- 1Laboratory Research on Biological Systems and Geomatics, Faculty of Nature and Life Sciences, University of Mascara, Mascara, Algeria
- 2Instituto de Biología Molecular y Celular del Cáncer, CSIC-IBSAL-Universidad de Salamanca, Salamanca, Spain
The Severe Acute Respiratory Syndrome-related Coronavirus 2 (SARS-CoV-2) or novel coronavirus (COVID-19) infection has been declared world pandemic causing a worrisome number of deaths, especially among vulnerable citizens, in 209 countries around the world. Although several therapeutic molecules are being tested, no effective vaccines or specific treatments have been developed. Since the COVID-19 outbreak, different traditional herbal medicines with promising results have been used alone or in combination with conventional drugs to treat infected patients. Here, we review the recent findings regarding the use of natural products to prevent or treat COVID-19 infection. Furthermore, the mechanisms responsible for this preventive or therapeutic effect are discussed. We conducted literature research using PubMed, Google Scholar, Scopus, and WHO website. Dissertations and theses were not considered. Only the situation reports edited by the WHO were included. The different herbal products (extracts) and purified molecules may exert their anti-SARS-CoV-2 actions by direct inhibition of the virus replication or entry. Interestingly, some products may block the ACE-2 receptor or the serine protease TMPRRS2 required by SARS-CoV-2 to infect human cells. In addition, natural products were shown to inhibit the SARS-CoV-2 life-cycle related proteins such as papain-like or chymotrypsin-like proteases. In conclusion, we suggest that natural products could be used alone or in combination as alternative medicines to treat/prevent COVID-19 infection. Moreover, their structures may offer clues for the development of anti-SARS-CoV-2 drugs.
Introduction
The Severe Acute Respiratory Syndrome-related Coronavirus 2 or novel coronavirus (COVID-19) infection has been declared world pandemic resulting in thousands of deaths in 216 countries around the world. The disease appeared in late December 2019 in Wuhan (China) as a result of zoonotic transmission (Mackenzie and Smith, 2020). Actually, the Severe Acute Respiratory Syndrome-related Coronavirus 2 (SARS-CoV-2) was shown to share 96% of the genomic identity with the related bat coronavirus (Zhou et al., 2020). Moreover, the SARS-CoV-2 genome was found to be 91.02% identical to that of the Pangolin-CoV, raising the possibility that the latter acted as an intermediate zoonotic host between bats and humans (Zhang T. et al., 2020). Till now, no vaccines or specific treatments for SARS-CoV-2 have been developed, although extraordinary efforts are being made (Amanat and Krammer, 2020). Some therapeutic approaches have been suggested such as nucleoside analogs, Remdesivir, anti-inflammatory drugs or Lopinavir/Ritonavir to treat COVID-19. At present, more than 200 clinical trials, some of them analyzing these drugs or others, have been registered in clinicaltrials.gov. Nevertheless, the clinical usefulness of these drugs against COVID-19 infection remains unclear (Li et al., 2020).
Herbal traditional medicines have been used in China since the first days of the COVID-19 outbreak. Indeed, these traditional medicines were shown to result in the recovery of 90% of the 214 patients treated (Hong-Zhi et al., 2020). Furthermore, some traditional herbal medicines prevented SARS-CoV-2 infection of healthy persons and improved the health state of patients with mild or severe symptoms (Hong-Zhi et al., 2020). Similar promising results were reported in Zhejiang Province – China (Xu K. et al., 2020). Chinese traditional medicines known as Shu Feng Jie Du and Lianhuaqingwen have been recommended due to their demonstrated efficacy against previous influenza A (H1N1) or SARS-CoV-1 (Lu, 2020). A group of experts from the Zhongnan Hospital of Wuhan University included the use of traditional medicines in the guidelines for the treatment and prevention of COVID-19. Several methods using medicinal plants were recommended for the prevention of COVID-19. Moreover, to treat the disease, the experts recommended the use of different herbal mixtures according to the disease-stage (Jin et al., 2020).
This review focuses on the possible uses of herbal traditional medicines and natural products in the prevention and treatment of COVID-19 infection (Table 1).
SARS-CoV-2
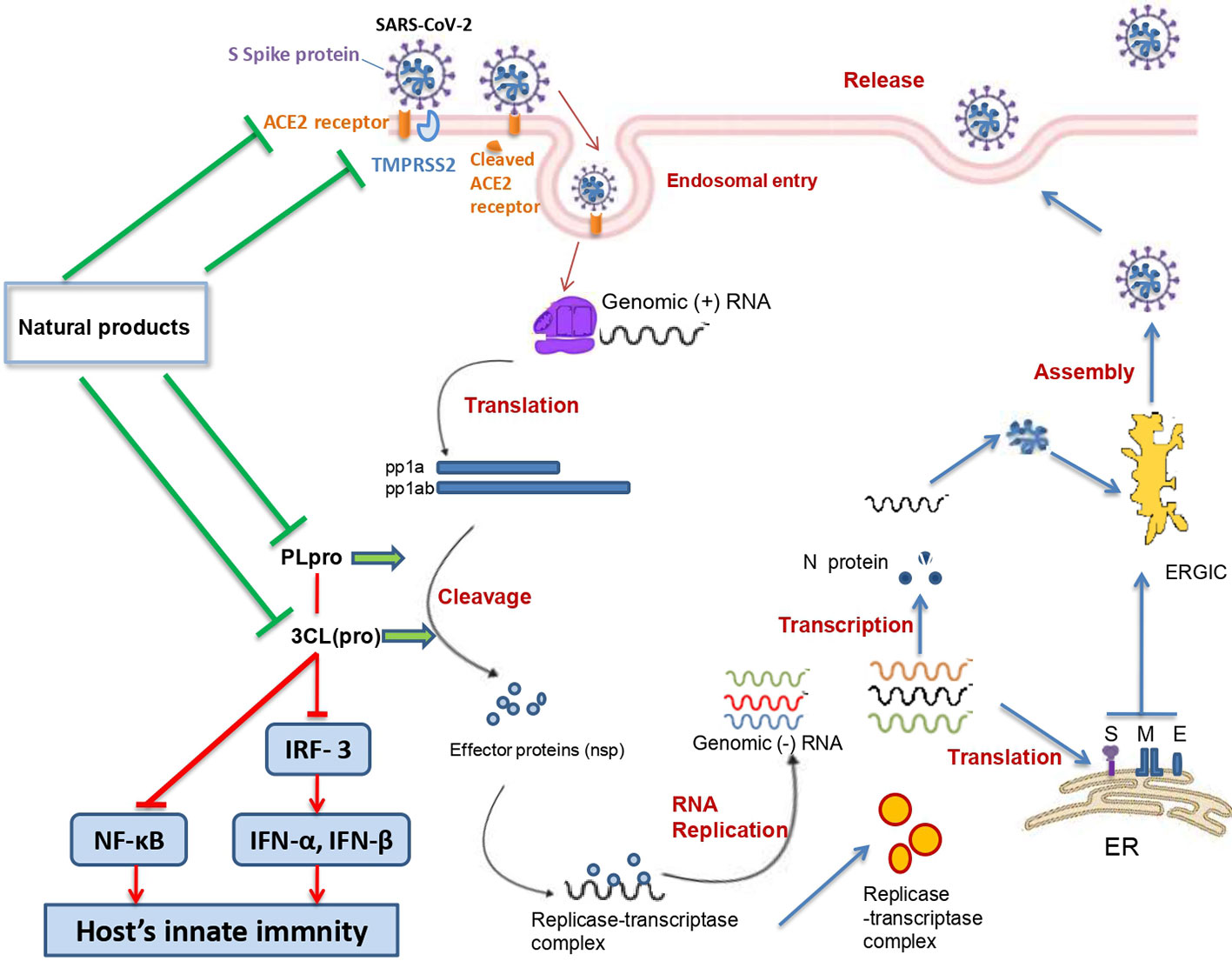
SARS-CoV-2 belongs to the β genus, Nidovirales order of the Coronaviridae family. SARS-CoV-2 is an enveloped, single (+) stranded RNA, with symmetric helical nucleocapsid (Khan et al., 2020). The virus encodes twenty different proteins including four main structural proteins (S: spike; E: envelope; M: membrane; N: nucleocapsid), and several nonstructural proteins such as RNA-dependent RNA polymerase (RdRp), coronavirus main protease (3CLpro), and papain-like protease (PLpro) (Chen et al., 2020).
The angiotensin converting enzyme II (ACE2) was found to be a key functional receptor for the SARS-CoV-2 allowing its attachment to human and bat cells and therefore its replication (Walls et al., 2020; Zhou et al., 2020). SARS-CoV-2 binds the host cells through interaction between the receptor binding motif of the spike protein—receptor binding domain (RBD) and the ACE2 receptor. This interaction will trigger conformational changes of the C-terminal S2 subunit (responsible for virus-cell membrane fusion) of the spike protein. The complex S protein-ACE2 is then proteolytically processed by the host cell-type 2II transmembrane serine protease TMPRSS2 leading to the ACE2 cleavage and therefore to viral entry into the host cell (Jiang et al., 2020; Rabi et al., 2020). After entry and uncoating, the genomic RNA is translated into two polyproteins (pp1a and pp1ab) which undergo a proteolytic cleavage generating 15–16 nonstructural proteins. The double-membrane vesicle is then formed from the rearrangement of cellular membrane induced by the nonstructural proteins. On the other hand, the genomic RNA is transcribed into subgenomic RNA which in turn leads to the synthesis of structural (spike, envelope, membrane, and nucleocapsid) and accessory proteins. Finally, virions are assembled in the ER-Golgi intermediate complex, and then released via the secretory pathway (Fung and Liu, 2020).
SARS-CoV-2 shares several genetic and clinical similarities with other coronaviruses of the β genus such as SARs-CoV and NL63 (Fani et al., 2020). Indeed, the entry of both viruses needs their interaction with the ACE2 receptor. However, some differences have been reported among these strains such as the length of the S protein and the structure of the receptor binding region (Ceccarelli et al., 2020). On the other hand, a high nucleotides homology has been found between SARS-CoV-2 and SARS-CoV in addition to a high homology (95–100%) that has been demonstrated between the proteins of the two strains. Actually, S2 and N proteins of SARS-CoV-2 and SARS-CoV share 99 and 90% similarities, respectively (Xu J. et al., 2020).
