NANOSPRESSO: toward personalized, locally produced nucleic acid nanomedicines
Explainer
Front Sci, 26 June 2025
This explainer is part of an article hub, related to lead article https://doi.org/10.3389/fsci.2025.1458636
Assembling nanomedicines in-hospital could close the treatment gap for rare diseases
Despite their name, rare diseases are surprisingly common. While each condition affects fewer than one in 2,000 people, there are over 5,000 such diseases affecting an estimated 300 million people.
However, the rarity of each disorder means there is a lack of research and development to produce effective treatments. Low patient numbers (occasionally just a single patient), extremely high drug development costs, and complex regulatory processes discourage pharmaceutical companies from investing in new treatments, leaving many conditions without effective therapies. This has led to them being known as orphan diseases.
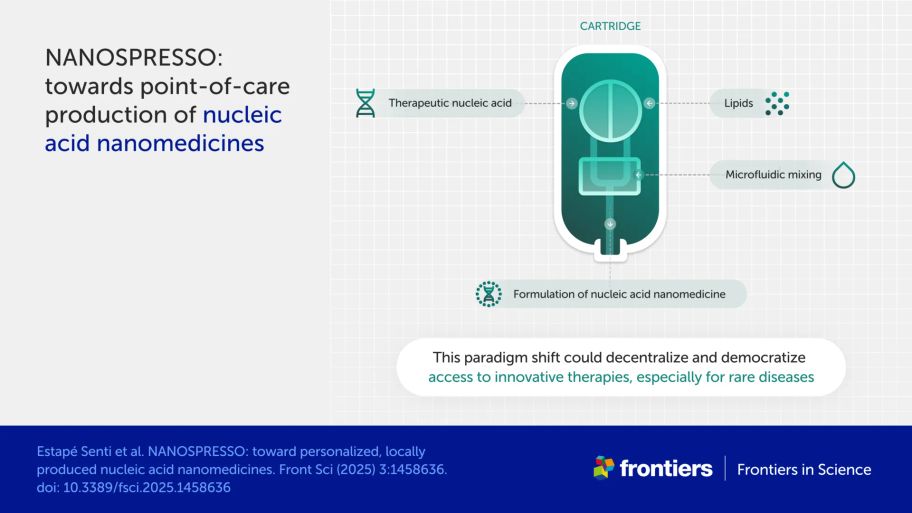
In their Frontiers in Science lead article, Estapé Senti et al. propose a new approach to treating rare diseases. Their project, called NANOSPRESSO, aims to avoid the constraints of large, one-size-fits-all drug manufacturing by enabling hospital pharmacists to assemble tailored-from-template cartridges of bespoke medicine in-house.
This “plug-and-play” approach could in future deliver personalized nanomedicines directly to patients, even in resource-poor settings. The portable, inexpensive NANOSPRESSO cartridges draw inspiration from coffee capsules, which offer simple and convenient customization for home brewing.
This explainer summarizes the article’s main points.
How can nucleic acid therapeutics be used to treat diseases?
Many human diseases are caused by problems with the way our cells produce proteins such as enzymes.
Proteins help our cells to function and are important for our overall health. They are created by our cells according to instructions within our genes. This means mistakes in genes can cause cells to produce too many, too few, non-functional, or even harmful proteins.
These mistakes account for many diseases, including rare ones. For example, cancers might thrive in the absence of tumor-suppressive proteins, and non-functional enzymes might cause metabolic diseases that affect the way we break down fats or sugars.
Nucleic acid therapeutics can help treat such conditions by finding and fixing the faulty genetic code. They can fix the mistakes by, for example, stimulating cells to produce missing proteins, or by blocking the production of harmful ones such as the pro-inflammatory proteins involved in autoimmune diseases.
This type of therapeutic is based on chains of DNA or RNA that can be tailored by changing their length and sequence. This means nucleic acids can act as templates that pharmacists can adjust to create bespoke therapeutics based on what each patient needs.
