Explore article hub
- 1Department of Biomedical Engineering, Tufts University, Medford, MA, United States
- 2Department of Food Science, University of Massachusetts, Amherst, MA, United States
Abstract
The industrial scale use of animals to produce food for humans, such as meat, egg, and dairy products, has serious environmental, health, and ethical implications. Livestock production is a major source of greenhouse gas emissions and drives soil depletion, water pollution, deforestation, and biodiversity loss. There are also concerns about its negative impacts on human health and animal welfare. To feed future generations, it will be important to produce nutritious foods in a more sustainable, ethical, and environmentally friendly manner. In this article, we examine several protein-rich food sources as alternatives to traditional animal proteins, including plants, insects, mycelia, cultured animal cells, and microbial fermentation products. Each of these alternative protein sources has advantages and disadvantages in terms of their organoleptic properties, nutritional profile, consumer acceptance, affordability, and scalability. We then consider combining different alternative protein sources to form affordable, scalable, delicious, nutritious, and sustainable hybrid foods that may compete with conventional meat products, including meat–plant, cultivated meat–plant, mycelium–plant, and insect–plant foods. However, these hybrid products are still relatively new, and significant challenges, including cost reduction, scalability, regulatory approval, and consumer acceptance, need to be addressed before they become commercially viable. Future research should therefore focus on optimizing protein sources, developing scalable production methods, conducting environmental and economic analyses, and leveraging artificial intelligence for innovation. To make hybrid food products viable and sustainable, more efficient collaboration across academia, industry, and regulatory bodies is urgently needed.
Key points
- Protein sources such as plants, cultured cells, insects, mycelia, and microbes are increasingly being explored as alternatives to animal-derived proteins due to sustainability, human health, and environmental concerns.
- Hybrid food products, which combine alternative protein sources, are emerging as a promising solution to animal-derived proteins, with enhanced sensory appeal, nutritional profile, affordability, scalability, and consumer acceptance, but designing them requires careful consideration of their organoleptic, health, and safety properties.
- To make hybrid foods commercially viable, challenges such as environmental impact, scalability, affordability, and regulatory approval must be addressed collaboratively by key stakeholders.
- The successful adaptation of hybrid food products depends on the following critical steps: (i) optimizing individual alternative protein sources (including using artificial intelligence approaches); (ii) developing combinatorial technologies; (iii) creating large-scale manufacturing facilities for economic and scalable production; (iv) improving consumer acceptability via marketing, sensory, nutrition, and cost optimization; and (v) employing life cycle and techno-economic analyses to identify the most sustainable and commercially viable hybrid foods.
Introduction
The food industry is exploring the potential of replacing traditional animal-derived foods, such as dairy, egg, meat, and seafood products, with foods created from alternative protein-rich sources, such as plants, cultured cells, microbes, insects, or mycelia (1, 2). There are several reasons for this push toward alternative protein-rich food sources, including the potentially adverse effects of some animal-derived foods (especially beef produced on intensive factory farms) on the environment, animal welfare, human health, antibiotic resistance, and zoonotic disease transmission (3). Moreover, the continued growth in the global population, as well as the “nutrition transition” (where more people in developing countries are switching to a meat-rich diet), will make it challenging to produce sufficient protein-rich foods to feed future generations from animal-derived sources alone (4). Consequently, there is an urgent need to produce alternatives to meat, seafood, egg, and dairy products from non-animal protein sources. These kinds of products are currently the main focus of the food industry because most consumers in developed countries are omnivores, rather than vegans or vegetarians. These products should have sensory attributes that consumers find desirable (5), as well as being affordable, convenient, healthy, and sustainable (6, 7). Considerable progress has been made in the development of many kinds of alternative protein products, especially those made from plant proteins and mycelia, where there are already successful commercial meat, seafood, egg, and dairy analogs on the market. However, the majority of the population in most countries is still not incorporating this new generation of alternative protein products into their diets (8).
The limited availability and adoption of alternative protein products can be partly attributed to the disadvantages inherent to each technology (2, 9). For instance, plant proteins are highly abundant, relatively inexpensive, and have been successfully scaled, but the products made from them are often lacking in the sensorial and nutritional attributes desired by consumers (10). In contrast, cultivated meat (CM) products have desirable sensory and nutritional attributes because they have properties similar to animal meat, but they are currently too expensive and difficult to produce at a sufficiently large scale (11). For these reasons, there are advantages to creating hybrid products that combine the desirable traits of different sources of alternative proteins (Figure 1) while overcoming the undesirable ones (12). For instance, researchers have proposed that combining CM cells with plant-derived ingredients can create hybrid products that overcome the limitations of both cultured meat and plant-based meat analogs (13). The presence of the CM cells provides sensory and nutritional attributes, whereas the presence of the plant-derived ingredients provides texture, reduces costs, and increases scalability. Hybrid products can also be generated by combining animal-derived foods (meat, fish, egg, or dairy products) with alternative protein-rich sources, such as cultured cells, insects, mycelia, or plant proteins, in order to reduce the total amount of meat in the diet and resulting in environmental, human health, and animal welfare benefits (14).
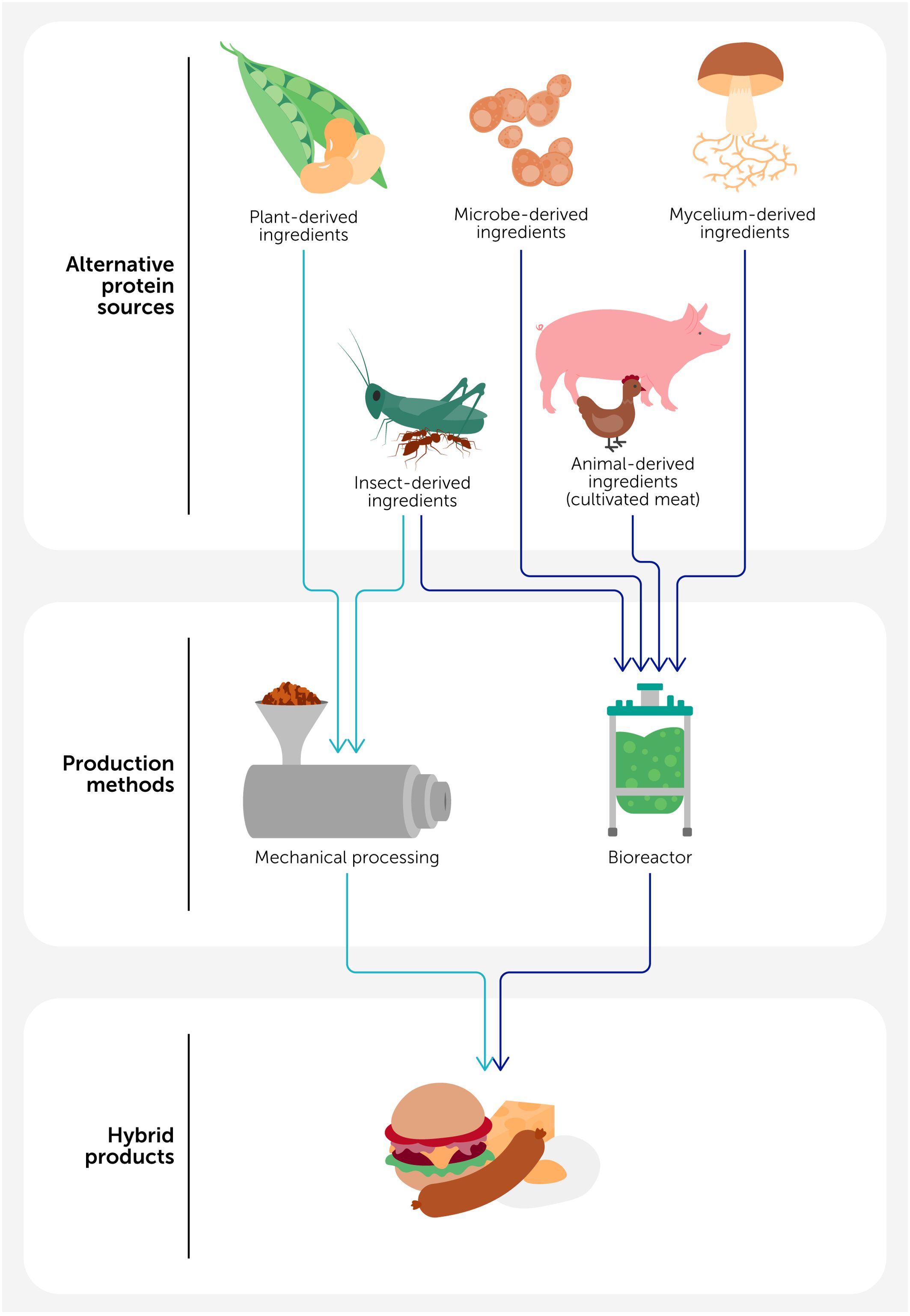
Figure 1. Hybrid meat products can be generated by combining different kinds of protein sources, including those derived from plants, insects, mycelia, microbes, and animals (cultivated meat). Depending on the product, the ingredients in these products are processed either mechanically (e.g., extrusion, shear cell, spinning, or additive manufacturing technologies) or they are cultivated in bioreactors.
This article focuses on the development of hybrid meat alternatives, but much of the material presented is also applicable to the development of hybrid seafood, egg, or dairy alternatives. Initially, a brief overview of the different sources of alternative proteins is provided, including a discussion of their potential benefits and limitations. Then, some of the factors that should be considered when combining different sources of alternative proteins are highlighted, followed by an overview of previous studies on the development and testing of hybrid alternative-protein products. Finally, we present areas where further research and development are needed to facilitate the commercial success of hybrid products.
Sources of alternative proteins: benefits and drawbacks
Alternative proteins derived from various non-animal sources such as plants, mycelium, cultured cells, microbes, and insects (Figure 1) are gaining increasing attention due to their potential to address global food challenges. The search for these alternative proteins began in response to the need to establish sustainable food systems to feed the growing global population, as well as rising concerns about the adverse environmental impacts of traditional animal agriculture. Early innovations focused on plant-based solutions, but recent technological advances have led to the possibility of using cultured cells and microbial fermentation as other viable sources of alternative proteins. The following sections provide an overview of alternative protein sources and the products derived from each, as well as an outline of current limitations and possible solutions (Table 1). Table 1 also provides a rough estimation of the technology readiness levels (TRLs) of the different alternative protein products.
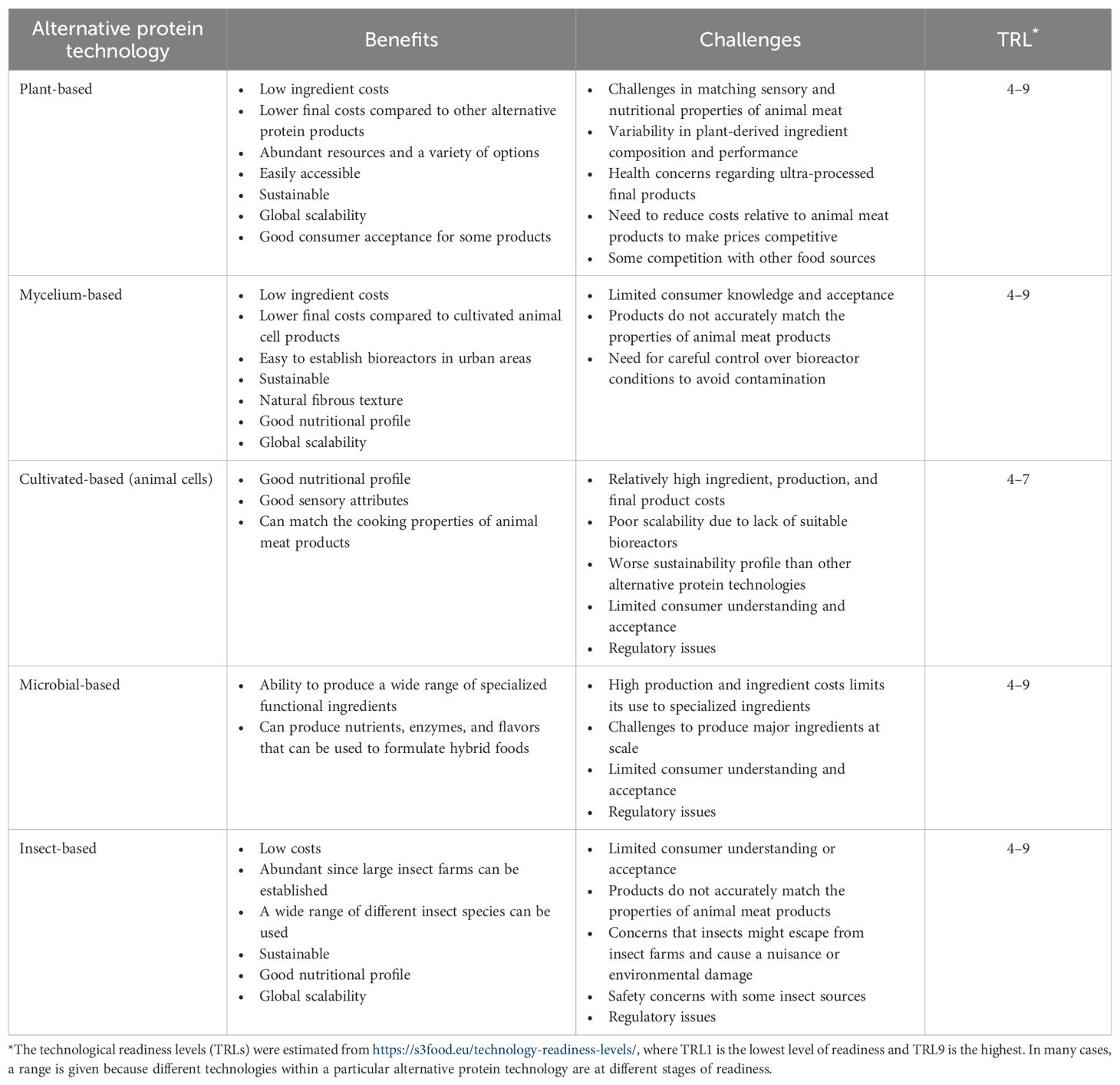
Table 1. Comparison of the benefits, challenges, and overall technology readiness levels (TRLs) of different alternative protein technologies.
