- School of Environment and Geography, Qingdao University, Qingdao, China
Electron shuttles play a critical role in mediating reductive dechlorination of tetrachloroethylene (PCE), significantly influencing the biogeochemical cycling of key elements in terrestrial ecosystems (e.g., soil). Despite their significance, the stimulatory effects of electron shuttles on the dechlorination of PCE in situ remediation remained underexplored. This study investigated the efficacy of PCE dechlorination and associated redox cycling of biogenic elements (Fe, S, and N) in three distinct habitats from Qingdao—river sediment (RS), farmland soil (FS), and chloroform-contaminated soil (CS)—using anthraquinone-2,6-disulfonate (AQDS) as an electron shuttle. The results indicated that: AQDS enhanced soil reductive capacity, lowering redox potential below −150 mV to create optimal conditions for dechlorinating microbial communities. The PCE removal efficiency was significantly improved with AQDS, increasing from 87.87% to 95.04% in RS (21 days), from 79.61% to 94.78% in FS (28 days), and from 81.48% to 89.40% in CS (35 days). Heterogeneous biogeochemical responses to PCE stress were observed across habitats. During PCE dechlorination, Fe(III) reduction was enhanced in FS and CS, but suppressed in RS, while reduction was inhibited in RS and FS but unaffected in CS. reduction accompanied PCE dechlorination across all habitats with AQDS significantly stimulating reduction. CH4 production was delayed in FS and CS (0–14 days), whereas RS exhibited the fastest production rates —1172.19 mmol/L-day. AQDS supplementation significantly increased CH4 production rates in RS (from 149.77 to 364.02 mmol/L-day during days 14-28) and CS (from 61.22 to 64.41 mmol/L-day during days 28-35), alongside elevated CO2 production, while FS displayed divergent trends. The regulatory effects of AQDS and PCE on microbial communities across different habitats further demonstrate their associated biogeochemical redox processes and PCE reductive dechlorination status. Microbial community analysis revealed that AQDS and PCE reshaped the microbiome, enriching for key functional genera such as Pelotomaculum_B, Clostridium_AF, and Desulfitobacterium, whose specific roles were habitat-dependent. These findings demonstrate that while electron shuttles can accelerate the bioremediation of chlorinated solvents, their performance and collateral effects on elemental cycling are dictated by the indigenous biogeochemical and microbial characteristics of the target environments. This underscores the critical need for habitat-specific assessments prior to the field application of electron shuttle-amended remediation strategies.
1 Introduction
Tetrachloroethylene (PCE), a chlorinated solvent extensively utilized in dry cleaning, metal degreasing, and other industrial applications, poses significant environmental and human health risks due to its carcinogenicity, teratogenicity, and mutagenicity (1, 2). Owing to its chemical stability, volatility, and persistence in subsurface environments, PCE is designated a priority pollutant in many regulatory frameworks (3, 4). In anaerobic conditions, PCE is primarily degraded through microbial reductive dechlorination, a process wherein chlorine atoms are sequentially replaced with hydrogen atoms, using electrons typically derived from hydrogen or fermentable carbon substrates (5). Although hydrogen serves as the direct electron donor, its low solubility and diffusivity under natural conditions limit its availability to dechlorinating microorganisms. Therefore, fermentable electron donors such as sodium lactate are commonly used to stimulate hydrogen production in situ (6–9). However, the efficiency of reductive dechlorination is often constrained by limited electron transfer rates, especially in soils with heterogeneous geochemical conditions (10). To address this, recent research has focused on strategies to enhance microbial electron transfer, including the use of redox mediators such as electron shuttles (11).
Humic substances (HS), a class of complex organic compounds ubiquitously present in soils, aquatic systems, and sediments, play critical roles in mediating redox reactions. HS can function either as electron shuttles between microorganisms and contaminants (12) or as terminal electron acceptors for anaerobic microbial respiration, thereby facilitating environmental electron transfer processes. Due to the structural complexity and variability of environmental HS, model compounds such as anthraquinone-2,6-disulfonate (AQDS) are widely used to investigate the mechanistic role of quinone-based electron shuttles (13). AQDS has been shown to accelerate dechlorination kinetics by facilitating electron transfer from donors (e.g., hydrogen, lactate) to acceptors (e.g., chlorinated solvents), and to promote the growth and activity of organohalide-respiring bacteria (14–16).
Reductive dechlorination in natural soils rarely occurs in isolation. It is intricately linked to the redox cycling of biogenic elements such as carbon (C), nitrogen (N), sulfur (S), and iron (Fe). Microorganisms capable of PCE dechlorination often coexist and compete with functional guilds involved in iron reduction, nitrate/nitrite reduction, sulfate reduction, methanogenesis, and acetogenesis (17–19). Conversely, active redox processes in habitats utilize H2 or other electron donors, where competition for these donors governs chlorinated hydrocarbon degradation kinetics. Organic carbon, as the principal electron donor source, directly governs activity of dechlorinating microorganisms through its bioavailability (20). Nitrogen and sulfur cycle derivatives may act as competitive electron acceptors, potentially interfering with PCE reduction (21). Iron, a pivotal redox-active element, undergoes valence transitions (Fe(III)/Fe(II)) that not only regulate electron transfer but also influence microbial adhesion and activity via iron mineral formation (22). Notably, nitrate () and nitrite () exhibit pronounced competitive effects on chlorinated hydrocarbon reduction in groundwater systems by diverting electron flow from dechlorination pathways (23–25). Thus, evaluating the interplay between PCE dechlorination and redox-sensitive elemental cycling is critical for optimizing bioremediation strategies that employ electron transfer mediators.
This study employs three kinds of habitats in Qingdao (river sediment, farmland soil, chloroform-contaminated soil) to investigate AQDS-mediated PCE dechlorination. While the principle of electron shuttle-mediated dechlorination is established, a predictive understanding of how this strategy performs across different geochemical landscapes is lacking. The efficacy of electron shuttles and their unintended consequences on coupled biogeochemical cycles have not been systematically compared in diverse, complex environments. To address this research gap, this study investigated AQDS-mediated PCE dechlorination in three distinct habitats: river sediment (RS), farmland soil (FS), and a historically chloroform-contaminated soil (CS). Using sodium lactate as the electron donor/carbon source and AQDS as the electron shuttle, we systematically analyzed: (1) the effects of AQDS addition in different habitats on PCE dechlorination; (2) the coupling mechanisms between PCE dechlorination and biogeochemical element cycling and (3) the interaction mechanisms between microbial communities and biogenous elements. These findings establish a mechanistic basis for implementing electron shuttle-assisted bioremediation at sites impacted by chlorinated solvent contamination.
2 Materials and methods
2.1 Anaerobic incubation
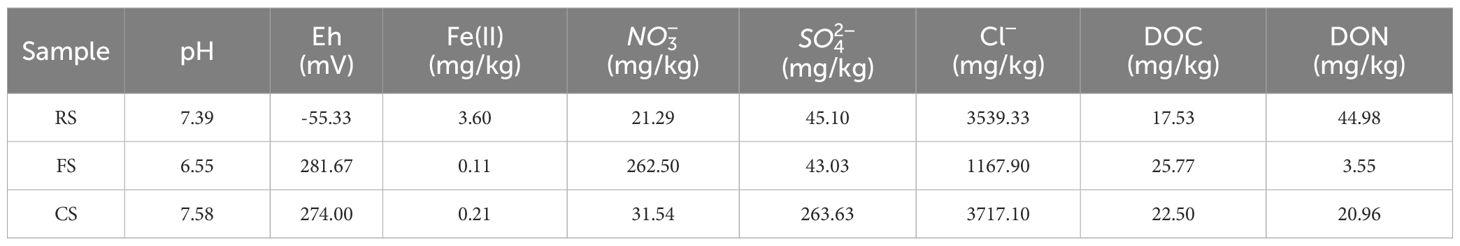
Three different habitat samples were used in this study. The habitat samples were obtained from Qingdao City, Shandong Province, China. The first habitat sample (denoted RS) was obtained at a depth of 0.30 - 0.50m below ground surface (BGS) and is from Licun River. The second habitat sample (denoted FS) was collected in the farmland area in Jimo District. The third soil material (denoted CS) was collected from a CAHs-contaminated plots that used to be an industrial site for a chemical factory. After removing gravel and impurities, samples were sieved through a 40-mesh screen and stored in the refrigerator at 4°C for future use. The physicochemical properties of river sediment, farmland soil, and chloroform-contaminated soil are shown in Table 1.
Microcosms were constructed by placing 20 g of samples and 80 ml of reduced anaerobic medium into 150-ml serum bottles, Nitrogen was introduced for 10 min to keep the bottle in an anaerobic state, and sealing the bottles with PTFE -lined butyl rubber stoppers and aluminum seals. The reduced anaerobic medium used in these tests was identical to that reported previously (26), and the anaerobic culture medium (1 L) contains the following components: 10 mL mineral stock solution (100.00 g/L NaCl, 50.00g/L MgCl2·6H2O, 30.00 g/L NH4Cl, 30.00 g/L KCl, 20.00 g/L KH2PO4, and 1.50 g/L CaCl2·2H2O); 1 mL trace element solution (1.500 g/L FeCl2·4H2O, 0.190 g/L CoCl2·6H2O, 0.100 g/L MnCl2·4H2O, 0.070 g/L ZnCl2, 0.006 g/L H3BO3,0.036 g/L Na2MoO4·2H2O, 0.024 g/L NiCl2·6H2O, 0.002 g/L CuCl2·2H2O, 0.006 g/L Na2SeO3·5H2O, and 0.008 g/L Na2WO4·2H2O); 0.25 mL (0.1%) resazurin solution (oxygen indicator); 2.292 g Tris-ethanesulfonic acid (buffer reagent); 0.048 g Na2S·9H2O, 0.242 g L-cysteine, and 0.0771 g DL-dithiothreitol (reducing agent); along with 2.52 g NaHCO3 (buffer reagent). The pH was adjusted to neutral using HCl and NaOH. Each bottle of medium was supplemented with 0.1 mL vitamin solution.
