- Institute of Archaeology, University College London, London, United Kingdom
Sulfur isotopic composition (δ34S) is used in archaeological research to reconstruct past mobility patterns and diet using environmental baselines. Human and faunal collagen δ34S is generally interpreted as reflecting environmental baselines derived from geological sulfur and marine sulfur near the coast. However, recent studies have highlighted that the δ34S of bioavailable sulfur is modified in wetlands and waterlogged environments as a result of microbial sulfate reduction and sulfide oxidation. These processes are driven by fluctuating redox conditions resulting from flooding, and can significantly alter bioavailable δ34S baselines in the biosphere, and subsequently in animal tissues, through the food chain. This challenges conventional approaches that assume local geology and coastal proximity alone determine bioavailable baseline δ34S. This study considers sulfur cycling within terrestrial ecosystems and its implications for archaeological interpretation. We explore how plant δ34S is influenced by anoxic soil conditions, hydrological controls and plant waterlogging adaptations, integrating these factors into a theoretical framework and simple numerical models of plant δ34S in wetland and waterlogged environments. The models simulate the effect of different hydrological regimes on plant δ34S (in flow-through, standing water, drained post-flooding, and simultaneously flooded and drained landscapes). We suggest how δ34S could change for crop plants grown using different past water management techniques, and discuss effects of wetland utilization and resource use on animal and human collagen δ34S. This study demonstrates that different systems can result in multiple “wetland” sulfur isotope signals that can deviate both positively and negatively from local baselines that are driven by geological and marine sulfur. δ34S has the potential to be a useful tool for exploring ancient wetland use and water management strategies. Such strategies have long been argued to be critical to early agricultural development and the establishment of complex societies.
1 Introduction
Sulfur stable isotopic composition has been used in archaeological research to investigate dietary resource use and mobility of people and animals in the past (e.g., Czére et al., 2022; Ebert et al., 2021; Paladin et al., 2020). Recent developments in analytical techniques for δ34S have significantly reduced the amount of sample material needed for analysis (Sayle et al., 2019). This has led to an increasingly large number of publications presenting δ34S of bioarchaeological material, mostly faunal and human bone collagen, and often including complementary information from carbon, nitrogen, oxygen and strontium isotopes. Such research has been built upon the assumption that human and animal bone and dentine collagen δ34S reflects the isotopic composition of bioavailable sulfur at the base of the food chain (plant δ34S) driven by the local geology. This is because the minimal trophic enrichment during plant and animal uptake of sulfur means that the environmental signals are largely conserved (Krajcarz et al., 2019; Nehlich, 2015; Raoult et al., 2024).
In terrestrial environments underlying bedrock provides sulfur-bearing parent material, while lithology exerts physical and chemical controls which affect the movement of sulfur into soil and on to plants, often via interactions with groundwater and microbiology. Proximity to the ocean (due to sea spray) can mix a marine signal with the bioavailable sulfur in soil derived from the geology (Bataille et al., 2020; Guiry and Szpak, 2020). Estimates of geological baselines of bioavailable sulfur δ34S form the basis for archaeological geographic assignment and mobility studies using collagen δ34S (Ebert et al., 2021; Lamb et al., 2023; Paladin et al., 2020; Zavodny et al., 2022). While fish consumption may also influence consumer δ34S (e.g., Nehlich et al., 2010; Privat et al., 2007), here we focus on sulfur isotope variation in plants, and the implications for sulfur isotopic composition of collagen from terrestrial animals that do not consume fish.
Recent studies indicate that the interpretation of δ34S is potentially complex because the δ34S of sulfur available to plants can be influenced by microbial sulfate reduction and sulfide oxidation in soil and sediments (Guiry et al., 2021; Lamb et al., 2023; Nitsch et al., 2019; Stevens et al., 2022, 2025). These important processes in the global sulfur cycle are mediated by soil Bacteria and Archaea, and the soil or sediment iron content. Microbial sulfate reduction is a widespread phenomenon in terrestrial (and marine) environments and is stimulated by waterlogged conditions, for example, in wetlands, on poorly drained soils (Canfield, 2001), and in thermokarst environments (Ren et al., 2023), and is often driven by poor permeability in the underlying lithology. In these environments isotope effects from microbial sulfate reduction followed by sulfide oxidation change the δ34S of soil sulfur available to plants originally derived from the geology. This modified isotopic signal can be incorporated into plants and passed on to animals to be stored in animal proteins. Sulfur in bioarchaeological material produced in these environments can, therefore, reflect a modified isotopic composition due to the effect of coeval microbial sulfur cycling (Lamb et al., 2023). We refer to environmental δ34S baselines of bioavailable sulfur derived from geological and/or marine sources on well-drained land as “dryland” baselines, in order to differentiate them from environmental δ34S baselines that have been altered due to waterlogging and sulfur cycling.
Evidence of early human activity has been found in wetland environments, where access to fresh drinking water and fertile soil would have made these locations attractive for resource use and settlement. These environments include marshes, fens, meres, swamps, lagoons, floodplains, lakesides, riverbanks and riparian zones, saltmarshes, estuaries and deltas, as well as land on the margins of these places. Such locations appear to have been used to provide wild food (e.g., Abu Hureyra, riparian marshland, Hillman et al., 2001), for animal husbandry and to grow crops (e.g., Neolithic Çatalhöyük, Konya plain, Turkey, Ayala et al., 2022; Sherratt, 1980; Must Farm, Cambridgeshire, UK, Ballantyne et al., 2024; Swifterbant, Neolithic wetland creek system, The Netherlands, Huisman and Raemaekers, 2014; Eye, Over, and Langtoft, fenland, Cambridgeshire, UK, Lamb et al., 2023; and Middle Yellow River Valley, China, Wang et al., 2023). Such environments were likely instrumental in the development of farming, with floodplain archaeobotanical assemblages suggesting the presence of crop plants growing alongside wetland plants (e.g., Hillman et al., 2001; Motuzaite-Matuzeviciute et al., 2012).
In archaeological applications, where the sulfur isotopic composition of bioarchaeological material deviates from the local dryland δ34S baseline, it may suggest movement of people or animals in the past. However, it could also indicate a wetland δ34S baseline signal incorporated via the consumption of crops grown on wetlands and wetland derived resources from the local area (and/or beyond) (Guiry et al., 2021, 2022; Lamb et al., 2023; Nitsch et al., 2019). Such signals in human, animal and plant archaeological material could potentially provide unique insights into ancient wetland resource use and early agriculture. Temporal sequences of such samples may also provide information on natural hydrological changes, seasonal resource use, and human manipulation of local hydrology.
The aim of this study is to explore how different hydrological conditions can impact terrestrial sulfur cycling and overwrite primary dryland δ34S baselines derived from parent geology. We use conceptual models to explore wetland sulfur cycling effects. We discuss parameters controlling plant-available sulfur δ34S, assess the effect of coeval sulfur cycling on biosphere mapping and palaeo-baselines, and explore factors contributing to wetland isotopic signals in plants. We consider how these factors may have interacted with ancient water management practices to affect the δ34S of crops and wild plants grown in these environments, and explore the influence of the wetland signal on archaeological collagen δ34S.
2 Drivers of bioavailable δ34S baselines
Almost all biologically incorporated sulfur in terrestrial environments is derived from geological reservoirs in rocks, as bedrock provides parent material from which sulfur is released into soil and groundwater. Therefore, the site specific δ34S of geological sulfur is the essential starting point for interpreting δ34S in archaeological collagen and plant material from a particular site. There are two broad categories of geological sulfur deposits, sulfate that reflects the δ34S of coeval ocean water sulfate with a range of 10‰ to 30‰, and sulfide formed from microbial sulfate reduction, usually with significantly lower δ34S values (Bottrell and Newton, 2006; Bottrell and Raiswell, 2000; Claypool et al., 1980; Paytan and Gray, 2012; Pellerin et al., 2019). Together, this results in a very wide range of geological sulfur δ34S values spanning c. 70‰, with offshore pyrite sulfide δ34S values reported down to −55‰ (Pasquier et al., 2021).
Terrestrial geological sulfur is released from rock into soil via a series of physiochemical processes which weather the rock. This occurs most commonly with near-surface sedimentary rock when exposed to changes in temperature and moisture at the surface, or via interactions with groundwater in the subsurface. In this way, sulfur bearing the local geological δ34S reaches soil and groundwater. It is this weathered, dissolved sulfur that is available to be taken up by plants and animals. Geological weathering product δ34S found in these terrestrial reservoirs may reflect a mix of sulfur from different sulfur bearing rocks with varied δ34S values. This mix is affected by preferential weathering of some deposits over others due to chemical and structural differences. For example, during weathering, sulfate in evaporites and carbonates readily dissolves in water, often resulting in high concentrations reaching soil, groundwater and rivers, whereas sulfide in pyrite is insoluble, and requires exposure to oxygen to weather, which usually occurs via microbially mediated reactions.
This geological origin sulfur in soil may mix with sulfur from natural atmospheric sources, including wind blown dust, the biogenic gases dimethyl sulfide and hydrogen sulfide (H2S), as well as ash from forest fires and sulfur dioxide from volcanic eruptions and passive degassing (Aneja, 1990). When two or more inputs of sulfur with different isotopic compositions are mixed, the relative amounts of sulfur and the isotopic difference between each input determine the resulting δ34S of the mixture. In near-coast sediments and soils the geological signal is likely to be overwritten by the dominant isotopic signal from sulfate-rich ocean water in sea spray (δ34S c. 21‰; Paytan and Gray, 2012), and dimethyl sulfide produced by marine phytoplankton (Aneja, 1990). The distribution of this marine signal is controlled by prevailing weather patterns which affect the continental distribution of sea spray and dimethyl sulfide, and by hydrogeology, which controls the extent of saline intrusion into fresh groundwater in coastal regions.
Archaeological studies may use modern material, (for example from plants), to characterize δ34S baselines. However, in modern environments, soil sulfur of geological origin may mix with atmospheric sulfur dioxide from fossil fuel combustion, and sulfur in agricultural fertilizer. Sulfate originating from fossil fuel combustion has an isotopic composition close to 0‰ (e.g., Chung et al., 2019; δ34S 2.2 ± 1.6‰). Inputs from fossil fuel combustion are likely to be diffuse, dampening the range of soil sulfur derived from geology over the affected area (Stack and Rock, 2011), with a larger effect for geologically derived soil δ34S values that are furthest from the value of fossil fuels. Fertilizer δ34S values are highly variable (Vitòria et al., 2004) and their effect on soil bioavailable sulfur δ34S is likely to be localized and spatially heterogeneous. Sulfur fertilizers may be artificially produced or mined from geological settings far from the locations where they are used. Sulfur from fertilizers can be transported away from the area of application in runoff and groundwater. When anthropogenic sulfur inputs decline or are discontinued, there is likely to be a time lag before their contribution to the bioavailable sulfur pool decreases.
Microbial sulfur cycling can significantly alter δ34S values derived from geological sulfur parent material, marine and atmospheric sources, laying down fresh sulfide deposits in soils and sediments (Figure 1), leading to bioavailable δ34S values which can be lowered or raised. This can occur as a result of poorly draining soils or impervious lithology. Sulfur with δ34S values affected by microbial cycling may be taken up by plants and passed up the food chain. Sulfur cycling can occur at local scales and can change over relatively short time periods, for example with alteration in floodplain extent due to dynamic river braiding and meandering, or at vast spatial and temporal scales, for example during continent-wide permafrost thaw during the Late Pleistocene.
