- 1College of Stomatology, Binzhou Medical University, Yantai, Shandong, China
- 2Department of Nuclear Medicine, Weifang People's Hospital, Weifang, Shandong, China
Objectives: To evaluate whether periodontitis is associated with stroke and to update pooled estimates with recent studies.
Methods: We systematically searched Medline, Embase, and the Cochrane Database of Systematic Reviews from inception to July 2025. We included cohort and case–control studies of adults comparing periodontitis with no periodontitis and reporting any stroke (ischemic, hemorrhagic, or unspecified). Random-effects models were used. Case–control and cohort data were pooled separately. Prespecified subgroups included stroke subtype, study design, sex, and follow-up duration. Publication bias was assessed when ≥10 studies were available.
Results: Twenty-two studies were included (16 cohorts; 6 case–control). In case–control studies, periodontitis was associated with higher odds of stroke (OR, 2.22; 95% CI, 1.48–3.34; I2 = 33%). In cohorts, periodontitis was associated with increased incident stroke risk (RR, 1.49; 95% CI, 1.23–1.80; I2 = 96%). In subgroup analysis, prospective cohorts showed slightly higher and more stable estimates than retrospective cohorts (RR, 1.53 vs. 1.40; I2 = 61% vs. 98%). Studies with >10 years of follow-up showed a stronger, less heterogeneous association (RR, 1.57; 95% CI, 1.35–1.84; I2 = 32%). Funnel plots suggested limited publication bias.
Conclusions: Periodontitis is associated with increased stroke risk, most clearly for ischemic outcomes and in long-term prospective cohorts. Given high heterogeneity and potential residual confounding, the findings support association rather than causation. Standardized periodontal definitions, subtype-specific endpoints, and rigorous prospective and interventional studies are needed to test clinical impact.
Introduction
Stroke is the leading cause of long-term disability and the second leading cause of mortality across the world (1). The incidence continues to rise, with millions of new cases reported annually in both developed and developing countries (2). Traditional risk factors such as hypertension, diabetes, smoking, and obesity are well established, but they do not fully explain the occurrence of stroke. Increasing attention has been directed toward chronic inflammatory conditions as potential contributors to stroke (3, 4).
Periodontitis, one of the most prevalent chronic inflammatory diseases, affects more than 10% of people globally (5). It is initiated by a dysbiotic oral microbiota, notably pathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum, which release virulence factors that trigger systemic inflammation and endothelial dysfunction (6). Elevated levels of C-reactive protein, interleukin-6, and tumor necrosis factor-α in patients with periodontitis provide a biological link to atherosclerosis and thrombus formation (6, 7). Recent studies also implicate the oral–gut–brain axis, whereby oral microbial dysbiosis modulates gut homeostasis and immune responses, further amplifying neuroinflammation in stroke (6, 8, 9).
Epidemiology supports an association between periodontitis and stroke, with pooled relative risks ranging from 1.22 to 2.88 in previous meta-analyses (10, 11). However, findings are not uniform and heterogeneity is substantial, as limited numbers of studies and variability in study design, diagnostic criteria, and confounder adjustment have led to inconsistent results. These controversies highlight the need for an updated synthesis, especially as new studies have been published. Therefore, we conducted a systematic review and meta-analysis to quantify the association between periodontitis and stroke and to explore heterogeneity through subgroup analyses, with the aim of informing prevention and clinical practice.
Materials and methods
Data source and search strategy
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and was not previously registered. We systematically searched Medline (via Pubmed), Embase, and the Cochrane Database of Systematic Reviews (CDSR) from inception to July 2025. The search strategy was developed using the PICO framework: adults (Population), periodontitis (Exposure/Intervention), individuals without periodontitis (Comparison), and any stroke type (Outcome). Search terms included controlled vocabulary (e.g., MeSH, Emtree) and free-text words such as “periodontitis,” “periodontal disease,” “stroke,” “cerebrovascular accident,” “ischemic stroke,” and “hemorrhagic stroke.” The detailed strategies for each database are provided in the Supplementary Appendix. Additionally, reference lists of included studies and prior systematic reviews were screened manually to identify additional eligible publications. No restrictions on language or publication date were imposed.
Study eligibility and selection
We included observational studies that evaluated periodontitis as a risk factor for stroke and reported extractable association measures. Eligible designs were cohort (prospective or retrospective, with ≥1 year follow-up) and case–control studies. Participants had to be adults (≥18 years) with periodontitis defined by clinical diagnosis (e.g., probing depth, clinical attachment loss, radiographic bone loss), validated indices, ICD codes, or confirmed self-report. Controls were individuals without periodontitis or with periodontal health. Outcomes of interest were incident or prevalent stroke events (ischemic, hemorrhagic, or unspecified), confirmed by medical records, imaging, ICD codes, or validated questionnaires.
We excluded studies that: (1) involved predominantly (>50%) children or adolescent populations; (2) evaluated gingivitis, tooth loss without attribution to periodontitis, or mixed periodontal conditions without stratification; (3) exposure assessment was not performed prior to the outcome or within a very short time window (for case-control studies) thereafter (e.g., within one week); (4) assessed cardiovascular events without stroke-specific outcomes; (5) used cross-sectional, ecological, case series, or case reports; (6) were reviews, guidelines, editorials, conference abstracts, or non–peer-reviewed sources; or (7) lacked sufficient data to compute association measures. When duplicate publications existed, we retained the study with the largest sample or most comprehensive analysis.
Two authors independently screened titles, abstracts, and full texts. Disagreements were resolved through consensus or by consulting an external expert.
Data extraction and quality assessment
Two authors independently extracted data using a standardized form. Extracted information included: first author, publication year, country, study design, sample size, participant demographics, definition of periodontitis, number of teeth or sites examined, stroke subtype and diagnostic method, follow-up length, matching or adjustment variables, effect estimates (OR, RR, HR, and 95% CI), and funding source. When studies reported multiple models, we extracted the most fully adjusted estimates; if unavailable, unadjusted values were recorded.
Study quality was assessed using the Newcastle–Ottawa Scale (NOS) separately for cohort and case–control designs (12). The scale evaluates three domains: selection of study groups, comparability of groups, and ascertainment of exposure/outcome. Studies scoring >7 were considered high quality (low risk of bias), 5–7 moderate quality, and <5 low quality (high risk of bias). Disagreements were resolved by discussion or adjudication by an external expert.
