Abstract
Objective:
This study aims to evaluate the effects of non-invasive brain stimulation (NIBS) on balance control in patients with multiple sclerosis (MS) through a systematic review and meta-analysis. The goal is to identify the most effective treatment strategies and provide valuable evidence for clinical decision-making.
Methods:
PubMed, Web of Science, Embase, Scopus, Cochrane Library, ClinicalTrials.gov and CNKI Scholar were searched for randomized controlled trials (RCTs) on the effects of NIBS on balance control in patients with MS. The search period was from the inception of each database to August 11, 2025. The Timed Up and Go test (TUG) and the Berg Balance Scale (BBS) are the primary outcome measures, with adverse events being the secondary outcome measure. Two researchers independently performed literature screening, data extraction and quality assessment. The quality of the included trials was assessed using the Cochrane Risk of Bias tool (RoB 2.0) and the GRADE proGDT software was used to evaluate the evidence grading recommendation standards (GRADE) for outcomes. Data were analyzed using RevMan 5.4 and StataMP 18 software. For binary variables, the effect size is measured using the risk ratio (RR), while for continuous variables, the effect size is measured using the mean difference (MD), with a 95% confidence interval (CI). Heterogeneity was explored through subgroup analysis, meta-regression, and sensitivity analysis to assess the robustness of the results. A funnel plot was constructed, and Egger’s test was performed to evaluate potential publication bias.
Results:
A total of 17 RCTs with 514 patients were included. Meta-analysis showed that NIBS can shorten the time taken for TUG [MD = −1.03, 95% CI (−1.86, −0.20)] and improve BBS scores [MD = 3.35, 95% CI (1.31, 5.39)], indicating that NIBS may improve both dynamic and static balance. Subgroup analysis revealed that interventions lasting ≥4 weeks were associated with a reduction in TUG completion time and an increase in BBS scores. Furthermore, transcranial direct current stimulation (tDCS) demonstrated favorable effects on both TUG and BBS outcomes, while evidence supporting the efficacy of repetitive transcranial magnetic stimulation (rTMS) remained limited. Although adverse events such as itching, warmth, tension, and fatigue were reported in NIBS group, these were generally mild and transient.
Conclusion:
This study suggests that NIBS may serve as an effective adjunctive therapy for balance rehabilitation in patients with MS, showing benefits in both dynamic and static balance. However, its application is accompanied by mild and transient adverse effects, necessitating a careful balance between efficacy and safety in clinical practice. The current evidence is limited by heterogeneity among included studies and short follow-up durations. Future research should focus on large-scale, high-quality RCTs to further validate the long-term efficacy of NIBS, optimize stimulation parameters, and promote the development of individualized treatment strategies.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/, identifier: CRD420251121717.
1 Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated disease characterized by inflammatory demyelination in the central nervous system, and is one of the leading causes of disability in young adults worldwide (1, 2). The clinical symptoms include limb movement disorders, visual disturbances, sensory abnormalities, and cognitive dysfunction, with a high rate of disability and progressive patients may face paralysis, blindness, cognitive decline and other consequences (1). Ninety percent of MS patients will experience significant motor dysfunction within 25 years of diagnosis, leading to balance disorders, gait abnormalities, and reduced quality of life (3). According to the latest MS global monitoring data, approximately 2.8 million people were affected by MS in 2020, and the prevalence is increasing worldwide. The pathological changes in MS include demyelination, axonal loss, and neurodegeneration, which particularly affect the neural networks involved in balance control, leading to impairments in sensory integration and motor output (4, 5). Balance dysfunction in MS significantly increases the risk of falls, limits mobility, and reduces quality of life. Therefore, restoring or improving balance is crucial for the management of patients with MS (6, 7).
Currently, the treatment of MS primarily relies on disease-modifying therapies (DMTs) and immunomodulatory, but the efficacy of drugs is limited and associated with certain adverse effects (8). Traditional rehabilitation methods such as physical therapy and exercise programs have shown benefits in improving balance and mobility in MS patients (9, 10). However, the effectiveness of these interventions is often constrained by variability in disease severity, patient adherence, and response (11).
In recent years, non-invasive brain stimulation (NIBS), such as transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS), has emerged as a promising adjunct to conventional rehabilitation strategies due to its non-invasiveness, safety, and good tolerability (12). Studies have shown that the core mechanism of tDCS involves modulating the functional balance of distributed neural networks and enhancing neuroplasticity (13). Specifically, anodal tDCS has been found to improve motor performance during balance tasks and enhance overall balance ability (14–16). NIBS may improve motor learning and functional recovery by modulating cortical excitability and promoting neuroplasticity, particularly by enhancing balance control through its effects on sensorimotor networks (17–19).
Previous studies (20, 21) have mainly focused on spasticity, walking speed, or overall motor function improvement, with relatively few systematic and quantitative analyses focused specifically on balance control as the primary outcome. Due to high heterogeneity among studies, insufficient stratification of stimulation targets, and methodological differences, the exact efficacy of NIBS on balance function in MS patients has not been clearly established. In particular, there is still a lack of consensus regarding the modulatory effects of various stimulation parameters, such as stimulation site, duration, and intensity. Therefore, this study aims to overcome these limitations through a more comprehensive literature search and systematic quantitative analysis. We will not only comprehensively evaluate the overall effect of NIBS on balance function in MS but also explore the potential sources of the aforementioned heterogeneity in depth through detailed subgroup analyses (stratified by NIBS type, MS subtype, stimulation parameters, intervention duration, etc.) and meta-regression, clarifying the relationship between different stimulation parameters and efficacy, in order to provide more precise and valuable evidence-based support for clinical decision-making and future research design in this field.
2 Materials and methods
2.1 Study design and registration
This systematic review and meta-analysis have been registered with PROSPERO (CRD420251121717). This study followed the Preferred Reporting Project for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines (22, 23) (Supplementary Table S1).
2.2 Search strategy
Databases searched included PubMed, Web of Science, Embase, Scopus, Cochrane Library, ClinicalTrials.gov and CNKI Scholar. The search period was from the inception of each database to August 11, 2025. The search utilized both controlled vocabulary and free-text terms. The following keywords were used for the search: “multiple sclerosis,” “non-invasive brain stimulation,” “transcranial magnetic stimulation,” “transcranial direct current stimulation,” “transcranial alternating current stimulation,” “theta burst transcranial magnetic stimulation,” “balance,” and “randomized controlled trials,” among others. See Supplementary Table S2 for detailed search strategies.
2.3 Inclusion and exclusion criteria
2.3.1 Inclusion criteria
Population: Adults diagnosed with MS according to McDonald criteria (24), regardless of sex or disease duration.
Intervention: Received any of the following interventions, alone or in combination: rTMS, tDCS, theta burst stimulation (TBS), combined rehabilitation training (such as balance training, aerobic exercise, virtual reality training), or other NIBS techniques.
Comparison: Sham stimulation, conventional rehabilitation, or no treatment.
Outcomes: The primary outcome was at least one measure of balance function, specifically the Timed Up and Go (TUG) test or the Berg Balance Scale (BBS). The secondary outcome was adverse events (AEs).
Study design: Published randomized controlled trials (RCTs) literature.
2.3.2 Exclusion criteria
Studies with unavailable full text or incomplete reporting.
Studies using invasive brain stimulation techniques.
Studies with concurrent treatments that could affect balance (unless the same treatment was applied to the control group).
Non-RCT studies or conference abstracts.
2.4 Literature screening and data extraction
Two researchers independently managed the literature using EndNote 20 software. After deduplication, the title, abstract and full text were read, and the literature that met the inclusion criteria was screened and cross-checked. Disagreements were resolved through discussion with a third researcher. The data of the literature were extracted using a pre-formulated Excel table, and the extracted information included: title, first author, year of publication, sample size, age, course of disease, Expanded Disability Status Scale (EDSS), MS subtype, intervention measures, intervention protocol, stimulation site, stimulation intensity, duration of treatment, outcomes and AEs.
2.5 Quality assessment of included studies
Two researchers independently assessed the quality of the studies using the Cochrane Risk of Bias tool (RoB 2.0) (25) in the following domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The results were categorized as “low risk,” “some concerns,” or “high risk.” Disagreements were resolved through discussion with a third researcher.
2.6 Certainty of evidence
Two researchers assessed the certainty of evidence for the outcomes using the GRADE approach with GRADE pro GDT software (Grading of Recommendations, Assessment, Development and Evaluation) (26). The GRADE system evaluates the quality of evidence based on six domains: study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall quality of evidence is classified into four levels: high, moderate, low, and very low.
2.7 Statistical analysis
Statistical analysis was performed using RevMan 5.4 and StataMP 18 software. For dichotomous variables (adverse events), the effect size was expressed as risk ratio (RR). For continuous variables, the mean difference (MD) was used to estimate the effect size. Both were reported with 95% confidence intervals (CI). If the 95% CI did not include zero, the difference between groups was considered statistically significant. Heterogeneity between studies was assessed using the I2 statistic. If I2 ≤ 50%, low heterogeneity was assumed, and a fixed-effects model was used for analysis. If I2 > 50%, high heterogeneity was indicated, and a random-effects model was employed. For the meta-analysis, the between-study variance was estimated using the DerSimonian–Laird method. When significant heterogeneity was detected, subgroup analysis or meta-regression was conducted to explore its potential sources. Meta-regression was performed for continuous moderators (e.g., mean age, baseline EDSS) using restricted maximum likelihood (REML) estimation in StataMP 18. Sensitivity analysis was carried out by excluding individual studies one by one to test the robustness of the combined results. A funnel plot combined with Egger’s test was used to assess potential publication bias. The significance level was set at α = 0.05.
3 Results
3.1 Literature search results
A total of 2,048 relevant articles were identified through database searches. After removing duplicates, 1,337 articles remained. Based on the inclusion and exclusion criteria, 187 articles were selected for full-text review. After screening the full texts, 170 articles were excluded. Ultimately, 17 studies met the eligibility criteria and were included in the final analysis (Figure 1).
Figure 1

Flow chart of the study selection process.
3.2 Basic characteristics of the included studies
A total of 17 RCTs (27–43) involving 514 MS patients were included in this study. Among these, 269 patients were assigned to the treatment group, and 245 patients were in the control group. The sample sizes of individual studies ranged from 16 to 64 participants, with a mean age range of 35 to 55 years. The duration of the interventions varied from 1 week to 6 weeks. Thirteen studies used tDCS, two studies used rTMS, one study used repetitive transspinal magnetic stimulation (rTSMS), and one study used intermittent theta burst stimulation (iTBS). The interventions in the control group were rehabilitation training or sham stimulation. The detailed characteristics of the included studies are presented in Tables 1, 2.
