- 1State Key Laboratory of Traditional Chinese Medicine Syndrome, The First Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Department of Rheumatology, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Center for Health Data Science, Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
- 4Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 5Department of Allergy, Immunology & Rheumatology, Chung Shan Medical University Hospital, Taichung, Taiwan
- 6Department of Nursing, Chung Shan Medical University, Taichung, Taiwan
- 7Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan
- 8Office of Research and Development, Asia University, Taichung, Taiwan
Objective: To compare the effects of tofacitinib and adalimumab on the risk of adverse lipidaemia outcomes in patients with newly diagnosed rheumatoid arthritis (RA).
Methods: Data of adult patients newly diagnosed with RA who were treated with tofacitinib or adalimumab at least twice during a 3-year period from 1 January 2018 to 31 December 2020, were enrolled in the TriNetX US Collaborative Network. Patient demographics, comorbidities, medications, and laboratory data were matched by propensity score at baseline. Outcome measurements include incidental risk of dyslipidemia, major adverse cardiac events (MACE) and all-cause mortality.
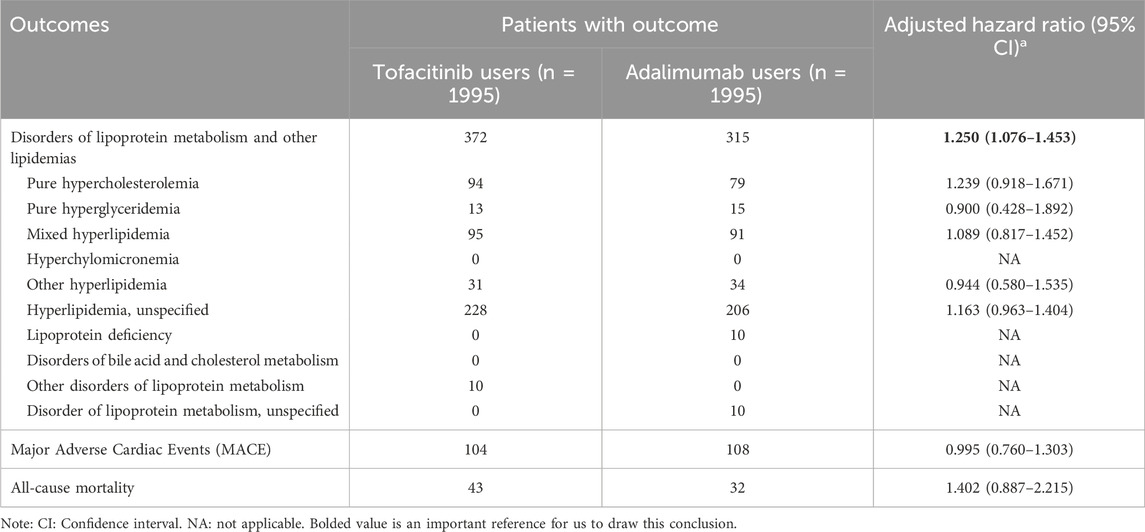
Results: A total of 7,580 newly diagnosed patients with RA (1998 receiving tofacitinib, 5,582 receiving adalimumab) were screened. After propensity score matching, the risk of dyslipidaemia outcomes were higher in the tofacitinib cohort, compared with adalimumab cohort (hazard ratio [HR] with 95% confidence interval [CI], 1.250 [1.076–1.453]). However, there is no statistically significant differences between two cohorts on MACE (HR, 0.995 [0.760–1.303]) and all-cause mortality (HR, 1.402 [0.887–2.215]).
Conclusion: Tofacitinib use in patients with RA may increase the risk of dyslipidaemia to some extent compared to adalimumab. However, there is no differences on MACE and all-cause mortality.
1 Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory autoimmune disease that requires long-term treatment to suppress inflammation and prevent progressive joint damage. Two of the most commonly prescribed RA medications with differing mechanisms are tofacitinib and adalimumab (Zullow et al., 2014; Zubkov et al., 2021; Xu et al., 2022; Eqbal et al., 2023; Li et al., 2023). Tofacitinib is an oral small molecule Janus kinase (JAK) inhibitor that interferes with inflammatory cytokine signaling pathways (Zhu et al., 2021). In contrast, adalimumab is an injectable tumor necrosis factor-alpha (TNF-α) inhibitor monoclonal antibody biologic that directly targets inflammatory cells and cytokines (Zullow et al., 2014; Panaccione et al., 2021; Huang et al., 2022; Zouboulis et al., 2023). Both drugs are widely used either alone or in combination strategies to manage RA symptoms and slow disease progression, often in side-by-side comparative studies (Zhang et al., 2014; Fleischmann et al., 2017; Deakin et al., 2023).
