Abstract
Objective:
This study aims to compare the efficacy of combined and single neuromuscular electrical stimulation (NMES), and traditional swallowing training (TST) for neurogenic dysphagia through a network meta-analysis (NMA).
Methods:
This meta-analysis has been prospectively registered on the PROSPERO (Registration number: CRD42025643351). Electronic databases, including Embase, PubMed, Web of science and the Cochrane Library, were searched up to March 1, 2025. All published randomized controlled trials (RCTs) comparing combined and/or single neuromuscular electrical stimulation with traditional swallowing therapy for the treatment of patients with neurogenic dysphagia were included. A network meta-analysis using STATA software synthesized data and ranked treatments by efficacy. The outcome measures included the Functional Oral Intake Scale (FOIS), Standardized Swallowing Assessment (SSA), Penetration-Aspiration Scale (PAS) and clinical efficacy (CE).
Results:
Twenty-two RCTs with a total sample size of 1,265 cases were ultimately included. The findings suggest that NMES combined with other therapies is more effective than single NMES or traditional swallowing therapy for patients with neurogenic dysphagia, demonstrating a higher clinical efficacy rate. Among the combined therapies, the integration of NMES with transcranial direct current stimulation (tDCS) and TST demonstrated the highest efficacy in improving FOIS scores and enhancing swallowing function [surface under cumulative ranking curve values (SUCRCV): 95.3%, standardized mean difference (SMD): 1.15, 95% confidence interval (CI): 0.34, to 1.97]. Additionally, NMES combined with acupuncture showed the most significant reduction in SSA scores (SUCRA: 87.6%, SMD: −1.49, 95% CI: −2.48 to −0.49). Furthermore, the combination of NMES combined with effortful swallowing (ES) and TST exhibited the most pronounced effects in lowering PAS scores and preventing aspiration (SUCRA: 81.2%, SMD: −1.06, 95% CI: −1.82 to −0.31).
Conclusions:
Ranking probabilities indicated that combined therapy had the highest likelihood of being the most effective intervention. However, large-scale, multi-center, high-quality studies are essential to further validate this conclusion.
1 Introduction
Neurogenic dysphagia, a prevalent complication of neurological disorders such as stroke, traumatic brain injury, and neurodegenerative diseases, significantly impairs patients' quality of life and increases the risk of aspiration pneumonia (1). Restoration of swallowing function in patients with neurogenic dysphagia depends on effective management strategies and preventing life-threatening complications. Neuromuscular electrical stimulation (NMES) has emerged as a promising adjunctive therapy for neurogenic dysphagia. NMES delivers electrical stimulation to muscles via surface electrodes, inducing muscle contraction through depolarization of nerve fibers, thereby enhancing swallowing muscle strength and sensory awareness to improve or restore swallowing function (2, 3). Traditional swallowing therapy, which includes exercises to strengthen swallowing muscles and improve swallowing mechanisms, remains the cornerstone of dysphagia rehabilitation. However, the relative efficacy of single NMES therapy, combined NMES therapy, and traditional swallowing therapy remains unclear. Although a comprehensive meta-analysis has confirmed that NMES combined with swallowing training is superior to swallowing training alone in improving swallowing function and reducing complications (4), this finding does not elucidate the comparative effectiveness of other NMES-based combination therapies. Moreover, the optimal pairing of NMES with other modalities, such as transcranial direct current stimulation (tDCS), acupuncture, or effortful swallowing, has not been systematically ranked.
Therefore, this study aims to compare the efficacy of combined and single NMES therapies with traditional swallowing therapy alone in the treatment of neurogenic dysphagia through a network meta-analysis, with the goal of providing evidence for optimizing treatment strategies for neurogenic dysphagia in clinical practice.
2 Methods
2.1 Protocol registration
This meta-analysis has been prospectively registered on the PROSPERO (Registration number: CRD42025643351). This systematic review adhered to the PRISMA-2020 guidelines.
2.2 Ethics
Since this treatment does not entail the recruitment of clients or the collection of personal info, ethical clearance is not essential.
2.3 Search strategy
We searched electronic databases, including Embase, PubMed, Web of Science, and the Cochrane Library, from their inception to March 1, 2025. The search was limited to studies published in English and Chinese. A combination of Medical Subject Headings (MeSH) terms and free-text words was employed. The search terms included: “dysphagia”, “Swallowing Disorders”, “Oropharyngeal Dysphagia”, “neuromuscular electrical stimulation”, “NMES”, “electrostimulation”, “randomized controlled trial”, “RCT”, and their related synonyms and variants in both English and Chinese. The detailed search strategy is illustrated in Supplementary Table 1.
2.4 Inclusion criteria
Literature search and testing procedures were conducted independently by two researchers to determine eligibility. Any differences in the results were discussed and resolved through consensus. Originally, titles and abstracts were screened to recognize pertinent studies. Ultimately, an in-depth examination of full texts was conducted to identify whether each research study satisfied the adhering to criteria:
-
(1) Literature type: studies were included if they were explicitly described as randomized controlled trials (RCTs), regardless of whether blinding was employed.
-
(2) Participants: ① Individuals aged 18 years or older. ② Patients diagnosed with neurogenic dysphagia secondary to neurological conditions, such as stroke, traumatic brain injury, Parkinson's disease, or multiple sclerosis. To address potential differences among these neurological conditions, only patients with clear diagnostic criteria and comparable treatment goals were included, ensuring the homogeneity and comparability of participants and reducing the impact of disease-specific variability on the results.
-
(3) Interventions: combined treatment group: neuromuscular electrical stimulation (NMES) combined with other therapies. Single treatment group: NMES alone or traditional swallowing therapy alone.
-
(4) Outcome indicators: the study included at least one of the following outcome measures: Functional oral intake scale (FOIS), standardized swallowing assessment (SSA), penetration-aspiration scale (PAS) and clinical efficacy. For the outcome of CE, it was defined as a significant improvement or recovery in swallowing function as per the original authors' criteria in each respective study, typically measured by a predefined clinically important improvement on standardized scales such as FOIS, SSA or a global clinician assessment as reported.
2.5 Exclusion criteria
-
(1) Studies published in languages other than English or Chinese.
-
(2) Studies involving children or adolescents (aged <18 years).
-
(3) Studies focusing on non-neurogenic dysphagia caused by conditions such as esophageal tumors or scleroderma.
-
(4) Animal studies, review articles, case reports, gray literature, dissertations, conference abstracts or studies other than RCTs.
-
(5) Studies with interventions or outcome measures that do not meet the inclusion criteria, or those with flawed experimental design or methodology.
-
(6) Studies with incomplete data or unavailable full-text articles.
2.6 Quality of literature
Two authors independently assessed the risk of bias using the Cochrane tool. Inter-rater reliability was evaluated with Cohen's kappa coefficient. The two scientists evaluated the literary works's efficiency on assessment criteria and made judgments of low, uncertain, and high danger, respectively, and then cross-checked them. If there were any kind of discrepancies, we would certainly review them. If agreement could not be gotten to, we would certainly review it with a third party scientist.
2.7 Interpretation of league tables
In a network meta-analysis, the league table is used to present the relative effectiveness of different interventions. The rows and columns represent various treatments, and the intersecting cells display the pairwise effect estimates along with their 95% confidence intervals. In this study, if a confidence interval does not include the null value (such as an odds ratio of 1 or a mean difference of 0), the comparison is considered statistically significant and is indicated accordingly in the table. The reported effect size reflects the relative efficacy of the treatment in the row compared to the treatment in the column.
2.8 Statistical analysis
The frequentist method used random effect models was employed to conduct network meta-analyses (NMAs) of randomized controlled trials. A common heterogeneity parameter was assumed in the network meta-analysis aimed to compare with the empirical distributions of heterogeneity. An assessment of global and local statistical heterogeneity was conducted with generalized Cochran's Q. Continuous variables (FOIS, PAS, SSA) were expressed as standardized mean differences (SMD) with their 95% confidence intervals (CI), while dichotomous variables (clinical effective rate) were expressed as risk ratios (RR) with their 95% CI. For studies involving three or more arms, the research will be split and error-corrected before inclusion in the website meta-analysis. Since control groups are prone to repeated use, their sample sizes will be proportionally reduced based on the number of splits and comparisons. To evaluate transitivity, we examined the distributions of key study characteristics across comparison groups; any detected differences were explored via meta-regression to assess their impact on the results. We assessed statistical inconsistency between direct and indirect evidence using both global and local tests.
As global approach, we used a design-by-treatment interaction model to investigate the inconsistency in the entire network. We evaluated local inconsistency based on the node-splitting method. We produced league tables and calculated the surface under the cumulative ranking curve (SUCRA) for each intervention. Publication bias was assessed using comparison-adjusted funnel plots. Regarding missing or incomplete data, studies with essential missing outcome data that could not be retrieved through author contact or calculated from available statistics were excluded during the full-text screening phase to ensure the integrity of the analysis. Statistical analyses were conducted using the network package based on Stata MP16.0 (StataCorp LP, College Station, TX, USA) and RStudio software (R Foundation for Statistical Computing). A significance level of two-sided α = 0.05 was set for all analyses.
3 Results
3.1 Study selection
A total of 576 records were retrieved from the following databases: PubMed (n = 200), Web of Science (n = 148), Embase (n = 56), and the Cochrane Library (n = 172). After removing 249 duplicate records, a stepwise screening process was conducted, resulting in the inclusion of 22 studies (2, 5–20). The detailed screening process is illustrated in Figure 1.
Figure 1

