- 1Department of Neurology, The Quzhou Affiliated Hospital of Wenzhou Medical University (Quzhou People’s Hospital), Quzhou, Zhejiang, China
- 2Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 3Department of Gerontology, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People's Hospital, Quzhou, China
Background: The monocyte-to-albumin ratio (MAR) integrates systemic inflammation and nutritional status derived from routine laboratory data. We assessed whether the admission MAR is associated with 3-month functional outcomes following acute ischemic stroke (AIS).
Methods: We conducted a single-center, retrospective cohort study of consecutive adults with AIS admitted within 3 days of symptom onset (October 2023–March 2024). MAR was calculated from the admission monocyte counts and serum albumin levels. The primary outcome was poor 3-month functional status, defined as a modified Rankin Scale (mRS) score ≥3. Associations between MAR and outcomes were examined using multivariable logistic regression (with and without adjustment), smooth curve fitting, and prespecified subgroup analyses (sex, age, smoking status, drinking status, hypertension, diabetes status, eGFR, and TOAST subtype).
Results: Among 395 patients (mean age 66.2 years; 34.7% female), 59 (14.9%) had poor outcomes. A higher admission MAR independently predicted poor outcomes: per 1-unit increase, the adjusted odds ratio (OR) was 1.13 (95% CI 1.07–1.20; p < 0.001). Compared with the low tertile, patients with the high tertile had significantly greater odds (OR 3.21; 95% CI 1.25–8.20) with a linear trend (P for trend = 0.006). Smooth curve fitting demonstrated a largely monotonic increase in risk across the observed MAR range. Associations were consistent across subgroups with no significant interactions (all interactions p > 0.05). With respect to the TOAST subtype, the MAR remained significant for large-artery atherosclerosis (OR 1.10; 95% CI 1.02–1.20) and small-artery occlusion (OR 1.23; 95% CI 1.07–1.42), but not for cardioembolism.
Conclusion: The admission MAR is independently and positively associated with poor 3-month functional outcomes after AIS. MAR is a promising tool for early risk assessment when it is integrated with established predictors.
1 Introduction
Acute ischemic stroke (AIS) remains a leading cause of death and long-term disability worldwide, imposing substantial individual and societal burdens through high mortality, recurrent events, and persistent functional impairment (1). With aging populations and persistent gaps in primary prevention, the global incidence and prevalence of AIS remain high, contributing to considerable years of life lost and disability-adjusted life years. Despite advances in reperfusion therapies and secondary prevention, early risk stratification to anticipate functional outcomes continues to be a clinical priority. Consequently, early and accurate risk stratification is a clinical imperative to guide acute management, optimize resource allocation, tailor secondary prevention strategies, and inform rehabilitation planning.
Accessible, inexpensive blood-based biomarkers that capture the host inflammatory response have attracted considerable interest in stroke prognostication (2, 3). Hematologic indices such as the remnant cholesterol inflammatory index are independent predictors of unfavorable 3-month outcomes (4). Among circulating leukocytes, monocytes are key effectors of innate immunity and inflammation after cerebral ischemia (5). These cells infiltrate ischemic tissues, amplify cytokine cascades, and contribute to tissue remodeling (5). Serum albumin appears to be neuroprotective during acute stroke, exerting multiple benefits including maintaining intravascular volume, exerting antioxidant and anti-inflammatory effects, and inhibiting platelet aggregation. Additionally, albumin serves as a marker of nutritional status and systemic inflammation, both of which are linked to adverse outcomes (6–8). Clinically, lower serum albumin levels predict worse functional outcomes and higher mortality after AIS (9).
Numerous studies have validated the prognostic utility of inflammation-based indices, such as the neutrophil-to-lymphocyte ratio (NLR), and nutrition-based indices, such as the prognostic nutritional index (PNI). However, these markers typically capture distinct aspects of the poststroke systemic response (10, 11). A biomarker that simultaneously integrates both the pro-inflammatory cellular response and the body’s nutritional and anti-inflammatory reserves could provide a more holistic prognostic assessment. The monocyte-to-albumin ratio (MAR) integrates these two complementary dimensions into a single metric calculated from routine tests. As a composite inflammatory index, the MAR has emerged as a prognostic marker across diverse conditions. A higher MAR independently predicted long-term mortality after percutaneous coronary intervention, underscoring its relevance in atherosclerotic cardiovascular disease (12). In acute brain injury, admission MAR was strongly associated with hematoma expansion after spontaneous intracerebral hemorrhage, a pathophysiological process similarly driven by early inflammatory responses (13). Beyond the cardiovascular and neurovascular fields, the MAR has also been linked to outcomes in patients with chronic liver disease, supporting its role as a general marker of systemic inflammation (14). Despite these advances, whether the MAR measured at admission is associated with functional prognosis after AIS still remains to be established.
Given the central role of monocyte-mediated inflammation in ischemic brain injury and the protective, anti-inflammatory functions of albumin, we hypothesized that a higher MAR would be related to worse 3-month functional outcomes after AIS. Accordingly, we investigated whether the admission MAR is associated with 3-month functional outcomes in AIS patients, with the aim of determining its incremental prognostic value and assessing its utility in identifying patients at increased risk of poor recovery.