Data synthesis and analysis
We synthesized odds ratios (ORs) from case–control studies and relative risks (RRs), hazard ratios (HRs), or incidence rate ratios (IRRs) from cohort studies. In cohort studies, these effect estimates were treated as equivalent measures of relative risk on the logarithmic scale and combined using the generic inverse-variance (I-V) method. If cohort studies reported ORs, we converted them to RRs using the control group risk whenever available; when baseline risk was not reported and stroke events were rare, ORs were considered approximations of RRs, in line with Cochrane recommendations. Meta-analyses of case–control studies and cohort studies were conducted separately.
Random-effects models (DerSimonian–Laird method) were used to account for between-study variability. Statistical heterogeneity was quantified using the Q statistic and I2, with I2 >75% considered substantial (13). Publication bias was assessed with funnel plot symmetry when ≥10 studies were available, supplemented by Egger's regression test (14). We further applied the Trim and Fill method to adjust for any potential publication bias. This method imputes missing studies and recalculates the pooled effect size, providing a more accurate estimate in the presence of small-study effects or publication bias. All statistical tests were two-sided with a significance level of 0.05. Meta-analyses were performed using Review Manager (RevMan, version 5.4; Cochrane Collaboration).
Subgroup analyses were performed by stroke subtype (ischemic, hemorrhagic, unspecified), study design (prospective vs. Retrospective), sex, and mean follow-up duration. Sensitivity analyses were conducted by restricting to studies with multivariable-adjusted estimates and studies with low risk of bias. An additional sensitivity analyses was conducted by restricting to studies that diagnosed periodontitis through clinical examination or radiographic assessment.
Results
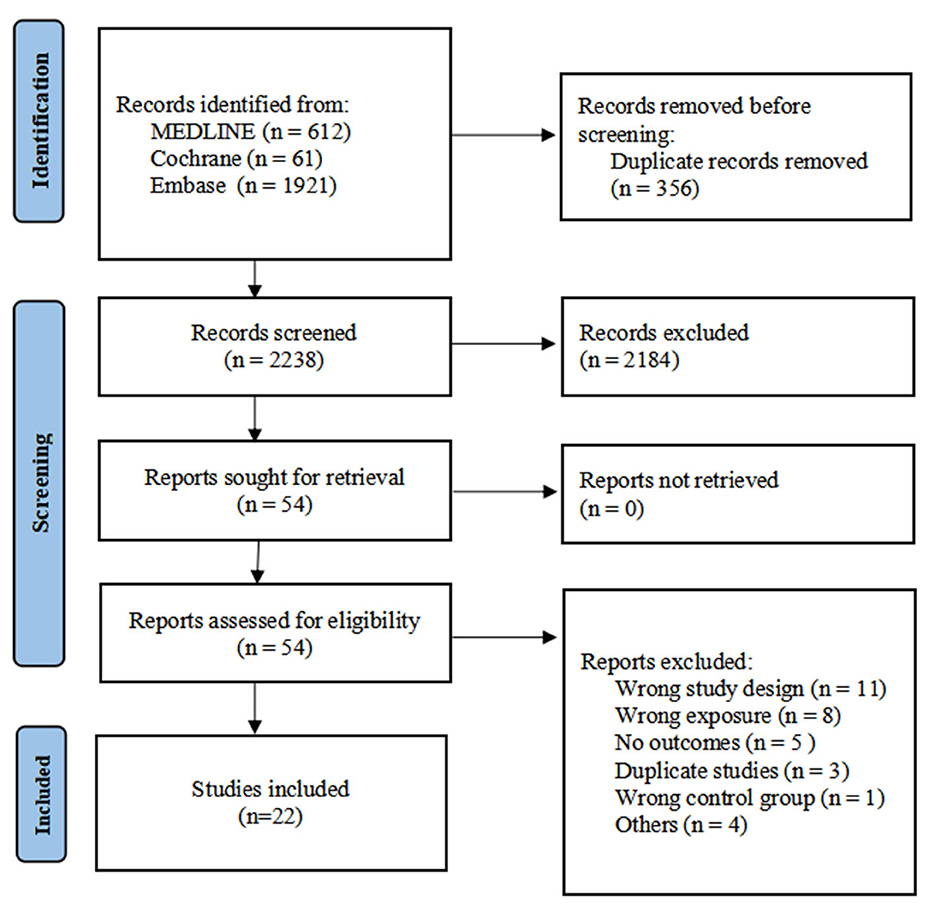
The database search identified 2,594 records. After removal of 356 duplicates, 2,238 records were screened, of which 2,184 were excluded based on title and abstract. Fifty-four full-text articles were assessed for eligibility. Twenty-two studies met the inclusion criteria and were included in the qualitative and quantitative synthesis (Figure 1).