Plant-based products
Plant-based meat analogs are generated by blending a variety of plant-derived ingredients, including proteins, polysaccharides, lipids, flavors, pigments, and preservatives (15, 16). These ingredients may be isolated from a variety of botanical sources, including cereals (such as corn, rice, wheat, barley, and oats), legumes (such as soybeans, peas, chickpeas, lentils, peanuts, and beans), pseudocereals (such as amaranth, quinoa, chia, and buckwheat), nuts (such as almonds, cashews, and hazelnuts), leaves (such as kale, spinach, lettuce, or duckweed), and seeds (such as flax, sunflower, or sesame) (17). In addition, functional ingredients may be isolated from marine “plants” (actually algae) like seaweed (such as carrageenan or alginate) or obtained through microbial fermentation (such as with gellan gum and xanthan gum) (18, 19). Plant-derived ingredients can also be isolated from agricultural or food industry waste streams, which may increase the sustainability of the food supply (20). It is extremely challenging to create meat analogs entirely from plant-derived ingredients because of the unique composition and structure of animal meat (3, 16). Whole meat has a complex structural architecture consisting of muscle, adipose, and connective tissues (21), which is difficult to replicate using only plant-derived ingredients. Indeed, one of the major factors holding back the more widespread adoption of plant-based meat analogs is that they do not accurately simulate the desirable structure, flavor, texture, and mouthfeel of animal meat products (22, 23).
Having said this, considerable progress has been made using both traditional and modern mechanical processing operations to convert plant-derived ingredients into fibrous meat analogs (24, 25). Currently, extrusion is the most common method of industrially producing meat analogs on a large scale, but other technologies have also shown promise, including shear cell, spinning, and additive manufacturing technologies (26). Many commercial plant-based meat analogs contain numerous ingredients (>20) to more accurately simulate the properties of animal meat products (27). However, the large number of ingredients and extensive processing operations required to formulate plant-based meat analogs have meant they are often considered to be “ultra-processed” foods, which can be perceived as unhealthy by consumers (28). Indeed, there is evidence from observational nutritional studies of a correlation between the amount of ultra-processed foods in the human diet and adverse health outcomes (29, 30). Consequently, there is interest in redesigning these products to make them healthier (31, 32). In addition, the need for multiple ingredients and processing operations to generate plant-based meat analogs can lead to relatively high costs compared to animal (livestock-derived) meat, which is also holding back their adoption (33, 34). However, as demonstrated by the products currently available on the market in Europe, it is possible to create plant-based products that are comparable or cheaper in price to those of conventional meat products.
Mycelium-based products
Commercially successful meat analogs have been generated using mycoproteins obtained using industrial fermentation processes (27). These products are mainly created from mycelium (Fusarium venenatum fungus, also used in bread, beer, and yogurt) grown in large bioreactors under appropriate conditions, including temperature, oxygen level, pH, and nutrient composition. A small quantity of mycelia is placed into a bioreactor, where it multiplies over time, leading to a large amount of biomass that can be collected, centrifuged, and then converted into a suitable food product. An RNA removal step is required during the production process, as there are concerns that high levels of nucleic acids in mycelium products could lead to health concerns, such as gout (35, 36). A major advantage of using mycelium for creating meat analogs is that it naturally has a fibrous structure that is somewhat similar to that found in meat, thereby improving its sensorial attributes (37). Moreover, mycelium is a good source of proteins, dietary fibers, vitamins, and minerals, and therefore naturally has a good nutritional profile (38, 39). Nevertheless, there are still challenges in converting mycelium into food products with sensory properties that accurately mimic those of animal meat (40, 41). Consequently, mycelia are usually combined with other ingredients, including binders, flavors, colors, and preservatives, to better simulate the desirable attributes of meat products. Other meat analogs can be produced from different kinds of mycelia, as they can also provide similar beneficial features as described above. For instance, Neurospora crassa has been used as an ingredient to formulate chicken nuggets.
Cultivated meat-based products
CM analogs are also created by growing cells within bioreactors under controlled conditions and then harvesting, purifying, and converting them into meatlike products (42, 43). The difference here is that the cells are the key ingredient in the food product and are used for the nutritional and sensory benefits mentioned earlier (44). The cells are usually isolated from animals, such as cows, pigs, chickens, or fish (45, 46), or sometimes from insects (46, 47). There are several stages involved in the production of meat analogs using this technology (42, 48): (i) isolate appropriate cells from animal tissues; (ii) proliferate precursor cells in a bioreactor using an appropriate growth medium, which contains nutrients and signaling factors; (iii) control bioreactor conditions so the precursor cells differentiate into the desired animal cell type, such as muscle or adipose tissue; (iv) harvest and wash the cells; and (v) combine the cells with other ingredients (such as plant and microbial fillers, binders, nutrients, flavors, or colors) to generate meat analogs. The bioreactor conditions require tight process control, including of temperature, oxygen levels, pH, osmolality, agitation conditions, and cell densities, to ensure efficient and safe production of the cells (48). This high level of process control, as well as the requirement for expensive recombinant growth factors to optimize cell doubling times and high cell densities in bioreactors, leads to high production costs. CM products based on immortalized chicken fibroblasts have received regulatory approval in the United States and Singapore, while other companies have pursued bovine and fish sources with regulatory approval still pending. Some products have also been approved in Israel and the United Kingdom for either human or animal consumption.
There are several technical, consumer, and regulatory challenges holding back the widespread availability and adoption of CM products (42, 44, 49, 50). Cell lines that exhibit robust proliferation and differentiation characteristics are required. The growth medium used to produce the cells should be food-grade, animal-free, and inexpensive, which is often challenging. Moreover, there is a need to optimize the operating conditions of industrial bioreactors so they can efficiently produce cells at the large scales needed for commercial production. More research is also required to determine any safety concerns associated with cells produced using this approach, such as contamination, toxicity, and allergenicity issues. In addition, none of these systems have been scaled to date, thus, many unknowns remain in terms of production efficiencies, impact on sustainability goals, and costs once scaling is achieved. There is also a need to generate CM-meat analogs with sensorial and nutritional attributes that satisfy consumers, and to overcome the reluctance of many consumers to accept products created using this novel technology (51, 52). Progress has been made toward these goals, particularly with porcine-derived adipose-derived stem cells in recent sensory analytical comparisons to livestock-derived fat tissue (53). In addition, these products are currently not allowed for consumption in many countries because of regulatory issues (45, 54). Consequently, there is a need to improve the marketing of these products and create a standardized regulatory framework. Moreover, there is a need to ensure that consumers will accept these novel products (55).
Microbial-based products
Rather than eating the whole cells, as is the case for CM analogs, it is possible to utilize precision fermentation methods to produce the proteins and other functional ingredients needed to formulate meat analogs (56, 57). These methods employ different kinds of microorganisms, including bacteria, yeast, or fungi, to produce these ingredients (58). Typically, the microbes are grown in bioreactors under optimized nutrient and environmental conditions. As they grow, the microbes produce proteins or other useful functional ingredients, such as lipids, polysaccharides, vitamins, pigments, or flavors, that can be used to formulate meat analogs once they are purified from the complex media and cells. These molecules may be secreted from the microbial cells or remain intracellular. The target molecules can be isolated from the microbial broth, either by simply separating them from the microbes or by breaking the microbial cell walls and then separating them. A series of downstream processing methods may be required to isolate and purify the target molecules, including selective precipitation, absorption, filtration, or centrifugation methods (56, 59, 60). In some cases, the microorganisms used may naturally produce the desired target molecule. In other cases, they may be genetically engineered to produce the target molecule of interest. In the case of proteins, the approach usually involves creating a DNA sequence that corresponds to the target protein, then inserting it into a plasmid, which is then introduced into the microbial cells. As a result, the target protein is produced by the molecular machinery inside the microorganisms during the fermentation process. At present, the biggest hurdle facing this precision fermentation technology is the difficulty in economically producing large quantities of the target molecules, due to the yields of target molecule per unit volume as well as the downstream purification steps required. For this reason, this approach is mainly used to produce high-value functional ingredients that can be utilized at relatively low levels in foods, such as the leghemoglobin that is used as a pigment in some plant-based burgers (61) or transglutaminase that is used as an enzymatic crosslinking agent in protein-based gels and foods (62). However, precision fermentation has also been used to produce meat, egg, and milk proteins, which have then been used to formulate food products (27).
Insect-based products
Despite being uncommon foods in many developed countries, insects are widely consumed in some countries, with over 2 billion people estimated to include them as part of their diets on a regular basis (63). Insects have many potential advantages as an alternative protein source in the human diet (64). According to a United Nations report (65), raising insects for food is much more environmentally friendly than raising livestock animals, with much lower greenhouse gas emissions, pollution, and land and water use. Moreover, many edible insects have good nutritional profiles (64). In general, the nutrient profile of insects depends on the species, development stage, and food processing operations used to generate the final food product. Certain insect species have been reported to have high levels of proteins, unsaturated fats, dietary fibers, vitamins (especially vitamin C), and minerals (especially iron and calcium), which means they can be utilized as nutritious alternatives to animal meat (66). Further, insects can be utilized as whole animal additions to foods, most often as insect flour, while insect cells can be isolated and utilized for CM goals, as described earlier (67). Of note is that, for CM, insect cells, due to their adaptability to a wide range of environmental variables (e.g., oxygen, nutrients, pH, and temperature), require lower process control than mammalian cells, thus, production costs are significantly reduced (47). In addition, insect cells have been scaled in the pharmaceutical industry to produce therapeutics, thus, there is precedence for advanced manufacturing. A major hurdle to incorporating insects into the food supply is that consumers in many countries find the idea of eating them highly undesirable (68, 69). This is partly due to food neophobia (the fear of trying new foods), as well as disgust at the idea of eating insects (70). To partly overcome this problem, the food industry is developing products that contain insect ingredients but that do not look like the insects themselves. For instance, the insects may be converted into flours or pastes that are then incorporated into foods like protein bars, baked snacks, burgers, nuggets, or sausages. The use of insect cells instead of whole insects can also help to reduce consumer-related insect phobia for food.
Designing hybrid products with desirable properties
A variety of factors need to be considered when designing hybrid meat products using the different kinds of protein sources described in the previous section. These factors include physicochemical, functional, sensory, nutritional, and safety properties and economic and regulatory matters (Figure 2), all of which have to be balanced to align with market demands.
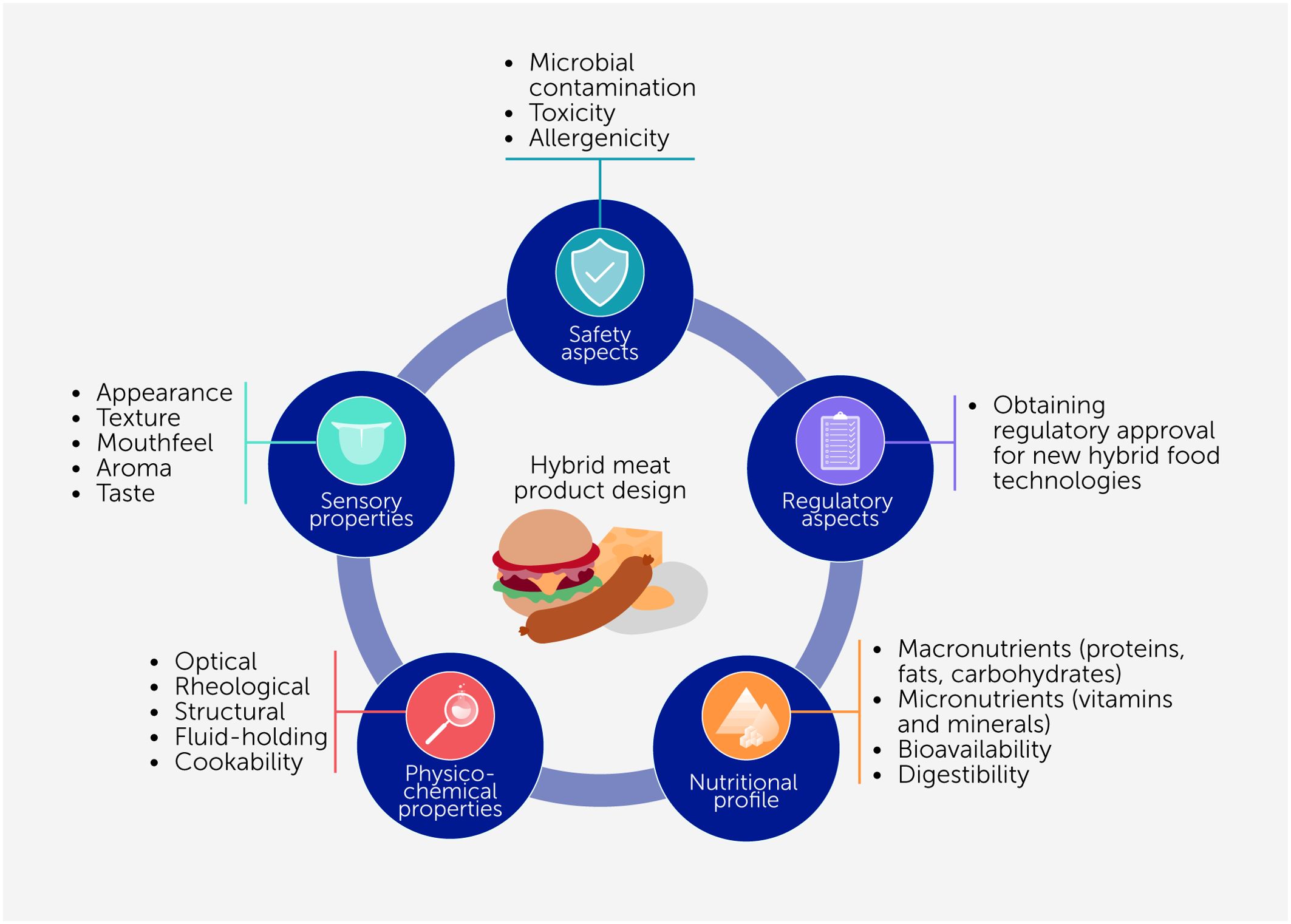
Figure 2. Several factors need to be considered when designing hybrid meat products, including their physicochemical, functional, sensory, and nutritional attributes, food safety issues, and economic and regulatory aspects.
