82:817–23. doi: 10.1007/s40265-022-01711-5
230. Lansita, JA, Mease, KM, Qiu, H, Yednock, T, Sankaranarayanan, S, and Kramer, S. Nonclinical development of Anx0005: a humanized anti-C1q antibody for treatment of autoimmune and neurodegenerative diseases. Int J Toxicol. (2017) 36:449–62. doi: 10.1177/1091581817740873
231. Tillmon, H, Soteros, BM, Shen, L, Cong, Q, Wollet, M, General, J, et al. Complement and microglia activation mediate stress-induced synapse loss in layer 2/3 of the medial prefrontal cortex in male mice. Nat Commun. (2024) 15:9803. doi: 10.1038/s41467-024-54007-5
232. Dailey, AT, Avellino, AM, Benthem, L, Silver, J, and Kliot, M. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci. (1998) 18:6713–22. doi: 10.1523/JNEUROSCI.18-17-06713.1998
233. Markiewski, MM, DeAngelis, RA, Benencia, F, Ricklin-Lichtsteiner, SK, Koutoulaki, A, Gerard, C, et al. Modulation of the antitumor immune response by complement. Nat Immunol. (2008) 9:1225–35. doi: 10.1038/ni.1655
234. Yang, S, Li, T, Jia, H, Gao, M, Li, Y, Wan, X, et al. Targeting C3b/C4b and Vegf with a bispecific fusion protein optimized for Neovascular age-related macular degeneration therapy. Sci Transl Med. (2022) 14:eabj2177. doi: 10.1126/scitranslmed.abj2177
235. Chung, EDP, Kim, D, Ryu, S, Chang, J, and Lee, BC. Knp-301, a dual inhibitor of the complement pathway and angiogenesis, effectively suppresses angiogenesis and atrophy. Invest Ophthalmol Vis Sci. (2021) 62:183. doi: 10.1038/s41467-024-54007-5
236. Lee, Y, Kim, D, Chung, PE, Lee, M, Kim, N, Chang, J, et al. Pre-clinical studies of a novel bispecific fusion protein targeting C3b and Vegf in Neovascular and nonexudative Amd models. Ophthalmol Therapy. (2024) 13:2227–42. doi: 10.1007/s40123-024-00982-3
237. Nielsen, J, MacLaren, RE, Heier, JS, Steel, D, Ivanova, T, Sivaprasad, S, et al. Preliminary results from a first-in-human phase I/ii gene therapy study (focus) of Subretinally delivered Gt005, an investigational Aav2 vector, in Patients with geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. (2022) 63:1504. doi: 10.1038/ni.1655
238. Giudice, V, Scala, P, Lamparelli, EP, Gorrese, M, Serio, B, Bertolini, A, et al. Biomimetic proteolipid vesicles for reverting Gpi deficiency in paroxysmal nocturnal hemoglobinuria. iScience. (2024) 27:109021. doi: 10.1016/j.isci.2024.109021
239. Mantegazza, R, O'Brien, FL, Yountz, M, and Howard, JF JrREGAIN study group. Consistent improvement with Eculizumab across muscle groups in myasthenia gravis. Ann Clin Transl Neurol. (2020) 7:1327–39. doi: 10.1002/acn3.51121
240. Muppidi, S, Utsugisawa, K, Benatar, M, Murai, H, Barohn, RJ, Illa, I, et al. Long-term safety and efficacy of Eculizumab in generalized myasthenia gravis. Muscle Nerve. (2019) 60:14–24. doi: 10.1002/mus.26447
241. Vu, T, Meisel, A, Mantegazza, R, Annane, D, Katsuno, M, Aguzzi, R, et al. Terminal complement inhibitor Ravulizumab in generalized myasthenia gravis. NEJM Evid. (2022) 1:EVIDoa2100066. doi: 10.1056/EVIDoa2100066
242. Jea, H. Aanem 2024 annual meeting abstracts guide. Muscle Nerve. (2024) 70:442–720. doi: 10.1002/mus.28233
243. Tuan Vu, PN, Peric, Stojic, and Shelly, Shahar, editor. Upstream targeting: rethinking Mg treatment through active C1 inhibition. (2025). doi: 10.1002/acn3.51121dont
244. Pittock, SJ, Fujihara, K, Palace, J, Berthele, A, Kim, HJ, Oreja-Guevara, C, et al. Eculizumab monotherapy for Nmosd: data from prevent and its open-label extension. Mult Scler. (2022) 28:480–6. doi: 10.1177/13524585211038291
245. Sean Pittock, MB, Bennett, J, Berthele, A, De Seze, J, Levy, M, Nakashima, I, et al. Efficacy and safety of Ravulizumab in adults with anti-Aquaporin-4 antibody-positive Neuromyelitis Optica Spectrum disorder (Aqp4+ Nmosd): interim analysis from the ongoing phase 3 Champion-Nmosd trial. Neurology. (2024) 102:2489. doi: 10.1212/WNL.0000000000204564
Keywords: complement, innate immunity, myasthenia gravis, aquaporin-4 IgG-positive neuromyelitis optica spectrum disorder, complement-targeted therapies
Citation: Chen JY, Sanghani N, Palmer K, Li Y, Lin F and Abbatemarco JR (2025) Complement activation in immunological neurological disorders: mechanisms and therapeutic strategies. Front. Neurol. 16:1695461. doi: 10.3389/fneur.2025.1695461
Edited by:
Guglielmo Lucchese, Universitätsmedizin Greifswald, GermanyReviewed by:
Shuhei Nishiyama, Tohoku University Hospital, JapanAnalisa Manin, Hospital J. M. Ramos Mejía, Argentina
Elle Levit, University of Vermont, United States
Copyright © 2025 Chen, Sanghani, Palmer, Li, Lin and Abbatemarco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin R. Abbatemarco, YWJiYXRlajJAY2NmLm9yZw==
†These authors have contributed equally to this work and share first authorship
 Jin Y. Chen
Jin Y. Chen Nirav Sanghani
Nirav Sanghani Kaitlyn Palmer
Kaitlyn Palmer Yuebing Li
Yuebing Li Feng Lin
Feng Lin Justin R. Abbatemarco
Justin R. Abbatemarco