Abstract
Opioid receptor agonists constitute a class of pharmaceuticals that are extensively employed in anesthesia and analgesia. Remifentanil (RF), a novel synthetic opioid receptor agonist, is characterized by rapid onset of action and a short half-life, attributed to its unique pharmacokinetic profile. Clinically, RF is often used for total intravenous anesthesia. In recent years, RF has garnered more attention due to its potential organ-protective effects. This review aims to consolidate the existing evidence regarding the neuroprotective properties of RF, encompassing various levels ranging from molecular mechanisms to clinical applications. Experimental studies demonstrate that RF exerts protective effects in brain injury models, with its mechanisms being associated with the attenuation of apoptosis, oxidative stress, and neuroinflammation. In clinical practice, RF serves as a safe and effective analgesic regimen for patients with traumatic brain injury (TBI), those undergoing neurosurgical procedures, and individuals requiring endotracheal intubation; furthermore, it confers certain benefits to the recovery of patients' neurological function. Compared with fentanyl, RF is capable of reducing the incidence of delirium and postoperative cognitive dysfunction (POCD) in patients. Moreover, remifentanil-induced hyperalgesia (RIH) during clinical administration is also discussed. In conclusion, RF is an anesthetic agent with significant neuroprotective potential. Future research should focus on elucidating its precise mechanism of action, optimizing clinical administration regimens, and exploring strategies in RIH management.
1 Introduction
Opioids are extensively utilized in clinical practice and exert their pharmacological effects through the activation of opioid receptors (OPRs), which are predominantly coupled with three major subtypes, namely, μ, κ, and δ (1). OPRs exhibit a broad distribution pattern across the cardiovascular system, central nervous system (CNS), and peripheral nerves. They are expressed in organs beyond the cardiovascular system, such as the gastrointestinal tract and kidneys (2). In the clinic, opioids are not only widely employed for the induction and maintenance of general anesthesia but also serve as pivotal agents for analgesic therapy in the management of acute pain and cancer-related pain. However, their clinical application is substantially constrained by the occurrence of adverse effects, including respiratory depression and addiction (3). In addition to their roles in anesthesia and analgesia, opioids have additional pharmacological effects, with distinct advantages, particularly in safeguarding organs against ischemia/reperfusion (I/R) injury. A number of opioids have been identified as candidate agents for clinical cardioprotection, with remifentanil being a case in point (4).
Remifentanil (RF) is an ultrashort-acting pharmacokinetic opioid developed by Feldman and his research team in 1991. It was synthesized by attaching a side chain containing a methyl ester to the piperidine ring nitrogen atom of fentanyl, to address the limitations of traditional opioids such as fentanyl—specifically, their prolonged duration of action and propensity for accumulation (5). RF shares a similar chemical structure to fentanyl, alfentanil, and sufentanil, and possesses analgesic potency comparable to fentanyl, being 100 times more potent than morphine (6, 7). As an opioid, RF exerts its pharmacological effects via the activation of OPRs. At clinical doses, RF has high affinity for μ-opioid receptors; when RF is administered at doses exceeding the clinical range, it can also interact with κ- and δ-opioid receptors (8). Unlike that of conventional opioids, the metabolic transformation of RF in vivo is independent of hepatic function. Instead, it undergoes rapid metabolism by nonspecific esterases present in the bloodstream and tissues, endowing it with the pharmacokinetic characteristics of rapid onset of action and a short half-life (9). Owing to these characteristics, RF is extensively employed in clinical practice for the induction and maintenance of anesthesia. With the discovery of the cardioprotective effects of opioids, many studies have reported the protective role of RF against organ ischemia–reperfusion injury, such as myocardial ischemia–reperfusion injury and cerebral ischemia–reperfusion injury. The mechanisms underlying its protective effects are associated with the attenuation of inflammation, the activation of antiapoptotic signaling pathways, and the inhibition of oxidative stress (4). In recent years, RF not only demonstrates distinct advantages in cardiovascular surgeries but also can be applied in anesthesia for various noncardiac surgical procedures, such as neurosurgical and spinal surgeries. Furthermore, it can be safely administered to patients across different age groups, including neonates, infants, and elderly individuals (10, 11).
In recent years, the neuroprotective effects of RF within the CNS have attracted the attention of researchers. This article systematically reviews the neuroprotective effects and underlying mechanisms of RF in acute brain injury and peripheral nerve-related disorders while further exploring the existing evidence and future challenges regarding its translation from basic research to clinical practice.
2 Literature search strategy
To comprehensively evaluate the neuroprotective effects of remifentanil, we conducted a systematic literature search. All literature published up to September 15, 2025, was retrieved from PubMed, Embase, Web of Science, and Google Scholar. The search query was: ((Neuroprotective Effects) OR (Neuroprotection) OR (brain protection)) AND ((Remifentanil) OR (Remifentanil hydrochloride) OR (Ultiva)). The initial database search yielded 86 records. After excluding review articles and duplicate records, 49 records were selected based on title and abstract screening. Following full-text reviewing of the most relevant literature, 28 studies were ultimately included for in-depth analysis and synthesis in this narrative review. Clinical study inclusion criteria: Randomized controlled trials conducted in patients with neurological disorders (e.g., traumatic brain injury) receiving remifentanil sedation/analgesia or at risk for neurological complications were included. Primary outcome measures comprised neurological function scores, visual analog scores, and postoperative delirium incidence. Basic research inclusion criteria: Mechanistic studies investigating remifentanil's neuroprotective or neurotoxic effects in experimental models of neurological disorders (e.g., animal or cellular models of cerebral ischemia-reperfusion injury, hypoxic-ischemic encephalopathy, neuroinflammation) were included.
3 Neuroprotective effects of remifentanil in basic research
3.1 Cerebral ischemia
Ischemic stroke refers to a neurological deficit syndrome caused by local cerebral circulatory disorders. As one of the major diseases endangering human health, cerebral ischemia can induce cerebral infarction, brain tissue necrosis, and focal neuronal damage (12). Significant neuronal injury occurs not only in the ischemic state but also after treatment with thrombolysis or mechanical thrombectomy. Therefore, the protection and regeneration of neurons have long been the focus of research on cerebral ischemia-related diseases. Studies have demonstrated that the neurological damage induced by cerebral ischemia is directly associated with neuronal loss, oxidative stress, and immune responses (12). Consequently, neurological damage caused by cerebral ischemia can be ameliorated through multiple approaches, such as enhancing neuronal protection, promoting neuronal repair and regeneration, directly mediating signaling pathways involved in neuronal survival and apoptosis, and regulating immune responses. In recent years, RF, an anesthetic and analgesic agent, has been shown to exert a protective effect against neuronal injury induced by cerebral ischemia–reperfusion (4) (Table 1).
Table 1
| Disorders | Animals/cells | Treatments (RE) | Functions | Mechamisms | Ref. |
|---|---|---|---|---|---|
| Cerebral ischemia | Male SD rats | 0.6 μg/kg/min for 5 min i.v. | Improved the spatial learning and memory; attenuated neuronal apoptosis in hippocampus | Activation of PI3K signaling pathway | (13) |
| Male SD rats | 5 μg/kg/min for 100 min, femoral vein | Improved the functional outcome and reduced the infarct volumes | Suppressed the phosphorylation of ERK 1/2 and TNF-α | (14) | |
| Adult male SD rats | 1.2 μg/kg/min i.v. | Improve brain function and decrease the brain damage | Inhibit TNF-α/TNFR1, JNK signal transduction pathways | (15) | |
| SD rats | 2 or 10 μg/kg, caudal veins | Decrease neuronal apoptosis level and cerebral infarct size, and increase the mitochondrial membrane potential | Repress the NR2B/CaMKIIα signaling pathway | (16) | |
| Male mongolian gerbils (11–13 weeks) | 0.02 mg/kg i.p. | Alleviated ischemia-induced memory impairment | The suppression of apoptotic neuronal cell death | (17) | |
| Neuroinflammation | Female Wistar Albino rats | 0.04 mg/kg for 40 min i.v. | Prevent neuroinflammation, and apoptosis | Regulating the PI3K/AKT/HIF-1α pathway;restoring blood–brain barrier | (27) |
| Male DDY mice; rat glioma cell line C6 | 10 μg/kg/min for mice; 100, 10, 1 μg/ml for cell | Suppressed increase in IL-6 mRNA levels in the brain in an inflammatory state | Inhibiting cyclic AMP synthesis | (29) | |
| BV2 microglia | 0.5, 1, 2 μM | Suppressed PC12 cell injury | Suppressed inflammatory releases, iNOS, NO and PGE2 stimulated | (31) | |
| Hypoxie-ischemic encephalopathy | HIBD rat model (SD, female, 6 weeks old) | 5 mg/min/kg for 5 min | Improved the learning memory ability, reduced neuronal cell damage and apoptosis, reduced inflammation | Suppressing the expression of BTB domain and CNC homolog 1 (BACH1) | (36) |
| Postnatal day 2 (P2) mice | 500 ng/g for 10-min i.p. | Reduce excitotoxicity, the size of ibotenate-induced brain lesion; prevention of some behavioral deficits | Decreased ROS production, cortical caspase activity, DNA fragmentation, interleukin-1β levels, and reactive astrogliosis. | (37) | |
| Postnatal day 7 SD rat | 5, 20, 80 μg/kg/h for 4 h | Reduces isoflurane-induced apoptosis | N.A. | (38) | |
| Cerebral slices from postnatal day 2 mice | 50 μM | Inhibited apoptosis and preserved mitochondrial integrity | Activate the opioid and NMDA receptors, and the mitochondrial-dependent apoptotic pathway | (39) | |
| Olfactory neurons from newborn SD rat | 2-, 0.2- or 0.02-mM | Reduce glutamate toxicity and to increase cell viability | N.A. | (40) | |
| Surgery-related brain injuryury | 94 healthy adult male SD rats | 1.2 μg/kg/min | Attenuated CPB-induced injury | Blocking AKT/NRF2 signal pathway | (44) |
The neuroprotective effects of RF in experimental studies.
