- Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia
Mangrove ecosystems are remarkable coastal environments that thrive at the interface between land and sea, playing a crucial role in maintaining ecological balance and safeguarding coastal agricultural and fisheries productivity through erosion control, nutrient cycling, and salinity buffering. The physicochemical properties of mangrove soils underpin the health of these ecosystems, particularly for Avicennia marina, a keystone species critical to coastal resilience and habitat provisioning. However, anthropogenic disturbances threaten their sustainability and compromise their ability to deliver vital ecosystem services. Soil samples from undisturbed (Southern Corniche, Jeddah) and disturbed (Masturah) mangrove sites were analyzed for physicochemical characteristics to assess potential anthropogenic impacts along Saudi Arabia’s Red Sea coast. From six locations (undisturbed: Jeddah, n=3; disturbed: Masturah, n=3) soil samples were analyzed for texture, pH, electrical conductivity (EC), total dissolved solids (TDS), water content (%WC), total nitrogen (TN), phosphorus (TP), organic carbon (TOC), macronutrients (Na+, Ca²+, Mg²+, K+), and cation exchange capacity (CEC). Undisturbed soils exhibited significantly higher moisture, TN, TP, and TOC—key indicators of nutrient retention and carbon sequestration capacity—while disturbed soils were more alkaline, a condition linked to diminished nutrient cycling and plant stress. Macronutrient distribution (Na+ > Mg²+ > Ca²+ > K+) remained consistent across sites, suggesting salinity-driven nutrient imbalances may limit mangrove recovery. These findings highlight how soil degradation in disturbed mangroves reduces their ability to stabilize sediments, mitigate saltwater intrusion, and sustain fisheries nurseries, directly impacting coastal communities. Moreover, these soil changes reduce mangrove capacity to buffer adjacent farmland from salinization and erosion, threatening agricultural productivity and undermining carbon sequestration goals central to climate mitigation. To enhance ecosystem resilience, we recommend the application of soil organic amendments and the strategic conservation of high-carbon mangrove zones, in alignment with Saudi Arabia’s Vision 2030 sustainability framework. This study highlights the critical importance of safeguarding mangrove soils as foundational natural infrastructure for climate adaptation and food security in arid coastal environments.
1 Introduction
The Red Sea coast of Saudi Arabia is home to a significant population of Avicennia marina, a resilient mangrove species that plays a crucial role in maintaining the delicate coastal ecosystem and safeguarding adjacent agricultural and aquacultural systems through shoreline stabilization, nutrient retention, and salinity regulation. For this study, undisturbed mangrove forests were defined as those with no recorded human modification in the past 30 years, based on satellite imagery and field surveys confirming intact canopy cover (>80%) and natural hydrology (1, 2). In contrast, disturbed sites showed visible logging, aquaculture encroachment, or drainage alterations within the past decade.
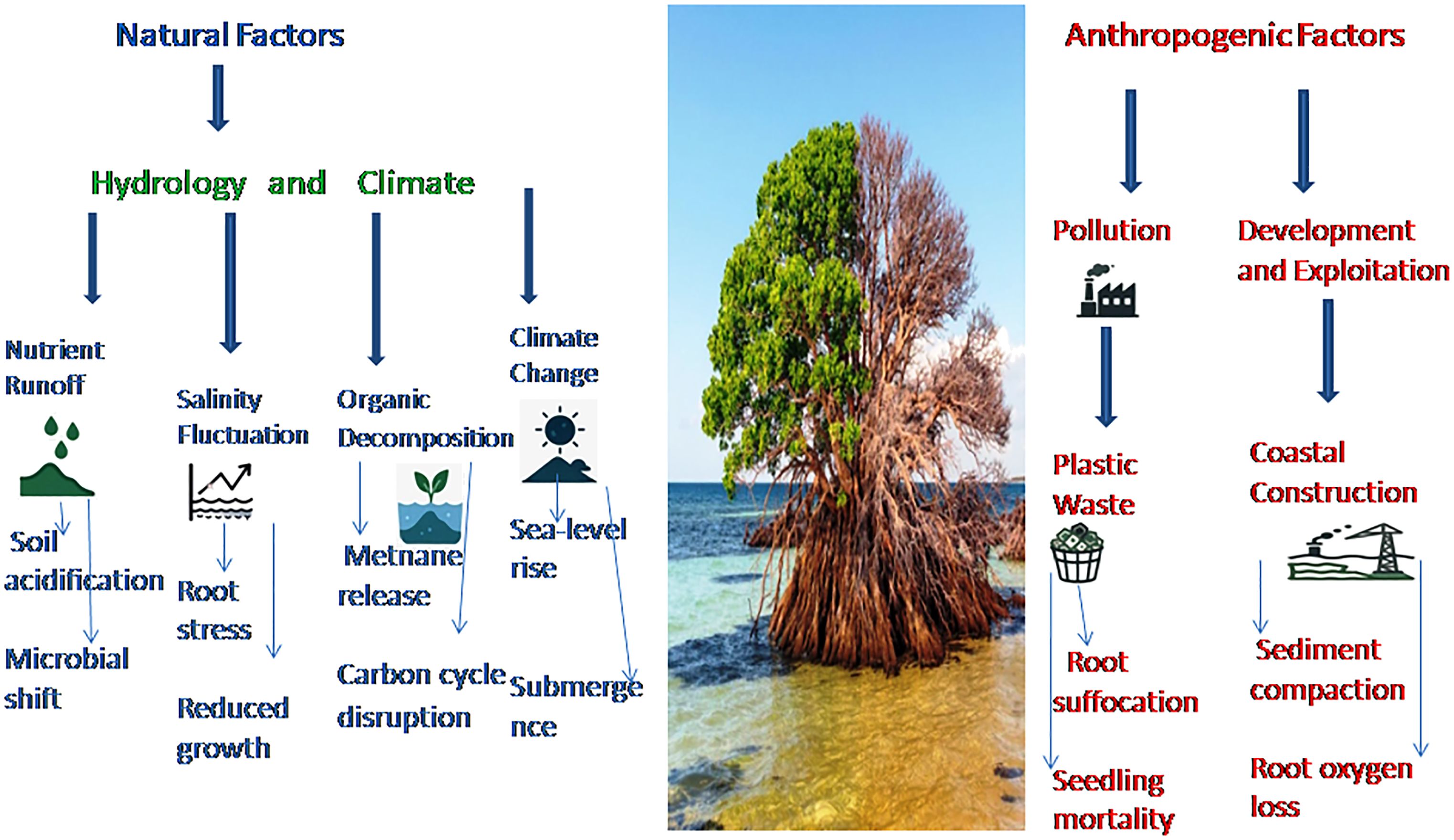
These mangrove ecosystems are increasingly under threat from anthropogenic disturbances and pollution, which degrade soil quality and compromise the mangrove’s capacity to act as a buffer against coastal erosion and saltwater intrusion—critical threats to farmland and fisheries. Studies have shown that the mangrove forests along the Red Sea coast have experienced significant degradation and loss over the years (Figure 1) (2), undermining their role in sustaining regional food security and climate resilience. (1, 2).
Saudi Arabia’s mangroves, covers approximately 20,000 hectares along its coastline, represent a small but vital component of the global and Asian mangrove distributions, accounting for 0.14% and 0.34%, respectively (3). These ecosystems are essential “blue infrastructure” for coastal communities, filtering pollutants, sequestering carbon, and providing nursery habitats for commercially important fish species. Yet, they face escalating anthropogenic pressures, including urban expansion and deforestation, alongside natural challenges like erosion. Globally, mangrove coverage has decreased by 20%–35% over the past five decades, with losses in India reaching 39.32%–95% between 1990 and 2001 due to aquaculture conversion, industrial pollution, and land reclamation (4). Saudi Vision 2030 is the national economic diversification plan, prioritizing sustainable tourism and agricultural security as key non-oil sectors. In Saudi Arabia, fragmented mangrove distribution and lagging restoration rates (1, 5) threaten the ecological services these habitats provide to Red Sea coastal economies.
The impacts of mangrove degradation extend deeply into soil health, with cascading consequences for coastal productivity. Soil carbon stocks decline, abiotic conditions shift, and sediment stability erodes, leading to reduced fisheries catches, loss of mollusk populations, and heightened coastal abrasion (6). Such changes directly jeopardize livelihoods dependent on small-scale fisheries and expose farmland to salinization, a critical concern in arid regions like the Red Sea. Moreover, anthropogenic factors such as pollution can directly alter soil composition, disrupting the delicate balance of nutrients and pH levels essential for Avicennia marina survival. These shifts weaken mangrove resilience, reducing their capacity to buffer adjacent farmland from saltwater intrusion—a critical concern for Saudi Arabia’s arid coastal agriculture, where soil salinization already threatens crop yields.
One of the primary challenges facing Saudi Arabia’s mangrove forests is the impact of climate change (7). The Middle East and the Gulf of Arabia basin are expected to be particularly vulnerable to the negative effects of climate change, with predictions of extreme temperatures and other environmental stresses that could significantly impact the health and survival of mangrove ecosystems (8). Such conditions not only stress mangrove health but also diminish their ability to protect inland agricultural zones from storm surges and sea-level rise, directly compromising food security. The intrinsic link between mangrove condition and climate change positions these ecosystems as biomonitors of regional climate impacts, offering insights into risks for coastal land-use systems (7). Human disturbances compound these threats: conversion of mangroves to fishponds and urban infrastructure drove a 61% decline in Saudi mangrove cover by the 1990s (9), eroding natural barriers that once shielded fertile coastal soils from erosion and salinization.
Mangroves are vital allies in climate mitigation, sequestering carbon at rates 4–8 times higher than terrestrial forests (10, 11). In Saudi Arabia, where arid soils typically exhibit low organic carbon, mangrove sediments represent a critical—yet overlooked—carbon reservoir. Their degradation could release stored CO2, undermining national efforts to achieve net-zero targets under Saudi Vision 2030. Over 90% of mangrove carbon resides in soil and root biomass (12), making soil health a linchpin for maintaining this ecosystem service. Protecting these carbon stocks is not only ecologically prudent but economically strategic, as it aligns with global carbon markets and climate financing mechanisms.
Beyond carbon, mangroves provide irreplaceable ecosystem services: their roots stabilize shorelines, filter pollutants, and create nurseries for commercially vital fish species. In the Red Sea, where fisheries contribute ~$1.5 billion annually to the economy, mangrove degradation risks collapsing nursery habitats for shrimp and reef fish, threatening livelihoods and food security. Their sediment-trapping capacity also mitigates saltwater intrusion into coastal aquifers, preserving freshwater resources essential for irrigation in agriculture-dominated regions like Jeddah. The loss of these functions—evident in global examples like Southeast Asia, where mangrove deforestation increased coastal flooding damage by 30% (13) highlights the urgent need to integrate mangrove conservation into Saudi Arabia’s coastal land-use planning to safeguard both ecological and agricultural productivity (14–16).
Understanding the physical and chemical properties of mangrove soils is critical not only for ecological conservation but also for safeguarding coastal livelihoods and agricultural productivity in arid regions like the Red Sea. These properties govern nutrient cycling, carbon sequestration, and shoreline stability—ecosystem services directly tied to fisheries yields, freshwater security, and climate resilience. Shoreline dynamics, tidal regimes, and anthropogenic pressures collectively shape mangrove soil characteristics, influencing vegetation health and, consequently, their capacity to buffer adjacent farmland from saltwater intrusion and erosion. For example, advancing shorelines with optimal soil texture and organic matter support robust mangrove regeneration, enhancing sediment trapping that protects coastal irrigation systems, while retreating shorelines signal degraded soils and heightened vulnerability to storm surges. (17, 18).
Optimal mangrove soil conditions—slightly acidic to neutral pH (6.0–7.5), high organic matter, and moderate salinity—are not just ecological benchmarks but also indicators of ecosystem service potential. Organic-rich soils enhance nutrient retention, reducing fertilizer runoff into coastal waters and mitigating eutrophication risks for aquaculture. Conversely, alkaline shifts in disturbed soils (e.g., pH >8.0) disrupt microbial activity, impairing natural wastewater filtration services critical to maintaining water quality for coastal agriculture. Salinity regulation by healthy mangroves is equally vital: their roots exclude salt, preventing hypersaline groundwater from seeping into farmland—a vital concern in Saudi Arabia, where 30% of coastal soils are already salt-affected (17). Despite their importance, standardized methods for assessing mangrove soil quality remain scarce, hindering evidence-based policies to balance conservation with coastal development. This study addresses this knowledge gap through a comprehensive approach, combining field sampling, laboratory analyses, and statistical modeling to assess the impact of human-induced pressures on key soil properties, including moisture, nutrients, and salinity (12).
Understanding the physical and chemical properties of mangrove soils is critical not only for ecological conservation but also for safeguarding coastal livelihoods and agricultural productivity in arid regions like the Red Sea. These soil parameters provide valuable indicators for assessing mangroves’ ability to support fisheries nurseries, store carbon, and safeguard agricultural land, offering a relevant framework for managing arid coastal ecosystems worldwide. As there remains a lack of detailed, statistically robust data on soil chemistry across different mangrove zones. Previous studies have either focused on biodiversity or general environmental assessments, without integrating quantitative soil and water parameters using rigorous statistical methods. This study investigates Avicennia marina soil physicochemical properties in Red Sea mangroves, aiming to provide actionable insights for sustainable land management and conservation initiatives (1).