Prior studies show somewhat conflicting results on how treatment with tofacitinib versus adalimumab differentially affects blood lipid levels, which can elevate cardiovascular disease risk if abnormal. Some systematic reviews and meta-analyses associate tofacitinib treatment with elevations in serum cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) levels that appear independent of dosage, while TNF-α antagonists like adalimumab have no significant lipid effects (Salgado et al., 2014; Souto et al., 2015). However, randomized controlled trials found no adverse changes in lipid profiles after up to 24 months of tofacitinib treatment (Kuo et al., 2018; Sands et al., 2021). Given the increased risks of cardiovascular and other diseases linked to dyslipidemia (Zweifler et al., 2011; Zysset et al., 2016; Zuzda et al., 2022), clarifying the real-world effects of these widely used RA medications on blood lipids is clinically important.
Since most evidence on the comparative lipid effects of tofacitinib versus adalimumab comes from literature reviews and randomized controlled trials rather than large-scale observational data, this retrospective cohort study aimed to compare dyslipidemia incidence with tofacitinib versus adalimumab treatment using the large-scale TriNetX electronic health records database.
2 Materials and methods
1) Study Design and Data Source
This study utilized de-identified electronic health records data for over 75 million patients across the TriNetX network, which represents numerous integrated delivery networks, hospitals, and administrative claims data across the United States (Yousaf et al., 2020; Raiker et al., 2021; Zhu et al., 2023). This real-world evidence source contains demographic details, diagnoses, procedures, medications, laboratory tests, and clinical notes restructured into a common format. The study period spanned January 2018 through December 2020.
2) Ethical Statements
