- 1Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia
- 2Prodia Clinical Laboratory, Jakarta, Indonesia
- 3Faculty of Pharmacy, University of 17 August 1945 Jakarta, Jakarta, Indonesia
- 4Prodia Diacro Laboratory, Jakarta, Indonesia
- 5Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia
- 6Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, West Java, Indonesia
- 7Center of Excellence for Pharmaceutical Care Innovation, Universitas Padjadjaran, Sumedang, West Java, Indonesia
Introduction: In recent years, diverse initiatives have been carried out to control the COVID-19 pandemic, ranging from measures restricting social activities to analyzing drugs and vaccines. Studies on herbal medicines are also increasingly conducted in various countries as an adjuvant therapy or supplement. Therefore, this systematic review aimed to investigate the efficacy of herbal medicines analyzed from various countries through clinical trials with the randomized controlled trial method. The outcomes of Length of Stay (LOS), Negative Conversion Time (NCT), and Negative Conversion Rate (NCR) were the main focus.
Methods: An extensive review of literature spanning from 2019 to 2023 was carried out using well-known databases including PubMed, Scopus, and Cochrane. The search included relevant keywords such as “randomized controlled trial,” “COVID-19,” and “herbal medicine.”
Results: A total of 8 articles were part of the inclusion criteria with outcomes of LOS, NCT, and NCR. In terms of LOS outcomes, all types of herbal medicines showed significant results, such as Persian Medicine Herbal (PM Herbal), Persian Barley Water (PBW), Jingyin Granules (JY granules), Reduning Injection, and Phyllanthus emblica (Amla). However, only JY granules showed significant results in NCR outcome, while JY granules and Reduning Injection showed significant results in reducing NCT.
Conclusion: These findings enrich our understanding of the potential benefits of herbal medicines in influencing LOS, NCR and NCT parameters in COVID-19 patients. Herbal medicines worked to treat COVID-19 through antiviral, anti-inflammatory, and immunomodulatory mechanisms.
1 Introduction
The COVID-19 pandemic, caused by the SARS-CoV-2 virus is a tremendous global challenge in the field of health (Filip et al., 2022). Over the past few years, various efforts have been made to reduce the pandemic, from policies related to social activity restrictions to studies on drugs and vaccines (Lazarus et al., 2022). Even though vaccination has been an important milestone in controlling the spread of the virus, the development of effective treatments is equally important to address the clinical impact (Davis et al., 2020; Machado et al., 2022). Various repurposing drugs have been studied regarding the efficacy and safety against COVID-19 (Babaei et al., 2021; Latarissa et al., 2023). Research on drugs for COVID-19 has made significant progress. However, there are still some challenges. For instance, Ritonavir has several drug interactions that require special evaluation before use (Igho-Osagie et al., 2023). In addition, the high financial cost of purchasing vaccines (Light and Lexchin, 2021). Therefore, there is a need to find more affordable alternatives that have a lorcwer risk of drug interactions. This calls for further research into the use of herbal medicine as a possible effective and economical alternative to deal with this pandemic (Liu et al., 2022).
Herbal medicines, with their long history of use in traditional medicine, offer the potential to contribute to symptom relief, accelerate recovery, and strengthen the immune system. While no herbal preparation has yet been consistently shown to be effective in treating COVID-19, recent studies have shown some promising candidates as potential adjunctive or alternative therapies (Al-kuraishy et al., 2022; Komariah et al., 2023; Kumar et al., 2023; Onyeaghala et al., 2023). This medicine has a long history across different cultures, and some plants have been recognized to possess antiviral, anti-inflammatory, and immunomodulatory properties relevant in the context of viral infections (Nugraha et al., 2020). Herbal medicines that have anti-inflammatory potential can help reduce inflammation in the respiratory tract and other organs due to coronavirus infection, through decreasing inflammatory indicators such as interleukin (IL)-6, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) (Zeng et al., 2020). Meanwhile, immunomodulatory properties found in herbal medicines can enhance the immune system’s response to infection, thus helping the body fight the virus (Das, 2022). In terms of antivirals, some herbal medicines show potential antiviral activity against various types of viruses, including the coronavirus (Jamal, 2022; Benzie and Wachtel-Galor, 2011) (19).
Evaluation of clinical trials of herbal medicines includes aspects of safety and efficacy, as well as specific parameters related to the characteristics of the disease. Some of the aspects evaluated are the ability of herbal medicines to relieve symptoms, reduce the severity of the disease, and accelerate patient recovery. In addition, this study also needs to pay attention to the potential of reducing the level of viral replication, inhibiting inflammation, and enhancing the patient’s immune response. An important parameter is the length of stay (LOS), which reflects the efficacy of the treatment in reducing hospital burden and accelerating patient recovery. Reduced length of hospitalization can be a positive indicator that herbal medicines have the potential to resolve symptoms and reduce the severity of the disease (Ang et al., 2022). In addition, Negative Conversion Rate (NCR) and Negative Conversion Time (NCT), including the length of time required for patients to achieve negative laboratory test results are also critical parameters in evaluating the effectiveness of herbal medicines. The faster the negative conversion occurs, the better the herbal medicines are considered to overcome the viral infection and accelerate the healing process (Jeon et al., 2022).
This systematic review aims to investigate the effects of herbal medicines on decreasing patient LOS, NCR, and NCT in the context of COVID-19. Evaluation of these parameters provides a more comprehensive understanding of the efficacy and potential of herbal medicines as part of the treatment strategy. The results of this systematic review have great scientific importance and significant relevance in the context of pandemic management. First, in terms of LOS outcomes, it can provide a deeper understanding of the effectiveness of herbal medicines in reducing patient LOS to optimize the use of limited health resources and accelerate patient recovery. Second, in terms of NCR outcomes, this review can provide a comprehensive analysis of how many patients are cured by using a particular herbal medicines to guide health practitioners in choosing the most effective therapy. Third, in terms of NCT outcomes, this review can provide insight into the potential of herbal medicines in accelerating the negative conversion time of patients infected with COVID-19. Meanwhile, the novelty of this review is that it focuses on specific outcomes so that readers can infer results based on these outcomes. Thus, this systematic review will be a very important tool for healthcare practitioners, researchers, and policymakers in making informed decisions and leading to a more holistic understanding of the role of herbal medicines in responding to the COVID-19 pandemic.
2 Methods
2.1 Article selection
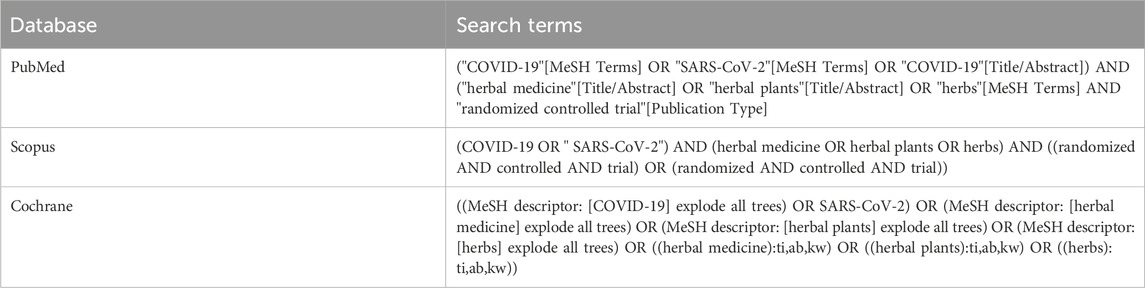
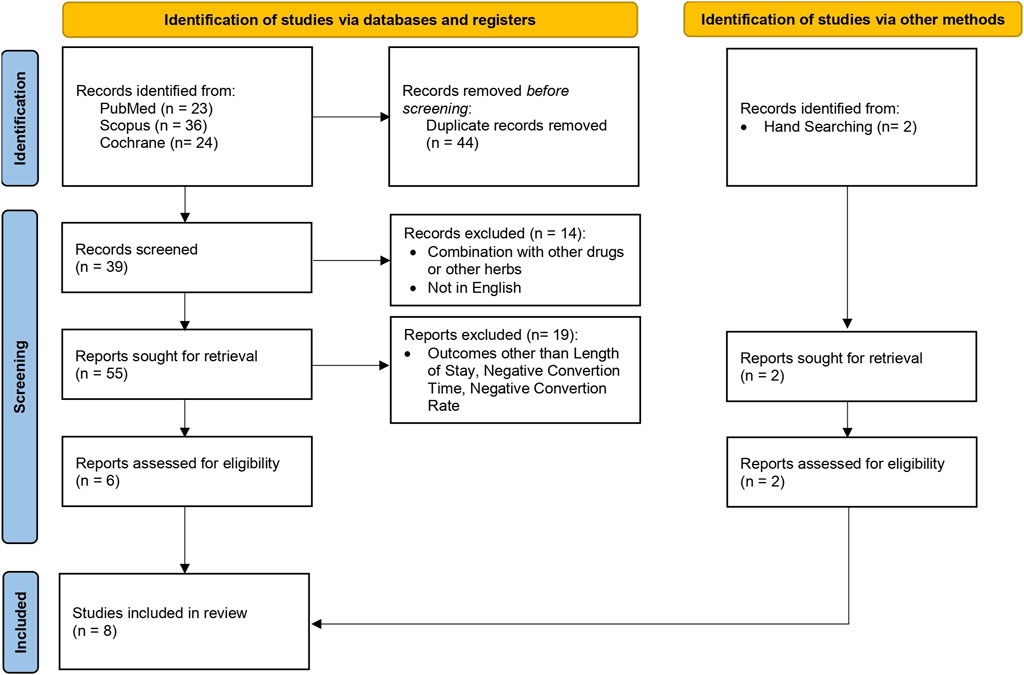
This systematic review included full original articles obtained through PubMed, Scopus, and Cochrane. During this search, original studies meeting the defined criteria were identified. Boolean operators such as “OR” and “AND” were used to expand the exploration within each concept and refine the results, respectively. Medical Subject Headings (MeSH) terms were also applied and the detailed search terms are outlined in Table 1. Furthermore, the original study published from 2019 to 2023 was used regarding clinical trials of herbal medicines with a randomized controlled method in English and the study subject was human. There were also study articles that did not clearly state the intervention, such as book chapters, article abstracts only, conference reports, reviews, posters, and discussion results. The articles identified for inclusion were screened and the findings were discussed to reach consensus. The procedure for selecting study articles is shown in Figure 1.
