- 1Department of Biomedical Engineering, Schulich School of Engineering, University of Calgary, Calgary, AB, Canada
- 2Cellular and Molecular Bioengineering Research Lab, University of Calgary, Calgary, AB, Canada
- 3Knight Cancer Institute, Oregon Health and Science University, Portland, OR, United States
- 4Arnie Charbonneau Cancer Institute, University of Calgary, Calgary, AB, Canada
- 5Department of Physiology and Pharmacology, University of Calgary, Calgary, AB, Canada
Young onset breast cancer (YOBC) is an increasing demographic with unique biology, limited screening, and poor outcomes. Further, women with postpartum breast cancers (PPBCs), cancers occurring up to 10 years after childbirth, have worse outcomes than other young breast cancer patients matched for tumor stage and subtype. Early-stage detection of YOBC is critical for improving outcomes. However, most young women (under 45) do not meet current age guidelines for routine mammographic screening and are thus an underserved population. Other challenges to early detection in this population include reduced performance of standard of care mammography and reduced awareness. Women often face significant barriers in accessing health care during the postpartum period and disadvantaged communities face compounding barriers due to systemic health care inequities. Blood tests and liquid biopsies targeting early detection may provide an attractive option to help address these challenges. Test development in this area includes understanding of the unique biology involved in YOBC and in particular PPBCs that tend to be more aggressive and deadly. In this review, we will present the status of breast cancer screening and detection in young women, provide a summary of some unique biological features of YOBC, and discuss the potential for blood tests and liquid biopsy platforms to address current shortcomings in timely, equitable detection.
Introduction
New evidence suggests incidence rates are increasing for breast cancer in young patients (aged 25–39 years) and outcomes are worse compared to older patients (1). Early-stage detection is a strong determinant of survival and quality of life, and the earlier a cancer is diagnosed, the lower the overall cancer-associated health care costs (2, 3). However, breast cancer screening has been limited to women over 50 or those at elevated risk of developing breast cancer. This review will present an overview of young onset breast cancer (YOBC), with a focus on postpartum breast cancer, current gaps in screening and diagnosis, and technologies relevant to early detection, including blood tests and liquid biopsy.
YOBC has typically been associated with poor outcomes due to lack of early diagnoses, poor clinicopathological features, and dense breast tissue affecting mammography sensitivity (1, 4–15). More specifically, this includes a higher proportion of aggressive cancer like triple negative breast cancer (TNBC), high risk of local recurrence, metastasis and lymph node involvement and larger tumor size (5, 6, 8, 10). Equitable care for breast cancer patients requires a deeper understanding of the specific mechanisms associated with breast cancer in young patients, specifically during the postpartum period, which will allow for development of improved diagnostic options for these groups. This review will examine some barriers postpartum women face when accessing breast cancer screening and early diagnosis, as well as the potential role for liquid biopsy in expanding access.
Young onset breast cancer: clinical and biological aspects
Although breast cancer is typically viewed as a disease of older women, women under 45 account for a considerable portion of overall breast cancer patients, ranging from 5% to 25%, with estimates varying based on study, country, and ethnicity (1, 16–26). The incidence and mortality rates for all early-onset cancers (women under 45) have increased over the past decade, with breast cancer leading the way (20). Despite improving outcomes in older patients through screening and better therapies, these advances have not improved outcomes in young patients. While there were improvements for young patients through the late 20th century, these trends have recently slowed (27) or even reversed (28). In addition, developing breast cancer under the age of 45 doubles the risk for metastasis and mortality, as compared to patients older than 45 (1, 29). This age-based discrepancy in outcome suggests that there is an unmet need in the care of patients under 45 with breast cancer.
Defining young-onset breast cancer (YOBC) necessitates considering the significant differences in hormone shifts with the start of menarche, pregnancy, postpartum and involution as well as perimenopause, menopause, and post-menopause (27, 30, 31). Women begin the shift from a reproductive to non-reproductive state in their mid- to late 40s, with an overall mean age of menopause at 49.9 (32, 33). Moreover, factors like hysterectomy (20% of women undergo by age 55), menopause hormone therapy (~20–35% in peri- and postmenopausal individuals) and use of oral contraceptive (62% of reproductive age women use worldwide) add layers of complexity to understanding the role of hormones (both natural and pharmaceutical), on breast cancer development and its detection (34–38). To encompass the most studies available, this review classifies YOBC as women under the age of 45 (37, 39).
YOBC often exhibits aggressive tumor biology and late-stage diagnosis, correlating with poor patient outcomes. Breast cancer in young women presents with higher prevalence of hormone receptor negative, triple negative and HER2+ tumors (29, 40), elevating risk of recurrence and metastases (41). Moreover, characteristics such as larger tumor diameter (>20mm), increased proliferation/Ki-67 expression, lymphovascular invasion and lymph node involvement are common and correlate to increased mortality (10, 42–44). In addition, factors relevant to young women, such as older age at first birth and not breastfeeding may contribute to increased risk of certain aggressive subtypes of YOBC, such as estrogen receptor negative (ER-) (45). These dynamics may be further amplified based on racial and ethnic predisposition. There is also increasing evidence that certain racial/ethnic groups, such as Black women, are at high risk of TNBC and represent a disproportionate number of cases diagnosed in young patients (15). In particular, young Black women (<50 years old) have a higher breast cancer incidence than young white women, a trend which reverses around menopause (46). Furthermore, Black women are more than twice as likely, and Hispanic women 1.2 times as likely, to be diagnosed with metastatic disease than white women in the US (47). Of the TNBCs diagnosed in young patients, cancers are often of a higher grade, are diagnosed at stage III or later, and have elevated Ki-67 as compared to their older counterparts. Young women diagnosed with Stage I/II cancer exhibit worse prognosis and higher mortality rates compared to their older counterparts, regardless of subtype (48). This may be further exacerbated by social and structural barriers, such as limited access to healthcare, which patients face in accessing a timely diagnosis, as reviewed extensively elsewhere (49–51). Globally, the average risk of dying from breast cancer before 40 years old is similar across continents except for Africa, which has more than double the risk (52). Approximately half of all young patients harbor a germline mutation in BRCA1, BRCA2 or TP53 that increases the risk of developing breast cancer (53, 54). As in older women, most breast cancers are invasive ductal carcinoma (IDC) as compared to invasive lobular carcinoma (ILC) (41). Epigenetic factors are also relevant, appearing to contribute to breast cancer risk in a manner dependent on ethnic and epidemiological factors as reviewed elsewhere (55, 56).
Postpartum breast cancer
Within YOBC, cases can be subdivided into broad categories. Breast cancer occurring in never-pregnant (nulliparous) patients and cases diagnosed during pregnancy, known as pregnancy-related breast cancer (PrBC), are associated with similar outcomes (57). In contrast, breast cancers diagnosed within 5, and up to 10 years postpartum have increased metastasis and mortality compared to diagnoses in nulliparous and PrBC patients (12, 44, 58–61). Women with PPBC are a unique and vulnerable population and like YOBC, defining a specific age range for this group poses challenges. Typically, ≤45 years has been used as a benchmark, but it is crucial to recognize that this may shift due to increasing age at first childbirth (62, 63). Notably, first pregnancy after 35 years old (classified as geriatric pregnancy or advanced maternal age) is considered a risk factor for breast cancer, with 50% increased risk compared to pregnancy at 20 years old (64, 65). It is stipulated that this is due to older women already possessing cancer-causing mutations or abnormal cells by the time of pregnancy and involution, thereby not benefiting from protective effects seen in younger pregnancies, and instead contributing to metastasis (64). The mechanism of the protective effect seen in pregnancies under 35 years old, remains unknown but is hypothesized to involve changes in hormone levels and the mechanical forces in the mammary gland (66–68). The poor outcomes of PPBC patients as compared to nulliparous and pregnant patients suggest that there are unique processes occurring in the breast after childbirth requiring further investigation.
The mammary gland is a unique and dynamic organ, as it largely develops postnatally and only reaches a mature state with lactation (69). During the time of puberty, the mammary gland undergoes cyclic proliferation, differentiation and death corresponding to hormone changes of the menstrual cycle (70, 71). Significant tissue expansion occurs with pregnancy and lactation, followed by regression at weaning (72). The cessation of lactation begins the process of involution, a remodeling of the mammary gland back to pre-pregnancy state. However, the immune signature developed during involution has been shown to persist up to 10 years post-childbirth (12, 73). The plasticity of the mammary gland is a key factor to influencing its vulnerability to the carcinogenesis process and has been reviewed elsewhere (74–76).
Breastfeeding has shown potential in reducing the risk of some breast cancer subtypes, but the mechanism of protection remains under-investigated (77, 78). One meta-analysis has shown breastfeeding associated with 10% risk reduction in estrogen receptor (ER) negative and progesterone receptor (PR) negative breast cancer, and a 20% risk reduction in TNBC (78–80). The risk of ER+ cancers also appears to be decreased in women who breastfed (80, 81). Additionally, the effectiveness of lactation may be reduced by the decline of breastfeeding duration and breastfeeding overall, with only 35.6% of females exclusively breastfeeding for at least 6 months in Canada as per WHO and Health Canada recommendations (82). Although a temporary increase in breast cancer risk follows childbirth (13, 65), it is succeeded by a long-term protective effect and reduced risk. However as discussed, this positive impact diminishes with a later age at first childbirth, posing a unique and significant challenge as the trend toward delayed pregnancies and increased child-bearing age continues to increase (13, 83). As women delay pregnancy and the age of first childbirth increases, with a historically high average age of first childbirth of 27.3 years old in the US in 2021, 29.4 years old in Europe in 2019 (84), 29.7 in Asia in 2003 (85), the incidence of PPBC is expected to rise, leading to a subsequent rise in morbidity rates (65, 86). These trends necessitate an increased focus on early diagnosis for patients with PPBC.
Current detection technologies and barriers to detection
The primary obstacle to detecting YOBC and PPBC is the lack of diagnostic technology and regular screening procedures with demonstrated efficacy in this population. Commonly used metrics for evaluating performance of breast cancer screening/diagnostic tests, such as mammography and MRI, include clinical sensitivity and specificity. Clinical sensitivity is the ability of the test to accurately detect cancer when present; a low sensitivity indicates a higher rate of missed cancers. Clinical specificity is the ability to correctly determine a patient as disease-free when cancer is not present; low specificity can lead to unnecessary downstream procedures and patient anxiety. These parameters are determined in clinical studies using a “gold standard” or longitudinal follow-up of participants to capture the true positives and true negatives. Comparing the performance of different technologies remains difficult. For review see Hollingsworth (2019) (87). Further, the population recruited in a study may have different characteristics including varying breast density, racial and ethnic predisposition, and more, that could affect performance. This produces performance metrics that may be higher or lower than other published studies, depending on the benchmark and population used. The gold standard for breast screening sensitivity is MRI. When compared to MRI, mammography sensitivity is lower (≤ 40%) (88, 89) due to MRI’s lower limit of detection (ability to find smaller tumors) and effectiveness of contrast agent in enhancing visualization of breast cancer, including lobular breast cancers. The difficulty in comparing technologies is a barrier in development and clinical implementation of effective screening and/or diagnostic tools for YOBC.