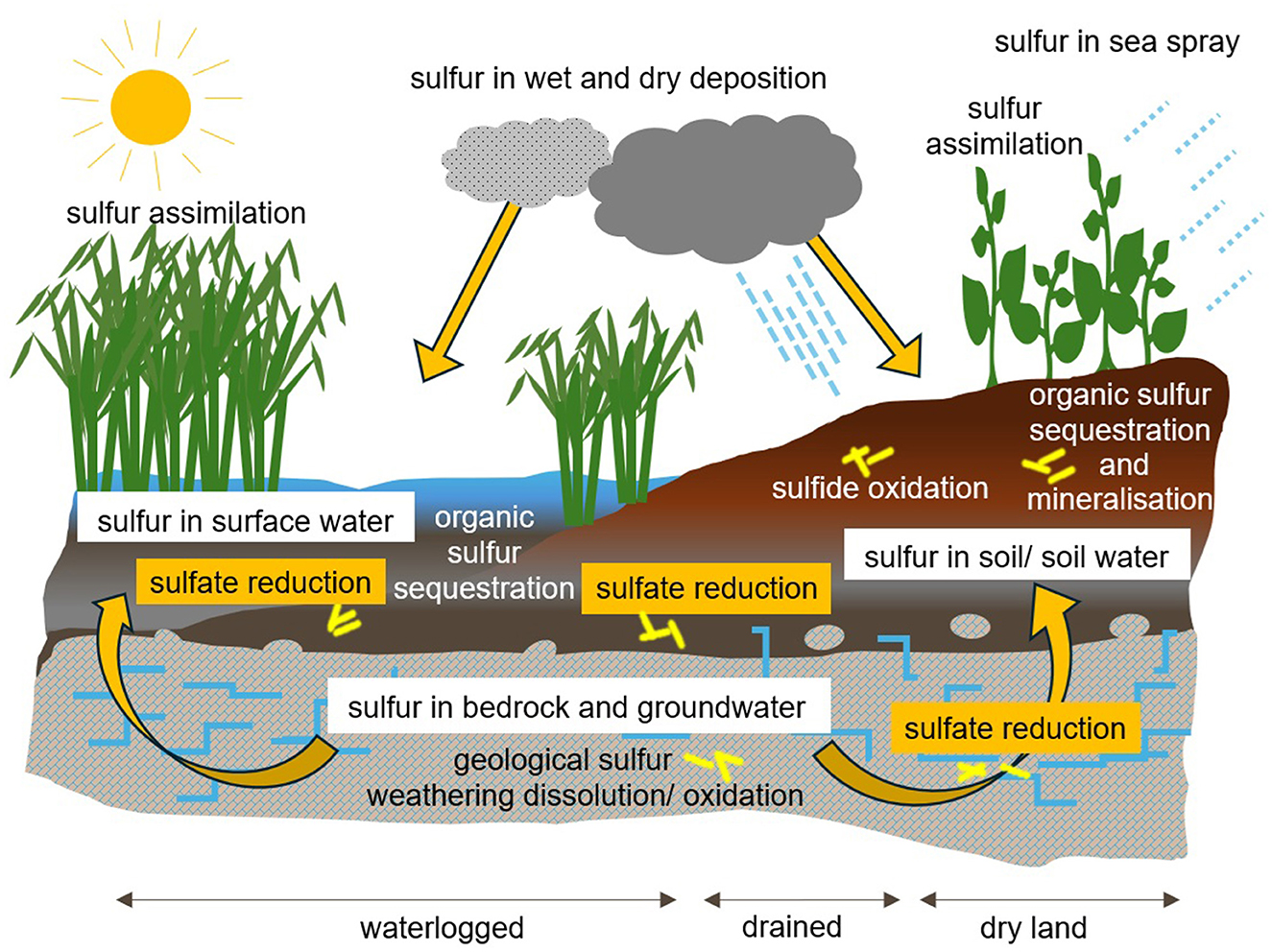
Figure 1. The terrestrial sulfur cycle, showing sources of sulfur and microbial sulfur cycling processes. Sulfate reduction is highlighted in yellow boxes as the main isotopically fractionating process.
Perturbations of geologically derived dryland δ34S baselines due to coeval sulfur cycling are relevant to archaeological studies using sulfur isotopic composition of human, animal or plant material. Such perturbations should be considered in archaeological settings to broaden the scope of interpretation and avoid misinterpretation of archaeological δ34S values. In particular, where archaeological sites were in wetlands, in periodically waterlogged, poorly draining or low-lying environments, or in thermokarst, saltmarshes or estuaries, dynamic sulfur cycling is likely to have affected bioavailable δ34S baselines significantly. However, in order to identify the effects of coeval sulfur cycling, the dryland baseline derived from the local geological parent material, (as well any sea spray influence), should be determined with good spatial resolution and a high degree of confidence. This is particularly important because of the very wide range of possible geological parent material baseline δ34S values. Distinguishing the influence of parent material from coeval sulfur cycling can be challenging, as some wetlands form on poorly permeable lithology that has a sulfur isotopic composition strongly affected by sulfate reduction over geological time scales, for example in lowlands underlain by Jurassic mudstones in central England (Lamb et al., 2023).
3 Sulfur cycling affects bioavailable δ34S baselines in wetlands and waterlogged environments
3.1 Sulfur cycling in soil
When considering the effects of waterlogging on plant δ34S, we refer to the partitioning of different amounts of sulfur in organic and inorganic compounds in the soil and their bioavailability to plants (the plant-available fraction), the isotopic composition of these different compounds (δ34S), and microbial sulfur cycling processes that may incur isotopic fractionations affecting the isotopic composition of the compounds. Around 95% of sulfur in soil is in organic form (Kilham, 1994), derived from biomass of plant, microbial, and animal origin. However, plants are not able to utilize this organic sulfur directly. It is broken down slowly and is only very gradually mineralized and oxidized to sulfate by soil microbes (Chaudhary et al., 2023), with negligible change in δ34S (e.g., Norman et al., 2002), to be replenished by organic sulfur in fresh biomass deposits. Breakdown of organic matter is suppressed if the soil is waterlogged (Dušek et al., 2020). Water also limits oxygen diffusion into waterlogged and flooded soils and sediments, which are often formed on poorly permeable lithologies. Under water, there is decreasing oxygen diffusion with depth and distance from the air-water surface interface. As remaining oxygen is used up by aerobic microbial respirers and plant roots, a steep vertical gradient of oxygen depletion develops, stimulating the activity of different anaerobic microbial communities and progressive electron acceptor depletion (Kilham, 1994). In the anoxic zone, microbial sulfate reducing activity is stimulated where sulfate and organic carbon are available. Within these redox gradients the boundary between well-oxidized conditions near the surface and anoxic conditions at depth can move up or down, for example, in response to water level fluctuations and bioturbation. This can result in dynamic switching between reducing and oxidizing conditions, stimulating rapid microbial cycling of sulfate and sulfide from the soil inorganic sulfur pool through sulfate reduction and sulfide oxidation.
Sulfate reduction in anoxic conditions is associated with a varied and often large isotopic fractionation (c. 3‰ to 68‰, Sim et al., 2011), which results in the production of sulfide with lower δ34S values than those of the original sulfate. The isotope effect associated with microbial sulfate reduction occurs via a series of fractionating, reversible enzymatic steps within the sulfate reducing organism. The magnitude of the sulfate reduction isotope effect in the environment is one of the main controls on wetland plant δ34S. Large isotope effects from wetland sulfur cycling have been reported, for example from peat wetland environments (11‰ to 51‰, Price and Casagrande, 1991), brackish marshland (c. 33‰, Stribling et al., 1998), and saltmarshes (25‰, Carlson and Forrest, 1982; 50‰, Peterson et al., 1986), although further studies are needed, especially from freshwater environments. At near neutral pH levels, sulfide produced from sulfate reduction can accumulate in the soil as hydrogen sulfide (H2S). Persistent large isotopic offsets between sulfate in water and sulfide in sediments are brought about via the formation of sulfide precipitates in the sediment which prevent the upward diffusion of dissolved sulfide to the surface where oxygen would enable its oxidation to sulfate. Most sulfide precipitates are formed with iron (Fe2+), as iron sulfide (FeS) and pyrite (FeS2), thus iron availability is key to the formation of sulfide precipitates and maintaining the large isotopic offsets found between sulfate in water and sulfide in sediments.
Factors causing variation in the isotope effect of sulfate reduction are not fully understood, and include the ratio of sulfate to organic carbon (a larger effect is found when the sulfate to carbon ratio is high), and non-linear effects from differences in sulfate concentration, temperature, microbial strain, the type of organic carbon, the cell specific sulfate reduction rate, and iron and ammonium availability (Sim et al., 2012, 2023). High groundwater levels, river flooding, heavy rainfall, poor soil permeability and impermeable lithology can cause soil saturation, which stimulates sulfate reduction. Soil waterlogging coupled with sulfate reduction may be a temporary or periodic phenomenon, for example in floodplains and estuaries, or a more permanent phenomenon, for example in marshes and fens. A wide diversity of wetlands are prime sulfate reducing environments, with low δ34S sulfide in soil and sediments.
3.2 Pathways for plant sulfur uptake and effects on plant δ34S
Sulfur is an essential nutrient for plants. It is taken up via sulfate transporter chemicals in roots, and used to make different sulfur containing compounds including the amino acids cysteine and methionine, the protective antioxidant glutathione, as well as many essential metabolites such as vitamins, enzymes, and compounds used for plant defense and stress response (Hill et al., 2022). If plants take up excess sulfate, it can be stored in cell vacuoles until it is needed, and if sulfate uptake is restricted due to low availability sulfur may be recycled within the plant from glucosinolates (secondary plant metabolites) (Maruyama-Nakashita, 2017).
Sulfate reduction results in sulfide with lowered δ34S. However, most plants are not sulfide tolerant and cannot take up sulfide directly. In order for a lowered δ34S from sulfide to be incorporated into plant biomass, sulfide must first be oxidized to sulfate. Here we use the term “lowered plant δ34S” as a relative term to refer to plant δ34S values that are lower than those expected from plants grown in non-waterlogged or flooded soil with the same geological parent material. For plants which are not sulfide tolerant, sulfide oxidation to sulfate can occur after waterlogged or flooded soil drains, allowing the ingress of air to oxygenate the soil, for example on seasonally draining floodplains. This enables the oxidizing microbial community to become active resulting in sulfide oxidation to sulfate, utilizing sulfide produced during the preceding period of submergence. Soil sulfide oxidation usually causes negligible isotopic fractionation (Canfield, 2001; Zerkle et al., 2009) so the newly oxidized sulfate retains the lowered δ34S from sulfide. This effect is enhanced by differences in the solubility of sulfate and sulfide. Sulfate is highly soluble and some soil sulfate is removed in water as it drains from temporarily waterlogged soil, as a function of soil water retention on draining, which varies with soil type. Sulfide precipitates such as iron sulfide and pyrite are insoluble and remain in the soil when the water drains away, after which they can be oxidized back to sulfate. The relative proportions of plant-available sulfate from soil oxidized sulfide to remaining sulfate in soil water after draining is referred to as the “soil sulfate source ratio” hereafter.
Some plants, primarily those adapted to wetland ecologies (e.g., wetland plants including the crops rice and taro), are sulfide tolerant. They have adapted to root zone (rhizosphere) waterlogging and can oxidize sulfide to sulfate in the root zone (Lamers et al., 2013; Simkin et al., 2013). This occurs through the development of a barrier along the root length to the tip which protects the plant from absorbing toxic sulfide along the root shaft, but allows leakage of oxygen into the rhizosphere from the root tip (Abiko and Miyasaka, 2020; de la Cruz Jiménez et al., 2021; Nishiuchi et al., 2012; Pan et al., 2021). The oxygen seeping from the root tip into the waterlogged rhizosphere (Ando et al., 1983), leads to localized root zone oxygenation and the development of a symbiotic bacterial community of sulfide oxidizers. This supports in situ microbial oxidation of sulfide with lowered δ34S, forming sulfate which can be then taken up and utilized by the plant. Internal sulfide oxidation by symbiotic bacterial colonies within the roots may also occur (Lamers et al., 2013). Wetland plants can also develop adventitious roots which form laterally at the soil/water interface, allowing uptake of oxygen and sulfate from overlying water (Parent et al., 2008; Steffens and Rasmussen, 2016).
A critical factor determining wetland plant δ34S is the relative amounts of sulfur taken up via these two pathways: the uptake ratio of sulfide oxidized in the root zone, to direct uptake of sulfate from overlying water; hereafter referred to as the “plant uptake ratio.” There are limited published data on wetland plant uptake ratios. The few studies that are available show saltmarsh plant uptake ratios ranging from 1.0: 0.0 to 0.6: 0.4 (Carlson and Forrest, 1982; Cornwell et al., 1995; Fry et al., 1982, 2023; Peterson et al., 1986; Price and Casagrande, 1991; Stribling and Cornwell, 1997; Stribling et al., 1998). In these sulfate rich environments sulfate δ34S in overlying water is as high as 21‰, but plant δ34S ranges from −14‰ to 13‰ due to root zone sulfide oxygenation. Although freshwater wetland plant uptake ratio studies are lacking, the range of δ34S from freshwater wetland plants reported recently is similar in magnitude to that of saltmarsh plants, although with lower absolute values (−31‰ to −1‰, Evans et al., 2023; Lamb et al., 2023).
Interestingly, adventitious roots (but not rhizosphere sulfide oxidation), can be induced by waterlogging stress in non-wetland species including the crop plants oats, barley, wheat, maize, and millet (Li et al., 2023; Matsuura et al., 2022; Pan et al., 2021; Yamauchi et al., 2013). This stress response may have enabled early cultivation of crop plants on floodplains and lowlands which would have been at risk of periodic inundation during the growing season.
4 The “wetland” sulfur isotope signal
Archaeologists are rapidly taking on board the concept that lowered δ34S is a marker of wetland habitat use. However, it is necessary to highlight that not all wetland and waterlogged environments will produce a distinctive isotopic signal. Whether such a signal is present depends on many variables, including the magnitude of the isotope effect, plant uptake ratios, the nature of the hydrologic system (flow-through, standing water), the duration of waterlogging and progression of sulfate reduction, the extent of drainage and the interplay between microbial sulfur cycling, isotopic fractionation, and plant adaptation. The hydrology of natural wetlands has traditionally been classified according to hydrogeomorphology, combining information about the dominant source of water, position in the landscape and water movement (Shaffer et al., 1999). Wetlands occur where these factors combine to support a seasonal or permanent high water table, which leads to the development of specific habitat types and wetland soils and sediments, and are often underlain by poorly permeable lithology. Soil waterlogging, while not necessarily leading to wetland development, supports microbial sulfate reduction and changes in δ34S, and is a function of elevation and/or soil type, with low lying areas, riparian zones and clay soil more likely to be temporarily or permanently waterlogged. Water plays two crucial roles in wetland and waterlogged soil sulfur cycling. First, it provides a barrier that limits oxygen diffusion and leads to soil anoxia, stimulating microbial sulfate reducing activity. Second, water carries dissolved sulfate for microbial sulfate reducers to use. Wetlands receive water directly via precipitation, and lose water via evapotranspiration. However, shallow groundwater is often the most important source of water for these systems, bringing in sulfate and other nutrients to support plant life. Here, we use the term shallow groundwater to include groundwater that originates from deeper circulation but becomes shallow once it has risen to the surface to provide baseflow to rivers and wetlands, and near-surface circulating groundwater that is recharged from surface runoff (Hiscock and Bense, 2021).