Physicochemical and functional attributes
The physicochemical and functional attributes of meat analogs play a critical role in determining their commercial success (3). As the majority of consumers in most developed countries are omnivores (71), hybrid products should be designed to accurately simulate the attributes of the animal meat products they are intended to replace, such as burgers, sausages, nuggets, or whole cuts. The most important physicochemical and functional attributes of these products are their appearance, texture, fluid-holding, and cookability properties, which can be quantified using a variety of standardized analytical methods (72, 73). Typically, the properties of the target meat product are first quantified using these methods, and then hybrid products are designed to exhibit similar properties. Optimized hybrid meat analogs can then be selected for sensory analysis.
Sensory attributes
The sensory attributes of meat analogs, such as their appearance, mouthfeel, aroma, and taste, also play a critical role in determining their desirability and consumer acceptance (74–76). Currently, the sensory attributes of most commercially available meat analogs formulated from alternative proteins do not accurately mimic those of the equivalent meat products (77). Some of these limitations may be overcome by combining different kinds of alternative protein sources to create hybrid products (9). For instance, the flavor and mouthfeel of plant-based foods may be enhanced by incorporating CM cells, as these may supply specific aromas, tastes, and mouthfeels associated with animal meat products. This strategy is already utilized by many CM companies.
Nutrition
It is important to ensure that meat analogs are designed to be at least as nutritious as the conventional meat products they are intended to replace, otherwise there could be adverse health consequences associated with switching from a traditional omnivore diet (7). Lean meat products are a good source of proteins, essential amino acids, vitamins, and minerals. Indeed, they typically contain sufficient levels of all the essential amino acids required to maintain human health and well-being, whereas most plant-based proteins do not. Some of the current generation of commercial meat analogs do not contain all of the desirable macronutrients (especially proteins) and micronutrients (specific vitamins and minerals) normally obtained from lean meat products (78, 79). Consequently, there could be some adverse nutritional effects associated with switching from lean to hybrid meat products. As mentioned earlier, concerns have also been expressed that some meat analogs are “ultra-processed” foods, which have been linked to adverse health effects (80–82). The harsh processing operations used to formulate meat analogs may disrupt plant cell walls, which makes the macronutrients (especially starches and lipids) more rapidly digestible and could have undesirable health consequences. For instance, rapid starch digestion and glucose absorption could result in the dysregulation of blood sugar and insulin levels, thereby increasing the risk of overeating, diabetes, and coronary heart disease (83).
Consequently, it is important to create hybrid meat analogs with nutritional profiles that are comparable or better than the corresponding livestock-derived meat products. For instance, in hybrid CM–plant meat analogs, plant proteins could be used as an economic source of essential amino acids, whereas cultured mammalian or avian cells could be used as a good source of vitamins and minerals, such as vitamins D and B12, as well as iron and zinc.
Having said this, there is good evidence that eating a predominantly plant-based diet can provide all of the required nutrients and have beneficial impacts on human health and well-being (4), which would support the movement away from the consumption of high levels of meat and other animal products from a nutritional perspective. Moreover, the nutritional impact of switching to hybrid products depends on the nature of the meat product that is being replaced. The consumption of traditional red meat and processed meat products has been linked to adverse health effects and so replacing them could have health benefits. In contrast, it may be more challenging to match the desirable nutritional profiles of lean meat products.
Safety
It is also important to consider any potential safety aspects when designing and producing hybrid foods. Some of the most important issues to consider are microbial contamination, chemical toxicity, and allergenicity (1, 84). Hybrid foods may be contaminated by harmful substances that come from any of the alternative protein sources they are formulated from, including pathogenic bacteria, viruses, heavy metals, pesticides, plasticizers, and other toxins, which could increase their food safety risks. There may be some new risks associated with hybrid foods because microbes different from those in either of the individual environments may grow in the composite environment. Hybrid foods may be formulated from food ingredients that cause allergies in certain individuals, such as soy, milk, or egg proteins, which may be from plants, animals, or precision fermentation. Consequently, it is important to consider all of the potential safety aspects associated with each alternative protein source used to formulate a hybrid food, as well as any new risks that might arise by combining them. In the case of CM, antibiotic requirements are often considerably reduced or eliminated in comparison to livestock-generated meats and, as a major health and safety concern due to growing antibiotic resistance, this would be a positive outcome for this new technology approach. This reduction in antibiotic needs is anticipated due to the controlled environment utilized in CM production. However, since scaled production has yet to be demonstrated, assessments of antibiotic requirements remain to be validated to support these claims.
Challenges to the formulation of hybrid products
There are several potential challenges that food manufacturers may experience when creating hybrid products from different sources of alternative proteins, which are briefly discussed in this section.
An “off” flavor, color, or mouthfeel
Some sources of alternative proteins contain components that adversely affect the flavor, color, or mouthfeel of food products. For instance, plant protein flours, concentrates, or isolates often contain relatively high levels of volatile or nonvolatile phytochemicals that lead to “off” colors (such as dark pigmentation) and/or “off” flavors (such as beany, bitter, or astringent flavors) (85–87). Research is therefore being carried out to identify and quantify the various types of “off” colors and flavors in these ingredients and to develop processing operations to remove them (85, 88, 89). Similarly, the ingredients and extracts obtained from insects often contain “off” flavors and colors and components that can lead to undesirable mouthfeels. For instance, many insects contain volatile substances that lead to unpleasant odors in foods (90–92). Moreover, the exoskeletons of insects contain high levels of chitin, which is present in food as small particles that lead to a gritty mouthfeel (93). Appropriate processing technologies are therefore needed to remove or reduce the undesirable flavors, colors, or mouthfeel attributes of alternative protein ingredients before they are combined. For example, fermentation (typically using yeast or bacteria) has been used to reduce or remove undesirable flavors from insect flours (94, 95), whereas deodorization has been used to reduce or remove them from insect oils (91). Grinding has been used to reduce the size of the insoluble particles in insect flours, which reduces their undesirable mouthfeel (93). More recently, insect cell isolation has been pursued to avoid the use of the chitinous exoskeleton (67). It is therefore important to identify any sources of undesirable components in the different sources of alternative proteins used to formulate a hybrid food and then either remove or deactivate them.
Antinutritional factors
Some of the alternative protein sources used to formulate hybrid products contain antinutritional factors (ANFs) that inhibit the digestion and/or absorption of key nutrients (96–98). For instance, some plant protein sources contain phytates, tannins, oxalates, or leptins. Phytates can bind to cationic mineral ions, such as calcium, iron, magnesium, or zinc, and form complexes that reduce their bioavailability. Tannins can bind to digestive enzymes, such as amylases, lipases, or proteases, thereby reducing macronutrient digestion. Oxalates can bind to calcium and reduce its bioavailability. Lectins can reduce nutrient absorption and cause inflammation of the gastrointestinal tract. Typically, ANFs are removed or deactivated during the production of plant-derived food ingredients but they may cause a problem if whole plant foods are used to formulate hybrid products (96, 97, 99). Consequently, it is important to consider the potential negative impacts of ANFs when formulating hybrid products. Further, additional processes required to remove these contaminants result in increased production costs. Future options may include the use of genetically modified plants where these ANFs are edited out of the genome to avoid the contaminants during plant growth.
Gastrointestinal effects
Combining multiple sources of alternative proteins may affect the gastrointestinal properties of the nutrients in a hybrid food, such as their digestion rate, bioavailability, or microbiome effects. In some cases, these effects may be advantageous, whereas in others they may be detrimental. For instance, the dietary fibers in mycelium, insect, or plant sources of alternative proteins may slow down the digestion of proteins, starches, and lipids from other alternative protein sources by increasing the viscosity of the gastrointestinal fluids, slowing down gastric emptying, or binding to key gastrointestinal constituents, such as bile salts, fatty acids, or calcium (100–102). Dietary fibers may affect the bioavailability of minerals co-ingested with them, depending on their molecular structure, physical form, and gastrointestinal fate (103–105). Dietary fibers can also impact the bioavailability of vitamins and nutraceuticals in a manner that depends on dietary fiber and food matrix type (105–107). Consequently, the fibers in some alternative protein sources may impact the micronutrient bioavailability in others, which should be considered when formulating hybrid products. When genetically modified organism-derived cells are used as ingredients in hybrid foods, the fate of DNA and other cellular constituents should be determined as an additional safety measure. The gastrointestinal properties of hybrid foods can be assessed using in vitro gastrointestinal tract and tissue models. For instance, the static INFOGEST digestion model can be used to monitor changes in a food as it passes through simulated oral, gastric, and small intestine conditions (108). In addition, more sophisticated dynamic in vitro digestion models are available to more realistically simulate the behavior of foods within the human gastrointestinal tract (109, 110). Moreover, human cell-derived bioengineered systems can be used to emulate the transport, absorption, and metabolism of food components (111).
Changes in pH and ionic composition
When multiple alternative protein sources are combined to formulate hybrid products, it is important to account for any changes in the pH and ionic composition of the aqueous phase, as each factor has important implications for ingredient solubility and functionality (112). Each protein source will contain different levels of acids, bases, buffers, and mineral ions, which determine the final properties of the aqueous phase after they are combined. A change in pH or ionic strength may alter the interactions and functionality of ingredients, which may impact their behavior in the hybrid food and gastrointestinal tract. For instance, proteins may precipitate around their isoelectric point or in the presence of oppositely charged polysaccharides, which may alter the physicochemical properties, functionality, and nutritional fate of hybrid foods. Consequently, it is important to take these changes into account and/or to adjust the pH to the required target value after forming the hybrid product.
Adhesion and binding effects
In many hybrid products, one or more of the protein sources may contain particulate materials, such as fat droplets, adipocytes, muscle cells, texturized proteins, connective tissue, or tissue fragments. The interactions of these particulate materials with the surrounding food matrix impact the texture, mouthfeel, and functionality of hybrid products. In general, particles in composite materials may act as either active or inactive fillers depending on whether they bind to the surrounding matrix or not (113). Various theoretical models have been developed to relate the mechanical properties of composite materials to the properties of the fillers they contain, such as their concentration, size, shape, interactions, and rigidity (114). These models are useful for assessing the importance of different factors on the formation and properties of hybrid meat products containing particulate materials.
Ingredient interactions
Interactions between ingredients from different alternative protein sources may lead to alterations in the structural, physical, and chemical properties of hybrid products (Figure 3). As an example, certain combinations of proteins and polysaccharides promote phase separation through either associative (complex coacervation) or segregative (thermodynamic incompatibility) mechanisms (115), which may be either desirable or undesirable when formulating hybrid meat analogs. For instance, phase separation can be used to create fibrous structures in meat analogs by shearing and setting the system (116–118). The presence of a particular component in one alternative protein source may impact the functionality of a component in another protein source. For instance, calcium ions may promote the crosslinking of anionic polysaccharides, thereby increasing the gel strength (119). Conversely, some components may interfere with the ability of biopolymers to form gels by binding to them, thereby inhibiting their ability to crosslink (120). The presence of transition metals or other prooxidants in an alternative protein source may promote the oxidation of unsaturated lipids in another one. Conversely, the presence of phenolic substances or other antioxidants may have the opposite effects. For instance, tannic acid (found in many plants) inhibits lipid oxidation because it can chelate iron ions, which are strong prooxidants (121). Similarly, quercetin, rutin, and chlorogenic acid (also found in many plants) can inhibit the oxidation of unsaturated lipids (122). Plant-derived ingredients also contain natural antioxidants (such as essential oils and some phytochemicals) that can inhibit the oxidation of lipids and proteins in meat products (123). The addition of myoglobin to plant-based (soy protein) burgers was found to alter their aroma profile, which was attributed to its ability to promote specific chemical reactions (124). Consequently, it is important to be aware of any chemical or physical interactions that may occur between the ingredients in different alternative protein sources.