2 Methods
2.1 Study design
This study is a single-center retrospective cohort study of consecutive adult AIS patients admitted from October 2023 to March 2024. The protocol complied with the Declaration of Helsinki and was approved by the Institutional Review Board. Owing to the retrospective design and de-identified data, the requirement for informed consent was waived.
2.2 Study population
The inclusion criteria were: age ≥18 years; AIS was identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 433 and 434, and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code I63 and confirmed by neuroimaging (CT or MRI) that excluded hemorrhage and identified an acute ischemic lesion consistent with the clinical presentation; admission within 3 days of symptom onset; availability of admission laboratory tests including monocyte count and serum albumin; documented National Institutes of Health Stroke Scale (NIHSS) score at admission; and documented the modified Rankin scale (mRS) score at admission and at 3 months. The exclusion criteria included active infection, hematologic disorders, hepatic insufficiency, and receipt of intravenous thrombolysis or mechanical thrombectomy.
2.3 Measurement of MAR
Peripheral venous blood samples were collected at admission as part of routine clinical care, generally within 24 h. The monocyte count was measured at 10^9/L using an automated hematology analyzer, and serum albumin concentration was measured in g/L using standardized biochemical methods in the hospital’s central laboratory. MAR was calculated as MAR = the ratio of monocyte count × 1,000 to the albumin level.
2.4 Outcomes
Functional status at 3 months post-stroke was evaluated using the mRS by trained personnel, either during an outpatient visit or through a standardized telephone interview. A poor outcome was defined as an mRS score ≥3 (i.e., 3–6), whereas a good outcome was defined as an mRS score of 0–2.
2.5 Covariates
Covariate selection was guided by clinical relevance and prior literature on AIS prognosis. The following variables were considered as potential confounders and/or effect modifiers and were extracted from the medical records at baseline unless stated otherwise: Demographics/behaviors: age, sex, current smoking status, and current drinking status. Vascular risk factors/comorbidities: diabetes mellitus, hypertension, and atrial fibrillation. Stroke characteristics: baseline NIHSS score; ischemic stroke etiology per TOAST classification, including large-artery atherosclerosis (LAA), cardioembolism (CE), small-artery occlusion (SAO), stroke of other determined etiology (SOE), and stroke of undetermined etiology (SUE). Renal function and lipid profile: estimated glomerular filtration rate (eGFR), triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels. Additional descriptive laboratory indices included white/red blood cell counts, platelet count, hemoglobin, globulin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen, creatinine, fasting blood glucose, and C-reactive protein (CRP).
2.6 Statistical analysis
Baseline features were summarized overall and across MAR tertiles. Depending on distribution, continuous variables are expressed as the mean ± SD or median (min–max) and were compared via one-way ANOVA or the Kruskal–Wallis test. Categorical data are summarized as counts (percentages) and were compared using χ2 tests. The amount of missing data for the variables included in the final regression models was minimal (<5%); therefore, any patient records with missing values for these variables were excluded from the analysis.
Associations between candidate variables and poor 3-month outcomes were first examined using univariate logistic regression. The association between the MAR and poor 3-month outcomes was assessed using logistic regression under three prespecified models: Model 1: unadjusted; Model 2: adjusted for age, sex, smoking status, and alcohol consumption status; Model 3: further adjusted for hypertension, diabetes mellitus, TOAST subtype, eGFR, baseline NIHSS, and LDL-C. The MAR was analyzed as a continuous predictor (per 1-unit increase) and by tertiles (low as reference). A linear trend across tertiles was evaluated by assigning each tertile to its median and entering that value as a continuous variable. To explore potential nonlinearity between continuous MAR and the probability of poor outcome, we fitted a smoothed curve using restricted cubic splines within Model 3. Prespecified stratified analyses examined the consistency of associations across strata of sex, age group (≤65 vs. >65 years), smoking status, alcohol consumption status, hypertension status, diabetes status, TOAST subtype, and renal function (eGFR).
3 Results
3.1 Baseline characteristics by MAR tertiles
In the final analytic sample of 395 patients with AIS, 336 (85.06%) had favorable 3-month outcomes (mRS ≤ 2), while 59 (14.94%) had unfavorable outcomes (mRS > 2). Patients were stratified by the admission MAR tertiles: Low, Middle, and High. The mean age was 66.20 ± 11.93 years, and the difference was not significant (p = 0.067). The proportion of female patients decreased across the MAR tertiles (51.9, 34.1, and 18.2%; p < 0.001). Smoking status and drinking status were more frequent in the higher MAR groups (p = 0.049). The prevalence of diabetes mellitus and hypertension was similar among the tertiles (Table 1).

Table 1. Baseline characteristics of acute ischemic stroke patients stratified by admission monocyte-to-albumin ratio tertiles (n = 395).
WBC and monocyte counts increased from the Low to High tertiles (both p < 0.001), whereas the serum ALB concentration was lower in the High group (p < 0.001). CRP was highest in the High tertile (p < 0.001). Renal indices were higher in the High group (p = 0.023), with comparable eGFRs and BUN levels across groups (p = 0.691 and p = 0.479), respectively. With respect to lipids, the TC level was lower in the High tertile (p = 0.042), while the HDL-C and LDL-C levels were not significantly different (p = 0.053 and p = 0.069). The full details are shown in Table 1.