Study characteristics
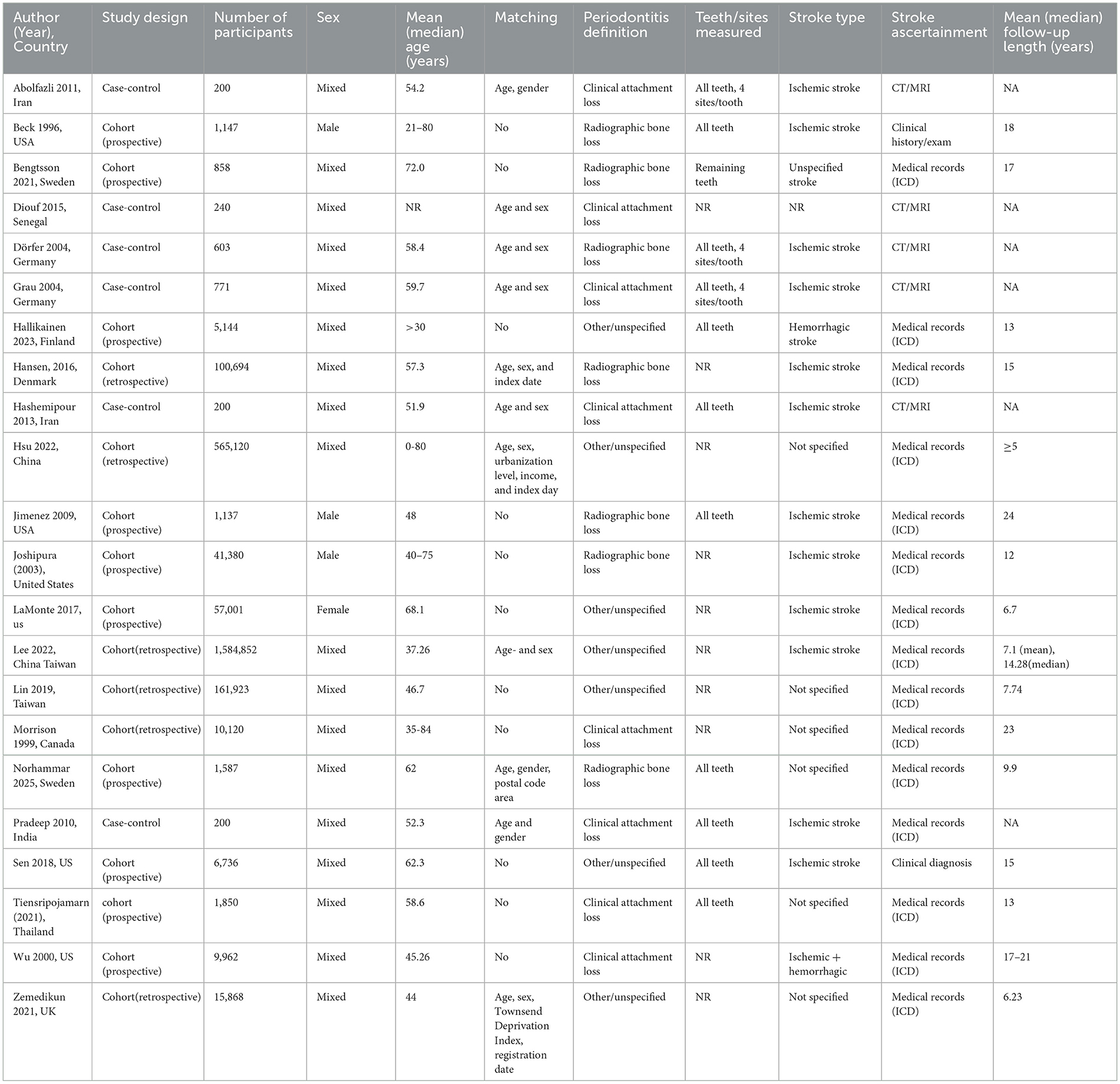
A total of 22 observational studies were included, comprising 16 cohort studies (15–30) and 6 case–control studies (31–36), with study populations ranging from 200 to over 1.5 million participants. Geographically, the studies were conducted across multiple regions: North America, (15, 20, 22, 25, 28, 29) Europe, (16, 18, 19, 26, 30, 33, 34) Asia, (17, 23, 24, 27, 31, 35, 36) and Africa (32).
In total, the included cohorts contributed more than 2.3 million participants with follow-up durations ranging from 6 to 24 years. Periodontitis was most frequently assessed using radiographic bone loss or clinical attachment loss, while some studies employed alternative criteria, including ICD codes, questionnaires, and general practitioner reports, which may introduce variability in the definition of periodontitis. The number of teeth or sites examined varied substantially across studies. Stroke outcomes included ischemic, hemorrhagic, and unspecified stroke types, confirmed primarily through medical records, ICD codes, or neuroimaging. Most case–control studies matched cases and controls on age and sex, whereas some cohort studies reported additional adjustments for cardiovascular risk factors, socioeconomic status, or comorbidities. Detailed characteristics of the included studies are presented in Table 1.
Quality assessment
Risk of bias was assessed using the Newcastle–Ottawa Scale (NOS). All 7 case–control studies were judged to be of high quality, with the main limitations related to non-response rate or ascertainment of exposure. For the 15 cohort studies, all achieved high quality ratings, with the main limitations related to non-response rate and selection of controls. A detailed summary of NOS assessments for each study is provided in Supplementary Tables 1, 2.
Association between periodontitis and stroke
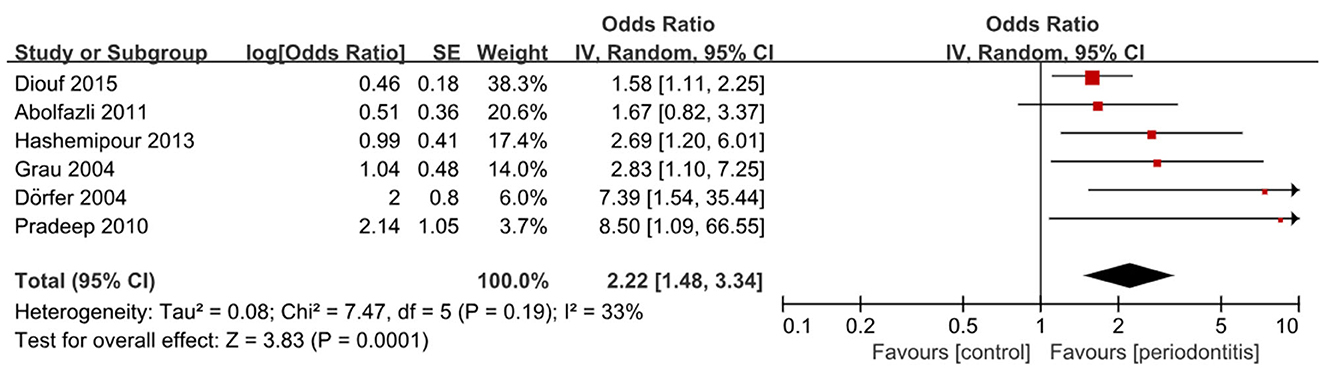
A total of 22 studies were eligible for quantitative synthesis, including 16 cohort and 6 case–control studies. Among the case–control studies, five demonstrated a significant association between periodontitis and stroke, (32–36) whereas one reported an OR greater than 1 that did not reach statistical significance (31). Pooled analysis confirmed that periodontitis was significantly associated with higher odds of stroke (6 studies; OR, 2.22; 95% CI, 1.48–3.34; P < 0.001; I2 = 33%) (Figure 2). Subgroup analysis by stroke sub-type demonstrated a stronger association for ischemic stroke (5 studies; OR, 2.66; 95% CI, 1.65–4.28; P <0.001; I2 = 12%) (Supplementary Table 3 and Supplementary Figure 1). More subgroup analysis are presented in Supplementary Figures 2, 3.
