- 1Department of Oncology, The First Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China
- 2Departerment of Oncology, Gaoxin Branch of the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
- 3Nanchang Key Laboratory of Tumor Gene Diagnosis and Innovative Treatment Research, Gaoxin Branch of the First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China
Background: TP53 mutation is a poor factor for non-small cell lung cancer (NSCLC), while the effect of TP53 on prognosis in epidermal growth factor receptor (EGFR)-mutated lung adenocarcinoma (LUAD) with brain metastasis remains elusive and needs further exploration.
Methods: We retrospectively analyzed 236 patients and tested for TP53- and EGFR-mutant status in metastasis LUAD patients who had received first-line EGFR-tyrosine kinase inhibitor (TKI) treatment. Survival rates were calculated by the Kaplan–Meier method. Furthermore, univariate and multivariate Cox analyses were performed to identify the independent prognostic factors.
Results: There were 114 patients with confirmed non-brain metastasis (NBM), 74 patients with preliminary diagnosis early brain metastasis (EBM), and 48 patients with late brain metastasis (LBM). TP53 and EGFR co-mutations were found in 35/236 patients (14.8%). The median progression-free survival (PFS) and overall survival (OS) in the EGFR mutation and TP53 wild-type group were significantly longer than those in the EGFR and TP53 co-mutation group in all advanced LUAD or NBM. Concurrently, PFS and OS were found to be not significant in EBM and LBM patients. Subgroup analysis revealed longer median PFS and OS in the TP53 wild-type group compared to the TP53 mutant group in L858R patients and not significant in EGFR Exon 19 deletion patients. In LBM patients, the time to brain metastasis in the EGFR mutation and TP53 wild-type group was longer than that in the EGFR and TP53 co-mutation group, and TP53 mutant status was an independent prognostic factor for brain metastasis. The TP53 wild-type group exhibited a higher objective remission rate (ORR) and disease control rate (DCR) than the TP53 mutant group in NBM, EBM, and LBM patients, irrespective of primary lung and brain metastatic lesions.
Conclusion: TP53/EGFR co-mutation patients receiving first-line EGFR-TKI treatment had poor prognoses in advanced LUAD, especially with L858R mutation. Moreover, TP53/EGFR co-mutation patients treated with EGFR-TKIs may more easy developed intracranial metastasis.
1 Introduction
Lung cancer remains the high incident cases in worldwide (1); lung adenocarcinoma (LUAD) accounts for 40%–50% of non-small cell lung cancer (NSCLC) (2). For early-stage NSCLC, the 5-year survival rate is more than 70% (3). However, there is a lack of awareness about routine physical examination in backward areas, and some individuals have no specific symptoms of peripheral LUAD; nearly 50%–60% of patients found distant metastasis at their first diagnosis (4). Nearly 30%–50% of the NSCLC patients progressed to brain metastasis following the preliminary diagnosis or after the anti-tumor treatment (5); even with whole brain radiation therapy (WBRT), stereotactic radiation therapy (SRT), and postoperation or epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), many patients still have a poor prognosis (4–7). The cellular and molecular mechanisms of brain metastasis were need further studied; however, the relationship between oncogenic driver mutations and LUAD of brain metastasis remains undefined (8).
EGFR-TKIs are the first- or further-line standard treatment in advanced NSCLC with EGFR mutation, and overall survival (OS) of such patients prolongs from 1 year to 20–30 months (9, 10). However, some patients develop brain or leptomeningeal metastases, having a median OS of 12 months or worse (11, 12). The studies showed that the patients of EGFR T790M-mutant NSCLC with central nervous system (CNS) metastases progressed on first- or second-generation EGFR-TKI and after osimertinib therapy had a high disease control rate (DCR) and objective remission rate (ORR) (13), and prolonged progression-free survival (PFS) (14, 15), since osimertinib has greater penetration of the blood–brain barrier and higher brain exposure compared with other EGFR-TKIs (16). Moreover, WBRT and chemotherapy are potential crucial treatment strategies for EGFR-wild type advanced NSCLC with CNS (17, 18).
TP53/EGFR co-mutations are present in nearly 17%–70% of advanced NSCLC, affecting cancer cell and non-cell-autonomous cancer features, resulting in genomic instability (19, 20). Li et al. reported that the median PFS and OS in TP53 wild type were the longest compared to exon 4 or 7 of TP53 and other TP53 mutations (p < 0.05), indicating TP53 as a promising predictive and prognostic indicator in EGFR-mutated advanced NSCLC on EGFR-TKIs (21). The study also demonstrated that TP53/EGFR co-mutations had lower response rates and shorter PFS than TP53 wild type in NSCLC with EGFR-TKI therapy; TP53 status did not impact the probability of developing CNS metastases either from diagnosis or from the start of TKIs at 5 years; however, this study included stages I–III (22). Jiao et al. suggested that the TP53 wild-type group had a better prognosis than the TP53 mutant group in EGFR wild type (23).
Many studies have stated that TP53 and EGFR co-mutation impact prediction and prognosis in advanced LUAD patients treated with EGFR-TKIs, while the effect on brain metastasis remains elusive and needs further investigation. Therefore, we performed a retrospective study to analyze the prognostic usefulness of TP53 and EGFR co-mutation in advanced LUAD with brain metastasis and EGFR-TKIs as the first-line treatment. It is critical to elucidate the predictive and prognostic TP53-mutated status in EGFR-mutated advanced LUAD patients with brain metastasis, treated with first-line EGFR-TKIs, and explore new molecular biomarkers and treatment approaches.
2 Patients and methods
2.1 Patients and data collection
We retrospectively identified patients with histologically proven TP53 and EGFR mutation status in metastasis LUAD and received EGFR-TKIs as first-line therapy between January 2014 and July 2021. The following patient characteristics were included: smoking index (400), TNM stages, EGFR and TP53-mutated status, gender, age, and blood biochemical indicators. This study included the following: (1) patients with TP53 and EGFR mutant status tested in metastasis LUAD with or without brain metastasis who had received EGFR-TKIs, in conjunction with WBRT or chemotherapy, and patients with data integrity and evaluable target lesions; (2) patients with LUAD without other malignant tumors; and (3) patients who have complete clinical data.
2.2 Post-treatment evaluation
The post-treatment evaluation included computed tomography (CT), bone scan, and craniocerebral nuclear magnetic resonance imaging (MRI). The short-term effects were evaluated by Response Evaluation Criteria in Solid Tumors 1.1 (RECIST1.1). This study of primary endpoint was mainly OS and PFS, which was measured from the starting date of first-line treatment. The LUAD with non-brain metastasis (NBM) means patients having no brain metastasis at preliminary or late diagnosis; early brain metastasis (EBM) means patients with brain metastasis at preliminary diagnosis and mainly received third-generation TKIs; late brain metastasis (LBM) refers to patients with brain metastasis. The patients received osimertinib for EBM or with T790M mutation. Moreover, a portion of patients received EGFR-TKIs combined with chemotherapy (platinum-based combination chemotherapy) in EGFR Exon 19 deletion or L858R mutation with resistant gene (such as her2, KRAS, met, etc.). Follow-up methods included short messages, outpatient visits, telephone conversations, and regular reviews. Furthermore, the follow-up time of the cutoff date was 30 December 2022, and the median follow-up period was 32 months.
2.3 Statistical analysis
Chi-square or Fisher’s exact test was used for categorical variables. Multivariate logistic regression analysis to found the independent risk factors (24). The Kaplan–Meier method was employed to calculate survival rates. Log-rank test was used for univariate prognostic analysis, and Cox proportional hazards models were used for multivariate prognostic analysis to identify independent prognostic factors (24). In statistical results, p < 0.05 was considered significant. Statistical analysis was performed using IBM SPSS 22 and GraphPad Prism version 8.0. The mean value of peripheral serum routine blood test and tumor biomarkers of normal value were taken as cutoff points for statistical analysis.