3.2 Univariate predictors of poor 3-month outcomes
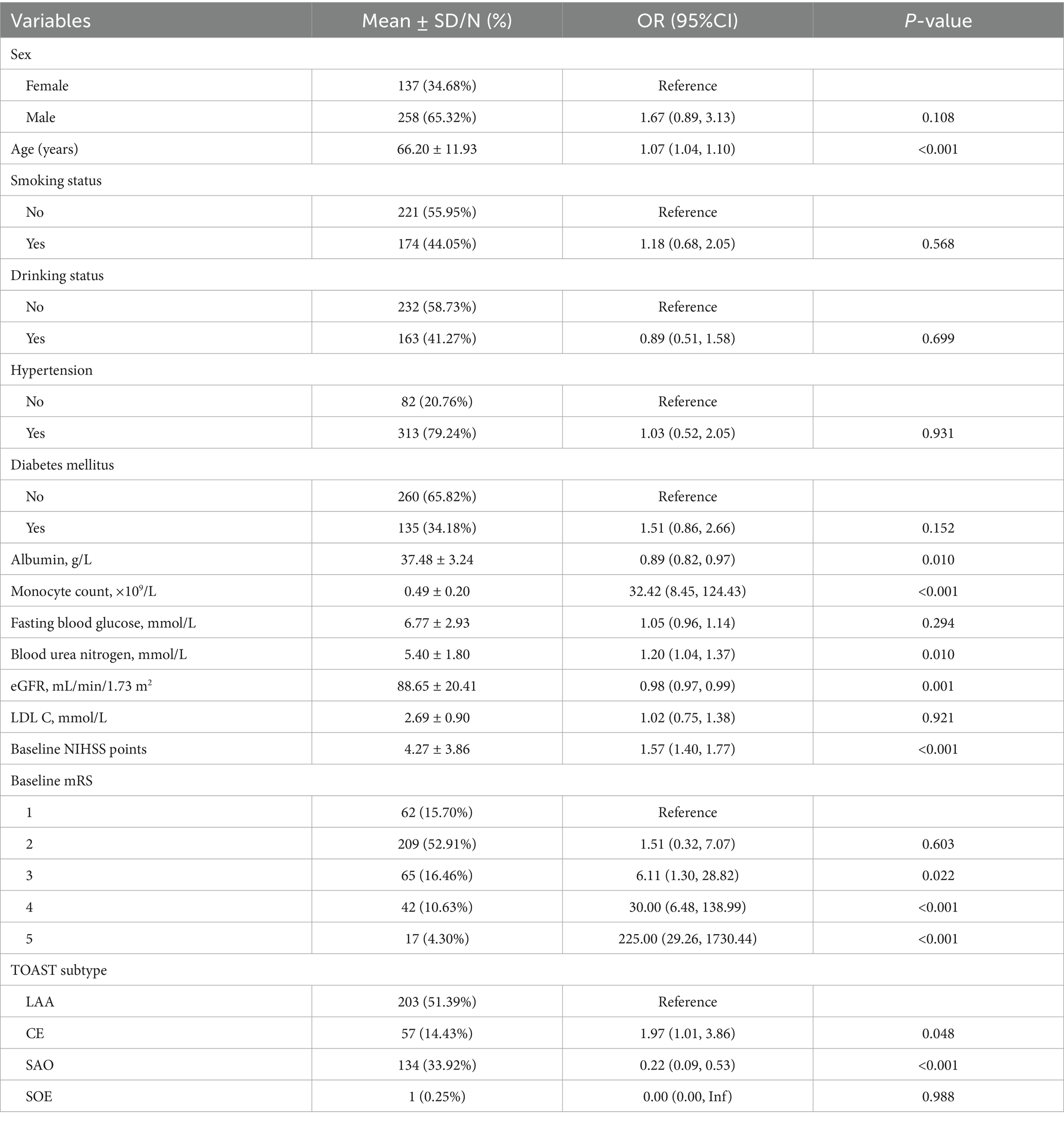
Based on univariable logistic regression (Table 2), older age was correlated with higher odds of poor outcomes (OR 1.07, 95% CI 1.04–1.10; p < 0.001). Compared with LAA, CE was linked to increased odds (OR 1.97, 95% CI 1.01–3.86; p = 0.048), whereas SAO was associated with lower odds (OR 0.22, 95% CI 0.09–0.53; p < 0.001). Low serum albumin levels (OR 0.89, 95% CI 0.82–0.97; p = 0.010) and high monocyte counts (OR 32.42, 8.45–124.43; p < 0.001) were significantly related to poor 3-month outcome. High BUN levels (OR 1.20, 95% CI 1.04–1.37; p = 0.010) and low eGFRs (OR 0.98, 95% CI 0.97–0.99; p = 0.001) were also related to poor outcomes. Baseline NIHSS score was strongly associated (OR 1.57, 95% CI 1.40–1.77; p < 0.001). Sex, smoking status, drinking status, hypertension status, diabetes status, LDL-C level, and fasting glucose level were not significantly associated with poor outcomes according to univariate analyses (all p > 0.05).