Flowchart of study screening process.
3.2 Study characteristics
In the selected 22 studies, the total sample size was 1,265 cases, and a total of 10 intervention measures were investigated, including neuromuscular electrical stimulation (abbreviated as NMES), traditional swallowing training (abbreviated as TST), neuromuscular electrical stimulation combined with traditional swallowing training (abbreviated as NMES+TST), neuromuscular electrical stimulation combined with transcranial direct current stimulation and traditional swallowing training (abbreviated as NMES+tDCS+TST), neuromuscular electrical stimulation combined with acupuncture (abbreviated as NMES+AP), neuromuscular electrical stimulation combined with upper cervical spine mobilization (abbreviated as NMES+UCSM), neuromuscular electrical stimulation combined with acupoint injection (abbreviated as NMES+AI), neuromuscular electrical stimulation combined with effortful swallowing and traditional swallowing training (abbreviated as NMES+ES+TST), neuromuscular electrical stimulation combined with eating training and traditional swallowing training (abbreviated as NMES+ET+TST), neuromuscular electrical stimulation combined with Swallowing Strengthening Exercises (abbreviated as NMES+SSE). Detailed characteristics of the included studies are presented in Table 1.
Table 1
| Study ID | Age, yr M/F | Sample size M/F | Duration of illness | Intervention | Disease | Treatment period | Outcome measure | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | T | C | T | C | T | C | T | C | T/C | T/C | |
| Huang 2014 | 64.5 ± 14.4 | 67.0 ± 10.1 | 11 | 8 | 16.6 ± 6.6 days | 25.2 ± 16.0 days | NMES | TST | Stroke | 3 weeks | FOIS, VFSS, PAS, FDS |
| 68.9 ± 9.8 | 10 | 23.5 ± 13.9 days | NMES+TST | ||||||||
| Lee 2021 | 68.9 ± 11.0 | 73.4 ± 6.7 | 26 | 23 | 44.9 ± 59.7 days | 40.2 ± 38.4 days | NMES+TST | TST | Stroke, brain tumor, encephalitis | Immediately | VDS, PAS |
| Permsirivanich 2009 | 64.50 ± 8.80 | 64.73 ± 9.39 | 12 | 11 | >2 weeks | NMES | TST | Stroke | 4 weeks | FOIS | |
| Bengisu 2024 | 68.0 ± 10.5 | 66.9 ± 12.5 | 10 | 10 | NMES+TST | TST | |||||
| 65.2 ± 11.5 | 63.1 ± 14.2 | 10 | 10 | 3.1 ± 2.5 days | NMES+tDCS +TST |
tDCS+TST | Stroke | 2 weeks | GUSS, VFSS, PAS, FOIS, DSRS | ||
| Cakmak 2023 | 62.9 ± 9.8 | 63.6 ± 10.0 | 17 | 17 | 48.3 ± 92.6 weeks | 52.2 ± 92.2 weeks | NMES+TST | TST | Stroke | 3 weeks | FOIS, EAT-10, SWAL-QOL, VRQOL |
| Cao 2023 | 61 ± 8 | 59 ± 7 | 30 | 30 | 34.0 ± 22.1 days | 36.8 ± 47.4 days | NMES+AP | NMES | Stroke | 4 weeks | SSA, PAS, CE |
| Chang 2014 | 46 ± 10 | 44 ± 11 | 38 | 36 | 16.6 ± 4.8 days | 17.3 ± 5.2 days | NMES+AP | NMES | Stroke | 4 weeks | SDSS, NTRR, CE |
| Jeon 2020 | 63.12 ± 13.50 | 64.47 ± 8.43 | 17 | 17 | 6 months-2 years | NMES+UCSM | NMES | Stroke | 4 weeks | CCFT, CVA, VDS, PAS | |
| Li 2019 | 23.8 ± 6.28 | 23.6 ± 6.29 | 30 | 30 | NMES+TST | TST | Wilson's disease | 8 weeks | WST, SSA | ||
| Liang 2021 | 62.74 ± 6.14 | 63.14 ± 5.49 | 36 | 36 | 31.27 ± 5.85 days | 31.85 ± 6.34 days | NMES+TST | TST | Stroke | 4 weeks | CE, SSA, KDWT |
| 51.2 ± 10.8 | 47.5 ± 10.5 | 40 | 32 | 24.4 ± 7.1 days | 23.8 ± 5.2 days | NMES | AI | ||||
| Ma 2014 | 50.6 ± 11.1 | 49.9 ± 11.8 | 35 | 40 | 25.3 ± 8.4 days | 25.2 ± 6.9 days | NMES+AI | TST | Stroke | 30 days | CE, WST |
| 51.5 ± 10.9 | 28 | 24.5 ± 5.7 days | Sham | ||||||||
| Ma 2015 | 66.8 ± 7.3 | 63.7 ± 8.4 | 40 | 40 | 25.7 ± 2.9 days | 27.2 ± 1.9 days | NMES | AP | Stroke | 2 weeks | CE, WST, SDSS |
| 66.5 ± 7.8 | 40 | 26.3 ± 2.6 days | NMES+AP | ||||||||
| Matos 2022 | 40-70 | 16 | 17 | < 24 hours | NMES+TST | TST | Stroke | 5 days | FOIS, FEES, DREP | ||
| Park 2018 | 63.44 ± 13.55 | 54.67 ± 13.82 | 9 | 9 | 36.44 ± 7.5 weeks | 36.11 ± 7.94 weeks | NMES+ES+TST | TST | Parkinson's disease | 4 weeks | VDS, PAS, VFSS |