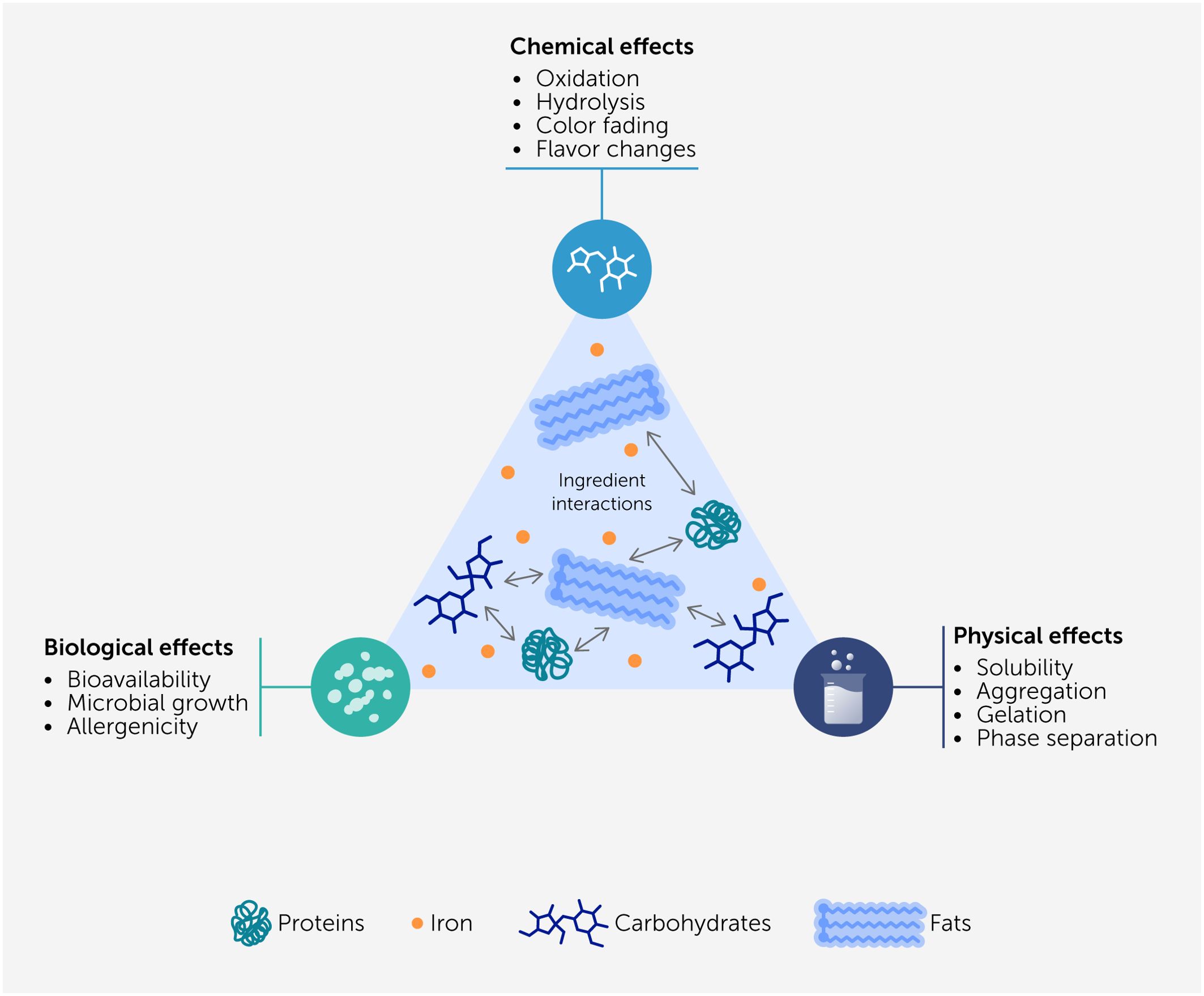
Figure 3. Interactions between the ingredients in different sources of alternative proteins could lead to desirable or undesirable physical, chemical, or biological effects in hybrid food products.
Development of hybrid products
In principle, many kinds of hybrid products can be created by combining different alternative protein sources together but, in reality, some combinations are much more practical than others. Due to their low cost, high availability, and good techno-functional properties, plant proteins are often used as key components to formulate hybrid foods. These ingredients can also be combined with polysaccharides to moderate costs due to the more expensive protein components. CM cells, precision-fermentation proteins, mycelia, or insects may then be used as another component due to their desirable flavor attributes and/or nutritional profiles. As a result, meat–plant, CM–plant, mycelium–plant, and insect–plant systems are the most commonly studied and marketed hybrid meat products. In this section, several examples of previous studies on these hybrid products are provided.
Livestock derived meat–plant hybrids
The adverse effects of livestock production on the environment, human health, and animal welfare can be reduced by developing meat–plant hybrids, where a substantial fraction of the meat is replaced with more sustainable plant-derived ingredients (13). These products are unsuitable for strict vegetarians or vegans (who do not consume any meat), but they would be suitable for omnivores or flexitarians (who intentionally reduce the amount of meat they consume), who make up the majority of consumers in most developed countries (71). Currently, meat–plant hybrids are the most widely studied type of hybrid products. The development of meat–plant hybrids has been reviewed recently (9), and so only a brief overview will be given here to highlight some of the issues involved.
Researchers have investigated the impact of meat-to-plant ratio on the sensory and textural attributes of hybrid meat–plant burgers (125). The fluid loss and shrinkage of the burgers during cooking increased as the meat-to-plant ratio increased. Sensory panelists could distinguish between the different products during mastication, especially during the earlier stages of oral processing. There were appreciable differences in the coarseness, crumbliness, elasticity, and oiliness of the products depending on their meat-to-plant ratios. The products containing higher animal meat contents were rated as having higher saltiness and flavor intensity scores.
In a recent study, researchers characterized the textural and sensory properties of animal meat burgers and hybrid meat–plant burgers (126). The hybrid burgers contained 50% meat (beef and pork) and 50% plant-derived ingredients (including texturized legume proteins). Texture profile analysis showed that the hybrid burgers were softer and less cohesive than the animal meat burgers, although sensory studies showed that the hybrid burgers were perceived as juicier. However, no significant difference in the overall liking of the animal meat versus the hybrid burgers was found. Other researchers have also reported no significant difference in the liking, appearance, odor, flavor, or texture of meat burgers, plant burgers, and hybrid meat–plant burgers (127). Another study reported that up to 25% of meat could be replaced with texturized pumpkin seed proteins in dry-cured hybrid sausages without significantly changing their desirable physicochemical and sensory attributes (128, 129). Research has also shown that various plant proteins (including those from peas, rice, or faba bean) can be incorporated into processed chicken products to reduce their meat content (130). Another study found that adding 20% of pea protein to pork sausages improved their nutritional profile but reduced their hardness (131). Other researchers reported that meatballs with good physiochemical and sensory properties could be created by blending texturized soy protein with beef (132). Indeed, these kinds of products have been commercially available in Europe for over two decades.
Interestingly, in some countries, many processed meat products can be considered to be hybrid products (12). For instance, the United Kingdom stipulates that a pork sausage only needs to contain a minimum of 42% pork, with the rest being fillers, extenders, or binders, which are often plant-based ingredients, such as those obtained from soy and wheat. These non-meat ingredients are used to reduce costs as well as to provide desirable functional attributes, such as binding, gelling, or emulsifying.
Overall, these studies suggest that the sensory desirability of plant-based meat analogs can be improved by adding some meat to them, due to the desirable colors, flavors, and mouthfeel provided by the meat components. When formulating a product, manufacturers should therefore identify the minimum amount of animal meat that they need to incorporate into meat–plant hybrids to obtain good consumer liking, as this would have the biggest environmental impact.
Cultivated meat–plant hybrids
As mentioned earlier, the main limitations of CM products are their relatively high costs and poor scalability, whereas the main limitations of plant-based products are their poor flavor, texture, mouthfeel, and nutrient profiles. These challenges can be addressed by combining CM-based products with plant-based products (13). Indeed, hybrid CM–plant products, containing 3% CM, with the rest being mainly plant-derived ingredients, were recently made commercially available in Singapore supermarkets.
Hybrid products can be produced by growing meat cells and then mixing them with plant-derived ingredients or by using plant-derived ingredients to form a suitable scaffold that is then used to grow the cells around. For example, researchers have generated CM–plant hybrids using alginate fibers as a scaffold to grow model meat (mouse) cells (133). The alginate fibers were produced by a wet-spinning method that involved injecting a sodium alginate solution into a calcium solution. The model meat cells attached to the alginate fibers and proliferated to form meatlike hybrid structures. The researchers showed that these CM–plant hybrids could be cooked to form chicken-like products. Other researchers have used scaffolds assembled from texturized vegetable proteins (TVPs) coated with a mixture of fish gelatin and agar to cultivate model meat cells (134). The presence of the fish gelatin–agar coating increased the adhesion of the CM cells to the surfaces of the TVP matrix. The cooked CM–plant hybrids were reported to have physicochemical and sensory attributes (appearance, texture, and flavor profile) somewhat similar to those of animal meat.
The possibility of forming CM–plant hybrids by blending animal adipocytes grown in bioreactors with plant-based matrices has also been investigated. In this case, the adipocytes are used to simulate the fatty tissue (rather than the muscle tissue) in meat products. For instance, researchers have grown pork adipocytes within hydrogel scaffolds formulated from κ-carrageenan and konjac glucomannan (135). Other researchers used hydrogel scaffolds formulated from fibrinogen and konjac glucomannan to grow model muscle cells derived from mice (136). The authors of these studies suggested that these hybrid products may be able to mimic the attributes of animal meat products. Nevertheless, more research is clearly needed to examine the impact on the physicochemical, functional, and sensory attributes when incorporating different kinds of CM cells into different kinds of plant-based matrices. Several companies are currently pursuing commercialization of such hybrids. As an example, Mission Barns includes pork fat cells with plant components in their hybrid bacon and meatball products.
Mycelium–plant hybrids
Several studies have reported that meat analogs can be produced by incorporating mycelium grown by microbial fermentation into plant-based matrices. Mycelium is particularly useful for this purpose because it naturally has a fibrous structure that mimics the mouthfeel and texture of animal meat. In addition, it contains some valuable nutrients, such as specific vitamins and minerals, that may not be found in plant-derived ingredients. For instance, sensory studies have shown that the consumer acceptance of burger analogs produced from mycelium (Agaricus bisporus)–plant hybrids was higher than of those produced from plant-derived ingredients alone (40). Mycelium–plant hybrids with physicochemical properties similar to animal meat products have also been produced by incorporating mycelium (Pleurotus eryngii) into pea protein-based matrices (41). Other researchers have explored the potential of creating meatlike structures and textures using other kinds of mycelia, such as P. sapidus (137) and P. ostreatus (138), to form mycelia–plant hybrids. It should be noted that the vegan version of commercial meat analogs (Quorn™) based on mycelium (Fusarium venenatum) is held together by a plant protein matrix, so this can already be considered to be a mycelia–plant hybrid. Moreover, Matr Foods in Denmark uses a combination of plant-derived ingredients (vegetables, legumes, and grains) and mycelia to create hybrid food products with meatlike textures using solid-state fermentation.
Insect–plant hybrids
Several researchers have examined the impact of combining insects with plant-derived ingredients to form hybrid meat products. For instance, meat burger analogs have been generated from a blend of insect and plant proteins (139). The main texturizing and binding ingredients used to formulate these products were insect mealworm (Tenebrio molitor) flour, seitan, soy proteins, oat flakes, and sodium alginate. The physicochemical and sensory attributes of these hybrid burgers, such as their texture, mouthfeel, and flavor, could be controlled by altering the ratio of these different ingredients. Under optimized conditions, hybrid burgers with properties resembling aspects of animal (livestock-derived) meat burgers could be produced. In another study, insect–plant hybrid meat analogs were prepared from lesser mealworm (Alphitobius diaperinus), soy protein, and soy fiber using twin-screw high-moisture extrusion (140). Fibrous meat analogs were generated with a protein content and hardness similar to animal meat.
Hybrid insect–plant meat analogs can also be generated from more exotic ingredients. For example, the Javanese grasshopper (Valanga nigricornis) has been combined with kidney beans and elephant foot yam to produce high-protein meat patty analogs (141). These products were targeted at the Indonesian market, as a large fraction of this population has protein consumption levels below recommended levels. By varying the ratio of the different ingredients, it was possible to obtain appearances, textures, and fluid-holding properties resembling those found in animal meat burgers. Meat analogs with flavors and nutritional profiles similar to animal meat have also been generated by blending 30% of mealworm larva (Tenebrio molitor) with soy protein (142). Taken together, these studies suggest that there may be some advantages to combining insect and plant proteins together, but further research is required to optimize these formulations so that they match consumer expectations for specific hybrid food products.
