Explore article hub
- Stanford Cardiovascular Institute, Stanford University School of Medicine, Stanford, CA, United States
An Editorial on the Frontiers in Science Lead Article
Precision cardiovascular medicine: shifting the innovation paradigm
Key points
- Precision cardiovascular medicine reframes disease heterogeneity from an obstacle to a leveraged signal, shifting from treatments that work moderately well for most patients to those that work exceptionally well for molecularly defined subgroups.
- The convergence of systems biology, artificial intelligence, and emerging therapeutic modalities—including RNA medicines, gene editing, and biologics—now enables targeting of mechanisms once considered “undruggable” through upstream molecular intervention rather than only downstream protein inhibition.
- Realizing the potential of precision medicine demands interdisciplinary collaboration and proactive equity approaches, as the use of sophisticated diagnostics risks exacerbating disparities unless diverse populations are included—from drug discovery onward.
Cardiovascular diseases (CVDs) remain the leading global cause of mortality despite decades of progress (1). In their lead article, Aikawa et al. (2) outline a roadmap for precision cardiovascular medicine that recognizes and leverages fundamental heterogeneity in disease mechanisms and patient responses across molecular, cellular, and clinical scales.
Reframing heterogeneity: from obstacle to opportunity
Cardiovascular medicine has long struggled with the “fallacy of averages,” which is the assumption that treatments optimized for population averages will work uniformly across individuals (3). This one-size-fits-all paradigm has yielded important successes, from statins to proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, yet leaves substantial residual risk for many patients. The central insight in the lead article by Aikawa et al. is to reframe heterogeneity from challenges to possibilities, looking at the patient-to-patient variability as signals to be dissected rather than noise to be averaged.
This paradigm shift may represent one of the most important conceptual advances in cardiovascular therapeutics since the recognition of cholesterol as a modifiable risk factor. The authors document how heterogeneity manifests across multiple scales (Figure 1A): genomic variations and exposomic differences at the molecular level; diverse cellular responses revealed by state-of-the-art single-cell technologies; and variation in clinical presentations and therapeutic responses at the patient level. Rather than viewing this complexity as an impediment to therapeutic development, precision medicine treats it as valuable insight into why current strategies work for some patients but not others.
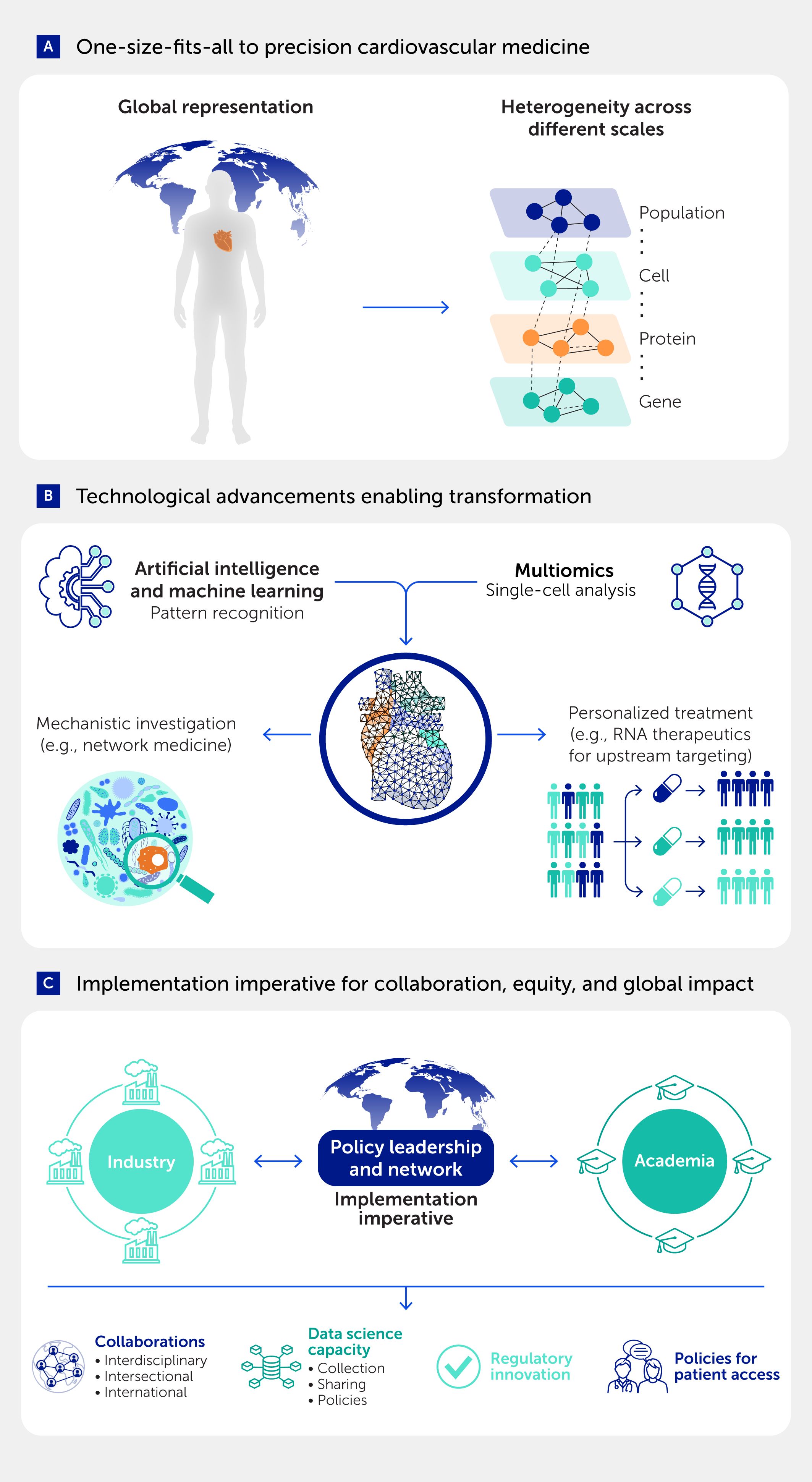