3.3 Associations between the MAR and the 3-month functional outcomes
The relationship between the admission MAR and poor 3-month functional outcomes (mRS ≥ 3) was evaluated using multivariable logistic regression. Higher MAR was significantly related to increased odds of poor outcomes across all models: per 1-unit increase, the ORs were 1.14 (95% CI 1.09–1.19; p < 0.001) in Model 1, 1.13 (95% CI 1.07–1.18; p < 0.001) following adjustment for age, sex, smoking status, and alcohol consumption status (Model 2), and 1.13 (95% CI 1.07–1.20; p < 0.001) with further adjustment for hypertension, diabetes, TOAST subtype, eGFR, NIHSS, and LDL-C (Model 3). Notably, when modeled by tertiles, the High MAR group had significantly greater odds of poor outcomes than the Low group did in Models 2 and 3, with a linear dose–response pattern that remained significant after full adjustment (P for trend = 0.006) (Table 3).

Table 3. Multivariable logistic regression analysis of the association between monocyte-to-albumin ratio (MAR) and poor 3-month functional outcome.
3.4 Subgroup and interaction analyses
In stratified analyses, the positive association between the admission MAR and poor 3-month outcomes persisted across the subgroups, and was independent of smoking status, alcohol consumption status, hypertension status, diabetes status, and renal function. Significant associations were observed in men and in participants aged >65 years. With respect to the TOAST subtype, the association remained significant for the large-artery atherosclerosis (LAA) subtype and small-artery occlusion (SAO) subtype, whereas that for the cardioembolism (CE) subtype did not reach significance. The stroke of other determined etiology (SOE) and stroke of undetermined etiology (SUE) subtypes were not evaluated because few or no cases were included. No significant interactions were detected for sex, age group, smoking status, alcohol consumption status, hypertension status, diabetes status, TOAST subtype, or eGFR (P for interaction >0.05), indicating that the associations were consistent across subgroups (Figure 1).

Figure 1. Forest plot depicting the association between monocyte-to-albumin ratio and 3-month modified Rankin Scale across clinical subgroups.
3.5 Dose–response relationship between the MAR and poor 3-month functional outcomes
Afterward, smoothed curve fitting was used to determine the dose–response relationships between continuous MAR and the probability of poor 3-month outcomes across the three adjustment schemes (Figures 2A–C), indicating that the risk increased with increasing MAR over the observed range.
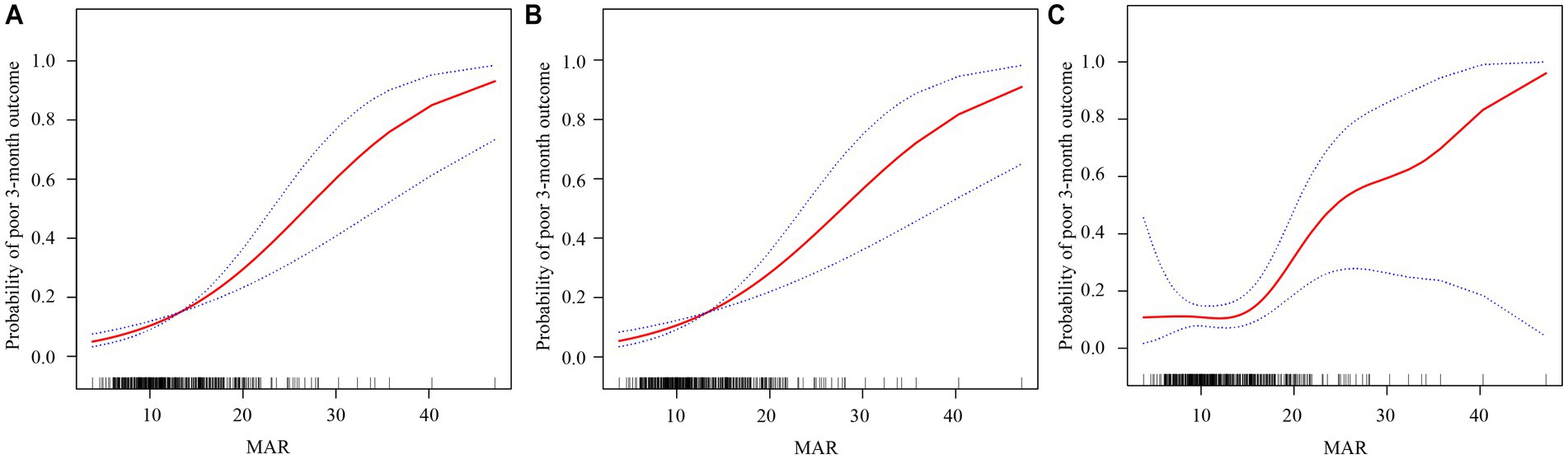
Figure 2. Dose–response relationships between the monocyte-to-albumin ratio (MAR) and a poor 3‑month functional outcome. The solid red line represents the smooth curve fit, and the blue bands indicate the 95% confidence interval. Three statistical models are presented: (A) Model 1 is the unadjusted analysis; (B) Model 2 is adjusted for age, sex, smoking status, and drinking status; and (C) Model 3 is further adjusted for hypertension, DM, TOST, eGFR, NIHSS, and LDL-C.