The TriNetX Analytics Network is compliant with the Health Insurance Portability and Accountability Act (HIPAA), the US federal law, which protects the privacy and security of healthcare data, and any additional data privacy regulations applicable to the contributing HCO. TriNetX is certified to the ISO 27001:2013 standard and maintains an Information Security Management System (ISMS) to ensure the protection of the healthcare data it has access to and to meet the requirements of the HIPAA Security Rule. The TriNetX Analytics Network was granted a waiver by the Western Institutional Review Board (WIRB) since it solely used aggregated counts and statistical summaries of de-identified data. Furthermore, the utilization of TriNetX for this study received approval from the Institutional Review Board of Chung Shan Medical University Hospital (CSMUH No: CS2-21176).
3) Cohort Selection
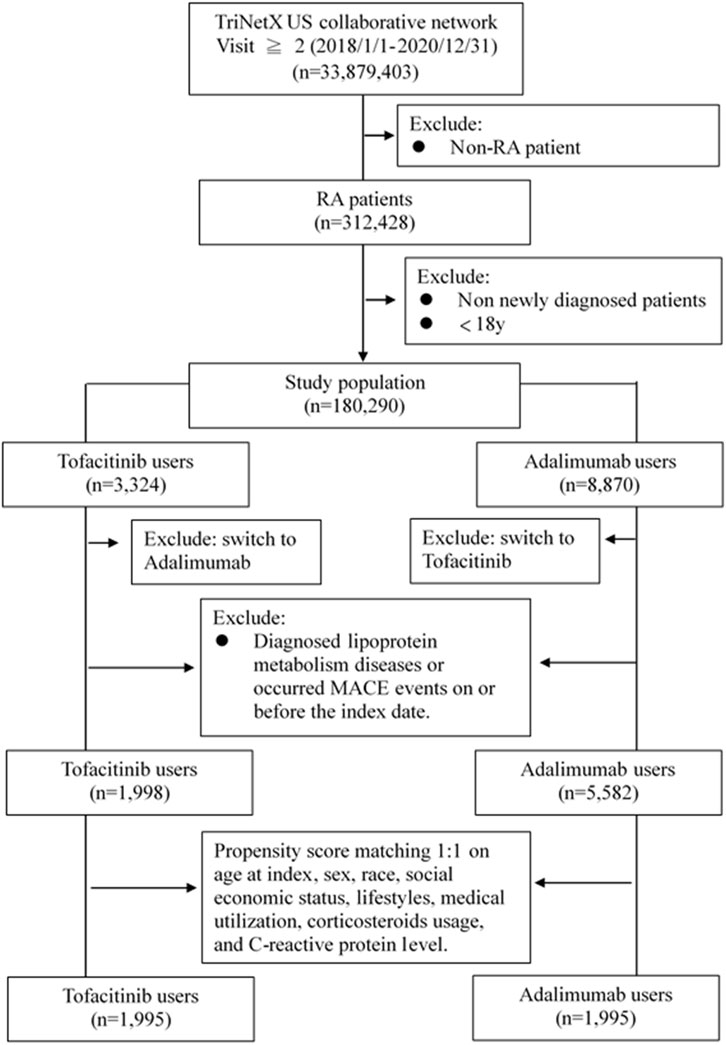
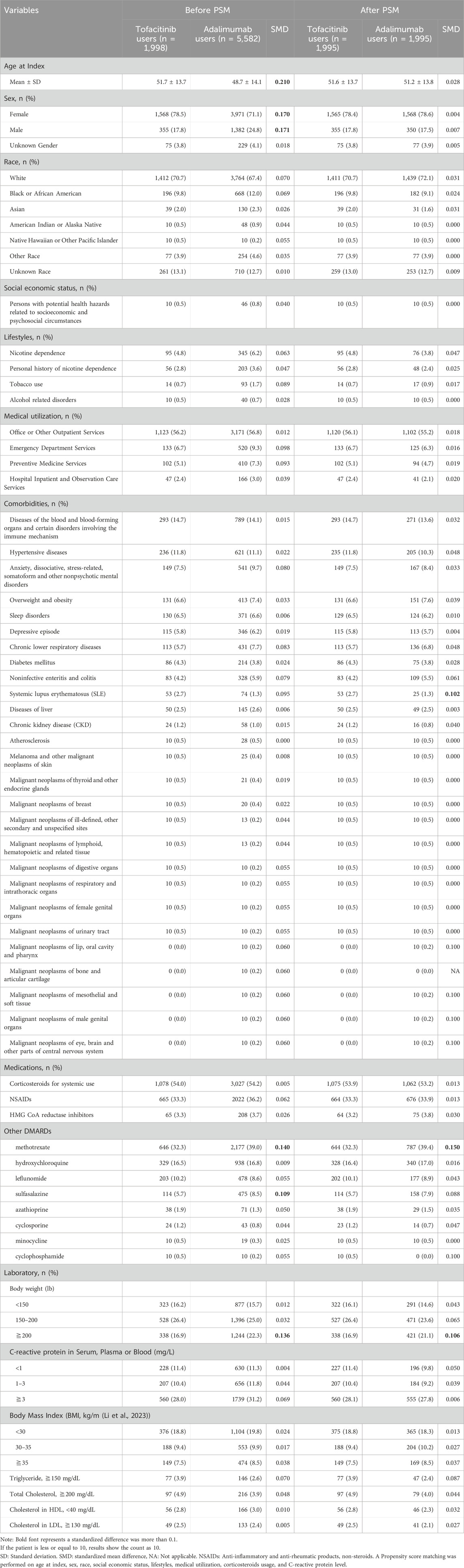
Adults newly diagnosed with RA between January 2018-December 2020 were included if treated with at least two doses of tofacitinib or adalimumab, without prior diagnoses of dyslipidemia or major adverse cardiac events (MACE). International Classification of Diseases, 10th Revision (ICD-10) codes were used to identify RA (M05-06), dyslipidemia (E78) and related comorbidities. RxNorm codes identified tofacitinib (1,357,536) and adalimumab (327,361) exposure. Patients were assigned to either the tofacitinib or adalimumab cohort based on which medication was received first after RA diagnosis. To balance baseline characteristics, propensity score matching at 1:1 ratio was performed for demographics, lifestyle factors, comorbid conditions, healthcare utilization, corticosteroids usage, and C-reactive protein level (proxy to classify the severity of RA). The participant screening flowchart for this study is shown in Figure 1.
4) Outcomes
The primary outcome was new diagnosis of dyslipidemia (ICD-10 E78) within 3 years of follow-up, including specific subtypes. Secondary outcomes assessed were all-cause mortality and MACE including acute coronary syndrome/unstable angina (ICD-10 I20.0), myocardial infarction (I21-I23), acute ischemic heart disease (I24), stroke (I60–I69, G45), heart failure (I50, I97.1, I11.0), cardiac shock/cardiac arrest (R57.0, I46), or coronary revascularization (coronary artery bypass grafting, defined by current procedural terminology, CPT code 33510–33536), and percutaneous coronary intervention (CPT code 92920–93572). All-cause mortality and MACE were evaluated over the 3-year follow-up period.