Non-plant-based hybrids
There are several examples of studies on hybrid products formulated without plant proteins. For instance, researchers have shown that incorporating insect proteins (5%) into beef burgers improved their phenolic acid content, which may have health benefits (143). Other researchers examined the impact of replacing some of the meat in burger patties with black soldier fly larvae (Hermetia illucens) (144). Cooked patties containing these insects had a softer texture, exhibited lower cooking losses, and had a darker appearance than the control beef patties. Hybrid products containing 25% insects had physicochemical properties most similar to those of beef patties. In another study, the impact on physicochemical and functional properties when replacing some of the meat in ground pork products with superworm (Zophobas morio larvae) was investigated (145). The incorporation of superworm decreased the hardness, water-holding, and cooking loss properties of the hybrid insect-meat products (145). The physicochemical properties of the hybrids were found to be most similar to those of the livestock-derived meat products when heated at a higher temperature (80°C instead of 70°C), but it was still difficult to completely match textural profiles. The same researchers reported that even replacing a small amount of meat in pork sausages with superworm (5–10%) led to an appreciable decrease in the textural attributes of the products (146). Numerous other studies have shown that different kinds of insects can be used as extenders in meat products, including grasshoppers in pork sausages (147), crickets in pork sausages (148), black soldier fly larvae in meat analogs (149), and silkworm pupae in meat batter (150). These and other studies demonstrate that products with properties somewhat similar to those of animal meat burgers can be produced by incorporating insect-derived ingredients up to a certain level, highlighting the potential for these products in reducing overall meat consumption, which, in turn, could have sustainability, environmental, and cost benefits. Further advances can also be considered by using insect cells as alternatives, akin to CM hybrids with plants, while also reducing costs compared to the use of mammalian-derived cells.
Current challenges and future directions for hybrid products
For hybrid alternative protein products to become commercially viable, there are several hurdles that need to be overcome, including their potential effects on human health and animal welfare, scalability, accessibility, affordability, desirability, and environmental impacts.
Ideally, we want hybrid alternative-protein products to significantly enhance our food supply systems while becoming more sustainable. Reducing our reliance on livestock production would improve overall food system sustainability by minimizing adverse environmental effects, such as greenhouse gas production, biodiversity loss, pollution, and water and land use. However, a number of challenges remain to be addressed. For instance, techno-economic analysis (TEA) and life cycle analysis (LCA) have shown that the media components (such as serum and growth factors) used to produce CM are major contributors to their high costs and negative environmental impacts. Thus, there is a pressing need to identify media constituents that are more cost effective, abundant, and sustainable, as this will provide immediate benefits to the economic and environmental impacts of any hybrid foods formulated from CM. Moreover, there is currently limited bioreactor capacity to produce cultivated cell products on a scale large enough to significantly reduce meat consumption. Consequently, it will be important to greatly increase capacity to have the desirable impact on the sustainability and environmental footprint of our modern food supply. This will require considerable investment from both the public and private sector, as well as a long-term commitment to the successful implementation of this technology.
The impact on human health and animal welfare is another important aspect to consider. Cultivated cell-based hybrid products could improve human and animal health by providing safer and more nutritious foods, but, as with any new technology, it is important to ensure that alternative food products do not have any unintended harmful effects on human health and well-being. Generally, there should be a decreased risk of disease transmission for food products containing cultivated cells when compared to conventional livestock production because of the closed production systems typically used to support cell growth and processing. Engineering cells to enhance their nutritional content is also feasible with CM, e.g., by controlling cell media composition or using cell engineering (151). For instance, it is possible to reduce the saturated fat level and increase the polyunsaturated fat level using these approaches (152). Animal and human health would also benefit from the anticipated reduced need for antibiotics, steroids, and other compounds currently used in industrial or factory farms. However, rigorous studies are still needed to systematically compare the nutritional and health consequences of switching from meat products to hybrid alternatives.
Fluctuations in the global economy also pose challenges for implementing new hybrid food technologies, potentially resulting in reduced investment. However, academic research has continued to develop new fundamental tools, and public and private funding has been supporting research innovation in this field (153–157). Naturally, continued funding will be critical for the further development of commercially viable products that can have an appreciable impact on food sustainability and human health.
Finally, difficulties in obtaining regulatory approval for new hybrid food technologies (particularly cultivated cell-based products) present another major obstacle. However, several CM and hybrid food products intended for either human or animal consumption have already received regulatory approval in countries such as Singapore, the United Kingdom, the United States, and Israel, which will pave the way for more products in the future. Still, it is important that regulatory approval is obtained for a wider range of hybrid products in a greater number of countries if this technology is going to be successful. Consumers will ultimately decide which hybrid options are most acceptable to them in terms of their desirability, affordability, convenience, healthiness, sustainability, and ethics. In the short term, plant–mycelium hybrids are likely to be the most economically viable due to their lower costs and scalability but, in the longer term, plant–CM hybrid products may become more desirable because of their potential for improved nutritional and sensorial properties. Even so, additional research is required to better understand consumer preferences so that appropriate products and marketing strategies can be developed and employed (55).
A road map for the future of hybrid products
The successful adoption of hybrid food products by consumers will depend on several critical steps being successfully implemented, thereby providing us with a clear road map (Figure 4):
● Optimization of individual alternative protein sources. It will be important to optimize the science and technology required to produce each individual alternative protein source, including plants, insects, mycelia, and cultivated cells. In particular, further research is needed on how to reduce costs, increase scalability, understand and control composition and structure, improve functional properties and nutritional profiles, and enhance sensory attributes.
● Development of hybrid combinatorial technologies. More research is required to rationally design hybrid products with desirable attributes based on knowledge of the properties of the individual alternative protein sources. At present, there is still a poor fundamental understanding of how the physicochemical, sensory, nutritional, and safety properties of hybrid foods depend on the nature of the different components they are assembled from.
● Economic and scalable production. Additional research and development is needed to create large-scale manufacturing facilities that can economically produce hybrid food products in the quantities required to satisfy the target consumer market. At present, many alternative protein products are too expensive and cannot be produced at a scale that will have an appreciable impact on the consumer meat market, thereby restricting their potential environmental and sustainability benefits.
● Sensory and consumer aspects. Further research is urgently needed to assess the consumer desirability and acceptability of different kinds of hybrid food products and determine effective communication and marketing strategies to enhance their potential success in the market. It will be critical to gain consumer confidence and trust in hybrid products to meet this goal, which will require transparency and honesty from food companies that manufacture and sell hybrid products.
● Comparative environmental and economic analyses. LCA and TEA are required on a broader range of hybrid food products to assess their environmental and economic impacts relative to conventional animal-derived food products. This information could then be used to identify the most commercially viable and sustainable hybrid foods. However, more research is still required to develop standardized methods and validated data to make meaningful comparisons between different products and processes.
● Knowledge integration technologies. Artificial intelligence approaches, including machine learning, deep learning, and computer vision, have great potential for promoting the expansion and efficiency of this field. For example, they can be used to identify the optimum type and combination of alternative protein ingredients and processing operations required to produce desirable, affordable, and sustainable hybrid food products. This could be achieved using AI to establish correlations between inputs (ingredients and processing operations) and outputs (nutritional profile, appearance, texture, mouthfeel, etc.).
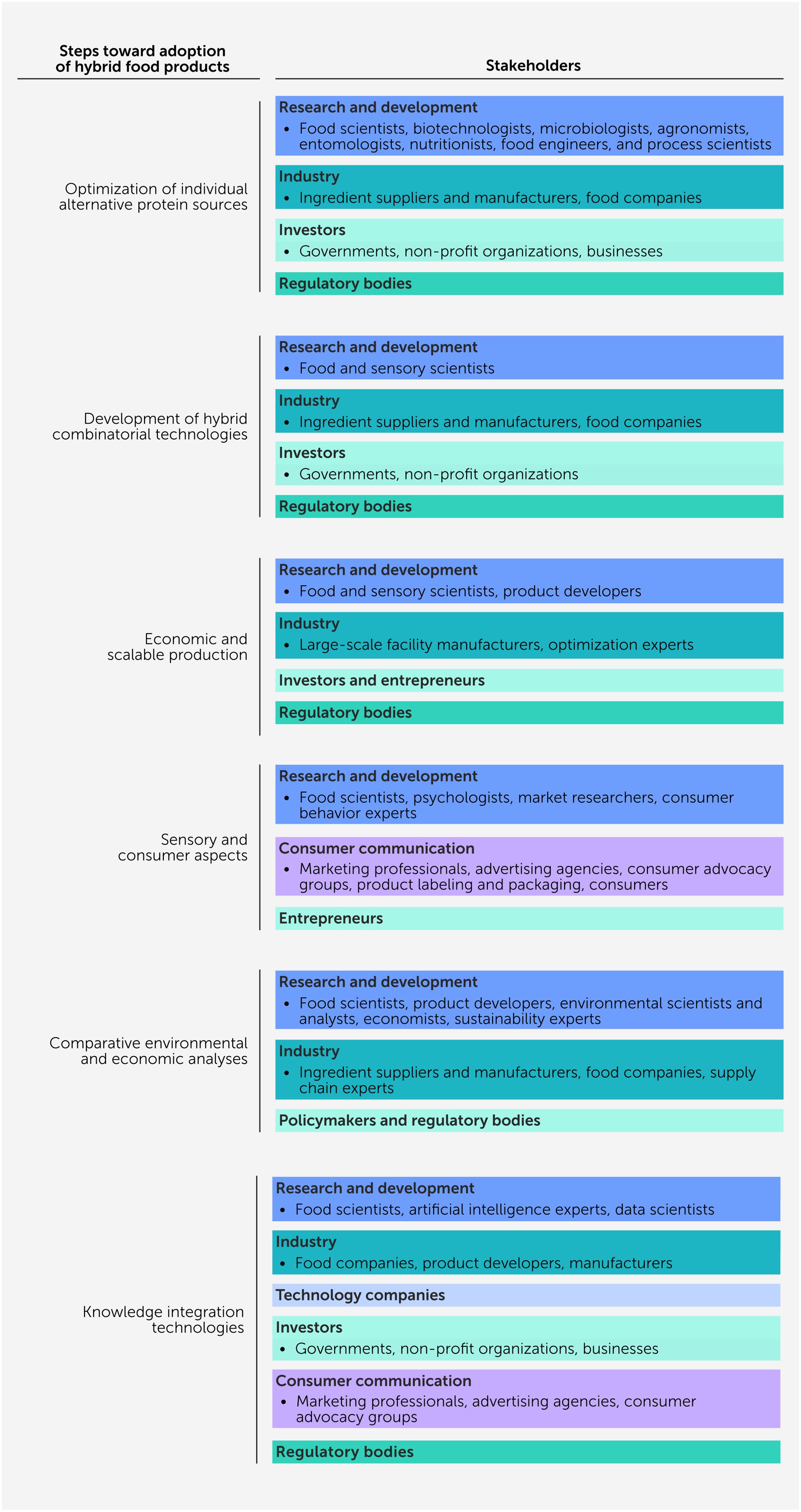
Figure 4. Successful adaptation of hybrid food products involves several critical steps and requires collaboration across key stakeholders, including from research and academia, industry, investors, relevant governmental regulatory bodies, marketing experts, and tech companies.
The successful implementation of these steps will require the concerted efforts of a diverse group of individuals and organizations, including academics, industry experts, entrepreneurs, investors, regulators, politicians, and the media.
Statements
Author contributions
DLK: Writing – original draft, Visualization, Funding acquisition, Writing – review & editing.
DJM: Funding acquisition, Visualization, Writing – original draft, Conceptualization, Writing – review & editing.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Funding
The authors declared financial support was received for this work and/or its publication.
DJM received funding from the United States Department of Agriculture (USDA) through the National Institute of Food and Agriculture (2020-03921, 2022-09185, and 2021-05678), the Massachusetts Agricultural Experiment Station (MAS00559) programs, and from the nonprofit think tank Good Food Institute (2022).
DLK received funding from the USDA (2021–05678), the Advanced Research Projects Agency – Energy, the nonprofit think tank Good Food Institute, and the nonprofit foundation New Harvest.
The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
DJM serves on the scientific advisory board of several food companies, including a plant-based cheese company, a tempeh company, a cannabis edibles company, and an eye health company. As part of his services, he may receive shares in these companies. He owns some patents on the development of colloidal delivery systems for bioactive agents. He has received funding from the federal government and non-profit organizations to carry out research on plant-based foods. He has also written several books on next-generation foods that include a discussion of alternative proteins.
DLK declared that this work was conducted in the absence of financial relationships that could be construed as a potential conflict of interest.
The reviewer MJP declared a past co-authorship with the author DLK to the handling editor.
Generative AI statement
The authors declared that no generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Professor Lutz Grossmann (University of Massachusetts Amherst, Amherst, MA, United States) for valuable discussions on the manuscript.
This material was partly based upon work supported by the National Institute of Food and Agriculture, the United States Department of Agriculture (USDA), Massachusetts Agricultural Experiment Station (MAS00559) and the USDA, Agriculture and Food Research Initiative (2020-03921, 2022-09185, and 2021-05678) grants as well as the Good Food Institute.