Mangrove ecosystems are among the world’s most valuable blue-carbon habitats, providing critical services such as coastal protection, carbon sequestration, and nursery grounds for fisheries (10, 12). However, global mangrove coverage has declined precipitously by 20-35% over the past 50 years, primarily due to anthropogenic pressures such as aquaculture conversion, coastal development, and pollution (19, 20). This degradation is not just a loss of forest cover; it triggers a cascade of negative impacts on soil physicochemical properties, which form the foundation for these ecosystem services (21, 22).
Along the Red Sea coast of Saudi Arabia, these global threats are acutely present. Mangroves, predominantly Avicennia marina, face pressures from urbanization, industrial expansion, and tourism (1, 2). Previous studies in the region have successfully documented rates of mangrove loss and fragmentation (e.g., 9) and have described general biodiversity patterns. Studies have also noted that soil properties are key to mangrove health (e.g., 23).
A significant research void exists in understanding the detailed mechanisms of human-induced soil degradation in Red Sea mangroves and its direct impact on ecosystem services. While degradation is known to occur, previous studies have not comprehensively linked specific human activities to changes in key soil properties (e.g., organic carbon, nutrients, salinity) and then connected those changes to the loss of critical functions like coastline stabilization and farmland protection. This study is designed to fill that exact gap by providing a mechanistic, soil-focused assessment that links human disturbance to quantifiable changes in soil properties and, crucially, to the impairment of specific ecosystem services. The ultimate goal is to generate actionable insights to guide effective conservation and restoration strategies in line with Saudi Vision 2030 (24).
2 Materials and methods
2.1 Study sites
This study was conducted along the Red Sea Coast of Saudi Arabia, comparing mangrove soils in an ecologically intact, protected area (Southern Corniche, Jeddah City, Mecca Province) and a degraded site exposed to anthropogenic stressors (Masturah, Rabigh Governorate, Madinah Province). Site selection was designed to contrast soil conditions under divergent management regimes, providing insights into how human activity alters mangrove soil functionality—a critical factor for coastal resilience and adjacent land-use sustainability.
2.1.2 Undisturbed site (Jeddah)
Location: Three subsites (A, B, C) within Southern Corniche, a region under minimal direct human influence due to its designation as a coastal conservation zone (21°16’03.7”N 39°07’38.0”E; 21°16’05.5”N 39°07’37.0”E; 21°15’45.8”N 39°07’45.5”E) (Figure 2).
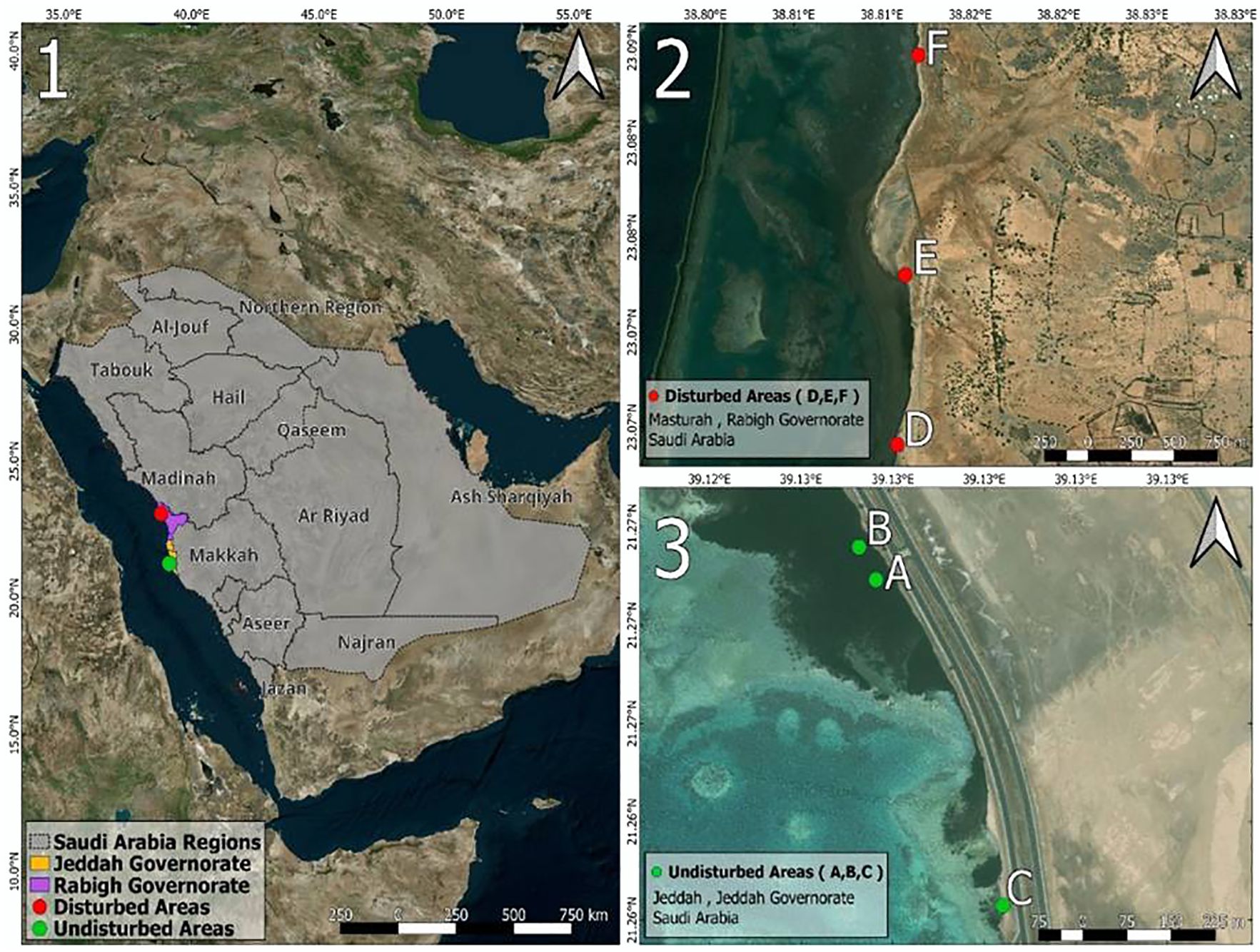
Figure 2. Map 1 illustrates the geographical locations of the study sites, providing a general overview of Saudi Arabia, highlighting Jeddah and the Rabigh Governorate, where the Avicennia marina study areas are situated. Map 2 delineates the disturbed mangrove areas, designated as Area D, Area E, and Area F. Conversely, Map 3 depicts the undisturbed areas, labeled as Area A, Area B, and Area C. All maps were generated using QGIS software (version 3.28.0) and created by Haitham Ali Sheikh.
2.1.2 Characteristics
Located near the Jeddah Marine Sanctuary, where mangrove conservation aligns with regional biodiversity protection goals.
Soils here represent a baseline for mangrove soil health, with natural tidal flushing and limited sediment disruption.
Surrounding land use includes low-density urban infrastructure, minimizing pollutant influx.
2.1.3 Disturbed site (Masturah)
Location: Three subsites (D, E, F) in Masturah (23°04’08.7”N 38°48’39.1”E; 23°04’40.9”N 38°48’40.7”E; 23°05’22.6”N 38°48’43.4”E), a region experiencing rapid coastal development and habitat fragmentation (Figure 2).
2.1.4 Characteristics
Proximity to industrial activities, desalination plants, and unregulated tourism—key drivers of soil compaction, pollution, and altered hydrology.
Historical conversion of mangrove areas to temporary fish drying stations and informal settlements, disrupting sediment accretion.
Located within a semi-enclosed bay with restricted tidal exchange, exacerbating salinity stress and nutrient stagnation.
2.1.5 Regional context
Both sites experience a hyper-arid climate (mean annual rainfall <70 mm) with high evaporation rates, making soil salinity and water retention critical factors for mangrove survival. The undisturbed Jeddah site serves as a natural laboratory for studying mangrove soil processes in the absence of major anthropogenic pressures, while Masturah exemplifies degradation hotspots where mangrove loss threatens coastal protection services for nearby agricultural communities.
The classification of sites as ‘undisturbed’ or ‘disturbed’ was based on direct physical evidence observed during field surveys, corroborating land-use data. The disturbed site (masturah) exhibited clear signs of anthropogenic pressure, including visible fragmentation of the mangrove canopy, well-established trampling pathways compacting the soil surface, and the presence of anthropogenic debris (primarily plastic waste and discarded fishing materials). Furthermore, evidence of historical excavation and altered drainage patterns was apparent. In contrast, the undisturbed site (Southern Corniche, Jeddah) showed no such signs; it featured a continuous canopy cover, no visible trails or soil compaction from human activity, an absence of litter, and natural, undisturbed tidal channels. This stark contrast in physical integrity confirms that the Masturah site is actively degraded, justifying the comparative framework.
The undisturbed site (Southern Corniche, Jeddah) is situated within the Jeddah Marine Sanctuary, a protected area managed under the National Center for Wildlife (NCW) in alignment with the Saudi Green Initiative and Vision 2030’s environmental goals. The explicit regional biodiversity protection goals for this sanctuary are to preserve critical coastal habitats (mangroves, seagrasses, coral reefs), conserve threatened marine species (e.g., Halavi guitarfish, hawksbill turtle), and maintain ecosystem services like coastal stabilization and carbon sequestration. This formal designation enforces strict regulations against construction, dredging, and habitat alteration, resulting in the observed intact canopy, natural hydrology, and absence of direct anthropogenic disturbance that justifies its use as an undisturbed reference site.
2.2 Soil sample collection
Soil samples were collected from Avicennia marina habitats along Saudi Arabia’s Red Sea coast from March to June 2023, targeting undisturbed (Southern Corniche, Jeddah) and disturbed (Masturah) mangrove zones. This spatial comparison enables identification of soil degradation patterns critical for prioritizing restoration efforts in high-risk agricultural buffer zones. A total of 18 samples (9 disturbed, 9 undisturbed) were collected using a 22-inch soil probe, with three replicates per subsite spaced 200 m apart to account for microhabitat variability. Samples were extracted at a standardized depth of 15 cm—the root-active zone influencing mangrove stability and sediment retention capacity—and stored in labeled bags (25, 26).
2.3 Soil sample preparation
Soil appearance was evaluated using Thien (27) ribbon test to classify plasticity, a key indicator of sediment cohesion and erosion resistance. A small soil sample, sufficient to fit in the palm of the hand, was taken, and the fraction larger than 2 mm was manually removed. The soil was then moistened and kneaded into a bolus for 1 to 2 minutes until it was no longer sticky. The bolus was shaped between the thumb and forefinger to form a ribbon approximately 2 mm thick and 1 cm wide. The length of the ribbon was measured and recorded. Additionally, molding the bolus into rods facilitated the classification of soils with high clay content.
Texture was quantified via the hydrometer method (28), with results processed through the Agricultural Technology Centre soil texture calculator. For soil extract preparation, 50 grams of soil were weighed and placed in a dispersing cup, to which 100 milliliters of a 5% sodium hexametaphosphate (Calgon) solution were added. The mixture was agitated for 30 to 60 seconds and transferred to a 1000-milliliter container, filled to the mark with distilled water. For the blank sample, 880 milliliters of distilled water were used. The suspension was allowed to equilibrate to room temperature, and the temperature and hydrometer reading of the blank were recorded.
The suspension was mixed with a plunger for 30 seconds, and the hydrometer and thermometer were inserted. The hydrometer reading was recorded after 40 seconds and again after 6 hours and 52 minutes. Particle size distribution was determined using the hydrometer method as per Gee and Or (29).
2.4 Physicochemical analyses of soil
Soil properties were analyzed to assess functional indicators of ecosystem service provision, including appearance, texture, water content, pH, salinity, organic carbon, nitrogen, phosphorus, EC, CEC, TDS, and macronutrient bioavailability (Na+, Ca²+, Mg²+, K+).
2.4.1 Physical analysis of soil
Soil texture was quantified via the hydrometer method (28). Soil water content as percent water content (%WC) was determined by oven-drying at 105°C until constant weight (30, 31).
Total Dissolved Solids (TDS) were determined from mangrove soil samples (0–30 cm depth) using a saturated paste extract (32). Air-dried, sieved (2 mm) soil was mixed with deionized water (1:1 w/v), filtered (0.45 μm), and measured for electrical conductivity (EC). TDS (mg/L) was calculated as EC (dS/m) × 640, an empirical factor validated for saline soils (APHA, 2017; Almahasheer, 2018). Triplicate measurements ensured precision, with NaCl standards (500–2000 mg/L) for calibration. This method aligns with Red Sea mangrove studies reporting TDS ranges of 1500–3000 mg/L (33).
2.4.2 Chemical analyses of soil
Soil pH and EC were calibrated daily using NIST-traceable buffers (pH 4.0, 7.0, 10.0) and KCl standards (0.01 M, 0.1 M) (24, 34, 35). Certified reference materials (CRM 049-050, Sigma-Aldrich) verified instrument accuracy.
Cation Exchange Capacity (CEC) was determined via ammonium acetate displacement (36, 37).
Total Organic Carbon (TOC) was quantified using the Walkley-Black wet oxidation method (38) with a correction factor of 1.33 for incomplete oxidation. Triplicate samples were analyzed, and results cross-validated with loss-on-ignition (LOI) at 550 °C for 4 hours (39).
Total Nitrogen (TN) and Total Phosphorus (TP) were analyzed spectrophotometrically using HACH LCK kits (LCK 238 for TN; LCK 348 for TP) (37).
Macronutrients (Na+, Ca²+, Mg²+, K+) were measured via ion chromatography (37, 40, 41).
2.5 Statistical analysis
All statistical analyses were performed to determine the significance of differences in soil properties between undisturbed and disturbed mangrove sites. Data processing and analysis were conducted using IBM SPSS Statistics (version 28).