2.2 Subject characteristics
Subjects involved in the studies that were included in this systematic review were patients with positive reverse transcription polymerase chain reaction (RT-PCR) test for COVID-19, who had mild or moderate symptoms. Inclusion criteria included patients >18 years of age, and there were no gender restrictions. Patients with severe symptoms were excluded from this study.
2.3 Outcomes
The studies included in this analysis evaluated the efficacy of herbal medicines based on specific outcomes such as LOS, NCR and NCT. The LOS for inpatients with COVID-19 refers to the duration of time that individuals diagnosed with COVID-19 spend in hospital care, from admission to discharge (Wen et al., 2022). NCT for inpatients with COVID-19 refers to the duration it takes for a patient to transition from testing positive for the virus to testing negative (Bob-Manuel et al., 2021). Meanwhile, NCR refers to the proportion of patients who transition from testing positive for the virus to testing negative within a certain timeframe (Hu et al., 2020).
2.4 Data extraction and risk of bias
IRL and NW screened all relevant articles by evaluating their titles and abstracts. The full articles deemed suitable were reviewed and approved by IRL. Data extraction was conducted utilizing a review matrix created with Microsoft Excel and included author names, the active compound, country, sample size, patient characteristics, design study and outcomes (LOS, NCT and NCR). The risk of bias was conducted utilizing the Jadad score, which focused on three main guidelines for evaluating articles: randomization, double blinding, and the description of withdrawals and dropouts. Five questions need to be answered within a 10-min timeframe: Was the study described as randomized (using terms like random, randomly, or randomization)? Was the method for generating the randomization sequence adequately described and suitable? Was the study identified as double-blind? Was the method of double blinding adequately described and appropriate? Was there a description provided for withdrawals and dropouts?
The Jadad score was chosen for the systematic review because of its advantage in providing a simple yet systematic assessment of the methodological quality of clinical studies. The tool focuses on three main elements: randomization, double blinding, and missing data reports, which are considered important indicators in evaluating the quality of a study. With a clear and standardized approach, the Jadad score allows researchers to directly compare the methodological quality of different studies, making it possible to make a more objective assessment of the evidence. In addition, the simplicity of the tool also facilitates its implementation in systematic analyses involving a large number of studies. (Jadad et al., 1996; Olivo et al., 2008).
3 Results
3.1 Outcomes on length of stay
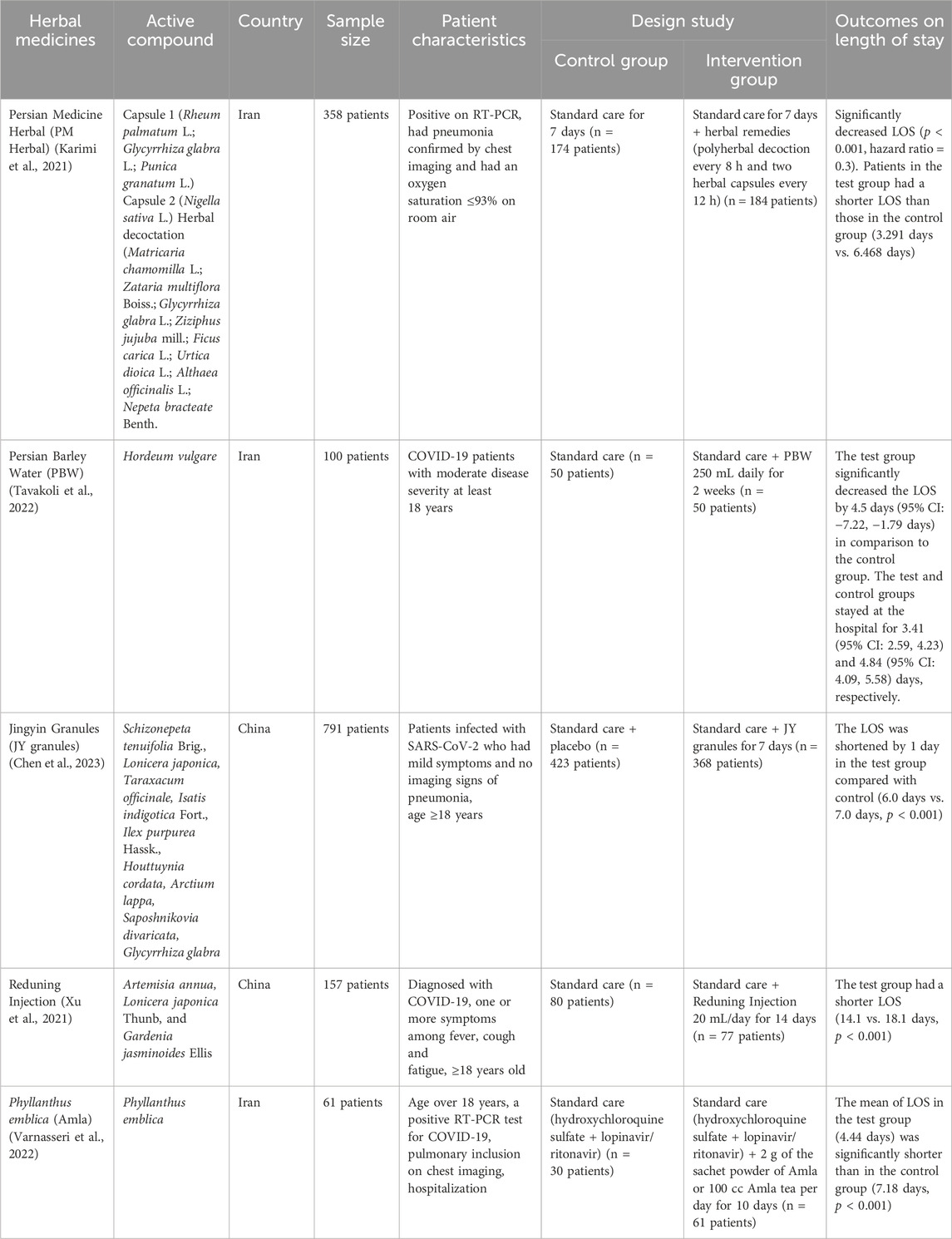
The 5 herbs in the inclusion criteria gave positive results where the LOS in the test group was better than the control and showed significant differences (Karimi et al., 2021; Xu et al., 2021; Tavakoli et al., 2022; Varnasseri et al., 2022; Chen et al., 2023). Meanwhile, the standard care used for each herbal medicine was adjusted to the value of each country. The duration of administration ranged from 7 days for Persian Medicine Herbal (PM Herbal) and Jingyin Granules (JY granules), 10 days for Phyllanthus emblica (Amla), and 14 days for Persian Barley Water (PBW) and Reduning Injection. A summary of the results for the efficacy of herbal medicines on LOS outcomes is provided in Table 2 and Figure 2.