References
1. Banach JL, van der Berg JP, Kleter G, Van Bokhorst-van De Veen H, Bastiaan-Net S, Pouvreau L, et al. Alternative proteins for meat and dairy replacers: food safety and future trends. Crit Rev Food Sci Nutr (2023) 63(32):11063–80. doi: 10.1080/10408398.2022.2089625
2. Lee HJ, Yong HI, Kim M, Choi YS, and Jo C. Status of meat alternatives and their potential role in the future meat market - a review. Asian-Australas J Anim Sci (2020) 33(10):1533–43. doi: 10.5713/ajas.20.0419
3. McClements DJ and Grossmann L. Next-generation plant-based foods: design, production, and properties. New York, NY: Springer Scientific (2022). doi: 10.1007/978-3-030-96764-2
4. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, et al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet (2019) 393(10170):447–92. doi: 10.1016/S0140-6736(18)31788-4
5. Waehrens SS, Faber I, Gunn L, Buldo P, Bom Frøst MB, and Perez-Cueto FJA. Consumers’ sensory-based cognitions of currently available and ideal plant-based food alternatives: a survey in Western, Central and northern Europe. Food Qual Prefer (2023) 108:104875. doi: 10.1016/j.foodqual.2023.104875
6. Azarkamand S, Fernández Ríos AF, Batlle-Bayer L, Bala A, Sazdovski I, Roca M, et al. Calculating the true costs of protein sources by integrating environmental costs and market prices. Sustain Prod Consum (2024) 49:28–41. doi: 10.1016/j.spc.2024.06.006
7. McClements IF and McClements DJ. Designing healthier plant-based foods: fortification, digestion, and bioavailability. Food Res Int (2023) 169:112853. doi: 10.1016/j.foodres.2023.112853
8. Nezlek JB and Forestell CA. Meat substitutes: current status, potential benefits, and remaining challenges. Curr Opin Food Sci (2022) 47:100890. doi: 10.1016/j.cofs.2022.100890
9. Grasso S and Goksen G. The best of both worlds? Challenges and opportunities in the development of hybrid meat products from the last 3 years. LWT Food Sci Technol (2023) 173:114235. doi: 10.1016/j.lwt.2022.114235
10. Tachie C, Nwachukwu ID, and Aryee ANA. Trends and innovations in the formulation of plant-based foods. Food Prod Process Nutr (2023) 5(1):1–14. doi: 10.1186/s43014-023-00129-0
11. Jahir NR, Ramakrishna S, Abdullah AAA, and Vigneswari S. Cultured meat in cellular agriculture: advantages, applications and challenges. Food Biosci (2023) 53:102614. doi: 10.1016/j.fbio.2023.102614
12. Grasso S and Jaworska S. Part meat and part plant: are hybrid meat products fad or future? Foods (2020) 9(12):1888. doi: 10.3390/foods9121888
13. Alam AN, Kim CJ, Kim SH, Kumari S, Lee SY, Hwang YH, et al. Trends in hybrid cultured meat manufacturing technology to improve sensory characteristics. Food Sci Anim Resour (2024) 44(1):39–50. doi: 10.5851/kosfa.2023.e76
14. Baune M-C, Broucke K, Ebert S, Gibis M, Weiss J, Enneking U, et al. Meat hybrids-an assessment of sensorial aspects, consumer acceptance, and nutritional properties. Front Nutr (2023) 10:1101479. doi: 10.3389/fnut.2023.1101479
15. Vila-Clarà G, Vila-Martí A, Vergés-Canet L, and Torres-Moreno M. Exploring the role and functionality of ingredients in plant-based meat analogue burgers: a comprehensive review. Foods (2024) 13(8):1258. doi: 10.3390/foods13081258
16. McClements DJ and Grossmann L. The science of plant-based foods: constructing next-generation meat, fish, milk, and egg analogs. Compr Rev Food Sci Food Saf (2021) 20(4):4049–100. doi: 10.1111/1541-4337.12771
17. Thakur S, Pandey AK, Verma K, Shrivastava A, and Singh N. Plant-based protein as an alternative to animal proteins: a review of sources, extraction methods and applications. Int J Food Sci Technol (2024) 59(1):488–97. doi: 10.1111/ijfs.16663
18. Liao YC, Chang CC, Nagarajan D, Chen CY, and Chang JS. Algae-derived hydrocolloids in foods: applications and health-related issues. Bioengineered (2021) 12(1):3787–801. doi: 10.1080/21655979.2021.1946359
19. Dev MJ, Warke RG, Warke GM, Mahajan GB, Patil TA, and Singhal RS. Advances in fermentative production, purification, characterization and applications of gellan gum. Bioresour Technol (2022) 359:127498. doi: 10.1016/j.biortech.2022.127498
20. Hicks TM and Verbeek CJR. Protein-rich by-products: production statistics, legislative restrictions, and management options. In: Dhillon GS, editor. Protein Byproducts. Cambridge: Academic Press (2016) 1–18. doi: 10.1016/B978-0-12-802391-4.00001-X
21. Listrat A, Lebret B, Louveau I, Astruc T, Bonnet M, Lefaucheur L, et al. How muscle structure and composition determine meat and flesh quality. Sci World J (2016) 2016(1):3182746. doi: 10.1155/2016/3182746
22. Appiani M, Cattaneo C, and Laureati M. Sensory properties and consumer acceptance of plant-based meat, dairy, fish and eggs analogs: a systematic review. Front Sustain Food Syst (2023) 7:1268068. doi: 10.3389/fsufs.2023.1268068
23. Szenderák J, Fróna D, and Rákos M. Consumer acceptance of plant-based meat substitutes: a narrative review. Foods (2022) 11(9):1274. doi: 10.3390/foods11091274
24. Imran M and Liyan Z. Production of plant-based meat: functionality, limitations and future prospects. Eur Food Res Technol (2023) 249(9):2189–213. doi: 10.1007/s00217-023-04287-w
25. Wang Y, Cai W, Li L, Gao YE, and Lai KH. Recent advances in the processing and manufacturing of plant-based meat. J Agric Food Chem (2023) 71(3):1276–90. doi: 10.1021/acs.jafc.2c07247
26. Nowacka M, Trusinska M, Chraniuk P, Drudi F, Lukasiewicz J, Nguyen NP, et al. Developments in plant proteins production for meat and fish analogues. Molecules (2023) 28(7):2966. doi: 10.3390/molecules28072966
28. Wickramasinghe K, Breda J, Berdzuli N, Rippin H, Farrand C, and Halloran A. The shift to plant-based diets: are we missing the point? Glob Food Sec Agric Policy Econ Environ (2021) 29:100530. doi: 10.1016/j.gfs.2021.100530
29. Lane MM, Davis JA, Beattie S, Gómez-Donoso C, Loughman A, O’Neil A, et al. Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev (2021) 22(3):e13146. doi: 10.1111/obr.13146
30. Liang SM, Zhou YS, Zhang Q, Yu S, and Wu SS. Ultra-processed foods and risk of all-cause mortality: an updated systematic review and dose-response meta-analysis of prospective cohort studies. Syst Rev (2025) 14(1):53. doi: 10.1186/s13643-025-02800-8
31. McClements DJ. Ultraprocessed plant-based foods: designing the next generation of healthy and sustainable alternatives to animal-based foods. Compr Rev Food Sci Food Saf (2023) 22(5):3531–59. doi: 10.1111/1541-4337.13204
32. Liu C, Shi JL, Wang J, Dai Y, and Raghavan V. Effects of different processing degrees of plant-based meat on the blood biochemical level, inflammation and intestinal microorganisms in mice. Food Res Int (2023) 173(2):113398. doi: 10.1016/j.foodres.2023.113398
33. Fechner D, Grün B, and Dolnicar S. Identifying segment-specific barriers to ordering environmentally sustainable plant-based meat dishes in restaurants. J Sustain Tourism (2025) 33(2):333–56. doi: 10.1080/09669582.2024.2342982
34. Kuosmanen S, Niva M, Pajari AM, Korhonen K, Muilu T, and Konttinen H. Barriers associated with pulse and plant-based meat alternative consumption across sociodemographic groups: a capability, opportunity, motivation, behaviour model approach. Front Nutr (2023) 10:1186165. doi: 10.3389/fnut.2023.1186165
35. Trevelyan WE. Determination of uric acid precursors in dried yeast and other forms of single-cell protein. J Sci Food Agric (1975) 26(11):1673–80. doi: 10.1002/jsfa.2740261108
36. Li K, Qiao K, Xiong J, Guo H, and Zhang Y. Nutritional values and bio-functional properties of fungal proteins: applications in foods as a sustainable source. Foods (2023) 12(24):4388. doi: 10.3390/foods12244388
37. Ahmad MI, Farooq S, Alhamoud Y, Li CB, and Zhang H. A review on mycoprotein: history, nutritional composition, production methods, and health benefits. Trends Food Sci Technol (2022) 121:14–29. doi: 10.1016/j.tifs.2022.01.027
38. Holt RR, Munafo JP, Salmen J, Keen CL, Mistry BS, Whiteley JM, et al. Mycelium: a nutrient-dense food to help address world hunger, promote health, and support a regenerative food system. J Agric Food Chem (2024) 72(5):2697–707. doi: 10.1021/acs.jafc.3c03307
39. Strong PJ, Self R, Allikian K, Szewczyk E, Speight R, O’Hara I, et al. Filamentous fungi for future functional food and feed. Curr Opin Biotechnol (2022) 76:102729. doi: 10.1016/j.copbio.2022.102729
40. Kim K, Choi B, Lee I, Lee H, Kwon S, Oh K, et al. Bioproduction of mushroom mycelium of Agaricus bisporus by commercial submerged fermentation for the production of meat analogue. J Sci Food Agric (2011) 91(9):1561–8. doi: 10.1002/jsfa.4348
41. Mandliya S, Pratap-Singh A, Vishwakarma S, Dalbhagat CG, and Mishra HN. Incorporation of mycelium (Pleurotus eryngii) in pea protein based low moisture meat analogue: effect on its physicochemical, rehydration and structural properties. Foods (2022) 11(16):2476. doi: 10.3390/foods11162476
42. Stout AJ, Kaplan DL, and Flack JE. Cultured meat: creative solutions for a cell biological problem. Trends Cell Biol (2023) 33(1):1–4. doi: 10.1016/j.tcb.2022.10.002
43. Olenic M, Deelkens C, Heyman E, De Vlieghere E, Zheng X, Van Hengel J, et al. Review: livestock cell types with myogenic differentiation potential: considerations for the development of cultured meat. Animal (2025) 19(Suppl 1):101242. doi: 10.1016/j.animal.2024.101242
44. Pajčin I, Knežić T, Savic Azoulay IS, Vlajkov V, Djisalov M, Janjušević L, et al. Bioengineering outlook on cultivated meat production. Micromachines (2022) 13(3):402. doi: 10.3390/mi13030402
45. Post MJ, Levenberg S, Kaplan DL, Genovese N, Fu JA, Bryant CJ, et al. Scientific, sustainability and regulatory challenges of cultured meat. Nat Food (2020) 1(7):403–15. doi: 10.1038/s43016-020-0112-z
46. Rubio NR, Fish KD, Trimmer BA, and Kaplan DL. Possibilities for engineered insect tissue as a food source. Front Sustain Food Syst (2019) 3:24. doi: 10.3389/fsufs.2019.00024
47. Ashizawa R, Rubio N, Letcher S, Parkinson A, Dmitruczyk V, and Kaplan DL. Entomoculture: a preliminary techno-economic assessment. Foods (2022) 11(19):3037. doi: 10.3390/foods11193037
48. Fasciano S, Wheba A, Ddamulira C, and Wang S. Recent advances in scaffolding biomaterials for cultivated meat. Biomater Adv (2024) 162:213897. doi: 10.1016/j.bioadv.2024.213897
49. O’Neill EN, Cosenza ZA, Baar K, and Block DE. Considerations for the development of cost-effective cell culture media for cultivated meat production. Compr Rev Food Sci Food Saf (2021) 20(1):686–709. doi: 10.1111/1541-4337.12678
50. Bomkamp C, Skaalure SC, Fernando GF, Ben-Arye T, Swartz EW, and Specht EA. Scaffolding biomaterials for 3D cultivated meat: prospects and challenges. Adv Sci (Weinh) (2022) 9(3):e2102908. doi: 10.1002/advs.202102908
51. Bryant C and Barnett J. Consumer acceptance of cultured meat: an updated review (2018-2020). Appl Sci Basel (2020) 10(15):5201. doi: 10.3390/app10155201
52. Lewisch L and Riefler P. Cultured meat acceptance for global food security: a systematic literature review and future research directions. Agric Food Econ (2023) 11(1):48. doi: 10.1186/s40100-023-00287-2
53. Lew ET, Yuen JSK, Zhang KL, Fuller K, Frost SC, and Kaplan DL. Chemical and sensory analyses of cultivated pork fat tissue as a flavor enhancer for meat alternatives. Sci Rep (2024) 14(1):17643. doi: 10.1038/s41598-024-68247-4
54. Broucke K, Van Pamel E, Van Coillie E, Herman L, and Van Royen G. Cultured meat and challenges ahead: a review on nutritional, technofunctional and sensorial properties, safety and legislation. Meat Sci (2023) 195:109006. doi: 10.1016/j.meatsci.2022.109006
55. Hanan FA, Karim SA, Aziz YA, Ishak FAC, and Sumarjan N. Consumer’s cultured meat perception and acceptance determinants: a systematic review and future research agenda. Int J Con Stud (2024) 48(5):e13088. doi: 10.1111/ijcs.13088