To further explore the dose–response relationship between the MAR and poor functional outcomes, a threshold effect analysis was performed using a piecewise linear regression model (Table 4). Below this threshold (MAR = 13.75), no significant association was observed between the MAR and poor outcomes (OR 0.97, 95% CI 0.81–1.17, p = 0.765). In contrast, above the inflection point, the association became markedly stronger, with each unit increase in MAR conferring a 19.4% increase in the odds of poor outcome (OR 1.19, 95% CI 1.09–1.31, p < 0.001). These findings suggest that the prognostic value of the MAR is most pronounced when values exceed approximately 13.75.
4 Discussion
In this consecutive AIS cohort, a higher admission MAR independently predicted a greater risk of poor 3-month functional outcome, and the association persisted after stepwise adjustment for demographics, lifestyle, comorbidities, renal function, baseline stroke severity, and lipid profile. Smoothing analyses suggested a monotonic, dose–response relationship between the MAR and unfavorable outcome risk. Subgroup analyses stratified by smoking, drinking, hypertension, and eGFR yielded broadly consistent results, and interaction tests indicated that the association did not differ significantly by sex, age, diabetes mellitus, or TOAST subtype. Together, these findings support the notion that the MAR represents a stable correlate of poststroke functional prognosis.
Inflammation is a key determinant of prognosis after AIS, shaping both early tissue injury and subsequent recovery (15, 16). Within minutes of arterial occlusion, damage-associated molecular patterns activate microglia and endothelial cells, triggering the release of pro-inflammatory cytokines (e.g., TNF-α and IL-6) and adhesion molecules that recruit peripheral leukocytes, notably neutrophils and monocytes (17). These effector cells generate reactive oxygen species and proteases, disrupt the blood–brain barrier, drive vasogenic edema, and increase the risk of hemorrhagic transformation (18). Nutrition is likewise a pivotal, yet often under-recognized, determinant of outcome after AIS (19). Pre-existing or early poststroke malnutrition compromises immune competence, delays tissue repair, and undermines neuroplasticity, thereby leading to complications (e.g., infections and pressure injuries) and poor functional recovery (20–22). Among circulating markers, serum albumin is a practical proxy for host nutritional and antioxidant reserves as well as capillary integrity (23). Albumin, synthesized in the liver, serves not only as a marker of nutritional status but also as an indicator of systemic inflammation and oxidative stress (24).
According to the above findings, the MAR, a ratio of monocyte and albumin that integrates inflammation and nutritional status, may be a potential predictor of functional prognosis after AIS. However, no relevant studies have explored the ability of the MAR to predict functional prognosis after AIS, leaving a major knowledge gap. In this study of 395 patients, multivariable models consistently demonstrated that higher admission MAR was independently associated with a greater likelihood of poor 3-month functional outcome, even after adjustment for demographics, vascular risk factors, stroke etiology, renal function, baseline severity, and lipid parameters. The stability of the association across progressively adjusted models and alternative parameterizations (continuous and categorical) suggests that the signal is not an artifact of confounding or model specification. Clinically, the per-unit effect appears modest, but the coherence of findings across models implies potential utility as part of a composite risk profile rather than a stand-alone predictor.
Our findings, which establish the MAR as a prognostic marker, align with the growing body of evidence highlighting the pivotal role of inflammation in the pathophysiology of ischemic stroke. Systemic inflammation contributes to endothelial dysfunction and arterial stiffness, both of which are key processes in the development and progression of cerebrovascular disease (25). This inflammatory state is often intertwined with metabolic comorbidities, creating a synergistic effect that can worsen vascular health and neurocognitive function (26, 27). Such a pro-inflammatory environment not only increases the risk of the initial ischemic event but may also amplify the secondary inflammatory cascade following stroke, thereby contributing to greater stroke severity and poorer functional outcomes. Indeed, the impact of inflammation on vascular health is common in various systemic conditions and has been linked to immune response genes, further underscoring its fundamental role in stroke susceptibility and pathogenesis (28, 29). Moreover, elevated monocyte counts reflect systemic immune activation, and circulating monocytes can infiltrate the ischemic brain parenchyma, where they interact with resident microglia to amplify neuroinflammatory cascades (30). Conversely, in addition to its nutritional role, albumin exerts multiple neuroprotective effects through several nononcotic mechanisms, including the molecular transportation and free radical scavenging, the modulation of capillary permeability, the regulation of neutrophil adhesion and activation, and hemostatic effects (31). Low albumin levels may therefore impair these protective mechanisms, rendering the brain more vulnerable to inflammatory injury. Therefore, the elevated MAR observed in patients with poor outcomes in our cohort may represent a confluence of both a preexisting inflammatory burden and an acute-phase reaction to the cerebral injury. Subgroup findings reinforce the generalizability of the association between MAR and outcome across common clinical strata. The effects were broadly consistent regardless of smoking status, alcohol use, hypertension, diabetes, or renal function, and interaction testing did not identify any meaningful effect modification. The stronger associations in men and in older adults are plausibly explained by differences related to sex and age. Sex differences in immune responses, partly driven by sex hormones and genetics, also shape monocyte phenotypes and downstream inflammatory signaling (32, 33). Aging is characterized by “inflammaging,” with chronically elevated cytokine and pro-inflammatory monocyte activity (34, 35). Older adults often have a higher baseline pro-inflammatory state (34). From an etiological perspective, in LAA and SAO, inflammation and microvascular injury are central to disease mechanisms. Since the MAR captures systemic inflammatory activity through monocytes and the overall inflammatory–nutritional balance through albumin, it is biologically aligned with these pathways and thus more likely to be correlated with prognosis. In contrast, cardioembolic stroke is driven primarily by emboli originating in the heart, often due to atrial fibrillation and atrial pathology. As a result, the association of the MAR with outcomes in patients with cardioembolic stroke may be weak or inconsistent.