| Park 2016 | 54.00 ± 11.93 | 55.80 ± 12.23 | 25 | 25 | 35.44 ± 5.63 weeks | 36.00 ± 6.05 weeks | NMES+ES+TST | TST | Stroke | 6 weeks | VDS, PAS, VFSS |
| Simonelli 2019 | 67.2 ± 16.2 | 72.4 ± 12.3 | 16 | 15 | 45.2 ± 22.3 days | 32.6 ± 18.1 days | NMES+TST | TST | Stroke | 8 weeks | FOIS, FEES, PAS, PS |
| Sproson 2018 | 76 ± 11.4 | 79 ± 11.4 | 12 | 12 | 9.1 ± 20.5 months | 17.3 ± 25.0 months | NMES+SSE | TST | Stroke | 4 weeks | FOIS, PAS, SWAL-QOL |
| Tarihci 2023 | 62.9 ± 9.8 | 63.6 ± 10.0 | 17 | 17 | 48.3 ± 92.6 weeks | 52.2 ± 92.2 weeks | NMES+TST | TST | Stroke | 3 weeks | FOIS, EAT-10, SWAL-QOL, VRQOL, FEES |
| Terre 2015 | 46 (28–60) | 51 (22–69) | 10 | 10 | 1-6 months | NMES+TST | TST | Stroke, brain injury | 4 weeks | FOIS, OTT, PDT, PTT | |
| Xia 2011 | 66.40 ± 15.63 | 65.32 ± 14.29 | 40 | 40 | 9.22 ± 3.88 days | 8.94 ± 3.62 days | NMES | TST | |||
| 65.85 ± 14.63 | 40 | 8.37 ± 3.12 days | NMES+TST | ||||||||
| Stroke | 4 weeks | sEMG, SSA, VFSS, SWAL-QOL | |||||||||
| Zhang 2016 | 62.2 ± 9.2 | 62.6 ± 8.7 | 27 | 27 | 20.6 ± 4.3 days | 21.3 ± 4.1 days | NMES+TST | TST | Stroke | 4 weeks | WST, SSA, FOIS, SWAL-QOL, MMSE |
| Zhang 2021 | 63.72 ± 6.29 | 62.31 ± 8.07 | 27 | 28 | 2.10 ± 0.54 months | 2.16 ± 0.50 months | NMES+ET+TST | TST | Stroke | 6 weeks | CE, DOSS, SAP |
| 63.14 ± 6.56 | 28 | 2.06 ± 0.54 months | ET+TST | ||||||||
Basic characteristics of the included trials.
T = treatment group, C = control group, M/F = male and female, yr = year, NMES = Neuromuscular Electrical Stimulation, TST = Traditional Swallowing Training, tDCS = Transcranial Direct Current Stimulation, AP = Acupuncture, UCSM = Upper Cervical Spine Mobilization, AI = Acupoint Injection, ES = Effortful Swallowing, SSE = Swallowing Strengthening Exercises, ET = Eating Training, FOIS = Functional Oral Intake Scale, VFSS = Videofluoroscopic Swallowing Study, PAS = Penetration–aspiration scale, FDS = Functional Dysphagia Scale, VDS = Video Fluoroscopic Dysphagia Scale, GUSS = Gugging Swallowing Screen, DSRS = Dysphagia Severity Rating Scale, EAT-10 = Eating Assessment Tool-10, SWAL-QOL = Swallow Quality of Life Questionnaire, VRQOL = Voice-related Quality of Life questionnaire, SSA = Standardized Swallowing Assessment, CE = Clinical Efficacy, SDSS = Swallowing Difficulty Scale Score, NTRR = Nasogastric Tube Removal Rate, CCFT = Craniocervical Flexion Test, CVA = Craniovertebral Angle, WST = Water Swallowing Test, FEES = Fiber Optic Endoscopic Evaluation of Swallowing, DREP = Dysphagia Risk Evaluation Protocol, PS = Pooling Score, OTT = Oral Transit Time, PDT = Pharyngeal Delay Time, PTT = Pharyngeal Transit Time, sEMG = Surface Electromyography, MMSE = Mini-Mental State Examination, DOSS = Dysphagia Outcome and Severity Scale, SAP = StrokeAssociated Pneumonia.
A full list of abbreviations and definitions are presented in Supplementary Table 2.
3.3 Risk of bias
The methodological quality assessment, as illustrated in Figures 2, 3, reveals that all included trials were rated as low risk in terms of random sequence generation. However, only 6 studies (7, 8, 18, 21–23) provided detailed allocation concealment methods and were consequently rated as low risk, while the remaining 16 studies (2, 5, 6, 9–17, 19, 20, 24) were categorized as unclear due to insufficient information. Regarding blinding procedures, all studies were rated as high risk for both participant and personnel blinding, as the nature of neuromuscular electrical stimulation (NMES) intervention makes complete blinding impractical. In contrast, 21 studies (5–7, 9–11, 13–21) were assessed as low risk for outcome assessment blinding, with 3 studies (2, 8, 12) rated as unclear due to lack of reporting on blinding procedures. Notably, none of the included studies exhibited incomplete outcome data, selective reporting, or other forms of bias.
Figure 2