3 Results
3.1 Correlation between LUAD brain metastasis and NBM in clinicopathological characteristics
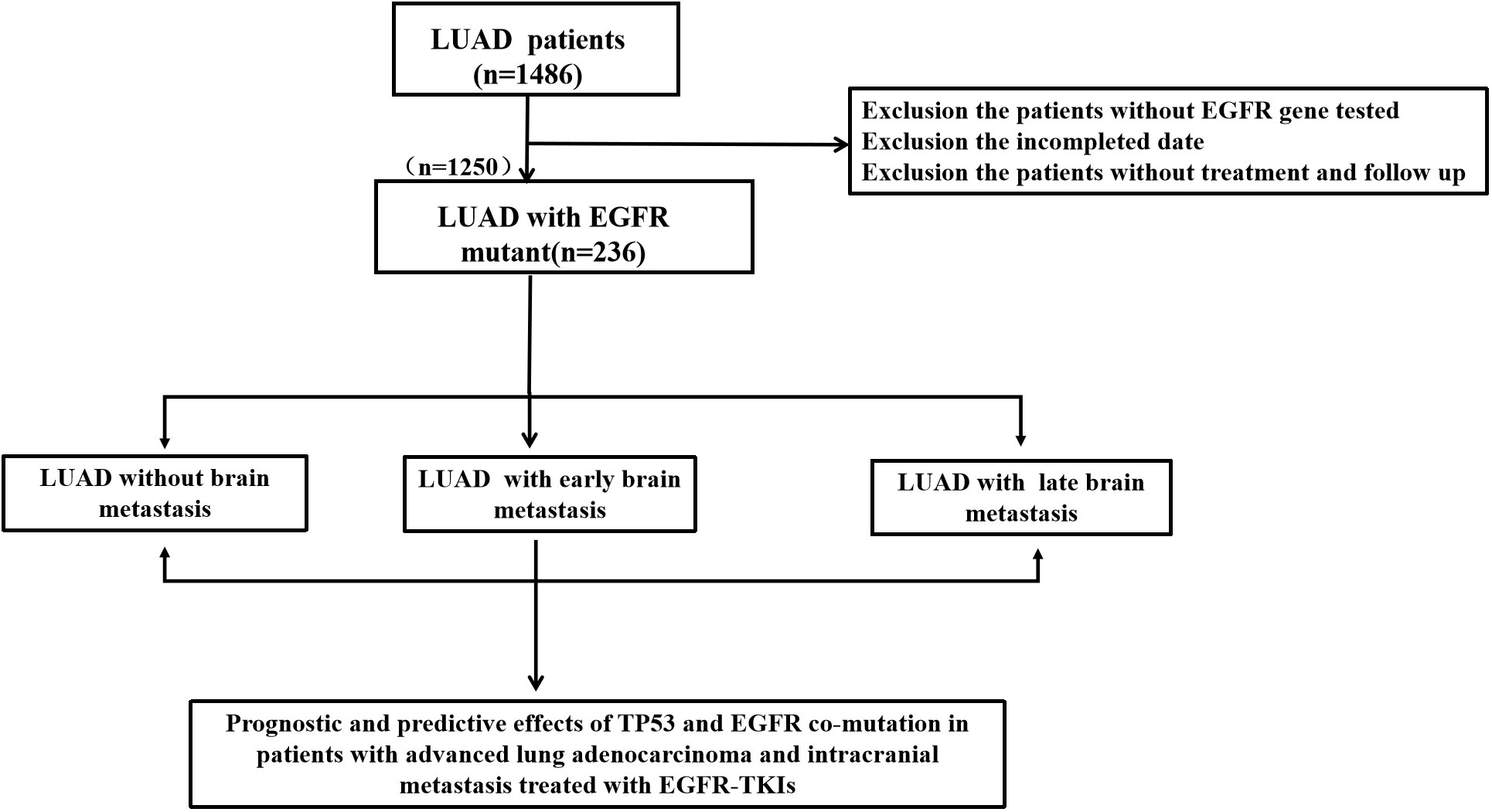
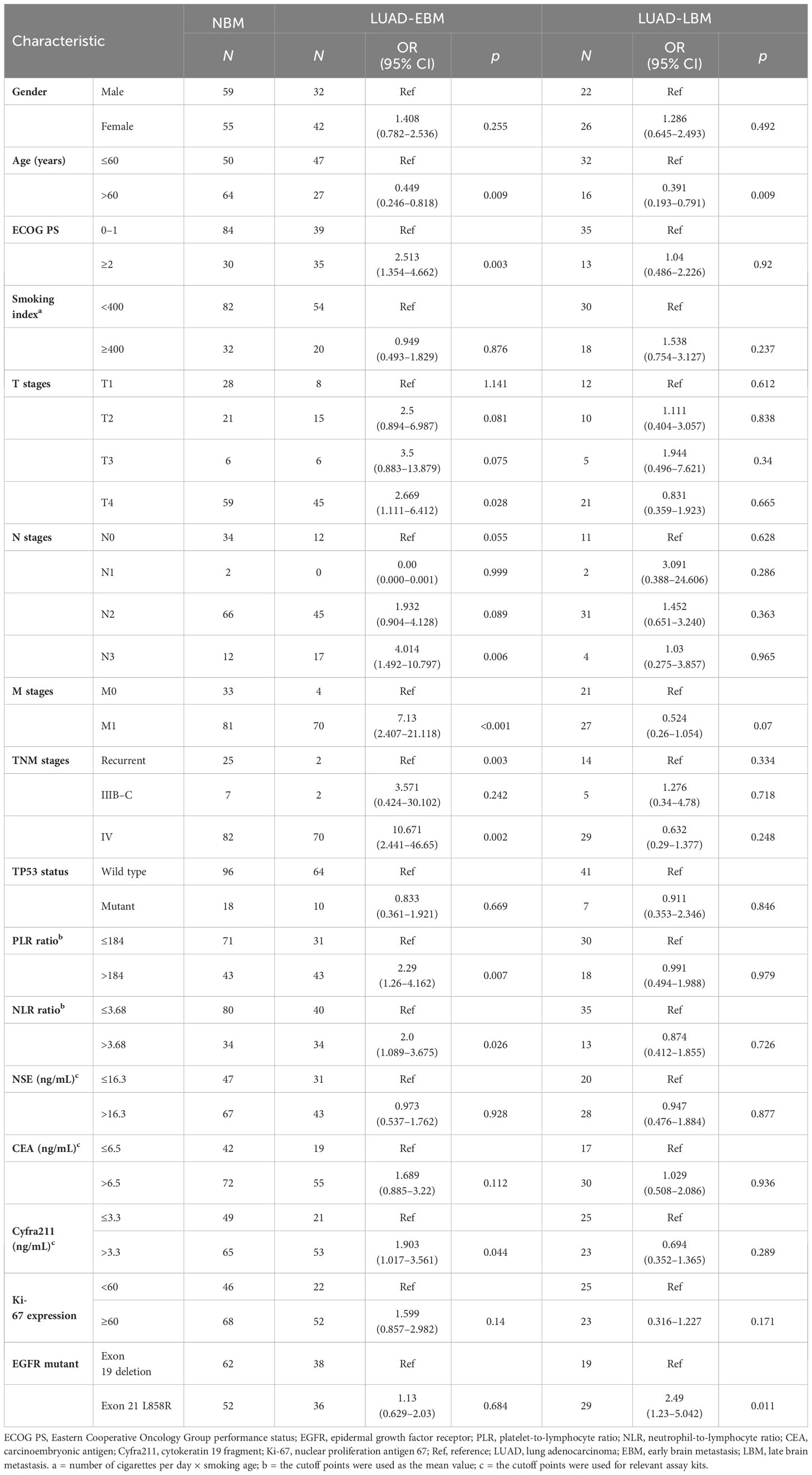
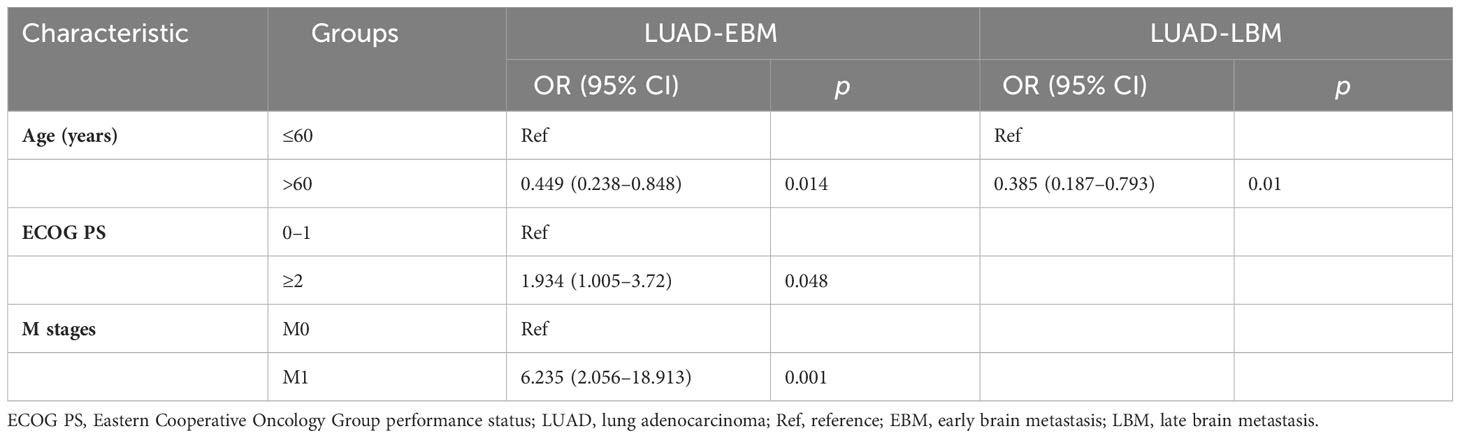
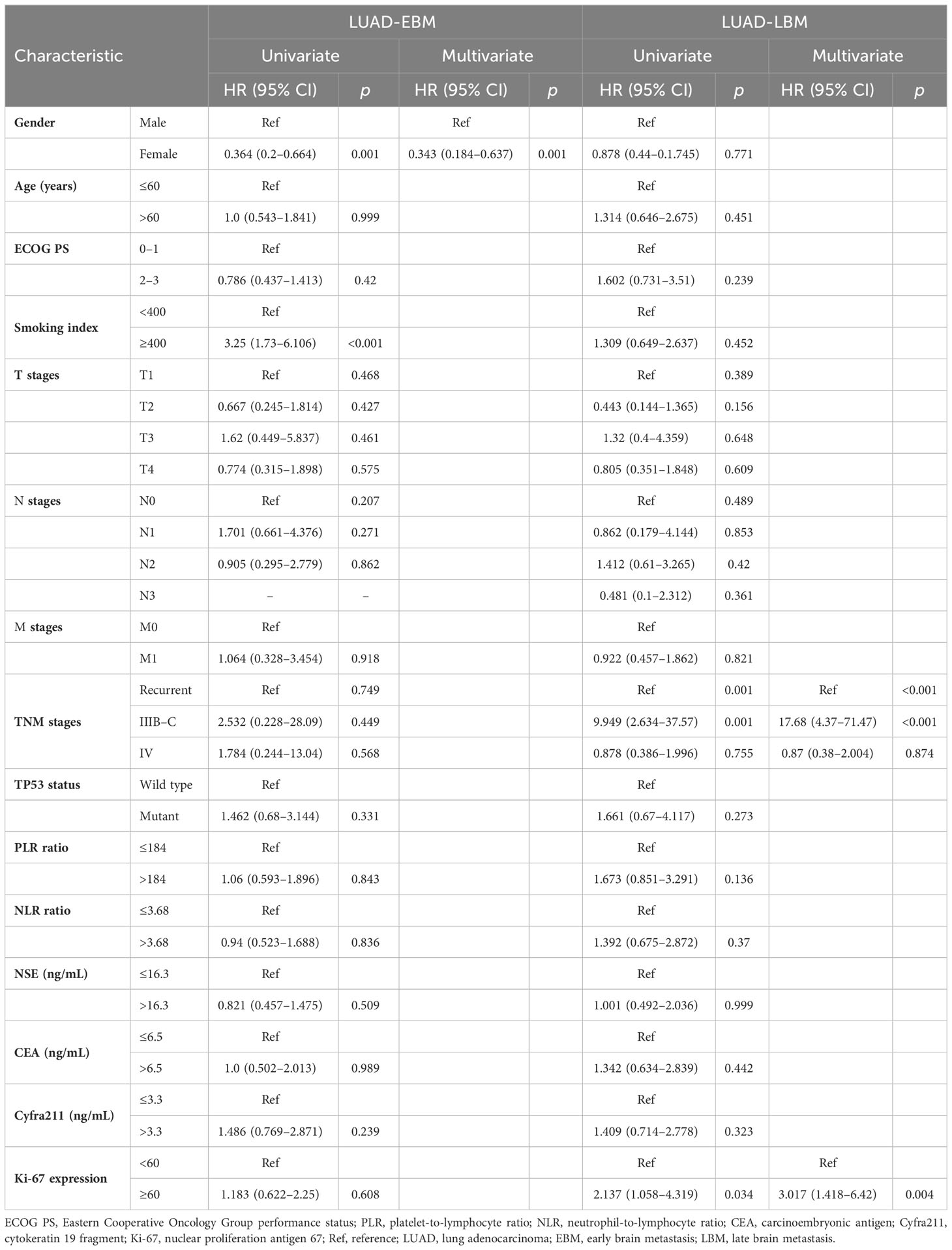
We performed a retrospective examination of 236 patients who were evaluated for TP53- and EGFR-mutant status and received first-line EGFR-TKIs for advanced or postoperatively recurrent LUAD. A flowchart for the study is shown in Figure 1. There were 114 patients with confirmed NBM, 74 with preliminary diagnosis EBM, and 48 with LBM (Figure 2A). TP53 and EGFR co-mutations were found in 35/236 (14.8%) patients; these TP53-mutant types belonged to missense patients, and nearly 10 non-missense patients were excluded; the different TP53 mutant statuses were as follows: Exon 4 mutation were 5 patients, Exon 5 mutation were 9 patients, Exon 6 mutation were 2 patients, Exon 7 mutation were 7 patients, Exon 8 mutation were 6 patients, Exon 9 mutation were 5 patients , Exon 10 mutation were 1 patients (Figure 2B). Moreover, EGFR exon 19 deletion was found in 119 patients, and EGFR L858R was found in 117 patients. There were 25 patients with T790M mutation (18 patients with EGFR exon 19 deletion and T790M mutation and 7 patients with EGFR L858R and T790M co-mutation). The univariate logistic analysis showed a significant relationship between age, performance status (PS), M stages, TNM stages, platelet-to-lymphocyte ratio (PLR) ratio, neutrophil-to-lymphocyte ratio (NLR) ratio, and serum cyfra211 and EBM in LUAD (p < 0.05); age and EGFR mutant were significantly related to LBM (Table 1). Multivariate logistic regression analysis revealed that age, ECOG PS, and M stages were independent risk factors associated with EBM, and age was an independent risk factor for LBM (Table 2).