An intriguing finding of our study is that the prognostic value of MAR was significant in patients with LAA and SAO, but not in those with CE. This differential association is pathophysiologically plausible. Atherosclerosis, the underlying cause of LAA and often implicated in SAO (microatheroma), is a chronic inflammatory disease in which monocytes play a critical role (36). Monocytes infiltrate the arterial wall, differentiate into macrophages, and contribute to all stages of plaque development, from initiation to rupture. Therefore, a higher MAR in these patients likely reflects a greater systemic inflammatory burden and a more unstable atherosclerotic process, which directly translates to a more severe stroke and poorer outcome. In contrast, the primary driver of a cardioembolic stroke is the dislodgment of a preformed thrombus from a cardiac source (e.g., in atrial fibrillation). While inflammation is a consequence of the resulting cerebral ischemia, the initial event is less dependent on the patient’s systemic inflammatory state as measured by admission MAR. The clinical outcomes in CE may be more strongly influenced by factors such as embolus size, collateral circulation patency, and underlying cardiac function, rather than peripheral inflammatory markers. Our findings therefore suggest that the utility of the MAR as a prognostic tool may be most pronounced in stroke etiologies with a strong, direct inflammatory component.
5 Strengths and limitations
This study utilized a consecutive, real-world cohort, thereby enhancing clinical relevance and reducing selection bias. Rigorous adjustment was performed for potential confounders, including demographics, vascular comorbidities, renal function, lipid profile, and baseline NIHSS score. In addition, a generalized additive mixed model was employed to graphically depict the dose–response relationship between the MAR and functional outcomes. The results were consistent across clinically relevant subgroups, supporting the robustness and generalizability of the study population. Because the MAR is derived from inexpensive, standardized admission laboratory tests, it is readily implementable across care settings and suitable for rapid bedside risk stratification.
Nonetheless, important limitations should be acknowledged. First, the single-center, retrospective design raises the possibility of selection bias and residual confounding. Second, reliance on a single admission measurement of the MAR prevents the assessment of temporal dynamics and trajectory-based risk; unmeasured influences on monocytes and albumin, such as intercurrent infection, hydration status, and hepatic function, cannot be fully excluded. Third, the relatively low number of patients with poor functional outcomes is a limitation, which may impact the statistical robustness of our multivariable models and the generalizability of our findings. Fourth, although the multivariable models included major vascular and demographic factors, prior stroke history, nutritional supplementation, and in-hospital infections, all of which can influence albumin levels and outcomes, they were not explored in this study and warrant investigation in future research. Finally, the exclusion of patients with hepatic dysfunction, active infections, hematologic diseases, or those receiving acute reperfusion therapies (intravenous thrombolysis or mechanical thrombectomy) limits the generalizability of our findings to the broader acute ischemic stroke population. Future studies should investigate whether the prognostic value of the MAR extends to patients undergoing these interventions, clarifying its utility across a broader, higher-risk patient population. Prospective, multicenter validation should also be prioritized to establish the generalizability and clinical transportability of MAR-based risk stratification.
6 Conclusion
Admission MAR is independently and positively associated with poor 3-month functional outcomes after AIS, demonstrating a largely monotonic dose–response relationship and consistent effects across key clinical subgroups. Given its simplicity and widespread availability, MAR shows promise for early risk assessment and stratified management. However, given the single-center retrospective design of this study, these findings should be considered as preliminary. Large-scale, multicenter prospective validation studies are urgently needed to validate the reproducibility and clinical utility of MAR before it can be recommended for routine clinical application.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University (Approval number: KY2023-R222). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin due to the retrospective nature of the study.
Author contributions
HL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MY: Data curation, Methodology, Writing – review & editing. YL: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. WJ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by Zhejiang Medical Association Clinical Research Fund Project (Grant No.: 2024ZYC-A526) and the Research Project of Quzhou People’s Hospital (Grant No.: KYQD2024-006).