3.2 Outcomes on NCT/NCR
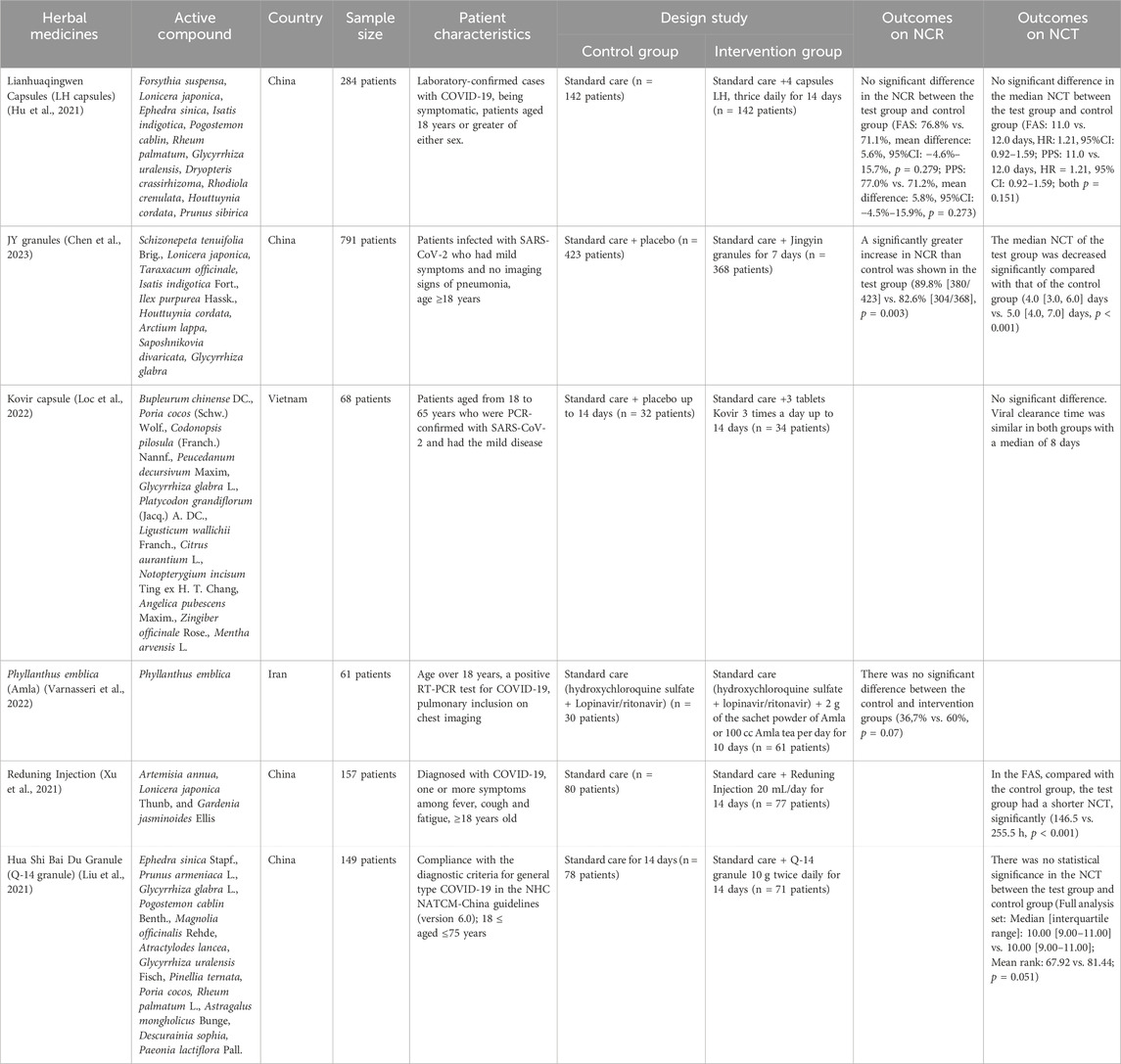
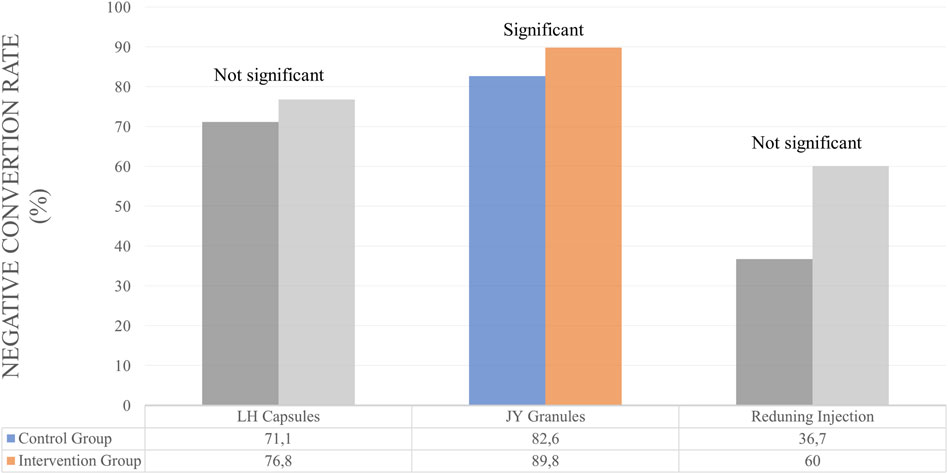
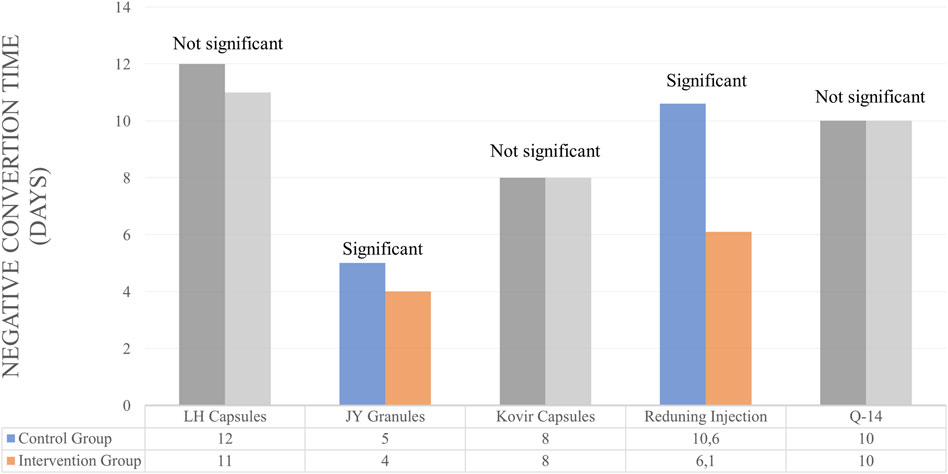
Several studies use NCR and NCT outcomes to determine the efficacy of herbal medicines. However, some only use one of the NCR or NCT outcomes. For Lianhuaqingwen Capsules (LH capsules), JY granules and Kovir capsule used NCR and NCT outcomes simultaneously. For LH capsules, the results showed no significant differences in the control and test groups for NCR and NCT parameters (Hu et al., 2021). Similarly, in the Kovir capsule, where the results showed no significant difference in the NCR or NCT parameters (Loc et al., 2022). JY granules showed good results, significantly increasing NCR and also decreasing the average of NCT (Chen et al., 2023).
Studies that only use NCR parameters to determine the efficacy of therapy is Amla. There was no significant difference between the control and test groups (Varnasseri et al., 2022). Herbs that use NCT as a parameter of therapeutic outcome are Reduning Injection and Hua Shi Bai Du Granule (Q-14 granule). Reduning Injection shows good therapeutic results where the herb can significantly reduce NCT (Xu et al., 2021). Meanwhile, the herbal Q-14 granule did not significantly differ in NCT parameters (Liu et al., 2021). The duration of herbal administration for these patients is 7, 10, and 14 days. A summary of the results for the efficacy of herbal medicines on NCT and NCR outcomes is provided in Table 3 and Figures 3, 4.
3.3 Quality assessment of the included studies
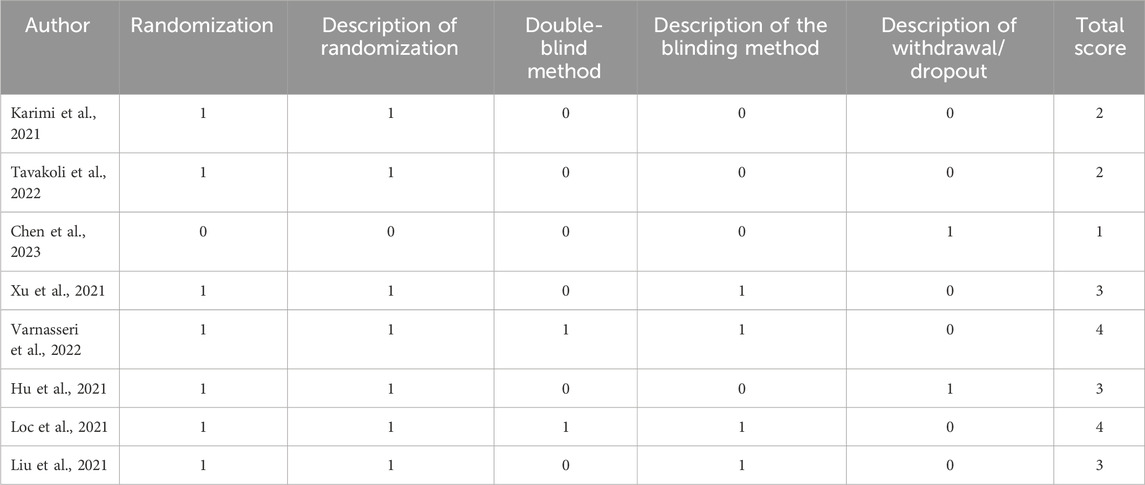
Quality assessment of the included studies is a critical step in evaluating the reliability and validity of research results. One of the commonly used methods to conduct such an assessment is by using the Jadad score. The Jadad score is a tool used to assess the methodological quality of clinical studies. The total value of the Jadad score ranges from 0 to 5, where higher values indicate higher methodological quality (Jadad et al., 1996). There were no complete scores in the reviewed studies. Two studies had the highest score of 4 (Loc et al., 2022; Varnasseri et al., 2022). The studies fully described the randomization and blinding methods used but did not explain the description of withdrawal/dropout. Meanwhile, a study by Chen et al., 2019 had the lowest Jadad score of 1, because it did not use randomization and blinding methods but a prospective cohort study (Chen et al., 2023). However, we still included this study in the review because it fell within the inclusion criteria. Two other studies did not use blinding methods and did not explain the description of withdrawal/dropout so they had a Jadad score of 2 (Karimi et al., 2021; Tavakoli et al., 2022). Three studies used a single-blind method and did not explain the description of withdrawal/dropout and therefore had a Jadad score of 3 (Hu et al., 2021; Liu et al., 2021; Xu et al., 2021). Table 4 provides an overview of the quality assessment by Jadad score.