Figure 1. The transformation from one-size-fits-all to precision cardiovascular medicine requires technological convergence and implementation imperatives. (A) Traditional cardiovascular medicine assumes global population homogeneity, whereas precision medicine leverages heterogeneity across multiple biological scales—from genomic and protein variation to cellular diversity and population-level differences—as informative signals for patient stratification and targeted therapeutics. (B) Technological advances, including artificial intelligence and machine learning for pattern recognition, multiomics platforms, and single-cell analysis technologies, enable mechanistic investigation through approaches such as network medicine and personalized treatment strategies, including RNA therapeutics for upstream molecular targeting. (C) Successful implementation requires collaboration between industry, academia, and policy leadership networks, supported by the following: interdisciplinary/intersectional/international collaboration; robust data science infrastructure with appropriate collection and sharing policies; regulatory innovation to accommodate precision medicine paradigms; and equitable policies ensuring patient access to precision therapeutics across diverse populations and healthcare systems. Adapted in part from Aikawa et al. (2).
The implications of this reframing extend beyond therapeutic development to fundamental questions about clinical trial design, regulatory approval, and clinical practice. Instead of looking for treatments that are just “effective enough” for most patients, precision approaches aim to identify treatments that work exceptionally well for specific patient subgroups, reaching patients who do not respond well to conventional therapies. This shift necessitates new methodologies for patient stratification, biomarker discovery, and ways of measuring different types of outcomes that reflect how molecular heterogeneity translates into clinical benefit.
The authors’ emphasis on systems biology and network medicine reflects a mature understanding that CVD cannot be simplified to a single pathway or target. Considering the genetic predisposition, environmental exposures, the cellular environment within an organism, and the complexity of CVD, newer approaches that can manage multidimensional data and search for emergent properties created from system-wide interactions are needed. A systems-level perspective not only manages complexity but also effectively organizes it, allowing identification of disease modules and therapeutic opportunities that reductionist approaches may overlook.
Technological convergence enabling transformation
The precision medicine revolution is taking place at an optimal time due to unprecedented technological advances that are coming together to permit a systems-level understanding of CVD (Figure 1B). High-throughput omics platforms enable simultaneous sampling across genomic, transcriptomic, proteomic, and metabolomic dimensions to help generate combined molecular snapshots. Single-cell technologies provide insights into cellular heterogeneity that bulk analyses mask, whereas spatial omics approaches can provide information about tissue context that is crucial for understanding disease mechanisms. Artificial intelligence (AI) algorithms extract patterns from datasets too complex for human interpretation alone, and network medicine approaches make use of computational frameworks to find disease modules and possible drug targets (4).