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that Generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2021 Stroke Risk Factor Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet Neurol. (2024) 23:973–1003. doi: 10.1016/s1474-4422(24)00369-7,
2. Zhang, L, Antabi, MA, Mattar, J, Bounkari, OE, Fang, R, Waegemann, K, et al. Circulating cytokine levels and 5-year vascular recurrence after stroke: a multicenter prospective cohort study. Eur Stroke J. (2025):23969873251360145. doi: 10.1177/23969873251360145
3. Ma, L, Ji, L, Cheng, Z, Geng, X, and Ding, Y. Developing an explainable prognostic model for acute ischemic stroke: combining clinical and inflammatory biomarkers with machine learning. Brain Behav. (2025) 15:e70673. doi: 10.1002/brb3.70673,
4. Yu, Y, Zhang, Y, Zhu, C, Duan, T, and Rao, Z. Remnant cholesterol inflammatory index, calculated from residual cholesterol to C-reactive protein ratio, and stroke outcomes: a retrospective study using the national institutes of health stroke scale and modified Rankin scale. Lipids Health Dis. (2025) 24:228. doi: 10.1186/s12944-025-02650-2,
5. Han, D, Liu, H, and Gao, Y. The role of peripheral monocytes and macrophages in ischemic stroke. Neurol Sci. (2020) 41:3589–607. doi: 10.1007/s10072-020-04777-9,
6. Manolis, AA, Manolis, TA, Melita, H, Mikhailidis, DP, and Manolis, AS. Low serum albumin: a neglected predictor in patients with cardiovascular disease. Eur J Intern Med. (2022) 102:24–39. doi: 10.1016/j.ejim.2022.05.004,
7. Mehta, A, De Paola, L, Pana, TA, Carter, B, Soiza, RL, Kafri, MW, et al. The relationship between nutritional status at the time of stroke on adverse outcomes: a systematic review and meta-analysis of prospective cohort studies. Nutr Rev. (2022) 80:2275–87. doi: 10.1093/nutrit/nuac034,
8. Thuemmler, RJ, Pana, TA, Carter, B, Mahmood, R, Bettencourt-Silva, JH, Metcalf, AK, et al. Serum albumin and post-stroke outcomes: analysis of UK regional registry data, systematic review, and Meta-analysis. Nutrients. (2024) 16:1486. doi: 10.3390/nu16101486,
9. Zhou, H, Wang, A, Meng, X, Lin, J, Jiang, Y, Jing, J, et al. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol. (2021) 6:458–66. doi: 10.1136/svn-2020-000676,
10. Deng, C, Liu, B, Wang, M, Zhu, C, Xu, Y, Li, J, et al. Analysis of the correlation between neutrophil percentage-to-albumin ratio, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with short-term prognosis in acute ischemic stroke patients undergoing intravenous thrombolysis. Front Neurol. (2025) 16:1512355. doi: 10.3389/fneur.2025.1512355,
11. Wang, J, Cao, X, Zeng, S, Zhou, L, Huang, J, Han, Y, et al. Nonlinear dose-response relationship between prognostic nutritional index and short-term outcome in acute ischemic stroke: a prospective cohort study. Front Nutr. (2025) 12:1529146. doi: 10.3389/fnut.2025.1529146,
12. Zhang, ZL, Guo, QQ, Tang, JN, Zhang, JC, Cheng, MD, Song, FH, et al. Monocyte-to-albumin ratio as a novel predictor of long-term adverse outcomes in patients after percutaneous coronary intervention. Biosci Rep. (2021) 41:BSR20210154. doi: 10.1042/bsr20210154,
13. Fu, J, Xu, Y, Chen, X, Li, J, and Peng, L. Monocyte-to-albumin ratio is associated with hematoma expansion in spontaneous intracerebral hemorrhage. Brain Behav. (2024) 14:e70059. doi: 10.1002/brb3.70059,
14. Yuan, M, Mao, WE, He, X, and Zhang, Q. Novel marker for predicting prognosis in hepatitis B virus-associated decompensated cirrhosis: monocyte-to-albumin ratio. Clin Lab. (2023) 69:2062–69. doi: 10.7754/Clin.Lab.2023.230204
15. Dash, UC, Bhol, NK, Swain, SK, Samal, RR, Nayak, PK, Raina, V, et al. Oxidative stress and inflammation in the pathogenesis of neurological disorders: mechanisms and implications. Acta Pharm Sin B. (2025) 15:15–34. doi: 10.1016/j.apsb.2024.10.004,
16. Xie, L, He, M, Ying, C, and Chu, H. Mechanisms of inflammation after ischemic stroke in brain-peripheral crosstalk. Front Mol Neurosci. (2024) 17:1400808. doi: 10.3389/fnmol.2024.1400808,
17. Fang, W, Zhai, X, Han, D, Xiong, X, Wang, T, Zeng, X, et al. CCR2-dependent monocytes/macrophages exacerbate acute brain injury but promote functional recovery after ischemic stroke in mice. Theranostics. (2018) 8:3530–43. doi: 10.7150/thno.24475,
18. Weiss, A, and Ding, Y. Beyond reperfusion: adjunctive therapies targeting inflammation, edema, and blood-brain barrier dysfunction in ischemic stroke. Cerebrovasc Dis. (2025) 54:1–10. doi: 10.1159/000547092,
19. de Man, AME, Gunst, J, and Reintam Blaser, A. Nutrition in the intensive care unit: from the acute phase to beyond. Intensive Care Med. (2024) 50:1035–48. doi: 10.1007/s00134-024-07458-9,