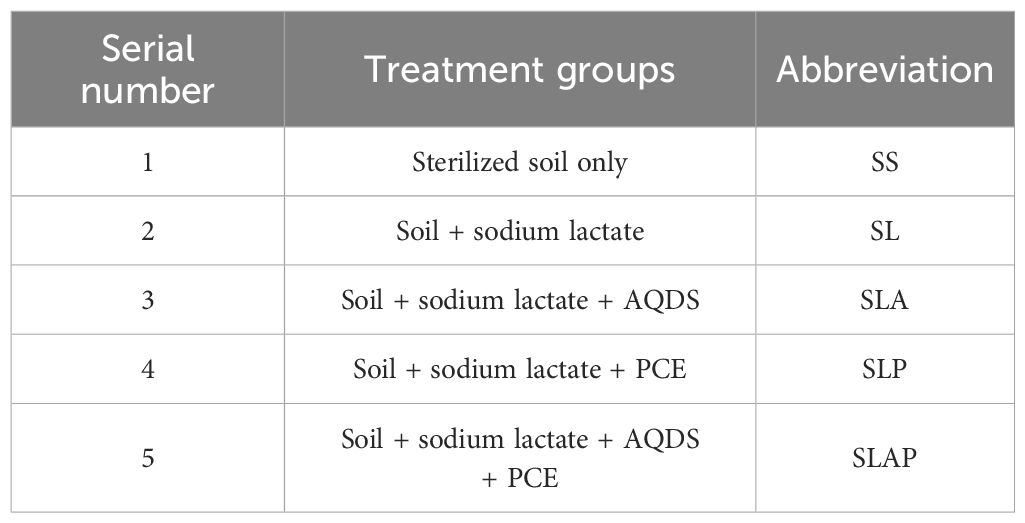
The design of the experiment was showed in Table 2. In this case, 20 mmol/L sodium lactate was used as electron donor, 100mg/L AQDS was used as electron shuttle, and 1 mmol/L PCE was used as pollutant. All treatments were performed in triplicate, and all bottles were incubated in dark with orbital shaking (30 °C, 150 rpm). Particularly, AQDS was only used as a model compound to simulate the redox behavior of redox-active natural organic matter (NOM) or humic substances (HS) in this study.
2.2 Analytical methods
2.2.1 PCE and dechlorination products analysis
Concentrations of PCE and its dechlorination products were determined by headspace gas chromatography (GC) (27). Chlorinated ethylenes were analyzed by sampling l00 μL of headspace gas with a 250-μl gas-tight glass syringe (Valco Co., CH) from 150 mL serum bottles (100 mL headspace). The samples were then analyzed using a flame ionization detector (FID) and a HP-5 capillary column (30-m, 0.32-mm, 0.25-μm) in an 8860A GC (Agilent Co., U.S). Nitrogen was used as the carrier gas at 5: 1 split ratio and 1 mL/min. The injector temperature was 220 °C and the detector temperature was set at 300 °C. The column temperature was initially 60 °C (hold 2 min) and gradually increased to 200 °C (hold 4 min) at a rate of 20 °C/min.
2.2.2 CH4 and CO2 analysis
CH4 and CO2 were analyzed by sampling l00 μL of headspace gas, and the samples then analyzed using a thermal conductivity detector (TCD) and a SE-30 column (30-m, 0.32-mm, 0.5-μm) in a SP-7802 GC (Lunan Ruihong Co., CN) (28). The column temperature was 70 °C, the injector temperature was 120 °C and the detector temperature was 120 °C; Nitrogen was used as the carrier gas at 30 mL/min.
2.2.3 Physicochemical and biogenic element analysis
The determination of physicochemical properties after the end of anaerobic culture was destructive sampling, and the specific determination methods were as follows:
1. Determination of Fe(II): We used a pipette to aspirate 2 mL of mud, placed it in a 50 mL centrifuge tube containing 45 mL of 0.5 mol/L HCl, extracted under 30°C for 24 hours, and centrifuged at 2000 rpm for 10 minutes. We collected the supernatant and filter through aqueous phase membrane (0.22-μm) to obtain the filtrate. Determined via 1,10-phenanthroline spectrophotometry at 510 nm (29).
2. Determination of //Cl−: We placed samples into a freeze dryer (−80°C) and freeze-dried it for 48 hours. We took 1.0 g of freeze-dried soil, added 25 mL of deionized water, and incubated at with an oscillator for 30 minutes (25°C, 250 rpm). After centrifugation at 3000 rpm for 10 minutes, we collected the supernatant and filter through water phase membrane (0.22-μm), and analyzed them by ion chromatography (Eco IC, Metrohm, Switzerland) (30).
3. Dissolved DOC and DON: Another 2.0 g of freeze-dried soil was added to a 50-mL centrifuge tube with 10 mL deionized water, shaken at 250 rpm for 60 min, and centrifuged at 4500 rpm for 10 min. The supernatant was collected and passed through organic phase filter membrane (0.45-μm), and then analyzed them by UV-VIS spectrophotometer (L4, youke, China) (17).
2.2.4 Microbial community analysis
After the cultivation experiment, the slurry was centrifuged at 3600 rpm for 5 minutes. The precipitate was frozen (−20°C) and stored for microbial DNA extraction and high-throughput sequencing. Total genomic DNA samples were extracted using the OMEGA Soil DNA Kit (M5635-02) (Omega Bio-Tek, USA). The quantity and quality of extracted DNAs were measured using a NanoDrop NC2000 spectrophotometer (Thermo Fisher Scientific, USA) and agarose gel electrophoresis, respectively. Primers 520F and 802R were employed to detect V3-V4 region of the16S rRNA gene. To identify amplicon sequence variants (ASVs), 16S rRNA gene amplicon reads were analyzed through the DADA2 pipeline. Performing biological information analysis using QIIME2, by conducting sequence comparisons with the Greengenes database. Then the sequencing analysis was performed on the Illumina MiSeq platform. The sequences have been submitted to NCBI and the link was PRJNA1290334.
3 Results and discussion
3.1 PCE reductive dechlorination capacity in three habitats
The variation of PCE concentration in river sediment (RS), farmland soil (FS)and chloroform-contaminated soil (CS) with culture time (Figure 1). In order to compare the degradation of PCE more clearly, linear regression analysis was carried out according to the variation of PCE concentration over time, and the results (Table 3). Except for SS, the PCE content in other treatments decreased with the increase of incubation time, which indicated that there was no air leakage in the culture system except for the loss caused by adsorption of a small amount of PCE, and the reductive transformation of PCE was mainly a microbial-mediated bio-reductive dechlorination.
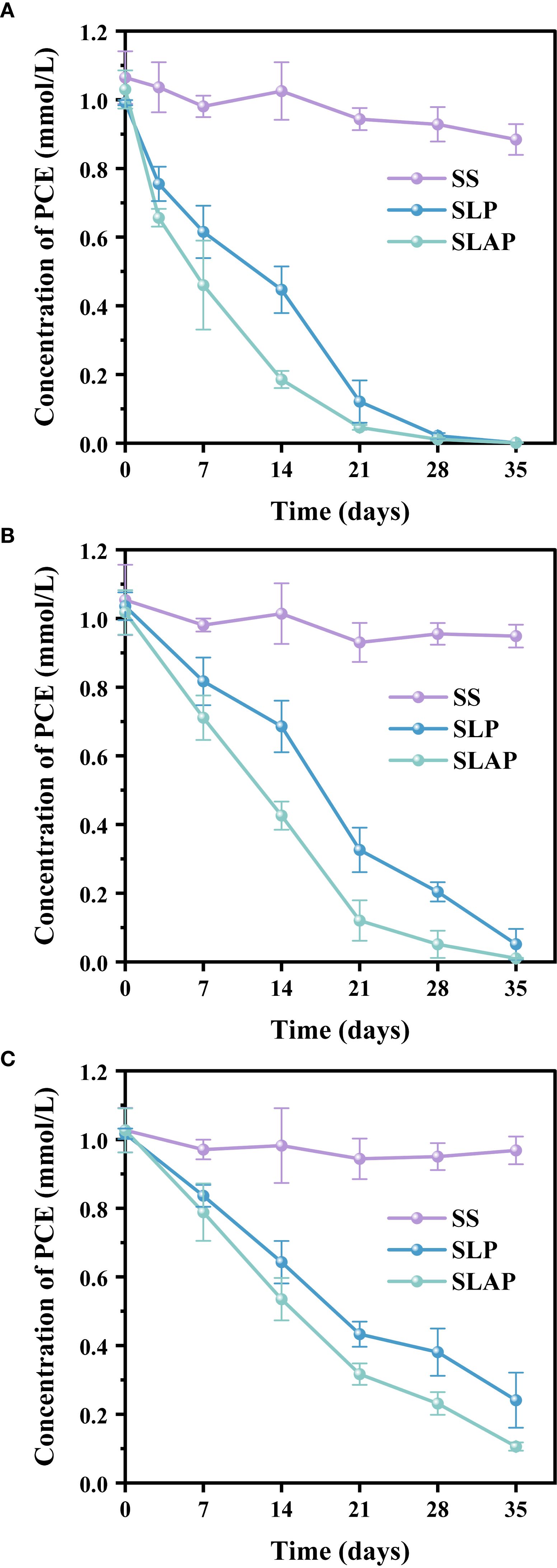
Figure 1. The concentration of PCE in river sediment (A), farmland soil (B) and chloroform-contaminated soil (C) during anaerobic cultivation. SS, sterilized sample only; SLP, soil+ sodium lactate + PCE; SLAP, soil + sodium lactate + AQDS + PCE.