With in-depth investigations into the neuroprotective effects of RF in cerebral ischemia, its underlying mechanisms have gradually become the focus of exploration. A study conducted by Hu et al. (13) demonstrated that RF administration could enhance spatial learning and memory abilities in rats after cerebral ischemia while attenuating hippocampal neuronal apoptosis. Further experiments revealed that the application of a PI3K inhibitor abrogated both the RF-mediated improvement in neurological function and the antiapoptotic properties of RF in postischemic rats, suggesting that the neuroprotective effect of RF against cerebral ischemia–reperfusion injury may be exerted through the activation of the PI3K pathway. Similarly, Jeong et al. (14) reported that RF could reduce cerebral infarct volume and decrease neurological deficit scores in rats following cerebral ischemia. Mechanistic studies indicated that RF significantly downregulated the expression of phosphorylated ERK 1/2 and TNF-α while increasing the expression of δ-opioid receptors in the rat brain. Zhang et al. (15) reported that RF treatment alleviated cerebral ischemia-induced brain damage in rats; in the RF-treated group, the protein expression levels of TNF-α, TNFR1, Cytc, caspase-3, caspase-9, and pJNK in rat brain tissues were downregulated. These findings suggest that RF may exert its neuroprotective effects by inhibiting the mitochondrial apoptotic pathway (TNF-α/TNFR1) and the JNK signaling pathway. By establishing a cerebral I/R rat model, Chen et al. (16) discovered that RF could reduce neuronal apoptosis and cerebral infarct size in rats, along with decreasing ROS levels and the MDA content. Further studies revealed that RF treatment significantly reduced the expression levels of cell death-related proteins (e.g., caspase-3) and proteins associated with the NR2B/CaMKIIα signaling pathway in rat brain tissues, indicating that RF can exert neuroprotective effects by inhibiting the NR2B/CaMKIIα signaling pathway and suppressing neuronal apoptosis in rats with cerebral ischemia–reperfusion injury (IRI). Research by Park et al. (17) revealed that RF could ameliorate cognitive impairment in adult male gerbils after transient cerebral ischemia. Memory function was evaluated via the step-down avoidance task, and the results revealed that RF treatment significantly shortened the latency period in gerbils, which was associated with RF-mediated inhibition of hippocampal apoptosis.
In addition to exerting protective effects against cerebral ischemia, RF also exerts protective effects against IRI in multiple other organs, such as the myocardium (18), liver (19), and intestine (20). Notably, RF can ameliorate the neurological dysfunction associated with ischemic injury in these noncerebral organs. One study revealed that the hepatoprotective effect of RF in rats with hepatic IRI is associated with the activation of the dorsal motor nucleus of the vagus nerve (DMV), rather than peripheral μ-opioid receptors, suggesting an interaction between RF and the central parasympathetic nervous system (21). Another study reported that RF protects against hepatic ischemia/reperfusion-induced injury to D1 medium spiny neurons (D1-MSNs) (22). Specifically, this protective effect manifests as downregulated expression of IL-1β and IL-18, attenuated oxidative stress, improved morphology and function of D1-MSNs, and reduced apoptosis. This protective mechanism is believed to be associated with RF-mediated upregulation of FGF18 expression, and FGF18 may serve as a potential therapeutic target for hepatic I/R-related neurological injury.
The aforementioned studies demonstrate that RF exerts a certain degree of protection against cerebral injury induced by cerebral ischemia/reperfusion (Table 1), and existing research has revealed that RF exerts its effects through multiple signaling pathways (Figure 1). Additionally, RF can ameliorate cognitive impairment induced by cerebral ischemia and protect against neurological injury associated with ischemia–reperfusion in other organs (4, 17).
Figure 1
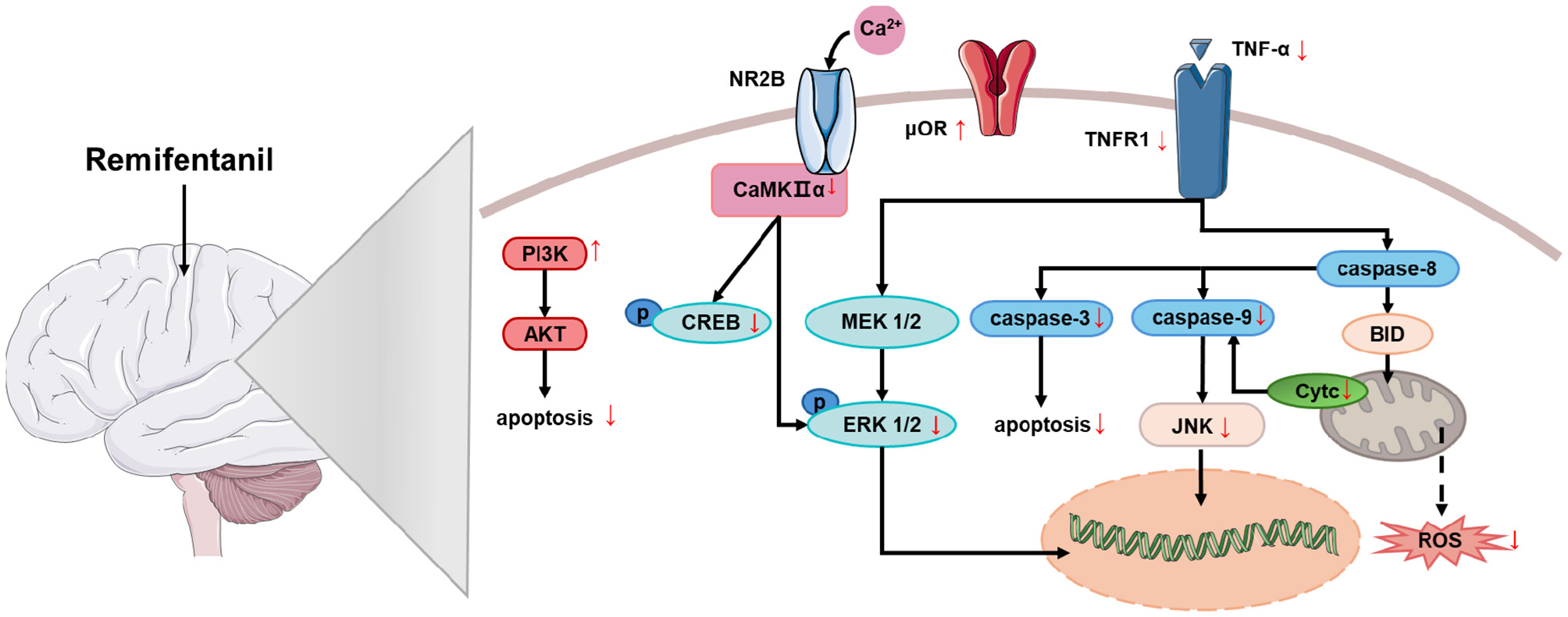
The mechanism of the neuroprotective effect of RF on cerebral ischemia. Remifentanil exerts neuroprotective effects against cerebral ischemia by mediating multiple signaling pathways that reduce apoptosis and oxidative stress. PI3K, phosphoinositide 3-kinase; Akt (PKB), protein kinase B; NR2B (GluN2B), NMDA receptor subtype 2B; CaMKIIα, calcium-dependent protein kinase II alpha; CREB, cAMP response element-binding protein; MEK 1/2 (MAP2K), mitogen-activated protein kinase kinase 1/2; ERK 1/2, extracellular signal-regulated kinase 1/2; μOR, mu-opioid receptor; TNFR1, tumor necrosis factor receptor 1; TNF-α, tumor necrosis factor-alpha; Caspase-3/8/9, cysteine-dependent aspartate-specific protease-3/8/9; JNK, c-Jun N-terminal kinase; Cyt c, cytochrome c; Bid, BH3-interacting domain death agonist; ROS, reactive oxygen species.
3.2 Neuroinflammation
Neuroinflammation constitutes a crucial pathological process in a spectrum of CNS disorders, such as Alzheimer's disease and Parkinson's disease (23). This process involves a cascade of complex responses, including the activation of neuroglial cells, the release of inflammatory mediators, and the generation of ROS (24). Under physiological conditions, neuroinflammation serves to protect the brain against pathogenic insults. However, sustained neuroinflammation also leads to neuronal damage (25). Notably, anesthetics and sedatives are recognized to affect cerebral inflammation. Opioids, ketamine, benzodiazepines, and propofol can elicit both neuroprotective and neurotoxic effects in the brain, with such outcomes being dependent on the duration of administration and dosage employed (26).