5) Other Covariates
Other covariates included: 1) demographic variables (age, sex, race, and potential health hazards related to socioeconomic and psychosocial circumstances); 2) lifestyle factors (tobacco use [ICD-10 Z72.0/Z87.891], nicotine dependence [ICD-10 F17], alcohol-related diseases [ICD-10 F10]); 3) medical utilisation (office or other outpatient services [CPT 1013626], preventive medicine services [CPT 1013829], emergency department services [CPT 1013711], or hospital inpatient services [CPT 1013659]); 4) comorbidities (hypertensive diseases [ICD-10 I10–16], atherosclerosis [ICD-10 I70], diabetes mellitus [ICD-10 E8–E13], obesity [ICD-10 E66], dyslipidaemia [ICD-10 E78.5], depression [ICD-10 F32], anxiety, traumatic past experiences, stress [ICD-10 F40–F48], sleep disorders [ICD-10 G47], chronic lower respiratory disease [ICD-10 J40–J47], non-infective enteritis and colitis [ICD-10 K50–K52], liver disease [ICD-10 K70–K77], systemic lupus erythematosus [ICD-10 M32], chronic kidney disease [ICD-10 N18], cancers [ICD-10 C00–C96], and blood diseases and disorders involving immune mechanisms [ICD-10 D50–D89]); 5) medication (anti-inflammatory and antirheumatic products, non-steroids [Anatomic Therapeutic Chemical (ATC) code M01A], corticosteroids for systemic use [ATC H02], statin [ATC C10AA], and other disease-modifying antirheumatic drugs [sulfasalazine (A07EC01), minocycline (J01AA08), cyclophosphamide (L01AA01), methotrexate (L01BA01), cyclosporine (L04AA01), leflunomide (L04AA13), azathioprine (L04AX01), methotrexate (L04AX03), and hydroxychloroquine (P01BA02)]); and 6) laboratory values (body weight, C-reactive protein in serum, body mass index (BMI), cholesterol; serum or plasma high-density lipoprotein cholesterol; serum or plasma low-density lipoprotein cholesterol; or serum, plasma, or blood triglyceride.
6) Statistical Analysis
Continuous variables were reported as means with standard deviations and categorical variables as counts with percentages. Propensity score matching quality was assessed by calculating absolute standardized differences, with <0.1 indicating good balance. Cox proportional hazards regression modeled time-to-event outcomes in the matched sample, generating hazard ratios (HRs) with 95% confidence intervals (CIs). Survival curves compared dyslipidemia incidence by treatment arm. Subgroup analyses evaluated dyslipidemia risks by sex, age, race, cholesterol, and BMI level. Sensitivity analyses were also conducted to examine the robustness of the results by modifying the initiation time of follow-up.
3 Results
1) Study Population and Baseline Characteristics
This study included 7,580 propensity score matched RA patients, with 1998 in the tofacitinib cohort and 5,582 in the adalimumab cohort (Table 1). The mean age was 51.7 and 48.7 years in the tofacitinib and adalimumab groups, respectively. Most patients were female in both treatment arms (78.5% tofacitinib vs. 71.1% adalimumab). After matching, the cohorts were well balanced on demographic factors, with absolute standardized differences <0.1.
At baseline, methotrexate and sulfasalazine were more commonly co-prescribed in the adalimumab group compared to tofacitinib (39.0% vs. 32.3% and 8.5% vs. 5.7%, respectively). These differences were small in magnitude after propensity score matching.
2) Dyslipidemia Risk
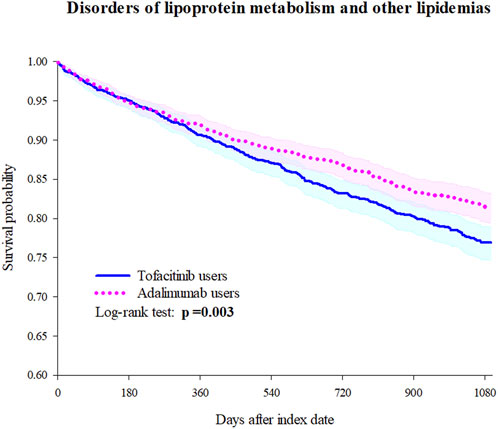
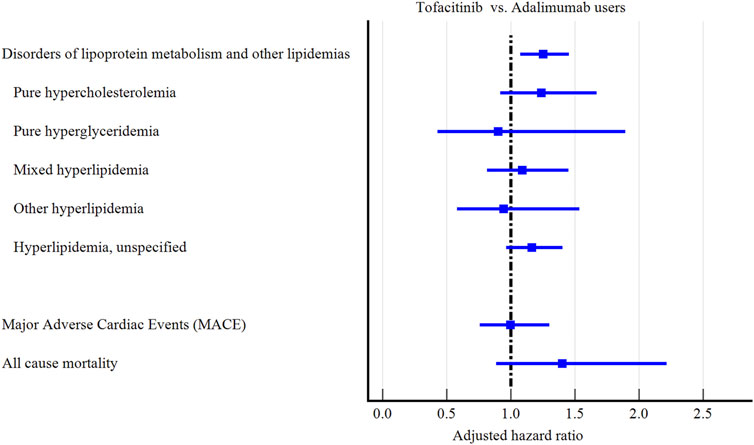
Tofacitinib use was associated with a higher 3-year risk of dyslipidemia versus adalimumab (Figure 2; Figure 3; Table 2). The adjusted hazard ratio (HR) for overall dyslipidemia disorders was 1.250 (95% CI 1.076–1.453) in tofacitinib users compared to adalimumab. No significant differences occurred between matched cohorts for major adverse cardiac events (MACE) (HR 0.995 [0.760–1.303]) or mortality (HR 1.402 [0.887–2.215]). After adjusting for various follow-up periods, the dyslipidemia risk associated with tofacitinib use increased over time, while MACE and mortality risks remained comparable to adalimumab (Supplementary Tables S1, S2).