Table 1
| Study | Country | Study type | Overall age | EDSS (T/C) | MS type | Treatment group | Control group | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (n) | Age (years) | Gender (M/F) | Disease duration (year) | Intervention | Sample size (n) | Age (years) | Gender (M/F) | Disease duration (year) | Comparison | |||||||
| Tramontano et al. (27) | Italy | RCT | 51.75 ± 7.9 | 5.75 ± 0.89 | SP PR |
8 | 51.9 ± 3.83 | 2/6 | 18.13 ± 8.24 | iTBS + vestibular rehabilitation (VR) | 8 | 51.6 ± 10.91 | 3/5 | 18.75 ± 9.62 | Sham iTBS + vestibular rehabilitation (VR) | BBS |
| Mohamed et al. (28) | Egypt | RCT | 33.75 ± 6.7 | 3.385 ± 0.64 | RR SP |
15 | 31.6 ± 5.6 | 6/9 | 5.8 ± 1.7 | rTMS + physiotherapy | 15 | 35.9 ± 7.2 | 6/9 | 5.3 ± 1.3 | rTMS + physiotherapy | BBS, AEs |
| Charehjou et al. (29) | Iran | RCT | 39.35 ± 11.2 | 6.25 ± 0.18 | NR | 10 | 39.9 ± 12.38 | 3/7 | NR | tDCS + virtual reality (VR) training | 10 | 40.0 ± 10.57 | 3/7 | NR | Virtual reality (VR) training | BBS |
| Baroni et al. (30) | Italy | RCT | 53.69 ± 13.01 | 4.59 ± 0.61 | RR PP SP |
8 | 55.25 ± 15.15 | 4/4 | 11.13 ± 6.99 | tDCS + TOCT | 8 | 52.13 ± 11.31 | 4/4 | 11.13 ± 9.95 | sham tDCS + TOCT | TUG, AEs |
| Mohammadkhanbeig et al. (31) | Iran | RCT | 37.57 ± 7.61 | 3.6 ± 1.42 | RR | 9 | 37.44 ± 7.89 | 0/9 | 9.44 ± 4.30 | tDCS | 10 | 37.70 ± 7.78 | 0/10 | 9.90 ± 6.90 | Sham tDCS | BBS, TUG |
| 38.89 ± 5.71 | 3.87 ± 1.51 | 9 | 37.44 ± 7.89 | 0/9 | 9.44 ± 4.30 | tDCS | 10 | 40.20 ± 2.43 | 0/10 | 9.50 ± 7.32 | Core stability exercises | |||||
| Akbari et al. (32) | Iran | RCT | 42.93 ± 7.95 | 4.16 ± 0.54 | RR | 16 | 42.87 ± 7.99 | 4/12 | NR | Cerebellar tDCS + postural training | 14 | 43.00 ± 8.20 | 6/8 | NR | Sham tDCS + postural training | BBS, TUG |
| 42.90 ± 8.09 | 4.09 ± 0.52 | 16 | 42.81 ± 8.26 | 10/6 | NR | DLPFC tDCS + postural training | 14 | 43.00 ± 8.20 | 6/8 | NR | Sham tDCS + postural training | |||||
| Muñoz-Paredes et al. (33) | Spain | RCT | 48.08 ± 8.55 | NR | RR SP |
12 | NR | NR | 0.36 ± 0.67 | tDCS + physiotherapy | 12 | NR | NR | 0.36 ± 0.67 | Sham tDCS + physiotherapy | TUG |
| Yassine et al. (34) | Egypt | RCT | 39.89 ± 6.05 | NR | RR | 20 | 39.4 ± 6.3 | 8/12 | 3.5 ± 2.8 | rTMS + rehabilitation program | 19 | 40.4 ± 5.9 | 6/13 | 3.2 ± 3.1 | rTMS + rehabilitation program | BBS, TUG, AEs |
| Rahimibarghani et al. (35) | Iran | RCT | 39.91 ± 6.78 | 4.76 ± 0.81 | RR PP SP |
21 | 40.0 ± 7.1 | 8/13 | 11.72 ± 3.70 | tDCS + aerobic exercise | 18 | 39.8 ± 6.6 | 7/11 | 9.32 ± 4.1 | Sham tDCS + aerobic exercise | TUG |
| Cinbaz et al. (36) | Turkey | RCT | 48.74 ± 7.82 | 4.08 ± 0.75 | RR PP |
11 | 49 ± 8.75 | 3/8 | 16 ± 4.25 | tDCS + exercise | 12 | 48.5 ± 7.25 | 3/9 | 20 ± 8.75 | Sham tDCS + exercise | TUG |
| 48.75 ± 9.26 | 4.35 ± 0.95 | 12 | 49 ± 11.25 | 4/8 | 13.5 ± 8 | tsDCS + exercise | 12 | 48.5 ± 7.25 | 3/9 | 20 ± 8.75 | Sham tDCS + exercise | |||||
| Marotta et al. (37) | Italy | RCT | 40.6 ± 14.4 | 3 ± 0.49 | RR | 9 | 43.22 ± 10.46 | 3/6 | NR | tDCS + physiotherapy | 8 | 39.75 ± 8.39 | 2/6 | NR | Sham tDCS + physiotherapy | BBS, TUG |
| Pagliari et al. (38) | Italy | RCT | 49.57 ± 10.21 | 4.50 ± 2.15 | NR | 20 | 51.60 ± 8.46 | 7/13 | 14.15 ± 9.42 | TR RS-AtDCS | 20 | 47.55 ± 11.56 | 10/10 | 15.30 ± 10.14 | Sham tDCS | TUG, AEs |
| 51.98 ± 8.91 | 4.50 ± 1.94 | 20 | 51.60 ± 8.46 | 7/13 | 14.15 ± 9.42 | TR RS-AtDCS | 30 | 52.23 ± 9.34 | 12/18 | 15.36 ± 7.17 | Usual care | |||||
| Pilloni et al. (39) | New York | RCT | 53.09 ± 10.74 | NR | RR SP |
9 | 52.1 ± 12.85 | NR | NR | tDCS + aerobic exercise | 8 | 54.2 ± 8.5 | NR | NR | Sham tDCS + aerobic exercise | TUG |
| Ghosh et al. (40) | Australia | RCT | 54 ± 10.9 | 3.24 ± 1.31 | RR PR PP |
19 | 52.21 ± 11.30 | 6/13 | 7 ± 4.25 | tDCS + physiotherapy | 21 | 55.62 ± 8.82 | 6/15 | 12 ± 9 | Sham tDCS + physiotherapy | BBS, TUG, AEs |
| Ehsani et al. (41) | Iran | RCT | 35.71 ± 2.51 | 2.16 ± 0.72 | NR | 10 | 35.88 ± 2.21 | 1/9 | NR | tDCS + postural training | 10 | 36.38 ± 2.74 | 0/10 | NR | Sham tDCS + postural training | BBS |
| Fawaz et al. (42) | Egypt | RCT | 35.81 ± 9.12 | NR | RR | 32 | NR | 19/13 | NR | rTSMS + physiotherapy | 32 | NR | 19/13 | NR | Sham rTSMS + physiotherapy | TUG |
| Nguemeni et al. (43) | Germany | RCT | 48.5 ± 9.71 | 3.77 ± 0.84 | NR | 12 | 49.83 ± 10.46 | 7/5 | NR | tDCS | 10 | 46.90 ± 9.00 | 3/7 | NR | Sham tDCS | TUG |
Basic characteristics of the included studies.
T, treatment group; C, control group; RCT, randomized controlled trial; iTBS, intermittent theta burst stimulation; rTMS, repeated transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; tsDCS, trans-spinal direct current stimulation; TOCT, task-oriented circuit training; DLPFC, dorsolateral prefrontal cortex; TR RS-AtDCS, telerehabilitation with active transcranial direct current stimulation; rTSMS, repetitive trans-spinal magnetic stimulation; M1, primary motor cortex; PP, primary progressive; RR, relapsing remitting; SP, secondary progressive; PR, progressive-relapsing; TUG, Timed Up and Go; BBS, Berg Balance Scale; AEs, adverse events; NR, not reported.
Table 2
| Study | Target electrode location | Intensity | Frequency | Treatment duration | Adverse event |
|---|---|---|---|---|---|
| Tramontano et al. (27) | Bilateral cerebellar hemispheres | NR | 40 min/per time, 5 times/week | 2 weeks | / |
| Mohamed et al. (28) | Bilateral cerebellar hemispheres | NR | 20 min/per time, 3 times/week | 2 weeks | Headache |
| Charehjou et al. (29) | +: Primary motor cortex (M1, C3) −: Right forehead |
2 mA | 20 min/per time, 3 times/week | 2 weeks | / |
| Baroni et al. (30) | +: Cerebellum right hemisphere −: Ipsilateral bucciNRtor |
2 mA | 2 h/per time, 5 times/week | 2 weeks | Tingling, skin redness, headache, trouble to concentrate, sleepiness, pain in the site of stimulation, mood fluctuations |
| Mohammadkhanbeig et al. (31) | +: Primary motor cortex (M1, Cz) −: Supraorbital area |
2 mA | 20 min/per time, 5 times/week | 6 weeks | / |
| Akbari et al. (32) | +: Cerebellum (1 cm below inion of occipital bone) −: Right bucciNRtor muscle |
1.5 mA | 20 min/per time, a 48-h interval between sessions | 4 weeks | / |
| +: Left dorsolateral prefrontal cortex −: Right supraorbital region |
/ | ||||
| Muñoz-Paredes et al. (33) | +: Left DLPFC −: Right supraorbital cortex |
2 mA | 20 min/per time, a total of 10 sessions | 4 weeks | / |
| Yassine et al. (34) | Bilateral cerebellar hemispheres | NR | 20 min/per time, a total of 12 sessions | 4 weeks | Nauseous, headache, dizziness |
| Rahimibarghani et al. (35) | +: Primary motor cortex (M1, C3) −: Contralateral shoulder |
1.5 mA | 20 min/per time, 2 times/week | 6 weeks | / |
| Cinbaz et al. (36) | +: Primary motor cortex (M1, C3) −: Supraorbital region |
2 mA | 20 min/per time, 3 times/week | 4 weeks | / |
| +: T10 spinous process −: Left posterior deltoid |
/ | ||||
| Marotta et al. (37) | +: Primary motor cortex (M1, C3) −: Fp2 (supraorbital margin) |
2 mA | 20 min/per time, 5 times/week | 2 weeks | / |
| Pagliari et al. (38) | +: Left DLPFC −: Right DLPFC |
2 mA | 45 min/per time with addition NRl 20 min of tDCS during the first week, 5 times/week | 6 weeks | Skin irritation, pain, burning sensation, heat sensation, itching, iron taste, fatigue |
| Pilloni et al. (39) | +: Primary motor cortex (M1, C3) −: Fp2 (supraorbital margin) |
2.5 mA | 20 min/per time | NR | / |
| Ghosh et al. (40) | +: Primary motor cortex (M1, Cz) −: Supraorbital area |
2 mA | 20 min/per time, 2 times/week | 6 weeks | Tingling, itching, burning, scalp pain, sleepiness, trouble concentrating |
| Ehsani et al. (41) | +: Cerebellum right hemisphere −: Right bucciNRtor muscle |
1.5 mA | 20 min/per time, 5 times/week | 2 weeks | / |
| Fawaz et al. (42) | C7 cervical vertebrae | 2 mA | 5 s/per time, 10-s intertrain interval, 30 trains in total. 5 times/week | 2 weeks | / |
| Nguemeni et al. (43) | +: Cerebellum (3 cm lateral to the inion) −: Ipsilateral bucciNRtor muscle |
2 mA | 15 min/per time, 3 times/week | 2 weeks | / |
Intervention details of the included studies.