Natural and engineered wetland systems span a variety of hydrologic regimes which support sulfate reduction and sulfide oxidation alongside plant life (Figure 2). Here we consider sulfur isotope cycling under four different theoretical hydrological regimes which can support a range of wet environments from permanent wetlands to temporarily waterlogged landscapes in natural and engineered systems; flow-through, standing water, drained post-waterlogging, and simultaneously flooded and drained; and model their potential impact on plant δ34S values (models presented in Supplementary Information). Our models represent a first step toward understanding controls on wetland plant δ34S, by presenting four simple scenarios which, in the real world, are expected to be more complex. For example, wetland landscapes are likely to be supported by heterogeneous hydrology that varies with season and rainfall patterns. Sulfate reduction isotope effects may vary with seasonal temperatures and sulfate and organic carbon availability, and sulfate δ34S values may vary with rainfall and hydrology. Furthermore, plant uptake ratios are poorly understood and could vary, for example, with changes in sulfate concentration, temperature, season or phase in the plant's growth cycle, and sulfide adapted and non-adapted plants may grow side by side at wetland margins. These scenarios need to be tested in situ to form the foundation for archaeological studies in wetland environments.
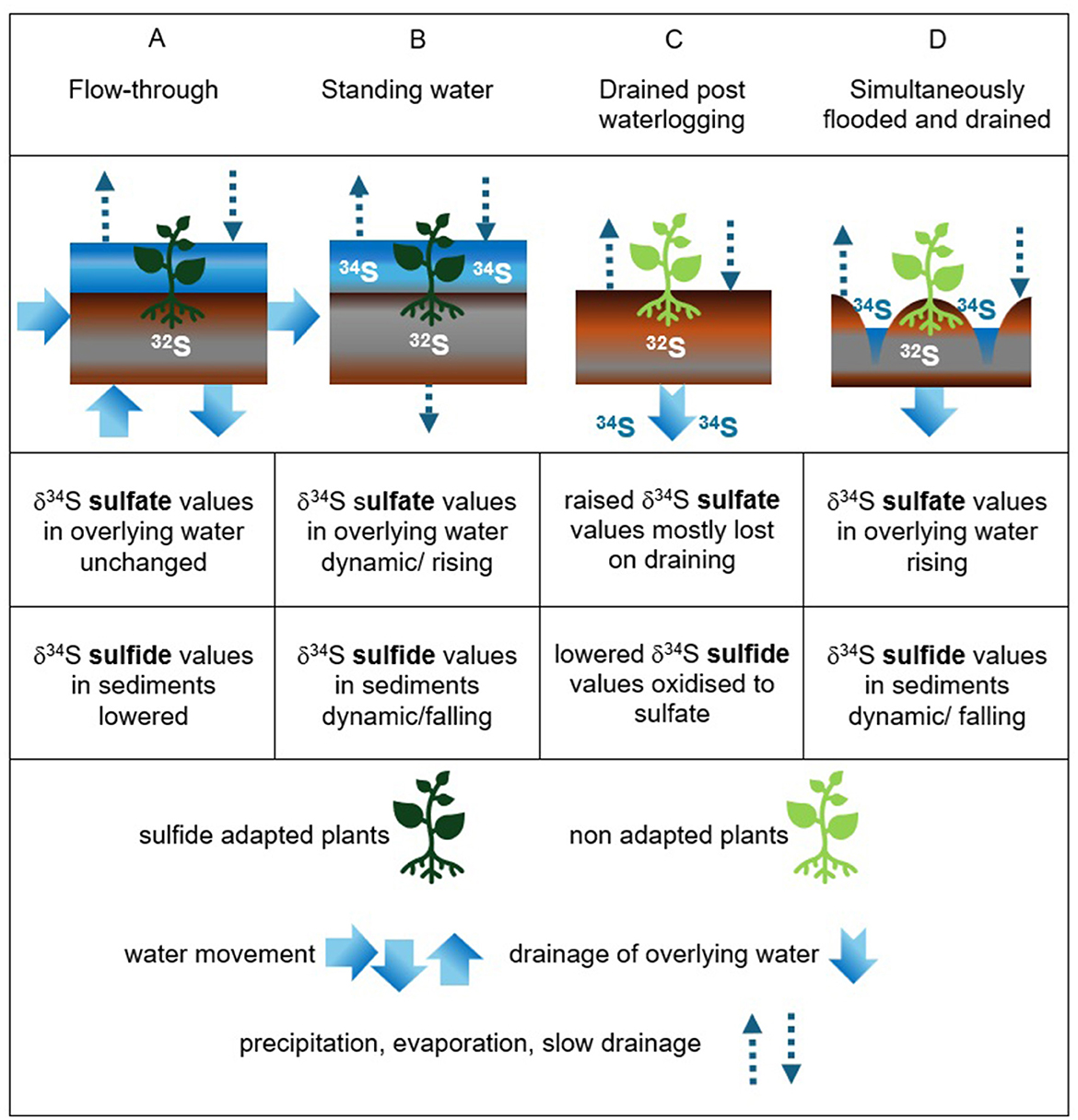
Figure 2. Four hydrological scenarios in natural and engineered wetland systems which support enhanced sulfate reduction and sulfide oxidation, including; (A) flow-through, (B) standing water, (C) drained post-waterlogging, and (D) simultaneously flooded and drained (standing water) hydrologies. Dashed arrows represent precipitation, evaporation, and very slow drainage, solid arrows represent water movement (shallow groundwater and surface water), and dark and light green plant symbols represent plants adapted to sulfide and unadapted plants, respectively. The partitioning of 34S in sulfate and 32S in sulfide due to isotopic fractionation during sulfate reduction is represented by white or blue font characters (color difference for readability only). These are absent from overlying water in (A) because no partitioning occurs there.
Omitted from these scenarios are ombrotrophic, nutrient poor, acid peat bogs, which are usually dominated by sphagnum. Evidence of early crop cultivation in such bogs is lacking and ombrotrophic bogs are unlikely to have been used for early crop cultivation due to their low level of nutrients and low pH. These bogs rely solely on precipitation for their water supply, and are disconnected from groundwater.
4.1 Flow-through hydrology
Plants living in wetland systems with flow-through hydrology (including natural wetland plants and the crops rice and taro) are sulfide adapted. The primary source of water and additional sulfate for many natural wetland environments is continuous lateral and/or vertical shallow groundwater flow (Winter, 1999) which supplies an ongoing input of sulfate derived from geological weathering and atmospheric sources for use by microbial sulfate reducers (Figure 2A). In such flow-through systems, sulfate which is reduced to sulfide in anoxic sediments is replenished with sulfate in the continuously flowing shallow groundwater. Such natural groundwater fed wetlands can be permanently waterlogged if shallow groundwater levels are stable, or temporarily waterlogged if shallow groundwater levels recede, and include riparian wetlands, marshes and fens. A similar hydrology existed where engineered flow-through systems were built, supplied with flowing water channeled from nearby sources such as springs and streams.
Referring to the partitioning of 32S and 34S within different reservoirs of sulfur during isotopic fractionation, flow-through wetlands maintain a near constant sulfate δ34S and sulfate concentration in overlying water because the continuous replenishment of sulfate from flowing water erases the effect of preferential removal of 32S by sulfate reduction (Figure 2A). 32S accumulates in sedimentary sulfide resulting in a lowered sulfide δ34S, which is passed on to plants via rhizosphere oxygenation and sulfide oxidation, resulting in plants with lowered δ34S. The difference between sulfate δ34S in the overlying water and sulfide δ34S in the sediment is stable if the magnitude of the isotope effect of sulfate reduction is stable (Högberg, 1997; Kendall and Caldwell, 1998; Mariotti et al., 1981), and is the first control on the lowered δ34S of the wetland plants. The magnitude of the isotope effect has been characterized for a limited number of wetlands (e.g., 11‰ to 51‰ Carlson and Forrest, 1982; Peterson et al., 1986; Price and Casagrande, 1991; Stribling et al., 1998). If minor fluctuations in groundwater sulfate δ34S occur as a result of changes in the relative proportions of sulfate source contributions, such as after recharge from rainfall or during drought, this could result in fluctuations in sulfate and sulfide δ34S values. As plant uptake ratios are not well known (see Section 2) and the sulfate reduction isotope effect is dependent on many factors (see Section 3.1), we use ranges from the literature, modeling plant uptake ratios of 0.6:0.4 to 0.8:0.2 and isotope effects of −10‰ to −40‰ (Figure 3A and Supplementary Information). Our models illustrate the effect of sulfate reduction as changes in plant δ34S (denoted by Δ34S) in different systems relative to baseline “initial sulfate,” driven by the geology, and are based on the simplifying assumption that there is no pre-existing sulfate or sulfide in the system. In the real world initial sulfate δ34S values could vary significantly depending on the underlying geology, which can encompass values from −20‰ to 30‰ (Seal, 2006).
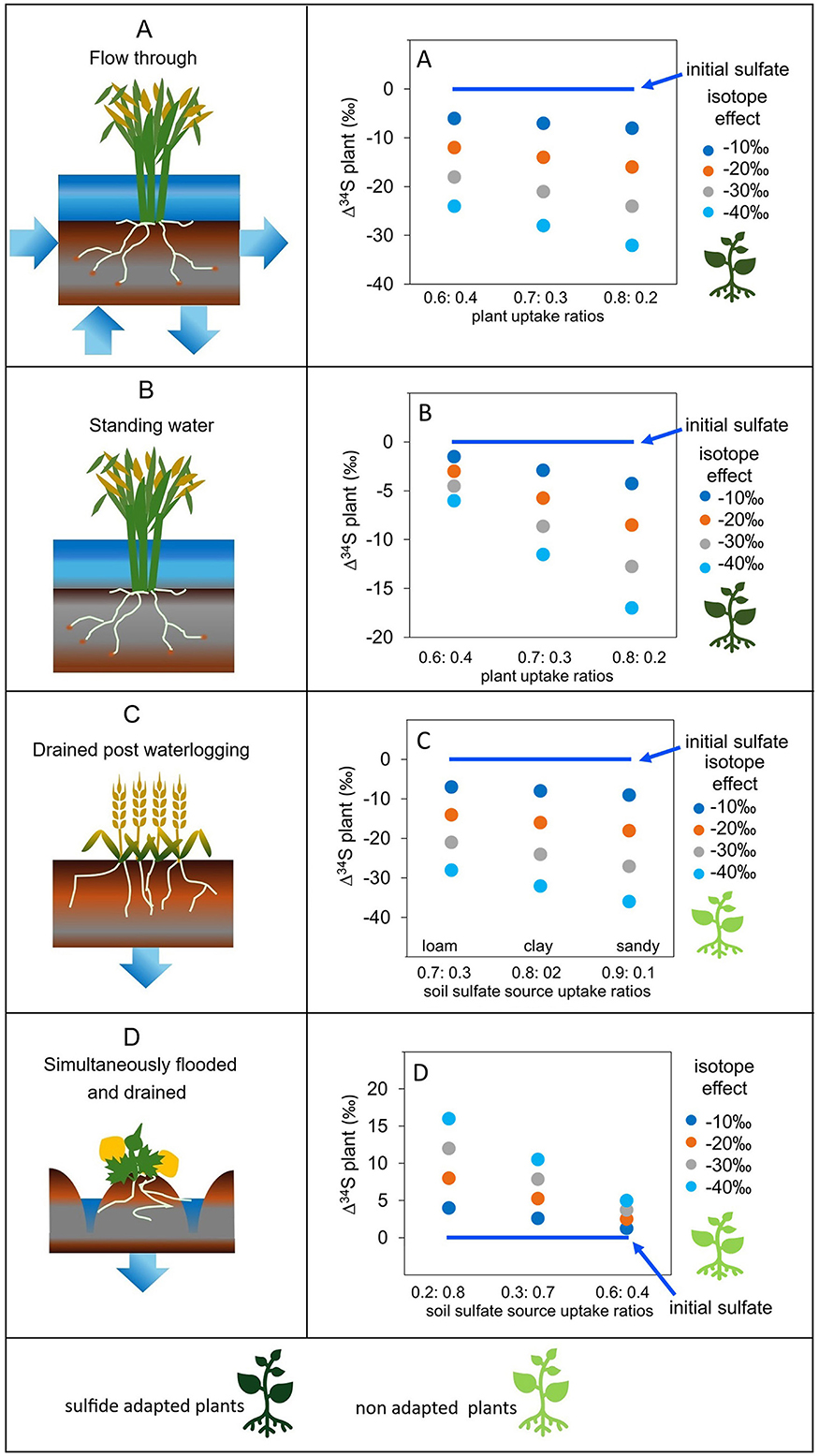
Figure 3. Representations of four wetland environments used for crop growing with different hydrologies; (A) flow-through, (B) standing water, (C) drained post flow-through waterlogging, and (D) simultaneously flooded with standing water and drained, with charts presenting ranges of plant δ34S. The plant changes in sulfur isotopic composition (Δ34S, where 0‰ refers to no change) relative to initial values were derived by combining modeled effects of sulfur fractionation associated with sulfate reduction (Högberg, 1997; Kendall and Caldwell, 1998; Mariotti et al., 1981), with four isotope effects for sulfate reduction; 10‰, 20‰, 30‰, 40‰, based on reported values from the literature (Carlson and Forrest, 1982; Peterson et al., 1986; Price and Casagrande, 1991; Stribling et al., 1998). These were combined with plant uptake ratios for emergent plants informed from the literature for (A) and (B) (Carlson and Forrest, 1982; Cornwell et al., 1995; Fry et al., 1982, 2023; Peterson et al., 1986; Price and Casagrande, 1991; Stribling and Cornwell, 1997; Stribling et al., 1998), and for soil sulfate source ratios for (C) and (D) using the proportion of sulfate in water remaining on draining based on soil porosity and the maximum available soil moisture on draining across three soil types (Kumar Rai et al., 2017; Marshall and Holmes, 1996). Initial values are not specified as they are controlled by the geological parent material and can span a range of at least 50‰ (e.g., Seal, 2006) (please refer to Supplementary Information for further details).