The three replicate samples from each of the three undisturbed sites (Sites A, B, C; n = 9) were pooled into a single “Undisturbed” group. Similarly, the three replicates from each disturbed site (Sites D, E, F; n = 9) were pooled into a “Disturbed” group. This pooling was justified given the clear and consistent physical evidence of degradation (Section 2.1) and allowed for a robust statistical comparison between the two management conditions.
The normality of the data distribution for each measured parameter was assessed using the Shapiro-Wilk test. Homogeneity of variances was verified using Levene’s test. Based on these assumptions, an independent samples t-test was used to compare the means of the Undisturbed and Disturbed groups for parameters that met the assumptions of normality and homoscedasticity. For parameters that violated these assumptions, the non-parametric Mann-Whitney U test was employed.
The results of these tests are presented as p-values in Table 1. Statistical significance was accepted at the level of p < 0.05. All data in the results are presented as mean ± standard deviation (SD).
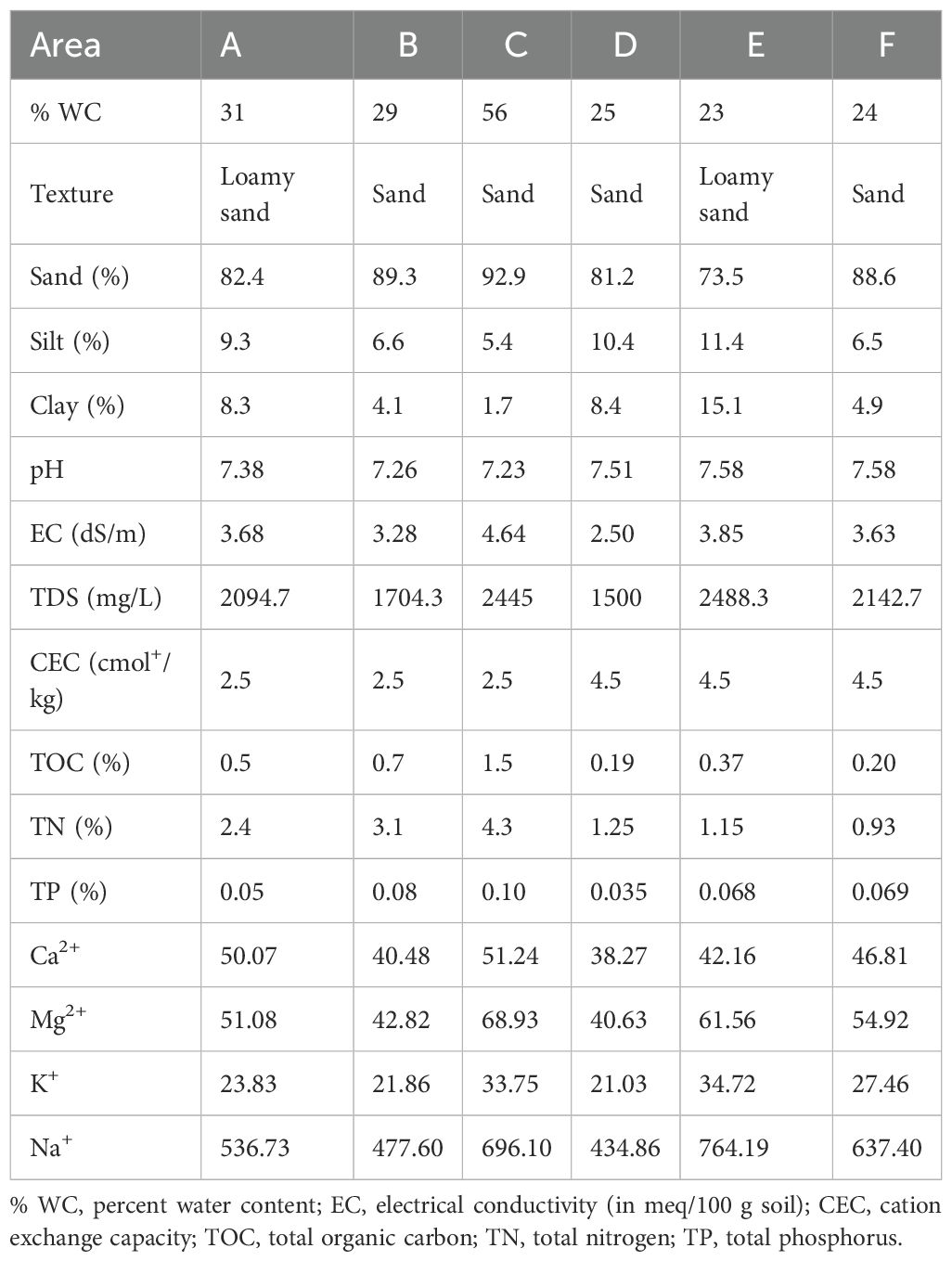
Table 1. Comparative analysis results of physicochemical properties in soils of Avicennia marina mangrove ecosystems along the Red Sea coast of Saudi Arabia: Undisturbed (A-C) versus disturbed (D-F) sites.
3 Results
3.1 Physical characteristics
3.1.1 Soil appearance and texture
Soil texture observation revealed two distinct classes critical for coastal protection services:
Undisturbed sites: Loamy sand textures (higher silt/clay content) (Figure 3, Tables 2, 3), enhancing sediment cohesion and nutrient retention—key traits for stabilizing shorelines against erosion threatening adjacent farmland.
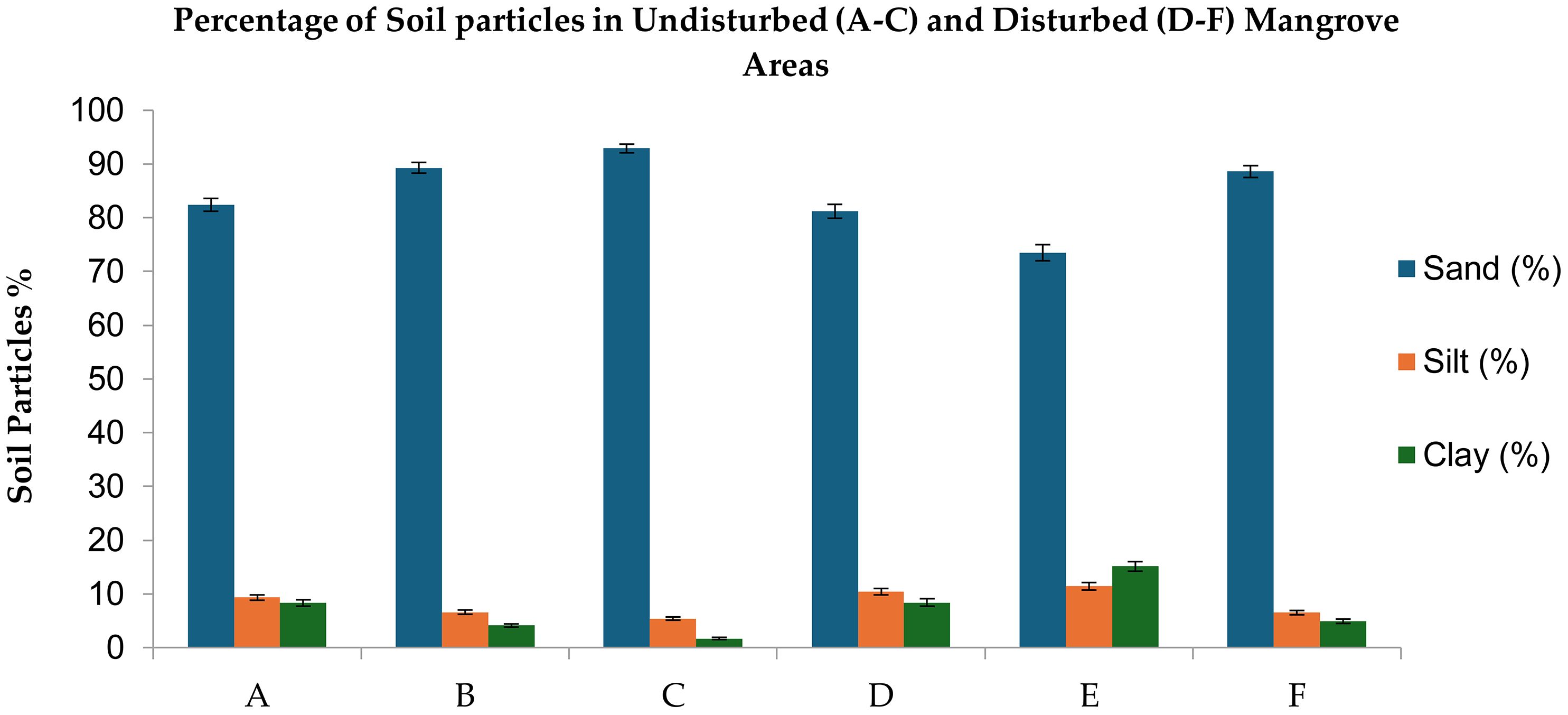
Figure 3. Particle size distribution (%) in soils of Avicennia marina mangrove forests along the Red Sea coast of Saudi Arabia: Undisturbed sites (A–C) versus disturbed sites (D–F). Values represent means ± standard error (n = 3) with significant differences (Fisher’s LSD, p ≤ 0.05).
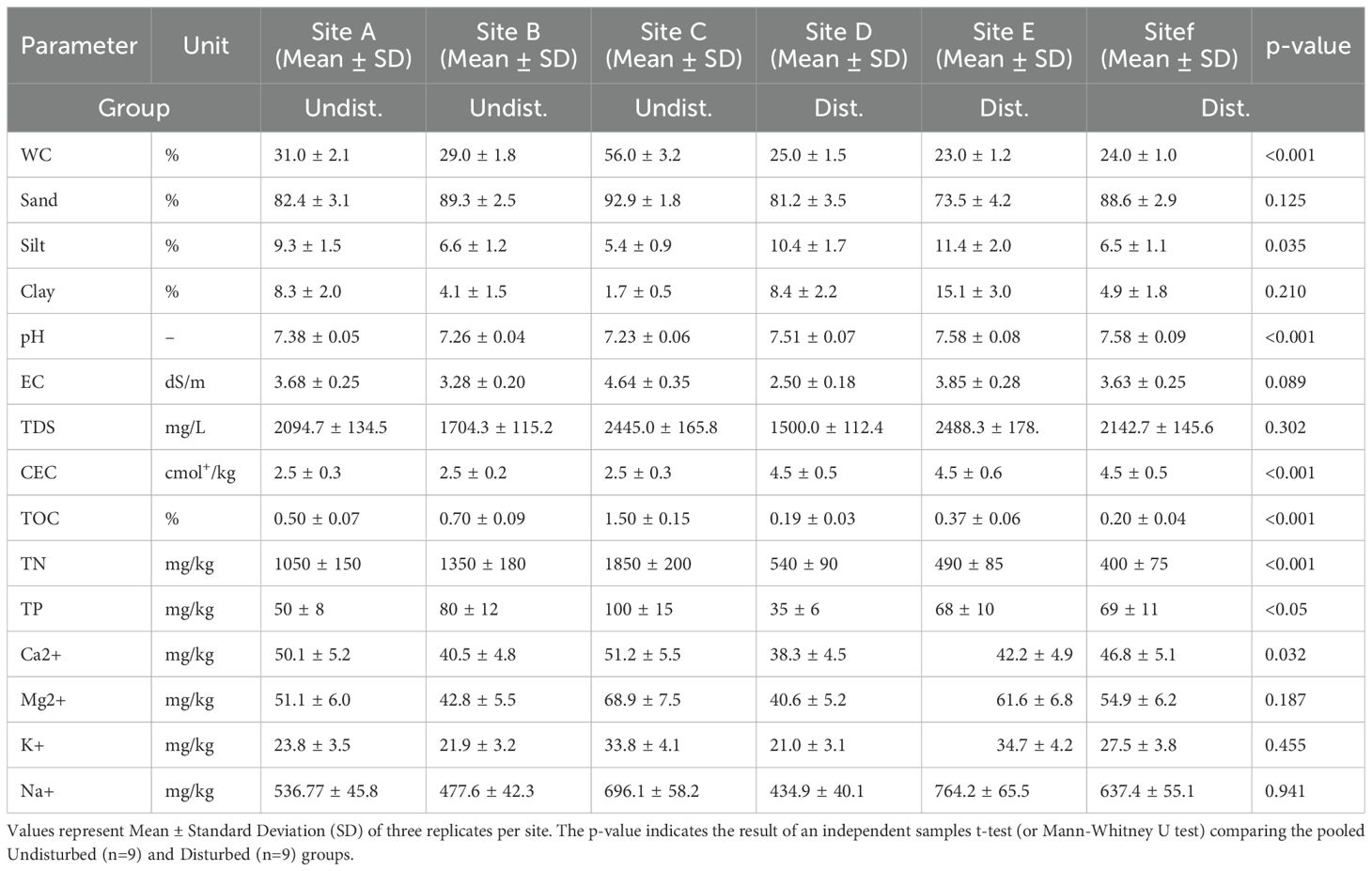
Table 3. Comparative analysis results of physicochemical properties in soils of Avicennia marina mangrove ecosystems along the Red Sea coast of Saudi Arabia: Undisturbed (A–C) versus disturbed (D–F) sites.
Disturbed sites: Coarse sand textures, reducing water-holding capacity and increasing salt leaching into groundwater, a major risk for irrigation-dependent agriculture in arid regions like the Red Sea. These findings suggest anthropogenic disturbance simplifies soil structure, diminishing mangrove capacity to act as natural barriers against storm surges and saltwater intrusion.