4 Discussion
4.1 Mechanism of herbal medicines for COVID-19
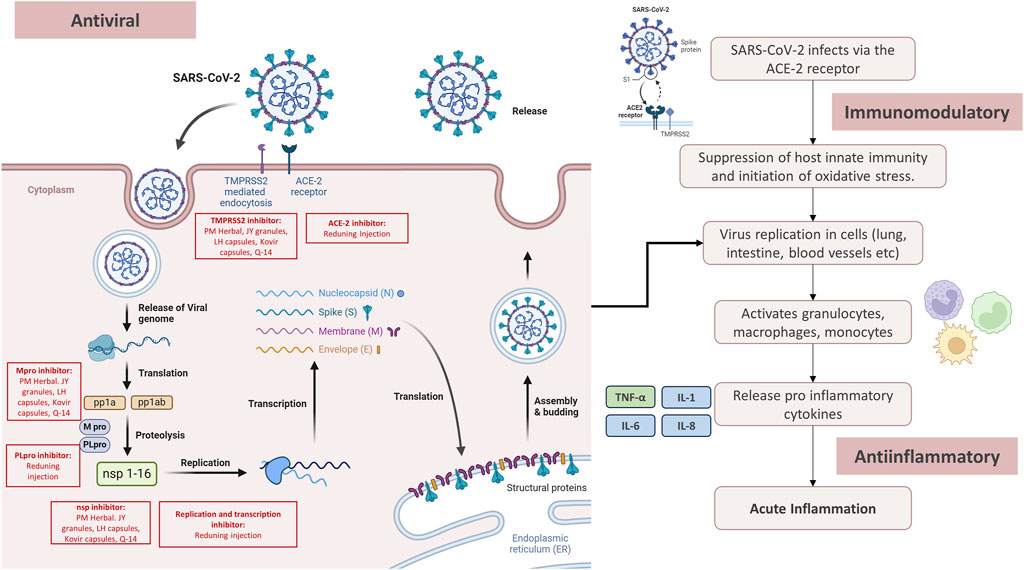
The pandemic has triggered intensive studies to obtain effective therapies for the disease. In addition, herbal medicines have also become the focus of attention as an alternative treatment (Demeke et al., 2021). Various medicinal plants have been investigated for their potential antiviral, anti-inflammatory, and immunomodulatory effects assisting the treatment of COVID-19 (Latarissa et al., 2021; Lim et al., 2021). The mechanism of herbal medicines for COVID-19 related to antiviral, anti-inflammatory and immunomodulatory is shown in Figure 5.
4.1.1 Antiviral
Several herbal medicines have shown antiviral capabilities that can reduce the replication of the SARS-CoV-2. The major SARS-CoV-2 protease (Mpro), also known as 3CL protease, plays a key role in processing viral polyproteins generated from SARS-CoV-2 Ribonucleic acid (RNA) translation. This stage has important significance in the virus replication cycle (Zhang and Wang, 2002). Glycyrrhizin, as one of the components in PM Herbal, JY granules, LH capsules, Kovir capsule, and Q-14 granule can reduce the replication of SARS-CoV-2 by inhibiting the main protease Mpro (van de Sand et al., 2021). In addition to inhibiting Mpro, glycyrrhizin can also reduce the human transmembrane serine protease (TMPRSS2) responsible for cleaving the SARS-CoV-2 spike protein and facilitating viral penetration into host cells (Hoffmann et al., 2020; van de Sand et al., 2021). The triterpenoid saponin A3 and glycyrrhizic acid found in Glycyrrhiza glabra show effectiveness in inhibiting SARS-CoV-2. This is achieved by targeting nsp7 and the receptor-binding domain of the spike protein (Yi et al., 2021). Antiviral activity is also shown by Artemisia annua which is one of the ingredients of Reduning Injection. Artemisia annua can inhibit SARS-CoV-2 attachment, membrane fusion and internalization into host cells, and reduce viral replication and transcription processes (Amini et al., 2022).
Molecular docking analyses showed that the components present in Reduning Injection possess the capability to spontaneously bind to papain-like protease (PLpro), Mpro, and angiotensin-converting enzyme-2 (ACE-2). This interaction serves to reduce SARS-CoV-2 from entering the body and replicating (Jia et al., 2021). Antiviral activity is also shown by compounds contained in Amla such as chlorogenic acid, quercitrin, and myricetin. The three compounds can inhibit the main viral protein n-CoV-2 after an in silico study (Chikhale et al., 2021).
4.1.2 Anti-inflammatory
COVID-19 is a multifaceted systemic illness, and individuals with moderate and severe cases often experience increased inflammation (Baranovskii et al., 2021; Casale et al., 2023). Cytochrome P450 1A (CYP1A) and Cytochrome P450 3A (CYP3A) are important in the generation of certain essential inflammatory factors, including the oxidative metabolites of arachidonic acid. Strong inhibition of CYP1A and CYP3A holds promise for eliciting anti-inflammatory effects (Tallima and El Ridi, 2018). The three plant extracts present in JY granules show anti-inflammatory properties by deactivating CYP3A. Specifically, Schizonepeta tenuifolia Brig. reversibly inhibits CYP3A-catalyzed testosterone 6β-hydroxylation, while the inhibition of Glycyrrhiza glabra and folium Ilex purpurea Hassk. depends on time, dose, and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) (Li et al., 2017; Zhang et al., 2022).
The potent docking capability of quercetin shows that the compound may effectively lower Interleukin-6 (IL-6) levels during inflammatory episodes. This suggests a potential therapeutic approach for addressing COVID-19 (Luo et al., 2022). In addition, β-glycyrrhizic acid acts as a potent anti-inflammatory compound. This regulates inflammation by inhibiting the accumulation of glucocorticoids and the production of reactive oxygen species (ROS) by neutrophils, which are significant mediators of tissue inflammation (Mittal et al., 2014).
Recent studies have shown that β-glucan in PBW can decrease systemic inflammation. This is achieved by reducing the production of leukocyte superoxide and tumor necrosis factor alpha (TNFα), as well as dampening the stimulation of interferon gene expression (Arcidiacono et al., 2019). Shanshan et al., 2019 showed that Reduning Injection had anti-inflammatory activity by inhibiting the overexpression of mitogen-activated protein kinase (MAPK), protein kinase C (PKC), and p65 nuclear factor-κB affecting cytokine levels in COVID-19 patients (Jia et al., 2021).
4.1.3 Immunomodulatory
Compounds with immunomodulatory activity have an important role with a good protective effect against SARS-CoV-2 by preventing the occurrence of cytokine storms (Al-Hajeri et al., 2022). Some of the compounds in herbal medicines have immunomodulatory activity improving the condition of COVID-19 patients. The effects of chamomile heteropolysaccharides found in PM Herbal have immune-stimulating effects on erythrocytes, activation of immune regulatory cells in peripheral blood, and increased sensitivity of helper cells (Gupta et al., 2010). Punica granatum L. also has immunomodulatory effects such as the growth stimulant effect of polysaccharides isolated from pomegranate on lymphocytes (Joseph et al., 2012). In addition, the plant is also effective in overcoming respiratory problems to improve the symptoms of COVID-19 (Aziz et al., 2016). Amla also has an immunomodulatory effect for repeated respiratory infections in humans. The extract acts as an adaptogen and enhances immunity through several mechanisms such as increased activity of interleukin-2 (IL-2), NK (natural killer) cells, Antibody-Dependent Cellular Cytotoxicity (ADCC), and Interferon-gamma (IFN-γ) production and inhibits apoptosis (Sai Ram et al., 2002; Sreeramulu and Raghunath, 2010; Antiretrovir and Mamidala, 2012). The active ingredients in LH capsules such as quercetin, luteolin oxalin, and kaempferol have effects as immunomodulators by targeting MAPK (Wang et al., 2021a). This can decrease the release of inflammatory mediators and lung tissue damage due to inflammation (Xu et al., 2008) [NO_PRINTED_FORM] In addition, LH capsules regulate lung immunity (Ding et al., 2017), considering that infections are related to immunity (Xu et al., 2008). Kovir capsule, an herbal originating from Vietnam has an immunostimulatory effect on SARS-CoV-2 in vitro to improve the immune system (Pham et al., 2023). This is because the Kovir capsule contains bioactive molecules such as flavonoids, alkaloids, flavanol glycosides, and withaferin with various biological activities to improve health in various aspects (Padhy, 2020).
4.2 Outcomes on length of stay
LOS is a parameter often used in clinical trials to determine the efficacy of a drug, and COVID-19 is no exception. Understanding the prognosis can be facilitated by examining the LOS (Alimohamadi et al., 2022). The 5 herbs showed significant differences in reducing LOS in COVID-19 patients. The main mechanisms are antiviral, anti-inflammatory, and immunomodulatory. Other mechanisms to accelerate the healing of patients which are seen from LOS parameters used as an antifever, bronchodilator, and antiasthma features, strengthen the body, and invigorate the lungs (Karimi et al., 2021). These mechanisms support providing improvements in the clinical condition of patients, specifically in cases seen from LOS parameters.