In subgroup analyses, both men and women and young patients (18–40 years old) treated with tofacitinib faced the highest dyslipidemia risks compared to those taking adalimumab (HR:1.404, 1.212, and 2.504, respectively, Supplementary Tables S3, S4). Dyslipidemia risk was higher in tofacitinib users compared to adalimumab user among White (HR:1.204 [1.009–1.436] and Black patients (1.218 [0.751–1.976] (Supplementary Table S5). Unexpectedly, elevated baseline cholesterol or BMI status did not clearly amplify the lipid abnormalities associated with tofacitinib (Supplementary Tables S6, S7).
After modified the initiation time of follow-up (start from 2 months, 12 months, 24 months after the index date and followed for 3 years), the dyslipidemia risk consistently associated with tofacitinib, while MACE and mortality risks remained similar to adalimumab (Supplementary Table S8).
4 Discussion
This large real-world study of over 7,500 well-matched RA patients aligns with prior evidence that tofacitinib therapy appears to increase the risk of dyslipidemia more than adalimumab (Salgado et al., 2014; Souto et al., 2015; Charles-Schoeman et al., 2016a; Charles-Schoeman et al., 2016b). Proposed mechanisms relate to tofacitinib decreasing inflammation-induced lipid clearance and cholesterol ester catabolism, which are otherwise accelerated in active RA (Charles-Schoeman et al., 2015; Pérez-Baos et al., 2017). Reassuringly, the higher cholesterol levels associated with tofacitinib did not clearly translate to increased MACE compared to adalimumab over 3-year follow-up (Kume et al., 2017).
The findings that women and young adults may warrant closer monitoring for tofacitinib-associated dyslipidemia could inform more tailored RA treatment approaches. No differences in lipid response to tofacitinib versus adalimumab were observed by race, contrasting with some cardiovascular studies showing higher event risks in minorities (Khosrow-Khavar et al., 2022; Kristensen et al., 2023a; Kristensen et al., 2023b; Schreiber et al., 2023).
This study was limited by potential misclassification bias, lack of treatment dose-response data, and inability to make conclusions about long-term MACE risks. Additionally, we were unable to surmount certain limitations of the study design, such as the challenge of detecting rare adverse events in small population groups or those with a delayed onset. Furthermore, we employed ICD10 codes to define the disease diagnoses and utilized ATC codes or RxNorm codes on at least two occasions to delineate the prescription of adalimumab or tofacitinib. Within the TriNetX system, we were unable to ascertain whether the diagnosis was rendered by any medical practitioners or specifically by rheumatologists. According to Kim et al. (2011), the Positive Predictive Values (PPVs) were 55.7% for at least two claims coded for RA, 65.5% for at least three claims for RA, and 66.7% for at least two rheumatology claims for RA. The PPVs of these algorithms in patients with at least one DMARD prescription rose to 86.2%–88.9% (Kim et al., 2011). In other words, the accuracy could be substantially enhanced with the incorporation of the drug code. Moreover, due to the constraints of the database platform, we were unable to illustrate the evolution in the utilization of OCS OVER TIME and could only offer data on whether or not OCS was employed in the year preceding the index date for two groups of cases.