Figure 2 The relation between TP53 and EGFR-TKI therapy in EGFR-mutant advanced LUAD. (A) The number of LUAD patients in NBM, EBM, and LBM. (B) Pie chart about TP53 missense mutant status. (C, D) The TP53 wild-type group had a high median PFS and OS than the TP53 mutant group in all advanced LUAD patients. (E, F) The TP53 mutant group had no effect on PFS and OS compared to the TP53 wild-type group in EGFR Exon 19 deletion patients. (G, H) Subgroup analysis revealed longer median PFS and OS in the TP53 wild-type group compared to the TP53 mutant group in L858R patients. EBM, early brain metastasis; LBM, late brain metastasis; and NBM, non-brain metastasis.
3.2 Univariate and multivariate survival analyses of TP53 and EGFR co-mutation in advanced LUAD with brain metastasis
Numerous studies demonstrated a connection between TP53 mutation and poor prognosis. Therefore, we analyzed the effect of EGFR-TKIs as the first-line treatment in advanced LUAD. In all advanced LUAD patients, the TP53 wild-type group had a high median PFS (9.4 vs. 6 months, p < 0.05, Figure 2C) and OS (20.8 vs. 10.9 months, p < 0.05, Figure 2D) compared to the TP53 mutant group. In EGFR Exon 19 deletion patients, the TP53 mutant group exhibited no effect on PFS (10.6 vs. 9.9 months, p > 0.05, Figure 2E) and OS (32.2 vs. 21.3 months, p > 0.05, Figure 2F) compared to the TP53 wild-type group. In L858R patients, the TP53 wild-type group displayed an effect on median PFS (7.2 vs. 4.9 months, p < 0.05, Figure 2G) and OS (20.4 vs. 8.0 months, p < 0.05, Figure 2H) compared to the TP53 mutant group.
We further investigated the relationship between TP53 mutant and brain metastasis in advanced LUAD treated with EGFR-TKIs. The median of PFS (7.7 vs. 10 vs. 9 months, p > 0.05, Figure 3A) in the EBM group was not significant compared to the NBM and LBM groups, while the median OS (15 vs. 23.5 vs. 20.4 months, p < 0.05, Figure 3B) in the EBM group was the shortest among all groups. Furthermore, the TP53 wild-type group had a higher median PFS (11 vs. 6 months, p < 0.05, Figure 3C) and OS (29.4 vs. 9.8 months, p < 0.05, Figure 3D) than the TP53 mutant group in NBM patients. In EBM and LBM patients, the TP53 wild-type group did not impact PFS and OS compared to the TP53 mutant group (p > 0.05, Figures 3E–H).