C3 and Cz are electrode positions according to the international 10–20 EEG system, corresponding to the left primary motor cortex and midline motor cortex, respectively.
3.3 Quality assessment of included studies
The quality of the 17 included studies was assessed using the Cochrane Risk of Bias tool. Fifteen described random sequence generation, 11 reported allocation concealment, and 9 implemented blinding of both participants and assessors. All studies provided complete data reporting and explained their outcomes. Overall, the methodological quality of the included studies was relatively high. The quality evaluation of the included literature is shown in Figure 2.
Figure 2
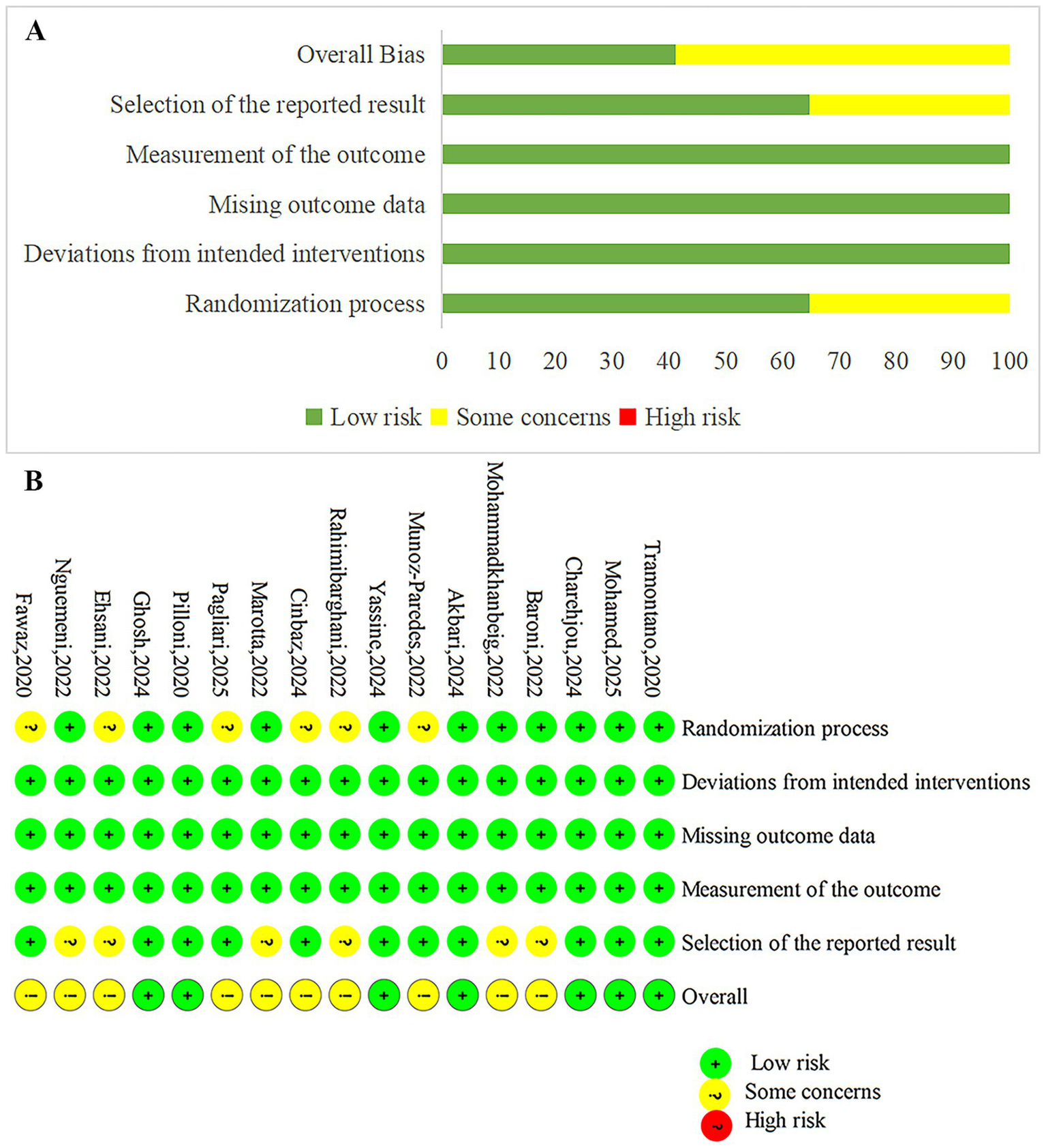
Risk of bias summary and graph. (A) The risk of bias profile across. (B) The detailed results of the risk of bias.
3.4 Meta-analysis results
3.4.1 Overall results
A total of 13 studies reported TUG results. Heterogeneity testing showed I2 = 96%, p < 0.00001, indicating substantial heterogeneity between studies. A random-effects model was used for the analysis. Meta-analysis showed that NIBS group had significantly shorter than that in the control group, and the difference was statistically significant [MD = −1.03, 95% CI (−1.86, −0.20), p = 0.01], as shown in Figure 3. A negative mean difference indicates a greater reduction in TUG completion time in the NIBS group, suggesting improved balance ability.
Figure 3
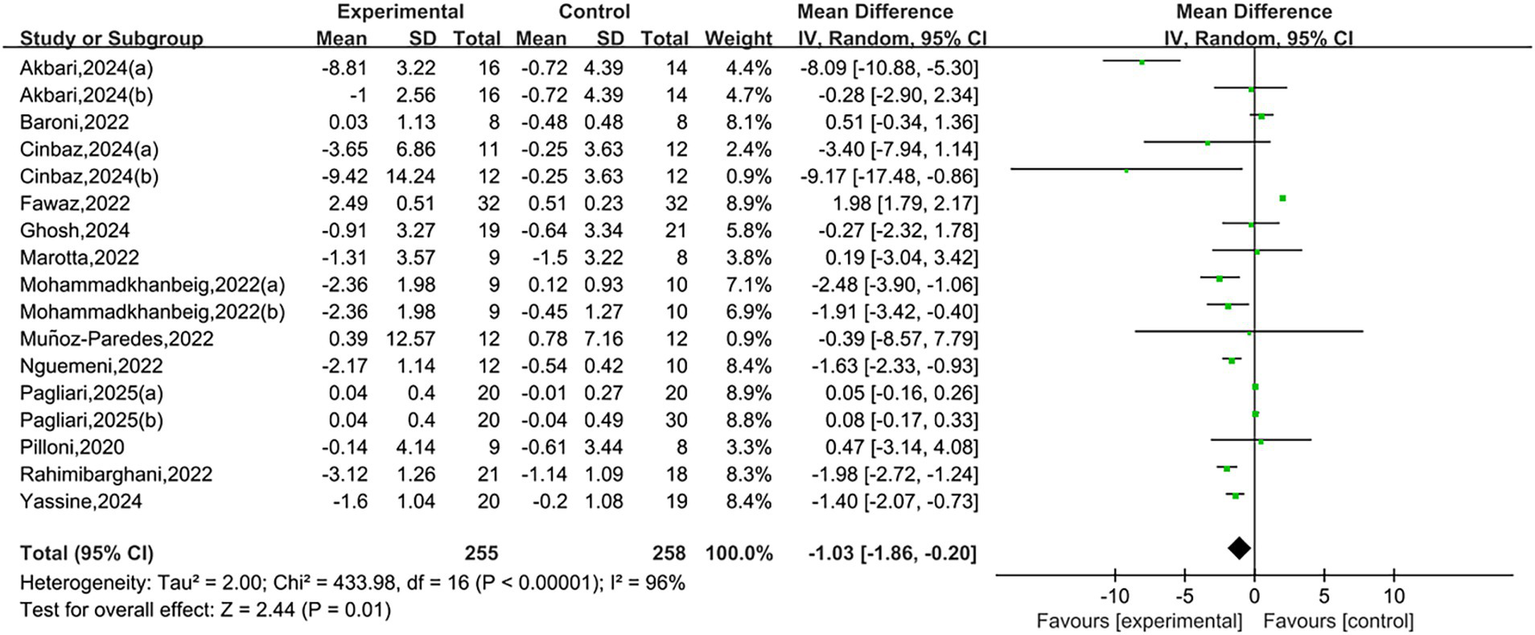
Forest plots of TUG.
A total of nine studies reported BBS. Heterogeneity testing showed I2 = 88%, p < 0.00001, indicating substantial heterogeneity between studies. A random-effects model was used for analysis. Meta-analysis results showed that NIBS group had significantly higher BBS scores than the control group, with a statistically significant difference [MD = 3.35, 95% CI (1.31, 5.39), p = 0.001], as shown in Figure 4. A positive mean difference indicates that the NIBS group had higher BBS scores, suggesting better balance ability and a lower risk of falls.
Figure 4
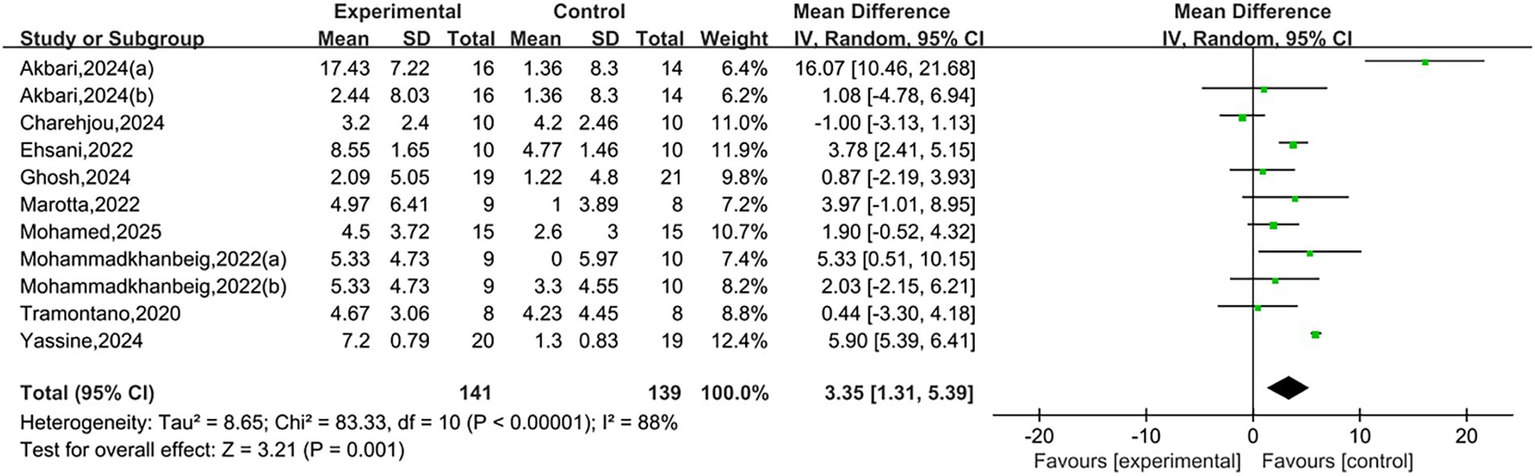
Forest plots of BBS.