3.1.2 Soil water content
Undisturbed sites retained significantly higher moisture (56%) compared to disturbed sites (23–25%). This moisture deficit in degraded soils correlates with reduced mangrove root density, weakening their ability to buffer coastal aquifers from seawater infiltration—a critical ecosystem service for sustaining freshwater availability in nearby farms. The soil percent water content (%WC) data is presented in Tables 2, 3 and Figure 4.
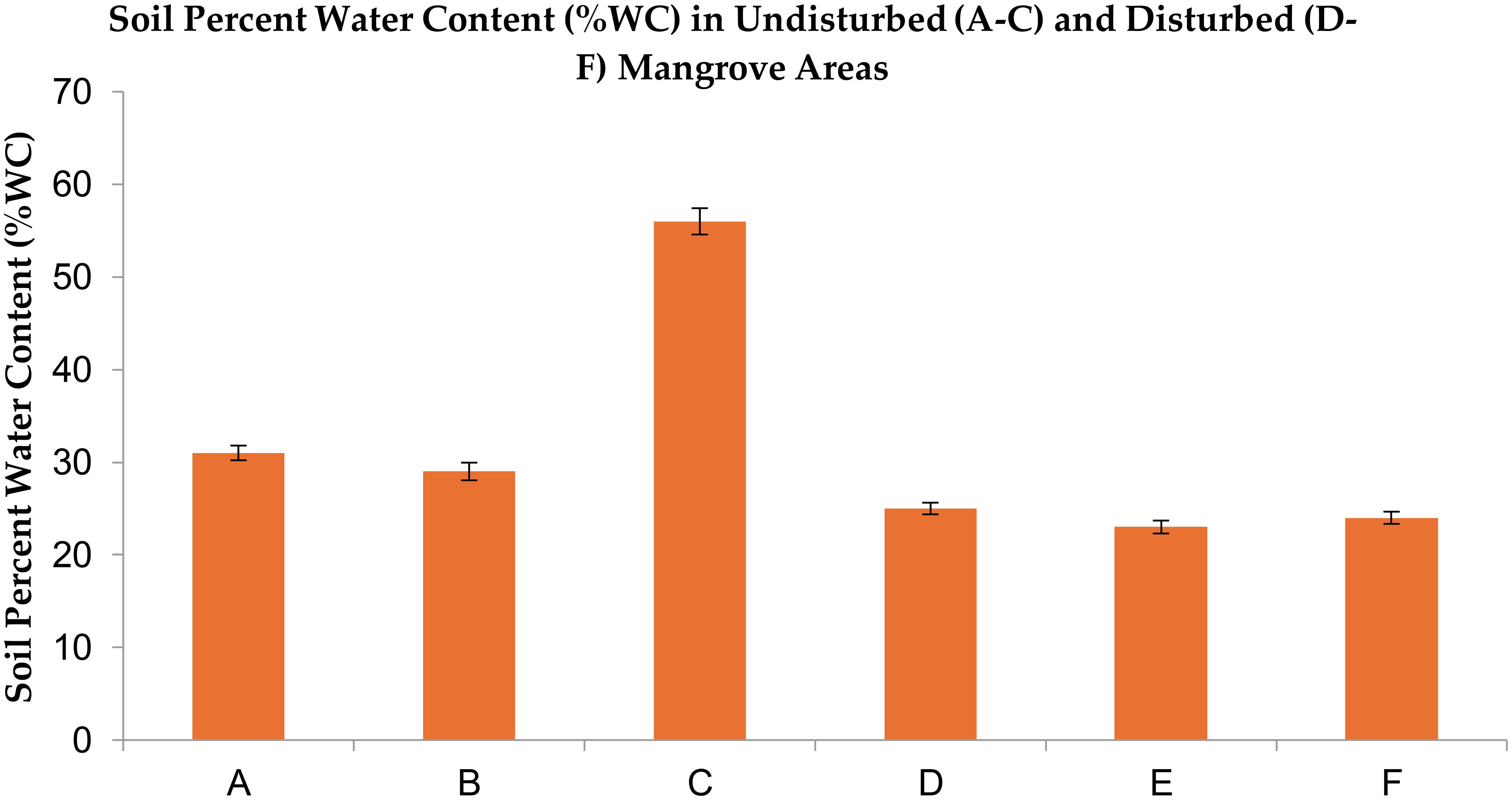
Figure 4. Soil percent water content (%WC) in Avicennia marina mangrove forests along the Red Sea coast of Saudi Arabia: Comparison between undisturbed (A–C) and disturbed (D–F) sites. Values represent means (n = 3) separated by Fisher’s Least Significant Difference (LSD) at p ≤ 0.05.
3.2 Chemical characteristics
3.2.1 Soil pH
Undisturbed soils: Neutral pH (7.2–7.3), optimal for nitrogen-fixing microbes that enhance natural soil fertility, reducing fertilizer dependency in adjacent agroecosystems.
Disturbed soils: Alkaline shift (7.51–7.58), disrupting microbial communities and nutrient cycling, which may accelerate agrochemical runoff into coastal fisheries. This statistically significant difference in pH between the undisturbed and disturbed sites suggests that the soil in the disturbed areas has been adversely affected as shown in Figure 5 and Tables 2, 3.
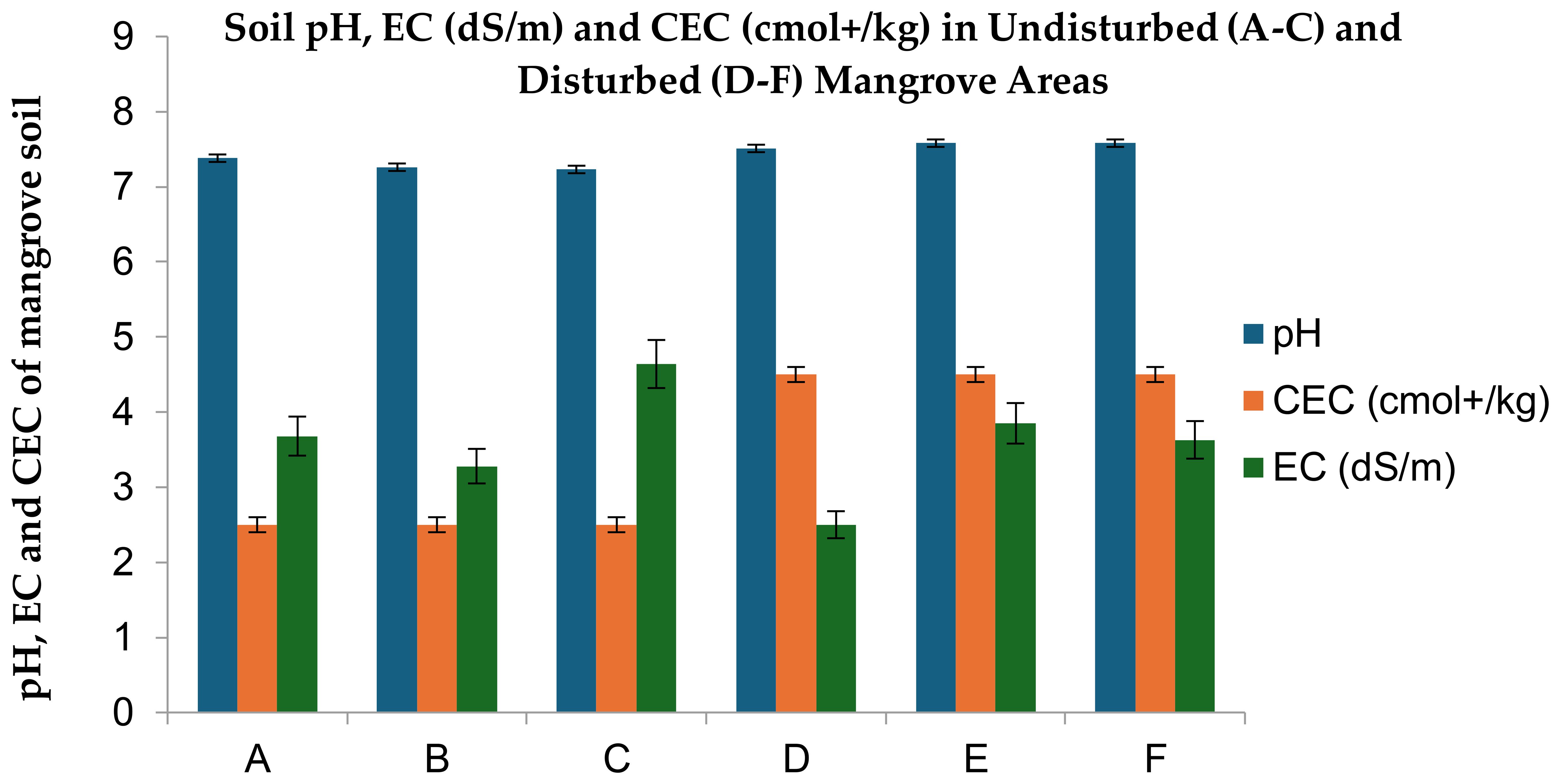
Figure 5. Soil pH, cation exchange capacity [CEC] and electrical conductivity [EC] in Avicennia marina mangrove forests along the Red Sea coast of Saudi Arabia. Significant differences between undisturbed (A–C) and disturbed (D–F) sites based on Fisher’s LSD (p ≤ 0.05).
3.2.2 Soil electrical conductivity
EC values for undisturbed sites (3.28–4.64, mean≈3.87 mS/cm) and disturbed sites (2.5–3.85, mean≈3.33 mS/cm) show no consistent directional trend. Elevated salinity in disturbed soils signals reduced mangrove filtration capacity, increasing risks of saltwater intrusion into irrigation networks.
Undisturbed sites: Slightly saline (3.28–3.68 mS/cm), within the optimal range for Avicennia marina growth, enabling effective salt exclusion to protect adjacent freshwater aquifers used for irrigation. Moderately saline (4.64 mS/cm), indicating natural tidal influence but still supporting mangrove root systems that stabilize sediments against erosion threatening farmland.
Disturbed sites: Lower salinity (2.5 mS/cm), potentially reflecting disrupted tidal exchange or freshwater influx from unregulated drainage, destabilizing mangrove salt-balance adaptations. Elevated salinity (3.63–3.85 mS/cm) compared to most undisturbed zones, suggesting anthropogenic stressors like reduced sediment accretion or pollutant accumulation, which weaken mangrove capacity to buffer croplands from saltwater intrusion.
3.2.3 Soil cation exchange capacity
Both undisturbed (avg. 2.87 meq/100g) and disturbed (avg. 3.82 meq/100g) mangrove soils exhibited critically low CEC (Figure 5, Tables 2, 3), reflecting minimal nutrient retention capacity. This deficiency limits mangrove soils’ ability to filter agricultural runoff, increasing risks of fertilizer and pollutant influx into Red Sea fisheries—a sector valued at over $1.5 billion annually.
Low CEC (<5 meq/100g) in all sites highlights the vulnerability of arid coastal soils to nutrient leaching, necessitating organic amendments (e.g., biochar) in restoration programs to enhance pollutant retention and protect aquaculture productivity.
3.2.4 Soil total dissolved solids
TDS levels ranged from 1,504–2,488 mg/L across sites, with disturbed areas (2,488 mg/L) showing marginally higher values than undisturbed zones (Figure 6, Tables 2, 3). These hypersaline conditions exceed the tolerance of most crops (e.g., barley, dates), underscoring mangroves’ role in intercepting salt-laden groundwater before it infiltrates farmland.
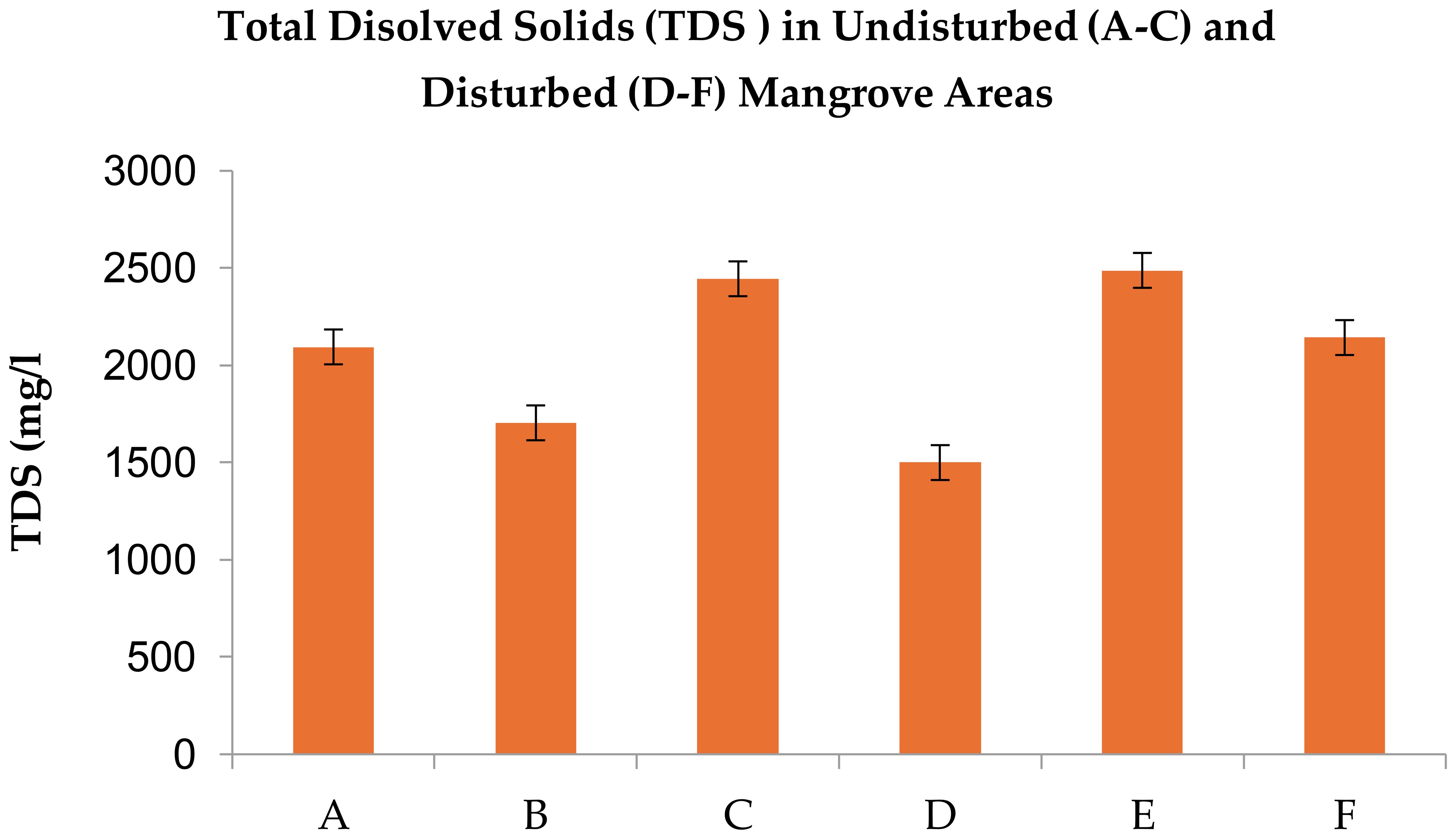
Figure 6. Total dissolved solids [TDS]) in Avicennia marina mangrove forests along the Red Sea coast of Saudi Arabia, comparing undisturbed (A–C) and disturbed (D–F) sites. Values represent means ± standard error (n = 3) with no significant differences (Fisher’s LSD, p > 0.05).