RF can ameliorate LPS-induced neuroinflammation (27). In the LPS-induced sepsis rat model, cerebral edema and inflammatory cell infiltration were observed, and the expression levels of PI3K, AKT, and HIF-1α were significantly elevated. However, all these pathological manifestations and molecular changes were markedly attenuated in the RF-treated group. As a crucial downstream substrate of the PI3K/AKT pathway, HIF-1α plays a pivotal role in regulating blood–brain barrier (BBB) permeability (28). These findings indicate that RF has a therapeutic effect on alleviating sepsis-associated neuroinflammation, and its underlying mechanism is associated with the downregulation of the PI3K/AKT/HIF-1α pathway and the restoration of BBB integrity. A study conducted by Maeda et al. (29) directly measured the expression level of the proinflammatory cytokine IL-6 in mice following LPS induction. The results demonstrated that RF significantly reduced the IL-6 mRNA levels in the hypothalamus, cortex, and plasma of the mice. In vitro cellular experiments revealed that RF inhibited IL-6 expression by suppressing the intracellular increase in cAMP in neuroglial cells. However, the mechanistic investigation has been insufficient, as the study failed to establish a direct causal link between RF-mediated cAMP inhibition and the reduction in IL-6 mRNA expression. Previous research has reported that the cAMP/PKA pathway mediates IL-1β-induced IL-6 synthesis, which is associated with the upregulation of the JAK2/STAT3 pathway (30). Therefore, subsequent studies could explore the role of the JAK2/STAT3 pathway in neuroglial cells following RF treatment to further clarify this mechanism. Another study similarly indicated that RF can inhibit the release of inflammatory factors from LPS-induced BV2 microglia (31). The findings of this study revealed that RF suppressed the LPS-induced increase in TNF-α, IL-6, iNOS, NO, and PGE2 in BV2 cells, and the inhibitory effect was positively correlated with RF concentration. Additionally, RF treatment led to significant reductions in the protein levels of p-NF-κB p65, p-IκBα, and COX2 in the cells. These results suggest that RF can reduce the release of inflammatory factors by inhibiting the NF-κB signaling pathway. Collectively, the aforementioned studies illustrate that RF mitigates LPS-induced neuroinflammation. Its protective mechanisms involve the downregulation of the PI3K/AKT/HIF-1α pathway and NF-κB signaling, the restoration of BBB integrity, and the suppression of intracellular cAMP expression in neuroglial cells (Table 1).
3.3 Neonatal hypoxic-ischemic encephalopathy (NHIE)
Neonatal encephalopathy (NE) refers to a spectrum of acute neurological dysfunctions characterized by altered states of consciousness, abnormal reflexes, convulsions, and other manifestations and occurs in neonates with a gestational age of ≥35 weeks (32). Hypoxia-ischemia is the most common etiological factor of NE; in addition, other factors, such as traumatic brain injury, bacterial or viral infections, and vascular disorders, may also induce NE (33, 34). Perinatal/neonatal hypoxic-ischemic encephalopathy (HIE) represents one of the most severe conditions encountered in neonatal neurology and is also among the leading causes of neonatal mortality and morbidity (35). Lu and Wang study (36) demonstrated that RF exerts neuroprotective effects by establishing a rat hypoxic-ischemic brain damage (HIBD) model. These effects specifically manifested as reductions in neuronal damage and apoptosis, decreases in inflammation, and improvements in the learning and memory abilities of HIBD rats. Further mechanistic investigations revealed that RF can downregulate the expression of BTB and CNC homology 1 (BACH1), thereby inhibiting the expression of TNF receptor-associated factor 3 (TRAF3). KEGG enrichment analysis revealed that TRAF3-related genes are enriched in the NF-κB signaling pathway. These findings suggest that RF can alleviate HIBD-induced cognitive impairment by downregulating BACH1 expression to inhibit the TRAF3/NF-κB signaling pathway. In another study, an ibotenate-induced neonatal brain injury model was established, and RF also reduced the production of ROS, the level of IL-1β, and the number of reactive astrocytes in the injured neonatal brain while enhancing relevant motor abilities, thus demonstrating a protective effect against neonatal brain injury (37). In a study conducted by Pan et al. (38), 7-day-old rats were subjected to continuous isoflurane exposure. The results showed that RF administration alone did not induce cell death; instead, it attenuated isoflurane-induced hippocampal apoptosis in developing rats. Tourrel et al. (39) investigated the effects of RF on the immature mouse brain. Brain slices isolated from postnatal day 2 (P2) mice were exposed to RF, and the results demonstrated that RF at a concentration of 50 μM had no necrotic effects but did have antiapoptotic effects in vitro. This antiapoptotic effect was abrogated by opioid receptor antagonists and NMDA receptor antagonists, suggesting that the underlying mechanism involves opioid ligands, NMDA receptors, and the mitochondria-dependent apoptotic pathway. Additionally, RF was found to attenuate glutamate-induced neuronal damage in the olfactory bulb of neonatal rats and increase neuronal cell viability (40). Collectively, these studies indicate that RF confers a certain degree of protection against neonatal-related encephalopathies (Table 1), with mechanisms involving the inhibition of BTB and CNC homology 1 (BACH1) expression, downregulation of the TRAF3/NF-κB signaling pathway, suppression of apoptosis, mitigation of oxidative stress, and alleviation of inflammation.
3.4 Surgery-related brain injury
Surgical intervention constitutes a prevalent therapeutic modality in the advancement of modern medicine, and surgical anesthesia is also recognized as a risk factor for cerebral injury. Patients who undergo surgery are susceptible to adverse outcomes such as stroke, postoperative delirium, and postoperative cognitive dysfunction, which are associated with factors such as surgical type and patient physical status. Research findings have revealed that the incidence of cerebral injury in patients undergoing cardiac surgery is significantly greater than that in those undergoing other noncardiac surgical procedures (41). Up to 40% of patients who undergo cardiopulmonary bypass (CPB) surgery develop perioperative neurological injury, which severely impairs postoperative recovery (42). A study conducted by Xiong et al. (43) demonstrated that the preoperative administration of RF to rats subjected to CPB mitigated surgery-induced oxidative stress, inflammation, and cerebral injury; shortened the latency of escape responses; improved memory function; and reduced hippocampal neuronal apoptosis in these rats. Further mechanistic investigations indicated that preoperative RF treatment reversed the CPB-induced downregulation of p-Akt and HO-1 expression and further inhibited the expression of Nrf2. These results suggest that the ameliorative effect of RF on CPB-induced cerebral injury in rats is associated with the Akt/Nrf2 signaling pathway. Studies have demonstrated that the PI3K/Akt/Nrf2/HO-1 signaling pathway plays a crucial role in cerebral injury associated with various neurological disorders, such as epilepsy and Alzheimer's disease (44, 45). The activation of this pathway is closely linked to pathological processes, including oxidative stress, inflammatory responses, and apoptosis (46). Currently, there are few research reports regarding the effects of RF on cerebral injury induced by other types of surgeries. Therefore, further basic research focusing on the neuroprotective mechanisms of RF in this field is warranted in future studies.
4 Neuroprotective effects of RF in clinical practice
4.1 Traumatic brain injury (TBI)
TBI refers to substantial damage to brain tissue, which can impair brain function either transiently or persistently. TBI often leads to severe neurological impairment and psychological distress, such as cognitive decline (47). The primary focus of initial TBI management is maintaining a patent airway, along with sustaining adequate ventilation, oxygenation, and blood pressure support. For patients with severe TBI, surgical intervention is frequently needed, followed by intensive postoperative monitoring and intracranial pressure surveillance. In recent years, therapeutic strategies involving pharmacotherapy for TBI have emerged as a research focus, aiming to enhance cognitive and neurological function recovery through neuroprotection and neural restoration (48). Endogenous opioids are considered to play a role in the pathophysiology of TBI, and the administration of opioids can influence brain injury and provide neuroprotection following TBI (49). A study conducted by Karabinis et al. (50) revealed that in patients with acute brain injury or those intubated post-neurosurgery, the administration of RF significantly shortened the time required for neurological assessment (P < 0.001 vs. fentanyl and morphine), and the extubation rate was notably faster than that in the morphine-treated group. These findings indicate that RF has superior analgesia-based sedative effects and enables more rapid awakening for neurological evaluation. Another study similarly demonstrated that in pediatric patients hospitalized due to TBI, continuous infusion of RF (at a median dose of 0.25 μg/kg/min) achieved target levels of sedation scores and neurological status, suggesting that RF can provide sedation with rapid onset and facilitate prompt recovery (51). A study by Engelhard et al. (52) also indicated that when used as an analgesic in TBI patients, RF did not exert adverse effects on cerebrovascular hemodynamics, cerebral perfusion pressure, or intracranial pressure. Collectively, these findings suggest that continuous RF infusion constitutes a safe and effective analgesic regimen for TBI patients or other patients intubated postneurosurgery. Moreover, RF has significant advantages in neurological assessment within clinical settings. In the future, developing an RF-centered analgesic regimen for TBI is promising, thereby enabling earlier intervention and improving the long-term neurological prognosis of patients (Table 2).
Table 2
| Disorders | Ages and genders | Control group | Remifentanil group | Clinical outcome | Ref. |
|---|---|---|---|---|---|
| TBI | Aged 18–80 years, n = 161 | Fentanyl (n = 37) or morphine (n = 40) | n = 84, before the propofol infusion started at 9 μg/kg/h | Permitted significantly faster and more predictable awakening for neurological assessment. | (50) |
| Median age was 9 years, n = 38 | Self-control | 0.25 μg/kg/min | Neurologic examinations remained reassuring during remifentanil infusion, a suitable sedative agent for use in children with TBI | (51) | |
| Aged 46 ± 18 years, n = 20 | Self-control | 0.5 mg/kg followed by 0.25 mg/kg/min for 20 min | No change in mean arterial blood pressure, intracranial pressure, and cerebral blood flow velocity | (52) | |
| Delirium | 752 patients >18 | Fentanyl (n = 376) | Intraoperative anesthetic dosage | Lower incidence of post-operative delirium in the early post-operative period | (57) |
| 80 children aged 3–7 years | Intraoperative remifentanil infusion (0.2 μg/kg/min) | Maintenance during the recovery phase 0.05 μg/kg/min | Attenuated the incidence of emergence delirium | (58) | |
| 95 nonoperative patients requiring ventilatory opioids >18 | Fentanyl, noradrenaline, midazolam, propofol, and dexmedetomidine | 0.029 μg/kg/min | Reduce delirium duration | (59) | |
| 84 patients aged 3–11 years | Sevoflurane and DEX (1 mg/kg) | Propofol (4 mg/kg/h) and remifentanil (0.03 mg/kg/h) | Recovery time was significantly shorter; incidence of emergence delirium decreased in both groups | (60) | |
| Spinal cord injury | 60 patients aged 14–28 | Alfentanil (1 mg/kg/min) | 0.2 mg/kg/min | Wake-up test can be conducted faster with remifentanil during PSF surgery. | (66) |
| Surgical-related brain injury and tracheal intubation | Aged 55–65 years, n = 40 | Saline | 0.6, 1.2, and 1.8 μg/kg/min | Played a protective role on brain damage, inhibition of the oxidative stress response | (70) |
| 2,760 patients underwent clipping of ruptured/unruptured ICA | Fentanyl only | Fentanyl+remifentanyl; anesthetic dosage | Reduced mortality | (71) | |
| 160 patients >18 | Saline | 0.5 and 0.05 μg/kg/min for 20 min | Significantly reduces the incidence of severe pain; the visual analog score is significantly lower. | (73) | |
| 161 patients aged 18–80 years | Fentanyl (n = 37) or morphine (n = 40) | 9–18 μg/kg/h | Significantly faster and more predictable awakening for neurological assessment | (50) | |
| 91 patients >18 | Fentanyl | 1 mg/kg and 1 mg/kg/min | Time to preoperative neurological recovery is faster | (74) | |
| Postoperative cognitive dysfunction | 70 patients undergoing radical surgery for cervical cancer | Fentanyl (n = 35) 0.3 μg/kg/min; maintenance dose of 1 μg/kg | 0.3 μg/kg/min; maintenance dose of 3 μg/kg | Low occurrence of postoperative cognitive dysfunction | (76) |
The neuroprotective effects of RF in clinical.