A total of five studies reported the occurrence of AEs. Heterogeneity testing showed I2 = 0%, p = 0.58, indicating low heterogeneity between the studies. A fixed-effect model was used for the pooled analysis. Meta-analysis results indicated that the incidence of AEs in NIBS group was higher than that in control group, with the difference being statistically significant [RR = 2.18, 95% CI (1.23, 3.85), p = 0.008]. It is noteworthy that the vast majority of reported AEs were mild, transient, and self-limiting, typically resolving shortly during or after the stimulation without the need for special intervention. Therefore, this result does not suggest that NIBS is a high-risk intervention, but rather objectively confirms the statistical significance of its known and commonly observed mild side effects. The forest plot is presented in Figure 5.
Figure 5
![Forest plot displaying risk ratios of five studies comparing experimental and control groups. Studies include Baroni (2022), Ghosh (2024), Mohamed (2025), Pagliari (2025), and Yassine (2024). Each study shows events, totals, and corresponding risk ratios with 95% confidence intervals. The overall risk ratio is 2.18 with a confidence interval of [1.23, 3.85]. Heterogeneity is low with I² = 0%. The plot visually illustrates individual study effects and the pooled effect, represented by a diamond.](https://www.frontiersin.org/files/Articles/1696343/xml-images/fneur-16-1696343-g005.webp)
Forest plots of AEs.
3.4.2 Subgroup analysis
Due to the high heterogeneity between studies, subgroup analyses were conducted to further explore potential sources of heterogeneity. Subgroup analysis of the TUG showed that among different NIBS types, tDCS reduced TUG time [MD = −1.14, 95% CI (−1.80, −0.49)], while rTMS showed no significant improvement. Regarding MS subtypes, patients with relapsing remitting (RR) MS showed a trend toward improvement [MD = −1.61, 95% CI [−3.77, 0.55]], although the difference did not reach statistical significance. Other mixed subtypes also did not demonstrate significant differences. This suggests that tDCS may be a more effective NIBS technique, and its efficacy may be associated with the MS subtype. However, given the limited number of studies within certain subgroups, these findings should be interpreted with caution. Variations in intervention duration (p = 0.13, I2 = 51.7%) and stimulation intensity (p = 0.12, I2 = 58.3%) were not statistically significant contributors to outcome differences. However, within-group analysis of intervention durations indicated that a 4-week intervention was more effective [MD = −3.42, 95% CI (−6.32, −0.53)], and a 6-week intervention also showed significant improvement [MD = −0.88, 95% CI (−1.55, −0.21)], while a 2-week intervention did not yield statistically significant benefits. These results suggest that a minimum of 4 weeks of NIBS intervention may be required to achieve meaningful improvements in balance function in MS patients. Therefore, these factors are unlikely to account for the observed heterogeneity. Subgroup analysis by stimulation site showed no significant differences in the cerebellum and left dorsolateral prefrontal cortex (DLPFC) groups. Stimulation targeting the primary motor cortex (M1) showed positive effects, particularly in the M1 (Cz) region [MD = −1.74, 95% CI (−2.90, −0.58)]. Due to the limited number of studies in each subgroup, the results may be unstable; however, stimulation site is likely a potential source of heterogeneity. See Table 3 and Supplementary Figures S1–S5.
Table 3
| Category | Subgroup | Number of included studies (n) | Heterogeneity test result | Effect model | Meta-analysis result | |||
|---|---|---|---|---|---|---|---|---|
| I 2 (%) | p | MD | 95% CI | p | ||||
| MS subtype | RR | 7 | 97 | <0.00001 | Random | −1.61 | [−3.77, 0.55] | 0.14 |
| RR + PP + SP | 3 | 90 | <0.0001 | −0.62 | [−2.49, 1.26] | 0.52 | ||
| RR + PP | 2 | 30 | 0.23 | −5.19 | [−10.42, 0.04] | 0.05 | ||
| RR + SP | 2 | 0 | 0.85 | 0.33 | [−2.97, 3.63] | 0.84 | ||
| NIBS types | tDCS | 15 | 86 | <0.00001 | Random | −1.14 | [−1.80, −0.49] | 0.0006 |
| rTMS | 2 | 99 | <0.00001 | 0.31 | [−3.01, 3.62] | 0.86 | ||
| Intervention duration | 2 weeks | 4 | 97 | <0.00001 | Random | 0.28 | [−1.83, 2.39] | 0.79 |
| 4 weeks | 6 | 81 | <0.0001 | −3.42 | [−6.32, −0.53] | 0.02 | ||
| 6 weeks | 6 | 89 | <0.00001 | −0.88 | [−1.55, −0.21] | 0.01 | ||
| Stimulation intensity | 1.5 mA | 3 | 90 | <0.0001 | Random | −3.34 | [−7.00, 0.32] | 0.07 |
| 2 mA | 11 | 97 | <0.00001 | −0.35 | [−1.29, 0.58] | 0.46 | ||
| Stimulation site | Cerebellum | 2 | 95 | <0.0001 | Random | −4.71 | [−11.04, 1.61] | 0.14 |
| Left DLPFC | 4 | 0 | 0.99 | 0.06 | [−0.10, 0.22] | 0.46 | ||
| M1 (C3) | 4 | 19 | 0.3 | −1.54 | [−2.79, −0.30] | 0.01 | ||
| M1 (Cz) | 3 | 34 | 0.22 | −1.74 | [−2.90, 0.58] | 0.003 | ||
Subgroup analysis results of TUG.
A total of 17 RCTs were included in this study; however, 3 of them contained multiple intervention groups, which were treated as independent comparisons in this subgroup analysis. The number of studies listed for each subgroup refers to the number of independent comparisons.
Subgroup analysis of BBS showed that both tDCS [MD = 3.57, 95% CI (0.84, 6.31)] and rTMS [MD = 4.08, 95% CI (0.18, 7.99)] were associated with improvements in BBS scores, suggesting that different types of NIBS may be effective in enhancing static balance function. Patients with RR MS demonstrated greater improvement [MD = 5.61, 95% CI (2.57, 8.65)], whereas those with RR + SP (secondary progressive) MS did not show significant benefits (p = 0.16). However, neither NIBS type nor MS subtype appeared to be significant sources of heterogeneity in BBS. No statistically significant differences were observed between subgroups based on intervention duration (p = 0.26, I2 = 26.5%), stimulation intensity (p = 0.21, I2 = 37.6%), or stimulation site (p = 0.79, I2 = 0%). Nevertheless, within-group analysis revealed that a 4-week intervention was particularly effective [MD = 7.57, 95% CI (0.99, 14.15)]. Stimulation targeting M1 (Cz) showed low heterogeneity (I2 = 15%), indicating that stimulation at other sites may be potential sources of inter-study heterogeneity. For further details, see Table 4 and Supplementary Figures S6–S10.
Table 4
| Category | Subgroup | Number of included studies (n) | Heterogeneity test result | Effect model | Meta-analysis result | |||
|---|---|---|---|---|---|---|---|---|
| I 2 (%) | p | MD | 95% CI | p | ||||
| MS subtype | RR | 6 | 74 | 0.002 | Random | 5.61 | [2.57, 8.65] | 0.0003 |
| RR + SP | 2 | 0 | 0.52 | 1.47 | [−0.56, 3.50] | 0.16 | ||
| NIBS types | tDCS | 8 | 82 | <0.00001 | Random | 3.57 | [0.84, 6.31] | 0.01 |
| rTMS | 2 | 90 | 0.002 | 4.08 | [0.18, 7.99] | 0.04 | ||
| Intervention duration | 2 weeks | 5 | 74 | 0.004 | Random | 1.74 | [−0.48, 3.95] | 0.12 |
| 4 weeks | 3 | 87 | 0.0005 | 7.57 | [0.99, 14.15] | 0.02 | ||
| 6 weeks | 3 | 15 | 0.31 | 2.21 | [−0.21, 4.63] | 0.07 | ||
| Stimulation intensity | 1.5 mA | 3 | 89 | <0.0001 | Random | 6.81 | [−0.70, 14.32] | 0.08 |
| 2 mA | 3 | 61 | 0.05 | 1.63 | [−1.16, 4.43] | 0.25 | ||
| Stimulation site | M1 (C3) | 2 | 69 | 0.07 | Random | 0.95 | [−3.80, 5.71] | 0.69 |
| M1 (Cz) | 3 | 15 | 0.31 | 2.21 | [−0.21, 4.63] | 0.07 | ||
| Bilateral cerebellar hemispheres | 3 | 89 | 0.0002 | 3.05 | [−0.59, 6.69] | 0.1 | ||
Subgroup analysis results of BBS.
3.4.3 Meta-regression
To investigate potential sources of the observed heterogeneity, meta-regression analyses were performed separately for TUG and BBS using the restricted maximum likelihood (REML) method, based on clinically relevant covariates. The included covariates were mean patient age and baseline EDSS score.
For TUG, a total of 13 studies were included in the meta-regression. The results indicated that neither age (β = 0.154, p = 0.214) nor EDSS score (β = −0.308, p = 0.821) was a significant predictor of the effect size. The overall model demonstrated low explanatory power, with an adjusted R2 of only 12.53%, and was not statistically significant [F (2, 10) = 0.88, p = 0.444]. After including these two covariates, substantial residual heterogeneity remained (I2_res = 76.51%, τ2 = 3.129). Therefore, this meta-regression did not identify age or EDSS score as significant contributors to the heterogeneity observed in the TUG meta-analysis (Figure 6).