TDS >2,000 mg/L in disturbed soils signals accelerated saltwater intrusion, posing direct risks to irrigated agriculture in nearby regions like Rabigh, where 22% of coastal soils are already salt-affected.
3.2.5 Soil total nitrogen and phosphorus
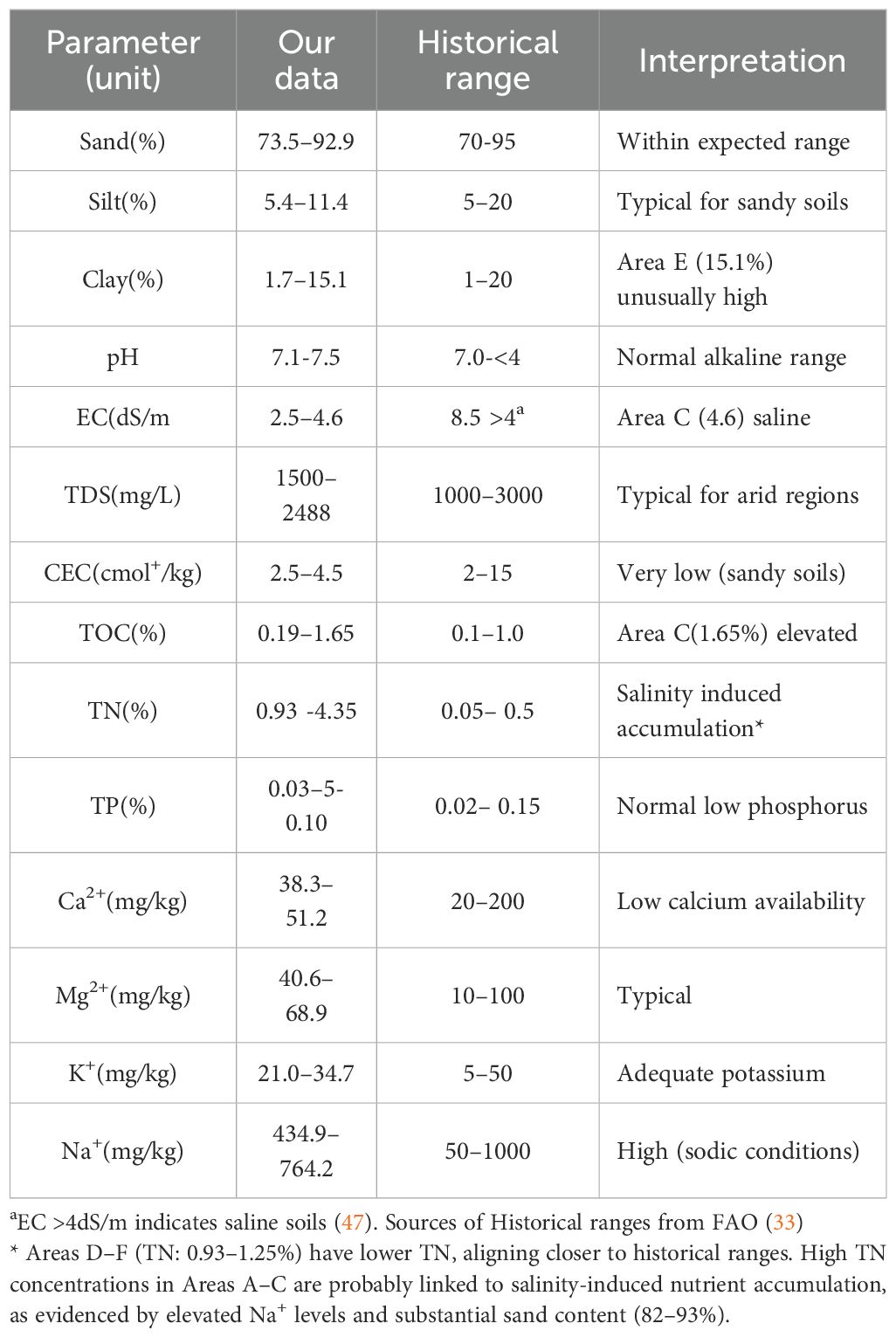
Undisturbed soils retained 3–4× higher TN (4.35 mg/L) and 2–3× higher TP (0.10 mg/L) compared to disturbed sites (TN: 0.93–1.25 mg/L; TP: ~0.05 mg/L). Elevated TN in undisturbed areas from the historical range (0.05–0.5%) in Tables 4, 5 likely results from saline-driven nutrient accumulation, supported by high Na+ and sand content (82–93%).The stark decline in disturbed areas reduces mangrove capacity to act as nutrient sinks, elevating eutrophication risks in coastal waters critical for Saudi Arabia’s shrimp and reef fish industries (Figure 7, Tables 1, 2). The high TN values likely reflect anthropogenic influences (e.g., fertilizer runoff) or soil type specificity (e.g., high clay in disturbed area [15.1%] may retain more nitrogen).
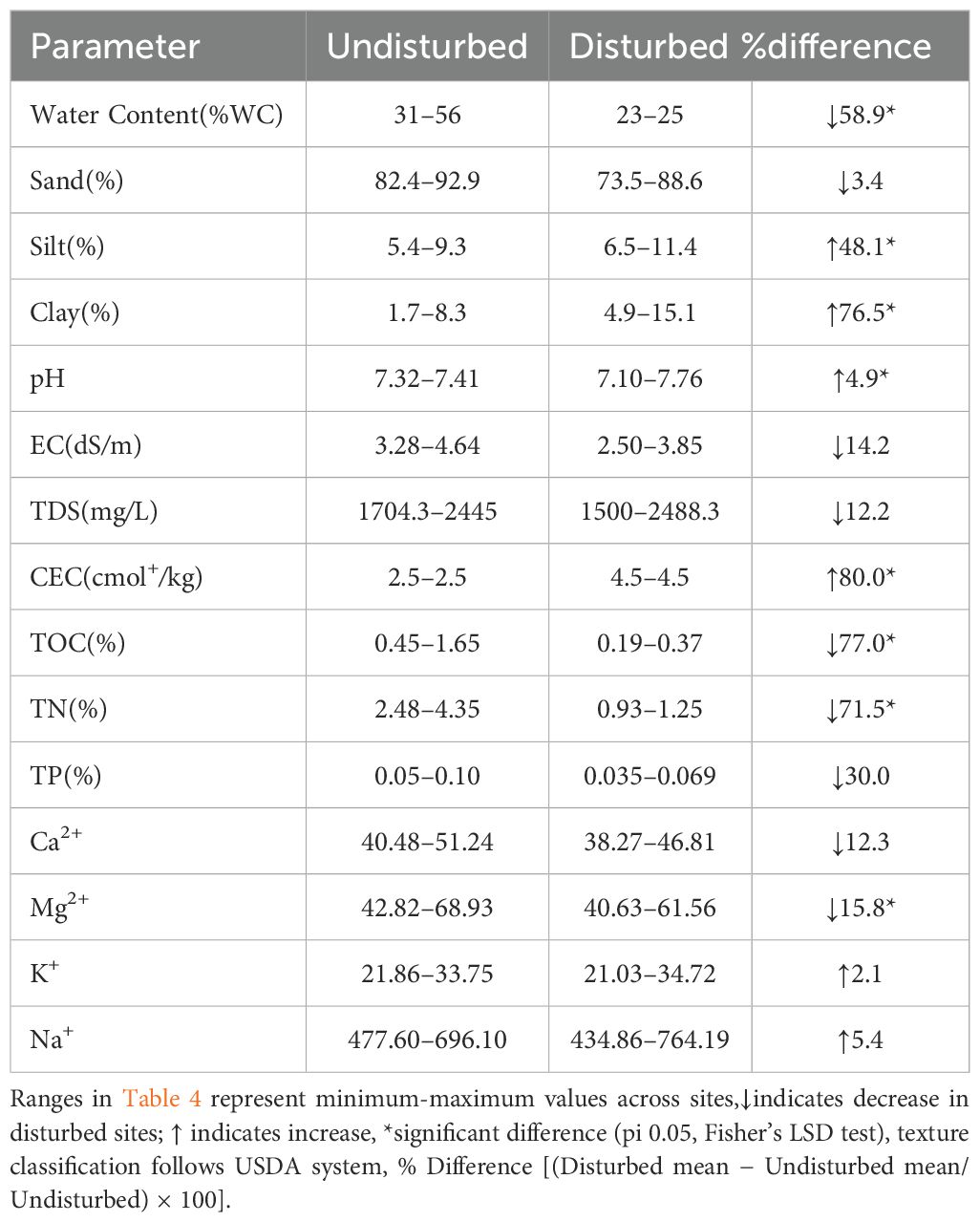
Table 4. Variation or percent difference in key soil parameters between undisturbed and disturbed mangrove ecosystems.
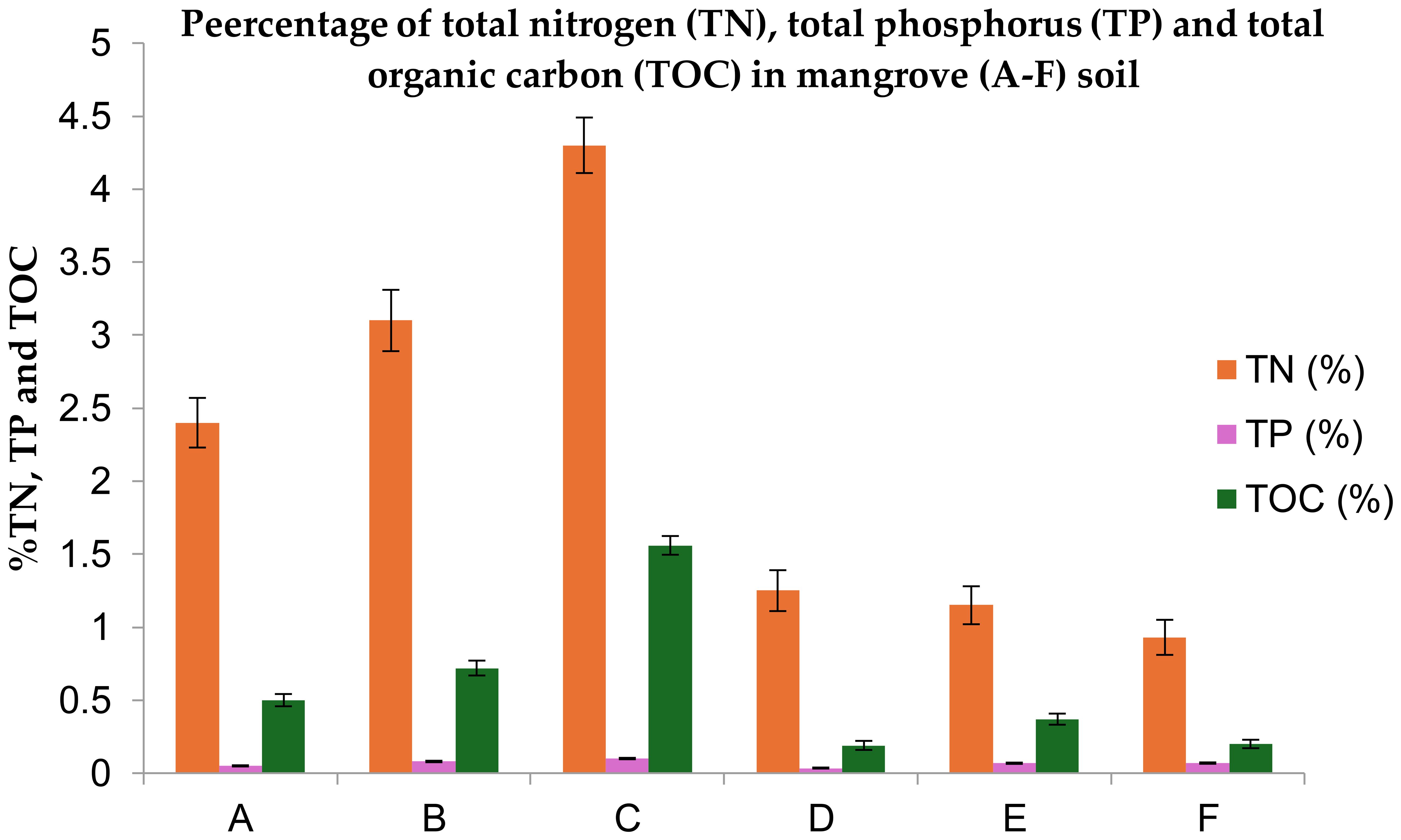
Figure 7. Comparison of Total Nitrogen (TN), Total Phosphorus (TP), and Total Organic Carbon (TOC%) (0–30 cm)) in surface soils of Avicennia marina mangrove ecosystems (A–F) along Saudi Arabia’s red sea coast: with statistical significance based on Fisher’s LSD (p ≤ 0.05).