4.2 Delirium
Delirium is an acute brain dysfunction characterized by impairments in cognitive ability, attention, and memory function, accompanied by symptoms such as disturbance of consciousness, disorientation, sensory confusion, and agitation. This condition is highly prevalent among hospitalized elderly patients, with an incidence of up to 50% following high-risk surgical procedures and as high as 75% in critically ill patients requiring mechanical ventilation (53). Currently, there is no single intervention or pharmacotherapeutic agent for the treatment of delirium; clinical management primarily involves addressing the underlying cause, controlling psychiatric symptoms, and promoting cognitive-enhancing factors (54). Opioids, as commonly used clinical analgesics, are a major contributing factor to the development of postanaesthetic delirium (55). A study investigating the impact of different opioids on delirium incidence revealed that the use of tramadol or pethidine was associated with an increased risk of delirium, whereas no such association was observed with the use of morphine, fentanyl, oxycodone, or codeine (56). As a novel synthetic opioid, RF has a lower incidence of delirium than other opioids, such as fentanyl (Table 2). An analysis of 752 clinical cases demonstrated that the use of different intraoperative anesthetic opioids was associated with variations in postoperative delirium incidence, with the RF group showing a lower rate of early postoperative delirium than the fentanyl group (57). A study by Choi et al. (58) reported that, in children who underwent strabismus surgery under sevoflurane anesthesia, maintaining low-dose RF infusion during both the intraoperative period and recovery phase effectively reduced the incidence of postoperative delirium compared with intraoperative RF infusion alone. In addition to postoperative delirium following conventional surgery, another study revealed that RF also has a beneficial effect on delirium associated with mechanical ventilation in nonsurgical ICU patients. Compared with non-RF anesthetics such as fentanyl, RF significantly shortens the duration of delirium and effectively alleviates agitation (59). A study by Oriby and Elrashidy (60) compared total intravenous anesthesia with propofol and RF vs. inhalational sevoflurane combined with dexmedetomidine in children undergoing strabismus surgery with respect to emergence delirium. The results revealed no difference in the incidence of emergence delirium between the two groups; however, the propofol-RF group presented a significantly shorter recovery time. Collectively, these studies indicate that, compared with other sedatives, including fentanyl, RF reduces the incidence of delirium and shortens its duration in patients. Furthermore, maintaining RF infusion during the postoperative recovery phase results in a lower incidence of delirium than does intraoperative infusion alone (Table 2). These findings have important implications for the clinical selection of analgesic agents and the development of administration strategies for patients at high risk of delirium.
4.3 Spinal cord injury
Spinal cord injury (SCI) is a severe CNS disorder caused by external physical force, impairing patients' sensory and motor functions while inducing multisystem complications (e.g., psychological disorders) with poor prognosis (61). Secondary SCI results from persistent spinal cord compression, involving damage to neural tissue or vascular structures, followed by hypoxia and localized ischemic infarction after blood supply disruption, exacerbating spinal cord tissue damage (62). Secondary injury is a complex process involving mechanisms such as inflammatory response, oxidative stress, and neuronal apoptosis (63). Current clinical management of SCI primarily focuses on acute decompression surgery and postoperative rehabilitation training, with limited neuroprotective interventions targeting neuropathic pain.
Experimental studies have demonstrated that RF induces hyperalgesia or pro-nociceptive effects in mice or rat by changing the expressions of genes or proteins in the spinal cord (64, 65). For example, RF exerted pro-nociceptive effects in mice, which were similar to surgical injury. DOR mRNA levels in the dorsal root ganglia were inhibited by either RF administration or surgery. Downregulating DOR contributes to RF and surgery-induced nociception, while enhancing enkephalin levels in the spinal cord and the periphery mostly reversed postoperative pain that is caused by RF (64). In a rat model with plantar incision surgery, the continuous infusion of RF resulted in elevated P2X4 and BDNF expression, which played essential roles in RF-induced hyperalgesia (65).
A randomized controlled trial involving 60 patients undergoing elective posterior thoracolumbar spinal fusion (PSF) found that intraoperative RF infusion significantly shortened patient recovery time compared to alfentanil (66) (Table 2). Although this study indicated potential benefits of RF for SCI, it compared only recovery time and quality of recovery without assessing commonly used neurological methods for detecting intraoperative spinal cord dysfunctions, e.g., somatosensory evoked potentials and motor evoked potentials, representing a limitation. A prospective, randomized, controlled clinical trial indicated that intraoperative anesthesia with dexmedetomidine and lidocaine in patients undergoing multi-segmental spinal fusion significantly reduced postoperative nausea and vomiting incidence and decreased morphine consumption within 24 h postoperatively. The fentanyl treatment and RF group further reduced the probability of requiring antihypertensive medications and shortened hospital stay (67). Another double-blind study indicated that intraoperative use of RF combined with low-dose ketamine during spinal fusion surgery improved hemodynamic stability and reduced postoperative morphine consumption (compared to RF infusion alone) (68). These studies demonstrate that RF, as a primary anesthetic agent in spinal surgery, provides superior hemodynamic stability, reduces the risk of secondary injury, significantly decreases postoperative opioid consumption, and delivers effective analgesia.
4.4 Surgery-related cerebral injury and tracheal intubation
Surgical cerebral injury refers to the unavoidable brain damage induced by various surgical manipulations during neurosurgical procedures, with specific contributing factors, including incisions, retraction, electrocautery-induced thermal injury, and intraoperative hemorrhage (69). In addition to these neurosurgical interventions, studies have indicated that cerebral injury is relatively common in patients undergoing cardiac surgery (41). A study by Zhang et al. (70) demonstrated that preoperative administration of RF in patients undergoing pump-assisted coronary artery bypass grafting mitigated oxidative stress responses, significantly reduced S-100β protein levels, and exerted a protective effect against cerebral injury. This finding is consistent with the aforementioned role of RF in attenuating CPB-induced cerebral injury in rats. A retrospective study compared postoperative complications, in-hospital mortality, and length of hospital stay among 1,380 pairs of patients who underwent intracranial aneurysm clipping. The results revealed that the in-hospital mortality rate in the RF + fentanyl group was significantly lower than that in the fentanyl-only group; although there was no significant difference in the incidence of postoperative complications between the two groups, the severity of hydrocephalus was alleviated in the RF + fentanyl group (71). This study revealed that RF administration is an independent factor contributing to the reduction in postoperative in-hospital mortality in ICA patients, which provides certain implications for the clinical selection of opioid analgesics in this type of surgery.
Neuromonitored tracheal intubation is a medical technique that integrates airway management with neurological function monitoring. It is utilized primarily in surgical procedures or intensive care settings to enable real-time assessment of patients' neurological status while ensuring airway patency and effective ventilation (72). Neurosurgical patients often experience significant pain and stress responses during tracheal intubation; thus, analgesic and sedative agents are commonly employed in this scenario. A study revealed that when neurosurgical patients received either RF or normal saline before extubation, the incidence of severe pain in the RF group during extubation was significantly reduced, with no statistically significant difference in immediate vital signs between the two groups. These findings indicate that prophylactic RF administration can safely and effectively reduce the incidence of pain associated with tracheal intubation in neurosurgical patients (73). Another study compared the effects of RF, fentanyl, and morphine on cerebral injury in patients with acute brain injury or those intubated after neurosurgery. The results showed that with RF use, the variability in patients' neurological assessment results (before and after evaluation) was significantly reduced, the average time required for neurological assessment was notably shorter, and the extubation speed was significantly faster than that in the morphine group (50). A study by Gelb et al. (74) demonstrated that RF serves as a safe alternative to fentanyl in supratentorial craniotomy. RF administration led to a reduction in patients' systolic blood pressure; although there was no difference in extubation time between the two groups, patients in the RF group achieved faster preoperative mental recovery. Collectively, these findings suggest that RF provides effective and safe analgesic effects for neurosurgical patients undergoing surgery and tracheal intubation. Compared with fentanyl, RF has potential advantages in alleviating neurological injury in patients (Table 2).