Perhaps nowhere is this technological revolution more evident than in the expansion of therapeutic modalities beyond traditional small molecules. The discussion of RNA therapeutics by Aikawa et al. highlights a particularly transformative frontier. With tools such as antisense oligonucleotides, small interfering RNAs (siRNAs), and clustered regularly spaced short palindromic repeats (CRISPR)-based approaches, researchers can now modulate targets previously considered “undruggable.” The recent successes of inclisiran for PCSK9 inhibition (5) and pelacarsen for lipoprotein(a) reduction are examples of promising RNA-based approaches that produce clinically meaningful outcomes in patients, while also having the potential to alleviate some traditional concerns around dosing frequency and patient adherence (6).
This expansion of available therapeutic options is more than simply having additional options; it is also a major step forward in targeting mechanisms of disease at their source. Rather than inhibiting proteins after they are produced, RNA therapeutics intervene earlier by preventing the translation of harmful proteins, offering longer-lasting effects with less frequent dosing, fundamentally reshaping the risk–benefit assessment for chronic cardiovascular disease.
The integration of AI into this technological ecosystem represents both tremendous promise and significant responsibility. AI applications span from diagnostic imaging and risk prediction to drug discovery and patient stratification, but the most powerful applications will work to enhance human capabilities to manage complexity rather than replace clinical judgment. Aikawa et al. (2) rightly point out that the success of AI will depend on the quality, representativeness of data, and algorithmic transparency. If algorithms are trained on homogeneous populations, they risk reinforcing rather than resolving disparities. Diversity is both an ethical imperative and a scientific requirement for developing rigorous, generalizable approaches.
The COVID-19 pandemic showed the possibilities available when technological capabilities supported urgent clinical needs with adequate resources (7, 8). The impressive speed and scale of the messenger RNA (mRNA) vaccines demonstrated that timelines for therapeutic development that used to take years could be shortened to months when regulatory latitude, robust funding, and historic stakeholder collaboration were channeled toward a common life-saving goal. The challenge for cardiovascular medicine is to maintain this level of integrated urgency for conditions that cause an even greater cumulative burden than COVID-19, albeit progressing over longer timescales rather than in acute surges.
The implementation imperative for collaboration, equity, and global impact
One of the most significant challenges facing precision cardiovascular medicine may not be technological but organizational and social (Figure 1C). Systems-based approaches are inherently complex and require unprecedented cooperation across disciplines that have historically operated in relative isolation. Molecular biologists must work closely with data scientists, clinicians must partner with engineers, and pharmaceutical companies must engage productively with academic researchers. The advocacy of Aikawa et al. for breaking down “silos” in research reflects hard-won experience showing that precision medicine cannot be fashioned out of traditional disciplinary compartmentalization.
The academia–industry collaboration paradigm discussed by these authors represents an especially important evolution. Standard academic research is typically good at exploring novel mechanisms and pursuing high-risk, high-reward approaches, whereas industry brings expertise in drug development, regulatory navigation, and manufacturing scale-up. Precision cardiovascular medicine will require new collaboration models that utilize their respective strengths while fulfilling scientific rigor and properly disclosing potential conflicts of interest. Aikawa et al. highlight several successful examples of partnerships, but broadening these models at scale across the research enterprise will require institutional adjustments to how academic and industry organizations measure their successes and distribute resources.
Perhaps the most difficult challenge is ensuring global equity in access to precision therapeutics. Sophisticated molecular diagnostics and personalized therapeutics will have little impact if they remain limited to affluent populations. The COVID-19 pandemic exposed stark global inequities in access to vaccines and therapeutics, underscoring the risk that precision medicine could deepen disparities unless it is proactively addressed. The authors accurately reveal the importance of a policy framework that supports equitable access while forging new pathways of access for further innovation.
Beyond access, the equity challenge also encompasses representation in research itself. The molecular signatures that define disease subtypes may vary across populations with different genetic backgrounds and environmental exposures. Models of precision medicine that have only been developed in populations primarily with European ancestry may not be applicable to other populations, potentially widening—not narrowing—health disparities (9). Addressing this challenge requires not only technology transfer and capacity building in low- and middle-income countries, but also collaborative research partnerships that are mindful of including diverse populations in drug discovery and development from the outset.