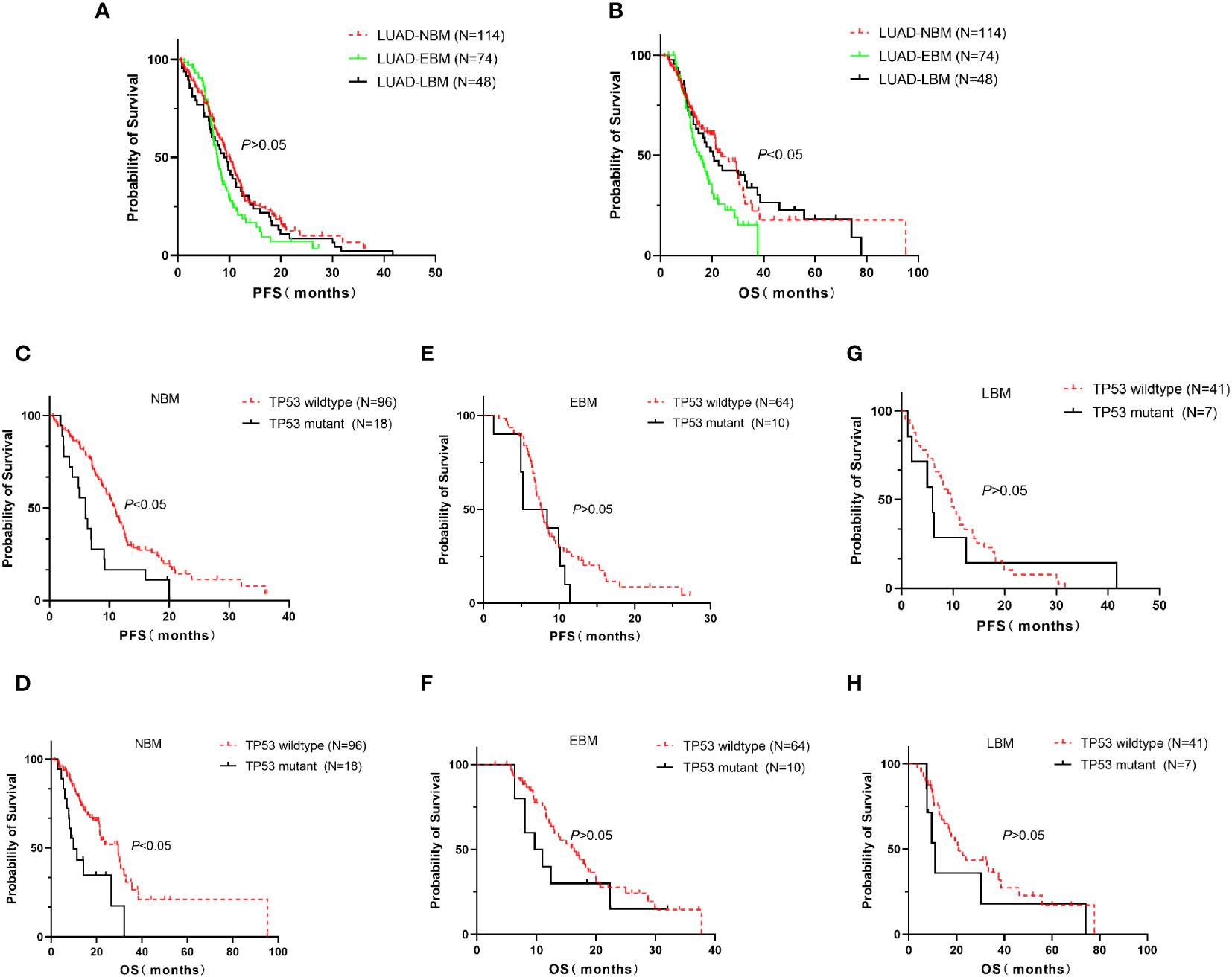
Figure 3 Correlation between TP53 mutation and brain metastasis in advanced LUAD first line treated with EGFR-TKIs. In all advanced LUAD patients, the median of PFS in the EBM group was insignificant compared to the NBM and LBM groups (A), while the median of OS in the NBM group was the longest among all groups (B). (C, D) The TP53 wild-type group had a higher median PFS and OS than the TP53 mutant group in NBM patients. (E–H) In EBM and LBM patients, the TP53 wild-type group did not affect PFS and OS compared to the TP53 mutant group. EBM, early brain metastasis; LBM, late brain metastasis; and NBM, non-brain metastasis.
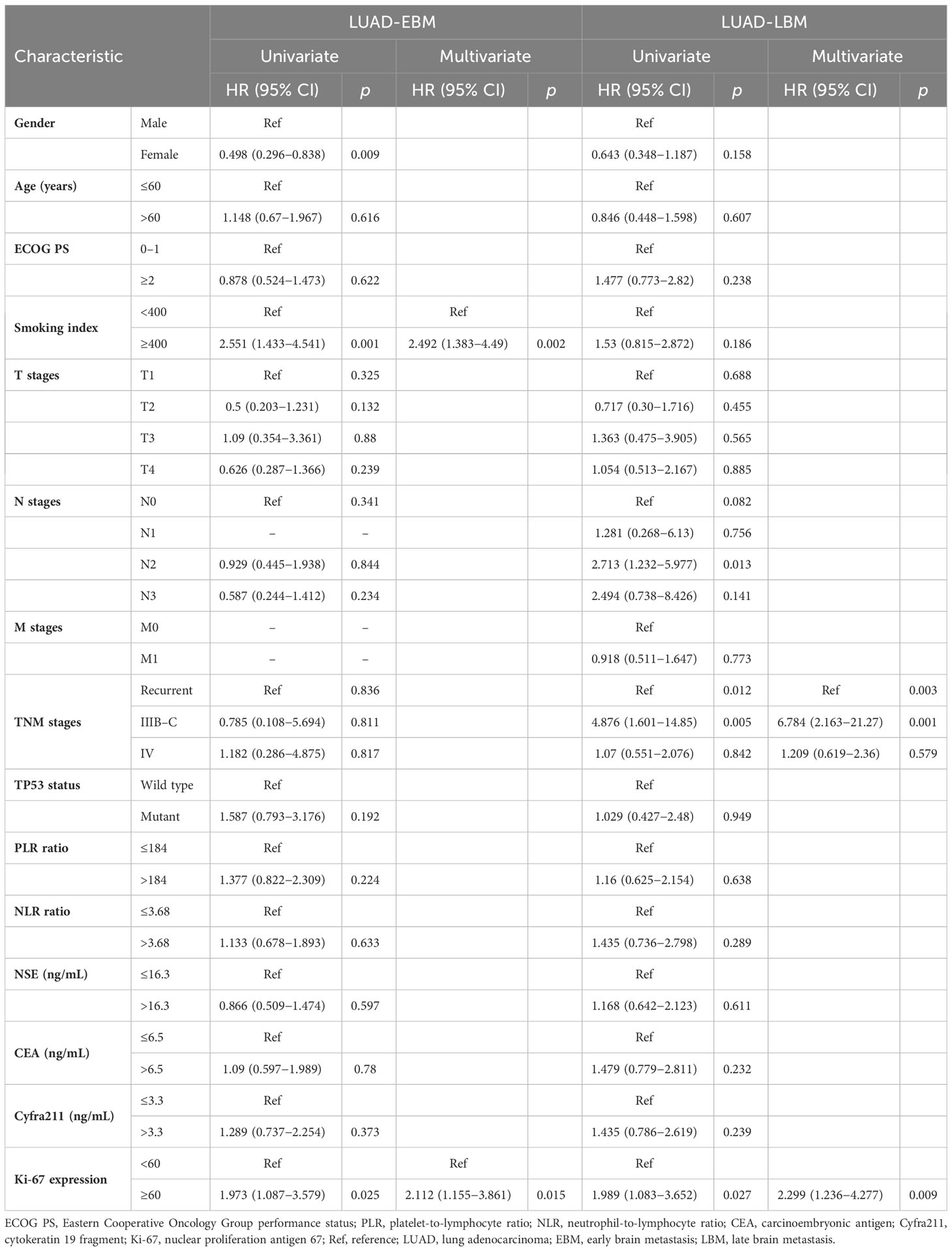
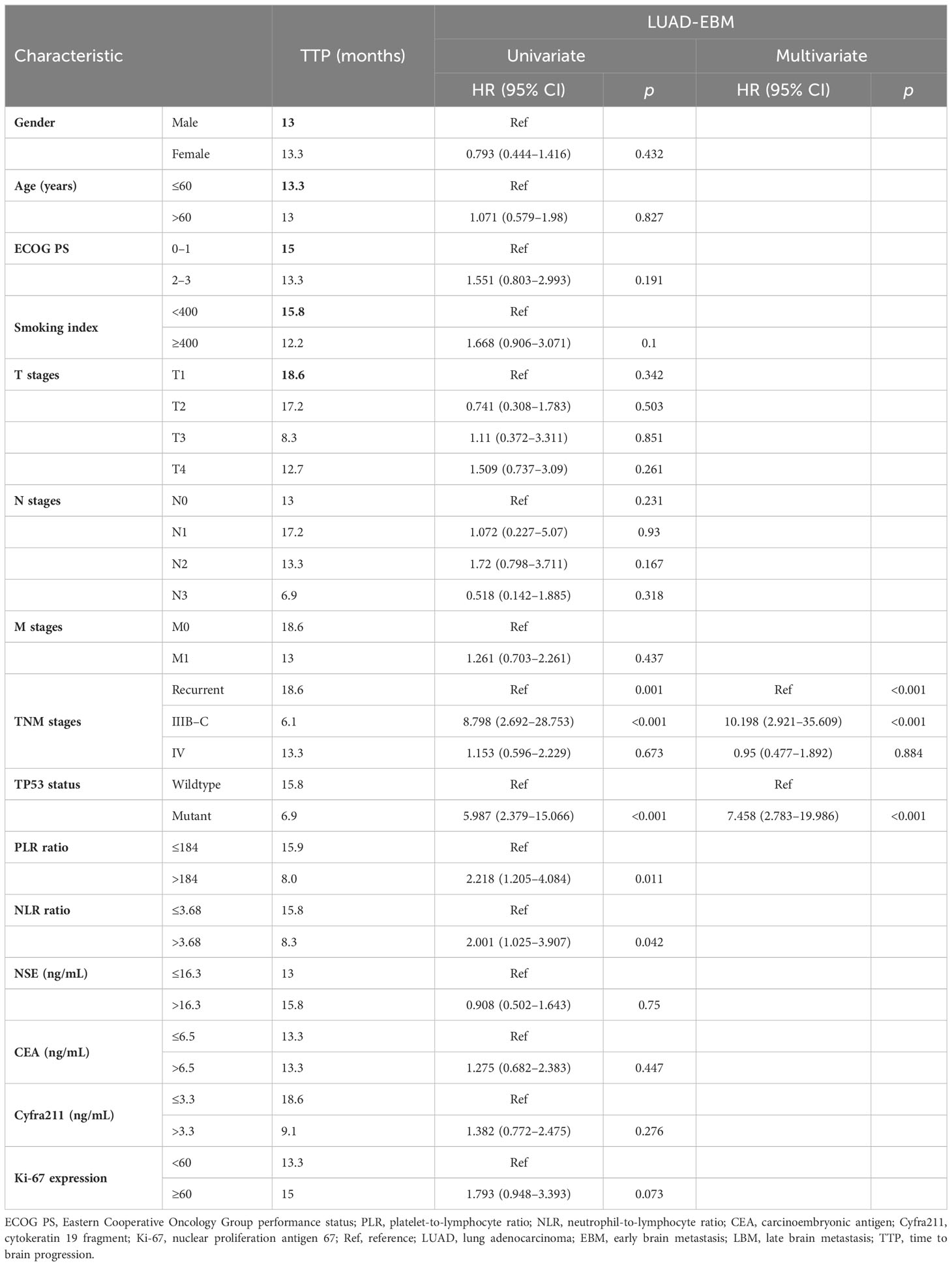
Additional evaluation of the association between clinicopathological information and prognosis in EBM and LBM patients was carried out. The univariate Cox analysis showed that gender, Ki-67 expression, and smoking index correlated with PFS in EBM patients; TNM stages and Ki-67 expression were associated with PFS in LBM patients. Multivariate Cox analysis (univariate analysis p < 0.05) showed that smoking index and Ki-67 expression were independent prognostic factors for PFS (p < 0.05) in EBM patients; TNM stages and Ki-67 expression were also independent prognostic factors for PFS (p < 0.05) in LBM patients (Table 3). Moreover, the univariate Cox analysis showed that gender and smoking index were associated with OS in EBM patients; TNM stages and Ki-67 expression were associated with OS in LBM patients. Multivariate Cox analysis (univariate analysis p < 0.05) showed that gender was an independent prognostic factor for OS in EBM patients (p < 0.05); TNM stages and Ki-67 expression were also independent prognostic factors for OS in LBM patients (p < 0.05) (Table 4).
3.3 Univariate and multivariate Cox analyses of EGFR and TP53 co-mutation status and time to brain metastasis in advanced LUAD