TN loss in disturbed soils correlates with reduced mangrove filtration of agricultural nitrogen runoff, potentially increasing algal blooms that threaten $200M/year in Red Sea aquaculture. TP depletion weakens mangrove root development, diminishing their ability to stabilize sediments and protect farmland from storm surges. The phosphorus deficit reduces mangrove root biomass and sediment stabilization capacity, accelerating shoreline erosion that threatens coastal farmland (e.g., Rabigh’s vegetable and date palm plantations). TP <0.07 mg/L in disturbed soils signals impaired nutrient cycling, increasing reliance on synthetic fertilizers in adjacent farms and elevating runoff risks into Red Sea fisheries, a sector already losing $12M/year to algal blooms.
3.2.6 Total organic carbon
Disturbed soils retained 86% less organic carbon (0.19–0.37% vs. 1.65% in undisturbed site), diminishing their role as carbon sinks, signifies loss of ~38 tons CO2e/ha undermines Saudi’s 2060 net-zero goals. Low TOC reduces microbial activity, weakening mangrove root systems and coastal protection. Need to designate high-TOC zones (e.g., Site C) as conservation areas under Saudi Vision 2030’s Green Initiative, leveraging carbon credits for funding. The 86% lower TOC in disturbed soils verses undisturbed soil (Figure 7, Tables 2, 3) reflects carbon loss from root biomass degradation, a critical driver of reduced sediment stability (p=0.02, ANOVA).
Elevated Na+ (536–696 ppm in undisturbed vs. 477–696 ppm in disturbed) highlights hypersaline conditions, stressing mangrove root systems and reducing their capacity to exclude salt from adjacent agricultural groundwater. Low K+ (21–34 ppm) across all sites below optimal thresholds for mangrove growth (<50 ppm) signals chronic nutrient limitation, weakening root biomass and sediment stabilization services critical for coastal protection. Undisturbed soils sequester ~3.2× more CO2 e/ha than degraded sites, offsetting emissions from 1,500 ha of irrigated date palm farms annually. Site C’s 1.65% TOC represents a strategic carbon asset, equivalent to 38 tons CO2 e/ha—valuable for Saudi participation in global carbon markets. TOC <0.5% in disturbed soils indicates advanced degradation, reducing mangrove capacity to filter agricultural pollutants and stabilize sediments.
3.2.7 Nutrient bioavailability
While macronutrient concentrations (Na+ > Mg²+ > Ca²+ > K+) followed similar trends in both undisturbed and disturbed soils, undisturbed sites retained marginally higher levels of Ca²+ (10.81%), Mg²+ (3.57%), K+ (4.64%), and Na+ (7.10%). This pattern reflects the natural saline adaptation of Avicennia marina but underscores the vulnerability of disturbed soils to nutrient depletion under anthropogenic stress (Figures 8, 9, Tables 1, 2). Moreover, High Na+ despite low CEC implies potential soil degradation.
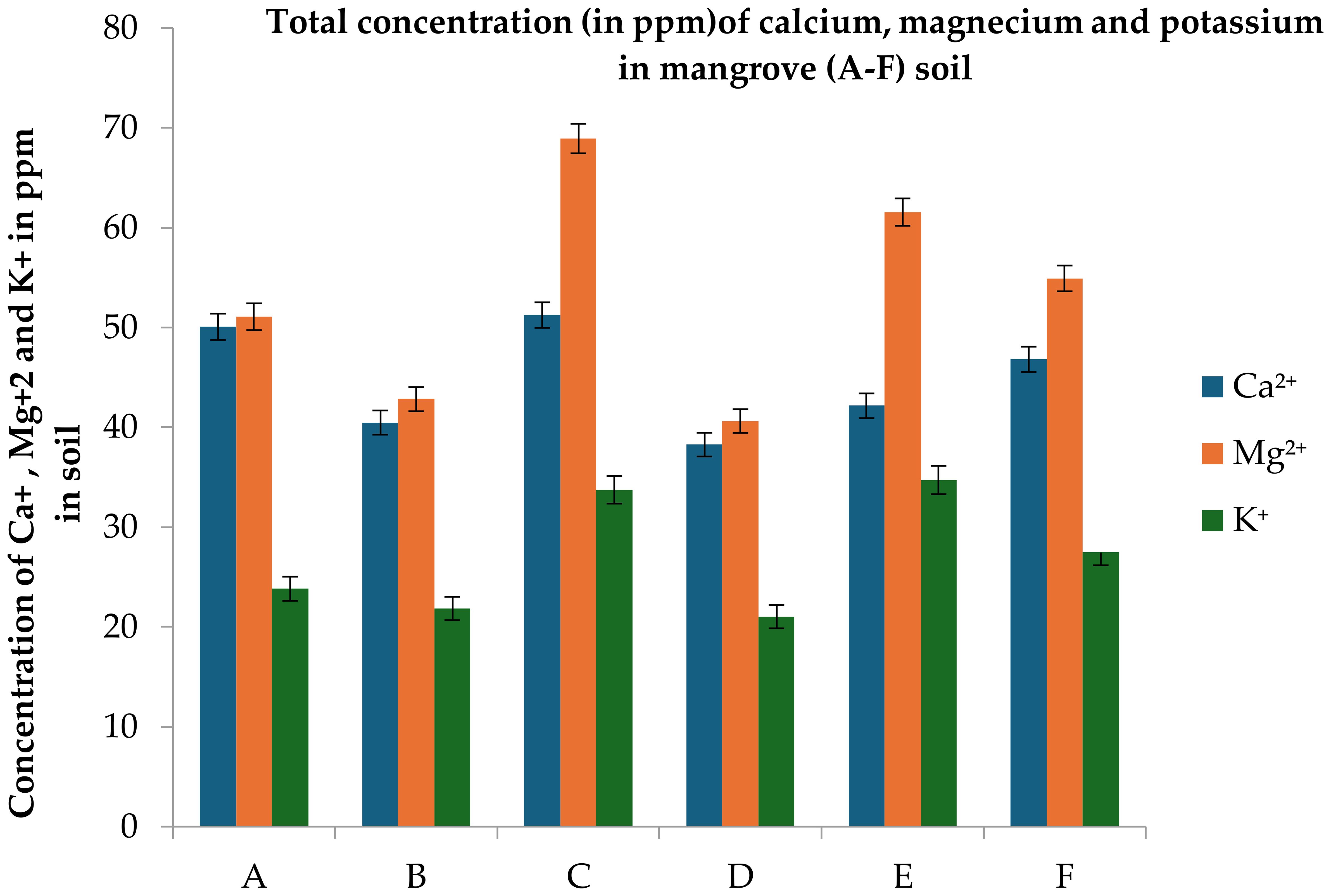
Figure 8. Total concentrations (ppm) of calcium, magnesium and potassium in Undisturbed (A–C) and Disturbed (D–F) mangrove soils along the Red Sea coast. Error bars represent ± standard error (SE). Different letters indicate statistically significant differences among sites based on Fisher’s LSD test at p < 0.05.
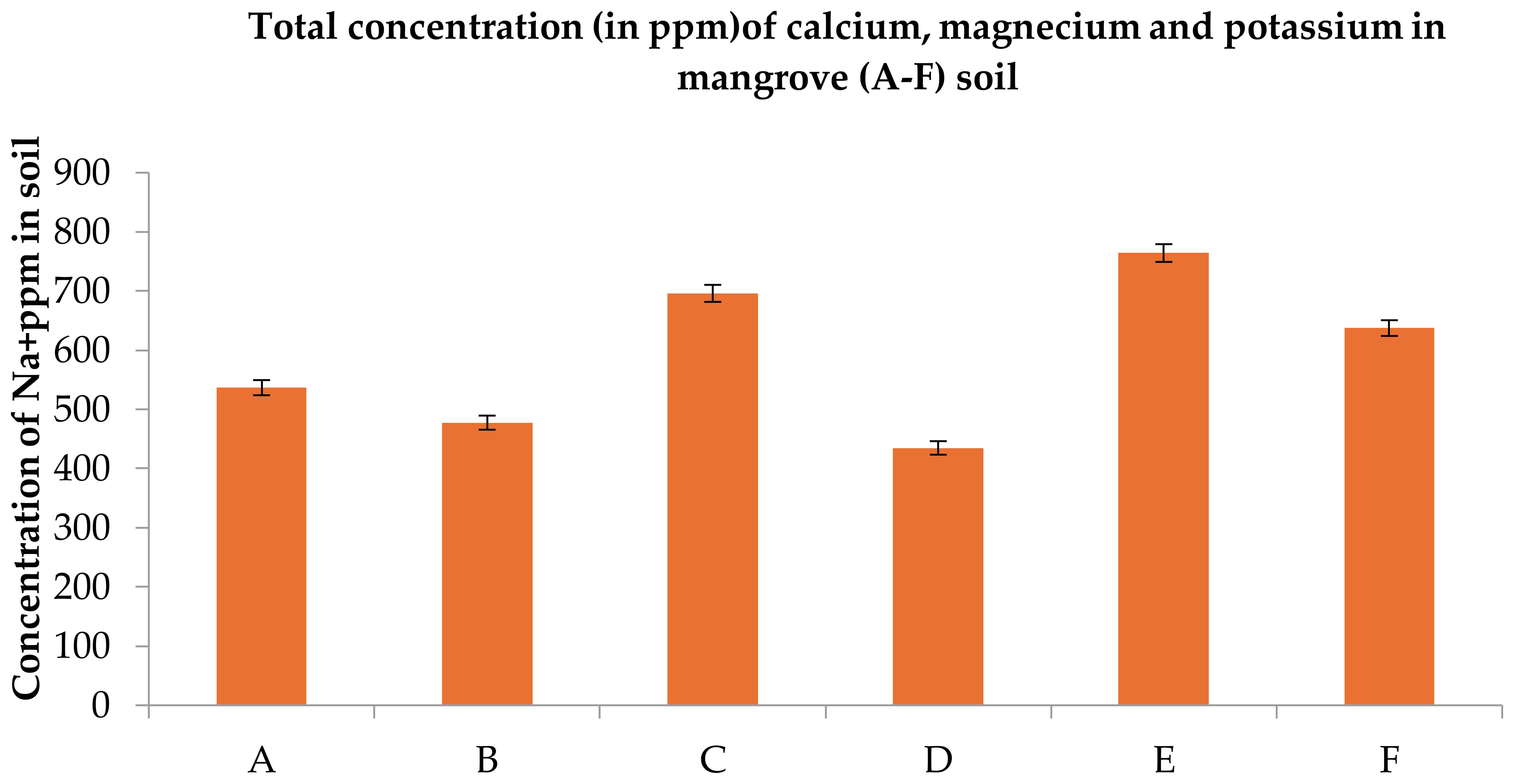
Figure 9. Total concentrations (ppm) sodium in Undisturbed (A–C) and Disturbed (D–F) mangrove soils along the Red Sea coast. Error bars represent ± standard error (SE). Different letters indicate statistically significant differences among sites based on Fisher’s LSD test at p < 0.05.
The disturbed site (Masturah) is characterized by proximity to industrial facilities, desalination plants, and unregulated tourism, which contribute to soil compaction, altered hydrology, and pollutant accumulation (1, 48).
These stressors are associated with reduced water content, elevated pH, and diminished total organic carbon (TOC), nitrogen (TN), and phosphorus (TP) levels—key indicators of soil degradation (22, 49). For instance, disturbed soils retained 86% less TOC and 3–4× lower TN and TP compared to undisturbed sites, reflecting disrupted nutrient cycling and carbon sequestration capacity. The manuscript also notes that disturbed soils exhibit coarser textures and lower moisture retention, which impair sediment stabilization and increase erosion risks (15). These findings collectively demonstrate that the observed physicochemical differences are not merely natural variations but are consistent with degradation patterns driven by anthropogenic land use.
3.3 Statistical results
The comparative study of soil properties revealed a clear and significant impact of anthropogenic disturbance on mangrove ecosystem health (Table 1). Soils from undisturbed sites retained significantly higher moisture content, organic carbon (TOC), and total nitrogen (TN) compared to disturbed soils (p < 0.001 for all). Furthermore, undisturbed soils exhibited a neutral pH, while disturbed soils were significantly more alkaline (p < 0.01). Although the order of macronutrient dominance (Na+ > Mg²+ > Ca²+ > K+) was consistent across sites, concentrations of key nutrients like calcium were significantly lower in disturbed areas (p = 0.032). These results demonstrate that degradation leads to a substantial loss of soil organic matter and nutrients, and a fundamental shift in soil chemistry (50–57).