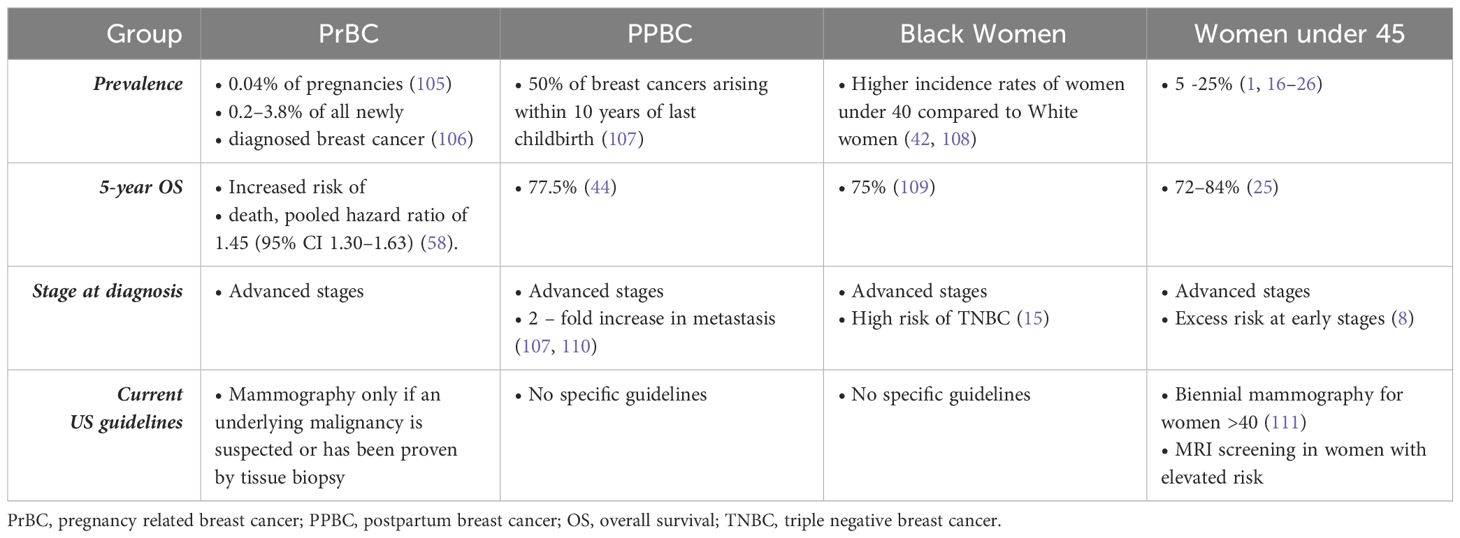
Mammography is the standard for breast cancer screening in many countries but has limited clinical utility in YOBC. Specifically, the sensitivity and specificity of mammography is reduced in young women due to increased prevalence of high breast density (15). Figure 1 compares the sensitivity of mammography to the breast density of women under and over 45. Elevated breast density [heterogeneously dense (C) or very dense (D)], decreases the sensitivity and specificity of mammography screening (92), resulting in detection when tumors are larger (94–96). Breast density is elevated in over 50% of women, and this is associated with a 2 to 5 times greater risk of breast cancer (90, 91, 97–100). Furthermore, Black women have the highest breast densities across all age groups (101, 102). Breast ultrasonography is often employed in young women with dense breast tissue; however, lower specificity and increased rates of operator error have hindered wider deployment as a stand-alone first screen (103, 104). MRI is used for screening in young women with elevated risk of breast cancer, however the need for contrast agent, cost, and access challenges limit participation and availability. Together, this evidence points to young patients being the most at risk due to high breast density but having limited screening options.
Figure 1 Comparison of BI-RADS classification to mammography sensitivity and percent of women under/over 45. The Breast Imaging Reporting and Data System (BI-RADS) uses mammographic density for classification of breasts based on percent of fibroglandular tissue. The four categories are (A) predominantly fatty (≤25%), (B) scattered fibroglandular (26–50%), (C) heterogeneously dense (51–75%), and (D) extremely dense (76–100%). The distribution of mammographic density was adapted from Checka et al. (90) and sensitivity from Lynge et al. (91). The sensitivity of mammography is decreased with dense tissue which is predominantly found in young women (92). The density of breast tissue decreases with age making mammography a suitable option for older women but presents a gap in screening of young women. Mammographic images originally open access published by Pawlak et al. (2023) (93).
US breast cancer screening guidelines reflect the higher performance of mammography and higher incidence of breast cancer in older women, with a gap in the screening and diagnosis of YOBC. A summary of the guidelines and statistics can be found in Table 1. Overall, breast cancer guidelines remain mostly consistent among 21 high-income countries (including the US, UK, Canada, etc.), with most countries recommending screening every 2 years between 50 to 69 years old for women of average risk (112). Women 40 to 49 may participate in screening based on individualized needs and a physician recommendation (111). Women with elevated risk may begin screening at earlier ages, typically using MRI. MRI has high screening sensitivity but is not viable for broader implementation due to high equipment and personnel costs, low availability, and lower specificity rates resulting in higher rates of follow-up procedures (113). The National Comprehensive Cancer Network (NCCN) guidelines recommend women begin MRI screening at age 25 to 40, depending on family history (first-degree relative with breast cancer) and genetic predisposition (BRCA1/2, p53 or pTEN mutations) (114). Similarly, the Canadian Task Force on Preventive Health Care recommends for women 40–49 years old to not screen with mammography and only undergo screening based on the “relative value a woman places on the possible benefits and harms from screening” (115). There are currently no recommendations for screening of women with dense breasts, leaving clinical diagnosis to occur following self-detection, indicating advanced disease progression, and contributing to unfavorable outcomes due to delayed intervention. Current guidelines do not address broad groups of individuals at risk of YOBC, including those with high breast density, recent childbirth, or racial/ethnic genetic predisposition.
Globally, there are population disparities for breast cancer incidence, diagnosis, access to new technologies, and consequent outcomes. Lebanon, for example, has the highest incidence of breast cancer in the Middle East, with diagnosis occurring at a younger age than its Western counterparts (52 years compared to 63 years respectively), as well as more aggressive and fatal outcomes (116). Access to and participation in screening technologies is a contributing factor to late detection in some geographical regions. Approximately 80% of deaths from breast cancer occur in low to middle income countries according to the World Health Organization (WHO), prompting the formation of the Global Breast Cancer Initiative Framework (117). Notably, among young women, regions across the world with comparable incidence rates have very different mortality rates, which is not the case for older women, where greatly different incidence rates have comparable mortality rates (52). There could be many reasons for this including differences in the availability of screening, healthcare and treatments (118). Current literature is limited regarding characterization, screening and treatment of YOBC, and the role of race and ethnicity. This is compounded by studies focusing on a specific demographic or being too broad as well as adopting varying definitions of YOBC and PPBC, making it difficult to compare patient outcomes (6, 8, 10, 26, 58, 119–121). More diversified studies using a population under 45 with information on parity status, breast density and ethnicity would highly benefit research within this area.
There are many barriers to implementation of screening in young women including physical access, procedural costs, and post-diagnosis care expenses. The deterrents for not seeking postpartum/postnatal care and breast cancer screening overlap, and depending on location, commonly include public transportation access, distance to facility, travel time, lack of trained professionals and lack of awareness (122–124). In higher income countries, immigrant and refugee women, in particular, face significant hurdles in access resulting in increased risk of mortality and morbidity related to pregnancy as compared to the rest of the population (125, 126). Furthermore, women of all racial/ethnic backgrounds living in rural areas have higher breast cancer mortality than women living in urban areas, illustrating the critical impact of accessibility on care and screening (47). Racial disparities in access also persist with Black women having three times the maternal mortality of White women in the United States (127). While it is difficult for early diagnosis and screening to eliminate these barriers, emphasis on community resources and education have been identified as an effective approach (128). Exploring the incorporation of early breast cancer screening alongside postnatal and postpartum care, which is widely implemented in many countries, could potentially enhance accessibility.
Early detection and liquid biopsy
Blood tests or liquid biopsies offer the potential to address many of the gaps in early detection of breast cancer for young women such as increased accessibility, higher participation, and complementarity to imaging. Liquid biopsy involves the use of a body fluid (such as blood, breast milk, nipple aspirate fluid or urine) for identifying the molecular characteristics of the disease. Typically, liquid biopsy tests have been used for treatment selection and risk monitoring of recurrence, but emerging multi-biomarker blood-based tests are focusing on early detection (129–137). Key criteria to consider in the implementation of liquid biopsy tests for early detection of breast cancer include analytical and clinical performance metrics in targeted patient populations, accessibility, and scalability. Detection technologies used in first pass screening typically have high diagnostic specificity with the intention of limiting the number of false positives and unnecessary downstream procedures. However, diagnostic sensitivity is also important due to the inevitable risk of false negatives. In most cases, stringent specimen collection and handling requirements have a significant effect on sample stability, integrity, and overall performance of the test. Molecular processing of samples in the laboratory is usually performed by certified professional laboratory users or automated solutions, with an effect on the cost and ability to scale operations.
Early liquid biopsy tests encompassed analysis of circulating tumor cells (CTCs), circulating tumor DNA (ctDNA) or other genetic material such as micro-RNAs from plasma for use in prognosis and treatment selection [for review see (129, 130, 132, 136, 137)]. Some tests have obtained FDA clearance including the CellSearch test by Veracyte that is indicated for cancer prognosis and the Guardant360DX test for treatment selection. The FoundationOne Liquid CDx (Foundation Medicine) test is an FDA-approved test indicated for breast cancer gene profiling for treatment selection (138, 139). As the CellSearch test is based on the expression of epithelial cell adhesion molecule (EpCAM) on CTCs and with EpCAM demonstrated to be downregulated in most aggressive breast cancer cells undergoing epithelial-to-mesenchymal transition (EMT), the ability of the test to accurately detect disease even in advanced stages might be limited (140, 141). First generation screening tests have shown limited sensitivity for breast cancer, particularly in the early stages, given the limited ctDNA and CTC that is shed from the breast tumor among other technical limitations (142–144). As an example, the ctDNA based multicancer early detection (MCED) screening blood test Galleri (Grail) showed limited detection of early breast cancer as part of breast screening. However the test identified cases of recurrence (145). TruCheck (Datar Genetics) is a blood test that measures 5 markers by immunofluorescence microscopy of CTCs isolated and expanded from a blood sample. Clinical performance results based on 141 participants (112 cancers, 29 no cancer) from women aged 18–81 revealed a specificity of 93.1% and a sensitivity of 94.64% (146). The Syantra DX™ Breast Cancer test (Syantra Inc.) is based on qPCR and machine-learning based software analysis of a proprietary panel of 12 target mRNA markers from whole blood. Clinical performance for this test revealed an overall accuracy of 92% (specificity of 94% and sensitivity of 79%) in blinded, independent clinical studies on a test set of 695 women (aged 30–75) screening for breast cancer (147).
To date, a limited number of studies have examined liquid biopsy technologies for breast cancer detection in young women. Lourenco et al. (148) examined the utility of a proteomic biomarker assay (Videssa Breast) to rule out breast cancer in women with inconclusive or suspicious imaging findings. The study focused on women under 50 with high breast density (BI-RADS 3 or 4), and reported sensitivity of 87.5% and specificity of 83.8% in a cohort of 545 women (148). The Syantra DX™ Breast Cancer test also evaluated clinical performance in women under 50, for which enhanced performance was reported with clinical sensitivity and specificity rates of 91.7% and 99.0%, respectively (147). Further clinical studies in this area will expand populations of women evaluated and evidence to support use in younger women.
Research-based approaches for breast cancer detection are demonstrating potential. Nipple aspirate fluid has been used for biomarker detection at the earliest stages of breast cancer, prior to a visible tumor mass (149). However, this strategy exhibits low yield, requires a local anesthetic, and cannot be collected from pregnant or lactating women. As a result, it is a promising option for the screening of young women in general, but not PrBC and PPBC. Jang et al. (150) demonstrated that miRNA multiplex analysis from plasma may be useful for diagnosing women under 50 with dense breasts. Most recently, Saura et al. (2023) demonstrated that breast milk contains ctDNA and surpasses the yield found in plasma (151). Interestingly, the samples with the highest ctDNA concentration demonstrated a loss-of-function variant of E-cadherin, consistent with decreased tumor cell junction tightness due to mutations in this gene. They also presented two cases in which breast cancer was detected via breast milk prior to diagnosis by imaging. Testing of breast milk did not produce any false positive results, though the numbers were small – limiting statistical analysis (n<30). These studies indicate that screening technologies for breast cancer in young women is an emerging field with great promise.