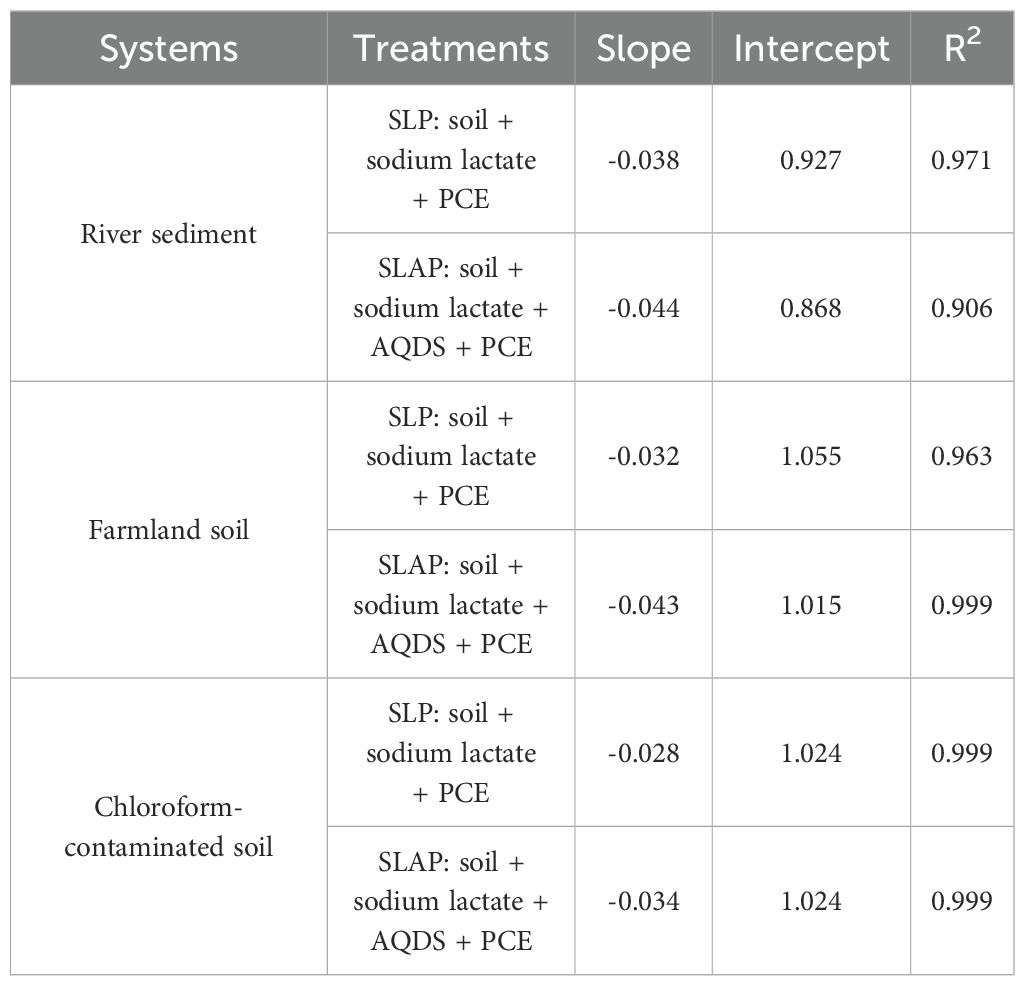
Table 3. Linear regression equations for PCE degradation rates of different treatments from days 0 to 21 in three systems.
In river sediment, the results showed that the dechlorination efficiencies of PCE exceeded 90% within 21 days in both sodium lactate + PCE (SLP) and sodium lactate + AQDS + PCE (SLAP) (Figure 1A). Throughout the culture process, the two treatments showed a consistent trend: the concentration of PCE decreased rapidly in the first 7 days, the rate of decline gradually decreased subsequently, and the degradation of PCE was basically constant by day 21. And the rates of PCE degradation within 21 days were 0.0379 and 0.0436 mmol/L-day in both SLP and SLAP. After 21 days of anaerobic culture, the removal rates of PCE in SLP and SLAP were 87.87% and 95.04% (P<0.05), respectively, and they were almost completely degraded. These results indicated that the river sediment had a good PCE degradation ability, and the addition of AQDS significantly improved its degradation effect on PCE.
In farmland soil, the degradation efficiencies of PCE reached 31.43% (SLP) and 57.40% (SLAP) by day 14 (P<0.05), increasing to 79.61% (SLP) and 94.78% (SLAP) by day 28 (P<0.05) (Figure 1B). Compared to river sediment, farmland soil exhibited slightly slower degradation rate (0.0323 and 0.0425 mmol/L-day) from days 0 to 21, yet achieved near-complete removal. AQDS increased its degradation rate of PCE reduction by 31.58%. Specifically, the residual concentration of PCE of SLAP treatment was 0.05 mmol/L by day 28, whereas SLP required 35 days to reach comparable levels. In this system, AQDS still showed a positive effect on the dechlorination and degradation process of PCE.
In contrast, the degradation rate of PCE in chloroform-contaminated soil was significantly lower than in the other two habitats (Figure 1C). After 21 days of anaerobic culture, the PCE content of SLP decreased by 0.57 mmol/L, and the PCE content of SLAP was not much different from that of SLP, which decreased by 0.68 mmol/L. During this period, AQDS increased the degradation rate of PCE from 0.0278 to 0.0340 mmol/L-day. At the end of the culture, the concentrations of PCE in the SLP and SLAP were 0.19 mmol/L and 0.11 mmol/L, respectively. The role of AQDS in the degradation effect of PCE is not obvious. The limited PCE degradation in this system is likely attributable to low microbial abundance, sandy soil texture, and suboptimal redox conditions. The deficiency of ion strength and nutrients in the soil and groundwater can lead to less PCE removal, and also decrease the abundance of dechlorinating microorganisms (31). And the microbial dechlorination effect on target pollutants can also be influenced by coexisting chlorinated hydrocarbons in a complex chlorinated hydrocarbon pollutant system (32).
3.2 Post-incubation physicochemical dynamics and redox-reactive element cycling
3.2.1 pH and Eh evolution
Figure 2A showed the change of pH in the culture system with different treatments in three different habitats. In the river sediment, the pH of the treatments with PCE decreased while that without PCE increased. This was due to the production of H+ by microorganisms during anaerobic reductive dechlorination (33). The dechlorination reaction of PCE is: PCE + H2→TCE + H+ + Cl−. Compared with the initial value, the pH of farmland soil increased while that of chloroform-contaminated soil decreased after incubation. After 35 days of culture, the pH of these three systems was basically stable at 7.0-7.6, which was suitable for the growth of anaerobic microorganisms.

Figure 2. Changes in soil pH (A) and Eh (B) before and after anaerobic cultivation. RS, river sediment; FS, farmland soil; CS, chloroform-contaminated soil; IS, initial soil; SS, sterilized soil; SL, soil + sodium lactate; SLA, soil + sodium lactate + AQDS; SLP, soil + sodium lactate + PCE; SLAP, soil + sodium lactate + AQDS + PCE. Different lowercase letters indicate significant difference (P<0.05).
After incubation, all three systems exhibited consistent trends in redox potential (Eh) dynamics (Figure 2B). Sterilized soils demonstrated the weakest reducing capacity, whereas all other treatments achieved Eh values below −150 mV, confirming establishment of pronounced reducing conditions.
Initial farmland and chloroform-contaminated systems exhibited oxidizing conditions, while river sediment showed mildly reducing properties with an Eh of −55 mV. Across all three systems, AQDS effectively lowered Eh. However, under PCE contamination, the Eh reduction was attenuated compared to PCE-free conditions. This likely stems from toxicity of PCE, which inhibits the activity of some microbes in the soil (functional microbes involved in reduction reactions) (34) and even destroys cell membranes and causes cell death (35). Moreover, as a preferential electron acceptor, PCE competes with other reductive processes (e.g., / reduction) for bioavailable electron donors (e.g., H2 and formic acid). This electron competition inhibits parallel reduction pathways, thereby constraining overall reducing intensity in the system.
These results demonstrated that AQDS enhanced reducing capacity by mediating electron transfer between microorganisms and electron donors/acceptors, thereby accelerating redox reaction kinetics. This process yielded reduced species that depressed Eh (36). Notably, the sodium lactate + AQDS treatment achieved optimal reducibility across all three habitats.
3.2.2 Changes of redox process of typical elements after culture
3.2.2.1 Fe(III) reduction
The variation of Fe(II) content across three systems are depicted in Figure 3A. In the river sediment, analysis of Fe(II) concentration reveals that the presence or absence of PCE dechlorination mediates diametrically opposed effects of AQDS on Fe(III) reduction processes. Absent PCE dechlorination, AQDS enhanced Fe(III) reduction by 27.88%. Conversely, during PCE dechlorination, AQDS decreasing Fe(II) extent by 19.18%.