The TP53 wild-type group had a longer OS than the TP53 mutant group in all LUAD, NBM, or L858R patients; therefore, we further analyzed the correlation between EGFR and TP53 co-mutation status and time to brain metastasis in LUAD patients. The TP53 wild-type group had a significantly longer time (15.8 vs. 6.9 months, p < 0.05, Figure 4A) to brain metastasis than the TP53 mutant group; the IIIB–C stage group had a significantly shorter time (6.1 vs. 18.6 vs. 13.3 months, p < 0.05, Figure 4B) to brain metastasis than the local recurrence and IV stage groups; the low PLR group had a significantly longer time (15.9 vs. 8 months, p < 0.05, Figure 4C) to brain metastasis than the high PLR group; the low NLR group had a significantly longer time (15.8 vs. 8.3 months, p < 0.05, Figure 4D) to brain metastasis than the high NLR group. Moreover, univariate and multivariate Cox analysis showed that TNM stages and TP53 mutant status were independent prognostic factors for time to brain metastasis in LUAD with LBM (Table 5; Figure 4E).
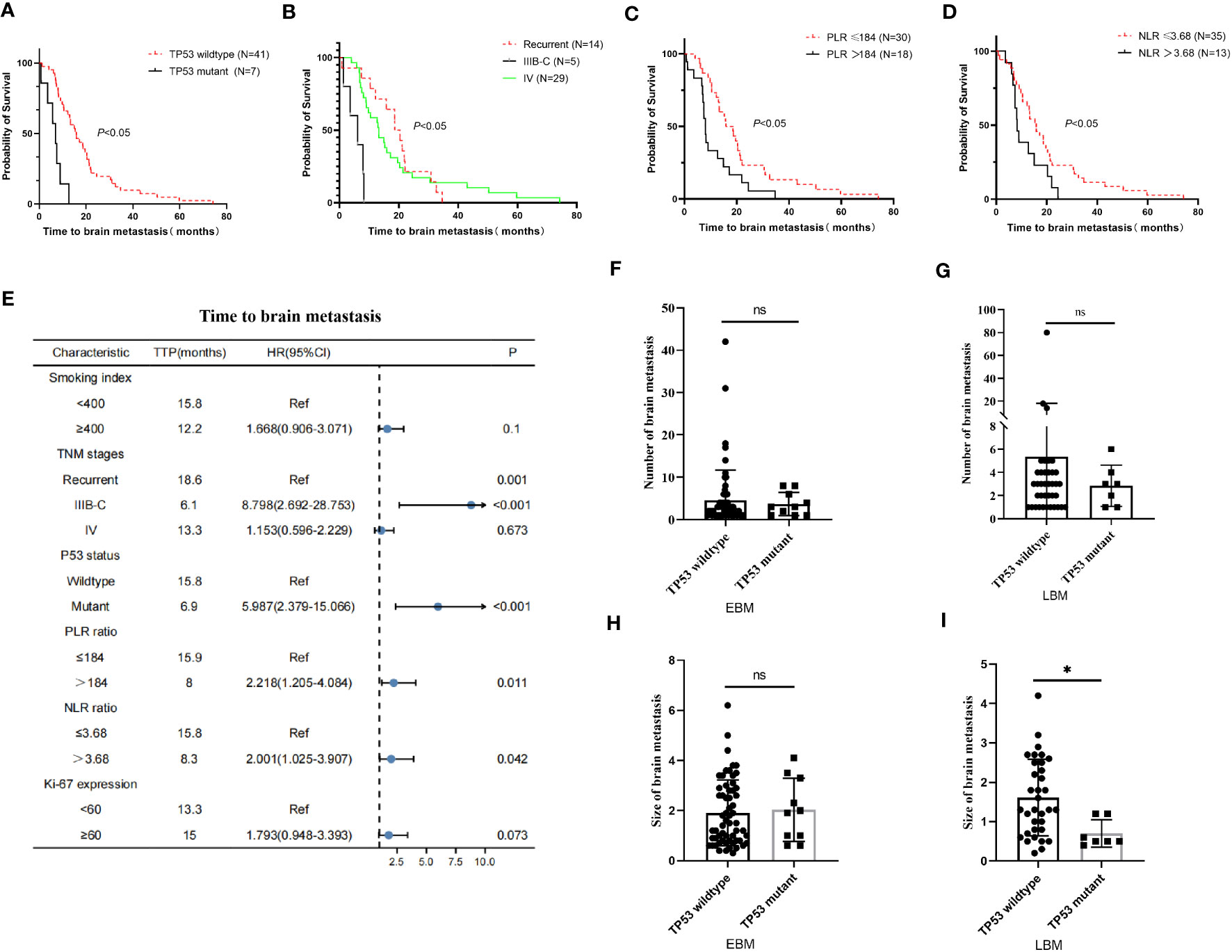
Figure 4 Relationship between clinical–pathological features and time to brain metastasis in EGFR-mutant advanced lung adenocarcinoma with EGFR-TKI therapy. (A) The TP53 wild-type group exhibited a significantly longer time to brain metastasis than the TP53 mutant group. (B) The IIIB–C group had a significantly shorter time to brain metastasis than the local recurrence and IV stage group. (C, D) The low PLR and NLR groups took significantly longer time to brain metastasis than the high PLR and NLR groups. (E) The univariate Cox analysis showed that gender, smoking index, and Ki-67 expression were correlated with time to brain metastasis in advanced lung adenocarcinoma (p < 0.05); these factors were drawn using a forest map. (F, G) The number of brain metastasis in the TP53 wild-type group was insignificant compared to the TP53 mutant group in EBM and LBM patients. The diameter of brain metastasis in the TP53 wild-type group was insignificant compared to the TP53 mutant group in EBM (H), while the TP53 wild-type group was greater than the TP53 mutant group in LBM (I).