PM Herbal is a system of medicine in the world that dates back 7,000 years with formulations that are beneficial for several viral and bacterial infections and respiratory diseases (Soleymani and Zargaran, 2018; Walsh, 2021). The results of the PM Herbal clinical trial to fight COVID-19 were evidenced by a significant difference in the decrease in LOS for the intervention group and the control group (3,291 days vs. 6,468 days) (Karimi et al., 2021). This is also supported by research showing that the compounds contained in PM Herbal are proven to fight several viruses such as influenza virus, Echovirus type 11, adenovirus, respiratory syncytial virus and coronavirus (Balzarini et al., 1992; Utsunomiya et al., 1997; Liu et al., 2004; Kumaki et al., 2011; Lazreg et al., 2011; Karimi et al., 2020).
PBW is a herb originating from Iran and has long been used as an antitussive, for flu, bronchitis, body pain, fever, and other respiratory symptoms (Kapadia, 2006; De Natale and Pollio, 2007). These effects result from the combination of vitamins, dietary fibers, unsaturated fatty acids, mineral elements, and antioxidants found in PBW (Jo et al., 2020). Its good efficacy against SARS-CoV-2 was also proven by an in vitro study where the ricin-based peptide from PBW was able to inhibit Mpro with the half-maximal inhibitory concentration (IC50) of 0.52 nM (Kashyap et al., 2022). Mpro is a cysteine protease that plays an important role in viral RNA replication and transcription (Liu et al., 2020). This in vitro study is in line with the results of clinical trials that can reduce patient LOS (Tavakoli et al., 2022).
The JY granules comprise various components that exhibit inhibitory effects on SARS-CoV-2. For instance, Radix glycyrrhizae is included to directly suppress SARS-CoV-2 by acting on the IL-6/signal transducers and activators of the transduction-3 (STAT3) pathway (Luo et al., 2022). In addition, Flos lonicera and its effective ingredients can also reduce the main protease activity of the new coronavirus (Gu et al., 2022). JY granules is an antiviral herbal product that has been used for more than 40 years in China to treat various respiratory problems such as fever, sore throat and cough (Wang et al., 2020). JY granules is known to have antiviral activity that has been proven in vitro and in vivo against the influenza virus (Wang et al., 2021b).
The antiviral activity of Reduning Injection, specifically for treating pneumonia, is also a potential treatment for COVID-19. Reduning Injection is used for indications of nausea, colds, fever, cough, yellow sputum, and upper respiratory tract infections (Zhang et al., 2013; Cao et al., 2020; Chen et al., 2020; Xie et al., 2020). Several studies have also proven that Ieduning Injection has effects as antipyretic, antiviral, and anti-inflammatory (Cao et al., 2020; Xie et al., 2020). Besides pneumonia, the activity of Reduning Injection against viruses is also proven by its efficacy against H1N1 A influenza (Chen et al., 2020).
The antiviral effect of Amla has been investigated to treat several diseases caused by viruses such as herpes simplex and those causing disorders in the reproductive and respiratory system (Xiang et al., 2011; Arjin et al., 2020). In addition, Amla also has therapeutic effects such as analgesic, antipyretic, anti-spasmolytic, expectorant, and antitussive. This can reduce the symptoms of patients affected by COVID-19 (Perianayagam et al., 2004; Krishnaveni and Mirunalini, 2010; Goel et al., 2014). In addition, Amla also showed antiviral potential against coxsackie virus B3 (CVB3) at concentrations of 7.8 μg/mL, 11.0 μg/mL, and 21.8 μg/mL. These results indicate that the highly oxygenated bisabolene sesquiterpenoid glycoside phyllaemblicins compounds found in Amla have potential against the Hepatitis B virus (Lv et al., 2015). The compound glochicoccinoside D which was also obtained from Amla has antiviral potential against influenza A virus strain H3N2 and hand, foot, mouth virus (enterovirus) EV71 (Perera et al., 2021).
4.3 Outcomes on negative conversion time (NCT)/Negative conversion rate (NCR)
NCT and NCR in COVID-19 are strongly related to disease progression and clinical outcomes in patients (Yang et al., 2021) There are only 2 herbs that show good therapeutic results in terms of NCT or NCR outcomes, namely, JY granules and Reduning Injection.
Other herbs showed results on NCT or NCR outcomes that were insignificantly different. However, improved results in NCT and NCR parameters are reported within the treatment group as opposed to the control without statistical significance. In certain clinical trials, a broader array of parameters such as symptom recovery, the mean increase in oxygen saturation (SpO2) level, reduction in the percentage of lung inclusion on CT, and improvement in CRP test results were considered. NCT/NCR parameter did not show a significant difference between the control and test groups.
4.4 Strengths and limitations
Herbal medicines have been an important part of traditional medicine practices in the community for centuries. Their use has empirically proven their benefits in overcoming various health problems. However, to increase the trust and validity of using herbal medicines, clinical studies have been conducted to scientifically validate their effectiveness. The summarized results of these clinical trials can be an important reference in developing phytopharmaceuticals, which are medicinal products derived from plant ingredients. Thus, the combination of empirical knowledge and scientific evidence from clinical studies can provide a strong basis for the use of herbal plants as a reliable therapeutic alternative in modern medicine. The strength of this study summarizes all clinical trials on herbal medicines using the randomized controlled trial method with specific outcomes. This review is exclusively a systematic review in nature and lacks statistical analysis, preventing a definitive conclusion regarding which herbs are most effective in terms of LOS, NCT, and NCR outcomes.
In addition, there are several potential biases and study heterogeneity that need to be considered in this systematic review. First, the variability of herbal medicine formulations can be a source of heterogeneity between studies. Various herbal medicines can have different compositions, both in active ingredients and concentrations, which can affect study results (Wang et al., 2023). Second, the method of administration of herbal medicines may also vary between studies, including dosage, frequency of administration, and mode of ingestion (e.g., oral, topical, or inhalation). These differences may affect the rate of absorption, distribution, metabolism, and excretion of herbs in the body, as well as the observed treatment outcomes (Sun et al., 2019). Third, heterogeneity in the patient population, such as differences in age, gender, premenopausal health conditions, history of chronic diseases, and use of other medications, may affect the response to herbal medicines. Studies that include demographically or clinically diverse populations may lead to heterogeneous results that are difficult to synthesize directly. These biases may affect the validity and reliability of the study results and may affect the interpretation and conclusions drawn in the systematic review.
4.5 Future studies
Study on herbal medicines related to COVID-19 is conducted as a supplementary therapy and the cases in several countries are currently increasing. The study provides an overview of beneficial herbal medicines with good effects on patients. In the future, a comprehensive systematic review/meta-analysis should be carried out with certain outcomes to compare herbal medicines that provide the best therapeutic results. In addition, the safety aspects of each herbal medicine must be thoroughly analyzed and this consideration becomes crucial in selecting a therapy for COVID-19, specifically in patients with comorbidities.