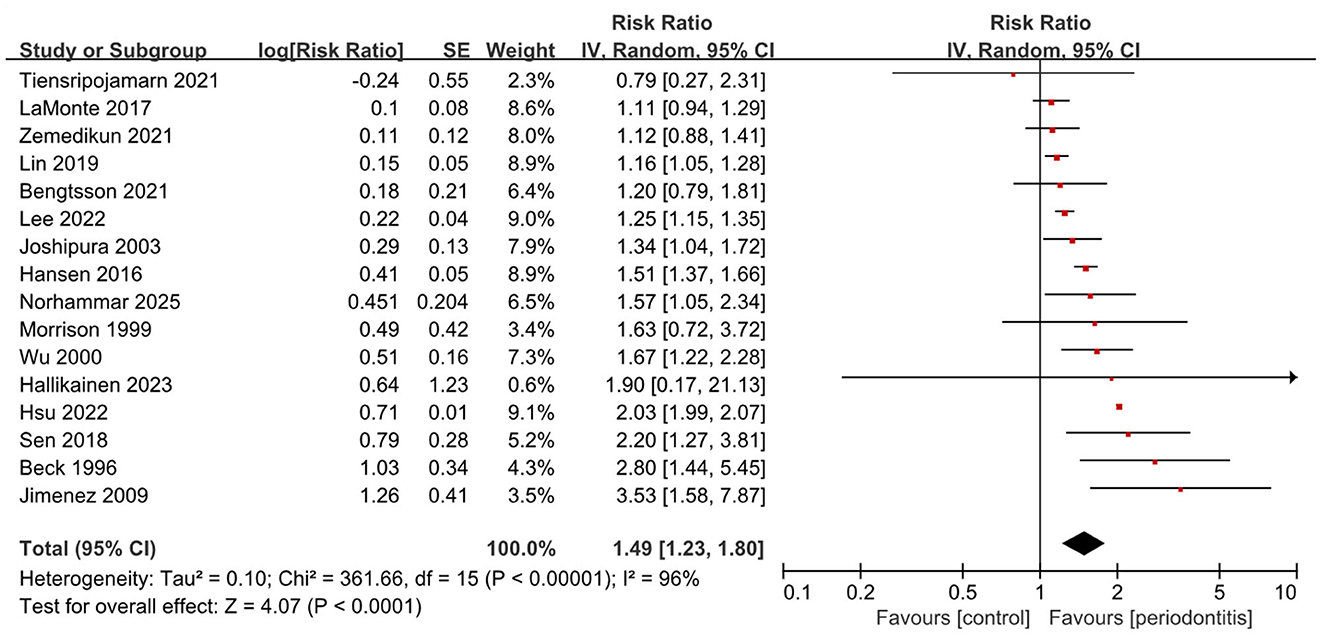
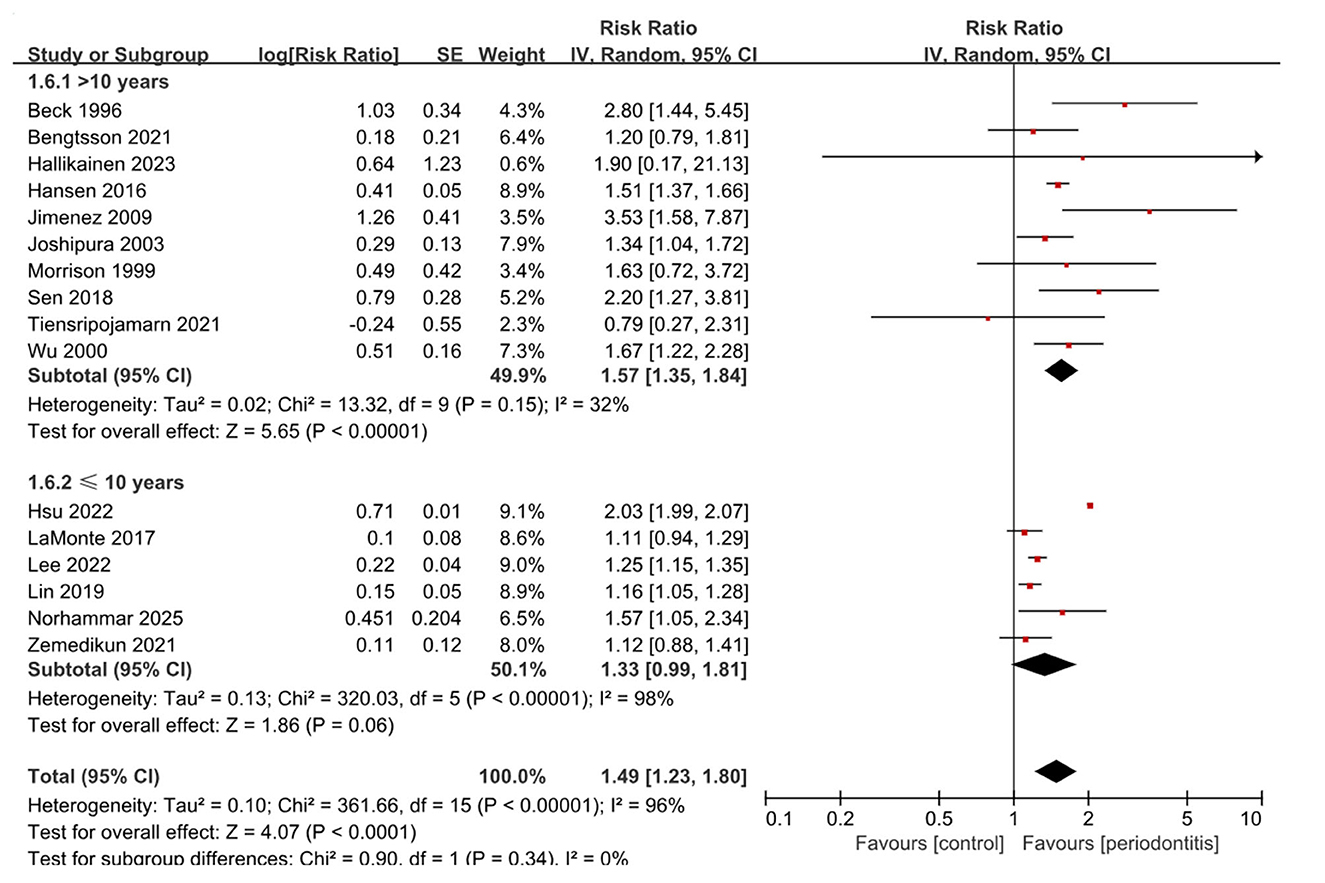
In the cohort studies, ten reported a significant association between periodontitis and the risk of incident stroke, (15, 17, 19–21, 23, 24, 26, 28, 29) while five suggested an elevated risk that did not reach significance (16, 18, 22, 25, 30). Notably, a single study conducted in Thailand involving 1,850 participants found a lower stroke risk among individuals with periodontitis compared with controls, although the difference was not statistically significant (27). Overall, meta-analysis of cohort studies indicated that periodontitis was consistently associated with an increased risk of stroke (16 studies; RR, 1.49; 95% CI, 1.23–1.80; P < 0.001; I2 = 96%) (Figure 3). Subgroup analysis by study design showed similar results in both prospective (10 studies; RR, 1.53; 95% CI, 1.23–1.90; P < 0.001; I2 = 61%) and retrospective cohorts (6 studies; RR, 1.40; 95% CI, 1.06–1.85; P = 0.02; I2 = 98%), with heterogeneity being lower in the prospective group (Supplementary Table 4 and Supplementary Figure 4). When stratified by stroke subtype (Supplementary Figure 5), the pooled association was significant for ischemic stroke (7 studies; RR, 1.44; 95% CI, 1.22–1.70; P < 0.001; I2 = 79%) and for mixed types (8 studies; RR, 1.40; 95% CI, 1.04–1.88; P = 0.03; I2 = 95%).
Notably, subgroup analysis by follow-up duration (Figure 4) highlighted a more stable association in studies with longer observation. Among 10 studies with follow-up longer than 10 years, periodontitis was associated with a 57% increased risk of stroke (RR, 1.57; 95% CI, 1.35–1.84; P < 0.001), with only moderate heterogeneity (I2 = 32%). By contrast, studies with ≤ 10 years of follow-up showed a weaker and nonsignificant association (6 studies; RR, 1.33; 95% CI, 0.99–1.81; P = 0.06; I2 = 98%). Subgroup analysis by participant sex is presented in Supplementary Figure 6.
Sensitivity analysis limiting to studies with low risk of bias did show the above results were stable. An additional sensitivity analysis excluding studies that used ICD codes or questionnaires to define periodontitis (Supplementary Figure 7) resulted in a slightly increased and less heterogeneous association between periodontitis and stroke in cohort studies (9 studies; RR, 1.53; 95% CI, 1.33–1.77; P < 0.001; I2 = 31%). This suggests that studies with more standardized diagnostic criteria for periodontitis provide more robust evidence. Other sensitivity analysis are presented in Supplementary Figures 8–10.