4.5 Postoperative cognitive disorders
Postoperative cognitive dysfunction (POCD) represents a prevalent CNS complication subsequent to surgery and anesthesia, with its core clinical manifestations encompassing memory impairment, attentional deficits, disorientation, reduced executive function, and diminished language fluency. Such cognitive impairments not only impede patients' postoperative recovery trajectory and prolong hospital stay but are also are closely associated with an elevated risk of postoperative mortality (75). A research team led by Lu et al. (76) compared the effects of intraoperative intravenous infusion of RF vs. fentanyl on postoperative cognitive function and inflammatory responses in patients undergoing radical hysterectomy for cervical cancer. The findings revealed that the postoperative awakening time in the RF group was significantly shorter than that in the fentanyl group, and the incidence of POCD in the RF group was notably lower than that in the fentanyl group (Table 2). Despite the aforementioned positive effects of RF on cognitive dysfunction, several studies have also documented its adverse impacts on cognitive function. One study reported that the administration of propofol, RF, or their combination in SD rats induced cognitive decline, which was specifically characterized by a significant prolongation of the latency period, as assessed by the Morris water maze test, and an increased level of Tau protein phosphorylation in the rat hippocampus (77). Another prospective, double-blind, randomized study reported no significant difference between RF and fentanyl in the incidence of POCD in elderly patients who underwent major abdominal surgery (78). Although the level of IL-6 in the RF group was significantly lower on the 7th postoperative day and the incidence of POCD in this group was the lowest among all patients, the absence of a direct correlation between inflammatory factor levels and POCD, coupled with the nonsignificant difference in POCD incidence, precluded the confirmation that RF reduces POCD in patients undergoing abdominal surgery. Studies focusing on opioids have demonstrated that morphine, fentanyl, and other opioids play pivotal roles in the inflammatory response (79). Notably, the aforementioned study (involving major abdominal surgery) indicated that RF has superior anti-inflammatory efficacy to fentanyl. This particular study enrolled 622 patients aged over 60 years who underwent major abdominal surgery as experimental subjects, indicating that the scale and research value of the scale are certain. In contrast, the study by Lu et al. (76) included 70 female patients who underwent radical hysterectomy for cervical cancer. The conflicting conclusions drawn from the two studies may be attributed to inconsistencies in the definition and assessment tools for POCD, as well as differences in the timing of evaluations. Furthermore, variations in clinical sample size, surgical procedures, patient gender, and age may have limited the statistical power and generalizability of the results, potentially introducing selection bias. Both studies compared the effects of RF and fentanyl on the incidence of POCD without conducting an in-depth exploration of other potential mechanisms or a broader range of neurocognitive indicators. Nevertheless, these studies still provide valuable references for the selection of intraoperative anesthetic and analgesic agents aimed at preventing POCD in different types of surgical procedures.
5 Remifentanil-induced hyperalgesia
Opioids are commonly used to treat neuropathic pain. Woller and Hook (80) found that clinical opioid use may increase pain progression, reduce motor recovery, and elevate infection risk. Opioids, including morphine (81), fentanyl (82), and heroin (83), can induce opioid-induced hyperalgesia (OIH), an adverse reaction that cannot be overcome by increasing drug dosage. In-depth investigation of OIH mechanisms and enhanced clinical management of opioid use are key research priorities.
Remifentanil-induced hyperalgesia (RIH) represents one of the most common adverse reactions associated with the clinical use of RF, and the mechanisms underlying RF-induced hyperalgesia have emerged as a research focus in recent years (84). Generally, the occurrence of RIH is reportedly associated with continuous RF infusion (at a rate of 0.3 mg/kg/min or higher) or abrupt discontinuation of RF (85). The mechanism of RIH involves sensitization of the central nervous system and is closely related to the activation of N-methyl-D-aspartate (NMDA) receptors (86). For example, the high-dose intravenous RF administration (bolus of 6.0 and 2.5 μg/kg/min for 2 h) induces hyperalgesia in rats, accompanied by elevated levels of phosphorylated CaMKII in the central nervous system, reflecting the interaction between NMDA receptors and pain mechanisms (87).
The core strategies for preventing and alleviating clinical RIH involve NMDA receptor antagonism and multimodal analgesia. A randomized controlled trial indicated that high-dose RF infusion (0.40 μg/kg/min) during major abdominal surgery led to postoperative hyperalgesia and increased morphine consumption, whereas low-dose ketamine (0.5 mg/kg) mitigated RIH by blocking NMDA receptors (88). A randomized controlled study involving 180 patients undergoing laparoscopic cholecystectomy found that propofol (0.3 μg/kg/min) suppressed NMDA glutamate subtype receptors and alleviated RIH by modulating NMDA receptor-mediated intracellular calcium influx (89). Beyond the NMDA pathway, a meta-analysis systematically evaluated preclinical studies on RIH mitigation, detailing comparative efficacy across different drug groups (RF dosage, animal models, etc.). Among these, Annexin A1-derived peptide (Anxa12-26), P2Y purinoceptor 1 antagonist (MRS2179), and α2-adrenergic agonist dexmedetomidine ranked highly and warrant inclusion in future clinical interventions for RIH (90).
RF has demonstrated to provide rapid recovery, reduce postoperative delirium, and mitigate secondary brain injury through hemodynamic stabilization in patients with TBI and surgery-related neurological damage. When considering RF for neuroprotection, its potential risk of RIH must be weighed. Thus, for clinical anesthesia and analgesia, RF administration requires careful dose selection, gradual tapering, and consideration of combination therapy (e.g., propofol, dexmedetomidine). For non-high-risk surgeries or chronic pain patients, dosage and duration must be cautiously evaluated.
6 Limitations
The main limitation of this review is related to the heterogeneity of clinical studies. This narrative review typically covers a broader range of literature concerning the neuroprotective effects of RF. However, each CNS disorder has its specific clinical manifestations, making it difficult to compare the neuroprotective effects of RF among different CNS diseases. The overall quality of those studies has not been evaluated qualitatively. Therefore, potential biases, such as literature selection bias, performance bias, detection bias, attrition bias, and reporting bias, cannot be completely avoided. To further confirm the neuroprotective effects of RN, more rigorously designed and large-scale prospective studies should be included for qualitative analysis and comparison.
Although RF has demonstrated significant neuroprotective effects in experimental models such as the rat MCAO model, these findings carry certain limitations. First, most preclinical studies utilize healthy animals, whose pathophysiological processes differ significantly from those of patients with multiple comorbidities (e.g., hypertension, diabetes), thereby limiting their clinical translation value. Future basic research must further investigate whether the neuroprotective signaling pathways of RF remain effective in complex models involving common clinical comorbidities such as advanced age, gender, metabolic disorders (e.g., hyperlipidemia and hyperglycemia), hypertension, and atherosclerosis.
7 Conclusion and perspective
As a synthetic ultrashort-acting opioid, RF has certain neuroprotective effects. Basic research has demonstrated that RF exerts protective effects against cerebral ischemia/reperfusion injury through mechanisms such as reducing inflammation, activating antiapoptotic signaling pathways, and inhibiting oxidative stress; RF also alleviates LPS-induced neuroinflammation both in vivo and in vitro and confers protective effects against neonatal encephalopathy and surgery-related cerebral injury. Clinical studies have shown that RF serves as a safe and effective analgesic regimen for patients with TBI, patients undergoing neurosurgical procedures, and patients requiring tracheal intubation while also providing certain benefits for the recovery of patients' neurological function (Figure 2). Additionally, compared with fentanyl, RF is associated with a reduced incidence of delirium and postoperative cognitive dysfunction in patients.
Figure 2
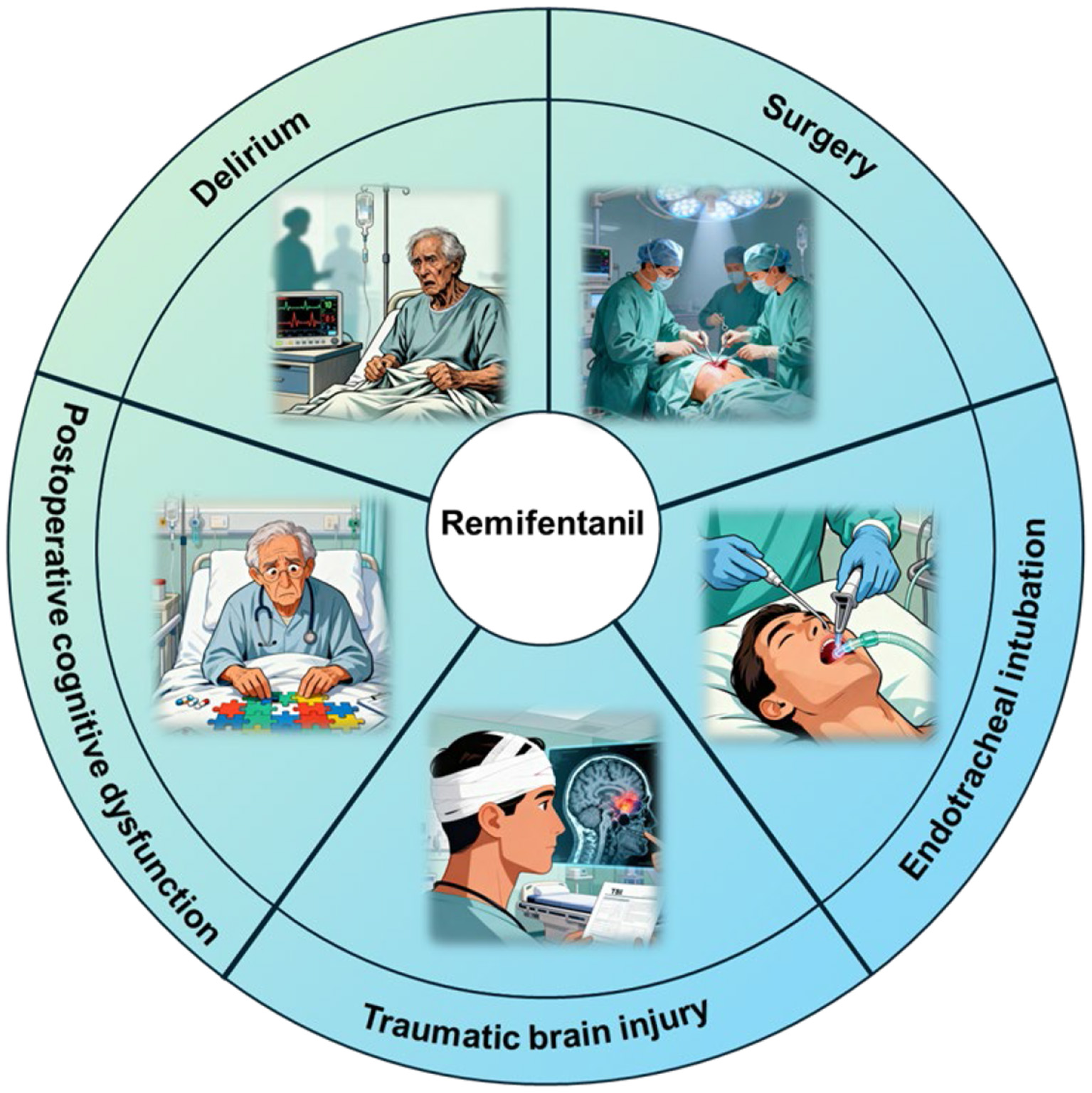
The neuroprotective effect of remifentanil in clinical applications. Remifentanil plays a neuroprotective role in traumatic brain injury, delirium, postoperative cognitive dysfunction, surgical procedures, and tracheal intubation.