5 Conclusion
In conclusion, valuable insights were provided in the investigation into the efficacy of herbal medicines in patients with COVID-19. The analysis of relevant literature, spanning from 2019 to 2023 through comprehensive searches in prominent databases, focused on key outcomes such as the LOS, NCT, and NCR. The findings contributed to the understanding of the potential benefits of herbal medicines in influencing critical parameters in patients. Herbal medicines worked to treat the pandemic through antiviral, anti-inflammatory, and immunomodulatory mechanisms. These findings can serve as a reference for practical implications that can be considered by healthcare practitioners or polic makers. Healthcare practitioners need to be aware that the use of herbal medicines as adjunctive therapy for COVID-19 may be an option for some patients, especially in countries where the use of herbs has become an integral part of the healthcare system. Therefore, it is important for practitioners to understand the effects, interactions, and appropriate doses of the herbs used, as well as to closely monitor patient responses. Policymakers also need to pay attention to the need for strict regulations related to the use of herbal medicines as adjunctive therapy for COVID-19. Clear and strict regulations will help ensure that the herbal products used are safe, quality, and effective, and limit the risk of misuse or unwanted side effects. However, further review of clinical studies was warranted to establish a strong evidence base and guide the integration of herbal medicines into comprehensive treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
IRL: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing–original draft, Writing–review and editing. AM: Methodology, Validation, Writing–review and editing. IPS: Writing–review and editing. ES: Writing–review and editing. NW: Writing–review and editing, Formal Analysis. MIB: Methodology, Writing–review and editing, Validation. KL: Conceptualization, Investigation, Methodology, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the Rector of Universitas Padjadjaran for facilitating the APC via the Directorate of Research and Community Engagement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Hajeri, H., Baroun, F., Abutiban, F., Al-Mutairi, M., Ali, Y., Alawadhi, A., et al. (2022). Therapeutic role of immunomodulators during the COVID-19 pandemic-a narrative review. Postgrad. Med. 134, 160–179. doi:10.1080/00325481.2022.2033563
Alimohamadi, Y., Yekta, E. M., Sepandi, M., Sharafoddin, M., Arshadi, M., and Hesari, E. (2022). Hospital length of stay for COVID-19 patients: a systematic review and meta-analysis. Multidiscip. Respir. Med. 17, 856. doi:10.4081/MRM.2022.856
Al-kuraishy, H. M., Al-Fakhrany, O. M., Elekhnawy, E., Al-Gareeb, A. I., Alorabi, M., De Waard, M., et al. (2022). Traditional herbs against COVID-19: back to old weapons to combat the new pandemic. Eur. J. Med. Res. 27, 186–211. doi:10.1186/s40001-022-00818-5
Amini, S. Z., Shakeri, N., Amini, S. M., Mokaberinejad, R., Hasheminasab, F. S., Azimi, M., et al. (2022). Evaluating the effect of a natural formulation based on hordeum vulgare on the recovery of COVID-19 patients: application of survival analysis in a clinical trial. Ann. Mil. Health Sci. Res. 20, 1–5. doi:10.5812/AMH-128009
Ang, L., Song, E., Zhang, J., Lee, H. W., and Lee, M. S. (2022). Herbal medicine for COVID-19: an overview of systematic reviews and meta-analysis. Phytomedicine 102, 154136–154139. doi:10.1016/J.PHYMED.2022.154136
Antiretrovir, J. A., and Mamidala, E. (2012). Human immunodeficiency virus (HIV-1) reverse transcriptase inhibition by extracts of the Phyllanthus emblica. J. Antivir. Antiretrovir, 01. doi:10.4172/1948-5964.S1.003
Arcidiacono, M. V., Carrillo-López, N., Panizo, S., Castro-Grattoni, A. L., Valcheva, P., Ulloa, C., et al. (2019). Barley-ß-glucans reduce systemic inflammation, renal injury and aortic calcification through ADAM17 and neutral-sphingomyelinase2 inhibition. Sci. Rep. 9, 17810–17814. doi:10.1038/S41598-019-54306-8
Arjin, C., Pringproa, K., Hongsibsong, S., Ruksiriwanich, W., Seel-Audom, M., Mekchay, S., et al. (2020). In vitro screening antiviral activity of Thai medicinal plants against porcine reproductive and respiratory syndrome virus. BMC Vet. Res. 16, 102–109. doi:10.1186/S12917-020-02320-8
Aziz, M. A., Adnan, M., Khan, A. H., Rehman, A. U., Jan, R., and Khan, J. (2016). Ethno-medicinal survey of important plants practiced by indigenous community at Ladha subdivision, South Waziristan agency, Pakistan. J. Ethnobiol. Ethnomed 12, 53–14. doi:10.1186/s13002-016-0126-7
Babaei, F., Mirzababaei, M., Nassiri-Asl, M., and Hosseinzadeh, H. (2021). Review of registered clinical trials for the treatment of COVID-19. Drug Dev. Res. 82, 474–493. doi:10.1002/DDR.21762
Balzarini, J., Neyts, J., Schols, D., Hosoya, M., Van Damme, E., Peumans, W., et al. (1992). The mannose-specific plant lectins from Cymbidium hybrid and Epipactis helleborine and the (N-acetylglucosamine)n-specific plant lectin from Urtica dioica are potent and selective inhibitors of human immunodeficiency virus and cytomegalovirus replication in vitro. Antivir. Res. 18, 191–207. doi:10.1016/0166-3542(92)90038-7
Baranovskii, D. S., Klabukov, I. D., Krasilnikova, O. A., Nikogosov, D. A., Polekhina, N. V., Baranovskaia, D. R., et al. (2021). Prolonged prothrombin time as an early prognostic indicator of severe acute respiratory distress syndrome in patients with COVID-19 related pneumonia. Curr. Med. Res. Opin. 37, 21–25. doi:10.1080/03007995.2020.1853510
Benzie, I. F. F., and Wachtel-Galor, S. (2011). Herbal medicine. Biomol. Clin. Aspects, 1–10. doi:10.1201/b10787-2
Bob-Manuel, M., Omunakwe, H., Uzosike, T., Enyinnaya, S., Wondah, F., and Alabi, A. (2021). Time to negative test result among patients with COVID-19: a retrospective study. Int. J. Trop. Dis. Health, 44–48. doi:10.9734/IJTDH/2021/V42I2230558
Cao, C., Zhen, Z., Kuang, S., and Xu, T. (2020). Reduning injection combined with western medicine for pneumonia: a protocol for systematic review and meta-analysis. Medicine 99, 227577–e22765. doi:10.1097/MD.0000000000022757
Casale, M., Dattilo, G., Imbalzano, E., de Fazio, M. G., Morabito, C., Mezzetti, M., et al. (2023). Thromboembolism in COVID-19: the unsolved problem. Panminerva Med. 65, 51–57. doi:10.23736/S0031-0808.20.03999-3
Chen, B., Yu, X., Zhang, L., Huang, W., Lyu, H., Xu, Y., et al. (2023). Clinical efficacy of Jingyin granules, a Chinese patent medicine, in treating patients infected with coronavirus disease 2019. Phytomedicine 108, 154496–154499. doi:10.1016/J.PHYMED.2022.154496
Chen, W., Ma, Y., Zhang, H., Guo, Y., Guan, M., and Wang, Y. (2020). Reduning plus ribavirin display synergistic activity against severe pneumonia induced by H1N1 influenza A virus in mice. J. Tradit. Chin. Med. 40, 803–811. doi:10.19852/J.CNKI.JTCM.2020.05.010
Chikhale, R. V., Sinha, S. K., Khanal, P., Gurav, N. S., Ayyanar, M., Prasad, S. K., et al. (2021). Computational and network pharmacology studies of Phyllanthus emblica to tackle SARS-CoV-2. Phytomedicine Plus 1, 100095–100099. doi:10.1016/J.PHYPLU.2021.100095
Das, K. (2022). Herbal plants as immunity modulators against COVID-19: a primary preventive measure during home quarantine. J. Herb. Med. 32, 100501–100509. doi:10.1016/J.HERMED.2021.100501
Davis, J. S., Ferreira, D., Denholm, J. T., and Tong, S. Y. C. (2020). Clinical trials for the prevention and treatment of COVID-19: current state of play. Med. J. Aust. 213, 86–93. doi:10.5694/MJA2.50673
Demeke, C. A., Woldeyohanins, A. E., and Kifle, Z. D. (2021). Herbal medicine use for the management of COVID-19: a review article. Metabol. Open 12, 100141–100146. doi:10.1016/J.METOP.2021.100141
De Natale, A., and Pollio, A. (2007). Plants species in the folk medicine of Montecorvino Rovella (inland Campania, Italy). J. Ethnopharmacol. 109, 295–303. doi:10.1016/J.JEP.2006.07.038
Ding, Y., Zeng, L., Li, R., Chen, Q., Zhou, B., Chen, Q., et al. (2017). The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 17, 130–211. doi:10.1186/S12906-017-1585-7
Filip, R., Gheorghita Puscaselu, R., Anchidin-Norocel, L., Dimian, M., and Savage, W. K. (2022). Global challenges to public health care systems during the COVID-19 pandemic: a review of pandemic measures and problems. J. Pers. Med. 12, 1295–1322. doi:10.3390/JPM12081295
Goel, B., Pathak, N., Nim, D., Singh, S., Dixit, R., and Chaurasia, R. (2014). Evaluation of analgesic activity of Emblica officinalis in albino rats. Int. J. Basic Clin. Pharmacol. 3, 365–368. doi:10.5455/2319-2003.IJBCP20140421
Gu, L., Xie, X., Wang, B., Jin, Y., Wang, L., Yin, G., et al. (2022). Chemical pattern recognition for quality analysis of lonicerae japonicae Flos and lonicerae Flos based on ultra-high performance liquid chromatography and anti-SARS-CoV2 main protease activity. Front. Pharmacol. 12, 810748–810815. doi:10.3389/FPHAR.2021.810748
Gupta, V., Mittal, P., Bansal, P., Khokra, S. L., and Kaushik, D. (2010). PHARMACOLOGICAL POTENTIAL OF MATRICARIA RECUTITA-A REVIEW. Int. J. Pharm. Sci. Drug Res. 2, 12–16.