Risk of bias graph.
Figure 3

Risk of bias summary.
3.4 Meta-analysis
3.4.1 Primary outcome
3.4.1.1 Clinical efficacy
Six studies (7, 8, 10–12, 21) reported the clinical efficacy (CE), involving six different interventions. The network plot indicated that the most frequent comparison was between NMES alone and NMES combined with AP, forming a closed loop (Figure 4). The inconsistency model test yielded a P-value of 0.19 (P > 0.05), suggesting no significant inconsistency. Local inconsistency was assessed using the node-splitting method; however, due to the incomplete network structure, the inconsistency between NMES and NMES+AP could not be evaluated. Other pairwise comparisons revealed no significant differences between direct and indirect comparisons (P > 0.05). The league table demonstrated that, compared to TST, NMES combined with ET and TST (1.42, 95% CI: 1.01, 2.00) showed statistically significant improvement in clinical efficacy for patients with neurogenic dysphagia, while NMES+AI (1.35, 95% CI: 0.98, 1.87), NMES+AP (1.32, 95% CI: 0.90, 1.93), NMES+TST (1.21, 95% CI: 0.91, 1.62), and NMES alone (1.19, 95% CI: 0.84, 1.67) exhibited no significant therapeutic differences (Figure 5). Based on SUCRA values, the ranking of interventions from highest to lowest in terms of clinical effective rate was: NMES+ET+TST (73.9%) > NMES+AI (69.3%) > NMES+AP (65.7%) > NMES+TST (47.2%) > NMES (36.2%) > TST (7.7%), indicating that the NMES+ET+TST regimen was the most effective in improving the clinical effective rate for patients with neurogenic dysphagia (Figure 6). The funnel plot for CE suggested potential publication bias, warranting cautious interpretation (Figure 7).
Figure 4
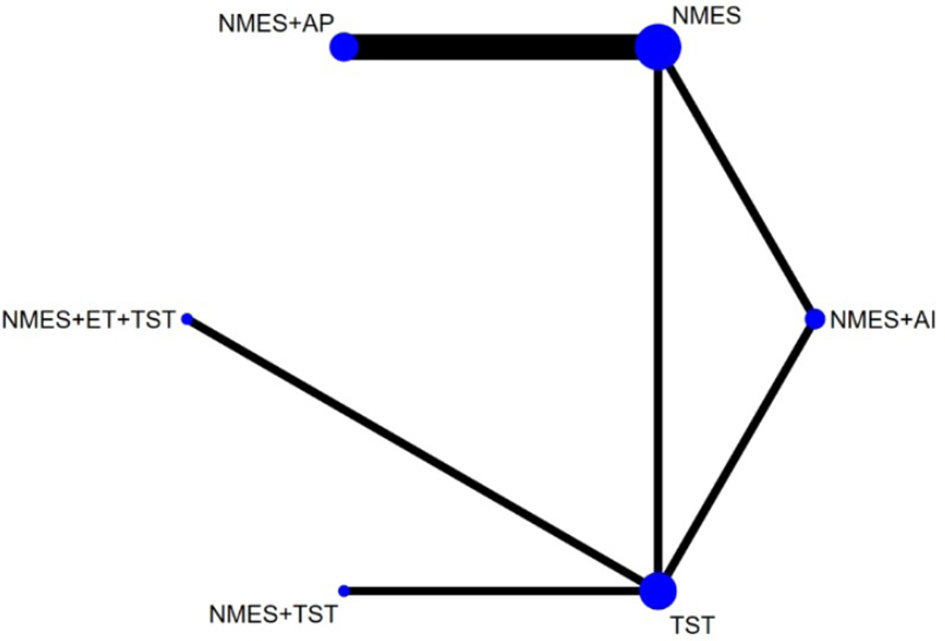
Network plot of clinical efficacy.
Figure 5

The league figure of clinical efficacy.
Figure 6

SUCRA probability ranking of clinical efficacy.
Figure 7

Comparative-corrected funnel plot of clinical efficacy.
3.4.1.2 FOIS
Ten RCTs (5, 6, 13, 16–18, 20, 23, 24) reported FOIS scores, encompassing five distinct interventions. The network plot indicated that the comparison between NMES combined with TST and TST alone was the most frequently investigated. Furthermore, the network plot formed two closed loops, as illustrated in Figure 8. Upon loop formation, initial assessment using an inconsistency model for FOIS scores yielded a P-value of 0.314 (P > 0.05). Subsequently, node-splitting analysis revealed no significant differences between direct and indirect comparisons across all nodes (P > 0.05), indicating good consistency between direct and indirect evidence. The league table suggested that, compared to TST alone, NMES combined with tDCS and TST showed a significant advantage in improving FOIS scores (1.15, 95% CI: 0.34, 1.97), as did NMES combined with TST (0.49, 95% CI: 0.23, 0.74). However, NMES combined with SSE (0.40, 95% CI: −0.41, 1.21) and NMES alone (0.16, 95% CI: −0.41, 0.73) demonstrated no significant therapeutic differences (Figure 9). The ranking of interventions based on SUCRA values for FOIS scores, from highest to lowest, was as follows: NMES+tDCS+TST (95.3%) > NMES+TST (62.4%) > NMES+SSE (51.5%) > NMES (29.2%) > TST (11.6%), suggesting that NMES+tDCS+TST appears to be the optimal combination for enhancing FOIS scores and improving swallowing function (Figure 10). Finally, the funnel plot for FOIS scores demonstrated good overall symmetry, indicating a low likelihood of publication bias in the included studies (Figure 11).
Figure 8