RF is most commonly used in surgical anesthesia. One study compared the use of RF, fentanyl, and alfentanil (each combined with propofol) for anesthetic induction and maintenance in patients undergoing craniotomy. The results revealed no significant differences among the three groups in terms of the required maintenance dose of propofol, heart rate, or mean arterial pressure. However, patients in the RF group presented a significantly shorter eye-opening time and achieved faster postoperative recovery (91). In conjunction with the aforementioned clinical findings, RF is associated with a lower incidence of postoperative delirium, surgery-related cerebral injury, and other adverse outcomes than fentanyl is. These differences are likely attributed to the significant pharmacokinetic disparities between RF and fentanyl. These studies suggest that in the clinical selection of anesthetic agents, RF may be considered one of the preferred analgesic regimens, depending on the type of surgery and the risk of neurological sequelae in patients. The clinical studies analyzed in this review demonstrate that RF offers clear advantages in specific clinical scenarios, such as intraoperative hemodynamic management and rapid recovery. However, adverse reactions with opioid medications, such as RIH, must be considered in clinical practice. Therefore, when RF is selected for clinical anesthetic analgesia, careful attention should be given to the injection dose, and considerations should be given to strategies such as gradual drug withdrawal and combined administration with other agents (e.g., propofol and dexmedetomidine). Future research should focus on in-depth investigations into the mechanisms of RF-induced hyperalgesia and the development of more effective and safe preventive approaches. Furthermore, it is essential to conduct in-depth studies on the relationship between RF dosage and its neuroprotective effects; explore the optimal timing (pretreatment, posttreatment, or full-course intervention), optimal dosage, and infusion mode for RF-mediated neuroprotection; and develop personalized medication regimens for RF in different populations (e.g., elderly individuals, neonates, and populations at high risk of neurodegenerative diseases).
Statements
Author contributions
XJ: Investigation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. MW: Conceptualization, Investigation, Methodology, Software, Writing – original draft. CY: Investigation, Software, Visualization, Writing – original draft. JL: Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CaMKIIα, calcium-dependent protein kinase II alpha; CNS, central nervous system; CREB, cAMP response element-binding protein; ERK 1/2, extracellular signal-regulated kinase 1/2; HIBD, hypoxic-ischemic brain damage; IL-1β, interleukin-1 beta; IL-6, interleukin-6; IRI, ischemia–reperfusion injury; JNK, c-Jun N-terminal kinase; MEK1/2 (MAP2K), mitogen-activated protein kinase kinase 1/2; NE, neonatal encephalopathy; NR2B, NMDA receptor subtype 2B; OPRs, opioid receptors; PI3K, phosphoinositide 3-kinase; POCD, postoperative cognitive dysfunction; RF, remifentanil; RIH, remifentanil-induced hyperalgesia; ROS, reactive oxygen species; SCI, spinal cord injury; TBI, traumatic brain injury; TNFR1, tumor necrosis factor receptor 1; TNF-α, tumor necrosis factor-alpha.
References
1.
Minami M Satoh M . Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci Res. (1995) 23:121–45. doi: 10.1016/0168-0102(95)00933-K
2.
Maslov LN Khaliulin I Oeltgen PR Naryzhnaya NV Pei JM Brown SA et al . Prospects for creation of cardioprotective and antiarrhythmic drugs based on opioid receptor agonists. Med Res Rev. (2016) 36:871–923. doi: 10.1002/med.21395
3.
Mercadante S . Prospects and challenges in opioid analgesia for pain management. Curr Med Res Opin. (2011) 27:1741–3. doi: 10.1185/03007995.2011.602057
4.
Yi S Cao H Zheng W Wang Y Li P Wang S et al . Targeting the opioid remifentanil: protective effects and molecular mechanisms against organ ischemia-reperfusion injury. Biomed Pharmacother Biomedecine Pharmacother. (2023) 167:115472. doi: 10.1016/j.biopha.2023.115472
5.
Feldman PL James MK Brackeen MF Bilotta JM Schuster SV Lahey AP et al . Design, synthesis, and pharmacological evaluation of ultrashort- to long-acting opioid analgetics. J Med Chem. (1991) 34:2202–8. doi: 10.1021/jm00111a041
6.
Stanley TH . The fentanyl story. J Pain. (2014) 15:1215–26. doi: 10.1016/j.jpain.2014.08.010
7.
Beers R Camporesi E . Remifentanil update: clinical science and utility. CNS Drugs. (2004) 18:1085–104. doi: 10.2165/00023210-200418150-00004
8.
Bürkle H Dunbar S Van Aken H . Remifentanil: a novel, short-acting, mu-opioid. Anesth Analg. (1996) 83:646–51. doi: 10.1097/00000539-199609000-00038
9.
Pan HL Chen SR . Sensing tissue ischemia: another new function for capsaicin receptors?Circulation. (2004) 110:1826–31. doi: 10.1161/01.CIR.0000142618.20278.7A
10.
Stroumpos C Manolaraki M Paspatis GA . Remifentanil, a different opioid: potential clinical applications and safety aspects. Expert Opin Drug Saf. (2010) 9:355–64. doi: 10.1517/14740331003672579
11.
Greco M Landoni G Biondi-Zoccai G Cabrini L Ruggeri L Pasculli N et al . Remifentanil in cardiac surgery: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. (2012) 26:110–6. doi: 10.1053/j.jvca.2011.05.007
12.
Zhao Y Zhang X Chen X Wei Y . Neuronal injuries in cerebral infarction and ischemic stroke: from mechanisms to treatment (review). Int J Mol Med. (2022) 49:15. doi: 10.3892/ijmm.2021.5070
13.
Hu X Xie C He S Zhang Y Li Y Jiang L . Remifentanil postconditioning improves global cerebral ischemia-induced spatial learning and memory deficit in rats via inhibition of neuronal apoptosis through the PI3K signaling pathway. Neurol Sci. (2013) 34:1955–62. doi: 10.1007/s10072-013-1419-4
14.
Jeong S Kim SJ Jeong C Lee S Jeong H Lee J et al . Neuroprotective effects of remifentanil against transient focal cerebral ischemia in rats. J Neurosurg Anesthesiol. (2012) 24:51–7. doi: 10.1097/ANA.0b013e3182368d70
15.
Zhang Y Li YW Wang YX Zhang HT Zhang XM Liang Y et al . Remifentanil preconditioning alleviating brain damage of cerebral ischemia reperfusion rats by regulating the JNK signal pathway and TNF-α/TNFR1 signal pathway. Mol Biol Rep. (2013) 40:6997–7006. doi: 10.1007/s11033-013-2819-5
16.
Chen CR Bi HL Li X Li ZM . Remifentanil protects neurological function of rats with cerebral ischemia-reperfusion injury via NR2B/CaMKIIα signaling pathway. J Biol Regul Homeost Agents. (2020) 34:1647–56. doi: 10.23812/20-169-A
17.
Park SW Yi JW Kim YM Kang JM Kim DO Shin MS et al . Remifentanil alleviates transient cerebral ischemia-induced memory impairment through suppression of apoptotic neuronal cell death in gerbils. Korean J Anesthesiol. (2011) 61:63–8. doi: 10.4097/kjae.2011.61.1.63
18.
Cheng L Wu Y Tang J Zhang C Cheng H Jiang Q Jian C . Remifentanil protects against myocardial ischemia/reperfusion injury via miR-205-mediated regulation of PINK1. J Toxicol Sci. (2021) 46:263–71. doi: 10.2131/jts.46.263
19.
Chen Y Zhang J Li F . Inhibitory role of remifentanil in hepatic ischemia-reperfusion injury through activation of Fmol/Parkin signaling pathway: a study based on network pharmacology analysis and high-throughput sequencing. Phytomedicine Int J Phytother Phytopharm. (2024) 128:155300. doi: 10.1016/j.phymed.2023.155300
20.
Shen J Zhan Y He Q Deng Q Li K Wen S et al . Remifentanil promotes PDIA3 expression by activating p38MAPK to inhibit intestinal ischemia/reperfusion-induced oxidative and endoplasmic reticulum stress. Front Cell Dev Biol. (2022) 10:818513. doi: 10.3389/fcell.2022.818513
21.