Moreover, we further analyzed the relationship between TP53 mutant status and number size of brain metastasis. In EBM and LBM patients, the number of brain metastasis in the TP53 wild-type group was insignificant compared to the TP53 mutant group (p > 0.05, Figures 4F, G). The diameter of brain metastasis in the TP53 wild-type group was not significant compared to the TP53 mutant group in EBM (p > 0.05, Figure 4H), while that of the TP53 wild-type group was greater than the TP53 mutant group in LBM (p < 0.05, Figure 4I).
3.4 Short-term therapeutic effects and TP53 expressions in EBM and LBM
Disease progression was evaluated through imaging examination every 2 months after 3–4 weeks of treatment. In NMB, EBM, and LBM patients with all tumor lesions, the TP53 wild-type group had a higher DCR than the TP53 mutant expression group (p < 0.05), but not ORR (p > 0.05) (Table 6, Figure 5). Additionally, we analyzed the TP53 mutant status and brain metastasis of the lesion. In EBM patients, the TP53 wild-type group had a higher CR rate (3.1% vs. 0%) and DCR (84.4% vs. 50%) than the TP53 mutant expression groups. In LBM patients, the TP53 wild-type group had a higher ORR (14.6% vs. 0%) and DCR (68.3% vs. 14.3%) than the TP53 mutant expression group (Table 7).
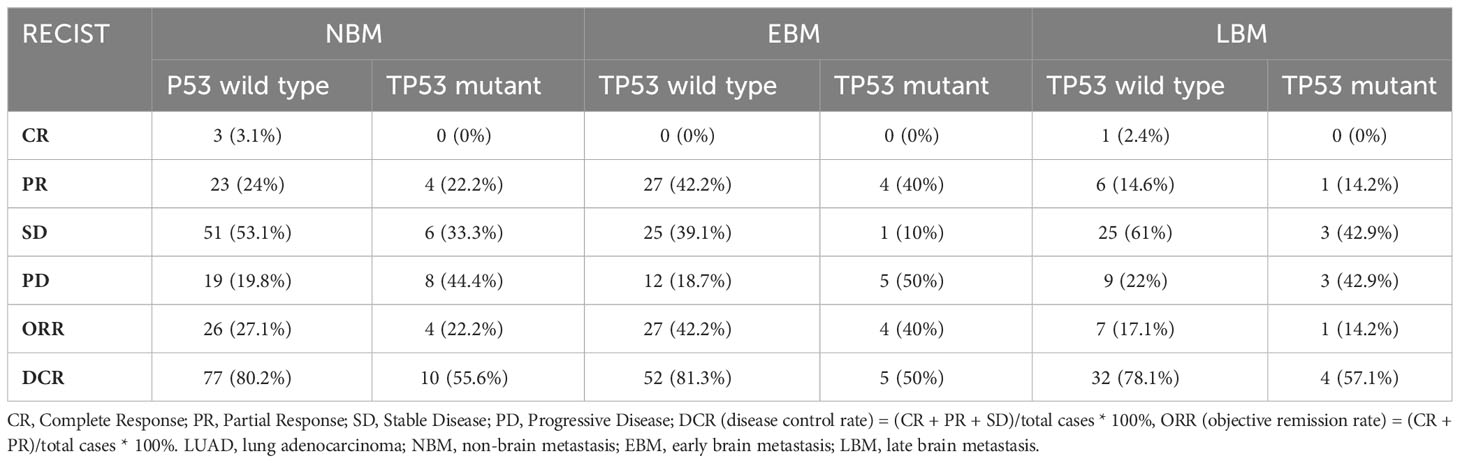
Table 6 The short-term efficacy comparison among TP53 mutant status with first-line therapy in all lesions of LUAD.
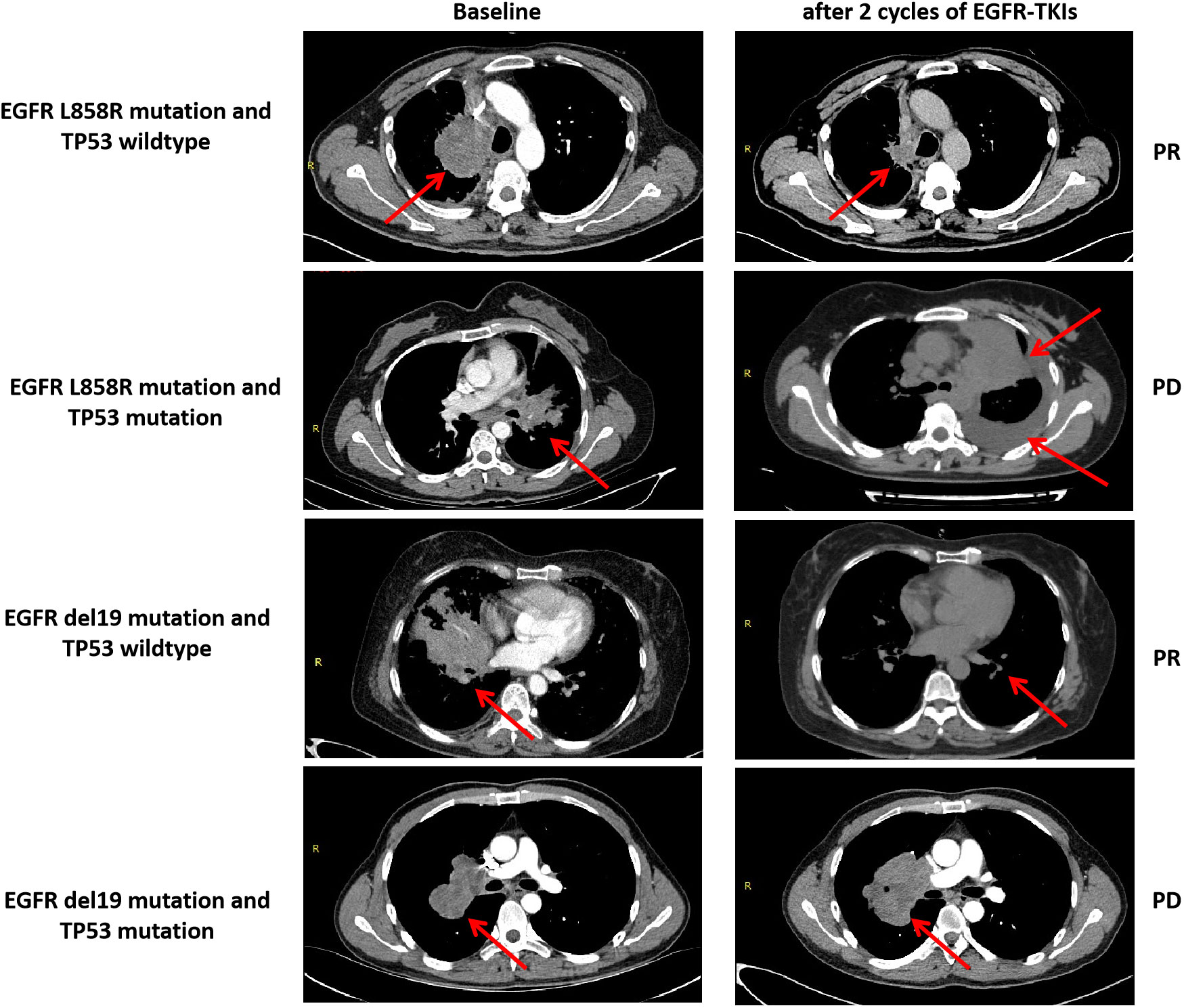
Figure 5 Anti-tumor activity and TP53 mutant status and EGFR-TKIs in four patients with EGFR-mutant advanced LUAD. PR, partial response; PD, progressive disease.