4.2 Standing water hydrology
Most plants growing in persistent standing water hydrology (including natural wetland plants and the crops rice and taro) are also sulfide adapted. Standing water systems receive water containing sulfate from rainfall and include seasonally and ephemerally waterlogged landscapes in low lying areas, and on poorly draining soil. In engineered standing water systems, pulses of water may have been diverted from local streams, lakes, or seasonal floodwaters, and retained by field edge structures, while poor soil permeability may have been enhanced by soil puddling (tillage under flooded conditions to reduce permeability), to prevent vertical drainage (Figure 2B). In these systems sulfate in overlying water slowly percolates into anoxic sediments to support microbial sulfate reduction. In the overlying water, sulfate δ34S and sulfate concentration undergo dynamic changes as sulfate reduction progresses, which in turn affect the δ34S of sulfide produced. In standing water systems which receive a single input of water, sulfate is progressively transferred from the overlying water to sulfide in the sediments where it reacts with iron to form insoluble precipitates. This results in an increase in 34S in the remaining sulfate in overlying water and in 32S in reduced sulfide in sediments as sulfate reduction proceeds. In this system, sulfate concentration in the overlying water decreases over time and the δ34S of sulfate rises well above the δ34S of the initial sulfate, while the δ34S of sulfide in the sediment rises gradually as more sulfide is sequestered, reaching a maximum δ34S sulfide value the same as that of initial sulfate. This point is reached when all sulfate from the single input has been reduced to sulfide (Högberg, 1997; Kendall and Caldwell, 1998; Mariotti et al., 1981) (Supplementary Information). Due to the fact that sulfide δ34S rises as sulfate reduction proceeds, there is a smaller difference between the initial sulfate δ34S and that of sulfide than the difference seen from flow-through systems (Figure 3B).
In some standing water systems, periodically repeated inputs of water supply fresh sulfate. Although the δ34S of sulfate and sulfide respond dynamically during sulfate reduction (Högberg, 1997; Kendall and Caldwell, 1998; Mariotti et al., 1981), the periodic inputs of sulfate buffer these effects, resulting in a similar effect to that seen from flow through systems. We also modeled plant δ34S with standing water hydrology and periodic inputs of “initial” sulfate in water, using sulfate and sulfide δ34S over time. The same plant uptake ratios and isotope effects are used as in the flow-through system. However, the range of resulting plant δ34S values is slightly smaller (Supplementary Information). Note that the plant uptake ratio is particularly sensitive in the standing water scenario as, if a large proportion of sulfate is taken up from overlying water after significant sulfate reduction, plant δ34S values could be raised.
Standing water hydrology and flow-through hydrology can occur on a continuum within the same wetland, often controlled by the seasonality of water inputs. For example, flow-through wetland systems may behave like standing water systems at their margins when water flow is reduced (e.g., Peterson et al., 1986), if they have poorly permeable sediments which restrict water movement, and with sediment depth (e.g., Carlson and Forrest, 1982; Price and Casagrande, 1991). In addition, many standing water systems receive sporadic inputs of water and sulfate from precipitation, flood events, high tides, or temporarily high groundwater levels (Supplementary Information).
4.3 Temporarily flooded and drained post-waterlogging
Plants grown after drainage of flooded soil conditions, (such plants include wild grasses and the crops barley, wheat, sorghum, and barnyard millet), are not able to oxidize sulfide but can tolerate transient flooded conditions. These plants have to take up their sulfur directly as sulfate from the environment. Many flow-through and standing water wetlands are temporarily waterlogged rather than permanently inundated. When flooded soil drains, the water which submerged the soil is mostly lost. This can occur via gradual drainage when flood waters recede and shallow groundwater levels drop, or, in engineered systems, as a result of intentional release (Figure 2C). Due to the high solubility of sulfate, when overlying water drains, much of the sulfate is lost via transport in drainage water. When drainage occurs, oxygen enters the soil triggering sulfide oxidation, creating low δ34S sulfate from the sulfide produced during waterlogging and stored in precipitates formed with iron in anoxic sediments (Figure 2C). Plants growing immediately after soils are drained incorporate sulfate primarily from sulfide which has been oxidized in the freshly aerobic soil. Where temporary wetlands are repeatedly flooded and drained, a cumulative effect can occur if conditions promote substantial sulfide oxidation each time the wetland is drained (for example if soils have high porosity, readily allowing oxygen to enter previously waterlogged pore space). Some wetlands, once drained, remain so permanently, whether as a result of natural processes (e.g., alterations in river courses), environmental change (e.g., lowered shallow groundwater levels as a results of decreases in recharging precipitation), or alteration in land use (e.g., marshland drained to create pasture or rice paddy fields drained to grow different crops). Permanent drainage enables oxygen to remain in the soil, which, as the soil is no longer waterlogged, no longer supports sulfate reduction. Within this drained soil, low δ34S sulfide (produced and stored in the soil before it was drained), is gradually oxidized to sulfate and is not replenished. This results in gradually decreasing inputs of low δ34S into the plant available sulfate pool from sulfide reserves near the soil surface; a process which may take many years (e.g., Chung et al., 2017). We have modeled δ34S for plants grown on drained soils after a flow-through hydrology (Figure 3C). We have estimated the proportion of sulfate lost in water on draining by considering soil water content under saturation and plant-available water on draining, for three soil types with different drainage; sandy, loam and clay soils. The different porosity of these soil types means they hold different proportions of water when saturated, and on draining, these soil properties affect how much of this water is available to plants (Kumar Rai et al., 2017; Marshall and Holmes, 1996) (Supplementary Information). Based on these estimates, we use soil sulfate source ratios (the relative proportions of plant-available sulfate from soil oxidized sulfide to remaining sulfate in soil water after draining), of 0.7:0.3, 0.8:0.2 to 0.9:0.1 for loam, clay and sandy soils, respectively. We use the same isotope effects as in the previous models. This system results in lowered plant δ34S. If before draining, the system had a standing water hydrology (rather than flow-through modeled here), we expect δ34S values of plants grown in drained soil to be lowered, but not lowered to the extent of plants growing in a drained flow-through system, reflecting the smaller signal expected from standing water systems in general (5.2). In landscapes that are repeatedly flooded and drained, we expect δ34S of plants grown in drained soil to be lowered by a similar amount as that expected from flow-through systems after draining.
4.4 Simultaneously flooded and drained hydrology
Plants grown in simultaneously flooded and drained systems (including the crops barley, wheat, squash, maize, beans) are not able to oxidize sulfide, but can tolerate partially saturated soil conditions. Such conditions occur through minor variations in elevation in waterlogged and flooded natural landscapes, and in some engineered wetlands (e.g., ridge and furrow, raised fields, floating fields) (Figure 2D). The hydrology in these systems may be standing water or flow-through. Such systems simultaneously support sulfate reduction in the waterlogged soil at depth, producing low δ34S sulfide values, and sulfate oxidation in the soil above the water, producing low δ34S sulfate (Figure 2D). However, plants grown on unsaturated soil above a high-water table are expected to incorporate sulfate primarily from roots reaching the upper level of saturated soil and the overlying water directly. The sulfate δ34S of these sources is controlled by the flow-through or standing water status of the water. We have modeled the plant δ34S with standing water hydrology over a range of soil and water sulfate source ratios of 0.4:0.6 to 0.2:0.8, at 50% reduction of sulfate to sulfide (but as discussed above the extent of reduction is dynamic). In simultaneously flooded and drained systems with standing water hydrology, plant δ34S could be raised above the initial sulfate δ34S, as illustrated in Figure 3D. However, if such systems have flow-through hydrology we would expect plant δ34S to be slightly below the initial sulfate δ34S of the water.
5 Sulfur wetland isotopic signals in archaeology
5.1 Ancient water management practices
These model results suggest that through analysis of archaeological crop δ34S we may be able to differentiate different water management practices, many of which played a critical role in subsistence agriculture and its development and intensification. In ancient societies, the ability to access, control and distribute water effectively often determined the success or failure of agricultural production, and increasingly sophisticated techniques often accompanied agricultural intensification (Fuller, 2020). Some of the earliest arable farmers harnessed the natural environment through “least-effort” cultivation on floodplains, as these areas offered fertile soils enriched by seasonal flooding, along with a reliable supply of water (Ma et al., 2016). Later, arable farmers developed infrastructure to manage water supply either to enable cultivation on land that required irrigation, or to utilize waterlogged or frequently flooded land through drainage (Langlie, 2018; Zhuang et al., 2025). This utilization or management of water is argued to have enabled early farmers not only to continue producing food in unpredictable climates but also to create agricultural surpluses that are thought to have underpinned the development of complex societies (Zhuang et al., 2014). While broad patterns of natural and managed water use in prehistoric agriculture are well-documented, the study of past water management regimes primarily relies on indirect evidence such as traces of irrigation infrastructure and weed ecology (Petrie and Bates, 2017; Zhuang et al., 2025). These methods often fall short in accurately reconstructing past water conditions, restricting interpretations to a coarse resolution. Analysis of δ34S values of archaeological crop remains may have the potential to elucidate past regimes of water management for cultivation and to determine the hydrological conditions under which agricultural production was taking place (Stevens et al., 2022), particularly if dryland bioavailable sulfur δ34S baselines derived from local geological and marine sulfur can be well-characterized.
In addition to supporting early agricultural development and intensification, the human manipulation of natural wet and waterlogged environments and the creation of engineered wetlands for crop production are likely to have resulted in environmental harms to the immediate environment and beyond. Such detrimental effects could have included biodiversity loss, the disruption of sediment cycling, and increases in greenhouse gas emissions (Erickson, 1992; Fuller et al., 2011; Marston and Scott Branting, 2016; Robinson and Lambrick, 1984). By studying archaeological crop δ34S we may be able to further our understanding of the timing and extent of these practices to clarify the likely extent of the environmental damage associated with managing water and wetlands for crop production.
The effects of past water management techniques on archaeological crop δ34S depends on the hydrology of the technique involved, and any waterlogging adaptation of the crop grown (Table 1). Here we suggest which model scenario (Section 4.) would best represent a particular past water management technique for specific crops. Rice and taro are probably the only truly flood adapted crops able to oxidize sulfide in the rhizosphere and to thrive when partially submerged (Abiko and Miyasaka, 2020; Nishiuchi et al., 2012). In the natural environment some wild rice and taro species would have grown in permanent wetlands which were likely to have included flow-through and standing water hydrology (Fullagar et al., 2006; Müller et al., 2010; Zheng et al., 2009; Zong et al., 2007) (Figures 3A, B). Under paddy cultivation, flow-through systems and standing water systems were likely used to grow wet rice and taro (Kay et al., 2019; Lee et al., 2014), depending on water source availability, with paddy drainage before harvest. Where such seasonal drainage occurred this would have removed much of the sulfate in overlying water and allowed oxidation of sulfide in the soil, which would then have become available for uptake by catch crops, as in the case of the rice-wheat system (Bates et al., 2017) (Figures 3A, B, followed by Figure 3C). Irrigation techniques which involved temporarily submerging soil, for example by engineered trapping of seasonal flood waters, would have suited cultivation of crops with some waterlogging tolerance such as dry rice, sorghum, and barnyard millet (Bates et al., 2017; Bhinda et al., 2023; Kay et al., 2019; Khalifa and Eltahir, 2023; Dal Corso et al., 2022; Neumann, 2018 (Figure 3C).