Network plot of FOIS.
Figure 9

The league figure of FOIS.
Figure 10
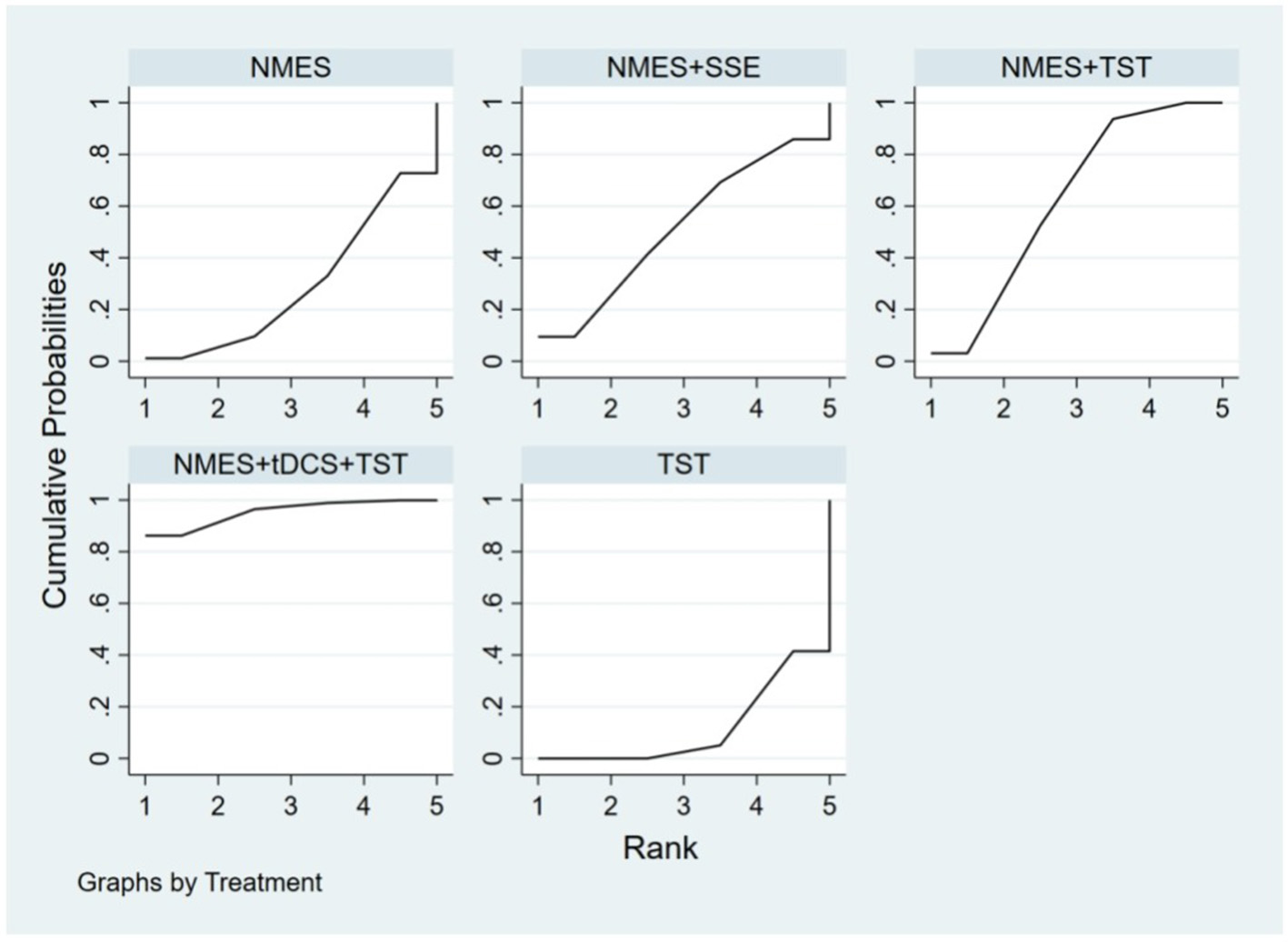
SUCRA probability ranking of FOIS.
Figure 11

Comparative-corrected funnel plot of FOIS.
3.4.1.3 SSA
Five studies (2, 7, 10, 19, 20) reported the standardized swallowing assessment scores, encompassing four distinct interventions. The network plot revealed that the most frequent comparison was between TST alone and NMES combined with TST, forming a closed loop (Figure 12). The inconsistency model test yielded a P-value of 0.61 (P > 0.05), indicating no significant inconsistency. Local inconsistency was assessed using the node-splitting method; however, due to insufficient data or an incomplete network structure, comparisons between NMES and NMES+AP, as well as NMES+TST and TST, were not available. Other pairwise comparisons showed no significant differences between direct and indirect comparisons (P > 0.05). The league table suggested that, compared to TST, NMES+AP (−1.49, 95% CI: −2.48, −0.49) and NMES+TST (−1.33, 95% CI: −1.70, −0.95) demonstrated superior efficacy in reducing SSA scores and improving swallowing function, whereas NMES (−0.20, 95% CI: −0.82, 0.43) showed no therapeutic difference (Figure 13). Based on SUCRA values, the ranking of interventions from highest to lowest was: NMES+AP (87.6%) > NMES+TST (79.0%) > NMES (24.6%) > TST (8.8%), suggesting that NMES+AP may offer significant advantages in improving swallowing function (Figure 14). The funnel plot for SSA indicated potential publication bias (Figure 15).
Figure 12

Network plot of SSA.
Figure 13

The league figure of SSA.
Figure 14

SUCRA probability ranking of SSA.
Figure 15

Comparative-corrected funnel plot of SSA.
3.4.2 Secondary outcome
3.4.2.1 PAS
Eight studies (5, 9, 14–17, 22, 24) reported Penetration-Aspiration Scale scores, involving seven distinct interventions. The network plot revealed that the comparison between TST alone and NMES combined with TST was the most frequently investigated, forming two closed loops (Figure 16). The inconsistency model test yielded a P-value of 0.25 (P > 0.05), indicating no significant inconsistency. Subsequent local inconsistency assessment using the node-splitting method showed no available data for the comparison between NMES and NMES combined with UCSM, potentially due to insufficient data or an incomplete network structure. For the remaining nodes, no significant differences were observed between direct and indirect comparisons (P > 0.05). The league table demonstrated that, compared to TST alone, NMES combined with ES and TST showed a significant advantage in reducing PAS scores and improving aspiration prevention (−1.06, 95% CI: −1.82, −0.31). In contrast, NMES+UCSM (−1.00, 95% CI: −2.35, 0.35), NMES+tDCS+TST (−0.75, 95% CI: −1.73, 0.23), NMES+TST (−0.45, 95% CI: −0.96, 0.05), NMES+Swallowing Strengthening Exercises (SSE) (−0.28, 95% CI: −1.30, 0.74), and NMES alone (−0.25, 95% CI: −1.22, 0.72) showed no significant therapeutic differences (Figure 17). The ranking of interventions based on SUCRA values for PAS scores, from highest to lowest, was as follows: NMES+ES+TST (81.2%) > NMES+UCSM (76.0%) > NMES+tDCS+TST (64.3%) > NMES+TST (46.6%) > NMES+SSE (37.1%) > NMES (31.8%) > TST (12.9%), suggesting that NMES+ES+TST may be the most effective combination for reducing aspiration risk and enhancing swallowing safety (Figure 18). The funnel plot for PAS scores exhibited good symmetry, indicating a low likelihood of publication bias (Figure 19).
Figure 16