Artificial intelligence (AI) is expanding in use in existing imaging modalities and emerging detection tests (152). Published in 2023, an AI model “Sybil” was developed to analyze low-dose CT scans and predict future lung cancer risk, achieving a success rate over 86% (153). This capability to predict an individual’s future cancer risk from a single scan supports personalized screening and monitoring. Improved and automated image analysis is a primary application of AI (154–156). Radiologists assisted with AI have improved sensitivity and specificity in making clinical decisions than either approach alone (157). Despite the progress in this area, challenges in cancer detection and clinical adoption persist, including model bias, data security, data size limitations and variable methodology standards, as discussed elsewhere (158). Additionally, AI assisted image analysis remains constrained to the sensitivity and specificity of current imaging modalities and has limited enhancement ability. Liquid biopsy tests often incorporate machine learning and advanced data analytics for performance enhancement. Advances in AI and data analytic technology hold promise for enhancing detection and management of breast cancer.
Biomarkers for screening/diagnosis of postpartum breast cancer
Previous research has identified PPBC as a unique population of breast cancer patients based on molecular phenotype and has suggested that this distinct phenotype may persist for up to 10 years (12, 59). As a result, biomarker identification and validation are important in this population. PPBC represents an opportunity to identify specific and sensitive biomarkers suitable for screening/diagnosis, based on processes solely associated with the postpartum period that are thought to promote breast cancer progression.
Involution may involve processes promoting tumor cell dissemination and upregulation of molecular markers associated with poor prognosis. In the initial phase of involution, there is an upregulation of acute immune response genes including STAT3 and interleukins (159–162). Leukocyte chemoattractants are also upregulated during this time, leading to the recruitment of large numbers of macrophages (163, 164). Mammary epithelial cells enter apoptosis and are further responsible for the continuation of this inflammatory, albeit immune regulatory phenotype. In the later phase of involution, there is an active T-cell presence, followed by T-cell exhaustion/suppression and resulting immune avoidance (59). Cycloxygenase-2 (COX2), a well-known inflammatory mediator, has been shown to mediate persistent lymphangiogenesis up to 10 years postpartum, suggesting that the unique immune signature present during mammary gland involution persists long after involution has concluded (12, 73). The increased lymphatic density observed during mammary gland involution is consistent with increased rates of lymph-node metastasis in PPBC, as compared to nulliparous cases.
Dynamics of the extracellular matrix (ECM) are equally important to the pro-tumorigenic effects of involution (Figure 2). Culturing of tumor cells on ECM isolated from involuting rat mammary glands leads to disruption of cell-cell adhesion junctions and loss of apical-basal polarity, consequently enhancing the invasive capacity of breast cancer cells. Furthermore, there is an increase in collagen, tenascin C, and proteolysis of collagen, fibronectin, and laminin (165–168). Increased fibrillar collagen density and radial alignment of collagen are observed in invasive breast tumors and observed in physiologically normal breast involution and in PPBC (169–171). These ECM-based mechanisms inherently improve the motility and invasiveness of breast cancer cells, however, there are also well-documented interactions between ECM dynamics and the immune system, suggesting further downstream effects of the local mechanical involution environment (172, 173). Beyond ECM dynamics, there is a distinct role for direct mechanical forces in regulating involution. Some groups have hypothesized that milk accumulation induces a stretching force on cells lining breast lobules, triggering the release of STAT3 and subsequent initiation of involution (174). Furthermore, there are likely changing fluid dynamics between interstitial fluid flow and inflammation, and the role of mechanical forces in regulating lymphatic expansion during involution. Consistently, many markers associated with the tumorigenic effects of mammary gland involution, such as fibronectin, Semaphorin 7A, matrix metalloproteinases, collagen I and more, have been shown to be flow-regulated by our group and others. Our group has previously demonstrated that fluid shear stress (FSS) upregulates S100 genes and fibronectin, and promotes epithelial-to-mesenchymal transition, motility, and adhesion of breast cancer cells (175, 176). These studies further highlight the important interactions between fluid dynamics and the immune microenvironment during involution (175, 177–186).
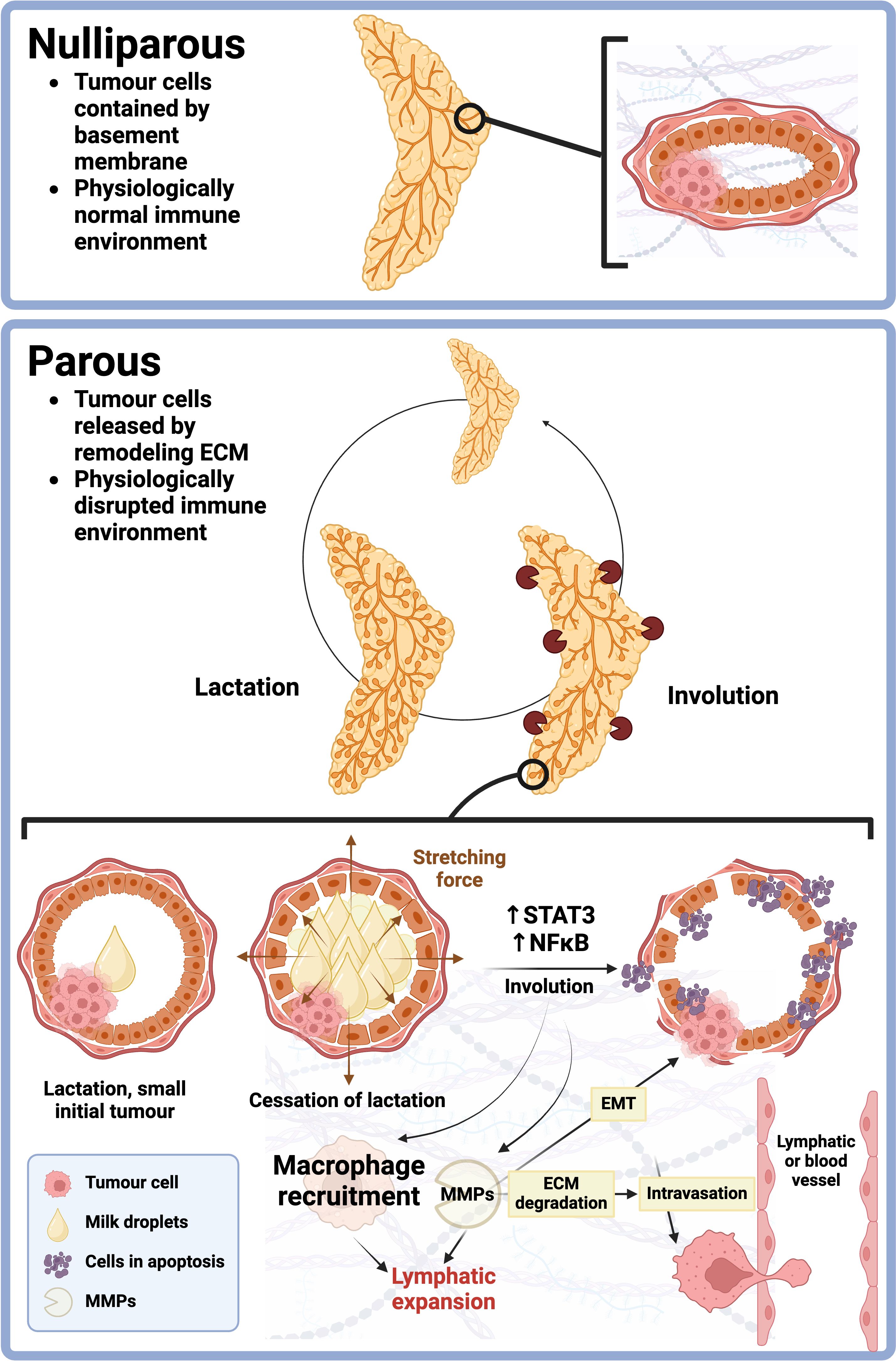
Figure 2 Visual representation of differences in the microenvironment of nulliparous YOBC and PrBC or PPBC. The top panel represents full view and ductal view of normal, undifferentiated (nulliparous) mammary gland, prior to pregnancy. As indicated, the basement membrane and basic structure of the duct remains intact, compared to the parous state. In the bottom panel, a full view and ductal view of the parous mammary gland is presented. The mammary gland undergoes cyclical remodeling prior and post-childbirth. Involution is a remodeling process that occurs post-lactation. It involves process such as matrix metalloproteinase (MMP) activation, immune cell recruitment and dysregulation, lymphatic expansion, extracellular matrix (ECM) degradation, epithelial-to-mesenchymal transition (EMT) and other activities that allow breast cancer cells to escape the primary tumor more easily, migrate into circulation and establish secondary sites in other locations.
Collectively, these data suggest a unique opportunity to identify biomarkers of PPBC by leveraging knowledge of mammary gland involution, focusing on a distinct inflammatory and wound healing signatures and the mechanical cell environment. Further research regarding the interactions between the mechanical and immune microenvironments during mammary gland involution present an excellent opportunity to develop diagnostic biomarkers exclusively associated with and aimed at sensitive detection of PPBC.
Accessing liquid biopsy for women in the postpartum period
As discussed above, one barrier to breast cancer screening participation is difficulty in accessing transportation to centralized imaging facilities (187–191). Further, the lack of awareness of the prevalence and risks of PPBC results in a decreased likelihood of women receiving referrals and access to traditional diagnostic methods like more sensitive diagnostic imaging or tissue biopsy. While these concerns cannot be directly addressed without a larger transition to community-based medicine, the existence of an early detection liquid biopsy test would be compatible with integration into a community-based approach and existing postpartum care (Figure 3). During pregnancy and the postpartum period, many patients will have more exposure to the health care system through their OB/GYN provider. This presents an opportunity to integrate early steps in the preventative cancer care pathway. Routine postpartum care already involves blood-based testing, providing an efficient and streamlined approach for incorporating PPBC screening especially with blood collection being available in many locations. Milk samples are also often collected as a part of routine postpartum care, particularly in cases of mastitis etc. Furthermore, if a patient is diagnosed with PPBC, liquid biopsy presents an opportunity for treatment surveillance and minimal residual disease (MRD) assessment (192), which may be beneficial given the unique reproductive concerns related to PrBC and PPBC (1, 107, 193). Additionally, there may be opportunities for integration into annual OB/GYN care for those who are not pregnant. Implementation of liquid biopsy tests in the context of breast cancer screening/diagnosis benefits from a sensitive imaging modality for downstream referrals in the event of a positive result. MRI is the gold-standard for sensitivity, however contrast-enhanced mammography and other imaging technologies are advancing. As we look to improve women’s health care, there are likely to be many combinations of technologies that may work to address the varied needs of women of different characteristics and life situations. Finally, limitations exist for disadvantaged communities in accessing all types of postpartum care, particularly evident in less developed countries and countries that operate without universal or public health care models, including the US (188). These systemic issues would need to be considered and addressed for liquid biopsy to fulfill its full potential in aiding the detection of YOBC across all communities.