These results add to the evidence base around the dyslipidemic effects of RA medications. While tofacitinib seems to have worse lipid profiles than adalimumab, especially in women and young patients, the real-world cardiovascular significance is uncertain. Further research should investigate whether dyslipidemia monitoring and preferential use of adalimumab over tofacitinib in certain higher-risk demographics can improve long-term cardiovascular outcomes. Cost-benefit analysis may also inform RA treatment decisions considering dyslipidemia risks. In conclusion, this study provides clinically useful real-world data to guide management of dyslipidemia as an important potential adverse effect of tofacitinib and adalimumab.
5 Conclusion
In this large real-world cohort of patients newly diagnosed with RA, tofacitinib use might be associated with a greater risk of dyslipidemia compared to adalimumab over 3 years, both in men and women and young adults. These observational findings can inform dyslipidemia monitoring and selective use of tofacitinib versus adalimumab to potentially mitigate lipid abnormalities in certain higher-risk RA populations. Further research should investigate the long-term cardiovascular safety of tofacitinib given its lipid effects.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
X-NM: Data curation, Formal Analysis, Validation, Writing–original draft. WF: Data curation, Formal Analysis, Validation, Writing–original draft. S-IW: Data curation, Formal Analysis, Validation, Writing–original draft. M-FS: Data curation, Investigation, Software, Writing–original draft, Writing–review and editing. X-QZ: Data curation, Investigation, Software, Writing–original draft. Q-PL: Data curation, Investigation, Software, Writing–review and editing. James C-CW: Conceptualization, Formal Analysis, Methodology, Project administration, Resources, Supervision, Writing–review and editing. C-SL: Conceptualization, Formal Analysis, Methodology, Project administration, Resources, Supervision, Writing–review and editing. QX: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this study was provided by Natural Science Foundation of Department of Education of Guangdong Province Grant 2022KTSCX030. Funding sources did not contribute to the study design, statistical analysis, interpretation, or manuscript preparation.
Acknowledgments
We gratefully acknowledge the English language editing of this work by Editage (www.editage.cn).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2024.1370661/full#supplementary-material
References
Charles-Schoeman, C., Fleischmann, R., Davignon, J., Schwartz, H., Turner, S. M., Beysen, C., et al. (2015). Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. 67 (3), 616–625. doi:10.1002/art.38974
Charles-Schoeman, C., Gonzalez-Gay, M. A., Kaplan, I., Boy, M., Geier, J., Luo, Z., et al. (2016a). Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin. Arthritis Rheum. 46 (1), 71–80. doi:10.1016/j.semarthrit.2016.03.004
Charles-Schoeman, C., Wicker, P., Gonzalez-Gay, M. A., Boy, M., Zuckerman, A., Soma, K., et al. (2016b). Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin. Arthritis Rheum. 46 (3), 261–271. doi:10.1016/j.semarthrit.2016.05.014
Deakin, C. T., De Stavola, B. L., Littlejohn, G., Griffiths, H., Ciciriello, S., Youssef, P., et al. (2023). Comparative effectiveness of adalimumab vs tofacitinib in patients with rheumatoid arthritis in Australia. JAMA Netw. Open 6 (6), e2320851. doi:10.1001/jamanetworkopen.2023.20851
Eqbal, A., Hilley, P., Choy, M., Srinivasan, A., and de Cruz, P. (2023). Outcomes out to 12 months after sequential use of high-dose tofacitinib following infliximab in acute severe ulcerative colitis. Intern Med. J. 53 (8), 1497–1500. doi:10.1111/imj.16192
Fleischmann, R., Mysler, E., Hall, S., Kivitz, A. J., Moots, R. J., Luo, Z., et al. (2017). Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 390 (10093), 457–468. doi:10.1016/S0140-6736(17)31618-5