56. Augustin MA, Hartley CJ, Maloney G, and Tyndall S. Innovation in precision fermentation for food ingredients. Crit Rev Food Sci Nutr (2024) 64(18):6218–38. doi: 10.1080/10408398.2023.2166014
57. Boukid F, Hassoun A, Zouari A, Tülbek MÇ, Mefleh M, Aït-Kaddour A, et al. Fermentation for designing innovative plant-based meat and dairy alternatives. Foods (2023) 12(5):1005. doi: 10.3390/foods12051005
58. Nadar CG, Fletcher A, Moreira BRA, Hine D, and Yadav S. Waste to protein: a systematic review of a century of advancement in microbial fermentation of agro-industrial byproducts. Compr Rev Food Sci Food Saf (2024) 23(4):e13375. doi: 10.1111/1541-4337.13375
59. Hilgendorf K, Wang Y, Miller MJ, and Jin YS. Precision fermentation for improving the quality, flavor, safety, and sustainability of foods. Curr Opin Biotechnol (2024) 86:103084. doi: 10.1016/j.copbio.2024.103084
60. Rangel AET, Ramírez JMG, and Barrios AFG. From industrial by-products to value-added compounds: the design of efficient microbial cell factories by coupling systems metabolic engineering and bioprocesses. Biofuels Bioprod Bioref (2020) 14:1228–38. doi: 10.1002/bbb.2127
61. Ahmad MI, Farooq S, Alhamoud Y, Li C, and Zhang H. Soy leghemoglobin: a review of its structure, production, safety aspects, and food applications. Trends Food Sci Technol (2023) 141:1–12. doi: 10.1016/j.tifs.2023.104199
62. Akbari M, Razavi SH, and Kieliszek M. Recent advances in microbial transglutaminase biosynthesis and its application in the food industry. Trends Food Sci Technol (2021) 110:458–69. doi: 10.1016/j.tifs.2021.02.036
63. Govorushko S. Global status of insects as food and feed source: a review. Trends Food Sci Technol (2019) 91:436–45. doi: 10.1016/j.tifs.2019.07.032
64. Liceaga AM, Aguilar-Toalá JE, Vallejo-Cordoba B, González-Córdova AF, and Hernández-Mendoza A. Insects as an alternative protein source. Annu Rev Food Sci Technol (2022) 13:19–34. doi: 10.1146/annurev-food-052720-112443
65. Food and Agriculture Organization of the United Nations. Looking at edible insects from a food safety perspective. Challenges and opportunities for the sector. Rome: FAO (2021). doi: 10.4060/cb4094en
66. Orkusz A. Edible insects versus meat-nutritional comparison: knowledge of their composition is the key to good health. Nutrients (2021) 13(4):1207. doi: 10.3390/nu13041207
67. Letcher SM, Rubio NR, Ashizawa RN, Saad MK, Rittenberg ML, McCreary A, et al. In vitro insect fat cultivation for cellular agriculture applications. ACS Biomater Sci Eng (2022) 8(9):3785–96. doi: 10.1021/acsbiomaterials.2c00093
68. La Barbera F, Verneau F, Amato M, and Grunert K. Understanding Westerners’ disgust for the eating of insects: the role of food neophobia and implicit associations. Food Qual Prefer (2018) 64:120–5. doi: 10.1016/j.foodqual.2017.10.002
69. Caparros Megido RC, Gierts C, Blecker C, Brostaux Y, Haubruge É, Alabi T, et al. Consumer acceptance of insect-based alternative meat products in Western countries. Food Qual Prefer (2016) 52:237–43. doi: 10.1016/j.foodqual.2016.05.004
70. Kornher L, Schellhorn M, and Vetter S. Disgusting or innovative-consumer willingness to pay for insect based burger patties in Germany. Sustainability (2019) 11(7):1878. doi: 10.3390/su11071878
71. World Population Review. Veganism by country [online] (2023). Available at: https://worldpopulationreview.com/country-rankings/vegetarianism-by-country
72. McClements DJ, Weiss J, Kinchla AJ, Nolden AA, and Grossmann L. Methods for testing the quality attributes of plant-based foods: meat- and processed-meat analogs. Foods (2021) 10(2):260. doi: 10.3390/foods10020260
73. Schreuders FKG, Schlangen M, Kyriakopoulou K, Boom RM, and van der Goot AJ. Texture methods for evaluating meat and meat analogue structures: a review. Food Control (2021) 127:108103. doi: 10.1016/j.foodcont.2021.108103
74. Fiorentini M, Kinchla AJ, and Nolden AA. Role of sensory evaluation in consumer acceptance of plant-based meat analogs and meat extenders: a scoping review. Foods (2020) 9(9):1334. doi: 10.3390/foods9091334
75. Ilić J, Djekic I, Tomasevic I, Oosterlinck F, and Van Den Berg MA. Materials properties, oral processing, and sensory analysis of eating meat and meat analogs. Annu Rev Food Sci Technol (2022) 13:193–215. doi: 10.1146/annurev-food-090821-032332
76. Fraeye I, Kratka M, Vandenburgh H, and Thorrez L. Sensorial and nutritional aspects of cultured meat in comparison to traditional meat: much to be inferred. Front Nutr (2020) 7:35. doi: 10.3389/fnut.2020.00035
77. NECTAR. Taste of the industry 2024: a sensory analysis of plant-based meats food systems innovations. NECTAR (2024). Available at: https://www.nectar.org/sensory-research/2024-taste-of-the-industry.
78. Craig WJ, Mangels AR, Fresán U, Marsh K, Miles FL, Saunders AV, et al. The safe and effective use of plant-based diets with guidelines for health professionals. Nutrients (2021) 13(11):4144. doi: 10.3390/nu13114144
79. Pellinen T, Päivärinta E, Isotalo J, Lehtovirta M, Itkonen ST, Korkalo L, et al. Replacing dietary animal-source proteins with plant-source proteins changes dietary intake and status of vitamins and minerals in healthy adults: a 12-week randomized controlled trial. Eur J Nutr (2022) 61(3):1391–404. doi: 10.1007/s00394-021-02729-3
80. Bohrer BM. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci Hum Wellness (2019) 8(4):320–9. doi: 10.1016/j.fshw.2019.11.006
81. Alcorta A, Porta A, Tárrega A, Alvarez MD, and Vaquero MP. Foods for plant-based diets: challenges and innovations. Foods (2021) 10(2):293. doi: 10.3390/foods10020293
82. Boukid F. Plant-based meat analogues: from niche to mainstream. Eur Food Res Technol (2021) 247(2):297–308. doi: 10.1007/s00217-020-03630-9
83. Pugh JE, Cai MZ, Altieri N, and Frost G. A comparison of the effects of resistant starch types on glycemic response in individuals with type 2 diabetes or prediabetes: a systematic review and meta-analysis. Front Nutr (2023) 10:1118229. doi: 10.3389/fnut.2023.1118229
84. Milana M, Van Asselt ED, and van der Fels-Klerx HJ. The chemical and microbiological safety of emerging alternative protein sources and derived analogues: a review. Compr Rev Food Sci Food Saf (2024) 23(4):e13377. doi: 10.1111/1541-4337.13377
85. Wang YQ, Tuccillo F, Lampi AM, Knaapila A, Pulkkinen M, Kariluoto S, et al. Flavor challenges in extruded plant-based meat alternatives: a review. Compr Rev Food Sci Food Saf (2022) 21(3):2898–929. doi: 10.1111/1541-4337.12964
86. Leonard W, Zhang PZ, Ying DY, and Fang ZX. Surmounting the off-flavor challenge in plant-based foods. Crit Rev Food Sci Nutr (2023) 63(30):10585–606. doi: 10.1080/10408398.2022.2078275
87. Tazeddinova D, Rahman MR, Hamdan SB, Matin MM, Bin Bakri MK, and Rahman MM. Plant based polyphenol associations with protein: a prospective review. BioResources (2022) 17(4):7110–34. doi: 10.15376/biores.17.4.Tazeddinova2
88. Saffarionpour S. Off-flavors in pulses and grain legumes and processing approaches for controlling flavor-plant protein interaction: application prospects in plant-based alternative foods. Food Bioproc Technol (2024) 17(5):1141–82. doi: 10.1007/s11947-023-03148-4
89. Mittermeier-Kleßinger VK, Hofmann T, and Dawid C. Mitigating off-flavors of plant-based proteins. J Agric Food Chem (2021) 69(32):9202–7. doi: 10.1021/acs.jafc.1c03398
90. Cheseto X, Baleba SBS, Tanga CM, Kelemu S, and Torto B. Chemistry and sensory characterization of a bakery product prepared with oils from African edible insects. Foods (2020) 9(6):800. doi: 10.3390/foods9060800
91. Tzompa-Sosa DA, Dewettinck K, Gellynck X, and Schouteten JJ. Replacing vegetable oil by insect oil in food products: effect of deodorization on the sensory evaluation. Food Res Int (2021) 141:110140. doi: 10.1016/j.foodres.2021.110140
92. Huseynli L, Parviainen T, Kyllönen T, Aisala H, and Vene K. Exploring the protein content and odor-active compounds of black soldier fly larvae for future food applications. Future Foods (2023) 7:100224. doi: 10.1016/j.fufo.2023.100224
93. Sipponen MH, Mäkinen OE, Rommi K, Heiniö RL, Holopainen-Mantila U, Hokkanen S, et al. Biochemical and sensory characteristics of the cricket and mealworm fractions from supercritical carbon dioxide extraction and air classification. Eur Food Res Technol (2018) 244(1):19–29. doi: 10.1007/s00217-017-2931-1
94. Kim J, Lee HE, Kim Y, Yang J, Lee S-J, and Jung YH. Development of a post-processing method to reduce the unique off-flavor of Allomyrina dichotoma: yeast fermentation. LWT Food Sci Technol (2021) 150:111940. doi: 10.1016/j.lwt.2021.111940
95. Lee HE, Kim J, Kim Y, Bang WY, Yang J, Lee SJ, et al. Identification and improvement of volatile profiles of Allomyrina dichotoma larvae by fermentation with lactic acid bacteria. Food Biosci (2021) 43:101257. doi: 10.1016/j.fbio.2021.101257
96. Kong X, Li Y, and Liu X. A review of thermosensitive antinutritional factors in plant-based foods. J Food Biochem (2022) 46(9):e14199. doi: 10.1111/jfbc.14199
97. Sakandar HA, Chen YF, Peng CT, Chen X, Imran M, and Zhang HP. Impact of fermentation on antinutritional factors and protein degradation of legume seeds: a review. Food Rev Int (2022) 39(3):1227–49. doi: 10.1080/87559129.2021.1931300
98. Samtiya M, Aluko RE, and Dhewa T. Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod Process Nutr (2020) 2(1):6. doi: 10.1186/s43014-020-0020-5
99. Rousseau S, Kyomugasho C, Celus M, Hendrickx MEG, and Grauwet T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit Rev Food Sci Nutr (2020) 60(5):826–43. doi: 10.1080/10408398.2018.1552243
100. McClements DJ. Food hydrocolloids: application as functional ingredients to control lipid digestion and bioavailability. Food Hydrocoll (2021) 111:106404. doi: 10.1016/j.foodhyd.2020.106404
101. Müller M, Canfora EE, and Blaak EE. Gastrointestinal transit time, glucose homeostasis and metabolic health: modulation by dietary fibers. Nutrients (2018) 10(3):275. doi: 10.3390/nu10030275
102. Capuano E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit Rev Food Sci Nutr (2017) 57(16):3543–64. doi: 10.1080/10408398.2016.1180501
103. Baye K, Guyot JP, and Mouquet-Rivier C. The unresolved role of dietary fibers on mineral absorption. Crit Rev Food Sci Nutr (2017) 57(5):949–57. doi: 10.1080/10408398.2014.953030
104. Agrizzi Verediano T, Agarwal N, Juste Contin Gomes M, Martino HSD, and Tako E. Effects of dietary fiber on intestinal iron absorption, and physiological status: a systematic review of in vivo and clinical studies. Crit Rev Food Sci Nutr (2023) 63(27):9017–32. doi: 10.1080/10408398.2022.2060933
105. Adams S, Sello CT, Qin G-X, Che D, and Han R. Does dietary fiber affect the levels of nutritional components after feed formulation? Fibers (2018) 6(2):1–15. doi: 10.3390/fib6020029
106. Kamiloglu S, Tomas M, Ozdal T, and Capanoglu E. Effect of food matrix on the content and bioavailability of flavonoids. Trends Food Sci Technol (2021) 117:15–33. doi: 10.1016/j.tifs.2020.10.030
107. Priyadarshani AMB. A review on factors influencing bioaccessibility and bioefficacy of carotenoids. Crit Rev Food Sci Nutr (2017) 57(8):1710–7. doi: 10.1080/10408398.2015.1023431
108. Brodkorb A, Egger L, Alminger M, Alvito P, Assunção R, Ballance S, et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc (2019) 14(4):991–1014. doi: 10.1038/s41596-018-0119-1
109. Dupont D, Alric M, Blanquet-Diot S, Bornhorst G, Cueva C, Deglaire A, et al. Can dynamic in vitro digestion systems mimic the physiological reality? Crit Rev Food Sci Nutr (2019) 59(10):1546–62. doi: 10.1080/10408398.2017.1421900