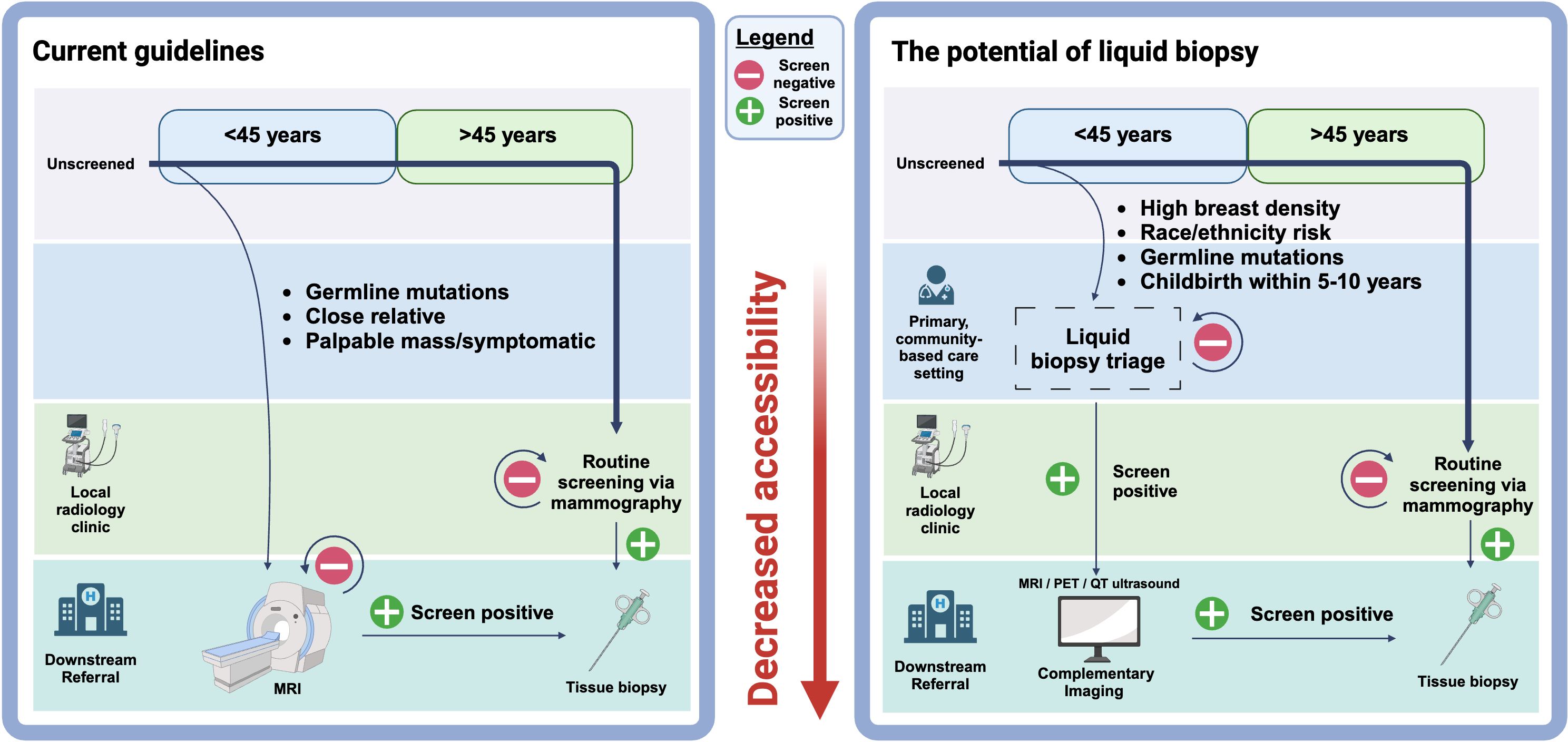
Figure 3 The potential of liquid biopsy to improve breast cancer screening accessibility through primary, community-based care. The current breast cancer screening guidelines do not have recommendations for several at-risk populations due to lack of diagnostic technology. Implementation of liquid biopsy early detection tests may allow for breast cancer screening to be accessed through a primary physician instead of a specialized clinic. This would address barriers of distance to facility, travel time and transportation. There is still a need for improvement of imaging modalities such as MRI, PET and QT ultrasound to be used complementary to liquid biopsy.
Few studies have examined the feasibility of collecting liquid biopsy specimens in routine, first-line clinical settings. Pilot studies during the pandemic have shown mobile collection of blood based liquid biopsy to be beneficial and cost-effective in delivering cancer care to patients (194, 195). In the case of milk-based liquid biopsies, there is a lack of guidelines for adequate storage and handling, appropriate collection containers, temperature control, and other factors (196). It is likely that immediate freezing would be required to prevent degradation of RNA and miRNA relevant to molecular diagnostics. Though milk-based liquid biopsies may be an attractive alternative to blood-based liquid biopsies for analytic reasons discussed, the practical implementation requires further investigation. For blood-based liquid biopsies, the National Cancer Institute currently recommends maximum storage times from 4 hours for EDTA tubes and up to 3 days for preservative tubes at ambient conditions determined from manufacturer stability tests (197–199). Longer times are available for samples that can be stored and transported at -20C or -80C. CTC-based assays continue to be limited in this context, with very few suitable collection tubes on the market (200). Current limitations associated with collection tubes could impact remote collection, depending on location, and would require efficient delivery of samples to processing locations. Other options to mitigate these issues include Point-of-Care (lab-on-chip) solutions, which have been reviewed extensively elsewhere (201–203).
Clinician education is critical to the implementation of any new technology into clinical practice, however implementation of liquid biopsy in the primary care setting is further complicated by the number of primary care physicians and their high workload/wide breadth of practice (200, 204). As outlined, liquid biopsy for early cancer detection is an emerging field with different technologies becoming available. Interpreting results within the context of patient characteristics alongside available tests and imaging data adds an additional layer of complexity. Clinicians must not only understand the indicated populations for use of liquid biopsy tests, but also the performance metrics and limitations of standard of care imaging for screening and diagnosis. This comprehensive understanding is essential for effective use of available technologies and facilitating early-stage detection. Many clinical blood tests report the amount of a biomarker, while cancer detection tests usually report a positive or negative signal informing a recommendation for follow-up diagnostic imaging. Appropriate options for downstream referral will also be paramount in providing primary care physicians with options for their patients in the event of a positive result. While patients with PPBC represent a uniquely suitable cohort for liquid biopsy early detection tests, clinician awareness of screening technology limitations is critical to quality care. This is particularly true in situations where OB-GYNs and other primary care providers with less knowledge of oncology are responsible for administering these tests and communicating with patients. Knowledge dissemination to clinicians to support this new aspect of postpartum care will facilitate widespread adoption.
The economics of liquid biopsy for breast screening appear promising but will vary based on target population, test performance, test cost and current standard of care. Emerging early detection liquid biopsy tests will likely enter the market with small target populations and low coverage by payers, and expand populations and coverage with time as more clinical validation and usage data is obtained. Liquid biopsy may benefit from an economy of scale model, wherein centralized labs are able to conduct large numbers of tests from multiple regional facilities (200). This model may lead to decreased costs, however the benefit may not trickle down to individual patients, depending on the market. Preliminary data suggests that liquid biopsies can be a cost-effective option with faster turnaround time, compared to conventional diagnostics (205–207). These estimates, however, can vary greatly, and further research is needed in this area (208). An understanding of the health economics of liquid biopsy for YOBC is currently limited by several factors. As previously discussed, the comparison of screening technology performance parameters, such as sensitivity, is difficult due to inconsistent “gold standards”. Additionally, when considering the population of women under 50, the availability and participation in screening are limited, resulting in inadequate performance data. Though the prevalence rates of breast cancer are lower in young women, liquid biopsy may be more cost effective as breast density is elevated in over 50% of young women, reducing mammography sensitivity, increasing risk of breast cancer, and delaying diagnosis (65–70). Liquid biopsy would likely improve early stage diagnosis thereby reducing disease burden which is increased with advanced stages (209). However, no studies have examined the cost-effectiveness of liquid biopsy in this specific population. Future studies to address this knowledge gap are imperative if we are to reverse the current trends of increased incidence of lethal breast cancer in young women worldwide.
Discussion/Conclusions
This review highlights the practical, clinical, and technological gaps that currently exist in early detection and screening of YOBC. Lack of detection options for young women, as well as their unique biology, make early-stage detection challenging. Challenges include lack of inclusion in screening recommendations, difficulties accessing screening, low awareness of breast cancer in women under 50 and screening technology limitations for this population. Emerging liquid biopsy tests may be able to address barriers in accessing screening through implementation of community-based (de-centralized) approaches in primary and integrated gynecological/postpartum care. Expanded clinical studies recruiting women under 50 for liquid biopsy early detection tests will support test approvals and reimbursement, increasing availability and awareness. Improved understanding of the mammary gland microenvironment during all stages of development may lead to identification of biomarkers particularly effective for this population for the next generation of tests. Further research investigating the cancer biology of YOBC, tests utilizing biological advancements, and clinical studies focused on YOBC and PPBC are needed to advance the available technologies and address the current gaps in care of these underserved populations.
Author contributions
MS-L: Writing – original draft, Writing – review & editing. JM: Writing – original draft, Writing – review & editing. SB: Writing – original draft, Writing – review & editing. PS: Writing – review & editing. KR: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported with funding from the Alberta Cancer Foundation, University of Calgary, and the Natural Sciences and Engineering Council of Canada.
Acknowledgments
We thank Dr. Alan B. Hollingsworth for his useful editorial comments and suggestions.
Conflict of interest
Author KR is a co-founder, director, and shareholder of the company Syantra, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ademuyiwa FO, Cyr A, Ivanovich J, Thomas MA. Managing breast cancer in younger women: challenges and solutions. Breast Cancer: Targets Ther. (2016) 8:1. doi: 10.2147/BCTT
2. McGarvey N, Gitlin M, Fadli E, Chung KC. Increased healthcare costs by later stage cancer diagnosis. BMC Health Serv Res. (2022) 22:1155. doi: 10.1186/s12913-022-08457-6
3. Reddy SR, Broder MS, Chang E, Paydar C, Chung KC, Kansal AR. Cost of cancer management by stage at diagnosis among Medicare beneficiaries. Curr Med Res Opinion. (2022) 38:1285–94. doi: 10.1080/03007995.2022.2047536
4. Bouferraa Y, Haibe Y, Chedid A, Jabra E, Charafeddine M, Temraz S, et al. The impact of young age (< 40 years) on the outcome of a cohort of patients with primary non-metastatic breast cancer: analysis of 10-year survival of a prospective study. BMC Cancer. (2022) 22:27. doi: 10.1186/s12885-021-09100-z
5. Narod SA. Breast cancer in young women. Nat Rev Clin Oncol. (2012) 9:460–70. doi: 10.1038/nrclinonc.2012.102
6. Fabiano V, Mandó P, Rizzo M, Ponce C, Coló F, Loza M, et al. Breast cancer in young women presents with more aggressive pathologic characteristics: Retrospective Analysis From an Argentine National Database. JCO Global Oncol. (2020) 6:639–46. doi: 10.1200/JGO.19.00228
7. Axelrod D, Smith J, Kornreich D, Grinstead E, Singh B, Cangiarella J, et al. Breast cancer in young women. J Am Coll Surg. (2008) 206:1193–203. doi: 10.1016/j.jamcollsurg.2007.12.026
8. Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PloS One. (2009) 4:e7695–e. doi: 10.1371/journal.pone.0007695
9. Azim HA, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. (2014) 16:427. doi: 10.1186/s13058-014-0427-5
10. Wang W, Tian B, Xu X, Zhang X, Wang Y, Du L, et al. Clinical features and prognostic factors of breast cancer in young women: a retrospective single-center study. Arch Gynecol Obstet. (2023) 307:957–68. doi: 10.1007/s00404-022-06670-6
11. Lyons TR, Schedin PJ, Borges VF. Pregnancy and breast cancer: When they collide. J Mammary Gland Biol Neoplasia. (2009) 14:87–98. doi: 10.1007/s10911-009-9119-7
12. Goddard ET, Bassale S, Schedin T, Jindal S, Johnston J, Cabral E, et al. Association between postpartum breast cancer diagnosis and metastasis and the clinical features underlying risk. JAMA Netw Open. (2019) 2:e186997. doi: 10.1001/jamanetworkopen.2018.6997
13. Albrektsen G, Heuch I, Tretli S, Kvåle G. Breast cancer incidence before age 55 in relation to parity and age at first and last births: a prospective study of one million Norwegian women. Epidemiology. (1994) 5:604–11. doi: 10.1097/00001648-199411000-00008
14. Ulery MA, Carter L, McFarlin BL, Giurgescu C. Pregnancy-associated breast cancer: significance of early detection. J Midwifery Women’s Health. (2009) 54:357–63. doi: 10.1016/j.jmwh.2008.12.007
15. Rossi L, Mazzara C, Pagani O. Diagnosis and treatment of breast cancer in young women. Curr Treat Opt Oncol. (2019) 20:86. doi: 10.1007/s11864-019-0685-7
16. Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. (2019) 30:1194–220. doi: 10.1093/annonc/mdz173
17. SEER Explorer. An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program NCI. National Cancer Institute (2023).
18. Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2023. Canadian Cancer Society (2023). Available at: https://cancer.ca/en/research/cancer-statistics.
19. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin. (2022) 72:7–33. doi: 10.3322/caac.21708
20. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
21. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: A Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
22. Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA: Cancer J Clin. (2020) 70:443–59. doi: 10.3322/caac.21637
23. Fernandes U, Guidi G, Martins D, Vieira B, Leal C, Marques C, et al. Breast cancer in young women: a rising threat: A 5-year follow-up comparative study. Porto BioMed J. (2023) 8:e213. doi: 10.1097/j.pbj.0000000000000213
24. Bellanger M, Zeinomar N, Tehranifar P, Terry MB. Are global breast cancer incidence and mortality patterns related to country-specific economic development and prevention strategies? J Global Oncol. (2018) 4. doi: 10.1200/JGO.17.00207
25. Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. (2009) 36:237–49. doi: 10.1053/j.seminoncol.2009.03.001
26. Zhang Z, Bassale S, Jindal S, Fraser A, Guinto E, Anderson W, et al. Young-Onset breast cancer outcomes by time since recent childbirth in Utah. JAMA Netw Open. (2022) 5:e2236763–e. doi: 10.1001/jamanetworkopen.2022.36763
27. Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. (2002) 94:606–16. doi: 10.1093/jnci/94.8.606
28. Surveillance, Epidemiology, and End Results (SEER). Surveillance Epidemiology and End Results (SEER) Program. Maryland, United States: National Cancer Institute (2020).
29. Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. (2008) 26:3324–30. doi: 10.1200/JCO.2007.14.2471
30. Satpathi S, Gaurkar SS, Potdukhe A, Wanjari MB. Unveiling the role of hormonal imbalance in breast cancer development: A comprehensive review. Cureus. (2023) 15:e41737. doi: 10.7759/cureus.41737
31. Folkerd E, Dowsett M. Sex hormones and breast cancer risk and prognosis. Breast. (2013) 22 Suppl 2:S38–43. doi: 10.1016/j.breast.2013.07.007
32. Appiah D, Nwabuo CC, Ebong IA, Wellons MF, Winters SJ. Trends in age at natural menopause and reproductive life span among US women, 1959–2018. Jama. (2021) 325:1328–30. doi: 10.1001/jama.2021.0278
33. Hoyt LT, Falconi AM. Puberty and perimenopause: Reproductive transitions and their implications for women’s health. Soc Sci Med. (2015) 132:103–12. doi: 10.1016/j.socscimed.2015.03.031
34. Pacello P, Baccaro LF, Pedro AO, Costa-Paiva L. Prevalence of hormone therapy, factors associated with its use, and knowledge about menopause: a population-based household survey. Menopause. (2018) 25:683–90. doi: 10.1097/GME.0000000000001066
35. Taha NH, Dizaye KF. Impact of zingiber officinale on symptoms and hormonal changes during the menopausal period–A clinical trial in Duhok, Iraq. J Natural Sci Biol Med. (2022) 13. doi: 10.4103/jnsbm.JNSBM_13_2_7
36. Zhang G-Q, Chen J-L, Luo Y, Mathur MB, Anagnostis P, Nurmatov U, et al. Menopausal hormone therapy and women’s health: An umbrella review. PloS Med. (2021) 18:e1003731. doi: 10.1371/journal.pmed.1003731
37. Group ECW. Hormones and breast cancer. Hum Reprod Update. (2004) 10:281–93. doi: 10.1093/humupd/dmh025
38. Vessey Mp, Villard-Mackintosh L, Mcpherson K, Coulter A, Yeates D. The epidemiology of hysterectomy: findings in a large cohort study. BJOG: Int J Obstetr Gynaecol. (1992) 99:402–7. doi: 10.1111/j.1471-0528.1992.tb13758.x
39. Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. (2019) 394:1159–68. doi: 10.1016/S0140-6736(19)31709-X
40. Shah AN, Carroll KJ, Gerratana L, Lin C, Davis AA, Zhang Q, et al. Circulating tumor cells, circulating tumor DNA, and disease characteristics in young women with metastatic breast cancer. Breast Cancer Res Treat. (2021) 187:397–405. doi: 10.1007/s10549-021-06236-1
41. Williams LA, Hoadley KA, Nichols HB, Geradts J, Perou CM, Love MI, et al. Differences in race, molecular and tumor characteristics among women diagnosed with invasive ductal and lobular breast carcinomas. Cancer Causes Control. (2019) 30:31–9. doi: 10.1007/s10552-018-1121-1
42. Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. (2008) 100:1643–8. doi: 10.1093/jnci/djn344
43. Zhu JW, Charkhchi P, Adekunte S, Akbari MR. What is known about breast cancer in young women? Cancers. (2023) 15:1917. doi: 10.3390/cancers15061917
44. Callihan EB, Gao D, Jindal S, Lyons TR, Manthey E, Edgerton S, et al. Postpartum diagnosis demonstrates a high risk for metastasis and merits an expanded definition of pregnancy-associated breast cancer. Breast Cancer Res Treat. (2013) 138:549–59. doi: 10.1007/s10549-013-2437-x
45. Bertrand KA, Bethea TN, Adams-Campbell LL, Rosenberg L, Palmer JR. Differential patterns of risk factors for early-onset breast cancer by ER status in African American women. Cancer Epidemiol Biomarkers Prev. (2017) 26:270–7. doi: 10.1158/1055-9965.EPI-16-0692
46. Wheeler SB, Reeder-Hayes KE, Carey LA. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncol. (2013) 18:986–93. doi: 10.1634/theoncologist.2013-0243
47. Thompson B, Hohl SD, Molina Y, Paskett ED, Fisher JL, Baltic RD, et al. Breast cancer disparities among women in underserved communities in the USA. Curr Breast Cancer Rep. (2018) 10:131–41. doi: 10.1007/s12609-018-0277-8
48. Gnerlich JL, Deshpande AD, Jeffe DB, Sweet A, White N, Margenthaler JA. Elevated breast cancer mortality in young women (<40 years) compared with older women is attributed to poorer survival in early stage disease. J Am Coll Surg. (2011) 208(3):341–7. doi: 10.1016/j.jamcollsurg.2008.12.001
49. Miller-Kleinhenz JM, Collin LJ, Seidel R, Reddy A, Nash R, Switchenko JM, et al. Racial disparities in diagnostic delay among women with breast cancer. J Am Coll Radiol. (2021) 18:1384–93. doi: 10.1016/j.jacr.2021.06.019
50. Yoda S, Theeke LA. A scoping review of factors contributing to late-stage diagnosis of breast cancer in racial and ethnic minority (African American and Hispanic) women. SAGE Open. (2022) 12:21582440221140297. doi: 10.1177/21582440221140297
51. Rebner M, Pai VR. Breast cancer screening recommendations: African American women are at a disadvantage. J Breast Imag. (2020) 2:416–21. doi: 10.1093/jbi/wbaa067
52. Sopik V. International variation in breast cancer incidence and mortality in young women. Breast Cancer Res Treat. (2021) 186:497–507. doi: 10.1007/s10549-020-06003-8
53. Paluch-Shimon S, Cardoso F, Partridge AH, Abulkhair O, Azim HA, Bianchi-Micheli G, et al. ESO–ESMO 4th international consensus guidelines for breast cancer in young women (BCY4). Ann Oncol. (2020) 31:674–96. doi: 10.1016/j.annonc.2020.03.284
54. Lalloo F, Varley J, Moran A, Ellis D, O’Dair L, Pharoah P, et al. BRCA1, BRCA2 and TP53 mutations in very early-onset breast cancer with associated risks to relatives. Eur J Cancer. (2006) 42:1143–50. doi: 10.1016/j.ejca.2005.11.032
55. Johansson A, Flanagan JM. Epigenome-wide association studies for breast cancer risk and risk factors. Trends Cancer Res. (2017) 12:19–28.
56. Joshi V, Lakhani SR, McCart Reed AE. NDRG1 in cancer: A suppressor, promoter, or both? Cancers. (2022) 14:5739. doi: 10.3390/cancers14235739
57. Amant F, von Minckwitz G, Han SN, Bontenbal M, Ring AE, Giermek J, et al. Prognosis of women with primary breast cancer diagnosed during pregnancy: results from an international collaborative study. J Clin Oncol. (2013) 31:2532–9. doi: 10.1200/JCO.2012.45.6335
58. Shao C, Yu Z, Xiao J, Liu L, Hong F, Zhang Y, et al. Prognosis of pregnancy-associated breast cancer: a meta-analysis. BMC Cancer. (2020) 20:746. doi: 10.1186/s12885-020-07248-8
59. Jindal S, Pennock ND, Sun D, Horton W, Ozaki MK, Narasimhan J, et al. Postpartum breast cancer has a distinct molecular profile that predicts poor outcomes. Nat Commun. (2021) 12:6341. doi: 10.1038/s41467-021-26505-3
60. Johansson AL, Andersson TML, Hsieh CC, Jirström K, Cnattingius S, Fredriksson I, et al. Tumor characteristics and prognosis in women with pregnancy-associated breast cancer. Int J cancer. (2018) 142:1343–54. doi: 10.1002/ijc.31174
61. Hartman EK, Eslick GD. The prognosis of women diagnosed with breast cancer before, during and after pregnancy: a meta-analysis. Breast Cancer Res Treat. (2016) 160:347–60. doi: 10.1007/s10549-016-3989-3
62. Mathews TJ, Hamilton BE. Mean age of mothers is on the rise: United States, 2000–2014. NCHS Data Brief. (2016) 232):1–8.
63. Zasloff E, Schytt E, Waldenström U. First time mothers’ pregnancy and birth experiences varying by age. Acta Obstet Gynecol Scand. (2007) 86:1328–36. doi: 10.1080/00016340701657209
64. Haricharan S, Dong J, Hein S, Reddy JP, Du Z, Toneff M, et al. Mechanism and preclinical prevention of increased breast cancer risk caused by pregnancy. Elife. (2013) 2:e00996. doi: 10.7554/eLife.00996
65. Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. (2006) 6:281–91. doi: 10.1038/nrc1839
66. Medina D. Breast cancer: the protective effect of pregnancy. Clin Cancer Res. (2004) 10:380s–4s. doi: 10.1158/1078-0432.CCR-031211
67. Innes KE, Byers TE. First pregnancy characteristics and subsequent breast cancer risk among young women. Int J cancer. (2004) 112:306–11. doi: 10.1002/ijc.20402
68. Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J cancer. (2005) 92:167–75. doi: 10.1038/sj.bjc.6602302
69. Hassiotou F, Geddes D. Anatomy of the human mammary gland: Current status of knowledge. Clin Anat. (2013) 26:29–48. doi: 10.1002/ca.22165
70. Ferguson JE. The Extracellular Matrix of the Normal Human Breast. United Kingdom: The University of Manchester (1991).
71. Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague-Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. (2000) 5:211–25. doi: 10.1023/a:1026447506666
72. Macias H, Hinck L. Mammary gland development. WIREs Dev Biol. (2012) 1:533–57. doi: 10.1002/wdev.35
73. Lyons TR, Borges VF, Betts CB, Guo Q, Kapoor P, Martinson HA, et al. Cyclooxygenase-2–dependent lymphangiogenesis promotes nodal metastasis of postpartum breast cancer. J Clin Invest. (2014) 124:3901–12. doi: 10.1172/JCI73777
74. Martinson HA, Lyons TR, Giles ED, Borges VF, Schedin P. Developmental windows of breast cancer risk provide opportunities for targeted chemoprevention. Exp Cell Res. (2013) 319:1671–8. doi: 10.1016/j.yexcr.2013.04.018
75. Schedin P, O’Brien J, Rudolph M, Stein T, Borges V. Microenvironment of the involuting mammary gland mediates mammary cancer progression. J mammary gland Biol neoplasia. (2007) 12:71–82. doi: 10.1007/s10911-007-9039-3
76. Avagliano A, Fiume G, Ruocco MR, Martucci N, Vecchio E, Insabato L, et al. Influence of fibroblasts on mammary gland development, breast cancer microenvironment remodeling, and cancer cell dissemination. Cancers. (2020) 12:1697. doi: 10.3390/cancers12061697
77. Schedin P, Palmer JR. Can breast cancer prevention strategies be tailored to biologic subtype and unique reproductive windows? JNCI: J Natl Cancer Instit. (2022) 114:1575–6. doi: 10.1093/jnci/djac114
78. Jung AY, Ahearn TU, Behrens S, Middha P, Bolla MK, Wang Q, et al. Distinct reproductive risk profiles for intrinsic-like breast cancer subtypes: pooled analysis of population-based studies. JNCI: J Natl Cancer Instit. (2022) 114:1706–19. doi: 10.1093/jnci/djac117
79. Fortner RT, Sisti J, Chai B, Collins LC, Rosner B, Hankinson SE, et al. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses’ Health Studies. Breast Cancer Res. (2019) 21:1–9. doi: 10.1186/s13058-019-1119-y
80. Faupel-Badger JM, Arcaro KF, Balkam JJ, Eliassen AH, Hassiotou F, Lebrilla CB, et al. Postpartum remodeling, lactation, and breast cancer risk: summary of a National Cancer Institute–sponsored workshop. J Natl Cancer Instit. (2013) 105:166–74. doi: 10.1093/jnci/djs505
81. Islami F, Liu Y, Jemal A, Zhou J, Weiderpass E, Colditz G, et al. Breastfeeding and breast cancer risk by receptor status—a systematic review and meta-analysis. Ann Oncol. (2015) 26:2398–407. doi: 10.1093/annonc/mdv379
82. Ricci C, Otterman V, Bennett T-L, Metcalfe S, Darling E, Semenic S, et al. Rates of and factors associated with exclusive and any breastfeeding at six months in Canada: an analysis of population-based cross-sectional data. BMC Pregnancy Childbirth. (2023) 23:56. doi: 10.1186/s12884-023-05382-2
83. MacMahon B, Cole P, Lin T, Lowe C, Mirra A, Ravnihar B, et al. Age at first birth and breast cancer risk. Bull World Health org. (1970) 43:209.
85. Liou J-D, Hsu J-J, Lo L-M, Chen S-F, Hung T-H. Advanced maternal age and adverse perinatal outcomes in an Asian population. Eur J Obstetr Gynecol Reprod Biol. (2010) 148:21–6. doi: 10.1016/j.ejogrb.2009.08.022
86. Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: final data for 2021. Centre Dis Control. (2023) 72(1).
87. Hollingsworth AB. Redefining the sensitivity of screening mammography: A review. Am J Surge. (2019) 218:411–8. doi: 10.1016/j.amjsurg.2019.01.039
88. Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. (2007) 57:75–89. doi: 10.3322/canjclin.57.2.75
89. Lo G, Scaranelo AM, Aboras H, Ghai S, Kulkarni S, Fleming R, et al. Evaluation of the utility of screening mammography for high-risk women undergoing screening breast MR imaging. Radiology. (2017) 285:36–43. doi: 10.1148/radiol.2017161103
90. Checka CM, Chun JE, Schnabel FR, Lee J, Toth H. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol. (2012) 198:W292–5. doi: 10.2214/AJR.10.6049
91. Lynge E, Vejborg I, Andersen Z, von Euler-Chelpin M, Napolitano G. Mammographic density and screening sensitivity, breast cancer incidence and associated risk factors in Danish breast cancer screening. J Clin Med. (2019) 8:2021. doi: 10.3390/jcm8112021
92. Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. (2005) 353:1773–83. doi: 10.1056/NEJMoa052911
93. Pawlak ME, Rudnicki W, Borkowska A, Skubisz K, Rydzyk R, Łuczyńska E. Comparative analysis of diagnostic performance of automatic breast ultrasound, full-field digital mammography and contrast-enhanced mammography in relation to breast composition. Biomedicines. (2023) 11:3226. doi: 10.3390/biomedicines11123226
94. Nickson C, Kavanagh AM. Tumour size at detection according to different measures of mammographic breast density. J Med screen. (2009) 16:140–6. doi: 10.1258/jms.2009.009054
95. Sharma S, Vicenty-Latorre FG, Elsherif S, Sharma S. Role of MRI in breast cancer staging: A case-based review. Cureus. (2021) 13. doi: 10.7759/cureus.20752
96. Wang J, Gottschal P, Ding L, van Veldhuizen DA, Lu W, Houssami N, et al. Mammographic sensitivity as a function of tumor size: A novel estimation based on population-based screening data. Breast. (2021) 55:69–74. doi: 10.1016/j.breast.2020.12.003
97. von Euler-Chelpin M, Lillholm M, Vejborg I, Nielsen M, Lynge E. Sensitivity of screening mammography by density and texture: a cohort study from a population-based screening program in Denmark. Breast Cancer Res. (2019) 21:1–7. doi: 10.1186/s13058-019-1203-3
98. Kerlikowske K, Grady D, Barclay J, Sickles EA, Ernster V. Effect of age, breast density, and family history on the sensitivity of first screening mammography. Jama. (1996) 276:33–8. doi: 10.1001/jama.276.1.33
99. Rebolj M, Assi V, Brentnall A, Parmar D, Duffy SW. Addition of ultrasound to mammography in the case of dense breast tissue: systematic review and meta-analysis. Br J cancer. (2018) 118:1559–70. doi: 10.1038/s41416-018-0080-3
100. Kim EY, Chang Y, Ahn J, Yun JS, Park YL, Park CH, et al. Mammographic breast density, its changes, and breast cancer risk in premenopausal and postmenopausal women. Cancer. (2020) 126:4687–96. doi: 10.1002/cncr.33138
101. Tran TXM, Kim S, Song H, Lee E, Park B. Association of longitudinal mammographic breast density changes with subsequent breast cancer risk. Radiology. (2022) 306:e220291. doi: 10.1148/radiol.220291
102. McCarthy AM, Keller BM, Pantalone LM, Hsieh M-K, Synnestvedt M, Conant EF, et al. Racial differences in quantitative measures of area and volumetric breast density. J Natl Cancer Instit. (2016) 108:djw104. doi: 10.1093/jnci/djw104
103. Yang L, Wang S, Zhang L, Sheng C, Song F, Wang P, et al. Performance of ultrasonography screening for breast cancer: a systematic review and meta-analysis. BMC Cancer. (2020) 20. doi: 10.1186/s12885-020-06992-1
104. Hlawatsch A, Teifke A, Schmidt M, Thelen M. Preoperative assessment of breast cancer: sonography versus MR imaging. AJR Am J roentgenol. (2002) 179:1493–501. doi: 10.2214/ajr.179.6.1791493
105. Alfasi A, Ben-Aharon I. Breast cancer during pregnancy—current paradigms, paths to explore. Cancers. (2019) 11:1669. doi: 10.3390/cancers11111669
106. Kieturakis AJ, Wahab RA, Vijapura C, Mahoney MC. Current recommendations for breast imaging of the pregnant and lactating patient. Am J Roentgenol. (2021) 216:1462–75. doi: 10.2214/AJR.20.23905
107. Lefrère H, Lenaerts L, Borges VF, Schedin P, Neven P, Amant F. Postpartum breast cancer: mechanisms underlying its worse prognosis, treatment implications, and fertility preservation. Int J gynecol Cancer. (2021) 31:412–22. doi: 10.1136/ijgc-2020-002072
108. Walsh SM, Zabor EC, Flynn J, Stempel M, Morrow M, Gemignani ML. Breast cancer in young black women. Br J Surge. (2020) 107:677–86. doi: 10.1002/bjs.11401
109. Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, Hudis CA, et al. The global breast cancer burden: variations in epidemiology and survival. Clin Breast cancer. (2005) 6:391–401. doi: 10.3816/CBC.2005.n.043
110. Paik PS, Choi JE, Lee SW, Lee YJ, Kang Y-J, Lee HJ, et al. Clinical characteristics and prognosis of postpartum breast cancer. Breast Cancer Res Treat. (2023) 202:275–86. doi: 10.1007/s10549-023-07069-w
111. U.S. Preventive Services Task Force. Breast Cancer: Screening. Maryland, United States: U.S. Preventive Services Task Force (2023).
112. Ebell MH, Thai TN, Royalty KJ. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. (2018) 39:7. doi: 10.1186/s40985-018-0080-0
113. Pediconi F, Catalano C, Roselli A, Dominelli V, Cagioli S, Karatasiou A, et al. The challenge of imaging dense breast parenchyma: is magnetic resonance mammography the technique of choice? A comparative study with x-ray mammography and whole-breast ultrasound. Invest radiol. (2009) 44:412–21. doi: 10.1097/RLI.0b013e3181a53654
114. Mann RM, Kuhl CK, Moy L. Contrast-enhanced MRI for breast cancer screening. J Magnet Resonance Imag. (2019) 50:377–90. doi: 10.1002/jmri.26654
115. Klarenbach S, Lewin G, Singh H, Thériault G, Tonelli M, Doull M, et al. Thombs. Recommendations on screening for breast cancer in women aged 40–74 years who are not at increased risk for breast cancer. Canadian Med Assoc J (CMAJ). (2018) 190(49). doi: 10.1503/cmaj.180463
116. Badr LK, Bourdeanu L, Alatrash M, Bekarian G. Breast cancer risk factors: a cross- cultural comparison between the west and the east. Asian Pac J Cancer Prev. (2018) 19:2109–16. doi: 10.22034/APJCP.2018.19.8.2109
117. World Health Organization - Noncommunicable Diseases, Rehabilitation and Disability (NCD). WHO launches new roadmap on breast cancer. Geneva, Switzerland: World Health Organization (2023).
