Explore article hub
A Viewpoint on the Frontiers in Science Lead Article
Precision cardiovascular medicine: shifting the innovation paradigm
Key points
- Precision cardiovascular medicine requires new experimental models that better mimic human diseases.
- Target definition must be adequately cell type-specific, as proteins can exert different functions in different cells.
- New treatments also need cell type-specific delivery approaches and methods to better understand and predict off-target effects.
In their excellent lead article “Precision cardiovascular medicine: shifting the innovation paradigm,” Aikawa et al. (1) highlight that, in addition to cardiovascular risk management, new and more effective therapies are needed to treat complex and heterogenous cardiovascular diseases (CVDs) to reduce overall morbidity and mortality among patients. There is no doubt that “precision medicine” has the potential to achieve such a goal in the near future. However, this will require progress in several aspects of basic and translational science (Figure 1), i.e., the creation of adequate animal models mimicking the complexity of human diseases, better definition of the time course and specific cells involved in disease development or progression, and improvement of cell type-specific targeting of therapeutic approaches and understanding of potential off-target effects.
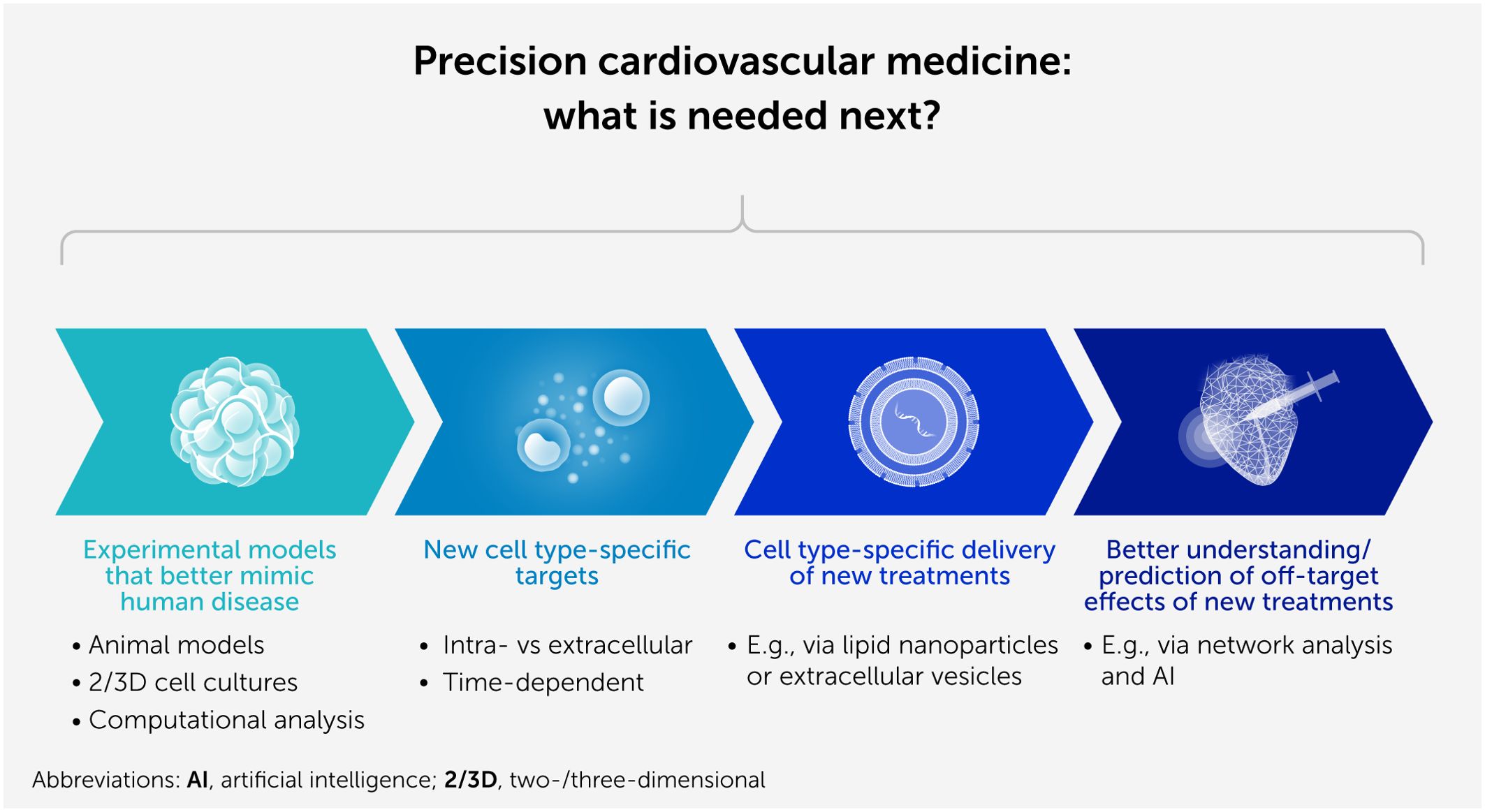
Figure 1. Progress in several aspects of basic and translational science is necessary to enable precision cardiovascular medicine.