The regulatory implications of precision cardiovascular medicine also demand careful consideration. Traditional drug development and approval processes rely on assumptions of homogeneity in patient populations and binary efficacy endpoints. Precision approaches may require new regulatory strategies to evaluate therapeutics intended for molecularly defined subgroups, companion diagnostics that direct treatment selection, and combination therapies that address disease through multiple pathways at once. The authors note encouraging precedents from precision oncology, but CVDs present unique challenges around endpoint selection, patient stratification, and long-term safety monitoring that may necessitate regulatory adaptation and new evaluation frameworks.
Looking forward, the success of precision cardiovascular medicine will ultimately be evaluated not by the sophistication of the technologies used, but through the lens of the clinical impact. Aikawa et al. offer compelling evidence that the tools and knowledge necessary for transformation are either in existence or are developing rapidly. What is now required is the collective courage and willingness to embrace greater complexity while maintaining scientific rigor, to engage in creative collaborations across traditional boundaries while honoring individual contributions, and to develop sophisticated therapeutic approaches while enabling equitable access. The path from one-size-fits-all to precision cardiovascular medicine will not be easy or quick, but with annual global CVD mortality projected to reach 26 million by 2030, we cannot afford to wait for perfect solutions. We must act on the knowledge we already possess while advancing the frontiers as outlined by Aikawa and colleagues.
The transition toward precision cardiovascular medicine is underway. The key challenge lies in how effectively and rapidly it can be implemented. Given the global burden of disease, sustained and coordinated efforts will be essential to maximize its impact.
Statements
Author contributions
SR: Writing – original draft, Writing – review & editing.
JCW: Writing – original draft, Writing – review & editing.
Funding
The authors declared that financial support was received for this work and/or its publication. This study was supported by research grants from the American Heart Association (no. 25CDA1449493 to SR) and from the National Institutes of Health (no. R01 HL150693, no. R01 HL163680 and no. UG3 AG097135 to JCW).
Conflict of interest
The authors declared that this work was conducted in the absence of financial relationships that could be construed as a potential conflict of interest.
The author JCW declared that he was an editorial board member of Frontiers at the time of submission. This had no impact on the review process and the final decision.
Generative AI statement
The authors declared that no generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of AI and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lindstrom M, Decleene N, Dorsey H, Fuster V, Johnson CO, Legrand KE, et al. Global burden of cardiovascular diseases and risks collaboration, 1990-2021. J Am Coll Cardiol (2022) 80(25):2372–425. doi: 10.1016/j.jacc.2022.11.001
2. Aikawa M, Sonawane AR, Chelvanambi S, Asano T, Halu A, Matamalas JT, et al. Precision cardiovascular medicine: shifting the innovation paradigm. Front Sci (2025) 3:1474469. doi: 10.3389/fsci.2025.1474469
3. Kravitz RL, Duan N, and Braslow J. Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages. Milbank Q (2004) 82(4):661–87. doi: 10.1111/j.0887-378X.2004.00327.x
4. Sonawane AR, Aikawa E, and Aikawa M. Connections for matters of the heart: network medicine in cardiovascular diseases. Front Cardiovasc Med (2022) 9:873582. doi: 10.3389/fcvm.2022.873582
5. Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, et al. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med (2020) 382(16):1507–19. doi: 10.1056/NEJMoa1912387
6. Tsimikas S, Moriarty PM, and Stroes ES. Emerging RNA therapeutics to lower blood levels of Lp(a): JACC focus seminar 2/4. J Am Coll Cardiol (2021) 77(12):1576–89. doi: 10.1016/j.jacc.2021.01.051
7. Lines K, Dzimadzi S, Lubega E, Mudimu-Matsangaise P, Rao V, Sebbanja JA, et al. COVID-19 vaccine rollout: data from informal settlements in Harare, Kampala, Lilongwe and Mumbai. Environ Urban (2023) 35(1):49–73. doi: 10.1177/09562478221149876
8. Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet (2021) 397(10278):1023–34. doi: 10.1016/S0140-6736(21)00306-8
Keywords: precision medicine, cardiovascular disease, disease heterogeneity, systems biology, network medicine, healthcare equity, clinical translation, global health policy
Citation: Rhee S and Wu JC. From one-size-fits-all to precision cardiovascular medicine. Front Sci (2025) 3:1703653. doi: 10.3389/fsci.2025.1703653
Received: 11 September 2025; Accepted: 19 September 2025;
Published: 07 October 2025.
Edited by:
Frontiers in Science Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2025 Rhee and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph C. Wu, am9ld3VAc3RhbmZvcmQuZWR1
 Siyeon Rhee
Siyeon Rhee Joseph C. Wu
Joseph C. Wu