Figure 6
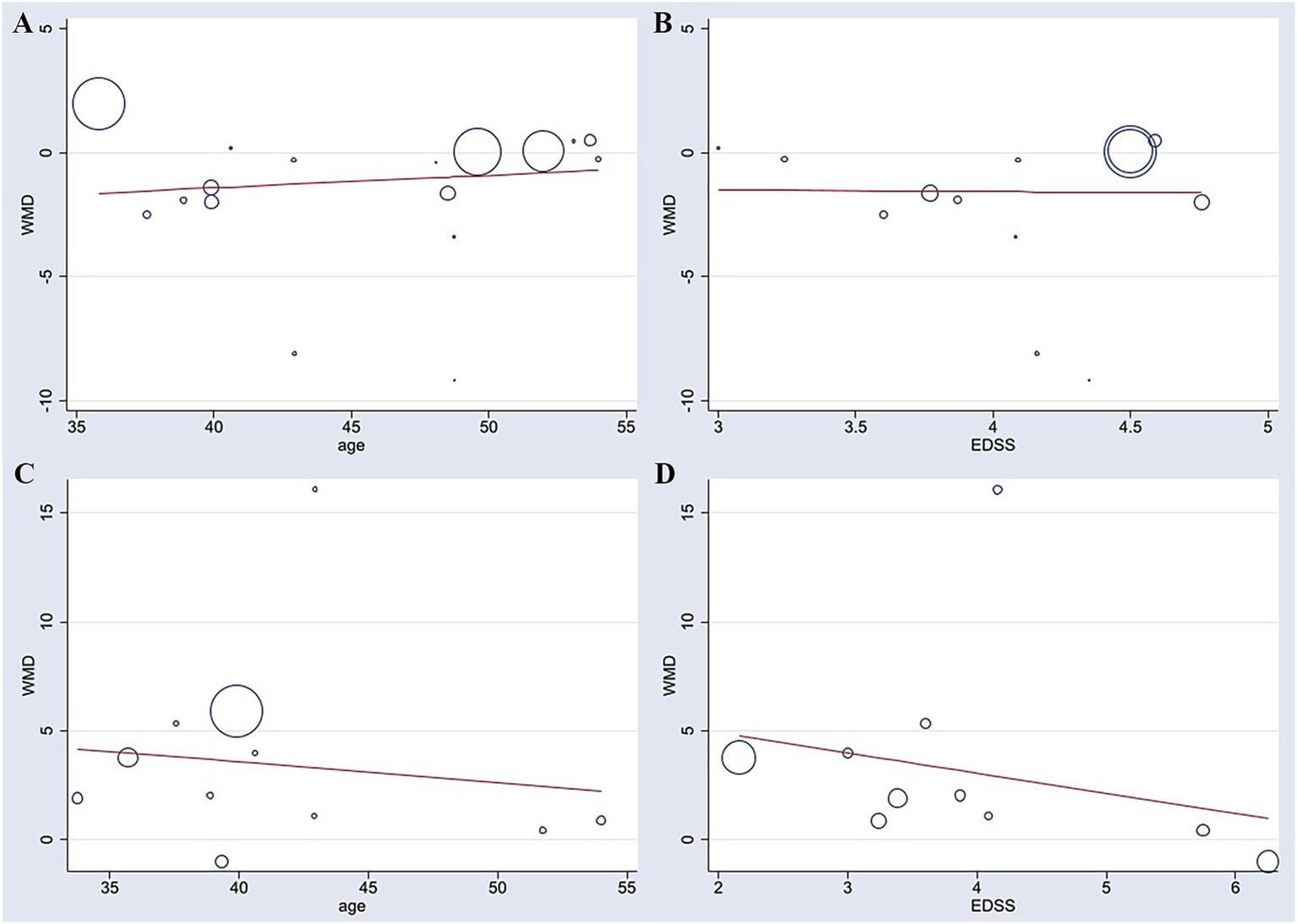
Meta-regression analyses of patient age and baseline EDSS score with improvements in TUG (A,B) and BBS (C,D).
For BBS, a total of 10 studies were included in the meta-regression. Similarly, age (β = −0.034, p = 0.897) and EDSS score (β = −0.875, p = 0.534) were not significant predictors of the effect size. The model showed poor explanatory power, with an adjusted R2 of −34.12%, and was not statistically significant [F (2, 7) = 0.27, p = 0.769]. Substantial residual heterogeneity remained after adjusting for the covariates (I2_res = 75.56%, τ2 = 18.06). These findings suggest that the heterogeneity may be attributable to other unknown study-level characteristics (Figure 6).
3.5 Sensitivity analysis
Due to some heterogeneity between studies, we sequentially excluded each study to assess the stability and reliability of the combined results. The results showed that the combined effect size did not change direction, indicating that despite the high heterogeneity among the included studies, the overall findings were robust. See Figures 7, 8.
Figure 7
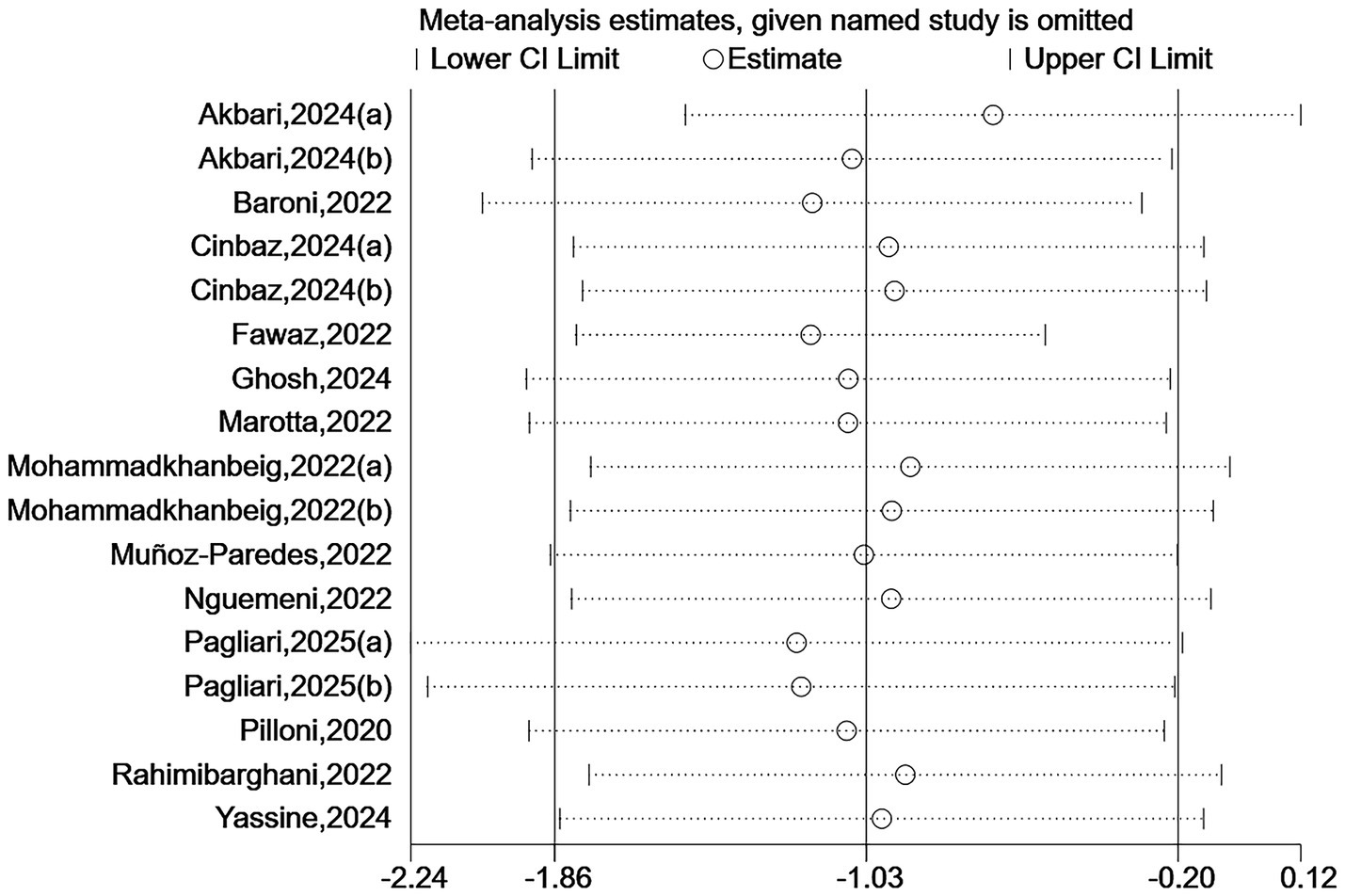
Sensitivity analysis for TUG.
Figure 8
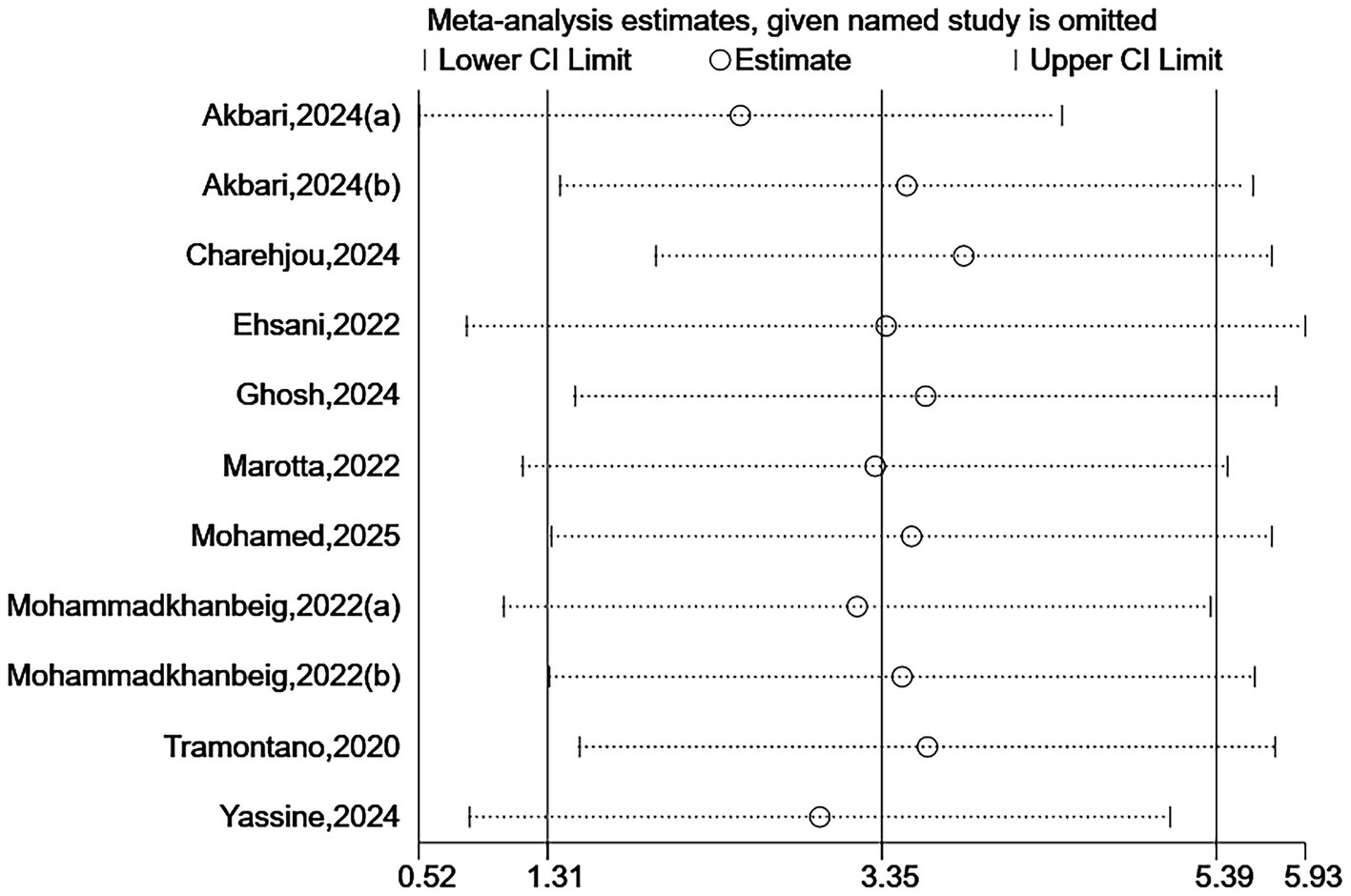
Sensitivity analysis for BBS.