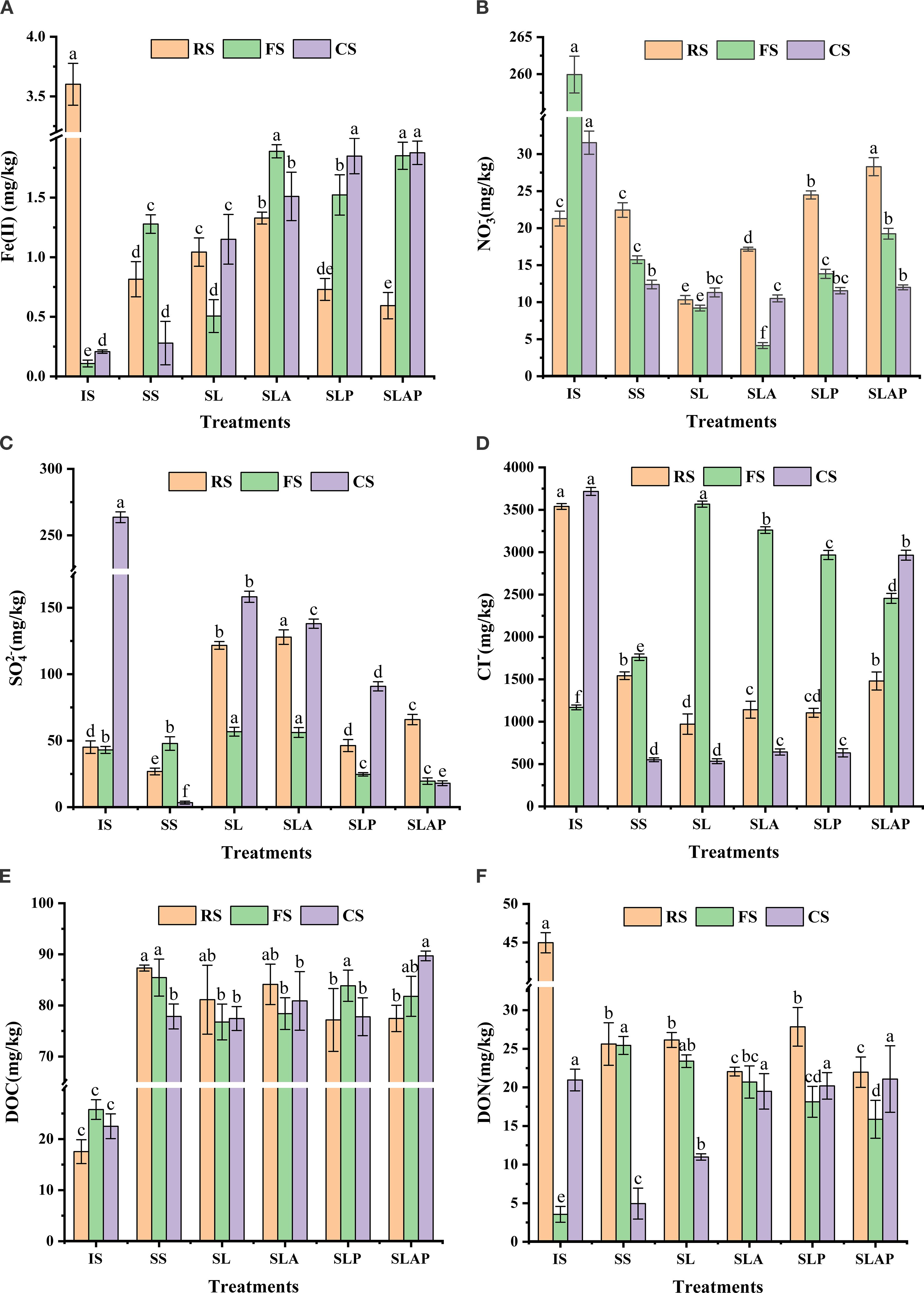
Figure 3. The changes of Fe(II) (A), (B), (C), CI− (D), DOC (E), and DON (F) in three habitats before and after anaerobic cultivation.
In the farmland and chloroform-contaminated soils, all treatments significantly enhanced Fe(II) generation. In the farmland soil, the Fe(II) content in the SS reached 1.28 mg/kg, significantly 12 times higher than initial levels. This pronounced increase primarily stems from: (1) Abundant native Fe(III) derived from iron-bearing minerals (e.g., hematite, limonite, magnetite, and goethite) through natural weathering processes (37); (2) Agricultural inputs including fertilizers and soil amendments serving as supplemental Fe (III) sources; (3) This higher concentration of Fe(II) might be due to changes provoked by heat during autoclaving (38).
Irrespective of PCE presence, the content of Fe(II) treated with AQDS was significantly higher than that without AQDS, indicating that AQDS as an electron shuttle can promote the reduction of Fe(III) in farmland soil system (39, 40). Without AQDS addition, Fe(II) content treated with PCE (1.52 mg/kg) exhibited significantly higher than that without PCE (0.51 mg/kg) (P<0.05), indicating synergistic/non-competitive coupling between PCE dechlorination and Fe(III) reduction. However, with AQDS addition, Fe(II) content showed no significant difference between treatments with PCE (1.85 mg/kg) and without PCE (1.89 mg/kg). This shift demonstrates that AQDS reconfigures electron transfer pathways, converting the PCE-Fe(III) relationship from synergistic/non-competitive into competitive/non synergistic. The PCE reduction dechlorination weakened the Fe(III) reduction and resulted in a smaller increase of its Fe(II).
In chloroform-contaminated soil, the Fe(II) content with PCE was significantly higher than that without PCE. The presence of PCE promoted Fe(III) reduction because PCE inhibits the biological reduction process of other electron acceptors (such as and CO2), thereby enhancing the activity of Fe(III) reduction. In the absence of PCE reductive dechlorination, AQDS enhanced Fe(II) content by 31.30%, demonstrating its role in stimulating Fe(III) reductive dissolution within this system. However, under PCE stress, AQDS failed to enhance Fe(III) reduction. This demonstrates preferential electron shuttling by AQDS toward PCE dechlorination, aligning with its enhanced dechlorination efficacy in Section 3.1.
3.2.2.2 reduction
The concentration of exhibited marked spatial variation: river sediment (21.29 mg/kg) and chloroform-contaminated soil (31.25 mg/kg) showed low levels, while content in the farmland soil was higher (262.50 mg/kg) (Figure 3B).
In river sediment, PCE addition inhibited reduction due to the thermodynamic hierarchy of electron acceptor utilization by microorganisms > Fe(III)> (41, 42). Although reductive dechlorination typically occurs after the depletion of inorganic electron acceptors, it may proceed concurrently when specific microbial consortia are present (43). The effective PCE reduction observed in river sediment confirmed the activity of specialized dechlorinating microorganisms. Crucially, PCE dechlorination competes with reduction for electrons, thereby suppressing reduction. Furthermore, irrespective of PCE presence, AQDS enhanced Fe(II) generation, indicating accelerated electron transfer from sodium lactate to Fe(III). This Fe(III) reduction competitively limited electron availability for reduction.
In both farmland and chloroform-contaminated soils, all treatments reduced the concentrations of to varying degrees, indicating significant SS reduction occurred. In chloroform-contaminated soil, the concentration of showed minimal variation across treatments, indicating neither PCE nor AQDS exerted significant effects on reduction.
In farmland soil, PCE addition inhibited reduction relative to the treatment without PCE—consistent with observations in river sediment. Furthermore, AQDS inhibited reduction, which reduced from 19.24 to 13.83 mg/kg (P<0.05). Conversely, without PCE, AQDS-added samples (4.14 mg/kg) had significantly lower than unamended controls (9.21 mg/kg) (P<0.05), revealing AQDS-enhanced reduction. This demonstrates that AQDS acts as an electron shuttle to accelerate reduction under non-PCE conditions. AQDS preferentially allocates electrons to PCE dechlorination, thereby failing to facilitate reduction. Instead, by competing for electron donors (e.g., sodium lactate), PCE dechlorination deprives reduction of essential electron donors, impairing electron flux toward reduction. Consequently, AQDS exerts inhibitory effects on this process.
3.2.2.3 reduction
Whether AQDS is added to farmland soil has little effect on content after reaction (Figure 3C). After the PCE degradation reaction, the content was lower than that in the treatment group without PCE, which may be attributed to the reduction-dechlorination function of some sulfate-reducing bacteria (SRB), which reduced sulfate to H2S by metabolizing chlorinated hydrocarbon such as PCE through respiration. And obtain the energy needed for their own growth (44). A large number of studies have conducted reduction dechlorination studies by screening and separating SRB or its enrichment, confirming that these microorganisms with sulfur reduction function can also directly participate in dechlorination (45).
In chloroform-contaminated soil, the content of treatments with sodium lactate as carbon source was significantly higher than that of SS, because the carbon source produced ATP and electrons in the catabolic process, and accelerated the reduction of and so on to S2- through electron transfer. SLA (138.00 mg/kg) < SL (158.25 mg/kg) and SLAP (17.99 mg/kg) < SLP (90.89 mg/kg) can also explain the addition of AQDS as an electron shuttle to accelerate the electron transport process.
3.2.2.4 Change of CI− content
During the 35 days of anaerobic culture, river sediment and chloroform-contaminated soil exhibited similar CI− depletion trends (Figure 3D), and CI− concentration decreased after culture. The cause of this phenomenon may be that Cl− in the RS combined with Fe(II) to form FeCl2 precipitate. This was consistent with the significant decrease in Fe(II) content observed in RS (Figure 3A). In the CS, the highly adsorptive porous structure of sandy soil substantially reduced CI− content. For farmland soil, the CI− concentration of all treatments increased after culture. On the one hand, due to the decomposition of chlorinated organic matter, in the PCE reduction of farmland soil, microorganisms break the C-Cl bond in PCE (C2Cl4), so that chlorine atoms are released into the soil solution in the form of CI−. On the other hand, the ion exchange balance may shift, that is, under anaerobic conditions, other anions (such as ) are consumed by microbial reduction or chemical reaction, which breaks the ion exchange balance, and CI− originally adsorbed on the surface of soil particles will be released into the soil solution, resulting in an increase in CI− concentration.