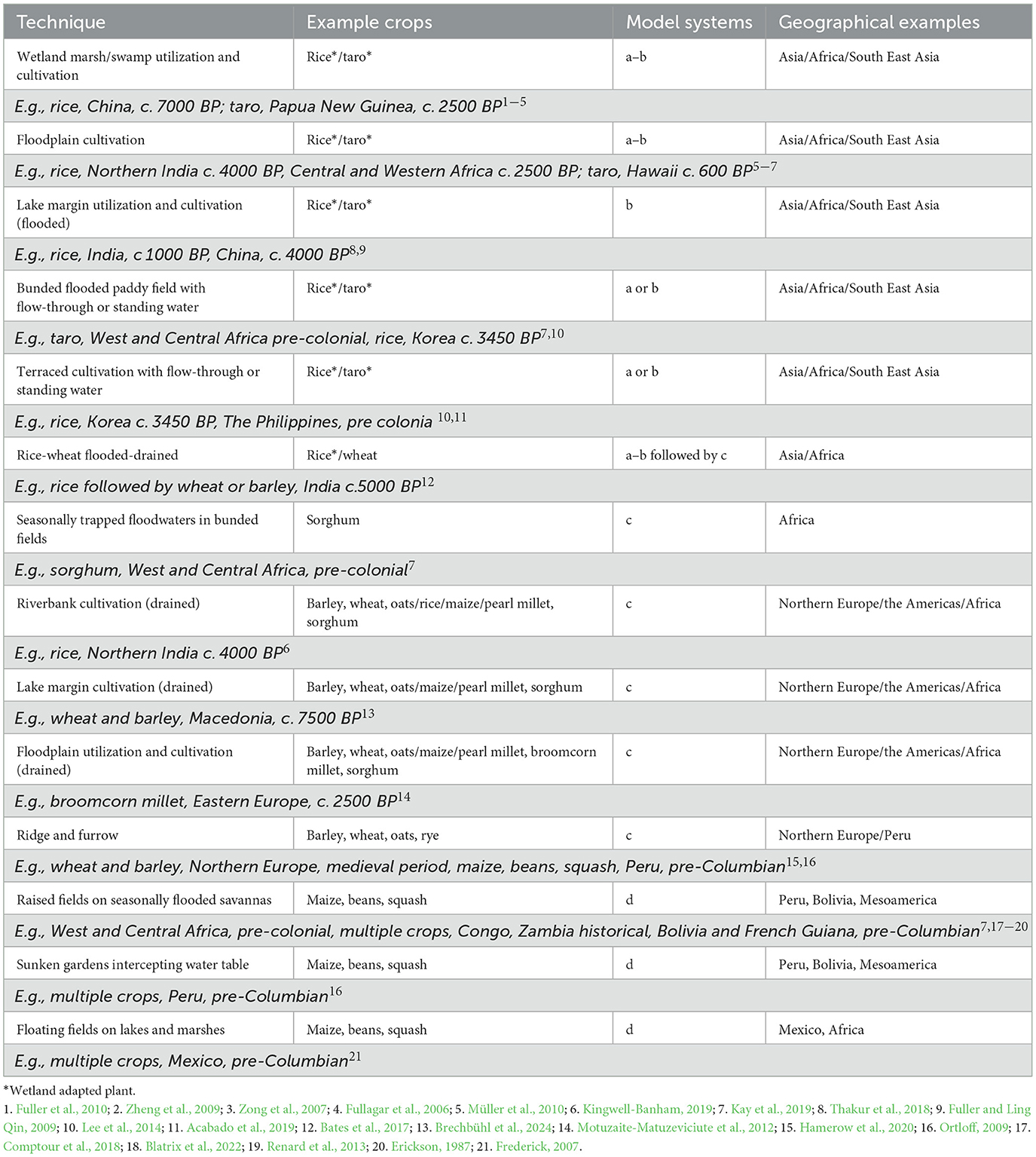
Table 1. Past wetland utilization and water management techniques for crop cultivation, showing example crops grown using these techniques, the hydrological type they correspond to (Figures 3A–D), and examples of geographical regions and periods associated with techniques were found (examples included in italics with references).
Similarly, crops grown after natural drainage of flood waters would have been cultivated in environments with seasonally high groundwater levels such as riverbanks, floodplains, lake margins, river valley lowlands, and ridge and furrow on low lying, poorly draining soil, and were likely to be able to tolerate temporary flooding and high groundwater levels in the root zone (Brechbühl et al., 2024; Hamerow et al., 2020; Kay et al., 2019; Kingwell-Banham, 2019; Motuzaite-Matuzeviciute et al., 2012; Ortloff, 2009). Where simultaneous flooding and drainage was engineered, cultivation may also have taken place at elevations above the reach of high groundwater levels, and crop plants may not have required flood tolerance (Figure 3D). Raised fields, floating fields, and sunken gardens, found in the Americas and parts of Africa (Blatrix et al., 2022; Comptour et al., 2018; Erickson, 1987; Frederick, 2007; Ortloff, 2009), would have enabled a steady supply of water to reach plant roots while protecting crops from waterlogging. It is possible, therefore, that in cases where dryland environmental δ34S baselines can be established, and in combination with other contextual information, analysis of δ34S in archaeological crop remains could be useful to differentiate between different wetland utilization strategies, as represented in our four modeled scenarios (Figures 3A–D).
5.2 Signals from past climate adaptation
Where changes in δ34S values are found in charred archaeological grains from the same site across different phases, this could suggest different water management practices or environmental change through time, especially if this can be supported by information from weed ecology or weed phytoliths. Since its domestication, rice has been cultivated using wet (flooded) and rainfed (dryland) methods. Switches between cultivation methods, and expansion into higher yielding wet rice cultivation have been linked to changes in climate in Iron Age North-eastern Thailand and the Lower Yangtze, China between 7000 and 6000 BP (Castillo et al., 2018; Patalano et al., 2015). These studies could be further elucidated by investigating variations in δ34S of charred archaeological rice from these sites which may be associated with changes in cultivation method, and to ascertain whether signals correspond in time to changes seen in climate proxies. We would expect significantly lower δ34S values from rice cultivated using wet techniques with flow-through hydrology than from rice grown using the rainfed method.
5.3 Sulfur isotopes in human and animal collagen and wetland resource use
Sulfur in human and animal bone and dentine collagen is derived through diet. At the base of the food chain, sulfur, an essential nutrient for plants, is taken up as sulfate from soil. Once within the plant, sulfur is used to make amino acids cysteine and methionine, and many other compounds (Hill et al., 2022; Li et al., 2022). Sulfur is passed up the food chain with minimal trophic enrichment when plants are eaten, so plant δ34S is broadly conserved during the transfer of sulfur from plants to animals (Cavallaro et al., 2022; Krajcarz et al., 2019; Nehlich, 2015; Raoult et al., 2024; Tcherkez and Tea, 2013). Collagen in bones and dentine primarily consists of type 1 collagen, and its sulfur content arises exclusively from methionine (Anné et al., 2019). Therefore, most sulfur preserved in archaeological collagen is derived from the essential amino acid methionine, which is only synthesized by plants. With respect to the transfer of δ34S from plants to animals, it is not known whether the overall (bulk) plant δ34S is close to, or is significantly different from plant methionine δ34S. It has been suggested that plant methionine δ34S could differ significantly from the δ34S of bulk plant material. This is because methionine is produced via a series of reactions which may cause isotopic fractionations in both directions, possibly resulting in an overall isotopic offset (Tcherkez and Tea, 2013). Clarification would require studies using compound-specific measurements of plant methionine δ34S in comparison to bulk plant δ34S from the same samples. However, until such studies are published, useful information can be found in two controlled animal feeding studies, which compared faunal bone collagen δ34S against plant material feed with characterized bulk plant δ34S (Tanz and Schmidt, 2010; Webb et al., 2017). These studies found a very small isotopic offset in animal bone collagen δ34S (which is primarily from methionine) relative to the bulk δ34S of plant-based feed (−0.5‰, Tanz and Schmidt, 2010; −1.5± 0.8‰; Webb et al., 2017). This suggests bulk plant δ34S is similar to plant methionine δ34S, and that bulk plant δ34S can be used to represent “base of the food chain” δ34S values in archaeological studies of bone and tooth collagen within a small margin of error. In this way, herbivore bone collagen δ34S can be seen as an integrator of environmental signals carried in plant δ34S as plant δ34S reflects the environment in which the plant grew. See Tanz and Schmidt (2010), and Tcherkez and Tea (2013), for further details on plant sulfur metabolism and isotopic fractionation, Anné et al. (2019) for discussion of collagen synthesis and sulfur preservation in archaeological bones and Devignes et al. (2022), for detailed information on the role of amino acids including methionine in bones.
Some studies also use δ34S of archaeological keratin from antlers, horns, hooves and hair. However, sulfur in keratin is mainly in the form of the non-essential amino acid cysteine which can be synthesized by mammals from various dietary sources of sulfur, such that keratin may be a less direct tracer of wetland environments than collagen δ34S. Despite this, evidence for a relatively minor effect from isotopic fractionation during sulfur uptake into keratin is also suggested by controlled feeding studies and wild animal tracking (Kabalika et al., 2023; Richards et al., 2003).
The incorporation of a wetland δ34S signal into bone and dentine collagen has the potential both to complicate and enhance the interpretation of archaeological human and faunal isotope data. In addition, wetland δ34S signals may overlap geologically driven δ34S baselines, particularly where geological δ34S reflects past large-scale microbial sulfate reduction. The incorporation of a wetland δ34S signal by mammals via diet is relevant for studies of hunter gathers, mobile pastoralists, and sedentary agricultural communities. For example, significant wild wetland resources use by hunter gatherers could potentially be seen in human collagen δ34S (Rand et al., 2021). Similarly, where a significant proportion of human dietary methionine intake originated from crops cultivated on wetlands and from livestock which grazed on or were foddered from the wetlands, a wetland δ34S signal from plants will be reflected in the consumer's collagen δ34S. This could be used to indicate the cultivation of wetlands by early agricultural communities.
Variations in the magnitude of a wetland signal in human and animal collagen δ34S could originate from differences in the proportion of dietary protein from a waterlogged or recently drained environment over time. For example, this could occur with different farming strategies for floodplain use including floodplain grazing, seasonal fodder using floodplain grown plants, and flood recession arable farming (Figures 4A–C).
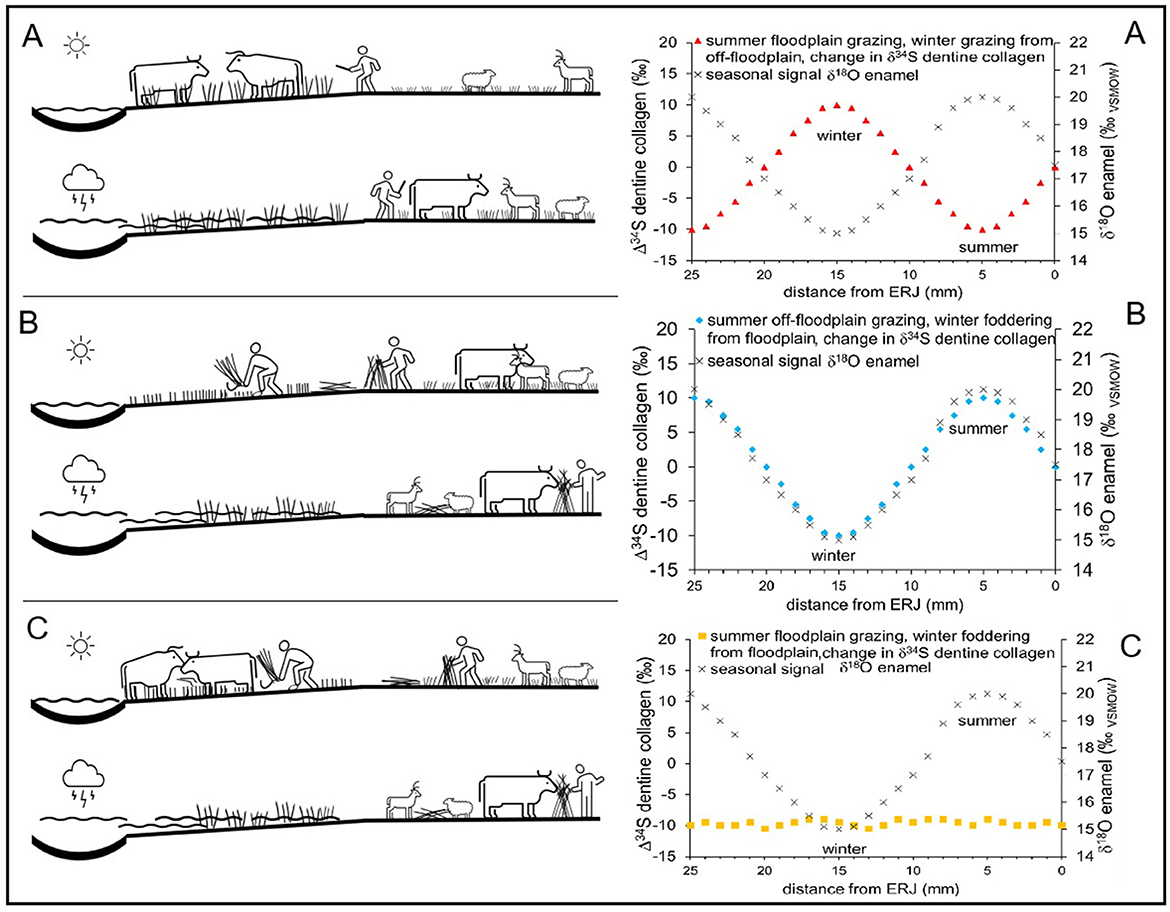
Figure 4. Illustrations of three possible floodplain utilization scenarios leading to different incorporation of sulfate reduction signals into cattle dentine collagen δ34S [left-hand panels (A–C)], with corresponding patterns of expected changes in sulfur isotopic composition (Δ34S) where 0‰ on the left hand axis refers to no change, with δ18O in millimeter increments of dentine and enamel [right-hand panels (A–C)], which may be revealed by serial analysis.