Huang, J. Y., Leong, P. Y., Ker, A., Chen, H. H., and Wei, J. C. (2022). The long-term persistence of tumor necrosis factor inhibitors in patients with moderate to severe immune-mediated rheumatic diseases: a nation-wide, population-based real-world study. Int. J. Rheum. Dis. 25 (11), 1295–1305. doi:10.1111/1756-185X.14423
Khosrow-Khavar, F., Kim, S. C., Lee, H., Lee, S. B., and Desai, R. J. (2022). Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann. Rheum. Dis. 81 (6), 798–804. doi:10.1136/annrheumdis-2021-221915
Kim, S. Y., Servi, A., Polinski, J. M., Mogun, H., Weinblatt, M. E., Katz, J. N., et al. (2011). Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res. Ther. 13 (1), R32. doi:10.1186/ar3260
Kristensen, L. E., Danese, S., Yndestad, A., Wang, C., Nagy, E., Modesto, I., et al. (2023a). Identification of two tofacitinib subpopulations with different relative risk versus TNF inhibitors: an analysis of the open label, randomised controlled study ORAL Surveillance. Ann. Rheum. Dis. 82 (7), 901–910. doi:10.1136/ard-2022-223715
Kristensen, L. E., Strober, B., Poddubnyy, D., Leung, Y. Y., Jo, H., Kwok, K., et al. (2023b). Association between baseline cardiovascular risk and incidence rates of major adverse cardiovascular events and malignancies in patients with psoriatic arthritis and psoriasis receiving tofacitinib. Ther. Adv. Musculoskelet. Dis. 15, 1759720x221149965. doi:10.1177/1759720X221149965
Kume, K., Amano, K., Yamada, S., Kanazawa, T., Ohta, H., Hatta, K., et al. (2017). Tofacitinib improves atherosclerosis despite up-regulating serum cholesterol in patients with active rheumatoid arthritis: a cohort study. Rheumatol. Int. 37 (12), 2079–2085. doi:10.1007/s00296-017-3844-9
Kuo, C. M., Tung, T. H., Wang, S. H., and Chi, C. C. (2018). Efficacy and safety of tofacitinib for moderate-to-severe plaque psoriasis: a systematic review and meta-analysis of randomized controlled trials. J. Eur. Acad. Dermatol Venereol. 32 (3), 355–362. doi:10.1111/jdv.14695
Li, M., Liao, P. H., Xiang-Zhang, G., Xin-Xie, Y., and James, W. C. (2023). Tofacitinib for Sjögren syndrome with renal tubular acidosis and psoriasis. Int. J. Rheum. Dis. 27, e14872. doi:10.1111/1756-185X.14872
Panaccione, R., Isaacs, J. D., Chen, L. A., Wang, W., Marren, A., Kwok, K., et al. (2021). Correction to: characterization of creatine kinase levels in tofacitinib-treated patients with ulcerative colitis: results from clinical trials. Dig. Dis. Sci. 66 (9), 3214–3215. doi:10.1007/s10620-020-06638-z
Pérez-Baos, S., Barrasa, J. I., Gratal, P., Larrañaga-Vera, A., Prieto-Potin, I., Herrero-Beaumont, G., et al. (2017). Tofacitinib restores the inhibition of reverse cholesterol transport induced by inflammation: understanding the lipid paradox associated with rheumatoid arthritis. Br. J. Pharmacol. 174 (18), 3018–3031. doi:10.1111/bph.13932
Raiker, R., DeYoung, C., Pakhchanian, H., Ahmed, S., Kavadichanda, C., Gupta, L., et al. (2021). Outcomes of COVID-19 in patients with rheumatoid arthritis: a multicenter research network study in the United States. Semin. Arthritis Rheum. 51 (5), 1057–1066. doi:10.1016/j.semarthrit.2021.08.010
Salgado, E., Maneiro, J. R., Carmona, L., and Gomez-Reino, J. J. (2014). Safety profile of protein kinase inhibitors in rheumatoid arthritis: systematic review and meta-analysis. Ann. Rheum. Dis. 73 (5), 871–882. doi:10.1136/annrheumdis-2012-203116
Sands, B. E., Colombel, J. F., Ha, C., Farnier, M., Armuzzi, A., Quirk, D., et al. (2021). Lipid profiles in patients with ulcerative colitis receiving tofacitinib-implications for cardiovascular risk and patient management. Inflamm. Bowel Dis. 27 (6), 797–808. doi:10.1093/ibd/izaa227
Schreiber, S., Rubin, D. T., Ng, S. C., Peyrin-Biroulet, L., Danese, S., Modesto, I., et al. (2023). Major adverse cardiovascular events by baseline cardiovascular risk in patients with ulcerative colitis treated with tofacitinib: data from the OCTAVE clinical programme. J. Crohns Colitis 17, 1761–1770. doi:10.1093/ecco-jcc/jjad104