3.2.2.5 Change of DOC content
In three habitats, the dissolved organic carbon (DOC) content of all treatments was higher than the initial content (Figure 3E). In river sediment, the addition of PCE reduced the content of DOC, which indicated that microorganisms used soluble organic carbon as electron donor to degrade PCE, and finally produced CO2, CI− and other products. On the contrary, in farmland soil, the addition of PCE increased the content of DOC. It is speculated that there are carbon-fixing microorganisms within this system, which facilitate the carbon cycle of CO2 and lead to the effective accumulation of DOC within the system. In Chloroform-contaminated soil, AQDS demonstrated an effective role in promoting the accumulation process of DOC during the PCE dechlorination reaction.
3.2.2.6 Change of DON content
In river sediment and chloroform-contaminated soil, dissolved organic nitrogen (DON) content decreased post-incubation, whereas it increased in farmland soil (Figure 3F). Therefore, we speculated that AQDS enhanced ammonification () and/or denitrification () in river sediment and farmland soil (46). This may concurrently stimulate the enrichment of DON-transforming microorganisms, such as Azospira, which facilitate organic nitrogen metabolism.
Chloroform-contaminated soil exhibited complex DON dynamics: Without PCE, AQDS moderately enhanced DON accumulation (from 10.98 to 19.49 mg/kg). With PCE, AQDS showed negligible promotion, which indicated that PCE hindered the process of inorganic nitrogen conversion to organic nitrogen. In addition, the dominant microbial with the function of degrading PCE will inhibit the activity of microorganisms involved in DON production, and PCE and its degradation intermediates (e.g., vinyl chloride) may be toxic to some microorganisms and inhibit nitrogen conversion function.
3.2.3 Dynamic changes of CH4 and CO2 during PCE reduction dechlorination
Methanogenesis is a process that can convert inorganic or organic matter into CH4 and CO2 under anaerobic fermentation conditions (47). Under anaerobic conditions, sodium lactate, as a carbon source and electron donor, can be metabolized by microorganisms such as anaerobic fermentant bacteria and acid-producing bacteria. The reaction formula is as follows: . In the condition of sufficient electron donors, methanogens will become major electronic competitors and produce large amounts of CH4 (24, 48).
River sediment exhibited the fastest response, with the final order of CH4 production in different treatments: SLAP > SLP > SLA > SL > SS. (Figure 4A). The addition of PCE increased the CH4 production rates from 90.87 mmol/L-day (SL) and 206.11 mmol/L-day (SLA) to 1172.19 mmol/L-day (SLP) and 934.69 mmol/L-day (SLAP) within 7–14 days. With AQDS supplementation, CH4 production rates rose from 149.77 to 364.02 mmol/L-day during days 14-28, and the final production of CH4 increased significantly from 11496.44 to 12491.82 mmol/L by day 35.
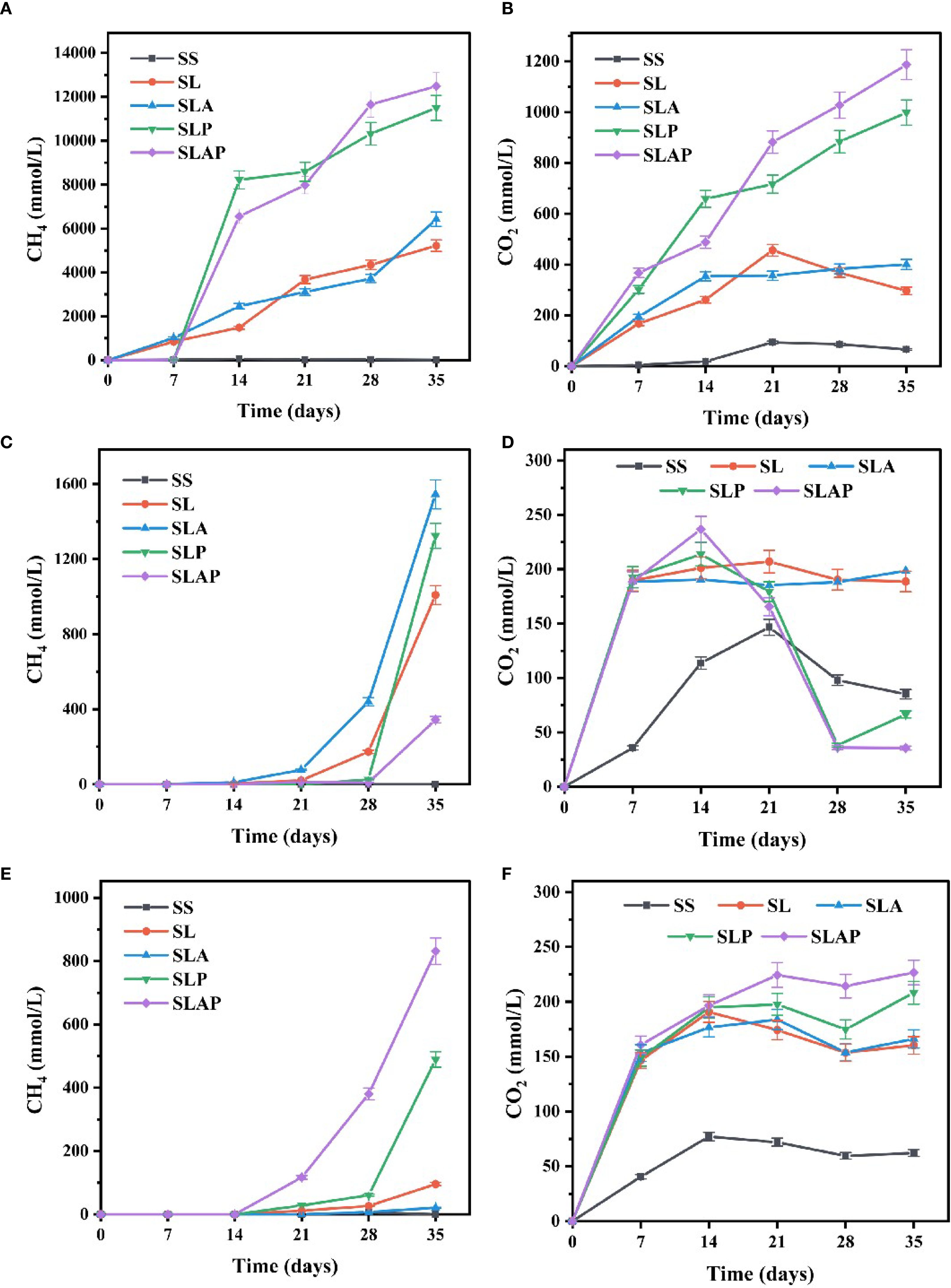
Figure 4. Changes of CH4 and CO2 accumulated in river sediment (A, B), farmland soil (C, D), and chloroform-contaminated soil (E, F) during the anaerobic cultivation.
The content of CH4 and CO2 produced by the two treatments with PCE was significantly higher than that without PCE, indicating that the growth of methanogens would be stimulated during dechlorination. The change trend of CO2 concentration in different treatments was similar to that of CH4 concentration at the end of culture (Figure 4B). It is also obvious from the figure that the addition of AQDS promotes the generation of CH4 and CO2 (49).
There was no CH4 production in farmland soil during 0–14 days (Figure 4C). After 14 days, CH4 was first produced in SL and SLA, while CH4 was only produced in SLP and SLAP after 28 days. In the absence of PCE, AQDS significantly increased the CH4 production rates, especially from days 28 to 35, where the CH4 production rates rose from 119.35 to 157.87 mmol/L-day. However, the presence of PCE altered this outcome, and AQDS did not play a positive role. The CO2 content produced in SLP and SLAP added in farmland soil showed a trend of first increasing (0–14 days) and then decreasing (14–28 days), which can be divided into two stages (Figure 4D). (1) The first stage is CO2 production, because when sodium lactate is degraded by anaerobic microorganisms, the initial products include CO2 and H2. At this stage, anaerobic bacteria (such as ferment bacteria and sulfate reducing bacteria) use the organic carbon in sodium lactate for metabolism, and CO2 is one of the main end products. And the redox potential is higher, which inhibits the activity of methanobacteria, so there is little or no methane production. (2) The second stage is CO2 decline and CH4 production, when the accumulation of organic acids and hydrogen from sodium lactate decomposition to a certain extent, the redox potential continues to decrease, then the activity of methanogens gradually increases. Methanogens begin to convert CO2 and H2 produced during anaerobic fermentation to CH4, or to produce CH4 through organic acid metabolism.
In chloroform-contaminated soil, consistent with the performance of farmland soil, CH4 production began after a period of environmental adaptation (0–14 days) (Figure 4E). The difference was that in the chloroform-contaminated soil, the CH4 production rates of SL (9.86 mmol/L-day) and SLA (2.01 mmol/L-day) were extremely low, while that of SLP (61.22 mmol/L-day) and SLAP (64.41 mmol/L-day) increased significantly during days 28-35. In the presence of PCE, the addition of AQDS significantly increased the final production of CH4 from 489.29 to 831.40 mmol/L. The order of CO2 production was almost the same as that of CH4 production at the end of culture (Figure 4F).
3.3 Composition and function of microbial communities in different habitats
3.3.1 River sediment system
The distribution of microbial community composition and relative abundance in the river sediment at genus level is shown in Figure 5A. The results showed that different treatments had significant effects on microbial community composition in river sediment. Five species of bacteria were detected in all treatments: Chlorobaculum, Azonexus, Acidaminobacter, Youngiibacter and Thiobacillus. Compared with SS, the relative abundance of Pelotomaculum_B, Azonexus, Clostridium_AF and Thiobacillus continuously increased under the other four treatments. Among them, the relative abundance of Pelotomaculum_B increased significantly from 1.89% (SL) and 0.62% (SLA) to 15.55% (SLP) and 16.23% (SLAP) after adding PCE, indicating that Pelotomaculum_B is a dominant bacterial genus closely related to dechlorination in this system. Pelotomaculum_B belongs to Firmicutes phylum, a strict anaerobic bacteria, which is widely involved in the anaerobic degradation and methanogenesis of organic matter in nature (50). Pelotomaculum_B can decompose complex carbon sources such as sodium lactate, and its products (acetic acid, H2, and CO2) can be provided as precursor substances to methanogens to accelerate the methanogenesis process (51), which is consistent with the results shown in Figure 4A. Pelotomaculum_B, while not directly involved in PCE dechlorination, can indirectly facilitate the dechlorination by providing an electron donor (H2) and maintaining an anaerobic metabolic network (52).