Natural Products With Anti-SARS-CoV-2 Effects
Runfeng et al. (2020) studied the inhibitory effects and anti-inflammatory potential of a Chinese herbal mixture called Lianhuaqingwen (a mixture of 11 medicinal species, a mineral medicine called gypsum and menthol) against SARS-CoV-2 (Table 2). Traditionally, Lianhuaqingwen has been widely used to treat fever, cough, fatigue, influenza, bronchitis, pneumonia, and early stage of measles (Ding et al., 2017), and has been included in phase II clinical trial in the USA (Gao et al., 2020). This herbal mixture was recommended by the Chinese National Health Commission to treat or manage COVID-19 (Yang Y. et al., 2020). The anti-SARS-CoV-2 activity was assessed in Vero E6 cells using cytopathic effect inhibition and plaque reduction assays. The herbal mixture inhibited SARS-CoV-2 replication in a dose-dependent manner with an IC50 of 411.2 µg/ml. Furthermore, the mixture was able to suppress the release of pro-inflammatory cytokines (TNF-α, IL-6, CCL-2/MCP-1, and CXCL-10/IP-10) in a dose-dependent manner (Runfeng et al., 2020). These results could be interesting since the cytokine storm has been shown to be one of the COVID-19 lethal complications. In a previous study, among the 61 molecules identified in this herbal mixture, seven (arctiin, forsythoside A, gallic acid, isoliquiritigenin, kaempferol, rutin, and secoxyloganin) exhibited important antiviral activities with IC50 ranging from 4.9 ± 0.1 (kaempferol) to 47.8 ± 1.5 µM (secoxyloganin) (Wang et al., 2016). Wang et al. (2020) reported results in four COVID-19 patients treated using a combination of lopinavir/ritonavir (Kaletra®) and arbidol with capsules of Shufeng Jiedu (a Chinese traditional medicine). After treatment, three patients were found COVID-19 negative and experienced significant improvements of the symptoms. In another study on 132 patients with COVID-19 living in northeast Chongqing (China), the traditional Chinese medicine was applied in almost 92% of them. The study concluded that the best therapeutic approach was a combination of Kaletra and the traditional medicine (Wan et al., 2020). Recently, Lung et al. (2020) demonstrated that theaflavin could be used as an important anti-SARS-CoV-2 drug using in silico approaches. Indeed, theaflavin showed promising docking affinities in the catalytic pocket of the SARS-CoV-2 RNA-dependent RNA polymerase. Nevertheless, it is worthy to point out that their bioavailability could limit their use since they are not absorbed in relevant amounts and that the theaflavin skeleton was found to resist to the degradation by the microbiota (Pereira-Caro et al., 2017). By searching single cohort studies undertaken regarding the efficacy of herbal medicines against SARS and H1N1 influenza viruses, it has been concluded that medicinal species, usually used as herbal formula, could be an interesting preventive approach for high-risk populations (medical staff and their families’ members, people living in COVID-19 outbreak areas, old populations). Six herbal species were found to be the most frequently used including Astragalus mongholicus Bunge, Glycyrrhiza glabra L., Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., Atractylodes lancea (Thunb.) DC., Atractylodes macrocephala Koidz., Lonicera japonica Thunb., and Forsythia suspensa (Thunb.) Vahl. These species are the ingredients of the Chinese traditional medicine Yupingfeng powder (Luo et al., 2020). On the other hand, the ethanol extract of Sambucus javanica subsp. chinensis (Lindl.) Fukuoka stem exerted promising anti-human coronavirus NL63 effects with IC50 ranging from 1.17 (virus yield) to 15.75 µg/ml (virus attachment). The extract significantly decreased virus yield, plaque formation, and virus attachment. Furthermore, three of its major phenolic acids (caffeic, chlorogenic, and gallic acid) were shown to inhibit the NL63 replication and virus attachment. Caffeic acid was the most potent phenolic acid (Weng et al., 2019). Phenolic acids are characterized by their metabolizing ability by the microbiota enhancing their bioavailability. Moreover, their antiviral potential could be increased with the alkyl chain length (Kumar and Goel, 2019). However, their efficacy is still controversial due to their low absorption and instability in alkaline and neutral media, which could limit their use in pure form. Therefore, the clinical utility of phenolic compounds as anti-SARS-CoV-2 agents remains debatable since their bioavailability, delivery mechanisms and efficient doses should be further studied using in vivo models.
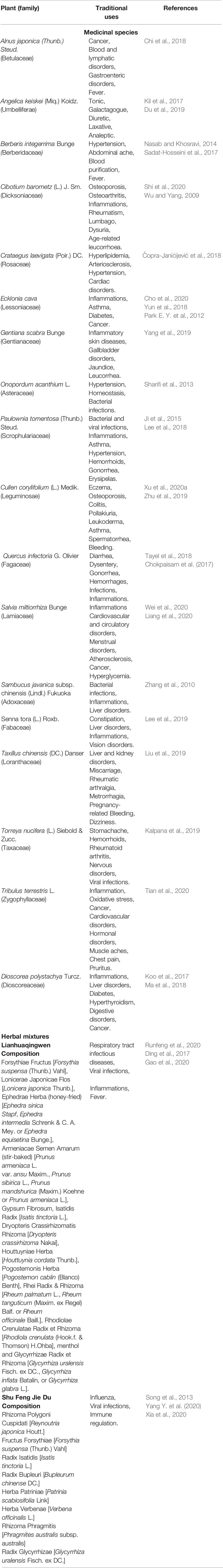
Table 2 Traditional uses of the medicinal species and mixtures with possible anti-SARS-CoV-2 effects.
Wen et al. (2011) evaluated 200 Chinese herbal extracts for their anti-SARS-CoV effect using a cell-based assay. Among them, six extracts [rhizomes of Gentiana scabra Bunge; tuber of Dioscorea polystachya Turcz.; seed of Senna tora (L.) Roxb.; stem and leaves of Taxillus chinensis (DC.) Danser; and two extracts of Cibotium barometz (L.) J.Sm. rhizome] were found to significantly inhibit SARS-CoV growth and replication. IC50 values ranged from 25 to 200 μg/ml. By using FRET assay, the study demonstrated that extracts obtained from tuber of Dioscorea polystachya Turcz. and rhizome of Cibotium barometz (L.) J.Sm. caused marked inhibition of SARS-CoV- 3CL protease with IC50 of 39 and 44 μg/ml, respectively.
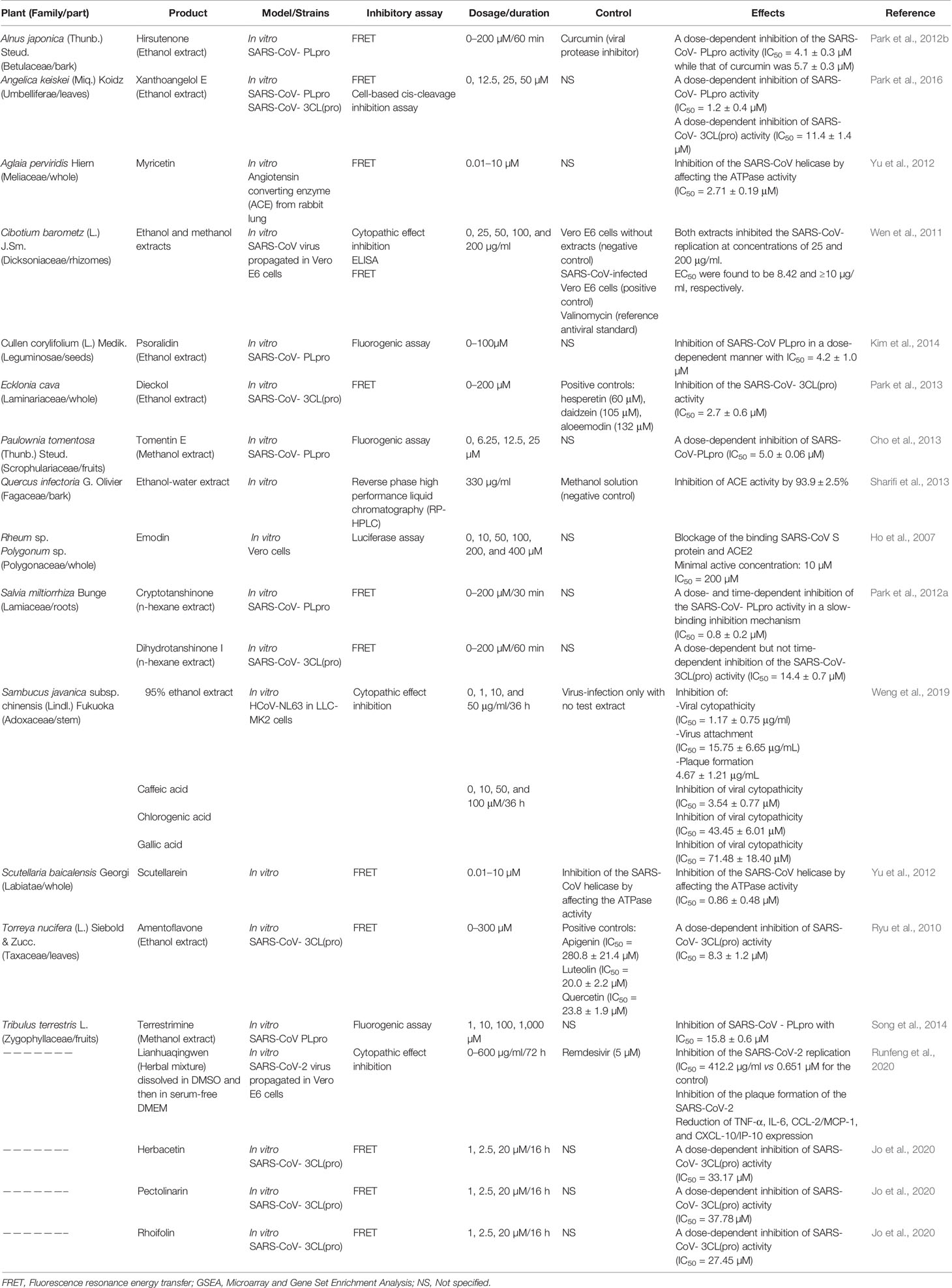
Owing to its importance as a key protein for SARS-CoV genome replication, SARS-CoV helicase still remains a target of novel antiviral drugs. Sixty-four natural molecules originated from 15 medicinal species were evaluated regarding their inhibitory activity of SARS-CoV helicase. Myricetin and scutellarein (Figure 1) significantly inhibited the SARS-CoV helicase activity. At 10 µM, myricetin (IC50 = 2.71 ± 0.19 µM) and scutellarein (IC50 = 0.86 ± 0.48 µM) were able to inhibit 90% of the ATPase activity of the SARS-CoV helicase. Accordingly, Myricetin and scutellarein were suggested to be promising future anti-SARS drugs (Yu et al., 2012).
SARS-CoV-2 life-cycle related proteins are considered promising targets of antiviral drugs. Therefore, molecules and/or products able to inhibit these proteins may be used to treat or even prevent the SARS-CoV-2 infections (Table 1).