3.6 Publication bias
To assess publication bias, funnel plots were created, and Egger’s test was used to evaluate the symmetry of the funnel plots. The results showed that there was publication bias for TUG (p = 0.041 < 0.05), suggesting that negative results may not have been published. However, trim-and-fill analysis [−1.144, 95% CI (−2.203, −0.005)] indicated that no additional studies were needed to make the funnel plot symmetrical, suggesting that the impact of publication bias on the overall results for TUG was minimal. No significant publication bias was found for BBS (p = 0.121). Due to the limited number of studies reporting AEs (n < 10), publication bias was not assessed. The funnel plots are shown in Figures 9, 10.
Figure 9
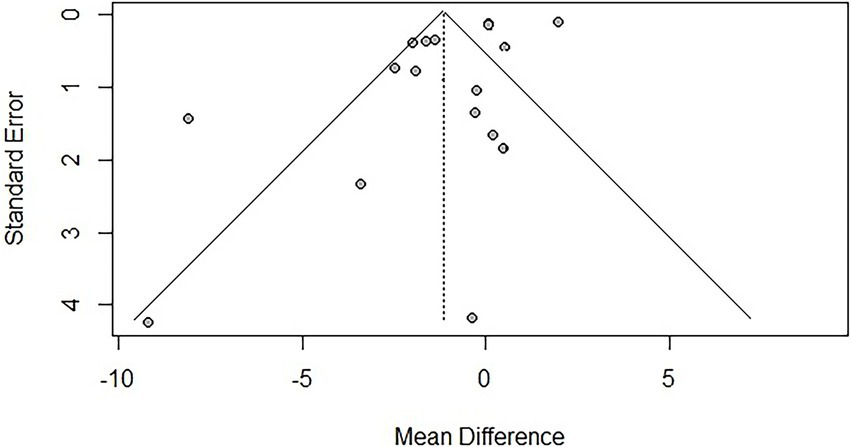
Funnel plot for TUG.
Figure 10
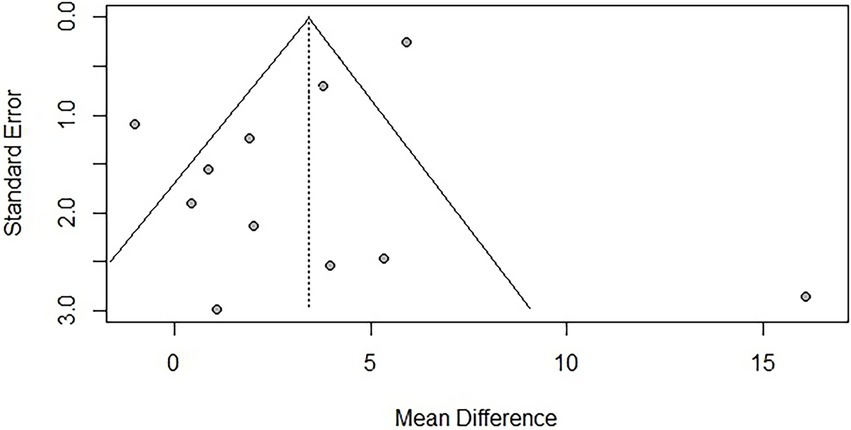
Funnel plot for BBS.
3.7 Quality of evidence assessment
The quality of evidence for the primary outcomes, TUG and BBS, was rated as moderate certainty. The downgrading was due to the risk of bias in the included studies (e.g., inadequate randomization and blinding in some trials) and substantial heterogeneity (I2 > 80%). However, the direction of effect was consistent across studies, and the results were robust.
The quality of evidence for AEs was rated as low certainty, primarily due to inconsistencies in the definition and reporting of adverse events across studies, as well as imprecision, reflected by wide confidence intervals and limited sample sizes. See Table 5 for details.
Table 5
| Outcome | Participants (studies) | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | I 2 (%) | 95% CI | Certainty (GRADE) |
|---|---|---|---|---|---|---|---|---|---|
| TUG | 448 (13 RCTs) | Serious ① | Serious ② | Not serious | Not serious | Possible | 96 | [−1.86, −0.20] | ⨁ ⨁ ⨁ ◯ Moderate |
| BBS | 257 (9 RCTs) | Serious ① | Serious ② | Not serious | Not serious | Unlikely | 88 | [1.31, 5.39] | ⨁ ⨁ ⨁ ◯ Moderate |
| AEs | 195 (5 RCTs) | Serious ③ | Not serious | Not serious | Serious ④ | Possible | 0 | [1.23, 3.85] | ⨁ ⨁ ◯ ◯ Low |
Assessment of quality of evidence.
① Some studies had a “some concerns” risk in terms of randomization and blinding procedures. ② There was substantial statistical heterogeneity across studies (I2 > 50%). ③ Definitions, monitoring methods, and reporting of adverse events varied greatly among studies, with incomplete reporting. ④ Wide confidence intervals and a limited number of studies with small sample sizes contributed to imprecision in the results.
4 Discussion
This study conducted a systematic review and meta-analysis to comprehensively evaluate the efficacy and safety of NIBS in improving balance function in patients with MS, providing relatively robust evidence to support clinical practice. The results show that NIBS has shown certain improvements in the balance abilities of MS patients, specifically reflected in the following aspects:
-
Meta-analysis revealed an association between NIBS intervention and a reduction in TUG completion time, suggesting that NIBS may help improve patients’ dynamic balance. In parallel, the increase in BBS scores indicated a potential improvement in static balance function.
-
Among different types of NIBS techniques, tDCS demonstrated consistent benefits for both dynamic and static balance, whereas the evidence supporting the efficacy of rTMS was relatively limited. In terms of MS subtypes, patients with RR MS appeared to benefit more from NIBS intervention, particularly with regard to improvements in static balance.
-
Subgroup analysis of intervention parameters indicated that both 4-week and 6-week interventions were associated with reductions in TUG time, and a 4-week intervention may also contribute to improvements in BBS. Regarding stimulation sites, targeting the M1, specifically the C3 and Cz regions, yielded relatively better outcomes, whereas stimulation of the cerebellum and prefrontal cortex did not show significant benefits. No statistically significant differences were observed across different NIBS stimulation intensities.
-
NIBS interventions were associated with AEs such as itching, warmth, burning sensations, and fatigue. However, most of these reactions were mild and transient. Since the control group did not receive electrical stimulation, adverse events were rarely reported in that group. These findings highlight the need to carefully weigh the therapeutic benefits of NIBS against its safety profile in clinical applications, and to enhance active monitoring and standardized reporting of AEs.
These improvements may stem from the multifaceted positive effects of NIBS on balance function in patients with MS. Individuals with MS often exhibit reduced excitability in M1 and impaired corticospinal tract conduction. Anodal tDCS or high-frequency rTMS can modulate neuronal membrane potentials, thereby enhancing M1 excitability and reversing pathological hypoexcitability (44, 45). Through this mechanism, NIBS strengthens motor commands from the brain to the spinal cord, improving motor output efficiency and muscle control precision, which in turn facilitates faster and more accurate postural adjustments (45). In addition, NIBS is believed to modulate the functional balance of distributed neural networks and enhance connectivity among different brain regions, such as the motor cortex, cerebellum, and somatosensory cortex (46, 47). This reorganization of neural networks may help compensate for MS-related neural pathway damage, optimizing the integration of sensory information and the generation of motor commands—both of which are fundamental to maintaining balance (46). It is worth noting that most of the included studies combined NIBS with conventional rehabilitation therapies (e.g., balance training, aerobic exercise). Therefore, NIBS may enhance cortical plasticity during rehabilitation, making concurrent motor learning more effective and thereby reinforcing and consolidating the effects of therapeutic interventions.
Furthermore, the heterogeneity in treatment response observed in our subgroup analyses may also be attributed to the intrinsic variability of MS itself, including phenotypic diversity and baseline disability levels. Patients with RRMS and lower EDSS scores typically retain greater neural plasticity and functional reserve capacity, potentially rendering them more responsive to neuromodulatory interventions like NIBS. Conversely, those with progressive forms (PPMS, SPMS) and higher EDSS scores often exhibit more extensive neurodegenerative changes and reduced brain reserve, which might constrain their capacity for functional reorganization following stimulation (48, 49). These considerations are supported by Iodice et al. (50), which systematically elaborated on how MS phenotypic heterogeneity and disability severity are critical determinants of rehabilitation outcomes, underscoring the necessity for individualized and stratified therapeutic approaches in this population. This underscores the importance of considering MS subtype and disability severity as key modifiers of NIBS effects, which should be carefully stratified in future clinical trials to identify the patient subgroups most likely to benefit.
The stimulation site is a critical factor influencing the efficacy of NIBS. M1 as the final common pathway for motor output, directly controls lower limb muscle contractions (51). Therefore, stimulation targeting the M1 region may more directly optimize the generation of motor commands required for balance control. In the international 10–20 EEG system, the central point (Cz) approximately corresponds to the cortical representation area of the lower limbs, making it a potentially more precise and targeted stimulation site (52). In contrast, cerebellar stimulation did not show consistent advantages in this meta-analysis. Several factors may account for this finding. First, due to the cerebellum’s deep anatomical location, there is ongoing debate over whether transcranial electrical stimulation can effectively penetrate the skull and reach the cerebellar cortex with sufficient field intensity (53). Second, the cerebellum is a commonly affected region in MS, and inherent structural damage, such as white and gray matter atrophy may disrupt functional connectivity. This disconnection could reduce the cerebellum’s responsiveness to neuromodulatory interventions such as TMS or tDCS (54). Third, the cerebellum primarily contributes to motor coordination, timing, prediction, and motor learning, rather than initiating movement or generating muscular force directly (55).