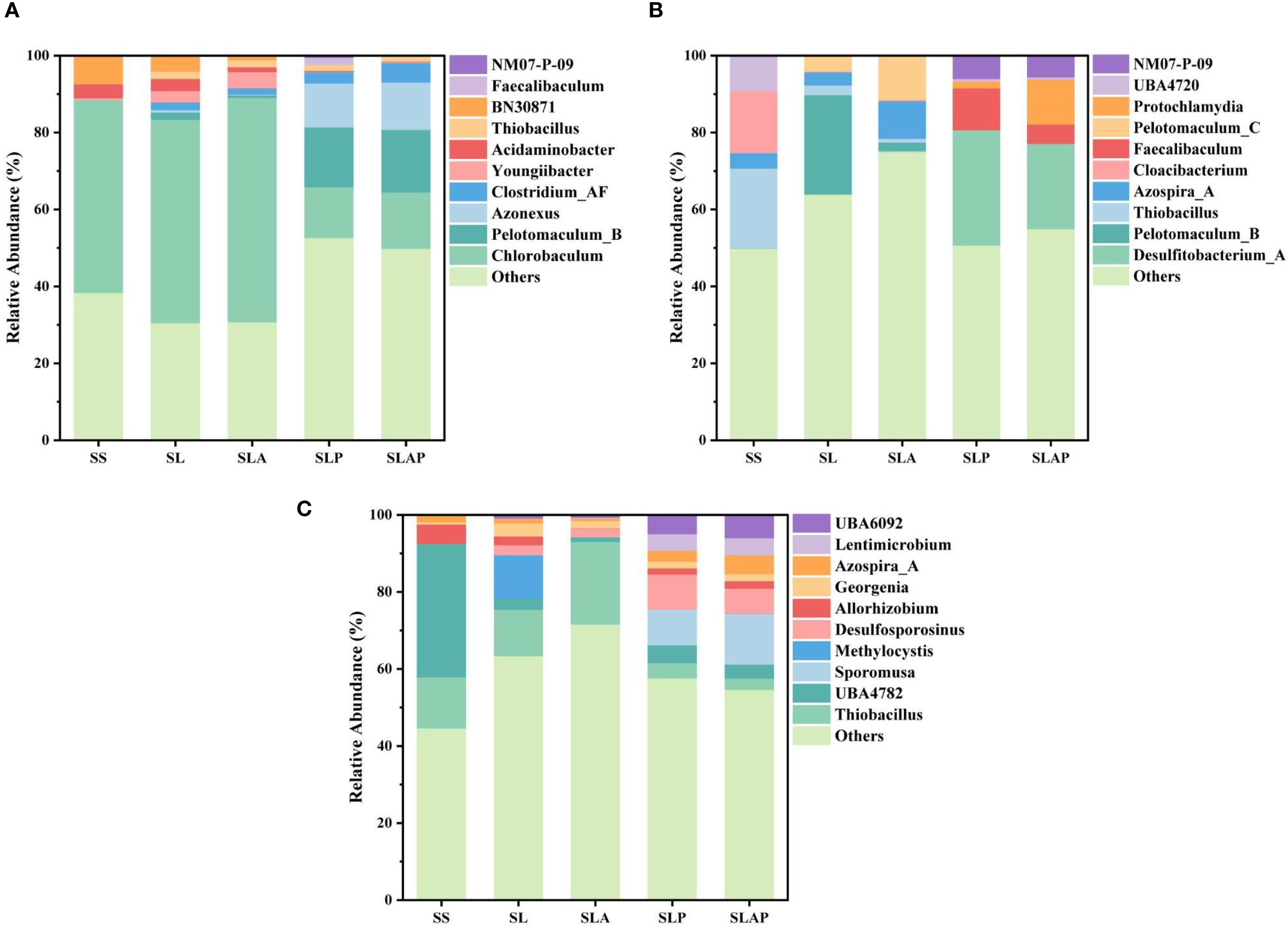
Figure 5. The relative abundance of bacterial community structure at the genus classification level in river sediment (A), farmland soil (B), and chloroform-contaminated soil (C).
Concurrent with PCE reductive dechlorination, a significant increase in Azonexus abundance was observed. Functionally characterized by its role in nitrogen cycling—specifically, biological nitrogen fixation under nitrogen-limited or anaerobic conditions where it converts atmospheric N2 into bioavailable nitrogen sources (53). Azonexus demonstrates probable tolerance to PCE and exhibits co-enrichment with dechlorinating microorganisms.
After being cultured in PCE, Thiobacillus abundance exhibited no significant shift, nor did AQDS exert discernible regulatory effects. As a bacterium capable of dissimilatory Fe(III) reduction, Thiobacillus has been documented to emerge as a dominant population under polycyclic aromatic hydrocarbon (PAH) stimulation (53), demonstrating its capacity for organic pollutant degradation under Fe(III) reduction conditions.
Clostridium_AF is a facultative anaerobic dechlorinator, which can carry out dechlorination reaction (29). AQDS reduced the redox potential in this system (Figure 1B), generating anaerobic conditions conducive to Clostridium_AF proliferation. Concurrently, during PCE degradation, AQDS mediated an increase in Clostridium_AF abundance from 2.95% to 5.08% (P< 0.05). This significant enrichment demonstrates that AQDS enhances reductive dechlorination capacity primarily by selectively stimulating Clostridium_AF populations within the microbial consortium.
In addition, Chlorobaculum, a distinctive genus of anaerobic anoxygenic phototrophs, drives energy and material cycles in anaerobic ecosystems via sulfur oxidation and carbon fixation through the reverse cycle. In treatments without PCE, Chlorobaculum maintained relative abundances exceeding 50%, with AQDS further elevating its prevalence. Conversely, PCE exposure drastically reduced Chlorobaculum abundances to 13.22% (SLP) and 14.73% (SLAP). Figure 3C indicated lower concentrations in PCE-added systems, suggesting that dechlorinating microorganisms (such as Clostridium_AF) outcompeted Chlorobaculum for electron donors during dechlorination, thereby suppressing its proliferation.
Although no obligate dechlorinators were detected in the river sediment system, the enrichment of Pelotomaculum_B, Clostridium_AF and Azonexus was effectively regulated by adding PCE. This demonstrates their enhanced ecological fitness under PCE stress, with AQDS further promoting their proliferation during dechlorination.
3.3.2 Farmland soil system
The composition and relative abundance of microbial communities in farmland soil under different treatments were analyzed at the genus level (Figure 5B). Analysis of non-PCE treatments revealed that AQDS differentially regulated abundances of Azospira, Desulfitobacterium, and Pelotomaculum: Azospira increased significantly from 3.42% to 9.77%; Pelotomaculum rose from 4.05% to 11.41%; Desulfitobacterium showed only a marginal increase from 0.13% to 0.36%.
Research findings indicate that the metabolic activities of Azospira are predominantly involved in nitrogen cycling and organic matter degradation. This genus demonstrates the capability to progressively reduce nitrate () through sequential steps to nitrite (), nitric oxide (NO), nitrous oxide (N2O), and dinitrogen gas (N2) (25). This reduction pathway aligns with the significant nitrate attenuation observed in Figure 4B. Furthermore, Azospira exhibits metabolic versatility in degrading diverse organic compounds (e.g., acetate, ethanol, lactate), thereby playing a significant role in carbon cycling.
Desulfitobacterium, a genus within the Firmicutes phylum, is a strictly anaerobic bacterium capable of reductive dechlorination and desulfurization. It utilizes a range of electron acceptors (e.g., , , Fe(III) and chlorinated organic compounds) to drive reduction reactions (54). Research has further demonstrated that Desulfitobacterium can utilize chlorinated alkenes (e.g., TCE and PCE) as electron acceptors, with reductive dehalogenases (RDases) catalyzing the dechlorination process (55). After the addition of PCE treatment, Desulfitobacterium became the dominant bacterial genus, with relative abundances of 29.97% (SLP) and 22.14% (SLAP), significantly higher than those in the untreated group. This was consistent with the reduction shown in Figure 3C, that was, the reduction effect of in SLP and SLAP was stronger than that in SL and SLA. However, this abundance shift (SLP < SLAP) contradicted PCE degradation, so AQDS showed no significant impact on Desulfitobacterium in this context.
3.3.3 Chloroform-contaminated soil system
In chloroform-contaminated soil, the dominant genera during PCE degradation included Lentimicrobium, Azospira, Desulfosporosinus and Sporomusa. (Figure 5C). Lentimicrobium (a fermentative bacterium capable of converting lactate to acetate, and H2) exhibited higher abundance in SLP (4.32%) and SLAP (4.76%), suggesting synergistic interactions with anaerobes in the dechlorination process. Genomic analysis demonstrated that Dehalobacter (Dhb) forms tight syntrophic partnerships with Lentimicrobium, Desulfovibrio, and Sedimentibacter via cobalamin, H2, and acetate exchange (56). AQDS elevated Lentimicrobium abundance from 0.06% (SL) to 0.21% (SLA), indicating its role in accelerating organic matter mineralization under anaerobic conditions. Desulfosporosinus (a dechlorination-capable sulfate-reducing bacterium) showed significantly increased abundance with PCE addition, confirming its functional role in reductive dechlorination (57), which was consistent with the significant enhancement of reduction effect in its system (Figure 3C).