Natural Products as ACE2-Blockers
The penetration of the SARS-CoV-2 genome into the host cells occurs as a result of the SARS-CoV-2 spike protein binding to host receptors (Sigrist et al., 2020). By using phylogenetic analysis and critical site of ACE2 structure, different animals such as cat, pigeon, and sheep were predicted to be important intermediate hosts for SARS-CoV-2 (Qiu et al., 2020). Hoffmann et al. (2020) demonstrated that the ACE2 receptor is used by SARS-CoV-2 to enter human cells. Moreover, they reported that the use of TMPRSS2 inhibitors may be a promising therapeutic approach against SARS-CoV-2. TMPRSS2 is a transmembrane serine protease that cleaves both ACE2 and the S protein. Recently, Ortega et al. (2020) used in silico approaches to understanding the relationship between changes in SARS-CoV-2 Spike protein and ACE2 receptor. They demonstrated superior affinity of SARS-CoV-2 spike protein towards human ACE2 as compared to that of the Bat-CoV spike and ACE2. This study supported the idea that the ACE2 receptor may be the key “bridge” used by SARS-CoV-2 to transmit among humans. Chen et al. (2020) confirmed that although SARS-CoV and SARS-CoV-2 RBD of spike glycoprotein had 72% of structural similarities, SARS-CoV-2 RBD exhibited higher interaction with ACE2. ACE2 inhibitors are thought to indirectly alter the RBD binding site and therefore block SARS-CoV-2 infection. Likewise, Wrapp et al. (2020) found that the SARS-CoV-2 spike exhibited a higher affinity to ACE2 than SARS-CoV. Adedeji et al. (2013) demonstrated that early blocking of SARS-CoV with ACE2 inhibitors was one of the mechanisms used by novel efficient anti-SARS drugs. Nonetheless, it has been shown in three recent studies on COVID-19 that hypertension and diabetes mellitus significantly enhanced the risk of COVID-19 infection, in spite of using ACE2 inhibitors (Guan et al., 2019; Yang X. et al., 2020; Zhang J. J. et al., 2020). ACE2 inhibitors, angiotensin II type-I receptor blockers, and ibuprofen lead to ACE2 upregulation which justifies the urgent need to use and/or identify alternative ACE2 blockers (Fang et al., 2020). Hence, medicinal plants-derived products or natural products able to selectively block the ACE2 receptor without inhibiting the enzyme activity may be useful to prevent and/or treat SARS-CoV-2 spread in humans without increasing ACE2 expression in patients and therefore increased risk for COVID-19.
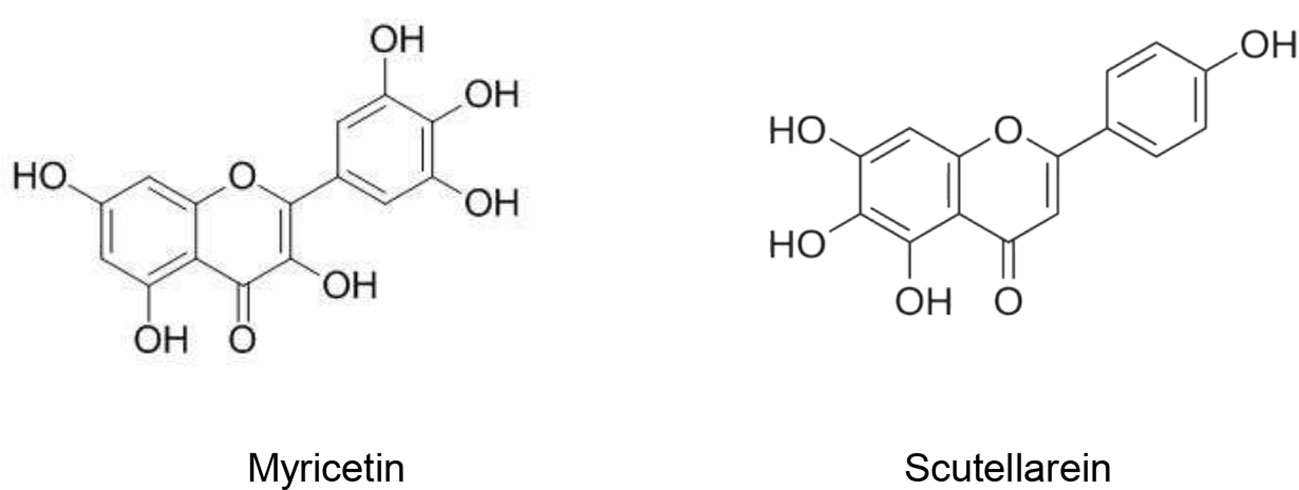
Since important similarities exist between the sequences of ACE and ACE2 (Guy et al., 2005), molecules with inhibitory effects toward ACE may exert the same effect toward ACE2 and thus lead to reduce the viral entry. However, further studies should be undertaken to evaluate this hypothesis. Patten et al. (2016) reviewed the medicinal plants for their inhibitory effects on ACE2. They reported 141 medicinal species belonging to 73 families and 49 purified natural compounds with documented ACE inhibitory potential. Moreover, 16 medicinal species (16 families) were found to be able to block the angiotensin type 1A receptor in vitro. Sharifi et al. (2013) identified four Iranian medicinal species able to inhibit more than 80% of ACE activity in vitro. These active species were: Berberis integerrima Bunge., Crataegus laevigata (Poir.) DC., Onopordum acanthium L., and Quercus infectoria G. Olivier. At 330 µg/ml, Quercus infectoria G. Olivier. was found to be the most active and caused 94% ACE inhibition. This important inhibitory activity might be attributed to its higher phenolic content and enhanced antioxidant potential. In spite of the important ACE inhibition and antioxidant activities exhibited by Q. Infectoria extract, the presence of condensed tannins compromised its usefulness due to their interference in the functions of ACE. On the other hand, B. integerrima, C. microphylla and O. acanthium were found to have both important ACE inhibitory activities and enhanced antioxidant potential without the presence of tannins (Sharifi et al., 2013). These species could be promising sources of antiviral molecules. Indeed, viral infections are accompanied by oxidative stress which in turn promotes virus replication. Antioxidant species decrease the ROS production in infected cells and target different oxidative stress-related signalling pathways resulting in reduction of viral spread (Fedoreyev et al., 2018). Walls et al. (2020) demonstrated that SARS-CoV-2 and SARS-CoV bind with similar affinities to ACE2. In another study, 25 Chinese herbal families were found to significantly inhibit the interaction SARS-CoV – ACE2. Among them, species belonging to Polygonaceae, Labiatae, Oleaceae, Magnoliaceae, Lauraceae, and Nelumbonaceae exhibited the most important inhibitory effects. These inhibitory effects were attributed to emodin (1,3,8-trihydroxy-6-methylanthraquinone) (Figure 2) produced in high levels in in genus Rheum and Polygonum. Emodin blocked the interaction SARS-CoV S protein and ACE2 in a dose-dependent manner with an IC50 of 200 µM (Ho et al., 2007).
Natural Products Targeting the TMPRSS2
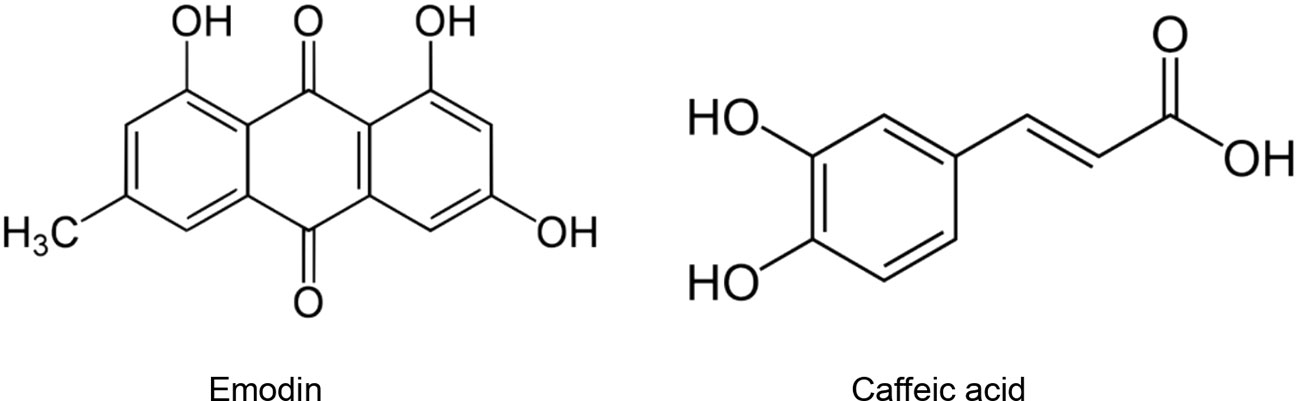
The type II transmembrane serine-proteinase serine type 2 RSS2 type II transmembrane serine protease that cleaves the S spike proteins of SARS-CoV and MERS and ACE2 (Iwata-Yoshikawa et al., 2019). Recently, Hoffmann et al. (2020) demonstrated that besides using ACE2 receptor to enter the host cells, SARS-CoV-2 uses also TMPRSS2 for S protein priming. After the interaction between the S spike protein (SARS-CoV-2) and the ACE2 (host cell), the complex is cleaved by the TMPRSS2 to facilitate viral entry (Rabi et al., 2020). Matsuyama et al. (2020) found that an important TMPRSS2 expression in cells makes them highly susceptible to SARS-CoV-2. Since SARS-CoV-2 viral entry is conditioned by its binding to the ACE2 receptor, and the latter should be cleaved by the TMPRSS, finding agents able to suppress or downregulate the TMPRSS2 expression in human cells could represent a promising therapeutic or preventive approach (Schlagenhauf et al., 2020). Several studies have demonstrated that natural products could downregulate or suppress TMPRSS2. It has been shown that kaempferol was able to inactivate TMPRSS2 expression by 49.14 and 79.48% at 5 and 15 μM, respectively (Da et al., 2019). Likewise, sulforaphane (an isothiocyanate) was found to downregulate the TMPRSS2 expression through the release and translocation of the Nrf2 (nuclear factor (erythroid-derived 2)-like 2) (Gibbs et al., 2009; Meyer and Jaspers, 2015). Mamouni et al. (2018) found that a standardized flavonoids formulation including luteolin, quercetin, and kaempferol significantly suppressed TMPRSS2 expression. In spite of the diverse biological effects attributed to the three flavonoids, this study has demonstrated that at low concentrations, they exhibited an important synergic effect. Nonetheless, the efficacy and safety of these compounds in COVID-19 patients is still unclear. Moreover, modes of administration, the health of the patients’ digestive system, and disease stage may limit the clinical usefulness of such formulations and compounds (Fuzimoto and Isidoro, 2020). Xu et al. (2012) demonstrated that cryptotanshinone at 0.5 µM effectively exhibited anti-TMPRSS2 activity (Figure 3).
Natural Products Targeting the Papain-Like Proteinase (Plpro)
PLpro is one of the nonstructural proteins encoded by the SARS-CoV-2 genome. This protease is vital for virus replication since it contributes to the cleavage of viral polyproteins (PP1A and PP1AB) into effector proteins (Jiang et al., 2020). Moreover, PLpro has been found to be an antagonist of the host’s innate immunity (Yuan et al., 2015; Li et al., 2016). Actually, PLpro was shown to target the interferon production by blocking the IRF3 phosphorylation, dimerization, and nuclear translocation and NF-κB signaling pathways (by preventing IκBα degradation) (Wong et al., 2016). These effects were shown to occur in Toll-like receptor 3 and retinoic acid-inducible gene 1 pathways. Moreover, it has been demonstrated that SARS-CoV PLpro inhibits the TLR7 pathway via inactivation of TRAF3/6-TBK1-IRF3/NF-κB/AP1 signalling pathways (Yuan et al., 2015).
Recently, Arya et al. (2020) screened FDA-approved drugs for their in silico inhibitory potential of PLpro. They demonstrated that sixteen FDA-approved drugs (Biltricide, Cinacalcet, Procainamide, Terbinafine, Pethidine, Labetalol, Tetrahydrozoline, Ticlopidine, Ethoheptazine, Levamisole, Amitriptyline, Naphazoline, Formoterol, Benzylpenicillin, Chloroquine, and Chlorothiazide) exhibited important binding affinity to SARS-CoV-2 PLpro suggesting their possible effectiveness as anti-SARS-CoV-2 agents. Likewise, Disulfiram (an alcohol-aversive drug) was found to be a competitive inhibitor of SARS-CoV PLpro (Lin et al., 2018).