20. Dal Bello, S, Ceccarelli, L, Tereshko, Y, Gigli, GL, D'Anna, L, Valente, M, et al. Prognostic impact of malnutrition evaluated via bioelectrical impedance vector analysis (BIVA) in acute ischemic stroke: findings from an inverse probability weighting analysis. Nutrients. (2025) 17:919. doi: 10.3390/nu17050919,
21. Jiang, TT, Zhu, XY, Yin, YW, Liu, HJ, and Zhang, GY. The prognostic significance of malnutrition in older adult patients with acute ischemic stroke. Front Nutr. (2025) 12:1529754. doi: 10.3389/fnut.2025.1529754,
22. Li, D, Liu, Y, Jia, Y, Yu, J, Li, F, Li, H, et al. Association between malnutrition and stroke-associated pneumonia in patients with ischemic stroke. BMC Neurol. (2023) 23:290. doi: 10.1186/s12883-023-03340-1,
23. Arques, S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. (2018) 52:8–12. doi: 10.1016/j.ejim.2018.04.014,
24. Zhao, J, Chen, M, Mo, J, Zhong, Y, Qiu, J, Qiu, Y, et al. Prognostic value of albumin-based malnutritional indices on short-term outcome in acute ischemic stroke patients undergoing reperfusion therapy. Front Nutr. (2025) 12:1659446. doi: 10.3389/fnut.2025.1659446,
25. Della Corte, V, Tuttolomondo, A, Pecoraro, R, Di Raimondo, D, Vassallo, V, and Pinto, A. Inflammation, endothelial dysfunction and arterial stiffness as therapeutic targets in cardiovascular medicine. Curr Pharm Des. (2016) 22:4658–68. doi: 10.2174/1381612822666160510124801,
26. Tuttolomondo, A, Petta, S, Casuccio, A, Maida, C, Corte, VD, Daidone, M, et al. Reactive hyperemia index (RHI) and cognitive performance indexes are associated with histologic markers of liver disease in subjects with non-alcoholic fatty liver disease (NAFLD): a case control study. Cardiovasc Diabetol. (2018) 17:28. doi: 10.1186/s12933-018-0670-7,
27. Tuttolomondo, A, Casuccio, A, Della Corte, V, Maida, C, Pecoraro, R, Di Raimondo, D, et al. Endothelial function and arterial stiffness indexes in subjects with acute ischemic stroke: relationship with TOAST subtype. Atherosclerosis. (2017) 256:94–9. doi: 10.1016/j.atherosclerosis.2016.10.044,
28. Tuttolomondo, A, Di Raimondo, D, Pecoraro, R, Casuccio, A, Di Bona, D, Aiello, A, et al. HLA and killer cell immunoglobulin-like receptor (KIRs) genotyping in patients with acute ischemic stroke. J Neuroinflammation. (2019) 16:88. doi: 10.1186/s12974-019-1469-5,
29. Zanoli, L, Ozturk, K, Cappello, M, Inserra, G, Geraci, G, Tuttolomondo, A, et al. Inflammation and aortic pulse wave velocity: a multicenter longitudinal study in patients with inflammatory bowel disease. J Am Heart Assoc. (2019) 8:e010942. doi: 10.1161/JAHA.118.010942,
30. Kanazawa, M, and Hatakeyama, M. Intercellular communication between peripheral monocytes and central nervous system cells in stroke. Neural Regen Res. (2025) 21. doi: 10.4103/nrr.Nrr-d-25-00738,
31. Evans, TW. Review article: albumin as a drug--biological effects of albumin unrelated to oncotic pressure. Aliment Pharmacol Ther. (2002) 16:6–11. doi: 10.1046/j.1365-2036.16.s5.2.x,
32. Regitz-Zagrosek, V, and Gebhard, C. Gender medicine: effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat Rev Cardiol. (2023) 20:236–47. doi: 10.1038/s41569-022-00797-4,
33. Sharma, S, Gibbons, A, and Saphire, EO. Sex differences in tissue-specific immunity and immunology. Science. (2025) 389:599–603. doi: 10.1126/science.adx4381,
34. Franceschi, C, Garagnani, P, Parini, P, Giuliani, C, and Santoro, A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. (2018) 14:576–90. doi: 10.1038/s41574-018-0059-4,
35. Klein, SL, and Flanagan, KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90,
Keywords: acute ischemic stroke, monocyte-to-albumin ratio, inflammation, prognosis, risk stratification
Citation: Lu H, Wang C, Yang M, Lin Y and Jiang W (2025) Admission monocyte-to-albumin ratio predicts 3-month functional outcomes after acute ischemic stroke: a retrospective cohort study. Front. Neurol. 16:1705544. doi: 10.3389/fneur.2025.1705544
Edited by:
Dalius Jatuzis, Vilnius University, LithuaniaReviewed by:
Antonino Tuttolomondo, Università degli Studi di Palermo, ItalyYang Liu, Fudan University, China
Dongming Zhang, Second Affiliated Hospital of Guangzhou Medical University, China
Copyright © 2025 Lu, Wang, Yang, Lin and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifeng Jiang, d2VpZmVuZ2ppYW5nQHdtdS5lZHUuY24=
 Huizhen Lu
Huizhen Lu Chuanliu Wang
Chuanliu Wang Ming Yang
Ming Yang Yuanshao Lin
Yuanshao Lin Weifeng Jiang
Weifeng Jiang