4 Conclusion
This study provides novel insights into the multifaceted functions of AQDS in the bioremediation of PCE-contaminated soils. Specifically, AQDS enhanced electron transfer efficiency, modulated redox cycling of biogenic elements (Fe, S, and N), and reshaped microbial community composition, collectively facilitating more effective PCE dechlorination. These findings establish a theoretical framework and offer a technical reference for advancing the bioremediation of complex contaminated environments. However, several limitations warrant consideration. The experimental design primarily focused on evaluating the impact of AQDS in the presence of sodium lactate as an electron donor, without fully revealing the individual contributions of each component. Key control treatments—such as soil + PCE + AQDS (without lactate), soil + PCE (without lactate), and soil + lactate + AQDS + PCE under sterilized conditions—were not included. Future work will incorporate these essential controls to rigorously assess the specific roles of AQDS and electron donors in driving dechlorination processes. Additionally, although AQDS serves as an effective model compound for probing fundamental mechanisms of electron shuttling, its high cost limits its viability for field-scale applications. In practical remediation scenarios, naturally occurring organic matter, humic substances, or their derivatives may represent more accessible and cost-effective alternatives. Future research should explore these materials as potential substitutes to facilitate sustainable and scalable remediation strategies.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI BioProject, accession PRJNA1290334.
Author contributions
XJ: Data curation, Writing – review & editing, Investigation, Software, Conceptualization, Methodology, Formal Analysis, Writing – original draft, Validation, Project administration. SH: Writing – original draft, Investigation, Formal Analysis, Project administration. AG: Writing – original draft, Validation, Data curation. HY: Writing – original draft, Investigation, Data curation, Project administration. YX: Resources, Supervision, Writing – review & editing. QL: Project administration, Writing – review & editing, Supervision. ZX: Supervision, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded in part by Science and Technology Project of Shinan District, Qingdao City (2023-1-013-CL) and Science and Technology Benefit the People Demonstration Project of Qingdao City (24-1-8-cspz-11-nsh). Research on Anaerobic Microbial Remediation Technology for Halogenated Hydrocarbon Contaminated Sites and Its Industrialization Application (24-1-8-cspz-11-nsh).
Acknowledgments
This work was supported by the Science and Technology Project of Shinan District, Qingdao City: Research and Industrial Application of Anaerobic Microbial Remediation Technology for Organochlorine Contaminated Soil (2023-1-013-CL); Science and Technology Benefit the People Demonstration Project of Qingdao City: Research on Anaerobic Microbial Remediation Technology for Halogenated Hydrocarbon.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang S, Chen S, Wang Y, Low A, Lu Q, and Qiu R. Integration of organohalide-respiring bacteria and nanoscale zero-valent iron (Bio-nZVI-RD): A perfect marriage for the remediation of organohalide pollutants? Biotechnol Adv. (2016) 34:1384–95. doi: 10.1016/j.biotechadv.2016.10.004
2. Vlaanderen J, Straif K, Pukkala E, Kauppinen T, Kyyrönen P, Martinsen JI, et al. Occupational exposure to trichloroethylene and perchloroethylene and the risk of lymphoma, liver, and kidney cancer in four Nordic countries. Occup Environ Med. (2013) 70:393–401. doi: 10.1136/oemed-2012-101188
3. Huang B, Lei C, Wei C, and Zeng G. Chlorinated volatile organic compounds (Cl-VOCs) in environment - sources, potential human health impacts, and current remediation technologies. Environ Int. (2014) 71:118–38. doi: 10.1016/j.envint.2014.06.013
4. Moran MJ, Zogorski JS, and Squillace PJ. Chlorinated solvents in groundwater of the United States. Environ Sci Technol. (2007) 26):41. doi: 10.1021/es061553y
5. Field JA and Sierra-Alvarez R. Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds. Rev Environ Sci Bio/Technology. (2004) 3:185–254. doi: 10.1007/s11157-004-4733-8
6. Löffler FE, Yan J, Ritalahti KM, Adrian L, Edwards EA, Konstantinidis KT, et al. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Systematic Evolutionary Microbiol. (2013) 63:625–35. doi: 10.1099/ijs.0.034926-0
7. Xiao Z, Jiang W, Chen D, and Xu Y. Bioremediation of typical chlorinated hydrocarbons by microbial reductive dechlorination and its key players: A review. Ecotoxicology Environ Saf. (2020) 202:110925. doi: 10.1016/j.ecoenv.2020.110925
8. Sung Y, Fletcher KE, Ritalahti KM, Apkarian RP, Ramos-Hernández N, Sanford RA, et al. Geobacter lovleyi sp. nov. strain SZ, a novel metal-reducing and tetrachloroethene-dechlorinating bacterium. Appl Environ Microbiol. (2006) 72:2775–82. doi: 10.1128/AEM.72.4.2775-2782.2006
9. Dell’Armi E, Rossi MM, Taverna L, Petrangeli Papini M, and Zeppilli M. Evaluation of the bioelectrochemical approach and different electron donors for biological trichloroethylene reductive dechlorination. Toxics. (2022) 10:37. doi: 10.3390/toxics10010037
10. Chen F, Li ZL, Yang JQ, Liang B, Lin XQ, Nan J, et al. Effects of different carbon substrates on performance, microbiome community structure and function for bioelectrochemical-stimulated dechlorination of tetrachloroethylene. Chem Eng J. (2018) 352:730–6. doi: 10.1016/j.cej.2018.07.082
11. Zhou L, Wu YL, Jiang Q, Sun SQ, Wang JT, Gao Y, et al. Pyrolyzed sediment accelerates electron transfer and regulates rhodamine B biodegradation. Sci Total Environ. (2023) 905:167126. doi: 10.1016/j.scitotenv.2023.167126
12. Pham AN, Rose AL, and Waite TD. Kinetics of Cu(II) reduction by natural organic matter. J Phys Chem C. (2012) 116:6590–9. doi: 10.1021/jp300995h
13. Van der Zee FR and Cervantes FJ. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: A review. Biotechnol Adv. (2009) 27:256–77. doi: 10.1016/j.biotechadv.2009.01.004
14. Wang Q, Zhang D, Li X, Wang Y, Wang H, Zhang Z, et al. Effects of humic electron mediators on reductive dechlorination of polychlorinated biphenyl by consortia enriched from terrestrial and marine environments. Front Microbiol. (2024) 15:1452787. doi: 10.3389/fmicb.2024.1452787
15. Ying Z, Chen H, He Z, Hu Y, Huang Z, Gao J, et al. Redox mediator-regulated microbial electrolysis cell to boost coulombic efficiency and degradation activity during gaseous chlorobenzene abatement. J Power Sources. (2022) 528:231214. doi: 10.1016/j.jpowsour.2022.231214
16. Aulenta F, Di Maio V, Ferri T, and Majone M. The humic acid analogue antraquinone-2,6-disulfonate (AQDS) serves as an electron shuttle in the electricity-driven microbial dechlorination of trichloroethene to cis-dichloroethene. Bioresource Technol. (2010) 101:9728–33. doi: 10.1016/j.biortech.2010.07.090
17. Xu Y, He Y, Zhang Q, Xu J, and Crowley D. Coupling between pentachlorophenol dechlorination and soil redox as revealed by stable carbon isotope, microbial community structure, and biogeochemical data. Environ Sci Technol. (2015) 49:5425–33. doi: 10.1021/es505040c
18. Cheng J, Yuan J, Li S, Yang X, Lu Z, Xu J, et al. Promoted reductive removal of chlorinated organic pollutants co-occurring with facilitated methanogenesis in anaerobic environment: A systematic review and meta-analysis. Crit Rev Environ Sci Technol. (2022) 52:2582–609. doi: 10.1080/10643389.2021.1886890
19. Smidt H and de Vos WM. Anaerobic microbial dehalogenation. Annu Rev Microbiol. (2004) 58:43–73. doi: 10.1146/annurev.micro.58.030603.123600
20. He J, Ritalahti KM, Yang K-L, Koenigsberg SS, and Löffler FE. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature. (2003) 424:62–5. doi: 10.1038/nature01717
21. Aulenta F, Majone M, and Tandoi V. Enhanced anaerobic bioremediation of chlorinated solvents: environmental factors influencing microbial activity and their relevance under field conditions. J Chem Technol Biotechnol. (2006) 81:1463–74. doi: 10.1002/jctb.1567
22. Amos BK, Suchomel EJ, Pennell KD, and Loeffler FE. Microbial activity and distribution during enhanced contaminant dissolution from a NAPL source zone. Water Res. (2008) 42:2963–74. doi: 10.1016/j.watres.2008.03.015
23. Yu HY, Wang YK, Chen PC, Li FB, Chen MJ, Hu M, et al. Effect of nitrate addition on reductive transformation of pentachlorophenol in paddy soil in relation to iron(III) reduction. J Environ Manage. (2014) 132:42–8. doi: 10.1016/j.jenvman.2013.10.020