Several compounds have been shown to target the SARS-CoV PLpro (Figure 4).
Cinnamic Amides From Tribulus terrestris
Several natural compounds were found to possess promising PLpro inhibitory effects. Indeed, Song et al. (2014) demonstrated that six cinnamic amides (N-trans-Feruloyloctopamine, N-trans-Coumaroyltyramine, N-trans-Caffeoyltryamine, Terrestrimine, N-trans-Feruloyltryamine, and Terrestriamide) extracted from Tribulus terrestris L. fruits were able to inhibit SARS-CoV PLpro in a dose-dependent manner. PLpro inhibitory IC50 of these compounds were found to be 15.8–70.1 µM. Terrestrimine [(E)-N-(1-hydroxy-2-(4-hydroxyphenyl)-2- oxoethyl)-3-(4-hydroxy-3-methoxypheny) acrylamide] showed the best inhibitory activity of SARS-CoV PLpro with an IC50 of 15.8 ± 0.6 µM. The presence of a polar substituent (ketone or alcohol) on the methylene groups (C8’and C7’) was associated with enhanced inhibitory activity.
Flavonoids From Cullen corylifolium (L.) Medik.
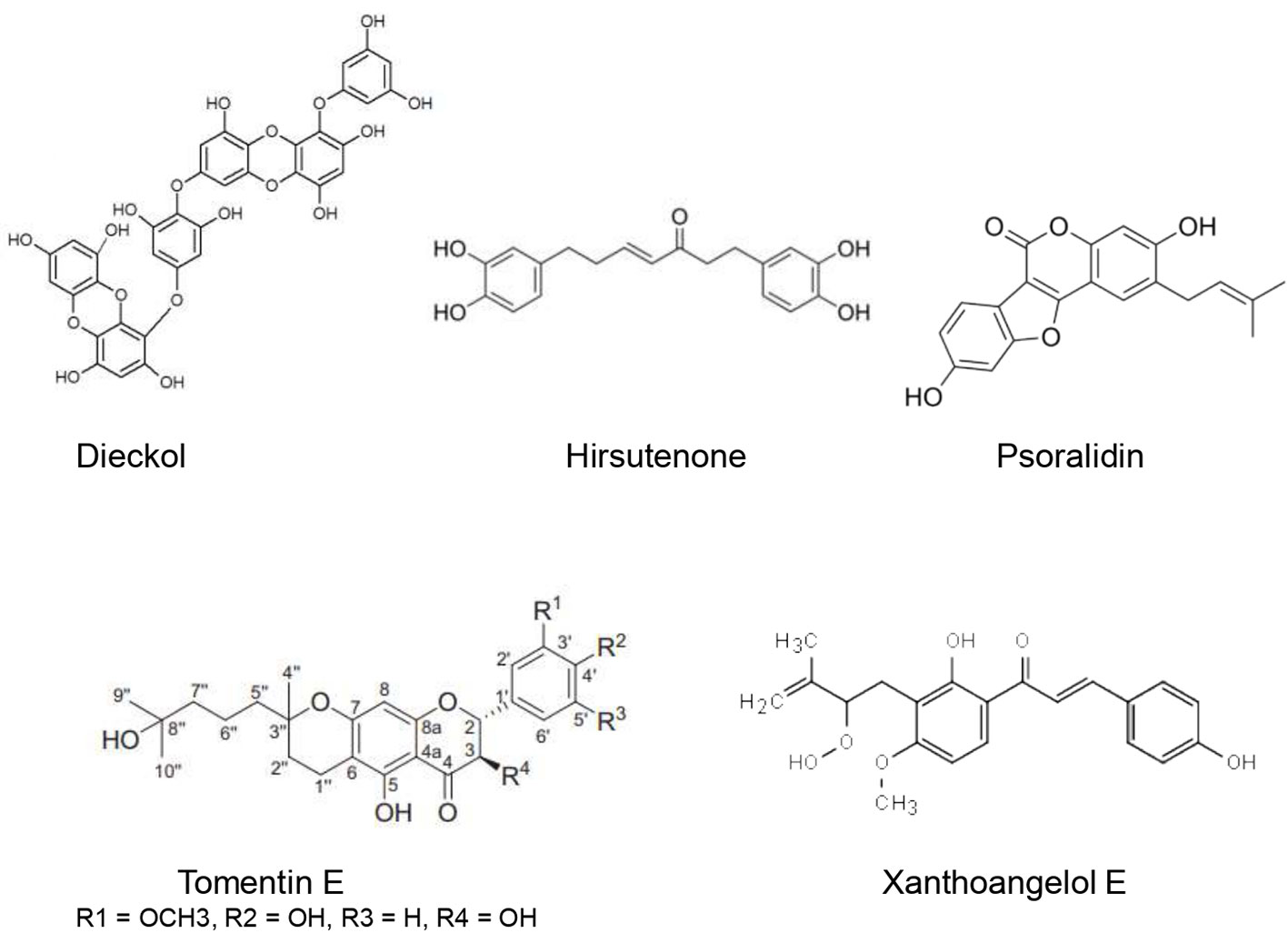
Ethanolic extract of Cullen corylifolium (L.) Medik. seeds showed an important inhibitory effect of SARS-CoV PLpro with an IC50 of 15 µg/ml. Furthermore, six flavonoids present in the extract (Bavachinin, neobavaisoflavone, isobavachalcone,40 –O-methylbavachalcone, psoralidin, and corylifol A) inhibited SARS-CoV PLpro activity in a dose-dependent manner with IC50 estimated to be 4.2–38.4 µM. The highest inhibitory effect was exerted by psoralidin (IC50 = 4.2 ± 1.0 µM) and isobavachalcone (IC50 = 7.3 ± 0.8 µM) (Kim et al., 2014).
Flavonoids From Paulownia tomentosa (Thunb.) Steud.
Cho et al. (2013) identified five new geranylated flavonones, tomentin A, tomentin B, tomentin C, tomentin D, tomentin E from the ethanolic extract of Paulownia tomentosa (Thunb.) Steud. fruits. These flavonoids besides seven other knowns resulted in significant inhibition of SARS-CoV PLpro in a dose-dependent manner with IC50 of 5.0 and 14.4 μM. Tomentin E exhibited the highest inhibitory effect with an IC50 of 5.0 ± 0.06 µM. It has been found that molecules with 3,4-dihydro-2H-pyran moiety possessed higher inhibition. The P. tomentosa flavonoids were found to be reversible, mixed inhibitors.
Chalcones From Angelica keiskei (Miq.) Koidz
Park et al. (2016) investigated the inhibitory potential of nine alkylated chalcones (isobavachalcone, 4-hydroxyderricin, xanthoangelol, xanthoangelol F, xanthoangelol D, xanthoangelol E, xanthoangelol B, xanthoangelol G, xanthokeistal A) and four coumarins extracted from the ethanolic extract of Angelica keiskei (Miq.) Koidz. The alkylated chalcones inhibited SARS-CoV PLpro in a significant dose-dependent manner with IC50 ranging from 1.2 ± 0.4 to 46.4 ± 7.8 µM. On the other hand, the analyzed coumarins did not show a significant inhibitory effect toward SARS-CoV PLpro. Kinetic studies revealed that isobavachalcone was a mixed inhibitor whereas the other chalcones were noncompetitive. Interestingly, xanthoangelol E, an –OOH substituted analogue, exhibited the most enhanced inhibitory effect (IC50 = 1.2 ± 0.4 µM), which was 40-fold higher when compared to other tested chalcones. This important inhibitory activity of xanthoangelol E was confirmed using in silico studies.
Tanshinones From Salvia miltiorrhiza Bunge
Salvia miltiorrhiza Bunge ethanolic extract (30 µg/ml) caused 88% inhibition of SARS-CoV PLpro. Moreover, seven bioactive tanshinones (tanshinone IIA, tanshinone IIB, methyl tanshinonate, cryptotanshinone, tanshinone I, dihydrotanshinone I, and rosmariquinone) were identified from the n-hexane fraction. These tanshinones were evaluated with regard to inhibition of SARS-CoV PLpro activity using a fluorometric assay. Both molecules exhibited important inhibitory time-dependent activities with IC50 of 0.8 to 30 µM. The dimethyl tetrahydronaphthalen structure was associated with enhanced inhibitory potential. The results obtained showed that cryptotanshinone was the most potent inhibitor of SARS-CoV PLpro with an IC50 of 0.8 ± 0.2 µM. Kinetic investigations demonstrated that rosmariquinone was a mixed-type inhibitor of SARS-CoV PLpro, whereas the other tanshinones were noncompetitive inhibitors (Park et al., 2012a).
Diarylheptanoids From Alnus japonica (Thunb.) Steud.
Park et al. (2012b) used activity-guided fractionation to identify nine diarylheptanoids (platyphyllenone, hirsutenone, platyphyllone, platyphyllonol-5-xylopyranoside, hirsutanonol, oregonin, rubranol, rubranoside B, and rubranoside A) from the ethanol extract of Alnus japonica (Thunb.) Steud. They evaluated their SARS-CoV PLpro inhibitory effect using a continuous fluorometric assay. The results showed that hirsutenone, hirsutanonol, oregonin, rubranol, rubranoside B, and rubranoside A exerted a significant dose-dependent inhibitory activity towards SARS-CoV PLpro with IC50 ranging from 3 to 44.5 µM. Hirsutenone possessed the most potent inhibitory effect with IC50 of 4.1 ± 0.3 µM which was less important than that of the reference inhibitor curcumin (5.7 µM). The enhanced inhibitory potential of diarylheptanoids seems to be related to the presence of α,β-unsaturated carbonyl, and catechol groups.
Natural Products Targeting the Chymotrypsin-Like Protease [3CL(pro)]
3CL(pro) belongs to the 16 nonstructural proteins of the SARS-CoV-2. Since it plays an important role in the SARS-CoV-2 replication process polyproteins, 3CL(pro) is considered a potential therapeutic target for anti-COVID-19 drugs (Zhang L. et al., 2020). Different natural compounds exhibited promising anti3CL(pro) activities (Figure 5).
Alkylated Chalcones From Angelica keiskei (Miq.) Koidz
Inhibitory potential toward SARS-CoV- 3CL(pro) of alkylated chalcones and coumarins extracted from Angelica keiskei (Miq.) Koidz was investigated using a fluorescence resonance energy transfer (FRET) method. Except for coumarins, alkylated chalcones exhibited marked inhibitory effects in a dose-dependent fashion. IC50 ranged from 11.4 ± 1.4 to 129.8 ± 10.3 µM. Moreover, xanthoangelol E (Figure 5) was found to be the most potent SARS-CoV- 3CL(pro) inhibitor. Kinetic studies showed that both alkylated chalcones were competitive inhibitors (Park O. K. et al., 2016). Since xanthoangelol E was also found to inhibit SARS-CoV- PLpro (Park O. K. et al., 2016) it could be a promising candidate in the therapeutic approach against COVID-19.