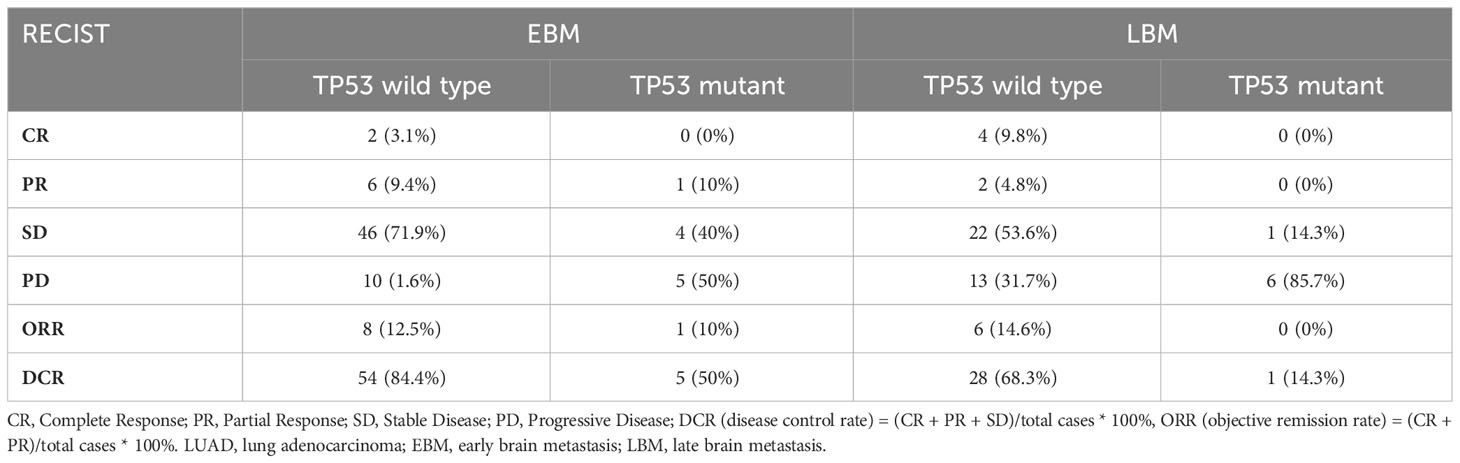
Table 7 The short-term efficacy comparison among TP53 mutant status in brain metastasis lesion of LUAD with EGFR-TKIs or brain radiotherapy.
4 Discussion
Nearly 30%–50% of lung cancer patients with a preliminary diagnosis or who have had anti-tumor therapy develop brain metastases, and their management and prognosis are poor (5–7). The studies showed that the median OS for the ALK/EGFR+ NSCLC brain metastasis was longer than that of the wild type (19.9 vs. 10.1 months, p = 0.028) (25). Osimertinib has greater blood–brain barrier penetration, higher brain exposure, and a good prognosis; hence, it has become an essential treatment for EGFR-mutated NSCLC patients with CNS metastases (13–16). Therefore, it is vital to analyze the correlation between driver gene and brain metastasis and explore a novel treatment strategies for brain metastasis.
TP53/EGFR co-mutation is commonly found in advanced NSCLC; the TP53 wild-type has a good prognosis and correlates with primary and acquired resistance to EGFR-TKIs (19–21). The study showed that nondisruptive TP53 mutations are related to poor survival (17.8 months vs. 28.4 months) compared to TP53 wild-type stage IIIB–IV EGFR-mutated NSCLC patients, and multivariate analyses suggested that nondisruptive TP53 mutations were independent prognostic factors associated with a shorter OS (26). Moreover, the mechanism and effect of TP53 in primary sensitivity and acquired resistance to EGFR-TKIs in NSCLC cells showed that TP53 mutations promote the epithelial-to-mesenchymal transition (EMT), activating EGFR mutations and enhancing resistance to osimertinib in H1975 cells (27). Furthermore, a phase III randomized trial (CTONG 0901) was conducted to analyze the relationship between TP53 and EGFR-TKIs in EGFR-mutated advanced NSCLC; the study found that the TP53 wild-type group had the longest median PFS (9.4 vs. 11.0 vs. 14.5 months, p = 0.009) and OS (15.8 vs. 20.0 vs. 26.1 months, p = 0.004) compared to exon 4 or 7 of TP53 or other TP53 mutations, indicating that TP53 could be a promising predictive and prognostic factor in EGFR-mutated NSCLC (21).
This study also evaluated the effect of TP53 on PFS and OS in EGFR-mutated advanced LUAD patients receiving EGFR-TKI therapy. The median PFS and OS in the TP53 wild-type group were longer than TP53 mutations in all advanced LUAD, and subgroup analysis revealed the relation between EGFR-mutant types and TP53; the effect of TP53-mutated status did not correlate with survival time in EGFR Exon 19 deletion patients. In contrast, TP53 mutation exhibited a shorter PFS and OS than the TP53 wild type in L858R patients. Moreover, the TP53 wild-type group had a longer DCR and ORR than the TP53 mutation. Therefore, the TP53 and EGFR L858R co-mutation may be an important indicator for the predictive effect of EGFR-TKIs in advanced LUAD patients.
Many studies reported that the median OS in NSCLC patients with brain or leptomeningeal metastases is 12 months or worse (11, 12). Labbé et al. found an insignificant association between TP53-mutant status from diagnosis and from the start of TKIs to developing brain metastases; results might be due to the small sample size of LUAD patients, and many patients were second- or further-line postoperatively relapsed patients receiving the EGFR-TKI therapy; these factors could significantly affect the outcomes (22). Our study retrospectively analyzed 236 patients and tested for TP53-mutated status (201 patients were wild type and 35 were mutated) in EGFR-mutant advanced LUAD patients undergoing EGFR-TKIs as the first-line treatment. The NBM patients in TP53 wild-type groups had a longer PFS and OS than TP53 mutations, while the TP53-mutant status had no effect on survival time in EBM or LBM patients. Moreover, the TP53 wild-type group had a higher CR rate and ORR than TP53 mutant groups in LBM and EBM patients receiving EGFR-TKI alone or in combination with craniocerebral radiotherapy. Notably, we interestingly found that the TP53 wild-type group had a significantly longer time to brain metastasis than the TP53-mutated group in advanced LUAD patients. However, the number of brain metastasis in the TP53 wild-type group was not significant compared to the TP53 mutant group.
Reportedly, serum systemic inflammatory reaction (SIR) of tumor immune infiltration microenvironment is critical for regulating the malignant biological behavior of tumor cells in multiple solid tumors (28–31). According to a study, high NLR and PLR were associated with shorter OS in NSCLC with brain metastases (32). Mansfield et al. found that the PD-L1 expression and tumor-infiltrating lymphocytes (TILs) of paired primary lung cancers and brain metastases were significantly different; the PD-L1 expression and TILs were higher in lung cancer tissues than in brain metastases (33). Moreover, the study suggested that high NLR (≥4.95) had significantly more brain metastases at diagnosis than those with low NLR, particularly in the group with adenocarcinoma (34). However, the relationship between TILs and EGFR-TKIs in EGFR-mutant advanced LUAD with brain metastasis is unknown. Therefore, in the current study, the univariate and multivariate Cox analysis showed that the PLR and NLR ratio did not correlate with PFS and OS in advanced LUAD patients receiving EGFR-TKI therapy, while high NLR and PLR were associated with shorter time to brain metastasis. The results may indicate that SIR might release immune cytokines and inflammatory factors into the peripheral blood, activating the inflammatory immune response and promoting the tumor cells’ distant metastasis.
There were some limitations in this study. First, many studies showed that TP53/EGFR co-mutations were found in nearly 17%–70% of advanced NSCLC, while our study found TP53 and EGFR co-mutations in only 14.8% (35/236 patients), which had an insufficient sample size to analyze the effect of TP53-specific exons on intracranial metastasis in EGFR-mutated advanced LUAD patients receiving EGFR-TKI therapy. Second, there were only 48 patients with LBM, and the fact that this is a single-center study with a small sample size and a retrospective design might have induced selective bias. Many patients received first-, second-, or third-generation TKI drugs; those who were uniformly treated may lead to variable therapeutic effects on brain metastases.