With well-characterized dryland baseline δ34S, serial analysis of δ34S in dentine collagen from archaeological herbivore teeth could provide high-resolution insights into ancient farming practices, including the movement of animals across regions with different geologies and the use of resources from wetlands or recently drained areas within landscapes. Dentine protein, which comprises c. 90% collagen (Goldberg et al., 2004), records a time series of isotopic inputs from diet which is unchanged after deposition. Dentine growth bands form in hypsodont herbivore teeth every few months (Zazzo et al., 2006), potentially recording seasonal signals in dentine collagen. Thus, incremental δ34S analysis of hypsodont tooth dentine collagen, as has been conducted for δ13C and δ15N (Díez-Canseco and Tornero, 2024), could be used to explore different floodplain utilization scenarios (e.g., Figures 4A–C), perhaps with seasonal climate signals constrained by incremental analysis of δ18O in enamel bioapatite adjusted for differences in timing and mineralization processes between dentine and enamel (Balasse, 2002).
6 Conclusions
This work highlights the dynamic nature of sulfur isotope baselines affected by sulfur cycling in wet and waterlogged environments, while acknowledging the complexity of establishing local dryland δ34S baselines derived from geological and marine sulfur against which to assess potential wetland signals for archaeological applications. Wetland effects on bioavailable sulfur δ34S can both confound and enrich the interpretation past human and animal movement and crop production, and provide exciting new avenues for archaeological investigation.
This study provides a step toward integrating palaeo sulfur cycling into archaeological isotope studies, but there is much further work to be done. The complex interactions between physiochemical conditions that support isotopically fractionating microbial sulfate reduction and subsequent sulfide oxidation, with varied isotope effects, hydrology and differences in plant sulfate and oxidized sulfide uptake, lead to potentially wide ranges of plant δ34S. Wetland signals may overlap geological δ34S and mimic the effects of movement and diet in faunal and human archaeological collagen δ34S. Factors affecting the isotope effect of sulfate reduction, plant uptake ratios and soil sulfur source ratios are not well-characterized, yet are key to our understanding of δ34S in bioarchaeological material. Further studies are needed to understand these interactions so they can be better resolved for use in archaeological studies in relation to natural and manipulated waterlogged environments. We emphasize the necessity of acknowledging possible effects from coeval sulfur cycling when interpreting δ34S values in bioarchaeological material. Where it is possible to resolve a wetland δ34S signal in collagen or archaeological crop remains, this could provide important new information about past resource use, early farming techniques, and animal feeding patterns. Wetlands, both natural and engineered, have long been integral to human subsistence and agricultural innovation, from the fertile floodplains of early farmers to the sophisticated irrigation and drainage systems that sustained complex societies. With further knowledge, δ34S analysis of bioarchaeological material could become a powerful tool for directly tracing past wetland use and water management strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
SKW: Writing – review & editing, Conceptualization, Writing – original draft, Methodology. RES: Writing – review & editing, Writing – original draft, Methodology, Conceptualization.
Funding
The authors declare that financial support was received for the research and/or publication of this article. NERC Pushing the Frontiers grant (NE/W000792/1).
Acknowledgments
We would like to thank Simon Bottrell, Delphine Frémondeau, and Daniel H. James for their comments on a draft of this article, and Brian Fry and Clement Bataille for helpful discussions on sulfur cycling and plant sulfur uptake and the implications for archaeology. This research was undertaken for the Iso-Wetlands project funded by NERC Pushing the Frontiers grant (NE/W000792/1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fearc.2025.1599779/full#supplementary-material
References
Abiko, T., and Miyasaka, S. C. (2020). Aerenchyma and barrier to radial oxygen loss are formed in roots of Taro (Colocasia esculenta) propagules under flooded conditions. J. Plant Res. 133, 49–56. doi: 10.1007/s10265-019-01150-6
Acabado, S. B., Koller, J. M., Liu, C., Lauer, A. J., Farahani, A., Barretto-Tesoro, G., et al. (2019). The short history of the Ifugao rice terraces: a local response to the Spanish conquest. J. Field Arch. 44, 195–214. doi: 10.1080/00934690.2019.1574159
Ando, T., Yoshida, S., and Nishiyama, I. (1983). Nature of oxidizing power of rice roots. Plant Soil 72, 57–71. doi: 10.1007/BF02185094
Aneja, V. P. (1990). Natural sulfur emissions into the atmosphere. J. Air Waste Man. Ass. 40, 469–476. doi: 10.1080/10473289.1990.10466701
Anné, J., Edwards, N. P., Brigidi, F., Gueriau, P., Harvey, V. L., Geraki, K., et al. (2019). Advances in bone preservation: identifying possible collagen preservation using sulfur speciation mapping. Palaeogeog. Palaeoclimatol. Palaeoecol. 520, 181–187. doi: 10.1016/j.palaeo.2019.01.030
Ayala, G., Bogaard, A., Charles, M., and Wainwright, J. (2022). Resilience and adaptation of agricultural practice in Neolithic Çatalhöyük, Turkey. World Archaeol. 54, 407–428. doi: 10.1080/00438243.2022.2125058
Balasse, M. (2002). Reconstructing dietary and environmental history from enamel isotopic analysis: time resolution of intra-tooth sequential sampling. Int. J. Osteoarchaeol. 12, 155–165. doi: 10.1002/oa.601
Ballantyne, R., Cooper, A., and Rajkovača, V. (2024). “Food,” in Must Farm Pile-Dwelling Settlement Volume 1. Landscape, Architecture and Occupation, eds M. Knight, R. Ballantyne, M. Brudenell, A. Cooper, D. Gibson, and I. Robinson Zeki. (McDonald Institute Monographs, University of Cambridge), 173–204. Available online at: https://www.repository.cam.ac.uk/handle/1810/365343
Bataille, C. P., Chartrand, M. M. G., Raposo, F., and St-Jean, G. (2020). Assessing geographic controls of hair isotopic variability in human populations: a case-study in Canada. PLoS ONE 15:e0237105. doi: 10.1371/journal.pone.0237105
Bates, J., Petrie, C. A., and Singh, R. N. (2017). Approaching rice domestication in South Asia: new evidence from Indus settlements in northern India. J. Archeol. Sci. 78, 193–201. doi: 10.1016/j.jas.2016.04.018
Bhinda, M. S., Joshi, D. C., Parihar, M., Meena, R. P., Joshi, P., Gupta, A., et al. (2023). “Genetics, breeding, and genomics of Indian barnyard millet (Echinochloa frumentacea): status and perspectives,” in Neglected and Underutilized Crops: Future Smart Food, eds M. Farooq, and K. H. M. Siddique (Amsterdam: Academic Press; Elsevier), 115–135. doi: 10.1016/B978-0-323-90537-4.00017-X
Blatrix, R., Aramayo, J. L., Zangerlé, A., Roux, B., Jouanne, M., Anselme, B., et al. (2022). Interpreting landscapes of pre-Columbian raised-field agriculture using high-resolution LiDAR topography. J. Archaeol. Sci. Report 42:103408. doi: 10.1016/j.jasrep.2022.103408
Bottrell, S. H., and Newton, R. J. (2006). Reconstruction of changes in global sulphur cycling from marine sulphate isotopes. Earth-Sci. Rev. 75, 59–83. doi: 10.1016/j.earscirev.2005.10.004
Bottrell, S. H., and Raiswell, R. (2000). “Sulphur isotopes and microbial sulphur cycling in sediments,” in Microbial Sediments, eds R. E., Riding, and S. M. Awramik (Berlin, Heidelberg: Springer), 96–104. doi: 10.1007/978-3-662-04036-2_12
Brechbühl, S., van Vugt, L., Gobet, E., Morales-Molino, C., Volery, J., Lotter, A. F., et al. (2024). Vegetation dynamics and land-use change at the Neolithic lakeshore settlement site of Ploča Mičov Grad, Lake Ohrid, North Macedonia. Veget. Hist. Archaeobot. 33, 247–267. doi: 10.1007/s00334-023-00931-3
Canfield, D. E. (2001). Biogeochemistry of sulfur isotopes. Rev. Min. Geochem. 43, 607–636. doi: 10.2138/gsrmg.43.1.607
Carlson, P. R., and Forrest, J. (1982). Uptake of dissolved sulfide by Spartina alterniflora: evidence from natural sulfur isotope abundance ratios. Science 216, 633–635. doi: 10.1126/science.216.4546.633
Castillo, C. C., Higham, C. F. W., Miller, K., Chang, N., Douka, K., Higham, T. F. G., et al. (2018). Social responses to climate change in Iron Age north-east Thailand: new archaeobotanical evidence. Antiquity 92, 1274–1291. doi: 10.15184/aqy.2018.198
Cavallaro, V., Maghrebi, M., Caschetto, M., Sacchi, G. A., and Nocito, F. F. (2022). Sulfur stable isotope discrimination in rice: a sulfur isotope mass balance study. Front. Plant Sci. 13:837517. doi: 10.3389/fpls.2022.837517
Chaudhary, S., Singh Sindhu, S., Rinku Dhanker, R., and Anju Kumari, A. (2023). Microbes-mediated sulphur cycling in soil: impact on soil fertility, crop production and environmental sustainability. Microbiol. Res. 271:127340. doi: 10.1016/j.micres.2023.127340
Chung, C. H., You, C. F., Hsu, S. C., and Liang, M. C. (2019). Sulfur isotope analysis for representative regional background atmospheric aerosols collected at Mt. Lulin, Taiwan. Sci. Rep. 9:19707. doi: 10.1038/s41598-019-56048-z
Chung, I. M., Lee, T. J., Oh, Y. T., Kumar Ghimire, B., Jang, I. B., and Kim, S. H. (2017). Ginseng authenticity testing by measuring carbon, nitrogen, and sulfur stable isotope compositions that differ based on cultivation land and organic fertilizer type, J. Ginseng Res., 41, 195–200. doi: 10.1016/j.jgr.2016.03.004
Claypool, G. E., Holser, W. T., Kaplan, I. R., Sakai, H., and Israel Zak, I. (1980). The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 28, 199–260. doi: 10.1016/0009-2541(80)90047-9
Comptour, M., Caillon, S., Rodrigues, L., and McKey, D. (2018). Wetland raised-field agriculture and its contribution to sustainability: ethnoecology of a present-day African system and questions about pre-Columbian systems in the American tropics. Sustainability 10:3120. doi: 10.3390/su10093120
Cornwell, J. C., Neill, C., and Stevenson, J. C. (1995). Biogeochemical origin of δ34S isotopic signatures in a prairie marsh. Can. J. Fish. Aquat. Sci. 52, 1816–1820. doi: 10.1139/f95-174
Czére, O., Lawson, J. A., Müldner, G., Evans, J., Boyle, A., and Britton, K. (2022). The bodies in the ‘bog': a multi-isotope investigation of individual life-histories at an unusual 6th/7th AD century group burial from a Roman latrine at Cramond, Scotland. Archaeol. Anthropol. Sci. 14:67. doi: 10.1007/s12520-022-01509-2
Dal Corso, M., Pashkevych, G., Filipović, D., Liu, X., Motuzaite-Matuzeviciute, G., Stobbe, A., et al. (2022). Between cereal agriculture and animal husbandry: millet in the early economy of the north Pontic Region. J. World Prehist. 35, 321–374. doi: 10.1007/s10963-022-09171-1
de la Cruz Jiménez, J., Pellegrini, E., Pedersen, O., and Nakazono, M. (2021). Radial oxygen loss from plant roots-methods. Plants 10:2322. doi: 10.3390/plants10112322
Devignes, C., Carmeliet, G., and Stegen, S. (2022). Amino acid metabolism in skeletal cells. Bone Rep. 17:101620. doi: 10.1016/j.bonr.2022.101620
Díez-Canseco, C., and Tornero, C. (2024). New methods for old challenges: a sampling protocol for sequential stable isotope analysis (δ13C and δ15N) of dentine collagen in high-crowned teeth. J. Archaeol. Sci. 162:105923. doi: 10.1016/j.jas.2023.105923
Dušek, J., Darenová, E., Pavelka, M., and Marek, M. V. (2020). “Methane and carbon dioxide release from wetland ecosystems,” in Climate Change and Soil Interactions, eds M. Narasimha, V. Prasad, and M. Pietrzykowski (Amsterdam: Elsevier), 509–553. doi: 10.1016/B978-0-12-818032-7.00019-9
Ebert, C. E., Rand, A. J., Green-Mink, K., Hoggarth, J. A., Freiwald, C., Awe, J. J., et al. (2021). Sulfur isotopes as a proxy for human diet and mobility from the preclassic through colonial periods in the Eastern Maya lowlands. PLoS ONE 16:e0254992. doi: 10.1371/journal.pone.0254992
Erickson, C. L. (1987). “The dating of raised field agriculture in the Lake Titicaca basin of Peru,” in Pre-Hispanic Agricultural Fields in the Andean Region, eds W. M. Denevan, K. Mathewson, and G. Knapp (Oxford: British Archaeological Reports, International Series, BAR Publishing), 373–383.