Phlorotannins From Ecklonia cava (Algae)
Park et al. (2013) isolated nine phlorotannins from the ethanolic extract of brown Alga Ecklonia cava. These phlorotannins were assessed regarding their inhibitory effects towards SARS-CoV- 3CL(pro) using a cell-free based assay. Eight phlorotannins (triphloretol A, eckol, dioxinodehydroeckol, 2-phloroeckol, 7-phloroeckol, fucodiphloroethol G, dieckol, and phlorofucofuroeckol A) were shown to be competitive inhibitors of SARS-CoV- 3CL(pro) in a dose dependent manner. IC50 ranged from 2.7 ± 0.6 (dieckol) to 164.7 ± 10.8 µM (triphloretol A). Moreover, six phlorotannins (dioxinodehydroeckol, 2-phloroeckol, 7-phloroeckol, fucodiphloroethol G, dieckol, and phlorofucofuroeckol A) resulted in a significant micromolar dose-dependent inhibition of SARS-CoV-3CL(pro) cis-cleavage activity. Of the tested molecules, diekcol (possessing two eckol groups linked by a diphenyl ether) exhibited the best inhibitory effect of SARS-CoV-3CL(pro). Molecular docking studies corroborated this result since diekcol possessed the lowest binding energy (11.51 kcal/mol) towards SARS-CoV-3CL(pro). Diekcol was shown to form strong H bonds to the catalytic dyad (Cys145 and His41). Nevertheless, the bioavailability of phlorotannins and the inter-individual differences regarding their metabolization is still a substantial limitation to validate their usefulness. Gut microbiota composition seems to play a critical role in determining their health benefits (Corona et al., 2016). Moreover, the complexity of their structures due to the diversity of structural linkages and the different structural and conformational isomers for the same molecular weight, in addition to the absence of analytical standards and the lack of clear relationship between their structure and bioactivity may be another limitation to their clinical use (Li et al., 2017).
Tanshinones From Salvia miltiorrhiza Bunge
Park O. K. et al. (2012) investigated the inhibitory potential of Salvia miltiorrhiza Bunge towards SARS-CoV-3CL(pro). They found that Salvia miltiorrhiza Bunge ethanolic extract (30 µg/ml) resulted in 60% inhibition of SARS-CoV-3CL(pro). Furthermore, they demonstrated that six tanshinones of the plant (lipophilic fraction) exerted marked inhibition of SARS-CoV-3CL(pro) in a dose- but not–time- dependent manner. IC50 was estimated at 14.4–89.1 µM. Dihydrotanshinone I exhibited the most important inhibitory effect with an IC50 of 14.4 ± 0.7 µM. With regard to the kinetic mechanism of SARS-CoV-3CL(pro) inhibition, Salvia miltiorrhiza Bunge tanshinones were found to be noncompetitive inhibitors.
Biflavonoids From Torreya nucifera (L.) Siebold & Zucc.
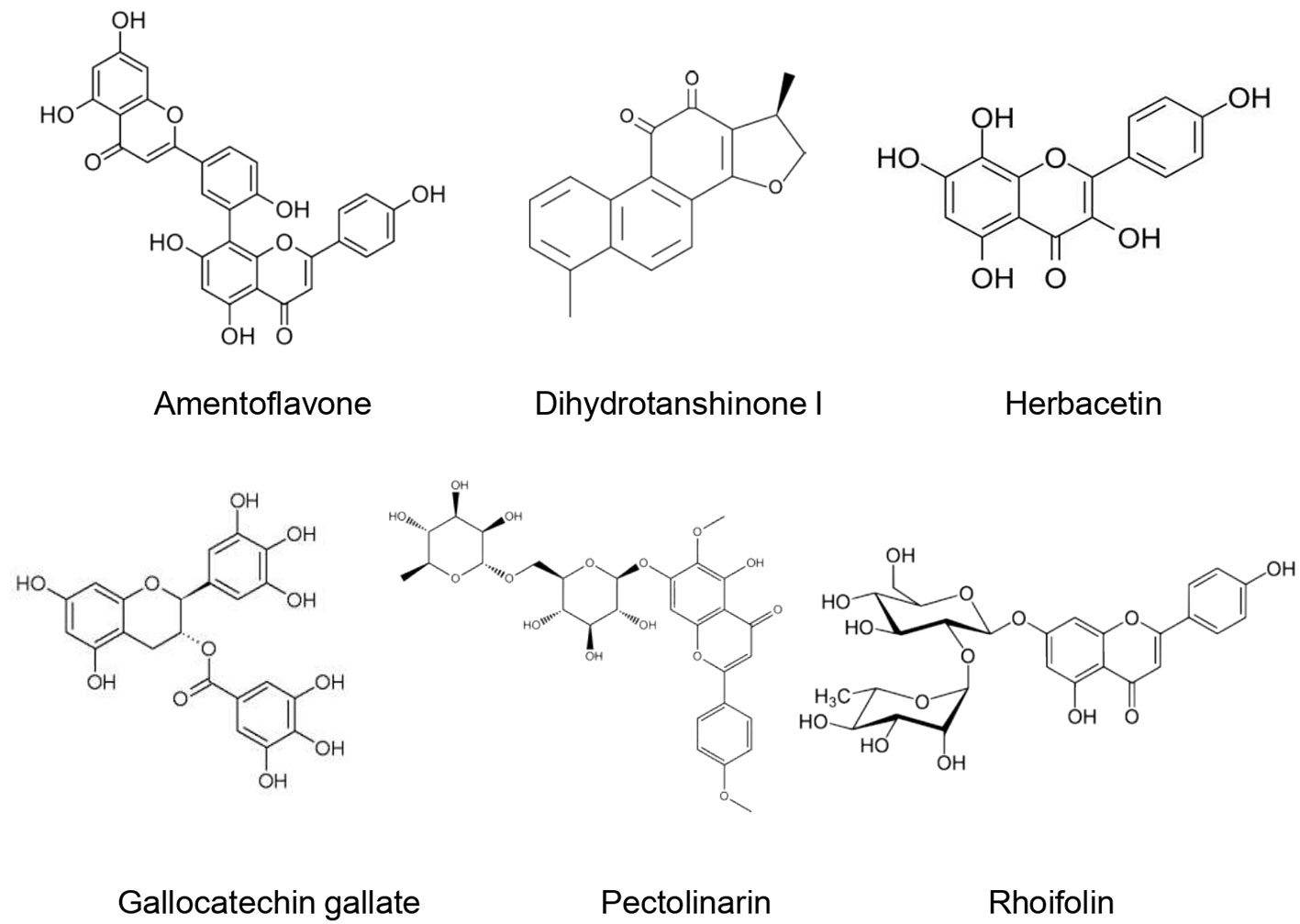
Four biflavonoids (amentoflavone, bilobetin, ginkgetin, and sciadopitysin) were isolated from the ethanol extract of Torreya nucifera (L.) Siebold & Zucc. leaves and evaluated for their SARS-CoV-3CL(pro) inhibitory effect by using a FRET method. All biflavonoids exhibited a marked inhibitory effect of SARS-CoV-3CL(pro) with IC50 of 8.3–72.3 µM. This inhibitory effect was stronger than that of eight diterpenoids isolated from the T. nucifera extract (IC50: 49.6–283.5 µM). Amentoflavone exerted the most important inhibitory activity since it possessed the lowest IC50 (8.3 ± 1.2 µM). Moreover, its inhibitory potential was more important than that of apigenin (IC50 = 280.8 ± 21.4 µM), quercetin (IC50 = 23.8 ± 1.9 µM) and luteolin (IC50 = 20.0 ± 2.2 µM). Molecular docking demonstrated that amentoflavone showed a good affinity with SARS-CoV-3CL(pro) and formed strong hydrogen bonds. An apigenin moiety at position C-30 of flavones was suggested to be responsible for a better inhibitory effect (Ryu et al., 2010).
Flavonoids
Seven flavonoids (Quercetin, Puerarin, Daidzein, gallocatechin gallate, epigallocatechin gallate, epigallocatechin, ampelopsi) were evaluated for their inhibitory effects of SARS-CoV-3CL(pro) expressed in Pichia pastoris GS115. At 200 µM, gallocatechin gallate, epigallocatechin gallate, and quercetin were able to inhibit the SARS-CoV-3CL(pro) activity by 91, 85, and 82%, respectively. Gallocatechin gallate was found to be a competitive inhibitor of SARS-CoV-3CL(pro) with IC50 of almost 47µM. Molecular docking studies confirmed the important inhibitory potential of gallocatechin gallate owing to the hydrophobic and H-bonds interaction formed with the active site of SARS-CoV-3CL(pro) (Nguyen et al., 2012). Nonetheless, it is difficult to predict how these H-bonds may contribute to the biological functions of the supposed active molecules (Chen et al., 2016). Furthermore, the strength of the H-bonds is not evaluated in this study which could limit the selectivity of the tested molecules since an important number of weak H-bonds often increase their affinity and therefore their interaction with off-target proteins.
Jo et al. (2020) evaluated the inhibitory effect of 64 flavonoids towards SARS-CoV-3CL(pro) using the FRET method. They found that at 20 µM, rhoifolin, herbacetin, and pectolinarin possessed the higher inhibitory effect, with IC50 values of 27.45, 33.17, and 37.78 μM, respectively. In addition, molecular docking revealed that flavonoids possessed an important binding affinity for SARS-CoV-3CL(pro) owing to their hydrophobic aromatic rings and hydrophilic hydroxyl groups.
Figure 6 summarizes the possible anti-SARS-CoV 2 actions of natural products.
Critical Considerations
In the present review, 15 in vitro studies were included. They were from Korea (9/15), Taiwan (3/15), China (2/15), and Iran (1/15). Most of the studies (10/15) were published between 2011 and 2018 whereas only two studies (Runfeng et al., 2020; Jo et al., 2020) were published in 2020 and one study (Weng et al., 2019) in 2019. Of them, only one study (Runfeng et al., 2020) investigated the antiviral activity against SARS-CoV-2, and another (Weng et al., 2019) on the human coronavirus NL63, whereas the other studies were not cell-based. Three studies used plant extracts and one study (Runfeng et al., 2020), a herbal mixture of 11 Chinese plants. Ethanol, methanol, and water were the solvents used to prepare the plant extracts included in these studies. Most of the studies were conducted with isolated natural compounds belonging to different phytochemical classes. Phenolic compounds were the most frequently reported.
When studies were analyzed using the best practice recommendations in phytopharmacological research (Heinrich et al., 2020), several concerns were detected especially regarding reporting data and outcomes. A large number of natural products reported in this review such as phenolic acids, quercetin, or kaempferol belong to phytochemical classes known to possess a broad spectrum of biological activities both in vitro and in vivo. Therefore, most of the studies failed to demonstrate the specificity of such products. In addition, all the included studies did not consider the drugability of the “active” compounds or extracts and presented an insufficient interpretation of the obtained data. Likewise, all the studies presented limitations regarding concepts and methods and also in the development of the project. In fact, all the studies investigating herbal extracts and isolated compounds did not consider the sustainable sourcing of the species nor the registration standards of both compounds or accepted plants’ names. Likewise, the included studies presented serious bias concerning the dose range and the toxic doses. On the other hand, eight studies did not report the use of control, whereas the other studies did not justify the choice of the positive controls used for comparison. This could be considered as a risk of bias resulting in possible limitations in the methodology of the studies. Besides, the lack of full taxonomic validity has been detected in all these studies.