Network plot of PAS.
Figure 17

The league figure of PAS.
Figure 18

SUCRA probability ranking of PAS.
Figure 19

Comparative-corrected funnel plot of PAS.
4 Discussion
Neurogenic dysphagia, resulting from neurological disorders including stroke and Parkinson's disease, involves central or peripheral nerve damage, neurotransmitter imbalance, impaired swallowing reflex pathways, and reduced muscular coordination. These pathophysiological changes primarily affect the oral, pharyngeal, and esophageal phases of swallowing. In the oral phase, common impairments include difficulty forming a bolus, food residue retention, and delayed oral transit time. The pharyngeal phase often presents difficulties such as impaired triggering of the swallowing reflex, delayed pharyngeal clearance, and increased swallowing attempts per bolus. Esophageal phase dysfunction is frequently characterized by esophageal motility disorders, such as lower esophageal sphincter dysfunction, impaired peristalsis, and esophageal obstruction, which can lead to symptoms like regurgitation, retrosternal discomfort, and aspiration (25). These dysphagic symptoms can predispose patients to serious complications including choking, aspiration pneumonia, and malnutrition, severely compromising their quality of life (26). Among various therapeutic interventions for dysphagia, NMES has emerged as a promising therapeutic modality. NMES facilitates the restoration of swallowing function through external electrical stimulation that activates axonal motor nerve terminals and muscle fibers, thereby promoting central nervous system recovery and strengthening impaired oropharyngeal musculature (27).
This study represents the first comprehensive network meta-analysis to systematically comparing the therapeutic efficacy of NMES combined with various interventions (including NMES+TST, NMES+tDCS+TST, NMES+AP, NMES+AI, NMES+UCSM, NMES+ES+TST, and NMES+ET+TST), standalone NMES and TST in the management of neurogenic dysphagia. The primary outcome measures included the FOIS, SSA, and clinical efficacy, with the PAS serving as a secondary outcome.
Our findings demonstrate that both combined NMES therapies, standalone NMES and TST are effective in improving swallowing function and reducing aspiration, but NMES combined with other therapies generally exhibiting superior clinical efficacy over either standalone NMES or TST. According to the efficacy ranking, NMES+tDCS+TST emerged as the most effective combination for enhancing FOIS scores. The therapeutic mechanism of tDCS involves delivering weak direct currents to the cerebral cortex through electrodes placed over targeted cortical areas, modulating brain function to enhance cortical excitability and neuroplasticity (28). Evidence suggests that tDCS particularly improves motor function in patients with post-stroke dysphagia, with anodal tDCS application over the pharyngeal motor cortex enhancing excitability to facilitate dysphagia recovery (29).
The combination of tDCS and NMES creates a synergistic effect by augmenting the brain's capacity to integrate sensory and motor inputs, promoting neuroplasticity at both cortical and peripheral levels (30). This dual-level approach enhances muscular strength while the incorporation of TST provides additional sensory feedback, improving coordination and establishing a comprehensive rehabilitation strategy.
Our findings align with and extend the current evidence base in neurogenic dysphagia management. The recent European Stroke Organization and European Society for Swallowing Disorders (ESO–ESSD) guideline (27) recognizes the potential of neuromodulation techniques like tDCS and peripheral stimulation, while underscoring the need for more robust evidence regarding their optimal application and combination. A systematic review by Wang et al. (4) concluded that transcutaneous neuromuscular electrical stimulation is a beneficial adjunctive therapy for post-stroke dysphagia, which is consistent with our observation that NMES-based therapies generally outperform traditional training alone. Furthermore, a broader literature review by Panebianco et al. (1) highlighted the complex pathophysiology of neurogenic dysphagia and the importance of multimodal interventions. Our network meta-analysis directly addresses these calls by providing a hierarchical comparison of various combination strategies, thereby offering preliminary evidence on which central-peripheral integrated approach might be most efficacious for specific swallowing outcomes.
The SSA score serves as a validated and reliable tool for evaluating swallowing function. Our findings demonstrate that the combination of NMES+AP exhibits superior efficacy in reducing SSA scores compared to both NMES+TST and standalone NMES or TST interventions. Acupuncture, an ancient Chinese medical technique, exerts its therapeutic effects through stimulation of specific acupoints, modulating the functional activities of both the central and autonomic nervous systems. This modulation enhances excitability in the swallowing centers, facilitates neural pathway restoration, and promotes circulatory improvement and tissue functional recovery (31–33). The therapeutic synergy between NMES and acupuncture can be attributed to their complementary mechanisms of action. While NMES directly enhances muscular function through peripheral nerve stimulation, acupuncture improves neural control via central nervous system regulation. This combined approach achieves dual modulation at both peripheral and central levels, synergistically promoting neuroplasticity. Consequently, this integrated therapeutic strategy results in more comprehensive improvement of swallowing function, as evidenced by the significant reduction in SSA scores.
The PAS serves as a standardized instrument for assessing the severity of aspiration. Our analysis revealed that the combined therapeutic regimen of NMES+TST+ES demonstrated significant superiority in reducing PAS scores. Effortful swallowing, a therapeutic maneuver, has been shown to effectively enhance hyoid bone movement and induce robust activation of hyoid musculature within a relatively short duration (34, 35). Training of the suprahyoid muscle group directly reduces aspiration risk and influences protective mechanisms during swallowing (36). The incorporation of effortful swallowing into NMES combined with TST creates a synergistic effect by further strengthening pharyngeal musculature and optimizing the timing of the swallowing reflex. This multimodal approach enhances both the sensitivity and coordination of the swallowing reflex, leading to significant reductions in PAS scores. Consequently, this therapeutic strategy effectively mitigates the risk of food or liquid entry into the airway and decreases the incidence of aspiration pneumonia (37).
Through this study, we conclude that central-peripheral combined stimulation therapy appears to be most effective treatment strategy. This is substantiated by the consistent superiority of combinations that simultaneously target both the central and peripheral nervous systems across primary and secondary outcomes. Specifically, the highest-ranked intervention for improving FOIS scores was NMES+tDCS+TST, where tDCS modulates cortical excitability and neuroplasticity centrally, while NMES acts peripherally to strengthen oropharyngeal musculature. Similarly, for SSA scores, the most effective regimen was NMES+AP, which integrates peripheral electrical stimulation with central neuromodulation via acupuncture. Furthermore, the combination of NMES+ES+TST, which ranked first for reducing PAS scores, incorporates a volitional central maneuver (effortful swallowing) to enhance the efficacy of peripheral stimulation and traditional training. These findings indicate that the top-performing interventions for each key outcome measure all embody the therapeutic principle of central-peripheral integration.