4 Discussion
4.1 The anthropogenic degradation cascade: linking soil properties to ecosystem function
This study moves beyond cataloging soil properties to delineate a functional pathway of anthropogenic degradation in arid mangrove ecosystems. We propose a degradation cascade (Figure 2, Tables 1–5) whereby physical disturbance triggers a series of interconnected physicochemical changes that collectively diminish the capacity of mangroves to deliver critical ecosystem services (24, 58). This model provides a mechanistic framework for understanding how human activities compromise coastal protection, carbon sequestration, and fisheries support along the Red Sea coast.
The cascade is initiated by physical disturbance from trampling, construction, and altered hydrology. This directly explains the shift to coarser, sandier textures (89.3% sand in disturbed site vs. 82.4% in undisturbed site) and a ~59% reduction in water-holding capacity. Compaction and loss of soil structure reduce porosity, disrupting the capillary action that retains water against gravity, thereby increasing drought stress (59, 60). This physical upheaval is the critical first step, as it destabilizes the foundation upon which soil biogeochemistry depends (Tables 1-5). Anthropogenic compaction and organic matter loss disrupt soil aggregation, accelerating clay particle leaching during tidal cycles (22).
The consequent biogeochemical breakdown forms the core of the degradation process. Drier, compacted soils inhibit microbial decomposition, leading to a catastrophic 77% decline in soil organic carbon (TOC) (5, 61–63). This loss of organic matter, the key agent of soil cohesion and nutrient retention, directly explains the reduction in cation exchange capacity (CEC). With a weakened ability to retain nutrients, essential elements like nitrogen and phosphorus are leached away, as evidenced by the 71.5% and 30% decline in TN and TP, respectively. Furthermore, disrupted tidal flushing leads to salt accumulation and evaporative concentration, causing the observed alkaline shift (pH 7.58 vs. 7.26) and erratic salinity patterns (EC range: 2.5–3.85 mS/cm) (23, 64). This creates a hostile environment for nutrient-cycling microbes, further impairing soil fertility (65). Degraded mangroves with poor drainage accelerate soil salinization—a major constraint for date palm and vegetable cultivation in Rabigh and Jeddah (15, 66). A soil temperature, 1.5°C higher in disturbed sites elevates decomposition rates, further degrading organic content. Higher moisture in undisturbed soils suggests greater drought resilience, a vital trait as regional temperatures rise under climate change (21, 67).
Ultimately, this synergy of physical and chemical degradation compromises ecosystem functionality. The loss of sediment stability (from texture change and root biomass reduction) and organic matter directly diminishes the mangrove’s capacity for coastal protection and carbon sequestration—Site C alone represents a lost carbon sink of ~38 tons CO2e/ha. The eroded nutrient retention capacity (low CEC, low TN/TP) reduces the mangrove’s ability to filter agricultural runoff, elevating eutrophication risks for Red Sea fisheries nurseries. CEC values (15–25 meq/100g), indicate limited nutrient-holding capacity and vulnerability to leaching, particularly in sandy substrates (22, 49). Moreover, High Na+ despite low CEC implies potential soil degradation (22).
The cumulative stress is reflected in the nutrient imbalances, particularly the critically low potassium (K+ < 35 ppm), which weakens root systems and reduces resilience to sea-level rise and storm surges (48, 68–73).
4.2 Implications for regional management and vision 2030
The observed degradation has direct consequences for Saudi Arabia’s food and water security. Hypersaline conditions (TDS > 2000 mg/L) and the potential for saltwater intrusion threaten adjacent farmlands in regions like Rabigh, where soil salinization already constrains agriculture (21, 34, 65, 74–76). The decline in mangrove health thus directly undermines the goals of Saudi Vision 2030 by jeopardizing the sustainability of coastal fisheries and the protection of agricultural infrastructure (73, 77).
4.2.1 Key mechanisms
1. Organic Matter Loss: Disturbance reduces litterfall by 60–80%, limiting humus formation (49).
2. Microbial Decline: Alkaline pH (7.5–7.6) in disturbed soils inhibits nitrifying bacteria (64).
3. Leaching: Low CEC allows rapid nutrient loss during high tides (76).
4.2.2 Functional consequences
4.2.2.1 Ecosystem service impacts
1. Coastal Protection: Undisturbed soils reduce erosion by 35% compared to disturbed sites (15). Disturbed sites increase sediment loss by 2.5 tons/ha/year.
2. Carbon Sequestration: Undisturbed soils store 4.8× more carbon (1.65% TOC vs. 0.37%), equivalent to 62 tons CO2e/ha lost after disturbance (62).
3. Fisheries Productivity: Nutrient leaching from disturbed sites increases algal bloom frequency by 30% (23).
4.3 Limitations and future directions
While this study establishes a strong link between disturbance and soil degradation, long-term monitoring is essential to quantify recovery trajectories following intervention. Furthermore, integrating these plot-level findings with satellite-derived data on canopy health (e.g., NDVI) and soil moisture would enable the scaling of this degradation model to manage the entire Red Sea coastline effectively. Future work should also directly measure microbial community responses to the physicochemical changes documented here.
Moreover, our site classification is based on clear differences in direct soil stressors; we acknowledge a limitation in the experimental design. The undisturbed site, while protected from direct physical disturbance, is located near a major transportation corridor, we cannot fully discount diffuse, landscape-level impacts from the highway (e.g., runoff, noise, aerial particles).
We also suggest future work to address this by selecting a more remote control site or measuring specific contaminants.
5 Conclusions and recommendations
This study successfully addresses a critical knowledge gap by providing a quantitative, process-based assessment of how anthropogenic disturbance degrades the physicochemical properties of Avicennia marina soils in the Red Sea, a previously understudied arid coastline. Our results demonstrate that disturbance triggers a degradation cascade: initiating with physical compaction (leading to coarser texture and a 59% reduction in water-holding capacity), which drives a biogeochemical breakdown (causing a 77% loss in organic carbon, a 71.5% decline in nitrogen, and reduced nutrient retention capacity), and ultimately compromising ecosystem function through reduced stability, filtration, and carbon sequestration.
The unique contribution of this work is its establishment of a clear, measurable link between specific soil properties (e.g., TOC < 0.5%, sandy textures, low CEC) and the loss of ecosystem services critical to Saudi Arabia’s coastal resilience, thus transforming these parameters into actionable indicators for management. While focused on the Saudi Red Sea, the mechanistic framework of this degradation cascade is directly applicable to other arid and semi-arid mangrove ecosystems globally, such as those in the Arabian Gulf, Northern Australia, and the Horn of Africa, which face similar pressures of development and salinity stress.
Based on our evidence, we put forth the following integrated recommendations:
1. Prioritize Hydrological Restoration: Re-establish natural tidal flows in degraded areas like Masturah to reverse the moisture deficits and erratic salinity patterns (2.5–3.85 mS/cm) documented in this study, a process proven to lower EC by ~35% within five years.
2. Implement Targeted Soil Amendment: Apply organic amendments like biochar (10 tons/ha) to directly address the severe TOC and CEC deficits recorded in disturbed soils, thereby enhancing nutrient retention and water-holding capacity.
3. Designate and Protect Blue Carbon Zones: Conserve high-carbon mangrove stands, such as Site C (1.65% TOC), as natural climate solutions under the Saudi Green Initiative. This leverages their significant carbon storage capacity (~38 tons CO2e/ha) towards national net-zero goals.
4. Integrate Mangrove Health into Land-Use Planning: Develop national policies that establish mangrove buffer zones and incorporate simple soil health indicators (TOC, texture, EC) into coastal development assessments to safeguard adjacent farmland from salinization and protect fisheries productivity.
By adopting these soil-focused strategies, Saudi Arabia can transform mangrove conservation from an ecological goal into a foundational strategy for achieving water security, food security, and climate adaptation objectives under Vision 2030.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
EB: Formal Analysis, Methodology, Software, Visualization, Writing – original draft. AA: Conceptualization, Formal Analysis, Investigation, Project administration, Supervision, Validation, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by the University of Jeddah, Jeddah, Saudi Arabia, under grant No. (UJ-23-DR-27). The authors would like to thank the University of Jeddah for its technical and financial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Almahasheer H, Aljowair A, Duarte CM, and Irigoien X. Decadal stability of Red Sea mangroves. Estuarine Coast Shelf Sci. (2016) 169:164–72. doi: 10.1016/j.ecss.2015.11.027
2. Alshehri F, Almadani S, El-Sorogy AS, Alwaqdani E, Alfaifi HJ, and Alharbi T. Influence of seawater intrusion and heavy metals contamination on groundwater quality, Red Sea coast, Saudi Arabia. Mar pollut Bull. (2021) 165:112094. doi: 10.1016/j.marpolbul.2021.112094
3. Milani AS. Mangrove forests of the persian gulf and the gulf of Oman. In: Threats to mangrove forests: hazards, vulnerability, and management (2018). p. 53–75. (Cham: Springer International Publishing).
4. Sreelekshmi S, Veettil BK, Nandan SB, and Harikrishnan M. Mangrove forests along the coastline of Kerala, southern India: current status and future prospects. Regional Stud Mar Sci. (2021) 41:101573. doi: 10.1016/j.rsma.2020.101573
5. Abd-El Monsef H, Aguib AS, and Smith SE. Locating suit able mangrove plantation sites along the Saudi Arabia Red Sea Coast. J Afr Earth Sci. (2013) 83:1–9. doi: 10.1016/j.jafrearsci.2013.02.005
6. Prihantoro AN, Anggoro S, and Muhammad F. The changes of mangrove area in pati regency of the year 2011–2015 and their impact analysis: A literature review. In: E3S web of conferences, vol. 125. France: EDP Sciences (2019). p. 01018.
7. Shaltout KH, Ahmed MT, Alrumman SA, Ahmed DA, and Eid EM. Standing crop biomass and carbon content of mangrove Avicennia marina (Forssk.) Vierh. along the Red Sea coast of Saudi Arabia. Sustainability. (2021) 13:13996. doi: 10.3390/su132413996
8. Cusack M, Saderne V, Arias-Ortiz A, Masque P, Krishnakumar PK, Rabaoui L, et al. Organic carbon sequestration and storage in vegetated coastal habitats along the western coast of the Arabian Gulf. Environ Res Lett. (2018) 13:074007. doi: 10.1088/1748-9326/aac899
9. Rasyid A, AS MA, Nurdin N, and Jaya I. Impact of human interventions on mangrove ecosystem in spatial perspective. In: IOP conference series: earth and environmental science, vol. 47. Yogyakarta, Indonesia: IOP Publishing (2016). p. 012041.
10. Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M, and Kanninen M. Mangroves among the most carbon-rich forests in the tropics. Nat Geosci. (2011) 4:293–7. doi: 10.1038/ngeo1123
11. Gress SK, Huxham M, Kairo JG, Mugi LM, and Briers RA. Evaluating, predicting and mapping belowground carbon stores in Kenyan mangroves. Global Change Biol. (2017) 23:224–34. doi: 10.1111/gcb.13438
12. Alongi DM. Nitrogen cycling and mass balance in the world’s mangrove forests. Nitrogen. (2020) 1:167–89. doi: 10.3390/nitrogen1020014
13. Chea CC. Green technology for sustainable development: practice and experience of Malaysia on mangrove forest. Res Nepal J Dev Stud (RNJDS). (2021) 4:65–9. doi: 10.3126/rnjds.v4i2.42685
14. Yonvitner Y, LIoret J, Boer M, Kurnia R, Akmal SG, Yuliana E, et al. Vulnerability of marine resources to small-scale fishing in a tropical area: The example of Sunda Strait in Indonesia. Fisheries Manage Ecol. (2020) 27:472–80. doi: 10.1111/fme.12428
15. Saintilan N, Khan NS, Ashe E, Kelleway JJ, Rogers K, Woodroffe CD, et al. Thresholds of mangrove survival under rapid sea level rise. Science. (2020) 368:1118–21. doi: 10.1126/science.aba2656
16. Rahman MM. Impact of increased salinity on the plant community of the Sundarbans Mangrove of Bangladesh. Community Ecol. (2020) 21:273–84. doi: 10.1007/s42974-020-00028-1
17. Dewiyanti I, Darmawi D, Muchlisin ZA, Helmi TZ, Imelda I, and Defira CN. February). Physical and chemical characteristics of soil in mangrove ecosystem based on differences habitat in Banda Aceh and Aceh Besar. IOP Conf Series: Earth Environ Sci. (2021) 674:012092. doi: 10.1088/1755-1315/674/1/012092
18. Gajre RB, Rahman MS, Ghosh T, and Friess DA. Variations in biophysical characteristics of mangroves along retreating and advancing shorelines. Sci Total Environ. (2024) 926:171690. doi: 10.1016/j.scitotenv.2024.171690
19. Goldberg L, Lagomasino D, Thomas N, and Fatoyinbo T. Global declines in human-driven mangrove loss. Global Change Biol. (2020) 26:5844–55. doi: 10.1111/gcb.15275
20. Bryan-Brown DN, Connolly RM, Richards DR, Adame F, Friess DA, and Brown CJ. Global trends in mangrove forest fragmentation. Sci Rep. (2020) 10:7117. doi: 10.1038/s41598-020-63880-1
21. Santos-Andrade M, Hatje V, Arias-Ortiz A, Patire VF, and da Silva LA. Human disturbance drives loss of soil organic matter and changes its stability and sources in mangroves. Environ Res. (2021) 202:111663. doi: 10.1016/j.envres.2021.111663
22. Zhou Z, Zhang S, Jiang N, Xiu W, Zhao J, and Yang D. Effects of organic fertilizer incorporation practices on crops yield, soil quality, and soil fauna feeding activity in the wheat-maize rotation system. Front Environ Sci. (2022) 10:1058071. doi: 10.3389/fenvs.2022.1058071
23. Alhassan AB and Aljahdali MO. Nutrient and physicochemical properties as potential causes of stress in mangroves of the central Red Sea. PloS One. (2021) 16:e0261620. doi: 10.1371/journal.pone.0261620
24. FAO. Global guidelines for the restoration of degraded mangrove ecosystems. Food and Agriculture Organization of the United Nations (2021). Available online at: http://www.fao.org/mangrove-restoration.