It has been reported that IC50 of chloroquine inhibition of SARS-CoV-2 replication was found to be 1.13 to 5.47 µM (Smit et al., 2020) whereas that of SARS-CoV was 8.8 µM (Keyaerts et al., 2004). On the other hand, IC50 of ivermectin was found to be ~2 μM (Caly et al., 2020). Therefore, molecules with IC50 ranging from 0 to 10 µM have been considered as possible active molecules against SARS-CoV-2. Accordingly, few natural molecules reported in the included studies could be considered active against SARS-CoV-2 including amentoflavone, dieckol, hirsutenone, cryptotanshinone, xanthoangelol E, tomentin E, psoralidin, scutellarein, myricetin, and caffeic acid. Nevertheless, most of these active molecules are phenolic compounds characterized by a low bioavailability and rapid elimination (Górniak et al., 2019) which could compromise their clinical usefulness in the context of COVID-19.
Despite the promising possible anti-SARS-CoV-2 effects exhibited by both plant extracts and natural molecules, several limitations should be considered. Overall, due to the recent outbreak, the clinical usefulness of these products needs to be demonstrated since the current data are still immature, and no final conclusions have been validated. As shown in Table 2, for some species, there was no relationship between the traditional ethnopharmacological uses and the anti-SARS-CoV-2 effects. In spite of that, these plants are currently used to treat or manage symptoms reported in SARS-CoV-2 disease such as fever, inflammations, or cardiovascular and circulatory disorders. Moreover, efficacy and safety of the active natural products should be further studied in vivo and clinically validated in COVID-19 patients. Importantly, bioavailability, modes of administration, safe doses, time of exposure, pharmacokinetic profile, the health of the patients’ digestive system, and disease stage are to be considered in the evaluation of the beneficial effects of natural products against SARS-CoV-2. On the other hand, further studies are needed to clarify the mechanisms and pathways targeted by such products, which will help to improve their clinical usefulness. Assessing the effects of combinations of active natural products with validated antiviral drugs could be a promising alternative to explore.
Conclusion
Medicinal plants and natural products are still considered promising alternatives to prevent or treat several diseases. Since the outbreak of the COVID-19 pandemic in December 2019, various traditional herbal medicines have been used and resulted in positive health effects among COVID-19 patients, mainly in China. In the present review, we have discussed the possible potential uses of medicinal plants and/or natural products to prevent or even treat COVID-19. Although the studies evaluating the anti-SARS-CoV-2 effects of medicinal plants are still insufficient and relatively immature, some natural products with IC50 below 10 µM could be considered as promising anti-SARS-CoV-2 agents since they were able to block its life-cycle related proteins such as the cellular receptor ACE2, papain-like or chymotrypsin-like proteinases. Nevertheless, several limitations have been detected in relation to the specificity of the action exerted by such products, sustainable sourcing of the species, doses range used, or the use of appropriate controls.
While available studies offer several indications that these plant-derived products may help in fighting COVID-19, further studies should be carried out to evaluate the clinical usefulness of such products against COVID-19 infection. Furthermore, the bioavailability of natural products with possible anti-SARS-CoV-2 effects such as tannins should be considered besides the need for clinical validation of their usefulness and safety. The herbal mixtures, medicinal plants, or natural products with possible anti-SARS-CoV-2 effects must be evaluated through prospective and interventional studies. A combination of natural products or herbal mixtures with validated anti-COVID-19 drugs may constitute a promising preventive and therapeutic alternative to be assessed.
Author Contributions
BB and AP contributed equally to the study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the General Directorate of Scientific Research and Technological Development (DGRSDT – Algeria).
References
Adedeji, A. O., Severson, W., Jonsson, C., Singh, K., Weiss, S. R., Sarafianos, S. G. (2013). Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J. Virol. 87 (14), 8017–8028. doi: 10.1128/JVI.00998-13
Amanat, F., Krammer, F. (2020). SARS-CoV-2vaccines: status report. J. Immunol. 52 (4), 583–589. doi: 10.1016/j.immuni.2020.03.007
Arya, R., Das, A., Prashar, V., Kumar, M. (2020). Potential inhibitors against papain-like protease of novel coronavirus (SARS-CoV-2) from FDA approved drugs. ChemRxiv. doi: 10.26434/chemrxiv.11860011.v2
Caly, L., Druce, J. D., Catton, M. G., Jans, D. A., Wagstaff, K. M. (2020). The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 178, 104787. doi: 10.1016/j.antiviral.2020.104787
Ceccarelli, M., Berretta, M., Rullo, E. V., Nunnari, G., Cacopardo, B. (2020). Editorial–Differences and similarities between Severe Acute Respiratory Syndrome (SARS)-CoronaVirus (CoV) and SARS-CoV-2. Would a rose by another name smell as sweet? Eur. Rev. Med. Pharmacol. Sci. 24, 2781–2783. doi: 10.26355/eurrev_202003_20551
Chen, D., Oezguen, N., Urvil, P., Ferguson, C., Dann, S. M., Savidge, T. C. (2016). Regulation of protein-ligand binding affinity by hydrogen bond pairing. Sci. Adv. 2 (3), e1501240. doi: 10.1126/sciadv.1501240
Chen, Y., Guo, Y., Pan, Y., Zhao, Z. J. (2020). Structure analysis of the receptor binding of 2019-nCoV. Biochem. Biophys. Res. Commun. 525 (1), 135–140. doi: 10.1016/j.bbrc.2020.02.071
Chi, J. H., Kim, Y. H., Sohn, D. H., Seo, G. S., Lee, S. H. (2018). Ameliorative effect of Alnus japonica ethanol extract on colitis through the inhibition of inflammatory responses and attenuation of intestinal barrier disruption in vivo and in vitro. BioMed. Pharmacother. 108, 1767–1774. doi: 10.1016/j.biopha.2018.10.050
Cho, J. K., Curtis-Long, M. J., Lee, K. H., Kim, D. W., Ryu, H. W., Yuk, H. J., et al. (2013). Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg. Med. Chem. 21 (11), 3051–3057. doi: 10.1016/j.bmc.2013.03.027
Cho, S. H., Kim, H. S., Lee, W., Han, E. J., Kim, S. Y., Fernando, I. S., et al. (2020). Eckol from Ecklonia cava ameliorates TNF-α/IFN-γ-induced inflammatory responses via regulating MAPKs and NF-κB signaling pathway in HaCaT cells. Int. Immunopharmacol. 82, 106146. doi: 10.1016/j.intimp.2019.106146
Chokpaisarn, J., Urao, N., Voravuthikunchai, S. P., Koh, T. J. (2017). Quercus infectoria inhibits Set7/NF-κB inflammatory pathway in macrophages exposed to a diabetic environment. Cytokine 94, 29–36. doi: 10.1016/j.cyto.2017.04.005
Čopra-Janićijević, A., Čulum, D., Vidic, D., Tahirović, A., Klepo, L., Bašić, N. (2018). Chemical composition and antioxidant activity of the endemic Crataegus microphylla Koch subsp. malyana KI Chr. & Janjić from Bosnia. Ind. Crops Prod. 113, 75–79. doi: 10.1016/j.indcrop.2018.01.016
Corona, G., Ji, Y., Anegboonlap, P., Hotchkiss, S., Gill, C., Yaqoob, P., et al. (2016). Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 115 (7), 1240–1253. doi: 10.1017/S0007114516000210
Da, J., Xu, M., Wang, Y., Li, W., Lu, M., Wang, Z. (2019). Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. Anal. Cell Pathol. (Amst) 2019, 1907698. doi: 10.1155/2019/1907698
Ding, Y., Zeng, L., Li, R., Chen, Q., Zhou, B., Chen, Q., et al. (2017). The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 17 (1), 130. doi: 10.1186/s12906-017-1585-7
Du, J., Hu, Z., Dong, W. J., Wang, Y., Wu, S., Bai, Y. (2019). Biosynthesis of large-sized silver nanoparticles using Angelica keiskei extract and its antibacterial activity and mechanisms investigation. Microchem. J. 147, 333–338. doi: 10.1016/j.microc.2019.03.046
Fang, L., Karakiulakis, G., Roth, M. (2020). Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 8 (4), e21. doi: 10.1016/S2213-2600(20)30116-8
Fani, M., Teimoori, A., Ghafari, S. (2020). Comparison of the COVID-2019 (SARS-CoV-2) pathogenesis with SARS-CoV and MERS-CoV infections. Future Virol. 15 (5), 317–323. doi: 10.2217/fvl-2020-0050
Fedoreyev, S. A., Krylova, N. V., Mishchenko, N. P., Vasileva, E. A., Pislyagin, E. A., Iunikhina, O. V., et al. (2018). Antiviral and Antioxidant Properties of Echinochrome A. Marine Drugs 16 (12), 509. doi: 10.3390/md16120509
Fung, T. S., Liu, D. X. (2020). Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 73, 529–557. doi: 10.1146/annurev-micro-020518-115759
Fuzimoto, A. D., Isidoro, C. (2020). The antiviral and coronavirus-host protein pathways inhibiting properties of herbs and natural compounds - Additional weapons in the fight against the COVID-19 pandemic? J. Trad. Complem. Med. 10 (4), 405–419. doi: 10.1016/j.jtcme.2020.05.003
Gao, D., Niu, M., Wei, S., Zhang, C., Zhou, Y., Yang, Z., et al. (2020). Identification of a Pharmacological Biomarker for the Bioassay-Based Quality Control of a Thirteen-Component TCM Formula (Lianhua Qingwen) Used in Treating Influenza A Virus (H1N1) Infection. Front. Pharmacol. 11, 746. doi: 10.3389/fphar.2020.00746
Gibbs, A., Schwartzman, J., Deng, V., Alumkal, J. (2009). Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc. Natl. Acad. Sci. U.S.A. 106 (39), 16663–16668. doi: 10.1073/pnas.0908908106
Górniak, I., Bartoszewski, R., Króliczewski, J. (2019). Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 18, 241–272. doi: 10.1007/s11101-018-9591-z