Adequate experimental models
While “big” clinical data are required to discover and test novel preventive and curative therapies for CVDs, new experimental models that better recapitulate human diseases are just as important. Translating basic science into clinical practice is challenging, especially for complex conditions such as CVDs, which often result from multiple risk factors and comorbidities. In the coming years, animal models need to be further refined and made more “human-like” using big datasets from human studies (2, 3). As well as predicting efficacy, it is also important to identify uncommon or “hidden” cardiotoxic effects of treatment modalities, which currently are often detected only after market launch when large numbers of patients are exposed to treatment (4). As CVD mechanisms and treatment responses potentially differ between males and females, the effect of the patient’s sex should also be considered in the design of experimental studies (5). Novel in vitro technologies, such as inducible pluripotent stem cells in two- or three-dimensional (so-called organoid) cultures or human slices and advanced computational analyses, will improve our experimental designs and better capture human pathophysiology—thereby reducing the number of laboratory animals required.
Adequate target definition
Different cell types might behave differently during disease development. This can be because a certain protein might have different functions:
(i) Within or outside the cell. For example, intracellular deletion of proprotein convertase subtilisin kexin type 9 (PCSK9) using genetic approaches reduced infarct sizes ex vivo, but inhibition of extracellular PCSK9 using antibodies did not (6). Thus, exact understanding of the intra- and extracellular function of a protein is required before target definition.
(ii) In different cell types. For example, uncoupling protein 2 contributes to the formation of reactive oxygen species (ROS) in many cell types, potentially causing harm under pathophysiological conditions. However, this is not the case in cardiomyocytes (7), where uncoupling protein 2 most likely functions by modifying substrate transport and usage and is potentially important for maintaining cardiomyocyte function. Therefore, targeting all cell types at once does not necessarily improve disease development and/or progression. This is seen on a larger scale when comparing the two sides of the heart during disease development: recent studies suggest that regulation of ROS formation, which is involved in many cardiovascular pathologies, differs in the left and right ventricle (8). Thus, any modification of the function of a target protein has to be cell-type-specific.
(iii) At different time points during disease development and/or progression. Time-dependent effects, either beneficial or detrimental, have been described for fibrosis development following myocardial infarction. Early interference with fibrosis development will cause harm due to the potential of increased ventricular rupture, while later inhibition will potentially attenuate heart failure development. Thus, the time course, rather than a snapshot in time, is required to understand protein function in disease development and progression.
(iv) Within subpopulations of the same cell type. Single-cell analyses of genetic, epigenetic, or proteome changes indicate that there are subpopulations within a given cell type (for example, cardiomyocytes or β cells) that behave differently during disease development. Here, a better understanding of the contribution of these different subpopulations of cells is required for adequate therapeutic target prediction.
Cell type-specific delivery of new treatments
Lipid nanoparticles (LNP) have been synthesized to protect messenger RNA (mRNA) therapeutic agents from degradation and, with adequate composition of the bilayer membrane, facilitate uptake into specific cellular compartments. However, even though there might be a certain cell-type specificity, LNPs are taken up to a small extent by almost every cell, raising the potential for adverse effects of the mRNA-encoded protein and/or protein inhibition (9). Moreover, LNPs might induce immune responses and local inflammation due to their synthetic components. Extracellular vesicles, which play an essential role in intercellular communication by facilitating the transport of bioactive molecules, may offer advantages over LNPs as delivery vehicles, being naturally derived and thus less likely to elicit immune responses. However, far more research is needed to develop standardized techniques for their isolation and purification, and to explore their therapeutic use (10).
Safety of new therapeutic approaches
Small interfering RNAs (siRNAs), microRNAs (miRNAs), and the RNA subtype of antisense oligonucleotides offer advantages over small-molecule drugs. These small RNAs can target any gene product, offering new, effective, and safe therapeutic approaches for a wide range of diseases. However, the use of these agents faces important challenges. Hybridization-dependent off-target effects remain a major hurdle, for example, as no established, standard methodology exists to minimize these, and the various proposed methods have limitations. Here, the inclusion of new techniques (including network theoretical algorithms and artificial intelligence) in the development process would enable examination of the entire, complex regulatory network—allowing more accurate prediction of potential off-target effects and more precise sequence design (11).