Erickson, C. L. (1992). Prehistoric landscape management in the Andean highlands: raised field agriculture and its environmental impact. Pop. Environ. 13, 285–300. doi: 10.1007/BF01271028
Evans, J. A., Mee, K., Chenery, C. A., and Marchant, A. P. (2023). Biosphere isotope domains GB (V2): interactive website. British Geological Survey (Dataset) (accessed February 10, 2025).
Frederick, C. D. (2007). “Chinampa cultivation in the basin of Mexico,” in Seeking a Richer Harvest. Studies in Human Ecology and Adaptation, vol 3, eds T. L. Thurston, and C. T. Fisher (Boston, MA: Springer), 107–124. doi: 10.1007/978-0-387-32762-4_5
Fry, B., O'Mara, K., Riekenberg, P. M., Wassenaar, L. I., and Cormier, N. (2023). Flexitraits, natural chemical tracers of plant competition and productivity in pacific mangroves. Wetlands 43:31. doi: 10.1007/s13157-023-01672-9
Fry, B., Scalan, R. S., Winters, K. J., and Parker, P. L. (1982). Sulphur uptake by salt grasses, mangroves, and seagrasses in anaerobic sediments. Geoch. Cosmochim. Acta 46, 1121–1124. doi: 10.1016/0016-7037(82)90063-1
Fullagar, R., Field, J., Denham, T., and Lentfer, C. (2006). Early and mid Holocene tool-use and processing of taro (Colocasia esculenta), yam (Dioscorea sp.) and other plants at Kuk Swamp in the highlands of Papua New Guinea. J. Archaeol. Sci. 33, 595–614. doi: 10.1016/j.jas.2005.07.020
Fuller, D. Q. (2020). Transitions in productivity: rice intensification from domestication to urbanisation. Archaeol. Int. 23, 88–103. doi: 10.14324/111.444.ai.2020.08
Fuller, D. Q., and Ling Qin, L. (2009). Water management and labour in the origins and dispersal of Asian rice. World Archaeol. 41, 88–111. doi: 10.1080/00438240802668321
Fuller, D. Q., Sato, Y. I., Castillo, C., Ling Qin, L., Weisskopf, A. R., Kingwell-Banham, E. J., et al. (2010). Consilience of genetics and archaeobotany in the entangled history of rice. Archaeol. Anthropol. Sci. 2, 115–131. doi: 10.1007/s12520-010-0035-y
Fuller, D. Q., van Etten, J., Manning, K., Castillo, C., Kingwell-Banham, E., Weisskopf, A., et al. (2011). The contribution of rice agriculture and livestock pastoralism to prehistoric methane levels: an archaeological assessment. Holocene 21, 743–759. doi: 10.1177/0959683611398052
Goldberg, M., Septier, D., Bourd, K., and Menashi, S. (2004). Role of matrix proteins in signalling and in dentin and enamel mineralisation. Comptes Rendus Palevol 3, 573–581. doi: 10.1016/j.crpv.2004.07.005
Guiry, E., Noël, S., and Fowler, J. (2021). Archaeological herbivore δ13C and δ34S provide a marker for saltmarsh use and new insights into the process of 15N-enrichment in coastal plants. J. Archaeol. Sci. 125:105295. doi: 10.1016/j.jas.2020.105295
Guiry, E. J., Orchard, T. J., Needs-Howarth, S., and Szpak, P. (2022). Freshwater wetland-driven variation in sulfur isotope compositions: implications for human paleodiet and ecological research. Front. Ecol. Evol. 10:953042. doi: 10.3389/fevo.2022.953042
Guiry, E. J., and Szpak, P. (2020). Seaweed-eating sheep show that δ34S evidence for marine diets can be fully masked by sea spray effects. RCMS 34:e8868. doi: 10.1002/rcm.8868
Hamerow, H., Bogaard, A., Charles, M., Forster, E., Holmes, M., McKerracher, M., et al. (2020). An integrated bioarchaeological approach to the Medieval ‘Agricultural Revolution': a case study from Stafford, England, c. ad 800-1200. Europ. J. Archaeol. 23, 585–609. doi: 10.1017/eaa.2020.6
Hill, C. R., Shafaei, A., Balmer, L., Lewis, J. R., Hodgson, J. M. A., et al. (2022). Sulfur compounds: from plants to humans and their role in chronic disease prevention. Crit. Rev. Food Sci. and Nutr. 63, 8616–8638. doi: 10.1080/10408398.2022.2057915
Hillman, G., Hedges, R., Moore, A., Colledge, S., and Pettitt, P. (2001). New evidence of Lateglacial cereal cultivation at Abu Hureyra on the Euphrates. Holocene 11, 383–393. doi: 10.1191/095968301678302823
Hiscock, K. M., and Bense, V. (2021). Hydrogeology: Principles and Practice, 3rd Edn. New Jersey: Wiley-Blackwell.
Högberg, P. (1997). Tansley review No. 95. 15N natural abundance in soil-plant systems. New Phytol. 137, 179–203. doi: 10.1046/j.1469-8137.1997.00808.x
Huisman, D. J., and Raemaekers, D. C. M. (2014). Systematic cultivation of the Swifterbant wetlands (The Netherlands). Evidence from Neolithic tillage marks (c. 4300–4000 cal. BC). J. Archaeol. Sci. 49, 572–584. doi: 10.1016/j.jas.2014.05.018
Kabalika, Z., Haydon, D. T., McGill, R. A. R., Morales, J. M., Thomas, A., Morrison, T. A., et al. (2023). Using sulfur stable isotope ratios (δ34S) for animal geolocation: estimating the delay mechanisms between diet ingestion and isotope incorporation in tail hair. Rapid Commun. Mass Spectrom. 38:e9674. doi: 10.1002/rcm.9674
Kay, A. U., Fuller, D. Q., Neumann, K., Eichhorn, B., Höhn, A., Morin-Rivat, J., et al. (2019). Diversification, intensification and specialization: changing land use in western Africa from 1800 BC to AD 1500. J. World Prehist. 32, 179–228. doi: 10.1007/s10963-019-09131-2
Kendall, C., and Caldwell, E. A. (1998). “Isotope tracers in catchment hydrology,” in Fundamentals of Isotope Geochemistry, eds C. Kendall, J.J. McDonnell (Amsterdam: Elsevier), 51–86. doi: 10.1016/B978-0-444-81546-0.50009-4
Khalifa, M., and Eltahir, E. A. B. (2023). Assessment of global sorghum production, tolerance, and climate risk. Front. Sustain. Food Syst. 7:1184373. doi: 10.3389/fsufs.2023.1184373
Kingwell-Banham, E. (2019). Dry, rainfed or irrigated? Reevaluating the role and development of rice agriculture in Iron Age-Early Historic South India using archaeobotanical approaches. Archaeol. Anthropol. Sci. 11, 6485–6500. doi: 10.1007/s12520-019-00795-7
Krajcarz, M. T., Krajcarz, M., Drucker, D. G., and Bocherens, H. (2019). Prey-to-fox isotopic enrichment of 34S in bone collagen: implications for paleoecological studies. RCMS 33, 1311–1317. doi: 10.1002/rcm.8471
Kumar Rai, R., Singh, V. P., and Upadhyay, A. (2017). Soil Analysis: Planning and Evaluation of Irrigation Projects. Cambridge, MA: Academic Press, 505–523. doi: 10.1016/B978-0-12-811748-4.00017-0
Lamb, A. L., Chenery, C. A., Madgwick, R., and Evans, J. A. (2023). Wet feet: developing sulfur isotope provenance methods to identify wetland inhabitants. R. Soc. Open Sci. 10:230391. doi: 10.1098/rsos.230391
Lamers, L. P., Govers, L. L., Janssen, I. C. J. M., Geurts, J. J. M., Van der Welle, M. E. W., Van Katwijk, M. M., et al. (2013). Sulfide as a soil phytotoxin: a review. Front. Plant Sci. 4:268. doi: 10.3389/fpls.2013.00268
Langlie, B. S. (2018). Building ecological resistance: late intermediate period farming in the south-central highland Andes (CE 1100–1450). J. Anthropol. Archaeol. 52, 167–179. doi: 10.1016/j.jaa.2018.06.005
Lee, H., French, C., and Macphail, R. I. (2014). Microscopic examination of ancient and modern irrigated paddy soils in South Korea, with special reference to the formation of silty clay concentration features. Geoarchaeology 29, 326–348. doi: 10.1002/gea.21478
Li, L., Dong, X., He, M., Huang, M., Cai, J., Zhou, Q., et al. (2023). Unravelling the role of adventitious roots under priming-induced tolerance to waterlogging stress in wheat. Environ. Exp. Bot. 216:105516. doi: 10.1016/j.envexpbot.2023.105516
Li, Q., Gao, Y., and Yang, A. (2022). Sulfur homeostasis in plants. Int. J. Mol. Sci. 21:8926. doi: 10.3390/ijms21238926
Ma, T., Zheng, Z., Rolett, B. V., Lin, G., Zhang, G., and Yue, Y. (2016). New evidence for Neolithic rice cultivation and Holocene environmental change in the Fuzhou Basin, southeast China. Veget. Hist. Archaeobot. 25, 375–386. doi: 10.1007/s00334-016-0556-0
Mariotti, A., Germon, J. C., Hubert, K., aiser, P., Letolle, R., Tardieux, A., and Tardieux, P. (1981). Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes. Plant Soil 62, 413–430. doi: 10.1007/BF02374138
Marshall, T. J., and Holmes, J. W. (1996). Soil Physics, 3rd Edn. CUP. Available online at: https://doi-org.libproxy.ucl.ac.uk/10.1017/CBO9781139170673 (Accessed April 21, 2025).
Marston, J. M., and Scott Branting, S. (2016). Agricultural adaptation to highland climate in Iron Age Anatolia. J. Arch. Sci. Rep. 9, 25–32. doi: 10.1016/j.jasrep.2016.06.050
Maruyama-Nakashita, A. (2017). Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant Biol. 39, 144–151. doi: 10.1016/j.pbi.2017.06.015
Matsuura, A., Kato, Y., Suzuki, T., Murata, K., and Ping, A. (2022). Hypoxia tolerance of four millet species is attributable to constitutive aerenchyma formation and root hair development of adventitious roots. Plant Prod. Sci. 25, 157–171. doi: 10.1080/1343943X.2021.2021092
Motuzaite-Matuzeviciute, G., Telizhenko, S., and Jones, M. K. (2012). Archaeobotanical investigation of two Scythian-Sarmatian period pits in eastern Ukraine: implications for floodplain cereal cultivation. J. Field Archaeol. 37, 51–61. doi: 10.1179/0093469011Z.0000000004
Müller, J. G., Ogneva-Himmelberger, Y., Lloyd, S., and Reed, J. M. (2010). Predicting prehistoric taro (Colocasia esculenta var. antiquorum) Lo'i Distribution in Hawaii. Econ. Bot. 64, 22–33. doi: 10.1007/s12231-010-9113-4
Nehlich, O. (2015). The application of sulphur isotope analyses in archaeological research: a review. Earth Sci. Rev. 142, 1–17. doi: 10.1016/j.earscirev.2014.12.002
Nehlich, O., Borić, D., Stefanović, S., and Richards, M. P. (2010). Sulphur isotope evidence for freshwater fish consumption: a case study from the Danube Gorges, SE Europe. J. Archaeol. Sci. 37, 1131–1139. doi: 10.1016/j.jas.2009.12.013
Neumann, K. (2018). Development of plant food production in the West African Savannas: archaeobotanical perspectives. Oxf. Res. Encycl. Afr. Hist. doi: 10.1093/acrefore/9780190277734.013.138
Nishiuchi, S., Yamauchi, T., Takahashi, H., Kotula, L., and Nakazono, M. (2012). Mechanisms for coping with submergence and waterlogging in rice. Rice 5:2. doi: 10.1186/1939-8433-5-2
Nitsch, E. K., Lamb, A. L., Heaton, T. H. E., Vaiglova, P., Fraser, R., Hartman, G., et al. (2019). The Preservation and interpretation of δ34S values in charred archaeobotanical remains. Archaeometry 61, 161–178. doi: 10.1111/arcm.12388
Norman, A. L., Giesemann, A., Krouse, H. R., and Jager, H. J. (2002). Sulphur isotope fractionation during sulphur mineralization: results of an incubation-extraction experiment with a Black Forest soil. Soil Biol. Biochem. 34, 1425–1438. doi: 10.1016/S0038-0717(02)00086-X
Ortloff, C. R. (2009). Water Engineering in the Ancient World: Archaeological and Climate Perspectives on Societies of Ancient South America, the Middle East, and South-East Asia. Oxford. doi: 10.1093/oso/9780199239092.001.0001
Paladin, A., Moghaddam, N., Stawinoga, A. E., Siebke, I., Depellegrin, V., Tecchiati, U., et al. (2020). Early medieval Italian Alps: reconstructing diet and mobility in the valleys. Archaeol. Anthropol. Sci. 12:82. doi: 10.1007/s12520-019-00982-6
Pan, J., Sharif, R., Xu, X., and Chen, X. (2021). Mechanisms of waterlogging tolerance in plants: research progress and prospects. Front. Plant Sci. 11:627331. doi: 10.3389/fpls.2020.627331
Parent, C., Capelli, N., Berger, A., Crevècoeur, M., and Dat, J. (2008). An overview of plant responses to soil waterlogging. Plant Stress 2, 20–27.