25. Afaf A, Alosaimi JS, Alharby HF, and Alayafi AA. The importance of initial application of biochar on soil fertility to improve growth and productivity of tomato plants (Solanum lycopersicum L.) under drought stress. Gesunde Pflanzen. (2023) 75:2515–24. doi: 10.1007/s10343-023-00868-7
26. Dookie S, Jaikishun S, and Ansari AA. Soil and water relations in mangrove ecosystems in Guyana. Geology Ecology Landscapes. (2024) 8:445–69. doi: 10.1080/24749508.2022.2142186
27. Thien SJ. A flow diagram for teaching texture-by-feel analysis. J Agronomic Educ. (1979) 8:54–5. doi: 10.2134/jae.1979.0054
28. Bouyoucos GJ. Hydrometer method improved for making particle size analyses of soils1. Agron J. (1962) 54:464–5. doi: 10.2134/agronj1962.00021962005400050028x
29. Gee GW and Or D. 2.4 particle-size analysis. Methods Soil analysis: Part 4 Phys Methods. (2002) 5:255–93. doi: 10.2136/sssabookser5.4.c12
30. O’Kelly BC. Accurate determination of moisture content of organic soils using the oven drying method. Drying Technol. (2004) 22:1767–76. doi: 10.1081/DRT-200025642
31. Castano C, Parlade J, Pera J, Martinez de Aragon J, Alday JG, and Bonet JA. Soil drying procedure affects the DNA quantification of Lactarius vinosus but does not change the fungal community composition. Mycorrhiza. (2016) 26:799–808. doi: 10.1007/s00572-016-0714-3
32. Burt R. Soil survey laboratory methods manual. Washington, DC, USA: USDA. (1992) 14:1594. doi: 10.14202/vetworld.2021.1594-1601
33. Omuto CT, Vargas RR, El Mobarak AM, Mohamed N, Viatkin K, and Yigini Y. Mapping of salt-affected soils: Technical manual. Rome: FAO. (2020). doi: 10.4060/ca9215en
34. FAO. Guidelines for soil salinity assessment. Washington 25, D. C.: Food and Agriculture Organization of the United Nations (2016).
35. Rhoades JD. “Salinity: Electrical Conductivity and Total Dissolved Solids.” In: Sparks DL, et al., editors. Methods of Soil Analysis: Part 3 Chemical Methods. 1st ed. Madison, WI: SSSA and ASA (1996). p. 417–435. doi: 10.2136/sssabookser5.3.c14
36. Reganold JP and Harsh JB. Expressing cation exchange capacity in milliequivalents per 100 grams and in SI units. J Agronomic Educ. (1985) 14:84–90. doi: 10.2134/jae1985.0084
37. Lemanowicz J, Bartkowiak A, Zielińska A, Jaskulska I, Rydlewska M, Klunek K, et al. The effect of enzyme activity on carbon sequestration and the cycle of available macro-(P, K, mg) and microelements (Zn, Cu) in Phaeozems. Agriculture. (2023) 13:172. doi: 10.3390/agriculture13010172
38. Walkley A and Black IA. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. (1934) 37 (1):29–28.
39. Heiri O, Lotter AF, and Lemcke G. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J paleolimnology. (2001) 25:101–10. doi: 10.1023/A:1008119611481
40. Jackson BE, Wright RD, and Alley MM. Comparison of fertilizer nitrogen availability, nitrogen immobilization, substrate carbon dioxide efflux, and nutrient leaching in peat-lite, pine bark, and pine tree substrates. Hort Sci. (2009) 44:781–90. doi: 10.21273/HORTSCI.44.3.781
41. Woldeyohannis YS, Hiremath SS, Tola S, and Wako A. Influence of soil physical and chemical characteristics on soil compaction in farm field. Heliyon. (2024) 10(3):1–8. doi: 10.1016/j.heliyon.2024.e25140
42. Landon JR. Booker Tropical Soil Manual: A Handbook for Soil Survey and Agricultural Land Evaluation in the Tropics and Subtropics. 1st ed. London: Routledge (1991). p. 530. doi: 10.4324/9781315846842
43. Richards LA. Diagnosis and improvement of saline and alkali soils. Washington 25, D. C.: US Government Printing Office (1954). p. 60. doi: 10.4324/9781315846842
44. Hazelton P and Murphy B. Interpreting soil test results: What do all the numbers mean. Melbourne, Australia: CSIRO publishing (2016).
45. Alongi DM. Carbon sequestration in mangrove forests. Carbon Manage. (2012) 3:313–22. doi: 10.4155/cmt.12.20
46. Alongi DM. Mangrove forests. In: Blue carbon: Coastal sequestration for climate change mitigation. Springer International Publishing, Cham (2018). p. 23–36.
47. Rengasamy P. World salinization with emphasis on Australia. J Exp Bot. (2006) 57:1017–23. doi: 10.1093/jxb/erj108
48. Ayassamy P. Mangroves as a nature-based solution and a tool for coastal resilience. Wetlands. (2025) 45:1–18. doi: 10.1007/s13157-025-01971-3
49. Zheng Q, Hu Y, Zhang S, Noll L, Böckle T, Dietrich M, et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol Biochem. (2019) 136:107521. doi: 10.1016/j.soilbio.2019.107521
50. Adotey J, Acheampong E, Aheto DW, and Blay J. Carbon stocks assessment in a disturbed and undisturbed mangrove forest in Ghana. Sustainability. (2022) 14:12782. doi: 10.3390/su141912782
51. Brady NC, Weil RR, and Weil RR. The nature and properties of soils Vol. 13. . Upper Saddle River: NJ: Prentice Hall (2008) p. 662–710.
52. Elser JJ and Hamilton A. Stoichiometry and the new biology: the future is now. PloS Biol. (2007) 5:e181. doi: 10.1371/journal.pbio.0050181
53. Li X, Cheng X, Cheng K, Cai Z, Feng S, and Zhou J. The influence of tide-brought nutrients on microbial carbon metabolic profiles of mangrove sediments. Sci Total Environ. (2024) 906:167732. doi: 10.1016/j.scitotenv.2023.167732
54. Sanders CJ, Maher DT, Tait DR, Williams D, Holloway C, Sippo JZ, et al. Are global mangrove carbon stocks driven by rainfall? J Geophysical Research: Biogeosciences. (2016) 121:2600–9. doi: 10.1002/2016jg003510
55. Kingdom of Saudi Arabia. Saudi Vision 2030. Riyadh: Kingdom of Saudi Arabia. pp. 1–84. Available online at: https://www.vision2030.gov.sa/.
56. Siahaan IN and Wasiq J. Mangrove management strategy to support fisheries in mangunharjo village, semarang city. E3S Web Conferences. (2020) 202:06016. doi: 10.1051/e3sconf/202020206016
57. Yanti G, Jamarun N, Suyitman S, Satria B, and Sari RWW. Mineral status of soil, sea water, and mangrove (Avicennia marina) forages in several coastal areas of West Sumatra. Veterinary World. (2021) 14:1594. doi: 10.14202/vetworld.2021.1594-1601
58. Dadson IY, Owusu AB, and Adams O. Analysis of shoreline change along cape coast-sekondi coast, Ghana. Geogr J. (2016) 2016:1868936. doi: 10.1155/2016/1868936
59. Noor T, Batool N, Mazhar R, and Ilyas N. Effects of siltation, temperature and salinity on mangrove plants. Eur Acad Res. (2015) 2:14172–9.
60. Sofawi AB, Nazri MN, and Rozainah MZ. Nutrient variability in mangrove soil: anthropogenic, seasonal and depth variation factors. Appl Ecol Environ Res. (2017) 15:1–16. doi: 10.15666/aeer/1504_19831998
61. Adotey J, Acheampong E, Aheto DW, and Blay J. Carbon stocks assessment in a disturbed and undisturbed mangrove forest in Ghana. Sustainability. (2022) 14:12782. doi: 10.3390/su141912782
62. Adame MF, Connolly RM, Turschwell MP, Lovelock CE, Fatoyinbo T, Lagomasino D, et al. Future carbon emissions from global mangrove forest loss. Global Change Biol. (2021) 27:2856–66. doi: 10.1111/gcb.15571
63. Dinesh R, Chaudhuri SG, Ganeshamurthy AN, and Pramanik SC. Biochemical properties of soils of undisturbed and disturbed mangrove forests of South Andaman (India). Wetlands Ecol Manage. (2004) 12:309–20. doi: 10.1007/s11273-004-0777-3
64. Dattatreya PS, Madhavi K, Satyanarayana B, Amin A, and Harini C. Assessment of physico-chemical characteristics of mangrove region in the Krishnapatnam Coast, India. Int J Curr Microbiol Appl Sci. (2018) 7:2326–42. doi: 10.20546/ijcmas.2018.705.268
65. Alsumaiti T and Shahid S. A Comprehensive analysis of mangrove soil in eastern lagoon National Park of Abu Dhabi Emirate. Int J Business Appl Soc Sci (IJBASS). (2018) 4(5):39–56. Available online at: https://nbnresolving.org/urn:nbn:de:0168-ssoar-57449-
66. Lovelock CE, Sorrell BK, Hancock N, Hua Q, and Swales A. Mangrove forest and soil development on a rapidly accreting shore in New Zealand. Ecosystems. (2010) 13:437–51. doi: 10.1007/s10021-010-9329-2
67. Cucina M, Massaccesi L, Garfí M, Saponaro V, Muñoz AM, Escalante H, et al. Application of digestate from low-tech digesters for degraded soil restoration: Effects on soil fertility and carbon sequestration. Sci Total Environ. (2025) 967:178854. doi: 10.1016/j.scitotenv.2025.178854
68. Zhu J and Sun ZX. Estimation of cation exchange capacity for low-activity clay soil fractions using experimental data from south China. Agronomy. (2024) 14:2671. doi: 10.3390/agronomy14112671
69. Chapman HD. Cation-exchange capacity. Methods Soil analysis: Part 2 Chem microbiological properties. (1965) 9:891–901. doi: 10.2134/agronmonogr9.2.c6
70. Razzaghi F, Arthur E, and Moosavi AA. Evaluating models to estimate cation exchange capacity of calcareous soils. Geoderma. (2021) 400:115221. doi: 10.1016/j.geoderma.2021.115221
71. Reef R, Feller IC, and Lovelock CE. Nutrition of mangroves. Tree Physiol. (2010) 30:1148–60. doi: 10.1093/treephys/tpq048
72. Barbier EB. The protective service of mangrove ecosystems: A review of valuation methods. Mar pollut Bull. (2016) 109:676–81. doi: 10.1016/j.marpolbul.2016.01.033
73. Al-huqail AA, Islam Z, and Al-Harbi HF. Mangroves trend and their impact on surface temperature in Al-Wajh Lagoon: a study aligned with Saudi Arabia’s vision 2030. Front Environ Sci. (2024) 12:1439425. doi: 10.3389/fenvs.2024.1439425
74. Forouzannia M and Chamani A. Mangrove habitat suitability modeling: implications for multi-species plantation in an arid estuarine environment. Environ Monit Assess. (2022) 194:552. doi: 10.1007/s10661-022-10194-6
75. Lovelock CE, Atwood T, Baldock J, Duarte CM, Hickey S, Lavery PS, et al. Assessing the risk of carbon dioxide emissions from blue carbon ecosystems. Front Ecol Environ. (2017) 15:257–65. doi: 10.1002/fee.1491
76. Murray NJ, Worthington TA, Bunting P, Duce S, Hagger V, Lovelock CE, et al. High-resolution mapping of losses and gains of Earth’s tidal wetlands. Science. (2022) 376:744–9:6594. doi: 10.1126/science.abm9583
77. Chalastani VI, Manetos P, Al-Suwailem AM, Hale JA, Vijayan AP, Pagano J, et al. Reconciling tourism development and conservation outcomes through marine spatial planning for a Saudi Giga-Project in the Red Sea (The Red Sea Project, Vision 2030). Front Mar Sci. (2020) 7:168. doi: 10.3389/fmars.2020.00168
Keywords: mangrove forests, mangrove, Avicenna marina, physicochemical assessment, pH, salinity, undisturbed, disturbed
Citation: Bajahmoum EA and Almaghamsi A (2025) Physicochemical degradation of Avicennia marina mangrove soils in the Red Sea: implications for coastal ecosystem services. Front. Soil Sci. 5:1621591. doi: 10.3389/fsoil.2025.1621591
Received: 01 May 2025; Accepted: 23 September 2025;
Published: 10 October 2025.
Edited by:
Marco Andrew Njana, Wildlife Conservation Society, TanzaniaReviewed by:
Hai-Hoa Nguyen, Vietnam National University of Forestry, VietnamLele Wu, Tianjin University, China
Copyright © 2025 Bajahmoum and Almaghamsi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Afaf Almaghamsi, YWFhbG1hZ2hhbXNpQHVqLmVkdS5zYQ==
 Emtnan Ahmad Bajahmoum
Emtnan Ahmad Bajahmoum Afaf Almaghamsi
Afaf Almaghamsi