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., He, J. X., et al. (2019). Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 382 (18), 1708–1720. doi: 10.1056/NEJMoa2002032
Guy, J. L., Lambert, D. W., Warner, F. J., Hooper, N. M., Turner, A. J. (2005). Membrane-associated zinc peptidase families: comparing ACE and ACE2. Biochim Biophys Acta (BBA) -. Prot. Proteom. 175 (1), 2–8. doi: 10.1016/j.bbapap.2004.10.010
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best practice in research - Overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi: 10.1016/j.jep.2019.112230
Ho, T. Y., Wu, S. L., Chen, J. C., Li, C. C., Hsiang, C. Y. (2007). Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 74 (2), 92–101. doi: 10.1016/j.antiviral.2006.04.014
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 181, 1–10. doi: 10.1016/j.cell.2020.02.052
Hong-Zhi, D. U., Hou, X. Y., Miao, Y. H., Huang, B. S., Liu, D. H. (2020). Traditional Chinese Medicine: an effective treatment for 2019 novel coronavirus pneumonia (NCP). Chin. J. Nat. Med. 18 (3), 226–230. doi: 10.1016/S1875-5364(20)30022-4
Iwata-Yoshikawa, N., Okamura, T., Shimizu, Y., Hasegawa, H., Takeda, M., Nagata, N. (2019). TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J. Virol. 93 (6), e01815–e01818. doi: 10.1128/JVI.01815-18
Ji, P., Chen, C., Hu, Y., Zhan, Z., Pan, W., Li, R., et al. (2015). Antiviral activity of Paulownia tomentosa against enterovirus 71 of hand, foot, and mouth disease. Biol. Pharm. Bull. 38 (1), 1–6. doi: 10.1248/bpb.b14-00357
Jiang, S., Hillyer, C., Du, L. (2020). Neutralizing Antibodies against SARS-CoV-2and Other Human Coronaviruses. Trends Immunol. 41 (5), 355–359. doi: 10.1016/j.it.2020.04.008
Jin, Y. H., Cai, L., Cheng, Z. S., Cheng, H., Deng, T., Fan, Y. P., et al. (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (COVID-19) infected pneumonia (standard version). Mil. Med. Res. 7 (1), 4. doi: 10.1186/s40779-020-0233-6
Jo, S., Kim, S., Shin, D. H., Kim, M. S. (2020). Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 35 (1), 145–151. doi: 10.1080/14756366.2019.1690480
Kalpana, D., Han, J. H., Park, W. S., Lee, S. M., Wahab, R., Lee, Y. S. (2019). Green biosynthesis of silver nanoparticles using Torreya nucifera and their antibacterial activity. Arab. J. Chem. 12 (7), 1722–1732. doi: 10.1016/j.arabjc.2014.08.016
Keyaerts, E., Vijgen, L., Maes, P., Neyts, J., Van Ranst, M. (2004). In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem. Biophys. Res. Commun. 323 (1), 264–268. doi: 10.1016/j.bbrc.2004.08.085
Khan, S., Siddique, R., Shereen, M. A., Ali, A., Liu, J., Bai, Q., et al. (2020). The emergence of a novel coronavirus (SARS-CoV-2CoV-2), their biology and therapeutic options. J. Clin. Microbiol. 58 (5), e00187–e00120. doi: 10.1128/jcm.00187-20
Kil, Y. S., Pham, S. T., Seo, E. K., Jafari, M. (2017). Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch. Pharm. Res. 40 (6), 655–675. doi: 10.1007/s12272-017-0892-3
Kim, D. W., Seo, K. H., Curtis-Long, M. J., Oh, K. Y., Oh, J. W., Cho, J. K., et al. (2014). Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J. Enzyme Inhib. Med. Chem. 29 (1), 59–63. doi: 10.3109/14756366.2012.753591
Koo, H. J., Lee, S., Chang, K. J., Sohn, E., Sohn, E. H., Kang, S. C., et al. (2017). Hepatic anti-inflammatory effect of hexane extracts of Dioscorea batatas Decne: Possible suppression of toll-like receptor 4-mediated signaling. BioMed. Pharmacother. 92, 157–167. doi: 10.1016/j.biopha.2017.05.036
Kumar, N., Goel, N. (2019). Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. (Amst) 24, e00370. doi: 10.1016/j.btre.2019.e00370
Lee, J. W., Seo, K. H., Ryu, H. W., Yuk, H. J., Park, H. A., Lim, Y., et al. (2018). Anti-inflammatory effect of stem bark of Paulownia tomentosa Steud. in lipopolysaccharide (LPS)-stimulated RAW264. 7 macrophages and LPS-induced murine model of acute lung injury. J. Ethnopharmacol. 210, 23–30. doi: 10.1016/j.jep.2017.08.028
Lee, M. J., Nho, J. H., Yang, B. D., Park, H., Lee, H. J., Lee, K. H., et al. (2019). Subchronic toxicity evaluation of ethanol extract of Cassia tora L. seeds in rats. Regulat. Toxicol. Pharmacol. 109, 104487. doi: 10.1016/j.yrtph.2019.104487
Li, S. W., Wang, C. Y., Jou, Y. J., Huang, S. H., Hsiao, L. H., Wan, L., et al. (2016). SARS coronavirus papain-like protease inhibits the TLR7 signaling pathway through removing Lys63-linked polyubiquitination of TRAF3 and TRAF6. Int. J. Mol. Sci. 17 (5), 678. doi: 10.3390/ijms17050678
Li, Y., Fu, X., Duan, D., Liu, X., Xu, J., Gao, X. (2017). Extraction and Identification of Phlorotannins from the Brown Alga, Sargassum fusiforme (Harvey) Setchell. Mar. Drugs 15 (2), 49. doi: 10.3390/md15020049
Li, H., Wang, Y. M., Xu, J. Y., Cao, B. (2020). Potential Antiviral Therapeutics for 2019 Novel Coronavirus. Chin. J. Tuberc. Respir. Dis. 43 (3), 170–172. doi: 10.3760/cma.j.issn.1001-0939.2020.03.004
Liang, J., Yan, C., Zhang, Y., Zhang, T., Zheng, X., Li, H. (2020). Rapid discrimination of Salvia miltiorrhiza according to their geographical regions by laser induced breakdown spectroscopy (LIBS) and particle swarm optimization-kernel extreme learning machine (PSO-KELM). Chemometrics Intelligent Lab. Syst. 197, 103930. doi: 10.1016/j.chemolab.2020.103930
Lin, M. H., Moses, D. C., Hsieh, C. H., Cheng, S. C., Chen, Y. H., Sun, C. Y., et al. (2018). Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antivir. Res. 150, 155–163. doi: 10.1016/j.antiviral.2017.12.015
Liu, R., Su, B., Huang, F., Ru, M., Zhang, H., Qin, Z., et al. (2019). Identification and analysis of cardiac glycosides in Loranthaceae parasites Taxillus chinensis (DC.) Danser and Scurrula parasitica Linn. and their host Nerium indicum Mill. J. Pharm. Biomedi. Anal. 174, 450–459. doi: 10.1016/j.jpba.2019.05.071
Lu, H. (2020). Drug treatment options for the 2019-new coronavirus (COVID-19). Biosci. Trends 14 (1), 69–71. doi: 10.5582/bst.2020.01020
Lung, J., Lin, Y. S., Yang, Y. H., Chou, Y. L., Shu, L. H., Cheng, Y. C., et al. (2020). The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J. Med. Virol. 10, 1002. doi: 10.1002/jmv.25761
Luo, H., Tang, Q. L., Shang, Y. X., Liang, S. B., Yang, M., Robinson, N., et al. (2020). Can Chinese Medicine Be Used for Prevention of Corona Virus Disease 2019 (COVID-19)? A Review of Historical Classics, Research Evidence and Current Prevention Programs. Chin. J. Integr. Med. 26 (4), 243–250. doi: 10.1007/s11655-020-3192-6
Ma, J., Kang, S. Y., Meng, X., Kang, A. N., Park, J. H., Park, Y. K., et al. (2018). Effects of rhizome extract of dioscorea batatas and its active compound, allantoin, on the regulation of myoblast differentiation and mitochondrial biogenesis in c2c12 myotubes. Molecules 23 (8), 2023. doi: 10.3390/molecules23082023
Mackenzie, J. S., Smith, D. W. (2020). COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don’t. Microbiol. Aust. 41 (1), 45–50. doi: 10.1071/MA20013
Mamouni, K., Zhang, S., Li, X., Chen, Y., Yang, Y., Kim, J., et al. (2018). A novel flavonoid composition targets androgen receptor signaling and inhibits prostate cancer growth in preclinical models. Neoplasia 20 (8), 789–799. doi: 10.1016/j.neo.2018.06.003
Matsuyama, S., Nao, N., Shirato, K., Kawase, M., Saito, S., Takayama, I., et al. (2020). Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U.S.A. 117 (13), 7001–7003. doi: 10.1073/pnas.2002589117
Meyer, M., Jaspers, I. (2015). Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. Am. J. Physiol. Lung Cell Mol. Physiol. 308 (12), 1189–1201. doi: 10.1152/ajplung.00028.2015
Nasab, F. K., Khosravi, A. R. (2014). Ethnobotanical study of medicinal plants of Sirjan in Kerman Province, Iran. J. Ethnopharmacol. 154 (1), 190–197. doi: 10.1016/j.jep.2014.04.003
Nguyen, T. T., Woo, H. J., Kang, H. K., Nguyen, V. D., Kim, Y. M., Kim, D. W., et al. (2012). Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol. Lett. 34 (5), 831–838. doi: 10.1007/s10529-011-0845-8
Ortega, J. T., Serrano, M. L., Pujol, F. H., Rangel, H. R. (2020). Role of changes in SARS-CoV-2 spike protein in the interaction with the human ACE2 receptor: An in silico analysis. Excli. J. 19, 410–417. doi: 10.17179/excli2020-1167
Park, O. K., Choi, J. H., Park, J. H., Kim, I. H., Yan, B. C., Ahn, J. H., et al. (2012). Comparison of neuroprotective effects of five major lipophilic diterpenoids from Danshen extract against experimentally induced transient cerebral ischemic damage. Fitoterapia 83, 1666–1674. doi: 10.1016/j.fitote.2012.09.020
Park, J. Y., Kim, J. H., Kim, Y. M., Jeong, H. J., Kim, D. W., Park, K. H., et al. (2012a). Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 20 (19), 5928–5935. doi: 10.1016/j.bmc.2012.07.038
Park, J. Y., Jeong, H. J., Kim, J. H., Kim, Y. M., Park, S. J., Kim, D., et al. (2012b). Diarylheptanoids from Alnus japonica Inhibit Papain-Like Protease of Severe Acute Respiratory Syndrome Coronavirus. Bioorg. Med. Chem. 5 (11), 2036–2042. doi: 10.1248/bpb.b12-00623
Park, E. Y., Kim, E. H., Kim, M. H., Seo, Y. W., Lee, J. I., Jun, H. S. (2012). Polyphenol-rich fraction of brown alga Ecklonia cava collected from Gijang, Korea, reduces obesity and glucose levels in high-fat diet-induced obese mice. Evid. Based Complement. Alternat. Med. 2012, 1–12. doi: 10.1155/2012/418912
Park, J. Y., Kim, J. H., Kwon, J. M., Kwon, H. J., Jeong, H. J., Kim, Y. M., et al. (2013). Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg. Med. Chem. 21 (13), 3730. doi: 10.1016/j.bmc.2013.04.026
Park, J. Y., Ko, J. A., Kim, D. W., Kim, Y. M., Kwon, H. J., Jeong, H. J., et al. (2016). Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzyme Inhib. Med. Chem. 31 (1), 23–30. doi: 10.3109/14756366.2014.1003215