Publication bias
Assessment of publication bias was performed only for cohort studies, as more than 10 studies were included in the meta-analysis. Visual inspection of the funnel plot did not suggest marked asymmetry (Supplementary Figure 11). Egger's regression test indicated a potential bias (p = 0.04). The Trim and Fill method (Supplementary Figure 12) imputed two missing smal studies. After adjustment, the pooled effect size was RR 1.40 (95% CI, 1.16–1.68), showing only a slight change from the original estimate, indicating minimal impact of publication bias.
Discussion
In this systematic review and meta-analysis of 22 studies, periodontitis was associated with higher stroke risk. The pooled effects were OR 2.22 (95% CI, 1.48–3.34; I2 = 33%) in case–control studies and RR 1.49 (1.23–1.80; I2 = 96%) in cohorts. Studies with >10-year follow-up showed a more stable estimate (RR 1.57; 1.35–1.84; I2 = 32%). Our review extends earlier work by including more recent studies and prespecifying subgroup analyses to explore sources of heterogeneity.
Our findings are broadly consistent with prior syntheses but refine the magnitude and certainty. The ischemic-stroke meta-analysis by Leira et al. (37) reported larger pooled effects (cohort RR 2.52; case–control RR 3.04), likely reflecting fewer studies and earlier exposure/outcome definitions than in our datasets. Fagundes et al. (10) similarly concluded that periodontitis increases stroke risk (overall RR 1.88; 95% CI, 1.55–2.29), especially for ischemic events (case–control RR 2.72; 95% CI, 2.00–3.71). By contrast, a 2023 cardiovascular meta-analysis that included stroke reported a smaller association (RR 1.26; 95% CI, 1.15–1.37), aligning with our more conservative cohort estimate and suggesting that broader, later cohorts tend to attenuate effects (38). A recent study by Asmat-Abanto et al. (39) found that periodontitis was a risk factor for ischemic stroke (overall OR 2.59; 95% CI, 1.90–3.53). The authors included only 16 primary studies and noted significant heterogeneity even after subgrouping. Evidence regarding hemorrhagic stroke remains sparse and inconclusive, reinforcing separation by stroke type in evidence appraisal (40). Our study adds to this literature by including a larger number of studies and multiple prespecified subgroup analyses.