24. Bennett P, Gandhi D, Warner S, and Bussey J. In situ reductive dechlorination of chlorinated ethenes in high nitrate groundwater. J Hazardous Materials. (2007) 149:568–73. doi: 10.1016/j.jhazmat.2007.06.092
25. Lee DJ, Wong BT, and Adav SS. Azoarcus Taiwanensis sp. nov., a denitrifying species isolated from a hot spring. Appl Microbiol Biotechnol. (2014) 98:1301–7. doi: 10.1007/s00253-013-4976-9
26. Löffler FE, Sanford RA, and Ritalahti KM. Enrichment, cultivation, and detection of reductively dechlorinating bacteria. Methods Enzymology. (2005) 397:77–111. doi: 10.1016/S0076-6879(05)97005-5
27. Mazur CS and Jones WJ. Hydrogen concentrations in sulfate-reducing estuarine sediments during PCE dehalogenation. Environ Sci Technol. (2001) 35:4783–8. doi: 10.1021/es0110372
28. Zhu M, Feng X, Qiu G, Feng J, Zhang L, Brookes PC, et al. Synchronous response in methanogenesis and anaerobic degradation of pentachlorophenol in flooded soil. J Hazardous Materials. (2019) 374:258–66. doi: 10.1016/j.jhazmat.2019.04.040
29. Xu Y, He Y, Feng X, Liang L, Xu J, Brookes PC, et al. Enhanced abiotic and biotic contributions to dechlorination of pentachlorophenol during Fe(III) reduction by an iron-reducing bacterium Clostridium beijerinckii Z. Sci Total Environ. (2014) 473:215–23. doi: 10.1016/j.scitotenv.2013.12.022
30. Cheng J, Xue L, Zhu M, Feng J, Shen-Tu J, Xu J, et al. Nitrate supply and sulfate-reducing suppression facilitate the removal of pentachlorophenol in a flooded mangrove soil. Environ pollut. (2019) 244:792–800. doi: 10.1016/j.envpol.2018.09.143
31. Dell’Armi E, Zeppilli M, Matturro B, Rossetti S, Petrangeli Papini M, and Majone M. Effects of the feeding solution composition on a reductive/oxidative sequential bioelectrochemical process for perchloroethylene removal. Processes. (2021) 9:405. doi: 10.3390/pr9030405
32. Duhamel M, Wehr SD, Yu L, Rizvi H, Seepersad D, Dworatzek S, et al. Comparison of anaerobic dechlorinating enrichment cultures maintained on tetrachloroethene, trichloroethene, cis-dichloroethene and vinyl chloride. Water Res. (2002) 36:4193–202. doi: 10.1016/S0043-1354(02)00151-3
33. Wang JJ, Li XY, Song YF, Yan J, and Yang Y. Effects of environmental factors on anaerobic microbial dehalogenation: a review. Microbiol China. (2022) 49:4357–81. doi: 10.13344/j.microbiol.china.220121
34. Chakraborty A and Picardal F. Neutrophilic, nitrate-dependent, Fe(II) oxidation by a Dechloromonas species. World J Microbiol Biotechnol. (2013) 29:617–23. doi: 10.1007/s11274-012-1217-9
35. Wang XX, Wang SH, Li W, Li Y, and Zhang YQ. Tolerant mechanisms of bacteria to organic solvents. Chin J Biotechnol. (2009) 25:641–9. doi: 10.3321/j.issn:1000-3061.2009.05.001
36. Liu CY, Xu XH, and Fan JL. Accelerated anaerobic dechlorination of DDT in slurry with Hydragric Acrisols using citric acid and anthraquinone-2, 6-disulfonate (AQDS). J Environ Sci. (2015) 38:87–94. doi: 10.1016/j.jes.2015.05.005
37. Jia R, Li LN, and Qu D. Erratum to: pH shift-mediated dehydrogenation and hydrogen production are responsible for microbial iron(III) reduction in submerged paddy soils. J Soils Sediments. (2015) 15:1619–9. doi: 10.1007/s11368-015-1116-4
38. Regenspurg S. Characterisation of schwertmannite - geochemical interactions with arsenate and chromate and significance in sediments of lignite opencast lakes. University of Bayreuth, Germany (Bayreuth (2002).
39. Cutting RS, Coker VS, Fellowes JW, Lloyd JR, and Vaughan DJ. Mineralogical and morphological constraints on the reduction of Fe(III) minerals by Geobacter sulfurreducens. Geochimica Et Cosmochimica Acta. (2009) 73:4004–22. doi: 10.1016/j.gca.2009.04.009
40. Liu D, Dong H, Zhao L, and Wang H. Smectite reduction by Shewanella species as facilitated by cystine and cysteine. Geomicrobiology J. (2014) 31:53–63. doi: 10.1080/01490451.2013.806609
41. Torres Alvarado R, Ramírez Vives F, Fernández FJ, and Barriga Sosa I. Methanogenesis and methane oxidation in wetlands: Implications in the global carbon cycle. Hidrobiológica. (2005) 15:327–49.
42. Löffler FE, Tiedje JM, and Sanford RA. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl Environ Microbiol. (1999) 65:4049–56. doi: 10.1128/AEM.65.9.4049-4056.1999
43. Xu Y, Xue L, Ye Q, Franks AE, Zhu M, Feng X, et al. Inhibitory effects of sulfate and nitrate reduction on reductive dechlorination of PCP in a flooded paddy soil. Front Microbiol. (2018) 9. doi: 10.3389/fmicb.2018.00567
44. Zhang Z, Zhang C, Yang Y, Zhang Z, Tang Y, Su P, et al. A review of sulfate-reducing bacteria: Metabolism, influencing factors and application in wastewater treatment. J Cleaner Production. (2022) 376:134109. doi: 10.1016/j.jclepro.2022.134109
45. Bao P, Hu Z, Wang X, Chen J, Ba Y, Hua J, et al. Dechlorination of p,p′-DDTs coupled with sulfate reduction by novel sulfate-reducing bacterium Clostridium sp BXM. Environ pollut. (2012) 162:303–10. doi: 10.1016/j.envpol.2011.11.037
46. Feng X, Zhu M, and He Y. Effects and mechanisms of soil redox processes on reductive dechlorination of chlorinated organic pollutants. Asian J Ecotoxicology. (2017) 12:151–61. doi: 10.7524/AJE.1673-5897.20170114004
47. Borek K, Romaniuk W, Roman K, Roman M, and Kubon M. The analysis of a prototype installation for biogas production from chosen agricultural substrates. Energies. (2021) 14:2132. doi: 10.3390/en14082132
48. Wei N and Finneran KT. Low and high acetate amendments are equally as effective at promoting complete dechlorination of trichloroethylene (TCE). Biodegradation. (2013) 24:413–25. doi: 10.1007/s10532-012-9598-x
49. Xia Q, Cheng J, Yang F, Yi X, Huang W, Lei Z, et al. Activated carbon and anthraquinone-2,6-disulfonate as electron shuttles for enhancing carbon and nitrogen removal from simultaneous methanogenesis, Feammox and denitrification system. Bioresource Technol. (2025) 418:131975. doi: 10.1016/j.biortech.2024.131975
50. Imachi H, Sakai S, Ohashi A, Harada H, Hanada S, Kamagata Y, et al. Pelotomaculum propionicicum sp nov., an anaerobic, mesophilic, obligately syntrophic propionate-oxidizing bacterium. Int J Systematic Evolutionary Microbiol. (2007) 57:1487–92. doi: 10.1099/ijs.0.64925-0
51. Zhang X, Zhang H, and Cheng L. Key players involved in methanogenic degradation of organic compounds: progress on the cultivation of syntrophic bacteria. Acta Microbiologica Sin. (2019) 59:211–23. doi: 10.13343/j.cnki.wsxb.20180181
52. Müller N, Worm P, Schink B, Stams AJ, and Plugge CM. Syntrophic butyrate and propionate oxidation processes: from genomes to reaction mechanisms. Environ Microbiol Rep. (2010) 2:489–99. doi: 10.1111/j.1758-2229.2010.00147.x
53. Salah ZB, Charles CJ, Humphreys PN, Laws AP, and Rout SP. Genomic insights into nvel, akalitolerant ntrogen fixing bacteria, Azonexus sp. Strain ZS02. J Genomics. (2019) 7:1–6. doi: 10.7150/jgen.28153
54. Pester M, Brambilla E, Alazard D, Rattei T, Weinmaier T, Han J, et al. Complete genome sequences of Desulfosporosinus orientis DSM765T, Desulfosporosinus youngiae DSM17734T, Desulfosporosinus meridiei DSM13257T, and Desulfosporosinus acidiphilus DSM22704T. J Bacteriology. (2012) 194:6300–1. doi: 10.1128/JB.01392-12
55. Fletcher KE, Ritalahti KM, Pennell KD, Takamizawa K, and Loffler FEJ. Resolution of Culture Clostridium bifermentans DPH-1 into Two Populations, a Clostridium sp. and Tetrachloroethene-Dechlorinating Desulfitobacterium hafniense Strain JH1. Appl Environ Microbiol. (2008) 74:6141–3. doi: 10.1128/AEM.00994-08
56. Li XC, Li XY, Jin HJ, Wang JJ, Yu L, Yan J, et al. Comparative proteogenomics reveals ecological and evolutionary insights into the organohalide-respiring Dehalobacter restrictus strain T. Appl Environ Microbiol. (2025) 91:e01719-24. doi: 10.1128/aem.01719-24
Keywords: tetrachloroethylene, sodium lactate, electron shuttles, biogeochemical elements, reductive dechlorination
Citation: Jiang X, He S, Guo A, Yu H, Xu Y, Li Q and Xiu Z (2025) Electron transfer-mediated enhancement of microbial reductive dechlorination of tetrachloroethylene and its impacts on key soil biogeochemical elements. Front. Soil Sci. 5:1636524. doi: 10.3389/fsoil.2025.1636524
Received: 29 May 2025; Accepted: 12 August 2025;
Published: 29 August 2025.
Edited by:
Tanvir Shahzad, Government College University, Faisalabad, PakistanReviewed by:
Shangwei Zhang, Beijing Normal University, ChinaEdgardo I. Valenzuela, University of Tübingen, Germany
Copyright © 2025 Jiang, He, Guo, Yu, Xu, Li and Xiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zongming Xiu, eGl1em9uZ21pbmdAMTYzLmNvbQ==
 Xinrui Jiang
Xinrui Jiang Yan Xu
Yan Xu Zongming Xiu
Zongming Xiu