In summary, the combination of NMES, which targets the peripheral nervous system, with other therapies that act on the central nervous system—referred to as combined central-peripheral stimulation—demonstrates significant advantages in improving swallowing function, reducing aspiration risk, and enhancing swallowing safety. This integrated approach may be considered a preferred therapeutic strategy for the management of neurogenic dysphagia.
5 Study limitations
Several limitations should be acknowledged in the present study. First, substantial heterogeneity was observed across included studies regarding NMES parameters (including treatment frequency, duration, intensity, and repetition), patient characteristics (such as underlying etiology, site of neural injury, and disease duration), intervention protocols, and outcome measurement tools. Second, the relatively small sample sizes in some studies may have compromised statistical power to detect significant differences. Due to the limited number of studies and insufficient data, we did not perform subgroup analyses. Third, the observed funnel plot asymmetry suggests potential publication bias, which may affect the robustness of our findings. Fifth, regarding the outcome of CE, although it was defined based on significant improvement according to the original authors' criteria in the included studies, we recognize that the specific operational definitions and assessment criteria for CE varied across trials. This variability, which could include differences in the choice of assessment tools like FOIS, SSA or the thresholds for “improvement”, may introduce heterogeneity and potentially bias the pooled estimate for this outcome. The interpretation of the findings for CE should therefore consider this limitation. It is worth noting that most of the included studies lacked blinding, and the inclusion of only English and Chinese language sources may introduce certain biases.
Future research should focus on standardizing both NMES protocols and traditional swallowing training regimens to minimize heterogeneity and enhance comparability across studies. Additionally, etiology-based subgroup analyses could help identify patient populations that derive maximal benefit from specific interventions. However, current research predominantly focuses on post-stroke dysphagia, with limited evidence available for neurogenic dysphagia resulting from other causes. Therefore, large-scale, high-quality randomized controlled trials involving diverse patient populations are warranted to strengthen the evidence base.
6 Conclusions
This network meta-analysis demonstrates that NMES combination therapy is superior to NMES or TST alone. The ranking plot suggests that central-peripheral combined stimulation therapy appears to be the most effective treatment for improving swallowing function and reducing aspiration rates. However, the funnel plot for some outcome indicators suggests potential bias, necessitating cautious interpretation of these findings.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SW: Writing – original draft. ZS: Writing – original draft. TW: Formal analysis, Writing – review & editing. CL: Software, Writing – review & editing. CH: Methodology, Writing – original draft. DS: Writing – review & editing, Project administration, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Traditional Chinese Medicine Science and Technology Program of Zhejiang Province (no. 2024ZL332) and Key discipline of Rehabilitation Medicine in Tongde Hospital of Zhejiang Province (no. 2D02208).
Acknowledgments
We thank all investigators for their significant contributions to the trials in our meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1700317/full#supplementary-material
Abbreviations
NMES, neuromuscular electrical stimulation; TST, traditional swallowing training; NMA, network meta-analysis; RCTs, randomized controlled trials; FOIS, functional oral intake scale; SSA, standardized swallowing assessment; PAS, penetration-aspiration scale; CE, clinical efficacy; tDCS, transcranial direct current stimulation; SUCRCV, surface under cumulative ranking curve values; SMD, standardized mean difference; CI, confidence interval; ES, effortful swallowing; PSRF, potential scale reduction factor; AP, acupuncture; UCSM, upper cervical spine mobilization; AI, acupoint injection; ET, eating training; SSE, swallowing strengthening exercises.
References
1.
Panebianco M Marchese-Ragona R Masiero S Restivo DA . Dysphagia in neurological diseases: a literature review. Neurol Sci. (2020) 41:3067–73. doi: 10.1007/s10072-020-04495-2
2.
Li XW Li LY . Efficacy of neuromuscular electrical stimulation on Wilson's disease patients with dysphagia. J Phys Ther Sci. (2019) 31:971–4. doi: 10.1589/jpts.31.971
3.
Oh DH Park JS Kim WJ . Effect of neuromuscular electrical stimulation on lip strength and closure function in patients with dysphagia after stroke. J Phys Ther Sci. (2017) 29:1974–5. doi: 10.1589/jpts.29.1974
4.
Wang Y Xu L Wang L Jiang M Zhao L . Effects of transcutaneous neuromuscular electrical stimulation on post-stroke dysphagia: a systematic review and meta-analysis. Front Neurol. (2023) 14:1163045. doi: 10.3389/fneur.2023.1163045
5.
Bengisu S Demir N Krespi Y . Effectiveness of conventional dysphagia therapy (CDT), neuromuscular electrical stimulation (NMES), and transcranial direct current stimulation (tDCS) in acute post-stroke dysphagia: a comparative evaluation. Dysphagia. (2024) 39:77–91. doi: 10.1007/s00455-023-10595-w
6.
Cakmak ET Sen EI Doruk C Sen C Sezikli S Yaliman A . The effects of neuromuscular electrical stimulation on swallowing functions in post-stroke dysphagia: a randomized controlled trial. Dysphagia. (2023) 38:874–85. doi: 10.1007/s00455-022-10512-7
7.
Cao X Zhang HJ Xu G Ma XX Pu XL Ma WJ et al . Post-stroke dysphagia treated with four-step acupuncture therapy for opening orifices and benefiting throat combined with neuromuscular electrical stimulation: a randomized controlled trial. Zhongguo Zhen Jiu. (2023) 43:611–4. doi: 10.13703/j.0255-2930.20220826-0002
8.
Chang L He PL Zhou ZZ Li YH . Efficacy observation of dysphagia after acute stroke treated with acupuncture and functional electric stimulation. Zhongguo Zhen Jiu. (2014) 34:737–40. doi: 10.13703/j.0255-2930.2014.08.006
9.
Jeon YH Cho KH Park SJ . Effects of neuromuscular electrical stimulation (NMES) plus upper cervical spine mobilization on forward head posture and swallowing function in stroke patients with dysphagia. Brain Sci. (2020) 10:478. doi: 10.3390/brainsci10080478
10.
Liang Y Lin J Wang H Li S Chen F Chen L et al . Evaluating the efficacy of vitalstim electrical stimulation combined with swallowing function training for treating dysphagia following an acute stroke. Clinics. (2021) 76:e3069. doi: 10.6061/clinics/2021/e3069
11.
Ma FX Cao GP Li WL . Post-stroke dysphagia treated with acupoint injection combined with neural electrical stimulation. Zhongguo Zhen Jiu. (2014) 34:1169–73. doi: 10.13703/j.0255-2930.2014.12.006