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181, 271–280. doi:10.1016/J.CELL.2020.02.052
Hu, K., Guan, W. jie, Bi, Y., Zhang, W., Li, L., Zhang, B., et al. (2021). Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine 85, 153242–153249. doi:10.1016/J.PHYMED.2020.153242
Hu, X., Xing, Y., Jia, J., Ni, W., Liang, J., Zhao, D., et al. (2020). Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci. Total Environ. 728, 138812–138817. doi:10.1016/J.SCITOTENV.2020.138812
Igho-Osagie, E., Puenpatom, A., Williams, M. G., Song, Y., Yi, D., Wang, J., et al. (2023). Prevalence of potential drug-drug interactions with ritonavir-containing COVID-19 therapy. J. Manag. Care Spec. Pharm. 29, 509–518. doi:10.18553/JMCP.2023.22366
Jadad, A. R., Moore, R. A., Carroll, D., Jenkinson, C., Reynolds, D. J. M., Gavaghan, D. J., et al. (1996). Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin. Trials 17, 1–12. doi:10.1016/0197-2456(95)00134-4
Jamal, Q. M. S. (2022). Antiviral potential of plants against COVID-19 during outbreaks—an update. Int. J. Mol. Sci. 23, 13564–13625. doi:10.3390/ijms232113564
Jeon, S. R., Kang, J. W., Ang, L., Lee, H. W., Lee, M. S., and Kim, T. H. (2022). Complementary and alternative medicine (CAM) interventions for COVID-19: an overview of systematic reviews. Integr. Med. Res. 11, 100842–100849. doi:10.1016/J.IMR.2022.100842
Jia, S., Luo, H., Liu, X., Fan, X., Huang, Z., Lu, S., et al. (2021). Dissecting the novel mechanism of reduning injection in treating Coronavirus Disease 2019 (COVID-19) based on network pharmacology and experimental verification. J. Ethnopharmacol. 273, 113871–113915. doi:10.1016/J.JEP.2021.113871
Jo, M., Jung, J. H., Kim, H. W., Lee, S. J., Chi, Y. M., Jee, H. S., et al. (2020). Polysaccharide isolated from fermented barley activates innate immune system and anti-tumor metastasis in mice. J. Cereal Sci. 92, 102919–102923. doi:10.1016/J.JCS.2020.102919
Joseph, M. M., Aravind, S. R., Varghese, S., Mini, S., and Sreelekha, T. T. (2012). Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 5, 489–496. doi:10.3892/MMR.2011.638
Kapadia, G. J. (2006). Medicinal plants of the World: chemical constituents, traditional and modern medicinal uses. Volume 3 By ivan A. Ross. Humana press, totowa, NJ. 2005. Xvii + 623 pp. 16.5 × 26 cm. ISBN 1-58829-129-4. $125.00. J. Med. Chem. 49, 3998. doi:10.1021/JM068020K
Karimi, A., Moradi, M. T., Rabiei, M., and Alidadi, S. (2020). In vitro anti-adenoviral activities of ethanol extract, fractions, and main phenolic compounds of pomegranate (Punica granatum L.) peel. Antivir. Chem. Chemother. 28, 2040206620916571–2040206620916576. doi:10.1177/2040206620916571
Karimi, M., Zarei, A., Soleymani, S., Jamalimoghadamsiahkali, S., Asadi, A., Shati, M., et al. (2021). Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial. Phytother. Res. 35, 6295–6309. doi:10.1002/PTR.7277
Kashyap, P., Bhardwaj, V. K., Chauhan, M., Chauhan, V., Kumar, A., Purohit, R., et al. (2022). A ricin-based peptide BRIP from Hordeum vulgare inhibits Mpro of SARS-CoV-2. Sci. Rep. 12, 12802–12811. doi:10.1038/S41598-022-15977-Y
Komariah, M., Amirah, S., Maulana, S., Abdurrahman, M. F., Ibrahim, K., Platini, H., et al. (2023). The efficacy of herbs as complementary and alternative therapy in recovery and clinical outcome among people with COVID-19: a systematic review, meta-analysis, and meta-regression. Ther. Clin. Risk Manag. 19, 611–627. doi:10.2147/TCRM.S405507
Krishnaveni, M., and Mirunalini, S. (2010). Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J. Basic Clin. Physiol. Pharmacol. 21, 93–105. doi:10.1515/JBCPP.2010.21.1.93
Kumaki, Y., Wandersee, M. K., Smith, A. J., Zhou, Y., Simmons, G., Nelson, N. M., et al. (2011). Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antivir. Res. 90, 22–32. doi:10.1016/J.ANTIVIRAL.2011.02.003
Kumar, V., Kumar, Y., Huria, R., Kumar, S., Kalson, T., Jangra, D., et al. (2023). Herbal medicines used for the management of COVID-19. Coronaviruses 4, 49–67. doi:10.2174/2666796704666230403101610
Latarissa, I. R., Barliana, M. I., Meiliana, A., and Lestari, K. (2021). Potential of quinine sulfate for COVID-19 treatment and its safety profile: review. Clin. Pharmacol. 13, 225–234. doi:10.2147/CPAA.S331660
Latarissa, I. R., Barliana, M. I., Meiliana, A., Sormin, I. P., Sugiono, E., Kartasasmita, C. B., et al. (2023). Efficacy of quinine sulfate in patients with mild-to-moderate COVID-19: a randomized controlled trial. Indonesian Biomed. J. 15, 366–374. doi:10.18585/INABJ.V15I6.2543
Lazarus, J. V., Romero, D., Kopka, C. J., Karim, S. A., Abu-Raddad, L. J., Almeida, G., et al. (2022). A multinational Delphi consensus to end the COVID-19 public health threat. Nature 611, 332–345. doi:10.1038/s41586-022-05398-2
Lazreg, A. H., Gaaliche, B., Fekih, A., Mars, M., Aouni, M., Chaumon, J. P., et al. (2011). In vitro cytotoxic and antiviral activities of Ficus carica latex extracts. Nat. Prod. Res. 25, 310–319. doi:10.1080/14786419.2010.528758
Li, G., Simmler, C., Chen, L., Nikolic, D., Chen, S. N., Pauli, G. F., et al. (2017). Cytochrome P450 inhibition by three licorice species and fourteen licorice constituents. Eur. J. Pharm. Sci. 109, 182–190. doi:10.1016/J.EJPS.2017.07.034
Light, D. W., and Lexchin, J. (2021). The costs of coronavirus vaccines and their pricing. J. R. Soc. Med. 114, 502–504. doi:10.1177/01410768211053006
Lim, X. Y., Teh, B. P., and Tan, T. Y. C. (2021). Medicinal plants in COVID-19: potential and limitations. Front. Pharmacol. 12, 611408–8. doi:10.3389/FPHAR.2021.611408
Liu, J., Manheimer, E., Shi, Y., and Gluud, C. (2004). Chinese herbal medicine for severe acute respiratory syndrome: a systematic review and meta-analysis. J. Altern. Complement. Med. 10, 1041–1051. doi:10.1089/ACM.2004.10.1041
Liu, J., Yang, W., Liu, Y., Lu, C., Ruan, L., Zhao, C., et al. (2021). Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial. Phytomedicine 91, 153671–153710. doi:10.1016/J.PHYMED.2021.153671
Liu, Y., Liang, C., Xin, L., Ren, X., Tian, L., Ju, X., et al. (2020). The development of Coronavirus 3C-Like protease (3CLpro) inhibitors from 2010 to 2020. Eur. J. Med. Chem. 206, 112711–112719. doi:10.1016/J.EJMECH.2020.112711
Liu, Y. X., Zhou, Y. H., Jiang, C. H., Liu, J., and Chen, D. Q. (2022). Prevention, treatment and potential mechanism of herbal medicine for Corona viruses: a review. Bioengineered 13, 5480–5508. doi:10.1080/21655979.2022.2036521
Loc, H. N., Lan, T. T. N., Huong, D. T. L., Tuyen, N. T., Quang, T. M., Dao, L. M., et al. (2022). Traditional Vietnamese medicine Kovir capsule in patients with mild COVID-19: a double-blind randomized controlled trial. Phytotherapy Res. 36, 2878–2888. doi:10.1002/PTR.7455
Luo, W., Ding, R., Guo, X., Zhan, T., Tang, T., Fan, R., et al. (2022). Clinical data mining reveals Gancao-Banxia as a potential herbal pair against moderate COVID-19 by dual binding to IL-6/STAT3. Comput. Biol. Med. 145, 105457–105512. doi:10.1016/J.COMPBIOMED.2022.105457
Lv, J. J., Yu, S., Xin, Y., Cheng, R. R., Zhu, H. T., Wang, D., et al. (2015). Anti-viral and cytotoxic norbisabolane sesquiterpenoid glycosides from Phyllanthus emblica and their absolute configurations. Phytochemistry 117, 123–134. doi:10.1016/J.PHYTOCHEM.2015.06.001
Machado, B. A. S., Hodel, K. V. S., Fonseca, LMDS, Pires, V. C., Mascarenhas, L. A. B., da Silva Andrade, L. P. C., et al. (2022). The importance of vaccination in the context of the COVID-19 pandemic: a brief update regarding the use of vaccines. Vaccines (Basel) 10, 591–625. doi:10.3390/VACCINES10040591
Mittal, M., Siddiqui, M. R., Tran, K., Reddy, S. P., and Malik, A. B. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 20, 1126–1167. doi:10.1089/ARS.2012.5149
Nugraha, R. V., Ridwansyah, H., Ghozali, M., Khairani, A. F., and Atik, N. (2020). Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evidence-based Complementary Altern. Med. 2020, 2560645–2560712. doi:10.1155/2020/2560645
Olivo, S. A., Macedo, L. G., Gadotti, I. C., Fuentes, J., Stanton, T., and Magee, D. J. (2008). Scales to assess the quality of randomized controlled trials: a systematic review. Phys. Ther. 88, 156–175. doi:10.2522/PTJ.20070147