118. Yazdani-Charati R, Hajian-Tilaki K, Sharbatdaran M. Comparison of pathologic characteristics of breast cancer in younger and older women. Caspian J Intern Med. (2019) 10:42–7. doi: 10.22088/cjim.10.1.42
119. Merlo DF, Ceppi M, Filiberti R, Bocchini V, Znaor A, Gamulin M, et al. Breast cancer incidence trends in European women aged 20–39 years at diagnosis. Breast Cancer Res Treat. (2012) 134:363–70. doi: 10.1007/s10549-012-2031-7
120. Kim JK, Kwak BS, Lee JS, Hong SJ, Kim HJ, Son BH, et al. Do very young Korean breast cancer patients have worse outcomes? Ann Surg Oncol. (2007) 14:3385–91. doi: 10.1245/s10434-006-9345-9
121. Carvalho FM, Bacchi LM, Santos PP, Bacchi CE. Triple-negative breast carcinomas are a heterogeneous entity that differs between young and old patients. Clinics. (2010) 65:1033–6. doi: 10.1590/S1807-59322010001000019
122. Somefun OD, Ibisomi L. Determinants of postnatal care non-utilization among women in Nigeria. BMC Res notes. (2016) 9:1–11. doi: 10.1186/s13104-015-1823-3
123. Akinyemiju TF. Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries: analysis of the World Health Survey. PloS One. (2012) 7:e48834. doi: 10.1371/journal.pone.0048834
124. Dhakal S, Chapman GN, Simkhada PP, Van Teijlingen ER, Stephens J, Raja AE. Utilisation of postnatal care among rural women in Nepal. BMC pregnancy childbirth. (2007) 7:1–9. doi: 10.1186/1471-2393-7-19
125. van den Akker T, van Roosmalen J. Maternal mortality and severe morbidity in a migration perspective. Best Pract Res Clin obstet gynaecol. (2016) 32:26–38. doi: 10.1016/j.bpobgyn.2015.08.016
126. World Health Organization. Report on the health of refugees and migrants in the WHO European Region. Geneva, Switzerland: World Health Organization (2018).
127. Mazul MC, Salm Ward TC, Ngui EM. Anatomy of good prenatal care: perspectives of low income African-American women on barriers and facilitators to prenatal care. J racial ethnic Health disparities. (2017) 4:79–86. doi: 10.1007/s40615-015-0204-x
128. Karcher R, Fitzpatrick DC, Leonard DJ, Weber S. A community-based collaborative approach to improve breast cancer screening in underserved African American women. J Cancer Educ. (2014) 29:482–7. doi: 10.1007/s13187-014-0608-z
129. Mazzitelli C, Santini D, Corradini AG, Zamagni C, Trerè D, Montanaro L, et al. Liquid biopsy in the management of breast cancer patients: where are we now and where are we going. Diagnostics. (2023) 13:1241. doi: 10.3390/diagnostics13071241
130. Lone SN, Nisar S, Masoodi T, Singh M, Rizwan A, Hashem S, et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol Cancer. (2022) 21:1–22. doi: 10.1186/s12943-022-01543-7
131. Yang J, Qiu L, Wang X, Chen X, Cao P, Yang Z, et al. Liquid biopsy biomarkers to guide immunotherapy in breast cancer. Front Immunol. (2023) 14:1303491. doi: 10.3389/fimmu.2023.1303491
132. Davidson BA, Croessmann S, Park BH. The breast is yet to come: current and future utility of circulating tumour DNA in breast cancer. Br J Cancer. (2021) 125:780–8. doi: 10.1038/s41416-021-01422-w
133. Pereira-Veiga T, Schneegans S, Pantel K, Wikman H. Circulating tumor cell-blood cell crosstalk: Biology and clinical relevance. Cell Rep. (2022) 40:111298. doi: 10.1016/j.celrep.2022.111298
134. Lawrence R, Watters M, Davies CR, Pantel K, Lu YJ. Circulating tumour cells for early detection of clinically relevant cancer. Nat Rev Clin Oncol. (2023) 20:487–500. doi: 10.1038/s41571-023-00781-y
135. Stadler JC, Belloum Y, Deitert B, Sementsov M, Heidrich I, Gebhardt C, et al. Current and future clinical applications of ctDNA in immuno-oncology. Cancer Res. (2022) 82:349–58. doi: 10.1158/0008-5472.CAN-21-1718
136. Alimirzaie S, Bagherzadeh M, Akbari MR. Liquid biopsy in breast cancer: A comprehensive review. Clin Genet. (2019) 95:643–60. doi: 10.1111/cge.13514
137. Alix-Panabières C, Pantel K. Liquid biopsy: from discovery to clinical application. Cancer Discovery. (2021) 11:858–73. doi: 10.1158/2159-8290.CD-20-1311
138. Woodhouse R, Li M, Hughes J, Delfosse D, Skoletsky J, Ma P, et al. Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PloS One. (2020) 15:e0237802. doi: 10.1371/journal.pone.0237802
139. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. (2019) 380:1929–40. doi: 10.1056/NEJMoa1813904
140. Wit Sd, Dalum Gv, Lenferink ATM, Tibbe AGJ, Hiltermann TJN, Groen HJM, et al. The detection of EpCAM+ and EpCAM– circulating tumor cells. Sci Rep. (2015) 5:12270. doi: 10.1038/srep12270
141. Eslami SZ, Cortés-Hernández LE, Alix-Panabières C. Epithelial cell adhesion molecule: an anchor to isolate clinically relevant circulating tumor cells. Cells. (2020) 9. doi: 10.3390/cells9081836
142. Croessmann S, Park BH. Circulating tumor DNA in early-stage breast cancer: New directions and potential clinical applications. Clin Adv Hematol Oncol. (2021) 19:155–61.
143. Panagopoulou M, Karaglani M, Balgkouranidou I, Biziota E, Koukaki T, Karamitrousis E, et al. Circulating cell-free DNA in breast cancer: size profiling, levels, and methylation patterns lead to prognostic and predictive classifiers. Oncogene. (2019) 38:3387–401. doi: 10.1038/s41388-018-0660-y
144. Venetis K, Cursano G, Pescia C, D’Ercole M, Porta FM, Blanco MC, et al. Liquid biopsy: Cell-free DNA based analysis in breast cancer. J Liquid Biopsy. (2023) 1:100002. doi: 10.1016/j.jlb.2023.100002
145. Schrag D, Beer TM, McDonnell CH, Nadauld L, Dilaveri CA, Reid R, et al. Blood-based tests for multicancer early detection (PATHFINDER): a prospective cohort study. Lancet. (2023) 402:1251–60. doi: 10.1016/S0140-6736(23)01700-2
146. Crook T, Leonard R, Mokbel K, Thompson A, Michell M, Page R, et al. Accurate screening for early-stage breast cancer by detection and profiling of circulating tumor cells. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14143341
147. Bundred N, Fuh K, Asgarian N, Brown S, Simonot D, Wang X, et al. Abstract P2–01-02: A whole blood assay to identify breast cancer: Interim analysis of the international identify breast cancer (IDBC) study evidence supporting the Syantra DX breast cancer test. Cancer Res. (2022) 82:P2–01-02. doi: 10.1158/1538-7445.SABCS21-P2-01-02
148. Lourenco AP, Benson KL, Henderson MC, Silver M, Letsios E, Tran Q, et al. A noninvasive blood-based combinatorial proteomic biomarker assay to detect breast cancer in women under the age of 50 years. Clin Breast cancer. (2017) 17:516–25.e6. doi: 10.1016/j.clbc.2017.05.004
149. Patuleia SI, Suijkerbuijk KP, van der Wall E, van Diest PJ, Moelans CB. Nipple aspirate fluid at a glance. Cancers. (2021) 14:159. doi: 10.3390/cancers14010159
150. Jang JY, Kim YS, Kang KN, Kim KH, Park YJ, Kim CW. Multiple microRNAs as biomarkers for early breast cancer diagnosis. Mol Clin Oncol. (2021) 14(2):31. doi: 10.3892/mco.2020.2193
151. Saura C, Ortiz C, Matito J, Arenas EJ, Suñol A, Martín Á, et al. Early-stage breast cancer detection in breast milk. Cancer Discovery. (2023) 13(10):OF1–OF12. doi: 10.1158/2159-8290.24249110.v1
152. Zhang B, Shi H, Wang H. Machine learning and AI in cancer prognosis, prediction, and treatment selection: A critical approach. J Multidiscip Healthc. (2023) 16:1779–91. doi: 10.2147/JMDH.S410301
153. Mikhael PG, Wohlwend J, Yala A, Karstens L, Xiang J, Takigami AK, et al. Sybil: A validated deep learning model to predict future lung cancer risk from a single low-dose chest computed tomography. J Clin Oncol. (2023) 41:2191–200. doi: 10.1200/JCO.22.01345
154. Ye M, Tong L, Zheng X, Wang H, Zhou H, Zhu X, et al. A classifier for improving early lung cancer diagnosis incorporating artificial intelligence and liquid biopsy. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.853801
155. Ginghina O, Hudita A, Zamfir M, Spanu A, Mardare M, Bondoc I, et al. Liquid biopsy and artificial intelligence as tools to detect signatures of colorectal Malignancies: A modern approach in patient’s stratification. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.856575
156. Foser S, Maiese K, Digumarthy SR, Puig-Butille JA, Rebhan C. Looking to the future of early detection in cancer: liquid biopsies, imaging, and artificial intelligence. Clin Chem. (2024) 70:27–32. doi: 10.1093/clinchem/hvad196
157. Cacciamani GE, Sanford DI, Chu TN, Kaneko M, De Castro Abreu AL, Duddalwar V, et al. Is artificial intelligence replacing our radiology stars? Not Yet! Eur Urol Open Sci. (2023) 48:14–6. doi: 10.1016/j.euros.2022.09.024
158. Hunter B, Hindocha S, Lee RW. The role of artificial intelligence in early cancer diagnosis. Cancers (Basel). (2022) 14. doi: 10.3390/cancers14061524
159. Hughes K, Watson CJ. The multifaceted role of STAT3 in mammary gland involution and breast cancer. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19061695
160. Sargeant TJ, Lloyd-Lewis B, Resemann HK, Ramos-Montoya A, Skepper J, Watson CJ. Stat3 controls cell death during mammary gland involution by regulating uptake of milk fat globules and lysosomal membrane permeabilization. Nat Cell Biol. (2014) 16:1057–68. doi: 10.1038/ncb3043
161. Humphreys RC, Bierie B, Zhao L, Raz R, Levy D, Hennighausen L. Deletion of Stat3 blocks mammary gland involution and extends functional competence of the secretory epithelium in the absence of lactogenic stimuli. Endocrinology. (2002) 143:3641–50. doi: 10.1210/en.2002-220224
162. Chapman RS, Lourenco PC, Tonner E, Flint DJ, Selbert S, Takeda K, et al. Suppression of epithelial apoptosis and delayed mammary gland involution in mice with a conditional knockout of Stat3. Genes Dev. (1999) 13:2604–16. doi: 10.1101/gad.13.19.2604
163. Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression. Breast Cancer Res. (2004) 6:1–18. doi: 10.1186/bcr754
164. Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy M-A, Heath VJ, et al. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. (2004) 6:1–17. doi: 10.1186/bcr753
165. McDaniel SM, Rumer KK, Biroc SL, Metz RP, Singh M, Porter W, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am J Pathol. (2006) 168:608–20. doi: 10.2353/ajpath.2006.050677
166. O’Brien J, Martinson H, Durand-Rougely C, Schedin P. Macrophages are crucial for epithelial cell death and adipocyte repopulation during mammary gland involution. Development. (2012) 139:269–75. doi: 10.1242/dev.071696
167. Schedin P, Strange R, Mitrenga T, Wolfe P, Kaeck M. Fibronectin fragments induce MMP activity in mouse mammary epithelial cells: evidence for a role in mammary tissue remodeling. J Cell Sci. (2000) 113:795–806. doi: 10.1242/jcs.113.5.795