110. Sensoy I. A review on the food digestion in the digestive tract and the used in vitro models. Curr Res Food Sci (2021) 4:308–19. doi: 10.1016/j.crfs.2021.04.004
111. Chen Y, Rudolph SE, Longo BN, Pace F, Roh TT, Condruti R, et al. Bioengineered 3D tissue model of intestine epithelium with oxygen gradients to sustain human gut microbiome. Adv Healthc Mater (2022) 11(16):2200447. doi: 10.1002/adhm.202200447
112. Grossmann L and Mcclements DJ. Current insights into protein solubility: a review of its importance for alternative proteins. Food Hydrocoll (2023) 137:108416. doi: 10.1016/j.foodhyd.2022.108416
113. Dickinson E. Emulsion gels: the structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll (2012) 28(1):224–41. doi: 10.1016/j.foodhyd.2011.12.017
114. McClements DJ. Biopolymer composite hydrogels: sustainable functional materials with a diverse range of applications. Adv Colloid Interface Sci (2024) 14:103278. doi: 10.1016/j.cis.2024.103278
115. Zeeb B. Interaction between components of plant-based biopolymer systems. Curr Opin Colloid Interface Sci (2021) 56:101524. doi: 10.1016/j.cocis.2021.101524
116. Zhang JC, Chen QL, Kaplan DL, and Wang Q. High-moisture extruded protein fiber formation toward plant-based meat substitutes applications: science, technology, and prospect. Trends Food Sci Technol (2022) 128:202–16. doi: 10.1016/j.tifs.2022.08.008
117. Hu X, Ju Q, Koo CKW, and McClements DJ. Influence of complex coacervation on the structure and texture of plant-based protein-polysaccharide composites. Food Hydrocoll (2024) 147:109333. doi: 10.1016/j.foodhyd.2023.109333
118. Ryu J, Xiang XK, Hu XY, Rosenfeld SE, Qin DK, Zhou HL, et al. Assembly of plant-based meat analogs using soft matter physics: a coacervation-shearing-gelation approach. Food Hydrocoll (2023) 142:108817. doi: 10.1016/j.foodhyd.2023.108817
119. Cao LQ, Lu W, Mata A, Nishinari K, and Fang YP. Egg-box model-based gelation of alginate and pectin: a review. Carbohydr Polym (2020) 242:116389. doi: 10.1016/j.carbpol.2020.116389
120. Morris ER, Rees DA, Robinson G, and Young GA. Competitive inhibition of interchain interactions in polysaccharide systems. J Mol Biol (1980) 138(2):363–74. doi: 10.1016/0022-2836(80)90292-2
121. Li RY, Dai TT, Zhou W, Fu GM, Wan Y, McClements DJ, et al. Impact of pH, ferrous ions, and tannic acid on lipid oxidation in plant-based emulsions containing saponin-coated flaxseed oil droplets. Food Res Int (2020) 136:109618. doi: 10.1016/j.foodres.2020.109618
122. Lorrain B, Dangles O, Loonis M, Armand M, and Dufour C. Dietary iron-initiated lipid oxidation and its inhibition by polyphenols in gastric conditions. J Agric Food Chem (2012) 60(36):9074–81. doi: 10.1021/jf302348s
123. Aguiar Campolina G, das Graças Cardoso M, Rodrigues-Silva-Caetano A, Lee Nelson D, and Mendes Ramos E. Essential oil and plant extracts as preservatives and natural antioxidants applied to meat and meat products: a review. Food Technol Biotechnol (2023) 61(2):212–25. doi: 10.17113/ftb.61.02.23.7883
124. Devaere J, De Winne A, Dewulf L, Fraeye I, Šoljić I, Lauwers E, et al. Improving the aromatic profile of plant-based meat alternatives: effect of myoglobin addition on volatiles. Foods (2022) 11(13):1985. doi: 10.3390/foods11131985
125. Chin SW, Baier SK, Stokes JR, and Smyth HE. Evaluating the sensory properties of hybrid (meat and plant-based) burger patties. J Texture Stud (2024) 55(1):e12819. doi: 10.1111/jtxs.12819
126. Petrat-Melin B and Dam S. Textural and consumer-aided characterisation and acceptability of a hybrid meat and plant-based burger patty. Foods (2023) 12(11):2246. doi: 10.3390/foods12112246
127. Sogari G, Grasso S, Caputo V, Gómez MI, Mora C, and Schouteten JJ. Sensory, emotional, and appropriateness of plant- and meat-based burgers. J Food Sci (2024) 89(5):2974–90. doi: 10.1111/1750-3841.17033
128. Ebert S, Jungblut F, Herrmann K, Maier B, Terjung N, Gibis M, et al. Influence of wet extrudates from pumpkin seed proteins on drying, texture, and appearance of dry-cured hybrid sausages. Eur Food Res Technol (2022) 248(6):1469–84. doi: 10.1007/s00217-022-03974-4
129. Ebert S, Michel W, Gotzmann L, Baune MC, Terjung N, Gibis M, et al. Acidification behavior of mixtures of pork meat and wet texturized plant proteins in a minced model system. J Food Sci (2022) 87(4):1731–41. doi: 10.1111/1750-3841.16080
130. Lin WL and Barbut S. Hybrid meat batter system: effects of plant proteins (pea, brown rice, faba bean) and concentrations (3–12%) on texture, microstructure, rheology, water binding, and color. Poult Sci (2024) 103(7):103822. doi: 10.1016/j.psj.2024.103822
131. Broucke K, Van Poucke C, Duquenne B, De Witte B, Baune MC, Lammers V, et al. Ability of (extruded) pea protein products to partially replace pork meat in emulsified cooked sausages. Innov Food Sci Emerg Technol (2022) 78:102992. doi: 10.1016/j.ifset.2022.102992
132. Grasso S, Smith G, Bowers S, Ajayi OM, and Swainson M. Effect of texturised soy protein and yeast on the instrumental and sensory quality of hybrid beef meatballs. J Food Sci Technol (2019) 56(6):3126–35. doi: 10.1007/s13197-018-3552-9
133. Ogilvie OJ, Bennie RZ, Trlin HJF, Loo LSW, Zhou HZ, Jin A, et al. Interdisciplinary methods for analysing food matrix structures of hybrid cell-based meats: a review. Food Struct (2024) 39:100361. doi: 10.1016/j.foostr.2023.100361
134. Lee M, Park S, Choi B, Kim J, Choi W, Jeong I, et al. Tailoring a gelatin/agar matrix for the synergistic effect with cells to produce high-quality cultured meat. ACS Appl Mater Interf (2022) 14(33):38235–45. doi: 10.1021/acsami.2c10988
135. Gu X, Hua SY, Huang YQ, Liu SQ, Wang YZ, Zhou M, et al. κ-carrageenan/konjac glucomannan composite hydrogel-based 3D porcine cultured meat production. Food Hydrocoll (2024) 151:109765. doi: 10.1016/j.foodhyd.2024.109765
136. Tang X, Deng GL, Yang L, Wang XH, Xiang W, Zou Y, et al. Konjac glucomannan-fibrin composite hydrogel as a model for ideal scaffolds for cell-culture meat. Food Res Int (2024) 187:114425. doi: 10.1016/j.foodres.2024.114425
137. Stephan A, Ahlborn J, Zajul M, and Zorn H. Edible mushroom mycelia of Pleurotus sapidus as novel protein sources in a vegan boiled sausage analog system: functionality and sensory tests in comparison to commercial proteins and meat sausages. Eur Food Res Technol (2018) 244(5):913–24. doi: 10.1007/s00217-017-3012-1
138. Zhang ZQ, Zang MW, Chen J, Zhang KH, Wang SW, Li D, et al. Effect of the mycelium of oyster mushrooms on the physical and flavor properties of a plant-based beef analogue. LWT Food Sci Technol (2024) 198:116029. doi: 10.1016/j.lwt.2024.116029
139. Ardila P, Honrado A, Marquina P, Beltrán JA, and Calanche JB. Innovative plant-based burger enriched with Tenebrio molitor meal: characterization and shelf-life. Foods (2023) 12(18):3460. doi: 10.3390/foods12183460
140. Smetana S, Ashtari Larki NA, Pernutz C, Franke K, Bindrich U, Toepfl S, et al. Structure design of insect-based meat analogs with high-moisture extrusion. J Food Eng (2018) 229:83–5. doi: 10.1016/j.jfoodeng.2017.06.035
141. Priyatnasari NS, Palupi E, Kamila F, Ardhiani KR, Khalisah P, Prilyadi GT, et al. Meat-analog made from Javanese Grasshopper, kidney beans, and elephant foot yam as a high-protein and low-cholesterol product. J Agric Food Res (2024) 16:101071. doi: 10.1016/j.jafr.2024.101071
142. Cho SY and Ryu GH. Effects of mealworm larva composition and selected process parameters on the physicochemical properties of extruded meat analog. Food Sci Nutr (2021) 9(8):4408–19. doi: 10.1002/fsn3.2414
143. Rocchetti G, Zengin G, Giuberti G, Cervini M, and Lucini L. Impact of in vitro gastrointestinal digestion on the phenolic bioaccessibility and bioactive properties of insect-containing beef burgers. Antioxid (Basel) (2024) 13(3):365. doi: 10.3390/antiox13030365
144. Bessa LW, Pieterse E, Marais J, and Hoffman LC. Black soldier fly larvae (Hermetia illucens) as a meat replacer in a burger patty. J Insects Food Feed (2023) 9(9):1211–22. doi: 10.3920/JIFF2021.0208
145. Scholliers J, Steen L, and Fraeye I. Structure and physical stability of hybrid model systems containing pork meat and superworm (Zophobas morio larvae). Innov Food Sci Emerg Technol (2020) 65:102452. doi: 10.1016/j.ifset.2020.102452
146. Scholliers J, Steen L, and Fraeye I. Partial replacement of meat by superworm (Zophobas morio larvae) in cooked sausages: effect of heating temperature and insect:meat ratio on structure and physical stability. Innov Food Sci Emerg Technol (2020) 66:102535. doi: 10.1016/j.ifset.2020.102535
147. Cruz-López SO, Escalona-Buendía HB, Román-Guerrero A, Domínguez-Soberanes J, and Alvarez-Cisneros YM. Characterization of cooked meat models using grasshopper (Sphenarium purpurascens) soluble protein extracted by alkalisation and ultrasound as meat-extender. Food Sci Anim Resour (2022) 42(3):536–55. doi: 10.5851/kosfa.2022.e22
148. Vlahova-Vangelova D, Balev D, and Kolev N. Cricket powder (Acheta domesticus) as a lean pork meat replacer in cooked sausages. Future Food J Food Agric Soc (2023) 11:1–12. doi: 10.17170/kobra-202210056951
149. Miron L, Montevecchi G, Macavei LI, Maistrello L, Antonelli A, and Thomas M. Effect of black soldier fly larvae protein on the texture of meat analogues. LWT Food Sci Technol (2023) 181:114745. doi: 10.1016/j.lwt.2023.114745
150. Park YS, Choi YS, Hwang KE, Kim TK, Lee CW, Shin DM, et al. Physicochemical properties of meat batter added with edible silkworm pupae (Bombyx mori) and transglutaminase. Korean J Food Sci Anim Resour (2017) 37(3):351–9. doi: 10.5851/kosfa.2017.37.3.351
151. Stout AJ, Mirliani AB, Rittenberg ML, Shub M, White EC, Yuen JSK, et al. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun Biol (2022) 5(1):466. doi: 10.1038/s42003-022-03423-8
152. Sugii S, Wong CYQ, Lwin AKO, and Chew LJM. Reassessment of adipocyte technology for cellular agriculture of alternative fat. Compr Rev Food Sci Food Saf (2022) 21(5):4146–63. doi: 10.1111/1541-4337.13021
153. Ben-Arye T, Shandalov Y, Ben-Shaul S, Landau S, Zagury Y, Ianovici I, et al. Textured soy protein scaffolds enable the generation of three-dimensional bovine skeletal muscle tissue for cell-based meat. Nat Food (2020) 1(4):210–20. doi: 10.1038/s43016-020-0046-5
154. Xiang N, Yuen JSK, Stout AJ, Rubio NR, Chen Y, and Kaplan DL. 3D porous scaffolds from wheat glutenin for cultured meat applications. Biomaterials (2022) 285:121543. doi: 10.1016/j.biomaterials.2022.121543
155. Yao Y, Yuen JS Jr., Sylvia R, Fennelly C, Cera L, Zhang KL, et al. Cultivated meat from aligned muscle layers and adipose layers formed from glutenin films. ACS Biomater Sci Eng (2024) 10(2):814–24. doi: 10.1021/acsbiomaterials.3c01500
156. Yen F-C, Glusac J, Levi S, Zernov A, Baruch L, Davidovich-Pinhas M, et al. Cultured meat platform developed through the structuring of edible microcarrier-derived microtissues with oleogel-based fat substitute. Nat Commun (2023) 14(1):2942. doi: 10.1038/s41467-023-38593-4
Keywords: plant-based foods, cultivated meat, precision fermentation, hybrid food products, insect foods
Citation: Kaplan DL and McClements DJ. Hybrid alternative protein-based foods: designing a healthier and more sustainable food supply. Front Sci (2025) 3:1599300. doi: 10.3389/fsci.2025.1599300
Received: 24 March 2025; Accepted: 10 July 2025;
Published: 30 September 2025.
Edited by:
Lovedeep Kaur, Massey University, New ZealandReviewed by:
Mike Gidley, The University of Queensland, AustraliaMark J. Post, Maastricht University, Netherlands
Copyright © 2025 Kaplan and McClements. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Julian McClements, bWNjbGVtZW5AdW1hc3MuZWR1
 David L. Kaplan
David L. Kaplan David Julian McClements
David Julian McClements