Biological pathways plausibly link periodontitis to stroke. Chronic oral dysbiosis sustains systemic inflammation (CRP, IL-6, TNF-α), impairs endothelial function, and promotes thrombosis; P. gingivalis gingipains can activate platelets and coagulation, providing a bridge to atherosclerotic events (41). Contemporary work on the oral–gut–brain axis further outlines how periodontal pathogens or their products (e.g., LPS, outer-membrane vesicles) translocate via blood or vagal routes, seed gut dysbiosis, and skew immune homeostasis, thereby amplifying cerebrovascular vulnerability (6). Experimental and translational studies indicate that P. gingivalis and F. nucleatum can promote atherosclerotic plaque and alter blood–brain barrier integrity, supporting a chain from periodontal inflammation to ischemic injury (42, 43). Genetic evidence adds specificity: Mendelian randomization suggests a potential causal effect of chronic periodontitis on cardioembolic stroke, consistent with a pro-thrombotic/inflammatory pathway (44). These lines of evidence are consistent with our stronger association in ischemic outcomes and in studies with longer follow-up.
Our subgroup analyses show a clearer pattern by study design: prospective cohorts yielded slightly higher and more stable estimates than retrospective studies (prospective RR = 1.53, 95% CI 1.23–1.90; I2 = 61% vs. retrospective RR = 1.40, 1.06–1.85; I2 = 98%). Longer observation strengthened this pattern (>10-year follow-up RR = 1.57, 1.35–1.84; I2 = 32%), supporting temporality and echoing earlier calls for better prospective evidence. By contrast, stroke subtype effects were broadly comparable in magnitude in our data (ischemic RR 1.44, 95%CI 1.22–1.70; mixed-type RR 1.40, 95%CI 1.04–1.88), whereas hemorrhagic stroke evidence remained sparse and imprecise, consistent with a recent focused review (40). These design-related differences likely reflect measurement and timing: retrospective cohorts often rely on single ICD codes or self-report and cannot track periodontal change, whereas prospective designs define exposure before the event and better capture latency (45). The stronger and more stable estimate with >10 years of follow-up argues against short-term reverse causation and is compatible with a slow process in which chronic inflammation and pro-thrombotic changes accumulate (46). Although the magnitudes for ischemic and other stroke were similar in our estimates, recent work suggests that not all ischemic subtypes behave the same: the association appears clearer for lacunar infarction with very low heterogeneity, which supports a small-vessel pathway (39). The higher estimate in male-only analyses should be interpreted with caution; residual confounding by smoking or socioeconomic status may remain even after adjustment.
Much of the between-study variation likely arises from how periodontitis and stroke were measured. Some cohorts used a single ICD code or self-report, while others used full-mouth clinical charts or radiographs. Newer European Federation of Periodontology/American Academy of Periodontology (EFP/AAP) 2018 criteria also differ from earlier case definitions and may change case finding. These differences can cause non-differential misclassification and pull effects toward the null (47, 48). Outcome ascertainment (incident vs. prevalent stroke; imaging vs. codes) and uneven adjustment for smoking, diabetes, BMI, socioeconomic status, and oral-hygiene behaviors may further contribute to heterogeneity. Publication bias appeared limited in our data, consistent with a null intercept on Egger's test (49).
For clinicians, periodontitis should be considered a modifiable associated factor in vascular risk management, especially in patients at high ischemic risk. Interventional studies show that periodontal therapy can improve endothelial function; a recent meta-analysis reported consistent gains in flow-mediated dilation after treatment (50). New trial data suggest that intensive periodontal therapy may slow carotid intima–media thickening, supporting a vascular mechanism, although longer multicenter trials are needed (51). Observational data from a nationwide Korean database further indicate that regular dental scaling is linked with lower stroke incidence among patients with moderate-to-severe periodontal disease (52). These findings align with our stronger association in long follow-up cohorts, but they should not be over-interpreted as proof of causality.
Future research should standardize and repeat periodontal assessments using the 2018 AAP/EFP framework, use incident stroke endpoints confirmed by imaging or reliable registries, and predefine stroke subtypes—including small-vessel disease—given the clearer lacunar signal in recent reviews. It should also test whether periodontal treatment reduces vascular events through randomized or quasi-experimental studies, together with Mendelian randomization and mediation analyses to clarify inflammatory and endothelial pathways.
In addition, future research should also consider exploring the relationship between gum disease and stroke outcomes. Specifically, it would be valuable to investigate whether periodontitis influences the prognosis of stroke patients after interventions such as thrombectomy, tissue plasminogen activator treatment, or craniotomy. Examining the potential impact of periodontitis on recovery and long-term health outcomes in stroke patients could provide important insights into its clinical relevance.
Our review has several limitations. First, heterogeneity was high in cohort syntheses (I2 up to 96%), reflecting wide variation including periodontitis definitions, teeth/sites examined, follow-up duration, and stroke ascertainment. Excluding studies with less precise diagnostic criteria (e.g., ICD codes, questionnaires) significantly reduced heterogeneity, highlighting that diagnostic variability is a major source of heterogeneity. Second, exposure misclassification is likely because most cohorts measured periodontal status once and did not track progression; non-differential error would bias effects toward the null, while differential error cannot be excluded. Third, residual confounding is unavoidable: adjustment sets differed across studies, and smoking, diabetes, BMI, socioeconomic status, and oral-hygiene behaviors were not uniformly controlled. Fourth, some effect measures required conversion (e.g., OR to RR for rare events); although results were robust in sensitivity analyses, model choice and rare-event handling may affect magnitude. Fifth, certain subgroups were underpowered (hemorrhagic stroke, female-only cohorts). Sixth, we analyzed study-level data rather than individual participant data, so consistent covariate adjustment and mediation testing were not possible. Finally, the review was not prospectively registered, which may introduce selection or reporting bias despite our predefined protocol and team adjudication. However, our study has strengths: this study comprised an ever large number of studies comparing with previous systematic review and meta-analysis. The pooled sample was large and geographically diverse.