Cui C Yu F Yin S Yang Y Jiao Y Cheung C et al . Remifentanil preconditioning attenuates hepatic ischemia-reperfusion injury in rats via neuronal activation in dorsal vagal complex. Mediators Inflamm. (2018) 2018:3260256. doi: 10.1155/2018/3260256
22.
You Y Xing X Tang B Deng H Lei E Wu Y . Remifentanil mitigates hepatic ischemia/reperfusion-induced D1-medium spiny neurons damage via fibroblast growth factor 18 upregulation. Antioxid Redox Signal. (2025) 43:709–26. doi: 10.1089/ars.2024.0892
23.
Shabab T Khanabdali R Moghadamtousi SZ Kadir HA Mohan G . Neuroinflammation pathways: a general review. Int J Neurosci. (2017) 127:624–33. doi: 10.1080/00207454.2016.1212854
24.
Yong HYF Rawji KS Ghorbani S Xue M Yong VW . The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol. (2019) 16:540–6. doi: 10.1038/s41423-019-0223-3
25.
Adamu A Li S Gao F Xue G . The role of neuroinflammation in neurodegenerative diseases: current understanding and future therapeutic targets. Front Aging Neurosci. (2024) 16:1347987. doi: 10.3389/fnagi.2024.1347987
26.
Dominguini D Steckert AV Michels M Spies MB Ritter C Barichello T et al . The effects of anaesthetics and sedatives on brain inflammation. Neurosci Biobehav Rev. (2021) 127:504–13. doi: 10.1016/j.neubiorev.2021.05.009
27.
Özcan MS Aşci H Karabacak P Özden ES Imeci OB Özmen Ö . Remifentanil ameliorates lipopolysaccharide-induced neuroinflammation by regulating the phosphatidylinositol 3-kinase/serine–threonine protein kinase/hypoxia-inducible factor 1 alpha pathway. Pharmacol Res Perspect. (2025) 13:e70071. doi: 10.1002/prp2.70071
28.
Tsao CC Baumann J Huang SF Kindler D Schroeter A Kachappilly N et al . Pericyte hypoxia-inducible factor-1 (HIF-1) drives blood-brain barrier disruption and impacts acute ischemic stroke outcome. Angiogenesis. (2021) 24:823–42. doi: 10.1007/s10456-021-09796-4
29.
Maeda S Andoh T Onishi R Tomoyasu Y Higuchi H Miyawaki T . Remifentanil suppresses increase in interleukin-6 mRNA in the brain by inhibiting cyclic AMP synthesis. J Anesth. (2018) 32:731–9. doi: 10.1007/s00540-018-2548-y
30.
Tanabe K Kozawa O Iida H . cAMP/PKA enhances interleukin-1β-induced interleukin-6 synthesis through STAT3 in glial cells. Cell Signal. (2016) 28:19–24. doi: 10.1016/j.cellsig.2015.10.009
31.
Huang Y Cai Q Liu H Wang Y Ma W . Remifentanil inhibits the inflammatory response of BV2 microglia and protects PC12 cells from damage caused by microglia activation. Bioengineered. (2022) 13:13944–55. doi: 10.1080/21655979.2022.2080421
32.
Schump EA . Neonatal encephalopathy: current management and future trends. Crit Care Nurs Clin North Am. (2018) 30:509–21. doi: 10.1016/j.cnc.2018.07.007
33.
Martinez-Biarge M Diez-Sebastian J Wusthoff CJ Mercuri E Cowan FM . Antepartum and intrapartum factors preceding neonatal hypoxic-ischemic encephalopathy. Pediatrics. (2013) 132:e952–9. doi: 10.1542/peds.2013-0511
34.
Nelson KB Bingham P Edwards EM Horbar JD Kenny MJ Inder T et al . Antecedents of neonatal encephalopathy in the Vermont Oxford network encephalopathy registry. Pediatrics. (2012) 130:878–86. doi: 10.1542/peds.2012-0714
35.
Ogawa Y Tsuji M Tanaka E Miyazato M Hino J . Bone morphogenetic protein (BMP)-3b gene depletion causes high mortality in a mouse model of neonatal hypoxic-ischemic encephalopathy. Front Neurol. (2018) 9:397. doi: 10.3389/fneur.2018.00397
36.
Lu WB Wang J . Remifentanil alleviates hypoxic-ischemic brain damage-induced cognitive impairment via BACH1. Neurosci Lett. (2022) 786:136802. doi: 10.1016/j.neulet.2022.136802
37.
Chollat C Lecointre M Leuillier M Remy-Jouet I Do Rego JC Abily-Donval L et al . Beneficial effects of remifentanil against excitotoxic brain damage in newborn mice. Front Neurol. (2019) 10:407. doi: 10.3389/fneur.2019.00407
38.
Pan B Huang S Sun S Wang T . The neuroprotective effects of remifentanil on isoflurane-induced apoptosis in the neonatal rat brain. Am J Transl Res. (2017) 9:4521–33. doi: 10.26355/eurrev_201805_14987
39.
Tourrel F de Lendeu PK Abily-Donval L Chollat C Marret S Dufrasne F et al . The antiapoptotic effect of remifentanil on the immature mouse brain: an ex vivo study. Anesth Analg. (2014) 118:1041–51. doi: 10.1213/ANE.0000000000000159
40.
Naldan ME Taghizadehghalehjoughi A . Remifentanil reduces glutamate toxicity in rat olfactory bulb neurons in culture. Braz J Anesthesiol Elsevier. (2021) 71:402–7. doi: 10.1016/j.bjane.2021.04.003
41.
Scolletta S Taccone FS Donadello K . Brain injury after cardiac surgery. Minerva Anestesiol. (2015) 81:662–77.
42.
Ramponi F Hon K Seco M Fanning JP Bannon PG Kritharides L et al . Chapter 43 - brain injury in cardiopulmonary bypass. In:KiraliKCoselliJSKalangosA, editors. Cardiopulmonary Bypass.New York, NY: Academic Press (2023). p. 659–73. doi: 10.1016/B978-0-443-18918-0.00043-7
43.
Xiong J Quan J Qin C Wang X Dong Q Zhang B . Remifentanil pretreatment attenuates brain nerve injury in response to cardiopulmonary bypass by blocking AKT/NRF2 signal pathway. Immunopharmacol Immunotoxicol. (2022) 44:574–85. doi: 10.1080/08923973.2022.2069577
44.
Ali T Kim T Rehman SU Khan MS Amin FU Khan M et al . Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer's disease. Mol Neurobiol. (2018) 55:6076–93. doi: 10.1007/s12035-017-0798-6
45.
Dai H Wang P Mao H Mao X Tan S Chen Z . Dynorphin activation of kappa opioid receptor protects against epilepsy and seizure-induced brain injury via PI3K/Akt/Nrf2/HO-1 pathway. Cell Cycle Georget Tex. (2019) 18:226–37. doi: 10.1080/15384101.2018.1562286
46.
Cui W Leng B Wang G . Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can J Physiol Pharmacol. (2019) 97:370–6. doi: 10.1139/cjpp-2018-0277
47.
Pavlovic D Pekic S Stojanovic M Popovic V . Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary. (2019) 22:270–82. doi: 10.1007/s11102-019-00957-9
48.
Xiong Y Zhang Y Mahmood A Chopp M . Investigational agents for treatment of traumatic brain injury. Expert Opin Investig Drugs. (2015) 24:743–60. doi: 10.1517/13543784.2015.1021919
49.
Rahimi S Dadfar B Tavakolian G Asadi Rad A Rashid Shabkahi A Siahposht-Khachaki A . Morphine attenuates neuroinflammation and blood-brain barrier disruption following traumatic brain injury through the opioidergic system. Brain Res Bull. (2021) 176:103–11. doi: 10.1016/j.brainresbull.2021.08.010
50.
Karabinis A Mandragos K Stergiopoulos S Komnos A Soukup J Speelberg B et al . Safety and efficacy of analgesia-based sedation with remifentanil versus standard hypnotic-based regimens in intensive care unit patients with brain injuries: a randomised, controlled trial [ISRCTN50308308]. Crit Care. (2004) 8:R268–80. doi: 10.1186/cc2896
51.
Hungerford JL O'Brien N Moore-Clingenpeel M Sribnick EA Sargel C Hall M et al . Remifentanil for sedation of children with traumatic brain injury. J Intensive Care Med. (2019) 34:557–62. doi: 10.1177/0885066617704390
52.
Engelhard K Reeker W Kochs E Werner C . Effect of remifentanil on intracranial pressure and cerebral blood flow velocity in patients with head trauma. Acta Anaesthesiol Scand. (2004) 48:396–9. doi: 10.1111/j.0001-5172.2004.00348.x
53.
Ormseth CH LaHue SC Oldham MA Josephson SA Whitaker E Douglas VC . Predisposing and precipitating factors associated with delirium: a systematic review. JAMA Netw Open. (2023) 6:e2249950. doi: 10.1001/jamanetworkopen.2022.49950
54.
Mattison MLP . Delirium. Ann Intern Med. (2020) 173:ITC49–64. doi: 10.7326/AITC202010060
55.
Sugiyama Y Tanaka R Sato T Sato T Saitoh A Yamada D et al . Incidence of delirium with different oral opioids in previously opioid-naive patients. Am J Hosp Palliat Care. (2022) 39:1145–51. doi: 10.1177/10499091211065171
56.
Swart LM van der Zanden V Spies PE de Rooij SE van Munster BC . The comparative risk of delirium with different opioids: a systematic review. Drugs Aging. (2017) 34:437–43. doi: 10.1007/s40266-017-0455-9
57.
Radtke FM Franck M Lorenz M Luetz A Heymann A Wernecke KD et al . Remifentanil reduces the incidence of post-operative delirium. J Int Med Res. (2010) 38:1225–32. doi: 10.1177/147323001003800403
58.