Conclusion
Taken together, precision medicine has enormous potential to improve therapy for CVDs and reduce patients’ morbidity and mortality. However, further research using a new generation of adequate experimental models is needed if we are to develop and deliver new, effective, and safe cell type-specific therapies.
Statements
Author contributions
RS: Conceptualization, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author declared that no financial support was received for this work and/or its publication.
Conflict of interest
The author declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author declared that they were an editorial board member of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author declared that no generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Aikawa M, Sonawane AR, Chelvanambi S, Asano T, Halu A, Matamalas JT, et al. Precision cardiovascular medicine: shifting the innovation paradigm. Front Sci (2025) 3:1474469. doi: 10.3389/fsci.2025.1474469
2. van der Velden J, Asselbergs FW, Bakkers J, Batkai S, Bertrand L, Bezzina CR, et al. Animal models and animal-free innovations for cardiovascular research: current status and routes to be explored. Consensus document of the ESC Working Group on Myocardial Function and the ESC Working Group on Cellular Biology of the Heart. Cardiovasc Res (2022) 118(15):3016–51. doi: 10.1093/cvr/cvab370
3. Heusch G, Bøtker HE, Ferdinandy P, and Schulz R. Primordial non-responsiveness: a neglected obstacle to cardioprotection. Eur Heart J (2023) 44(19):1687–9. doi: 10.1093/eurheartj/ehad160
4. Ferdinandy P, Baczkó I, Bencsik P, Giricz Z, Görbe A, Pacher P, et al. Definition of hidden drug cardiotoxicity: paradigm change in cardiac safety testing and its clinical implications. Eur Heart J (2019) 40(22):1771–7. doi: 10.1093/eurheartj/ehy365
5. Perrino C, Ferdinandy P, Bøtker HE, Brundel BJ, Collins P, Davidson SM, et al. Improving translational research in sex-specific effects of comorbidities and risk factors in ischaemic heart disease and cardioprotection: position paper and recommendations of the ESC Working Group on Cellular Biology of the Heart. Cardiovasc Res (2021) 117(2):367–85. doi: 10.1093/cvr/cvaa155
6. Schreckenberg R, Wolf A, Szabados T, Gömöri K, Szabó IA, Ágoston G, et al. Proprotein convertase subtilisin kexin type 9 (PCSK9) deletion but not inhibition of extracellular PCSK9 reduces infarct sizes ex vivo but not in vivo. Int J Mol Sci (2022) 23(12):6512. doi: 10.3390/ijms23126512
7. Schulz R and Schlüter KD. Importance of mitochondria in cardiac pathologies: focus on uncoupling proteins and monoamine oxidases. Int J Mol Sci (2023) 24(7):6459. doi: 10.3390/ijms24076459
8. Schlüter KD, Kutsche HS, Hirschhäuser C, Schreckenberg R, and Schulz R. Review on chamber-specific differences in right and left heart reactive oxygen species handling. Front Physiol (2018) 9:1799. doi: 10.3389/fphys.2018.01799
9. Schreckenberg R, Woitasky N, Itani N, Czech L, Ferdinandy P, and Schulz R. Cardiac side effects of RNA-based SARS-CoV-2 vaccines: hidden cardiotoxic effects of mRNA-1273 and BNT162b2 on ventricular myocyte function and structure. Br J Pharmacol (2024) 181(3):345–61. doi: 10.1111/bph.16262
10. Wang M, Chen Y, Xu B, Zhu X, Mou J, Xie J, et al. Recent advances in the roles of extracellular vesicles in cardiovascular diseases: pathophysiological mechanisms, biomarkers, and cell-free therapeutic strategy. Mol Med (2025) 31(1):169. doi: 10.1186/s10020-025-01200-x
Keywords: precision medicine, animal models, drug toxicity, microRNA, lipid nanoparticles, cardiovascular disease, cell type-specific therapy, RNA therapeutics
Citation: Schulz R. Toward precision cardiovascular medicine: progressing basic and translational science. Front Sci (2025) 3:1659045. doi: 10.3389/fsci.2025.1659045
Received: 03 July 2025; Accepted: 28 July 2025;
Published: 07 October 2025.
Edited and reviewed by:
Ichiro Manabe, Chiba University, JapanCopyright © 2025 Schulz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rainer Schulz, cmFpbmVyLnNjaHVsekBwaHlzaW9sb2dpZS5tZWQudW5pLWdpZXNzZW4uZGU=
 Rainer Schulz
Rainer Schulz