Conclusion
Periodontitis is associated with a higher risk of stroke in observational studies, with more stable estimates in long-term prospective cohorts. The association appears most evident for ischemic outcomes and is biologically plausible through inflammatory, endothelial, and pro-thrombotic pathways. Given high heterogeneity and potential residual confounding, these findings should be interpreted with caution. Standardized exposure definitions, subtype-specific endpoints, and well-designed interventional and causal studies are needed to determine whether improving periodontal health can reduce stroke risk.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XM: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. XC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2025.1700946/full#supplementary-material
References
1. Feigin VL, Nguyen G, Cercy K, Johnson CO, Alam T, Parmar PG, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
2. Cheng Y, Lin Y, Shi H, Cheng M, Zhang B, Liu X, et al. Projections of the stroke burden at the global, regional, and national levels up to 2050 Based on the Global Burden of Disease Study (2021). J Am Heart Assoc. (2024) 13:e036142. doi: 10.1161/JAHA.124.036142
3. Qiu X, Huang Y, Wei C, Wang Q. Association of systemic immune-inflammation index on the risk of stroke in American adults. Innov Aging. (2024) 8:1023. doi: 10.1093/geroni/igae098.3294
4. Khan A, Azzam MA. Inflammatory bowel disease and stroke: exploring hidden vascular risks. Cureus. (2025) 17:e79304. doi: 10.7759/cureus.79304
5. Chen MX, Zhong YJ, Dong QQ, Wong HM, Wen YF. Global, regional, and national burden of severe periodontitis, 1990-2019: an analysis of the Global Burden of Disease Study 2019. J Clin Periodontol. (2021) 48:1165–88. doi: 10.1111/jcpe.13506
6. Zhong Y, Kang X, Bai X, Pu B, Smerin D, Zhao L, et al. The oral-gut-brain axis: the influence of microbes as a link of periodontitis with ischemic stroke. CNS Neurosci Ther. (2024) 30:e70152. doi: 10.1111/cns.70152
7. Almeida A, Fagundes N, Maia LC, Lima RR. Is there an association between periodontitis and atherosclerosis in adults? a systematic review. Curr Vasc Pharmacol. (2018) 16:569–82. doi: 10.2174/1570161115666170830141852
8. Ma B, Chan T, Lo B. Unveiling the hidden culprit: how the brain-gut axis fuels neuroinflammation in ischemic stroke. Surg Neurol Int. (2024) 15:394. doi: 10.25259/SNI_703_2024
9. Kerstens R, Ng YZ, Pettersson S, Jayaraman A. Balancing the oral-gut-brain axis with diet. Nutrients. (2024) 16:3206. doi: 10.3390/nu16183206
10. Fagundes N, Almeida A, Vilhena K, Magno MB, Maia LC, Lima RR. Periodontitis as a risk factor for stroke: a systematic review and meta-analysis. Vasc Health Risk Manag. (2019) 15:519–32. doi: 10.2147/VHRM.S204097
11. Saleh A, Sahili M, Jrad I, Mortada F, El Masri J, Al Chaar S, et al. The association between periodontitis, gingivitis, tooth loss and stroke: an umbrella study with meta-analysis. Brain Sci. (2024) 15:10. doi: 10.3390/brainsci15010010
12. GA Wells BSDO. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2022). Available online at: http://wwwohrica/programs/clinical_epidemiology/oxfordasp (Accessed April 25, 2022).
13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
14. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
15. Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. (1996) 67 Suppl 10S:1123–37. doi: 10.1902/jop.1996.67.10s.1123
16. Bengtsson VW, Persson GR, Berglund JS, Renvert S. Periodontitis related to cardiovascular events and mortality: a long-time longitudinal study. Clin Oral Investig. (2021) 25:4085–95. doi: 10.1007/s00784-020-03739-x
17. Hsu PW, Shen YW, Syam S, Liang WM, Wu TN, Hsu JT, et al. Patients with periodontitis are at a higher risk of stroke: a Taiwanese cohort study. J Chin Med Assoc. (2022) 85:1006–10. doi: 10.1097/JCMA.0000000000000797
18. Hallikainen J, Pessi T, Vehkalahti M, Suominen AL, Pyysalo M, Frösen J. Unlike severe periodontitis, caries does not associate with intracranial aneurysms or aneurysmal subarachnoid hemorrhage. Acta Neurochir. (2023) 165:169–75. doi: 10.1007/s00701-022-05406-4
19. Hansen GM, Egeberg A, Holmstrup P, Hansen PR. Relation of Periodontitis to Risk of Cardiovascular and All-Cause Mortality (from a Danish Nationwide Cohort Study). Am J Cardiol. (2016) 118:489–93. doi: 10.1016/j.amjcard.2016.05.036
20. Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol. (2009) 66:505–12. doi: 10.1002/ana.21742
21. Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. (2003) 34:47–52. doi: 10.1161/01.STR.0000052974.79428.0C
22. LaMonte MJ, Genco RJ, Hovey KM, Wallace RB, Freudenheim JL, Michaud DS, et al. History of periodontitis diagnosis and edentulism as predictors of cardiovascular disease, stroke, and mortality in postmenopausal women. J Am Heart Assoc. (2017) 6:908. doi: 10.1161/JAHA.116.004518
23. Lee YT, Tsai CF, Yen YC, Huang LK, Chao SP, Hu LY, et al. Periodontitis is a potential risk factor for transient ischemic attack and minor ischemic stroke in young adults: a nationwide population-based cohort study. J Periodontol. (2022) 93:1848–56. doi: 10.1002/JPER.21-0528
24. Lin HW, Chen CM, Yeh YC, Chen YY, Guo RY, Lin YP, et al. Dental treatment procedures for periodontal disease and the subsequent risk of ischaemic stroke: a retrospective population-based cohort study. J Clin Periodontol. (2019) 46:642–49. doi: 10.1111/jcpe.13113
25. Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. (1999) 6:7–11. doi: 10.1177/204748739900600102
26. Norhammar A, Näsman P, Buhlin K, de Faire U, Ferrannini G, Gustafsson A, et al. Does periodontitis increase the risk for future cardiovascular events? long-term follow-up of the PAROKRANK study. J Clin Periodontol. (2025) 52:16–23. doi: 10.1111/jcpe.14064
27. Tiensripojamarn N, Lertpimonchai A, Tavedhikul K, Udomsak A, Vathesatogkit P, Sritara P, et al. Periodontitis is associated with cardiovascular diseases: a 13-year study. J Clin Periodontol. (2021) 48:348–56. doi: 10.1111/jcpe.13418
28. Sen S, Giamberardino LD, Moss K, Morelli T, Rosamond WD, Gottesman RF, et al. Periodontal disease, regular dental care use, and incident ischemic stroke. Stroke. (2018) 49:355–62. doi: 10.1161/STROKEAHA.117.018990
29. Wu T, Trevisan M, Genco RJ, Dorn JP, Falkner KL, Sempos CT. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med. (2000) 160:2749–55. doi: 10.1001/archinte.160.18.2749
30. Zemedikun DT, Chandan JS, Raindi D, Rajgor AD, Gokhale KM, Thomas T, et al. Burden of chronic diseases associated with periodontal diseases: a retrospective cohort study using UK primary care data. BMJ Open. (2021) 11:e048296. doi: 10.1136/bmjopen-2020-048296
31. Abolfazli N, Ghoreishizadeh A, Ayramlu H, Ghavimi M, Ghoreishizadeh M, Salehsaber F, et al. Periodontal Disease and Risk of Cerebral Ischemic Stroke. J Neurol Sci. (2011) 28:307–16.