Souto, A., Salgado, E., Maneiro, J. R., Mera, A., Carmona, L., and Gómez-Reino, J. J. (2015). Lipid profile changes in patients with chronic inflammatory arthritis treated with biologic agents and tofacitinib in randomized clinical trials: a systematic review and meta-analysis. Arthritis Rheumatol. 67 (1), 117–127. doi:10.1002/art.38894
Xu, Q., Huang, Z. S., Liu, Q. P., and Wei, J. C. (2022). Tofacitinib for sarcoidosis, a new potential treatment. Int. J. Rheum. Dis. 25 (11), 1217–1219. doi:10.1111/1756-185X.14441
Yousaf, A., Patterson, J., Hobbs, G., Davis, S. M., Yousaf, M., Hafez, M., et al. (2020). Smoking is associated with adrenal adenomas and adrenocortical carcinomas: a nationwide multicenter analysis. Cancer Treat. Res. Commun. 25, 100206. doi:10.1016/j.ctarc.2020.100206
Zhang, X., Liang, F., Yin, X., Xiao, X., Shi, P., Wei, D., et al. (2014). Tofacitinib for acute rheumatoid arthritis patients who have had an inadequate response to disease-modifying antirheumatic drug (DMARD): a systematic review and meta-analysis. Clin. Rheumatol. 33 (2), 165–173. doi:10.1007/s10067-013-2452-7
Zhu, K. J., Yang, P. D., and Xu, Q. (2021). Tofacitinib treatment of refractory cutaneous leukocytoclastic vasculitis: a case report. Front. Immunol. 12, 695768. doi:10.3389/fimmu.2021.695768
Zhu, K. Y., Karimi, A. H., Lavu, M., Burkhart, R. J., and Kamath, A. F. (2023). Impact of external beam radiation on total shoulder arthroplasty outcomes: a propensity-matched cohort study. Arch. Orthop. Trauma Surg. 144, 113–119. doi:10.1007/s00402-023-05048-w
Zouboulis, C. C., Hou, X., von Waldthausen, H., Zouboulis, K. C., and Hossini, A. M. (2023). HS 3D-SeboSkin model enables the preclinical exploration of therapeutic candidates for hidradenitis suppurativa/acne inversa. Pharmaceutics 15 (2), 619. doi:10.3390/pharmaceutics15020619
Zubkov, M. R., Waldman, R. A., Wu, R., and Grant-Kels, J. M. (2021). Duration of adalimumab therapy in hidradenitis suppurativa with and without oral immunosuppressants. Cutis 108 (4), 199–200. doi:10.12788/cutis.0366
Zullow, S., Flasar, M. H., Greenberg, D., Tracy, J. K., Rustgi, A., and Cross, R. K. (2014). A health survey of gastroenterologist prescribing practices of adalimumab for treatment of crohn’s disease: final results. Gastroenterol. Hepatol. (N Y) 10 (8), 503–509.
Zuzda, K., Grycuk, W., Małyszko, J., and Małyszko, J. (2022). Kidney and lipids: novel potential therapeutic targets for dyslipidemia in kidney disease? Expert Opin. Ther. Targets 26 (11), 995–1009. doi:10.1080/14728222.2022.2161887
Zweifler, R. M., McClure, L. A., Howard, V. J., Cushman, M., Hovater, M. K., Safford, M. M., et al. (2011). Racial and geographic differences in prevalence, awareness, treatment and control of dyslipidemia: the reasons for geographic and racial differences in stroke (REGARDS) study. Neuroepidemiology 37 (1), 39–44. doi:10.1159/000328258
Keywords: tofacitinib, adalimumab, lipidemias, risk factors, TriNetX
Citation: Ma X-N, Shi M-F, Wang S-I, Feng W, Chen S-L, Zhong X-Q, Liu Q-P, Cheng-Chung Wei J, Lin C-S and Xu Q (2024) Risk of dyslipidemia and major adverse cardiac events with tofacitinib versus adalimumab in rheumatoid arthritis: a real-world cohort study from 7580 patients. Front. Pharmacol. 15:1370661. doi: 10.3389/fphar.2024.1370661
Received: 15 January 2024; Accepted: 13 May 2024;
Published: 31 May 2024.
Edited by:
Anick Bérard, Montreal University, CanadaReviewed by:
Marc Henri De Longueville, UCB Pharma, BelgiumEugenia Piragine, University of Pisa, Italy
Copyright © 2024 Ma, Shi, Wang, Feng, Chen, Zhong, Liu, Cheng-Chung Wei, Lin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: James Cheng-Chung Wei, jccwei@gmail.com; Chang-Song Lin, linchs999@163.com; Qiang Xu, fjksg@163.com
†These authors have contributed equally to this work and share first authorship
 Xiao-Na Ma
Xiao-Na Ma Mei-Feng Shi
Mei-Feng Shi Shiow-Ing Wang
Shiow-Ing Wang Wei Feng
Wei Feng Shu-Lin Chen
Shu-Lin Chen Xiao-Qin Zhong
Xiao-Qin Zhong Qing-Ping Liu
Qing-Ping Liu James Cheng-Chung Wei
James Cheng-Chung Wei Chang-Song Lin
Chang-Song Lin Qiang Xu
Qiang Xu