In terms of stimulation parameters, although this study did not identify a significant effect of different stimulation intensities on balance improvement in MS, subgroup analysis of intervention duration yielded valuable insights: both 4-week and 6-week interventions were associated with reductions in TUG completion time, and the 4-week intervention also appeared to contribute to improvements in BBS. These findings suggest that the therapeutic effect of NIBS may stabilize once certain threshold parameters are met, and further increases in stimulation intensity or total treatment duration may not produce additional benefits. This phenomenon may be related to the biological characteristics of tDCS, whose ultimate effects are likely the result of complex interactions among multiple dose-related parameters, including current intensity, stimulation duration, electrode size and configuration, and the number of treatment sessions (56). The present findings are consistent with those of Emadi et al. (21), who reported that single-session stimulation is typically insufficient to induce sustained plastic changes. Instead, repeated sessions, such as those delivered over at least 4 weeks in the current study are crucial for achieving cumulative effects and long-term benefits. Therefore, ensuring an adequate intervention duration may be a key factor in eliciting clinically measurable improvements.
This study found that the incidence of adverse events was significantly higher in the NIBS intervention group compared to the control group. However, the vast majority of reported adverse events were mild and transient, such as skin tingling, itching at the stimulation site, mild headache, and a burning sensation. Serious adverse events were rarely observed. These discomforts may be related to factors such as locally elevated current density, the quality of the conductive medium, the method of current ramp-up/ramp-down, or individual differences in skin sensitivity (56, 57). Despite the increased risk of adverse events, their mild and temporary nature suggests that the overall safety profile of NIBS remains favorable.
Compared with previous studies, the study by Nombela-Cabrera et al. (20) focused solely on tDCS and found improvements in gait function, while improvements in static balance did not reach statistical significance. Additionally, no significant differences were observed when grouping by stimulation site. In contrast, the present study included a broader range of NIBS techniques (including rTMS, iTBS, etc.) and a larger number of studies, confirming that NIBS can improve both dynamic and static balance. This discrepancy may be attributed to the broader inclusion criteria and larger sample size in the present analysis, suggesting that different types of NIBS may exert synergistic effects through shared neuromodulatory mechanisms, rather than tDCS being the only effective modality. Emadi et al. (21) primarily investigated spasticity and gait speed, reporting that multi-session TMS effectively improved spasticity, while single-session tDCS had no significant impact on gait speed. However, their study did not analyze balance-specific outcomes, limiting the strength of its conclusions regarding balance function. In contrast, our study not only focused on key indicators of balance function but also assessed the safety of NIBS in this population by reporting the risk of adverse events—an aspect of high practical relevance for clinical decision-making. Furthermore, through subgroup analyses and meta-regression, we explored the potential impact of various factors such as stimulation targets, stimulation intensity, intervention parameters, and patients’ baseline characteristics on treatment efficacy. These analyses enhanced the interpretability of the results and provided important evidence to guide the individualization and precision of treatment strategies in future clinical practice.
Despite the comprehensive literature search and rigorous methodology employed in this meta-analysis, several limitations should be acknowledged. First, our search was restricted to articles published in English, which may have introduced language bias and led to the omission of potentially relevant studies published in other languages. Second, although subgroup analyses and meta-regression were conducted to explore the heterogeneity and stimulation parameters reported in previous studies, substantial residual heterogeneity remained. This may be due to variations in study design, types of concurrent rehabilitation programs, and stimulation parameters (e.g., session duration, electrode size, or coil type) across the included trials. The conclusions drawn from certain subgroups (e.g., cerebellar stimulation) require further validation through future studies. As highlighted by Iodice et al. (58) in their comprehensive review, this variability represents a fundamental challenge in the NIBS field for MS and may account for the inconsistent findings across studies. The analysis of safety outcomes was limited by the incomplete and inconsistent reporting of adverse events in the original studies. Only a small number of trials reported adverse events, and definitions, data collection methods, and reporting standards varied significantly. This inconsistency may introduce bias in the assessment of NIBS safety. Future research should adopt standardized adverse event reporting guidelines, such as the CONSORT extension for harms, and enhance monitoring and documentation during interventions. Standardizing the reporting process will allow for a more comprehensive and accurate evaluation of the safety profile of NIBS. Lastly, with respect to long-term outcomes, most of the included studies had relatively short follow-up periods and primarily assessed immediate or short-term effects. As a result, this meta-analysis cannot draw definitive conclusions regarding the durability of NIBS effects over time. Future research should prioritize well-designed, large-scale randomized controlled trials with long-term follow-up (e.g., at 3, 6, and 12 months post-intervention), focusing on optimizing strategies for sustained intervention effects during the maintenance phase.
5 Conclusion
As an adjunctive therapeutic approach, NIBS has shown certain beneficial effects in improving balance control in patients with MS. It provides a non-pharmacological, non-invasive treatment option for the rehabilitation of balance dysfunction in MS, particularly suitable for patients who are unresponsive to or intolerant of pharmacological therapies. However, the potential for mild adverse effects should not be overlooked. Future studies should prioritize large-scale, high-quality randomized controlled trials focusing on standardized and targeted stimulation protocols, long-term follow-up to assess the sustainability of effects, and comprehensive, standardized reporting of adverse events. These efforts are essential to validate the findings of this study, optimize stimulation parameters, and facilitate the integration of NIBS into individualized rehabilitation strategies for MS.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LZ: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft. YS: Data curation, Software, Writing – original draft. ZD: Validation, Writing – original draft. YJ: Data curation, Writing – original draft. YH: Data curation, Writing – original draft. YM: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Administration of Traditional Chinese Medicine High level Key Discipline Construction Project of Traditional Chinese Medicine: External treatment of Traditional Chinese Medicine (zyyzdxk-2023116); the Natural Science Foundation of Shandong Province (ZR2021MH373 and ZR2021LZY044); and the Jinan University 20 Funding Project (2020GXRC005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1696343/full#supplementary-material
References
1.
Compston A Coles A . Multiple sclerosis. Lancet. (2008) 372:1502–17. doi: 10.1016/s0140-6736(08)61620-7
2.
McGinley MP Goldschmidt CH Rae-Grant AD . Diagnosis and treatment of multiple sclerosis: a review. JAMA. (2021) 325:765–79. doi: 10.1001/jama.2020.26858
3.
Walton C King R Rechtman L Kaye W Leray E Marrie RA et al . Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler. (2020) 26:1816–21. doi: 10.1177/1352458520970841
4.
Fernández-Vázquez D Calvo-Malón G Molina-Rueda F López-González R Carratalá-Tejada M Navarro-López V et al . Kinematic gait analysis in people with mild-disability multiple sclerosis using statistical parametric mapping: a cross-sectional study. Sensors. (2023) 23:7671. doi: 10.3390/s23187671
5.
Sandgren S Novakova L Nordin A Sabir H Axelsson M Malmeström C et al . The effect of alemtuzumab on neurodegeneration in relapsing-remitting multiple sclerosis: a five-year prospective mono-center study. Mult Scler Relat Disord. (2024) 91:105894. doi: 10.1016/j.msard.2024.105894
6.
Cameron MH Lord S . Postural control in multiple sclerosis: implications for fall prevention. Curr Neurol Neurosci Rep. (2010) 10:407–12. doi: 10.1007/s11910-010-0128-0
7.
Nilsagård Y Lundholm C Denison E Gunnarsson LG . Predicting accidental falls in people with multiple sclerosis—a longitudinal study. Clin Rehabil. (2009) 23:259–69. doi: 10.1177/0269215508095087
8.
Hauser SL Cree BAC . Treatment of multiple sclerosis: a review. Am J Med. (2020) 133:1380–1390.e2. doi: 10.1016/j.amjmed.2020.05.049
9.
Sepehri Far S Amiri B Sahebozamani M Ebrahimi HA . Improvement in the multiple sclerosis functional composite score by multi-function swing suspension training program. Mult Scler Relat Disord. (2022) 67:104174. doi: 10.1016/j.msard.2022.104174
10.
Kupjetz M Joisten N Rademacher A Gonzenbach R Bansi J Zimmer P . Cycling in primary progressive multiple sclerosis (Cypro): study protocol for a randomized controlled superiority trial evaluating the effects of high-intensity interval training in persons with primary progressive multiple sclerosis. BMC Neurol. (2023) 23:162. doi: 10.1186/s12883-023-03187-6
11.
Dalgas U Stenager E . Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis?Ther Adv Neurol Disord. (2012) 5:81–95. doi: 10.1177/1756285611430719
12.
Padberg F Bulubas L Mizutani-Tiebel Y Burkhardt G Kranz GS Koutsouleris N et al . The intervention, the patient and the illness—personalizing non-invasive brain stimulation in psychiatry. Exp Neurol. (2021) 341:113713. doi: 10.1016/j.expneurol.2021.113713
13.
Brunoni AR Teng CT Correa C Imamura M Brasil-Neto JP Boechat R et al . Neuromodulation approaches for the treatment of major depression: challenges and recommendations from a working group meeting. Arq Neuropsiquiatr. (2010) 68:433–51. doi: 10.1590/s0004-282x2010000300021
14.
Lattari E Costa SS Campos C de Oliveira AJ Machado S Maranhao Neto GA . Can transcranial direct current stimulation on the dorsolateral prefrontal cortex improves balance and functional mobility in Parkinson’s disease?Neurosci Lett. (2017) 636:165–9. doi: 10.1016/j.neulet.2016.11.019
15.
Kaminski E Steele CJ Hoff M Gundlach C Rjosk V Sehm B et al . Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin Neurophysiol. (2016) 127:2455–62. doi: 10.1016/j.clinph.2016.03.018
16.
Yosephi MH Ehsani F Zoghi M Jaberzadeh S . Multi-session anodal tDCS enhances the effects of postural training on balance and postural stability in older adults with high fall risk: primary motor cortex versus cerebellar stimulation. Brain Stimul. (2018) 11:1239–50. doi: 10.1016/j.brs.2018.07.044
17.
Palm U Ayache SS Padberg F Lefaucheur JP . Non-invasive brain stimulation therapy in multiple sclerosis: a review of tDCS, rTMS and ECT results. Brain Stimul. (2014) 7:849–54. doi: 10.1016/j.brs.2014.09.014
18.
Kuo MF Nitsche MA . Effects of transcranial electrical stimulation on cognition. Clin EEG Neurosci. (2012) 43:192–9. doi: 10.1177/1550059412444975
19.
Beretta VS Santos PCR Orcioli-Silva D Zampier VC Vitório R Gobbi LTB . Transcranial direct current stimulation for balance rehabilitation in neurological disorders: a systematic review and meta-analysis. Ageing Res Rev. (2022) 81:101736. doi: 10.1016/j.arr.2022.101736
20.