What are the challenges of using nucleic acid therapeutics?
Nucleic acid therapeutics are large, negatively charged molecules. As a result, it is very difficult for them to enter cells and reach their targets. One way to overcome this is by encasing the nucleic acids in lipid nanoparticles. Lipid nanoparticles can easily pass through the outer membrane of cells and release their cargo inside. This approach was used successfully to quickly develop new mRNA COVID-19 vaccines in response to viral variants.
Nucleic acids also degrade quickly and must always be kept at –80°C to preserve their activity. This means a costly and complex cold chain must be maintained when transporting them between centralized manufacturing sites and hospitals. NANOSPRESSO’s decentralized approach eliminates the cold chain by bringing manufacturing inside hospitals.
How might NANOSPRESSO help treat orphan diseases?
NANOSPRESSO is developing a prototype device that could bring medicines within closer reach for rare disease patients. The device uses advanced techniques to mix nucleic acid therapeutics and lipid nanoparticles in a way that’s precise and reproducible.
At the hospital, medics would select a bespoke combination of nucleic acids tailored to each patient. Pharmacists would insert these nucleic acids, as well as lipid nanoparticles, into a mixer. The mixer would then dispense the result as fresh medicine.
Just as mRNA vaccines were easily adapted to emerging COVID-19 variants, these therapies could be tailored to the DNA sequences of individuals. The result would be a personalized treatment tailored to each patient’s condition.
The same device can then be re-used to deliver different therapies for different patients.
Transitioning to local production has already been achieved for other personalized therapies. For example, new technologies have enabled automated, localized manufacture of cancer-targeting immune cells called chimeric antigen receptor (CAR) T-cells, simplifying the process and reducing medicine delivery times.
What are the advantages of producing personalized nucleic acid therapeutics in the hospital?
Nucleic acid therapeutics for rare diseases can be prohibitively expensive to develop and deliver using conventional, centralized approaches.
This localized production could help to reduce the costs of developing and delivering new treatments for rare diseases—thereby democratizing access to these therapies, including in low-resource regions. It could also avoid the transportation and storage challenges associated with the cold chain distribution system.
What are the hurdles to crafting nucleic acid therapeutics at the point of care?
Current pharmaceutical regulations are mostly designed around centralized industrial production. NANOSPRESSO’s localized model challenges this and will instead fall under specific pathways to allow pharmacies to make patient-specific medicines with greater flexibility.
For example, the European Union’s pharmaceutical legislation has specific exemptions allowing non-routine preparation of advanced medicinal products in hospitals. Similarly, patent laws often have exemptions for therapeutics prepared for individual patients.
Nevertheless, medicines regulators and developers will need to work together to ensure that stringent quality control processes and requirements are developed and applied to this approach. The NANOSPRESSO project will apply a variety of new analytical techniques to ensure quality and consistency, and is in dialogue with regulatory authorities to ensure compliance with quality, safety, and efficacy standards.
Demonstrating the efficacy and cost-effectiveness of new treatments using evidence from large clinical trials is a critical part of making them available through healthcare systems. This is challenging for medicines produced locally or for rare diseases with smaller patient populations, where traditional large clinical trials may not be feasible.
Therefore, the authors argue that collaborations between scientists, healthcare providers, policymakers, and funders are pivotal for realizing the full potential of these therapies while ensuring they remain affordable and accessible.
What are the next steps for NANOSPRESSO and personalized nucleic acid therapeutics?
The NANOSPRESSO model could help transform the landscape of advanced nanomedicines and, more broadly, precision medicine and medical innovation. While the project focuses on orphan diseases at present, its personalized, localized model could also be applied to cancer medicine, and in vaccines to tackle infectious disease outbreaks. The model could also help to enhance access to nucleic acid therapies in lower-income countries.
To meet their ambitions for this paradigm shift, the NANOSPRESSO team again urges interdisciplinary collaborations to tackle the various scientific, technological, regulatory, and economic challenges.
They invite interested parties to engage with them by visiting their website.