In conclusion, TP53 wild type had a longer median OS than TP53 mutation in all LUAD or L858R patients. Moreover, the effect of median PFS and OS in the TP53 wild-type group was significantly longer than the TP53-mutant group in NBM patients. The TP53 wild-type group had a higher ORR and DCR than TP53 mutation with NBM, EBM, and LBM patients, regardless of the primary lung and brain metastatic lesions. Interestingly, it was found that TP53-mutated patients quickly progressed to brain metastasis. Therefore, it is imperative to clarify the predictive and prognostic TP53-mutated status in EGFR-mutant advanced LUAD patients with brain metastasis who underwent EGFR-TKI therapy and explore new molecular markers and treatment strategies for LUAD patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study has been reviewed and approved by the ethics institution committee of First Affiliated Hospital of Nanchang University, Nanchang, China, all patients provided written informed consent and compliance with the declaration of Helsinki. All the patients’ data were kept confidential. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WG: Data curation, Formal analysis, Funding acquisition, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. PL: Data curation, Formal analysis, Software. SW: Methodology, Validation, Writing – review & editing. JT: Conceptualization, Data curation, Writing – review & editing. JL: Methodology, Software, Writing – review & editing. JZ: Conceptualization, Data curation, Writing – review & editing. JX: Software, Writing – review & editing. JD: Investigation, Methodology, Writing – review & editing. FY: Data curation, Writing – review & editing. CS: Formal analysis, Software, Writing – review & editing. FQ: Methodology, Software, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the First Affiliated hospital of Nanchang University of Youth Talent Research and Cultivation Fund (YFYPY202244 to WG), Jiangxi Provincial Chinese Medicine Science (2023B0049 to WG), by Jiangxi Provincial Natural Science Foundation (20232BAB216086 to WG) and Nanchang Key Laboratory of Tumor Gene Diagnosis and Innovative Treatment Research (2021-NCZDSY-009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Takenaka T, Takenoyama M, Yamaguchi M, Toyozawa R, Inamasu E, Kojo M, et al. Impact of the epidermal growth factor receptor mutation status on the post-recurrence survival of patients with surgically resected non-small-cell lung cancer. Eur J Cardiothorac Surg (2015) 47(3):550–5. doi: 10.1093/ejcts/ezu227
4. Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer (2014) 86(1):78–84. doi: 10.1016/j.lungcan.2014.07.020
5. Berger LA, Riesenberg H, Bokemeyer C, Atanackovic D. CNS metastases in non-small-cell lung cancer: current role of EGFR-TKI therapy and future perspectives. Lung Cancer (2013) 80(3):242–8. doi: 10.1016/j.lungcan.2013.02.004
6. Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev (2017) 9(9):CD006121. doi: 10.1002/14651858.CD006121.pub4
7. Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer (2012) 118(9):2486–93. doi: 10.1002/cncr.26515
8. Jiang T, Fang Z, Tang S, Cheng R, Li Y, Ren S, et al. Mutational landscape and evolutionary pattern of liver and brain metastasis in lung adenocarcinoma. J Thorac Oncol (2021) 16(2):237–49. doi: 10.1016/j.jtho.2020.10.128
9. Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol (2015) 26(9):1877–83. doi: 10.1093/annonc/mdv276
10. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med (2017) 376(7):629–40. doi: 10.1056/NEJMoa1612674
11. Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A, Floyd SR, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer (2015) 88(1):108–11. doi: 10.1016/j.lungcan.2015.01.020
12. Li YS, Jiang BY, Yang JJ, Tu HY, Zhou Q, Guo WB, et al. Leptomeningeal metastases in patients with NSCLC with EGFR mutations. J Thorac Oncol (2016) 11(11):1962–9. doi: 10.1016/j.jtho.2016.06.029
13. Goss G, Tsai CM, Shepherd FA, Ahn MJ, Bazhenova L, Crinò L, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol (2018) 29(3):687–93. doi: 10.1093/annonc/mdx820
14. Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC, Ahn JS, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: the BLOOM study. J Clin Oncol (2020) 38(6):538–47. doi: 10.1200/JCO.19.00457
15. Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol (2018) 36(26):2702–9. doi: 10.1200/JCO.2018.77.9363
16. Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res (2016) 22(20):5130–40. doi: 10.1158/1078-0432.CCR-16-0399
17. Mak KS, Gainor JF, Niemierko A, Oh KS, Willers H, Choi NC, et al. Significance of targeted therapy and genetic alterations in EGFR, ALK, or KRAS on survival in patients with non-small cell lung cancer treated with radiotherapy for brain metastases. Neuro Oncol (2015) 17(2):296–302. doi: 10.1093/neuonc/nou146
18. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13(3):239–46. doi: 10.1016/S1470-2045(11)70393-X
19. Jin Y, Shi X, Zhao J, He Q, Chen M, Yan J, et al. Mechanisms of primary resistance to EGFR targeted therapy in advanced lung adenocarcinomas. Lung Cancer. (2018) 124:110–6. doi: 10.1016/j.lungcan.2018.07.039
20. Rachiglio AM, Fenizia F, Piccirillo MC, Galetta D, Crinò L, Vincenzi B, et al. The presence of concomitant mutations affects the activity of EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer (NSCLC) patients. Cancers (Basel) (2019) 11(3):341. doi: 10.3390/cancers11030341
21. Li XM, Li WF, Lin JT, Yan HH, Tu HY, Chen HJ, et al. Predictive and prognostic potential of TP53 in patients with advanced non-small-cell lung cancer treated with EGFR-TKI: analysis of a phase III randomized clinical trial (CTONG 0901). Clin Lung Cancer (2021) 22(2):100–109.e3. doi: 10.1016/j.cllc.2020.11.001
22. Labbé C, Cabanero M, Korpanty GJ, Tomasini P, Doherty MK, Mascaux C, et al. Prognostic and predictive effects of TP53 co-mutation in patients with EGFR-mutated non-small cell lung cancer (NSCLC). Lung Cancer (2017) 111:23–9. doi: 10.1016/j.lungcan.2017.06.014
23. Jiao XD, Qin BD, You P, Cai J, Zang YS. The prognostic value of TP53 and its correlation with EGFR mutation in advanced non-small cell lung cancer, an analysis based on cBioPortal data base. Lung Cancer (2018) 123:70–5. doi: 10.1016/j.lungcan.2018.07.003
24. Gu W, Hu M, Xu L, Ren Y, Mei J, Wang W, et al. The ki-67 proliferation index-related nomogram to predict the response of first-line tyrosine kinase inhibitors or chemotherapy in non-small cell lung cancer patients with epidermal growth factor receptor-mutant status. Front Med (Lausanne) (2021) 8:728575. doi: 10.3389/fmed.2021.728575
25. Balasubramanian SK, Sharma M, Venur VA, Schmitt P, Kotecha R, Chao ST, et al. Impact of EGFR mutation and ALK rearrangement on the outcomes of non-small cell lung cancer patients with brain metastasis. Neuro Oncol (2020) 22(2):267–77. doi: 10.1093/neuonc/noz155
26. Molina-Vila MA, Bertran-Alamillo J, Gascó A, Mayo-de-las-Casas C, Sánchez-Ronco M, Pujantell-Pastor L, et al. Nondisruptive p53 mutations are associated with shorter survival in patients with advanced non-small cell lung cancer. Clin Cancer Res (2014) 20(17):4647–59. doi: 10.1158/1078-0432.CCR-13-2391
27. Jung S, Kim DH, Choi YJ, Kim SY, Park H, Lee H, et al. Contribution of p53 in sensitivity to EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Sci Rep (2021) 11(1):19667. doi: 10.1038/s41598-021-99267-z
28. Kim CG, Hong MH, Kim KH, Seo IH, Ahn BC, Pyo KH, et al. Dynamic changes in circulating PD-1+CD8+ T lymphocytes for predicting treatment response to PD-1 blockade in patients with non-small-cell lung cancer. Eur J Cancer (2021) 143:113–26. doi: 10.1016/j.ejca.2020.10.028
29. Li X, Tan Q, Li H, Yang X. Predictive value of tumor-infiltrating lymphocytes for response to neoadjuvant chemotherapy and breast cancer prognosis. J Surg Oncol (2021) 123(1):89–95. doi: 10.1002/jso.26252
30. Loupakis F, Depetris I, Biason P, Intini R, Prete AA, Leone F, et al. Prediction of benefit from checkpoint inhibitors in mismatch repair deficient metastatic colorectal cancer: role of tumor infiltrating lymphocytes. Oncologist (2020) 25(6):481–7. doi: 10.1634/theoncologist.2019-0611
31. Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol (2017) 14(12):717–34. doi: 10.1038/nrclinonc.2017.101
32. Li A, Mu X, He K, Wang P, Wang D, Liu C, et al. Prognostic value of lymphocyte-to-monocyte ratio and systemic immune-inflammation index in non-small-cell lung cancer patients with brain metastases. Future Oncol (2020) 16(30):2433–44. doi: 10.2217/fon-2020-0423
33. Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol (2016) 27(10):1953–8. doi: 10.1093/annonc/mdw289
Keywords: lung cancer, brain metastasis, EGFR, TP53, prognosis
Citation: Gu W, Liu P, Tang J, Lai J, Wang S, Zhang J, Xu J, Deng J, Yu F, Shi C and Qiu F (2024) The prognosis of TP53 and EGFR co-mutation in patients with advanced lung adenocarcinoma and intracranial metastasis treated with EGFR-TKIs. Front. Oncol. 13:1288468. doi: 10.3389/fonc.2023.1288468
Received: 04 September 2023; Accepted: 28 November 2023;
Published: 05 February 2024.
Edited by:
Brian D. Adams, Brain Institute of America, United StatesReviewed by:
Priyanka Banerjee, Texas A&M Health Science Center, United StatesXiaodong Lu, Emory University, United States
Copyright © 2024 Gu, Liu, Tang, Lai, Wang, Zhang, Xu, Deng, Yu, Shi and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Yu, eXVmZW5nOTcyMzk3QDE2My5jb20=; Chao Shi, bmR5Znk0NTQwQG5jdS5lZHUuY24=; Feng Qiu, bmR5ZnkwMTE0OUBuY3UuZWR1LmNu
†These authors share first authorship
‡These authors have contributed equally to this work
 Weiguo Gu
Weiguo Gu Penghui Liu1†‡
Penghui Liu1†‡ Jianfei Lai
Jianfei Lai Jianxiong Deng
Jianxiong Deng