32. Diouf M, Basse A, Ndiaye M, Cisse D, Lo CM, Faye D. Stroke and periodontal disease in Senegal: case-control study. Public Health. (2015) 129:1669–73. doi: 10.1016/j.puhe.2015.02.033
33. Dörfer CE, Becher H, Ziegler CM, Kaiser C, Lutz R, Jörss D, et al. The association of gingivitis and periodontitis with ischemic stroke. J Clin Periodontol. (2004) 31:396–401. doi: 10.1111/j.1600-051x.2004.00579.x
34. Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, et al. Periodontal disease as a risk factor for ischemic stroke. Stroke. (2004) 35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D
35. Hashemipour MA, Afshar AJ, Borna R, Seddighi B, Motamedi A. Gingivitis and periodontitis as a risk factor for stroke: a case-control study in the Iranian population. Dent Res J. (2013) 10:613–9.
36. Pradeep AR, Hadge P, Arjun Raju P, Shetty SR, Shareef K, Guruprasad CN. Periodontitis as a risk factor for cerebrovascular accident: a case-control study in the Indian population. J Periodontal Res. (2010) 45:223–8. doi: 10.1111/j.1600-0765.2009.01220.x
37. Leira Y, Seoane J, Blanco M, Rodríguez-Yáñez M, Takkouche B, Blanco J, et al. Association between periodontitis and ischemic stroke: a systematic review and meta-analysis. Eur J Epidemiol. (2017) 32:43–53. doi: 10.1007/s10654-016-0170-6
38. Guo X, Li X, Liao C, Feng X, He T. Periodontal disease and subsequent risk of cardiovascular outcome and all-cause mortality: a meta-analysis of prospective studies. PLoS One. (2023) 18:e0290545. doi: 10.1371/journal.pone.0290545
39. Ajalcriña-Martensen AA, Minchón-Medina CA, Astigueta-Perez J, Gamboa-Vicente WG, Ganoza-Larrea LJ, Asmat-Abanto AS. Is periodontitis a risk factor for ischemic stroke? Systematic review and meta-analysis. J Clin Exp Dent. (2025) 17:e329–40 doi: 10.4317/jced.62538
40. Tsimpiris A, Tsolianos I, Grigoriadis A, Tsimtsiou Z, Goulis DG, Grigoriadis N, et al. Association of chronic periodontitis with hemorrhagic stroke: a systematic review and meta-analysis. Eur J Dent. (2025) 19:265–74. doi: 10.1055/s-0044-1793844
42. Park S, Kim I, Han SJ, Kwon S, Min EJ, Cho W, et al. Oral Porphyromonas gingivalis infection affects intestinal microbiota and promotes atherosclerosis. J Clin Periodontol. (2023) 50:1553–67. doi: 10.1111/jcpe.13864
43. Polak D, Wilensky A, Shapira L, Halabi A, Goldstein D, Weiss EI, et al. Mouse model of experimental periodontitis induced by Porphyromonas gingivalis/Fusobacterium nucleatum infection: bone loss and host response. J Clin Periodontol. (2009) 36:406–10. doi: 10.1111/j.1600-051X.2009.01393.x
44. Ma C, Wu M, Gao J, Liu C, Xie Y, Lv Q, et al. Periodontitis and stroke: a mendelian randomization study. Brain Behav. (2023) 13:e2888. doi: 10.1002/brb3.2888
45. Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. (2009) 113:c214-7 doi: 10.1159/000235241
46. Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. (2003) 34:2518–32 doi: 10.1161/01.STR.0000089015.51603.CC
47. Du M, Mo Y, Li A, Ge S, Peres MA. Assessing the surveillance use of 2018 EFP/AAP classification of periodontitis: a validation study and clustering analysis. J Periodontol. (2023) 94:1254–65. doi: 10.1002/JPER.23-0088
48. Botelho J, Machado V, Proenca L, Mendes JJ. The 2018 periodontitis case definition improves accuracy performance of full-mouth partial diagnostic protocols. Sci Rep. (2020) 10:7093. doi: 10.1038/s41598-020-63700-6
49. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
50. Lyu J, Zhang Y, Zhou R, Ding C, Ye H, Fang Q, et al. The effect of periodontal treatments on endothelial function in degrees of periodontitis patients: a systematic review and meta-analysis. PLoS ONE. (2024) 19:e0308793. doi: 10.1371/journal.pone.0308793
51. Orlandi M, Masi S, Lucenteforte E, Bhowruth D, Malanima MA, Darbar U, et al. Periodontitis treatment and progression of carotid intima-media thickness: a randomized trial. Eur Heart J. (2025) ehaf555. doi: 10.1093/eurheartj/ehaf555
Keywords: periodontitis, stroke risk assessment, systematic review, meta-analysis, ischemic stroke
Citation: Meng X and Chen X (2025) Periodontitis and risk of stroke: a systematic review and meta-analysis of observational studies. Front. Neurol. 16:1700946. doi: 10.3389/fneur.2025.1700946
Received: 08 September 2025; Accepted: 09 October 2025;
Published: 03 November 2025.
Edited by:
Wen-Jun Tu, Capital Medical University, ChinaReviewed by:
Minchon Carlos, Universidad Nacional de Trujillo, PeruMichael Feldman, University of Oklahoma, United States
Copyright © 2025 Meng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohua Chen, Mjc2MDM3MDU4MUBxcS5jb20=
 Xinyu Meng1
Xinyu Meng1 Xiaohua Chen
Xiaohua Chen