12.
Ma JN Wang ZL Ning LN Yang H Xiong J . Observation on therapeutic effects of acupuncture combined with cutaneous electrical stimulation for dysphagia in patients with cerebral infarction. Zhen Ci Yan Jiu. (2015) 40:238–41. doi: 10.13702/j.1000-0607.2015.03.013
13.
Matos KC de Oliveira VF de Oliveira PLC Carvalho FA de Mesquita MRM da Silva Queiroz CG et al . Combined conventional speech therapy and functional electrical stimulation in acute stroke patients with dyphagia: a randomized controlled trial. BMC Neurol. (2022) 22:231. doi: 10.1186/s12883-022-02753-8
14.
Park J-S Oh D-H Hwang N-K Lee J-H . Effects of neuromuscular electrical stimulation in patients with Parkinson's disease and dysphagia: a randomized, single-blind, placebo-controlled trail. NeuroRehabilitation. (2018) 42:457–63. doi: 10.3233/NRE-172306
15.
Park JS Oh DH Hwang NK Lee JH . Effects of neuromuscular electrical stimulation combined with effortful swallowing on post-stroke oropharyngeal dysphagia: a randomised controlled trial. J Oral Rehabil. (2016) 43:426–34. doi: 10.1111/joor.12390
16.
Simonelli M Ruoppolo G Iosa M Morone G Fusco A Grasso MG et al . A stimulus for eating. The use of neuromuscular transcutaneous electrical stimulation in patients affected by severe dysphagia after subacute stroke: A pilot randomized controlled trial. Neurorehabilitation. (2019) 44:103–10. doi: 10.3233/NRE-182526
17.
Sproson L Pownall S Enderby P Freeman J . Combined electrical stimulation and exercise for swallow rehabilitation post-stroke: a pilot randomized control trial. Int J Lang Commun Disord. (2018) 53:405–17. doi: 10.1111/1460-6984.12359
18.
Terre R Mearin F . A randomized controlled study of neuromuscular electrical stimulation in oropharyngeal dysphagia secondary to acquired brain injury. Eur J Neurol. (2015) 22:687–E44. doi: 10.1111/ene.12631
19.
Xia W Zheng C Lei Q Tang Z Hua Q Zhang Y et al . Treatment of post-stroke dysphagia by vitalstim therapy coupled with conventional swallowing training. J Huazhong Univ Sci Technolog Med Sci. (2011) 31:73–6. doi: 10.1007/s11596-011-0153-5
20.
Zhang M Tao T Zhang ZB Zhu X Fan WG Pu LJ et al . Effectiveness of neuromuscular electrical stimulation on patients with dysphagia with medullary infarction. Arch Phys Med Rehabil. (2016) 97:355–62. doi: 10.1016/j.apmr.2015.10.104
21.
Zhang Q Wu S . Effects of synchronized neuromuscular electrical stimulation (NMES) on the submental muscles during ingestion of a specified volume of soft food in patients with mild-to-moderate dysphagia following stroke. Med Sci Monit. (2021) 27:e928988. doi: 10.12659/MSM.928988
22.
Lee SY Park D Jang J Jang EG Lee JC Park Y et al . Compensatory effects of sequential 4-channel neuromuscular electrical stimulation for the treatment of acute, subacute, and chronic dysphagia in a prospective, double-blinded randomized clinical trial. Neurorehabil Neural Repair. (2021) 35:801–11. doi: 10.1177/15459683211029891
23.
Permsirivanich W Tipchatyotin S Wongchai M Leelamanit V Setthawatcharawanich S Sathirapanya P et al . Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: a randomized controlled study. J Med Assoc Thai. (2009) 92:259–65.
24.
Huang KL Liu TY Huang YC Leong CP Lin WC Pong YP . Functional outcome in acute stroke patients with oropharyngeal Dysphagia after swallowing therapy. J Stroke Cerebrovasc Dis. (2014) 23:2547–53. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.031
25.
Sebastian S Nair PG Thomas P Tyagi AK . Oropharyngeal dysphagia: neurogenic etiology and manifestation. Indian J Otolaryngol Head Neck Surg. (2015) 67:119–23. doi: 10.1007/s12070-014-0794-3
26.
McCarty EB Chao TN . Dysphagia and swallowing disorders. Med Clin North Am. (2021) 105:939–54. doi: 10.1016/j.mcna.2021.05.013
27.
Dziewas R Michou E Trapl-Grundschober M Lal A Arsava EM Bath PM et al ., European stroke organisation and European society for swallowing disorders guideline for the diagnosis and treatment of post-stroke dysphagia. Eur Stroke J. (2021) 6:Lxxxix-cxv. doi: 10.1177/23969873211039721
28.
Boes AD Kelly MS Trapp NT Stern AP Press DZ Pascual-Leone A . Noninvasive brain stimulation: challenges and opportunities for a new clinical specialty. J Neuropsychiatry Clin Neurosci. (2018) 30:173–9. doi: 10.1176/appi.neuropsych.17110262
29.
Gómez-García N Álvarez-Barrio L Leirós-Rodríguez R Soto-Rodríguez A Andrade-Gómez E Hernández-Lucas P . Transcranial direct current stimulation for post-stroke dysphagia: a meta-analysis. J Neuroeng Rehabil. (2023) 20:165. doi: 10.1186/s12984-023-01290-w
30.
Koseki T Kudo D Yoshida K Nito M Takano K Jin M et al . Combined neuromuscular electrical stimulation and transcutaneous spinal direct current stimulation increases motor cortical plasticity in healthy humans. Front Neurosci. (2022) 16:1034451. doi: 10.3389/fnins.2022.1034451
31.
Lei LM Wu L Hu YQ Luo BH Huang JJ Su SY et al . Effects of different acupuncture treatment on mean blood flow velocity of middle cerebral artery on the affected side and rehabilitation of hemiparalysis caused by cerebral infarction. Zhongguo Zhen Jiu. (2009) 29:517–20. doi: 10.13703/j.0255-2930.2009.07.003
32.
Wang LP Xie Y . Systematic evaluation on acupuncture and moxibustion for treatment of dysphagia after stroke. Zhongguo Zhen Jiu. (2006) 26:141–6. doi: 10.13703/j.0255-2930.2006.02.028
33.
Wang P Ma X Huang J Li J Ma L Xu D et al . Effect of acupuncture treatment on dysphagia caused by pseudobulbar paralysis after stroke: a systematic review and meta-analysis. Ann Palliat Med. (2022) 11:2257–64. doi: 10.21037/apm-21-3551
34.
Wheeler-Hegland KM Rosenbek JC Sapienza CM . Submental sEMG and hyoid movement during Mendelsohn maneuver, effortful swallow, and expiratory muscle strength training. J Speech Lang Hear Res. (2008) 51:1072–87. doi: 10.1044/1092-4388(2008/07-0016)
35.
Jang HJ Leigh JH Seo HG Han TR Oh BM . Effortful swallow enhances vertical hyolaryngeal movement and prolongs duration after maximal excursion. J Oral Rehabil. (2015) 42:765–73. doi: 10.1111/joor.12312
36.
Kim JH Kim YA Lee HJ Kim KS Kim ST Kim TS et al . Effect of the combination of Mendelsohn maneuver and effortful swallowing on aspiration in patients with dysphagia after stroke. J Phys Ther Sci. (2017) 29:1967–9. doi: 10.1589/jpts.29.1967
37.
Huckabee ML Steele CM . An analysis of lingual contribution to submental surface electromyographic measures and pharyngeal pressure during effortful swallow. Arch Phys Med Rehabil. (2006) 87:1067–72. doi: 10.1016/j.apmr.2006.04.019
Summary
Keywords
dysphagia, neuromuscular electrical stimulation, traditional swallowing training, network meta-analysis, neurorehabilitation
Citation
Wang S, Shi Z, Wu T, Li C, Huang C and Sun D (2025) Comparative efficacy of combined and single neuromuscular electrical stimulation and traditional swallowing training for neurogenic dysphagia: a network meta-analysis. Front. Neurol. 16:1700317. doi: 10.3389/fneur.2025.1700317
Received
06 September 2025
Revised
02 November 2025
Accepted
07 November 2025
Published
03 December 2025
Volume
16 - 2025
Edited by
Mariagiovanna Cantone, Gaspare Rodolico Hospital, Italy
Reviewed by
Vitriana Biben, Padjadjaran University, Indonesia
Pragna Landge, Drs Kiran and Pallavi Patel Global University, India
Daniela Vieira, Fernando Pessoa Foundation, Portugal
Updates
Copyright
© 2025 Wang, Shi, Wu, Li, Huang and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Di Sun, 94384321@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.