Nombela-Cabrera R Pérez-Nombela S Avendaño-Coy J Comino-Suárez N Arroyo-Fernández R Gómez-Soriano J et al . Effectiveness of transcranial direct current stimulation on balance and gait in patients with multiple sclerosis: systematic review and meta-analysis of randomized clinical trials. J Neuroeng Rehabil. (2023) 20:142. doi: 10.1186/s12984-023-01266-w
21.
Emadi A Halakoo S Delkhoush CT Ehsani F . The effects of neuromodulators on spasticity, balance, and gait in patients with MS: a systematic review and meta-analysis study. Eur Neurol. (2023) 86:144–54. doi: 10.1159/000528100
22.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
23.
Hutton B Salanti G Caldwell DM Chaimani A Schmid CH Cameron C et al . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/m14-2385
24.
Polman CH Reingold SC Banwell B Clanet M Cohen JA Filippi M et al . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. (2011) 69:292–302. doi: 10.1002/ana.22366
25.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . Rob 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
26.
Guyatt GH Oxman AD Vist G Kunz R Brozek J Alonso-Coello P et al . Grade guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. (2011) 64:407–15. doi: 10.1016/j.jclinepi.2010.07.017
27.
Tramontano M Grasso MG Soldi S Casula EP Bonnì S Mastrogiacomo S et al . Cerebellar intermittent theta-burst stimulation combined with vestibular rehabilitation improves gait and balance in patients with multiple sclerosis: a preliminary double-blind randomized controlled trial. Cerebellum. (2020) 19:897–901. doi: 10.1007/s12311-020-01166-y
28.
Mohamed HZA Elwishy AA Shalaby NM Shehata HSM Ali AS . Cerebellar repetitive transcranial magnetic stimulation combined with physiotherapy for balance in people with multiple sclerosis: a randomized controlled trial with six-week follow-up. Mult Scler Relat Disord. (2025) 102:106610. doi: 10.1016/j.msard.2025.106610
29.
Charehjou B Tabrizi Y Minoonejad H . The combination of transcranial direct current stimulation and virtual reality training on fatigue, balance and walking in patients with multiple sclerosis. Phys Treat. (2024) 14:171–82. doi: 10.32598/ptj.14.3.578.2
30.
Baroni A Magro G Martinuzzi C Brondi L Masiero S Milani G et al . Combined effects of cerebellar tDCS and task-oriented circuit training in people with multiple sclerosis: a pilot randomized control trial. Restor Neurol Neurosci. (2022) 40:85–95. doi: 10.3233/rnn-211245
31.
Mohammadkhanbeigi S Tabrizi Y Nabavi S Minoonejad H . The comparable effect of tDCS and core exercises on balance and mobility in patients with multiple sclerosis. Iran Rehabil J. (2023) 20:569–78. doi: 10.32598/irj.20.4.1699.1
32.
Akbari NJ Tahan N Naimi SS Baghban AA Moghadam NB Zoghi M . Comparing the effects of cerebellar and prefrontal anodal transcranial direct current stimulation concurrent with postural training on balance and fatigue in patients with multiple sclerosis: a double-blind, randomized, sham-controlled trial. Exp Brain Res. (2024) 242:1087–100. doi: 10.1007/s00221-024-06816-9
33.
Muñoz-Paredes I Herrero AJ Llamas-Ramos R Rodríguez-Pérez V Seco-Calvo J . The effect of combining transcranial direct current stimulation treatment and an exercise program on fragility in a population with multiple sclerosis: cross-over design trial. Int J Environ Res Public Health. (2022) 19:2747. doi: 10.3390/ijerph191912747
34.
Yassine IA Shehata H Hamdy S Abdel-Naseer M Hassan T Sherbiny M et al . Effect of high frequency repetitive transcranial magnetic stimulation (rTMS) on the balance and the white matter integrity in patients with relapsing-remitting multiple sclerosis: a long-term follow-up study. Mult Scler Relat Disord. (2024) 83:105471. doi: 10.1016/j.msard.2024.105471
35.
Rahimibarghani S Azadvari M Emami-Razavi SZ Harirchian MH Rahimi-Dehgolan S Fateh HR . Effects of nonconsecutive sessions of transcranial direct current stimulation and stationary cycling on walking capacity in individuals with multiple sclerosis. Int J MS Care. (2022) 24:202–8. doi: 10.7224/1537-2073.2021-004
36.
Cinbaz G Sarı Z Oğuz S Tombul T Hanoğlu L Fernández-Pérez JJ et al . Effects of transcranial and trans-spinal direct current stimulation combined with robot-assisted gait training on gait and fatigue in patients with multiple sclerosis: a double-blind, randomized, sham-controlled study. J Clin Med. (2024) 13:7632. doi: 10.3390/jcm13247632
37.
Marotta N de Sire A Marinaro C Moggio L Inzitari MT Russo I et al . Efficacy of transcranial direct current stimulation (tDCS) on balance and gait in multiple sclerosis patients: a machine learning approach. J Clin Med. (2022) 11:3505. doi: 10.3390/jcm11123505
38.
Pagliari C Tella SD Bonanno C Cacciante L Cioeta M De Icco R et al . Enhancing the effect of rehabilitation on multiple sclerosis: a randomized clinical trial investigating the impact of remotely-supervised transcranial direct current stimulation and virtual reality telerehabilitation training. Mult Scler Relat Disord. (2025) 94:106256. doi: 10.1016/j.msard.2024.106256
39.
Pilloni G Choi C Coghe G Cocco E Krupp LB Pau M et al . Gait and functional mobility in multiple sclerosis: immediate effects of transcranial direct current stimulation (tDCS) paired with aerobic exercise. Front Neurol. (2020) 11:310. doi: 10.3389/fneur.2020.00310
40.
Ghosh S Tucak C Eisenhauer J Jacques A Hathorn D Dixon J et al . Non-invasive brain stimulation enhances the effect of physiotherapy for balance and mobility impairment in people with multiple sclerosis. Mult Scler Relat Disord. (2024) 92:106149. doi: 10.1016/j.msard.2024.106149
41.
Ehsani F Ahmadi M Masoudian N Jaberzadeh S . Priming of postural training with cerebellar anodal transcranial direct current stimulation for its effects on postural balance and fear of falling in patients with multiple sclerosis: a randomized, double-blind, sham-controlled study. J Clin Neurosci. (2022) 99:294–301. doi: 10.1016/j.jocn.2022.03.025
42.
Fawaz SI Izumi SI Hamada SM Omara AA Wassef GO Saber HG et al . Role of cervical spinal magnetic stimulation in improving posture and functional ambulation of patients with relapsing remitting multiple sclerosis. Rehabil Res Pract. (2022) 2022:6009104. doi: 10.1155/2022/6009104
43.
Nguemeni C Hiew S Kögler S Homola GA Volkmann J Zeller D . Split-belt training but not cerebellar anodal tDCS improves stability control and reduces risk of fall in patients with multiple sclerosis. Brain Sci. (2021) 12:63. doi: 10.3390/brainsci12010063
44.
Zeng K Darmani G Fomenko A Xia X Tran S Nankoo JF et al . Induction of human motor cortex plasticity by theta burst transcranial ultrasound stimulation. Ann Neurol. (2022) 91:238–52. doi: 10.1002/ana.26294
45.
Kesikburun S . Non-invasive brain stimulation in rehabilitation. Turk J Phys Med Rehabil. (2022) 68:1–8. doi: 10.5606/tftrd.2022.10608
46.
Rektorová I Pupíková M Fleury L Brabenec L Hummel FC . Non-invasive brain stimulation: current and future applications in neurology. Nat Rev Neurol. (2025). doi: 10.1038/s41582-025-01137-z
47.
Polanía R Nitsche MA Paulus W . Modulating functional connectivity patterns and topological functional organization of the human brain with transcranial direct current stimulation. Hum Brain Mapp. (2011) 32:1236–49. doi: 10.1002/hbm.21104
48.
Prosperini L Di Filippo M . Beyond clinical changes: rehabilitation-induced neuroplasticity in MS. Mult Scler. (2019) 25:1348–62. doi: 10.1177/1352458519846096
49.
Moccia M Lanzillo R Palladino R Chang KC Costabile T Russo C et al . Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult Scler. (2016) 22:659–67. doi: 10.1177/1352458515599075
50.
Iodice R Aceto G Ruggiero L Cassano E Manganelli F Dubbioso R . A review of current rehabilitation practices and their benefits in patients with multiple sclerosis. Mult Scler Relat Disord. (2023) 69:104460. doi: 10.1016/j.msard.2022.104460
51.
Lemon RN . Descending pathways in motor control. Annu Rev Neurosci. (2008) 31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547
52.
Herwig U Satrapi P Schönfeldt-Lecuona C . Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. (2003) 16:95–9. doi: 10.1023/b:brat.0000006333.93597.9d
53.
Bahr-Hosseini M Bikson M . Neurovascular-modulation: a review of primary vascular responses to transcranial electrical stimulation as a mechanism of action. Brain Stimul. (2021) 14:837–47. doi: 10.1016/j.brs.2021.04.015
54.
Fritz NE Edwards EM Ye C Prince J Yang Z Gressett T et al . Cerebellar contributions to motor and cognitive control in multiple sclerosis. Arch Phys Med Rehabil. (2023) 104:842. doi: 10.1016/j.apmr.2023.02.001
55.
Manto M Bower JM Conforto AB Delgado-García JM da Guarda SN Gerwig M et al . Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum. (2012) 11:457–87. doi: 10.1007/s12311-011-0331-9
56.
Woods AJ Antal A Bikson M Boggio PS Brunoni AR Celnik P et al . A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. (2016) 127:1031–48. doi: 10.1016/j.clinph.2015.11.012
57.
Bikson M Grossman P Thomas C Zannou AL Jiang J Adnan T et al . Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. (2016) 9:641–61. doi: 10.1016/j.brs.2016.06.004
58.
Iodice R Manganelli F Dubbioso R . The therapeutic use of non-invasive brain stimulation in multiple sclerosis—a review. Restor Neurol Neurosci. (2017) 35:497–509. doi: 10.3233/rnn-170735
Summary
Keywords
non-invasive brain stimulation, multiple sclerosis, balance control, meta-analysis, randomized controlled trials, systematic review
Citation
Zhang L, Sun Y, Diao Z, Jiang Y, Hu Y and Ma Y (2025) Effects of non-invasive brain stimulation on balance control in patients with multiple sclerosis: a systematic review and meta-analysis. Front. Neurol. 16:1696343. doi: 10.3389/fneur.2025.1696343
Received
31 August 2025
Accepted
26 September 2025
Published
10 October 2025
Volume
16 - 2025
Edited by
Maria Grazia Grasso, Santa Lucia Foundation (IRCCS), Italy
Reviewed by
Raffaele Dubbioso, Federico II University Hospital, Italy
Shahab Lotfinia, Shahid Beheshti University of Medical Sciences, Iran
Updates
Copyright
© 2025 Zhang, Sun, Diao, Jiang, Hu and Ma.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxia Ma, mayuxia2025@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.