Pasquier, V., Bryant, R. N., Fike, D. A., and Halevy, I. (2021). Strong local, not global, controls on marine pyrite sulfur isotopes. Sci. Adv. 7:eabb7403. doi: 10.1126/sciadv.abb7403
Patalano, R., Wang, Z., Leng, Q., Liu, W., Zheng, Y., Sun, G., et al. (2015). Hydrological changes facilitated early rice farming in the lower Yangtze River Valley in China: a molecular isotope analysis. Geology 43, 639–642. doi: 10.1130/G36783.1
Paytan, A., and Gray, E. T. (2012). “Sulfur isotope stratigraphy,” in The Geologic Time Scale, eds F. M. Gradstein, J. G. Ogg, M. D. Schmitz, and G. M. Ogg (Boston, MA: Elsevier), 167–180. doi: 10.1016/B978-0-444-59425-9.00009-3
Pellerin, A., Antler, G., Holm, S. A., Findlay, A. J., Crockford, P. W., Turchyn, A. V., et al. (2019). Large sulfur isotope fractionation by bacterial sulfide oxidation. Sci. Adv. 5:eaaw1480. doi: 10.1126/sciadv.aaw1480
Peterson, B. J., Howarth, R. W., and Garritt, R. H. (1986). Sulfur and carbon isotopes as tracers of salt-marsh organic matter flow. Ecology 67, 865–874. doi: 10.2307/1939809
Petrie, C. A., and Bates, J. (2017). ‘Multi-cropping', intercropping and adaptation to variable environments in Indus South Asia. J. World Prehist. 30, 81–130. doi: 10.1007/s10963-017-9101-z
Price, F. T., and Casagrande, D. J. (1991). Sulfur distribution and isotopic composition in peats from the Okefenokee Swamp, Georgia and the Everglades, Florida. Int. J.Coal Geol. 17, 1–20. doi: 10.1016/0166-5162(91)90002-Z
Privat, K. L., O'Connell, T. C., and Hedges, R. E. M. (2007). The distinction between freshwater-and terrestrial-based diets: methodological concerns and archaeological applications of sulphur stable isotope analysis. J. Archaeol. Sci. 34, 1197–1204. doi: 10.1016/j.jas.2006.10.008
Rand, A. J., Freiwald, C., and Grimes, V. (2021). A multi-isotopic (δ13C, δ 15N, and δ34S) faunal baseline for Maya subsistence and migration studies. J. Archaeol. Sci. Rep. 37:102977. doi: 10.1016/j.jasrep.2021.102977
Raoult, V., Phillips, A. A., Nelson, J., Niella, Y., Skinner, C., Bell Tilcock, M., et al. (2024). Why aquatic scientists should use sulfur stable isotope ratios (δ34S) more often. Chemosphere 355:141816. doi: 10.1016/j.chemosphere.2024.141816
Ren, Z., Ma, K., Jia, X., Wang, Q., Zhang, C., and Li, X. (2023). Metagenomics unveils microbial diversity and their biogeochemical roles in water and sediment of thermokarst lakes in the yellow river source area. Microb. Ecol. 85, 904–915. doi: 10.1007/s00248-022-02053-1
Renard, D., Birk, J. J., Zangerlé, A., Lavelle, P., Glaser, B., Blatrix, R., et al. (2013). Ancient human agricultural practices can promote activities of contemporary non-human soil ecosystem engineers: a case study in coastal savannas of French Guiana. Soil Biol. Biochem. 62, 46–56. doi: 10.1016/j.soilbio.2013.02.021
Richards, M. P., Fuller, B. T., Sponheimer, M., Robinson, T., and Ayliffe, L. (2003). Sulphur isotopes in palaeodietary studies: a review and results from a controlled feeding experiment. Int. J. Osteoarchaeol. 13, 37–45. doi: 10.1002/OA.654
Robinson, M., and Lambrick, G. (1984). Holocene alluviation and hydrology in the upper Thames basin. Nature 308, 809–814. doi: 10.1038/308809a0
Sayle, K. L., Brodie, C. R., Cook, G. T., and Hamilton, W. D. (2019). Sequential measurement of δ15N, δ13C and δ34S values in archaeological bone collagen at the Scottish Universities Environmental Research Centre (SUERC): a new analytical frontier. RCMS 33, 1258–1266. doi: 10.1002/rcm.8462
Seal, R. R. (2006). Sulfur Isotope geochemistry of sulfide minerals. Rev. Min. Geochem. 61, 633–677. doi: 10.2138/rmg.2006.61.12
Shaffer, P. W., Kentula, M. E., and Gwin, S. E. (1999). Characterization of wetland hydrology using hydrogeomorphic classification. Wetlands 19:490504. doi: 10.1007/BF03161688
Sherratt, A. (1980). Water, soil and seasonality in early cereal cultivation. World Archaeol. 11, 313–330. doi: 10.1080/00438243.1980.9979770
Sim, M. S., Bosak, T., and Ono, S. (2011). Large sulfur isotope fractionation does not require disproportionation. Science 333, 74–77. doi: 10.1126/science.1205103
Sim, M. S., Ono, S., and Bosak, T. (2012). Effects of iron and nitrogen limitation on sulfur isotope fractionation during microbial sulfate reduction. Appl. Environ. Microbiol. 78, 8368–8376. doi: 10.1128/AEM.01842-12
Sim, M. S., Woo, D. K., Kim, B., Jeong, H., Joo, Y. J., Hong, Y. W., et al. (2023). What controls the sulfur isotope fractionation during dissimilatory sulfate reduction? ACS Environ. Au 3, 76–86. doi: 10.1021/acsenvironau.2c00059
Simkin, S. M., Bedford, B. L., and Weathers, K. C. (2013). Phytotoxic sulfide more important than nutrients for plants within a groundwater-fed wetland. Ecosystems 16, 1118–1129. doi: 10.1007/s10021-013-9671-2
Stack, P. E., and Rock, L. (2011). A δ34S isoscape of total sulphur in soils across Northern Ireland. Appl. Geochem. 26, 1478–1487. doi: 10.1016/j.apgeochem.2011.05.021
Steffens, B., and Rasmussen, A. (2016). The physiology of adventitious roots. Plant Physiol. 170, 603–617. doi: 10.1104/pp.15.01360
Stevens, R. E., Reade, H., Read, D. S., Bottrell, S. H., Frémondeau, D., and Wexler, S. (2022). Iso-Wetlands: unlocking wetland ecologies and agriculture in prehistory through sulfur isotopes. Archeol. Int. 25, 168–176. doi: 10.14324/111.444.ai.2022.11
Stevens, R. E., Reade, H., Sayle, K., Tripp, J., Frémondeau, D., Lister, A., et al. (2025). Major excursions in sulfur isotopes linked to permafrost change in Eurasia during the last 50,000 Years. Nat. Geosci. (in press).
Stribling, J. M., and Cornwell, J. C. (1997). Identification of important primary producers in a Chesapeake Bay tidal creek system using stable isotopes of carbon and sulfur. Estuaries 20, 77–85. doi: 10.2307/1352721
Stribling, J. M., Cornwell, J. C., and Currin, C. (1998). Variability of stable sulfur isotopic ratios in Spartina alterniflora. Mar. Ecol. Prog. Ser. 166, 73–81. doi: 10.3354/meps166073
Tanz, N., and Schmidt, H. L. (2010). δ34S-value measurements in food origin assignments and sulfur isotope fractionations in plants and animals. J. Agric. Food Chem. 58, 3139–3146. doi: 10.1021/jf903251k
Tcherkez, G., and Tea, I. (2013). 32S/34S isotope fractionation in plant sulphur metabolism. New Phytol. 200, 44–53. doi: 10.1111/nph.12314
Thakur, B., Saxena, A., and Singh, I. B. (2018). Paddy cultivation during early Holocene: evidence from diatoms in Lahuradewa lake sediments. Ganga Plain. Curr. Sci. 114, 2106–2115. doi: 10.18520/cs/v114/i10/2106-2115
Vitòria, L., Otero, N., Soler, A., and Canals, À. (2004). Fertilizer characterization: isotopic data (N, S, O, C, and Sr). Environ. Sci. Tech. 38, 3254–3262. doi: 10.1021/es0348187
Wang, J., He, Y., Tang, Y., Liu, L., Li, Y., Chen, X., et al. (2023). An interplay of dryland and wetland: millet and rice cultivation at the Peiligang site (8000–7600 BP) in the middle Yellow River Valley, China. Agronomy 13:2130. doi: 10.3390/agronomy13082130
Webb, E. C., Newton, J., Lewis, J., Stewart, A., Millerd, B., Tarlton, J. F., et al. (2017). Sulphur-isotope compositions of pig tissues from a controlled feeding study. Star Sci. Tech. Archaeo. Res. 3, 71–79. doi: 10.1080/20548923.2017.1368821
Winter, T. C. (1999). Relation of streams, lakes, and wetlands to groundwater flow systems. Hydrogeol. J. 7, 28–45. doi: 10.1007/s100400050178
Yamauchi, T., Shimamura, S., Nakazono, M., and Mochizuki, T. (2013). Aerenchyma formation in crop species: a review. Field Crops Res. 152, 8–16. doi: 10.1016/j.fcr.2012.12.008
Zavodny, E., McClure, S. B., Culleton, B. J., Podrug, E., Balen, J., Drnić, I., et al. (2022). Investigating past livestock mobility using δ34S stable isotopes: three preliminary case studies from prehistoric Croatia. Open Quat. 8, 1–15. doi: 10.5334/oq.113
Zazzo, A., Balasse, M., and Patterson, W. P. (2006). The reconstruction of mammal individual history: refining high-resolution isotope record in bovine tooth dentine. J. Archaeol. Sci. 33, 1177–1187. doi: 10.1016/j.jas.2005.12.006
Zerkle, A. L., Farquhar, J., Johnston, D. T., Cox, R. P., and Canfield, D. E. (2009). Fractionation of multiple sulfur isotopes during phototrophic oxidation of sulfide and elemental sulfur by a green sulfur bacterium. Geochim. Cosmoch. Acta. 73, 291–306. doi: 10.1016/j.gca.2008.10.027
Zheng, Y., Sun, G., Qin, L., Li, C., Wu, X., and Chen, X. (2009). Rice fields and modes of rice cultivation between 5000 and 2500 BC in east China. J. Archaeol. Sci. 36, 2609–2616. doi: 10.1016/j.jas.2009.09.026
Zhuang, Y., Ding, P., and French, C. (2014). Water management and agricultural intensification of rice farming at the late-Neolithic site of Maoshan, Lower Yangtze River, China. Holocene 24, 531–545. doi: 10.1177/0959683614522310
Zhuang, Y., Ding, P., Zhuang, L., Wang, Y., Wu, W., Wang, X., et al. (2025). Geoarchaeological study of the evolution of rice farming fields in prehistoric Yangtze Delta and Huai River regions of China. Quat. Sci. Rev. 356:109293. doi: 10.1016/j.quascirev.2025.109293
Keywords: sulfur isotope baselines, microbial sulfate reduction, wetlands, waterlogging, plant uptake, archaeology, archaeobotany
Citation: Wexler SK and Stevens RE (2025) Wetland sulfur isotope signals and dynamic isotope baselines: implications for archaeological research. Front. Environ. Archaeol. 4:1599779. doi: 10.3389/fearc.2025.1599779
Received: 25 March 2025; Accepted: 11 June 2025;
Published: 22 July 2025.
Edited by:
Xinyi Liu, Washington University in St. Louis, United StatesReviewed by:
Angela L. Lamb, British Geological Survey, The Lyell Centre, United KingdomOlaf Nehlich, Esarom GmbH, Austria
Melissa M. Ritchey, Washington University in St. Louis, United States
Copyright © 2025 Wexler and Stevens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah K. Wexler, cy53ZXhsZXJAdWNsLmFjLnVr
 Sarah K. Wexler
Sarah K. Wexler Rhiannon E. Stevens
Rhiannon E. Stevens