Patten, G. S., Abeywardena, M. Y., Bennett, L. E. (2016). Inhibition of Angiotensin Converting Enzyme, Angiotensin II Receptor Blocking, and Blood Pressure Lowering Bioactivity across Plant Families. Crit. Rev. Food Sci. Nutr. 56 (2), 181–214. doi: 10.1080/10408398.2011.651176
Pereira-Caro, G., Moreno-Rojas, J. M., Brindani, N., Del Rio, D., Lean, M. E., Hara, Y., et al. (2017). Bioavailability of black tea theaflavins: Absorption, metabolism, and colonic catabolism. J. Agri. Food Chem. 65 (26), 5365–5374. doi: 10.1021/acs.jafc.7b01707
Qiu, Y., Zhao, Y. B., Wang, Q., Li, J. Y., Zhou, Z. J., Liao, C. H., et al. (2020). Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARSCoV-2. Microbes Infect. 22 (4–5), 221–225. doi: 10.1016/j.micinf.2020.03.003
Rabi, F., Al Zoubi, M. S., Kasasbeh, G. A., Salameh, D. M., Al-Nasser, A. D. (2020). SARS-CoV-2CoV-2 and Coronavirus Disease 2019: What We Know So Far. Pathogens 9 (3), 231. doi: 10.3390/pathogens9030231
Runfeng, L., Yunlong, H., Jicheng, H., Weiqi, P., Qinhai, M., Yongxia, S., et al. (2020). Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 156, 104761. doi: 10.1016/j.phrs.2020.104761
Ryu, Y. B., Jeong, H. J., Kim, J. H., Kim, Y. M., Park, J. Y., Kim, D., et al. (2010). Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 18 (22), 7940–7947. doi: 10.1016/j.bmc.2010.09.035
Sadat-Hosseini, M., Farajpour, M., Boroomand, N., Solaimani-Sardou, F. (2017). Ethnopharmacological studies of indigenous medicinal plants in the south of Kerman, Iran. J. Ethnopharmacol. 199, 194–204. doi: 10.1016/j.jep.2017.02.006
Schlagenhauf, P., Grobusch, M. P., Maier, J. D., Gautret, P. (2020). Repurposing antimalarials and other drugs for COVID-19. Trav. Med. Infect. Dis. 34, 101658. doi: 10.1016/j.tmaid.2020.101658
Sharifi, N., Souri, E., Ziai, S. A., Amin, G., Amanlou, M. (2013). Discovery of new angiotensin converting enzyme (ACE) inhibitors from medicinal plants to treat hypertension using an in vitro assay. Daru 21 (1), 74. doi: 10.1186/2008-2231-21-74
Shi, Y., Wang, X., Wang, N., Li, F. F., You, Y. L., Wang, S. Q. (2020). The effect of polysaccharides from Cibotium barometz on enhancing temozolomide–induced glutathione exhausted in human glioblastoma U87 cells, as revealed by 1H NMR metabolomics analysis. Int. J. Biol. Macromol. 156, 471–484. doi: 10.1016/j.ijbiomac.2020.03.243
Sigrist, C. J., Bridge, A., Le Mercier, P. (2020). A potential role for integrins in host cell entry by SARS-CoV-2. Antivir. Res. 177, 104759. doi: 10.1016/j.antiviral.2020.104759
Smit, C., Peeters, M. Y. M., van den Anker, J. N., Knibee, C. A. (2020). Chloroquine for SARS-CoV-2: Implications of Its Unique Pharmacokinetic and Safety Properties. Clin. Pharmacokinet. 59, 659–669. doi: 10.1007/s40262-020-00891-1
Song, J., Zhang, F., Tang, S., Liu, X., Gao, Y., Lu, P., et al. (2013). A module analysis approach to investigate molecular mechanism of TCM formula: a trial on shu-feng-jie-du formula. Evid-Based Compl. Alt. Med. 2013, 1–14. doi: 10.1155/2013/731370
Song, Y. H., Kim, D. W., Curtis-Long, M. J., Yuk, H. J., Wang, Y., Zhuang, N., et al. (2014). Papain-like protease (PLpro) inhibitory effects of cinnamic amides from Tribulus terrestris fruits. Biol. Pharm. Bull. 37 (6), 1021–1028. doi: 10.1248/bpb.b14-00026
Tayel, A. A., El-Sedfy, M. A., Ibrahim, A., II, Moussa, S. H. (2018). Application of Quercus infectoria extract as a natural antimicrobial agent for chicken egg decontamination. Rev. Argent. Microbiol. 50 (4), 391–397. doi: 10.1016/j.ram.2017.12.003
Tian, C., Chang, Y., Liu, X., Zhang, Z., Guo, Y., Lan, Z., et al. (2020). Anti-inflammatory activity in vitro, extractive process and HPLC-MS characterization of total saponins extract from Tribulus terrestris L. fruits. Ind. Crops Prod. 150, 112343. doi: 10.1016/j.indcrop.2020.112343
Walls, A. C., Park, Y. J., Tortorici, M. A., Wall, A., McGuire, A. T., Veesler, D. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181 (2), 281–292. doi: 10.1016/j.cell.2020.02.058
Wan, S., Xiang, Y., Fang, W., Zheng, Y., Li, B., Hu, Y., et al. (2020). Clinical Features and Treatment of COVID-19 Patients in Northeast Chongqing. J. Med. Virol. 10, 1002. doi: 10.1002/jmv.25783
Wang, C. H., Zhong, Y., Zhang, Y., Liu, J. P., Wang, Y. F., Jia, W. N., et al. (2016). A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Mol. Syst. Biol. 12 (2), 606–613. doi: 10.1039/C5MB00448A
Wang, Z., Chen, X., Lu, Y., Chen, F., Zhang, W. (2020). Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci. Trends 14 (1), 64–68. doi: 10.5582/bst.2020.01030
Wei, T., Deng, K., Gao, Y., Chen, L., Song, W., Zhang, Y., et al. (2020). SmKSL overexpression combined with elicitor treatment enhances tanshinone production from Salvia miltiorrhiza hairy roots. Biochem. Engin. J. 158, 107562. doi: 10.1016/j.bej.2020.107562
Wen, C. C., Shyur, L. F., Jan, J. T., Liang, P. H., Kuo, C. J., Arulselvan, P., et al. (2011). Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Trad. Compl. Med. 1 (1), 41–50. doi: 10.1016/S2225-4110(16)30055-4
Weng, J. R., Lin, C. S., Lai, H. C., Lin, Y. P., Wang, C. Y., Tsai, Y. C., et al. (2019). Antiviral activity of Sambucus FormosanaNakai ethanol extract and related phenolic acid constituents against human coronavirus NL63. Virus Res. 273, 197767. doi: 10.1016/j.virusres.2019.197767
Wong, L. Y., Lui, P. Y., Jin, D. Y. (2016). A molecular arms race between host innate antiviral response and emerging human coronaviruses. Virol. Sin. 31 (1), 12–23. doi: 10.1007/s12250-015-3683-3
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C. L., Abiona, O., et al. (2020). Cryo-EM structure of the COVID-19 spike in the prefusion conformation. Science 367, 1260–1263. doi: 10.1126/science.abb2507
Wu, Q., Yang, X. W. (2009). The constituents of Cibotium barometz and their permeability in the human Caco-2 monolayer cell model. J. Ethnopharmacol. 125 (3), 417–422. doi: 10.1016/j.jep.2009.07.017
Xia, R., Hu, X., Fei, Y., Willcox, M., Wen, L. Z., Yu, M. K., et al. (2020). Shufeng Jiedu capsules for treating acute exacerbations of chronic obstructive pulmonary disease: a systematic review and meta-analysis. BMC Complement. Med. Ther. 20, 151. doi: 10.1186/s12906-020-02924-5
Xu, D., Lin, T. H., Li, S., Da, J., Wen, X. Q., Ding, J., et al. (2012). Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett. 316 (1), 11–22. doi: 10.1016/j.canlet.2011.10.006
Xu, K., Cai, H., Shen, Y., Ni, Q., Chen, Y., Hu, S., et al. (2020). Management of Corona Virus disease-19 (COVID-19): The Zhejiang Experience. J. Zhejiang Univ. Med. Sci. 49 (1), 0.
Xu, J., Zhao, S., Teng, T., Abdalla, A. E., Zhu, W., Xie, L., et al. (2020). Systematic Comparison of Two Animal-to-Human Transmitted Human Coronaviruses: SARS-CoV-2 and SARS-CoV. Virus 12 (2), 244. doi: 10.3390/v12020244
Yang, B., Kim, S., Kim, J. H., Lim, C., Kim, H., Cho, S. (2019). Gentiana scabra Bunge roots alleviates skin lesions of contact dermatitis in mice. J. Ethnopharmacol. 233, 141–147. doi: 10.1016/j.jep.2018.12.046
Yang, Y., Islam, M. S., Wang, J., Li, Y., Chen, X. (2020). Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2CoV-2): A Review and Perspective. Int. J. Biol. Sci. 16 (10), 1708. doi: 10.7150/ijbs.45538
Yang, X., Yu, Y., Xu, J. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8 (5), 475–481. doi: 10.1016/S2213-2600(20)30079-5
Yu, M. S., Lee, J., Lee, J. M., Kim, Y., Chin, Y. W., Jee, J. G., et al. (2012). Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 22 (12), 4049–4054. doi: 10.1016/j.bmcl.2012.04.081
Yuan, L., Chen, Z., Song, S., Wang, S., Tian, C., Xing, G., et al. (2015). p53 degradation by a coronavirus papain-like protease suppresses type I interferon signaling. J. Biol. Chem. 290, 3172–3182. doi: 10.1074/jbc.M114.619890
Yun, J. W., Kim, S. H., Kim, Y. S., You, J. R., Cho, E. Y., Yoon, J. H., et al. (2018). Enzymatic extract from Ecklonia cava: Acute and subchronic oral toxicity and genotoxicity studies. Regulat. Toxicol. Pharmacol. 92, 46–54. doi: 10.1016/j.yrtph.2017.10.034
Zhang, T., Zhu, M., Chen, X., Bi, K. (2010). Simultaneous analysis of seven bioactive compounds in Sambucus chinensis Lindl by HPLC. Annalyt. Lett. 43, 2525–2533. doi: 10.1080/00032711003731399
Zhang, T., Wu, Q., Zhang, Z. (2020). Probable pangolin origin of SARS-CoV-2CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 30, 1–6. doi: 10.1016/j.cub.2020.03.022
Zhang, J. J., Dong, X., Cao, Y. Y. (2020). Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan, China. Allergy. 75 (7), 1730–1741. doi: 10.1111/all.14238
Zhang, L., Lin, D., Sun, X., Curth, U., Drosten, C., Sauerhering, L., et al. (2020). Crystal structure of SARS-CoV-2main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368 (6489), 409–412. doi: 10.1126/science.abb3405
Zhou, P., Yang, X. L., Wang, X. G., Hu, B., Zhang, L., Zhang, W., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273. doi: 10.1038/s41586-020-2012-7
Keywords: Severe Acute Respiratory Syndrome-related Coronavirus 2 (SARS-CoV-2), Coronavirus Disease 2019 (COVID-19), plants, natural products, ACE2
Citation: Benarba B and Pandiella A (2020) Medicinal Plants as Sources of Active Molecules Against COVID-19. Front. Pharmacol. 11:1189. doi: 10.3389/fphar.2020.01189
Received: 15 May 2020; Accepted: 22 July 2020;
Published: 07 August 2020.
Edited by:
Michael Heinrich, UCL School of Pharmacy, United KingdomReviewed by:
Ulrike Grienke, University of Vienna, AustriaVerena Spiegler, University of Münster, Germany
Copyright © 2020 Benarba and Pandiella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bachir Benarba, bachirsb@yahoo.fr
 Bachir Benarba
Bachir Benarba Atanasio Pandiella
Atanasio Pandiella