Choi EK Lee S Kim WJ Park SJ . Effects of remifentanil maintenance during recovery on emergence delirium in children with sevoflurane anesthesia. Paediatr Anaesth. (2018) 28:739–44. doi: 10.1111/pan.13446
59.
Haruna J Sasaki A Kazuma S . Remifentanil use in critically Ill patients requiring mechanical ventilation is associated with increased delirium-free days: a retrospective study. Int J Emerg Med. (2025) 18:58. doi: 10.1186/s12245-025-00846-y
60.
Oriby ME Elrashidy A . Comparative effects of total intravenous anesthesia with propofol and remifentanil versus inhalational sevoflurane with dexmedetomidine on emergence delirium in children undergoing strabismus surgery. Anesthesiol Pain Med. (2021) 11:e109048. doi: 10.5812/aapm.109048
61.
Lee W Jeong S Lee BS Lim JC Kim O . Association between functional outcomes and psychological variables in persons with spinal cord injury. Sci Rep. (2023) 13:23092. doi: 10.1038/s41598-023-50252-8
62.
Sterner RC Sterner RM . Immune response following traumatic spinal cord injury: pathophysiology and therapies. Front Immunol. (2022) 13:1084101. doi: 10.3389/fimmu.2022.1084101
63.
Zhu J Wang S Zhang Y Zhou C . Identification and validation of biomarkers associated with mitochondrial dysfunction and ferroptosis in rat spinal cord injury. Front Neurol. (2025) 16:1526966. doi: 10.3389/fneur.2025.1526966
64.
Cabañero D Célérier E García-Nogales P Mata M Roques BP Maldonado R et al . The pro-nociceptive effects of remifentanil or surgical injury in mice are associated with a decrease in delta-opioid receptor mRNA levels: prevention of the nociceptive response by on-site delivery of enkephalins. Pain. (2009) 141:88–96. doi: 10.1016/j.pain.2008.10.011
65.
Song F Wang A Feng G Wang L Zhang L Deng L . Dexmedetomidine alleviates remifentanil-induced hyperalgesia in rats by modulating the P2 X 4/BDNF pathway. Neurochem Res. (2025) 50:130. doi: 10.1007/s11064-025-04377-z
66.
Imani F Jafarian A Hassani V Khan ZH . Propofol–alfentanil vs propofol–remifentanil for posterior spinal fusion including wake-up test. Br J Anaesth. (2006) 96:583–6. doi: 10.1093/bja/ael075
67.
Barakat H Al Nawwar R Abou Nader J Aouad M Yazbeck Karam V Gholmieh L . Opioid-free versus opioid-based anesthesia in major spine surgery: a prospective, randomized, controlled clinical trial. Minerva Anestesiol. (2024) 90:482–90. doi: 10.23736/S0375-9393.24.17962-X
68.
Hadi BA Al Ramadani R Daas R Naylor I Zelkó R . Remifentanil in combination with ketamine versus remifentanil in spinal fusion surgery–a double blind study. Int J Clin Pharmacol Ther. (2010) 48:542–8. doi: 10.5414/CPP48542
69.
Travis ZD Sherchan P Hayes WK Zhang JH . Surgically-induced brain injury: where are we now?Chin Neurosurg J. (2019) 5:29. doi: 10.1186/s41016-019-0181-8
70.
Zhang TZ Zhou J Jin Q Sun YJ Diao YG Zhang YN et al . Protective effects of remifentanil preconditioning on cerebral injury during pump-assisted coronary artery bypass graft. Genet Mol Res. (2014) 13:7658–65. doi: 10.4238/2014.September.26.3
71.
Uchida K Yasunaga H Sumitani M Horiguchi H Fushimi K Yamada Y . Effects of remifentanil on in-hospital mortality and length of stay following clipping of intracranial aneurysm: a propensity score-matched analysis. J Neurosurg Anesthesiol. (2014) 26:291–8. doi: 10.1097/ANA.0000000000000039
72.
Battaglini D Siwicka Gieroba D Brunetti I Patroniti N Bonatti G Rocco PRM et al . Mechanical ventilation in neurocritical care setting: a clinical approach. Best Pract Res Clin Anaesthesiol. (2021) 35:207–20. doi: 10.1016/j.bpa.2020.09.001
73.
Wu YX Chen H Li Q Hao JJ Zhao LH He X et al . The prophylactic use of remifentanil for delayed extubation after elective intracranial operations: a prospective, randomized, double-blinded trial. J Neurosurg Anesthesiol. (2017) 29:281–90. doi: 10.1097/ANA.0000000000000311
74.
Gelb AW Salevsky F Chung F Ringaert K McTaggart-Cowan RM Wong T et al . Remifentanil with morphine transitional analgesia shortens neurological recovery compared to fentanyl for supratentorial craniotomy. Can J Anaesth J Can Anesth. (2003) 50:946–52. doi: 10.1007/BF03018745
75.
Zhao Q Wan H Pan H Xu Y . Postoperative cognitive dysfunction-current research progress. Front Behav Neurosci. (2024) 18:1328790. doi: 10.3389/fnbeh.2024.1328790
76.
Lu XY Chen M Chen DH Li Y Liu PT Liu Y . Remifentanil on T lymphocytes, cognitive function and inflammatory cytokines of patients undergoing radical surgery for cervical cancer. Eur Rev Med Pharmacol Sci. (2018) 22:2854–9.
77.
Zhi XL Li CY Xue M Hu Y Ji Y . Changes in cognitive function due to combined propofol and remifentanil treatment are associated with phosphorylation of Tau in the hippocampus, abnormal total water and calcium contents of the brain, and elevated serum S100β levels. Eur Rev Med Pharmacol Sci. (2016) 20:2156–62.
78.
De Cosmo G Sessa F Fiorini F Congedo E . Effect of remifentanil and fentanyl on postoperative cognitive function and cytokines level in elderly patients undergoing major abdominal surgery. J Clin Anesth. (2016) 35:40–6. doi: 10.1016/j.jclinane.2016.07.016
79.
Odunayo A Dodam JR Kerl ME DeClue AE . Immunomodulatory effects of opioids. J Vet Emerg Crit Care San Antonio Tex 2001. (2010) 20:376–85. doi: 10.1111/j.1476-4431.2010.00561.x
80.
Woller SA Hook MA . Opioid administration following spinal cord injury: implications for pain and locomotor recovery. Exp Neurol. (2013) 247:328–41. doi: 10.1016/j.expneurol.2013.03.008
81.
Mangutov E Pradhan AA . Tiam1 is part of a novel mechanism for morphine tolerance and hyperalgesia. Brain J Neurol. (2024) 147:2264–6. doi: 10.1093/brain/awae170
82.
Mauermann E Filitz J Dolder P Rentsch KM Bandschapp O Ruppen W . Does fentanyl lead to opioid-induced hyperalgesia in healthy volunteers?: A double-blind, randomized, crossover trial. Anesthesiology. (2016) 124:453–63. doi: 10.1097/ALN.0000000000000976
83.
Compton P Canamar CP Hillhouse M Ling W . Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. J Pain. (2012) 13:401–9. doi: 10.1016/j.jpain.2012.01.001
84.
Zhu K Wen X Mei X Fang F Zhang T . Mechanisms of remifentanil-induced postoperative hyperalgesia: a comprehensive review. Drug Des Devel Ther. (2025) 19:7445–57. doi: 10.2147/DDDT.S550335
85.
Vitin AA Egan TD . Remifentanil-induced hyperalgesia: the current state of affairs. Curr Opin Anaesthesiol. (2024) 37:371–8. doi: 10.1097/ACO.0000000000001400
86.
Angst MS Koppert W Pahl I Clark DJ Schmelz M . Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. (2003) 106:49–57. doi: 10.1016/S0304-3959(03)00276-8
87.
Wang Q Zhao X Li S Han S Peng Z Li J . Phosphorylated CaMKII levels increase in rat central nervous system after large-dose intravenous remifentanil. Med Sci Monit Basic Res. (2013) 19:118–25. doi: 10.12659/MSMBR.883866
88.
Joly V Richebe P Guignard B Fletcher D Maurette P Sessler DI et al . Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. (2005) 103:147–55. doi: 10.1097/00000542-200507000-00022
89.
Su X Zhu W Tian Y Tan L Wu H Wu L . Regulatory effects of propofol on high-dose remifentanil-induced hyperalgesia. Physiol Res. (2020) 69:157–64. doi: 10.33549/physiolres.934133
90.
Koponen ME Naray E Hales TG Forget P . Pharmacological interventions for remifentanil-induced hyperalgesia: a systematic review and network meta-analysis of preclinical trials. PLoS ONE. (2024) 19:e0313749. doi: 10.1371/journal.pone.0313749
91.
Coles JP Leary TS Monteiro JN Brazier P Summors A Doyle P et al . Propofol anesthesia for craniotomy: a double-blind comparison of remifentanil, alfentanil, and fentanyl. J Neurosurg Anesthesiol. (2000) 12:15–20. doi: 10.1097/00008506-200001000-00004
Summary
Keywords
remifentanil, neuroprotection, apoptosis, neuroinflammation, ischemia–reperfusion injury
Citation
Jiang X, Wu M, Yan C and Liu J (2025) A narrative review of neuroprotective effects of remifentanil: from basic research to clinical practice. Front. Neurol. 16:1707972. doi: 10.3389/fneur.2025.1707972
Received
18 September 2025
Revised
14 November 2025
Accepted
17 November 2025
Published
10 December 2025
Volume
16 - 2025
Edited by
Luodan Yang, South China Normal University, China
Reviewed by
James W. Grau, Texas A and M University, United States
Igor Seror Cuiabano, Centro Universitario de Varzea Grande Curso de Medicina, Brazil
Updates
Copyright
© 2025 Jiang, Wu, Yan and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Liu, jialiu96@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.