Onyeaghala, A. A., Anyiam, A. F., Husaini, D. C., Onyeaghala, E. O., and Obi, E. (2023). Herbal supplements as treatment options for COVID-19: a call for clinical development of herbal supplements for emerging and re-emerging viral threats in Sub-Saharan Africa. Sci. Afr. 20, e01627. doi:10.1016/J.SCIAF.2023.E01627
Padhy, M. (2020). A review on medicinal plants withania somnifera and nyctanthes arbortristis: boosting of immune system during SARS-CoV-2. Lett. Appl. NanoBioScience 9, 1538–1546. doi:10.33263/LIANBS94.15381546
Perera, WPRT, Liyanage, J. A., Dissanayake, K. G. C., Gunathilaka, H., Weerakoon, WMTDN, Wanigasekara, D. N., et al. (2021). Antiviral potential of selected medicinal herbs and their isolated natural products. Biomed. Res. Int. 2021, 7872406–7872418. doi:10.1155/2021/7872406
Perianayagam, J. B., Sharma, S. K., Joseph, A., and Christina, A. J. M. (2004). Evaluation of anti-pyretic and analgesic activity of Emblica officinalis Gaertn. J. Ethnopharmacol. 95, 83–85. doi:10.1016/J.JEP.2004.06.020
Pham, M. N., Nguyen-Dung, P. T., Nguyen, T. K. N., Tran, V. H., Phan, N. T. T., Do, T. H. T., et al. (2023). Antiviral and immunostimulatory effects of Ssanti-Covir, a mixed herbal formulation, in cyclophosphamide-treated mice. Pharmacol. Res. - Mod. Chin. Med. 9, 100329–100410. doi:10.1016/J.PRMCM.2023.100329
Sai Ram, M., Neetu, D., Yogesh, B., Anju, B., Dipti, P., Pauline, T., et al. (2002). Cyto-protective and immunomodulating properties of Amla (Emblica officinalis) on lymphocytes: an in-vitro study. J. Ethnopharmacol. 81, 5–10. doi:10.1016/S0378-8741(01)00421-4
Silveira, D., Prieto-Garcia, J. M., Boylan, F., Estrada, O., Fonseca-Bazzo, Y. M., Jamal, C. M., et al. (2020). COVID-19: is there evidence for the use of herbal medicines as adjuvant symptomatic therapy? Front. Pharmacol. 11, 581840–581844. doi:10.3389/FPHAR.2020.581840
Soleymani, S., and Zargaran, A. (2018). A historical report on preparing sustained release dosage forms for addicts in medieval persia, 16th century AD. Subst. Use Misuse 53, 1726–1729. doi:10.1080/10826084.2018.1432648
Sreeramulu, D., and Raghunath, M. (2010). Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res. Int. 43, 1017–1020. doi:10.1016/J.FOODRES.2010.01.009
Sun, S., Wang, Y., Wu, A., Ding, Z., and Liu, X. (2019). Influence factors of the pharmacokinetics of herbal resourced compounds in clinical practice. Evid. Based Complement. Altern. Med. 2019, 1983780–1983816. doi:10.1155/2019/1983780
Tallima, H., and El Ridi, R. (2018). Arachidonic acid: physiological roles and potential health benefits – a review. J. Adv. Res. 11, 33–41. doi:10.1016/J.JARE.2017.11.004
Tavakoli, A., Molavi Vardanjani, H., Namjouyan, F., Cramer, H., and Pasalar, M. (2022). Efficacy of Persian barley water on clinical outcomes of hospitalized moderate-severity COVID-19 patients: a single-blind, add-on therapy, randomized controlled clinical trial. Eur. Rev. Med. Pharmacol. Sci. 26, 1033–1041. doi:10.26355/EURREV_202202_28013
Utsunomiya, T., Kobayashi, M., Pollard, R. B., and Suzuki, F. (1997). Glycyrrhizin, an active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob. Agents Chemother. 41, 551–556. doi:10.1128/AAC.41.3.551
van de Sand, L., Bormann, M., Alt, M., Schipper, L., Heilingloh, C. S., Steinmann, E., et al. (2021). Glycyrrhizin effectively inhibits SARS-CoV-2 replication by inhibiting the viral main protease. Viruses 13, 609–610. doi:10.3390/V13040609
Varnasseri, M., Siahpoosh, A., Hoseinynejad, K., Amini, F., Karamian, M., Yad, M. J. Y., et al. (2022). The effects of add-on therapy of Phyllanthus Emblica (Amla) on laboratory confirmed COVID-19 Cases: a randomized, double-blind, controlled trial. Complement. Ther. Med. 65, 102808–102817. doi:10.1016/J.CTIM.2022.102808
Walsh, M. (2021). Effectiveness of Chinese herbal medicine and Persian medicine against viral infections: a systematic review. Syst. Rev. Pharm., 65–81. doi:10.31838/SRP.2021.2.6
Wang, B., Sun, X., Kong, X., and Gao, Y. (2021b). Systematic elucidation of the mechanism of jingyin granule in the treatment of novel coronavirus (COVID-19) pneumonia via network pharmacology. Int. J. Med. Sci. 18, 1648–1656. doi:10.7150/IJMS.53575
Wang, C., Sun, S., and Ding, X. (2021a). The therapeutic effects of traditional Chinese medicine on COVID-19: a narrative review. Int. J. Clin. Pharm. 43, 35–45. doi:10.1007/S11096-020-01153-7
Wang, H., Chen, Y., Wang, L., Liu, Q., Yang, S., and Wang, C. (2023). Advancing herbal medicine: enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 14, 1265178–1265216. doi:10.3389/FPHAR.2023.1265178
Wang, Y. L., Zhang, C. C., Zhang, F., Huang, J., Wang, D. D., Zhao, Y. F., et al. (2020). Chemical profiling and tissue distribution study of Jingyin Granules in rats using UHPLC-Q-Exactive Orbitrap HR-MS. China J. Chin. materia medica 45, 5537–5554. doi:10.19540/J.CNKI.CJCMM.20200903.201
Wen, Y., Rahman, M. F., Zhuang, Y., Pokojovy, M., Xu, H., McCaffrey, P., et al. (2022). Time-to-event modeling for hospital length of stay prediction for COVID-19 patients. Mach. Learn. Appl. 9, 100365–100368. doi:10.1016/J.MLWA.2022.100365
Xiang, Y., Pei, Y., Qu, C., Lai, Z., Ren, Z., Yang, K., et al. (2011). In vitro anti-herpes simplex virus activity of 1,2,4,6-tetra-O-galloyl-β-D-glucose from Phyllanthus emblica L. (Euphorbiaceae). Phytother. Res. 25, 975–982. doi:10.1002/PTR.3368
Xie, X., Muruato, A., Lokugamage, K. G., Narayanan, K., Zhang, X., Zou, J., et al. (2020). An infectious cDNA clone of SARS-CoV-2. Cell. Host Microbe 27, 841–848. doi:10.1016/j.chom.2020.04.004
Xu, M., Yang, X., Yang, X. A., Zhou, L., Liu, T. Z., Fan, Z., et al. (2008). Experimental investigation of the effect of LianhuaqingwenCapsule on the rat models of chronic obstructive pulmonary disease. Fudan Univ. J. Med. Sci. 35, 441–444. doi:10.1007/S13238-016-0264-7
Xu, X., Zhang, J., Zheng, W., Yang, Z., Zhao, X., Wang, C., et al. (2021). Efficacy and safety of Reduning injection in the treatment of COVID-19: a randomized, multicenter clinical study. Ann. Palliat. Med. 10, 5146–5155. doi:10.21037/APM-20-2121
Yang, Y., Hu, X., Xiong, L., Fu, P., Feng, W., Li, W., et al. (2021). Clinical characteristics of hospitalized mild/moderate COVID-19 patients with a prolonged negative conversion time of SARS-CoV-2 nucleic acid detection. BMC Infect. Dis. 21, 141–148. doi:10.1186/S12879-021-05851-Z
Yi, Y., Li, J., Lai, X., Zhang, M., Kuang, Y., Bao, Y. O., et al. (2021). Natural triterpenoids from licorice potently inhibit SARS-CoV-2 infection. J. Adv. Res. 36, 201–210. doi:10.1016/J.JARE.2021.11.012
Zeng, F., Huang, Y., Guo, Y., Yin, M., Chen, X., Xiao, L., et al. (2020). Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int. J. Infect. Dis. 96, 467–474. doi:10.1016/J.IJID.2020.05.055
Zhang, F., Liu, W., Huang, J., Chen, Q. long, dan, W. D., Zou, L. wei, et al. (2022). Inhibition of drug-metabolizing enzymes by Jingyin granules: implications of herb-drug interactions in antiviral therapy. Acta Pharmacol. Sin. 43, 1072–1081. doi:10.1038/S41401-021-00697-2
Zhang, G., Zhao, J., He, L., Yan, S., Zhuo, Z., Zheng, H., et al. (2013). Reduning injection for fever, rash, and ulcers in children with mild hand, foot, and mouth disease: a randomized controlled clinical study. J. Tradit. Chin. Med. 33, 733–742. doi:10.1016/S0254-6272(14)60005-4
Keywords: herbal medicines, randomized controlled trial, clinical trial, antiviral, antiinflammatory, immunomodulatory
Citation: Latarissa IR, Meiliana A, Sormin IP, Sugiono E, Wathoni N, Barliana MI and Lestari K (2024) The efficacy of herbal medicines on the length of stay and negative conversion time/rate outcomes in patients with COVID-19: a systematic review. Front. Pharmacol. 15:1383359. doi: 10.3389/fphar.2024.1383359
Received: 21 February 2024; Accepted: 10 May 2024;
Published: 30 May 2024.
Edited by:
Michał Tomczyk, Medical University of Bialystok, PolandReviewed by:
Nazim Uddin Emon, University of Arkansas, United StatesSafaet Alam, Bangladesh Council of Scientific and Industrial Research (BCSIR), Bangladesh
Copyright © 2024 Latarissa, Meiliana, Sormin, Sugiono, Wathoni, Barliana and Lestari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keri Lestari, lestarikd@unpad.ac.id
 Irma Rahayu Latarissa
Irma Rahayu Latarissa Anna Meiliana
Anna Meiliana Ida Paulina Sormin
Ida Paulina Sormin Erizal Sugiono
Erizal Sugiono Nasrul Wathoni
Nasrul Wathoni Melisa Intan Barliana
Melisa Intan Barliana Keri Lestari
Keri Lestari