168. O’Brien JH, Vanderlinden LA, Schedin PJ, Hansen KC. Rat mammary extracellular matrix composition and response to ibuprofen treatment during postpartum involution by differential GeLC–MS/MS analysis. J Proteome Res. (2012) 11:4894–905. doi: 10.1021/pr3003744
169. Lyons TR, O’Brien J, Borges VF, Conklin MW, Keely PJ, Eliceiri KW, et al. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. (2011) 17:1109–15. doi: 10.1038/nm.2416
170. Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. (2006) 4:1–15. doi: 10.1186/1741-7015-4-38
171. Guo Q, Sun D, Barrett AS, Jindal S, Pennock ND, Conklin MW, et al. Mammary collagen is under reproductive control with implications for breast cancer. Matrix Biol. (2022) 105:104–26. doi: 10.1016/j.matbio.2021.10.006
172. Kalli M, Stylianopoulos T. Toward innovative approaches for exploring the mechanically regulated tumor-immune microenvironment. APL bioeng. (2024) 8. doi: 10.1063/5.0183302
173. Zhu P, Lu H, Wang M, Chen K, Chen Z, Yang L. Targeted mechanical forces enhance the effects of tumor immunotherapy by regulating immune cells in the tumor microenvironment. Cancer Biol Med. (2023) 20:44. doi: 10.20892/j.issn.2095-3941.2022.0491
174. Quaglino A, Salierno M, Pellegrotti J, Rubinstein N, Kordon EC. Mechanical strain induces involution-associated events in mammary epithelial cells. BMC Cell Biol. (2009) 10:1–13. doi: 10.1186/1471-2121-10-55
175. Fuh KF, Withell J, Shepherd RD, Rinker KD. Fluid flow stimulation modulates expression of S100 genes in Normal breast epithelium and breast cancer. Cell Mol Bioeng. (2022) 15:115–27. doi: 10.1007/s12195-021-00704-w
176. Fuh KF, Shepherd RD, Withell JS, Kooistra BK, Rinker KD. Fluid flow exposure promotes epithelial-to-mesenchymal transition and adhesion of breast cancer cells to endothelial cells. Breast Cancer Res. (2021) 23:1–17. doi: 10.1186/s13058-021-01473-0
177. Kunnen SJ, Leonhard WN, Semeins C, Hawinkels LJ, Poelma C, Ten Dijke P, et al. Fluid shear stress-induced TGF-β/ALK5 signaling in renal epithelial cells is modulated by MEK1/2. Cell Mol Life Sci. (2017) 74:2283–98. doi: 10.1007/s00018-017-2460-x
178. Kunnen SJ, Malas TB, Semeins CM, Bakker AD, Peters DJM. Comprehensive transcriptome analysis of fluid shear stress altered gene expression in renal epithelial cells. J Cell Physiol. (2018) 233:3615–28. doi: 10.1002/jcp.26222
179. Reichenbach M, Mendez PL, da Silva Madaleno C, Ugorets V, Rikeit P, Boerno S, et al. Differential impact of fluid shear stress and YAP/TAZ on BMP/TGF-β Induced osteogenic target genes. Adv Biol. (2021) 5:2000051. doi: 10.1002/adbi.202000051
180. Zabinyakov N. Shear Stress Modulates Gene Expression in Normal Human Dermal Fibroblasts. Calgary, Canada: Master’s thesis, University of Calgary (2017). doi: 10.11575/PRISM/27775
181. Kang H, Duran CL, Abbey CA, Kaunas RR, Bayless KJ. Fluid shear stress promotes proprotein convertase-dependent activation of MT1-MMP. Biochem Biophys Res Commun. (2015) 460:596–602. doi: 10.1016/j.bbrc.2015.03.075
182. Kang H, Kwak H-I, Kaunas R, Bayless KJ. Fluid shear stress and sphingosine 1-phosphate activate calpain to promote membrane type 1 matrix metalloproteinase (MT1-MMP) membrane translocation and endothelial invasion into three-dimensional collagen matrices *. J Biol Chem. (2011) 286:42017–26. doi: 10.1074/jbc.M111.290841
183. Utsunomiya T, Ishibazawa A, Yoshioka T, Song Y-S, Yoshida K. Assessing effects of mechanical stimulation of fluid shear stress on inducing matrix Metalloproteinase-9 in cultured corneal epithelial cells. Exp Eye Res. (2023) 234:109571. doi: 10.1016/j.exer.2023.109571
184. Kang H, Bayless KJ, Kaunas R. Fluid shear stress modulates endothelial cell invasion into three-dimensional collagen matrices. Am J Physiology-Heart Circulatory Physiol. (2008) 295:H2087–H97. doi: 10.1152/ajpheart.00281.2008
185. Urbich C, Dernbach E, Reissner A, Vasa M, Zeiher AM, Dimmeler S. Shear stress–induced endothelial cell migration involves integrin signalling via the fibronectin receptor subunits α5 and β1. Arteriosclerosis thrombosis Vasc Biol. (2002) 22:69–75. doi: 10.1161/hq0102.101518
186. Steward RL, Cheng C-M, Ye JD, Bellin RM, LeDuc PR. Mechanical stretch and shear flow induced reorganization and recruitment of fibronectin in fibroblasts. Sci Rep. (2011) 1:147. doi: 10.1038/srep00147
187. Rogers HJ, Hogan L, Coates D, Homer CSE, Henry A. Responding to the health needs of women from migrant and refugee backgrounds-Models of maternity and postpartum care in high-income countries: A systematic scoping review. Health Soc Care Commun. (2020) 28:1343–65. doi: 10.1111/hsc.12950
188. Bellerose M, Rodriguez M, Vivier PM. A systematic review of the qualitative literature on barriers to high-quality prenatal and postpartum care among low-income women. Health Serv Res. (2022) 57:775–85. doi: 10.1111/1475-6773.14008
189. Alam N, Chowdhury ME, Kouanda S, Seppey M, Alam A, Savadogo JR, et al. The role of transportation to access maternal care services for women in rural Bangladesh and Burkina Faso: a mixed methods study. Int J Gynecol Obstet. (2016) 135:S45–50. doi: 10.1016/j.ijgo.2016.09.003
190. Rodin D, Silow-Carroll S, Cross-Barnet C, Courtot B, Hill I. Strategies to promote postpartum visit attendance among medicaid participants. J women’s Health. (2019) 28:1246–53. doi: 10.1089/jwh.2018.7568
191. Pistella CY, Synkewecz CA. Community postpartum care needs assessment and systems development for low income families. J Health Soc policy. (1999) 11:53–64. doi: 10.1300/J045v11n01_04
192. Stergiopoulou D, Markou A, Strati A, Zavridou M, Tzanikou E, Mastoraki S, et al. Comprehensive liquid biopsy analysis as a tool for the early detection of minimal residual disease in breast cancer. Sci Rep. (2023) 13:1258. doi: 10.1038/s41598-022-25400-1
193. Ruggeri M, Pagan E, Bagnardi V, Bianco N, Gallerani E, Buser K, et al. Fertility concerns, preservation strategies and quality of life in young women with breast cancer: baseline results from an ongoing prospective cohort study in selected European Centers. breast. (2019) 47:85–92. doi: 10.1016/j.breast.2019.07.001
194. Napolitano S, Caputo V, Ventriglia A, Martini G, Della Corte CM, De Falco V, et al. Liquid biopsy at home: delivering precision medicine for patients with cancer during the COVID-19 pandemic. Oncol. (2022) 27:e633–e41. doi: 10.1093/oncolo/oyac071
195. Farncombe KM, Wong D, Norman ML, Oldfield LE, Sobotka JA, Basik M, et al. Current and new frontiers in hereditary cancer surveillance: Opportunities for liquid biopsy. Am J Hum Genet. (2023) 110:1616–27. doi: 10.1016/j.ajhg.2023.08.014
196. McGuire MK, Seppo A, Goga A, Buonsenso D, Collado MC, Donovan SM, et al. Best practices for human milk collection for COVID-19 research. Breastfeed Med. (2021) 16:29–38. doi: 10.1089/bfm.2020.0296
197. Greytak SR, Engel KB, Parpart-Li S, Murtaza M, Bronkhorst AJ, Pertile MD, et al. Harmonizing cell-free DNA collection and processing practices through evidence-based guidance. Clin Cancer Res. (2020) 26:3104–9. doi: 10.1158/1078-0432.CCR-19-3015
198. Grölz D, Hauch S, Schlumpberger M, Guenther K, Voss T, Sprenger-Haussels M, et al. Liquid biopsy preservation solutions for standardized pre-analytical workflows—venous whole blood and plasma. Curr pathobiol Rep. (2018) 6:275–86. doi: 10.1007/s40139-018-0180-z
199. Van Paemel R, De Koker A, Caggiano C, Morlion A, Mestdagh P, De Wilde B, et al. Genome-wide study of the effect of blood collection tubes on the cell-free DNA methylome. Epigenetics. (2021) 16:797–807. doi: 10.1080/15592294.2020.1827714
200. Ignatiadis M, Sledge GW, Jeffrey SS. Liquid biopsy enters the clinic — implementation issues and future challenges. Nat Rev Clin Oncol. (2021) 18:297–312. doi: 10.1038/s41571-020-00457-x
201. Dincer C, Bruch R, Kling A, Dittrich PS, Urban GA. Multiplexed point-of-care testing – xPOCT. Trends Biotechnol. (2017) 35:728–42. doi: 10.1016/j.tibtech.2017.03.013
202. Singh S, Podder PS, Russo M, Henry C, Cinti S. Tailored point-of-care biosensors for liquid biopsy in the field of oncology. Lab Chip. (2023) 23:44–61. doi: 10.1039/D2LC00666A
203. Moro G, Fratte CD, Normanno N, Polo F, Cinti S. Point-of-Care Testing for the Detection of MicroRNAs: Towards Liquid Biopsy on a Chip. Weinheim, Germany: Angewandte Chemie International Edition (2023). doi: 10.1002/ange.202309135
204. Febbo PG, Allo M, Alme EB, Carter GC, Dumanois R, Essig A, et al. Recommendations for the equitable and widespread implementation of liquid biopsy for cancer care. JCO Precis Oncol. (2024) 8):e2300382. doi: 10.1200/PO.23.00382
205. Ezeife DA, Spackman E, Juergens RA, Laskin JJ, Agulnik JS, Hao D, et al. The economic value of liquid biopsy for genomic profiling in advanced non-small cell lung cancer. Ther Adv Med Oncol. (2022) 14:17588359221112696. doi: 10.1177/17588359221112696
206. Zheng Y, Vioix H, Liu FX, Singh B, Sharma S, Sharda D. Diagnostic and economic value of biomarker testing for targetable mutations in non-small-cell lung cancer: a literature review. Future Oncol. (2022) 18:505–18. doi: 10.2217/fon-2021-1040
207. Fagery M, Khorshidi HA, Wong SQ, Vu M, Ijzerman M. Health economic evidence and modeling challenges for liquid biopsy assays in cancer management: A systematic literature review. PharmacoEconomics. (2023) 41:1229–48. doi: 10.1007/s40273-023-01292-5
208. van der Poort EKJ, van Ravesteyn NT, van den Broek JJ, de Koning HJ. The early detection of breast cancer using liquid biopsies: model estimates of the benefits, harms, and costs. Cancers. (2022) 14:2951. doi: 10.3390/cancers14122951
Keywords: breast cancer, young women, postpartum, involution, liquid biopsy, molecular diagnostics, early detection
Citation: Stibbards-Lyle M, Malinovska J, Badawy S, Schedin P and Rinker KD (2024) Status of breast cancer detection in young women and potential of liquid biopsy. Front. Oncol. 14:1398196. doi: 10.3389/fonc.2024.1398196
Received: 11 March 2024; Accepted: 01 May 2024;
Published: 21 May 2024.
Edited by:
Edward Van Der Horst, Sensei Biotherapeutics, United StatesReviewed by:
Jyothi S. Prabhu, St. John’s Research Institute, IndiaAdana A. M. Llanos, Columbia University, United States
Copyright © 2024 Stibbards-Lyle, Malinovska, Badawy, Schedin and Rinker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristina D. Rinker, kdrinker@ucalgary.ca
 Maya Stibbards-Lyle
Maya Stibbards-Lyle Julia Malinovska
Julia Malinovska Seleem Badawy
Seleem Badawy Pepper Schedin3
Pepper Schedin3 Kristina D. Rinker
Kristina D. Rinker